Abstract
Circadian clocks drive daily rhythms of physiology and behavior in multiple organisms and synchronize these rhythms to environmental cycles of light and temperature. The basic mechanism of the clock consists of a transcription-translation feedback loop, in which key clock proteins negatively regulate their own transcription. Although much of the focus with respect to clock mechanisms has been on the regulation of transcription and on the stability and activity of clock proteins, it is clear that other regulatory processes also have to be involved to explain aspects of clock function. Here, we review the role of alternative splicing in circadian clocks. Starting with a discussion of the Drosophila clock and then extending to other major circadian model systems, we describe how the control of alternative splicing enables organisms to maintain their circadian clocks as well as to respond to environmental inputs, in particular to temperature changes.
Keywords: clock genes, splicing, Drosophila, feedback loop, temperature entrainment
INTRODUCTION TO CIRCADIAN CLOCKS
A rotation of planet Earth around its axis takes 24 h and generates predictable changes in light and temperature. In the course of evolution, most organisms developed mechanisms, called circadian rhythms, not only to respond to these daily changes but also to effectively anticipate them. Circadian rhythms are generated by endogenous molecular clocks that drive daily cycles in behavior, metabolism, and physiology. Circadian clocks entrain to environmental cycles, pre-dominantly light and temperature, and maintain rhythms when released into constant conditions (free-run).
While circadian clocks have most likely evolved independently in different lineages, eukaryotic clocks share a similar structural framework (Rosbash, 2009): an autoregulatory transcription-translation feedback loop (TTFL; Fig. 1). At its most basic level, the TTFL consists of “positive” elements that drive the expression of “negative” elements, which feed back onto the positive elements to inhibit their own expression. The positive elements (transcriptional activators) also drive expression of a significant proportion of the transcriptome, so their inhibition by the negative elements in a time-of-day–specific manner generates circadian cycles of gene expression. For this clock system to sustain itself and to set the period of rhythmic oscillations at ~24 h, nontranscriptional mechanisms that tune the pace of the clock are required. While the basic plan of the molecular mechanism that underlies daily rhythm generation has been established, key aspects of how the clock is sustained remain unclear.
Figure 1. Conserved structure of the transcription-translation feedback loop (TTFL).

The circadian TTFL consists of PER-ARNT-SIM (PAS) domain transcription factors (TFs) that control the expression of clock proteins. The clock proteins accumulate in a regulated manner to repress TF-mediated expression. Positive regulation is depicted in red; negative regulation is shown in blue (color online). Core clock components in fungi (Neurospora), plants (Arabidopsis), insects (Drosophila), and mammals are shown.
CIRCADIAN CLOCKS IN DROSOPHILA MELANOGASTER
Studies in Drosophila have been instrumental in uncovering the molecular basis of circadian rhythms (reviewed in Sehgal, 2017). Here, we provide an overview of circadian clocks in fruit flies and highlight the importance of delays in the circadian TTFL.
PERIOD (PER) and TIMELESS (TIM) are the negative elements that inhibit CLOCK (CLK) and CYCLE (CYC) transcription factors, the corresponding positive clock elements in the TTFL in Drosophila. CLK/CYC-mediated expression of per and tim peaks in the early night. Relative to the mRNAs peak, accumulation of PER and TIM proteins is delayed by ~6 h. In the mid to late night, PER and TIM change their localization from cytoplasmic to predominantly nuclear. Once in the nucleus, PER/TIM repress CLK/CYC activity and decrease per and tim expression. Degradation of TIM in the morning by light destabilizes PER, contributing to its degradation in the nucleus and resetting the TTFL (reviewed in Dubowy and Sehgal, 2017; Zheng and Sehgal, 2012; Fig. 1). Light input is sensed both cell autonomously through the flavin-based photoreceptor CRYPTOCHROME (CRY) and through the visual system (Stanewsky et al., 1998). CRY interacts with TIM and promotes its degradation by an F-box protein JETLAG (JET; Naidoo et al., 1999; Ceriani et al., 1999; Koh et al., 2006).
To prevent circadian molecular oscillators from reaching equilibrium, temporal delays are necessary to separate the phase of active transcription from the phase of transcriptional repression. Thus, both PER and TIM need to be dynamically regulated on multiple posttranscriptional levels to maintain circadian rhythms and to pace the clock so that it runs with ~24-h periodicity. The role of posttranslational regulation of PER and TIM has, by far, received the most attention. Diurnal profiles of PER and TIM, as observed on western blots, slow dramatic shifts in protein weight, indicating the circadian nature of posttranslational modifications (Edery et al., 1994; Zeng et al., 1996). A concept of “phospho-timer” was coined to reflect the role of phosphorylation in setting the pace of the circadian clock (reviewed in Duvall and Taghert, 2011). Phosphorylation of PER and TIM controls their stability, nuclear accumulation, and repressor activity (reviewed in Zheng and Sehgal, 2012). Mutations in kinases and phosphatases that act on clock proteins, or directly in the clock protein phosphorylation sites, can lead to dramatic changes in circadian behavior (Price et al., 1998; Martinek et al., 2001; Lin et al., 2002; Akten et al., 2003; Fang et al., 2007; Sathyanarayanan et al., 2004; Kivimäe et al., 2008; Chiu et al., 2011; Garbe et al., 2013; Top et al., 2016; Top et al., 2018).
REGULATION OF RNA IN THE DROSOPHILA CIRCADIAN CLOCK
A comparison of nascent RNA transcripts and steady-state mRNA profiles around the clock indicates more robust steady-state cycling compared with the transcriptional cycling for a large number of rhythmic genes in Drosophila (Rodriguez et al., 2013). This finding, along with the previous reports that demonstrated a delay between per and tim transcription and their mRNA accumulation (So and Rosbash, 1997), suggests that posttranscriptional regulation is important, likely even to introduce a delay in the circadian clock. Molecular mechanisms that act at the level of mRNA splicing, stabilization, and translation contribute to the ~24-h periodicity of the clock (reviewed in Kojima et al., 2011; Bartok et al., 2013).
Translational control contributes to setting PER levels in the pigment-dispersing factor–positive circadian neurons that drive rest:activity rhythms under free-running conditions in Drosophila (Lim et al., 2011; Bradley et al., 2012; Lim and Allada, 2013; Zhang et al., 2013; Lee et al., 2017). PER translation is enhanced by an atypical translation factor NAT1, as well as a complex of the RNA-binding protein ATAXIN2 (ATX2) with a protein called TWENTY FOUR (TYF) and LIKE-SM 12 (LSM12; Bradley et al., 2012; Lim et al., 2011; Lim and Allada, 2013; Zhang et al., 2013; Lee et al., 2017). Notably, loss-of-function mutations or downregulation of these translational regulators (tyf, atx2, nat1, or lsm12) causes long and weak rest:activity rhythms under free-running conditions. Whether translational regulation of core clock components other than PER is necessary remains an open question.
TIM protein can regulate per RNA through a mechanism that is still largely unclear (Suri et al., 1999). Heat-shock–mediated induction of TIM in a tim0 mutant background leads to an increase in PER, supposedly via posttranscriptional mechanisms that increase per mRNA levels (Suri et al., 1999). Interestingly, the TIM-regulated per mRNA increase requires PER protein, indicating a potential involvement of PER-TIM heterodimers. While this mechanism requires further investigation, one hypothesis is that TIM and PER interact with posttranscriptional regulators (discussed above) to form a positive feedback loop that sustains PER protein accumulation.
BRIEF OVERVIEW OF ALTERNATIVE SPLICING
On the most basic level, pre-mRNA splicing ensures removal of intronic sequences from newly transcribed mRNA sequences. Alternative splicing mechanisms enhance the complexity and scale of the eukaryotic transcriptome by allowing for selective intron retention and exon reshuffling. The major spliceosome consists of 5 small nuclear ribonucleoprotein complexes (snRNPs; U1, U2, U4, U5, and U6) and more than 150 additional regulatory proteins associated with snRNPs. First, 5ʹ and 3ʹ splice sites that frame introns are recognized by the U1 and U2 snRNP, respectively. This initial event triggers intron excision and exon ligation by U4, U5, and U6 snRNP. Auxiliary splicing factors, such as Serine Arginine Rich (SR) proteins and heterogeneous nuclear ribonucleoproteins (hnRNPs) act on cis-regulatory elements (exonic and intronic enhancers and silencers) to provide an additional layer of control (reviewed in Wahl et al., 2009). Typically, alternative splicing is thought of as a highly regulated process, but it is possible that retention of an intron sometimes represents inefficient processing, which could be the case in some of the examples described below.
ALTERNATIVE SPLICING IN DROSOPHILA
Splicing of per
Splicing of an intron (also known as dmpi8) in the 3ʹ-UTR of per is one of the most extensively studied alternative splicing events in the circadian field (Cheng et al., 1998; Majercak et al., 1999). Mutations that lead to dmpi8 retention were reported to decrease per mRNA and delay PER protein accumulation (Cheng et al., 1998). In wild-type flies, splicing of dmpi8 is regulated by multiple modalities including the endogenous circadian clock, light, and temperature (Majercak et al., 2004; Collins et al., 2004), such that dmpi8 splicing is increased by shorter photoperiods and colder temperatures. According to Cheng et al. (1998), protein levels accumulate earlier under these conditions, which accounts for an advanced phase of the rhythm. On the other hand, higher dmpi8 retention is associated with hotter days and longer photoperiods; it delays the onset of evening activity and allows flies to have a mid-day “siesta,” a likely adaptation against desiccation during hot summer days. However, a second study indicated that mutant flies that either permanently retain or splice dmpi8 have similar phenotypes of delayed PER accumulation and delayed cold-induced phase advance in behavior, as compared with the flies expressing the wild-type per construct (Majercak et al., 1999). Majercak and colleagues argue that it is not the outcome of the splicing choice at dmpi8 that influences PER levels but the active splicing process itself that is necessary for appropriate expression.
Although splicing of dmpi8 was first described in the laboratory setting (Cheng et al., 1998), it was later shown to occur under natural conditions as well (Montelli et al., 2015). Different Drosophila species have different polymorphisms that modify the strength of the dmpi8 splice site to affect splicing (Low et al., 2008). Interestingly, 2 Drosophila species, Drosophila yakuba and Drosophila santomea, that inhabit equatorial regions with limited temperature fluctuation (particularly in the cold range), do not display temperature-sensitive splicing of dmpi8. On the other hand, in species that colonized temperate climates (for example, in Drosophila melanogaster), weak splice sites at the dmpi8 locus might promote temperature sensitivity in splicing, which likely evolved as an adaptation to seasonal weather changes (Low et al., 2008).
The molecular machinery that regulates splicing of dmpi8 has not been conclusively established. A recent report implicated SR-related matrix protein of 160 kDa (SRm160), an alternative splicing regulator, in circadian rhythm maintenance, potentially via per splicing (Beckwith et al., 2017). However, this study did not demonstrate altered splicing of dmpi8 in SRm160 mutants. B52/SRp55 is yet another splicing factor that regulates dmpi8 splicing. Downregulation of B52 decreases the splicing efficiency of dmpi8 in Drosophila embryonic cell culture (S2) and in vivo (Zhang et al., 2018).
Splicing of tim
tim has 9 predicted transcripts (Flybase). Thus, compared with per with its 2 transcripts (Cheng et al., 1998), tim is a major target for alternative splicing mechanisms; nevertheless, until recently, the splicing of tim was largely overlooked by the Drosophila scientific community. The first splicing event described in tim was the retention of the last (~850 bp) intron that generates the tim-cold transcript (Boothroyd et al., 2007). As the name indicates, retention of this intron occurs predominantly at lower temperatures, and because of a stop codon in the retained intron, an isoform 3.5 kDa smaller than full-length TIM is produced. Despite the identification of this smaller isoform, however, very limited insights have been provided into its function. Mechanistic analysis of tim-cold assessed its role in combination with the ls-tim or s-tim alleles (Montelli et al., 2015). ls-tim and s-tim alleles differ in their usage of alternative translation initiation sites to generate either the long and short isoforms (ls-TIM) or only the shorter (s-TIM) isoform, with functional differences between the isoforms reflected in their differential sensitivity to light (Rosato et al., 1997; Tauber et al., 2007). According to Montelli and colleagues (2015), retention of the tim-cold intron increases affinity of TIM for CRY but not for PER. The strength of this affinity is further increased if tim-cold retention is paired with the s-TIM variant.
Three recent works, including one from our lab, bring tim splicing into the spotlight and implicate distinct splicing events in clock responses to temperature changes as well as in the fundamental mechanisms of circadian clock maintenance (Shakhmantsir et al., 2018; Foley et al., 2018; Evantal et al., 2018). In our recent work, we reveal a new mechanism for circadian clock delays that relies on alternative splicing of tim (Shakhmantsir et al., 2018). Alternative splicing of the intron that we call tim-tiny regulates the daily profile of TIM protein accumulation in constant darkness after entrainment to light:dark cycles as well as under temperature cycles. The retention of tim-tiny produces a truncated TIM protein that is likely unstable as it is undetectable by western blot analysis. tim-tiny transcripts constitute a larger proportion of the tim mRNA pool when the RNA first starts to accumulate in a daily cycle, thereby reducing the expression of TIM protein at this point. Thus, retention of tim-tiny contributes to the delay between the expression of tim mRNA and the accumulation of TIM protein, which in turn delays accumulation of PER as PER requires TIM for stability. We also report that Pre-mRNA Processing factor 4 (PRP4), a conserved component of the spliceosomal U4/U6.U5 triple small nuclear ribonucleoprotein (tri-snRNP) complex, is a regulator of tim-tiny splicing and is necessary to maintain robust rhythms and the 24-h period of the circadian clock. While tim-tiny splicing likely accounts for some of the prp4 knockdown phenotype, given the modest period lengthening effect of tim-tiny retention (~2 h), it cannot fully explain the period phenotype of prp4 knockdown (~3–6 h, depending on the RNAi line). A few explanations could account for this discrepancy. prp4 knockdown affects splicing of multiple targets, which could potentially feed into the circadian clock and regulate its speed and rhythmicity. Alternatively, prp4 knockdown affects the levels of multiple transcripts (without affecting their splicing in a significant way) that could also exacerbate the phenotype of tim-tiny retention.
A recent study by Evantal and colleagues (2018) focused on tim splicing, with particular emphasis on its temperature dependence. Evantal et al. analyzed 4 major RNA isoforms from the tim locus: tim-cold, tim-short&cold (tim-sc), tim-Medium (tim-M), and tim-L (full length). In addition to full-length tim and the previously described tim-cold isoform, the study highlights the previously uncharacterized tim-short&cold (tim-sc) isoform and a tim-Medium (tim-M) isoform, which corresponds to the tim-tiny isoform described by us (Shakhmantsir et al., 2018). In terms of temperature dependence, colder temperatures (18 °C) increase the production of both tim-cold and tim-sc isoforms, while hot temperatures (29 °C) increase tim-M (consistent with our findings). Temperature sensitivity of tim splicing is independent of the circadian clock and persists in cell culture, which suggests that tim intronic sequences have in-built temperature sensors or that tim can interact with temperature sensors in cultured cells. Therefore, in fruit flies, tim may mediate not only light input into the circadian clock but perhaps also temperature signals that can further adjust circadian behavior and physiology.
A study to identify RNA-binding and RNA-associated proteins that regulate circadian clocks led to the discovery of P-element somatic inhibitor (PSI), an alternative splicing regulator (Foley et al., 2018). Downregulation of psi shortens circadian period in free-running conditions and affects tim splicing. While PSI has several targets in addition to tim (cwo for example), the authors conclude that the circadian period phenotype of psi knockdown is tim-dependent. This conclusion is based on lack of a period-shortening effect on psi knockdown in tim-null flies rescued with a largely intronless tim transgenic construct.
psi knockdown leads to a selective increase in tim-cold and tim-sc transcripts, 2 of the transcripts enriched under cold exposure (Evantal et al., 2018). Conversely, tim-M (or tim-tiny), a transcript that is highest at hot temperatures, is decreased upon psi knockdown. These observations imply a role for PSI in setting the tim splicing profile prevalent at warm temperatures. How PSI selectively affects some splicing events over others remains to be determined. It is possible that PSI has a preferential binding affinity to some tim splicing motifs over others or that it selectively interacts with other splicing factors that discriminate between tim splicing events. While PSI regulates the relative ratio of tim isoforms, it does not affect the temperature sensitivity of tim splicing; in other words, despite their relative overall levels, the temperature-regulated splicing events still change with temperature (Foley et al., 2018). Given the findings of Evantal and colleagues (2018), the most parsimonious explanation is that the splice site strength and/or the pre-mRNA structure of tim transcripts enables temperature-dependent splicing sensitivity.
A common theme that begins to arise is that the shift to noncanonical isoforms of tim provides a way to regulate full-length TIM protein levels. For example, at lower temperature (18 °C), TIM levels are decreased independently of changes in total tim mRNA levels (i.e., in tim transcription). Therefore, as proposed by Evantal and colleagues (2018), the cold temperature–dependent shift in tim splicing to tim-sc and tim-cold spliceoforms could be a mechanism to affect overall TIM levels. Similarly, tim-tiny retention controls the rate of TIM accumulation to set the total TIM levels (Shakhmantsir et al., 2018). Recently, scaling of the amplitude of TIM oscillations was proposed as one of the steps contributing to the robustness of temperature compensation in the Drosophila circadian clock (Kidd et al., 2015). Thus, it is possible that temperature-modulated splicing mechanisms of tim are important for temperature compensation of the clock. In the future, it would be worth dissecting the physiologic contribution of each of the 9 different tim isoforms to maintaining fundamental clock features such as rhythmicity and temperature compensation.
Heterogeneity of Splicing in the Drosophila Brain
Heterogeneity in splicing across cells and tissues is yet another important aspect of alternative splicing that has been recently brought to light in the context of circadian neurons in Drosophila (Wang et al., 2018). RNA sequencing of transcripts isolated from different circadian clock neurons revealed a striking difference in the expression of various spliceoforms. The expression of splicing factors also differed between circadian neuronal clusters, which could account for cell-specific heterogeneity in the splicing of clock transcripts in the Drosophila brain (Wang et al., 2018).
ALTERNATIVE SPLICING IN PLANTS
Brief Overview of Circadian Clocks in Plants (Arabidopsis thaliana)
The structure of plant circadian clocks is quite complex and involves multiple clock components expressed at different times of the day, forming multiple interconnected TTFLs (Fig. 1). LATE ELONGATED HYPOCOTYL and CIRCADIAN CLOCK ASSOCIATED 1 (LHY and CCA1, respectively) are MYB domain-containing transcription factors that interact to repress TIMING OF CAB EXPRESSION 1 (TOC1). As LHY and CCA1 levels decrease around dusk, TOC1 gets expressed, ultimately repressing LHY and CCA1 expression at the end of the day. There are 3 homologs of TOC1, PSEUDO-RESPONSE REGULATOR 9 (PRR9), PRR7 AND PRR5 that can also repress LHY and CCA1 transcription (reviewed in Sanchez and Kay, 2016).
Alternative Splicing in Arabidopsis
The transcripts of most Arabidopsis core clock genes, including LHY, CCA1, TOC1, PRR5, PRR7, and PRR9 get alternatively spliced (Filichkin et al., 2010; Sanchez et al., 2010; James et al., 2012; Kwan et al., 2014). Alternative splicing of clock transcripts, LHY and CCA1 in particular, has been heavily studied in the context of environmental regulation, such as temperature changes (James et al., 2012). Colder temperatures (in the 4–12 °C range) lead to the retention of the “long” intron in LHY yet promote the splicing of the fourth intron in CCA1 (James et al., 2012). While the consequences of these splicing events differ (discussed below), it is clear that pre-mRNA splicing allows plants to rapidly shift their clock protein profiles to adjust to environmental stressors, such as cold.
Alternative Splicing Coupled to Nonsense-Mediated Decay in Plants
In plants, many clock genes adjust their transcript and corresponding protein levels via alternative splicing followed by the nonsense-mediated decay (NMD). LHY exhibits retention of the “long” intron 5 under colder temperatures, which results in a premature termination codon (PTC) and likely leads to NMD of the RNA (James et al., 2012). This type of intron retention can reach 20% of the total LHY transcripts at 4°C at dawn and could be important for temperature adaptation (James et al., 2012). Another example of nonproductive splicing that is induced by the reduction in temperature is the skipping of exon 4 and the retention of intron 3 in PRR7, both of which introduce PTCs. Skipping of exon 4 in PRR7 can lead to NMD (James et al., 2012). The importance of such a cold-induced decrease of LHY and PRR7 mRNA expression, which likely results in decreased LHY and PRR7 proteins, is unclear. One could speculate that a decrease in LHY is offset by increased CCA1 levels (see below). While LHY and CCA1 are proposed to be partially redundant, a shift from LHY-CCA1 heterodimers to CCA1 homodimers under cold temperature exposure could be a potential mechanism to ensure clock robustness in response to environmental temperature changes. Future studies are necessary to test this hypothesis.
Dominant Negative Role of Alternatively Spliced Transcripts in Plants
While most alternatively spliced transcripts contain PTCs that can trigger NMD, some transcripts, those with retained introns in particular, can escape the NMD to generate proteins. Such truncated proteins can interfere with the function of their normally spliced protein counterparts, usually in a dominant negative way. An illustrative example of such regulation is the retention of a fourth intron in CCA1 that results in the production of the CCA1-β isoform (Seo et al., 2012). This isoform is similar to the full-length functional CCA1-α isoform but lacks the MYB DNA binding motif and so acts as a dominant negative inhibitor of the normal function of CCA1-α. On a mechanistic level, CCA1-β effectively binds to CCA1-α and LHY to form dimers, sequestering CCA1-α and LHY and preventing their binding to DNA.
The retention of the fourth intron in CCA1, which is conserved in at least 4 plant species, also allows for regulation of CCA1-α activity by temperature (James et al., 2012; Filichkin et al., 2010). Cold temperatures prevent retention of intron 4, thereby decreasing CCA1-β levels and increasing CCA1-α activity. Because CCA1-α is known to induce expression of C-repeat binding factor genes that are necessary for cold acclimation, the regulation of CCA1 splicing promotes tolerance under freezing conditions.
Discovery of Splicing Factors That Regulate Clocks in Plants
Identification of splicing factors that control circadian rhythms has been a prolific area of exploration in Arabidopsis (Sanchez et al., 2010; Jones et al., 2012; Wang et al., 2012; Schlaen et al., 2015; Marshall et al., 2016; Table 1). Notably, mutations in splicing factors or their upstream regulators affect circadian rhythms, generally by lengthening the free-running period. This is the case for PRMT5, an arginine methyltransferase that methylates Sm and LSm spliceosomal proteins (Sanchez et al., 2010). A hypomorphic mutation in an SM-like (LSM) gene, LSM5, a core component of the U6 snRNP, also lengthens the circadian period in Arabidopsis (Perez-Santangelo et al., 2014). A mutation in SNW/Ski-interacting protein (SKIP), another spliceosome component, not only lengthens the circadian period but also affects the light sensitivity of the plant clock (Wang et al., 2012). In addition, SPLICEOSOMAL TIMEKEEPER LOCUS1 (STIPL1), a putative RNA binding protein involved in spliceosomal disassembly, regulates the length of circadian period (Jones et al., 2012).
Table 1. Alternative splicing in clock transcripts is affected by multiple splicing factor/regulator mutations in Arabidopsis.
| Splicing Factor or Splicing Regulator | Clock Phenotype Associated with Mutation | Alternatively Spliced Core Clock Transcripts | Citation |
|---|---|---|---|
| PRMT5 (Atprmt5) | Lengthening of circadian period | PRR7, PRR9 | Sanchez et al., 2010 |
| SKIP (skip-1) | Lengthening of circadian period; temperature compensation defect; increased light sensitivity | PRR7, PRR9 | Wang et al., 2012 |
| STIPL1 | Lengthening of circadian period | CCA1, LHY1, TОC1, PRR9 | Jones et al., 2012 |
| LSM4 and LSM5 | Lengthening of circadian period | CCA1, TОC1 | Perez-Santangelo et al., 2014 |
| GEMIN2 | Shortening of circadian period at 22 °C; temperature compensation defect | TОC1 | Schlaen et al., 2015 |
| SR45 | N/A | CCA1 | Filichkin et al., 2015 |
GEM NUCLEAR ORGANELLE ASSOCIATED PROTEIN 2 (GEMIN2), a spliceosomal small nuclear ribonucleoprotein assembly factor, is an additional regulator of circadian clock speed in Arabidopsis, potentially by acting on intron 4 in TOC1. As opposed to the majority of splicing factor mutations that affect clocks in Arabidopsis, GEMIN2 mutants exhibit a shorter circadian period at normal conditions (22 °C; Schlaen et al., 2015). However, GEMIN2 mutants do not maintain their short periods in cold conditions: at 12 °C, their rhythms get longer and match those of wild-type plants. Normally, the circadian period is temperature compensated, which means that changes in temperature do not perturb the ~24-h pace of the clocks (Pittendrigh, 1954). The phenotype of GEMIN2 mutants points to the disruption of this critical circadian clock property, suggesting that splicing by GEMIN2, likely at intron 4, maintains ~24-h periods at ambient temperature and prevents lengthening at cold temperatures. In addition to GEMIN2, SKIP is necessary for proper temperature compensation in plants: the long period of SKIP mutants at 17 °C does not persist at 27 °C (Wang et al., 2012). Thus, in both of these cases, the loss of splicing factors causes period shortening with temperature increase, which may be expected of a biochemical reaction that is not temperature compensated. Thus, splicing factors may counter the normal effects of temperature on the activity of circadian components, perhaps by decreasing or changing the function of the relevant proteins via production of different RNA isoforms.
Alternative splicing can happen co-transcriptionally and is tightly coupled to transcriptional elongation (reviewed in Bentley, 2014; Kornblihtt et al., 2004). Splicing factors can promote transcription elongation, and the speed of transcriptional elongation, in turn, can affect splicing efficiency. For example, slow polymerase dynamics favor weaker splicing sites and lead to inclusion of alternative exons and introns. Therefore, it is possible that splicing changes in some of the splicing factor plant mutants are accompanied by transcriptional changes. However, unfortunately, most studies of splicing factor mutants in Arabidopsis do not comprehensively evaluate the overall changes in transcription nor how these correlate with the production of alternatively spliced isoforms.
It is becoming clear that some of the evolutionary conserved splicing factors, initially identified as regulating clocks in Arabidopsis, play a conserved circadian role in other systems, from Drosophila to mammals (Sanchez et al., 2010; Perez-Santangelo et al., 2014). However, because of complex splicing interactions and networks, the function of any given splicing regulator is often difficult to trace to a concrete set of splicing events. Drawing straightforward mechanistic relationships between splicing factors and clock genes has proven to be a hard task and requires further investigation (Wang et al., 2012; Jones et al., 2012; Perez-Santangelo et al., 2014; Schlaen et al., 2015; Table 1).
ALTERNATIVE SPLICING IN FUNGI
Brief Overview of Circadian Clocks in Fungi (Neurospora crassa)
The circadian feedback loop in Neurospora crassa is composed of the White Collar Complex (WCC), which includes WC-1 and WC-2 transcription factors that activate the core clock component frequency (frq) and are subject to rhythmic feedback by the FRQ protein (Fig. 1). As in the case of CLK-CYC in Drosophila, the WCC also targets many other genes in the Neurospora genome. FRQ inhibition of WCC activity is regulated by phosphorylation and allows for genome-wide rhythmic expression of clock-controlled mRNAs (reviewed in Liu, 2003; Dunlap and Loros, 2004; Schafmeier et al., 2005).
Alternative Splicing in Neurospora
Thermosensitive splicing of frq, a central component of the Neurospora circadian clock, determines the protein profile of FRQ. FRQ can be produced as either a long (l) or a short (s) isoform, depending on the selection of the translation initiation site in the 5ʹ-UTR of its RNA. The selection of the translation start site is a consequence of an alternative splicing choice at intron 6 of frq (also referred to as intron 2 in Colot et al., 2005; Liu et al., 1997; Diernfellner et al., 2005). Splicing of this intron, which contains the initiation codon for l-FRQ, selectively generates the s-FRQ isoform. Interestingly, since the splicing of this intron is more efficient at lower temperatures, the ratio of l-FRQ to s-FRQ decreases as the temperature drops (Liu et al., 1997). It is speculated that at lower temperatures, the spliceosome machinery can better recognize nonconsensus splicing motifs at intron 6 of frq, which thereby acts as a molecular temperature sensor of the Neurospora clock (Diernfellner et al., 2005).
The ratio of l-FRQ to s-FRQ is important because it establishes the overall robustness of the circadian free-running rhythm in response to temperature changes (Liu et al., 1997). Circadian clocks are functional only within a set temperature range for any given organism, such that oscillations are lost outside this temperature range. The loss of either l-frq or s-frq expression is detrimental to the maintenance of circadian rhythmicity, especially at the extremes of the normal temperature range. Therefore, the expression of both frq isoforms is necessary to confer onto circadian clocks a broad temperature range of rhythmicity, an adaptation that likely promotes environmental fitness in Neurospora (Liu et al., 1997). The ratio of l-FRQ to s-FRQ also fine-tunes the circadian period length (Diernfellner et al., 2007). In particular, mutants that selectively express s-FRQ exhibit period lengthening, while selective l-FRQ expression causes modest period shortening (Diernfellner et al., 2007). Interestingly, temperature compensation, an ability of clocks to maintain a stable period length across a physiological temperature range, does not seem to be disrupted in strains that selectively express l-frq or s-frq (Diernfellner et al., 2007). Thus, while the splicing of frq is sensitive to temperature and the splicing outcome affects the range of permissive temperatures for clock function, the ratio of l-frq and s-frq spliced forms is dispensable for temperature compensation. Clocks interact with temperature on many levels, and it appears that alternative splicing contributes mechanistically to some of the interactions but perhaps not to all.
ALTERNATIVE SPLICING IN MAMMALS
Brief Overview of Circadian Clocks in Mammals
In mammals, CLOCK and BMAL1 form a transcriptional complex that activates the circadian transcriptome (Fig. 1). A particularly important set of targets of CLOCK/BMAL1 are the Period (Per1, Per2, and Per3) and Cryptochrome (Cry1 and Cry2) genes, which are the canonical negative regulators of the mammalian clock. Because PER and CRY are negative regulators of CLOCK and BMAL1, an increase in Per and Cry production eventually leads to the inhibition of CLOCK/BMAL1 transcriptional activity. Reinitiation of transcription requires destabilization and degradation of PER and CRY by multiple post-translational mechanisms. Thus, CLOCK/BMAL1 and PER/CRY together form a central autoregulatory TTFL in mammals (Lowrey and Takahashi, 2000). An additional TTFL that includes the nuclear repressor Rev-erbα drives the cycling of Bmal1 transcription (Preitner et al., 2002).
Light resets the central pacemaker in the suprachiasmatic nucleus and enables brain clocks to run in synchrony with environmental light:dark schedules. Light induces expression of immediate-early genes such as Per1, which contribute to light-induced phase shifts of behavioral rhythms (advances in the early morning and delays in the evening; Shigeyoshi et al., 1997).
Alternative Splicing in Mammals
Approximately 80% and 95% of all multiexon genes in Mus musculus and Homo sapiens, respectively, undergo alternative splicing (Wang et al., 2008; Mollet et al., 2010). Alternative splicing exhibits tissue-specific circadian rhythms, in particular in genes that are transcribed in a circadian fashion (McGlincy et al., 2012). Alternatively spliced genes include core clock genes, which are predicted to encode multiple spliceoforms, although the roles of the different spliced forms have not been studied mechanistically.
U2-auxiliary-factor 26 (U2AF26) is a splicing factor that undergoes temperature-driven diurnal skipping of exons 6 and 7 to generate the U2AF26deltaE67 isoform (Preuβner et al., 2014). Unexpectedly, the reading frame of U2AF26deltaE67 translates far into the 3ʹ-UTR and generates a C terminus with close homology to the key Drosophila circadian component TIMELESS (TIM). Both U2AF26 and U2AF26deltaE67 interact with PER1; however, only U2AF26deltaE67 selectively destabilizes PER1. Interestingly, while U2AF26-deficient mice have normal circadian rhythms in free-running conditions, they exhibit faster adaptation to ~4-h phase advances. Thus, a proposed role for U2AF26, specifically in the context of U2AF26deltaE67 production, is to buffer the circadian clock against unpredicted changes in light conditions by limiting the induction of PER1 (Preuβner et al., 2014).
Splicing of U2af26 responds robustly to small changes in ambient temperature. Drops in body temperature correlate with increased exon skipping in U2af26. In addition to U2af26, modest daily changes in body temperature entrain alternative splicing oscillations in hundreds of genes in mice. Temperature fluctuations also drive rhythms in the phosphorylation of SR proteins, which in turn regulate alternative splicing in mammalian peripheral clocks. For example, alternative splicing of U2af26 is regulated by temperature-dependent phosphorylation of 2 SR proteins, SRSF2 and SRSF7 (Preuβner et al., 2017).
CONCLUSION
In the past decade, alternative splicing has emerged as an important contributor to circadian regulation, helping to maintain clock function and also to modulate responses of clocks to the environment, in particular to temperature. Alternative splicing mechanisms impinge on the clockwork either by regulating the abundance of full-length clock proteins or by producing truncated isoforms, which sometimes have a dominant-negative function (Fig. 2). The abundance of full-length clock proteins is generally modified via changes in RNA stability (with alternatively spliced unstable transcripts shuttled into the NMD pathway) and/or via translational control. Temporal control of alternative splicing can thereby provide a mechanism to drive the cyclic expression of proteins. Splicing of many genes is under circadian control, in a tissue-specific manner, but the mechanisms that drive the cycling and the impact they have on protein expression are largely unknown.
Figure 2. Model for how alternative splicing affects the circadian clock.
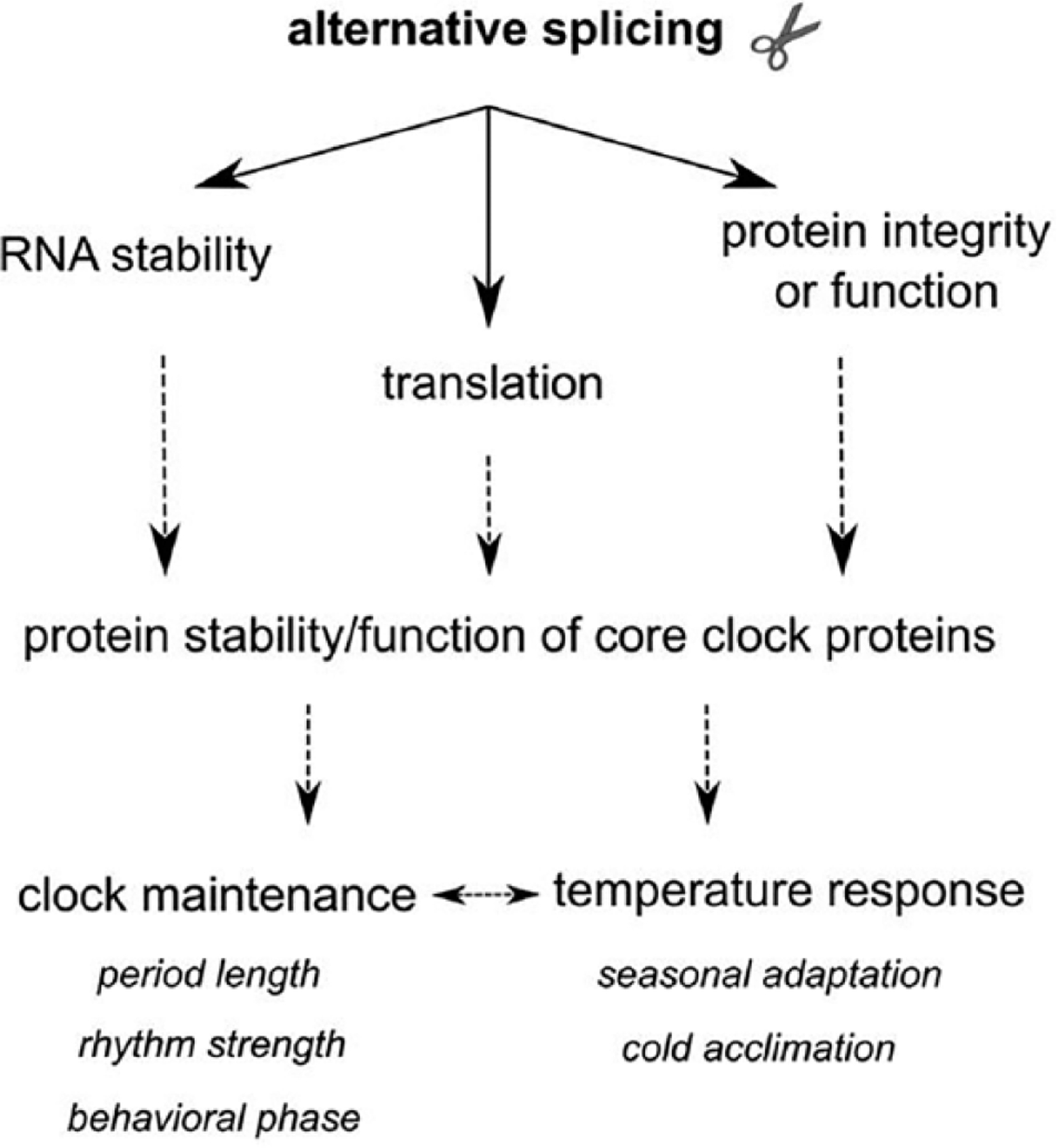
In summary, regulated splicing of clock genes is important for maintenance of core molecular feedback loops, in part by contributing to critical delays within these loops, and also for interactions of the clock with temperature. For instance, alternative splicing is implicated in entrainment to temperature as well as temperature compensation. Our overview of clock-relevant splicing mechanisms across multiple organisms also emphasizes the gap currently present in the mammalian studies. One of the key lessons learned from fruit flies, plants, and Neurospora is that most of the core circadian components undergo some form of alternative splicing that is relevant for the maintenance of proper circadian clock function. Therefore, it is safe to hypothesize that regulated splicing of mammalian core clock genes will be important as well. A recently identified 5ʹ splice site mutation in human CRY1, which is implicated in familial delayed sleep phase disorder, highlights the need to extend our understanding of the mammalian alternative splicing landscape, both under normal conditions and in disease states (Patke et al., 2017). Finally, given how tissue specific some alternative splicing mechanisms are, it would be important to understand if alternative splicing mechanisms can confer tissue specificity onto circadian clocks. Alternative splicing is clearly an area of circadian biology that is ripe for future investigation and will undoubtedly provide new insights into our understanding of biological rhythms.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- Akten B, Jauch E, Genova GK, Kim EY, Edery I, Raabe T, and Jackson FR (2003) A role for CK2 in the Drosophila circadian oscillator. Nat Neurosci 6(3):251–257. [DOI] [PubMed] [Google Scholar]
- Bartok O, Kyriacou CP, Levine J, Sehgal A, and Kadener S (2013) Adaptation of molecular circadian clockwork to environmental changes: a role for alternative splicing and miRNAs. Proc Biol Sci 280(1765):20130011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith EJ, Hernando CE, Polcownuk S, Bertolin AP, Mancini E, Ceriani MF, and Yanovsky MJ (2017) Rhythmic behavior is controlled by the SRm160 splicing factor in Drosophila melanogaster. Genetics 207(2): 593–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley DL (2014) Coupling mRNA processing with transcription in time and space. Nat Rev Genet 15(3): 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothroyd CE, Wijnen H, Naef F, Saez L, and Young MW (2007) Integration of light and temperature in the regulation of circadian gene expression in Drosophila. PLoS Genet 3(4):e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley S, Narayanan S, and Rosbash M (2012) NAT1/DAP5/p97 and atypical translational control in the Drosophila Circadian Oscillator. Genetics 192(3):943–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceriani MF, Darlington TK, Staknis D, Mas P, Petti AA, Weitz CJ, and Kay SA (1999) Light-dependent sequestration of TIMELESS by CRYPTOCHROME. Science 285(5427):553–556. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Gvakharia B, and Hardin PE (1998) Two alternatively spliced transcripts from the Drosophila period gene rescue rhythms having different molecular and behavioral characteristics. Mol Cell Biol 18(11):6505–6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu JC, Ko HW, and Edery I (2011) NEMO/NLK phosphorylates PERIOD to initiate a time-delay phosphorylation circuit that sets circadian clock speed. Cell 145(3):357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colot HV, Loros JJ, and Dunlap JC (2005) Temperature-modulated alternative splicing and promoter use in the Circadian clock gene frequency. Mol Biol Cell 16(12):5563–5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins BH, Rosato E, and Kyriacou CP 2004. Seasonal behavior in Drosophila melanogaster requires the photoreceptors, the circadian clock, and phospholipase C. Proc Natl Acad Sci U S A 101(7):1945–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diernfellner AC, Colot HV, Dintsis O, Loros JJ, Dunlap JC, and Brunner M (2007) Long and short isoforms of Neurospora clock protein FRQ support temperature-compensated circadian rhythms. FEBS Lett 581(30):5759–5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diernfellner AC, Schafmeier T, Merrow MW, and Brunner M (2005) Molecular mechanism of temperature sensing by the circadian clock of Neurospora crassa. Genes Dev 19(17):1968–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubowy C and Sehgal A (2017) Circadian rhythms and sleep in Drosophila melanogaster. Genetics 205(4):1373–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap JC and Loros JJ (2004) The neurospora circadian system. J Biol Rhythms 19(5):414–424. [DOI] [PubMed] [Google Scholar]
- Duvall LB and Taghert PH (2011) Circadian rhythms: biological clocks work in phospho-time. Curr Biol 21(9):305. [DOI] [PubMed] [Google Scholar]
- Edery I, Zwiebel LJ, Dembinska ME, and Rosbash M (1994) Temporal phosphorylation of the Drosophila period protein. Proc Natl Acad Sci U S A 91(6):2260–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evantal N, Anduaga AM, Bartok O, Patop IL, Weiss R, and Kadener S (2018) Thermosensitive alternative splicing senses and mediates temperature adaptation in Drosophila. bioRxiv doi: 10.1101/503409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Sathyanarayanan S, and Sehgal A (2007) Post-translational regulation of the Drosophila circadian clock requires protein phosphatase 1 (PP1). Genes Dev 21(12):1506–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filichkin SA, Cumbie JS, Dharmawardhana P, Jaiswal P, Chang JH, Palusa SG, Reddy AS, Megraw M, and Mockler TC (2015) Environmental stresses modulate abundance and timing of alternatively spliced circadian transcripts in Arabidopsis. Mol Plant 8(2):207–227. [DOI] [PubMed] [Google Scholar]
- Filichkin SA, Priest HD, Givan SA, Shen R, Bryant DW, Fox SE, Wong WK, and Mockler TC (2010) Genome-wide mapping of alternative splicing in Arabidopsis thaliana. Genome Res 20(1):45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley L, Ling J, Joshii R, Evantal N, Kadener S, and Emery P (2018) PSI controls tim splicing and circadian period in Drosophila. bioRxiv doi: 10.1101/504282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbe DS, Fang Y, Zheng X, Sowcik M, Anjum R, Gygi SP, and Sehgal A (2013) Cooperative interaction between phosphorylation sites on PERIOD maintains circadian period in Drosophila. PLoS Genet 9(9):e1003749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AB, Syed NH, Bordage S, Marshall J, Nimmo GA, Jenkins GI, Herzyk P, Brown JW, and Nimmo HG (2012) Alternative splicing mediates responses of the Arabidopsis circadian clock to temperature changes. Plant Cell 24(3):961–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MA, Williams BA, McNicol J, Simpson CG, Brown JW, and Harmer SL (2012) Mutation of Arabidopsis spliceosomal timekeeper locus1 causes circadian clock defects. Plant Cell 24(10):4066–4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd PB, Young MW, and Siggia ED (2015) Temperature compensation and temperature sensation in the circadian clock. Proc Natl Acad Sci U S A 112(46):E6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivimäe S, Saez L, and Young MW (2008) Activating PER repressor through a DBT-directed phosphorylation switch. PLoS Biol 6(7):e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh K, Zheng X, and Sehgal A (2006) JETLAG resets the Drosophila circadian clock by promoting light-induced degradation of TIMELESS. Science 312(5781): 1809–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Shingle DL, and Green CB (2011) Post-transcriptional control of circadian rhythms. J Cell Sci 124(Pt 3):311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblihtt AR, de la Mata M, Fededa JP, Munoz MJ, and Nogues G (2004) Multiple links between transcription and splicing. RNA 10(10):1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y, Park M, Kim S, Baldwin IT, and Park C (2014) Alternative splicing and nonsense-mediated decay of circadian clock genes under environmental stress conditions in Arabidopsis. BMC Plant Biol 14(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Yoo E, Lee H, Park K, Hur J, and Lim C (2017) LSM12 and ME31B/DDX6 define distinct modes of posttranscriptional regulation by ATAXIN-2 protein complex in Drosophila circadian pacemaker neurons. Mol Cell 66(1):140.e7. [DOI] [PubMed] [Google Scholar]
- Lim C and Allada R (2013) ATAXIN-2 activates PERIOD translation to sustain circadian rhythms in Drosophila. Science 340(6134):875–879. [DOI] [PubMed] [Google Scholar]
- Lim C, Lee J, Choi C, Kilman VL, Kim J, Park SM, Jang SK, Allada R, and Choe J (2011) The novel gene twenty-four defines a critical translational step in the Drosophila clock. Nature 470(7334):399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JM, Kilman VL, Keegan K, Paddock B, Emery-Le M, Rosbash M, and Allada R (2002) A role for casein kinase 2 alpha in the Drosophila circadian clock. Nature 420(6917):816–820. [DOI] [PubMed] [Google Scholar]
- Liu Y (2003) Molecular mechanisms of entrainment in the Neurospora circadian clock. J Biol Rhythms 18(3): 195–205. [DOI] [PubMed] [Google Scholar]
- Liu Y, Garceau NY, Loros JJ, and Dunlap JC (1997) Thermally regulated translational control of FRQ mediates aspects of temperature responses in the neurospora circadian clock. Cell 89(3):477–486. [DOI] [PubMed] [Google Scholar]
- Low KH, Lim C, Ko HW, and Edery I (2008) Natural variation in the splice site strength of a clock gene and species-specific thermal adaptation. Neuron 60(6): 1054–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrey PL and Takahashi JS (2000) Genetics of the mammalian circadian system: photic entrainment, circadian pacemaker mechanisms, and posttranslational regulation. Annu Rev Genet 34:533–562. [DOI] [PubMed] [Google Scholar]
- Majercak J, Chen WF, and Edery I (2004) Splicing of the period gene 3ʹ-terminal intron is regulated by light, circadian clock factors, and phospholipase C. Mol Cell Biol 24(8):3359–3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majercak J, Sidote D, Hardin PE, and Edery I (1999) How a circadian clock adapts to seasonal decreases in temperature and day length. Neuron 24(1):219–230. [DOI] [PubMed] [Google Scholar]
- Marshall CM, Tartaglio V, Duarte M, and Harmon FG (2016) The Arabidopsis sickle mutant exhibits altered circadian clock responses to cool temperatures and temperature-dependent alternative splicing. Plant Cell 28(10):2560–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinek S, Inonog S, Manoukian AS, and Young MW (2001) A role for the segment polarity gene shaggy/GSK-3 in the Drosophila circadian clock. Cell 105(6): 769–779. [DOI] [PubMed] [Google Scholar]
- McGlincy NJ, Valomon A, Chesham JE, Maywood ES, Hastings MH, and Ule J (2012) Regulation of alternative splicing by the circadian clock and food related cues. Genome Biol 13(6):r54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollet IG, Ben-Dov C, Felicio-Silva D, Grosso AR, Eleuterio P, Alves R, Staller R, Silva TS, and Carmo-Fonseca M (2010) Unconstrained mining of transcript data reveals increased alternative splicing complexity in the human transcriptome. Nucleic Acids Res 38(14):4740–4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montelli S, Mazzotta G, Vanin S, Caccin L, Corra S, De Pitta C, Boothroyd C, Green EW, Kyriacou CP, and Costa R (2015) period and timeless mRNA splicing profiles under natural conditions in Drosophila melanogaster. J Biol Rhythms 30(3):217–227. [DOI] [PubMed] [Google Scholar]
- Naidoo N, Song W, Hunter-Ensor M, and Sehgal A (1999) A role for the proteasome in the light response of the timeless clock protein. Science 285(5434):1737–1741. [DOI] [PubMed] [Google Scholar]
- Patke A, Murphy PJ, Onat OE, Krieger AC, Ozcelik T, Campbell SS, and Young MW (2017) Mutation of the human circadian clock gene CRY1 in familial delayed sleep phase disorder. Cell 169(2):215.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Santangelo S, Mancini E, Francey LJ, Schlaen RG, Chernomoretz A, Hogenesch JB, and Yanovsky MJ (2014) Role for LSM genes in the regulation of circadian rhythms. Proc Natl Acad Sci U S A 111(42): 15166–15171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh CS (1954) On temperature independence in the clock system controlling emergence time in Drosophila. Proc Natl Acad Sci U S A 40(10):1018–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, and Schibler U (2002) The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110(2):251–260. [DOI] [PubMed] [Google Scholar]
- Preuβner M, Goldammer G, Neumann A, Haltenhof T, Rautenstrauch P, Muller-McNicoll M, and Heyd F (2017) Body temperature cycles control rhythmic alternative splicing in mammals. Mol Cell 67(3):446.e4. [DOI] [PubMed] [Google Scholar]
- Preuβner M, Wilhelmi I, Schultz AS, Finkernagel F, Michel M, Moroy T, and Heyd F (2014) Rhythmic U2af26 alternative splicing controls PERIOD1 stability and the circadian clock in mice. Mol Cell 54(4):651–662. [DOI] [PubMed] [Google Scholar]
- Price JL, Blau J, Rothenfluh A, Abodeely M, Kloss B, and Young MW (1998) double-time is a novel Drosophila clock gene that regulates PERIOD protein accumulation. Cell 94(1):83–95. [DOI] [PubMed] [Google Scholar]
- Rodriguez J, Tang CH, Khodor YL, Vodala S, Menet JS, and Rosbash M (2013) Nascent-Seq analysis of Drosophila cycling gene expression. Proc Natl Acad Sci U S A 110(4):275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosato E, Trevisan A, Sandrelli F, Zordan M, Kyriacou CP, and Costa R (1997) Conceptual translation of timeless reveals alternative initiating methionines in Drosophila. Nucleic Acids Res 25(3):455–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosbash M (2009) The implications of multiple circadian clock origins. PLoS Biol 7(3):e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez SE and Kay SA (2016) The plant circadian clock: from a simple timekeeper to a complex developmental manager. Cold Spring Harb Perspect Biol 8(12):a027748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez SE, Petrillo E, Beckwith EJ, Zhang X, Rugnone ML, Hernando CE, Cuevas JC, Godoy Herz MA, Depetris-Chauvin A, Simpson CG, et al. (2010) A methyl transferase links the circadian clock to the regulation of alternative splicing. Nature 468(7320):112–116. [DOI] [PubMed] [Google Scholar]
- Sathyanarayanan S, Zheng X, Xiao R, and Sehgal A (2004) Posttranslational regulation of Drosophila PERIOD protein by protein phosphatase 2A. Cell 116(4):603–615. [DOI] [PubMed] [Google Scholar]
- Schafmeier T, Haase A, Kaldi K, Scholz J, Fuchs M, and Brunner M (2005) Transcriptional feedback of Neurospora circadian clock gene by phosphorylation-dependent inactivation of its transcription factor. Cell 122(2):235–246. [DOI] [PubMed] [Google Scholar]
- Schlaen RG, Mancini E, Sanchez SE, Perez-Santangelo S, Rugnone ML, Simpson CG, Brown JW, Zhang X, Chernomoretz A, and Yanovsky MJ (2015) The spliceosome assembly factor GEMIN2 attenuates the effects of temperature on alternative splicing and circadian rhythms. Proc Natl Acad Sci U S A 112(30): 9382–9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal A (2017) Physiology flies with time. Cell 171(6): 1232–1235. [DOI] [PubMed] [Google Scholar]
- Seo PJ, Park MJ, Lim MH, Kim SG, Lee M, Baldwin IT, and Park CM (2012) A self-regulatory circuit of CIRCADIAN CLOCK-ASSOCIATED1 underlies the circadian clock regulation of temperature responses in Arabidopsis. Plant Cell 24(6):2427–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakhmantsir I, Nayak S, Grant GR, and Sehgal A (2018) Spliceosome factors target timeless (tim) mRNA to control clock protein accumulation and circadian behavior in Drosophila. eLife 7. doi: 10.7554/eLife.39821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeyoshi Y, Taguchi K, Yamamoto S, Takekida S, Yan L, Tei H, Moriya T, Shibata S, Loros JJ, Dunlap JC, et al. (1997) Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell 91(7):1043–1053. [DOI] [PubMed] [Google Scholar]
- So WV and Rosbash M (1997) Post-transcriptional regulation contributes to Drosophila clock gene mRNA cycling. EMBO J 16(23):7146–7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanewsky R, Kaneko M, Emery P, Beretta B, Wager-Smith K, Kay SA, Rosbash M, and Hall JC (1998) The cryb mutation identifies cryptochrome as a circadian photo-receptor in Drosophila. Cell 95(5):681–692. [DOI] [PubMed] [Google Scholar]
- Suri V, Lanjuin A, and Rosbash M (1999) TIMELESS-dependent positive and negative autoregulation in the Drosophila circadian clock. EMBO J 18(3):675–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber E, Zordan M, Sandrelli F, Pegoraro M, Osterwalder N, Breda C, Daga A, Selmin A, Monger K, Benna C, et al. (2007) Natural selection favors a newly derived timeless allele in Drosophila melanogaster. Science 316(5833):1895–1898. [DOI] [PubMed] [Google Scholar]
- Top D, Harms E, Syed S, Adams EL, and Saez L (2016) GSK-3 and CK2 kinases converge on Timeless to regulate the master clock. Cell Rep 16(2):357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Top D, O’Neil JL, Merz GE, Dusad K, Crane BR, and Young MW (2018) CK1/Doubletime activity delays transcription activation in the circadian clock. eLife 7. doi: 10.7554/eLife.32679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl MC, Will CL, and Luhrmann R (2009) The spliceosome: design principles of a dynamic RNP machine. Cell 136(4):701–718. [DOI] [PubMed] [Google Scholar]
- Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP, and Burge CB (2008) Alternative isoform regulation in human tissue transcriptomes. Nature 456(7221):470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Abruzzi KC, Rosbash M, and Rio DC (2018) Striking circadian neuron diversity and cycling of Drosophila alternative splicing. eLife 7. doi: 10.7554/eLife.35618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wu F, Xie Q, Wang H, Wang Y, Yue Y, Gahura O, Ma S, Liu L, Cao Y, et al. (2012) SKIP is a component of the spliceosome linking alternative splicing and the circadian clock in Arabidopsis. Plant Cell 24(8): 3278–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Qian Z, Myers MP, and Rosbash M (1996) A light-entrainment mechanism for the Drosophila circadian clock. Nature 380(6570):129–135. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ling J, Yuan C, Dubruille R, and Emery P (2013) A role for Drosophila ATX2 in activation of PER translation and circadian behavior. Science 340(6134):879–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Cao W, and Edery I (2018) The SR protein B52/SRp55 regulates splicing of the period thermosensitive intron and mid-day siesta in Drosophila. Sci Rep 8(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X and Sehgal A (2012) Speed control: cogs and gears that drive the circadian clock. Trends Neurosci 35(9):574–585. [DOI] [PMC free article] [PubMed] [Google Scholar]


