Abstract
The remarkable ability of biological systems to sense and adapt to complex environmental conditions has inspired new materials and novel designs for next-generation wearable devices. Hydrogels are being intensively investigated for their versatile functions in wearable devices due to their superior softness, biocompatibility, and rapid stimulus response. This review focuses on recent strategies for developing bioinspired hydrogel wearable devices that can accommodate mechanical strain and integrate seamlessly with biological systems. We will provide an overview of different types of bioinspired hydrogels tailored for wearable devices. Next, we will discuss the recent progress of bioinspired hydrogel wearable devices such as electronic skin and smart contact lenses. Also, we will comprehensively summarize biosignal readout methods for hydrogel wearable devices as well as advances in powering and wireless data transmission technologies. Finally, current challenges facing these wearable devices are discussed, and future directions are proposed.
Keywords: bioinspired hydrogels, wearable devices, flexible electronics, diagnostics, biosensors, biosignal computing
Graphical Abstract
1. INTRODUCTION
Wearable electronics are emerging technologies that consist of devices capable of quantifying biochemical analytes, monitoring physiological parameters, detecting human movements, and generating interactions between users and external environmental stimuli. Despite the remarkable advances in recent decades, the intrinsic differences between soft skin and rigid synthetic systems have been among the most daunting challenges in developing wearable devices to be more compatible and effective for the skin.
Hydrogels, similar to natural biological soft tissues, comprise a three-dimensional polymer network that can retain water and have emerged as promising candidates for wearable devices used for wearable diagnostics and biosensors. The copolymerization of 2-hydroxyethyl methacrylate was first reported via ethylene glycol dimethacrylate in 1960.1 Hydrogels are typically synthesized by cross-linking hydrophilic polymer chains or polymerizing water-soluble monomers with cross-linkers. Synthetic hydrogels have enabled the realization of smart hydrogels to respond to changes in the environment (i.e., pH, temperature, and electric or magnetic field). Furthermore, other features such as self-healing, superb adhesion, and superwettability have also been achieved.2,3 However, there are remaining opportunities to develop novel synthetic hydrogels with desirable properties like multiple stimuli responses to mimic biological substances or structures from nature.
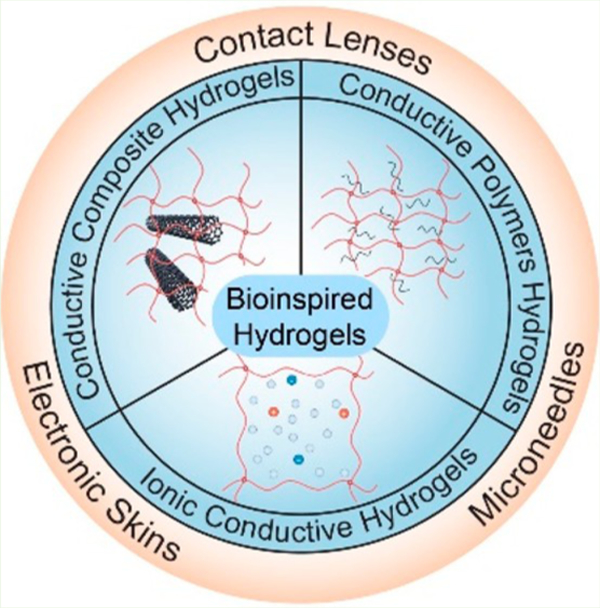
In recent years, significant research progress has been made in fabricating various bioinspired hydrogels. Based on these advances, this review paper summarizes the recent progress in hydrogels inspired by natural materials and structures for wearable diagnostics and biosensing (Figure 1). We start with a comprehensive overview of the rational guidelines for designing hydrogels such as ionic conductive hydrogels, conductive polymer (CP) hydrogels, and micro/nanocomposite conductive hydrogels for wearable diagnostics and biosensors. These state-of-the-art hydrogel-based wearable devices can be categorized into three classes: electronic skin, smart contact lens, and minimally invasive devices (typically microneedles (MNs)). These devices will be first summarized, followed by the discussions on biological readouts for bioinspired hydrogel-based wearable devices, such as geometrical, optical, and electrical readouts. Finally, we will highlight the need for wearable devices, including wearable powering (i.e., flexible battery or supercapacitor) and wireless data transmission (i.e., Bluetooth, WIFI, and near-field communication (NFC)) technologies.
Figure 1.
Bioinspired hydrogel materials and their applications in recently developed wearable devices. They can be divided into the ionic conductive hydrogel, conductive polymer hydrogel, and conductive micro/nanocomposite hydrogel. Current hydrogel wearable devices are mainly “electronic skin”, reproduced with permissions from refs 4 and 5. Copyright 2020 Royal Society of Chemistry and copyright 2020 Wiley, respectively; and “microneedle”, reproduced with permissions from refs 6 and 7. Copyright 2019 Wiley, respectively, and “contact lens”, reproduced with permission from refs 8 and 9. Copyright 2021 Elsevier and copyright 2020 American Society for Pharmacology and Experimental Therapuetics, respectively.
2. BIOINSPIRED HYDROGELS FOR WEARABLE DEVICES
Conventional wearable devices consist of conductive films (e.g., copper) or a combination of conductive materials (e.g., carbon-based compounds, metal nanoparticles) integrated with elastomeric substrates such as polydimethylsiloxane (PDMS),10 poly(ether imide) (PEI),11 polyurethane (PU),12 styrene-ethylene-butylene-styrene (SEBS),13 and Ecoflex.14 Inspired by skin, these devices are designed to be stimuli-responsive, meaning that they can convert environmental stimuli such as pH, temperature, moisture, and pressure to electrical signals. These stimuli-responsive electrical signals can be employed as wearable sensors to monitor health status or detect human movements. The performance of these devices depends on both the flexibilities of the elastomer substrates and the electrical conductivity and sensitivity of conductive networks. Thereby, there are several limitations in the fabrication of these traditional wearable devices: (i) the fabrication process (e.g., spin coating, photolithography, or patterning) is often complicated, limiting commercialization; (ii) the mismatch between the rigid conductive network and flexible elastomer matrix results in displacement during deformation; therefore, the device generates a poor electrical signal; (iii) the lack of flexibility and stretchability in the conductive network causes irreversible damage to the device under continuous stretching and compression stress, thus lowering the efficacy; (iv) the conventional elastomer substrate suffers from poor biocompatibility to the human tissues; (v) the lack of sufficient stretchability hinders proper attachment of the elastomer substrate to the skin.15
Hydrogels are promising candidates as an alternative to conventional elastomers in wearable technology due to their high flexibility and stretchability.16 Hydrogels are three-dimensional (3D) hydrophilic polymers with high water content. These polymers are physically or chemically cross-linked to form stable gels that retain their structures in the presence of water. Hydrogels can be divided into two categories: natural or synthetic. Natural hydrogels are mainly composed of repeatable building blocks of proteins (e.g., collagen,17 gelatin,18 and silk19), carbohydrates (e.g., alginate,20 cellulose,21 and chitosan22), or DNA.23 They contain multiple functional groups such as carboxyl, hydroxyl, or amine groups that can modify their physicochemical and mechanical characteristics.24 Taking advantage of these functional groups, researchers designed conductive natural hydrogels with tunable mechanical properties favorable for wearable devices.25 Natural conductive hydrogels have superior advantages over conventional elastomers since they have essential properties such as conductivity and stretchability, which are required for wearable devices; thereby, there is no concern about the mismatch between elastomer substrate and the conductive rigid network. Besides, natural hydrogels are highly biocompatible, and their synthesis procedure is simple and cost-effective.24 Additional features, including viscoelasticity, permeability to biomolecules (e.g., proteins and ions), self-healing, and antibacterial properties,26,27 make hydrogels promising materials for numerous applications such as sensors,28 optics,29 actuators,30 drug delivery,31 and tissue regeneration.32
In contrast to natural hydrogels, synthetic hydrogels are amorphous, display isotropic structures, and are still simple and have limited functionality.33 Given that natural hydrogels contain a considerable amount of water to ensure their functionality, there is an opportunity to manipulate synthetic hydrogels with properties analogous to those of natural hydrogels while mimicking biological structures and demonstrating all the key features of natural hydrogels.34 In most cases, hydrogels are not inherently conductive and should be carefully incorporated with sensitive conductive moieties to prevent degradation of their mechanical properties. Additionally, the additive conductive moieties should be sensitive enough to detect subtle stimuli and transduce them into electrical signals. In addition to their stability, all these features need to be considered while designing bioinspired hydrogels for wearable devices.15
Recently, several attempts have been made to fabricate conductive hydrogels by mimicking biological systems. In this review, we will describe various examples about the fabrication of bioinspired synthetic conductive hydrogels and encourage readers to refer to the review by Cui et al.24 to learn about conductive natural hydrogels used for wearable devices. Herein, for ease of understanding, we divide bioinspired hydrogels into three categories based on their conductive materials: ionic conductive hydrogels, conductive polymer (CP) hydrogels, and micro/nanocomposite conductive hydrogels. Finally, we will provide recent examples for each category.
2.1. Ionic Conductive Hydrogels.
In this category, conductive components such as salts35 and strong polyelectrolytes36 are added to the hydrogels to transmit ions/electrons. The ions are either chemically bound to the hydrogels or physically added to the prepolymer as free ions. The physical mixing of strong polyelectrolytes with a hydrogel is the most convenient way to increase its conductivity because it supplies numerous anions and cations to the hydrogels.37 Conductivity occurs via the transfer of electrons/ions through the water in the hydrogel. The water in hydrogels either is bound to the polymer chain or exists in free water formed between the spaces of the polymer chains. Water loss in the hydrogel significantly reduces the ionic conductivity as ions cannot diffuse well without water. Nonetheless, hydrogels should contain a high amount of free water and remain moist to retain good conductivity.
Table 1 summarizes the properties of typical bioinspired hydrogels incorporated either physically or chemically with ionic conductors to fabricate wearable devices. In an example, Zhang et al. physically mixed lithium chloride (LiCl) to the tough, hydrophobic hexadecyl methacrylate (HMA)-acrylamide (AMM) hydrogel to increase its conductivity (Figure 2a).38 Inspired by the delamination structure of the skin, including the outer tough epidermis and inner elastic dermis, the authors further developed a supportive layer under the tough hydrogel, composed of acrylate thymine (Ata)-acrylate adenine (AAa) to endow adhesiveness and stretchability to the bilayer hydrogel. The upper tough hydrogel layer exhibited a maximum conductivity of 0.039 S/cm for the highest concentration of LiCl (1.96 mol/L). In addition, the lower layer stuck to the skin and insulated the skin from electrical current attacks.
Table 1.
Summary of the Bio-Inspired Hydrogels in Wearable Diagnostics and Biosensors
| type | hydrogel | electrolyte | biomimicry | properties | sensing category | ref |
|---|---|---|---|---|---|---|
| Physical mixing | HMA-AMM/Ata-AAa | LiCl | Human skin | Conductivity (0.039 S/cm); Adhesive strength (275.6 N/m); Stretchability (1784%); Toughness (~1750 kJ/m3); Self-recovery | Strain | 38 |
| TOCNF/PAA–PPy | Fe3+ | Human skin | Stretchability (890%); Viscoelasticity (G′ 27.1 kPa); Conductibility (3.9 S m−1); Self-healing (98–99%); Gauge factor (7.3) | Strain | 53 | |
| G/HPAAM/Dopa-Fe3+ | LiCl | Mussel | Stretchability (1150%); Tensile strength (112 kPa); Conductivity (0.0720 S cm−1); Adhesion (100 N m−1); Self-healing | Strain | 16 | |
| OCS-AMP/PAAM | NaCl | DNA | Stretchability (500%); elastic modulus (22.9 kPa); Toughness (1457.6 kJ m−3); Conductivity (0.028 S cm−1); Self-recovery | Strain Pressure | 43 | |
| PAAM/PDA | Sodium casein | Mussle | Stretchability (2100%); Adhesion (2200 N/m); Conductivity (3.5 mS cm−1); Self-healing | Strain Pressure | 54 | |
| PAA/PVA/PAAc | H2SO4 | N/A | Stretchability (2600%); Fracture strength (3.1 MPa); Toughness (18.7 MJ m−3); Self-recover | Stretchable electronics | 55 | |
| Chemical bonding | ACC/PAA/Alg | Ca2CO3 | Shrimp shell | Shrimp shell Stretchability (1000%); Self-recovery (83%); Pressure sensitivity (0.17 kPa−1); Self-healing | Strain Pressure | 56 |
| PAA/DOPADHA | Fe(NO3)3 | Human skin | Stretchability (800%); Adhesion to porcine skin (12.6 kPa); Thermoplastic; Electroconductive; Self-healing (98% recovery) | Strain | 57 | |
| LiCl/agar/PAAM | LiCl | Human skin | Stretchability (1600%); Toughness 2.2 MJ/m3; Gauge factor (~1.8); Bending sensibility | Temperature Strain | 58 | |
| Guar gum | Na2B4O7 | Hydrogen bond | Adhesive; Antifreezing; Self-healing; Injectability; Conductive | Strain | 59 | |
| PNaAMPS/PAAM | Fe3+ | Skeletal muscles | Toughness/elastic modulus (~1 MPa)/self-growth | Soft robots | 60 |
Figure 2.
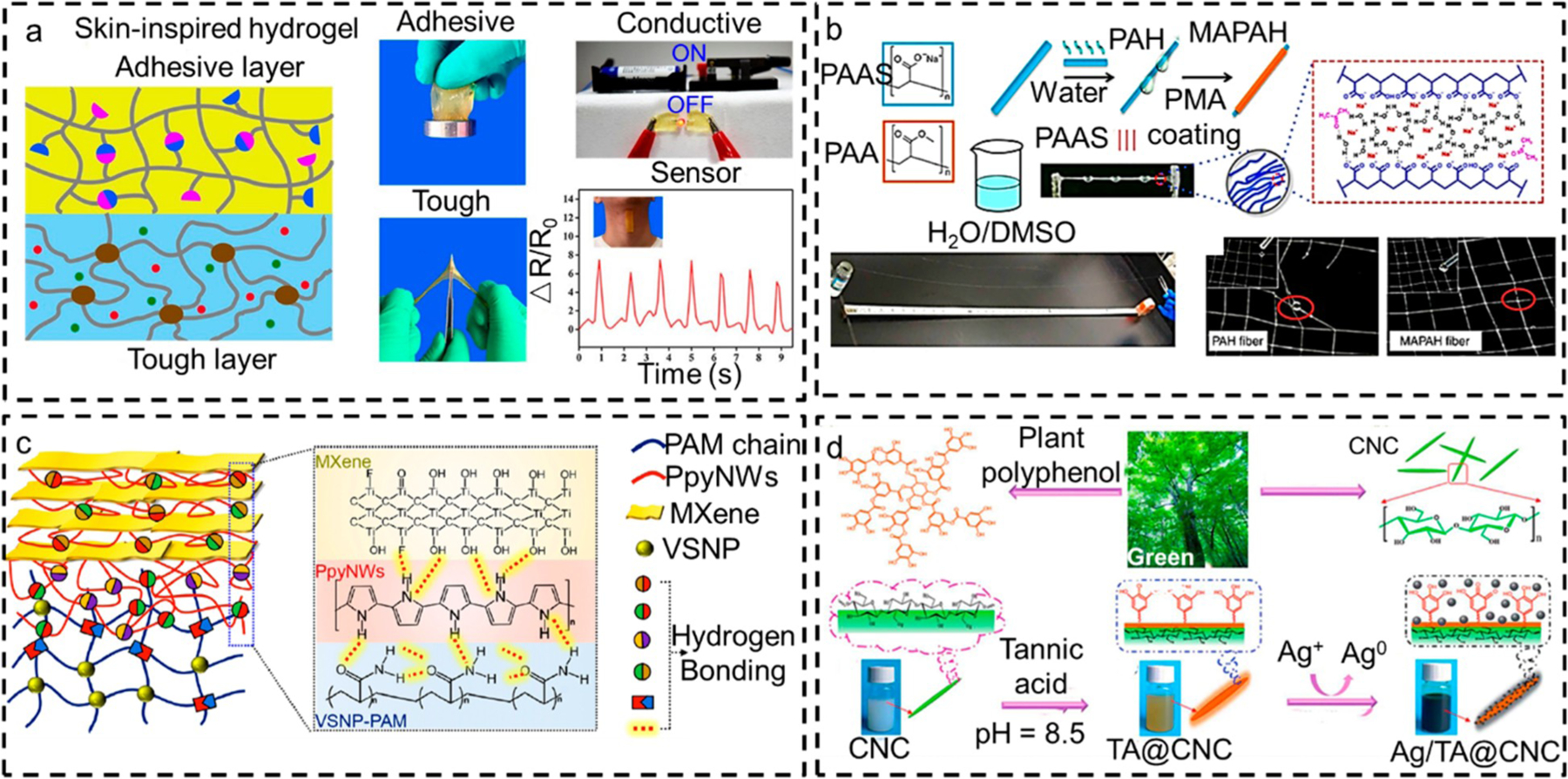
Bioinspired hydrogel materials for wearable devices. (a) Schematic illustration of the skin-inspired bilayer conductive hydrogel. The hydrogel is made of a tough conductive layer containing hexadecyl methacrylate (HMA)-acrylamide (AMM) and a nonconductive adhesive layer comprising acrylate thymine (Ata)-acrylate adenine (AAa). The images display the adhesive property, conductivity, and toughness of the hydrogel and its application in real-time monitoring of neck movements. Reprinted from ref 38 with permission from 2019 Elsevier. (b) Schematic illustration demonstrating the fabrication process of PAH and MAPAH fibers, and the picture of PAH fiber exhibits bead formation on the spider-like string. Adapted from ref 39 with permission. Copyright 2018 from Nature Research. (c) Schematic illustration demonstrating MXene-PVP/PVA double-network hydrogel sensor fabrication process and its application for simulating Morse code and handwriting verification. Adapted from ref 40 with permission. Copyright 2020, from AAAS. (d) Schematic illustration showing the fabrication process of Ag/TA incorporated CNC hydrogel with dynamic borate ester bonds and its structure in SEM image. The hydrogel was used as electronic skin to dial a number on a smartphone. Reprinted from ref 21 with permission. Copyright 2019 Royal Society of Chemistry.
Another example is designing a skin-mimicking mechanical sensor using DNA-inspired hydrogel. Such DNA-inspired hydrogels with high biocompatibility have been of interest because their properties can switch from gel to sol in response to chemical or physical stimuli. Various functional motifs such as aptamers, DNAzymes, i-motifs, or antisense DNA can also be incorporated into DNA-hydrogel to boost their biological sensing.41 In addition, abundant hydrogen bonding interactions in the structure of nucleobases offer excellent self-adhesiveness to the tissue.42 Taking advantage of these properties, Zhang et al. engineered a DNA-inspired hydrogel made of adenosine monophosphate (AMP) cross-linked quaternized chitosan (QCS) polymer and polyacrylamide (PAAm) network to mimic skin-like sensing. Sodium chloride (NaCl) was physically mixed in the hydrogel to provide ionic conductivity.43 High concentration of AMP improved the mechanical strength and strain of the hydrogel, which could withstand the strain of 500%, resembling skin-like mechanical behavior. The incorporation of NaCl increased the conductivity of the hydrogel to ca. 0.028 S/cm, which did not affect by increasing the AMP content. The authors could successfully use this hydrogel as a wearable sensor for detecting human motions such as joint movement, facial expression, and voice signals.
In addition to physically mixing ionic conductors into the gels, several researchers have also used ionic conductors to chemically cross-link the hydrogels while improving their electrical conductivity. Examples include ferric ions (Fe3+), which were used to cross-link hyaluronic acid (HA),44 and calcium ions (Ca2+), which were used to cross-link alginate.45 It is important to note that although adding ionic conductors to the hydrogels is a simple way to improve its conductivity, the gauge factor is low (1–3) in ionic conductive devices,15 and there is a specific concentration limit beyond which these electrolytes can be toxic for human tissues.
2.2. Conductive Polymer (CP) Hydrogels.
CPs are synthetic polymers that are inherently conductive. These polymers display tunable conductivity, allowing communication with different tissues such as muscle, cardiac, and nerve tissues.46 However, their hydrophobicity and rigid structure hinder their usage as a standalone polymer for wearable devices.27 Therefore, researchers combined CPs with hydrogels to improve their flexibility. CP hydrogels are usually made by several methods. (i) The first method is electropolymerization of CPs in prefabricated hydrophilic hydrogels. In this method, the prefabricated insulating hydrogel is loaded on the working electrode (e.g., gold electrode) and subsequently immersed in the CP monomer precursors (e.g., pyrrole or aniline). Upon applying electricity, CP grows in the pores of the hydrogel and forms the CP hydrogel.47 For instance, inspired by the dynamic network in the human dermis formed by the interaction between collagen and elastin fibers, Gao et al.48 prepared CP hydrogels in which polypyrrole (PPy) was grown within the voids of pentaerythritol ethoxylate (PEE) hydrogels using an electrochemical method. The hybrid hydrogel cross-linked by hydrogen bonds and electrostatic interactions provides a dynamic network that can drastically improve the flexibility and mechanics of the CP hydrogels, which also display superior electrical conductivity (115 S cm−1).48 (ii) The second method is chemical polymerization where the precursors of CP and insulating hydrogel are mixed and chemically cross-linked, or the prefabricated hydrogel is soaked in the CP precursors, which promoted CPs growth in the pores of hydrogel using oxidants. Inspired by the animal dermis, Li et al.49 developed hybrid hydrogels comprising soft poly(vinyl alcohol) (PVA) polymer and rigid polyaniline (PANI) CPs via dynamic boronate bond. The functional boronic acid groups on PANI cross-linked with PVA form a stretchable (250%) and conductive (0.1 S cm−1) CP hydrogel. In another study, a biologically derived CP hydrogel composed of methacrylate modified heparin and PANI was engineered.50 The authors first prepared modified heparin hydrogel by photo-cross-linking, then soaked it in monomer precursors of PANI, and then applied oxidative polymerization. The fabricated CP hydrogel showed a maximum impedance of 2 × 104 Ω at 0.01 Hz and a storage modulus of ~900 Pa. It is important to note that in both electro- and chemical polymerization, growing CP monomers in the prefabricated hydrogel is a physical entrapment with weak interaction. Therefore, there is a possibility that, in a wet environment, the hydrogel may lose its conductivity due to the leaching of the CPs, and it might cause toxicity issues.51 (iii) The third method was developed to address the drawbacks of electro- and chemical polymerization. In this method, the CP is used as a standalone polymer hydrogel, and it is fabricated by self-assembly or the addition of chemical cross-linkers. The typical CP polymer used as sole CP hydrogel is poly(ethylene dioxythiophene) (PEDOT). Alkoxysulfonate functionalized PEDOT hydrogels are synthesized using sulfonate PEDOT monomer precursors in the presence of ammonium peroxide (APS) or iron chloride (FeCl3) as oxidants.52 The conductivity of these hydrogels varied between 100 and 102 S cm−1 depending on the type of the oxidant and precursor concentration.
CP hydrogels are made in two-dimensional (2D) or 3D structures using molding methods.61,62 The traditional molding fabrication leads to a random arrangement of the polymer chains. This random arrangement deteriorates the mechanical properties and the stretchability of the hydrogel. Inspired by the unique spinning of silk from spiders, Zhao et al. fabricated conductive polymer-based hydrogels by simply spinning the polyelectrolyte sodium polyacrylate (PAAS) to form an oriented and reversible alignment of PAAS.39 For this purpose, 4 wt % PAAS was added to a mixture of water and dimethyl sulfoxide (DMSO; 20 wt %) and stirred at 80 °C to get a homogeneous viscous solution. When the solution was cooled to ambient temperature, the PAAS filaments were withdrawn from the cold solution, and the remaining solution aggregated in the form of PASS hydrogel (PAH) fibers. The thermally responsive nature of PAAS provided good spinnability and ordered alignment of the filaments during the spinning and withdrawing process. By tilting the withdrawn filaments, the excess liquid was either removed from the PAH fibers in the form of droplets or solidified in the horizontally oriented fibers to form beads that imitate spiders’ threads (Figure 2b). The long fibers with a length of >1 m were prepared to mimic a spider web. The diameter (200 ± 20 μm) of the fibers was controlled by regulating the withdrawing speed. To increase the stability of the hydrophilic PAH fibers in the presence of water, the fibers were coated with a thin layer of poly(methyl acrylate) to fabricate hydrophobic core–shell MAPAH fibers by quick immersion in 5% PMA/ethyl acetate solution followed by evaporation of the ethyl acetate. Due to the coexistence of crystalline and amorphous domains in the MAPAH fibers, they showed a tensile strength of 5.6 ± 0.6 MPa and extensive stretchability of 1180 ± 100%, and 26.8 ± 3.1 MJ m−3 energy was required to break the fibers. Along with the high stretchability, the MAPAH fibers demonstrated good electrical conductivity of ~2 S m−1 due to the polyelectrolyte nature of PAAS, which dissociated in the water to form a charged polymer. The highest conductivity was observed at stretchability of 300%, and the fibers exhibited electrical conductivity at subzero temperatures. Conductivity was reduced to 0.1 S m−1 at −35 °C due to the low transmission rate of ions at subzero temperature. This highly stretchable antifreezing conductive hydrogel can be used for strain and temperature detection in wearable devices.
2.3. Conductive Micro/Nanocomposite Hydrogels.
In wearable device applications, various conductive micro/nanomaterials are combined with hydrogels as strain and/or pressure sensors. The main classes of these micro/nanomaterials include carbon-based micro/nanocomposites, MXene-based nanocomposites, and polymer or other nanofiller-based nanocomposites.15 Because of their small size, stability at different environmental conditions, easy and low-cost processing, these micro/nanomaterials are good candidates for improving the mechanical properties and the conductivity of hydrogels.27 Among various carbon-based nanomaterials, carbon nanotubes (CNTs) and graphene oxides (GOs) are abundantly used materials, which are mixed or self-assembled for applications in wearable devices.27
CNTs are soft and highly conductive, and they demonstrate tremendous potential in wearable device applications.63 However, due to their hydrophobicity in water, their combination with hydrophilic polymers is challenging and rather limited.64 To address this problem, CNTs have been functionalized with hydrophilic moieties (e.g., hydroxyl and carboxyl groups) to improve their solubility in water and increase their interactions with blending hydrogels.15 Modified CNTs have been used with several hydrogels, including PAAM,64 poly(l-lactide) (PLA),65 poly(N-isopropylacrylamide) (PNIPAM),66 nanoclay (Laponite),67 and gelatin methacryloyl (GelMA).68
For example, mussel-inspired modified CNTs were prepared by conjugating polydopamine with CNTs (PDA-CNTs) to increase the water solubility. The modified PDA-CNTs were then mixed with PAAM hydrogel using glycerol/water as a solvent to fabricate antifreezing and antiheating conductive hydrogel, which was essential to monitor arm motions with a wide range of temperatures (from −20 to 60 °C). Furthermore, due to the catechol moieties of PDA, the CNTs/PAAM hydrogels demonstrated excellent self-adhesion for electrocardiogram (ECG) monitoring applications. Although chemical functionalization of CNTs is found to increase solubility, it can impair the conductivity of CNTs. Alternative methods, such as using a surfactant (e.g., sodium dodecyl sulfate (SDS)),69 also require large amounts of CNTs, which may have eventual biocompatibility issues.15 Therefore, designing alternative methods which increase the solubility of CNTs without harming their structure and properties remains warranted.
PDA has been used to coat other nanomaterials such as GO. The mussel-inspired modified GO was engineered by Han and his colleagues.70 They oxidized dopamine monomers in an alkaline environment to form PDA and subsequently reduced GO in the presence of PDA. The conductive hydrogel, which exhibited good conductivity, was prepared by mixing the PAAM monomers with the modified GO. Both the GO and PDA could interact with the PAAM network via a hydrogen bond, π–π stacking, and electrostatic interactions. These interactions endowed the hydrogels with excellent toughness, stretchability, conductivity, self-healing, and adhesion, all of which are fundamental characteristics of hydrogels for applications in wearable and implantable bioelectronics.
Recently, MXene, a known class of 2D inorganic material, has attracted significant attention from the research community for applications in wearable devices.71,72 MXene is derived from a bulk crystal called MAX. M is transition-metal, A is group IIIA or IVA element, and X is C or N.73 Overall, MXene is a transition-metal carbide, carbonitride, and/or nitrides. Outstanding properties (i.e., electrical conductivity, mechanical strength, water solubility, and antioxidant) suggest that MXene is an ideal alternative to carbon-based micro/nanomaterials.73 Various corrosive agents such as hydrochloric acid (HCl), hydrofluoric acid (HF), and fluoride salts.73 have been used to etch layers from the MAX phase to form MXene nanosheets. The etching process exposes several hydrophilic functional groups on the surface and edges of the MXene and results in high dispersity in the water while maintaining conductivity.15 Inspired by human skin structure, Lu et al. prepared an electronic skin sensor composed of MXene-(Ti3C2Tx-)/PVA/PVP double-network hydrogel.40 As shown in Figure 2c, the composite hydrogel was prepared using a microwave-assisted aldol condensation reaction followed by a freeze–thawing process. Incorporating the double-network, comprising hard PVP segments and soft PVA segments, generates excellent mechanical strength to the composite hydrogel achieving a stretchability of ~2400%. This bioinspired hydrogel pressure sensor exhibited ultrahigh sensitivity (10.75 kPa−1) with a low detection limit. This sensor could monitor various human motions (e.g., swallowing, blinking, mouth movement), writing sensation for different letters, and simulation of Morse code.
The other type of micro/nanocomposite hydrogel is polymer nanofillers such as CNFs, cellulose nanocrystals (CNCs), PANI,74 and PPy,75 which are explored in the field of wearable sensors due to their mechanical strength, biocompatibility, and ease of functionalization.15 Bioinspired from the synergistic “soft and hard” hierarchical network from the biological soft tissues, Liu et al. engineered a wearable strain sensor consisting of hierarchical CNCs and PVA/polyvinylpyrrolidone (PVP) network structure.76 They combined dynamic hard iron cross-linked CNC hydrogel with soft PVA/PVP matrix where Fe3+ provided electrical conductivity. Several dynamic physical interactions between soft and hard networks provided the device with self-healing capabilities, excellent flexibility, and high sensitivity to detect human physiological signals. In another study, skin-inspired hydrogels, composed of CNC-conjugated tannic acid (TA) and silver nanoparticles (Ag NPs) incorporated PVA sensor, were designed as artificial electronic skins for monitoring human movement (Figure 2d).21 The sensor exhibited self-healing properties and outstanding stretchability (>4000%), and its conductivity varied in the range of 0.16–4.61 S m−1 depending on the concentration of Ag/TA in the hydrogel. It is important to note that despite the extensive application of polymer nanofillers in the fabrication of micro/nanocomposite hydrogels, the incompatibility between the nanofillers and hydrogel and the exact mechanism of sensing remain be addressed.
3. BIOINSPIRED HYDROGEL WEARABLE DEVICES FOR DIAGNOSTICS AND BIOSENSING
The emergence of advanced wearable biosensing platforms within the past decade is poised to redefine our relationship with health and disease.77 Their ability to provide personalized healthcare monitoring using rapid and minimally invasive sampling techniques represents a paradigm shift in healthcare. Wearable sensors could enable massive health metrics to be monitored and analyzed while encouraging more preventative healthcare measures than palliative.78 This shift could improve patient outcomes and lower the overall financial burden of the healthcare system. There have been upward of 425 clinical trials involving hydrogels for diagnostics of therapeutic indications within the past decade.79 Of the 425 trials, 202 have been for soft contact lens applications, while the remaining 223 are a mix of topical bandages, skin interfaces for electrodes, and engineered tissue applications. While hydrogels are classified in the “device” category by the FDA, standard functions such as drug-eluting systems render them “combinations” products which lengthens the regulatory process to around 7–10 years.
Nonetheless, researchers have long been inspired by nature to achieve these therapeutic and diagnostic goals when designing materials and engineering complex systems. Several recent reviews have summarized the impact of specific bioinspired animal architectures on sensors and actuators, including fish,80 butterflies,81 and adhesive capabilities like geckos, mussels, and octopi.82 This section will focus on wearable biosensors that incorporate bioinspired hydrogels and structures into their design.
3.1. Electronic Skin.
Human skin represents the body’s largest organ and is responsible for diverse functions, including temperature sensing and regulation, tactile sensing, and providing barrier properties.83 Artificial skin, also known as electronic skin (e-skin) in wearable device applications, ultimately seeks to recreate these diverse functions for applications in prosthetics and robotics to improve human–robot interactions. In either application, a primary challenge lies in creating wearable devices that can maintain high sensitivity over the wide range of mechanical motions human skin can experience. This subsection will discuss recent advances in mechanically compliant bioinspired hydrogel devices toward fully functional artificial skin.
3.1.1. Tactile and Temperature Sensors.
To mimic native skin sensing capabilities, artificial skin should be capable of sensitive tactile and temperature sensing. Multiple sensing mechanisms have been developed throughout the years to transduce the human body’s mechanical movement into readily transferable electrical signals. To date, these tactile devices can be classified as piezoresistive, piezoelectric, or capacitive, while temperature sensing typically relies on resistance changes in temperature-sensitive metals and polymers. As previously discussed, ionic hydrogels were inspired by biological tissues, and they are up-and-coming candidates for artificial skin due to their soft, biocompatible, and stretchable characteristics. Still, many ionic hydrogels rely on covalently cross-linked polyacrylamide (PAAm) and therefore suffer from a lack of recoverability and fatigue resistance, limiting their long-term wearability. Xia et al. recently developed a novel cross-linking approach using core–shell hybrid latex particles (HLPs), which could covalently bind to a lauryl methacrylate (LMA)-acrylamide copolymer.84 The resultant hydrogel matrix exhibited skin-link characteristics in low modulus, namely, high strength, rapid recoverability, and high sensitivity of 0.13 kPa−1 up to 15 kPa−1. They further demonstrated the ability of a piezoresistive pressure sensor device to detect a variety of body movements repeatedly and robustly, such as finger bending, speaking, and walking (Figure 3a). In addition to tactile sensing, integrated temperature sensors are required to impart electrical skins with the same functions as natural skin. Such sensors require higher than typical sensitivities to detect subtle temperature changes experienced by human skin over physiological ranges (32–42 °C). Accordingly, Oh et al. developed a highly temperature-sensitive electronic skin integrated with bioinspired octopus microstructures to improve skin adhesion.85 The thermoresponsive hydrogel is based on a polymer composite electrode comprising poly(N-isopropylacrylamide) (pNI-PAM)/poly(3,4-ethylene dioxythiophene):poly-(styrenesulfonate) (PEDOT:PSS)/carbon nanotubes (Figure 3b). Ultimately, the device detected temperature changes as low as 0.5 °C, and the octopus-inspired microstructures enabled long-term wearability (12 h) even with multiple attachment/detachment cycles.
Figure 3.

Bioinspired hydrogel wearable devices. (a) Skin-inspired physical cross-linking ionic hydrogel with core–shell hybrid latex particles (HLPs) displayed excellent mechanical adaptability and could distinguish between various body movements and vocal patterns. Reproduced with permission from ref 84. Copyright 2019 American Chemical Society. (b) Octopus-inspired PDMS microstructure with integrated multilayered pNIPAM/PEDOT:PSS/CNT composite for skin compliant temperature sensing. Reproduced with permission from ref 85. Copyright 2018 American Chemical Society. (c) 3D bioprinting of skin-inspired Ca-PAA-SA-CNT ionic hydrogel for on-body strain sensing. Reproduced with permission from ref 86. Copyright 2021 American Chemical Society. (d) Schematic diagram of biological skin and self-powered biomimetic artificial skin for highly sensitive static and dynamic pressure sensing. Reproduced with permission from ref 87. Copyright 2020 American Chemical Society. (e) Schematic illustration of multifunctional MN array with a mussel-inspired PDA base, polymyxa derived poylmxin loaded in the PEDGA/SA tips, and octopus-inspired suction cups surrounding each needle for improved skin adhesion. Reproduced with permission from ref 88. Copyright 2020, AAAS. (f) Schematic illustration for fabricating drug-laden biomimetic hydrogels via imprinting by polymerizing the HEMA polymer in the presence of functional monomers inspired by the human carbonic anhydrase active site. Reproduced with permission from ref 89. Copyright 2011 American Chemical Society. (g) Schematic of contractive hydrogel mechanism inspired by a frog jumping for storing and releasing chemical energy by incorporating permanent and reversible bonding throughout the hydrogel network. Reproduced with permission from ref 90. Copyright 2020 AAAS. (h) Thermophilic bacteria-inspired PAAc hydrogel with temperature-dependent hydrophobic and ionic interactions for instant thermal hardening. Reproduced with permission from ref 91. Copyright 2020 Wiley.
3.1.2. Other Properties: Self-Healing, Self-Powered, Scalability.
In addition to ensuring robust, accurate, and sensitive sensing, researchers have drawn know-how from nature to develop artificial skin with other properties to make it self-healing and self-powered. Lei et al. drew inspiration from the biomineralization process of shrimp shells to design a mineral hydrogel capacitive ionic skin composed of amorphous calcium carbonate nanoparticles cross-linked with polyacrylic and alginate chains.56 In addition to acting as a highly sensitive pressure sensor up to 1 kPa (S = 0.17 kPa−1), the device could autonomously self-heal within 20 min at room temperature and even retain >90% capacitive recovery after 10 drying–swelling cycles. In another study, Wei et al. synthesized a self-healing hydrogel inspired by the strong interaction between aspartic acid-rush proteins and calcium ions during the formation of biominerals.86 The hydrogel was formed by incorporating carbon nanotubes into a chelate of calcium ions with sodium alginate and poly(acrylic acid) (Figure 3c). In capacitive mode, the sensor demonstrated a high sensitivity of 1.25 kPa−1 up to 0.3 kPa. Moreover, the hydrogel could be printed with a commercial bioprinter, which allows the scalable fabrication of such devices. Self-powered sensors are also highly desired to minimize overall device complexity and costs. Sun et al. recently developed an ultrasensitive self-powered piezoresistive sensor with microstructures inspired by the natural structure of the epidermis.87 They used commercially available sandpaper as a template to fabricate graphite/PDMS films as the cathodic pressure-sensing layer (Figure 3d). The device’s power generation relied on redox-induced electricity realized by structuring the graphite/PDMS films between an aluminum cathode and separated by a NaCl electrolyte. This unique signal transduction approach allowed the sensor to have an ultrahigh sensitivity of 4.2 × 103 kPa−1 up to 4 kPa and 1.1 × 103 kPa−1 up to 30 kPa. Finally, enabled by this ultrahigh sensitivity, the authors demonstrated on-body high-resolution wrist pulse waveform monitoring in real-time.
3.2. Microneedles (MNs).
MNs are a class of microscale needles used in various applications, including drug delivery, biosensing, wound healing, and cosmetics.92 They have received considerable attention due to their relatively pain-free administration and lower risk of infection than standard hypodermic needles.93 MNs are designed to penetrate the outer layer of the skin (stratum corneum), which is the primary barrier for transdermal drug delivery, and access the interstitial fluid (ISF) for biosensing. At present, most MN designs fall into five classes: solid MNs, hollow MNs, coated MNs, dissolving MNs, and hydrogel-based MNs. While a diverse set of materials has been selected for MN fabrication, solid/hollow MNs are generally metal, dissolving/hydrogel MNs are polymer-based, and coated MNs are often a combination of both. Such MN platforms are formed as arrays and can have different needle shapes, dimensions, and materials depending on the application. In general, MN lengths are between 500 and 1000 μm with tip diameters on the order of 10 s of micrometers and interneedle spacings of 50–150 μm. In many cases, researchers have drawn inspiration from nature when engineering MNs in terms of material and structural design. In the following sections, we will investigate recent advances in the field of bioinspired hydrogel-based MNs and their applications in transdermal drug delivery, biosensing, and wound healing.
3.2.1. Hydrogel Microneedles for Transdermal Delivery.
The development of MN arrays for transdermal drug delivery represents their most common application.94 The study of hydrogel MNs has gained recent traction due to their excellent biocompatibility and tunable drug release profiles. To date, a wide array of therapeutic agents have been delivered, including oligonucleotides, vaccines, antibiotics, hormones, and small molecule drugs.95 A primary challenge is to engineer MNs with high drug delivery efficacy, mainly to deliver precise amounts of molecules and optimize their therapeutic window. Recently, Bok et al. developed a multifunctional dissolvable MN patch that combined iontophoresis and ultrasound to maximize drug delivery.96 The patch was constructed out of hyaluronic acid (HA), a frequently used biodegradable hydrogel found extensively throughout epithelial, connective, and neural tissue. Ultimately, the team demonstrated that by incorporating ultrasound and an electric field treatment for 10 min, the drug delivery efficiency was increased 83.4% compared to passive dissolution.
Hydrogel-based MNs represent the MN class and consist of swellable cross-linked polymers that serve as unblockable pathways for improved drug delivery.97 This is in contrast to hollow MNs, whose channels can be physically occluded by bodily debris upon skin insertion or during fluid extraction/delivery. They differ from conventional hydrogel MNs by readily uptaking surrounding interstitial fluid (ISF) and swell instead of dissolving upon insertion. Furthermore, hydrogel-based MNs leave no polymeric residue at the insertion site, as is often not the case for dissolving MNs. This swelling phenomenon has been leveraged in rat models to deliver various therapeutics transdermally, including analgesic esketamine98 for treatment-resistant depression and metformin HCl99 for type II diabetes mellitus management.
3.2.2. Hydrogel Microneedles for Biosensing.
In addition to drug delivery, the swellability of hydrogel-forming microneedles has enabled ISF extraction for specific biomarker analysis. ISF is gaining increasing attention as a biofluid of interest, because it can be sampled minimally invasively and often has a more similar composition to plasma than other biofluids.100 As a highly prevalent natural hydrogel, HA is still a prime material candidate to develop bioinspired hydrogel-based MNs for ISF extraction. However, in its unmodified state, HA is water-soluble and thus cannot be used directly. Chang et al. discovered that modifying HA with methacrylate could induce further covalent cross-linking under UV light via free radical polymerization.101 They further demonstrated the ability of the patch to readily extract ISF and subsequently perform offline analysis of metabolites such as glucose and cholesterol.
Other bioinspired hydrogels have been formulated to optimize MN characteristics, which are often inversely related (i.e., swelling time and dissolution time). In many cases, hydrogel-based MNs with strongly cross-linked networks require postprocessing chemical or mechanical extraction to detect biomarkers of interest. He et al. developed a poly(vinyl alcohol) (PVA)/chitosan (CS) hydrogel matrix, which could readily extract ISF while only requiring mild temperatures (60 °C) to dissolve and release target biomarkers.102 CS is a naturally occurring linear polysaccharide extracted from the chitin shells of crustaceans. CS is used for its biocompatibility, strong immobilization properties, and high porosity. The authors demonstrated the ability of MNs to take up sufficient ISF in vitro and in vivo for subsequent colorimetric quantification of glucose, lactate, chlorine, and bovine serum albumin (BSA). In another work, Sulaiman et al. developed a hydrogel-coated MN array capable of specific nucleic acid detection.103 They coated poly(l-lactide) MNs with alginate polymers, a polysaccharide derived from brown algae, and functionalized with peptide nucleic acid (PNA) probes for sequence-specific DNA detection. Exposure to UV light released the bound DNA duplex and thus enabled in situ fluorescent detection. These works demonstrated the potential hydrogel-based MNs have for wearable biosensing applications. Future work may include engineered materials for simpler extraction/analysis of target biomarkers and the integration of portable fluorescent/colorimetric cameras for rapid online quantification.
3.2.3. Others (Wound Healing and Adhesion).
An ongoing challenge for developing mechanically compliant MN patches lies in ensuring stable adhesion to the skin tissue. In this regard, researchers have drawn extensive inspiration from natural adhesive mechanisms in terms of needle geometry and modification with chemical moieties. Zhang et al. have reported a multifunctional MN array using poly(ethylene glycol) diacrylate (PEGDA)/sodium alginate as the tip material and polydopamine (PDA) as the base substrate.88 PDA was selected as the base material because its molecular structure is similar to the adhesive protein found in mussel byssus (Figure 3e). They also integrated octopus-like microstructures surrounding each MN for improved adhesion in dry and wet conditions. Finally, the authors loaded the needle tips with the antibiotic polymyxin from polymyxa for sustained bacteria-killing while maintaining high cell viability. This multimaterial bioinspired approach to hydrogel MN design could ensure long-term wearability and sustained drug release. In another more recent work by Zhang et al., the authors fabricated eagle-claw-inspired slanted PEGDA MNs for improved tissue adhesion.104 They further encapsulated liquid metal electrodes within the MN array to deliver electrical stimulation for improved wound healing with minimal scar formation. In a similar effort, inspired by the backward-facing barbs of honeybee stingers, Han et al. developed a 3D printing approach to mimic such structures on MNs.105 Ultimately, they showed that the tissue adhesion with barbed needles is 18 times more robust than barbless MNs. These works highlight how many aspects of the MNs can be tailored, namely, the needle material and geometry as well as the base substrate structure and material.
3.3. Contact Lens.
The first-generation contact lenses for vision correction were developed in the 1930s and constructed out of glass or rigid plastic poly(methyl methacrylate) (PMMA). However, demand for new lenses with improved comfort, oxygen permeation, wettability, and resistance to lipid deposition led to the development of bioinspired hydrogel contact lenses.106 With recent advancements in drug-laden hydrogels, flexible electronics, and biosensing, researchers have become increasingly interested in leveraging the wearability of contact lenses to provide meaningful health status data and targeted drug delivery. Human tear biofluid is produced by the lacrimal glands and contains various electrolytes, lipids, and proteins that could be monitored.107 The precise chemical composition varies according to three types of tears: basal, reflex, and psychic. Accordingly, these tear types serve different biological functions, namely, lubrication (basal), irritant removal (reflex), and response to a strong emotional reaction (psychic).108 Despite the rich source of biochemical analytes present in tears, rigorous correlation studies are still required to ensure similar trends compared to blood plasma concentrations. For this reason, researchers have primarily focused on developing drug-eluting lenses for targeted delivery and integrated biosensor lenses for monitoring established biomarkers, such as glucose for diabetes management. Other physical characteristics (i.e., intraocular pressure) are of interest for their association with glaucoma.
3.3.1. Drug-Eluting Contact Lenses.
The accessibility of the ocular surface and the ubiquitous use of contact lenses makes them an attractive platform for targeted drug delivery. However, the complex anatomy of the eye reduces the bioavailability of delivered molecules.109 Various strategies have been developed to prolong the ocular bioavailability of target molecules, including in situ gelling hydrogels,110 nanosuspensions,111 and even physical methods112 for penetrating the physiological barriers in the eye. Bioinspired approaches primarily focused on tuning the contact lens material properties to selectively bind to the molecule of interest, often based on the physiological properties (functional groups, charges, and size of the native bioreceptors). Thus, researchers aimed to develop contact lenses with higher drug loading concentrations and extended-release profiles.
A common approach for improving the lens-drug affinity involves molecularly imprinting the lens substrate by polymerizing the backbone monomers in the presence of monomers bearing the same functional groups as the native amino acids involved with binding. For example, Ribeiro et al. reported poly(2-hydroxyethyl methacrylate) (PHEMA) hydrogels polymerized in the presence of monomers mimicking chemical groups with high affinity to carbonic anhydrase inhibitor drugs (acetazolamide and ethoxzolamide) (Figure 3f).89 Compared to non-imprinted controls, the monomer-imprinted PHEMA hydrogels yielded superior release profiles. However, the authors found no clear trends regarding PHEMA hydrogels polymerized directly in the presence of acetazolamide, indicating certain limitations when molecularly imprinting target molecules directly. Gonzalez-Chomon et al. used a similar strategy to load PHEMA contact lenses with the antihistamine drug, olopatadine, used to treat allergic conjunctivitis.113 However, they reported a drug-eluting hydrogel with slower release rates, which were also polymerized in the presence of olopatadine, compared to nondrug imprinted controls. Most biomimetic formulations could still release olopatadine concentrations high enough to inhibit the response of sensitized mast cells in vitro.
Although the polymerization of backbone monomers in the presence of the drug molecules and functional monomers yields molecularly imprinted hydrogels with adequate drug release properties in many cases, some molecules such as antioxidants are incompatible with the polymer synthesis conditions. Varela-Garcia et al. investigated the use of cytosine-functionalized PHEMA hydrogels to load the antioxidant transferulic acid.114 They proposed that with the addition of a nitrogenous base, the hydrogel is endowed with a complementary chemical structure to the antioxidant in terms of hydrogen bonding and π–π stacking. In vitro loading studies confirm that cytosine functionalized PHEMA hydrogels could host up to 2.5 times the transferulic acid than non-functionalized control groups. Finally, using an ex vivo bovine eye model, they demonstrate that the transferulic-loaded hydrogel performed similarly to free transferulic solution delivery in terms of permeation through and accumulation into the sclera.
3.3.2. Electronic-Based Hydrogel Contact Lens Biosensors.
Electronic and electrochemical sensing approaches are promising forms of transducing biological phenomena on a contact lens substrate. They often require traditional clean-room processes such as photolithography and metal deposition to integrate electronic sensing and readout components to the lens. Despite the known concerns of low oxygen permeability and discomfort associated with ridged plastic substrates and elastomers, many contact lens sensors still rely on these materials because of standardized processing techniques. Recently, Kim et al. developed integrated glucose and intraocular pressure sensors on a commercial hydrogel contact lens.115 They utilized a combination of 1D (Ag nanowires) and 2D (graphene) nanomaterials to improve the conductivity, transparency, and flexibility of the contact lens. Using graphene/Ag nanowire interconnects and a glucose oxidase modified graphene channel, they demonstrated glucose monitoring via a field-effect transistor (FET) in vivo. They further incorporated wireless power transmission and data communication capabilities using a resistance (R), inductance (L), capacitance (C), or RLC resonant circuit.
Additionally, the lens monitored in vitro pressure changes by placing a polymer dielectric (Ecoflex) between two graphene/Ag nanowire electrodes and detecting the changes in capacitance and inductance. While these results are promising, the short readout distance (1 cm) inherent in near-field wireless communication could limit its use in practical long-term monitoring applications. More recent work by Keum et al. used an inductive resonant circuit to wirelessly power and transmit amperometric glucose data with a readout distance of 1 cm.116 The authors further integrated a flexible drug delivery system (f-DDS) to deliver genistein selectively, an antiangiogenic used to treat diabetic retinopathy, in an in vivo rabbit model. This combination of sensing and therapy represents the promise of contact lenses being developed as personalized wearable theragnostic devices.
Despite the improved gas permeability of bioinspired hydrogel lenses, the frequent use of gas-impermeable electronic and interfacial adhesion materials such as graphene, Parylene, and Ecoflex could undercut this advantage. Toward this end, Wei et al. recently developed a highly gas-permeable hydrogel contact lens sensor using porous Au nanomesh electrodes and electrochemically deposited PEDOT:PSS as the interfacial adhesion material.117 They found that the PEDOT:PSS outgrowth could anchor the Au nanomesh electrodes to the PHEMA hydrogel substrate. The lens could still retain high gas permeability and biocompatibility by optimizing deposition time. Then, the authors demonstrated that the sensor could conduct in vivo full-field electroretinogram (ERG) recordings, which are used in ophthalmic diagnostics to measure the electrical activity of the retina in response to light. The device yielded comparable signal amplitude to gold-standard commercial devices, making it a promising candidate for longer-term wearable applications.
3.3.3. Optical-Based Hydrogel Contact Lens Biosensors.
While electronic-based contact lens sensors have received considerable attention, they require complex fabrication, power sources, and readout systems. The demand for unpowered self-reporting contact lens biosensors has spurred the development of optical sensors, seen by the naked eye, or using ubiquitous smartphone cameras. Wang et al. reported a structurally colored contact lens sensor to monitor eye hydration and intraocular pressure.118 To circumvent problems associated with fluorescent dyes (i.e., uncontrolled diffusion and low stability), the authors incorporated colloidal silica nanoparticles into PHEMA hydrogel lenses. Various sizes of silica nanoparticles imbued the lenses with tunable colors, which changed as the contact lens dried or was under pressure. The working mechanism was attributed to changes in the periodicity of the silica nanoparticles as the lens underwent structural deformation during drying and with applied pressure. This structural color approach is promising for developing contact lens biosensors with less complex internal/external components; however, the authors noted that the contact lens color shift was difficult to distinguish within physiological pressure ranges (1–4 kPa) by the naked eye. Perhaps if used in combination with a readily available smartphone camera, this approach could be used in practical point-of-care applications.
3.4. Other Wearables (Stimuli-Responsive).
Although the previously discussed bioinspired wearable sensors represent many ongoing research efforts, unique challenges and applications for hydrogels are constantly being discovered. Stimuli-responsive hydrogels are one class of materials that have drawn increased attention for their applications as actuators and sensors in tissue engineering, artificial muscles, and soft robotics.119 The scope of their applications is extensive because hydrogels can be tuned to respond to various physical (electrical, temperature, light, magnetic fields) and chemical (pH, ions) stimuli. For example, Ma et al. recently developed a high power density (15.3 kJ/m3) elastic-driven hydrogel, which could store and subsequently release high contractile forces (40 kPa).90 The working mechanism of the PAAm/PAAc hydrogel was inspired by the energy conversion mechanism in the elastic actuation of animals such as frogs, in which chemical energy is converted to mechanical potential energy and finally kinetic energy (Figure 3g). Upon stretching, the hydrogel could store energy with the addition of Fe3+ and carboxylic groups to form coordination bonds, and two triggering mechanisms (UV and pH) were demonstrated to break and release the stored energy. Finally, the authors demonstrated multiple applications of the EDG hydrogel, including acting as programmable artificial muscles and smart wound dressing.
Thermoresponsive hydrogels have also been inspired mechanistically by nature. Pu et al. drew inspiration from sweating to design a thermosensitive hydrogel capable of providing heat dissipation via evaporative cooling for portable electronic devices.120 They synthesized a poly(N-isopropylacrylamide) (PNIPA) hydrogel capable of cooling a virtual reality device with a high heat-flux of 1555.5 W/m2 up to 27 °C. Furthermore, the device yielded excellent material reliability by maintaining its water retention properties even at high temperatures (90 °C) and high relative humidity (85%) for 450 h. As wearable devices and integrated electronics serve more functions and become more complex, researchers should consider similar potential challenges like heat generation and power consumption. In addition to deriving mechanistic inspiration from nature, thermoresponsive hydrogels have been inspired at the molecular level. Nonoyama et al. referenced the high thermal stability of thermophilic proteins when designing a thermal stiffing hydrogel by exploiting a combination of hydrophobic interactions and ionic bonding (Figure 3h).91 The gel comprised poly(acrylic acid) and calcium acetate (PAAc/CaAc) and could undergo rapid hardening at 60 °C, demonstrating 1800-, 80-, and 20-fold increased stiffness and strength and toughness, respectively. The authors demonstrated a potential application as a protective thermohardening racing suit by reinforcing woven glass fabrics with the PAAc/CaAc hydrogel fibers. The reinforced suit activated upon contact with asphalt due to frictional heating and yielded minimal structural damage compared to control groups. These examples demonstrate the sheer versatility and applicability of tunable bioinspired hydrogel devices outside the scope of their traditional platforms.
4. BIOSIGNAL READOUTS FOR WEARABLE DEVICES
Wearable sensors can transform biophysical, biochemical, and environmental targets related to the human body into detectable readouts for noninvasive monitoring applications.125–127 Compared with other materials, hydrogels have high biocompatibility, high permeability, tunable biodegradability, and adjustable functionality.128 Meanwhile, most biological tissues are soft, deformable, and contain water, thus acting as a biological “hydrogel”. Therefore, natural tissues and animals are always an essential inspiration for researchers to develop bioinspired hydrogel sensors.129 The Young’s moduli of hydrogels can be adjusted to match the mechanical requirements of biological tissues specific for different applications.130,131 All these advantages make hydrogels promising materials for wearable sensing applications. Targets, receptors, and sensing readouts are three major components for wearable sensors. Hydrogel wearable sensors can respond to biological targets using stimulus-responsive hydrogels or using immobilized bioreceptors (functional molecular, nanoparticles, biomaterials, and cells).132,133 These biological interactions can induce hydrogel sensors to generate readable readouts. This section classifies these readouts into four different types (Table 2): geometrical, optical, electrical, and biological readouts.
Table 2.
Summary of the Biosignal Readouts for Wearable Devices
| output | principle | advantages | typical applications |
|---|---|---|---|
| Geometrical | Changing conformational/cross-linking properties | Easy to fabricate | Strain sensor134 |
| Cost-efficient | Soft actuators, robots121,135 | ||
| Optical | Photonic structures | Cost-efficient | Bionic electronic skin136,137 |
| Structure color | Naked-eye readout | Colorimetric/florescent sensors138,139 | |
| Florescent/color additives | Instrument-free | ||
| Electrical | Intrinsically conductive hydrogels or embedded conductive micro/nanomaterials | Real-time measurement | Hydrogel electronics140,141 |
| High sensitivity | Wearable sensors123,142 | ||
| Integrated | Hydrogel skin143,144 | ||
| Biological | Biological events in hydrogel | Biocompatible | Microneedles124,145,146 |
| Air and nutrient permeable Wearable sensors147 | Air and nutrient permeable Wearable sensors147 |
4.1. Geometrical Readouts.
The geometry of a hydrogel is sensitive to physical and chemical signals. Variations in temperatures, pH, ionic strength, electric fields, and even biomolecules affect conformational and cross-linking properties that change hydrogel geometries.148–152 For example, poly(N-isopropylacrylamide) (PNIPAM) is widely used as a temperature sensor. The polymer chains of PNIPAM can collapse or extend under different temperatures.153 The hydrogel can admit or release protons under different pH, which leads to the swelling of hydrogel sensors. The swelling properties of anionic acrylic acid hydrogels are also sensitive to external electric fields. When immobilized with functional bioreceptors, the geometry of such hydrogels is even sensitive to biomolecules, such as proteins, soluble and cell-surface expressed molecules.154,155 The geometrical readout is a universal and powerful signal readout method, yet geometrical changes are often accompanied by other readouts.134
Hydrogels show necessary reconfigurability and multifunctionality for developing soft actuators and robots.121,135 Inspired by the functions of native soft tissues with complex 3D morphologies, a hydrogel with programmed morphologies and motions was created by using temporal control of polymerization and cross-linking reactions (Figure 4a).156 However, mechanical outputs of hydrogels lack sufficient “quality” in resolution, sensitivity, and specificity. The accuracy, reproducibility, and durability of a hydrogel are comparatively lower than traditional rigid materials.128,157 To improve the mechanical properties, hydrogel matrices are widely functionalized with active units or nanomaterials.158 For example, hydrogels filled with magnetic particles can respond to an external magnetic field.159 Meanwhile, hydrogel robots can achieve continuous movement by tuning the magnetic field.
Figure 4.
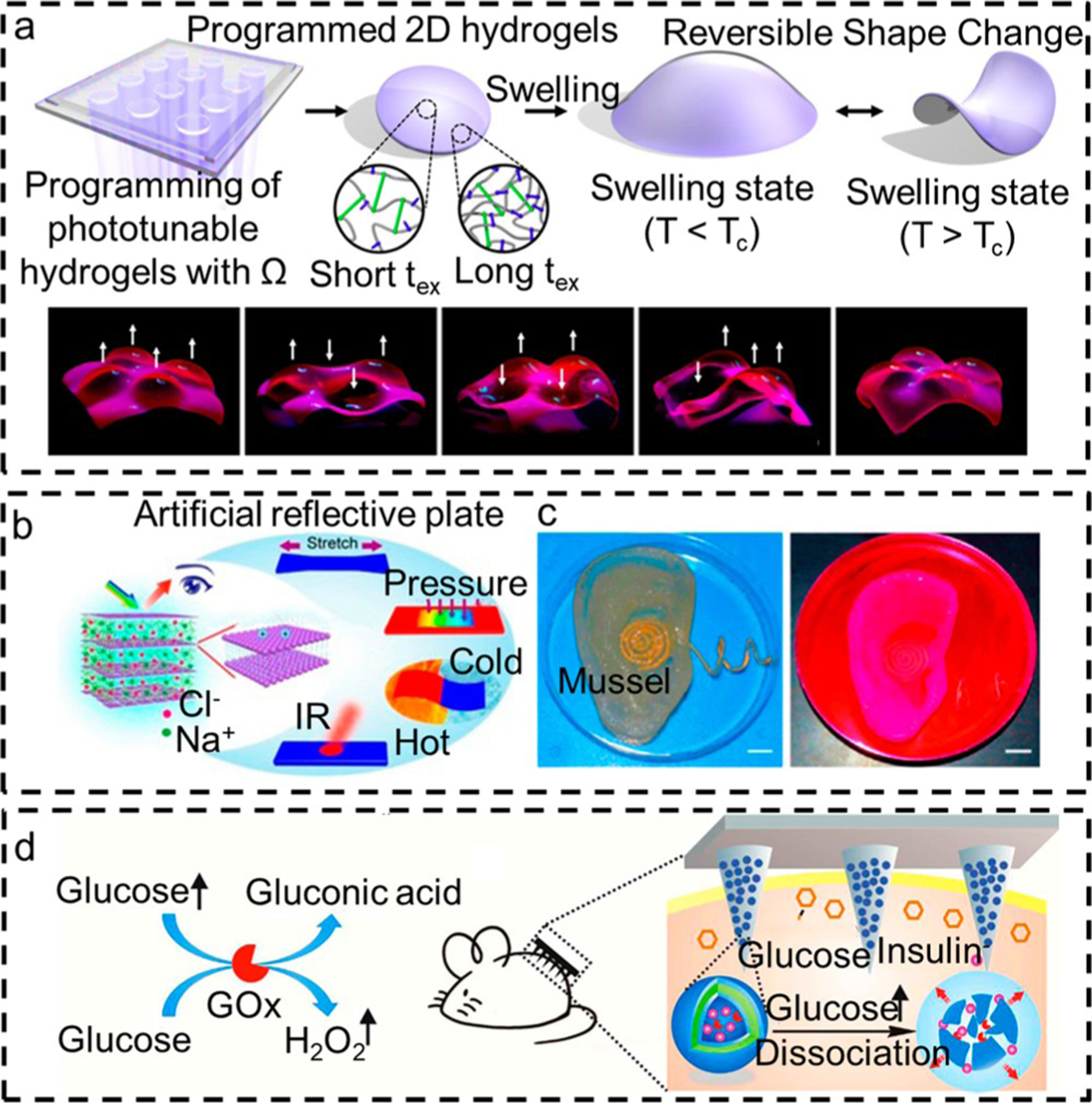
Represented hydrogel sensor with different signal readouts. (a) Hydrogel with programmed morphologies and motions which is realized by temporal control of polymerization and cross-linking reactions. Reproduced with permission from ref 121. Copyright 2018 Nature Publishing Group. (b) Photonic hydrogel skin can respond to multiple stimuli, including tension, pressure, and temperature, into an optical readout. Reproduced with permission from ref 122. Copyright 2021 American Chemical Society. (c) Hydrogel sensors with auditory abilities. The sound signal can be converted into a readable electrical signal by the hydrogel ear. Reproduced with permission from ref 123. Copyright 2013 American Chemical Society. (d) Hydrogel sensors with controllable and insulin release ability. Reproduced with permission from ref 124. Copyright 2017 American Chemical Society.
4.2. Optical Readouts.
Optical readouts allow real-time, rapid, instrument-free, and cost-effective demonstration of biological events. Optical readouts constitute the most widely used means of obtaining signals from hydrogel sensors.160,161 Hydrogels can absorb and release different molecules, leading to distinct volumes and optical color change. Meanwhile, many hydrogels are optically transparent, which benefits the conduction and display of optical signals. Photonic structures embedded in hydrogels are standard methods for developing photonic band gap, plasmonic, and reflection/refractive index modulation-based hydrogel sensors.162–165 These approaches are widely used in hydrogel-based biomedical devices. As functional bioreceptors can be physically embedded or chemically anchored to the hydrogel matrix, the biological targets can change the equilibrium state of swelling, resulting in changes in the polymer composition of cross-linking density.
Structural color in nature has inspired many researchers to design wearable hydrogel sensors with optical readouts.137,166,167 Inspired by cephalopods, researchers have developed a chronotropic ionic skin that can respond to multiple stimuli, including tension, pressure, and temperature.136 This is realized by a multifunctional ionic-conductive photonic hydrogel with anisotropic electrostatic repulsion (Figure 4b). As most polymers are supramolecular, the aggregation state can lead to substantial changes in the optical properties of these kinds of hydrogels.168,169 For example, recently developed photonic crystal hydrogels change their optical properties when their volume changes.163,170 A bionic electronic skin was developed using hydroxypropyl cellulose, poly(acrylamide-co-acrylic acid), and carbon nanotubes composited liquid-crystal hydrogel.137 The cholesteric liquid-crystal structures of the hydrogel could be changed in response to pressure, tension, and temperature variations.
Biological interactions in a biosystem cause swelling alteration, which may translate to a macroscopic response that results in fluorescence change.138,139 Fluorescence is very common in nature, and it is widely used to develop optical sensors with high sensitivity and selectivity. In general, the fluorescent signal can be derived from self-luminescent hydrogels and the generation of fluorescent molecules. Ions, such as Eu3+, Tb3+, Fe2+, Ru2+, and Ir2+, are essential for fluorescent hydrogels.171 Their fluorescent properties can be easily adjusted in response to stimuli. Hydrogels with fluorescence readouts aggregation-induced emission (AIE) and aggregation-caused quenching (ACQ) effects caused by intramolecular motion restriction provide a promising platform for developing hydrogel sensors.172–174 These sensors can respond to stimuli differently, such as fluorescence, brightness, and shape.
4.3. Electrical Readouts.
Conductive materials are essential for wearable hydrogel sensors. The conductivity of hydrogel sensors arises from the use of intrinsically conductive hydrogels or embedded micro/nanoparticles.175 Compared with rigid materials, hydrogel-based electronics are more biocompatible with tissues due to their similarity with the mechanical, biological, and electrical properties of tissues. Meanwhile, hydrogels provide an adjustable polymer matrix for the chemical and physical cross-linking of functional components. Hydrogel-based electronics provide seamless interfacing between biological surfaces and electronics, which benefits bioelectrical signal recording.140,141 Many electrical signals are recorded and transmitted by hydrogels, such as ECG, electromyography (EMG), and electrooculogram (EOG).176–178 Combined with appropriate stimulation and/or drug delivery systems, hydrogel sensors can provide feedback according to the detected signal to realize an accurate and automated diagnosis and treatment.179
Electronic activity in the nervous system is the principal constituent of our daily lives. Our sensory abilities, such as sight, hearing, smell, taste, and touch, are recorded and processed by biological electrical signals. Inspired by these biological activities, researchers have designed and created many hydrogel-based wearable devices to detect signals of these activities. Hydrogel sensors with visual, olfactory, tactile, gustatory, and auditory abilities have been developed (Figure 4c).123,142
Hydrogel-based wearable sensors can transduce external stimuli into electrical readouts, such as strain, temperature, and pressure.143,144 Using two polyacrylamide (PAAm) hydrogel layers and a dielectric layer, an electronic skin with sensitivity to stress and strain was developed.137,180–183 However, challenges remain in meeting the mechanical and sensory demands for human skin fully. The electronic-skin interface is the most significant challenge in the development of next-generation of electronic skins. Inspired by the gecko, sensors with nanoarchitectures allow exquisite sensing of various mechanical forces.184 In addition, the nanostructures of the gecko also enhanced the signal-to-noise ratio.
4.4. Biological Readouts.
Our body has an extracellular matrix that surrounds the cells and has very similar properties to hydrogels. The exchange of molecules between organelles, cells, or organisms and their environment is critical for life. Here, we treat these kinds of activities as biological interactions. Many hydrogel-based sensors are designed according to this principle.185–187 Inspired by the glucose-responsive mechanism, wearable hydrogel sensors with controllable insulin release ability were developed.124,145,146 When the blood glucose level rises, glucose oxidase (GOx) can catalyze glucose to H2O2. H2O2-sensitive polymeric vesicles can become water-soluble after reacting with the generated H2O2. The preloaded insulin is released during this progress (Figure 4d). Cells can sense their local environments and secrete biomolecules in response to stimuli. The hydrogel matrix also provides an ideal scaffold for cell growth. Meanwhile, the permeability of air, water, and nutrients through hydrogels ensures the viability and functionality of cells.188,189 Inspired by this phenomenon, hydrogel sensors with encapsulated cells can respond to these kinds of stimuli.147
Hydrogel-based wearable devices have witnessed significant progress in recent years. In this section, we have summarized and discussed bioinspired hydrogel-based wearable devices from a readout perspective. The versatility, tunability, and controllability of hydrogels can be exploited in analytical sensing systems. The similarity between human tissue and hydrogels makes them an ideal platform for wearable devices. Novel hydrogel materials, functions, and structures are needed to develop the next generation of hydrogel-based wearable devices in the future. Researchers can develop hydrogel-based sensors that imitate the function of natural tissues and animals. Therefore, there is plenty of room for hydrogel-based wearable sensors.
5. POWERING AND WIRELESS TRANSMISSION FOR WEARABLE DEVICES
To realize the full potential of wearable devices, suitable powering and data transfer units must be integrated into the systems.190 However, current power sources for wearable systems have multiple challenges, including low energy densities, slow recharging, and incompatible mechanical properties.191 Similarly, the present data transfer technology also has bottlenecks in reducing power consumption, increasing data transfer rate, and extending the working distance. In recent years, researchers from different fields have been addressing the challenges in wearable power sources and wearable data transfer units. There have been many discoveries and improvements in the field. To date, the most developed power sources in wearable applications are wearable batteries and wearable supercapacitors,192 which are discussed in this section. Besides, we also list several popular wireless data transfer protocols and highlight their specific advantages and limitations in wearable applications.
5.1. Wearable Batteries.
Due to existing mature fabrication techniques and impressive electrical performance, batteries have been widely explored for biosensing applications and remain the most common power source for wearable devices.193 Conventional batteries are suitable choices when the size of the target device is quite large. The batteries must be encapsulated with a biocompatible layer to guarantee safety in wearable applications (Figure 5a).194 This coating makes the device bulky and is not flexible enough for compliance in small wearable devices. Furthermore, the weight of traditional batteries is usually higher than the device; it is supposed to power, which can reduce the comfort of long-term wearable devices. Researchers have made significant progress in the past decade toward developing flexible and stretchable batteries for wearables to address these issues. To date, four main types of deformable batteries have been proposed:195 the fiber-like battery, which is intrinsically flexible due to the cable structure (Figure 5b);196–198 the bridge-island battery, which consists of islands of rigid battery components and serpentine interconnect networks on elastomeric substrates (Figure 5c);192,199 the strain engineering battery, which refers to batteries that use patterns200–202 or microstructure203–206 processing technique to enhance strain capability of batteries (Figure 5d); the intrinsically stretchable battery, which can be achieved by creating new stretchable active materials (Figure 5e).207–210
Figure 5.
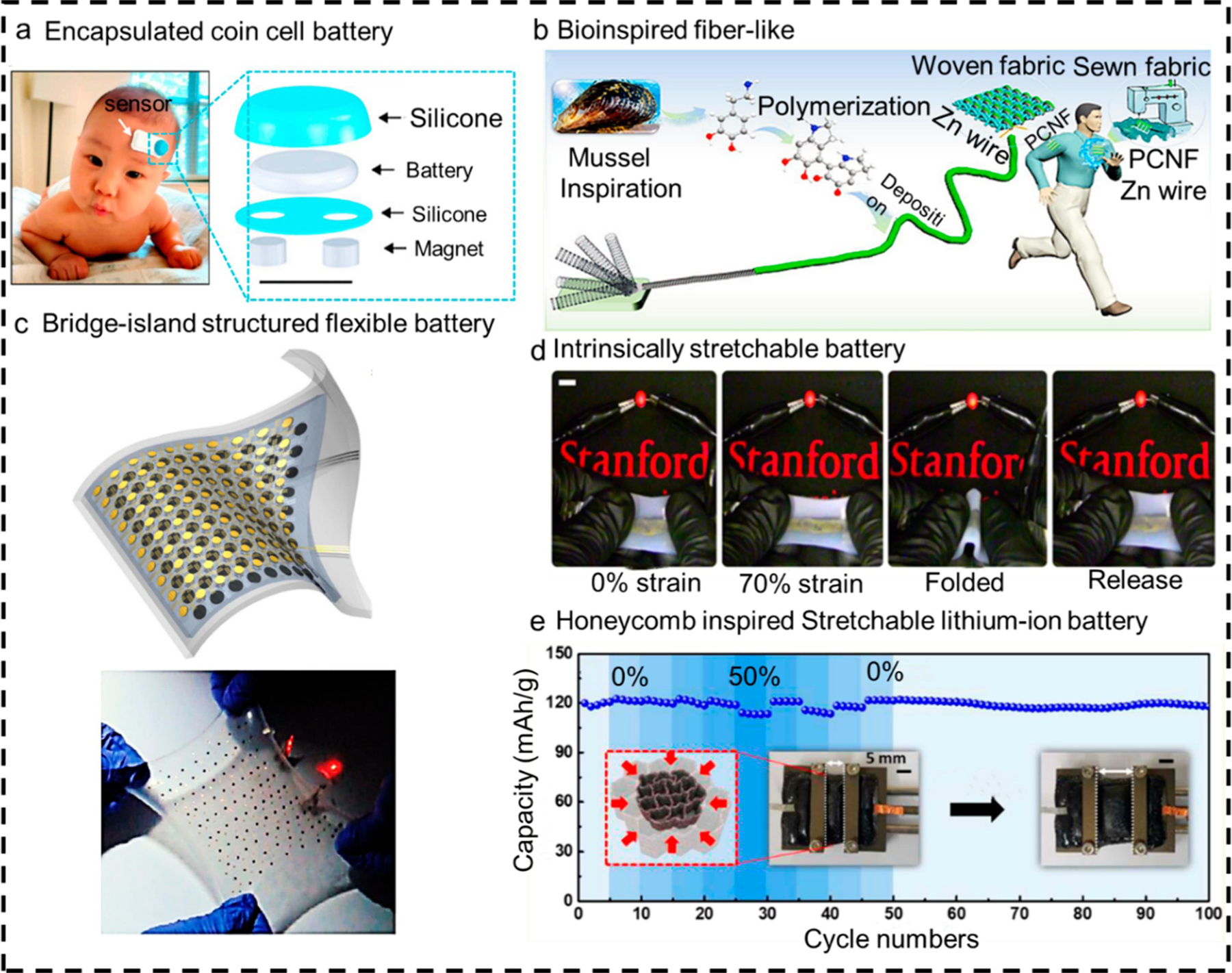
Various wearable battery solutions. (a) Encapsulated coin cell batteries are suitable solutions to power wearable devices for the cases which require the entire stretchability is not very high. Reproduced with permission from ref 212. Copyright 2020 the Proceedings of the National Academy of Sciences. (b) A mussel-inspired fiber-like battery to display high flexibility. Reproduced with permission from ref 213. Copyright 2020 Wiley. (c) Typical bridge-island structured stretchable battery. Reproduced with permission from ref 214. Copyright 2016 American Chemical Society. (d) Stretchable battery providing power to a red LED under no strain, stretched 70%, folded, and returned to its original position. Reproduced with permission from ref 215. Copyright 2019 Nature Publishing Group. (e) Honeycomb-inspired wearable battery based on microstructure strain engineering technique. Reproduced with permission from ref 216. Copyright 2020 American Chemical Society.
Additionally, bioinspired materials can also be used to improve the performance of wearable batteries. Wang et al. proposed a bioinspired interfacial design of the quinone-rich polydopamine as organic redox-active cathodes and nano-binders on the carbon substrate simultaneously. They demonstrated a wearable soft fiber zinc battery. Their work further improved the specific capacity (372.3 mAh/g at 50 mA/g) and term cyclic durability (80% retention over 1700 cycles at 1000 mA/g) of the fiber-like wearable battery.211
New wearable battery technology still faces problems regarding low charge rates and poor power density, but progress in this field has been encouraging. Continued research has the potential to yield high-performance deformable batteries for long-term wearable use.
5.2. Wearable Supercapacitors.
Supercapacitors have shown great potential for promising wearable energy storage due to their high rate of charge transfer, long cycle life, and safety.217 Supercapacitors are utilized to be integrated onto wearable energy harvesting devices such as body motion nanogenerators,218,219 wearable solar cells,220,221 and wearable biofuel cells.222,223 It is ideal for integrating supercapacitors into the wearable device, which requires high power density and high-speed charge/discharge capability. However, it is challenging to keep the traditional supercapacitors attached to the soft human body for a long time.224 Recently, various strategies have been explored to create wearable supercapacitors that are stretchable.225–231 Like the wearable batteries, the stretchable supercapacitors can also be made by strain engineering structures such as the fiber-shape design structures,225,228 wavy-shape structures,230,231 and folding structures.226,227,229 In addition to improving mechanical properties, several advances have been explored to improve the electrical performance of the wearable supercapacitors.232,233 To date, supercapacitors based on carbon nanomaterials have attracted the most attention due to the high electrical conductivity and outstanding stability.234 Also, various bioinspired materials have been reported to exhibit the potential to improve the properties of carbon-based supercapacitors. For instance, Fisher et al. reported a bioinspired leaves-on-branchlet structure design for carbon-based supercapacitors, further enhancing the areal capacitance, the rate capability, and cyclic stability.235 For the next generation of wearable sensing platforms, wearable supercapacitors might be combined with wearable batteries and implemented to store energy from multiple wearable power harvesting devices during sleep time, which builds a more energy-efficient platform.
5.3. Wireless Data Transmission.
Real-time data monitoring and remote control are essential properties for wearable devices. For a genuinely standalone wearable sensing platform, the device must communicate wirelessly between users and contain multiplexed sensors or different devices.191 Wireless communication can be achieved by using radiofrequency (RF), optical, and acoustic signals. The most common approach is RF data transmission technology,236 which includes various communication protocols, such as the Bluetooth Low Energy (BLE),194,236,237 Wi-Fi,238 Zigbee,239 and NFC.240–243 Each protocol has its advantages and limitations, and selecting a particular communication approach depends on the required data transfer rate, power consumption, and communication range. Among these RF protocols, BLE has been explored the most for long-term wearable sensor applications due to its acceptable power consumption, low cost, and high compatibility; however, BLE has a short working range of 100 m, limiting the working distance the sensor can be from the data receiver.190 For more extended working distance applications, Wi-Fi performs better than BLE. Wi-Fi uses multiple parts of the IEEE 802 protocol family and usually has a longer working distance and higher data transmission rate.244
Nevertheless, the high-power consumption of Wi-Fi devices consequently requires larger power sources, limiting the degree of skin conformability. Zigbee is a low-power wireless data communication protocol supporting mesh networking, so Zigbee is the most reasonable one for multinode sensor network applications.245 Unlike the above protocols, NFC can be inductively powered by the wireless reader and has less hardware requirement, making this technology widely used in versatile battery-free wearable applications. However, the shortcoming of NFC is also realized by the need for proximity for signal reception and low data transmission speed. These make the system cumbersome for users and limit the continuous monitoring capability of sensors. Considering these challenges of RF wireless communication methods, the next-generation wireless technologies for wearable devices should handle lower power consumption, longer working distance, and higher data transmission rate at low cost.
6. CONCLUSIONS AND OUTLOOK
Over the past two decades, looking to nature for inspiration when developing novel hydrogels has made remarkable progress in wearable devices, especially for wearable diagnostics and biosensors. By analyzing biosystems from the material design to macroscopic geometry, numerous bioinspired hydrogels with diverse functionalities have been reported. Hydrogels are an ideal candidate for integrating biological and engineering systems due to the similarity between hydrogels and living tissues in terms of mechanical, chemical, and biological characteristics.
Despite the great promise and progress of bioinspired hydrogels, customized materials are still in their infancy compared with natural hydrogels, which provide ample room for future research and development. Here, we propose several possible directions for reference as summarized below:
6.1. New Hydrogel Materials for Wearable Devices.
Thus far, we have focused on the surface-level possibilities in mimicking biological structures. The precise control of polymer or system still has a long way to construct hierarchical structures with specific geometries, enabling wearable devices to fulfill multiple functions (i.e., fast stimuli-response, broad sensitive range, high sensitivity, and long-term reliability). One typical challenge that should be noted is that most hydrogels are susceptible to dehydration in the air, limiting their scalable applications in wearable devices. Some research employs a hydrophobic coating as an encapsulated layer or utilizes nonvolatile organic solvents to replace water that existed in the hydrogel network to address this issue.246,247 Future research to develop antihydration hydrogels to improve the reliability of hydrogel wearable devices is recommended.
Another current problem is that hydrogels exhibit inferior performance in terms of signal transmission efficiency. The electrical conductivity of conductive hydrogels is around 10−3–103 S/m, far lower than that of the copper used in current electronic devices (107 S/m). In addition, designing multiple stimuli-responsive hydrogels is highly demanded. One strategy is to expand hydrogels’ functions by incorporating functional nanomaterials that offer additional optical, electronic, and mechanical properties. Therefore, it is necessary to dive deeply into developing new hydrogel materials with better electrical properties to improve the hydrogel wearable device performance.
6.2. Artificial Intelligence Integrated Hydrogel Wearable Devices.
Machine learning has recently emerged as a promising technique in controlling various devices in the biomedical field. Assisted by machine learning, intelligent devices could learn from data, identify patterns, and make decisions with minimal human intervention. Incorporating machine learning into the control systems is a promising direction for hydrogel wearable devices to improve data accuracy. For example, the machine learning technique could transform long-term data collected from hydrogel wearable devices into scientifically and/or clinically meaningful information, enabling the classification of human behaviors, characterizing human habits, and diagnosis abnormalities and diseases. In addition, the intelligent hydrogel wearable devices could obtain feedback from sensors, developing a closed-loop platform for the diagnosis and therapy of specific diseases.
6.3. Fully Integrated Hydrogel Wearable Device.
Given the multiple complex mechanisms to consider when designing wearable diagnostics to be non- or minimally invasive to the user, an attractive strategy is to devise a fully integrated sensing system. An ideal system could automatically and in real-time wirelessly extract the multiplexed information available from the human body. However, such efforts require multidisciplinary research, including material science, sensor design and fabrication, onboard power supply systems, and wireless data transmission techniques. This would accelerate their transition to full-scale manufacturing and commercial deployment. Therefore, a fully integrated hydrogel wearable device represents a technology class with powerful potential in personalized medicine by seamlessly bridging the biological body with electronics to continuously monitor the human physiological health state.
6.4. Instrument-Free Rapid Colorimetric Detection.
One of the challenges in wearable devices is developing instrument-free, rapid detection home-testing devices that reduce sampling-related discomfort and healthcare cost. Developing hydrogel biosensors integrated with plasmonic metal nanoparticles that offer unique optical properties such as gold or silver nanoparticles hold great promise for a new generation of instrument-free colorimetric biosensors. These wearable biosensors are very sensitive to chemical environments, and small changes in the size and morphology of the nanoparticles can be visually realized as color changes in the device with the naked eye. Integration of anisotropic shapes of nanoparticles endow the device with multiple color changes, which is beneficial for visual detection as the human eye is more sensitive to light changes in frequency than in intensity.248
6.5. Operating in Harsh Environmental Conditions.
Some of the sensors require open access to the environment to operate efficiently. This is also applicable for in vivo applications of wearable and implantable biosensors such as health monitoring, diagnosis of diseases, and drug delivery. The challenge in this field is to fabricate sensors resistant to harsh environmental conditions such as underwater, high humidity, or highly acidic or alkaline environments. Future integration of a new generation of environmentally resistance hydrogels in the design of wearable devices is one of the strategies for point-of-care diagnosis and treatment, which is helpful for laboratory purposes and actual clinical practices. We hope that this review will inspire researchers to investigate engineering environmentally resistance hydrogels for biosensing applications intensively.
ACKNOWLEDGMENTS
The authors gratefully acknowledge funding by the National Institutes of Health (CA214411, AR074234, GM126571, TR003148) and the Office of the Secretary of Defense and were accomplished under Agreement Number W911NF-17–3-003. The views and conclusions contained in this document are those of the authors. They should not be interpreted as representing the official policies, either expressed or implied, of the Office of the Secretary of Defense or the U.S. Government. The U.S. Government is authorized to reproduce and distribute reprints for Government purposes, notwithstanding any copyright notation.
Footnotes
The authors declare no competing financial interest.
Contributor Information
Yangzhi Zhu, Terasaki Institute for Biomedical Innovation, Los Angeles, California 90064, United States.
Reihaneh Haghniaz, Terasaki Institute for Biomedical Innovation, Los Angeles, California 90064, United States.
Martin C. Hartel, Terasaki Institute for Biomedical Innovation, Los Angeles, California 90064, United States; Department of Bioengineering, University of California-Los Angeles, Los Angeles, California 90095, United States
Lei Mou, Terasaki Institute for Biomedical Innovation, Los Angeles, California 90064, United States.
Xinyu Tian, Department of Nanoengineering, University of California, San Diego, La Jolla, California 92093, United States.
Pamela Rosario Garrido, Terasaki Institute for Biomedical Innovation, Los Angeles, California 90064, United States; Department of Electric and Electronic Engineering, Technological Institute of Merida, Merida, Yucatan 97118, Mexico.
Zhuohong Wu, Department of Nanoengineering, University of California, San Diego, La Jolla, California 92093, United States.
Taige Hao, Department of Nanoengineering, University of California, San Diego, La Jolla, California 92093, United States.
Shenghan Guan, Terasaki Institute for Biomedical Innovation, Los Angeles, California 90064, United States; Mork Family Department of Chemical Engineering and Materials Science, University of Southern California, Los Angeles, California 90089, United States.
Samad Ahadian, Terasaki Institute for Biomedical Innovation, Los Angeles, California 90064, United States.
Han-Jun Kim, Terasaki Institute for Biomedical Innovation, Los Angeles, California 90064, United States.
Vadim Jucaud, Terasaki Institute for Biomedical Innovation, Los Angeles, California 90064, United States.
Mehmet R. Dokmeci, Terasaki Institute for Biomedical Innovation, Los Angeles, California 90064, United States
Ali Khademhosseini, Terasaki Institute for Biomedical Innovation, Los Angeles, California 90064, United States.
REFERENCES
- (1).Peppas NA; Moynihan HJ; Lucht LM The structure of highly crosslinked poly(2-hydroxyethyl methacrylate) hydrogels. J. Biomed. Mater. Res 1985, 19 (4), 397–411. [DOI] [PubMed] [Google Scholar]
- (2).Daly AC; Davidson MD; Burdick JA 3D bioprinting of high cell-density heterogeneous tissue models through spheroid fusion within self-healing hydrogels. Nat. Commun 2021, 12 (1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Zhang C; Zhou Y; Han H; Zheng H; Xu W; Wang Z Dopamine-Triggered Hydrogels with High Transparency, Self-Adhesion, and Thermoresponse as Skinlike Sensors. ACS Nano 2021, 15 (1), 1785–1794. [DOI] [PubMed] [Google Scholar]
- (4).Zhang Q; Liu X; Duan L; Gao G Nucleotide-driven skin-attachable hydrogels toward visual human–machine interfaces. J. Mater. Chem. A 2020, 8 (8), 4515–4523. [Google Scholar]
- (5).Ding H; Xin Z; Yang Y; Luo Y; Xia K; Wang B; Sun Y; Wang J; Zhang Y; Wu H; Fan S; Zhang L; Liu K Ionic Sensing Hydrogels: Ultrasensitive, Low-Voltage Operational, and Asymmetric Ionic Sensing Hydrogel for Multipurpose Applications (Adv. Funct. Mater. 12/2020). Adv. Funct. Mater 2020, 30 (12), 2070080. [Google Scholar]
- (6).Yin M; Xiao L; Liu Q; Kwon S-Y; Zhang Y; Sharma PR; Jin L; Li X; Xu B Microneedle Patches: 3D Printed Microheater Sensor-Integrated, Drug-Encapsulated Microneedle Patch System for Pain Management (Adv. Healthcare Mater. 23/2019). Adv. Healthcare Mater 2019, 8 (23), 1970093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Zhang X; Chen G; Bian F; Cai L; Zhao Y Encoded Microneedles: Encoded Microneedle Arrays for Detection of Skin Interstitial Fluid Biomarkers (Adv. Mater. 37/2019). Adv. Mater 2019, 31 (37), 1970267. [DOI] [PubMed] [Google Scholar]
- (8).Jones L; Hui A; Phan C-M; Read ML; Azar D; Buch J; Ciolino JB; Naroo SA; Pall B; Romond K; Sankaridurg P; Schnider CM; Terry L; Willcox M CLEAR - Contact lens technologies of the future. Contact Lens and Anterior Eye 2021, 44 (2), 398–430. [DOI] [PubMed] [Google Scholar]
- (9).Gote V; Sikder S; Sicotte J; Pal D Ocular Drug Delivery: Present Innovations and Future Challenges. J. Pharmacol. Exp. Ther 2019, 370 (3), 602–624. [DOI] [PubMed] [Google Scholar]
- (10).Zhu Y; Kim S; Ma X; Byrley P; Yu N; Liu Q; Sun X; Xu D; Peng S; Hartel MC; Zhang S; Jucaud V; Dokmeci MR; Khademhosseini A; Yan R Ultrathin-shell epitaxial Ag@Au core-shell nanowires for high-performance and chemically-stable electronic, optical, and mechanical devices. Nano Res 2021, 14, 4294. [Google Scholar]
- (11).Yang JC; Kim J-O; Oh J; Kwon SY; Sim JY; Kim DW; Choi HB; Park S Microstructured Porous Pyramid-Based Ultrahigh Sensitive Pressure Sensor Insensitive to Strain and Temperature. ACS Appl. Mater. Interfaces 2019, 11 (21), 19472–19480. [DOI] [PubMed] [Google Scholar]
- (12).Zhang S; Liu H; Yang S; Shi X; Zhang D; Shan C; Mi L; Liu C; Shen C; Guo Z Ultrasensitive and Highly Compressible Piezoresistive Sensor Based on Polyurethane Sponge Coated with a Cracked Cellulose Nanofibril/Silver Nanowire Layer. ACS Appl. Mater. Interfaces 2019, 11 (11), 10922–10932. [DOI] [PubMed] [Google Scholar]
- (13).Xu Y; Sun B; Ling Y; Fei Q; Chen Z; Li X; Guo P; Jeon N; Goswami S; Liao Y; Ding S; Yu Q; Lin J; Huang G; Yan Z Multiscale porous elastomer substrates for multifunctional on-skin electronics with passive-cooling capabilities. Proc. Natl. Acad. Sci. U. S. A 2020, 117 (1), 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Wang C; Xia K; Wang H; Liang X; Yin Z; Zhang Y Advanced Carbon for Flexible and Wearable Electronics. Adv. Mater 2019, 31 (9), 1801072. [DOI] [PubMed] [Google Scholar]
- (15).Sun X; Yao F; Li J Nanocomposite hydrogel-based strain and pressure sensors: a review. J. Mater. Chem. A 2020, 8 (36), 18605–18623. [Google Scholar]
- (16).Gao Z; Li Y; Shang X; Hu W; Gao G; Duan L Bio-inspired adhesive and self-healing hydrogels as flexible strain sensors for monitoring human activities. Mater. Sci. Eng., C 2020, 106, 110168. [DOI] [PubMed] [Google Scholar]
- (17).Patel A; Zaky SH; Schoedel K; Li H; Sant V; Beniash E; Sfeir C; Stolz DB; Sant S Design and evaluation of collagen-inspired mineral-hydrogel nanocomposites for bone regeneration. Acta Biomater 2020, 112, 262–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Zambuto SG; Clancy KBH; Harley BAC A gelatin hydrogel to study endometrial angiogenesis and trophoblast invasion. Interface Focus 2019, 9 (5), 20190016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Dorishetty P; Balu R; Athukoralalage SS; Greaves TL; Mata J; de Campo L; Saha N; Zannettino ACW; Dutta NK; Choudhury NR Tunable Biomimetic Hydrogels from Silk Fibroin and Nanocellulose. ACS Sustainable Chem. Eng 2020, 8 (6), 2375–2389. [Google Scholar]
- (20).Jahanban-Esfahlan R; Derakhshankhah H; Haghshenas B; Massoumi B; Abbasian M; Jaymand M A bio-inspired magnetic natural hydrogel containing gelatin and alginate as a drug delivery system for cancer chemotherapy. Int. J. Biol. Macromol 2020, 156, 438–445. [DOI] [PubMed] [Google Scholar]
- (21).Lin F; Wang Z; Shen Y; Tang L; Zhang P; Wang Y; Chen Y; Huang B; Lu B Natural skin-inspired versatile cellulose biomimetic hydrogels. J. Mater. Chem. A 2019, 7 (46), 26442–26455. [Google Scholar]
- (22).Alam MN; Christopher LP Natural Cellulose-Chitosan Cross-Linked Superabsorbent Hydrogels with Superior Swelling Properties. ACS Sustainable Chem. Eng 2018, 6 (7), 8736–8742. [Google Scholar]
- (23).Li F; Lyu D; Liu S; Guo W DNA Hydrogels and Microgels for Biosensing and Biomedical Applications. Adv. Mater 2020, 32 (3), 1806538. [DOI] [PubMed] [Google Scholar]
- (24).Cui C; Fu Q; Meng L; Hao S; Dai R; Yang J Recent Progress in Natural Biopolymers Conductive Hydrogels for Flexible Wearable Sensors and Energy Devices: Materials, Structures, and Performance. ACS Applied Bio Materials 2021, 4 (1), 85–121. [DOI] [PubMed] [Google Scholar]
- (25).Long T; Li Y; Fang X; Sun J Salt-Mediated Polyampholyte Hydrogels with High Mechanical Strength, Excellent Self-Healing Property, and Satisfactory Electrical Conductivity. Adv. Funct. Mater 2018, 28 (44), 1804416. [Google Scholar]
- (26).Zhou D; Chen F; Handschuh-Wang S; Gan T; Zhou X; Zhou X Biomimetic Extreme-Temperature- and Environment-Adaptable Hydrogels. ChemPhysChem 2019, 20 (17), 2139–2154. [DOI] [PubMed] [Google Scholar]
- (27).Rong Q; Lei W; Liu M Conductive Hydrogels as Smart Materials for Flexible Electronic Devices. Chem. - Eur. J 2018, 24 (64), 16930–16943. [DOI] [PubMed] [Google Scholar]
- (28).Zhai D; Liu B; Shi Y; Pan L; Wang Y; Li W; Zhang R; Yu G Highly sensitive glucose sensor based on pt nanoparticle/polyaniline hydrogel heterostructures. ACS Nano 2013, 7 (4), 3540–6. [DOI] [PubMed] [Google Scholar]
- (29).Kim CC; Lee HH; Oh KH; Sun JY Highly stretchable, transparent ionic touch panel. Science 2016, 353 (6300), 682–7. [DOI] [PubMed] [Google Scholar]
- (30).Sun Z; Yamauchi Y; Araoka F; Kim YS; Bergueiro J; Ishida Y; Ebina Y; Sasaki T; Hikima T; Aida T An Anisotropic Hydrogel Actuator Enabling Earthworm-Like Directed Peristaltic Crawling. Angew. Chem., Int. Ed 2018, 57 (48), 15772–15776. [DOI] [PubMed] [Google Scholar]
- (31).Dreiss CA Hydrogel design strategies for drug delivery. Curr. Opin. Colloid Interface Sci 2020, 48, 1–17. [Google Scholar]
- (32).Mantha S; Pillai S; Khayambashi P; Upadhyay A; Zhang Y; Tao O; Pham HM; Tran SD Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12 (20), 3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Fan H; Gong JP Fabrication of Bioinspired Hydrogels: Challenges and Opportunities. Macromolecules 2020, 53 (8), 2769–2782. [Google Scholar]
- (34).Amoli V; Kim SY; Kim JS; Choi H; Koo J; Kim DH Biomimetics for high-performance flexible tactile sensors and advanced artificial sensory systems. J. Mater. Chem. C 2019, 7 (47), 14816–14844. [Google Scholar]
- (35).Zhao X; Chen F; Li Y; Lu H; Zhang N; Ma M Bioinspired ultra-stretchable and anti-freezing conductive hydrogel fibers with ordered and reversible polymer chain alignment. Nat. Commun 2018, 9 (1), 3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Xia S; Song S; Gao G Robust and flexible strain sensors based on dual physically cross-linked double network hydrogels for monitoring human-motion. Chem. Eng. J 2018, 354, 817–824. [Google Scholar]
- (37).Tang L; Wu S; Qu J; Gong L; Tang J A Review of Conductive Hydrogel Used in Flexible Strain Sensor. Materials 2020, 13 (18), 3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Zhang Q; Liu X; Duan L; Gao G Ultra-stretchable wearable strain sensors based on skin-inspired adhesive, tough and conductive hydrogels. Chem. Eng. J 2019, 365, 10–19. [Google Scholar]
- (39).Zhao X; Chen F; Li Y; Lu H; Zhang N; Ma M Bioinspired ultra-stretchable and anti-freezing conductive hydrogel fibers with ordered and reversible polymer chain alignment. Nat. Commun 2018, 9 (1), 3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Lu Y; Qu X; Zhao W; Ren Y; Si W; Wang W; Wang Q; Huang W; Dong X Highly Stretchable, Elastic, and Sensitive MXene-Based Hydrogel for Flexible Strain and Pressure Sensors. Research 2020, 2020, 2038560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Khajouei S; Ravan H; Ebrahimi A DNA hydrogel-empowered biosensing. Adv. Colloid Interface Sci 2020, 275, 102060–102060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Chen B; Wang W; Yan X; Li S; Jiang S; Liu S; Ma X; Yu X Highly Tough, Stretchable, Self-Adhesive and Strain-Sensitive DNA-Inspired Hydrogels for Monitoring Human Motion. Chem. - Eur. J 2020, 26 (50), 11604–11613. [DOI] [PubMed] [Google Scholar]
- (43).Zhang Q; Liu X; Duan L; Gao G A DNA-inspired hydrogel mechanoreceptor with skin-like mechanical behavior. J. Mater. Chem. A 2021, 9 (3), 1835–1844. [Google Scholar]
- (44).Vorvolakos K; Isayeva IS; Luu H-MD; Patwardhan DV; Pollack SK Ionically cross-linked hyaluronic acid: wetting, lubrication, and viscoelasticity of a modified adhesion barrier gel. Med. Devices: Evidence Res 2010, 4, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Sun H; Zhou K; Yu Y; Yue X; Dai K; Zheng G; Liu C; Shen C Highly Stretchable, Transparent, and Bio-Friendly Strain Sensor Based on Self-Recovery Ionic-Covalent Hydrogels for Human Motion Monitoring. Macromol. Mater. Eng 2019, 304 (10), 1900227. [Google Scholar]
- (46).Lu H; Zhang N; Ma M Electroconductive hydrogels for biomedical applications. Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol 2019, 11 (6), e1568. [DOI] [PubMed] [Google Scholar]
- (47).Mawad D; Lauto A; Wallace GG Conductive Polymer Hydrogels. In Polymeric Hydrogels as Smart Biomaterials, Kalia S, Ed.; Springer International Publishing: Cham, 2016; pp 19–44. DOI: 10.1007/978-3-319-25322-0_2. [DOI] [Google Scholar]
- (48).Gao F; Zhang N; Fang X; Ma M Bioinspired Design of Strong, Tough, and Highly Conductive Polyol-Polypyrrole Composites for Flexible Electronics. ACS Appl. Mater. Interfaces 2017, 9 (7), 5692–5698. [DOI] [PubMed] [Google Scholar]
- (49).Li W; Gao F; Wang X; Zhang N; Ma M Strong and Robust Polyaniline-Based Supramolecular Hydrogels for Flexible Supercapacitors. Angew. Chem., Int. Ed 2016, 55 (32), 9196–9201. [DOI] [PubMed] [Google Scholar]
- (50).Ding H; Zhong M; Kim YJ; Pholpabu P; Balasubramanian A; Hui CM; He H; Yang H; Matyjaszewski K; Bettinger CJ Biologically derived soft conducting hydrogels using heparin-doped polymer networks. ACS Nano 2014, 8 (5), 4348–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Lin J; Tang Q; Wu J; Li Q A multifunctional hydrogel with high-conductivity, pH-responsive, and release properties from polyacrylate/polyptrrole. J. Appl. Polym. Sci 2009, 116 (3), 1376–1383. [Google Scholar]
- (52).Du R; Xu Y; Luo Y; Zhang X; Zhang J Synthesis of conducting polymer hydrogels with 2D building blocks and their potential-dependent gel–sol transitions. Chem. Commun 2011, 47 (22), 6287–6289. [DOI] [PubMed] [Google Scholar]
- (53).Chen Y; Lu K; Song Y; Han J; Yue Y; Biswas SK; Wu Q; Xiao H A Skin-Inspired Stretchable, Self-Healing and Electro-Conductive Hydrogel with A Synergistic Triple Network for Wearable Strain Sensors Applied in Human-Motion Detection. Nanomaterials 2019, 9 (12), 1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Xu J; Wang G; Wu Y; Ren X; Gao G Ultrastretchable Wearable Strain and Pressure Sensors Based on Adhesive, Tough, and Self-healing Hydrogels for Human Motion Monitoring. ACS Appl. Mater. Interfaces 2019, 11 (28), 25613–25623. [DOI] [PubMed] [Google Scholar]
- (55).Wang MX; Chen YM; Gao Y; Hu C; Hu J; Tan L; Yang Z Rapid Self-Recoverable Hydrogels with High Toughness and Excellent Conductivity. ACS Appl. Mater. Interfaces 2018, 10 (31), 26610–26617. [DOI] [PubMed] [Google Scholar]
- (56).Lei Z; Wang Q; Sun S; Zhu W; Wu P A Bioinspired Mineral Hydrogel as a Self-Healable, Mechanically Adaptable Ionic Skin for Highly Sensitive Pressure Sensing. Adv. Mater 2017, 29 (22), 1700321. [DOI] [PubMed] [Google Scholar]
- (57).Zhang Z; Gao Z; Wang Y; Guo L; Yin C; Zhang X; Hao J; Zhang G; Chen L Eco-Friendly, Self-Healing Hydrogels for Adhesive and Elastic Strain Sensors, Circuit Repairing, and Flexible Electronic Devices. Macromolecules 2019, 52 (6), 2531–2541. [Google Scholar]
- (58).Yang B; Yuan W Highly Stretchable and Transparent Double-Network Hydrogel Ionic Conductors as Flexible Thermal–Mechanical Dual Sensors and Electroluminescent Devices. ACS Appl. Mater. Interfaces 2019, 11 (18), 16765–16775. [DOI] [PubMed] [Google Scholar]
- (59).Pan X; Wang Q; Ning D; Dai L; Liu K; Ni Y; Chen L; Huang L Ultraflexible Self-Healing Guar Gum-Glycerol Hydrogel with Injectable, Antifreeze, and Strain-Sensitive Properties. ACS Biomater. Sci. Eng 2018, 4 (9), 3397–3404. [DOI] [PubMed] [Google Scholar]
- (60).Matsuda T; Kawakami R; Namba R; Nakajima T; Gong JP Mechanoresponsive self-growing hydrogels inspired by muscle training. Science 2019, 363 (6426), 504–508. [DOI] [PubMed] [Google Scholar]
- (61).Wang K; Zhang X; Li C; Sun X; Meng Q; Ma Y; Wei Z Chemically Crosslinked Hydrogel Film Leads to Integrated Flexible Supercapacitors with Superior Performance. Adv. Mater 2015, 27 (45), 7451–7457. [DOI] [PubMed] [Google Scholar]
- (62).Hur J; Im K; Kim SW; Kim J; Chung D-Y; Kim T-H; Jo KH; Hahn JH; Bao Z; Hwang S; Park N Polypyrrole/Agarose-Based Electronically Conductive and Reversibly Restorable Hydrogel. ACS Nano 2014, 8 (10), 10066–10076. [DOI] [PubMed] [Google Scholar]
- (63).Dugasani SR; Gnapareddy B; Kesama MR; Jeon S; Jeong J-H; Park SH Optoelectronic properties of DNA thin films implanted with titania nanoparticle-coated multiwalled carbon nanotubes. AIP Adv 2019, 9 (1), 015011. [Google Scholar]
- (64).Sun X; Qin Z; Ye L; Zhang H; Yu Q; Wu X; Li J; Yao F Carbon nanotubes reinforced hydrogel as flexible strain sensor with high stretchability and mechanically toughness. Chem. Eng. J 2020, 382, 122832. [Google Scholar]
- (65).Zhang Z-J; Cui W; Xu H; Xie L; Liu H; Zhu L-M; Li H; Ran R A free radical assisted strategy for preparing functionalized carbon nanotubes as a highly efficient nucleating agent for poly(l-lactide). RSC Adv 2015, 5, 16604. [Google Scholar]
- (66).Pandey S; Siva Prasanna SRV; Karakoti M; Tewari C; SanthiBhushan B; Pandey JK; Srivastava A; Rana S; Sahoo NG Dispersion and stability study of carbon nanotubes in pH and temperature responsive polymeric matrix: Experiment and dispersion-corrected DFT study. Materials Today Communications 2018, 17, 187–193. [Google Scholar]
- (67).Deng Z; Hu T; Lei Q; He J; Ma PX; Guo B Stimuli-Responsive Conductive Nanocomposite Hydrogels with High Stretchability, Self-Healing, Adhesiveness, and 3D Printability for Human Motion Sensing. ACS Appl. Mater. Interfaces 2019, 11 (7), 6796–6808. [DOI] [PubMed] [Google Scholar]
- (68).Shin SR; Jung SM; Zalabany M; Kim K; Zorlutuna P; Kim SB; Nikkhah M; Khabiry M; Azize M; Kong J; Wan KT; Palacios T; Dokmeci MR; Bae H; Tang XS; Khademhosseini A Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS Nano 2013, 7 (3), 2369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Qin Z; Sun X; Yu Q; Zhang H; Wu X; Yao M; Liu W; Yao F; Li J Carbon Nanotubes/Hydrophobically Associated Hydrogels as Ultrastretchable, Highly Sensitive, Stable Strain, and Pressure Sensors. ACS Appl. Mater. Interfaces 2020, 12 (4), 4944–4953. [DOI] [PubMed] [Google Scholar]
- (70).Han L; Lu X; Wang M; Gan D; Deng W; Wang K; Fang L; Liu K; Chan CW; Tang Y; Weng LT; Yuan H A Mussel-Inspired Conductive, Self-Adhesive, and Self-Healable Tough Hydrogel as Cell Stimulators and Implantable Bioelectronics. Small 2017, 13 (2), 1601916. [DOI] [PubMed] [Google Scholar]
- (71).Kumar R; Pal S; Prajapati YK; Saini JP Sensitivity Enhancement of MXene Based SPR Sensor Using Silicon: Theoretical Analysis. Silicon 2021, 13, 1887. [Google Scholar]
- (72).Liu H; Duan C; Yang C; Shen W; Wang F; Zhu Z A novel nitrite biosensor based on the direct electrochemistry of hemoglobin immobilized on MXene-Ti3C2. Sens. Actuators, B 2015, 218, 60–66. [Google Scholar]
- (73).Lei D; Liu N; Su T; Wang L; Su J; Zhang Z; Gao Y Research progress of MXenes-based wearable pressure sensors. APL Mater 2020, 8 (11), 110702. [Google Scholar]
- (74).Ge G; Lu Y; Qu X; Zhao W; Ren Y; Wang W; Wang Q; Huang W; Dong X Muscle-Inspired Self-Healing Hydrogels for Strain and Temperature Sensor. ACS Nano 2020, 14 (1), 218–228. [DOI] [PubMed] [Google Scholar]
- (75).Han L; Yan L; Wang M; Wang K; Fang L; Zhou J; Fang J; Ren F; Lu X Transparent, Adhesive, and Conductive Hydrogel for Soft Bioelectronics Based on Light-Transmitting Polydopamine-Doped Polypyrrole Nanofibrils. Chem. Mater 2018, 30 (16), 5561–5572. [Google Scholar]
- (76).Liu Y-J; Cao W-T; Ma M-G; Wan P Ultrasensitive Wearable Soft Strain Sensors of Conductive, Self-healing, and Elastic Hydrogels with Synergistic “Soft and Hard” Hybrid Networks. ACS Appl. Mater. Interfaces 2017, 9 (30), 25559–25570. [DOI] [PubMed] [Google Scholar]
- (77).Kim J; Campbell AS; de Ávila BE-F; Wang J Wearable biosensors for healthcare monitoring. Nat. Biotechnol 2019, 37 (4), 389–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Andreu-Perez J; Leff DR; Ip HMD; Yang G From Wearable Sensors to Smart Implants-–Toward Pervasive and Personalized Healthcare. IEEE Trans. Biomed. Eng 2015, 62 (12), 2750–2762. [DOI] [PubMed] [Google Scholar]
- (79).Mandal A; Clegg JR; Anselmo AC; Mitragotri S Hydrogels in the clinic. Bioeng Transl Med 2020, 5 (2), e10158–e10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Duraisamy P; Kumar Sidharthan R; Nagarajan Santhanakrishnan M Design, Modeling, and Control of Biomimetic Fish Robot: A Review. Journal of Bionic Engineering 2019, 16 (6), 967–993. [Google Scholar]
- (81).Osotsi MI; Zhang W; Zada I; Gu J; Liu Q; Zhang D Butterfly wing architectures inspire sensor and energy applications. National Science Review 2021, DOI: 10.1093/nsr/nwaa107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Baik S; Lee HJ; Kim DW; Kim JW; Lee Y; Pang C Bioinspired Adhesive Architectures: From Skin Patch to Integrated Bioelectronics. Adv. Mater 2019, 31 (34), 1803309. [DOI] [PubMed] [Google Scholar]
- (83).Chortos A; Bao Z Skin-inspired electronic devices. Mater Today 2014, 17 (7), 321–331. [Google Scholar]
- (84).Xia S; Zhang Q; Song S; Duan L; Gao G Bioinspired Dynamic Cross-Linking Hydrogel Sensors with Skin-like Strain and Pressure Sensing Behaviors. Chem. Mater 2019, 31 (22), 9522–9531. [Google Scholar]
- (85).Oh JH; Hong SY; Park H; Jin SW; Jeong YR; Oh SY; Yun J; Lee H; Kim JW; Ha JS Fabrication of High-Sensitivity Skin-Attachable Temperature Sensors with Bioinspired Microstructured Adhesive. ACS Appl. Mater. Interfaces 2018, 10 (8), 7263–7270. [DOI] [PubMed] [Google Scholar]
- (86).Wei J; Xie J; Zhang P; Zou Z; Ping H; Wang W; Xie H; Shen JZ; Lei L; Fu Z Bioinspired 3D Printable, Self-Healable, and Stretchable Hydrogels with Multiple Conductivities for Skin-like Wearable Strain Sensors. ACS Appl. Mater. Interfaces 2021, 13 (2), 2952–2960. [DOI] [PubMed] [Google Scholar]
- (87).Sun Q-J; Zhao X-H; Yeung C-C; Tian Q; Kong K-W; Wu W; Venkatesh S; Li W-J; Roy VAL Bioinspired, Self-Powered, and Highly Sensitive Electronic Skin for Sensing Static and Dynamic Pressures. ACS Appl. Mater. Interfaces 2020, 12 (33), 37239–37247. [DOI] [PubMed] [Google Scholar]
- (88).Zhang X; Chen G; Yu Y; Sun L; Zhao Y Bioinspired Adhesive and Antibacterial Microneedles for Versatile Transdermal Drug Delivery. Research 2020, 2020, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Ribeiro A; Veiga F; Santos D; Torres-Labandeira JJ; Concheiro A; Alvarez-Lorenzo C Bioinspired imprinted PHEMA-hydrogels for ocular delivery of carbonic anhydrase inhibitor drugs. Biomacromolecules 2011, 12 (3), 701–709. [DOI] [PubMed] [Google Scholar]
- (90).Ma Y; Hua M; Wu S; Du Y; Pei X; Zhu X; Zhou F; He X Bioinspired high-power-density strong contractile hydrogel by programmable elastic recoil. Science Advances 2020, 6 (47), eabd2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Nonoyama T; Lee YW; Ota K; Fujioka K; Hong W; Gong JP Instant Thermal Switching from Soft Hydrogel to Rigid Plastics Inspired by Thermophile Proteins. Adv. Mater 2020, 32 (4), 1905878. [DOI] [PubMed] [Google Scholar]
- (92).Teymourian H; Tehrani F; Mahato K; Wang J Lab under the Skin: Microneedle Based Wearable Devices. Adv. Healthcare Mater 2021, 10, 2002255. [DOI] [PubMed] [Google Scholar]
- (93).Dharadhar S; Majumdar A; Dhoble S; Patravale V Microneedles for transdermal drug delivery: a systematic review. Drug Dev. Ind. Pharm 2019, 45 (2), 188–201. [DOI] [PubMed] [Google Scholar]
- (94).Waghule T; Singhvi G; Dubey SK; Pandey MM; Gupta G; Singh M; Dua K Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother 2019, 109, 1249–1258. [DOI] [PubMed] [Google Scholar]
- (95).Ma G; Wu C Microneedle, bio-microneedle and bio-inspired microneedle: A review. J. Controlled Release 2017, 251, 11–23. [DOI] [PubMed] [Google Scholar]
- (96).Bok M; Zhao Z-J; Jeon S; Jeong J-H; Lim E Ultrasonically and Iontophoretically Enhanced Drug-Delivery System Based on Dissolving Microneedle Patches. Sci. Rep 2020, 10 (1), 2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Donnelly RF; Singh TRR; Garland MJ; Migalska K; Majithiya R; McCrudden CM; Kole PL; Mahmood TMT; McCarthy HO; Woolfson AD Hydrogel-Forming Microneedle Arrays for Enhanced Transdermal Drug Delivery. Adv. Funct. Mater 2012, 22 (23), 4879–4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Courtenay AJ; McAlister E; McCrudden MTC; Vora L; Steiner L; Levin G; Levy-Nissenbaum E; Shterman N; Kearney M-C; McCarthy HO; Donnelly RF Hydrogel-forming microneedle arrays as a therapeutic option for transdermal esketamine delivery. J. Controlled Release 2020, 322, 177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Migdadi EM; Courtenay AJ; Tekko IA; McCrudden MTC; Kearney M-C; McAlister E; McCarthy HO; Donnelly RF Hydrogel-forming microneedles enhance transdermal delivery of metformin hydrochloride. J. Controlled Release 2018, 285, 142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Madden J; O’Mahony C; Thompson M; O’Riordan A; Galvin P Biosensing in dermal interstitial fluid using microneedle based electrochemical devices. Sensing and Bio-Sensing Research 2020, 29, 100348. [Google Scholar]
- (101).Chang H; Zheng M; Yu X; Than A; Seeni RZ; Kang R; Tian J; Khanh DP; Liu L; Chen P; Xu C A Swellable Microneedle Patch to Rapidly Extract Skin Interstitial Fluid for Timely Metabolic Analysis. Adv. Mater 2017, 29 (37), 1702243. [DOI] [PubMed] [Google Scholar]
- (102).He R; Niu Y; Li Z; Li A; Yang H; Xu F; Li F A Hydrogel Microneedle Patch for Point-of-Care Testing Based on Skin Interstitial Fluid. Adv. Healthcare Mater 2020, 9 (4), 1901201. [DOI] [PubMed] [Google Scholar]
- (103).Al Sulaiman D; Chang JYH; Bennett NR; Topouzi H; Higgins CA; Irvine DJ; Ladame S Hydrogel-Coated Microneedle Arrays for Minimally Invasive Sampling and Sensing of Specific Circulating Nucleic Acids from Skin Interstitial Fluid. ACS Nano 2019, 13 (8), 9620–9628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).Zhang X; Chen G; Sun L; Ye F; Shen X; Zhao Y Claw-inspired microneedle patches with liquid metal encapsulation for accelerating incisional wound healing. Chem. Eng. J 2021, 406, 126741. [Google Scholar]
- (105).Han D; Morde RS; Mariani S; La Mattina AA; Vignali E; Yang C; Barillaro G; Lee H 4D Printing of a Bioinspired Microneedle Array with Backward-Facing Barbs for Enhanced Tissue Adhesion. Adv. Funct. Mater 2020, 30 (11), 1909197. [Google Scholar]
- (106).Athreya PK; Bhardwaj GK Contact Lens Materials and Modalities. Trend in Opfthalomology 2018, 1 (1), 1–5. [Google Scholar]
- (107).Ma X; Ahadian S; Liu S; Zhang J; Liu S; Cao T; Lin W; Wu D; de Barros NR; Zare MR; Diltemiz SE; Jucaud V; Zhu Y; Zhang S; Banton E; Gu Y; Nan K; Xu S; Dokmeci MR; Khademhosseini A Smart Contact Lenses for Biosensing Applications. Advanced Intelligent Systems 2021, 3 (5), 2000263. [Google Scholar]
- (108).Murube J Basal, Reflex, and Psycho-emotional Tears. Ocular Surface 2009, 7 (2), 60–66. [DOI] [PubMed] [Google Scholar]
- (109).Srinivasarao DA; Lohiya G; Katti DS Fundamentals, challenges, and nanomedicine-based solutions for ocular diseases. Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol 2019, 11 (4), e1548. [DOI] [PubMed] [Google Scholar]
- (110).Kirchhof S; Goepferich AM; Brandl FP Hydrogels in ophthalmic applications. Eur. J. Pharm. Biopharm 2015, 95, 227–238. [DOI] [PubMed] [Google Scholar]
- (111).Abrego G; Alvarado HL; Egea MA; Gonzalez-Mira E; Calpena AC; Garcia ML Design of Nanosuspensions and Freeze-Dried PLGA Nanoparticles as a Novel Approach for Ophthalmic Delivery of Pranoprofen. J. Pharm. Sci 2014, 103 (10), 3153–3164. [DOI] [PubMed] [Google Scholar]
- (112).Huang D; Chen Y-S; Rupenthal ID Overcoming ocular drug delivery barriers through the use of physical forces. Adv. Drug Delivery Rev 2018, 126, 96–112. [DOI] [PubMed] [Google Scholar]
- (113).González-Chomón C; Silva M; Concheiro A; Alvarez-Lorenzo C Biomimetic contact lenses eluting olopatadine for allergic conjunctivitis. Acta Biomater 2016, 41, 302–311. [DOI] [PubMed] [Google Scholar]
- (114).Varela-Garcia A; Concheiro A; Alvarez-Lorenzo C Cytosine-functionalized bioinspired hydrogels for ocular delivery of antioxidant transferulic acid. Biomater. Sci 2020, 8 (4), 1171–1180. [DOI] [PubMed] [Google Scholar]
- (115).Kim J; Kim M; Lee M-S; Kim K; Ji S; Kim Y-T; Park J; Na K; Bae K-H; Kyun Kim H; Bien F; Young Lee C; Park J-U Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nat. Commun 2017, 8 (1), 14997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Keum DH; Kim S-K; Koo J; Lee G-H; Jeon C; Mok JW; Mun BH; Lee KJ; Kamrani E; Joo C-K; Shin S; Sim J-Y; Myung D; Yun SH; Bao Z; Hahn SK Wireless smart contact lens for diabetic diagnosis and therapy. Science Advances 2020, 6 (17), eaba3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (117).Wei S; Yin R; Tang T; Wu Y; Liu Y; Wang P; Wang K; Mei M; Zou R; Duan X Gas-Permeable, Irritation-Free, Transparent Hydrogel Contact Lens Devices with Metal-Coated Nanofiber Mesh for Eye Interfacing. ACS Nano 2019, 13 (7), 7920–7929. [DOI] [PubMed] [Google Scholar]
- (118).Wang Y; Zhao Q; Du X Structurally coloured contact lens sensor for point-of-care ophthalmic health monitoring. J. Mater. Chem. B 2020, 8 (16), 3519–3526. [DOI] [PubMed] [Google Scholar]
- (119).Ding M; Jing L; Yang H; Machnicki CE; Fu X; Li K; Wong IY; Chen PY Multifunctional soft machines based on stimuli-responsive hydrogels: from freestanding hydrogels to smart integrated systems. Materials Today Advances 2020, 8, 100088. [Google Scholar]
- (120).Pu S; Su J; Li L; Wang H; Chen C; Hu X Bioinspired sweating with temperature sensitive hydrogel to passively dissipate heat from high-end wearable electronics. Energy Convers. Manage 2019, 180, 747–756. [Google Scholar]
- (121).Yuk H; Lin S; Ma C; Takaffoli M; Fang NX; Zhao X Hydraulic hydrogel actuators and robots optically and sonically camouflaged in water. Nat. Commun 2017, 8 (1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (122).Wang Y; Cao X; Cheng J; Yao B; Zhao Y; Wu S; Ju B; Zhang S; He X; Niu W Cephalopod-Inspired Chromotropic Ionic Skin with Rapid Visual Sensing Capabilities to Multiple Stimuli. ACS Nano 2021, 15 (2), 3509–3521. [DOI] [PubMed] [Google Scholar]
- (123).Mannoor MS; Jiang Z; James T; Kong YL; Malatesta KA; Soboyejo WO; Verma N; Gracias DH; McAlpine MC 3D Printed Bionic Ears. Nano Lett 2013, 13 (6), 2634–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (124).Hu X; Yu J; Qian C; Lu Y; Kahkoska AR; Xie Z; Jing X; Buse JB; Gu Z H2O2-Responsive Vesicles Integrated with Transcutaneous Patches for Glucose-Mediated Insulin Delivery. ACS Nano 2017, 11 (1), 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (125).Ray TR; Choi J; Bandodkar AJ; Krishnan S; Gutruf P; Tian L; Ghaffari R; Rogers JA Bio-integrated wearable systems: A comprehensive review. Chem. Rev 2019, 119 (8), 5461–5533. [DOI] [PubMed] [Google Scholar]
- (126).Amjadi M; Kyung KU; Park I; Sitti M Stretchable, skin-mountable, and wearable strain sensors and their potential applications: a review. Adv. Funct. Mater 2016, 26 (11), 1678–1698. [Google Scholar]
- (127).Zhang YS; Khademhosseini A Advances in engineering hydrogels. Science 2017, 356 (6337), 1 DOI: 10.1126/science.aaf3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Liu X; Liu J; Lin S; Zhao X Hydrogel machines. Mater Today 2020, 36, 102–124. [Google Scholar]
- (129).Sun X; Agate S; Salem KS; Lucia L; Pal L HydrogelBased Sensor Networks: Compositions, Properties, and Applications—A Review. ACS Applied Bio Materials 2021, 4 (1), 140–162. [DOI] [PubMed] [Google Scholar]
- (130).Lin S; Liu J; Liu X; Zhao X Muscle-like fatigue-resistant hydrogels by mechanical training. Proc. Natl. Acad. Sci. U. S. A 2019, 116 (21), 10244–10249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Shiblee MNI; Ahmed K; Khosla A; Kawakami M; Furukawa H 3D printing of shape memory hydrogels with tunable mechanical properties. Soft Matter 2018, 14 (38), 7809–7817. [DOI] [PubMed] [Google Scholar]
- (132).Tavakoli J; Tang Y Hydrogel based sensors for biomedical applications: An updated review. Polymers 2017, 9 (8), 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (133).Thoniyot P; Tan MJ; Karim AA; Young DJ; Loh XJ Nanoparticle–hydrogel composites: Concept, design, and applications of these promising, multi-functional materials. Advanced Science 2015, 2 (1–2), 1400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (134).Li Z; Davidson-Rozenfeld G; Vázquez-González M; Fadeev M; Zhang J; Tian H; Willner I Multi-triggered supramolecular DNA/bipyridinium dithienylethene hydrogels driven by light, redox, and chemical stimuli for shape-memory and self-healing applications. J. Am. Chem. Soc 2018, 140 (50), 17691–17701. [DOI] [PubMed] [Google Scholar]
- (135).Lee Y; Song W; Sun J-Y Hydrogel soft robotics. Materials Today Physics 2020, 15, 100258. [Google Scholar]
- (136).Wang Y; Cao X; Cheng J; Yao B; Zhao Y; Wu S; Ju B; Zhang S; He X; Niu W Cephalopod-Inspired Chromotropic Ionic Skin with Rapid Visual Sensing Capabilities to Multiple Stimuli. ACS Nano 2021, 15 (2), 3509–3521. [DOI] [PubMed] [Google Scholar]
- (137).Zhang Z; Chen Z; Wang Y; Zhao Y Bioinspired conductive cellulose liquid-crystal hydrogels as multifunctional electrical skins. Proc. Natl. Acad. Sci. U. S. A 2020, 117 (31), 18310–18316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (138).Wei S; Lu W; Le X; Ma C; Lin H; Wu B; Zhang J; Theato P; Chen T Bioinspired Synergistic Fluorescence-Color-Switchable Polymeric Hydrogel Actuators. Angew. Chem., Int. Ed 2019, 58 (45), 16243–16251. [DOI] [PubMed] [Google Scholar]
- (139).Ma C; Lu W; Yang X; He J; Le X; Wang L; Zhang J; Serpe MJ; Huang Y; Chen T Bioinspired anisotropic hydrogel actuators with on–off switchable and color-tunable fluorescence behaviors. Adv. Funct. Mater 2018, 28 (7), 1704568. [Google Scholar]
- (140).Liu H; Li M; Ouyang C; Lu TJ; Li F; Xu F Biofriendly, stretchable, and reusable hydrogel electronics as wearable force sensors. Small 2018, 14 (36), 1801711. [DOI] [PubMed] [Google Scholar]
- (141).Liu Y; Liu J; Chen S; Lei T; Kim Y; Niu S; Wang H; Wang X; Foudeh AM; Tok JB-H Soft and elastic hydrogel-based microelectronics for localized low-voltage neuromodulation. Nature Biomedical Engineering 2019, 3 (1), 58–68. [DOI] [PubMed] [Google Scholar]
- (142).Jung YH; Park B; Kim JU; Kim T. i. Bioinspired electronics for artificial sensory systems. Adv. Mater 2019, 31 (34), 1803637. [DOI] [PubMed] [Google Scholar]
- (143).Jeong K-H; Park D; Lee Y-C Polymer-based hydrogel scaffolds for skin tissue engineering applications: a mini-review. J. Polym. Res 2017, 24 (7), 1–10. [Google Scholar]
- (144).Han L; Lu X; Liu K; Wang K; Fang L; Weng L-T; Zhang H; Tang Y; Ren F; Zhao C Mussel-inspired adhesive and tough hydrogel based on nanoclay confined dopamine polymerization. ACS Nano 2017, 11 (3), 2561–2574. [DOI] [PubMed] [Google Scholar]
- (145).Lee H; Song C; Hong YS; Kim MS; Cho HR; Kang T; Shin K; Choi SH; Hyeon T; Kim D-H Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Science Advances 2017, 3 (3), e1601314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (146).Rizwan M; Yahya R; Hassan A; Yar M; Azzahari AD; Selvanathan V; Sonsudin F; Abouloula CN pH sensitive hydrogels in drug delivery: Brief history, properties, swelling, and release mechanism, material selection and applications. Polymers 2017, 9 (4), 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (147).Lim JY; Goh SS; Loh XJ Bottom-Up Engineering of Responsive Hydrogel Materials for Molecular Detection and Biosensing. ACS Materials Letters 2020, 2 (8), 918–950. [Google Scholar]
- (148).Yang T; Wang M; Jia F; Ren X; Gao G Thermoresponsive shape memory sensors based on tough, remolding and anti-freezing hydrogels. J. Mater. Chem. C 2020, 8 (7), 2326–2335. [Google Scholar]
- (149).Guo W; Lu CH; Orbach R; Wang F; Qi XJ; Cecconello A; Seliktar D; Willner I pH-Stimulated DNA Hydrogels Exhibiting Shape-Memory Properties. Adv. Mater 2015, 27 (1), 73–78. [DOI] [PubMed] [Google Scholar]
- (150).Shang J; Theato P Smart composite hydrogel with pH-, ionic strength-and temperature-induced actuation. Soft Matter 2018, 14 (41), 8401–8407. [DOI] [PubMed] [Google Scholar]
- (151).Shang J; Le X; Zhang J; Chen T; Theato P Trends in polymeric shape memory hydrogels and hydrogel actuators. Polym. Chem 2019, 10 (9), 1036–1055. [Google Scholar]
- (152).Miyata T; Asami N; Uragami T A reversibly antigen-responsive hydrogel. Nature 1999, 399 (6738), 766–769. [DOI] [PubMed] [Google Scholar]
- (153).Kolberg A; Wenzel C; Hackenstrass K; Schwarzl R; Rüttiger C; Hugel T; Gallei M; Netz RR; Balzer BN Opposing temperature dependence of the stretching response of single PEG and PNiPAM polymers. J. Am. Chem. Soc 2019, 141 (29), 11603–11613. [DOI] [PubMed] [Google Scholar]
- (154).Galateanu B; Dimonie D; Vasile E; Nae S; Cimpean A; Costache M Layer-shaped alginate hydrogels enhance the biological performance of human adipose-derived stem cells. BMC Biotechnol 2012, 12 (1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (155).Kim HJ; Choi W; Kim J; Choi J; Choi N; Hwang KS Highly sensitive three-dimensional interdigitated microelectrode biosensors embedded with porosity tunable hydrogel for detecting proteins. Sens. Actuators, B 2020, 302, 127190. [Google Scholar]
- (156).Nojoomi A; Arslan H; Lee K; Yum K Bioinspired 3D structures with programmable morphologies and motions. Nat. Commun 2018, 9 (1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (157).Banerjee H; Suhail M; Ren H Hydrogel actuators and sensors for biomedical soft robots: brief overview with impending challenges. Biomimetics 2018, 3 (3), 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (158).Xue P; Bisoyi H; Chen Y; Zeng H; Yang J; Yang X; Lv P; Zhang X; Priimagi A; Wang L Near-Infrared Light-Driven Shape-Morphing of Programmable Anisotropic Hydrogels Enabled by MXene Nanosheets. Angew. Chem., Int. Ed 2021, 60 (7), 3390–3396. [DOI] [PubMed] [Google Scholar]
- (159).Li C; Lau GC; Yuan H; Aggarwal A; Dominguez VL; Liu S; Sai H; Palmer LC; Sather NA; Pearson TJ Fast and programmable locomotion of hydrogel-metal hybrids under light and magnetic fields. Science Robotics 2020, 5 (49), 1 DOI: 10.1126/scirobotics.abb9822. [DOI] [PubMed] [Google Scholar]
- (160).Elsherif M; Hassan MU; Yetisen AK; Butt H Glucose sensing with phenylboronic acid functionalized hydrogel-based optical diffusers. ACS Nano 2018, 12 (3), 2283–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (161).Zhang J; Mou L; Jiang X Hydrogels incorporating Au@polydopamine nanoparticles: robust performance for optical sensing. Anal. Chem 2018, 90 (19), 11423–11430. [DOI] [PubMed] [Google Scholar]
- (162).Qin J; Dong B; Cao L; Wang W Photonic hydrogels for the ultratrace sensing of divalent beryllium in seawater. J. Mater. Chem. C 2018, 6 (15), 4234–4242. [Google Scholar]
- (163).Chen J; Xu L; Yang M; Chen X; Chen X; Hong W Highly stretchable photonic crystal hydrogels for a sensitive mechanochromic sensor and direct ink writing. Chem. Mater 2019, 31 (21), 8918–8926. [Google Scholar]
- (164).Li S; Zeng Y; Hou W; Wan W; Zhang J; Wang Y; Du X; Gu Z Photo-responsive photonic hydrogel: in situ manipulation and monitoring of cell scaffold stiffness. Mater. Horiz 2020, 7 (11), 2944–2950. [Google Scholar]
- (165).Choi SH; Duzik AJ; Kim H-J; Park Y; Kim J; Ko H-U; Kim H-C; Yun S; Kyung K-U Perspective and potential of smart optical materials. Smart Mater. Struct 2017, 26 (9), 093001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (166).Fu F; Chen Z; Zhao Z; Wang H; Shang L; Gu Z; Zhao Y Bio-inspired self-healing structural color hydrogel. Proc. Natl. Acad. Sci. U. S. A 2017, 114 (23), 5900–5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (167).Fu F; Shang L; Chen Z; Yu Y; Zhao Y Bioinspired living structural color hydrogels. Science Robotics 2018, 3 (16), 1 DOI: 10.1126/scirobotics.aar8580. [DOI] [PubMed] [Google Scholar]
- (168).Tavakoli J; Gascooke J; Xie N; Tang BZ; Tang Y Enlightening freeze–thaw process of physically cross-linked poly (vinyl alcohol) hydrogels by aggregation-induced emission fluorogens. ACS Applied Polymer Materials 2019, 1 (6), 1390–1398. [Google Scholar]
- (169).Qin J; Li X; Cao L; Du S; Wang W; Yao SQ Competition-Based Universal Photonic Crystal Biosensors by Using Antibody–Antigen Interaction. J. Am. Chem. Soc 2020, 142 (1), 417–423. [DOI] [PubMed] [Google Scholar]
- (170).Jung S; MacConaghy KI; Kaar JL; Stoykovich MP Enhanced optical sensitivity in thermoresponsive photonic crystal hydrogels by operating near the phase transition. ACS Appl. Mater. Interfaces 2017, 9 (33), 27927–27935. [DOI] [PubMed] [Google Scholar]
- (171).Li Y; Young DJ; Loh XJ Fluorescent gels: a review of synthesis, properties, applications and challenges. Materials Chemistry Frontiers 2019, 3 (8), 1489–1502. [Google Scholar]
- (172).Ji X; Li Z; Hu Y; Xie H; Wu W; Song F; Liu H; Wang J; Jiang M; Lam JW Bioinspired hydrogels with muscle-like structure for AIEgen-guided selective self-healing. CCS Chemistry 2021, 3, 1146–1156. [Google Scholar]
- (173).Li Z; Liu P; Ji X; Gong J; Hu Y; Wu W; Wang X; Peng HQ; Kwok RT; Lam JW Bioinspired simultaneous changes in fluorescence color, brightness, and shape of hydrogels enabled by AIEgens. Adv. Mater 2020, 32 (11), 1906493. [DOI] [PubMed] [Google Scholar]
- (174).Li P; Zhang D; Zhang Y; Lu W; Zhang J; Wang W; He Q; Théato P; Chen T Aggregation-caused quenching-type naphthalimide fluorophores grafted and ionized in a 3D polymeric hydrogel network for highly fluorescent and locally tunable emission. ACS Macro Lett 2019, 8 (8), 937–942. [DOI] [PubMed] [Google Scholar]
- (175).Peng Q; Chen J; Wang T; Peng X; Liu J; Wang X; Wang J; Zeng H Recent advances in designing conductive hydrogels for flexible electronics. InfoMat 2020, 2 (5), 843–865. [Google Scholar]
- (176).Xu S; Li T; Ren H; Mao X; Ye X; Liang B PEDOT: PSS Hydrogel based Flexible Electrodes for Wearable ECG Monitoring; 2020 IEEE Sensors; IEEE: 2020; pp 1–4. [Google Scholar]
- (177).Yuk H; Lu B; Zhao X Hydrogel bioelectronics. Chem. Soc. Rev 2019, 48 (6), 1642–1667. [DOI] [PubMed] [Google Scholar]
- (178).Han L; Lu X; Wang M; Gan D; Deng W; Wang K; Fang L; Liu K; Chan CW; Tang Y A mussel-inspired conductive, self-adhesive, and self-healable tough hydrogel as cell stimulators and implantable bioelectronics. Small 2017, 13 (2), 1601916. [DOI] [PubMed] [Google Scholar]
- (179).Kiaee G; Mostafalu P; Samandari M; Sonkusale S A pH-Mediated Electronic Wound Dressing for Controlled Drug Delivery. Adv. Healthcare Mater 2018, 7 (18), 1800396. [DOI] [PubMed] [Google Scholar]
- (180).Huang H; Han L; Fu X; Wang Y; Yang Z; Pan L; Xu M Multiple stimuli responsive and identifiable zwitterionic ionic conductive hydrogel for bionic electronic skin. Advanced Electronic Materials 2020, 6 (7), 2000239. [Google Scholar]
- (181).Han L; Cui S; Yu H-Y; Song M; Zhang H; Grishkewich N; Huang C; Kim D; Tam KMC Self-healable conductive nanocellulose nanocomposites for biocompatible electronic skin sensor systems. ACS Appl. Mater. Interfaces 2019, 11 (47), 44642–44651. [DOI] [PubMed] [Google Scholar]
- (182).Huang J; Liu Y; Chi X; Jiang Y; Xu Z; Qu G; Zhao Y; Li Z; Chen C; Chen G Programming electronic skin with diverse skin-like properties. J. Mater. Chem. A 2021, 9 (2), 963–973. [Google Scholar]
- (183).Shit A; Heo SB; In I; Park SY Mineralized Soft and Elastic Polymer Dot Hydrogel for a Flexible Self-Powered Electronic Skin Sensor. ACS Appl. Mater. Interfaces 2020, 12 (30), 34105–34114. [DOI] [PubMed] [Google Scholar]
- (184).Mahdavi A; Ferreira L; Sundback C; Nichol JW; Chan EP; Carter DJ; Bettinger CJ; Patanavanich S; Chignozha L; Ben-Joseph E; et al. A biodegradable and biocompatible gecko-inspired tissue adhesive. Proc. Natl. Acad. Sci. U. S. A 2008, 105 (7), 2307–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (185).Liu X; Tang T-C; Tham E; Yuk H; Lin S; Lu TK; Zhao X Stretchable living materials and devices with hydrogel–elastomer hybrids hosting programmed cells. Proc. Natl. Acad. Sci. U. S. A 2017, 114 (9), 2200–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (186).Liu X; Yuk H; Lin S; Parada GA; Tang TC; Tham E; de la Fuente-Nunez C; Lu TK; Zhao X 3D printing of living responsive materials and devices. Adv. Mater 2018, 30 (4), 1704821. [DOI] [PubMed] [Google Scholar]
- (187).Choi M; Choi JW; Kim S; Nizamoglu S; Hahn SK; Yun SH Light-guiding hydrogels for cell-based sensing and optogenetic synthesis in vivo. Nat. Photonics 2013, 7 (12), 987–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (188).Zhan H; Löwik DW A hybrid peptide amphiphile fiber PEG hydrogel matrix for 3D cell culture. Adv. Funct. Mater 2019, 29 (16), 1808505. [Google Scholar]
- (189).Tam RY; Smith LJ; Shoichet MS Engineering cellular microenvironments with photo-and enzymatically responsive hydrogels: toward biomimetic 3D cell culture models. Acc. Chem. Res 2017, 50 (4), 703–713. [DOI] [PubMed] [Google Scholar]
- (190).Kim J; Campbell AS; de Avila BE; Wang J Wearable biosensors for healthcare monitoring. Nat. Biotechnol 2019, 37 (4), 389–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (191).Bandodkar AJ; Jeerapan I; Wang J Wearable Chemical Sensors: Present Challenges and Future Prospects. ACS Sensors 2016, 1 (5), 464–482. [Google Scholar]
- (192).Mackanic DG; Chang TH; Huang Z; Cui Y; Bao Z Stretchable electrochemical energy storage devices. Chem. Soc. Rev 2020, 49 (13), 4466–4495. [DOI] [PubMed] [Google Scholar]
- (193).Wang D; Han C; Mo F; Yang Q; Zhao Y; Li Q; Liang G; Dong B; Zhi C Energy density issues of flexible energy storage devices. Energy Storage Materials 2020, 28, 264–292. [Google Scholar]
- (194).Rwei AY; Lu W; Wu C; Human K; Suen E; Franklin D; Fabiani M; Gratton G; Xie Z; Deng Y; Kwak SS; Li L; Gu C; Liu A; Rand CM; Stewart TM; Huang Y; Weese-Mayer DE; Rogers JA A wireless, skin-interfaced biosensor for cerebral hemodynamic monitoring in pediatric care. Proc. Natl. Acad. Sci. U. S. A 2020, 117 (50), 31674–31684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (195).Mackanic DG; Kao M; Bao Z Enabling Deformable and Stretchable Batteries. Adv. Energy Mater 2020, 10 (29), 2001424. [Google Scholar]
- (196).Zhou Y; Wang CH; Lu W; Dai L Recent Advances in Fiber-Shaped Supercapacitors and Lithium-Ion Batteries. Adv. Mater 2020, 32 (5), e1902779. [DOI] [PubMed] [Google Scholar]
- (197).Sun H; Zhang Y; Zhang J; Sun X; Peng H Energy harvesting and storage in 1D devices. Nature Reviews Materials 2017, 2 (6), 1 DOI: 10.1038/natrevmats.2017.23. [DOI] [Google Scholar]
- (198).Li X; Tang Y; Lv H; Wang W; Mo F; Liang G; Zhi C; Li H Recent advances in flexible aqueous zinc-based rechargeable batteries. Nanoscale 2019, 11 (39), 17992–18008. [DOI] [PubMed] [Google Scholar]
- (199).Xu S; Zhang Y; Cho J; Lee J; Huang X; Jia L; Fan JA; Su Y; Su J; Zhang H; Cheng H; Lu B; Yu C; Chuang C; Kim TI; Song T; Shigeta K; Kang S; Dagdeviren C; Petrov I; Braun PV; Huang Y; Paik U; Rogers JA Stretchable batteries with self-similar serpentine interconnects and integrated wireless recharging systems. Nat. Commun 2013, 4, 1543. [DOI] [PubMed] [Google Scholar]
- (200).Weng W; Sun Q; Zhang Y; He S; Wu Q; Deng J; Fang X; Guan G; Ren J; Peng H A gum-like lithium-ion battery based on a novel arched structure. Adv. Mater 2015, 27 (8), 1363–9. [DOI] [PubMed] [Google Scholar]
- (201).Shi C; Wang T; Liao X; Qie B; Yang P; Chen M; Wang X; Srinivasan A; Cheng Q; Ye Q; Li AC; Chen X; Yang Y Accordion-like stretchable Li-ion batteries with high energy density. Energy Storage Materials 2019, 17, 136–142. [Google Scholar]
- (202).Liu W; Chen J; Chen Z; Liu K; Zhou G; Sun Y; Song M-S; Bao Z; Cui Y Stretchable Lithium-Ion Batteries Enabled by Device-Scaled Wavy Structure and Elastic-Sticky Separator. Adv. Energy Mater 2017, 7 (21), 1701076. [Google Scholar]
- (203).Zhu H-W; Ge J; Peng Y-C; Zhao H-Y; Shi L-A; Yu S-H Dip-coating processed sponge-based electrodes for stretchable Zn-MnO2 batteries. Nano Res 2018, 11 (3), 1554–1562. [Google Scholar]
- (204).Song W-J; Park J; Kim DH; Bae S; Kwak M-J; Shin M; Kim S; Choi S; Jang J-H; Shin TJ; Kim SY; Seo K; Park S Jabuticaba-Inspired Hybrid Carbon Filler/Polymer Electrode for Use in Highly Stretchable Aqueous Li-Ion Batteries. Adv. Energy Mater 2018, 8 (10), 1702478. [Google Scholar]
- (205).Shin M; Song W-J; Son HB; Yoo S; Kim S; Song G; Choi N-S; Park S Highly Stretchable Separator Membrane for Deformable Energy-Storage Devices. Adv. Energy Mater 2018, 8 (23), 1801025. [Google Scholar]
- (206).Kang S; Hong SY; Kim N; Oh J; Park M; Chung KY; Lee SS; Lee J; Son JG Stretchable Lithium-Ion Battery Based on Re-entrant Micro-honeycomb Electrodes and Cross-Linked Gel Electrolyte. ACS Nano 2020, 14 (3), 3660–3668. [DOI] [PubMed] [Google Scholar]
- (207).Mackanic DG; Yan X; Zhang Q; Matsuhisa N; Yu Z; Jiang Y; Manika T; Lopez J; Yan H; Liu K; Chen X; Cui Y; Bao Z Decoupling of mechanical properties and ionic conductivity in supramolecular lithium ion conductors. Nat. Commun 2019, 10 (1), 5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (208).Lopez J; Sun Y; Mackanic DG; Lee M; Foudeh AM; Song MS; Cui Y; Bao Z A Dual-Crosslinking Design for Resilient Lithium-Ion Conductors. Adv. Mater 2018, 30 (43), e1804142. [DOI] [PubMed] [Google Scholar]
- (209).Guo X; Zhang L; Ding Y; Goodenough JB; Yu G Room-temperature liquid metal and alloy systems for energy storage applications. Energy Environ. Sci 2019, 12 (9), 2605–2619. [Google Scholar]
- (210).Ding Y; Guo X; Qian Y; Gao H; Weber DH; Zhang L; Goodenough JB; Yu G In Situ Formation of Liquid Metals via Galvanic Replacement Reaction to Build Dendrite-Free Alkali-Metal-Ion Batteries. Angew. Chem., Int. Ed 2020, 59 (29), 12170–12177. [DOI] [PubMed] [Google Scholar]
- (211).Wang C; He T; Cheng J; Guan Q; Wang B Bioinspired Interface Design of Sewable, Weavable, and Washable Fiber Zinc Batteries for Wearable Power Textiles. Adv. Funct. Mater 2020, 30 (42), 2004430. [Google Scholar]
- (212).Rwei AY; Lu W; Wu C; Human K; Suen E; Franklin D; Fabiani M; Gratton G; Xie Z; Deng Y; Kwak SS; Li L; Gu C; Liu A; Rand CM; Stewart TM; Huang Y; Weese-Mayer DE; Rogers JA A wireless, skin-interfaced biosensor for cerebral hemodynamic monitoring in pediatric care. Proc. Natl. Acad. Sci. U. S. A 2020, 117 (50), 31674–31684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (213).Wang C; He T; Cheng J; Guan Q; Wang B Bioinspired Interface Design of Sewable, Weavable, and Washable Fiber Zinc Batteries for Wearable Power Textiles. Adv. Funct. Mater 2020, 30 (42), 2004430. [Google Scholar]
- (214).Fu KK; Cheng J; Li T; Hu L Flexible Batteries: From Mechanics to Devices. ACS Energy Letters 2016, 1 (5), 1065–1079. [Google Scholar]
- (215).Mackanic DG; Yan X; Zhang Q; Matsuhisa N; Yu Z; Jiang Y; Manika T; Lopez J; Yan H; Liu K; Chen X; Cui Y; Bao Z Decoupling of mechanical properties and ionic conductivity in supramolecular lithium ion conductors. Nat. Commun 2019, 10 (1), 5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (216).Kang S; Hong SY; Kim N; Oh J; Park M; Chung KY; Lee S-S; Lee J; Son JG Stretchable Lithium-Ion Battery Based on Re-entrant Micro-honeycomb Electrodes and Cross-Linked Gel Electrolyte. ACS Nano 2020, 14 (3), 3660–3668. [DOI] [PubMed] [Google Scholar]
- (217).Qiu Y; Li G; Hou Y; Pan Z; Li H; Li W; Liu M; Ye F; Yang X; Zhang Y Vertically Aligned Carbon Nanotubes on Carbon Nanofibers: A Hierarchical Three-Dimensional Carbon Nanostructure for High-Energy Flexible Supercapacitors. Chem. Mater 2015, 27 (4), 1194–1200. [Google Scholar]
- (218).Zhou D; Wang F; Zhao X; Yang J; Lu H; Lin LY; Fan LZ Self-Chargeable Flexible Solid-State Supercapacitors for Wearable Electronics. ACS Appl. Mater. Interfaces 2020, 12 (40), 44883–44891. [DOI] [PubMed] [Google Scholar]
- (219).Pu X; Li L; Liu M; Jiang C; Du C; Zhao Z; Hu W; Wang ZL Wearable Self-Charging Power Textile Based on Flexible Yarn Supercapacitors and Fabric Nanogenerators. Adv. Mater 2016, 28 (1), 98–105. [DOI] [PubMed] [Google Scholar]
- (220).Thekkekara LV; Gu M Bioinspired fractal electrodes for solar energy storages. Sci. Rep 2017, 7, 45585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (221).O’Connor TF; Zaretski AV; Savagatrup S; Printz AD; Wilkes CD; Diaz MI; Sawyer EJ; Lipomi DJ Wearable organic solar cells with high cyclic bending stability: Materials selection criteria. Sol. Energy Mater. Sol. Cells 2016, 144, 438–444. [Google Scholar]
- (222).Jia W; Wang X; Imani S; Bandodkar AJ; Ramírez J; Mercier PP; Wang J Wearable textile biofuel cells for powering electronics. J. Mater. Chem. A 2014, 2 (43), 18184–18189. [Google Scholar]
- (223).Jia W; Valdes-Ramirez G; Bandodkar AJ; Windmiller JR; Wang J Epidermal biofuel cells: energy harvesting from human perspiration. Angew. Chem., Int. Ed 2013, 52 (28), 7233–6. [DOI] [PubMed] [Google Scholar]
- (224).Yu Z; Tetard L; Zhai L; Thomas J Supercapacitor electrode materials: nanostructures from 0 to 3 dimensions. Energy Environ. Sci 2015, 8 (3), 702–730. [Google Scholar]
- (225).Yu J; Lu W; Smith JP; Booksh KS; Meng L; Huang Y; Li Q; Byun J-H; Oh Y; Yan Y; Chou T-W A High Performance Stretchable Asymmetric Fiber-Shaped Supercapacitor with a Core-Sheath Helical Structure. Adv. Energy Mater 2017, 7 (3), 1600976. [Google Scholar]
- (226).Nam I; Kim G-P; Park S; Han JW; Yi J All-solid-state, origami-type foldable supercapacitor chips with integrated series circuit analogues. Energy Environ. Sci 2014, 7 (3), 1095. [Google Scholar]
- (227).Lv Z; Tang Y; Zhu Z; Wei J; Li W; Xia H; Jiang Y; Liu Z; Luo Y; Ge X; Zhang Y; Wang R; Zhang W; Loh XJ; Chen X Honeycomb-Lantern-Inspired 3D Stretchable Supercapacitors with Enhanced Specific Areal Capacitance. Adv. Mater 2018, 30 (50), e1805468. [DOI] [PubMed] [Google Scholar]
- (228).Li M; Zu M; Yu J; Cheng H; Li Q Stretchable Fiber Supercapacitors with High Volumetric Performance Based on Buckled MnO2 /Oxidized Carbon Nanotube Fiber Electrodes. Small 2017, 13 (12), 1602994. [DOI] [PubMed] [Google Scholar]
- (229).Jiao S; Zhou A; Wu M; Hu H Kirigami Patterning of MXene/Bacterial Cellulose Composite Paper for All-Solid-State Stretchable Micro-Supercapacitor Arrays. Adv. Sci. (Weinh) 2019, 6 (12), 1900529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (230).Gu T; Wei B High-performance all-solid-state asymmetric stretchable supercapacitors based on wrinkled MnO2/CNT and Fe2O3/CNT macrofilms. J. Mater. Chem. A 2016, 4 (31), 12289–12295. [Google Scholar]
- (231).Chang TH; Zhang T; Yang H; Li K; Tian Y; Lee JY; Chen PY Controlled Crumpling of Two-Dimensional Titanium Carbide (MXene) for Highly Stretchable, Bendable, Efficient Supercapacitors. ACS Nano 2018, 12 (8), 8048–8059. [DOI] [PubMed] [Google Scholar]
- (232).Poonam; Sharma K; Arora A; Tripathi SK Review of supercapacitors: Materials and devices. Journal of Energy Storage 2019, 21, 801–825. [Google Scholar]
- (233).Krishnamoorthy K; Pazhamalai P; Mariappan VK; Nardekar SS; Sahoo S; Kim SJ Probing the energy conversion process in piezoelectric-driven electrochemical self-charging supercapacitor power cell using piezoelectrochemical spectroscopy. Nat. Commun 2020, 11 (1), 2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (234).Wang Q; Yan J; Fan Z Carbon materials for high volumetric performance supercapacitors: design, progress, challenges and opportunities. Energy Environ. Sci 2016, 9 (3), 729–762. [Google Scholar]
- (235).Xiong G; He P; Lyu Z; Chen T; Huang B; Chen L; Fisher TS Bioinspired leaves-on-branchlet hybrid carbon nanostructure for supercapacitors. Nat. Commun 2018, 9 (1), 1 DOI: 10.1038/s41467-018-03112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (236).Chung HU; Rwei AY; Hourlier-Fargette A; Xu S; Lee K; Dunne EC; Xie Z; Liu C; Carlini A; Kim DH; Ryu D; Kulikova E; Cao J; Odland IC; Fields KB; Hopkins B; Banks A; Ogle C; Grande D; Park JB; Kim J; Irie M; Jang H; Lee J; Park Y; Kim J; Jo HH; Hahm H; Avila R; Xu Y; Namkoong M; Kwak JW; Suen E; Paulus MA; Kim RJ; Parsons BV; Human KA; Kim SS; Patel M; Reuther W; Kim HS; Lee SH; Leedle JD; Yun Y; Rigali S; Son T; Jung I; Arafa H; Soundararajan VR; Ollech A; Shukla A; Bradley A; Schau M; Rand CM; Marsillio LE; Harris ZL; Huang Y; Hamvas A; Paller AS; Weese-Mayer DE; Lee JY; Rogers JA Skin-interfaced biosensors for advanced wireless physiological monitoring in neonatal and pediatric intensive-care units. Nat. Med 2020, 26 (3), 418–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (237).Park Y; Kwon K; Kwak SS; Kwak JW; Luan H; Chung TS; San Chun K; Kim JU; Jang H; Ryu H Wireless, skin-interfaced sensors for compression therapy. Science Advances 2020, 6 (49), eabe1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (238).Zhang Z; He T; Zhu M; Sun Z; Shi Q; Zhu J; Dong B; Yuce MR; Lee C Deep learning-enabled triboelectric smart socks for IoT-based gait analysis and VR applications. npj Flexible Electronics 2020, 4 (1), 1 DOI: 10.1038/s41528-020-00092-7. [DOI] [Google Scholar]
- (239).Seshadri DR; Li RT; Voos JE; Rowbottom JR; Alfes CM; Zorman CA; Drummond CK Wearable sensors for monitoring the internal and external workload of the athlete. NPJ. Digit Med 2019, 2, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (240).Tian X; Lee PM; Tan YJ; Wu TLY; Yao H; Zhang M; Li Z; Ng KA; Tee BCK; Ho JS Wireless body sensor networks based on metamaterial textiles. Nature Electronics 2019, 2 (6), 243–251. [Google Scholar]
- (241).Lin R; Kim HJ; Achavananthadith S; Kurt SA; Tan SCC; Yao H; Tee BCK; Lee JKW; Ho JS Wireless battery-free body sensor networks using near-field-enabled clothing. Nat. Commun 2020, 11 (1), 444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (242).Zulqarnain M; Stanzione S; Rathinavel G; Smout S; Willegems M; Myny K; Cantatore E A flexible ECG patch compatible with NFC RF communication. npj Flexible Electronics 2020, 4 (1), 1 DOI: 10.1038/s41528-020-0077-x. [DOI] [Google Scholar]
- (243).Madhvapathy SR; Wang H; Kong J; Zhang M; Lee JY; Park JB; Jang H; Xie Z; Cao J; Avila R Reliable, low-cost, fully integrated hydration sensors for monitoring and diagnosis of inflammatory skin diseases in any environment. Science Advances 2020, 6 (49), eabd7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (244).Song EY; Lee KB IEEE 1451.5 standard-based wireless sensor networks. In Advances in wireless sensors and sensor networks; Springer: 2010; pp 243–271. [Google Scholar]
- (245).Liang T; Yuan Y Wearable medical monitoring systems based on wireless networks: A Review. IEEE Sens. J 2016, 1–1. [Google Scholar]
- (246).Wang M; Chen Q; Li H; Ma M; Zhang N Stretchable and Shelf-Stable All-Polymer Supercapacitors Based on Sealed Conductive Hydrogels. ACS Applied Energy Materials 2020, 3 (9), 8850–8857. [Google Scholar]
- (247).Zhu T; Jiang C; Wang M; Zhu C; Zhao N; Xu J Skin-Inspired Double-Hydrophobic-Coating Encapsulated Hydrogels with Enhanced Water Retention Capacity. Adv. Funct. Mater 2021, 31 (27), 2102433. [Google Scholar]
- (248).Boselli L; Pomili T; Donati P; Pompa PP Nanosensors for Visual Detection of Glucose in Biofluids: Are We Ready for Instrument-Free Home-Testing? Materials 2021, 14 (8), 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


