Abstract
Cardiac amyloidosis is a type of amyloidosis that deserves special attention as organ involvement significantly worsens the prognosis. Cardiac amyloidosis can be grouped under three main headings: immunoglobulin light chain (AL) amyloidosis that is dependent on amyloidogenic monoclonal light chain production; hereditary Transthyretin (TTR) amyloidosis that results from accumulation of mutated TTR; and wild-type (non-hereditary) TTR amyloidosis formerly known as senile amyloidosis. Although all three types cause morbidity and mortality due to severe heart failure when untreated, they contain differences in their pathogenesis, clinical findings, and treatment. In this article, the clinical features, pathogenesis, diagnosis, and treatment methods of cardiac amyloidosis will be explained with an overview, and an awareness will be raised in the diagnosis of this disease.
Keywords: Amyloid, Cardiac amyloidosis, Transthyretin, Hereditary amyloidosis, Senile amyloidosis
DEFINITION and HISTORY
Amyloidoses are a group of diseases that exhibit heterogeneity. Their common feature is that they have accumulations of abnormal proteins that result in tissue and organ damage. Historically, the term “amyloid” (a normal amylous component in plants) was first suggested in 1838 by German botanist Matthias Schleiden (1). He named it “corpora amylacea”, describing the small round structures in the nervous system that give a characteristic color reaction (brown to blue) with sulfuric acid andiodine, which is typical for starch (2). In 1861, Dr. T. Grainger Stewart described the deposits in the kidney (in Bright’s disease) as a “waxy or amyloid form” (3). Pathologist John W. Budd was the first scientist to report one of the cardiac amyloidosis (CA) cases in the literature, saying, “Although primary amyloid disease of the heart is very rare, the cases reported in the literature show that hyaline material can accumulate in the epicardium, myocardium, endocardium, valves or walls of adjacent blood vessels” (4).
INCIDENCE
The reported incidence for CA is 18-55 per 100,000 person-years. In fact, its prevalence is difficult to determine precisely, because the disease is often overlooked as it often presents with non-specific symptoms (5). A population-based autopsy study suggests that 25% of people aged 80-85 years have cardiac amyloid deposition (6). The UK National Amyloidosis Center database report, which includes data on 11006 patients between 1997 and 2019, reports that the number of patients suffering from amyloidosis increased by 670% from 1987-1999 to 2010-2019 (7). Again, the same report emphasizes that the incidence of CA, which was less than 3% of all cases in the 1987-2009 period, increased to 14% in the 2010-2015 period and to 25% in the last 4 years. It is conceivable that this increased incidence may be the result of increased awareness of amyloidosis and diagnostic cardiac imaging.
CLINICAL FEATURES
The disease usually first presents with shortness of breath secondary to exertion, which is partly rapidly progressing and results in peripheral edema and/or ascites. Left ventricular diastolic dysfunction results in dyspnea. However, significant hardening of the auricles likely contributes to exertional dyspnea. The first signs of the disease include cardiac arrhythmias (atrial/ventricular) and heart block of varying degrees (8,9). Deposits in the atrium cause dysfunction, and thrombi can form even if the heart is operating in sinus rhythm. This causes thromboembolism to be seen as an early sign of the disease. If the clinician is not aware of the phenomenon of left atrial systolic dysfunction that is the source of neurological or systemic embolism, the disease can easily be overlooked (10). Electrical conduction disturbances in the heart due to amyloid deposition, embolic events, and syncope are some of the reasons for hospital admission for affected individuals. However, patients often come to the health institution with cardiogenic shock. In recent years, it can be said that awareness of cardiac dysfunction caused by amyloidosis has increased (11). In addition, it is reported that toxic infiltrative cardiomyopathy from cardiac amyloidosis is much more common than previously believed and is an underrecognized cause of diastolic heart failure in particular (12).
PATHOGENESIS
Among the amyloidoses, CA has a special importance because organ involvement significantly worsens the prognosis. Cardiac amyloidoses are grouped under three headings: AL amyloidosis due to amyloidogenic monoclonal light chain production of a plasma cell clone; hereditary TTR amyloidosis (ATTRv) caused by accumulation of mutated Transthyretin (TTR); and wild-type (non-hereditary) TTR amyloidosis (ATTRwt) (13). Rarely, cardiac involvement can be seen in secondary amyloidosis (14).
AL amyloidosis is a multi-organ disease, although involvement of one organ is usually predominant. The kidney is the first organ to be affected, and it manifests itself with nephrotic syndrome. The second most affected organ is the heart. There is a slight male dominance. Although the disease can be seen at any age from the fourth decade, it often occurs after the fifth decade (14). AL amyloidosis is known to be the most severe of CAs. If the disease is not treated, the patient is expected to die within about 6 months after the onset of heart failure (15). Although there is greater accumulation in the left ventricle in TTR amyloidosis, heart failure has been shown to be more severe in AL amyloidosis than in TTR amyloidosis (14,16). Studies focusing on the pathogenesis of cardiac AL amyloidosis have shown that amyloidogenic light chains cause an increase in reactive oxygen products (ROP) and upregulation of heme oxygenase in rat cardiac muscle cells. Unfortunately, this process results in the deterioration of contraction and relaxation (14,17). The initial response to amyloid deposition appears as lysosomal dysfunction. This leads to generation of highly ROP, functional loss in cells, impaired calcium homeostasis, and cell death, with disruption of autophagy (14,18). The unbranched amyloid fibrils not only consist of precursor protein units, but also contain serum amyloid P and proteoglycans. Evidence has shown that amyloid can be reabsorbed, albeit slowly, after fibrillogenesis is stopped but generally the other proteins it contains are extremely resistant to degradation (14,19,20). AL CA studies suggest that AL amyloidosis may have infiltrative as well as toxic effects (21). In the light of the evidence, it would be more accurate to consider AL cardiac amyloidosis both as an infiltrative heart disease and as an infiltrative toxic cardiomyopathy.
Transthyretin amyloidosis: The main function of Transthyretin (prealbumin) produced by the liver is to transport thyroxine and retinol. The gene encoding the protein is located on chromosome 18. Although it can acquire monomeric amyloidogenic properties in conditions such as genetic damage and aging, it is a homotetramer in its normal state. It has been shown that monomeric amyloidogenic intermediates can subsequently spontaneously revert to amyloid fibrils (22). Point mutations that destabilize the tetramer are involved in hereditary amyloidogenesis (23). More than 100 amyloidogenic mutations have been reported in TTR. However, the most common cause of familial amyloid cardiomyopathy is the isoleucine mutation at position 122, which is also seen in 3.9% of the Afro-Caribbean population (V122I) (24). Caucasian variant mutations have also been identified: Leu111Met (Denmark), Ile68Leu (Italy), and Thr60Ala (Appalachian and Irish regions) (25).
The mechanism of heart failure in individuals carrying the V1221 allele has not been clearly elucidated. However, it is suggested that individuals carrying this allele have an increased risk of heart failure and death, particularly at the age of 60 to 65 years (26). Connors et al.’s study showed that ATTRv patients with the V122I mutation were older, had more pronounced ventricular hypertrophy, had lower left ventricular ejection fractions, and had more atrial dilatation on echocardiography (ECO) than Black Americans with AL amyloidosis. However, despite these findings, the patients’ symptoms are less severe (27,28). An isolated cardiomyopathy is often an expected finding in individuals with the V122I mutation. It is not surprising that cardiac involvement and familial amyloid polyneuropathy are more common in individuals with TTR gene mutations, which are endemic in Europe and Asia. (28). Applications to healthcare institutions with non-cardiac symptoms such as purpura, easy bruising, carpal tunnel syndrome, and peripheral polyneuropathy are substantial (8). When examining the mechanism of cardiac damage, as mentioned above, it is well known that light chains have direct toxicity to myocytes from those affected by AL amyloidosis (21). However, it has been shown that mutant transthyretin fibrils can trigger different mechanisms that cause damage in cardiac muscle cells. These mechanisms include triggering the proinflammatory cascade mainly by NF-kB activation, disruption of calcium metabolism, and downregulation of proteosomal activity (28,29).
Transthyretin-associated nonhereditary amyloidosis (ATTRwt): The main reason ATTRwt is called senile systemic amyloidosis (SSA) is that the disease often begins after the age of 70. Similar to AL amyloidosis, it has a strong male predominance (6,20). The prevalence of ATTRwt amyloidosis is not known precisely because the diagnosis cannot be made in many cases. Prolongation of life expectancy and the availability of modern diagnostic tools such as cardiovascular magnetic resonance imaging (CMR) and Technetium-99m-3,3-diphosphono-1,2-propanodicarboxylic acid (99m Tc-DPD) scintigraphy can be counted as the reason for the increasing prevalence today (7,10,20,30). ATTRwt type CA is thought to result from misfolding in the TTR due to advancing age (12). Oxidative modifications of proteins in aging-related proteogenesis and damage repair mechanisms are thought to contribute to the degradation and fibrillation of native TTR (31). Cardiac symptoms in ATTRwt patients usually present as shortness of breath, fatigue, and malaise, and these symptoms are generally not considered to be related to old age and further investigation is not performed. This is one of the most important reasons for the delay in diagnosis (6,8,9). Cardiac vascular amyloid deposition is a major cause of anginal chest pain, which can occur even in patients without obstructive coronary stenosis. Patients have heart failure but typically preserved ejection fraction (32). Studies suggest that mean left ventricular wall thickness is greater in ATTRwt than in ATTRv (33). Figure 1 shows the increase in ventricular thickness in the explant material of the patient who underwent cardiac transplantation due to ATTRv at Ege University (Figure 1). ATTRwt is expected to have a much better natural course than other amyloid cardiomyopathies (12,34). In one of the first studies, the median survival from admission with symptoms of heart failure was 60 months, compared to 5.4 months in patients with cardiomyopathy from AL amyloidosis (33).
Figure 1.
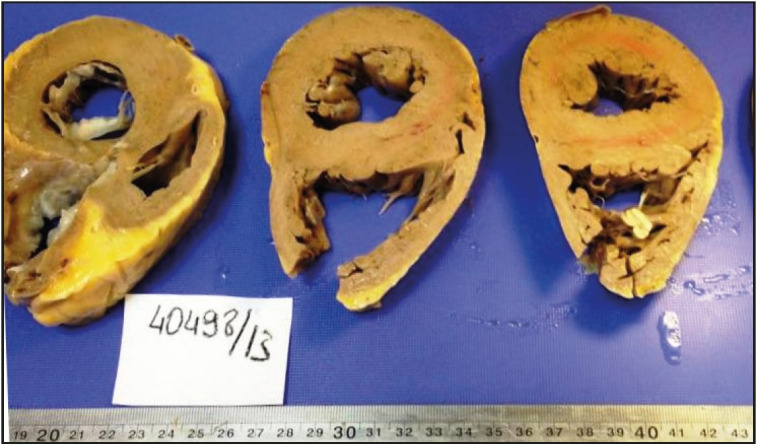
Ventricular thickness increase due to amyloid deposition in the explant material of the patient who underwent heart transplantation due to ATTRv.
The distinction between ATTRwt and ATTRv needs to be made carefully as the families of patients diagnosed with ATTRv should be given genetic counseling and their family members should be screened for this disease. Also, if the individual has a V122I mutation, closer monitoring is required as aggressive progression may occur. In addition, a confirmed diagnosis of ATTRv is essential for the patient to receive only treatments approved for ATTRv (35). Although some authors propose a diagnostic algorithm including NT pro-BNP level and age at diagnosis to distinguish senile ATTR cardiac amyloidosis from AL primary cardiac amyloidosis, it should not be forgotten that the definitive distinction can only be made with endomyocardial biopsy (EMB) (36,37).
DIAGNOSIS
The first steps on the way to the correct diagnosis of cardiac amyloidosis is obtaining a detailed history, evaluating the symptoms, and clinically suspecting the disorder as a result of the examination. This suspicion is followed by laboratory studies and cardiac imaging.
Scanning
International experts (ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI) recommend the appropriate use of ECO, CMR, and radionuclide imaging in the diagnosis of cardiac amyloidosis and/or in the evaluation of patients with cardiac amyloidosis (38). ECO has the ability to offer clues to further testing, and CMR has the ability to show an infiltrative process. However, 99m technetium pyrophosphate scintigraphy allows for non-invasive diagnosis os CA, although the mechanism of attachment of radioactive material to amyloid deposits cannot be fully elucidated. In this respect, it is considered as an important milestone in the clinical diagnosis of cardiac amyloid (35,39). It has been reported that cardiac amyloidosis can be diagnosed by evaluating the patient together with non-cardiac biopsy, Tc-99m pyrophosphate scintigraphy and positron emission tomography data using molecules targeting myocardial amyloid uptake, as well as echocardiographic and CMR findings in cases where the disease cannot be proven with endomyocardial biopsy (38) (Table 1). In addition, it was suggested by the same authors that the need for invasive endomyocardial or extracardiac biopsy is eliminated if there are consistent echo or CMR findings in patients without monoclonal plasma cell increase, as well as findings consistent with ATTR cardiac amyloidosis on 99m Tc-PYP/DPD/HMDP scintigraphy.
Table 1.
Diagnostic Criteria for Cardiac Amyloidosis (Dorbala, 2019)
|
Diagnostic Criteria |
Subtype |
|
Histology of Endomyocardial biopsy | |
|
1. Endomyocardial biopsy showing birefringent amyloid deposition in apple green to polarized light in Congo red. Immunohistochemistry and/or mass spectrometry may be preferred for subtyping. |
AL, ATTR, other subtype |
|
Histology of Extracardiac biopsy | |
|
1. The following conditions should be sought in the diagnosis of ATTR amyloidosis: a) Demonstration of ATTR amyloidosis on extracardiac biopsy, and b) Presence of typical cardiac imaging features described below |
ATTR |
|
2. The following conditions should be sought for the diagnosis of AL amyloidosis: a) Demonstration of AL amyloidosis on extracardiac biopsy, and b) Presence of typical cardiac imaging features described below c) Abnormality of age-adjusted NT pro BNP or abnormal Troponin T/I/HS Troponin levels; after exclusion of all causes to explain the abnormality of these markers |
AL |
|
Clinical Diagnosis of ATTR Cardiac Amyloidosis: 99m Tc-PYP/DPD/HMDP | |
|
3. The diagnosis of ATTR amyloidosis can be made in the presence of the following conditions: a) Presence of Grade 2 or 3 myocardial involvement in 99m Tc-PYP/DPD/HMDP, and b) Demonstration of clonal plasma cell absence by serum FLCs and serum and urine immunofixation, and c) Presence of typical cardiac imaging features described below |
ATTR |
|
Typical Imaging Features in the Diagnosis of Cardiac Amyloidosis | |
|
Typical cardiac echocardiography or CMR or PET features: All other diseases (including hypertension) that may produce the following imaging findings should be excluded | |
|
1. Echocardiography • Left ventricular wall thickness >12mm • Relative apical preservation of global LS ratio (apical LS mean/combined mean+basal LS >1 mean) • ≥ Grade 2 diastolic dysfunction |
ATTR/AL |
|
2. Cardiac MRI a) LV wall thickness above the upper limit of normal for gender in unstable condition b) Overall ECV >0.40 c) Diffuse late gadolinium increase d) Abnormal gadolinium kinetics typical for amyloidosis, resetting myocardium before blood pool reset |
ATTR/AL |
|
3. PET: 18F-florbetapir or 18F-florbetaben PET a) Target - background (LV myocardium - blood pool) ratio >1.5 b) b. Retention index >0.030 min-1 |
ATTR/AL |
Laboratory
Patients with suspected CA after evaluation should first be evaluated for monoclonal gammopathy for AL-CA (40). Laboratory tests should include serum-free kappa and lambda light chains, as well as immune-fixed serum and urine protein electrophoresis (SPEP/UPEP with IFE). The sensitivity of serum plasma electrophoresis for AL amyloidosis is lower than that of serum IFE (~70% and >90%, respectively) (41). Troponin, BNP (brain natriuretic peptide) and NT-proBNP (N-terminal probrain natriuretic peptide) are other markers whose usefulness in the diagnosis and for predicting prognosis has been investigated. In AL-CA, increased production of monoclonal AL also activates the MAP kinase signaling pathway, increasing natriuretic peptide production. This results in elevated brain natriuretic peptide (BNP) and N-terminal proBNP level (42). However, the utility of these tests in the diagnosis of TTR-CA is very limited. Patients with ATTR amyloidosis often require EMB to confirm the diagnosis. When ATTR amyloidosis is detected, genetic testing for the TTR gene mutation should be performed. In addition, it has been reported that circulating retinol-binding protein 4 may be useful in identifying patients with ATTR-CA with V122I mutant heart failure (43). It should not be forgotten that a high troponin level can be used to predict a worse prognosis in both AL-CA and ATTR-CA, even if it is not in the diagnosis (37).
Biopsy
In patients with suspected cardiac amyloidosis, scintigraphy or biopsy should be performed after monoclonal protein tests (44). While biopsy is valid for all forms of cardiac amyloidosis, noninvasive criteria are acceptable for ATTR only (45). In cases with monoclonal proteinemia, the accumulated amyloid form can be detected in biopsy taken from affected organ (such as endomyocardial, abdominal fat, bone marrow) (46). Because of its invasive nature, endomyocardial biopsy carries a small risk of complications, which can be serious. Its implementation requires technical expertise. Fat pad biopsy is less invasive and carries less risk, but its sensitivity in ATTR-CA is highly variable (47). All types of cardiac amyloidosis cause extracellular amyloid deposition and the accumulating form of amyloid is not expected to be distinguished by light microscopy. Although the type of amyloid deposition cannot be clearly differentiated from the deposition pattern, AL amyloidosis predominantly presents with pericellular, endocardial, and arterial and/or arteriolar deposits; nodular deposits are usually seen in ATTR (Figure 2) (48). Regardless of left ventricular wall thickness, the diagnosis of CA is confirmed when amyloid deposition is demonstrated on endomyocardial biopsy with Congo red (Figure 2). Once amyloid deposition is detected, the next step should be the classification of the amyloid fibril protein. The gold standard in classification is mass spectrometry, immunohistochemistry, or immunoelectron microscopy, which are routinely used in specialized centers (Figure 2) (49). In a study of 117 patients with amyloid, immunohistochemical analysis was reported to have 96% sensitivity and 100% specificity in identifying hereditary amyloidoses (50). In Ege University, where the first cardiac transplantation was performed in 1998, myocardial biopsy has been used for the diagnosis of cardiac diseases for approximately 25 years. In cases where clinical, laboratory data or histopathology arouses suspicion, amyloid accumulation in the tissue is investigated with histochemical congo red. When cardiac amyloidosis accumulation is detected, immunohistochemical subtyping is performed with Amyloid A, C4d, Fibrinogen, Pre Albumin/Transthyretin (TTR), Lambda, Lysozyme, and Kappa. Evaluation using these antibodies provides sensitivity and specificity rates similar to the study of Schönland et al. in detecting hereditary amyloidosis in our center.
Figure 2.
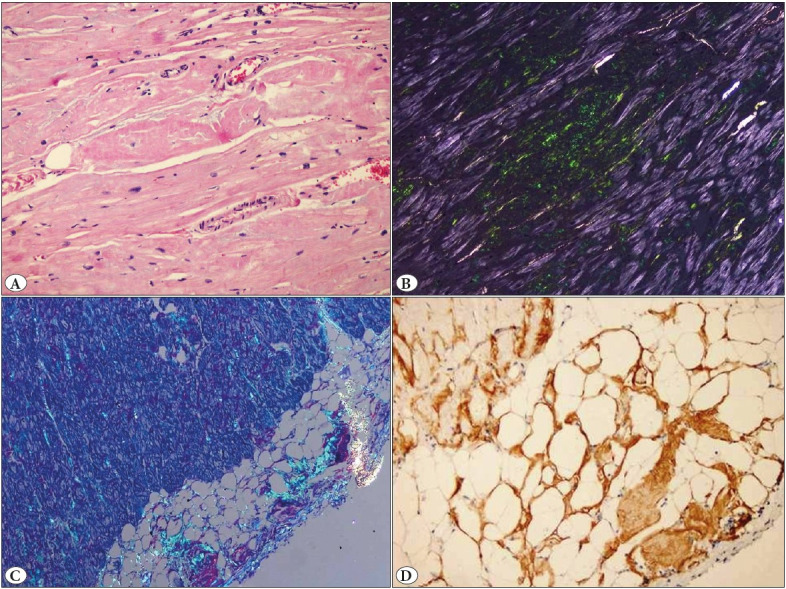
Histopathological features of ventricular muscle samples taken from the explant material of the patient who underwent heart transplantation due to ATTRv. A) Pericellular nodular deposits with hematoxylin-eosin stain, x10; B) Pericellular nodular amyloid deposits with apple green reflectors on Congo red staining, x10; C) Subendocardial and pericellular amyloid deposits with apple green reflectors on Congo red staining, x4: D) Positivity with immunohistochemical Transthyretin, x10.
TREATMENT
Medical Treatments
In patients with cardiac amyloid, the two ventricles are usually affected together; moreover, the left ventricle is greatly shrunk secondary to muscle hypertrophy. Therefore, durable left ventricular assist devices are often not a good option (51). Amyloid deposition, which causes damage to organs, is seen in both ATTR and AL amyloidosis, but the treatment regimens of these diseases are different (52). The main agent used in the treatment of ATTR is TTR silencing, which is involved in the synthesis of TTR in the liver, TTR stabilization that prevent misfolding by binding to the tetramer, and TTR disruption that ensures the clearance of amyloid from the organism (35). The TTR stabilizer tafamidis, which was approved in 2019, has taken its place in the treatment of amyloid cardiomyopathy (7,53). In addition, new treatments such as patisiran and inotersen, which reduce hepatic TTR production in hereditary ATTR amyloidosis, are also promising (7,54,55).
Since light chain toxicity causes cardiac damage in AL amyloidosis, correction of the relevant light chain should be the main goal (52). Autologous stem cell transplantation (ASCT) is among the emerging treatments for AL amyloidosis patients and has been shown to have very good long-term results. However, it has been reported that it may be an appropriate treatment option in a small proportion of patients with CA (56). The advent of effective anti-plasma cell therapies has changed the definition of hematological response from a complete response to a modified, strict, and absolutely relevant free light chain response. The agents used in anti-plasma cell therapy are mainly alkylating agents, immunomodulators, corticosteroids, and proteasome inhibitors (52).
Orthotopic Cardiac Transplantation
Cardiac transplantation (CTx) may be considered in cardiac AL amyloidosis responding to light chain suppressive therapies and in cases with end-stage heart failure secondary to ATTR. In the past, cardiac amyloidosis, especially the AL type, was a contraindication for heart transplantation (HTx) because the disease is systemic and carries a high risk of death. However, effective therapies used today, including proteasome inhibitors, have made heart transplantation an option for AL-CA patients as well (57–59). Cardiac amyloidosis patients have a higher heart transplant waiting list mortality than cardiomyopathy from other causes (51,60). The 2018 UNOS heart allocation scheme gives priority clearance for CTx to patients with CA as they are at high risk of mortality (51,61). For patients with ATTRwt-CA or ATTRv-CA with the V122I mutation, heart transplantation alone is usually sufficient, but may need to be considered for dual heart/liver transplantation in the presence of other variants such as Thr60Ala (52). However, follow-up studies unfortunately show that disease recurrence and improvement in extracardiac manifestations may occur after Tx (62). Cardiac transplantation was performed on a patient with ATTRwt-CA mutation in our Ege University heart transplant program. However, the patient died from Candida sepsis at 6 weeks postoperatively.
CONCLUSION
Cardiac amyloidosis is one of the causes of restrictive cardiomyopathy. It is characterized by the extracellular accumulation of abnormal proteins that show birefringence in Congo red-polarized light. Regardless of the subtype of the deposited amyloid, the disease results in progressive heart failure if left untreated. Its incidence has increased with the development of diagnostic methods in recent years and the increased sensitivity of clinicians to disease symptoms. This has enabled both the understanding of the pathogenesis of the disease and the development of new treatment options. A growing number of studies on the disease have made significant advances. This situation raises our hope that there may be significant changes in the diagnosis, treatment, and follow-up of cardiac amyloidosis in the coming years.
Conflict of Interest
The authors declare no potential conflicts of interest regarding the research, authorship and/or publication of this article. Only the authors are responsible for the content and writing of the article.
References
- Kyle R. A. Amyloidosis: a convoluted story. Sep;2001 Br J Haematol. 114:529–538. doi: 10.1046/j.1365-2141.2001.02999.x. [DOI] [PubMed] [Google Scholar]
- Kyle R. A. Amyloidosis: a brief history. Jun;2011 Amyloid. 18 Suppl 1:6–7. doi: 10.3109/13506129.2011.574354001. [DOI] [PubMed] [Google Scholar]
- Stewart TG. 1861On the Waxy or Amyloid Form of Bright's Disease. 6:710–728. [PMC free article] [PubMed] [Google Scholar]
- Amarelli Cristiano, Limongelli Giuseppe. Cardiac amyloidosis: Watching the tip of the iceberg emerging from the "heart of the sea". Sep;2021 Int J Cardiol. 338:226–228. doi: 10.1016/j.ijcard.2021.06.001. [DOI] [PubMed] [Google Scholar]
- Rubin Jonah, Maurer Mathew S. Cardiac Amyloidosis: Overlooked, Underappreciated, and Treatable. Jan;2020 Annu Rev Med. 71:203–219. doi: 10.1146/annurev-med-052918-020140. [DOI] [PubMed] [Google Scholar]
- Tanskanen Maarit, Peuralinna Terhi, Polvikoski Tuomo, Notkola Irma-Leena, Sulkava Raimo, Hardy John, Singleton Andrew, Kiuru-Enari Sari, Paetau Anders, Tienari Pentti J., Myllykangas Liisa. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: a population-based autopsy study. 2008Ann Med. 40:232–239. doi: 10.1080/07853890701842988. [DOI] [PubMed] [Google Scholar]
- Ravichandran Sriram, Lachmann Helen J., Wechalekar Ashutosh D. Epidemiologic and Survival Trends in Amyloidosis, 1987-2019. Apr;2020 N Engl J Med. 382:1567–1568. doi: 10.1056/NEJMc1917321. [DOI] [PubMed] [Google Scholar]
- Dharmarajan Kumar, Maurer Mathew S. Transthyretin cardiac amyloidoses in older North Americans. Apr;2012 J Am Geriatr Soc. 60:765–774. doi: 10.1111/j.1532-5415.2011.03868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng Belinda, Connors Lawreen H., Davidoff Ravin, Skinner Martha, Falk Rodney H. Senile systemic amyloidosis presenting with heart failure: a comparison with light chain-associated amyloidosis. Jun;2005 Arch Intern Med. 165:1425–1429. doi: 10.1001/archinte.165.12.1425. [DOI] [PubMed] [Google Scholar]
- Santarone M., Corrado G., Tagliagambe L. M., Manzillo G. F., Tadeo G., Spata M., Longhi M. Atrial thrombosis in cardiac amyloidosis: diagnostic contribution of transesophageal echocardiography. Jun;1999 J Am Soc Echocardiogr. 12:533–536. doi: 10.1016/s0894-7317(99)70091-x. [DOI] [PubMed] [Google Scholar]
- Liao Ronglih, Ward Jennifer E. Amyloid Cardiomyopathy: Disease on the Rise. Jun;2017 Circ Res. 120:1865–1867. doi: 10.1161/CIRCRESAHA.117.310643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruberg Frederick L., Grogan Martha, Hanna Mazen, Kelly Jeffery W., Maurer Mathew S. Transthyretin Amyloid Cardiomyopathy: JACC State-of-the-Art Review. Jun;2019 J Am Coll Cardiol. 73:2872–2891. doi: 10.1016/j.jacc.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharoubi Mounira, Bézard Mélanie, Galat Arnault, Le Bras Fabien, Poullot Elsa, Molinier-Frenkel Valérie, Fanen Pascale, Funalot Benoit, Moktefi Anissa, Lefaucheur Jean-Pascal, Abulizi Mukedaisi, Deux Jean-François, Lemonnier François, Guendouz Soulef, Chalard Coraline, Zaroui Amira, Audard Vincent, Bequignon Emilie, Bodez Diane, Itti Emmanuel, Hittinger Luc, Audureau Etienne, Teiger Emmanuel, Oghina Silvia, Damy Thibaud. History of extracardiac/cardiac events in cardiac amyloidosis: prevalence and time from initial onset to diagnosis. Dec;2021 ESC Heart Fail. 8:5501–5512. doi: 10.1002/ehf2.13652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk Rodney H., Alexander Kevin M., Liao Ronglih, Dorbala Sharmila. AL (Light-Chain) Cardiac Amyloidosis: A Review of Diagnosis and Therapy. Sep;2016 J Am Coll Cardiol. 68:1323–1341. doi: 10.1016/j.jacc.2016.06.053. [DOI] [PubMed] [Google Scholar]
- Kyle R. A., Linos A., Beard C. M., Linke R. P., Gertz M. A., O'Fallon W. M., Kurland L. T. Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989. Apr;1992 Blood. 79:1817–1822. [PubMed] [Google Scholar]
- Dubrey S. W., Cha K., Skinner M., LaValley M., Falk R. H. Familial and primary (AL) cardiac amyloidosis: echocardiographically similar diseases with distinctly different clinical outcomes. Jul;1997 Heart. 78:74–82. doi: 10.1136/hrt.78.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner Daniel A., Jain Mohit, Pimentel David R., Wang Bo, Connors Lawreen H., Skinner Martha, Apstein Carl S., Liao Ronglih. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Apr;2004 Circ Res. 94:1008–1010. doi: 10.1161/01.RES.0000126569.75419.74. [DOI] [PubMed] [Google Scholar]
- Mishra Shikha, Guan Jian, Plovie Eva, Seldin David C., Connors Lawreen H., Merlini Giampaolo, Falk Rodney H., MacRae Calum A., Liao Ronglih. Human amyloidogenic light chain proteins result in cardiac dysfunction, cell death, and early mortality in zebrafish. Jul;2013 Am J Physiol Heart Circ Physiol. 305:H95–103. doi: 10.1152/ajpheart.00186.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmanandam Vikram, McGraw Sloane, Mirza Omer, Desai Ankit A., Farzaneh-Far Afshin. Regression of cardiac amyloidosis after stem cell transplantation assessed by cardiovascular magnetic resonance imaging. Jun;2014 Circulation. 129:2326–2328. doi: 10.1161/CIRCULATIONAHA.114.009135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Naharro Ana, Hawkins Philip N., Fontana Marianna. Cardiac amyloidosis. Apr;2018 Clin Med (Lond) 18:s30–s35. doi: 10.7861/clinmedicine.18-2-s30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrey S., Mendes L., Skinner M., Falk R. H. Resolution of heart failure in patients with AL amyloidosis. Sep;1996 Ann Intern Med. 125:481–484. doi: 10.7326/0003-4819-125-6-199609150-00009. [DOI] [PubMed] [Google Scholar]
- Kelly J. W. Mechanisms of amyloidogenesis. Oct;2000 Nat Struct Biol. 7:824–826. doi: 10.1038/82815. [DOI] [PubMed] [Google Scholar]
- Saraiva M. J. Transthyretin mutations in health and disease. 1995Hum Mutat. 5:191–196. doi: 10.1002/humu.1380050302. [DOI] [PubMed] [Google Scholar]
- Jacobson D. R., Pastore R. D., Yaghoubian R., Kane I., Gallo G., Buck F. S., Buxbaum J. N. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. Feb;1997 N Engl J Med. 336:466–473. doi: 10.1056/NEJM199702133360703. [DOI] [PubMed] [Google Scholar]
- Rapezzi Claudio, Quarta Candida Cristina, Obici Laura, Perfetto Federico, Longhi Simone, Salvi Fabrizio, Biagini Elena, Lorenzini Massimiliano, Grigioni Francesco, Leone Ornella, Cappelli Francesco, Palladini Giovanni, Rimessi Paola, Ferlini Alessandra, Arpesella Giorgio, Pinna Antonio Daniele, Merlini Giampaolo, Perlini Stefano. Disease profile and differential diagnosis of hereditary transthyretin-related amyloidosis with exclusively cardiac phenotype: an Italian perspective. Feb;2013 Eur Heart J. 34:520–528. doi: 10.1093/eurheartj/ehs123. [DOI] [PubMed] [Google Scholar]
- Buxbaum Joel, Alexander Alice, Koziol James, Tagoe Clement, Fox Ervin, Kitzman Dalane. Significance of the amyloidogenic transthyretin Val 122 Ile allele in African Americans in the Arteriosclerosis Risk in Communities (ARIC) and Cardiovascular Health (CHS) Studies. May;2010 Am Heart J. 159:864–870. doi: 10.1016/j.ahj.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors Lawreen H., Prokaeva Tatiana, Lim Amareth, Théberge Roger, Falk Rodney H., Doros Gheorghe, Berg Alan, Costello Catherine E., O'Hara Carl, Seldin David C., Skinner Martha. Cardiac amyloidosis in African Americans: comparison of clinical and laboratory features of transthyretin V122I amyloidosis and immunoglobulin light chain amyloidosis. Oct;2009 Am Heart J. 158:607–614. doi: 10.1016/j.ahj.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Mankad Anit K., Shah Keyur B. Transthyretin Cardiac Amyloidosis. Aug;2017 Curr Cardiol Rep. 19:97–97. doi: 10.1007/s11886-017-0911-5. [DOI] [PubMed] [Google Scholar]
- Ton Van-Khue, Mukherjee Monica, Judge Daniel P. Transthyretin cardiac amyloidosis: pathogenesis, treatments, and emerging role in heart failure with preserved ejection fraction. 2014Clin Med Insights Cardiol. 8:39–44. doi: 10.4137/CMC.S15719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-López Esther, Gallego-Delgado Maria, Guzzo-Merello Gonzalo, Haro-Del Moral F. Javier, Cobo-Marcos Marta, Robles Carolina, Bornstein Belén, Salas Clara, Lara-Pezzi Enrique, Alonso-Pulpon Luis, Garcia-Pavia Pablo. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Oct;2015 Eur Heart J. 36:2585–2594. doi: 10.1093/eurheartj/ehv338. [DOI] [PubMed] [Google Scholar]
- Zhao Lei, Buxbaum Joel N., Reixach Natàlia. Age-related oxidative modifications of transthyretin modulate its amyloidogenicity. Mar;2013 Biochemistry. 52:1913–1926. doi: 10.1021/bi301313b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Hiroyuki, Yokochi Tomoki. Transthyretin cardiac amyloidosis: an update on diagnosis and treatment. Dec;2019 ESC Heart Fail. 6:1128–1139. doi: 10.1002/ehf2.12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapezzi Claudio, Merlini Giampaolo, Quarta Candida C., Riva Letizia, Longhi Simone, Leone Ornella, Salvi Fabrizio, Ciliberti Paolo, Pastorelli Francesca, Biagini Elena, Coccolo Fabio, Cooke Robin M. T., Bacchi-Reggiani Letizia, Sangiorgi Diego, Ferlini Alessandra, Cavo Michele, Zamagni Elena, Fonte Maria Luisa, Palladini Giovanni, Salinaro Francesco, Musca Francesco, Obici Laura, Branzi Angelo, Perlini Stefano. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Sep;2009 Circulation. 120:1203–1212. doi: 10.1161/CIRCULATIONAHA.108.843334. [DOI] [PubMed] [Google Scholar]
- Mankad Anit K., Sesay Isata, Shah Keyur B. Light-chain cardiac amyloidosis. 2017Curr Probl Cancer. 41:144–156. doi: 10.1016/j.currproblcancer.2016.11.004. [DOI] [PubMed] [Google Scholar]
- Kittleson Michelle M., Maurer Mathew S., Ambardekar Amrut V., Bullock-Palmer Renee P., Chang Patricia P., Eisen Howard J., Nair Ajith P., Nativi-Nicolau Jose, Ruberg Frederick L., On behalf of the American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology Cardiac Amyloidosis: Evolving Diagnosis and Management: A Scientific Statement From the American Heart Association. Jul;2020 [ Jan 20; 2024 ];Circulation. 142 doi: 10.1161/CIR.0000000000000792. [DOI] [PubMed] [Google Scholar]
- Pinney Jennifer H., Whelan Carol J., Petrie Aviva, Dungu Jason, Banypersad Sanjay M., Sattianayagam Prayman, Wechalekar Ashutosh, Gibbs Simon D. J., Venner Christopher P., Wassef Nancy, McCarthy Carolyn A., Gilbertson Janet A., Rowczenio Dorota, Hawkins Philip N., Gillmore Julian D., Lachmann Helen J. Senile systemic amyloidosis: clinical features at presentation and outcome. Apr;2013 J Am Heart Assoc. 2:e000098–e000098. doi: 10.1161/JAHA.113.000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roof Logan, Coker Woodrow J., Lazarchick John, Kang Yubin. Senile transthyretin cardiac amyloidosis in patients with plasma cell dyscrasias: importance of cardiac biopsy for making the correct diagnosis. 2014Aperito J Cell Mol Biol. 1:102–102. [PMC free article] [PubMed] [Google Scholar]
- Dorbala Sharmila, Ando Yukio, Bokhari Sabahat, Dispenzieri Angela, Falk Rodney H., Ferrari Victor A., Fontana Marianna, Gheysens Olivier, Gillmore Julian D., Glaudemans Andor W. J. M., Hanna Mazen A., Hazenberg Bouke P. C., Kristen Arnt V., Kwong Raymond Y., Maurer Mathew S., Merlini Giampaolo, Miller Edward J., Moon James C., Murthy Venkatesh L., Quarta C. Cristina, Rapezzi Claudio, Ruberg Frederick L., Shah Sanjiv J., Slart Riemer H. J. A., Verberne Hein J., Bourque Jamieson M. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI Expert Consensus Recommendations for Multimodality Imaging in Cardiac Amyloidosis: Part 2 of 2-Diagnostic Criteria and Appropriate Utilization. Nov;2019 J Card Fail. 25:854–865. doi: 10.1016/j.cardfail.2019.08.002. [DOI] [PubMed] [Google Scholar]
- Perugini Enrica, Guidalotti Pier Luigi, Salvi Fabrizio, Cooke Robin M. T., Pettinato Cinzia, Riva Letizia, Leone Ornella, Farsad Mohsen, Ciliberti Paolo, Bacchi-Reggiani Letizia, Fallani Francesco, Branzi Angelo, Rapezzi Claudio. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. Sep;2005 J Am Coll Cardiol. 46:1076–1084. doi: 10.1016/j.jacc.2005.05.073. [DOI] [PubMed] [Google Scholar]
- Addison Daniel, Slivnick Jeremy A., Campbell Courtney M., Vallakati Ajay, Jneid Hani, Schelbert Erik. Recent Advances and Current Dilemmas in the Diagnosis and Management of Transthyretin Cardiac Amyloidosis. May;2021 J Am Heart Assoc. 10:e019840–e019840. doi: 10.1161/JAHA.120.019840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchtar Eli, Gertz Morie A., Kyle Robert A., Lacy Martha Q., Dingli David, Leung Nelson, Buadi Francis K., Hayman Suzanne R., Kapoor Prashant, Hwa Yi Lisa, Fonder Amie, Hobbs Miriam, Gonsalves Wilson, Kourelis Taxiarchis V., Warsame Rahma, Russell Stephen, Lust John A., Lin Yi, Go Ronald S., Zeldenrust Steven, Rajkumar S. Vincent, Kumar Shaji K., Dispenzieri Angela. A Modern Primer on Light Chain Amyloidosis in 592 Patients With Mass Spectrometry-Verified Typing. Mar;2019 Mayo Clin Proc. 94:472–483. doi: 10.1016/j.mayocp.2018.08.006. [DOI] [PubMed] [Google Scholar]
- Palladini Giovanni, Campana Carlo, Klersy Catherine, Balduini Alessandra, Vadacca Giovanbattista, Perfetti Vittorio, Perlini Stefano, Obici Laura, Ascari Edoardo, d'Eril Gianvico Melzi, Moratti Remigio, Merlini Giampaolo. Serum N-terminal pro-brain natriuretic peptide is a sensitive marker of myocardial dysfunction in AL amyloidosis. May;2003 Circulation. 107:2440–2445. doi: 10.1161/01.CIR.0000068314.02595.B2. [DOI] [PubMed] [Google Scholar]
- Arvanitis Marios, Koch Clarissa M., Chan Gloria G., Torres-Arancivia Celia, LaValley Michael P., Jacobson Daniel R., Berk John L., Connors Lawreen H., Ruberg Frederick L. Identification of Transthyretin Cardiac Amyloidosis Using Serum Retinol-Binding Protein 4 and a Clinical Prediction Model. Mar;2017 JAMA Cardiol. 2:305–313. doi: 10.1001/jamacardio.2016.5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nativi-Nicolau Jose, Maurer Mathew S. Amyloidosis cardiomyopathy: update in the diagnosis and treatment of the most common types. Sep;2018 Curr Opin Cardiol. 33:571–579. doi: 10.1097/HCO.0000000000000547. [DOI] [PubMed] [Google Scholar]
- McVeigh Todd, Tennyson Carolina. Understanding and recognizing cardiac amyloidosis. Oct;2020 JAAPA. 33:16–20. doi: 10.1097/01.JAA.0000697236.11386.3a. [DOI] [PubMed] [Google Scholar]
- Grogan Martha, Dispenzieri Angela, Gertz Morie A. Light-chain cardiac amyloidosis: strategies to promote early diagnosis and cardiac response. Jul;2017 Heart. 103:1065–1072. doi: 10.1136/heartjnl-2016-310704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer Mathew S., Bokhari Sabahat, Damy Thibaud, Dorbala Sharmila, Drachman Brian M., Fontana Marianna, Grogan Martha, Kristen Arnt V., Lousada Isabelle, Nativi-Nicolau Jose, Cristina Quarta Candida, Rapezzi Claudio, Ruberg Frederick L., Witteles Ronald, Merlini Giampaolo. Expert Consensus Recommendations for the Suspicion and Diagnosis of Transthyretin Cardiac Amyloidosis. Sep;2019 Circ Heart Fail. 12:e006075–e006075. doi: 10.1161/CIRCHEARTFAILURE.119.006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen Brandon T., Mereuta Oana M., Dasari Surendra, Fayyaz Ahmed U., Theis Jason D., Vrana Julie A., Grogan Martha, Dogan Ahmet, Dispenzieri Angela, Edwards William D., Kurtin Paul J., Maleszewski Joseph J. Correlation of histomorphological pattern of cardiac amyloid deposition with amyloid type: a histological and proteomic analysis of 108 cases. Apr;2016 Histopathology. 68:648–656. doi: 10.1111/his.12793. [DOI] [PubMed] [Google Scholar]
- Garcia-Pavia Pablo, Rapezzi Claudio, Adler Yehuda, Arad Michael, Basso Cristina, Brucato Antonio, Burazor Ivana, Caforio Alida L. P., Damy Thibaud, Eriksson Urs, Fontana Marianna, Gillmore Julian D., Gonzalez-Lopez Esther, Grogan Martha, Heymans Stephane, Imazio Massimo, Kindermann Ingrid, Kristen Arnt V., Maurer Mathew S., Merlini Giampaolo, Pantazis Antonis, Pankuweit Sabine, Rigopoulos Angelos G., Linhart Ales. Diagnosis and treatment of cardiac amyloidosis: a position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Apr;2021 Eur Heart J. 42:1554–1568. doi: 10.1093/eurheartj/ehab072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönland Stefan O., Hegenbart Ute, Bochtler Tilmann, Mangatter Anja, Hansberg Marion, Ho Anthony D., Lohse Peter, Röcken Christoph. Immunohistochemistry in the classification of systemic forms of amyloidosis: a systematic investigation of 117 patients. Jan;2012 Blood. 119:488–493. doi: 10.1182/blood-2011-06-358507. [DOI] [PubMed] [Google Scholar]
- Vaidya Gaurang N., Patel Jignesh K., Kittleson Michelle, Chang David H., Kransdorf Evan, Geft Dael, Czer Lawrence, Vescio Robert, Esmailian Fardad, Kobashigawa Jon A. Intermediate-term outcomes of heart transplantation for cardiac amyloidosis in the current era. Jun;2021 Clin Transplant. 35:e14308–e14308. doi: 10.1111/ctr.14308. [DOI] [PubMed] [Google Scholar]
- Griffin Jan M., Rosenblum Hannah, Maurer Mathew S. Pathophysiology and Therapeutic Approaches to Cardiac Amyloidosis. May;2021 Circ Res. 128:1554–1575. doi: 10.1161/CIRCRESAHA.121.318187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer Mathew S., Schwartz Jeffrey H., Gundapaneni Balarama, Elliott Perry M., Merlini Giampaolo, Waddington-Cruz Marcia, Kristen Arnt V., Grogan Martha, Witteles Ronald, Damy Thibaud, Drachman Brian M., Shah Sanjiv J., Hanna Mazen, Judge Daniel P., Barsdorf Alexandra I., Huber Peter, Patterson Terrell A., Riley Steven, Schumacher Jennifer, Stewart Michelle, Sultan Marla B., Rapezzi Claudio, ATTR-ACT Study Investigators Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. Sep;2018 N Engl J Med. 379:1007–1016. doi: 10.1056/NEJMoa1805689. [DOI] [PubMed] [Google Scholar]
- Adams David, Gonzalez-Duarte Alejandra, O'Riordan William D., Yang Chih-Chao, Ueda Mitsuharu, Kristen Arnt V., Tournev Ivailo, Schmidt Hartmut H., Coelho Teresa, Berk John L., Lin Kon-Ping, Vita Giuseppe, Attarian Shahram, Planté-Bordeneuve Violaine, Mezei Michelle M., Campistol Josep M., Buades Juan, Brannagan Thomas H., Kim Byoung J., Oh Jeeyoung, Parman Yesim, Sekijima Yoshiki, Hawkins Philip N., Solomon Scott D., Polydefkis Michael, Dyck Peter J., Gandhi Pritesh J., Goyal Sunita, Chen Jihong, Strahs Andrew L., Nochur Saraswathy V., Sweetser Marianne T., Garg Pushkal P., Vaishnaw Akshay K., Gollob Jared A., Suhr Ole B. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. Jul;2018 N Engl J Med. 379:11–21. doi: 10.1056/NEJMoa1716153. [DOI] [PubMed] [Google Scholar]
- Benson Merrill D., Waddington-Cruz Márcia, Berk John L., Polydefkis Michael, Dyck Peter J., Wang Annabel K., Planté-Bordeneuve Violaine, Barroso Fabio A., Merlini Giampaolo, Obici Laura, Scheinberg Morton, Brannagan Thomas H., Litchy William J., Whelan Carol, Drachman Brian M., Adams David, Heitner Stephen B., Conceição Isabel, Schmidt Hartmut H., Vita Giuseppe, Campistol Josep M., Gamez Josep, Gorevic Peter D., Gane Edward, Shah Amil M., Solomon Scott D., Monia Brett P., Hughes Steven G., Kwoh T. Jesse, McEvoy Bradley W., Jung Shiangtung W., Baker Brenda F., Ackermann Elizabeth J., Gertz Morie A., Coelho Teresa. Inotersen Treatment for Patients with Hereditary Transthyretin Amyloidosis. Jul;2018 N Engl J Med. 379:22–31. doi: 10.1056/NEJMoa1716793. [DOI] [PubMed] [Google Scholar]
- Grogan Martha, Gertz Morie, McCurdy Arleigh, Roeker Lindsey, Kyle Robert, Kushwaha Sudhir, Daly Richard, Dearani Joseph, Rodeheffer Richard, Frantz Robert, Lacy Martha, Hayman Suzanne, McGregor Christopher, Edwards Brooks, Dispenzieri Angela. Long term outcomes of cardiac transplant for immunoglobulin light chain amyloidosis: The Mayo Clinic experience. Jun;2016 World J Transplant. 6:380–388. doi: 10.5500/wjt.v6.i2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtenberg Barry H., Kamble Rammurti T., Rice Lawrence, Araujo-Gutierrez Raquel, Bhimaraj Arvind, Guha Ashrith, Park Myung H., Hussain Imad, Bruckner Brian A., Suarez Erik E., Victor David W., Adrogue Horacio E., Baker Kelty R., Estep Jerry D. Delayed autologous stem cell transplantation following cardiac transplantation experience in patients with cardiac amyloidosis. Oct;2019 Am J Transplant. 19:2900–2909. doi: 10.1111/ajt.15487. [DOI] [PubMed] [Google Scholar]
- Kristen Arnt V., Kreusser Michael M., Blum Patrick, Schönland Stefan O., Frankenstein Lutz, Dösch Andreas O., Knop Benjamin, Helmschrott Matthias, Schmack Bastian, Ruhparwar Arjang, Hegenbart Ute, Katus Hugo A., Raake Philip W. J. Improved outcomes after heart transplantation for cardiac amyloidosis in the modern era. May;2018 J Heart Lung Transplant. 37:611–618. doi: 10.1016/j.healun.2017.11.015. [DOI] [PubMed] [Google Scholar]
- Griffin Jan M., Chiu Leonard, Axsom Kelly M., Bijou Rachel, Clerkin Kevin J., Colombo Paolo, Cuomo Margaret O., De Los Santos Jeffeny, Fried Justin A., Goldsmith Jeff, Habal Marlena, Haythe Jennifer, Helmke Stephen, Horn Evelyn M., Latif Farhana, Hi Lee Sun, Lin Edward F., Naka Yoshifumi, Raikhelkar Jayant, Restaino Susan, Sayer Gabriel T., Takayama Hiroo, Takeda Koji, Teruya Sergio, Topkara Veli, Tsai Emily J., Uriel Nir, Yuzefpolskaya Melana, Farr Maryjane A., Maurer Mathew S. United network for organ sharing outcomes after heart transplantation for al compared to ATTR cardiac amyloidosis. Oct;2020 Clin Transplant. 34:e14028–e14028. doi: 10.1111/ctr.14028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panhwar Muhammad S., Al-Kindi Sadeer G., Tofovic David, Oliveira Guilherme H., Ginwalla Mahazarin. Waitlist Mortality of Patients With Amyloid Cardiomyopathy who Are Listed for Heart Transplantation and Implications for Organ Allocation. Sep;2019 J Card Fail. 25:767–771. doi: 10.1016/j.cardfail.2019.04.011. [DOI] [PubMed] [Google Scholar]
- Colvin M., Smith J. M., Ahn Y., Skeans M. A., Messick E., Goff R., Bradbrook K., Foutz J., Israni A. K., Snyder J. J., Kasiske B. L. OPTN/SRTR 2019 Annual Data Report: Heart. Feb;2021 Am J Transplant. 21 Suppl 2:356–440. doi: 10.1111/ajt.16492. [DOI] [PubMed] [Google Scholar]
- Rosenbaum Andrew N., AbouEzzeddine Omar F., Grogan Martha, Dispenzieri Angela, Kushwaha Sudhir, Clavell Alfredo, Daly Richard C., Edwards Brooks S. Outcomes After Cardiac Transplant for Wild Type Transthyretin Amyloidosis. Nov;2018 Transplantation. 102:1909–1913. doi: 10.1097/TP.0000000000002240. [DOI] [PubMed] [Google Scholar]


