Abstract
After six years without any detection of poliomyelitis cases, Angola reported a case of circulating vaccine-derived poliovirus type 2 (cVDPV2) with paralysis onset date of 27 March 2019. Ultimately, 141 cVDPV2 polio cases were reported in all 18 provinces in 2019–2020, with particularly large hotspots in the south-central provinces of Luanda, Cuanza Sul, and Huambo. Most cases were reported from August to December 2019, with a peak of 15 cases in October 2019. These cases were classified into five distinct genetic emergences (emergence groups) and have ties with cases identified in 2017–2018 in the Democratic Republic of Congo. From June 2019 to July 2020, the Angola Ministry of Health and partners conducted 30 supplementary immunization activity (SIA) rounds as part of 10 campaign groups, using monovalent OPV type 2 (mOPV2). There were Sabin 2 vaccine strain detections in the environmental (sewage) samples taken after mOPV2 SIAs in each province. Following the initial response, additional cVDPV2 polio cases occurred in other provinces. However, the national surveillance system did not detect any new cVDPV2 polio cases after 9 February 2020. While reporting subpar indicator performance in epidemiological surveillance, the laboratory and environmental data as of May 2021 strongly suggest that Angola successfully interrupted transmission of cVDPV2 early in 2020. Additionally, the COVID-19 pandemic did not allow a formal Outbreak Response Assessment (OBRA). Improving the sensitivity of the surveillance system and the completeness of AFP case investigations will be vital to promptly detect and interrupt viral transmission if a new case or sewage isolate are identified in Angola or central Africa.
Keywords: Angola, Poliomyelitis, Vaccine-derived, Type 2, Vaccination campaigns
1. Introduction
Since the Global Polio Eradication Initiative (GPEI) was established in 1988 [1], two of the three wild poliovirus (WPV) serotypes (types 2 and 3) have been eradicated globally [2]. Transmission of WPV type 1 (WPV1) remains uninterrupted only in Afghanistan and Pakistan. The African Regional Commission for the Certification of the Eradication of Polio declared the absence of transmission of indigenous WPV in the World Health Organization African Region countries on 24 August 2020. Nonetheless, outbreaks of circulating vaccine-derived poliovirus type 2 (cVDPV2) have become a growing problem in multiple African and Asian countries [3]. This is partially due to a 2016 global policy decision, known as ‘‘the switch,” to remove type 2 oral polio vaccine (OPV) from routine immunization schedules. One dose of inactivated polio vaccine (IPV) would also be added to routine immunization schedules to protect against type 2 poliovirus [4]. While this move was intended to reduce the number of cVDPV2 outbreaks, unanticipated issues with the switch have actually led to a marked increase in such outbreaks in countries around the world.
One of the largest vaccine-derived outbreaks was identified in Angola, where 141 cVDPV2 polio cases were identified (138 in 2019 and 3 in 2020). The outbreak was classified into five distinct emergences (emergence groups) but will be considered as a single outbreak. Along with the rest of OPV-using countries in the world, in April 2016, Angola withdrew all type 2 poliovirus vaccines from use during the switch from trivalent OPV (tOPV, containing serotypes 1, 2, and 3 Sabin strains) to bivalent OPV (bOPV, containing serotypes 1 and 3 Sabin strains). However, low vaccination coverage rates with tOPV in the years prior to the switch (less than 60 % third dose tOPV among children aged less than 1 year) and low coverage with the single dose of trivalent inactive poliovirus vaccine (IPV) after the switch (56–51 %) according to the 2018–2019 WHO and UNICEF estimates of national immunization coverage (WUENIC) [5] left the population vulnerable to type 2 poliovirus infection and paralytic disease. We report the epidemiological and genetic information about the 2019–2020 vaccine-derived outbreak in Angola and describe the response operations that the Ministry of Health (MoH) of Angola undertook to curtail transmission. We use GPEI threshold values for vaccination coverage and surveillance performance to assess whether transmission of cVDPV2 has been interrupted in Angola.
2. Methods
2.1. Vaccination coverage
To interrupt person-to-person transmission, GPEI guidelines state that all poliovirus outbreaks should trigger a vaccination response with an appropriate type-specific Oral Poliovirus Vaccine (OPV) within 14 days of laboratory notification, which is defined as the communication of the final classification of the sample by the reference laboratory to the country (i.e., Day 0). A vaccination response must include two, high-quality and timely supplementary immunization activities (SIAs) but may be preceded by a rapid vaccination response (i.e., Round 0) to ensure vaccination occurs within the 14 days following laboratory notification to the extent possible. Mop-up rounds are scheduled after the second SIA to focus on areas identified with suboptimal SIA quality, and SIAs are discontinued once no subsequent breakthrough cases (additional polio cases detected in an area covered by vaccination, more than 28 days after the last SIA) have been identified [1]. Lot Quality Assurance Sampling (LQAS), a rapid survey method, is used to assess the quality of vaccination coverage following SIAs in pre-defined areas (i.e., lots), using a small sample size. In each lot, 60 children are sampled in six different clusters (e.g., villages, settlements)—10 children from each cluster. If more than three children out of the 60 are found to have been missed by SIA activities, this is considered a low-quality result and the SIA must be repeated in the area [6].
2.2. Epidemiological surveillance
To measure acute flaccid paralysis (AFP) surveillance performance, GPEI developed a series of indicators to be calculated monthly. The two principal indicators and minimum levels for standard surveillance are:
Sensitivity of surveillance: At least one case of non-polio AFP (NPAFP) should be detected annually per 100,000 population younger than 15 years of age. In endemic regions, to ensure even higher sensitivity, this rate should be two per 100,000. In the Africa region (including Angola), the target is three per 100,000 population younger than 15 years of age.
Stool adequacy rate: All AFP cases should have a full clinical and virological investigation with at least 80 % of AFP cases having ‘adequate’ stool specimens collected. ‘Adequate’ is defined as two stool specimens of sufficient quantity for laboratory analysis, collected at least 24 h apart, within 14 days after the onset of paralysis, and arriving in the laboratory by reverse cold chain and with proper documentation.
Data on AFP surveillance, environmental sampling, reported cVDPV2 cases and SIAs are available through the Polio Information System (Pol IS) database maintained by the World Health Organization (WHO). On 28 June 2021, we retrieved all relevant data that was entered between January 2019 and December 2020. Any missing information was supplied by the WHO Country Office in Angola.
2.3. Environmental sampling
The examination of composite human fecal samples through environmental surveillance links poliovirus isolates from unknown individuals to populations served by the wastewater system. Environmental surveillance can provide valuable supplementary information, particularly in urban populations where AFP surveillance is absent or questionable, persistent virus circulation is suspected, or frequent virus re-introduction is perceived. Angola has a robust system of environmental sampling since 2014, with sewage collection points in the capital city of Luanda (4 collection points; an additional ad hoc site was established in Chitato district, following the confirmation of the first cVDPV2 case in May 2019) Benguela province (2 points), Huambo province (1 point), and Lunda Norte province (1 point). One liter of raw sewage is collected at each sampling site. The samples are sent to a WHO-accredited regional reference laboratory, where they are differentiated as wild or vaccine-like, ideally within 14 days of detection. Reporting of laboratory results from environmental surveillance to the Ministry of Health of a country and WHO must follow the reporting guidelines for clinical surveillance, as well as immediate reporting of wild poliovirus isolation [7].
Robustness of environmental surveillance is assessed via the detection rate for non-polio enteroviruses in collected samples. As non-polio enteroviruses are a common finding in human stool, detecting them in environmental surveillance samples indicates that the collection site (as well as the procedures for sample transport and laboratory analysis) can be relied upon to also identify polioviruses if they were present. The global benchmark for a well-functioning environmental surveillance site is the detection of non-polio enteroviruses in at least 50 % of annual samples collected.
2.4. Laboratory surveillance
The surveillance and investigation protocol of the Angola MoH calls for the collection of two stool samples from each child with a case of acute flaccid paralysis (AFP), ideally within 14 days after the onset of paralysis, in accordance GPEI guidelines [1]. The samples are shipped regularly to the National Institute for Communicable Diseases (NICD) in Johannesburg (South Africa). NICD serves as the designated National Polio Laboratory for Angola; it is a Regional Reference Laboratory that is a member of the Global Polio Laboratory Network (GPLN), accredited for performing virus isolation, intratypic differentiation, and genetic sequencing of the VP1 capsid region following WHO laboratory manual protocols. Sequencing analysis results are shared with the U.S. Centers for Disease Control and Prevention (CDC), which serves as a GPLN global specialized laboratory [8]. The sequencing data reported in this article result from this collaboration. We conducted phylogenetic analyses of VP1 sequences [9 8], using the bioinformatics software Geneious Prime 2020.2.4 (www.geneious.com). Phylogenetic tree inference was performed, using a Bayesian approach [10,9] as implemented in Geneious. VP1 sequences were deposited in GenBank under accession numbers OK634089 – OK634280.
3. Results
3.1. cVDPV2 cases
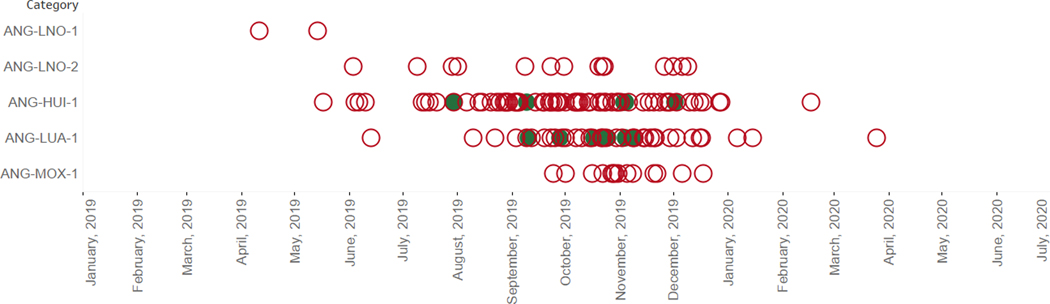
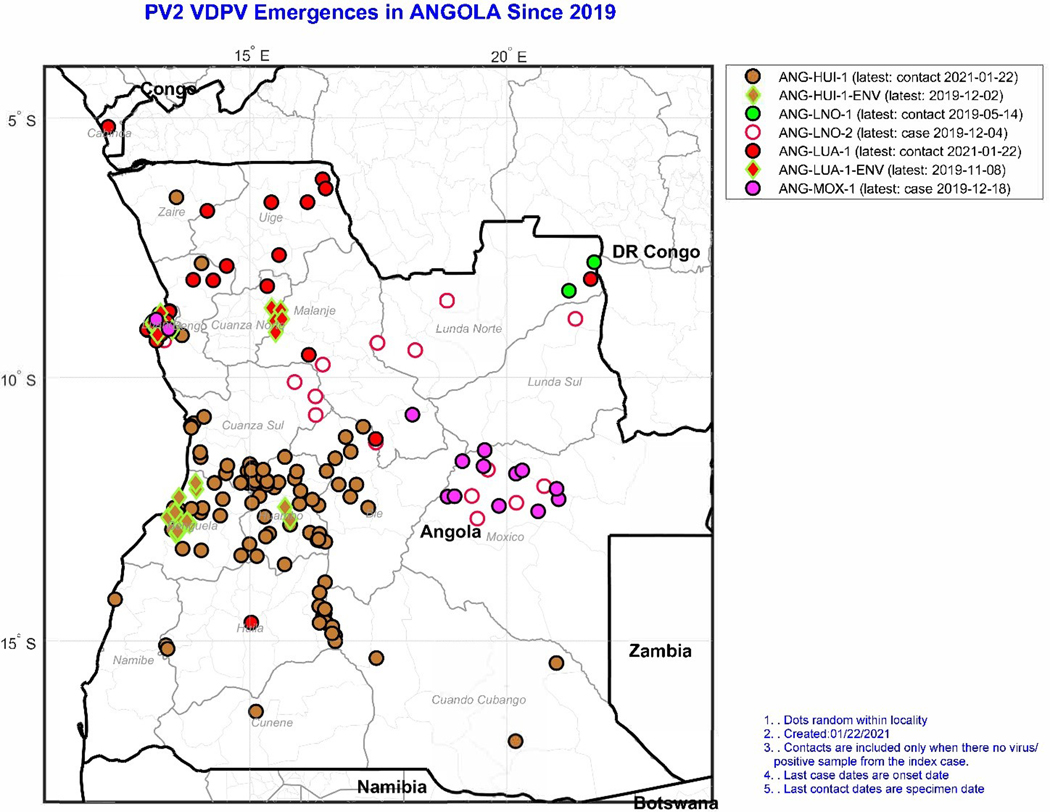
On 7 May 2019, six years after the last case of poliomyelitis was detected, Angola identified one cVDPV2 case in Lunda Norte province with the paralysis onset date of 7 March [11,10]. Angola MoH and partners launched an outbreak investigation and collected additional samples from the case for laboratory analysis. Laboratory notification was made on 7 May 2019, and the Ministry of Health of Angola launched the first wave of response operations on 10 June, while declaring the outbreak on 13 June. From January 2019 to December 2020, Angola field staff and health care facilities reported 956 AFP cases, of which 141 were cVDPV2 cases (Fig. 1). Along with the cVDPV cases, 22 AFP cases were classified as polio-compatible—meaning, AFP cases without adequate samples but with residual paralysis 60 days after onset [1]. The outbreak affected all 18 Angolan provinces, with large hotspots in Luanda, Cuanza Sul, and Huambo provinces (with 23–25 cVDPV2 cases each (Fig. 2). Overall, 33 % of the cVDPV2 cases had a reported onset date between August and December 2019, with a peak of 15 cases with onset during epidemiological week 42 in October.
Fig. 1.
Post-switch cVDPV2 distribution in Angola, by emergence group and specimen date.* *AFP cases in red circles, environmental samples in green circles.
Fig. 2.
Location of isolations of cVDPV2 by emergence group (color), and specimen source (outline shape: circles for AFP cases or contacts/community; diamond for environmental surveillance [ENV]), Angola, 2019–2020.
3.2. Genetic sequencing
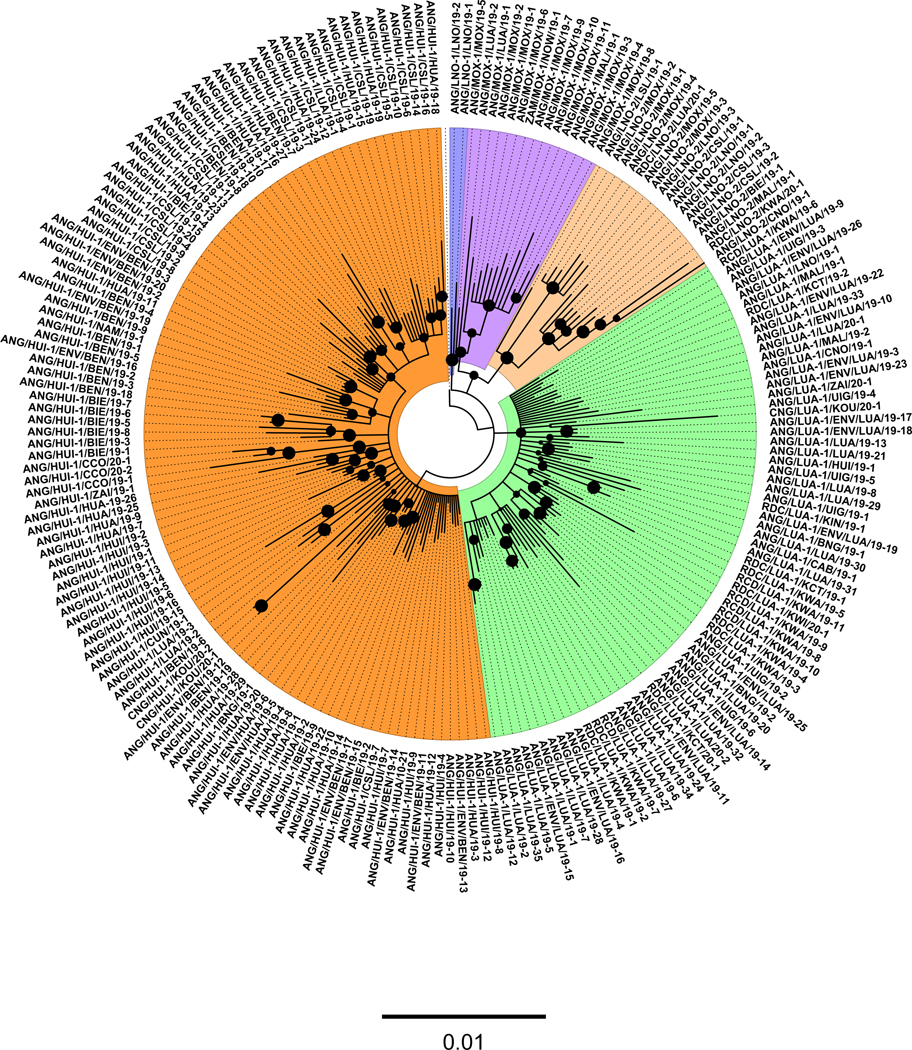
All PV2 isolates from AFP cases, contacts, and those from environmental samples in Angola were sequenced in the VP1 capsid region (903 nucleotides). According to the GPLN guidelines for VDPV classification [12], PV2 isolates with equal or more than six nucleotide differences from the parental Sabin 2 strain were classified as VDPV2. VDPV2 cases are classified as cVPDV2 when there is evidence of circulation defined by the isolation of genetically-related poliovirus for another AFP case, a non-household community member, or through environmental surveillance. Overall, 54 % of VDPV2 isolates from AFP cases and contacts had less than ten VP1 nucleotide differences from Sabin 2 (range: 6–20), suggesting detection soon after emergence. Phylogenetic analysis of VP1 resolved five distinct and independent cVDPV2 emergences (emergence groups) (Table 1, Fig. 3). cVDPV2 emergences were labeled according to the province where each emergence group was first detected or characterized; ANG-HUI-1 (Huila province), ANG-LNO-1 (Lunda Norte province), ANG-LNO-2 (Lunda Norte province), ANG-LUA-1 (Luanda province), and ANG-MOX-1 (Moxico province) (Fig. 2).
Table 1.
Summary of the cVDPV2 outbreaks reported in Angola in 2019 and 2020.
| Emergence group | Outbreak serotype | Total isolates (all sources) | AFP | Environmental Sampling | Other (Human) | Summary | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nb. confirmed positive AFP case | Onset of 1st case | Onset of last case | Nb. positive ES samples | 1st collection date | Last collection date | Nb. other positive samples | 1st collection date | Last collection date | Most recent virus isolation | Interval between 1st and last isolation (days) | Nucleotide* change range | |||
| ANG-LNO-1 | VDPV Type 2 | 2 | 1 | 05–04-19 | - | 0 | – | – | 1 | 14–05-19 | - | 14–05-19 | 8–10 | |
| ANG-LNO-2 | VDPV Type 2 | 15 | 14 | 01–06-19 | 04–12-19 | 0 | – | – | 1 | 01–08-19 | - | 04–12-19 | 10–20 | |
| ANG-LUA-1 | VDPV Type 2 | 52 | 35 | 07–06-19 | 09–02-20 | 14 | 10–09-19 | 08–11-19 | 3 | 12–09-19 | 31–10-19 | 09–02-20 | 6–14 | |
| ANG-HUI-1 | VDPV Type 2 | 98 | 80 | 10–05-19 | 09–02-20 | 13 | 09–09-19 | 30–07-19 | 15 | 04–06-19 | 27–11-19 | 09–02-20 | 6–16 | |
| ANG-MOX-1 | VDPV Type 2 | 14 | 12 | 16–09-19 | 18–12-19 | 0 | – | – | 2 | 28–10-19 | 30–10-19 | 16–09-19 | 7–15 | |
Nucleotide differences in the VP1 capsid region (903 nucleotides) from parental Sabin 2.
Fig. 3.
Bayesian phylogenetic tree of VP1 sequences (903 nucleotides) of 2019–2020.**191 isolates representing the five emergence groups identified in Angola since April 2019. The tree includes cVDPV2 sequences of isolates from DRC (18), Congo (4), and Zambia (1). The five independent cVDPV2 emergences are color-coded. Sequence names start by three-letter country code followed by emergence name, province, and year. Bold circles at interior nodes represent posterior values > 0.7. The scale at the bottom of the tree represents the number of substitutions per site.
Sequence analysis of the isolates from polio cases reported in the southern province of Huila and in the northern province of Lunda Norte (on the border with the Democratic Republic of the Congo—DRC) during April–June 2019 showed that the VDPV2s were closely related to Sabin 2 and represented VDPV2 cases that were distinct from cVDPV2 emergences previously reported in DRC [3]. While ANG-LNO-1 was represented by only one case and an isolate from a community member in Lunda Norte, ANG-LNO-2 spread to six provinces south of Lunda Norte with most cases (n = 5) detected in the southeast province of Moxico. ANG-HUI-1 was the most widely disseminated cVDPV2 emergence group in Angola; it was associated with most of the polio cases (n = 80), detected in most of the provinces (n = 11), mostly in Benguela, Cuanza Sul, and Huambo provinces. During September-November 2019, Luanda province detected most of the cases associated with ANG-LUA-1, which spread north to Uíge and Zaire provinces (on the border with the DRC) until the most recent detection in Zaire province in February 2020. Most of the polio cases in the southern provinces of Angola were associated with ANG-HUI-1. In contrast, Lunda Norte, Luanda, and Cuanza Norte reported polio cases associated with three distinct emergence groups, reflecting a wide circulation among the northern and central provinces of Angola, including municipalities close to Angola’s capital (Luanda).
Between September 2019 and February 2020, cVDPV2 sequences of isolates from DRC (18), Congo (4), and Zambia (1) were associated with Angola cVDPV2 emergences (Fig. 3), reflecting frequent cross-border transmission. The cVDPV2 cases associated with ANG-LUA-1 caused an outbreak with seven reported cases in the southern DRC province of Kwango, which shares an extended border with the northern Angola provinces of Lunda Norte, Malanje, and Uíge. All cVDPV2 isolates had reverted the determinant of the attenuated phenotype of Sabin 2 in VP1 (coding amino acid 143; VP1143). Most of the pairwise sequence comparisons between closely related isolates within each cVDPV2 emergence group showed that there were at least four and up to 12 shared nucleotide substitutions in VP1 from parental Sabin 2. In addition to VP1143, most (99 %) of the isolates in all Angola emergences had a shared nucleotide substitution from Sabin 2 at nucleotide site 700, a nucleotide substitution without replacement of the corresponding original amino acid in the parental Sabin 2 strain. Sequence relationships were inferred in a single VP1 tree, including all five emergence groups and the sequence for Sabin 2 as an outgroup (Fig. 3). The five distinct emergences were inferred to be originated at deep nodes close to the Sabin 2 sequence, suggesting rapid transmission soon after the circulation of Sabin 2 strains among the unimmunized populations in 2018. Several chains of transmission were inferred from the VP1 tree and visualized as groups of isolates grouped in common branches (lineages) of the tree. Most lineages were clustered geographically, indicating intense local circulation within each province or between adjacent provinces. For example, the branching pattern of the tree for ANG-LUA-1 is compatible with several lines of transmission within Luanda and dissemination into the adjacent province of Bengo and into the northern provinces of Zaire and Uíge; further spread occurred across the border into the DRC provinces Kwango, Kwilu, and Kasai Central.
The estimated dates of the originating OPV2 doses for the five Angola cVDPV2 emergence groups were calculated from the extent of divergence of VP1 sequences from the Sabin 2 sequence assuming a constant rate of evolution of 1.1 %/year [13,11]. The estimated dates showed that all five emergences originated after the 2016 switch from tOPV to bOPV. The estimated mean date of cVDPV2 emergence for ANG-LNO-1 (March 2018; 95 % interval August 2017-August 2018), ANG-LNO-2 (February 2018; 95 % interval August 2017-July 2018), ANG-LUA-1 (May 2018; 95 % interval December 2017-October 2018) and ANG-MOX-1 (May 2018; 95 % interval December 2017-October 2018) was within the first half of 2018, while ANG-HUI-1 (July 2018; 95 % interval February 2018-November 2018) emerged within the third quarter of 2018. The reported dates represent estimated mean values around a distribution of overlapping estimated dates among all cVDPV2 emergences.
3.3. Environmental sampling
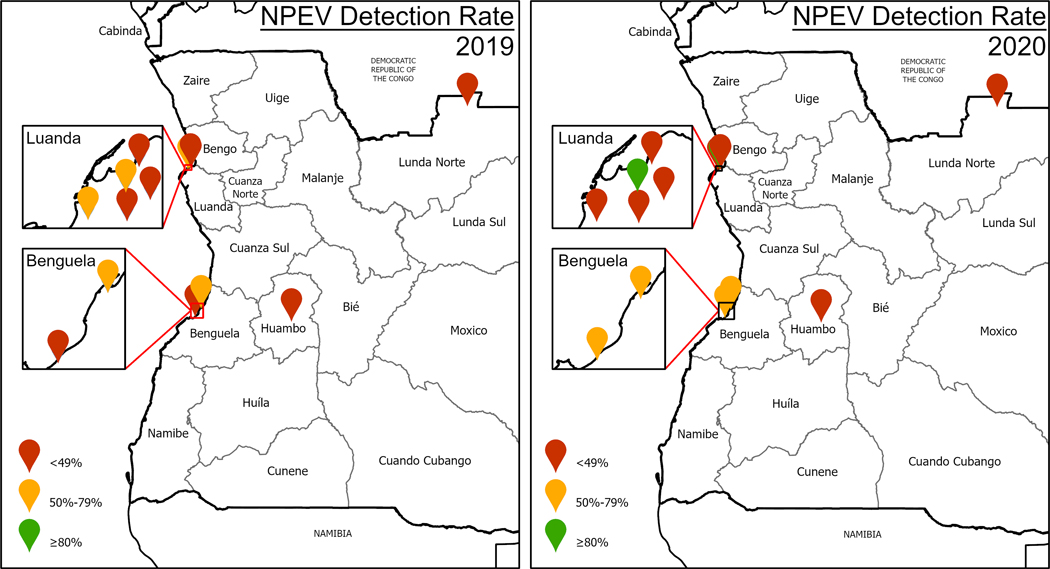
From January 2019 to December 2020, 199 samples were collected from the nine sampling sites, and cVDPV2 was detected in 15 (8 %) of the samples. There were ultimately 27 cVDPV2 isolates, resulting in the 27 cVDPV2 sequences (Table 1), genetically linked to ANG-HUI-1 and ANG-LUA-1 (Fig. 3). All positive environmental samples were collected in 2019 - the first one being identified during epi week 31 (July). The last positive sample was collected during epi week 49 (December). As expected, all Sabin 2 detections in the environmental samples occurred after the launch of monovalent OPV type 2 (mOPV2) Round 0 in each province. Of the 9 active environmental surveillance sites in Angola in 2019 and 2020, only one achieved a non-polio enterovirus detection rate of 50 % (Centro Medico Bethel in Luanda, in 2020) (Fig. 4). Although other sites did detect cVDPV2, the absence of cVDPV2 in samples from these sites could not provide reliable information on the progress of the outbreak.
Fig. 4.
Location of environmental surveillance sites and their annual non-polio enterovirus detection rates, Angola, 2019 and 2020.
3.4. Performance of the AFP surveillance system
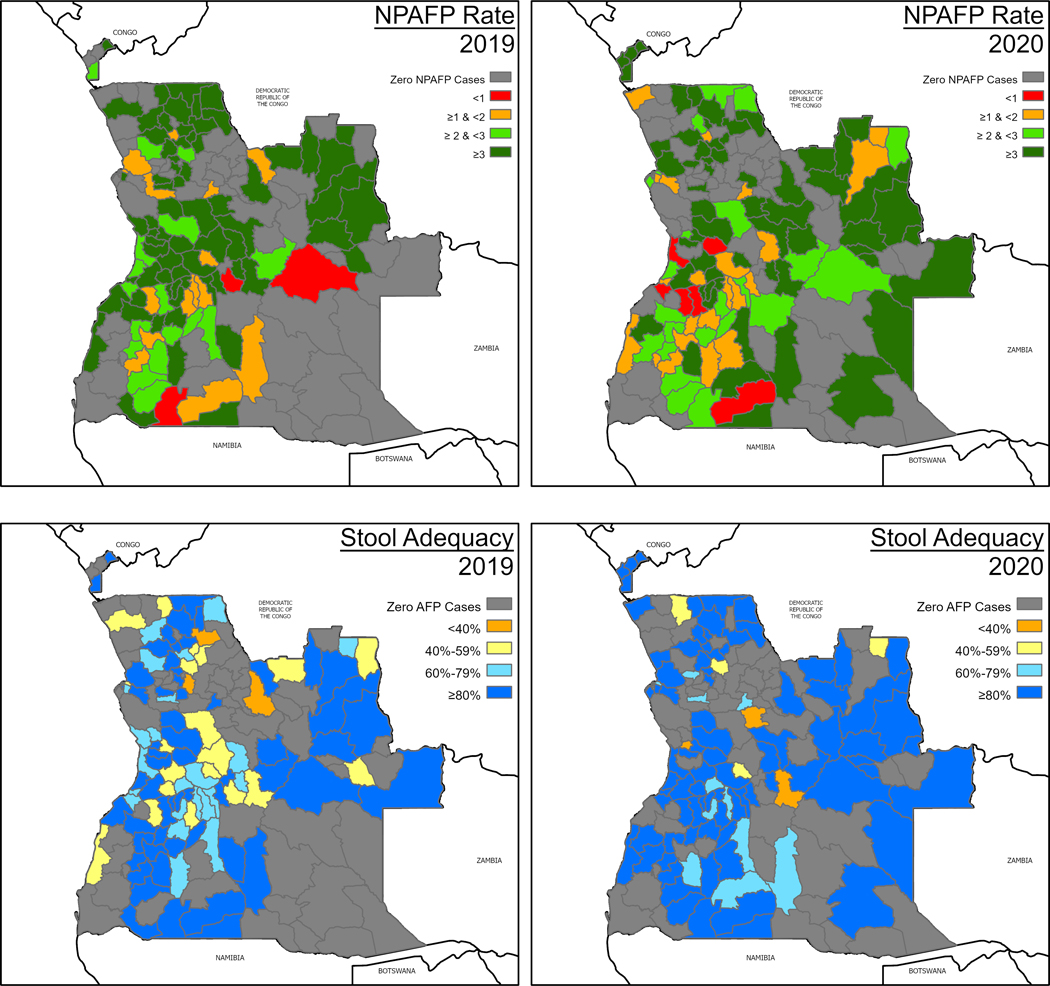
In accordance with GPEI guidelines, Angola reports its AFP surveillance indicators to the WHO Country Office each week [14,12]. Of the 956 reported AFP cases during 2019–2020, 590 (61.7 %) had onset in 2019 and 366 (38.3 %) in 2020. The national NPAFP rate did not reach 3 per 100,000 population younger than 15 at any time during 2019 or 2020 and has considerably declined since the start of the COVID-19 pandemic. The country’s national stool adequacy rate also did not reach the target 80 % threshold in 2019 or 2020 (Fig. 5).
Fig. 5.
NPAFP and stool adequacy rates by district, Angola, 2019 and 2020.
3.5. SIA implementation
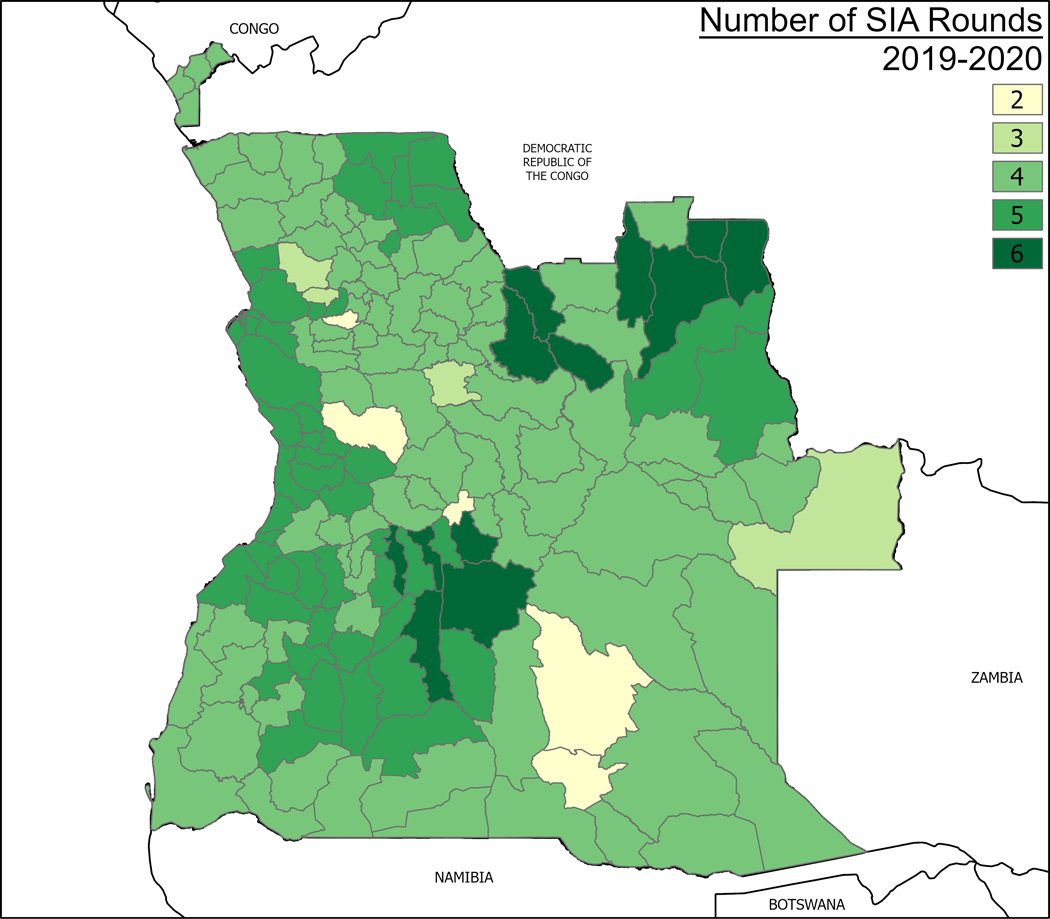
In response to the five cVDPV2 emergences, the Angola MoH conducted 30 mOPV2 SIAs within 10 groups of campaigns based on sequential identification of the affected areas from June 2019 to December 2020 across 171 districts (Fig. 6, Supplemental file 1). Round 0 of the first SIA was launched in Luanda Norte province on 10 June 2019—two months after the identification of the first cVDPV2 case. During this same period, four of the outbreak’s five genetic emergences were detected (i.e., ANG-HUI-1; ANG-LNO-1; ANG-LNO-2; ANG-LUA-1). Response operations started by addressing four concurrent emergence groups in Huíla and Lunda Norte provinces and expanded to the remaining 16 provinces as new cases emerged. Multiple campaigns were implemented across the provinces and in different municipalities at the same time. Therefore, it was not always possible to assign sequential numbers to individual rounds or SIAs. Additionally, the MoH implemented ‘‘Round 3” and ‘‘Round 4” to revaccinate for the third and fourth time in Capenda Camulemba, Cuango, Xa Muteba, and Lovua districts in Lunda Norte province, as well as Muconda and Saurimo districts in Lunda Sul. These rounds are not contemplated in the GPEI guidelines but had to be implemented because of the poor coverage rates recorded during the previous three rounds.
Fig. 6.
Cumulative mOPV2 SIA rounds performed in Angola, by district, 2019–2020.
This protracted response created challenges at several levels and consumed considerable human, financial, and logistical resources. Many of the vaccination rounds coincided with the rainy season (November to April), resulting in impassable roads and difficult terrain for house-to-house operations. Heavy rainfall impacted communications among the vaccination teams and the MoH Polio EOC in Luanda, delaying results reporting and complicating responses to requests for specialized transportation (e.g., 4×4 vehicles and boats to reach isolated populations). Social mobilization activities before most SIAs were a critical component of Angola’s polio response; however, the selection criteria for mobilizers and training quality standards were not applied uniformly in all areas. These variations resulted in instances of vaccination refusal due to religious beliefs, government mistrust, or confusion around the need for multiple campaigns with the same vaccine.
According to the administrative coverage data, almost all of the rounds reached 90 % vaccination coverage rate. The one exception was Round 0 on 18–20 October 2019 in Bengo, Luanda, and Uíge provinces, which achieved 25 % coverage. LQAS results were available for 88 % of the districts that conducted Round 0 vaccination activities. This proportion increased to 89 % for Round 1 and to 96 % for Rounds 2. Over time, the number of districts with adequate LQAS results increased. During Round 0, 31 % of districts reported adequate coverage while during Rounds 1 and 2, the proportion of successful districts were 47 % and 75 %, respectively. However, 9 districts that failed Round 1 and 13 districts that failed Round 2 did not conduct mop-up activities. These districts are listed in Table 2.
Table 2.
Results of the Lot Quality Assurance Sampling (LQAS) evaluation of vaccination coverage by vaccination round—Angola, From June 2019 to July 2020.
| Round 0: 54 of 61 districts provided LQAS data (88%) |
Round 1: 151 of 168 districts provided LQAS data (89%) |
Round 2: 146 of 152 districts provided LQAS data (96%) |
Round 3: 13 of 26 districts provided LQAS data (50%) |
Round 4: 3 of 4 districts provided LQAS data (75%) |
|---|---|---|---|---|
| Passed: 17/54 | Passed: 71/151 | Passed: 110/146 | Passed: 2/13 | Passed: 2/3 |
| Cusp: 11/54 | Cusp: 38/151 | Cusp: 29/146 | Cusp: 8/13 | Cusp: 0 |
| Failed: 26/54 | Failed: 42/151 | Failed: 16/146 | Failed: 3/13 | Failed: 1/3 |
| Failed and did not receive revaccination: 0 | Failed and did not receive revaccination: 9 Cunene: Cahama, Cuanhama, Curoca, Namacunde, Ombadja Cuando Cubango: Dirico Namibe: Bibala Zaire: Mbanza Congo, Soyo |
Failed and did not receive revaccination: 13 Benguela: Baia Farta, Catumbela, Chongoroi Bié: Cunhinga Cabinda: Cacongo Huila: Caluquembe, Gambos Luanda: Icolo e Bengo Malanje: Quirima Moxico: Bundas, Cameia, Luchazes, Luena |
Failed and did not receive revaccination: 2 Bié: Chinguar Cuando Cubango: Menongue Cunene: Cuvelai |
Failed and did not receive revaccination: 1 Lunda Norte: Cuango |
At the writing of this publication, the last identified cases of cVDPV2 polio in Angola occurred in Mbanza Congo district in the Zaire province and in the Mavinga district of the Cuando Cubango province, both with a symptom onset date of 9 February 2020. The number of cVDPV2 cases nationwide started to decline in October 2019, after five SIA campaigns, and continued to decline until February 2020. No new cases have been identified to date. Three cases did not meet the GPEI definition of a breakthrough case (i.e., detection of cVDPV2 (onset of collection) 28 days after 2 or more rounds [15,13]) but are worth noting:
Mussende district, Cuanza Sul province: Round 2 was completed on 20 October 2019, and one case reported paralysis onset on 11 November 2019 (22 days after completion of Round 2). A mop-up vaccination response was conducted 6–8 November 2020 in Mussende, with 100.9 % administrative coverage.
Cuito district, Bié province: Round 2 was completed on 1 September 2019, and one case reported paralysis onset on 15 November 2019 (76 days after completion of Round 2). A mop-up round in Cuito took place on October 30–1 November 2020 to address this issue, with 113.2 % administrative coverage.
Cazenga district, Luanda province: In this densely populated area of the capital with suboptimal sanitary services, Round 2 was completed on 15 December 2019. One case reported paralysis onset on 27 December (12 days following Cazenga’s third round of mOPV2).
4. Discussion
After 6 years with no reported WPV or cVDPV cases, Angola experienced a large outbreak of cVDPV2 in 2019 and 2020. While the GPEI recommends a maximum of 14 days between notification and outbreak declaration, 37 days elapsed between the laboratory notification of the first cVDPV2 case (7 May 2019) and the official declaration of the outbreak (13 June 2019). Genetic sequence analysis indicated five distinct emergences and suggested rapid transmission soon after emergence during 2018 among the unimmunized population. The origins of this outbreak remain unclear.
There were 141 cVPDPV2 polio cases across all 18 provinces, and 75 (54 %) were identified in Huambo, Luanda, and Lunda Norte. About 38 % of the Angolan population lives in these three provinces, according to the 2014 Angolan Census [16,14]. Cross-border transmission may have been facilitated by commercial bus services, which transport passengers from Luanda to DRC. Most cases were identified in the second part of 2019 and were met with extensive response operations in the form of 30 vaccination rounds within 10 SIA campaigns across all 18 provinces.
The performance of the national AFP surveillance system appears to be subpar. During 2019 and 2020, the national NPAFP rate remained below 3 per 100,000 population younger than 15 (the GPEI threshold set for the African continent). At the subnational level, the threshold was reached in Huambo, Luanda, and Lunda Norte provinces, where the largest outbreaks occurred. Angola reached the 80 % threshold for sample adequacy 20 times during the 97 epidemiological weeks of the outbreak (that is, the rolling annualized sample adequacy rate was 80 % or greater during 20 of the 97 outbreak weeks)—possibly aided by the house-to-house SIAs that helped identify AFP cases, which would have remained undetected otherwise. However, in the three most affected provinces, this threshold was rarely met. In the remaining 15 provinces, stool adequacy and NPAFP rate performance indicators reflect poor performance. Therefore, the Type 2 virus may still circulate undetected in these areas because of the low sensitivity of the surveillance system. These indicators suggest that Angola’s AFP surveillance system requires additional strengthening across the country.
Conversely, NPEV cases continued to be identified consistently throughout this same period, suggesting that the sensitivity of the environmental sampling system remained constant over time. Therefore, any changes in cVDPV2 detection are likely due to a true decrease of the virus in the environment. Further, all Sabin 2 isolates were detected after SIA operations with mOPV2 had been launched in the same districts where the environmental samples had been collected. This finding supports the statement that Sabin 2 was introduced by response operations alone.
5. Conclusions
The MoH Polio Emergency Operations Center (EOC) launched a multi-pronged response strategy to address cVDPV2 cases as they emerged. Given the sequential identification of cases across the country, vaccination rounds were launched simultaneously in multiple provinces.
The environmental surveillance data strongly suggest that the aggressive multi-province response by MoH and its partners ended the cVDPV2 outbreak that affected Angola in 2019 and 2020. After 13 months from the onset of the most recent case, the Emergency Committee of the Polio Public Health Emergency of International Concern considers Angola ‘‘no longer infected” but vulnerable to reinfection [17,15].
Nonetheless, based on the suboptimal performance of Angola’s AFP surveillance system, it is possible that existing or newly emerged cVDPV2 lineages may still be circulating undetected in some communities. Furthermore, Angola remains at risk of virus importation from neighboring countries with cVDPV2 outbreaks. The COVID-19 pandemic is expected to continue to interrupt both routine and emergency vaccination activities until 2022; therefore, future large-scale response operations could be complicated to implement. We recommend that surveillance strengthening operations focus on the districts where mop-up rounds were not completed, especially where additional cases were detected following the SIA campaigns. Depending on the resources available, reinforcement operations can be extended to the entire country.
Supplementary Material
Acknowledgments
This article was published as part of a supplement supported by Centers for Disease Control and Prevention Global Immunization Division. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or World Health Organization or UNICEF or Bill and Melinda Gates Foundation. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors.
Footnotes
CRediT authorship contribution statement
Alda Morais: Conceptualization, Methodology, Investigation, Resources, Writing – review & editing, Supervision, Project administration. Joana Morais: Resources, Writing – review & editing. Miguel Felix: Conceptualization, Methodology, Investigation, Writing – review & editing. Zoraima Neto: Conceptualization, Methodology, Investigation, Writing – review & editing. Valódia Madaleno: Conceptualization, Methodology, Investigation, Writing – review & editing. Abubakar Sadiq Umar: Conceptualization, Methodology, Investigation, Resources, Writing – review & editing, Supervision, Project administration. Nirakar Panda: Conceptualization, Methodology, Investigation, Data curation, Writing – review & editing, Supervision, Project administration. Fekadu Lemma: Conceptualization, Methodology, Investigation, Writing – review & editing. José Alexandre Lifande Chivale: Conceptualization, Methodology, Investigation, Writing – review & editing, Supervision. Danielle Graça Cavalcante: Formal analysis, Investigation, Data curation, Writing – review & editing. Elizabeth Davlantes: Investigation, Resources, Writing – review & editing, Visualization, Supervision. Margherita Ghiselli: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration. Catherine Espinosa: Formal analysis, Investigation, Resources, Writing – review & editing, Supervision. Ari Whiteman: Formal analysis, Data curation, Visualization. Jane Iber: Methodology, Formal analysis, Data curation, Writing – review & editing, Supervision. Elizabeth Henderson: Methodology, Formal analysis, Data curation, Writing – review & editing. Kelley Bullard: Methodology, Formal analysis, Data curation, Writing – review & editing. Jaume Jorba: Methodology, Formal analysis, Data curation, Writing – review & editing, Visualization. Cara C. Burns: Methodology, Formal analysis, Data curation, Writing – review & editing, Visualization. Ousmane Diop: Conceptualization, Methodology, Resources, Writing – review & editing, Supervision, Project administration. Nicksy Gumede: Methodology, Formal analysis, Data curation, Writing – review & editing. Lerato Seakamela: Formal analysis, Data curation, Writing – review & editing. Wayne Howard: Formal analysis, Data curation, Writing – review & editing. Alean Frawley: Resources, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2023.02.035.
Data availability
Data will be made available on request.
References
- [1].Global Polio Eradication Initiative (GPEI). Standard operating procedures: responding to a poliovirus event or outbreak, version 3.1. Geneva: World Health Organization; 2020. Licence: CC BY-NC-SA 3.0 IGO. Available at:. [Google Scholar]
- [2].Chard AN et al. Progress Toward Polio Eradication - Worldwide, January 2018-March 2020. MMWR Morb Mortal Wkly Rep 2020;69(25):784–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Alleman MM et al. Update on Vaccine-Derived Poliovirus Outbreaks - Worldwide, July 2019-February 2020. MMWR Morb Mortal Wkly Rep 2020;69(16):489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Garon J et al. Polio endgame: the global switch from tOPV to bOPV. Expert Rev Vaccines 2016;15(6):693–708. [DOI] [PubMed] [Google Scholar]
- [5].WHO-UNICEF estimates of IPV1 coverage. Retrieved on 2 May 2021 from: https://apps.who.int/immunization_monitoring/globalsummary/timeseries/tswucoverageipv1.html.
- [6].Global Polio Eradication Initiative (GPEI). Assessing Vaccination Coverage Levels Using Clustered Lot Quality Assurance Sampling – Field Manual (27 April 2012). Retrieved on 2 April 2021 from: https://polioeradication.org/wp-content/uploads/2016/09/Assessing-Vaccination-Coverage-Levels-Using-Clustered-LQAS_Apr2012_EN.pdf.
- [7].Department of Vaccines and Biologicals, World Health Organization (WHO). Guidelines for environmental surveillance of poliovirus circulation (March 2003). Retrieved on 5 April 2021 from: https://polioeradication.org/wp-content/uploads/2016/07/WHO_V-B_03.03_eng.pdf.
- [8].US Centers for Disease Control and Prevention (CDC). Poliovirus Laboratory Testing. Retrieved on 1 March 2021 from: https://www.cdc.gov/polio/what-is-polio/lab-testing/index.html.
- [9].Jorba J. Phylogenetic Analysis of Poliovirus Sequences. Methods Mol Biol 2016;1387:227–37. [DOI] [PubMed] [Google Scholar]
- [10].Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001;17(8):754–5. [DOI] [PubMed] [Google Scholar]
- [11].Jorba J et al. Update on Vaccine-Derived Poliovirus Outbreaks - Worldwide, January 2018-June 2019. MMWR Morb Mortal Wkly Rep 2019;68(45):1024–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Initiative GPE. Classification and reporting of vaccine-derived polioviruses (VDPV). Geneva: World Health Organization; 2016. Available at: https://polioeradication.org/wp-content/uploads/2016/09/Reporting-and-Classification-of-VDPVs_Aug2016_EN.pdf. [Google Scholar]
- [13].Jorba J et al. Calibration of multiple poliovirus molecular clocks covering an extended evolutionary range. J Virol 2008;82(9):4429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Global Polio Eradication Initiative (GPEI). Surveillance Indicators – Acute Flaccid Paralysis (AFP) Surveillance. Retrieved on 1 March 2021 from: https://polioeradication.org/polio-today/polio-now/surveillance-indicators/.
- [15].Bigouette JP et al. Progress Toward Polio Eradication — Worldwide, January 2019–June 2021. MMWR Morb Mortal Wkly Rep 2021;70(34):1129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Censo 2014. Retrieved on 2 April 2021 from: http://censo.ine.gov.ao/xportal/xmain?xpid=censo2014.
- [17].Statement following the Twenty-Eighth IHR Emergency Committee for Polio. Retrieved on 19 June 2021 from: https://www.who.int/news/item/21-05-2021-statement-following-the-twenty-eighth-ihr-emergency-committee-for-polio.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.