Abstract
Crohn’s disease (CD) is caused by immune, environmental, and genetic factors. It can involve the entire gastrointestinal tract, and although its prevalence is rapidly increasing its etiology remains unclear. Emerging biological and small-molecule drugs have advanced the treatment of CD; however, a considerable proportion of patients are non-responsive to all known drugs. To achieve a breakthrough in this field, innovations that could guide the further development of effective therapies are of utmost urgency. In this review, we first propose the innovative concept of pan-lymphatic dysfunction for the general distribution of lymphatic dysfunction in various diseases, and suggest that CD is the intestinal manifestation of pan-lymphatic dysfunction based on basic and clinical preliminary data. The supporting evidence is fully summarized, including the existence of lymphatic system dysfunction, recognition of the inside-out model, disorders of immune cells, changes in cell plasticity, partial overlap of the underlying mechanisms, and common gut-derived fatty and bile acid metabolism. Another benefit of this novel concept is that it proposes adopting the zebrafish model for studying intestinal diseases, especially CD, as this model is good at presenting and mimicking lymphatic dysfunction. More importantly, the ensuing focus on improving lymphatic function may lead to novel and promising therapeutic strategies for CD.
Keywords: Inflammatory bowel disease, Crohn’s disease, Lymphatic system, Inside-out model, Immune cells, Zebrafish
Core Tip: The lymphatic system plays an active role in the pathogenesis, progression, and complications of certain diseases. Our review proposes an innovative concept of pan-lymphatic dysfunction and suggests Crohn’s disease (CD) as the intestinal manifestation of pan-lymphatic dysfunction using basic and clinical preliminary data, which may bring new perspectives to both the scientific study and clinical management of CD.
INTRODUCTION
The lymphatic system in humans consists of lymphatic vessels and lymphoid tissues and organs, allowing the unidirectional transport of fluid, cells, and molecules[1], and thereby playing a functional role in fluid homeostasis, immune cell trafficking, and lipid absorption[2,3]. Lymphatic system dysfunction has been demonstrated in various conditions including obesity, cardiovascular disease, chronic inflammation, atherosclerosis, neurological disorders, hypertension, and elephantiasis[4,5]. Recently emerging novel technologies, such as single-cell analysis and intravital imaging, have revealed novel characteristics of lymphatic dysfunction in various diseases and the extensive heterogeneity of lymphatic vessels[6]. Moreover, interventions targeting the lymphatic system have yielded ground-breaking results. Nevertheless, the prevalence and impact of disease-associated lymphatic dysfunction remain underestimated. Therefore, we propose an innovative concept of disease-associated pan-lymphatic dysfunction as an updated theory using the gut, which acts as a reservoir of bacteria and immune cells and has a lymphatic system which plays a predominant role in inflammation and immune regulation, as an example[4,7,8]. Among the various gut-associated diseases, the importance of the lymphatic system in Crohn’s disease (CD) has received increasing attention, but has not yet been systematically summarized. Here, we review the molecular, cellular, and clinical evidence for lymphatic dysfunction in CD and propose that CD is the intestinal manifestation of pan-lymphatic dysfunction. The benefits of this concept for animal model development and clinical management are also discussed.
RATIONALE FOR CONSIDERING CD AS THE INTESTINAL MANIFESTATION OF PAN-LYMPHATIC DYSFUNCTION
CD is a form of inflammatory bowel disease (IBD) characterized by transmural damage and skip lesions. It can involve the entire gastrointestinal (GI) tract but the most common affected segments are the terminal ileum and the colon[9]. Although the mechanism underlying the development of CD remains unknown, a dysregulated immune system, altered microbiota, genetic susceptibility, and environmental factors are major contributors to its onset and progression[10]. The long-term therapeutic effects of commonly used drugs are unsatisfactory, and approximately 80% of patients require surgery 20 years after disease onset[11]. Furthermore, these drugs are associated with various adverse effects and uncontrolled immunogenicity[2,4]. Because mucosal healing is the optimal therapeutic outcome[10], it is crucial to understand the pathogenesis and processes of mucosal pathological alterations in CD. We provide evidence to suggest that CD is the intestinal manifestation of pan-lymphatic dysfunction (Figure 1), which may lead to the development of novel therapeutic strategies and the optimization of CD management.
Figure 1.
Flowchart for Crohn’s disease as the intestinal manifestation of pan-lymphatic dysfunction. The following factors are considered: inside-out model, fatty acid/bile acid metabolism, cellular/molecular mechanisms, and zebrafish as a novel fine animal model. CD: Crohn’s disease; PBA: Primary bile acid; SBA: Second bile acid.
Pathological changes of the lymphatic system supporting its fundamental involvement in CD
The gut lymphatic system is composed of lymphatic vessels, mesenteric lymphatic nodes (MLNs), and gut-associated lymphatic tissues, including Peyer’s patches (PPs) and isolated lymphoid follicles. Lacteals (also called lymphatic capillaries) and submucosal and mesenteric lymphatic vessels represent the three structural levels of the intestinal lymphatic vasculature[12]. Loss or dysfunction of the intestinal lymphatics causes severe gut inflammation, infection, and sepsis, with 100% lethality by 60 h in a mouse model[12,13]. Several functional and morphological alterations in the lymphatic system are well-recognized features of CD, including lymphangiogenesis, lymphadenopathy, and lymphatic vessel dysfunction[5,14]. Patients with CD have a higher density of lymphatic vessels than controls[15]. However, their functional fluid drainage and anti-inflammatory capacities are impaired[16], demonstrating the lymphatic phenomenon of “increased quantity but decreased quality” in CD. Lymphatic vessel dysfunction, including lymphangiectasia and lymphangitis, leads to lymphatic hyperpermeability, interstitial edema, lymphostasis, granulomatous inflammatory response, and lymphatic vasculature obstruction[17,18]. Button junctions, which are closely associated with lymphatic capillary permeability and intestinal material exchange, are lost during inflammation[12]. The submucosal and mesenteric collecting lymphatic vessels are also damaged during intestinal inflammation, as shown by increased junction permeability, flawed valves, and decreased smooth muscle pumping activity[12,19].
Previous studies have revealed several intriguing phenomena suggesting a lymphatic basis of CD. Initial lesions in CD occur in the lymphoid follicles and PPs in the colon and ileum, respectively[20]. Moreover, intestinal lymphatics are intimately associated with the gross segmental distribution of intestinal lesions, with longer CD segments in the ileum consistent with longer lymphatic collecting ducts[14]. The microscopic characteristics of CD further support the active involvement of lymphatics, as granulomas appear in and around lymphatic vessels[21]. Furthermore, several studies have indicated that granulomas in the MLNs of patients with CD may cause lymphatic obstruction[21] and increase the risk of recurrence after ileocolonic resection[22]. From a clinical standpoint, previous studies have indicated that decreased lymphatic vessel density is associated with disease and the postoperative endoscopic recurrence of CD[23].
Inside-out model of CD pathogenesis reveals the long-ignored pathological effect of the lymphatic system in CD
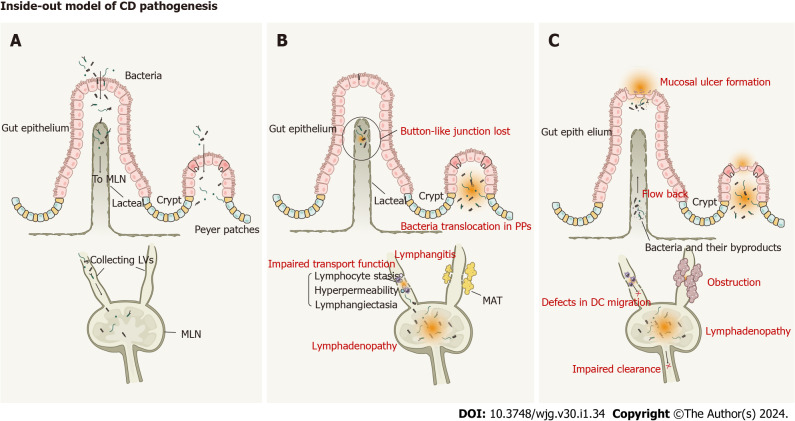
Currently, there are two opposing models of CD pathogenesis: outside-in and inside-out[24]. The conventional outside-in model regards luminal bacteria-induced mucosal damage as the initial event in CD and submucosal injury as the ensuing terminal event. However, some evidence contradicts this theory of a primary mucosal injury. A study on nucleotide-binding oligomerization domain-2 (NOD2)-/- mice revealed that macrophages, rather than epithelial cells, are the predominant abnormal cell type during gut inflammation[25]. Therefore, the long-overlooked inside-out model, in which infection or inflammation by intracellular bacteria and their metabolites in the intestinal lymphatic system is the initial event in CD, leading to mucosal inflammation, may be better supported by the evidence. This model, which emphasizes the significance of the lymphatic system, supports our hypothesis of CD as a pan-lymphatic dysfunction and can be divided into three phases (Figure 2).
Figure 2.
Inside-out model of Crohn’s disease pathogenesis. A: Phase I, intracellular bacteria infect into lymphatic system with few signs of intestinal mucosal injury; B: Phase II, Pathogens infect and persist in intestinal lymphatic tissues, which causes an ‘immunological scar’ in the intestinal lymphatic system. The impaired transport function, lymphangitis, lymphadenopathy, loss of button-like junctions, bacteria translocation to mesenteric lymph nodes and Peyer’s patches, and mesenteric adipose tissue formation have been found in the pathogenesis of Crohn’s disease (CD); C: Phase III, Mucosal injury as the terminal event of CD. Lymphatic dysfunction, including impaired clearance, defective dendritic cells migration, and obstruction, provides opportunities for bacteria and their by-products flowing back to the draining lymphatic vessels and causing mucosal lesions. DC: Dendritic cells; MLN: Mesenteric lymph nodes; PPs: Peyer’s patches.
Phase I: Infection by intracellular bacteria: The inside-out model proposes that infection by intracellular bacteria and their metabolites occurs as the first stage of CD pathogenesis without obvious mucosal pathology. The importance of gut bacteria in CD was shown by a previous study in which ileitis and colitis did not occur in the absence of bacterial flora[14]. Genetic studies have revealed several specific genes associated with CD, including NOD2, ATG16L1, and IRGM[26]. The proteins encoded by these genes are involved in intracellular pathogen elimination via autophagy, indicating that this process is impaired in CD. Several studies have shown that MLN dysbiosis in CD is characterized by an overabundance of Proteobacteria, such as Escherichia, Shigella, Helicobacter, and Salmonella[20,27]. Intriguingly, Salmonella can invade PPs and MLNs with few signs of intestinal mucosa injury[28]. Adherent invasive Escherichia coli strains isolated from CD patients can survive within macrophages without inducing cell death[29]. These findings suggest that intestinal bacteria can invade the mucosa without resultant inflammation. Importantly, a study on postoperative recurrence in patients with CD showed significant inflammatory cell infiltration of the lamina propria, whereas only small intestinalulcers were detected in the ileal mucosa[24]. Based on this evidence, we suspect that bacterial invasion of the lymphatic system occurs prior to the development of mucosal lesions.
Phase II: Pathogens infect and persist in intestinal lymphatic tissues: The inside-out model states that pathogen invasion of MLNs through lymphatic vessels causing persistent infection is critical for CD progression. Pathological bacterial translocation (PBT) to MLNs is well established in CD[20]; detection of bacterial DNA in MLNs using high-throughput sequencing indicates that PBT is a non-selective process[30]. A study on acute intestinal infection by Yersinia pseudotuberculosis revealed that an ‘immunological scar’ persists in the gut lymphatics even after pathogen eradication[31]. Remodeling of the lymphatic system and a deviation of the immune response disrupts communication between the tissue and the immune system, compromising homeostasis. Notably, there are many similarities between Y. pseudotuberculosis infection and CD. Lymphangitis and lymphatic vascular dysfunction are also common in CD. More importantly, PBT to MLNs[20,32] and mesenteric adipose tissue[20,33] as well as defects in dendritic cell (DC) migration[33,34] have been described in CD. Moreover, CD-induced lymphadenopathy remains after the resolution of acute intestinal inflammation in a dextran sodium sulfate (DSS) model of colitis[35]. Therefore, we speculate that such an ‘immunological scar’ also exists in CD, which alters GI immunity and the lymphatic system, ultimately leading to persistent intestinal lesions.
Phase III: Mucosal injury as the terminal event of CD: Mucosal injury is considered the terminal event in the inside-out model. A breakthrough study used formalin injections to block certain segments of the mesenteric lymphatics of the small intestine, establishing experimental enteritis resembling CD[24]. This indicates that intestinal lymphatic blockage or malfunction are essential for the initiation of mucosal injuries. Bacteria and their by-products are retained in the MLNs. During intestinal inflammation, mesenteric collecting vessel function is impaired owing to increased junction permeability, defective valves, and reduced smooth muscle cell pumping activity[19]. This lymphatic dysfunction provides opportunities for bacteria and their by-products to flow back into the draining lymphatic vessels, aggravating intestinal inflammation and causing mucosal lesions. Moreover, the compromised integrity and hyperpermeability of lymphatic vessels prevent the routine drainage of antigens and immune cells into the MLN. In SAMP1/YitFc mice, inhibiting DC trafficking to lymph nodes induced ileitis resembling CD[36].
Interactions between immune cells and the lymphatic system and the immune dysfunction caused by CD further support the importance of the lymphatic system
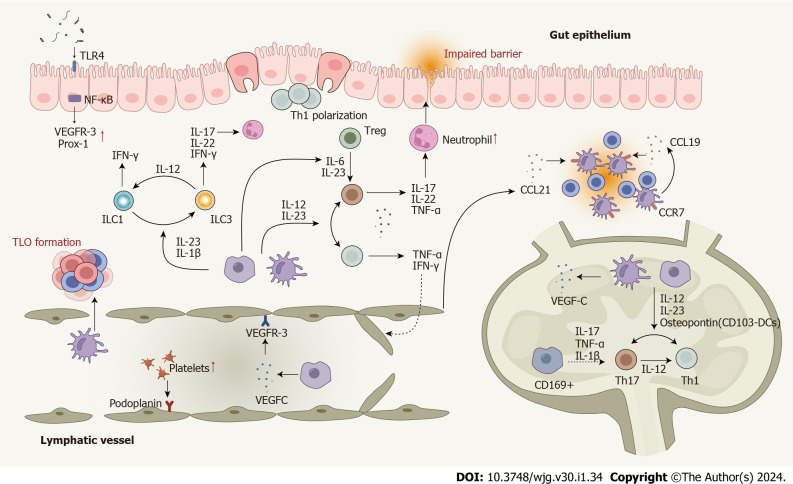
The gut lymphatic system and the immune cells within it not only play a critical role in the pathogenesis of CD[37] but are also influenced by the immune dysfunction induced in CD, revealing a two-way interaction. In addition, immune and non-immune cells have been shown to exhibit increased plasticity in CD to compensate for lymphatic dysfunction, with enhanced lymphatic system contact, lymphatic phenotype changes, and expression of lymphocyte surface markers. A partial overlap between the molecular mechanisms involved in lymphatic function and CD was also identified; this complex network is summarized at both the cellular and molecular levels in Figure 3.
Figure 3.
Dysregulated cells, cytokines, and enhanced cell plasticity in the lymphatic system in Crohn’s disease. T cells are polarized to Th1 and Th17 cells in response to pro-inflammatory cytokines produced by macrophages and dendritic cells (DCs). Several cytokines play roles in Th17-Th1 and Treg-Th17 trans-differentiation. DCs, macrophages, T cells, and B cells contribute to lymphangiogenesis by mediating the expression of lymphangiogenic factors. Impaired lymphatic function and dysregulated chemokines lead mature DCs and T cells away from their usual mesenteric lymph nodes. Innate lymphoid cells (ILCs) also contribute to Crohn’s disease (CD) pathogenesis and enhanced plasticity between ILC1 and ILC3 has been observed. Several signaling pathways involved in the lymphatic system actively participate in the CD pathogenesis. Nuclear factor-kappa B is involved in lymphatic remodeling by directly up-regulating the expression of VEGFR-3 and Prox-1. The mTOR signaling affects leukocyte trafficking through the lymphatic barrier in CD. CD: Crohn’s disease; DC: Dendritic cells; IFN: Interferon; IL: Interleukin; ILC: Innate lymphoid cell; NF-κB: Nuclear factor-kappa B; Prox-1: Prospero homeobox protein 1; Th: T helper; TLO: Tertiary lymphoid organ; TLR4: Toll-like receptor 4; TNF: Tumor necrosis factor; Treg: Regulatory T cell; VEGF: Vascular endothelial growth factor; VEGFR: Vascular endothelial growth factor receptor; MAT: Mesenteric adipose tissue.
Immune cell disorders and changes of cell plasticity further support the importance of the lymphatic system in CD: Lymphatic dysfunction contributes to CD pathogenesis by altering the influx of immune cells. Importantly, CD initiation is tightly correlated with B and T cell trafficking between the bloodstream, intestinal mucosa, and lymphatic system[38,39]. An increasing number of studies have suggested that the unique cytokine milieu produced by immune cells in MLNs leads to a dysregulated T helper (Th)1/Th17 immune response in CD[7,38,40]. Th1 cells mainly secrete tumor necrosis factor (TNF)-α and interferon (IFN)-γ while Th17 cells primarily produce interleukin (IL)-17, IL-22, and TNF-α[41]. Th1 polarization has been detected in the PPs of patients with active CD[42] and an increase in specific pro-inflammatory DC subsets has been observed in the MLNs of both animal models of colitis and patients with CD[43,44]. Moreover, long-lasting ‘immunological scars’ promote DC migration and increase susceptibility to further infections[35]. The impairment of DC migration due to lymphatic dysfunction and dysregulated chemokines in CD leads to mature DCs moving away from their usual MLNs and causes the proliferation of T cells, contributing to the formation of tertiary lymphoid tissue[34,45]. Macrophages are involved in both acute inflammation and inflammation-associated lymphangiogenesis[46]. CD169+ macrophages differ from M1 and M2 macrophages and are located in MLNs, where they produce high levels of pro-inflammatory cytokines and may participate in Th17 responses[7]. Type 3 innate lymphoid cells (ILCs), the inappropriate activation of which causes IL-17 and IL-22 overproduction, are responsible for CD pathogenesis and progression[47]. Importantly, ILC3 also play a critical role in the generation of lymphoid structures[48], further supporting the role of the lymphatic system in CD.
Disordered immune cells are directly associated with alterations in the lymphatic vasculature. The observed increase in tissue macrophages, T cells, and neutrophils in CD reflects the failure of the direct clearance of inflammatory cells due to impaired lymphatic function[49]. Structural (dilated torturous lymphatic vessels) and functional (greater submucosal edema, higher immune cell burden) changes were associated with elevated colonic neutrophil, macrophage, and T cell infiltration in a mouse model of experimental colitis[50]. DCs, monocytes, mast cells, macrophages, T cells, and B cells are actively involved in the induction of lymphangiogenesis during inflammation by mediating the expression of lymphangiogenic factors[50]. B cells and ILCs have been observed in the inflamed bowel walls of patients with CD and may contribute to lymphatic remodeling[34]. Impaired lymphatic transport exacerbates the accumulation of inflammatory cells[49], forming a vicious cycle of lymphatic dysfunction in CD.
Several cell types show enhanced cell plasticity with altered characteristics and functions when exposed to an inflammatory milieu. For instance, lymphocytes from MLNs display Th1 and Th17 characteristics in CD, while regulatory T cell (Treg)-Th17 and Th17-Th1 trans-differentiation has also been observed[51]. In addition, an imbalance between ILC3 and ILC1 was detected in the gut mucosa of patients with CD, and the possibility of ILC3 deviating towards ILC1 was identified[47]. Interestingly, monocytes can present lymphatic phenotypes and express lymphatic endothelial markers such as lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1), prospero homeobox protein 1 (Prox-1), and podoplanin in certain inflammatory environments[52]. Finally, macrophages play a critical role in promoting lymphangiogenesis in response to lymphatic dysfunction caused by inflammation[46]; lymphangiogenic macrophages may differentiate into lymphatic endothelial cells (LECs) and be directly incorporated into lymphatic vessels[50].
Cells other than immune cells also interact with the lymphatic system and play pathological roles in CD. Platelet aggregation in lymphatic vessels may prevent blood flow. Increased platelet levels are often observed in patients with severe CD[53], suggesting that aggregation of platelets in these patients may decrease the unidirectional flow of lymph fluid. Moreover, platelets can interact with podoplanin and inhibit the proliferation of LECs, suppressing the formation of lymphatic vessels and lymphatic-venous connections[54,55]. Intestinal stem cells (ISCs) maintain the epithelium by replacing cells through self-differentiation[12]. A previous study showed that depletion of ISC niche factors alters epithelial differentiation in a mouse model of CD-like ileitis[56]. Interestingly, crypt lymphatics act as an important source of niche factors for ISC[57], consistent with the critical role of LECs in ISC homeostasis and injury-mediated regeneration[58]. Therefore, lymphatic dysfunction and obstruction can directly influence ISCs, leading to epithelial barrier impairment.
Partial overlap of lymphatic system and CD molecular mechanisms reinforces crosstalk: Several regulatory pathways in the intestinal lymphatic system are involved in the pathogenesis of CD. For instance, vascular endothelial growth factor (VEGF)-C-VEGF receptor (VEGFR)3 signaling is critical to maintain intestinal lymphatic function during inflammation. VEGFR3 inhibition aggravates colitis symptoms in animal models[59]. In contrast, VEGF-C has a protective function in experimental colitis, causing increased inflammatory cell mobilization and bacterial antigen clearance from the inflamed site to the draining lymph nodes by signal transducer and activator of transcription 6-dependent macrophages[60]. The lacteals play significant roles in dietary fat absorption and the gut immune response[50], and are continuously regenerated through cell proliferation mediated by signaling via Notch and its ligand delta-like-4 (DLL4)[12,61]. Notably, the expression levels of Notch signaling-related genes and DLL4 were decreased in SAMP1 mice that spontaneously develop CD-like ileitis[56]. The VEGFR3-phosphoinositide 3-kinase (PI3K) signaling pathway is another important regulatory axis in lymphatic vasculature development, and activation of the PI3K-AKT-mammalian target of rapamycin (mTOR) pathway is involved in CD pathogenesis[62]. The mTOR signaling pathway plays a pivotal role in CD[63] and a recent study revealed its active involvement in intestinal lymphatic function through effects on leukocyte trafficking in patients with CD[64]. Additionally, angiopoietin-TIE signaling contributes to whole-body lymphatic vessel development and is important for mesenteric lymphatic development and maintenance[65,66]. Intriguingly, dysregulated angiopoietin levels have been observed in patients with CD[67] and the targeting of this pathway with thalidomide represents a potential treatment strategy[68].
Toll-like receptor 4 (TLR4) is an important component of inflammatory signaling pathways in the GI tract[69]. A previous study suggested that TLR4 expressed on enterocytes plays an important role in phagocytosis and bacterial translocation across the intestinal barrier[70]. TLR4 activation also mediates mesenteric lymphatic alterations in a DSS mouse model of colitis[71]. The major downstream component of the TLR4 signaling pathway, nuclear factor-kappa B (NF-κB), also plays a significant role in inflammation regulation, and its inappropriate activation has been described in CD[72]. Furthermore, NF-κB contributes to lymphatic remodeling by directly up-regulating VEGFR3 and Prox-1 expression[73]. Sphingosine-1-phosphate (S1P) is a pleiotropic lipid mediator that plays a significant role in inflammation by regulating lymphocyte trafficking[74]. The reduction of lymphocyte gut homing by S1P receptor agonists has shown profound therapeutic effects in both colitis models and patients with CD[75-77].
Inflammatory cytokines produced by various cells play central roles in intestinal inflammation caused by CD and contribute to lymphatic structure dysregulation. IL-17 recruits neutrophils, thereby enhancing intestinal permeability[47]. TNF is a significant factor in CD and its close relationship with the lymphatic system has been demonstrated. TNF can also disrupt lymph flow and immune cell migration through impaired lymphatic valves and ileitis-associated tertiary lymphoid organs[78]. The NOD2 mutation associated with CD affects IL-1β expression and processing. IL-1β stimulates lymphatic proliferation but down-regulates angiopoietin 1 expression[15], contributing to the impaired integrity of, and leakage from, lymphatic vessels. Also, IL-1β and IL-23 participate in ILC3-ILC1 plasticity[47]. IL-3 is another critical regulator of lymphatic development that controls LYVE-1 and podoplanin expression. Notably, a reduction in monocyte-derived IL-3 in the lamina propria has been observed in patients with CD[79]. IL-6, produced by monocytes, macrophages, and endothelial cells, is pivotal for intestinal inflammation in CD, as it controls the balance between pro-inflammatory T cells and Tregs[80]. The presence of IL-6 and/or IL-23 can stimulate the differentiation of Tregs into Th17 cells[41]. Prox1 is a lymphatic-specific transcription factor, and Prox1 knockout mice do not develop any lymphatic vascular structures and experience increased lymph leakage[81]. Decreased Prox1 Levels are associated with the postoperative recurrence of CD[82]. Higher levels of CCL21 and CCL19 have been detected at the site of inflammation in patients with CD; these chemokines recruit mature DCs and proliferating T cells through their ligand, CCR7[45]. The chemokine decoy receptor D6 can limit inflammation by scavenging inflammatory chemokines; a significant increase in the levels of this receptor has been reported in the lymphatic vessels of patients with CD[8]. D6-/- mice have higher levels of pro-inflammatory chemokines than controls, and are more susceptible to experimental colitis[83]. Glucagon-like peptide 1 (GLP-1) is the classic incretin involved in glucose regulation, and its postprandial levels are higher in the gut lymph than in the blood[84]. Abnormal postprandial GLP-1 secretion has been observed in patients with CD[85].
Gut-derived fatty and bile acid metabolism mediate lymphatic dysfunction in CD
The pathogenesis of CD is closely related to dysbiosis of the intestinal microbiota. Metabolomic analysis revealed that the intestinal microbiota imbalance is characterized by changes in bile and fatty acid metabolism[86]. Recently, bile and fatty acid dysregulation has been emphasized in CD because they are closely related to intestinal homeostasis and inflammation[87,88]. Moreover, their association with the lymphatic system has been suggested.
Lymphatic vessels play a significant role in lipid absorption[5] and provide the main cholesterol drainage route from the interstitium[89]. A previous study showed that cholesterol synthesis and absorption is altered by the low serum levels of total cholesterol, low-density lipoprotein cholesterol, and high-density lipoprotein (HDL) cholesterol detected in active CD[90], reflecting the impaired drainage of lymphatic vessels. HDL cholesterol exerts anti-inflammatory effects in CD models[91] and affects LECs in the presence of pro-inflammatory cytokines[5]. Importantly, abnormal lymphatic drainage can lead to chylomicron leakage and fat accumulation around the mesenteric tissues[34], forming creeping fat, a recently recognized feature of CD. Creeping fat plays a significant role in intestinal fibrosis and stricture formation in CD[92]. It is also a vital target site for PBT in CD[20], serving as a reservoir for pathological bacteria that results in persistent inflammation. MAT hypertrophy in patients with CD also influences lymphoid dysfunction through the activated NF-κB signaling pathway[93]. Additionally, the aberrant interaction of perinodal adipose tissue (PAT) and the lymphatic system might contribute to CD by reducing the polyunsaturated fatty acid supply from PAT to MLNs[94]. Intriguingly, adipocytes surrounding draining lymph nodes perform immunological functions after subcutaneous bacterial infection[95].
Bile acids (BAs) are primarily reabsorbed in the terminal ileum and colon, and malabsorption of BAs is a well-established characteristic of patients with CD. Further metabolomic studies have shown impaired BA metabolism in patients with CD, with an increase in primary BAs (PBAs) and a reduction in secondary BAs (SBAs). Moreover, interactions between BAs and various immune cells, including ILC3, Th17 cells, DCs, monocytes, and macrophages, have been reported[87]. For example, SBAs and their derivatives can inhibit Th17 differentiation and promote macrophage and DC differentiation[87,96], which ultimately suppresses gut immune responses. Intriguingly, previous studies have demonstrated that bile flow obstruction leads to mucosal injury with intestinal hyperpermeability and bacterial overgrowth, and increased bacterial translocation to MLNs[97]. However, the role of the lymphatic system in BA-related CD pathogenesis requires further investigation.
RECOGNIZING THE IMPORTANCE OF THE LYMPHATIC SYSTEM IN CD USING ZEBRAFISH AS A RESEARCH MODEL
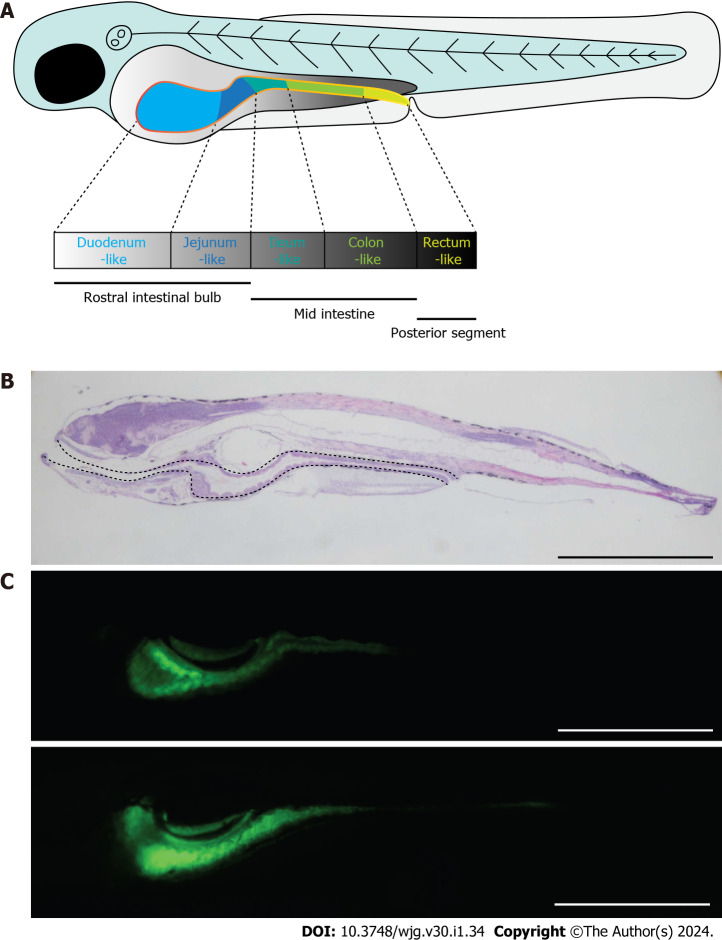
Owing to their rapid development, transparent embryos and larvae, small size, and strong reproductive ability, zebrafish have become a popular model for studying embryonic development and human disease. Importantly, zebrafish are similar to mammals, not only in embryonic development, gene regulation networks, and organ morphology and physiology, but also in the pathological processes of many human diseases. Moreover, high-throughput genetic and drug screening can be easily performed in zebrafish[98]. In addition, the cellular composition and architectural organization of the intestinal tract are highly conserved between zebrafish and mammals. Similar to mammals, the enteric nervous system (ENS) of zebrafish is derived from neural crest cells and is comparable to mouse and human ENS based on gene expression and functional studies[99]. Therefore, zebrafish are ideal for studying human intestinal diseases (Figure 4).
Figure 4.
Zebrafish as a good candidate model for the study of Crohn’s disease. A: Schematic diagram showing the morphology of zebrafish embryo at 5 dpf. The solid yellow line indicates the intestine of the embryo; B: Morphology of zebrafish embryo visualized by hematoxylin and eosin staining of embryonic sections at 5 dpf. Sections were cut along the sagittal plane. The dotted line indicates the whole intestine; C: Fluorescence signals of ET33J1: enhanced green fluorescent protein zebrafish reporter (top) and dichloro-dihydro-fluorescein diacetate treatment (bottom) showing the morphology of the whole intestines at 5 dpf (top) and 7 dpf (bottom). Scale bar: 200 µm. dpf: Days post fertilization.
The advantages of studying the lymphatic system in zebrafish[100] are well recognized, and recent studies using zebrafish models have shed light on IBD and drug development[101]. Our work on the zebrafish Feingold syndrome 1 model showed that Mycn loss-of-function leads to severe intestinal developmental defects resulting from proliferation arrest caused by abnormal ribosome biogenesis, which can be rescued through the mTOR pathway with leucine supplementation[102]. Similar to other vertebrates, zebrafish harbor commensal bacteria within their digestive tracts, and the use of gnotobiotic techniques allows for the study of microbial-host interactions. The importance of intestinal alkaline phosphatase in promoting mucosal tolerance to commensal bacteria by dephosphorylating and detoxifying the endotoxin component of lipopolysaccharide was first identified in a zebrafish model[103]. However, despite the accumulation of preliminary data, zebrafish have seldom been used to study CD because CD is not recognized as the intestinal manifestation of a pan-lymphatic disease. Therefore, our proposal may encourage the increased use of powerful zebrafish models for the study of CD.
A zebrafish model of IBD has been established through drug treatment and genetic modifications. Treating zebrafish embryos at 3-6 d post fertilization with 2,4,6-trinitrobenzene sulfonic acid (TNBS), DSS, or nonsteroidal anti-inflammatory drugs can result in bacterial overgrowth, neutrophil infiltration into the gut, upregulation of pro-inflammatory genes, or disruption of epithelial barrier function[104]. Zebrafish lacking macrophage-stimulating protein (MSP) develop spontaneous intestinal inflammation, which supports human genome-wide association study data showing that MSP is an IBD susceptibility factor[105]. Recent research has shown that PI3K deficiency in zebrafish can induce IBD-like features with intestinal injury and inflammation, as well as the suppression of barrier-function-related IBD susceptibility genes[106]. Another study showed that Trmt5-deficient zebrafish spontaneously develop an IBD-like phenotype, including epithelial destruction, goblet cell failure, and an overactive immune system[107]. These zebrafish IBD models not only provide a better understanding of the pathological processes involved in IBD, but also provide powerful models for screening potential drug treatments. Combining publicly available databases, zebrafish chemical screening, machine learning, and mouse preclinical models, a recent study identified environmental factors that control intestinal inflammation, and revealed that the AHR-NF-κB-C/EBPβ signaling axis in T cells and DCs promotes intestinal inflammation and can be targeted by propyzamide[108]. Overall, the use of zebrafish as a model has greatly accelerated the study of intestinal diseases and the identification of potential treatment strategies.
CLINICAL BENEFITS OF CONSIDERING CD AS THE INTESTINAL MANIFESTATION OF PAN-LYMPHATIC DYSFUNCTION
The acknowledgment of CD as the intestinal manifestation of pan-lymphatic dysfunction may lead to the development of novel treatments targeting the lymphatic system. Studies targeting the lymphatic system are summarized in Tables 1 and 2[55,60,71,76,109-116]. Owing to the significant role of VEGFC-VEGFR3 signaling in lymphangiogenesis, VEGFC administration enhances lymphatic function and ameliorates acute and chronic colitis in animal models[60,117]. However, another study showed that Cartilage Oligomeric Matrix Protein – Angiopoietin-1 (COMP-Ang1) treatment alleviated DSS-induced colitis by diminishing inflammation-associated lymphangiogenesis and reducing macrophage infiltration, which are involved in the inhibition of VEGF-C and VEGF-D expression[109]. Moreover, a study showed that artemisinin ameliorated inflammation-induced lymphangiogenesis through the VEGF-C/VEGFR3 pathway and reduced colitis in animal models[110]. Therefore, the specific role of lymphangiogenesis in CD requires further investigation. Disruption of PBT is another treatment option for CD, and agents aimed at lymphocyte trafficking may have wide applicability[7]. Ozanimod, a new drug targeting the S1P1/5 receptor, significantly reduced the severity of colitis in mice treated with TNBS[118] and also successfully completed phase 2 clinical trials in patients with IBD[75,76]. In addition, phase 2/3 clinical trials of etrasimod, a S1P1/4/5 receptor modulator, are underway in patients with CD (NCT04173273). Considering the concept of pan-lymphatic dysfunction, disrupting lymphocyte trafficking via the S1P pathway may be crucial. TLR4 has been shown to play several roles within mesenteric lymphatic vessels, and its inhibitor C34 significantly reduced the severity of DSS-induced colitis in a mouse model[71]. In addition, a study on the bacterial FimH blocker EB8018, which can inhibit the activation of TLR4, is ongoing (NCT03709628). Furthermore, an anti-Mycobacterium Avium Ssp. Paratuberculosis agent, RHB-104, showed a therapeutic effect in patients with CD, causing lower disease activity and reduced CD activity index scores (NCT03009396).
Table 1.
Current studies (published articles and finished/on-going clinical trials) investigating the effect of targeting lymphatic circulation and related pathophysiology processes, providing strong evidence to support our hypothesis
|
Agent class
|
Agent
|
Mechanism/pathway
|
Drug stage
|
Efficacy
|
Ref.
|
| Targets of lymphangiogenic factors | VEGFC/Ad-hVEGF-C | VEGFC-VEGFR3 signaling pathway; increase lymph drainage and bacteria antigen clearance | Animal model | Reduced severity of chronic colitis in terms of histological examination, body weight, endoscopic evaluation, and CDAI than control group | [60] |
| COMP-Ang1 | Reduce inflammation-induced lymphangiogenesis and M1 and M2 macrophage infiltration by inhibiting VEGF-C/D expression | Animal model | Less weight loss, fewer clinical signs of colitis, and longer colons than control group | [109] | |
| TLR4 inhibitor | C34 | TLR4-PAMP/DAMP discriminatory mechanism | Animal model | Less weight loss, reduced disease activity score and reduced colon shortening in treatment group | [71] |
| Anti-trafficking therapies: S1P receptor modulators | Ozanimod | S1P1/5 receptor modulator; induce internalization and degradation of S1P1/5 receptor subtypes | Phase 2 clinical studies in patients with CD | Endoscopic, histological, and clinical improvements within 12 wk of initiating ozanimod therapy in patients with moderate to severe CD | [76] |
| Phase 3 clinical studies | Recruiting | NCT03440372, NCT03440385, NCT03464097, NCT03467958 | |||
| Etrasimod | S1P1/4/5 receptor modulator | Phase 2/3 clinical studies in patients with CD | Recruiting | NCT04173273 | |
| Antiplatelet antibody | GPIb inhibitor | Promote lymphangiogenesis and increase lymphatic vessel densities | Animal model | Suppressed colitis with reduced thickness of the submucosal layer, reduced inflammatory cell infiltration, and reduced histological score | [55] |
| Anti-bacterial and its related metabolites or secretions1 | EB8018 | Inhibit bacterial lectin (FimH) to stop the activation of TLR4 and ensuing TNF-α production | Open-label, multicenter, pharmacokinetic Study | Finished without results disclosure | NCT03709628 |
| RHB-104 | Anti-MAP (Mycobacterium Avium Ssp. Paratuberculosis | Phase 3 study to assess the efficacy and safety of fixed-dose combination RHB-104 | Number of patients in remission at week 16 was higher in RHB-104 compared with placebo | NCT03009396 | |
| A randomized, double blind, placebo-controlled, multicenter, parallel group study | Reduction of the total CDAI score to less than 150 at week 26 was significantly higher in RHB-104 than placebo | NCT01951326 | |||
| Chemical compound | Artemisinin | Ameliorate inflammation-driven lymphangiogenesis via the VEGFC/VEGFR3 signaling pathway | Animal model | Reduced symptoms of colitis with improved tissue histology, relieved inflammatory edema, and decreased infiltration of inflammatory cells | [110] |
These studies specifically support the inside-out model of the pathogenesis of Crohn’s disease.
CD: Crohn’s disease; CDAI: Crohn’s disease activity index; COMP-Ang1: Cartilage Oligomeric Matrix Protein – Angiopoietin-1; DAMP: damage-associated molecular pattern; PAMP: Pathogen-associated molecular pattern; S1P: Sphingosine-1-phosphate; TLR: Toll-like receptor; TNF: Tumor necrosis factor; VEGF: Vascular endothelial growth factor; VEGFR: Vascular endothelial growth factor receptor.
Table 2.
Current studies (published articles and finished/on-going clinical trials) investigating the effect of targeting lymphatic circulation and related pathophysiology processes, providing moderately strong evidence to support our hypothesis
|
Agent class
|
Agent
|
Mechanism/pathway
|
Drug stage
|
Efficacy
|
Ref.
|
| JAK inhibitors | Filgotinib/GLPG0634 | Selective JAK1 inhibitor; inhibiting JAK-STAT pathway | Phase 2 clinical studies in patients with CD | Filgotinib induced clinical remission in significantly more patients with active CD compared with placebo (47% vs 23%) | [111] |
| Phase 3 clinical studies | Recruiting or finished without result disclosure | NCT02914600, NCT02914561, NCT02048618 | |||
| MT-1303 | Selective JAK1 inhibitor; inhibiting JAK-STAT pathway | Phase II, open-label, multicenter studies for moderate to severe active CD | Finished without results disclosure | NCT02389790, NCT02378688 | |
| Upadacitinib1 | Selective JAK1 inhibitor; Inhibiting JAK-STAT pathway | Multicenter, randomized, double-blind, placebo-controlled induction study of its efficacy and safety in moderate to severe active patients with CD | Upadacitinib induced CDAI remission at week 12 in significantly more patients with active CD compared with placebo (49.5% vs 29.1%) | NCT03345849, NCT03345836 | |
| Anti-trafficking therapies: target of adhesion molecules | AS101 | Inhibit lymphocyte trafficking by blocking ligand for α4β7 integrin, MAdCAM-1 and Il-1β. Regulate the intestinal epithelial barrier by the PI3K/AKT pathway | Animal model | Suppressed colitis with reduced colonic inflammatory cytokine levels, reduced histopathology score and fewer clinical symptoms | [112] |
| Vedolizumab | Inhibit lymphocyte trafficking by block the ligand for α4β7 integrin, MAdCAM-1 | Induction and maintenance study for active CD | The rate of clinical remission is significantly higher in treatment group (300 mg) at week 6 | [113] | |
| Firategrast | α4β7 and α1β7 inhibitor | Randomized, double-blind, placebo-controlled, parallel-group study | Finished without results disclosure | NCT00101946 | |
| Abrilumab | Inhibit lymphocyte trafficking by blocking the ligand for α4β7 integrin, MAdCAM-1 | Phase 1, randomized, double-blind, placebo-controlled, ascending multiple dose study | Finished without results disclosure | NCT01290042 | |
| CCX282-B | Anti CCR9 and its related Ca2+ mobilization and inflammatory cell attraction | Pilot, double-blind, placebo-controlled, parallel group study | Finished without results disclosure | NCT00102921 | |
| Risankizumab | Monoclonal antibody against the p19 subunit of IL-23 | Phase 2 clinical studies in patients with CD | Effective in clinical remission, response, and endoscopic remission at week 12 compared to placebo | [114,115] | |
| NCT03105128 | |||||
| IL-23 inhibitors | Ustekinumab | Monoclonal antibody against p40 subunit of IL-12/IL-23 | Induction and maintenance study for active CD | Rate of response was significantly higher in treatment group (dosage as 130 mg or 6 mg/kg) in both UNITI 1 and UNITI | [116] |
| Brazikumab | Monoclonal antibody against the p19 subunit of IL-23 | Phase 1 clinical trial in healthy people | Finished without results disclosure | NCT05033431 | |
| Other IL inhibitors | Semapimod | IL-1β/IL-6 inhibitors | Open label single arm study for CD | Finished without results disclosure | NCT00740103 |
There are two clinical trials on upadacitinib from the same leading team but with different designs and recruiting patient numbers. For the word limitations of this article, please refer to the relative number of clinical trials required.
CD: Crohn’s disease; CDAI: Crohn’s disease activity index; IL: Interleukin; JAK: Janus kinase; MAdCAM: Mucosal vascular addressin cell adhesion molecule; PI3K: Phosphoinositide 3-kinase.
Antiplatelet agents could be promising targets for CD based on the involvement of platelets in intestinal inflammation. A previous study showed that an antiplatelet antibody, a GPIb inhibitor, ameliorated DSS-induced colitis by promoting lymphangiogenesis and increasing lymphatic vessel density[55]. Other therapeutic agents, such as Janus kinase inhibitors, anti-adhesion molecules, and anti-IL-1β/6/23, could impact the lymphatic system. However, it remains unclear whether changes observed in the lymphatic system are the cause or result of disease improvement. As MLNs are the primary site of CD pathogenesis, excision of MLNs, as well as the mesentery, has been correlated with decreased recurrence of ileocolic CD[27].
The clinical management of the long-term consequences of CD, such as intestinal fibrosis and obstruction, is challenging, and the lack of understanding of the underlying mechanisms has contributed to a shortage of effective therapies. It has been claimed that fibrosis in CD is a consequence of chronic gut edema, which may be caused by lymphatic dysfunction, as decreased lymph flow during chronic inflammation has been observed[119]. Additionally, fatty acids released from creeping fat have been reported to promote muscle proliferation[92], indicating a potential role of creeping fat in fibrosis and obstruction in CD. Therefore, treatments targeting lymphatic dysfunction offer a viable means of managing fibrosis in patients with CD.
CONCLUSION
Based on the evidence presented above, it is reasonable to conclude that the lymphatic system plays an active role in the pathogenesis, progression, and complications of certain diseases. Intriguingly, immune-mediated inflammatory diseases, including IBD, rheumatoid arthritis, and sclerosing cholangitis, emphasize the disease-driving mechanisms and therapeutic strategies shared by these diseases[39]. Similarly, the concept of CD as the intestinal manifestation of pan-lymphatic dysfunction may change our understanding of disease initiation, progression, and treatment. For instance, it has been shown that increased lymphatic vessel density could occur even in non-inflamed areas. Clinical management of the extraintestinal manifestations (EIMs) of CD has long been challenging[10]; the concept of pan-lymphatic dysfunction may provide a new perspective on EIM formation and management. However, unresolved problems require further investigation.
We propose this concept based on accumulating evidence of lymphatic system involvement in CD, including the existence of lymphatic system dysfunction, recognition of the inside-out model, disorders of immune cells, changes in cell plasticity, partial overlap of underlying mechanisms, and gut-derived fatty and bile acid metabolism. This hypothesis offers a larger potential role for zebrafish as a model of CD because of the ability to study lymphatic dysfunction in this model. Importantly, the ensuing focus on improving lymphatic function may lead to the development of novel therapeutic strategies and improve the clinical management of patients with CD.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: September 27, 2023
First decision: October 25, 2023
Article in press: December 27, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ertan A, United States; Gravina AG, Italy S-Editor: Gong ZM L-Editor: A P-Editor: Yuan YY
Contributor Information
Yu-Wei Zhou, Department of Gastroenterology, The First Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China.
Yue Ren, Department of Gastroenterology, The Second Hospital of Jiaxing, Jiaxing 314000, Zhejiang Province, China.
Miao-Miao Lu, Endoscopy Center, Children’s Hospital of Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China.
Ling-Ling Xu, Department of Gastroenterology, The Second People’s Hospital of Yuhang District, Hangzhou 310000, Zhejiang Province, China.
Wei-Xin Cheng, Department of Gastroenterology, The First Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China.
Meng-Meng Zhang, Department of Gastroenterology, Hangzhou Shangcheng District People’s Hospital, Hangzhou 310003, Zhejiang Province, China.
Lin-Ping Ding, Department of Gastroenterology, The First Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China.
Dong Chen, Department of Colorectal Surgery, The First Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China.
Jian-Guo Gao, Department of Gastroenterology, The First Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China.
Juan Du, Department of Gastroenterology, The First Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China.
Ci-Liang Jin, Department of Gastroenterology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325000, Zhejiang Province, China.
Chun-Xiao Chen, Department of Gastroenterology, The First Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China.
Yun-Fei Li, Women’s Hospital and Institute of Genetics, Zhejiang University School of Medicine, Hangzhou 310000, Zhejiang Province, China.
Tao Cheng, Women’s Hospital and Institute of Genetics, Zhejiang University School of Medicine, Hangzhou 310000, Zhejiang Province, China.
Peng-Lei Jiang, Center of Stem Cell and Regenerative Medicine, and Bone Marrow Transplantation Center, The First Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China.
Yi-Da Yang, Department of Infectious Disease, The First Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China.
Peng-Xu Qian, Center of Stem Cell and Regenerative Medicine, and Bone Marrow Transplantation Center, The First Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China.
Peng-Fei Xu, Women’s Hospital and Institute of Genetics, Zhejiang University School of Medicine, Hangzhou 310000, Zhejiang Province, China.
Xi Jin, Department of Gastroenterology, The First Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China. jxfl007@zju.edu.cn.
References
- 1.Swartz MA. The physiology of the lymphatic system. Adv Drug Deliv Rev. 2001;50:3–20. doi: 10.1016/s0169-409x(01)00150-8. [DOI] [PubMed] [Google Scholar]
- 2.Liao S, von der Weid PY. Inflammation-induced lymphangiogenesis and lymphatic dysfunction. Angiogenesis. 2014;17:325–334. doi: 10.1007/s10456-014-9416-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang X, Nicolls MR, Tian W, Rockson SG. Lymphatic Dysfunction, Leukotrienes, and Lymphedema. Annu Rev Physiol. 2018;80:49–70. doi: 10.1146/annurev-physiol-022516-034008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hokari R, Tomioka A. The role of lymphatics in intestinal inflammation. Inflamm Regen. 2021;41:25. doi: 10.1186/s41232-021-00175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliver G, Kipnis J, Randolph GJ, Harvey NL. The Lymphatic Vasculature in the 21(st) Century: Novel Functional Roles in Homeostasis and Disease. Cell. 2020;182:270–296. doi: 10.1016/j.cell.2020.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeda A, Hollmén M, Dermadi D, Pan J, Brulois KF, Kaukonen R, Lönnberg T, Boström P, Koskivuo I, Irjala H, Miyasaka M, Salmi M, Butcher EC, Jalkanen S. Single-Cell Survey of Human Lymphatics Unveils Marked Endothelial Cell Heterogeneity and Mechanisms of Homing for Neutrophils. Immunity. 2019;51:561–572.e5. doi: 10.1016/j.immuni.2019.06.027. [DOI] [PubMed] [Google Scholar]
- 7.Nikolakis D, de Voogd FAE, Pruijt MJ, Grootjans J, van de Sande MG, D'Haens GR. The Role of the Lymphatic System in the Pathogenesis and Treatment of Inflammatory Bowel Disease. Int J Mol Sci. 2022;23 doi: 10.3390/ijms23031854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vetrano S, Borroni EM, Sarukhan A, Savino B, Bonecchi R, Correale C, Arena V, Fantini M, Roncalli M, Malesci A, Mantovani A, Locati M, Danese S. The lymphatic system controls intestinal inflammation and inflammation-associated Colon Cancer through the chemokine decoy receptor D6. Gut. 2010;59:197–206. doi: 10.1136/gut.2009.183772. [DOI] [PubMed] [Google Scholar]
- 9.Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet. 2017;389:1741–1755. doi: 10.1016/S0140-6736(16)31711-1. [DOI] [PubMed] [Google Scholar]
- 10.Roda G, Chien Ng S, Kotze PG, Argollo M, Panaccione R, Spinelli A, Kaser A, Peyrin-Biroulet L, Danese S. Crohn's disease. Nat Rev Dis Primers. 2020;6:22. doi: 10.1038/s41572-020-0156-2. [DOI] [PubMed] [Google Scholar]
- 11.Feuerstein JD, Cheifetz AS. Crohn Disease: Epidemiology, Diagnosis, and Management. Mayo Clin Proc. 2017;92:1088–1103. doi: 10.1016/j.mayocp.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Bernier-Latmani J, Petrova TV. Intestinal lymphatic vasculature: structure, mechanisms and functions. Nat Rev Gastroenterol Hepatol. 2017;14:510–526. doi: 10.1038/nrgastro.2017.79. [DOI] [PubMed] [Google Scholar]
- 13.Jang JY, Koh YJ, Lee SH, Lee J, Kim KH, Kim D, Koh GY, Yoo OJ. Conditional ablation of LYVE-1+ cells unveils defensive roles of lymphatic vessels in intestine and lymph nodes. Blood. 2013;122:2151–2161. doi: 10.1182/blood-2013-01-478941. [DOI] [PubMed] [Google Scholar]
- 14.Van Kruiningen HJ, Colombel JF. The forgotten role of lymphangitis in Crohn's disease. Gut. 2008;57:1–4. doi: 10.1136/gut.2007.123166. [DOI] [PubMed] [Google Scholar]
- 15.Alexander JS, Chaitanya GV, Grisham MB, Boktor M. Emerging roles of lymphatics in inflammatory bowel disease. Ann N Y Acad Sci. 2010;1207 Suppl 1:E75–E85. doi: 10.1111/j.1749-6632.2010.05757.x. [DOI] [PubMed] [Google Scholar]
- 16.Tonelli F, Giudici F, Liscia G. Is lymphatic status related to regression of inflammation in Crohn's disease? World J Gastrointest Surg. 2012;4:228–233. doi: 10.4240/wjgs.v4.i10.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui Y, Lu SY, Xu J, Peng YS, Miao Q, Wang XQ, Chen XY, Ran ZH. Microscopic features of small bowel mucosa of patients with Crohn's disease. BMC Gastroenterol. 2019;19:232. doi: 10.1186/s12876-019-1138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Ocansey DKW, Liu L, Olovo CV, Zhang X, Qian H, Xu W, Mao F. Implications of lymphatic alterations in the pathogenesis and treatment of inflammatory bowel disease. Biomed Pharmacother. 2021;140:111752. doi: 10.1016/j.biopha.2021.111752. [DOI] [PubMed] [Google Scholar]
- 19.von der Weid PY, Rehal S, Ferraz JG. Role of the lymphatic system in the pathogenesis of Crohn's disease. Curr Opin Gastroenterol. 2011;27:335–341. doi: 10.1097/MOG.0b013e3283476e8f. [DOI] [PubMed] [Google Scholar]
- 20.Caparrós E, Wiest R, Scharl M, Rogler G, Gutiérrez Casbas A, Yilmaz B, Wawrzyniak M, Francés R. Dysbiotic microbiota interactions in Crohn's disease. Gut Microbes. 2021;13:1949096. doi: 10.1080/19490976.2021.1949096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sura R, Colombel JF, Van Kruiningen HJ. Lymphatics, tertiary lymphoid organs and the granulomas of Crohn's disease: an immunohistochemical study. Aliment Pharmacol Ther. 2011;33:930–939. doi: 10.1111/j.1365-2036.2011.04605.x. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Stocchi L, Liu X, Rui Y, Liu G, Remzi FH, Shen B. Presence of Granulomas in Mesenteric Lymph Nodes Is Associated with Postoperative Recurrence in Crohn's Disease. Inflamm Bowel Dis. 2015;21:2613–2618. doi: 10.1097/MIB.0000000000000541. [DOI] [PubMed] [Google Scholar]
- 23.Rahier JF, Dubuquoy L, Colombel JF, Jouret-Mourin A, Delos M, Ferrante M, Sokol H, Hertogh GD, Salleron J, Geboes K, Desreumaux P. Decreased lymphatic vessel density is associated with postoperative endoscopic recurrence in Crohn's disease. Inflamm Bowel Dis. 2013;19:2084–2090. doi: 10.1097/MIB.0b013e3182971cec. [DOI] [PubMed] [Google Scholar]
- 24.Behr MA. The path to Crohn's disease: is mucosal pathology a secondary event? Inflamm Bowel Dis. 2010;16:896–902. doi: 10.1002/ibd.21171. [DOI] [PubMed] [Google Scholar]
- 25.Casanova JL, Abel L. Revisiting Crohn's disease as a primary immunodeficiency of macrophages. J Exp Med. 2009;206:1839–1843. doi: 10.1084/jem.20091683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ventham NT, Kennedy NA, Nimmo ER, Satsangi J. Beyond gene discovery in inflammatory bowel disease: the emerging role of epigenetics. Gastroenterology. 2013;145:293–308. doi: 10.1053/j.gastro.2013.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiernan MG, Coffey JC, McDermott K, Cotter PD, Cabrera-Rubio R, Kiely PA, Dunne CP. The Human Mesenteric Lymph Node Microbiome Differentiates Between Crohn's Disease and Ulcerative Colitis. J Crohns Colitis. 2019;13:58–66. doi: 10.1093/ecco-jcc/jjy136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barthel M, Hapfelmeier S, Quintanilla-Martínez L, Kremer M, Rohde M, Hogardt M, Pfeffer K, Rüssmann H, Hardt WD. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun. 2003;71:2839–2858. doi: 10.1128/IAI.71.5.2839-2858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glasser AL, Boudeau J, Barnich N, Perruchot MH, Colombel JF, Darfeuille-Michaud A. Adherent invasive Escherichia coli strains from patients with Crohn's disease survive and replicate within macrophages without inducing host cell death. Infect Immun. 2001;69:5529–5537. doi: 10.1128/IAI.69.9.5529-5537.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Brien CL, Pavli P, Gordon DM, Allison GE. Detection of bacterial DNA in lymph nodes of Crohn's disease patients using high throughput sequencing. Gut. 2014;63:1596–1606. doi: 10.1136/gutjnl-2013-305320. [DOI] [PubMed] [Google Scholar]
- 31.Fonseca DM, Hand TW, Han SJ, Gerner MY, Glatman Zaretsky A, Byrd AL, Harrison OJ, Ortiz AM, Quinones M, Trinchieri G, Brenchley JM, Brodsky IE, Germain RN, Randolph GJ, Belkaid Y. Microbiota-Dependent Sequelae of Acute Infection Compromise Tissue-Specific Immunity. Cell. 2015;163:354–366. doi: 10.1016/j.cell.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diehl GE, Longman RS, Zhang JX, Breart B, Galan C, Cuesta A, Schwab SR, Littman DR. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX(3)CR1(hi) cells. Nature. 2013;494:116–120. doi: 10.1038/nature11809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peyrin-Biroulet L, Gonzalez F, Dubuquoy L, Rousseaux C, Dubuquoy C, Decourcelle C, Saudemont A, Tachon M, Béclin E, Odou MF, Neut C, Colombel JF, Desreumaux P. Mesenteric fat as a source of C reactive protein and as a target for bacterial translocation in Crohn's disease. Gut. 2012;61:78–85. doi: 10.1136/gutjnl-2011-300370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Randolph GJ, Bala S, Rahier JF, Johnson MW, Wang PL, Nalbantoglu I, Dubuquoy L, Chau A, Pariente B, Kartheuser A, Zinselmeyer BH, Colombel JF. Lymphoid Aggregates Remodel Lymphatic Collecting Vessels that Serve Mesenteric Lymph Nodes in Crohn Disease. Am J Pathol. 2016;186:3066–3073. doi: 10.1016/j.ajpath.2016.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rehal S, Stephens M, Roizes S, Liao S, von der Weid PY. Acute small intestinal inflammation results in persistent lymphatic alterations. Am J Physiol Gastrointest Liver Physiol. 2018;314:G408–G417. doi: 10.1152/ajpgi.00340.2017. [DOI] [PubMed] [Google Scholar]
- 36.Mikulski Z, Johnson R, Shaked I, Kim G, Nowyhed H, Goodman W, Chodaczek G, Pizarro TT, Cominelli F, Ley K. SAMP1/YitFc mice develop ileitis via loss of CCL21 and defects in dendritic cell migration. Gastroenterology. 2015;148:783–793.e5. doi: 10.1053/j.gastro.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi S, Kawamura T, Kanda Y, Taniguchi T, Nishizawa T, Iiai T, Hatakeyama K, Abo T. Multipotential acceptance of Peyer's patches in the intestine for both thymus-derived T cells and extrathymic T cells in mice. Immunol Cell Biol. 2005;83:504–510. doi: 10.1111/j.1440-1711.2005.01361.x. [DOI] [PubMed] [Google Scholar]
- 38.Koboziev I, Karlsson F, Grisham MB. Gut-associated lymphoid tissue, T cell trafficking, and chronic intestinal inflammation. Ann N Y Acad Sci. 2010;1207 Suppl 1:E86–E93. doi: 10.1111/j.1749-6632.2010.05711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zundler S, Günther C, Kremer AE, Zaiss MM, Rothhammer V, Neurath MF. Gut immune cell trafficking: inter-organ communication and immune-mediated inflammation. Nat Rev Gastroenterol Hepatol. 2023;20:50–64. doi: 10.1038/s41575-022-00663-1. [DOI] [PubMed] [Google Scholar]
- 40.Sakuraba A, Sato T, Kamada N, Kitazume M, Sugita A, Hibi T. Th1/Th17 immune response is induced by mesenteric lymph node dendritic cells in Crohn's disease. Gastroenterology. 2009;137:1736–1745. doi: 10.1053/j.gastro.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 41.Li N, Shi RH. Updated review on immune factors in pathogenesis of Crohn's disease. World J Gastroenterol. 2018;24:15–22. doi: 10.3748/wjg.v24.i1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kudo T, Nagata S, Aoyagi Y, Suzuki R, Matsuda H, Ohtsuka Y, Shimizu T, Okumura K, Yamashiro Y. Polarized production of T-helper cell type 1 cells in Peyer's patches in Crohn's disease. Digestion. 2004;70:214–225. doi: 10.1159/000082892. [DOI] [PubMed] [Google Scholar]
- 43.Bsat M, Chapuy L, Baba N, Rubio M, Panzini B, Wassef R, Richard C, Soucy G, Mehta H, Sarfati M. Differential accumulation and function of proinflammatory 6-sulfo LacNAc dendritic cells in lymph node and colon of Crohn's versus ulcerative colitis patients. J Leukoc Biol. 2015;98:671–681. doi: 10.1189/jlb.5A1014-509RR. [DOI] [PubMed] [Google Scholar]
- 44.Verstege MI, ten Kate FJ, Reinartz SM, van Drunen CM, Slors FJ, Bemelman WA, Vyth-Dreese FA, te Velde AA. Dendritic cell populations in colon and mesenteric lymph nodes of patients with Crohn's disease. J Histochem Cytochem. 2008;56:233–241. doi: 10.1369/jhc.7A7308.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Middel P, Raddatz D, Gunawan B, Haller F, Radzun HJ. Increased number of mature dendritic cells in Crohn's disease: evidence for a chemokine mediated retention mechanism. Gut. 2006;55:220–227. doi: 10.1136/gut.2004.063008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Becker F, Kurmaeva E, Gavins FN, Stevenson EV, Navratil AR, Jin L, Tsunoda I, Orr AW, Alexander JS, Ostanin DV. A Critical Role for Monocytes/Macrophages During Intestinal Inflammation-associated Lymphangiogenesis. Inflamm Bowel Dis. 2016;22:1326–1345. doi: 10.1097/MIB.0000000000000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeng B, Shi S, Ashworth G, Dong C, Liu J, Xing F. ILC3 function as a double-edged sword in inflammatory bowel diseases. Cell Death Dis. 2019;10:315. doi: 10.1038/s41419-019-1540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stehle C, Rückert T, Fiancette R, Gajdasik DW, Willis C, Ulbricht C, Durek P, Mashreghi MF, Finke D, Hauser AE, Withers DR, Chang HD, Zimmermann J, Romagnani C. T-bet and RORα control lymph node formation by regulating embryonic innate lymphoid cell differentiation. Nat Immunol. 2021;22:1231–1244. doi: 10.1038/s41590-021-01029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Becker F, Potepalov S, Shehzahdi R, Bernas M, Witte M, Abreo F, Traylor J, Orr WA, Tsunoda I, Alexander JS. Downregulation of FoxC2 Increased Susceptibility to Experimental Colitis: Influence of Lymphatic Drainage Function? Inflamm Bowel Dis. 2015;21:1282–1296. doi: 10.1097/MIB.0000000000000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ocansey DKW, Pei B, Xu X, Zhang L, Olovo CV, Mao F. Cellular and molecular mediators of lymphangiogenesis in inflammatory bowel disease. J Transl Med. 2021;19:254. doi: 10.1186/s12967-021-02922-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao J, Lu Q, Liu Y, Shi Z, Hu L, Zeng Z, Tu Y, Xiao Z, Xu Q. Th17 Cells in Inflammatory Bowel Disease: Cytokines, Plasticity, and Therapies. J Immunol Res. 2021;2021:8816041. doi: 10.1155/2021/8816041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Changming W, Xin L, Hua T, Shikun W, Qiong X, Zhigeng Z, Xueying W. Monocytes can be induced to express lymphatic phenotypes. Lymphology. 2011;44:48–53. [PubMed] [Google Scholar]
- 53.Welsh JD, Kahn ML, Sweet DT. Lymphovenous hemostasis and the role of platelets in regulating lymphatic flow and lymphatic vessel maturation. Blood. 2016;128:1169–1173. doi: 10.1182/blood-2016-04-636415. [DOI] [PubMed] [Google Scholar]
- 54.Osada M, Inoue O, Ding G, Shirai T, Ichise H, Hirayama K, Takano K, Yatomi Y, Hirashima M, Fujii H, Suzuki-Inoue K, Ozaki Y. Platelet activation receptor CLEC-2 regulates blood/lymphatic vessel separation by inhibiting proliferation, migration, and tube formation of lymphatic endothelial cells. J Biol Chem. 2012;287:22241–22252. doi: 10.1074/jbc.M111.329987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sato H, Higashiyama M, Hozumi H, Sato S, Furuhashi H, Takajo T, Maruta K, Yasutake Y, Narimatsu K, Yoshikawa K, Kurihara C, Okada Y, Watanabe C, Komoto S, Tomita K, Nagao S, Miura S, Hokari R. Platelet interaction with lymphatics aggravates intestinal inflammation by suppressing lymphangiogenesis. Am J Physiol Gastrointest Liver Physiol. 2016;311:G276–G285. doi: 10.1152/ajpgi.00455.2015. [DOI] [PubMed] [Google Scholar]
- 56.Lee C, Hong SN, Kim ER, Chang DK, Kim YH. Depletion of Intestinal Stem Cell Niche Factors Contributes to the Alteration of Epithelial Differentiation in SAMP1/YitFcsJ Mice With Crohn Disease-Like Ileitis. Inflamm Bowel Dis. 2021;27:667–676. doi: 10.1093/ibd/izaa314. [DOI] [PubMed] [Google Scholar]
- 57.Niec RE, Chu T, Schernthanner M, Gur-Cohen S, Hidalgo L, Pasolli HA, Luckett KA, Wang Z, Bhalla SR, Cambuli F, Kataru RP, Ganesh K, Mehrara BJ, Pe'er D, Fuchs E. Lymphatics act as a signaling hub to regulate intestinal stem cell activity. Cell Stem Cell. 2022;29:1067–1082.e18. doi: 10.1016/j.stem.2022.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goto N, Goto S, Imada S, Hosseini S, Deshpande V, Yilmaz ÖH. Lymphatics and fibroblasts support intestinal stem cells in homeostasis and injury. Cell Stem Cell. 2022;29:1246–1261.e6. doi: 10.1016/j.stem.2022.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jurisic G, Sundberg JP, Detmar M. Blockade of VEGF receptor-3 aggravates inflammatory bowel disease and lymphatic vessel enlargement. Inflamm Bowel Dis. 2013;19:1983–1989. doi: 10.1097/MIB.0b013e31829292f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.D'Alessio S, Correale C, Tacconi C, Gandelli A, Pietrogrande G, Vetrano S, Genua M, Arena V, Spinelli A, Peyrin-Biroulet L, Fiocchi C, Danese S. VEGF-C-dependent stimulation of lymphatic function ameliorates experimental inflammatory bowel disease. J Clin Invest. 2014;124:3863–3878. doi: 10.1172/JCI72189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bernier-Latmani J, Cisarovsky C, Demir CS, Bruand M, Jaquet M, Davanture S, Ragusa S, Siegert S, Dormond O, Benedito R, Radtke F, Luther SA, Petrova TV. DLL4 promotes continuous adult intestinal lacteal regeneration and dietary fat transport. J Clin Invest. 2015;125:4572–4586. doi: 10.1172/JCI82045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Long SH, He Y, Chen MH, Cao K, Chen YJ, Chen BL, Mao R, Zhang SH, Zhu ZH, Zeng ZR, Hu PJ. Activation of PI3K/Akt/mTOR signaling pathway triggered by PTEN downregulation in the pathogenesis of Crohn's disease. J Dig Dis. 2013;14:662–669. doi: 10.1111/1751-2980.12095. [DOI] [PubMed] [Google Scholar]
- 63.Powell JD, Pollizzi KN, Heikamp EB, Horton MR. Regulation of immune responses by mTOR. Annu Rev Immunol. 2012;30:39–68. doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ungaro F, Garlatti V, Massimino L, Spinelli A, Carvello M, Sacchi M, Spanò S, Colasante G, Valassina N, Vetrano S, Malesci A, Peyrin-Biroulet L, Danese S, D'Alessio S. mTOR-Dependent Stimulation of IL20RA Orchestrates Immune Cell Trafficking through Lymphatic Endothelium in Patients with Crohn's Disease. Cells. 2019;8 doi: 10.3390/cells8080924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shen B, Shang Z, Wang B, Zhang L, Zhou F, Li T, Chu M, Jiang H, Wang Y, Qiao T, Zhang J, Sun W, Kong X, He Y. Genetic dissection of tie pathway in mouse lymphatic maturation and valve development. Arterioscler Thromb Vasc Biol. 2014;34:1221–1230. doi: 10.1161/ATVBAHA.113.302923. [DOI] [PubMed] [Google Scholar]
- 66.Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, Martin C, Witte C, Witte MH, Jackson D, Suri C, Campochiaro PA, Wiegand SJ, Yancopoulos GD. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev Cell. 2002;3:411–423. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 67.Oikonomou KA, Kapsoritakis AN, Kapsoritaki AI, Manolakis AC, Tiaka EK, Tsiopoulos FD, Tsiompanidis IA, Potamianos SP. Angiogenin, angiopoietin-1, angiopoietin-2, and endostatin serum levels in inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:963–970. doi: 10.1002/ibd.21410. [DOI] [PubMed] [Google Scholar]
- 68.Wang L, Wang S, Xue A, Shi J, Zheng C, Huang Y. Thalidomide Inhibits Angiogenesis via Downregulation of VEGF and Angiopoietin-2 in Crohn's Disease. Inflammation. 2021;44:795–807. doi: 10.1007/s10753-020-01378-8. [DOI] [PubMed] [Google Scholar]
- 69.Hayashi Y, Nakase H. The Molecular Mechanisms of Intestinal Inflammation and Fibrosis in Crohn's Disease. Front Physiol. 2022;13:845078. doi: 10.3389/fphys.2022.845078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Neal MD, Leaphart C, Levy R, Prince J, Billiar TR, Watkins S, Li J, Cetin S, Ford H, Schreiber A, Hackam DJ. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol. 2006;176:3070–3079. doi: 10.4049/jimmunol.176.5.3070. [DOI] [PubMed] [Google Scholar]
- 71.Stephens M, Liao S, von der Weid PY. Mesenteric Lymphatic Alterations Observed During DSS Induced Intestinal Inflammation Are Driven in a TLR4-PAMP/DAMP Discriminative Manner. Front Immunol. 2019;10:557. doi: 10.3389/fimmu.2019.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Atreya I, Atreya R, Neurath MF. NF-kappaB in inflammatory bowel disease. J Intern Med. 2008;263:591–596. doi: 10.1111/j.1365-2796.2008.01953.x. [DOI] [PubMed] [Google Scholar]
- 73.Flister MJ, Wilber A, Hall KL, Iwata C, Miyazono K, Nisato RE, Pepper MS, Zawieja DC, Ran S. Inflammation induces lymphangiogenesis through up-regulation of VEGFR-3 mediated by NF-kappaB and Prox1. Blood. 2010;115:418–429. doi: 10.1182/blood-2008-12-196840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nagahashi M, Abe M, Sakimura K, Takabe K, Wakai T. The role of sphingosine-1-phosphate in inflammation and cancer progression. Cancer Sci. 2018;109:3671–3678. doi: 10.1111/cas.13802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sandborn WJ, Feagan BG, D'Haens G, Wolf DC, Jovanovic I, Hanauer SB, Ghosh S, Petersen A, Hua SY, Lee JH, Charles L, Chitkara D, Usiskin K, Colombel JF, Laine L, Danese S True North Study Group. Ozanimod as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. 2021;385:1280–1291. doi: 10.1056/NEJMoa2033617. [DOI] [PubMed] [Google Scholar]
- 76.Feagan BG, Sandborn WJ, Danese S, Wolf DC, Liu WJ, Hua SY, Minton N, Olson A, D'Haens G. Ozanimod induction therapy for patients with moderate to severe Crohn's disease: a single-arm, phase 2, prospective observer-blinded endpoint study. Lancet Gastroenterol Hepatol. 2020;5:819–828. doi: 10.1016/S2468-1253(20)30188-6. [DOI] [PubMed] [Google Scholar]
- 77.Mizushima T, Ito T, Kishi D, Kai Y, Tamagawa H, Nezu R, Kiyono H, Matsuda H. Therapeutic effects of a new lymphocyte homing reagent FTY720 in interleukin-10 gene-deficient mice with colitis. Inflamm Bowel Dis. 2004;10:182–192. doi: 10.1097/00054725-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 78.Czepielewski RS, Erlich EC, Onufer EJ, Young S, Saunders BT, Han YH, Wohltmann M, Wang PL, Kim KW, Kumar S, Hsieh CS, Scallan JP, Yang Y, Zinselmeyer BH, Davis MJ, Randolph GJ. Ileitis-associated tertiary lymphoid organs arise at lymphatic valves and impede mesenteric lymph flow in response to tumor necrosis factor. Immunity. 2021;54:2795–2811.e9. doi: 10.1016/j.immuni.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gröger M, Loewe R, Holnthoner W, Embacher R, Pillinger M, Herron GS, Wolff K, Petzelbauer P. IL-3 induces expression of lymphatic markers Prox-1 and podoplanin in human endothelial cells. J Immunol. 2004;173:7161–7169. doi: 10.4049/jimmunol.173.12.7161. [DOI] [PubMed] [Google Scholar]
- 80.Waldner MJ, Neurath MF. Master regulator of intestinal disease: IL-6 in chronic inflammation and cancer development. Semin Immunol. 2014;26:75–79. doi: 10.1016/j.smim.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 81.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 82.Shen W, Li Y, Cao L, Cai X, Ge Y, Zhu W. Decreased Expression of Prox1 Is Associated With Postoperative Recurrence in Crohn's Disease. J Crohns Colitis. 2018;12:1210–1218. doi: 10.1093/ecco-jcc/jjy091. [DOI] [PubMed] [Google Scholar]
- 83.Martinez de la Torre Y, Locati M, Buracchi C, Dupor J, Cook DN, Bonecchi R, Nebuloni M, Rukavina D, Vago L, Vecchi A, Lira SA, Mantovani A. Increased inflammation in mice deficient for the chemokine decoy receptor D6. Eur J Immunol. 2005;35:1342–1346. doi: 10.1002/eji.200526114. [DOI] [PubMed] [Google Scholar]
- 84.Lu WJ, Yang Q, Sun W, Woods SC, D'Alessio D, Tso P. The regulation of the lymphatic secretion of glucagon-like peptide-1 (GLP-1) by intestinal absorption of fat and carbohydrate. Am J Physiol Gastrointest Liver Physiol. 2007;293:G963–G971. doi: 10.1152/ajpgi.00146.2007. [DOI] [PubMed] [Google Scholar]
- 85.Lucotti P, Lovati E, Lenti MV, Valvo B, Sprio E, Aronico N, Giuffrida P, Dell'Aera D, Pasini A, Ubezio C, Delliponti M, Tinelli C, Corazza GR, Di Sabatino A. Abnormal post-prandial glucagon-like peptide release in patients with Crohn's disease. Clin Res Hepatol Gastroenterol. 2021;45:101533. doi: 10.1016/j.clinre.2020.08.011. [DOI] [PubMed] [Google Scholar]
- 86.Gallagher K, Catesson A, Griffin JL, Holmes E, Williams HRT. Metabolomic Analysis in Inflammatory Bowel Disease: A Systematic Review. J Crohns Colitis. 2021;15:813–826. doi: 10.1093/ecco-jcc/jjaa227. [DOI] [PubMed] [Google Scholar]
- 87.Thomas JP, Modos D, Rushbrook SM, Powell N, Korcsmaros T. The Emerging Role of Bile Acids in the Pathogenesis of Inflammatory Bowel Disease. Front Immunol. 2022;13:829525. doi: 10.3389/fimmu.2022.829525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Piotrowska M, Binienda A, Fichna J. The role of fatty acids in Crohn's disease pathophysiology - An overview. Mol Cell Endocrinol. 2021;538:111448. doi: 10.1016/j.mce.2021.111448. [DOI] [PubMed] [Google Scholar]
- 89.Randolph GJ, Miller NE. Lymphatic transport of high-density lipoproteins and chylomicrons. J Clin Invest. 2014;124:929–935. doi: 10.1172/JCI71610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hrabovský V, Zadák Z, Bláha V, Hyspler R, Karlík T, Martínek A, Mendlová A. Cholesterol metabolism in active Crohn's disease. Wien Klin Wochenschr. 2009;121:270–275. doi: 10.1007/s00508-009-1150-6. [DOI] [PubMed] [Google Scholar]
- 91.Gerster R, Eloranta JJ, Hausmann M, Ruiz PA, Cosin-Roger J, Terhalle A, Ziegler U, Kullak-Ublick GA, von Eckardstein A, Rogler G. Anti-inflammatory Function of High-Density Lipoproteins via Autophagy of IκB Kinase. Cell Mol Gastroenterol Hepatol. 2015;1:171–187.e1. doi: 10.1016/j.jcmgh.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mao R, Kurada S, Gordon IO, Baker ME, Gandhi N, McDonald C, Coffey JC, Rieder F. The Mesenteric Fat and Intestinal Muscle Interface: Creeping Fat Influencing Stricture Formation in Crohn's Disease. Inflamm Bowel Dis. 2019;25:421–426. doi: 10.1093/ibd/izy331. [DOI] [PubMed] [Google Scholar]
- 93.Shen W, Li Y, Zou Y, Cao L, Cai X, Gong J, Xu Y, Zhu W. Mesenteric Adipose Tissue Alterations in Crohn's Disease Are Associated With the Lymphatic System. Inflamm Bowel Dis. 2019;25:283–293. doi: 10.1093/ibd/izy306. [DOI] [PubMed] [Google Scholar]
- 94.Westcott ED, Mattacks CA, Windsor AC, Knight SC, Pond CM. Perinodal adipose tissue and fatty acid composition of lymphoid tissues in patients with and without Crohn's disease and their implications for the etiology and treatment of CD. Ann N Y Acad Sci. 2006;1072:395–400. doi: 10.1196/annals.1326.034. [DOI] [PubMed] [Google Scholar]
- 95.Caputa G, Matsushita M, Sanin DE, Kabat AM, Edwards-Hicks J, Grzes KM, Pohlmeyer R, Stanczak MA, Castoldi A, Cupovic J, Forde AJ, Apostolova P, Seidl M, van Teijlingen Bakker N, Villa M, Baixauli F, Quintana A, Hackl A, Flachsmann L, Hässler F, Curtis JD, Patterson AE, Henneke P, Pearce EL, Pearce EJ. Intracellular infection and immune system cues rewire adipocytes to acquire immune function. Cell Metab. 2022;34:747–760.e6. doi: 10.1016/j.cmet.2022.04.008. [DOI] [PubMed] [Google Scholar]
- 96.Di Vincenzo F, Puca P, Lopetuso LR, Petito V, Masi L, Bartocci B, Murgiano M, De Felice M, Petronio L, Gasbarrini A, Scaldaferri F. Bile Acid-Related Regulation of Mucosal Inflammation and Intestinal Motility: From Pathogenesis to Therapeutic Application in IBD and Microscopic Colitis. Nutrients. 2022;14 doi: 10.3390/nu14132664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gadaleta RM, van Erpecum KJ, Oldenburg B, Willemsen EC, Renooij W, Murzilli S, Klomp LW, Siersema PD, Schipper ME, Danese S, Penna G, Laverny G, Adorini L, Moschetta A, van Mil SW. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut. 2011;60:463–472. doi: 10.1136/gut.2010.212159. [DOI] [PubMed] [Google Scholar]
- 98.Grunwald DJ, Eisen JS. Headwaters of the zebrafish -- emergence of a new model vertebrate. Nat Rev Genet. 2002;3:717–724. doi: 10.1038/nrg892. [DOI] [PubMed] [Google Scholar]
- 99.Kuil LE, Chauhan RK, Cheng WW, Hofstra RMW, Alves MM. Zebrafish: A Model Organism for Studying Enteric Nervous System Development and Disease. Front Cell Dev Biol. 2020;8:629073. doi: 10.3389/fcell.2020.629073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim JD, Jin SW. A tale of two models: mouse and zebrafish as complementary models for lymphatic studies. Mol Cells. 2014;37:503–510. doi: 10.14348/molcells.2014.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xie Y, Meijer AH, Schaaf MJM. Modeling Inflammation in Zebrafish for the Development of Anti-inflammatory Drugs. Front Cell Dev Biol. 2020;8:620984. doi: 10.3389/fcell.2020.620984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li YF, Cheng T, Zhang YJ, Fu XX, Mo J, Zhao GQ, Xue MG, Zhuo DH, Xing YY, Huang Y, Sun XZ, Wang D, Liu X, Dong Y, Zhu XS, He F, Ma J, Chen D, Jin X, Xu PF. Mycn regulates intestinal development through ribosomal biogenesis in a zebrafish model of Feingold syndrome 1. PLoS Biol. 2022;20:e3001856. doi: 10.1371/journal.pbio.3001856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bates JM, Akerlund J, Mittge E, Guillemin K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe. 2007;2:371–382. doi: 10.1016/j.chom.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao X, Pack M. Modeling intestinal disorders using zebrafish. Methods Cell Biol. 2017;138:241–270. doi: 10.1016/bs.mcb.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 105.Witte M, Huitema LF, Nieuwenhuis EE, Brugman S. Deficiency in macrophage-stimulating protein results in spontaneous intestinal inflammation and increased susceptibility toward epithelial damage in zebrafish. Zebrafish. 2014;11:542–550. doi: 10.1089/zeb.2014.1023. [DOI] [PubMed] [Google Scholar]
- 106.Zhao S, Xia J, Wu X, Zhang L, Wang P, Wang H, Li H, Wang X, Chen Y, Agnetti J, Li Y, Pei D, Shu X. Deficiency in class III PI3-kinase confers postnatal lethality with IBD-like features in zebrafish. Nat Commun. 2018;9:2639. doi: 10.1038/s41467-018-05105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhao Q, Chang H, Zheng J, Li P, Ye L, Pan R, Li D, Shao JZ, Zhao RC, Chen Y. A novel Trmt5-deficient zebrafish model with spontaneous inflammatory bowel disease-like phenotype. Signal Transduct Target Ther. 2023;8:86. doi: 10.1038/s41392-023-01318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sanmarco LM, Chao CC, Wang YC, Kenison JE, Li Z, Rone JM, Rejano-Gordillo CM, Polonio CM, Gutierrez-Vazquez C, Piester G, Plasencia A, Li L, Giovannoni F, Lee HG, Faust Akl C, Wheeler MA, Mascanfroni I, Jaronen M, Alsuwailm M, Hewson P, Yeste A, Andersen BM, Franks DG, Huang CJ, Ekwudo M, Tjon EC, Rothhammer V, Takenaka M, de Lima KA, Linnerbauer M, Guo L, Covacu R, Queva H, Fonseca-Castro PH, Bladi MA, Cox LM, Hodgetts KJ, Hahn ME, Mildner A, Korzenik J, Hauser R, Snapper SB, Quintana FJ. Identification of environmental factors that promote intestinal inflammation. Nature. 2022;611:801–809. doi: 10.1038/s41586-022-05308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee AS, Sung MJ, Kim W, Jung YJ. COMP-angiopoietin-1 ameliorates inflammation-induced lymphangiogenesis in dextran sulfate sodium (DSS)-induced colitis model. J Mol Med (Berl) 2018;96:459–467. doi: 10.1007/s00109-018-1633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee AS, Hur HJ, Sung MJ. The Effect of Artemisinin on Inflammation-Associated Lymphangiogenesis in Experimental Acute Colitis. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21218068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vermeire S, Schreiber S, Petryka R, Kuehbacher T, Hebuterne X, Roblin X, Klopocka M, Goldis A, Wisniewska-Jarosinska M, Baranovsky A, Sike R, Stoyanova K, Tasset C, Van der Aa A, Harrison P. Clinical remission in patients with moderate-to-severe Crohn's disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet. 2017;389:266–275. doi: 10.1016/S0140-6736(16)32537-5. [DOI] [PubMed] [Google Scholar]
- 112.Halpert G, Eitan T, Voronov E, Apte RN, Rath-Wolfson L, Albeck M, Kalechman Y, Sredni B. Multifunctional activity of a small tellurium redox immunomodulator compound, AS101, on dextran sodium sulfate-induced murine colitis. J Biol Chem. 2014;289:17215–17227. doi: 10.1074/jbc.M113.536664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, Lukas M, Fedorak RN, Lee S, Bressler B, Fox I, Rosario M, Sankoh S, Xu J, Stephens K, Milch C, Parikh A GEMINI 2 Study Group. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2013;369:711–721. doi: 10.1056/NEJMoa1215739. [DOI] [PubMed] [Google Scholar]
- 114.Deepak P, Sandborn WJ. Ustekinumab and Anti-Interleukin-23 Agents in Crohn's Disease. Gastroenterol Clin North Am. 2017;46:603–626. doi: 10.1016/j.gtc.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 115.Feagan BG, Panés J, Ferrante M, Kaser A, D'Haens GR, Sandborn WJ, Louis E, Neurath MF, Franchimont D, Dewit O, Seidler U, Kim KJ, Selinger C, Padula SJ, Herichova I, Robinson AM, Wallace K, Zhao J, Minocha M, Othman AA, Soaita A, Visvanathan S, Hall DB, Böcher WO. Risankizumab in patients with moderate to severe Crohn's disease: an open-label extension study. Lancet Gastroenterol Hepatol. 2018;3:671–680. doi: 10.1016/S2468-1253(18)30233-4. [DOI] [PubMed] [Google Scholar]
- 116.Feagan BG, Sandborn WJ, Gasink C, Jacobstein D, Lang Y, Friedman JR, Blank MA, Johanns J, Gao LL, Miao Y, Adedokun OJ, Sands BE, Hanauer SB, Vermeire S, Targan S, Ghosh S, de Villiers WJ, Colombel JF, Tulassay Z, Seidler U, Salzberg BA, Desreumaux P, Lee SD, Loftus EV Jr, Dieleman LA, Katz S, Rutgeerts P UNITI-IM-UNITI Study Group. Ustekinumab as Induction and Maintenance Therapy for Crohn's Disease. N Engl J Med. 2016;375:1946–1960. doi: 10.1056/NEJMoa1602773. [DOI] [PubMed] [Google Scholar]
- 117.Wang X, Zhao J, Qin L. VEGF-C mediated enhancement of lymphatic drainage reduces intestinal inflammation by regulating IL-9/IL-17 balance and improving gut microbiota in experimental chronic colitis. Am J Transl Res. 2017;9:4772–4784. [PMC free article] [PubMed] [Google Scholar]
- 118.Scott FL, Clemons B, Brooks J, Brahmachary E, Powell R, Dedman H, Desale HG, Timony GA, Martinborough E, Rosen H, Roberts E, Boehm MF, Peach RJ. Ozanimod (RPC1063) is a potent sphingosine-1-phosphate receptor-1 (S1P1) and receptor-5 (S1P5) agonist with autoimmune disease-modifying activity. Br J Pharmacol. 2016;173:1778–1792. doi: 10.1111/bph.13476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cromer W, Wang W, Zawieja SD, von der Weid PY, Newell-Rogers MK, Zawieja DC. Colonic Insult Impairs Lymph Flow, Increases Cellular Content of the Lymph, Alters Local Lymphatic Microenvironment, and Leads to Sustained Inflammation in the Rat Ileum. Inflamm Bowel Dis. 2015;21:1553–1563. doi: 10.1097/MIB.0000000000000402. [DOI] [PMC free article] [PubMed] [Google Scholar]