Abstract
Background.
Treatments for cognitive dysfunction in neuropsychiatric conditions are urgently needed. Cognitive training and transcranial direct current stimulation (tDCS) hold promise, and there is growing interest in combined or multi-modal treatments though studies to date have small samples and inconsistent results.
Methods.
A systematic review and meta-analysis was completed. Retained studies included cognitive training combined with active or sham tDCS in a neuropsychiatric population and reported a post-treatment cognitive outcome. Meta-analyses included effect sizes comparing cognitive training + active tDCS and cognitive training + sham tDCS in five cognitive domains. Risk of bias in included studies and across studies were explored.
Results.
Fifteen studies were included; ten in neurodegenerative disorders and five in psychiatric disorders (n=629). There were several tDCS montages though two-thirds of studies placed the anode over left dorsolateral prefrontal cortex. A wide variety of cognitive training types and outcome measures were reported. There was a small, statistically significant effect of combined treatment on measures of attention/working memory, as well as small and non-statistically significant effects favoring combined treatment on global cognition and language. There was no evidence of bias in individual studies, but some evidence of non-reporting or small-study bias across studies.
Conclusions.
These results may provide preliminary support for the efficacy of combined cognitive training and tDCS on measures of attention/working memory. More data are needed, particularly via studies that explicitly align the cognitive ability of interest, stimulation target, training type, and outcome measures.
Keywords: brain stimulation, neuromodulation, cognitive rehabilitation, cognition, dementia, schizophrenia
Introduction
Cognitive impairment is a major characteristic of many neuropsychiatric disorders, interfering with diverse aspects of daily functioning and contributing to chronic disability (e.g., 1-3). While pharmacological agents have shown minimal benefit in improving cognition to date (e.g., 4,5), non-pharmacological or behavioral interventions have been developed and tested in neuropsychiatric disorders (e.g., mild cognitive impairment and dementia, Parkinson’s disease, schizophrenia, major depression, among others). Theoretically, such interventions capitalize on neuroplastic change though the distinct approach varies. Some focus on “drill and practice” or rehearsal-based cognitive exercises (e.g., 6,7), some emphasize internal compensatory approaches or mnemonic strategies that may strengthen neural networks (e.g., 8), some feature external compensatory aids and environmental modifications as ‘work-arounds’ for everyday cognitive difficulties (e.g., 9), and many incorporate multiple approaches (for comprehensive reviews see 10-17). The empirical evidence base for these treatments is promising but modest, with effect sizes in the small to medium range on cognitive outcome variables and uncertainty regarding durability and generalization (7,9,16,18,19).
Given these modest effects, recent work has augmented cognitive training with an add-on treatment using transcranial direct current stimulation (tDCS), a cost effective, safe, and easy to administer technology (20,21). An exhaustive review of tDCS is outside the scope of this paper (see for example, 22-26); briefly, laboratory studies show that the transcranial application of weak electrical currents induces intracerebral current flow sufficient to alter neuronal activity and behavior (24; but see also 27). Particularly relevant for cognitive training, tDCS has been shown to modulate cortical excitability in electrophysiological studies, and there is some indication that tDCS can improve a variety of cognitive functions in both healthy participants and those with neuropsychiatric conditions (28-31). One recent expert review of the therapeutic efficacy of tDCS in neurological and psychiatric disorders concluded that tDCS may be effective for depression, Parkinson’s disease, epilepsy, and schizophrenia, among others (32; but see also 33) The evidence in mild cognitive impairment and dementia of the Alzheimer’s type is more mixed (28,34-38). Although tDCS may be a viable treatment for cognitive dysfunction, much work remains given small sample sizes and heterogeneity in populations, stimulation parameters, and outcomes of interest.
Given the need for robust treatments for cognitive dysfunction in neuropsychiatric conditions, there is growing interest in whether combined or multi-modal treatments can enhance benefit through additive or synergistic effects. Toward this end, a recent literature describing the combination of tDCS with cognitive training has emerged, though samples are small and power is limited. We therefore completed a systematic review and meta-analysis to provide a preliminary snapshot of the field. Specifically, the key comparison of interest was cognitive training plus active tDCS versus cognitive training plus sham tDCS among those with well-described cognitive impairments (e.g., schizophrenia, depression, mild cognitive impairment, dementia). To reduce heterogeneity in our sample, we elected to exclude neuropsychiatric syndromes with focal lesions, such as stroke and traumatic brain injury.
Methods and Materials
This review and meta-analysis was conducted in accordance with the Preferred Reporting for Systematic Reviews and Meta-Analyses (PRISMA) statement (39,40). Detailed eligibility criteria are outlined in the supplement.
Search Strategy and Study Selection
Search and selection procedures are fully described in the supplementary materials. Briefly, Ovid Medline, PsycINFO, Scopus, CINAHL, and Cochrane Central databases were systematically searched, and retained studies (1) were primary research, (2) included human participants, (3) focused on a ‘neuropsychiatric’ population with primary psychiatric disorders or neurodegenerative conditions (based on clinical phenotype with or without biomarker/pathology confirmation), (4) administered a combined intervention that included both tDCS and cognitive training, and (5) were published in English.
Data Extraction and Items
Data were extracted by the lead author, in consultation with co-authors to arrive at consensus nominations for principal data items. These included cognitive outcomes measured at post-treatment for both intervention and sham-control groups. If the data needed to compute an effect size were not reported in the published text, the corresponding authors were contacted and invited to provide their data directly to the lead author; three were requested and all were received.
Statistical Analysis
The Cochrane Risk of Bias tool for randomized (n=13) and crossover (n=2) studies evaluated risk of bias in individual studies (42). See supplementary materials for details. Effect sizes were calculated using Review Manager 5.4 (43) from means and standard deviations for each outcome variable; for all analyses, comparison groups included those who received cognitive training with active brain stimulation and those who received cognitive training with sham stimulation. Standard guidelines were used to interpret the magnitude of effect, where an effect size of 0.20 represents a small effect, 0.50 represents a medium effect, and 0.80 represents a large effect (44). The standardized difference in means was used as the effect size; for the current analyses, a positive effect size (greater than 0) favored the active stimulation condition. The 95% confidence intervals are also presented. The meta-analysis was also conducted using Review Manager 5.4, with a random effects model applied given anticipated heterogeneity among the study effect sizes. Heterogeneity was evaluated using the chi-square heterogeneity statistic I2 and by manual inspection of forest plots. Following Cochrane procedures, the following interpretive guidelines for heterogeneity as indexed by I2 were applied: 0%-40% might not be important, 30%-60% may represent moderate heterogeneity, 50%-90% may represent substantial heterogeneity, and 75%-100% represented considerable heterogeneity (45). For evidence of between-study heterogeneity (I2 ≥ 30), fixed-effect and random-effects estimates were compared for similarity (indicating that small-study effects likely had little effect on the intervention effect estimate) or dissimilarity (perhaps suggesting that the results of smaller studies were disseminated selectively) (46).
Risk of Bias across Studies
The extent of missing results was explored via visual inspection of funnel plots, where the effect estimates were plotted on the horizontal axis against the standard error of the effect estimate on the vertical axis. Although this method is vulnerable to subjectivity, particularly with few studies, there was an insufficient number of studies in each meta-analysis to use statistical tests for funnel plot asymmetry (e.g., Egger test).
Results
Search Results
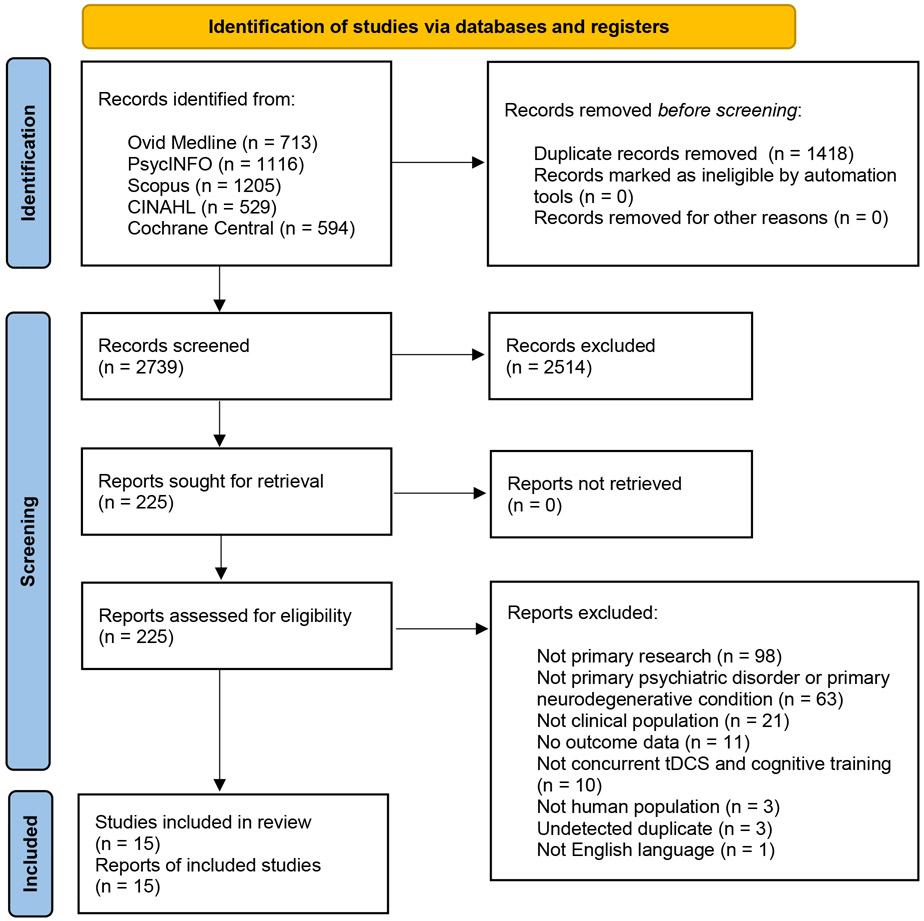
The search strategies retrieved a total of 4,157 results and after removing duplicates 2,739 records remained for screening. At the title and abstract level 2,514 records were excluded and 225 were advanced for full text review. The PRISMA flow diagram (Figure 1) documents the exclusion rationale for the 210 articles excluded during full text review. The study selection process yielded 15 studies that met the inclusion criteria.
Figure 1.
PRISMA 2020 Flow Diagram
Study Characteristics
Details of the 15 included studies are summarized in Table 1. Briefly, ten of the studies involved primary neurodegenerative disorders (47-56), and five included primary psychiatric disorders (57-61). Twelve studies used a design including cognitive training with randomization to active or sham tDCS (of which two were crossover studies with at least two months between tDCS conditions), and three included additional treatment comparison groups (e.g., tDCS alone or tDCS with ‘control’ training). tDCS was most often delivered to left dorsolateral prefrontal cortex (F3 according to the international 10-20 EEG system) at 2mA (range 1mA - 2mA) for 20 minutes (range 20 minutes – 30 minutes ). Sham tDCS typically included brief ramp up and ramp down time to mimic active stimulation, and ranged from 30 seconds to 2 minutes total. The number of tDCS sessions varied widely from 2 to 28, mostly over 2-4 weeks and up to 14 weeks. Most studies used computerized cognitive training administered concurrently with tDCS and specified outcome variables distinct from but in the same domain as training tasks, though there was a wide array of training programs and neuropsychological outcome measures.
Table 1.
Study Characteristics and Results
| Authors | Year | Sample size |
Population | Design | tDCS ‘dose’ |
tDCS montage |
tDCS intensity & duration |
Cognitive training type |
CT and tDCS timing |
Primary outcome |
Secondary outcome(s) |
Main result(s) reported |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cotelli et al (52) | 2014a | 36 | AD | atDCS+CT, stDCS+CT, atDCS+motor training | 10 sessions over 2 weeks | anode left DLPFC, cathode right deltoid | 2mA, 25 min | Individualized computerized memory training (selected from baseline performance on FNAT) | Fully concurrent | FNAT | Picture Naming Task; BADA; Rivermead Memory; RAVLT; Rey copy; Trails | Improvement in FNAT, selectively for trained stimuli, with individualized CT with or without tDCS. No effects on other outcomes. |
| Segrave et al (59) | 2014 | 27 | MDD | atDCS+CT, stDCS+CT, atDCS+sCT | 5 sessions over 1 week | anode F3, cathode F8 | 2mA, 24 min | Computerized (modified Wells Attentional Training paradigm and adaptive Paced Serial Addition Task) | Fully concurrent | MADRS | Affective and non-affective 2-back | No difference in cognitive control in active vs sham |
| Cotelli et al (47) | 2014b | 16 | PPA | CT plus active or sham tDCS | 10 sessions over 2 weeks | anode left DLPFC (BA 8/9), cathode right arm | 2mA, 25 min | Computerized (ICAT) | Fully concurrent | AAT, BAAD | Experimental naming; communication & functional abilities | Untrained naming improved in active versus sham |
| Vanderhasselt et al (60) | 2015 | 33 | MDD | CT plus active or sham tDCS | 10 sessions over 2 weeks | anode F3, cathode F4 | 2mA, 30 min | Computerized (modified PASAT with adaptive ISI) | Partially concurrent (CT during last 15 min of stimulation) | Ruminative Responses Scale | PASAT | Both CT alone and combined with tDCS reduced depressive brooding (i.e., no added effect from tDCS) |
| Biundo et al (48) | 2015 | 24 | PD-MCI | CT plus active or sham tDCS | 16 sessions over 4 weeks | anode left DLPFC, cathode right supraorbital | 2mA, 20 min | Computerized (Rehacom) | Fully concurrent | RBANS | Motor | Active worse than sham on coding. No other differences. |
| Nienow et al (57) | 2016 | 10 | SZ | CT plus active or sham tDCS | 28 sessions over 14 weeks | anode F3, cathode right supraorbital | 1mA, 20 min | Computerized (CogRehab, BrainTrain, and word/picture n-back tasks) | Partially concurrent (stimulation during first 20 minutes of CT for 2 of 3 sessions per week) | Non-trained word 2-back, non-trained picture 2-back, MCCB composite | BPRS | Improvement on word & picture 2-back in active versus sham. No effect on MCCB composite or BPRS. |
| Orlov et al (58) | 2017 | 49 | SZ | CT plus active or sham tDCS | 2 sessions total, day 1 and day 14 | anode F3, cathode Fp2 | 2mA, 30 min | Computerized (letter n-back, language learning) | Partially concurrent (stimulation during 2 of 8 training sessions) | Working memory & implicit learning | CogState performance | No same-day benefit of tDCS; improved working memory in active versus sham next-day. No differences in implicit learning or generalization. |
| Roncero et al (56) | 2017 | 10 | anomic AD or FTD | CT plus active or sham tDCS (crossover with 2-mos between) | 10 daily sessions of both active and sham | anode P3, cathode right fronto-orbital | 2mA, 30 min | Picture naming using items from a standardized set | Fully concurrent | Spontaneous naming (trained and untrained items) | Digit span, verbal fluency, MoCA, MMSE | Improvement on trained items, untrained items, and digit span in active versus sham |
| Manenti et al (55) | 2018 | 22 | PD | CT plus active or sham tDCS | 10 sessions over 2 weeks | anode F3, cathode right supraorbital | 2mA, 25 min | Computerized (BrainHQ) | Fully concurrent | BDI and cognition | Motor & other symptoms | Cognitive improvement in both groups; greater change in phonemic fluency for active vs sham |
| Inagawa et al (54) | 2019 | 20 | Mild or Major NCD | CT plus active or sham tDCS | 10 sessions over 5 days | anode F3, cathode Fp2 | 2mA, 20min | Paper & pencil calculation and reading tasks | Fully concurrent | Attrition | MMSE, ADAS-Cog, FAB, CDR | No improvement in MMSE or ADAS-Cog for active vs sham |
| Das et al (51) | 2019 | 22 | MCI | CT plus active or sham tDCS | 8 sessions over 4 weeks | anode IFG, cathode contralateral shoulder | 2mA, 20min immediately before CT session | SMART aka 'gist reasoning training' | No overlap (tDCS immediately prior to CT) | Cognition | N/A | Cognitive gains in sham but not active. Irrespective of stimulation type, some gains over time |
| Lu et al (53) | 2019 | 201 | NCD-AD | atDCS+CT, stDCS+CT, atDCS | 12 sessions over 4 weeks | anode T3, cathode contralateral upper limb | 2mA, 20min | Computerized (Adaptive N-back) | Fully concurrent | Global cognition & N-back | Domain-specific cognition | Improvement in delayed recall and working memory in active vs sham |
| Martin et al (50) | 2019 | 68 | aMCI | CT plus active or sham tDCS | 15 sessions over 5 weeks | anode F3, cathode F8 | 2mA, 30min | Computerized (COGPACK) | Partially concurrent | CVLT-II | Other cognitive, functional, symptom | No differences between active and sham |
| de Sousa et al (49) | 2020 | 16 | MCI | CT plus active or sham tDCS (crossover with 3-mos between) | 3-day training 'paired' with either active or sham tDCS | anode T6, cathode left supraorbital | 1mA, 20 min | Computerized (visuospatial memory training) | Fully concurrent | Training task performance immediately after training | One-month training task performance | Improvement in active vs sham; reached similar improvement rates as healthy participants who did sham or active |
| Xu et al (61) | 2021 | 75 (women only) | SUD (MA) | atDCS+CT, stDCS+CT, TAU | 20 sessions over 4 weeks | anode F4, cathode F3 | 1.5mA, 20 min | Computerized (CCAT) | Fully concurrent | Cue-induced craving | DPT, SST, DDT, Cogstate shopping list task, Cogstate 2-back, Cogstate SEC | Improvement in 2-back accuracy in active vs sham |
Abbreviations: AAT (Aachener Aphasie Test); AD (Alzheimer’s Disease); ADAS-Cog (Alzheimer’s Disease Assessment Scale – Cognitive); BA (Brodmann Area); BAAD/BADA (Battery for Analysis of Aphasic Deficits); BDI (Beck Depression Inventory); BPRS (Brief Psychiatric Rating Scale); CCAT (Computerized Cognitive Addiction Therapy); CDR (Clinical Dementia Rating); Cogstate SEC (Cogstate Social Emotional Cognition task); CT (Cognitive Training); sCT (sham Cognitive Training); CVLT-II (California Verbal Learning Test, Second Edition); DDT (Delay-Discounting Task); DLPFC (dorsolateral prefrontal cortex); DPT (Dot Probe Task); FAB (Frontal Assessment Battery); FNAT (Face-Name Association Memory Task); FTD (Frontotemporal dementia); ICAT (Individualized Computerized Anomia Training); IFG (inferior frontal gyrus); ISI (interstimulus interval); MA (methamphetamine); MADRS (Montgomery-Asberg Depression Rating Scale); MCCB (MATRICS Consensus Cognitive Battery); MDD (Major Depressive Disorder); MCI (Mild Cognitive Impairment); aMCI (amnestic Mild Cognitive Impairment); MMSE (Mini Mental State Examination); MoCA (Montreal Cognitive Assessment); NCD (Neurocognitive Disorder); NCD-AD (Mild Neurocognitive Disorder due to AD); PASAT (Paced Auditory Serial Addition Test); PD (Parkinson’s Disease); PD-MCI (Mild Cognitive Impairment in Parkinson’s Disease); PPA (Primary Progressive Aphasia); RAVLT (Rey Auditory Verbal Learning Test); RBANS (Repeatable Battery for the Assessment of Neuropsychological Status); SMART (Strategic Memory and Advanced Reasoning Training); SST (Stop Signal Task); SUD (Substance Use Disorder); SZ (Schizophrenia); TAU (treatment as usual); atDCS (anodal transcranial direct current stimulation [tDCS]); stDCS (sham tDCS).
Risk of Bias within Studies
Overall, the 15 individual studies consistently yielded low risk of bias. Most reports described adequate randomization/allocation procedures, participant and rater blinding, and appropriate outcome measurement.
Risk of Bias due to Missing Results
Though limited by the small number of included studies, visual inspection of the funnel plots suggested asymmetry across outcomes (Supplemental Figure 1). In particular, for global cognition, attention/working memory, and language, data points were missing from the lower left quadrants where studies with larger standard error (i.e., smaller samples) and negative results would appear. For episodic memory and executive functioning, the funnel plots showed missing data points in the lower right quadrants, suggesting instead a lack of studies with small samples and positive results. This asymmetry indicates some evidence of non-reporting bias, but may also reflect broader “small-study effects” where intervention effects in smaller studies differ from those estimated in larger studies due to lower methodological quality, true heterogeneity, statistical artifact, and/or chance (46).
Meta-analytic Results
Given the range of outcome variables reported in the reviewed studies, we elected to group outcomes by cognitive domain and conduct separate meta-analyses for any domain with at least three sources of data. Domains considered included ‘global’ cognition (e.g., a broad cognitive screening instrument or summary score derived from a standardized battery), processing speed, attention/working memory, episodic memory, executive functioning, language, and visuospatial functioning. Processing speed and visuospatial variables were reported in only two studies each and were excluded from further analyses. Domains and variables for each study are included in Table 2. Because there were insufficient numbers of studies to separately analyze primary psychiatric and primary neurodegenerative disorders, they were combined. The domain of attention/working memory included five studies in each, so these were analyzed together and then separately, in an exploratory subgroup analysis.
Table 2.
Domains and Outcome Measures
| Global cognition | Attention/Working Memory |
Memory | Executive Functioning |
Language | |
|---|---|---|---|---|---|
| Cotelli et al., 2014a (52) | RAVLT delayed recall | Trails B (reversed) | PNT objects correct | ||
| Segrave et al., 2014 (59) | 2-back task % accuracy, neutral | ||||
| Cotelli et al., 2014b (47) | AAT accuracy | ||||
| Vanderhasselt et al., 2015 (60) | PASAT median ISI | ||||
| Biundo et al., 2015 (48) | RBANS total change | RBANS digit span change score | RBANS list recall change score | RBANS language index change score | |
| Nienow et al., 2016 (57) | MCCB composite | Word 2-back d-prime | |||
| Orlov et al., 2017 (58) | N-back d-prime | ||||
| Roncero et al., 2017 (56) | Total digit span | Raw accuracy on trained items | |||
| Manenti et al., 2018 (55) | Mini Mental Parkinson | Forward digit span | RAVLT delayed recall | Trails B (reversed) | Semantic verbal fluency |
| Inagawa et al., 2019 (54) | MMSE change score | FAB change score | |||
| Das et al., 2019 (51) | TOSL episodic memory | DKEFS CWI | |||
| Lu et al., 2019 (53) | ADAS-Cog (reversed) | Forward digit span | Delayed recall | Trails B (reversed) | CVFT |
| Martin et al., 2019 (50) | Mean reaction time (reversed) | CVLT-II LDFR z-score | |||
| de Sousa et al., 2020 (49) | OLM | ||||
| Xu et al., 2021 (61) | 2-back | ISLT total correct | SST Reaction Time (reversed) | ||
| # of studies in domain | 5/15 | 10/15 | 8/15 | 6/15 | 6/15 |
Abbreviations: AAT (Aachener Aphasie Test); ADAS-Cog (Alzheimer’s Disease Assessment Scale – Cognitive); CVFT (category verbal fluency test), CVLT-II (California Verbal Learning Test, Second Edition); DKEFS (Delis-Kaplan Executive Functioning System); FAB (Frontal Assessment Battery); ISLT (International Shopping List Task); LDFR (Long Delay Free Recall); MCCB (MATRICS Consensus Cognitive Battery); MMSE (Mini Mental State Examination); OLM (Object-Location Memory); PASAT (Paced Auditory Serial Addition Test); PNT (Picture Naming Task); RAVLT (Rey Auditory Verbal Learning Test); RBANS (Repeatable Battery for the Assessment of Neuropsychological Status); SST (Stop Signal Task); TOSL (Test of Strategic Learning).
‘Global’ cognition (5 studies; n=202).
Study characteristics.
Four of the five studies with a measure of global cognition were in neurodegenerative disorders and one was in schizophrenia. The majority had the anode placed at F3 and the cathode at right supraorbital, and included general computerized cognitive training.
Analyses.
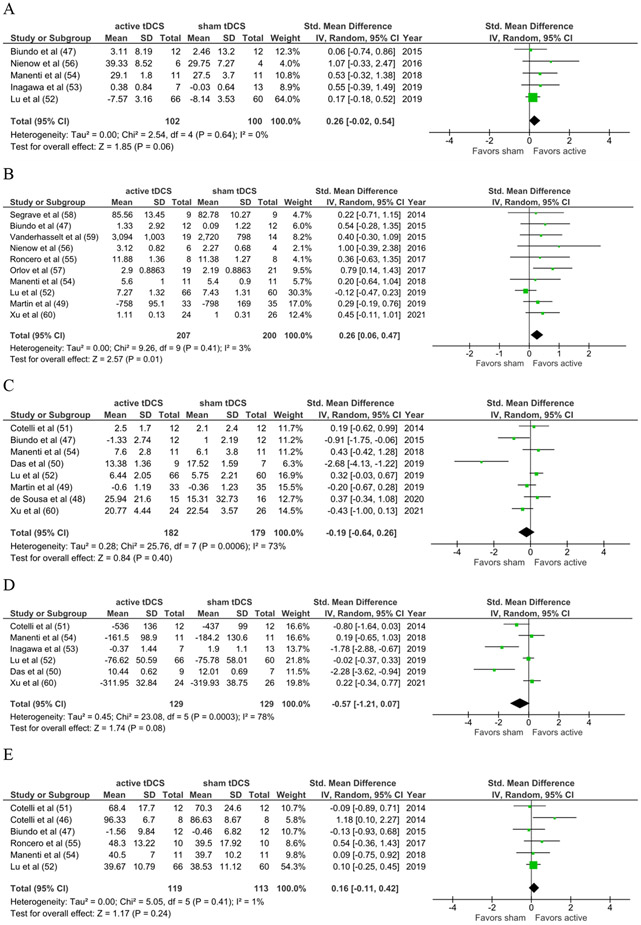
The effect for active versus sham tDCS combined with cognitive training on global/screening measures of cognition just missed the 0.05 threshold for statistical significance (SMD=0.26; 95%CI −0.02, 0.54; z=1.85; p=0.06; Figure 2A).
Figure 2.
Meta-Analytic Results
Figure 2A. Global Cognition
Figure 2B. Attention & Working Memory
Figure 2C. Episodic Memory
Figure 2D. Executive Functioning
Figure 2E. Language
Attention/working memory (10 studies; n=407).
Study characteristics.
Five studies were in neurodegenerative disorders and five were in psychiatric disorders. Seven of the 10 had the anode placed at F3 and cathode contralaterally (F4, F8, Fp2, right supraorbital). Seven studies included targeted working memory training.
Analyses.
There was a statistically significant effect favoring the combined intervention on measures of attention/working memory for all studies combined (SMD=0.26; 95%CI 0.06, 0.47; z=2.57; p=0.01; Figure 2B). Exploratory subgroup analysis revealed a statistically significant effect favoring active versus sham tDCS for primary psychiatric disorders alone (n=151; SMD=0.53; 95%CI 0.20, 0.85; z=3.14; p=0.002), but not neurodegenerative disorders (n=248; SMD=0.10; 95%CI −0.14, 0.35; z=0.83; p=0.41).
Episodic memory (8 studies; n=361).
Study characteristics.
Seven studies were in neurodegenerative disorders and one was in a psychiatric disorder. Half had the anode placed at F3, with one extracephalic cathode placement (right deltoid), two right supraorbital, and the other F8. Three included targeted memory training (e.g., face-name association memory, object-location memory).
Analyses.
There was no statistically significant effect of the combined intervention on measures of episodic memory (SMD=−0.19; 95%CI −0.64, 0.26; z=0.84; p=0.40; Figure 2C).
Executive functioning (6 studies; n=258).
Study characteristics.
Five studies were in neurodegenerative disorders and one was in a psychiatric disorder. Three included the anode at F3, with one cathode on right deltoid, one right supraorbital, and one Fp2. Three training types were targeted (memory and working memory) and three were more general.
Analyses.
There was no statistically significant effect of cognitive training combined with active versus sham tDCS on measures of executive functioning (SMD=−0.57, 95%CI −1.21, 0.07; z=1.74; p=0.08; Figure 2D).
Language (6 studies; n=232).
Study characteristics.
All six studies were in neurodegenerative disorders. Four studies had anode placed at F3, with cathode on right deltoid, right arm, or right supraorbital. Two included targeted language (naming) training.
Analyses.
There was no statistically significant effect of the combined intervention on measures of language (SMD=0.16; 95%CI −0.11, 0.42; z=1.17; p=0.24; Figure 2E).
Omnibus analysis (all measures, all studies).
To examine the overall effect of active versus sham stimulation, the effect sizes for each outcome variable in a study were averaged to ‘collapse’ across cognitive domains and entered into an omnibus meta-analysis. The resulting effect size was small and not statistically significant (SMD=0.17; 95%CI −0.11, 0.45; z=1.21; p=0.23; Supplemental Figure 2), with evidence of substantial heterogeneity (I2=52%).
Heterogeneity was not statistically significant and quite low for global cognition (I2=0%), attention/working memory (I2=3%), and language (I2=1%), but was statistically significant and in the substantial to considerable range for episodic memory (I2=73%) and executive functioning (I2=78%). However, the fixed-effect results were similar to the random-effects results for both episodic memory (fixed effect SMD=−0.02; 95%CI −0.24, 0.19; z=0.22; p=0.82) and executive functioning (fixed effect SMD=−0.19, 95%CI −0.45, 0.06; z=1.50; p=0.13), suggesting that small-study effects probably had little effect on the intervention effect estimate.
Discussion
Summary of Evidence
Overall, these findings provide preliminary support for the effect of cognitive training and active tDCS on measures of attention and working memory at post-treatment, where there was a small but statistically significant effect favoring the combined intervention. For tasks of global cognition and language, as well as the overall effect collapsing across cognitive domains, the small effect sizes were in the direction favoring active stimulation though they did not reach statistical significance. There were also non-significant effects favoring cognitive training and sham stimulation on measures of episodic memory and executive functioning, with evidence of substantial heterogeneity between studies. These effect sizes (ES) are on the lower end yet generally within the ranges of those found in prior studies of cognitive training (ES 0.20-0.47, with most in the 0.4 range) (7,9,16,18,19) and tDCS in clinical populations (ES 0.20-1.20, with most in the 0.3 to 0.4 range) (29,32,34,36-38). Interestingly, they are also modestly lower yet broadly in line with a number of common treatments in general medicine and psychiatry (e.g., statins and aspirin for prevention of major vascular events, medications for schizophrenia, major depression, and Alzheimer’s disease) (62), though it is certainly premature to speculate about the clinical significance of these findings.
Considering that two-thirds of included studies administered stimulation with the anode placed over left dorsolateral prefrontal cortex (DLPFC) (e.g., F3, Brodmann area 8/9) -- an area often implicated in working memory – these results provide some evidence of functional specificity of these anatomical regions. Though speculative, there could be greater expectation of a benefit of tDCS targeting left DLPFC on tasks of attention/working memory, rather than other cognitive abilities like language or episodic memory that are not strongly mediated by these regions.
Considerations and limitations of included data
Disease types and baseline cognition/symptom severity.
Though this review and meta-analysis was deliberately intended to survey studies in individuals with neuropsychiatric disorders with cognitive impairments, there is of course heterogeneity among these disease types. With the exception of attention/working memory, there were not enough included studies to separately analyze cognitive outcomes for primary neurodegenerative disorders and primary psychiatric disorders. It could be the case that people with neurodegenerative disorders do not benefit from combination therapy for attention/working memory, but we would not make this conclusion from our data with the small number of studies available for analysis and the extent of the variation in electrode placement, training type, and outcome measurement. It remains an empirical question, therefore, for whom cognitive training combined with tDCS is most effective and why.
Another source of sample heterogeneity is in baseline cognitive ability. Although all of these neuropsychiatric conditions are strongly associated with impaired cognition, and in many cases the diagnostic criteria for study entry involved cognitive impairment by definition, the degree of deficit prior to treatment was not pre-defined or well characterized in the individual studies. Accordingly, there could have been participants with very little room for cognitive improvement before even receiving treatment, which could have suppressed a treatment effect. Similarly, severity of other baseline symptoms like depression or psychosis could have directly or indirectly (via interaction with cognitive functioning, for example) affected treatment outcomes.
Types of training.
In addition, the wide range of cognitive training types introduces a major source of heterogeneity in the data and may obscure the findings. An outstanding question is whether tDCS may be more likely to benefit rehearsal-based interventions that recruit a narrower range of brain functions, versus targeted internal compensatory strategy training, versus broader external strategy training, which each require successively larger domains of cognitive functioning.
Stimulation parameters.
The range of stimulation strength was restricted and did not exceed 2mA in any studies. While these parameters are commonly accepted in research samples, they are largely based on presumed safety considerations rather than scientific rationale or established evidence (e.g., 63). However, there is neither safety (21) nor tolerability data to suggest that higher scalp-based amplitudes are problematic. For example, high-definition tDCS was well-tolerated and safe in older adults at 3mA (64). Similarly, all of the included studies used conventional pad-based tDCS with large electrodes (e.g., 35cm2) where the current delivery is diffuse (65,66) and may penetrate poorly to intended brain regions due to a variety of underlying anatomy/tissue factors (low conductivity of skull bone and surrounding tissue, cortical morphology, hair thickness, sweat) (67,68). Two of the included studies mentioned some kind of neuro-navigation assistance to identify the target anatomical area: one described an “infrared-guided neuro-navigation system” (48) and the other used the participant’s MRI and a TMS neural-navigation device via the Brainsight software package (56). Otherwise researchers relied on traditional standardized methods of head measurement to determine electrode placement. It is therefore difficult to confirm whether the intended target was the actual target and whether there was alignment between the cerebral target, cognitive domain, and cognitive training paradigm. Moreover, although the pattern of delivered current between the large pads is the active ingredient, there were a number of different montages and cathode placements, which strongly influence the electrical fields applied. Similarly the inhibitory/excitatory effects of most configurations are not well understood, as they do not readily correspond to the notion that “anodal” stimulation is always excitatory and “cathodal” stimulation is always inhibitory (69). Indeed, cathodal tDCS has been shown to have nonlinear modulatory effects depending on scalp-based intensity and duration (70), which accordingly could facilitate, impede, or have no effect on the desired outcome. Finally, whether there is any relationship between tDCS timing (before, during, or after training) or ‘dose’ (e.g., frequency, intensity, duration, total number of sessions) and cognitive outcomes is not well understood, and represent important factors to consider in future work. These limitations in stimulation type and intensity may lead to false-negative errors, where there may be a ‘true’ effect of active stimulation but greater intensity and/or more focal delivery is necessary to reveal it. In more recent work, focality has been improved with high definition electrodes (71,72), which may reveal greater between-group differences on cognitive outcomes in future studies, though there are complex relationships between area of stimulation, focality, and intensity in both conventional and high-definition approaches that remain under investigation.
Outcome measures.
There was a wide variety of outcome measures in the included studies. This is an ongoing challenge for the field, as there is little consensus or standardization in cognitive measurement, which leads to difficulty comparing effects across studies. This may be especially true for arguably ‘broader’ domains of cognition like executive functioning, memory, or language, where there may be a number of interrelated yet theoretically distinct sub-functions of interest (e.g., abstract reasoning, novel problem-solving, set-shifting, and response inhibition are all commonly considered under the term executive functioning). Indeed, the current meta-analyses indicated significant heterogeneity for executive functioning and episodic memory, which may have undermined the ability to show effects.
Study design.
Although all included studies reported comparisons between participants who received active or sham tDCS in addition to cognitive training, only two publications included an active tDCS group without cognitive training in the study design. Unfortunately, this precludes analysis of synergistic effects, or whether a combined intervention is more efficacious than either one alone. This is particularly important as the field moves toward multi-modal interventions and increasingly personalized medicine, where treatment decision-making is guided by the incremental benefit of two or more treatments rather than simply a ‘mash-up’ of interventions that may or may not work well together.
Additionally, most studies had no clear description or unification of the pathway from the cognitive construct of interest to the stimulation target/montage, to the cognitive training type, to the primary outcome measure. In many cases, a broad neuropsychological battery was included with no specific hypotheses about what was expected to change versus what wasn’t. This misalignment between intervention and outcome limits the interpretability of findings, as these results may provide discriminant validity for a combined intervention (i.e., an effect on attention/working memory when most studies ‘targeted’ left DLPFC), but they do not demonstrate a lack of efficacy because the domain was often out of alignment with the intervention method and target.
Transfer and real-world functioning.
As an initial step, it is clearly important to show the effect of tDCS on a training task itself to demonstrate proof-of-concept. Ultimately, however, for these to become viable treatments there needs to be evidence of benefit on increasingly distal outcomes, including perhaps more ecologically valid cognitive tasks but most importantly to real-world functioning. Many studies included cognitive outcome measures other than trained tasks, enabling some understanding of transfer, but only four included quality of life questionnaires and only two included measures of everyday functioning.
Considerations and limitations of this meta-analysis
This meta-analysis has several limitations. For example, although there was consensus among the study team regarding outcome cognitive domains and assignment of variables to those domains, this remains a source of some judgment and subjectivity. As mentioned, there are cognitive domains that are understood to encapsulate a number of subcomponent skills (borrowing the earlier example of executive functioning, for instance), though these are not universally agreed upon and the presumed divisions between cognitive domains are arbitrary. We acknowledge that the way we grouped outcomes and variables is predicated on these ambiguous distinctions, and while suitable it is an imperfect approach. We also examined post-treatment performance only, which does not allow for the possibility of consolidation of treatment gains over time; future analyses should consider follow-up data to better evaluate durability of treatment gains, loss of treatment gains, or late emergence of treatment benefit. There were also few studies overall of combined cognitive training and tDCS interventions in neuropsychiatric samples, highlighting the preliminary nature of these findings and the anticipated growth in publications in the coming years. Finally, given these limited data and some evidence of heterogeneity, it is premature to make conclusions about the efficacy of these combined interventions in domains other than attention/working memory.
Unanswered questions and topics for future research
Following from the considerations above, there are a number of remaining questions and future directions for this work. Future research may benefit from more systematic evaluation of the dose-response relationship between stimulation strength and outcome, use of more focal stimulation techniques (e.g., high-definition or HD-tDCS), and 2x2 factorial designs where possible so that some participants receive one treatment or the other, some receive both, and some receive neither. In addition, ‘futility designs’ or alternative statistical methods where the null rather than the alternative hypothesis assumes a benefit of treatment compared to control may be an appealing option; rather than demonstrating efficacy, the futility design identifies treatments that do not warrant further investigation in superiority trials (73). There is also ongoing debate about the optimal timing or sequencing of ‘combined’ interventions, and whether they should be done fully concurrently, overlapping at the beginning, at the end, or not at all. Future research will benefit from systematically investigating these timing considerations to enhance benefit.
Conclusions
This meta-analysis provides a preliminary snapshot of a rapidly growing field; the present findings broadly support the use of active tDCS during cognitive training to improve attention/working memory among individuals with neuropsychiatric disorders. Although based on these results there is insufficient evidence to conclude whether or not a combined intervention improves cognitive abilities in other domains or clinical populations, there are abundant opportunities to align methodologies and outcomes to advance scientific understanding and deploy effective treatments in clinical settings.
Supplementary Material
Acknowledgments:
The authors would like to acknowledge Maya Akhoury for her contributions to title/abstract screening. This work was supported by the University of Michigan Eisenberg Family Depression Center. The funder had no role in study design, analyses and interpretation of data, report writing, or decision to submit the manuscript for publication. An abstract presenting a portion of this work (systematic review only) was presented at the annual meeting of the International Neuropsychological Society in February 2022.
Footnotes
Disclosures and Conflict of Interest:
Dr. Burton reported no biomedical financial interests or potential conflicts of interest. Dr. Garnett reported no biomedical financial interests or potential conflicts of interest. Ms. Capellari reported no biomedical financial interests or potential conflicts of interest. Dr. Chang reported no biomedical financial interests or potential conflicts of interest. Dr. Tso reported no biomedical financial interests or potential conflicts of interest. Dr. Hampstead reports research funding from the Department of Veterans Affairs, the Department of Defense, and the National Institutes of Health; he has received honoraria for research lectures and professional services from Wayne State University, the International Neuropsychological Society, Pennsylvania State University, and the Great Valley Publication Company. Dr. Taylor reports funding from Boehringer-Ingelheim.
References
- 1.Cloutier M, Aigbogun MS, Guerin A, Nitulescu R, Ramanakumar A v, Kamat SA, et al. (2016): The Economic Burden of Schizophrenia in the United States in 2013. J Clin Psychiatry 77: 764–71. [DOI] [PubMed] [Google Scholar]
- 2.Vossius C, Larsen JP, Janvin C, Aarsland D (2011): The economic impact of cognitive impairment in Parkinson’s disease. Movement Disorders 26: 1541–1544. [DOI] [PubMed] [Google Scholar]
- 3.Wong W (2020): Economic Burden of Alzheimer Disease and Managed Care Considerations. American Journal of Managed Care 26: S177. [DOI] [PubMed] [Google Scholar]
- 4.Cummings JL, Tong G, Ballard C (2019): Treatment Combinations for Alzheimer’s Disease: Current and Future Pharmacotherapy Options. Journal of Alzheimer’s Disease 67: 779–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harvey PD, Bowie CR (2012): Cognitive Enhancement in Schizophrenia: Pharmacological and Cognitive Remediation Approaches. 10.1016/j.psc.2012.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prikken M, Konings MJ, Lei WU, Begemann MJH, Sommer IEC (2019): The efficacy of computerized cognitive drill and practice training for patients with a schizophrenia-spectrum disorder: A meta-analysis. Schizophrenia Research 204: 368–374. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H, Huntley J, Bhome R, Holmes B, Cahill J, Gould RL, et al. (2019): Effect of computerised cognitive training on cognitive outcomes in mild cognitive impairment: a systematic review and meta-analysis. BMJ Open 9. 10.1136/bmjopen-2018-027062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hampstead BM, Sathian K, Phillips PA, Amaraneni A, Delaune WR, Stringer AY (2012): Mnemonic Strategy Training Improves Memory for Object Location Associations in Both Healthy Elderly and Patients With Amnestic Mild Cognitive Impairment: A Randomized, Single-Blind Study. Neuropsychology 26: 385–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allott K, Van-Der-El K, Bryce S, Parrish EM, Mcgurk SR, Hetrick S, et al. (2020): Compensatory Interventions for Cognitive Impairments in Psychosis: A Systematic Review and Meta-Analysis. Schizophrenia Bulletin 46: 869–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bahar-Fuchs A, Clare L, Woods B (2013): Cognitive training and cognitive rehabilitation for persons with mild to moderate dementia of the Alzheimer’s or vascular type: a review. Alzheimer’s Research & Therapy 5: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bender A, Spat-Lemus J (2019): Cognitive Training and Rehabilitation in Aging and Dementia. In: Ravdin LD, Katzen HL, editors. Handbook on the Neuropsychology of Aging and Dementia. Springer Nature Switzerland, pp 365–387. [Google Scholar]
- 12.Hampstead BM, Gillis MM, Stringer AY (2014): Cognitive rehabilitation of memory for mild cognitive impairment: A methodological review and model for future research. Journal of the International Neuropsychological Society, vol. 20. Cambridge University Press, pp 135–151. [DOI] [PubMed] [Google Scholar]
- 13.Keshavan MS, Vinogradov S, Rumsey J, Sherrill J, Wagner A, Bar-Haim Y (2014): Cognitive Training in Mental Disorders: Update and Future Directions. Am J Psychiatry 171: 510–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim EJ, Bahk Y-C, Oh H, Lee W-H, Lee JS, Choi K-H (2018): Current Status of Cognitive Remediation for Psychiatric Disorders: A Review. Frontiers in Psychiatry. 10.3389/fpsyt.2018.00461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rabipour S, Raz A (2012, July): Training the brain: Fact and fad in cognitive and behavioral remediation. Brain and Cognition, vol. 79. pp 159–179. [DOI] [PubMed] [Google Scholar]
- 16.Wykes T, Huddy V, Cellard C, McGurk S, Czobor P (2011): A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry 168: 472–485. [DOI] [PubMed] [Google Scholar]
- 17.Kurtz MM (2016): Cognitive Remediation for Psychological Disorders: An Overview. In: Medalia A, Bowie CR, editors. Cognitive Remediation to Improve Functional Outcomes. Oxford University Press, pp 1–23. [Google Scholar]
- 18.Sherman DS, Mauser J, Nuno M, Sherzai D (2017): The Efficacy of Cognitive Intervention in Mild Cognitive Impairment (MCI): a Meta-Analysis of Outcomes on Neuropsychological Measures. Neuropsychology Review 27: 440–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sitzer DI, Twamley EW, Jeste D v. (2006): Cognitive training in Alzheimer’s disease: A meta-analysis of the literature. Acta Psychiatrica Scandinavica 114: 75–90. [DOI] [PubMed] [Google Scholar]
- 20.Antal A, Alekseichuk I, Bikson M, Brockmöller J, Brunoni AR, Chen R, et al. (2017, September 1): Low intensity transcranial electric stimulation: Safety, ethical, legal regulatory and application guidelines. Clinical Neurophysiology, vol. 128. Elsevier Ireland Ltd, pp 1774–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, et al. (2016, September 1): Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain Stimulation, vol. 9. Elsevier Inc., pp 641–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, et al. (2012, July): Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimulation, vol. 5. pp 175–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lefaucheur JP, Wendling F (2019, September 1): Mechanisms of action of tDCS: A brief and practical overview. Neurophysiologie Clinique, vol. 49. Elsevier Masson SAS, pp 269–275. [DOI] [PubMed] [Google Scholar]
- 24.Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. (2008): Transcranial direct current stimulation: State of the art 2008. Brain Stimulation 1: 206–223. [DOI] [PubMed] [Google Scholar]
- 25.Nitsche MA, Paulus W (2011): Transcranial direct current stimulation-update 2011. Restorative Neurology and Neuroscience 29: 463–492. [DOI] [PubMed] [Google Scholar]
- 26.Sudbrack-Oliveira P, Razza LB, Brunoni AR (2021): Non-invasive cortical stimulation: Transcranial direct current stimulation (tDCS). International Review of Neurobiology, vol. 159. Academic Press Inc., pp 1–22. [DOI] [PubMed] [Google Scholar]
- 27.Horvath JC, Forte JD, Carter O (2015, January 1): Evidence that transcranial direct current stimulation (tDCS) generates little-to-no reliable neurophysiologic effect beyond MEP amplitude modulation in healthy human subjects: A systematic review. Neuropsychologia, vol. 66. Elsevier Ltd, pp 213–236. [DOI] [PubMed] [Google Scholar]
- 28.Ciullo V, Spalletta G, Caltagirone C, Banaj N, Vecchio D, Piras F, Piras F (2021, June 1): Transcranial Direct Current Stimulation and Cognition in Neuropsychiatric Disorders: Systematic Review of the Evidence and Future Directions. Neuroscientist, vol. 27. SAGE Publications Inc., pp 285–309. [DOI] [PubMed] [Google Scholar]
- 29.Brunoni AR, Vanderhasselt M-A (2014): Working memory improvement with non-invasive brain stimulation of the dorsolateral prefrontal cortex: A systematic review and meta-analysis. 10.1016/j.bandc.2014.01.008 [DOI] [PubMed] [Google Scholar]
- 30.Mancuso LE, Ilieva IP, Hamilton RH, Farah MJ (2016): Does Transcranial Direct Current Stimulation Improve Healthy Working Memory?: A Meta-analytic Review. 10.1162/jocn_a_00956 [DOI] [PubMed] [Google Scholar]
- 31.Shin Y il, Foerster Á, Nitsche MA (2015): Transcranial direct current stimulation (tDCS) - Application in neuropsychology. Neuropsychologia 69: 154–175. [DOI] [PubMed] [Google Scholar]
- 32.Fregni F, El-Hagrassy MM, Pacheco-Barrios K, Carvalho S, Leite J, Simis M, et al. (2021, April 1): Evidence-Based Guidelines and Secondary Meta-Analysis for the Use of Transcranial Direct Current Stimulation in Neurological and Psychiatric Disorders. International Journal of Neuropsychopharmacology, vol. 24. Oxford University Press, pp 256–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aust S, Brakemeier EL, Spies J, Herrera-Melendez AL, Kaiser T, Fallgatter A, et al. (2022): Efficacy of Augmentation of Cognitive Behavioral Therapy with Transcranial Direct Current Stimulation for Depression: A Randomized Clinical Trial. JAMA Psychiatry 79: 528–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cruz Gonzalez P, Fong KNK, Chung RCK, Ting KH, Law LLF, Brown T (2018, October 16): Can transcranial direct-current stimulation alone or combined with cognitive training be used as a clinical intervention to improve cognitive functioning in persons with mild cognitive impairment and dementia? A systematic review and meta-analysis. Frontiers in Human Neuroscience, vol. 12. Frontiers Media S.A. 10.3389/fnhum.2018.00416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inagawa T, Narita Z, Sugawara N, Maruo K, Stickley A, Yokoi Y, Sumiyoshi T (2019): A Meta-Analysis of the Effect of Multisession Transcranial Direct Current Stimulation on Cognition in Dementia and Mild Cognitive Impairment. Clinical EEG and Neuroscience 50: 273–282. [DOI] [PubMed] [Google Scholar]
- 36.Teselink J, Bawa KK, Koo GK, Sankhe K, Liu CS, Rapoport M, et al. (2021, December 1): Efficacy of non-invasive brain stimulation on global cognition and neuropsychiatric symptoms in Alzheimer’s disease and mild cognitive impairment: A meta-analysis and systematic review. Ageing Research Reviews, vol. 72. Elsevier Ireland Ltd. 10.1016/j.arr.2021.101499 [DOI] [PubMed] [Google Scholar]
- 37.Wang T, Guo Z, Du Y, Xiong M, Yang Z, Ren L, et al. (2021): Effects of Noninvasive Brain Stimulation (NIBS) on Cognitive Impairment in Mild Cognitive Impairment and Alzheimer Disease A Meta-Analysis. Retrieved from www.alzheimerjournal.com [DOI] [PubMed] [Google Scholar]
- 38.Xu Y, Qiu Z, Zhu J, Liu J, Wu J, Tao J, Chen L (2019): The modulation effect of non-invasive brain stimulation on cognitive function in patients with mild cognitive impairment: A systematic review and meta-analysis of randomized controlled trials. BMC Neuroscience 20. 10.1186/s12868-018-0484-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moher D, Liberati A, Tetzlaff J, Altman DG (2009): Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Page MJ, Mckenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. (2021): The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clare L, Woods RT (2004): Cognitive training and cognitive rehabilitation for people with early-stage Alzheimer’s disease: A review. Neuropsychological Rehabilitation 14: 385–401. [Google Scholar]
- 42.Sterne J, Savovic J, Page M, Elbers R, Blencowe N, Boutron I, et al. (2019): RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366: 14898. [DOI] [PubMed] [Google Scholar]
- 43.Review Manager (RevMan) [no. 5.4] (2020): The Cochrane Collaboration. [Google Scholar]
- 44.Cohen J (2013): Statistical Power Analysis for the Behavioral Sciences, Second. Routledge. [Google Scholar]
- 45.Deeks J, Higgins J, Altman D (2022): Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, Welch V, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3. www.training.cochrane.org/handbook. [Google Scholar]
- 46.Page M, Higgins J, Sterne J (2022): Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, Welch V, editors. Cochrane Handbook for Systematic Reviews of Interventions, version 6.3. [Google Scholar]
- 47.Cotelli M, Manenti R, Petesi M, Brambilla M, Cosseddu M, Zanetti O, et al. (2014): Treatment of primary progressive aphasias by transcranial direct current stimulation combined with language training. Journal of Alzheimer’s Disease 39: 799–808. [DOI] [PubMed] [Google Scholar]
- 48.Biundo R, Weis L, Fiorenzato E, Gentile G, Giglio M, Schifano R, et al. (2015, November 1): Double-blind randomized trial of t-DCS versus sham in Parkinson patients with mild cognitive impairment receiving cognitive training. Brain Stimulation, vol. 8. Elsevier Inc., pp 1223–1225. [DOI] [PubMed] [Google Scholar]
- 49.de Sousa AVC, Grittner U, Rujescu D, Külzow N, Flöel A (2020): Impact of 3-Day Combined Anodal Transcranial Direct Current Stimulation-Visuospatial Training on Object-Location Memory in Healthy Older Adults and Patients with Mild Cognitive Impairment. Journal of Alzheimer’s Disease 75: 223–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin DM, Mohan A, Alonzo A, Gates N, Gbadeyan O, Meinzer M, et al. (2019): A Pilot Double-Blind Randomized Controlled Trial of Cognitive Training Combined with Transcranial Direct Current Stimulation for Amnestic Mild Cognitive Impairment. Journal of Alzheimer’s Disease 71: 503–512. [DOI] [PubMed] [Google Scholar]
- 51.Das N, Spence JS, Aslan S, Vanneste S, Mudar R, Rackley A, et al. (2019): Cognitive training and transcranial direct current stimulation in mild cognitive impairment: A randomized pilot trial. Frontiers in Neuroscience 13. 10.3389/fnins.2019.00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cotelli M, Manenti R, Petesi M, Brambilla M, Rosini S, Ferrari C, et al. (2014): Anodal tDCS during face-name associations memory training in Alzheimer’s patients. Frontiers in Aging Neuroscience 6. 10.3389/fnagi.2014.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu H, Chan SSM, Chan WC, Lin C, Cheng CPW, Linda Chiu Wa L (2019): Randomized controlled trial of TDCS on cognition in 201 seniors with mild neurocognitive disorder. Annals of Clinical and Translational Neurology 6: 1938–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Inagawa T, Yokoi Y, Narita Z, Maruo K, Okazaki M, Nakagome K (2019): Safety and Feasibility of Transcranial Direct Current Stimulation for Cognitive Rehabilitation in Patients With Mild or Major Neurocognitive Disorders: A Randomized Sham-Controlled Pilot Study. Frontiers in Human Neuroscience 13. 10.3389/fnhum.2019.00273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manenti R, Cotelli MS, Cobelli C, Gobbi E, Brambilla M, Rusich D, et al. (2018): Transcranial direct current stimulation combined with cognitive training for the treatment of Parkinson Disease: A randomized, placebo-controlled study. Brain Stimulation 11: 1251–1262. [DOI] [PubMed] [Google Scholar]
- 56.Roncero C, Kniefel H, Service E, Thiel A, Probst S, Chertkow H (2017): Inferior parietal transcranial direct current stimulation with training improves cognition in anomic Alzheimer’s disease and frontotemporal dementia. Alzheimer’s and Dementia: Translational Research and Clinical Interventions 3: 247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nienow TM, MacDonald AW, Lim KO (2016, April 1): TDCS produces incremental gain when combined with working memory training in patients with schizophrenia: A proof of concept pilot study. Schizophrenia Research, vol. 172. Elsevier B.V., pp 218–219. [DOI] [PubMed] [Google Scholar]
- 58.Orlov ND, Tracy DK, Joyce D, Patel S, Rodzinka-Pasko J, Dolan H, et al. (2017): Stimulating cognition in schizophrenia: A controlled pilot study of the effects of prefrontal transcranial direct current stimulation upon memory and learning. Brain Stimulation 10: 560–566. [DOI] [PubMed] [Google Scholar]
- 59.Segrave RA, Arnold S, Hoy K, Fitzgerald PB (2014): Concurrent cognitive control training augments the antidepressant efficacy of tDCS: A pilot study. Brain Stimulation 7: 325–331. [DOI] [PubMed] [Google Scholar]
- 60.Vanderhasselt MA, de Raedt R, Namur V, Lotufo PA, Bensenor IM, Boggio PS, Brunoni AR (2015): Transcranial electric stimulation and neurocognitive training in clinically depressed patients: A pilot study of the effects on rumination. Progress in Neuro-Psychopharmacology and Biological Psychiatry 57: 93–99. [DOI] [PubMed] [Google Scholar]
- 61.Xu X, Ding X, Chen L, Chen T, Su H, Li X, et al. (2021): The transcranial direct current stimulation over prefrontal cortex combined with the cognitive training reduced the cue-induced craving in female individuals with methamphetamine use disorder: A randomized controlled trial. Journal of Psychiatric Research 134: 102–110. [DOI] [PubMed] [Google Scholar]
- 62.Leucht S, Helfer B, Gartlehner G, Davis JM (2015): How effective are common medications: a perspective based on meta-analyses of major drugs. BMC Medicine. 10.1186/s12916-015-0494-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, et al. (2016): A technical guide to tDCS, and related non-invasive brain stimulation tools guide Methodology review Safety Design h i g h l i g h t s. Clinical Neurophysiology 127: 1031–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reckow J, Rahman-Filipiak A, Garcia S, Schlaefflin S, Calhoun O, DaSilva AF, et al. (2018): Tolerability and blinding of 4×1 High-Definition transcranial direct current stimulation (HD-tDCS) at two and three milliamps. Brain Stimulation 11: 991–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, et al. (2005): How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? European Journal of Neuroscience 22: 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nitsche MA, Doemkes S, Karaköse T, Antal A, Liebetanz D, Lang N, et al. (2007): Shaping the effects of transcranial direct current stimulation of the human motor cortex. Journal of Neurophysiology 97: 3109–3117. [DOI] [PubMed] [Google Scholar]
- 67.Datta A, Truong D, Minhas P, Parra LC, Bikson M, Brunoni AR, et al. (2012): Inter-individual variation during transcranial direct current stimulation and normalization of dose using MRI-derived computational models. Frontiers in Psychiatry 3: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Horvath JC, Carter O, Forte JD, Lebedev M, Vicario CM (2014): Transcranial direct current stimulation: five important issues we aren’t discussing (but probably should be). Frontiers in System Neuroscience 8: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garnett EO, Malyutina S, Datta A, Den Ouden Dirk-Bart ; (2015): On the Use of the Terms Anodal and Cathodal in High-Definition Transcranial Direct Current Stimulation: A Technical Note. 10.1111/ner.12320 [DOI] [PubMed] [Google Scholar]
- 70.Mosayebi Samani M, Agboada D, Jamil A, Kuo MF, Nitsche MA (2019): Titrating the neuroplastic effects of cathodal transcranial direct current stimulation (tDCS) over the primary motor cortex. Cortex 119: 350–361. [DOI] [PubMed] [Google Scholar]
- 71.Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M (2009): Gyri-precise head model of transcranial direct current stimulation: Improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimulation 2. 10.1016/j.brs.2009.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuo HI, Bikson M, Datta A, Minhas P, Paulus W, Kuo MF, Nitsche MA (2013): Comparing cortical plasticity induced by conventional and high-definition 4 × 1 ring tDCS: A neurophysiological study. Brain Stimulation 6: 644–648. [DOI] [PubMed] [Google Scholar]
- 73.Fridriksson J, Rorden C, Elm J, Sen S, George MS, Bonilha L (2018): Transcranial Direct Current Stimulation vs Sham Stimulation to Treat Aphasia After Stroke A Randomized Clinical Trial. JAMA Neurol 75: 1470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.