Abstract
The role of Enterococcus faecalis in polymicrobial peritonitis is still debated. Virulence factors expressed in some enterococcal strains might be involved in the pathogenicity of these organisms. To clarify their role, three of these virulence factors (cytolysin, gelatinase, and aggregation substance) were studied in six isogenic strains of E. faecalis expressing various combinations of these factors. Since the pathogenic effects of enterococci are only moderate, the expression of their virulence might vary from one animal species to another and from one type of infection to another. Therefore, we evaluated these effects in two animal models, i.e., a systemic infection in mice in which we assessed the virulence of the strains in 50% lethal dose studies and a model of compartmentalized infection in rats in which the microbiologic and inflammatory effects of the strains were evaluated in monomicrobial or polymicrobial infection. In mice, significant differences were observed in the cumulative survival curves depending on the virulence factors (P < 0.0001 [log rank test]). In rats, monomicrobial infection induced only mild changes. In polymicrobial peritonitis, the virulence factors mainly increased the inflammatory response while the changes observed in the microbiologic response were minimal. The combination of two virulence factors did not significantly increase the severity of infection either in the mice model or the polymicrobial rat model. These data argue for species and model dependence of the role of the virulence factors studied here and suggest that other important factors may be involved in the pathogenicity of enterococci.
Many issues remain unsolved regarding the pathogenic role of enterococci in the course of intraabdominal infection. From the previous reports, it appears that the enterococcal infections are rarely monomicrobial in nature, especially in surgical patients, suggesting the role of bacterial synergy (30, 31). Among the factors that might be involved, the virulence factors could be of interest but have been minimally studied (23, 25). Plasmids and conjugative transposons, which play a key role in the acquisition of drug resistance, may also carry these virulence traits (6), but their clinical relevance remains unclear.
Three virulence factors of Enterococcus faecalis might have the potential to increase the severity of intraabdominal sepsis. The plasmid-encoded cytolysin-bacteriocin (Cly) is the best studied of these factors. In vitro, this factor induces lysis of erythrocytes, polymorphonuclear neutrophils, and macrophages and could lead to a reduction in phagocytosis (29). Aggregation substance (Agg), which is closely linked to mating response to enterococcal sex pheromones (7), mediates adhesion of the bacteria to host cells, such as intestinal epithelial cells (34), renal tubular cells (27), and heart endothelial cells (17), and could be involved in the persistence of the organisms within the host tissues and fluids. Gelatinase (Gel) is a non-plasmid-encoded potential virulence factor. Although Gel strains of E. faecalis have been mainly studied in dental diseases (16), the properties given by this extracellular metalloendopeptidase (hydrolyzis of collagen, gelatin, and small peptides [18]) could increase bacterial dissemination. The expression of these virulence factors might vary from one animal species to another and from one type of infection to another, especially because the pathogenic role of E. faecalis seems to be moderate (36, 40, 41) and expressed only in polymicrobial infections (3, 10, 28, 30).
To address these issues, we evaluated the effects of six isogenic strains of E. faecalis containing various combinations of virulence factors in two animal models, i.e., a model of systemic infection in mice in which we assessed the virulence of enterococcal strains in 50% lethal dose studies and a model of compartmentalized infection in rats in which the microbiologic and inflammatory effects of the strains were evaluated in monomicrobial or polymicrobial infection in combination with Escherichia coli and Bacteroides fragilis, two species frequently associated in clinical samples.
MATERIALS AND METHODS
Microorganisms.
Six derivatives of E. faecalis OG1 were obtained from the University of Texas Medical School (Houston, Tex.) and the University of Michigan (Ann Arbor, Mich.). The four OG1x derivatives used in the present study are isogenic strains produced by mutagenesis with nitrosoguanidine from strain OG10 (14, 39). The Tn917 transposon was inserted into the PAD1 plasmid, leading to the following different phenotypes of OG1x: pAM714 with Cly and Agg (20, 21) and pAM9058 with Agg and pAM944 with Cly (5, 15). The two remaining strains (OG1RF and OG1SSP) are not isogenic with the previous four strains. OG1RF is a spontaneous mutant of OG1 with a Gel phenotype (14, 32). The OG1SSP strain is a derivative of OG1. The Tn915 transposon was inserted into the PCF10 plasmid, leading to the Gel and Agg phenotypes of the OG1SSP strain (12, 13, 35). All of these strains of E. faecalis had an intrinsic low-level resistance to aminoglycosides, and their characteristics are listed in Table 1. The resistance phenotype, which was encoded on the same plasmid as the virulence factor, was verified in each experiment. Gel and Cly expression was verified in vitro before inoculation according to the method described by Coque et al. (8). The strains of B. fragilis (AIP5-86) and E. coli (CB1496) used in the current study have previously been used in this model (30).
TABLE 1.
Microbiologic characteristics of the derivatives of E. faecalis inoculated
| Strain | Plasmid content | Virulence factor | Antimicrobial resistancea |
|---|---|---|---|
| OG1X | None | None | None |
| OG1RF | None | Gel | RI + FA |
| OG1X | pAM 9058 (Tn917) | Agg | EM |
| OG1X | pAM 944 (Tn917) | Cly | EM |
| OG1X | pAM 714 (Tn917) | Cly + Agg | EM |
| OG1SSP | pCF10 (Tn915) | Gel + Agg | TC |
All of these strains of E. faecalis had an intrinsic low-level chromosomal resistance to aminoglycosides. OG1RF had a constitutive chromosomal resistance to rifampin and to fucidic acid. Resistance to erythromycin and tetracycline was plasmid encoded. RI, rifampin; FA, fusidic acid; EM, erythromycin; and TC, tetracycline.
Animals.
Female OF1 mice (Iffa Credo, L’Arbresles, France), weighing 20 to 25 g and housed 10 per cage, were used for 50% lethal dose (LD50) evaluation of the six strains of E. faecalis, which were injected intraperitoneally. Male Sprague-Dawley rats (Charles River, St-Aubin-les-Elbeufs, France), weighing 250 to 300 g and housed 5 per cage, were used for the peritonitis model. All animals had access to chow and water ad libitum throughout the experiment. All of these experiments were performed according to current European regulations.
Preparation of the microorganisms.
B. fragilis was grown and diluted anaerobically in prereduced thioglycolate broth. E. coli and the six strains of E. faecalis were grown in brain-heart infusion broth. The final inoculum was made when the bacteria were in the log phase of growth. The inocula were adjusted spectrophotometrically and bacteria were diluted to give the number of microorganisms required for bacterial challenge. Purity was assessed and counts were validated for each strain immediately before inoculation (mice model) or before mixing (rat model).
LD50 in mice.
The virulence of each E. faecalis strain was evaluated by inoculation of increasing concentrations of microorganisms (107 to 1011 CFU per ml) in mice. Nine groups of 10 animals were studied for each bacterium. The animals received intraperitoneally a 0.5-ml injection of the bacterial suspension. After inoculation, the animals were returned to their cages and daily mortality was recorded until day 7. Lethal dose curves were plotted, and LD50s were calculated according to the method described by Ike et al. (21).
Intraabdominal infection in rats.
Semisolid agar medium was prepared by adding 2% (wt/vol) agar to the diluted broth cultures associated with barium sulfate (10% [wt/vol]). Aliquots (0.5 ml) of the final product were placed in double gelatin capsules for peritoneal implantation. Each E. faecalis strain was studied twice: once as a monomicrobial infection (108 CFU/ml, 12 animals in each group) and once as a polymicrobial infection (20 animals in each group) associated with E. coli (108 CFU/ml) and B. fragilis (109 CFU/ml).
Implantation of inoculum.
The rats were anesthetized with an intramuscular injection of ketamine (30 mg/kg of body weight [Parke-Davis, Courbevoie, France]), and the gelatin capsule was inserted into the pelvic peritoneal cavity through a midline abdominal incision (40). The wound was closed with a musculoperitoneal layer and a skin layer by using interrupted nylon sutures.
Assessment of spontaneous outcome.
After implantation of the inoculum, the animals were returned to separate cages; they were observed and weighed daily until sacrifice. No death was observed within 6 h of capsule implantation. In the six groups of animals receiving a monomicrobial inoculum, sacrifice was performed at 24 h after inoculation for four animals and at day 3 for four animals. In the groups receiving a polymicrobial inoculum, eight animals were sacrificed at 24 h and eight animals were sacrificed at day 3. In addition, an early sacrifice (6 h after inoculation) was planned in both models (monomicrobial and polymicrobial) for four animals in each group. The following pathogenicity criteria were studied: clinical criteria (body weight and mortality), microbiological criteria (positivity of blood cultures and bacterial counts in the peritoneal fluid), and the inflammatory response (peritoneal concentrations of phagocytes, tumor necrosis factor alpha [TNF-α], and interleukin-6 [IL-6] and serum concentrations of α1-acid glycoprotein).
Sacrifice.
Animals were killed with chloroform. Blood samples were obtained by aseptic percutaneous transthoracic cardiac puncture for qualitative blood culture on days 1 and 3 and for measurements of α1-acid glycoprotein concentrations in serum on day 3. After injection of 10 ml of cold phosphate-buffered saline (PBS) intraperitoneally, a midline laparotomy was performed and peritoneal fluid samples were recovered from all regions of the peritoneal cavity for bacterial and cell counts (days 1 and 3). Peritoneal fluid cytokine concentrations were specifically evaluated at 6 h after bacterial challenge. A dilution factor taking into account the fluid present in the peritoneal cavity prior to the injection of the 10 ml of PBS was applied in all calculations according to a technique previously described (26, 31). Blood cultures were inoculated immediately after collection (NR-7A; Becton-Dickinson, Le-Pont-de-Claix, France) and analyzed daily until day 5 for identification. Serial dilutions of the peritoneal fluid were made, and 0.1 ml of each dilution was spread on agar plates for colony counts. The limit of detection for each microbiological test was <1 log10 CFU/ml. In cases of values below this threshold, the results were listed as ≤1 log10 CFU/ml. In the statistical analysis, these results were treated as 1 log10 CFU/ml. Plates were incubated under appropriate conditions (aerobic and anaerobic) for 2 to 5 days. The selective medium used for detection of B. fragilis was Columbia agar base (Bio-mérieux, Charbonnières-les-Bains, France) with 5% sheep blood containing 75 μg of kanamycin, 7.5 μg of vancomycin, and 4 μg of pefloxacin per ml. The selective media used for aerobic culture were Drigalski agar (Diagnostics Pasteur, Marnes-la Coquette, France) and bile-esculine-azide agar (Diagnostics Pasteur). Mueller-Hinton agar (Diagnostics Pasteur) was used for E. faecalis counts in the monomicrobial model.
Peritoneal cell counts.
Total cell counts (polymorphonuclear neutrophils and macrophages) were made on an aliquot of the original peritoneal fluid by using a Malassez counting chamber.
Cytokine assay.
Four 1- ml samples of the original peritoneal fluid recovered from all of the regions of the peritoneal cavity were centrifuged (300 × g for 15 min) and then divided into 200-μl aliquots and stored at −80°C until assaying. The samples were assayed in duplicate. TNF-α activity was measured with a Factor-Test-X rat TNF-α enzyme-linked immunosorbent assay kit (Genzyme Diagnostics, Cambridge, Mass.) according to a previously described technique (37). The sensitivity of the test, which was defined as the lowest concentration of standard which shows greater absorbance than the mean absorbance of the 0-pg/ml sample ± 2 standard deviations (SD), was 10 pg/ml. IL-6 activity was measured by means of a bioassay with the murine hybridoma cell line B9 according to a previously described technique (1). The limit of detection in peritoneal fluid was 0.2 ng/ml. The specificity of the response for IL-6 was assessed by using polyclonal rabbit antimurine IL-6 antibodies (Genzyme, Brussels, Belgium).
α1-acid glycoprotein assay.
Blood samples were obtained by cardiac puncture and transferred to sterile glass tubes. After coagulation, the sera were collected and centrifuged at 500 × g for 5 min. The supernatant was divided into four aliquots and stored at −20°C until the assay. Radial immunodiffusion was used for the assay which involved agar containing 3% (wt/vol) immune serum and 3% (wt/vol) polyethylene glycol 6000 (Fluka, Malakov, France). The values of α1-acid glycoprotein were determined with a monoclonal rabbit anti-rat α1-acid glycoprotein antibody (4). A value of less than 200 mg/liter was considered normal. The limit of detection was 25 mg/liter.
Statistical analysis.
Results are expressed as means ± SD. The LD50s in mice were compared by a Kaplan-Meier analysis by using a log rank test. Continuous parameters were compared by an analysis of variance analysis, followed (in the case of significance) by limited comparisons between the control group without virulence factor (E. faecalis OG1X) and the other groups by using Fisher’s least significant procedures. A chi square test was used for quantitative data. A P value of ≤ 0.05 was considered significant.
RESULTS
LD50s in mice.
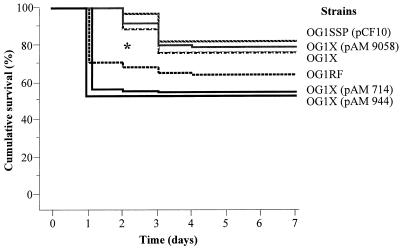
E. faecalis was classified into three groups according to the LD50 (Table 2). The first group, which was characterized by the lowest LD50, included E. faecalis OG1X(pAM 714) and OG1X(pAM 944). An intermediate LD50 was evidenced for E. faecalis OG1RF. Finally, the highest LD50s were observed with E. faecalis OG1X, OG1X(pAM 9058), and OG1SSP(pCF10). With a Kaplan-Meier model, a statistically significant difference was observed within the groups of animals in the delay before death (Fig. 1). Mice inoculated with E. faecalis OG1X(pAM 714), OG1X(pAM 944), and OG1RF strains had a significantly higher and earlier mortality than those receiving E. faecalis OG1X, OG1X(pAM 9058), and OG1SSP(pCF10), in which a low and delayed mortality was observed.
TABLE 2.
LD50s in mice according to the derivative of E. faecalis inoculated
| Mouse groupa | E. faecalis strain | Virulence factor | LD50b |
|---|---|---|---|
| 1 | OG1X | None | 10.5 |
| 2 | OG1RF | Gel | 9.9 |
| 3 | OG1X(pAM 9058) | Agg | 10.5 |
| 4 | OG1X(pAM 944) | Cly | 9.4 |
| 5 | OG1X(pAM 714) | Cly + Agg | 9.6 |
| 6 | OG1SSP(pCF10) | Gel + Agg | 10.6 |
Each group was composed of 90 mice.
Expressed in log10 CFU per milliliter.
FIG. 1.
Cumulative survival curves of mice (expressed as percentages of survivors) after inoculation of log10 CFU of each E. faecalis strain per ml ∗, P < 0.0001 by a Kaplan-Meier analysis with a log rank test.
Peritonitis model in rats. (i) Effect of inoculum on survival.
No mortality was observed in the rat peritonitis model.
(ii) Effect of E. faecalis strains on body weight.
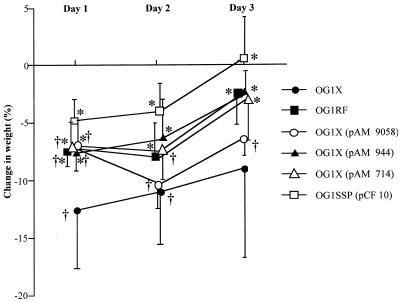
In the polymicrobial model, maximal loss of weight occurred in most groups on day 1, except for the animals receiving the OG1X(pAM 9058) and OG1RF strains, in which maximal weight loss was reported on day 2 (Fig. 2). Progressive recovery was observed afterward. The greatest weight loss was observed in animals receiving the OG1X strain, while the least weight loss was seen with the OG1SSP(pCF10) strain. In the monomicrobial model, there was no difference in the variations of weight within the groups (data not shown). When comparing polymicrobial and monomicrobial models, a significant difference was observed in weight variations in the 3 days of the study (P < 0.01 in every case).
FIG. 2.
Changes in body weight expressed as percentages of baseline (means ± SD) in rats receiving one of the strains of E. faecalis combined with E. coli and B. fragilis. ∗, P < 0.01 compared with changes in the animals receiving the OG1X strain; †, P < 0.01 compared with changes in the animals receiving the OG1SSP(pCF 10) strain.
(iii) Effect of E. faecalis strains on blood cultures.
All animals had positive blood cultures at day 1. A significant decrease in the frequency of positive blood cultures was noted between days 1 and 3 (Table 3). However, the different strains of E. faecalis did not modify the frequency of bacteremia of the other organisms. The frequency of bacteremia due to E. faecalis was more marked in the polymicrobial model than in the monomicrobial one; 75 of 90 rats (80%) had E. faecalis-positive blood cultures in the polymicrobial model versus 26 of 48 rats (55%) in the monomicrobial model (P < 0.01). In the monomicrobial model, the different strains of E. faecalis had similar frequencies of positive culture (data not shown).
TABLE 3.
Numbers of infected animals with positive blood cultures at sacrifice in the polymicrobial model according to the bacterium inoculated
| Bacterium | No. of infected animals positive (% of total)
|
|
|---|---|---|
| Day 1 | Day 3 | |
| E. coli | 45 (98) | 35 (73)c |
| B. fragilis | 48 (100) | 39 (81)c |
| E. faecalisa | 57 (81) | 44 (62)b |
Including all of the strains of E. faecalis.
P < 0.05 compared to day 1.
P < 0.01 compared to day 1.
(iv) Effect of E. faecalis strains on peritoneal cultures.
The bacterial titers within the peritoneal cavity between days 1 and 3 are displayed in Table 4. In the monomicrobial model, no difference was observed on day 1 or 3 or between days 1 and 3 in the different groups of animals. The peritoneal concentrations of E. faecalis were significantly higher on day 1 in the polymicrobial model than in the monomicrobial model in animals receiving E. faecalis OG1X, OG1X(pAM 9058), OG1X(pAM 714), and OG1SSP(pCF10) (data not shown). On day 3, only the animals inoculated with E. faecalis OG1X(pAM 9058) had significantly higher E. faecalis peritoneal concentrations in the polymicrobial model than in the monomicrobial model.
TABLE 4.
Bacterial titers within the peritoneal cavity at sacrifice in animals receiving a polymicrobial inoculum
| Groupb | Titer
|
|||||
|---|---|---|---|---|---|---|
| Day 1 studya
|
Day 3 studya
|
|||||
| E. coli | B. fragilis | E. faecalis | E. coli | B. fragilis | E. faecalis | |
| OG1X | 6.5 ± 1.6 | 5.8 ± 1.3 | 5.6 ± 0.5 | 4.1 ± 1.7c | 2.8 ± 1.8c | 4.7 ± 0.9c |
| OG1RF | 4.1 ± 0.7 | 5.2 ± 0.5 | 4.5 ± 0.9 | 3.8 ± 0.9 | 4.8 ± 1.4 | 4 ± 0.5 |
| OG1X(pAM 9058) | 4.9 ± 1.3 | 5.5 ± 0.6 | 4.7 ± 0.7 | 3.6 ± 0.8c | 4.5 ± 1.3 | 4 ± 0.5c |
| OG1X(pAM 944) | 4.6 ± 1.1 | 5.8 ± 0.6 | 4.5 ± 1.1 | 3.5 ± 0.9c | 4.8 ± 1.2d | 4 ± 0.6 |
| OG1X(pAM 714) | 6.4 ± 1.8 | 6.2 ± 1.3 | 5.2 ± 0.4 | 3.5 ± 1.9d | 4.6 ± 1.7 | 3.6 ± 1.2d |
| OG1SSP(pCF10) | 4.9 ± 0.8 | 5.5 ± 0.8 | 4.5 ± 0.8 | 3.7 ± 0.6d | 4.7 ± 1.5 | 3.9 ± 0.5 |
Bacterial titers are expressed in log10 CFU per milliliter ± SD.
Each group was composed of eight rats on day 1 and eight rats on day 3.
P < 0.05 versus day 1 study for the same bacterium.
P < 0.01 versus day 1 study for the same bacterium.
(v) Effect of E. faecalis strains on peritoneal cell counts.
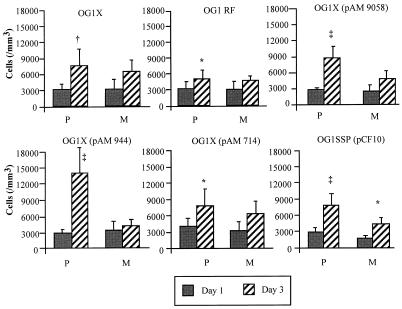
In the polymicrobial model, an increased peritoneal cell count was observed between days 1 and 3 for all groups (Fig. 3), while an increase was noted only for the OG1SSP(pCF10) strain in the monomicrobial model. At day 1, the peritoneal cell counts were similar in each group in the polymicrobial and monomicrobial models, except for the group of animals receiving the OG1SSP(pCF10) strain, in which an increased cell count was noted in the polymicrobial model compared to the monomicrobial inoculum (P < 0.05). On day 3 in the polymicrobial model, the peritoneal cell count was significantly increased in animals receiving the OG1X(pAM 944) strain compared to the other groups (P < 0.01). Moreover, increased peritoneal cell counts were noted at day 3 in the polymicrobial model compared to the monomicrobial model in the groups receiving the OG1X(pAM 9058), OG1X(pAM 944), and OG1SSP(pCF10) strains (P < 0.05, P < 0.01, and P < 0.05, respectively).
FIG. 3.
Peritoneal cell counts expressed in cells per cubic millimeter (means ± SD) measured at days 1 and 3, according to the strain of E. faecalis inoculated in rats receiving a polymicrobial (P) inoculum (one of the six strains of E. faecalis plus E. coli plus B. fragilis) or a monomicrobial (M) inoculum (one of the six strains of E. faecalis). ∗, P < 0.05 changes observed between days 1 and 3; †, P < 0.01 between days 1 and 3; ‡, P < 0.001 between days 1 and 3.
(vi) Effect of E. faecalis strains on cytokine levels.
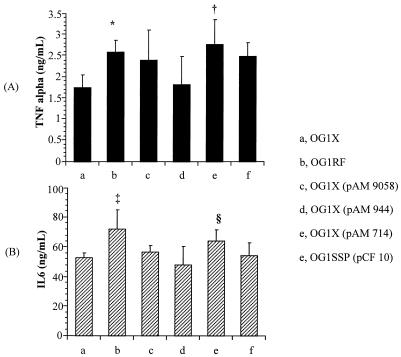
The animals inoculated with E. faecalis OG1X(pAM 714) and OG1RF had higher concentrations of TNF-α in the peritoneal fluid than those receiving E. faecalis OG1X (P < 0.05, P < 0.05) and OG1X(pAM 944) (P < 0.05, P < 0.05) (Fig. 4). The animals receiving E. faecalis OG1RF had higher peritoneal concentrations of IL-6 than those inoculated with E. faecalis OG1X (P < 0.01), OG1X(pAM 944) (P < 0.001), OG1X(pAM 9058) (P < 0.05), and OG1SSP(pCF10) (P < 0.05). Moreover, animals given E. faecalis OG1X(pAM 714) had higher concentrations of IL-6 in the peritoneal fluid than those receiving E. faecalis OG1X(pAM 944) (P < 0.05).
FIG. 4.
Concentrations of TNF-α (A) and IL-6 (B) within the peritoneal fluid expressed in nanograms per milliliter (means ± SD) 6 h after inoculation of a polymicrobial inoculum (one of the six strains of E. faecalis plus E. coli plus B. fragilis). ∗, P < 0.05 compared to E. faecalis OG1X and OG1X(pAM 944) strains; †, P < 0.05 compared to E. faecalis OG1X and OG1X(pAM944) strains; ‡, P < 0.05 compared to E. faecalis OG1X, OG1X(pAM 944), OG1X(pAM 9058), and OG1SSP(pCF10) strains; §, P < 0.05 compared to E. faecalis OG1X(pAM 944).
(vii) Effect of E. faecalis strains on α1-acid glycoprotein concentrations.
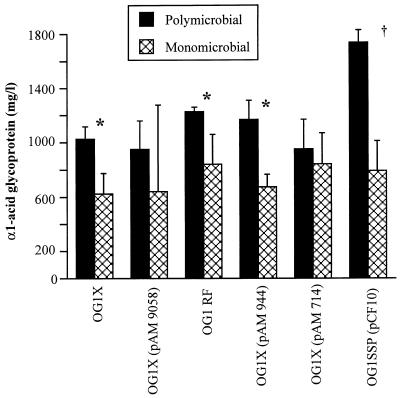
The concentrations of α1-acid glycoprotein in plasma were significantly higher in animals receiving a polymicrobial inoculum than in those receiving E. faecalis alone (P < 0.01), except for animals inoculated with E. faecalis OG1X(pAM 9058) and OG1X(pAM 714) (Fig. 5). In the polymicrobial model, only the animals given E. faecalis OG1SSP(pCF10) had higher α1-acid glycoprotein concentrations than the other groups (P < 0.05). There was no difference within groups in the concentrations of α1-acid glycoprotein in plasma in the monomicrobial model.
FIG. 5.
Concentrations of α1-acid glycoprotein in serum expressed in milligrams per liter (means ± SD) measured at day 3 after inoculation of one of the six isogenic strains of E. faecalis according to whether the strain of E. faecalis was inoculated alone (monomicrobial) or combined in a polymicrobial inoculum. ∗, P < 0.05 compared to E. coli and B. fragilis (polymicrobial); †, P < 0.01 compared to polymicrobial inoculum.
DISCUSSION
The literature on the pathogenic role of E. faecalis strains is somewhat unclear. Their potential pathogenic effects are quite subtle and are not evidenced in nondiscriminative models of infection. In addition, the results of an experimental model of enterococcal infection seem to differ from one model to another, as illustrated by our results.
The direct injection of microorganisms within the peritoneum represents a model of systemic sepsis induced within a very short period of time (9, 33). This model allows a good approach to identify quickly the most pathogenic strains. However, this experimental design may not resemble the development of infection in patients, which commonly occurs over days. Therefore, the information provided by the peritoneal implantation of a septic capsule is of interest. This model, which represents a compartmentalized sepsis with prolonged infectious and inflammatory responses, allows study over a prolonged period of time of the relationships between the host and the offending organisms and among the various organisms inoculated. Taking these features into consideration, we chose these two models in order to study different aspects of the role of virulence factors of enterococci.
Several experimental investigations, mainly performed with rats, have demonstrated the synergistic role of enterococci (2, 11, 28, 30). In these studies, the enterococcal strains did not express any known virulence factor. Nevertheless, increased bacteremia (10, 30) or increased mortality (2, 10) was reported when enterococcus was part of the inoculum. Our current results confirm these observations of bacterial synergy. Moreover, we demonstrated that the pathogenicity of E. faecalis in rats is minimal when inoculated alone, an issue frequently suggested in clinical studies but rarely assessed. All of the parameters that we tested were in agreement in demonstrating that polymicrobial infection and monomicrobial infection significantly differed. On the other hand, there was divergence between the parameters according to the strain tested in the rat polymicrobial model.
Our observations with the mouse model poorly predicted the effects of enterococci in rats. To the best of our knowledge, the virulence of a microorganism is rarely assessed in two different models in the same study, raising difficulties in the interpretation of the results of previous studies. The rat model has been largely used to evaluate the mechanisms of bacterial synergy (2, 11, 28, 30), and the information obtained seems to be clinically relevant (40, 41). Since we have previously demonstrated that enterococcal pathogenicity is expressed in a dose-dependent fashion (30, 31), the results reported with the rat model could be related to an insufficient concentration of enterococci in the inoculum. In addition, virulence factors might not be expressed in rats or might not be important in the model. Only few studies assessing the virulence of enterococci are available, and these are always performed with monomicrobial infections in mouse and rabbit models (5, 21, 24). It is not possible from the data available to determine whether virulence factors are expressed in intraabdominal infections or at what level and whether this expression is similar in poly- and monomicrobial infections.
In the mouse model, the highest mortality was observed for animals given the strain with the cytolysin factor. This is in agreement with the results obtained by Ike et al. (21), who reported that inoculation of a Cly-producing strain of E. faecalis caused an increased mortality and a 90% decrease in LD50 compared to a control strain. Moreover, in previous studies performed with models of endophthalmitis (24, 38) or endocarditis, disease severity was markedly increased after inoculation of an enterococcal strain producing Cly (5). In clinical practice, the Cly phenotype, although difficult to detect, seems to be common, as reported by Ike et al. (22), who tested 97 clinical isolates of E. faecalis and showed that 60% were hemolytic. The data reported by Huycke et al. also suggested the pathogenicity of Cly in cases of enterococcal bacteremia in which a fivefold increased risk of death was observed in patients infected with an E. faecalis strain demonstrating a Cly phenotype (19).
In our models of the mouse and rat, Agg did not seem to exert a significant virulence. In the mouse model, the mortality level induced by strains mating Agg and no Cly was low and delayed. The effects of adherence and persistent infection, which were linked to the properties of Agg (17, 27, 34), were not observed in our rat model, suggesting that Agg has only a minor role to play in the severity of peritoneal infection. Agg is the most difficult factor to assess in vivo, since there is no clinical marker of its activity. Only a molecular approach could demonstrate its activation, as previously performed by other authors (8).
Gelatinase is probably the least-studied virulence factor. In the mouse model, this factor induced an increased mortality compared to the control strain OG1X, as well as a 68% decrease of the LD50. In the rat model, the Gel strain seemed to be the least pathogenic, with only moderate weight loss and a mild peritoneal cellular reaction. The pathogenicity of Gel strains of E. faecalis in intraabdominal infections is purely hypothetical and has never been demonstrated. However, since a relationship between Gel and gentamicin resistance has been reported in one study (8), therapeutic difficulties could be expected in the presence of this factor.
The effect obtained with the OG1X strain (control strain) in the polymicrobial model is unclear. Significant and prolonged weight loss combined with an increased frequency of positive blood cultures was noted in animals receiving this strain. Reversion of this mutant to a wild expression of gelatinase might be involved, although this strain acts differently from the OG1RF strain. A lower level of interaction of the other OG1 derivatives with E. coli and B. fragilis might also be involved. In this setting, the gelatinase may damage the virulence factors of these organisms. Even though the cytolysin demonstrates bacteriocin activities that might be deleterious to other bacteria, such properties have not been reported with gelatinase. On the other hand, OG1X was generated with nitrosoguanidine, a technique which could induce a mutation in other genes.
The combination of two virulence factors (Cly and Agg or Agg and Gel) gave results different from those reported with a single factor. A combination of Cly and Agg generated the lowest LD50 in the mouse model. These results suggest a possible interaction between Cly and Agg, the mechanism of which remains to be elucidated. Similar synergy has been previously reported with the combination of Cly and Agg, which caused an increased level of mortality in a rabbit model of endocarditis (5). In contrast, the combination of Agg and Gel did not seem to result in increased pathogenicity. In the rat model, the effects of the combinations were minimal, but the inflammatory response was increased, as assessed by the concentrations of α1-acid glycoprotein in plasma.
In conclusion, our study confirms the previously reported mechanisms of bacterial synergy between enterococci and other organisms. However, the marked discrepancy in results obtained from mice and rats renders any extrapolation to clinical practice difficult. The use of one virulence factor only minimally influenced the course of the disease. In contrast, the combination of two factors (Cly and Agg) seemed to be responsible for the more severe peritoneal infection which resulted in mice. In view of our results, other components shared by these strains may have a greater influence on the pathogenicity of enterococci. A good candidate could be the bacterial wall and, more specifically, some of its constituents, such as peptidoglycan or lipoteichoic acid, as has previously been suggested with experimental peritonitis (31).
ACKNOWLEDGMENTS
We thank B. Murray, Texas University, Houston, and D. B. Clewell, Michigan University, Ann Arbor, for providing the strains of E. faecalis; and C. Poüs, Faculté de Pharmacie de Chatenay-Malabry, for performing the α1-acid glycoprotein assay.
This work was supported by a grant from the French Foundation for Medical Research.
REFERENCES
- 1.Aarden L A, De Groot E R, Schaap O L, Lansdorp P M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987;17:1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- 2.Arai S, Hayashi S. Therapeutic effects of cefpirome (HR810) on experimental mixed infections with Enterococcus faecalis and Escherichia coli in mice. Infection. 1990;18:186–190. doi: 10.1007/BF01642112. [DOI] [PubMed] [Google Scholar]
- 3.Barie P S, Christou N V, Patchen-Dellinger E, Rout W R, Stone H H, Waymack J P. Pathogenicity of the Enterococcus in surgical infections. Ann Surg. 1990;212:155–159. doi: 10.1097/00000658-199008000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biou D, Monnet M, Millet F, Feger J, Durand G. An immunochemical procedure to evaluate the degree of desialylation of α1-acid glycoprotein in rat serum. J Immunol Methods. 1984;74:267–271. doi: 10.1016/0022-1759(84)90293-x. [DOI] [PubMed] [Google Scholar]
- 5.Chow J W, Tall L A, Perri M B, Vasquez J A, Donadebian S M, Clewell D B, Zervos M J. Plasmid-associated hemolysin and aggregation substance production contribute to virulence in experimental enterococcal endocarditis. Antimicrob Agents Chemother. 1993;37:2474–2477. doi: 10.1128/aac.37.11.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clewell D B. Bacterial sex pheromone-induced plasmid transfer. Cell. 1993;73:9–12. doi: 10.1016/0092-8674(93)90153-h. [DOI] [PubMed] [Google Scholar]
- 7.Clewell D B, Weaver K E. Sex pheromones and plasmid transfer in Enterococcus faecalis. Plasmid. 1989;21:175–184. doi: 10.1016/0147-619x(89)90041-3. [DOI] [PubMed] [Google Scholar]
- 8.Coque T M, Patterson J E, Steckelberg J M, Murray B E. Incidence of hemolysin, gelatinase, and aggregation substance among enterococci isolated from patients with endocarditis and other infections and from feces of hospitalized and community-based persons. J Infect Dis. 1995;171:1223–1229. doi: 10.1093/infdis/171.5.1223. [DOI] [PubMed] [Google Scholar]
- 9.Cross A C, Opal S M, Sadoff J C, Gemsli P. Choice of bacteria in animal models of sepsis. Infect Immun. 1993;61:2741–2747. doi: 10.1128/iai.61.7.2741-2747.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalhoff A. Influence of Escherichia coli on Streptococcus faecalis in mixed cultures and experimental animal infections. Eur J Clin Microbiol. 1982;1:17–21. doi: 10.1007/BF02014135. [DOI] [PubMed] [Google Scholar]
- 11.Dalhoff A. Therapy of infections caused by mixed cultures of Escherichia coli and Streptococcus faecalis. Curr Microbiol. 1982;7:275–280. doi: 10.1007/BF02014135. [DOI] [PubMed] [Google Scholar]
- 12.Dunny G, Yuhasz M, Ehrenfeld E. Genetic and physiological analysis of conjugation in Streptococcus faecalis. J Bacteriol. 1982;151:855–859. doi: 10.1128/jb.151.2.855-859.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunny G M. Genetic function and cell-cell interactions in the pheromone-inducible plasmid transfer system of Enterococcus faecalis. Mol Microbiol. 1990;4:689–696. doi: 10.1111/j.1365-2958.1990.tb00639.x. [DOI] [PubMed] [Google Scholar]
- 14.Dunny G M, Brown B L, Clewell D B. Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc Natl Acad Sci USA. 1978;75:3479–3483. doi: 10.1073/pnas.75.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehrenfeld E E, Clewell D B. Transfer functions of the Streptococcus faecalis pAD1: organization of plasmid DNA encoding response to sex pheromone. J Bacteriol. 1987;169:3473–3481. doi: 10.1128/jb.169.8.3473-3481.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gold O, Jordan H V, Van Houte J. The prevalence of enterococci in the human mouth and their pathogenicity in animal models. Arch Oral Biol. 1975;20:473–477. doi: 10.1016/0003-9969(75)90236-8. [DOI] [PubMed] [Google Scholar]
- 17.Guzman C A, Pruzzo C, Lipira G, Calegari L. Role of adherence in pathogenesis of Enterococcus faecalis urinary tract infection and endocarditis. Infect Immun. 1989;57:1834–1838. doi: 10.1128/iai.57.6.1834-1838.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hase C C, Finkelstein R A. Bacterial extracellular zinc-containing metalloproteases. Microbiol Rev. 1993;57:823–837. doi: 10.1128/mr.57.4.823-837.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huycke M K, Spiegel C A, Gilmore M S. Bacteremia caused by hemolytic, high-level gentamicin-resistant Enterococcus faecalis. Antimicrob Agents Chemother. 1991;35:1626–1634. doi: 10.1128/aac.35.8.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ike Y, Clewell D B. Genetic analysis of the pAD1 pheromone response in Streptococcus faecalis, using transposon tn917 as an insertional mutagen. J Bacteriol. 1984;158:777–783. doi: 10.1128/jb.158.3.777-783.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ike Y, Hashimoto H, Clewell D. Hemolysin of Streptococcus faecalis subspecies zymogenes contributes to virulence in mice. Infect Immun. 1984;45:528–530. doi: 10.1128/iai.45.2.528-530.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ike Y, Hashimoto H, Clewell D. High incidence of hemolysin production by Enterococcus (Streptococcus) faecalis strains associated with human parenteral infections. J Clin Microbiol. 1987;25:1524–1528. doi: 10.1128/jcm.25.8.1524-1528.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jett B D, Huycke M M, Gilmore M S. Virulence of enterococci. Clin Microbiol Rev. 1994;7:462–478. doi: 10.1128/cmr.7.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jett B D, Jensen H G, Nordquist R E, Gilmore M S. Contribution of the pAD1-encoded cytolysin to the severity of experimental Enterococcus faecalis endophthalmitis. Infect Immun. 1992;60:2445–2452. doi: 10.1128/iai.60.6.2445-2452.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson A P. The pathogenicity of enterococci. J Antimicrob Chemother. 1994;33:1083–1089. doi: 10.1093/jac/33.6.1083. [DOI] [PubMed] [Google Scholar]
- 26.Kelton J G, Ulan R, Stiller C, Holmes E. Comparison of chemical composition of peritoneal fluid and serum. Ann Int Med. 1978;89:67–70. doi: 10.7326/0003-4819-89-1-67. [DOI] [PubMed] [Google Scholar]
- 27.Kreft B, Marre A, Schamm U, Wirth R. Aggregation substance of Enterococcus faecalis mediates adhesion to cultured renal tubular cells. Infect Immun. 1992;60:25–30. doi: 10.1128/iai.60.1.25-30.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matlow A G, Bohnen J M A, Nohr C, Christou N, Meakins J. Pathogenicity of enterococci in a rat model of fecal peritonitis. J Infect Dis. 1989;160:142–145. doi: 10.1093/infdis/160.1.142. [DOI] [PubMed] [Google Scholar]
- 29.Miyazaki S, Ohno A, Kobayashi I, Uji T, Yamaguchi K, Goto S. Cytotoxic effect of hemolytic culture supernatant from Enterococcus faecalis on mouse polymorphonuclear neutrophils and macrophages. Microbiol Immunol. 1993;37:265–270. doi: 10.1111/j.1348-0421.1993.tb03209.x. [DOI] [PubMed] [Google Scholar]
- 30.Montravers P, Andremont A, Massias L, Carbon C. Investigation of the potential role of Enterococcus faecalis in the pathophysiology of experimental peritonitis. J Infect Dis. 1994;169:821–830. doi: 10.1093/infdis/169.4.821. [DOI] [PubMed] [Google Scholar]
- 31.Montravers P, Mohler J, Saint-Julien L, Carbon C. Evidence of the proinflammatory role of Enterococcus faecalis in polymicrobial peritonitis in rats. Infect Immun. 1997;65:144–149. doi: 10.1128/iai.65.1.144-149.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray B E, Singh K V, Ross R P, Heath J D, Dunny G M, Weinstock G M. Generation of restriction map of Enterococcus faecalis strain OG1 and investigation of growth requirements and regions encoding biosynthetic function. J Bacteriol. 1993;175:5216–5223. doi: 10.1128/jb.175.16.5216-5223.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Natanson C, Hoffman W D, Suffredini A F, Eichacker P Q, Danner R L. Selected treatment strategies for septic shock based on proposed mechanisms of pathogenesis. Ann Intern Med. 1994;120:771–783. doi: 10.7326/0003-4819-120-9-199405010-00009. [DOI] [PubMed] [Google Scholar]
- 34.Olmsted S B, Dunny G M, Erlandsen S L, Wells C L. A plasmid-encode surface protein on Enterococcus faecalis augments its internalization by cultured intestinal epithelial cells. J Infect Dis. 1994;170:1549–1556. doi: 10.1093/infdis/170.6.1549. [DOI] [PubMed] [Google Scholar]
- 35.Olmsted S B, Erlandsen S L, Dunny G M, Wells C L. High-resolution visualization by field emission scanning electron microscopy of Enterococcus faecalis surface proteins encode by the pheromone-inducible conjugative plasmid pCF10. J Bacteriol. 1993;175:6229–6237. doi: 10.1128/jb.175.19.6229-6237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Onderdonk A B, Bartlett J G, Louie T, Sullivan-Seigler N, Gorbach S L. Microbial synergy in experimental intra-abdominal abscess. Infect Immun. 1976;13:22–26. doi: 10.1128/iai.13.1.22-26.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seu P, Imagawa D K, Wasef E, Olthoff K M, Hart J, Stephens S, Dempsey R A, Busuttil R W. Monoclonal anti-tumor necrosis factor-alpha antibody treatment of rat cardiac allografts: synergism with low dose cyclosporine and immunohistological studies. J Surg Res. 1991;50:520–528. doi: 10.1016/0022-4804(91)90035-k. [DOI] [PubMed] [Google Scholar]
- 38.Stevens S X, Jensen H G, Jett B D, Gilmore M S. A hemolysin-encoding plasmid contributes to bacterial virulence in experimental Enterococcus faecalis endophtalmitis. Investig Ophthalmol Vis Sci. 1992;33:1650–1656. [PubMed] [Google Scholar]
- 39.Su Y A, Sulavik M C, He P, Makinen K K, Makinen P L, Fiedler S, Wirth R, Clewell D B. Nucleotide sequence of the gelatinase gene (gelE) from Enterococcus faecalis var. liquefaciens OG1-10. Infect Immun. 1991;59:415–420. doi: 10.1128/iai.59.1.415-420.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinstein W M, Onderdonk A B, Bartlett J G, Gorbach S L. Experimental intra-abdominal abscesses in rats: development of an experimental model. Infect Immun. 1974;10:1250–1255. doi: 10.1128/iai.10.6.1250-1255.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weinstein W M, Onderdonk A B, Bartlett J G, Louie T J, Gorbach S L. Antimicrobial therapy of experimental intraabdominal sepsis. J Infect Dis. 1975;132:282–286. doi: 10.1093/infdis/132.3.282. [DOI] [PubMed] [Google Scholar]