Abstract
Background:
The heavy metals cause repro-toxicity via oxidative stress and suppress the antioxidant enzymes. Kaempferol and vitamin E possess antioxidant properties that can counteract the deleterious heavy metals effects.
Aim:
The present study was directed to investigate the protective role of kaempferol, alone or with vitamin E, on testicular toxicity mediated by lead acetate in male rats.
Methods:
Fifty adult male rats were randomly grouped into five groups (n = 10): the control group received 5 ml distilled water, and the Pb group was intraperitoneally injected with 20 mg/kg of lead acetate. The Pb + Vitamin E group received Pb with 100 mg/kg of vitamin E, the Pb + KAF group received Pb with 50 mg/kg of kaempferol, the Pb + KAF + Vitamin E group received Pb with kaempferol and vitamin E for 6 weeks.
Results:
The testicular levels of superoxide dismutase, catalase, steroidogenic enzyme, serum testosterone, follicle-stimulating hormone, interleukin (IL)-10, and sperm function were significantly decreased in the Pb group compared with all experimental groups. These parameters were significantly elevated in the Pb + KAF + Vitamin E group compared to other experimental groups. Lead acetate caused elevation in testicular malondialdehyde, nitric oxide, IL-6, IL-1β, tumor necrosis factor-α, nuclear factor kappa, and sperm abnormality compared to all treatment groups. All these parameters were significantly declined in the Pb + KAF + Vitamin E group and Pb + KAF group compared with the Pb group. The fold changes of pituitary follicle-stimulating hormone beta, gonadotropin-releasing hormone receptor, and luteinizing hormone beta, and testicular CYP11A1, LH receptor, and FHr gene expression were significantly upregulated in Pb + KAF + Vitamin E group compared with all experimental groups. In addition, KAF + Vitamin E has the potential to improve testicular regeneration in seminiferous tubules, Leydig, and Sertoli cells.
Conclusion:
Administration of kaempferol alone or with vitamin E can mitigate lead acetate-induced testicular toxicity in rats via its antioxidant and anti-inflammatory properties. The current research is the first to demonstrate that kaempferol can exert a preventive role in testicular dysfunction.
Keywords: Antioxidant, Anti-inflammatory, Kaempferol and Vitamin E, Lead acetate, Testicular toxicity
Introduction
Lead is a common heavy metal found in the environment. It can be utilized in various industries, including paints, building materials, batteries, cars, gasoline, hair dyes, cosmetics, pesticides, and fertilizer (Karimfar et al., 2016). Exposure to lead can occur when consuming grains, vegetables, and fruits grown on soil containing high quantities of lead (Wani et al., 2015). As a result of industrial activities and the emissions from vehicles, lead has gained recognition as a significant environmental pollutant. Human systems can absorb lead through weather, water, and food and accumulate in different tissues, as recorded by Abdulnabi (2016), who found that Iraqi water was contaminated by lead at a concentration of 0.9229 mg/l; furthermore, even minimal exposure to lead can cause organs dysfunction. Lead, Pb+2, can enter the body via dermal contact, ingestion, and inhalation and deposit in different tissues such as the brain, lung, liver, kidney, vas deferens, seminal vesicle, and testis (Wani et al., 2015, Karimfar et al., 2016). Lead ions can interfere with various cations, such as Mg+2, Fe+2, and Zn+2, altering cellular pathway signaling and disturbing gene expression of enzymes and hormones (Talpur et al., 2018). Furthermore, the hypothalamic-pituitary-testicular axis is the primary site of lead’s adverse effects on the male reproductive system, causing reduced sperm count, altered sperm maturation, and changes in sperm morphology that can result in infertility (Li et al., 2018, Oyeyemi et al., 2022). Lead exposure causes impairment in male reproductive function via apoptosis, testicular oxidative stress, and declined testicular antioxidants (Wahab et al., 2019). Besides, a study reported that the lead ions can elevate testicular caspase-3 protein expression, cause cellular lipid peroxidation, and suppress antioxidant activities, which results in excessive reactive oxygen species (ROS) production and cellular oxidative stress in rats (Wani et al., 2015). Overproduction of ROS and oxidative stress are the central pathological mechanisms of lead acetate-induced testicular dysfunction, which negatively impacts sperm DNA, testicular metabolism, sperm function, and fertilization failure, resulting in infertility (Li et al., 2018).
Vitamin E comprises a group of lipid-soluble molecules and consists of at least 10 homologous derivatives of 6 chromanol, including tocotrienols, tocomonoenols, and tocopherols. The most biologically active homologous of vitamin E is α- tocopherols (Azzi, 2019). Vitamin E possesses antioxidant activities and prevents subcellular and cellular phospholipid peroxidation (Oyeyemi et al., 2015). Vitamin E is present in eggs, wholemeal cereal, vegetable oil, green leafy, seafood, and fruits (Azzi, 2019). Vitamin E is biologically essential due to its ability to scavenge ROS and reactive nitrogen species (RNS) and reduce oxidative stress (Aboubakr et al., 2023). Vitamin E regulates immunity, cellular signaling, gene expression, and hormone biosynthesis. Its antioxidant and protective effects on testicles exposed to fluoxetine (Tohid et al., 2014) and cadmium (Amanpour et al., 2020) have been demonstrated. Besides, vitamin E can attenuate testicular toxicity induced by lead acetate in rats by preventing lipid peroxidation via antioxidant properties (Oyeyemi et al., 2022).
Kaempferol, ″3,5,7-trihydroxy-2-(4-hydroxyphenyl) -4H- chromen-4-one″ is a type of flavonoids, a polyphenol and lipophilic, also known as kaempferol flavonol, kaempferide, kaempferol-3 (Jin et al., 2023), it can be extracted from tea and many vegetables and fruits such as green cabbage, broccoli, olive oil, apple, chives, cucumber, strawberries, lettuce, onion, grapes, spinach, tomatoes, horseradish, and cowpea (Alam et al., 2020). Studies in vivo and in vitro reported that kaempferol possesses therapeutic effects in neurodegenerative disease, colitis, and in acute and chronic inflammatory disease, including acute lung injury and postmenopausal bone loss (Ren et al., 2019, Liu et al., 2022, Lim et al., 2023), as well as, kaempferol has a protective role in liver injury and prevents metabolic disorders, furthermore, anticancer role in breast cancer, and hepatocellular carcinoma (Wang et al., 2019, Sharma et al., 2021, Fan et al., 2023).
Recently, Liu et al. (2022) reported that kaempferol exerts anti-inflammatory and antioxidant effects against heat stress-induced Sertoli cell injury in vitro, which prevents spermatogenic disorders; however, the protective effects and mechanisms of kaempferol alone and with vitamin E on lead acetate-induced hypothalamic-hypophyseal-testicular dysfunction in vivo have not been reported. Based on this limitation, the present research aimed to investigate the possible mechanism(s) by which kaempferol, separately or combined with vitamin E, improves testicular function in rats exposed to lead acetate.
Materials and Methods
Experimental animals
Adult Wistar male rats weighing 251–315 g at age 13 weeks had been used in this study, and housed under controlled laboratory conditions with 12-hour (light and dark cycle) at 27°C ± 1°C. They had provided ad libitum drinking water and standard feed. All experiments were conducted at Veterinary Medicine/Al-Qasim Green University from 20 August 2022 to 23 April 2023. Before beginning the experiment, the rats were allowed 14 days to acclimate.
Experimental design
Fifty Wistar rats were assigned into five groups. Each group contains 10 animals. The first group (control) was administered 5 ml of normal saline orally. The second group (Pb) was injected intraperitoneally (I.P) 20 mg/kg.b.w. of lead acetate (PVT, Ltd., India) single dose per day for 6 weeks to cause reproductive toxicity, the third group (Pb + Vitamin E) was injected 20 mg/kg/day of lead acetate followed by giving 100 mg/kg. b.w. of vitamin E (CENTURY®, USA). Fourth (Pb + KAF): rats were injected with 20 mg/kg. I.P of lead acetate followed by administrating 50 mg/kg.b.w. of kaempferol, KAF (Violet Herbs Co., USA), and fifth groups (Pb + KAF + Vitamin E) injected daily 20 mg/kg. I.P. of lead acetate followed by administering orally 50 mg/kg. b.w. of kaempferol and 100 mg/kg.b.w. of vitamin E. Vitamin E and KAF were administered orally by gavage tube and daily for 6 weeks consecutively. The doses of lead acetate, vitamin E, and kaempferol were used depending on previous research (Aithamadouche et al., 2013, Oyeyemi et al., 2015, Alkhalidy et al., 2018).
After 6 weeks, blood samples were collected and centrifugated at 5,000 rpm for 10 minutes to obtain sera for determining the reproductive hormone levels. After being sacrificed, the left testis was collected for histological study. The pituitary and right testis was taken to assess the fold changes levels of pituitary luteinizing hormone beta subunit (LHβ), follicle-stimulating hormone beta subunit (FSHβ), gonadotropin-releasing hormone receptor (GnRHr), and testicular FSH receptor (FSHr), LH receptor (LHr), and cytochrome P450 A1 genes.
Analysis of seminal fluid function
To calculate the sperm count, the epididymal tail was put in 2 ml of saline at 37°C and cut into tiny pieces. A drop suspension was placed in the Neubauer chamber to assess the count using Raji’s method (Raji et al., 2006).
To evaluate sperm motility, 10 µl of the epididymal suspension was placed in the warm slide and covered with a glass slip; the sperm motility was observed under a light microscope (Atashfaraz et al., 2013).
The percentage of sperm morphology was determined according to Narayana’s method (Narayana et al., 2005). One drop of the epididymal suspension was smeared in the slide and stained by eosin-nigrosine, then observed under a microscope.
The method of (Björndahl et al., 2003) was used to determine the percentage of sperm viability by mixing a drop of semen suspension with an eosin-nigrosine dye and examined under a microscope, 400 sperm were calculated since the heads of live sperm were unstained whereas heads of dead sperm were stained.
Determination of the reproductive hormone levels
The sera obtained at the end of the experiment were used to determine the levels of luteinizing hormone (LH), testosterone, and Follicle stimulating hormone (FSH) by using ELIZA kits “Elabscience, Company, USA” according to the manufacturer’s instruction.
Preparation of testis homogenate
From each rat, 100 µg of right testis homogenized in 50 mM TrisHCl at pH 7.4 containing KCl (1.15%) for preparing a 20% (w/v 1/5) testicular tissue homogenate using Potter–Elvehjem homogenizer. The homogenates were centrifugated via cold centrifuge at 4°C for 10 minutes at 10,000 g. The testicular supernatants were used to assess levels/activities of oxidants/antioxidants, pro and anti-inflammatory cytokines, and steroidogenic enzymes/proteins (Udefa et al., 2020).
Determination of oxidant and antioxidant markers
The activities of testicular catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx), as well as malondialdehyde (MDA) and nitric oxide (NO), were determined according to the method described by Nishikimi et al. (1972); Satoh (1978); Green et al. (1982); Aebi (1984); Mohandas et al. (1984). According to the kit manufacturer’s instructions, the supernatant was used to determine antioxidant and oxidant markers.
Determination of steroidogenic acute regulatory (StAR) protein and steroidogenic enzyme activities
The testicular supernatant was used to assess the testicular StAR protein and steroidogenic enzymes, 3β-Hydroxysteroid dehydrogenase (3β-HSD), and 17β-Hydroxysteroid dehydrogenase(17β-HSD), according to the kit manufacturer’s instructions of ELISA (Elabscience®, USA).
Determination of cytokines
The levels of interleukin (IL)-1β, IL-6, IL 10, nuclear factor kappa B (NF-KB), tumor necrosis factor-α (TNF-α), and C-reactive protein were assessed by using ELISA kits (Elabscience®, USA) following the manufacturer’s instruction.
Quantification of gene expression
Extraction of RNA, cDNA synthesis, and analysis Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
Total RNAs were extracted from tissue samples (testicular and pituitary tissues) by adding 1 µl of TRIzol reagent to 100 mg of the tissue sample. This was performed according to the manufacturer’s instructions for the Bioneer kit provided by Korea. The nano-drop-spectrophotometer “Thermos, USA” was used at absorbance 260 and 280 nm to determine the quantity and purity of extracted RNA.
The extracted RNA samples were treated with DNase-I-enzyme to remove any genome that may be present; this was performed according to the manufacturer’s protocol of the Promega kit provided by the USA. The RNA samples were treated with cDNA synthesis depending on the protocol explained by AccuPower®, Korea kit. The SYBER Green was performed to detect gene amplification by a real-time PCR machine. The primers used in the present experiment were designed by the NCBI Gene bank database and primer three tools and illustrated in Table 1. The relative expression “fold change” of target genes in testicular and pituitary tissues was calculated by ∆∆Ct method explained by Livak and Schmittgen (2001), which depended on the target gene relative to GAPDH (housekeeping gene).
Table 1. Primer sequences using real-time qPCR.
| NCBI reference | Pb product | Sequence (5′-3′) | Primer | Genes |
|---|---|---|---|---|
| NM_031038 | 109 | ″CAC GAG TCC TTC ATC AGG ACC″ ″GAG GTG GCA AAT GCC ACT GT″ |
Forward Reverse |
GnRHr |
| NM_001007597 | 111 | ″CAT AAG ATT GCC TGG CTG TGC″ ″CTT ACA GTG CAG TCG GTG CT″ |
Forward Reverse |
FSHβ |
| NM_012858 | 104 | ″GAG TAC TGC CAG CTG CCT TG″ ″GTC TAC ACC AGG TGG GCA GC″ |
Forward Reverse |
LHβ |
| NM_199237 | 119 | ″CCA GGT CAA CAT ACC GCT TG″ ″GAT TTG CCG CTT CAA GTT TGC” |
Forward Reverse |
FSHr |
| NM_012978 | 109 | ″CTG GAG AAG ATG CAC AGT GG″ ″GAA TGG ACT CCA GCC CGT G″ |
Forward Reverse |
LHr |
| NM_017286 | 106 | ″GAC CAT CTT CCT CAT CAA CGT G″ ″GAT AGG CTT CTC AGG CAT CAG G″ |
Forward Reverse |
CYP11A1 |
| NM_017008 | 109 | ″CAA GAA GGT GGT GAA GCA GG″ ″GGT GGA AGA ATG GGA GTT GCT″ |
Forward Reverse |
GAPDH |
Histological study
The left testis was fixed in 10% buffered formaldehyde for 48 hours and processed histologically depending on the method adopted by Mescher and Junqueira (2013), as follows: dehydration, clearing, embedded in paraffin and stained by hematoxylin and eosin (H&E) after sectioning into 5-µm slices.
Statistical analysis
The results of the present experiment were statistically analyzed using SPSS version 16. The variances among the experimental groups were evaluated by using one-way ANOVA. The data is represented as the mean (M) ± standard deviation (SD). The variances at p < 0.05 are significant statistically (Schefler, 1980).
Ethical approval
The ethical committee and scientific committee of Veterinary Medicine at Al-Qasim Green University granted approval for the current experiment, ensuring proper care and use of laboratory animals (ESCVM, NO. 1192022).
Results
Effects of kaempferol, vitamin E, and lead acetate on oxidative stress and antioxidants markers in male rats
The levels of testicular MDA, NO, CAT, SOD, and GPx in all experimental rats are shown in Table 2. Lead acetate exposure caused significant (p < 0.05) elevation in MDA and NO levels, as well as significant (p < 0.05) decrement in SOD, CAT, and GPx activities in the Pb group compared to all experimental groups (Table 2).
Table 2. Effects of kaempferol, vitamin E, and lead acetate on testicular oxidant stress and antioxidant status markers in male rats.
| Pb + KAF + Vitamin E | Pb + KAF | Pb + Vitamin E | Pb | C | Parameters |
|---|---|---|---|---|---|
| 0.289 ± 13.779a | 0.206 ±7.415c | 0.183 ± 6.345d | 0.224± 2.382e | 0.155 ± 8.570b | SOD (U/mg protein) |
| 1.497± 89.945a | 0.712± 67.928c | 0.998± 62.231d | 1.874± 36.103e | 1.248 ± 75.145b | CAT (U/mg protein) |
| 0.260± 6.822a | 0.169± 3.665c | 0.133± 2.932d | 0.046± 1.068e | 0.130± 5.261b | GPx (U/minute/mg protein) |
| 0.181± 1.544e | 0.312± 3.615c | 0.313 ± 4.823b | 0.626± 10.196a | 0.190 ± 2.684d | MDA (nmol/mg protein) |
| 0.052± 2.179e | 0.046± 5.881c | 0.337 ± 6.731b | 0.298± 15.151a | 0.041 ± 5.111d | NO (µmol/g protein) |
Values are expressed as Mean ± SD. Various letters were referred to statistical differences among groups at level (p < 0.05). (C): Control group, (Pb): Lead acetate group, (Pb + Vitamin E): Lead acetate + 100 mg/kg.b.w of vitamin E group, (Pb + KAF): Pb+ 50 mg/kg.b.w. of kaempferol group, and (Pb + KAF + Vitamin E): Pb + 50 mg/kg.b.w. of kaempferol + 100 mg/kg.b.w. of vitamin E group. SOD: superoxide dismutase, CAT: catalase, GPx: glutathione peroxidase, and MDA: malondialdehyde.
On the other hand, the testicular GPx, CAT, and SOD activities were significantly (p < 0.05) increased; in contrast, MDA and NO were significantly (p < 0.05) decreased in the Pb + KAF + vitamin E group compared with Pb, Pb + KAF, Pb + vitamin E, and control rats. The testicular GPx, SOD, and CAT activities registered a significant (p < 0.05) elevation in the Pb + KAF group compared to the Pb + vitamin E and Pb groups. SOD, GPx, and CAT activities were significantly (p < 0.05) elevated in Pb + vitamin E group compared to Pb rats. In addition, MDA and NO levels were significantly (p < 0.05) reduced in Pb + KAF and Pb + vitamin E relative to Pb rats (Table 2).
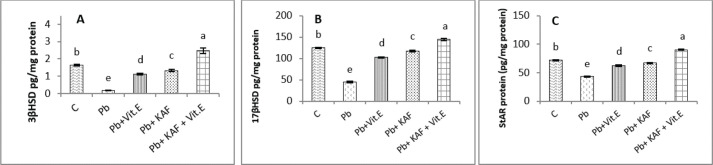
Effects of kaempferol, vitamin E, and lead acetate on testicular StAR protein, 3β-HSD, and 17β-HSD activities in male rats
The activities of 3βHSD and 17βHSD were significantly (p < 0.05) increased in rats co-administration of kaempferol and vitamin E with Pb compared with Pb, Pb + KAF, and Pb + vitamin E, and control groups, whereas, in comparison with all treatment groups, injection of lead acetate, Pb group, for 6 weeks caused a decline (p < 0.05) in the activities of 3β-HSD and 17β-HSD. The actions of 17β-HSD and 3β-HSD were increased significantly (p < 0.05) in Pb plus KAF compared with the Pb group and Pb plus vitamin E. These steroidogenic enzymes increased significantly (p < 0.05) in Pb cotreated with vitamin E compared to Pb rats (Fig. 1A and B).
Fig. 1. Testicular steroidogenic enzyme activities and StAR protein levels in experimental rats. (A) 3β-HSD: 3β-Hydroxysteroid dehydrogenase, (B) 17β-HSD: 17β- Hydroxysteroid dehydrogenase, and (C) StAR protein: Steroidogenic acute regulatory protein. Various letters were referred to statistical differences among groups at level (p < 0.05). (C): Control group, (Pb): Lead acetate group, (Pb + Vitamin E): Lead acetate + 100 mg/kg.b.w of vitamin E group, (Pb + KAF): Pb + 50 mg/kg.b.w. of kaempferol group, and (Pb + KAF + Vitamin E): Pb + 50 mg/kg.b.w. of kaempferol + 100 mg/kg.b.w. of vitamin E group.
The StAR protein level was elevated significantly (p < 0.05) in the Pb co-treated with kaempferol and vitamin E group compared to other experimental groups. It was significantly (p < 0.05) raised in Pb + KAF and Pb + vitamin E groups compared to Pb rats. The StAR protein levels declined significantly (p < 0.05) in Pb rats compared with all experimental groups (Fig. 1C).
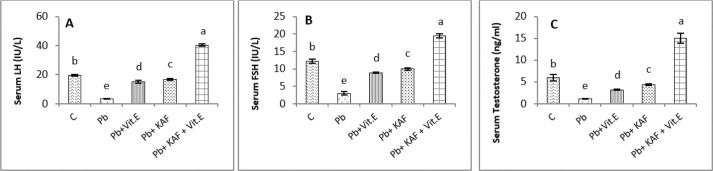
Effects of kaempferol, vitamin E, and lead acetate on reproductive hormones in male rats
The concentration of FSH, testosterone, and LH in all experimental rats is shown in Figure 2. The injection of lead acetate for 6 weeks (Pb group) caused a significant (p < 0.05) decrement in testosterone, FSH, and LH concentrations when compared with all treatment rats (Fig. 2), whereas when co-administration of 50 mg/kg.b.w of KAF and 100/kg.b.w of vitamin E with Pb can restore and elevate FSH, LH, and testosterone levels significantly (p < 0.05) compared to Pb, Pb plus KAF, Pb plus vitamin E, and control groups (Fig. 2).
Fig. 2. Serum reproductive hormones in experimental rats. (A) LH, (B) FSH, and (C) Testosterone concentrations. Various letters were referred to statistical differences among groups at level (p < 0.05). (C): Control group, (Pb): Lead acetate group, (Pb + Vitamin E): Lead acetate + 100 mg/kg.b.w of vitamin E group, (Pb + KAF): Pb + 50 mg/kg.b.w. of kaempferol group, and (Pb + KAF + Vitamin E): Pb + 50 mg/kg.b.w. of kaempferol + 100 mg/kg.b.w. of vitamin E group.
The concentrations of LH, FSH, and testosterone in the Pb plus KAF group were significantly (p < 0.05) elevated compared to that in the Pb plus vitamin E and Pb groups and declined significantly (p < 0.05) relative to the control group (Fig. 2). As well as, the concentrations of these hormones in the Pb plus vitamin E group showed a significant increment compared to the Pb group and a significant decrement compared to the control group, kaempferol plus Pb group, and kaempferol and vitamin E with Pb group (Fig. 2).
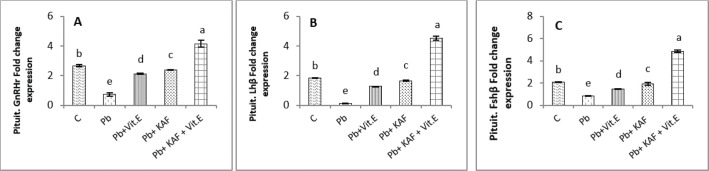
Effects of kaempferol, vitamin E, and lead acetate on fold change of pituitary FSHβ, LHβ, and GnRHr in male rats
The fold changes levels of hypophyseal GnRHr, LHβ, and FSHβ expression were significantly (p < 0.05) upregulated in Pb-treated with KAF plus vitamin E male rats compared to that in other experimental groups. Whereas the exposure to lead acetate, the Pb group caused a significant (p < 0.05) downregulation and suppression of the gene expression (the fold changes) of pituitary GnRHr, FSHβ, and LHβ in comparison with other treatment groups Figure 3.
Fig. 3. Pituitary fold change expression in experimental rats. (A): Pituitary GnRHr fold change, (B): LHβ fold change, and (C): pituitary FSHβ fold change fold changes expression. Various letters were referred to statistical differences among groups at level (p < 0.05). (C): Control group, (Pb): Lead acetate group, (Pb + Vitamin E): Lead acetate + vitamin E group, (Pb + KAF): Pb + 50 mg/kg.b.w. of kaempferol group, and (Pb + KAF + Vitamin E): Pb + 50 mg/kg.b.w. of kaempferol + 100 mg/kg.b.w. of vitamin E group.
The levels of hypophyseal GnRHr, FSHβ, and LHβ fold changes expression in Pb plus vitamin E rats were significantly upregulated compared to the Pb group and downregulated compared to the control and Pb plus KAF groups, Pb + KAF + Vitamin E (Fig. 3). Compared with Pb group and vitamin E plus Pb group, the gene expression (fold changes) levels of pituitary GnRHr, FSHβ, and LHβ elevated significantly in KAF plus Pb male rats (Fig. 3).
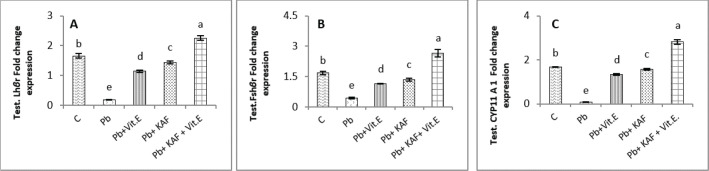
Effects of kaempferol, vitamin E, and lead acetate on fold change of testicular FSHr, LHr, and CYP11A1 genes expression in male rats
The testicular gene expression of LHr, FSHr, and CYP11A1 downregulated significantly (p < 0.05) in rats exposed to lead acetate (Pb rats) compared to that in other experimental rats (Fig. 4). Interestingly, the Pb co-administration of kaempferol with vitamin E significantly (p < 0.05) upregulated and enhanced the expression levels of testicular LHr, FSHr, and CYP11A1 genes relative to other experimental rats. The current results recorded significant (p < 0.05) upregulated gene expression levels of testicular CYP11A1, LHr, and FSHr in rats Pb co-administrated with KAF compared to the Pb plus vitamin E and Pb groups (Fig. 4).
Fig. 4. Testicular fold change expression in experimental rats. (A): Testicular LHβ receptor fold changes, (B): testicular FSHβ receptor fold changes, and (C): testicular CYP11A1 fold changes expression. Various letters were referred to statistical differences among groups at level (p < 0.05). (C): Control group, (Pb): Lead acetate group, (Pb + Vitamin E): Lead acetate + 100 mg/kg.b.w of vitamin E group, (Pb + KAF): Pb + 50 mg/kg.b.w. of kaempferol group, and (Pb + KAF + Vitamin E): Pb + 50 mg/kg.b.w. of kaempferol + 100 mg/kg.b.w. of vitamin E group.
The gene expression levels of testicular CYP11A1, LHr, and FSHr in Pb-treated with vitamin E male rats were significantly (p < 0.05) upregulated compared with the Pb group and significantly (p < 0.05) downregulated when compared to that in the control group, Pb-treated with KAF, and Pb-treated with KAK plus vitamin E group (Fig. 4).
Effects of kaempferol, vitamin E, and lead acetate on testicular pro and anti-inflammatory parameters in male rats
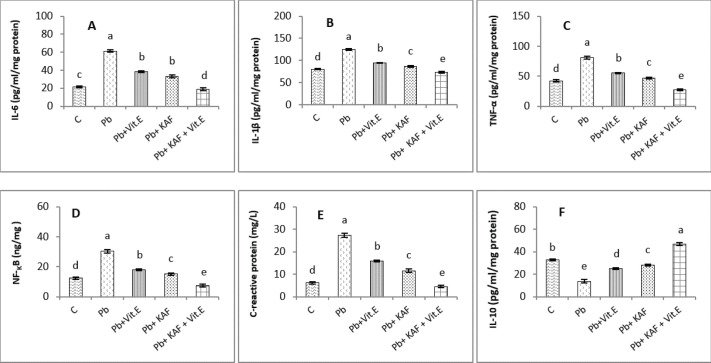
The level of IL-6 significantly (p < 0.05) declined in the Pb + KAF + vitamin E group compared to that in all experimental rats (Fig. 5A). IL-6 level was elevated significantly (p < 0.05) in Pb rats relative to the Pb + KAF, Pb + vitamin E, Pb + KAF +vitamin E, and control groups. In comparison with the Pb group, the IL-6 levels showed a significant (p < 0.05) decline in Pb co-treated with the vitamin E group and KAF group (Fig. 5A).
Fig. 5. Testicular markers of inflammation in experimental rats. (A) IL-6: interleukin-6, (B) IL-1β: interleukin-1β, (C) TNF-α: tumor necrosis factor-α, (D) NF-KB: nuclear factor kappa B, (E) C. reactive protein, (F) IL-10: interleukin-10. Various letters were referred to statistical differences among groups at level (p < 0.05). (C): Control group, (Pb): Lead acetate group, (Pb + Vitamin E): Lead acetate + 100 mg/kg.b.w of Vitamin E group, (Pb + KAF): Pb + 50 mg/kg.b.w. of kaempferol group, and (Pb + KAF + Vitamin E): Pb + 50 mg/kg.b.w. of kaempferol + 100 mg/kg.b.w. of vitamin E group.
The levels of IL-1β, TNF-α, NF-kB, and C-reactive protein were significantly (p < 0.05) increased in Pb rats compared with that in all experimental groups. Interestingly, proinflammatory mediators were significantly (p < 0.05) declined in the Pb + KAF + vitamin E group compared to all experimental rats (Fig. 5B–E).
The level of IL-10 lowered significantly (p < 0.05) in Pb and Pb + vitamin E groups compared to Pb + KAF + vitamin E, control, and Pb + KAF groups (Fig. 5F). It was elevated significantly (p < 0.05) in rats that received KAF and vitamin E after exposure to lead acetate compared to all experimental groups (Fig. 5F). Compared with Pb and Pb + vitamin E, the level of IL-10 was raised (p < 0.05) in the Pb + KAF group (Fig. 5F).
Effects of kaempferol, vitamin E, and lead acetate on seminal function analysis in male rats
The sperm count and percentage of sperm viability and motility were significantly (p < 0.05) declined, whereas the percentage of abnormal sperm was significantly (p < 0.05) raised in rats exposed to lead acetate (Pb group) compared to all experimental rats (Table 3). Interestingly, the administration of kaempferol at a dose of 50 mg/kg with vitamin E at a dose of 100 mg/kg after exposure to lead acetate can enhance and significantly increase (p < 0.05) the sperm viability, count, and motility, accompanied by significant (p < 0.05) lowering in the abnormal sperm in comparison with the control, Pb + KAF, Pb + vitamin E, and Pb group. In comparison with the Pb group and Pb + vitamin E, the percentages of motility and viability, as well as sperm count, were elevated significantly (p < 0.05) in rats administered KAF following exposure to Pb, whereas abnormal morphology of sperm significantly (p < 0.05) declined compared to Pb + vitamin E, control, and Pb groups (Table 3).
Table 3. Effects of kaempferol, vitamin E, and lead acetate on seminal function analysis in male rats.
| Pb + KAF + Vitamin E | Pb + KAF | Pb + Vitamin E | Pb | C | Parameters |
|---|---|---|---|---|---|
| 1.114 ± 91.022a | 1.070 ± 66.352c | 1.542 ± 61.513d | 1.129± 38.269e | 1.433 ±70.707b | Sperm motility % |
| 1.102 ± 90.530a | 0.916 ± 64.726c | 0.903± 58.070d | 0.384± 37.493e | 0.988 ± 68.116b | Sperm viability % |
| 1.543 ± 72.698a | 1.081± 48.386c | 1.644 ± 42.124d | 1.157 ± 26.372e | 1.842 ± 54.076b | Sperm count (million/ml) |
| 0.558± 5.259e | 1.018 ± 9.134d | 0.808 ± 12.613c | 1.560 ± 48.810a | 1.152 ±16.358b | Abnormal sperm% |
Values are expressed as Mean ± SD. Various letters were referred to statistical differences among groups at level (p < 0.05). (C): Control group, (Pb): Lead acetate group, (Pb+ Vitamin E): Lead acetate + vitamin E group, (Pb + KAF): Pb + 50 mg/kg.b.w. of kaempferol group, and (Pb + KAF + Vitamin E): Pb + 50 mg/kg.b.w. of kaempferol + 100 mg/kg.b.w. of vitamin E group.
The co-administration of lead acetate plus vitamin E can elevate sperm motility, count, and viability compared to Pb. Administered vitamin E with Pb can decrease (p < 0.05) abnormal spermatozoa compared to Pb and control rats (Table 3).
Histopathological changes
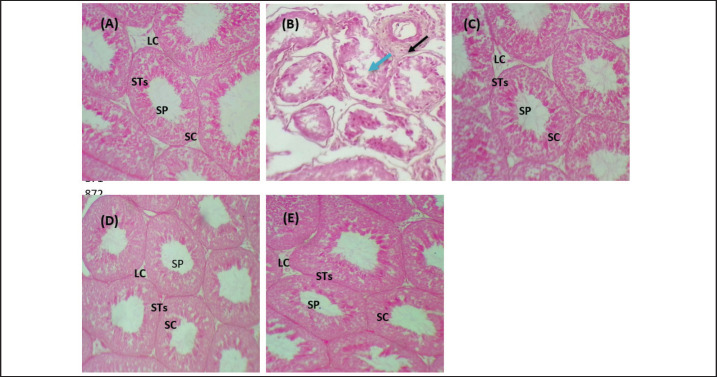
The histological findings of lead acetate-treated male rats’ testes showed the irregular form of seminiferous tubules, marked cell loss, empty lumen, with edema and degeneration of Sertoli cells and seminiferous tubules with arrested spermatogenesis, compared to the control group that showed a typical architecture of the Sertoli cells, interstitial cells and seminiferous tubules (Fig. 6A and B).
Fig. 6. Photomicrographs of testicular tissues in experimental groups. (A): Control group: showed a typical feature of testicular architecture with normal Sertoli cells and Leydig cells as well as complete spermatogenesis series. (B) Pb group: showed congestion (black raw), edema (blue raw), and degenerative seminiferous tubules and Sertoli cells with losing spermatogenic cells. (C): Pb + Vitamin E: showed that the Sertoli cells and Leydig cells returned to their normal structure and were occupied with little spermatozoa. (D): Pb + KAF: showed that the seminiferous tubules, germinal epithelia, and Sertoli cells return to normal texture. (E): Pb + KAF + Vitamin E: showed regeneration of the testicular cells and return to normal architecture as normal Leydig cells, and Sertoli cells with active spermatogenesis series. H&E, (100×). LC: Leydig cells, SC: Sertoli cells, STs: seminiferous tubules, and SP: spermatozoa.
Importantly, the administration of kaempferol and vitamin E with Pb can enhance the regeneration and recovery of the testicular tissue and appear to possess a typical architecture as that shown in control male rats, with normal appearance of the Sertoli cells, Leydig, germ cells, and seminiferous tubules occupied with different stages of normal spermatocytes, and the spermatogenesis series were evident (Fig. 6E).
The histological findings of rat’s testes co-treated with KAF plus lead acetate group and vitamin E plus lead acetate group showed that the seminiferous epithelia and interstitial cells return to normal architecture of Sertoli cells and seminiferous epithelia with moderate spermatogenesis (Fig. 6C and D).
Discussion
Lead acetate exposure can induce testicular damage via an imbalance between the production of antioxidant/removal-free free radicles, leading to testicular oxidative stress. The present experiment was conducted to study the effect of kaempferol on reproductive toxicity induced by lead acetate and compare its action with the impact of vitamin E in male rats.
Lead exposure generates testicular oxidative stress via the downregulation of antioxidants and upregulation of ROS production, resulting in oxidative stress and, subsequently, apoptosis (Li et al., 2018). In current study showed that exposure to lead acetate led to increase testicular NO and MDA with decreased testicular CAT, SOD, and GPx, which is consistent with a previous study (Udefa et al., 2020). It had been observed that lead acetate caused tissue damage via two distinct mechanisms, including excessive generation and accumulation of ROS in testicular tissues, which includes singlet oxygen, hydrogen peroxides, hydroxy radicals, and RNS, as well as direct exhaustion of antioxidant pool (Talpur et al., 2018, Elsheikh et al., 2022). Pb has a higher affinity to bind with sulfhydryl groups and suppress the functional thiol;-SH groups (sulfhydryl) groups, of SOD, GPx, and CAT; and it can replace the divalent bio-elements such as selenium, zinc, and magnesium that serve as an essential cofactor of antioxidant enzymes that caused a significant depletion of these antioxidant enzymes in testicular postmitochondrial and mitochondrial fractions in rats exposed to lead acetate (Udefa et al., 2020, Elsheikh et al., 2022). The current experiment shows an increase in the testicular NO levels in rats treated with lead acetate that may be due to elevated biosynthesis and activity of testicular nitric oxide synthase (iNOS) resulting from lead acetate accumulation in testicular tissues; the present results are consistent with that results obtained from Matović et al. (2015) who reported that exposure to lead acetate led to raise the cellular NO level resulting in fragmentation of cellular lipid and DNA.
Importantly, the impacts of kaempferol have not yet been completely identified on the reproductive system. Current results revealed that the administration of kaempferol following exposure to lead caused elevation in the levels of CAT, GPx, and SOD, whereas MDA and NO were reduced relative to their levels in rats treated with vitamin E and/or lead only; this may be attributed to the antioxidant ability of kaempferol to suppress the activities of pro-oxidant enzymes such as xanthine oxidase that responsible for generating ROS (Lim et al., 2023). Kaempferol, like other flavonoids, can chelate with cuprous or ferrous ions in Fenton’s reaction, ultimately leading to prevent lipid peroxidation of cytomembrane, as well as; kaempferol increases the expression and activity of CAT, GPx, and SOD, as found by (Lim et al., 2023). Furthermore, kaempferol possesses a double bond at C3-C2 and hydroxyl groups at C5, C3, and C4, and an oxo group at C4 which might explain its powerful antioxidant properties (Imran et al., 2019); the current results consistent with (Albrakati et al., 2021) who concluded that kaempferol can suppress lipid peroxidation and NO production with increase generation of antioxidants in neurotoxic rats.
The results of the current study are consistent with (Oyeyemi et al., 2022), who reported that vitamin E could reduce testicular MDA and NO concentrations when co-administered with lead acetate due to suppress phospholipid membrane peroxidation and protect testicular cell membranes via its lipophilic properties (Malmir et al., 2021); in addition, tocopherols-OH can replace their hydrogen atom to a single electron of free radicle and removing it before the free radicle binds with cell membranes, so vitamin E considers as a nonenzymatic defense mechanism in testicular mitochondria (Ahmed et al., 2020, Malmir et al., 2021) that effects on the testicular NO and MDA levels in Pb cotreated with vitamin E.
The ability of kaempferol with vitamin E to reduce MDA and NO whereas enhancing the antioxidant enzymes in rats indicates that both kaempferol and vitamin E acted additively to reduce the testicular oxidative stress, that consistent with the findings of (Shrivastava et al., 2017, Oyeyemi et al., 2022). Importantly, kaempferol lowered the testicular NO level via its ability to scavenge free radicals by a strong antioxidant capacity due to its phenolic hydroxyl groups, four hydroxyl groups on the benzene ring that can react with free radicals, converting it to a more stable semiquinone free radicals that prevent oxidation chain reaction (Akefe et al., 2020, Lim et al., 2023). These differences in chemical structure and number of hydroxyl groups between kaempferol and vitamin E may explain the significant differences in antioxidant activities between rats that received kaempferol and those that received Vitamin E (Li et al., 2018).
The testicular steroidogenic cells require some steroidogenic enzymes, 3β-HSD, 17β-HSD, and CYP11A1, to synthesize steroid hormones (Falvo et al., 2018). The results of this study reported a decline in activities of testicular steroidogenic enzymes 3β-HSD, 17β-HSD, and StAR protein levels, as well as suppressed testicular CYP11A1 expression in rats exposed to lead acetate (Figs. 1 and 4C). This decrement could be attributed to the accumulation of lead acetate in testicular tissues, which causes the excessive generation of ROS in testicular tissues and disturbs its function (Elsheikh et al., 2022), and inhibits the testicular expression of StAR protein, leading to a decrease in cholesterol transferring to mitochondria, accompanied by a reduction of testicular CYP11A1 gene expression and a decline in the activities of 3β-HSD and 17β-HSD causing impaired steroidogenesis which is the reason for the decline in serum testosterone concentration observed in the Pb group (Fig. 2C), these results are consistent with findings of (Udefa et al., 2020).
The kaempferol exhibited the antioxidant properties to improve the Leydig cells membranes and facilitate cholesterol transfer into mitochondria (Alam et al., 2020), subsequently improving steroidogenesis as StAR protein levels and CYP11A1, 3β-HSD, and 17β-HSD activities and testosterone concentration were upregulated (Liu et al., 2022) as reported in the current research which illustrated that kaempferol administration caused testicular upregulation 3β-HSD, 17β-HSD, and CTP11A1 enzymes and StAR protein gene expression which positively effect on testosterone levels in rats cotreated with kaempferol. Furthermore, the administration of vitamin E following exposure to lead acetate caused elevation of testicular 13β-and 17β- HSD activities and StAR levels due to the ability of vitamin E to promote gene expression of StAR protein and mRNA steroidogenic enzyme in Leydig cells that can upregulate testosterone concentration (Malmir et al., 2021).
The current results showed a significant decline in gene fold change of hypophyseal GnRHr, FSHβ, and LHβ, as well as testicular LHβr, FSHβr, and CYP11A1 in rats exposed to lead acetate, Pb group, that may be attributed to the lead acetate ability to damage pituitary and hypothalamus which are the central site of lead neurotoxic action (Wani et al., 2015), thereby, results in impaired mRNA expression of hypothalamic gonadotropin-releasing hormone (GnRH), as well as down-regulation of gene expression of hypophyseal FSHβ, LHβ, and GnRHr that negatively reflected on synthesis and secretion LH, and FSH (Udefa et al., 2020). At the testicular level, lead acetate caused Leydig and Sertoli cell dysfunctions, which in turn impaired the testicular LH and FSHr expression, resulting in impaired steroidogenesis and spermatogenesis (Udefa et al., 2020), as well as disturbed feedback response to testosterone in plasma results from GnRH and LH dysregulation (Oyeyemi et al., 2022).
Importantly, the effects of kaempferol on the reproductive system have not yet been fully reported. Kaempferol, like other flavonoids that affect LH-and FSH-secreting gonadotroph cells by incrementing their secretory granules (Zhang et al., 2023), which results from the ability of kaempferol to upregulate hypophyseal GnRHr, FSHβ, and LHβ genes due to kaempferol’s antioxidant, antiapoptotic, and anti-inflammatory effects (Liu et al., 2022). In addition, kaempferol has the ability to upregulate the testicular receptor expression of LH and FSH due to its powerful antioxidant properties that protect Leydig and Sertoli cells from Pb oxidative damage (Lee et al., 2023), which promotes testicular steroidogenic enzyme expression which enhanced testosterone biosynthesis, as well as improvement the count, and viability of sperm (Kluska et al., 2022, Liu et al., 2022).
Vitamin E has antioxidant properties that can protect the hypothalamic and pituitary cells from oxidative stress that promotes GnRH, FSH, and LH biosynthesis via upregulated gene expression of hypophyseal GnRHr, LHβ, and FSHβ and testicular FSHr, LHr, and CYP11A1 that enhance serum FSH and LH concentrations in turn that improving the testosterone levels and spermatogenesis (Oyeyemi et al., 2022). Interestingly, the improvement the steroidogenesis and spermatogenesis in Pb co-administered with kaempferol and vitamin E resulted from the prevention of oxidative stress and chelating activities of kaempferol and vitamin E, which ameliorate lead acetate-induced testicular toxicity, thereby improving the levels of LH and FSH that supported steroidogenesis and spermatogenesis in Pb co-treated with kaempferol and vitamin E (Akefe et al., 2020, Malmir et al., 2021).
Inflammation has occurred following oxidative stress in tissue. The current results concluded that exposure to lead acetate caused testicular inflammation as the levels of anti- and pro-inflammatory cytokines were significantly reduced and elevated, respectively, in the Pb group compared with all experimental groups (Fig. 5). The occurrence of inflammation in testicles might be attributed to the elevation of testicular NO levels in rats exposed to lead acetate (Udefa et al., 2020). Elevated NO levels cause an increase in the NF-kB level; the latter, in turn, initiates an inflammatory signaling cascade with subsequent secretion of several inflammatory cytokines (Alam et al., 2020).
Interestingly, following administration of kaempferol, the results reported that all the proinflammatory cytokines declined (p < 0.05), and IL-10 was elevated in rats co-treated with Pb due to the presence of one hydroxyl group in the B ring at 4́ position, that suppresses lipoxygenase; therefore, kaempferol can act as an anti-inflammatory substance (Sroka et al., 2017). Importantly, kaempferol upregulates IL-10 expression and suppresses expression of NF-kB and TNF-α, thereby, resulting in the inhibition of the NF-kB signaling cascade as well as downregulating the release of pro-inflammatory mediators, such as IL-8, IL-1β, IL-6, TNF-α, and COX-2 (Zhang et al., 2019, Alam et al., 2020). The current results are consistent with the findings of (Lee et al., 2023). As well as, kaempferol can reduce C-reactive protein and lowered lipopolysaccharide-induced expression of IL-1β and TNF-α by elevating the activated macrophages (Sroka et al., 2017).
In addition, vitamin E has a major role in immunity and acts as an anti-inflammatory and antioxidant substance via the reduction of the levels of proinflammatory with elevation of anti-inflammatory cytokines, that consistent with a previous study (Fan et al., 2023) found that the administration of 30 IU/kg of vitamin E can reduce the TNF-α, IL-18, IL-6, and IL-12 in rats treated with dextran sulfate sodium-induced ulcerative colitis.
The current experiment reported that exposure to lead acetate (Pb) caused an increase in abnormal morphology of sperm accompanied by a decrease in sperm motility, viability, and count, which are consistent with previous studies (Oyeyemi et al., 2022). Furthermore, lead exposure can cause an increase in immature spermatozoa due to spermatogenesis disruption. Moreover, Li et al. (2018) concluded that the presence of high quantities of lead acetate in drinking water caused a decline in sperm viability, density, and motility with elevated abnormal morphological spermatozoa, as well as impairment of DNA structure and integrity. The exposure and accumulation of lead acetate in testicular tissues cause damage to cellular membranes through lipid peroxidation, which in turn decreases the spermatozoa count, causes the displacement of the testicular Ca+2, and decreases the motility of sperm (Oyeyemi et al., 2020), which results in a complete arrest of spermatogenesis (Mustafa, 2023). The current histopathological alteration in Pb testicular rats reported a reduction in cellularity with irregular thickening in the basement membrane accompanied by abnormal spermatozoa morphology and loss of the interstitial cells (Mabrouk, 2018).
These alterations were improved and enhanced by administering kaempferol and vitamin E due to their antioxidant and anti-inflammatory properties (Sukmawati et al., 2019, Liu et al., 2022). In addition, the administration of kaempferol in the current experiment exhibits potent antioxidant and anti-inflammatory activities in testicular tissues via reducing the production of pro-inflammatory mediators and oxidative stress, which can reduce edema and degenerative changes in testicular tissues, protect DNA from oxidative stress (Akefe et al., 2020), enhance the regeneration process in the testicular tissues, seminiferous tubules, Sertoli and Leydig cells as in (Fig. 6D), thereby improving and enhancing steroidogenesis (Fig. 1) and the semen quantity and quality (Table 3) of present study.
The current study revealed that the administered vitamin E could restore and recover the seminiferous tubules, Sertoli cells, and Leydig cells damaged due to its anti-inflammatory effects (Sukmawati et al., 2019). Other studies also reported similar findings, that vitamin E declined the degeneration of the seminiferous tubules and repair testicular tissues via its antioxidant activity to eliminate free radicals and protect DNA of cells from oxidative damage (Ramah et al., 2015) and preventing apoptosis in spermatogonia, Leydig, and Sertoli cells, which improve the sperm viability as well as their count via improvement the spermatogenesis and stabilized cellular membranes (Oyeyemi et al., 2015).
Interestingly, the results of the current study were reported for the first time that co-administration of kaempferol, separately or with vitamin E, can enhance and restore the seminal functions via downregulation of the oxidative stress and pro-inflammatory cytokines, and upregulation of antioxidant enzymes and anti-inflammatory marker, that indicating that the additive effects of kaempferol and vitamin E to improve antioxidant defense system and ameliorate the testicular toxicity caused by oxidative stress in rats (Akefe et al., 2020, Oyeyemi et al., 2022).
Conclusion
The present research is the first to demonstrate kaempferol’s preventive function in testicular damage induced by lead acetate via its antioxidant and anti-inflammatory properties. In addition, lead acetate co-treated with kaempferol and vitamin E can act additively as protective agents for the hypothalamic-hypophyseal-testis axis to improve the reproductive parameters in male rats with heavy metal toxicity via their antioxidant and anti-inflammatory activities. A single kaempferol administration exhibits a more potent mitigative impact than vitamin E, whereas their combination produces additive effects. Further research is needed to understand the mechanisms of action and manifested kaempferol’s efficacy in treating other diseases and disorders related to female postmenopausal and polycystic ovaries.
Acknowledgments
The author appreciates the efforts of the dean of Veterinary Medicine at Al-Qasim Green University in s upporting the present experiment.
Conflict of interest
The author has stated that there are no conflicts of interest.
Funding
There is no specific grant provided for this research.
Data availability
All data supporting the findings of this study are available within the manuscript.
References
- Abdulnabi Z.A. Assessment of some toxic element levels in Iraqi marine water. Mesopot. J. Mar. Sci. 2016;31:85–94. [Google Scholar]
- Aboubakr M, Elmahdy A.M, Taima S, Emam M.A, Farag A, Alkafafy M, Said A.M, Soliman A. Protective effects of N acetylcysteine and vitamin E against acrylamide-induced neurotoxicity in rats. Pak. Vet. J. 2023;43:262–268. [Google Scholar]
- Aebi H. Cambridge, MA: Academic Press; 1984. [13] Catalase in vitro, in: methods in enzymology, oxygen radicals in biological systems; pp. 121–126. [Google Scholar]
- Ahmed A.E, Alshehri A, Al-Kahtani M.A, Elbehairi S.E.I, Alshehri M.A, Shati A.A, Alfaifi M.Y, Al-Doais A.A, Taha R, Morsy K, El-Mansi A.A. Vitamin E and selenium administration synergistically mitigates ivermectin and doramectin-induced testicular dysfunction in male Wistar albino rats. Biomed. Pharmacother. 2020;124:109841. doi: 10.1016/j.biopha.2020.109841. [DOI] [PubMed] [Google Scholar]
- Aithamadouche N.A, Sadi N, Kharoubi O, Slimani M, Aoues A. The protective effect of vitamin E against genotoxicity of lead acetate intraperitoneal administration in male rat. Arch. Biol. Sci. 2013;65:1435–1445. [Google Scholar]
- Akefe I.O, Ayo J.O, Sinkalu V.O. Kaempferol and zinc gluconate mitigate neurobehavioral deficits and oxidative stress induced by noise exposure in Wistar rats. PLoS One. 2020;15:e0236251. doi: 10.1371/journal.pone.0236251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam W, Khan H, Shah M.A, Cauli O, Saso L. Kaempferol as a dietary anti-inflammatory agent: current therapeutic standing. Molecules. 2020;25(18):4073. doi: 10.3390/molecules25184073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrakati A, Albarakati A, Lokman M, Theyab A, Algahtani M, Menshawi S, Alamri O, Omairi N, Essawy E, Kassab R, Abdel Moneim A. Possible role of kaempferol in reversing oxidative damage, inflammation, and apoptosis-mediated cortical injury following cadmium exposure. Neurotox. Res. 2021;39:1–12. doi: 10.1007/s12640-020-00300-2. [DOI] [PubMed] [Google Scholar]
- Alkhalidy H, Moore W, Wang A, Luo J, McMillan R.P, Wang Y, Zhen W, Hulver M.W, Liu D. Kaempferol ameliorates hyperglycemia through suppressing hepatic gluconeogenesis and enhancing hepatic insulin sensitivity in diet-induced obese mice. J. Nutr. Biochem. 2018;58:90–101. doi: 10.1016/j.jnutbio.2018.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanpour P, Khodarahmi P, Salehipour M. Protective effects of vitamin E on cadmium-induced apoptosis in rat testes. Naunyn. Schmiedebergs. Arch. Pharmacol. 2020;393:349–358. doi: 10.1007/s00210-019-01736-w. [DOI] [PubMed] [Google Scholar]
- Atashfaraz E, Farokhi F, Najafi G. Protective effect of ethyl pyruvate on epididymal sperm characteristics, oxidative stress and testosterone level in methotrexate treated mice. J. Reprod. Infertil. 2013;14:190–196. [PMC free article] [PubMed] [Google Scholar]
- Azzi A. Tocopherols, tocotrienols and tocomonoenols: many similar molecules but only one vitamin E. Redox. Biol. 2019;26:101259. doi: 10.1016/j.redox.2019.101259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björndahl L, Söderlund I, Kvist U. Evaluation of the one-step eosin-nigrosin staining technique for human sperm vitality assessment. Hum. Reprod. 2003;18:813–816. doi: 10.1093/humrep/deg199. [DOI] [PubMed] [Google Scholar]
- Elsheikh N.A.H, Omer N.A, Li-lian G, Wang G.L. Lead induced oxidative stress and affected the expression of steroidogenesis -related genes in testis of male mice. Indian. J. Forensic. Med. Toxicol. 2022;16(3):248–254. [Google Scholar]
- Falvo S, Chieffi Baccaria G, Spaziano G, Rosati L, Venditti M, Di Fiore M.M, Santillo A. StAR protein and steroidogenic enzyme expressions in the rat harderian gland. C. R. Biol. 2018;341:160–166. doi: 10.1016/j.crvi.2018.02.001. [DOI] [PubMed] [Google Scholar]
- Fan X, Yin J, Yin J, Weng X, Ding R. Comparison of the anti-inflammatory effects of vitamin E and vitamin D on a rat model of dextran sulfate sodium-induced ulcerative colitis. Exp. Ther. Med. 2023;25(2):98. doi: 10.3892/etm.2023.11797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L.C, Wagner D.A, Glogowski J, Skipper P.L, Wishnok J.S, Tannenbaum S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Imran M, Salehi B, Sharifi-Rad J, Aslam Gondal T, Saeed F, Imran A, Shahbaz M, Tsouh Fokou P.V, Umair Arshad M, Khan H, Guerreiro S.G, Martins N, Estevinho L.M. Kaempferol: a key emphasis to its anticancer potential. Molecules. 2019;24(12):2277. doi: 10.3390/molecules24122277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S, Zhang L, Wang L. Kaempferol, a potential neuroprotective agent in neurodegenerative diseases: from chemistry to medicine. Biomed. Pharmacother. 2023;165:115215. doi: 10.1016/j.biopha.2023.115215. [DOI] [PubMed] [Google Scholar]
- Karimfar M.H, Bargahi A, Moshtaghi D, Farzadinia P. Long-term exposure of lead acetate on rabbit renal tissue. Iran. Red. Crescent. Med. J. 2016;18:e22157. doi: 10.5812/ircmj.22157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluska M, Juszczak M, Żuchowski J, Stochmal A, Woźniak K. Effect of kaempferol and its glycoside derivatives on antioxidant status of HL-60 cells treated with etoposide. Molecules. 2022;27:333. doi: 10.3390/molecules27020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Yoon S, Moon J.O. Kaempferol suppresses carbon tetrachloride-induced liver damage in rats via the MAPKs/NF-κB and AMPK/Nrf2 signaling pathways. Int. J. Mol. Sci. 2023;24:6900. doi: 10.3390/ijms24086900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Zhao K, Zhang H, Liu L, Xiong F, Wang K, Chen B. Lead exposure reduces sperm quality and DNA integrity in mice. Environ. Toxicol. 2018;33:594–602. doi: 10.1002/tox.22545. [DOI] [PubMed] [Google Scholar]
- Lim H.J, Prajapati R, Seong S.H, Jung H.A, Choi J.S. Antioxidant and antineuroinflammatory mechanisms of kaempferol-3-O-β-d-glucuronate on lipopolysaccharide-stimulated BV2 microglial cells through the Nrf2/HO-1 signaling cascade and MAPK/NF-κB pathway. ACS. Omega. 2023;8(7):6538–6549. doi: 10.1021/acsomega.2c06916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D.L, Liu S.J, Hu S.Q, Chen Y.C, Guo J. Probing the potential mechanism of quercetin and kaempferol against heat stress-induced sertoli cell injury: through integrating network pharmacology and experimental validation. Int. J. Mol. Sci. 2022;23:11163. doi: 10.3390/ijms231911163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J, Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mabrouk A. Therapeutic effect of thymoquinone against lead-induced testicular histological damage in male Wistar rats. Andrologia. 2018;50:e13014. doi: 10.1111/and.13014. [DOI] [PubMed] [Google Scholar]
- Malmir M, Mehranjani M.S, Faraji T, Noreini S.N. Antioxidant effect of vitamin E on the male rat reproductive system by a high oral dose of bisphenol-A. Toxicol. Res. Appl. 2021;5:23978473211005562. [Google Scholar]
- Matović V, Buha A, Ðukić-Ćosić D, Bulat Z. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food. Chem. Toxicol. 2015;78:130–140. doi: 10.1016/j.fct.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Mescher A.L, Junqueira L.C.U. McGraw-Hill’s AccessMedicine. 13th. New York, NY: McGraw-Hill Medical; 2013. Junqueira’s basic histology: text and atlas. [Google Scholar]
- Mohandas J, Marshall J.J, Duggin G.G, Horvath J.S, Tiller D.J. Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney. Possible implications in analgesic nephropathy. Biochem. Pharmacol. 1984;33:1801–1807. doi: 10.1016/0006-2952(84)90353-8. [DOI] [PubMed] [Google Scholar]
- Mustafa H.N. Ameliorative potential of the quercetin on lead-induced testicular damage: morphohistometric and biochemical analysis. Afr. J. Urol. 2023;29:36. [Google Scholar]
- Narayana K, Prashanthi N, Nayanatara A, Kumar H.H.C, Abhilash K, Bairy K.L. Effects of methyl parathion (o,o-dimethyl o-4-nitrophenyl phosphorothioate) on rat sperm morphology and sperm count, but not fertility, are associated with decreased ascorbic acid level in the testis. Mutat. Res. 2005;588:28–34. doi: 10.1016/j.mrgentox.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Nishikimi M, Appaji N, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972;46:849–854. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- Oyeyemi W.A, Akinola A.O, Daramola O.O, Aikpitanyi I, Durotoluwa O.T, Alele P.G.O, Ogieriakhi I.O, Okoro T.D. Vitamin E and quercetin attenuated the reproductive toxicity mediated by lead acetate in male Wistar. Bull. Natl. Res. Cent. 2022;46:22. [Google Scholar]
- Oyeyemi W.A, Daramola O.O, Akinola A.O, Idris A.O, Aikpitanyi I. Hepatic and reproductive toxicity of sub-chronic exposure to dichlorvos and lead acetate on male Wistar rats. Asian. Pac. J. Rep. 2020;9:283. [Google Scholar]
- Oyeyemi W.A, Shittu S.T, Kolawole T.A, Ubanecheand P, Akinola A.O. Protective effect of vitamin E on nicotine induced reproductive toxicity in male rats. Nig. J. Basic. Appl. Sci. 2015;23:7–13. [Google Scholar]
- Raji Y, Oloyo A.K, Morakinyo A.O. Effect of methanol extract of Ricinus communis seed on reproduction of male rats. Asian. J. Androl. 2006;8(1):115–121. doi: 10.1111/j.1745-7262.2006.00055.x. [DOI] [PubMed] [Google Scholar]
- Ramah A, EL-shwarby R, Nabila M.A, El-shewey E. The effect of lead toxicity on male albino rats reproduction with ameliorate by vitamin E and pumpkin seeds oil. Benha. Vet. Med. J. 2015;28:43–52. [Google Scholar]
- Ren J, Lu Y, Qian Y, Chen B, Wu T, Ji G. Recent progress regarding kaempferol for the treatment of various diseases. Exp. Ther. Med. 2019;18:2759–2776. doi: 10.3892/etm.2019.7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta. 1978;90:37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- Schefler W.C. Reading, Mass. Don Mills: Addison-Wesley Pub. Co; 1980. Statistics for the biological sciences. [Google Scholar]
- Sharma N, Biswas S, Al-Dayan N, Alhegaili A.S, Sarwat M. Antioxidant role of kaempferol in prevention of hepatocellular carcinoma. Antioxidants (Basel) 2021;10:1419. doi: 10.3390/antiox10091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava S, Uthra C, Reshi M.S, Shukla S. Protective role of kaempferol against acrylamide intoxication. Free. Radic. Antioxid. 2017;7:36–42. [Google Scholar]
- Sroka Z, Sowa A, Dryś A. Inhibition of lipoxygenase and peroxidase reaction by some flavonols and flavones: the structure-activity relationship. Nat. Prod. Commun. 2017;12(11):1705–1708. [Google Scholar]
- Sukmawati Y, Arisanty D, Tofrizal A, Amir A. Vitamin E ameliorates testicular histological features and androgen binding protein levels in testicle of rats induced by allethrin. J. Adv. Vet. Anim. Res. 2019;6:486–491. doi: 10.5455/javar.2019.f372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talpur S, Afridi H.I, Kazi T, Talpur F.N. Interaction of lead with calcium, iron, and zinc in the biological samples of malnourished children. Biol. Trace. Elem. Res. 2018;183:209–217. doi: 10.1007/s12011-017-1141-9. [DOI] [PubMed] [Google Scholar]
- Tohid J, Arash K, Zahra G, Mahdi I.A, Farzam H. A study of the therapeutic effects of vitamin E on testicular tissue damage caused by fluoxetine. Crescent. J. Med. Biol. Sci. 2014;1:37–41. [Google Scholar]
- Udefa A.L, Amama E.A, Archibong E.A, Nwangwa J.N, Adama S, Inyang V.U, Inyaka G.U, Aju G.J, Okpa S, Inah I.O. Antioxidant, anti-inflammatory and anti-apoptotic effects of hydro-ethanolic extract of Cyperus esculentus L. (tigernut) on lead acetate-induced testicular dysfunction in Wistar rats. Biomed. Pharmacother. 2020;129:110491. doi: 10.1016/j.biopha.2020.110491. [DOI] [PubMed] [Google Scholar]
- Wahab O.A, Princely A.C, Oluwadamilare A.A, Oore-oluwapo D.O, Blessing A.O, Alfred E.F. Clomiphene citrate ameliorated lead acetate-induced reproductive toxicity in male Wistar rats. JBRA. Assist. Reprod. 2019;23:336–343. doi: 10.5935/1518-0557.20190038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Yang Y, An Y, Fang G. The mechanism of anticancer action and potential clinical use of kaempferol in the treatment of breast cancer. Biomed. Pharmacother. 2019;117:109086. doi: 10.1016/j.biopha.2019.109086. [DOI] [PubMed] [Google Scholar]
- Wani A.L, Ara A, Usmani J.A. Lead toxicity: a review. Interdiscip. Toxicol. 2015;8:55–64. doi: 10.1515/intox-2015-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Guo Z, Wang Y, Geng J, Han S. The protective effect of kaempferol on heart via the regulation of Nrf2, NF-κβ, and PI3K/Akt/GSK-3β signaling pathways in isoproterenol-induced heart failure in diabetic rats. Drug. Dev. Res. 2019;80:294–309. doi: 10.1002/ddr.21495. [DOI] [PubMed] [Google Scholar]
- Zhang X, Tang Y, Lu G, Gu J. Pharmacological activity of flavonoid quercetin and its therapeutic potential in testicular injury. Nutrients. 2023;15:2231. doi: 10.3390/nu15092231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data supporting the findings of this study are available within the manuscript.