Abstract
Earlier studies implied a role for Mycoplasma arthritidis surface protein MAA2 in cytadherence and virulence and showed that it exhibited both size and phase variability. Here we report the further analysis of MAA2 and the cloning and sequencing of the maa2 gene from two M. arthritidis strains, 158p10p9 and H606, expressing two size variants of MAA2. Triton X-114 partitioning and metabolic labeling with [3H]palmitic acid suggested lipid modification of MAA2. Surface exposure of the C terminus was indicated by cleavage of monoclonal antibody-specific epitopes from intact cells by carboxypeptidase Y. The maa2 genes from both strains were highly conserved, consisting largely of six (for 158p10p9) or five (for H606) nearly identical, 264-bp tandem direct repeats. The deduced amino acid sequence predicted a largely hydrophilic, highly basic protein with a 29-amino-acid lipoprotein signal peptide. The maa2 gene was expressed in Escherichia coli from the lacZ promoter of vector pGEM-T. The recombinant product was approximately 3 kDa larger than the native protein, suggesting that the signal peptide was not processed in E. coli. The maa2 gene and upstream DNA sequences were cloned from M. arthritidis clonal variants differing in MAA2 expression state. Expression state correlated with the length of a poly(T) tract just upstream of a putative −10 box. Full-sized recombinant MAA2 was expressed in E. coli from genes derived from both ON and OFF expression variants, indicating that control of expression did not include alterations within the coding region.
Mycoplasma arthritidis is a natural pathogen of rats, causing an acute, self-limited, septic arthritis under natural and experimental conditions. Although an extensive body of descriptive literature exists concerning the clinical, microbiological, and pathological aspects of this disease (reviewed in reference 13), mechanisms of pathogenesis are still not clearly understood. Several potential virulence factors are currently under investigation (10, 39, 46, 50, 53), all of which probably work synergistically to cause disease. It will probably not be possible to assign a relative level of importance to any one factor until all have been characterized and the tools for genetic manipulation of mycoplasmas have been vastly improved. However, there is no doubt that cytadhesins play a critical role, especially in the early stages of infection, since a close association between host and parasite is most likely required before any of the other virulence factors can come into play.
We have identified two M. arthritidis surface proteins, designated MAA1 and MAA2, that may be involved in cytadherence (53). Both proteins are immunogenic for rats and are recognized by convalescent-phase rat sera. Both are capable of eliciting immune responses in rats that protect against challenge with the virulent M. arthritidis strain 158p10p9 (55), suggesting a role in the disease process. MAA2 exhibits size variability among strains and is an important marker for a group of M. arthritidis strains antigenically related to 158p10p9 (54). In addition to size variation, MAA2 is also phase variable; that is, its expression state rapidly vacillates between ON and OFF within individual clonal isolates (54). The ability to alter the antigenic and expression states of immunogenic surface components is common to many pathogens across a broad phylogenetic spectrum (9, 17, 25, 37, 41, 42, 48, 56). Mycoplasma species are no exception, and phase and antigenic variation are frequently proposed as mechanisms for avoidance of host immune mechanisms and persistence of mycoplasmal pathogens in chronic infections (reviewed in references 19 and 59).
In the present study, we have undertaken a further analysis of MAA2 at the molecular level in order to begin to identify more precisely its function in M. arthritidis and its role in host-parasite interaction. We have cloned and sequenced the maa2 gene from two strains of M. arthritidis expressing two different size variants of MAA2 and identified the basis for size variation. We have expressed recombinant versions of both maa2 genes in Escherichia coli, and we have examined clonal isolates of M. arthritidis 158p10p9 exhibiting two different MAA2 expression states for evidence of mechanisms controlling the ON/OFF switch.
MATERIALS AND METHODS
Mycoplasmal strains, culture conditions, and metabolic labeling.
M. arthritidis strains and culture and storage conditions were described previously (54). Concentrated stock cultures were prepared by inoculating 100 ml of Edward broth (EB) with 100 μl from a thawed stock culture, expanding the culture to 1 liter after 48 h of incubation at 37°C with agitation, incubation for an additional 18 to 24 h under similar conditions, centrifugation at 12,000 × g for 20 min at 4°C, and resuspending the mycoplasmas from 1 liter in 20 ml of EB prepared with 15% (wt/vol) sucrose in place of horse serum. These concentrated stocks contained approximately 3 × 1010 to 4 × 1010 CFU/ml and were stored in 1-ml aliquots at −70°C. Most of the work in this study was done with M. arthritidis 158p10p9 (12) and H606 (30). These strains differ in virulence (20, 50, 52), in restriction fragment length polymorphisms in their chromosomal DNAs (54), and in lysogenization with M. arthritidis bacteriophage MAV1 (50) but are closely related antigenically and share strain-specific surface markers, including MAA2 (54). M. arthritidis PG6 (34) was used as an MAA2-negative control (54) for PCR. Additional strains used in Southern hybridization experiments are described in detail elsewhere (54).
M. arthritidis 158p10p9 lipoproteins were labeled with [3H]palmitic acid as described by Bricker et al. (8). Briefly, 20 ml of EB was inoculated with 20 μl of stock culture; after 48 h of incubation at 37°C, mycoplasmas were recovered by centrifugation, washed once with phosphate-buffered saline (PBS; pH 7.4), resuspended in 2 ml of EB containing 1 mCi of [3H]palmitic acid (NEN Research Products, Du Pont Co., Boston, Mass.), and incubated an additional 18 h at 37°C. Cells were washed once with 100 mM Tris-HCl–150 mM sodium chloride (pH 7.4), and partitioned into aqueous and hydrophobic fractions by Triton X-114 (TX-114) extraction (6). A sample of each fraction was subjected to polyacrylamide gel electrophoresis (PAGE) on a sodium dodecyl sulfate (SDS)–7.5% (wt/vol) polyacrylamide gel; the gel was dried, and labeled bands were visualized by fluorography.
Carboxypeptidase Y treatment of intact cells.
Samples (200 μl) from a 48-h broth culture of M. arthritidis were centrifuged at 10,000 × g for 10 min and resuspended in 15 μl of 50 mM sodium citrate buffer (pH 5.3) containing 1.52, 1, 0.5, 0.25, and 0.075 mg of carboxypeptidase Y (Boehringer Mannheim Corp., Indianapolis, Ind.) per ml. Reaction mixtures were incubated for 1 h at 37°C, solubilized, electrophoresed, and immunoblotted against pooled monoclonal antibodies (MAbs) specific for MAA1 and MAA2 (designated A9a and 7a, respectively [53]).
Isolation of clonal lineages differing in phenotypic expression of MAA2.
A 48-h broth culture of M. arthritidis 158p10p9 was filtered through a 0.45-μm-pore-size filter; the filtrate was diluted 1:10,000, plated onto Edward agar (54), and incubated at 37°C. After 72 h, the MAA2 expression state was determined by colony blotting as described previously (26, 54). To facilitate location of colonies in which expression of MAA2 was switched OFF, duplicate blots were prepared; one was stained with the MAA2-specific MAb 7a, and the other was stained with polyclonal rabbit antiserum against M. arthritidis 158p10p9 whole cells. One colony expressing the ON and two expressing the OFF phenotype were each subcultured to 2 ml of EB. Subcultures were incubated for approximately 72 h, split into two 1-ml aliquots, and stored frozen at −70°C. Subsequent colony blotting from these stock cultures indicated that 98% of the colonies from the ON variant retained the ON phenotype, while >99% of colonies from the OFF variants retained the OFF phenotype. Templates for PCR were prepared from each variant as described by Theiss et al. (49); briefly, cells from 1 ml of frozen stock culture were pelleted by centrifugation at 10,000 × g for 10 min, washed twice with PBS, resuspended in 75 μl of PBS, and boiled for 10 min.
Isolation and N-terminal sequencing of MAA2; design of oligonucleotide probes.
A concentrated stock culture of M. arthritidis 158p10p9 containing approximately 16 mg of protein was extracted with TX-114, and the entire hydrophobic fraction was separated by SDS-PAGE on a 6% (wt/vol) gel. The gel was stained with 0.3 M CuCl2 (29), and the MAA2 band was excised, crushed, and extracted with 0.1% (wt/vol) SDS–50 mM Tris (pH 8.8)–0.1 mM EDTA–200 mM NH4HCO3. Protein was separated from gel fragments by filtration through a 0.2-μm-pore-size filter. The remaining gel was reextracted with 10 mM Tris (pH 7.5)–0.5% (wt/vol) SDS, and the second filtrate added to the first. The filtrates were dried under vacuum and redissolved in 100 μl of deionized water. Protein was precipitated by overnight exposure to 900 μl of acetone at −20°C (16). The precipitate was collected by centrifugation at 14,000 × g for 20 min, dried, and redissolved in equal volumes (40 μl of each) of PBS and SDS-PAGE solubilizing solution (50 mM Tris [pH 6.8], 2% [vol/vol] glycerol, 0.6% [vol/vol] 2-mercaptoethanol, 2% [wt/vol] SDS). Samples were reelectrophoresed on an SDS–6% (wt/vol) polyacrylamide gel and transferred electrophoretically (Transblot apparatus; Bio-Rad Laboratories, Hercules, Calif.) to a polyvinylidene difluoride membrane in CAPS (cyclohexylaminopropane sulfonic acid) buffer (10 mM CAPS, 3.2 mM dithiothreitol, 15% [vol/vol] methanol [pH 10.5]) (3). One lane was immunostained with the MAA2-specific MAb 7a for confirmation of the identity of the extracted protein, while a second was stained with 0.1% (wt/vol) Coomassie blue-R in 50% (vol/vol) methanol and destained with 10% (vol/vol) acetic acid–50% (vol/vol) methanol. The protein band corresponding to MAA2 was sliced from the Coomassie blue-stained membrane. N-terminal sequencing was performed by the Mayo Protein Core Facility, Department of Biochemistry and Molecular Biology, Mayo Medical School, Rochester, Minn.
From this amino acid sequence (Ala-X-Asp-Asn-Glu-Glu-Lys-Pro-Thr-Pro/Thr-Glu-Gln-Asp), a degenerate 23-base oligonucleotide was designed for use in screening an M. arthritidis genomic DNA library. The oligonucleotide sequence was 5′-GATAATGAAGAAAAACCWACWCC-3′, where W represented either an A or a T. After the authentic nucleotide sequence of maa2 was determined, a corrected 24-mer probe, designated NT, was prepared from the same region (see Fig. 3). NT was used as a probe for the Southern hybridization experiments described below and as a primer for PCR. Oligonucleotides and primers used in this study were synthesized by GIBCO BRL Life Technologies (Grand Island, N.Y.).
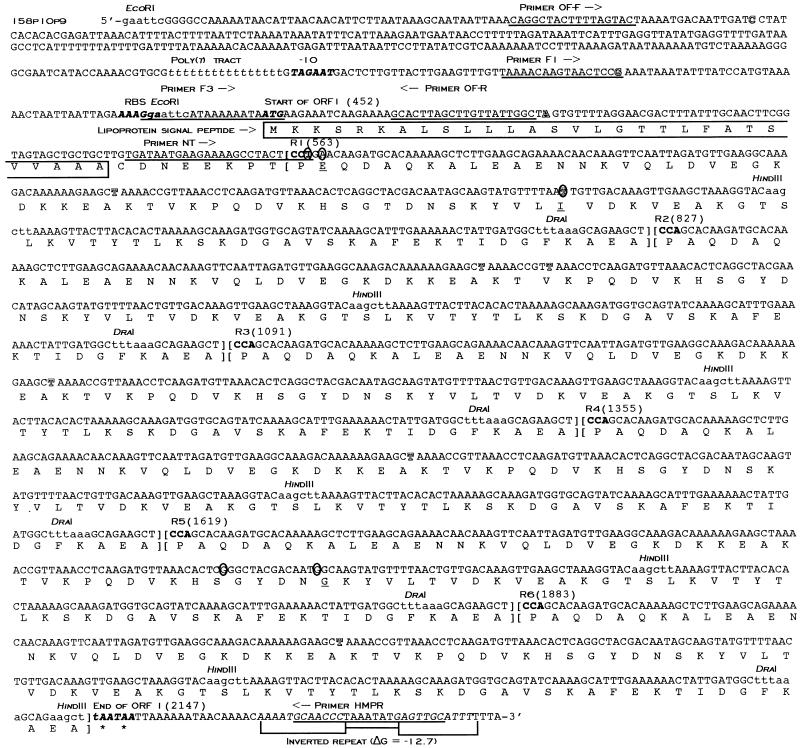
FIG. 3.
Complete nucleotide and deduced amino acid sequences of ORF 1 (maa2) from M. arthritidis 158p10p9. Nucleotides are numbered sequentially from the 5′-terminal EcoRI site. Sites at which DNA sequences differ from those of the maa2 gene from strain H606 are shown as outlined letters. Restriction sites for EcoRI, HindIII, and DraI are printed in lowercase letters. DNA sequences used for preparation of oligonucleotide primers for sequencing and PCR and for use as probes in Southern blotting are underlined. A putative −10 box, ribosome binding site (RBS), and translation terminator are shown in italicized boldface lettering. Repeat units are set apart in brackets and numbered R1 to R6; the first codon of each is printed in boldface. Nucleotides in R1 and R5 that differ from the consensus sequence are circled; amino acids in R1 and R5 that differ from the consensus sequence are underlined. The predicted lipoprotein signal peptide amino acid sequence is boxed. The poly(T) tract is printed in italicized lowercase letters. The inverted repeat downstream from the TAA stop codons is italicized in uppercase letters.
Mycoplasmal chromosomal and recombinant DNA preparation and Southern hybridization.
For preparation of chromosomal DNA, mycoplasmas contained in 1 ml of concentrated stock culture were pelleted by centrifugation at 10,000 × g for 10 min and resuspended in 0.5 ml of 10 mM Tris-HCl–1 mM EDTA (pH 8.0) (TE buffer). Cells were then lysed with 1% (wt/vol, final concentration) SDS, and RNase A (Sigma Chemical Co., St. Louis, Mo.) was added to a final concentration of 100 μg/ml; the sample was then incubated for 30 min at 37°C and extracted twice with Tris-buffered phenol (pH 8.0), once with phenol-chloroform (1:1), and once with chloroform. After ethanol precipitation, the DNA was redissolved in 20 to 25 μl of TE buffer and stored at −20°C until use (50).
For routine screening of recombinant plasmids isolated from E. coli, boiling minipreps of plasmid DNA were prepared as described by Ausubel et al. (3). For DNA sequencing and PCR, plasmid preparations were purified chromatographically, using Qiagen (Chatsworth, Calif.) columns according to the manufacturer’s instructions.
For Southern hybridization (44), 9-μg samples of mycoplasmal chromosomal DNA or 0.25- to 0.5-μg samples of recombinant plasmid DNA were digested with the appropriate restriction endonucleases, electrophoresed on agarose gels according to standard procedures (3), and transferred by vacuum blotting to nylon membranes (Hybond-N+; Amersham Life Sciences, Arlington Heights, Ill.) as instructed by the manufacturer (Bio-Rad). Blots were hybridized either with oligonucleotide probes or with a 264-bp HindIII fragment subcloned from the repeat region of maa2 (see below). The oligonucleotide probe was end labeled with [γ-32P]ATP, using T4 polynucleotide kinase as instructed by the supplier (Promega, Madison, Wis.) (3), while the 264-bp HindIII fragment was labeled with [α-32P]dATP or [α-32P]TTP, using an Amersham Life Sciences random primed labeling kit.
Preparation and screening of an M. arthritidis genomic DNA library and subcloning of fragments for DNA sequencing.
Randomly sized fragments of M. arthritidis 158p10p9 genomic DNA were prepared for molecular cloning by digestion with EcoRI under conditions favoring “star” activity (2). The Lambda Zap II/Gigapack II cloning and packaging kits (Stratagene, La Jolla, Calif.) were used according to the manufacturer’s instructions. Plaques on lawns of E. coli SOLR (Stratagene) were screened for expression of MAA2, using a monospecific polyclonal rabbit antiserum (55); the pBlueScript phagemid was excised as described by the supplier (Stratagene) from a subset of positive plaques and transformed into E. coli JM101 (3) for further screening with the degenerate oligonucleotide probe described above. One positive clone containing a 5-kb insert was identified for further study. For initial sequencing, several restriction fragments from this insert were subcloned into pUC19 as follows. Chromatographically purified recombinant plasmid and pUC19 DNA were digested with the appropriate restriction enzymes (see Results) and electrophoresed on 0.8% (wt/vol) low-melting-point agarose gels. Bands were sliced from the gel, heated for 3 to 4 min at 65°C, combined in a 1:10 (vector/insert) ratio in a 12-μl reaction mix, and ligated overnight at 15°C with T4 ligase (Promega). The gelled reaction mixture was remelted at 65°C, and DNA was extracted once with Tris-buffered phenol and precipitated with absolute ethanol. The pellets were washed once with 70% ethanol, dried under vacuum, and redissolved in 10 μl of water. Recombinant plasmid DNA was then introduced into E. coli XL1-Blue MRF′ by electroporation (Cell-Porator; Bethesda Research Laboratories Life Technologies, Inc., Bethesda, Md.).
PCR and cloning of amplified DNA products.
Three sets of primer pairs were used for amplification of DNA sequences from M. arthritidis 158p10p9 (see also Fig. 3). (i) For determination of the number of repeats in the maa2 gene and for sequencing through the repeat region, primers were NT (forward primer, beginning 115 bp downstream of the maa2 translation start site [5′-GATAATGAAGAAAAGCCTACTCCT-3′]) and HMPR (reverse primer, 43 bp downstream of the second TAA stop codon [5′-GCAACTCATATTTAGGGTTGCATTTT-3′]). (ii) For amplification of the entire maa2 coding region plus a small amount of flanking DNA, primers were F1 (forward primer, beginning 75 bp upstream of the maa2 translation start site [5′-TAAAACAAGTAACTCCG-3′]) and HMPR. (iii) For analysis of the putative promoter region of maa2, primers were OF-F (forward primer, beginning 392 bp upstream of the maa2 translation start site [5′-ATTAAACAGGCTACTTTTAGTAC-3′]) and OF-R (reverse primer, starting 38 bp downstream from the start codon [5′-GCCAATAACAAGCTAAGTGC-3′]). F1 and HMPR were also used as sequencing primers.
In the first two PCRs, DNA samples were amplified with Taq DNA polymerase (2.5 U/reaction mix; Promega) in 50-μl reaction mixtures containing 1.5 mM magnesium chloride, 1× reaction buffer (Promega), 10 mM deoxynucleoside triphosphates (Pharmacia Biotech, Piscataway, N.J.), 5 μM each primer, and 100 ng of chromosomal or 5 ng of plasmid DNA or 10 μl of boiled mycoplasmal suspension from the MAA2 ON/OFF expression variants described above. This is a modification of the method described by Ausubel et al. (3). Thermal cycler settings were as follows: denaturation for 1 min at 94°C, annealing for 1 min at 52°C, and polymerization for 3 min at 72°C for 30 cycles followed by 1 cycle of 1 min at 94°C, 1 min at 52°C, and 10 min at 72°C. For the third reaction (amplification of upstream DNA), Pfu DNA polymerase (Stratagene) was used (2.5 U/reaction mix) in an otherwise identical reaction mixture; thermal cycler settings were also the same except that the annealing temperature was decreased to 42°C.
Because the maa2 coding region could not be amplified from M. arthritidis H606 by using primer F1, a different forward primer, F3, was designed for this purpose. The 3′ end of F3 overlapped the ATG start codon (5′-GGATTCATAAAAAATAATG-3′) (see Fig. 3). Primer F3 was used with reverse primer HMPR under conditions identical to those in the first two reactions described above.
Products amplified with Taq DNA polymerase were cloned directly into PCR cloning vector pGEM-T or pGEM-T Easy (Promega). Products amplified with Pfu polymerase were incubated an additional 10 min at 72°C with Taq polymerase and dATP to add the terminal adenine residues prior to cloning into pGEM-T Easy. Recombinant plasmids were inserted into E. coli XL1-Blue MRF′ by electroporation.
DNA sequencing.
DNA sequencing was performed by the Iowa State University DNA Synthesis and Sequencing Facility, Ames. DNA and deduced amino acid sequence data were analyzed by using the MacDNASIS Pro version 3.6 software package (Hitachi Software Engineering Co., Ltd., South San Francisco, Calif.). For sequencing through the repeat regions of maa2, sequences amplified by PCR from forward and reverse primers NT and HMPR were cloned into vector pGEM-T as described above, and a series of nested deletions was prepared by using the Erase-A-Base system (Promega).
Expression of MAA2 in E. coli.
Recombinant MAA2 was expressed from the lacZ promoter of vector pGEM-T. Transcriptional fusions were prepared by cloning the PCR-amplified full-length coding region of maa2 into this vector as described above; the recombinant plasmids were inserted into E. coli XL1-Blue MRF′ by electroporation. A convenient EcoRI site just upstream from the ATG start codon allowed us to determine the orientation of the inserts by using an EcoRI/PstI double digest. The PstI site was located within the vector multiple cloning site; there were no internal PstI sites within the insert. E. coli colonies containing inserts in both orientations were transferred to 1 ml of LB broth medium (3) and grown overnight with agitation; 0.25 ml of each broth culture was transferred to 40 ml of LB and incubated with agitation until the absorbance at 590 nm reached 0.50. The cultures were then split into two flasks, and 80 μl of 100 mM isopropyl-1-thio-β-d-galactoside (IPTG) was added to one. Both cultures were incubated an additional 2 h at 37°C without agitation. Bacteria were recovered by centrifugation and resuspended in 10 ml of PBS. Samples (100 μl) of these suspensions were lysed with SDS-PAGE solubilizer, electrophoresed on SDS–7.5% (wt/vol) polyacrylamide gels, and subjected to Western immunoblotting against MAb 7a.
Nucleotide sequence accession numbers.
GenBank accession numbers for nucleotide and deduced amino acid sequences for maa2 from strain 158p10p9, maa2 from strain H606, and open reading frame (ORF) 2 from strain 158p10p9 are AF021925, AF021927, and AF021926, respectively.
RESULTS
TX-114 partitioning and metabolic labeling of M. arthritidis lipoproteins with [3H]palmitic acid.
Western immunoblotting of TX-114-extracted M. arthritidis 158p10p9 confirmed that MAA1 and MAA2 surface antigens both partitioned into the hydrophobic phase. In addition, autoradiography of electrophoretically separated M. arthritidis TX-114-extracted proteins indicated that proteins comigrating with MAA1 and MAA2, in addition to several other detergent-phase proteins, could be metabolically labeled with [3H]palmitic acid (data not shown). These results indicated that MAA1 and MAA2 were both integral membrane proteins and provided preliminary evidence for lipid modification.
Carboxypeptidase Y treatment of intact cells.
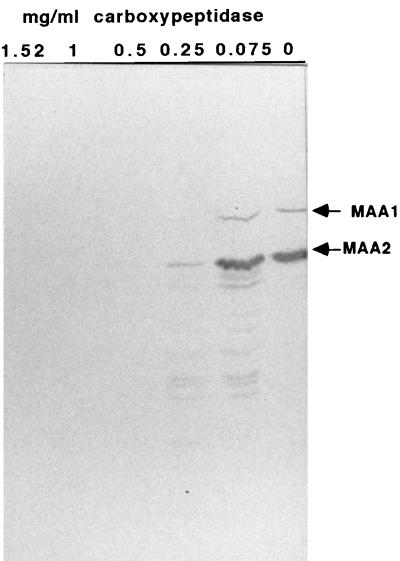
Intact M. arthritidis 158p10p9 cells were treated with various concentrations of carboxypeptidase Y for 1 h. Cells were then lysed and subjected to SDS-PAGE and Western immunoblotting against pooled MAbs A9a (specific for MAA1) and 7a (specific for MAA2) (Fig. 1). Epitopes recognized by both MAbs were removed from the cell surface by carboxypeptidase Y, which hydrolyzes those peptide bonds closest to the C terminus of a protein, indicating that the C termini of both proteins were exposed to the extracellular environment.
FIG. 1.
Western immunoblot of electrophoretically separated M. arthritidis proteins after treatment of intact cells with carboxypeptidase Y concentrations of 0, 0.075, 0.25, 0.5, 1, and 1.52 mg/ml for 1 h, stained with pooled MAA1- and MAA2-specific MAbs A9a and 7a, respectively. Proteins were electrophoresed on SDS–7.5% (wt/vol) polyacrylamide gels.
In addition, a ladder pattern is apparent under the full-length MAA2 band on some lanes of this immunoblot. This pattern is derived from MAA2 and not MAA1, because it appears on immunoblots stained only with MAb 7a (not shown), on which MAA1-derived ladders would not be visible. A similar MAA2 ladder pattern also appears occasionally on Western immunoblots in which lysates from untreated mycoplasmas are overloaded onto SDS-polyacrylamide gels (not shown) and may represent the expression of a variety of MAA2 size variants by different members of a mixed population.
Molecular cloning, mapping, and sequencing of an M. arthritidis DNA fragment containing the maa2 gene.
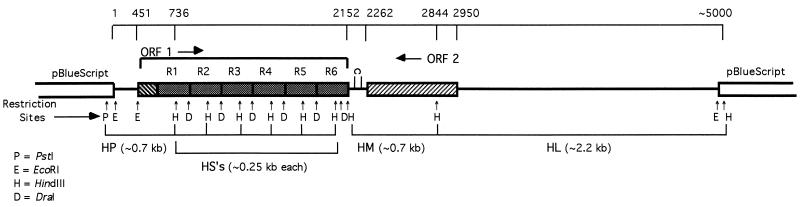
A genomic library from M. arthritidis 158p10p9 was constructed as described in Materials and Methods, and plaques were screened with a polyclonal monospecific rabbit antiserum against MAA2. Recombinant phage from eight plaques showing various degrees of reactivity with the antiserum were expanded; the pBluescript phagemids were then excised and transformed into E. coli JM101. Because it was possible that restriction sites at the fragment ends could not be recut with EcoRI due to the use of star activity during cloning, recombinant plasmids were digested with two enzymes with sites within the vector that flanked the inserts. HindIII and PstI were chosen for this purpose. The resulting fragments were subjected to Southern hybridization with the 32P-labeled degenerate oligonucleotide probe derived from the MAA2 N-terminal amino acid sequence. One plasmid contained an approximately 700-bp HindIII/PstI (HP) fragment that hybridized specifically with the probe (not shown). This plasmid was designated pM8-2. Restriction mapping indicated that the insert consisted of a 2.2-kb (HL), a 0.7-kb (HM), and an indeterminate number of ∼0.25-kb (HS) HindIII fragments, in addition to the 0.7-kb HP fragment (Fig. 2). Although it was not possible to determine the number of HS fragments from restriction analysis alone, the size of the entire cloned fragment, approximately 5 kb, was sufficient to allow for the presence of five to six. The insert contained one internal EcoRI site. There were no internal PstI sites, indicating that sequences hybridizing with the MAA2 N-terminus-derived oligonucleotide were located proximal to the vector-encoded PstI site at one end of the cloned fragment.
FIG. 2.
Map of ∼5-kb M. arthritidis 158p10p9 DNA insert in plasmid pM8-2 (derived from pBlueScript) containing sequences hybridizing with the oligonucleotide probe derived from the MAA2 N-terminal amino acid sequence. Base pairs are numbered beginning with the terminal EcoRI site at the 5′ end of the insert. The ORF 1 tandem direct repeat units are indicated as shaded boxes and numbered R1 to R6. A putative Rho-independent transcription terminator between ORFs 1 and 2 is indicated as a hairpin loop. Restriction sites are indicated by arrows. The ∼0.7-kb HP fragment and ∼2.2-kb, ∼0.7-kb, and ∼0.25-kb (HL, HM, and HS, respectively) fragments from this insert that were subcloned to pUC19 for DNA sequencing are also shown.
HP, HL, HM, and the mixture of comigrating HS fragments were sliced from agarose gels and ligated into pUC19 for sequencing as described in Materials and Methods. Using vector primers, complete forward and reverse DNA sequences were obtained from HP, HM, and six copies of HS, and partial sequence was obtained from the 2.2-kb HL. All six copies of the subcloned HS fragments were identical and contained 264 bp of mycoplasmal DNA. The beginning of an ORF (ORF 1) was found 451 bp downstream of the pBluescript-derived PstI site on fragment HP, oriented away from the vector and reading into the insert (Fig. 2 and 3). A sequence homologous to that of the degenerate oligonucleotide probe used to identify this fragment was located 92 nucleotides (nt) downstream of the ATG start codon. Upstream of the start site was a sequence resembling a ribosome binding site (AAAGGA), although its ability to function as such is uncertain, considering its distance from the ATG start codon. A putative −10 box (TAGAAT) was also found immediately downstream of a 16-nt poly(T) tract. No obvious −35 box was seen. The positions of these putative regulatory sites are shown in Fig. 3.
To confirm previous sequence data and the positions of the HS fragments, two additional sequencing primers were designed. The forward primer (primer F1) was located 75 nt upstream from the ATG start codon, while the reverse primer (primer HMPR) was designed from sequence at the 5′ end of the HM fragment (Fig. 3). These primers were used with template plasmid pM8-2, containing the entire 5-kb cloned fragment from the genomic library. DNA sequence data from both primers indicated the presence of a series of tandem 264-bp nearly identical direct repeats beginning 112 bp downstream of the start codon and ending with two tandem TAA translation termination codons. Each repeat contained a single central HindIII site and a single DraI site near the 3′ end. The consensus repeat sequence was represented by repeat 2 (R2), R3, R4, and R6; minor substitutions were found in R1 and R5. All six HS copies exactly matched two halves of the consensus sequence. It was possible to obtain reliable DNA sequence data through the first two and the last two repeats by using primers from outside the repeat region. The strategy used to generated DNA sequence through the middle repeats is described below.
A possible Rho-independent transcription terminator with a calculated ΔG of −12.7 kcal was found just downstream of the TAA stop codons (Fig. 3). In addition, a second ORF was located in the opposite orientation beginning in the HL fragment (Fig. 2) and ending 99 nt downstream of the second ORF 1 TAA stop codon. Its placement suggested the possibility that the two ORFs use the same terminator, although no definite conclusions can be drawn from the data available at this time.
Determining the number of repeat units in strains expressing two different size variants of MAA2.
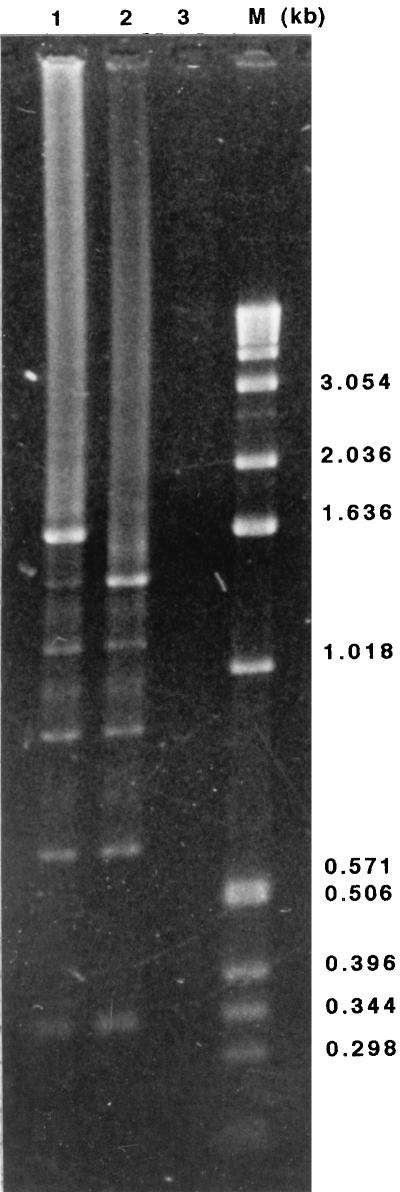
MAA2 is a major strain-specific marker for a group of M. arthritidis strains antigenically related to 158p10p9. This includes the avirulent strain H606; however, H606 expresses a variant of MAA2 that is approximately 10 kDa smaller than the 158p10p9 version (54), almost exactly what would be expected if one of the 264-bp repeat units were missing from H606. To determine how many repeats were contained within ORF 1 and whether this number differed between strains, the entire repeat region was amplified by PCR from pM8-2 recombinant plasmid DNA and from M. arthritidis H606 chromosomal DNA, using primers NT and HMPR (primer sequences and locations are shown in Fig. 3). These reactions amplified major products of 1.6 and 1.3 kb from pM8-2 and H606, respectively, enough to account for the presence of six and five 264-bp repeats, respectively. In addition, five and four minor products decreasing progressively in size in approximately 260-bp increments were amplified from pM8-2 and H606, respectively (Fig. 4). All of the products hybridized under highly stringent conditions with one of the 264-bp HS fragments subcloned from the ORF 1 repeat region (not shown). Using the same primers and PCR conditions, the same major and minor products seen with the pM8-2 template were also amplified from M. arthritidis 158p10p9 chromosomal DNA (not shown). No product was amplified from chromosomal DNA from M. arthritidis PG6 (Fig. 4), which does not express MAA2 (54).
FIG. 4.
PCR products (10 μl) amplified from recombinant plasmid pM8-2 (lane 1) and from chromosomal DNA from M. arthritidis H606 (lane 2) and M. arthritidis PG6 (lane 3) by using primers NT and HMPR were electrophoresed on a 1.5% (wt/vol) agarose gel and stained with ethidium bromide. PCR products differed in size in approximately 260-bp increments. Size markers are shown in lane M.
Sequencing through the entire repeat region of ORF 1.
A nested deletion strategy was used to obtain the DNA sequence through the entire repeat region of 158p10p9. The full-length ORF 1 plus a small amount of flanking DNA was amplified by PCR from the pM8-2 plasmid template, using primers F1 and HMPR. The amplified product was cloned into vector pGEM-T, and a series of nested deletions was made by using the Erase-A-Base system as described in Materials and Methods. The deleted products were religated and inserted into E. coli XL1-Blue MRF′ by electroporation. Three appropriately sized recombinant plasmids were selected for DNA sequencing, and data from overlapping regions were combined to provide the complete sequence of ORF 1 (Fig. 3). Comparison of the repeat regions showed substitutions in R1 and R5 with respect to the consensus sequence (R2, R3, R4, and R6) (Fig. 3): R1 had an A→T substitution at nt 3, a C→A substitution at nt 5, and a C→T substitution at nt 146; R5 had A→G substitutions at nt 117 and 130. These substitutions were confirmed by comparison of DNA sequence data from pM8-2 and several of the major and minor PCR products described above.
Comparison of full-length coding regions of ORF 1 from strains 158p10p9 and H606.
Partial DNA sequence of the H606 version of ORF 1 was obtained from the PCR-amplified 1.3-kb product described above containing only the repeats. To confirm these data and to obtain the DNA sequence of the 5′ end of the gene, the entire coding region was amplified by PCR using primers F3 and HMPR. Primer F3 was used in this reaction instead of F1, because we were unable to amplify a product from H606 with F1. Again, the reaction mixture contained one major and four minor products, and no product was amplified from strain PG6 (not shown). The full-sized H606 product was cloned into vector pGEM-T and sequenced from both directions, using vector-derived primers. To obtain the DNA sequence upstream of the ATG start codon, H606 template DNA was amplified by PCR from primers OF-F and OF-R (Fig. 3); the single 441-bp product was cloned into vector pGEM-T Easy and sequenced in both directions.
Sites at which ORF 1 from H606 and its associated upstream DNA differ from the corresponding regions in 158p10p9 are indicated on Fig. 3 by outlined letters. Comparison of the two sequences showed that this region was very highly conserved between the two strains, with the exception of the missing repeat unit in H606. There were two nucleotide substitutions in the upstream DNA, including one corresponding to the 3′-most nucleotide of primer F1, which may explain why we were not able to amplify a product from H606 with this primer. While it is not clear from this experiment alone whether the upstream substitutions were authentic or the result of PCR-induced errors, the latter is unlikely, because a DNA polymerase (Pfu) containing 3′-5′ proofreading activity was used for this reaction.
Within the coding region itself, there were a total of eight substitutions, including a T→C change at nt 84 in each of the five H606 repeat units. As for strain 158p10p9, R1 from H606 differed from the consensus sequence by the substitution of a T for an A at position 3 and an A for a C at position 5. In addition, R2 from H606 had a C→T substitution at position 93. These substitutions were confirmed by compilation of DNA sequence data from several PCRs. Changes in the predicted amino acid sequence resulting from these substitutions are described below.
The G+C contents of the coding region are 34.85 and 35.42 mol% for 158p10p9 and H606, respectively, compared with a previously reported 30 to 33 mol% for the entire genome (19, 21). The G+C content of upstream DNA is much lower, 21.8 mol%, as is typical of other mycoplasmal intergenic regions (19).
Analyses of deduced amino acid sequences of ORF 1 and ORF 2.
The deduced amino acid sequence of ORF 1 from strain 158p10p9 is shown in Fig. 3. The first 29 amino acids constitute a typical prokaryotic lipoprotein signal peptide with a predicted cleavage site for signal peptidase II (47). The single Cys residue located at the N terminus of the mature protein is a likely site for lipid modification and membrane anchorage. The 11 amino acids immediately following the Cys residue (Asp-Asn-Glu-Glu-Lys-Pro-Thr-Pro-Glu-Gln-Asp) exactly matched the sequence obtained from N-terminal sequencing of gel-purified MAA2, indicating that the prolipoprotein is processed in M. arthritidis. The 264-bp tandem repeats translated in frame into five and six, for H606 and 158p10p9, respectively, 88-amino-acid nearly identical direct repeats. MAA2 was predicted to be highly basic (predicted pI = 9.43) with an unusually high concentration of lysine, >18 mol%. Aside from the signal peptide, the protein was also predicted to be largely hydrophilic, suggesting surface exposure of the bulk of the molecule, >90% of which consisted of the 88-amino-acid repeats. The nucleotide differences between the 158p10p9 and H606 versions of ORF 1 resulted in only two amino acid changes, one within the signal peptide (amino acid 14, Ser→Gly) and one within R1 (amino acid 86, Ile→Thr).
The predicted molecular weights for the MAA2 prolipoproteins of strains 158p10p9 and H606 were 61,399 and 51,805, respectively, and those for the mature proteins were 58,469 and 48,904. The predicted molecular weight of 58,469 for MAA2 from 158p10p9 was less than the 66,000 calculated from mobility on SDS-PAGE (54); however, lipid modification can cause alterations in electrophoretic migration patterns.
Comparison of deduced amino acid sequences of the 11 repeat units (6 from 158p10p9 and 5 from H606) showed that the nucleotide substitutions resulted in only three amino acid changes with regard to the consensus sequence (altered residues are underlined on Fig. 3). They were Ala→Glu in the 2nd residue of R1 from both 158p10p9 and H606, Ser→Gly in the 44th residue of 158p10p9 R5, and Thr→Ile in the 49th residue of 158p10p9 R1. The 84th nt C→T substitution that characterized all five of the H606 repeats did not result in an amino acid change.
GenBank searches did not reveal any significant similarities to other known proteins.
The deduced amino acid sequence of ORF 2 predicted a very basic (pI = 9.63), 25,584-Da protein with a putative 26-amino-acid lipoprotein-like signal peptide (not shown; this sequence was deposited in GenBank with accession number AF021926). GenBank searches revealed no significant homology to other known proteins. It is not known whether this ORF is transcribed or translated in M. arthritidis.
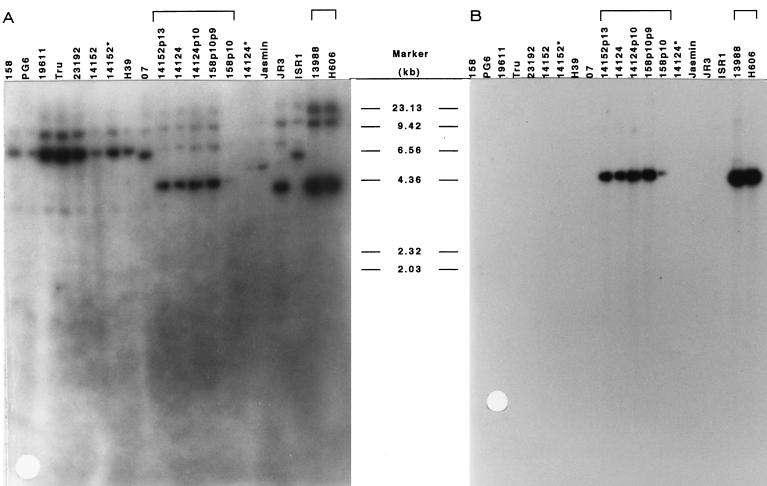
Hybridization of chromosomal DNA from 20 M. arthritidis strains with probes homologous to two different regions of ORF 1.
M. arthritidis chromosomal DNA samples from 20 different strains (54) were digested with EcoRI and subjected to Southern hybridization with the 32P-labeled oligonucleotide probe NT (Fig. 5A). NT was designed from authentic sequence near the 5′ end of ORF 1, replacing the original degenerate oligonucleotide probe derived from the MAA2 N-terminal amino acid sequence (Fig. 3). After hybridization with NT, the blot was stripped and reprobed with one of the cloned 264-bp HS fragments from within the repeat region (Fig. 5B). All 20 strains contained at least one copy of a sequence hybridizing with the NT probe, and several contained two to three such sequences; however, only seven strains, 14152p13, 14124, 14124p10, 158p10p9, 158p10, 13988, and H606, contained sequences hybridizing with the 264-bp HS fragment from within the repeats. The EcoRI fragment on which these sequences were located also hybridized with the NT probe. These seven strains were the only ones found to express the MAA2 epitope recognized by MAb 7a in an earlier study (54).
FIG. 5.
Southern hybridization of M. arthritidis chromosomal DNA from 20 strains with probes prepared from DNA sequences in two different regions of ORF 1. Nine-microgram samples of chromosomal DNA were digested with EcoRI, electrophoresed on a 0.8% (wt/vol) agarose gel, transferred to nylon membrane, and hybridized with 32P-labeled oligonucleotide probe NT, from near the 5′ end of the coding sequence (A), and a 32P-labeled 264-bp HS fragment from within the ORF 1 repeat region (B). All 20 strains contained at least one copy of sequences homologous to NT, while only seven strains contained copies of the sequence from within the repeat region. These seven strains were shown in an earlier study to express the epitope recognized by MAA2-specific MAb 7a, while the others did not (54).
The HS-probe-positive EcoRI fragments from M. arthritidis H606 and 13988 (a version of H606 that we recently acquired from the American Type Culture Collection [54]) were slightly smaller than those from the other five strains. Although the bands were to diffuse to allow accurate migration calculations, this size difference appears to be approximately 200 to 300 bp and could be accounted for by the absence of one of the tandem repeat units that make up the bulk of ORF 1. These data provided additional evidence that ORF 1 corresponded to the maa2 gene and that MAA2 size variation among strains was due to addition or deletion of repeat units.
Expression of recombinant MAA2 in E. coli.
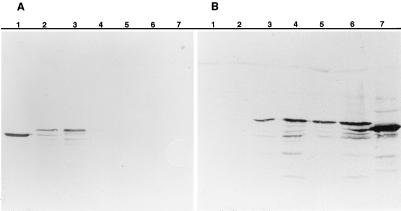
It is often difficult to express recombinant mycoplasmal proteins in other bacterial hosts because of the use by these organisms of an alternative genetic code in which the universal stop codon UGA is used to encode tryptophan (19, 61). However, deduced amino acid sequence data showed that MAA2 contained no tryptophan residues, indicating that it should be possible to express the entire protein in E. coli. The entire ORF 1 coding region plus flanking DNA was amplified by PCR from recombinant plasmid pM8-2, using primers F1 and HMPR (Fig. 3). The amplified product was cloned into pGEM-T and introduced into E. coli XL1-Blue MRF′ by electroporation, as described above for use in the production of nested deletions. E. coli electroporants containing recombinant plasmids with inserts in both orientations were grown in the presence and absence of IPTG as described in Materials and Methods. Equal amounts of E. coli from each culture were centrifuged, lysed, and subjected to SDS-PAGE and Western immunoblotting against MAA2-specific MAb 7a in comparison with a whole-cell lysate of M. arthritidis 158p10p9 and untransformed E. coli. The MAb recognized a product in both IPTG-induced and uninduced E. coli cultures containing the insert in the forward orientation with respect to the lacZ promoter (Fig. 6A). Induction with IPTG was not necessary for expression of the recombinant product but appeared to increase the amount of product detected. No product was detected when the insert was in the reverse orientation or in untransformed E. coli. The recombinant product was approximately 3 kDa larger than the authentic M. arthritidis protein; this size difference is sufficient to account for the presence of the 29-amino-acid signal peptide. It is therefore likely that recombinant MAA2 is not processed in E. coli.
FIG. 6.
Expression of recombinant MAA2 in E. coli. (A) E. coli cells containing the maa2 gene ligated into vector pGEM-T in both orientations with respect to the lacZ promoter were grown in the presence and absence of IPTG, lysed, electrophoresed by SDS-PAGE on 7.5% (wt/vol) gels, transferred to nitrocellulose, and stained with MAA2-specific MAb 7a. Lanes contain M. arthritidis 158p10p9 whole-cell lysate (lane 1), E. coli containing the cloned insert in the forward orientation with respect to the lacZ promoter, in the absence (lane 2) and presence (lane 3) of IPTG, E. coli containing the cloned insert in the reverse orientation with respect to the lacZ promoter, in the absence (lane 4) and presence (lane 5) of IPTG, and untransformed E. coli in the absence (lane 6) and presence (lane 7) of IPTG. (B) E. coli cells containing the maa2 gene from M. arthritidis 158p10p9 clonal variants in which expression of MAA2 was switched ON or OFF were grown in the presence or absence of IPTG, lysed, electrophoresed and immunoblotted as described above. Lanes contain E. coli in which maa2 from an OFF variant was ligated into vector pGEM-T in the reverse orientation with respect to the lacZ promoter, in the absence (lane 1) and presence (lane 2) of IPTG, E. coli in which maa2 from an OFF variant was ligated into vector pGEM-T in the forward orientation with respect to the lacZ promoter, in the absence (lane 3) and presence (lane 4) of IPTG, E. coli in which maa2 from an ON variant was ligated into vector pGEM-T in the forward orientation with respect to the lacZ promoter, in the absence (lane 5) and presence (lane 6) of IPTG, and a whole-cell lysate of M. arthritidis strain 158p10p9 (lane 7).
The full-length maa2 gene was amplified from strain H606 chromosomal DNA by PCR with primers F3 and HMPR and cloned into pGEM-T Easy as described above. E. coli containing recombinant plasmids with inserts in both orientations were tested for ability to express recombinant MAA2 as described for 158p10p9. Essentially identical results were obtained; a recombinant MAA2 product was produced in E. coli only when the insert was in the forward orientation with respect to the lacZ promoter, and the recombinant product was approximately 10 kDa smaller than the recombinant 158p10p9 product (not shown).
These data constitute final proof that ORF 1 encodes the MAA2 protein.
Possible mechanism for phase variation.
Phase variation of MAA2 could be controlled by any of several different mechanisms at the levels of transcription or translation or both. Differences in the putative promoter region between variants in which expression states differed might suggest that control was at the transcriptional level. To test this, 441 bp of DNA immediately upstream of the ATG start codon, plus 38 bp from the 5′ end of the coding region, were amplified by PCR from two clonal variants of 158p10p9 in which expression of MAA2 was switched OFF and one variant in which it was switched ON. The Pfu DNA polymerase, containing 3′-5′ proofreading activity, was used in this experiment for greater fidelity. The PCR products were cloned into pGEM-T Easy and sequenced in both directions. The poly(T) tract just upstream from the putative −10 box contained 16 T residues in the original insert from recombinant plasmid pM8-2 (Fig. 3) and in PCR products amplified from the ON variant, but only 14 T residues were present in the corresponding region of DNA amplified from the two OFF variants (not shown). Otherwise the DNA sequences from the three variants were identical. It is possible that these small changes in spacing within the promoter region might influence the binding of RNA polymerase or of positive or negative transcription regulators. Similar mechanisms have been proposed for control of expression of a series of variable lipoproteins in the swine pathogen Mycoplasma hyorhinis (62), a fimbrial gene in Bordetella pertussis (56), and the Opc outer membrane protein in Neisseria meningitidis (40).
While these results suggested that control of expression of MAA2 may occur at the level of transcription, they did not rule out additional levels of control. To determine whether there were any alterations within the actual coding region that might also influence expression, the full-length maa2 gene was amplified by PCR using primers F1 and HMPR from one OFF and one ON variant. The resulting products were cloned into pGEM-T and inserted into E. coli XL1-Blue MRF′ by electroporation. A full-sized recombinant MAA2 product was expressed from both cloned inserts (Fig. 6B), indicating that the ON/OFF switching mechanism does not involve genetic recombination within the coding region.
DISCUSSION
M. arthritidis-induced arthritis has been extensively studied and characterized over the last several decades (11, 13, 51); however, until recently virulence factors have remained obscure. In recent years, more information regarding mechanisms of host-parasite interaction has become available, and a number of factors that could play important roles in the disease process have been identified. These include the possibility of autoimmunity, triggered either by the M. arthritidis superantigen MAM (10) or by cross-reactivity between M. arthritidis and rat joint tissues (39, 46), activation of other inflammatory pathways via MAM-induced nonspecific T-cell activation (10), the as yet uncharacterized factor(s) supplied by virulence-associated temperate bacteriophage MAV1 (50), and M. arthritidis surface proteins involved directly or indirectly in mycoplasma-host cell interaction (53).
The main focus of investigation in our laboratory in recent years has been M. arthritidis surface antigens, including those involved in cytadherence. Attachment to host tissues is one of the most critical components of an infectious process, enabling many of the later-acting inflammatory and tissue-damaging processes. Two putative M. arthritidis cytadhesins, designated MAA1 and MAA2, were identified in an earlier study on the basis of attachment-inhibition experiments with MAbs prepared against M. arthritidis membrane antigens (53). Subsequently, we showed that MAA2 was a major strain-specific marker for a group of seven M. arthritidis strains related to 158p10p9 and that it exhibited both size and phase variation (54). More recently, we reported that both MAA1 and MAA2 were capable of inducing protective immunity in rats (55), which suggested a possible role for these proteins in pathogenesis, based on the assumption that protective immunity in most bacterial infections is directed against the most important virulence factors.
In this study, we undertook further characterization of MAA2 at the molecular level. A chromosomal fragment encoding MAA2 was cloned from M. arthritidis 158p10p9, mapped, and sequenced. Two ORFs were found in opposite orientation to each other on the cloned fragment, their translation stop codons separated by a 99-bp intergenic region containing an inverted repeat that may function as a Rho-independent transcriptional termination site for both ORFs. Of the 1,695 bp constituting ORF 1, all but 111 were contained within six tandem 264-bp direct repeats.
The deduced amino acid sequence of ORF 1 predicted a largely hydrophilic 565-amino-acid product with a 29-amino-acid N-terminal signal peptide consistent with those found in other prokaryotic lipoproteins, with a potential cleavage site for signal peptidase II (AAA↓C) that nearly matched the L(S,A)(A,G)↓C consensus sequence used by other gram-positive and gram-negative bacteria (7, 47). The first 11 amino acids of the deduced sequence following the Cys residue exactly matched the N-terminal amino acid sequence derived from the gel-purified protein, indicating that the signal peptide was in fact cleaved in M. arthritidis. The modified Cys residue at the N terminus of the mature protein is most likely the site of membrane anchorage and is probably the only portion of the protein embedded in the membrane (60). Lipid modification is extremely common among mycoplasmal surface proteins and provides a means by which these proteins can be anchored to the plasma membrane in the absence of a rigid cell wall (19, 57).
The mature protein was predicted to be highly basic, with a pI of 9.43. This is partly due to an unusually high concentration of Lys residues (>18 mol%), which in turn may reflect the A-T bias in all three codon positions shown by many mycoplasmas (19). The predicted size of the mature lipoprotein was about 8,000 Da smaller than the size calculated by mobility on SDS-PAGE; this may be due to the lipid moiety. Such discrepancies are not uncommon for posttranslationally modified proteins or proteins with unusual secondary structure (5, 35, 36, 43, 62–64).
The 264-bp direct repeats translated in frame into six 88-amino-acid tandem direct repeats that constituted >98% of the mature protein. These repeats were highly conserved, with slight changes from the consensus sequence resulting in only three amino acid substitutions, two in R1 and one in R5 of MAA2 from strain 158p10p9. While repetitive domains are common in surface-exposed bacterial proteins, particularly those involved in host-pathogen cell-cell interaction (reviewed in reference 18), the proportion of MAA2 consisting of repeat sequences seems extreme compared to that reported for other mycoplasmal proteins (14, 28, 43, 62, 64).
An oligonucleotide probe derived from sequence near the 5′ end of ORF 1 hybridized with at least one EcoRI chromosomal fragment from all 20 M. arthritidis strains tested, while a probe derived from within the repeat region hybridized only with those strains expressing the MAb-recognized MAA2 epitope. This constituted additional evidence that ORF 1 encoded MAA2. Conclusive evidence for this was obtained by expression in E. coli of full-sized recombinant MAA2 from the pGEM-T lacZ promoter; the recombinant product was definitively identified with an MAA2-specific MAb. The Southern hybridization experiment further suggested that all 20 strains may produce an MAA2-like protein with a conserved N terminus and an antigenically variable C terminus. Hypervariability among surface-exposed epitopes is common among pathogenic bacteria and constitutes a mechanism for immune avoidance and adaptability.
An additional factor in immune avoidance and adaptation to changing conditions within a host may be the tendency for many proteins containing repeated domains to undergo size variation. A few examples include the Vlp proteins and protein P3 of M. hyorhinis (15, 38), the Vsp proteins of Mycoplasma bovis (4), Streptococcus pyogenes M protein (22), the LMP1 protein of Mycoplasma hominis (23), the V-1 and Vsa proteins of Mycoplasma pulmonis (5, 43), and the MB antigen of Ureaplasma urealyticum (64). MAA2 shows size variability between strains (54), the basis for which is the loss of one of the 264-bp repeat regions from strain H606. There is also evidence for size variation within a single strain, in the ladder pattern seen on overloaded Western immunoblots and in the presence of multiple PCR products differing in size by multiples of ∼260 bp amplified from chromosomal DNA by using primers flanking the maa2 coding region (Fig. 4). The most likely explanation for these multiple products is the presence in any given M. arthritidis population of minor subpopulations in which one or more copies of the maa2 repeat sequence have been deleted. A similar phenomenon appears to have occurred during replication of recombinant plasmids containing the MAA2 coding region, resulting in mixed populations in the E. coli host. Size variation involving deletion or addition of repetitive sequences is often attributed to slipped-strand mispairing during DNA replication (31). However, there is a bias toward the full-sized products in both strains 158p10p9 and H606, as indicated by the fact that the smaller PCR products were less abundant, and we saw no products that were larger than full sized (Fig. 4). In addition, in an earlier study, 11 and 10 progeny populations filter-cloned from strains 158p10p9 and H606, respectively, all expressed the same-size version of MAA2 as their respective parent strains (54). Size variation of MAA2 may be under functional constraint, which could involve the need to maintain a particular secondary structure or a certain distance between the C terminus and the bacterial surface. Both could be important in mycoplasma-host cell interaction.
Variation in the level of expression of surface proteins (phase variation) is also a common phenomenon among pathogenic bacteria (1, 9, 17, 25, 33, 37, 41, 42, 48, 56). This has been well documented within the class Mollicutes as well as is seen in M. bovis (4), M. fermentans (49, 58), M. pulmonis (43), M. hyorhinis (38), M. pneumoniae (reviewed in reference 27), and M. genitalium (32), among others. The mechanisms of phase variation are diverse and not well understood for all these organisms. Of particular relevance to MAA2 is a mechanism involving changes in the length of homopolymeric nucleotide tracts. Differences as small as 2 to 3 bp can affect spacing either within promoters, affecting the binding of RNA polymerases or transcription regulators, or within coding regions, affecting reading frames. For example, changes in the length of a poly(G) tract within the coding region of the pilC gene of Neisseria gonorrhoeae (24) or a poly(C) tract within the bvgS gene of B. pertussis (45) are known to affect translation by altering reading frames. In addition, variations within the length of a poly(A) tract between the −10 and −35 sites in the Vlp promoter region of M. hyorhinis presumably affect ON/OFF expression state at the level of transcription (62), as do changes within poly(C) tracts at similar locations in the opc promoter of N. meningitidis (56) and the fim promoter of B. pertussis (40), although in the latter two cases no −35 boxes were seen, and variation is suggested to affect the binding of transcription activators. We have shown that the length of a poly(T) tract just upstream of a putative −10 box correlates with ON/OFF expression of MAA2. No −35 box was seen upstream of this poly(T) tract, but, as suggested for B. pertussis and N. meningitidis (40, 56), phase variation may be controlled by changes in binding of other transcription regulators. We have shown that PCR-amplified maa2 from a clonal variant expressing the OFF phenotype could be transcribed and translated under control of the pGEM-T lacZ promoter, indicating that the ON/OFF switch does not involve changes within the gene itself.
Further examination of the precise mechanisms of phase variation and the biological function of this and other virulence-associated M. arthritidis surface proteins is under way.
ACKNOWLEDGMENTS
We thank Richard Duman and Denise Riley for valuable technical assistance. We also gratefully acknowledge the participation of Wendy J. Maury, Department of Microbiology, University of South Dakota, and F. Chris Minion, Iowa State University, in technical consultations concerning PCR and DNA sequencing, respectively.
This work was supported by grants from the National Institutes of Health (RO1 AR42553), the National Science Foundation EPSCoR program (OSR-9108733), the South Dakota Future Fund, and the University of South Dakota School of Medicine Parson’s Endowment Fund.
REFERENCES
- 1.Abraham J M, Freitag C S, Clements J R, Eisenstein B I. An invertible element of DNA controls phase variation of type 1 fimbriae of Escherichia coli. Proc Natl Acad Sci USA. 1985;82:5724–2727. doi: 10.1073/pnas.82.17.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson B, McDonald G. Construction of DNA libraries of A-T rich organisms using EcoRI star activity. Anal Biochem. 1993;211:325–327. doi: 10.1006/abio.1993.1278. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1993. [Google Scholar]
- 4.Behrens A, Heller M, Kirchhoff H, Yogev D, Rosengarten R. A family of phase- and size-variant membrane surface lipoprotein antigens (Vsps) of Mycoplasma bovis. Infect Immun. 1994;62:5075–5084. doi: 10.1128/iai.62.11.5075-5084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhugra B, Voelker L L, Zou N, Yu H, Dybvig K. Mechanism of antigenic variation in Mycoplasma pulmonis: interwoven, site-specific DNA inversions. Mol Microbiol. 1995;18:703–714. doi: 10.1111/j.1365-2958.1995.mmi_18040703.x. [DOI] [PubMed] [Google Scholar]
- 6.Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981;256:1604–1606. [PubMed] [Google Scholar]
- 7.Braun V, Wu H C. Lipoproteins, structure, function, biosynthesis, and model for protein export. In: Ghuysen J-M, Hakenbeck R, editors. New comprehensive biochemistry. Vol. 27. Amsderdam, The Netherlands: Elsevier Science; 1994. pp. 319–341. [Google Scholar]
- 8.Bricker T M, Boyer M J, Keith J, Watson-McKown R, Wise K S. Association of lipids with integral membrane surface proteins of Mycoplasma hyorhinis. Infect Immun. 1988;56:295–301. doi: 10.1128/iai.56.2.295-301.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunham R C, Plummer F A, Stephens R S. Bacterial antigenic variation, host immune response, and pathogen-host coevolution. Infect Immun. 1993;61:2273–2276. doi: 10.1128/iai.61.6.2273-2276.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole B C, Atkin C L. The Mycoplasma arthritidis T-cell mitogen, MAM: a model superantigen. Immunol Today. 1991;12:271–276. doi: 10.1016/0167-5699(91)90125-D. [DOI] [PubMed] [Google Scholar]
- 11.Cole B C, Ward J R. Mycoplasmas as arthritogenic agents. In: Tully J G, Whitcomb R F, editors. The mycoplasmas. II. Human and animal mycoplasmas. New York, N.Y: Academic Press; 1979. pp. 367–398. [Google Scholar]
- 12.Cole B C, Ward J R, Jones R S, Cahill J F. Chronic proliferative arthritis of mice induced by Mycoplasma arthritidis. I. Induction of disease and histopathological characteristics. Infect Immun. 1971;4:344–355. doi: 10.1128/iai.4.4.344-355.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole B C, Washburn L R, Taylor-Robinson D. Mycoplasma-induced arthritis. In: Razin S, Barile M F, editors. The mycoplasmas. IV. Mycoplasma pathogenicity. New York, N.Y: Academic Press; 1985. pp. 108–160. [Google Scholar]
- 14.Dallo S F, Chavoya A, Baseman J B. Characterization of the gene for a 30-kilodalton adhesin-related protein of Mycoplasma pneumoniae. Infect Immun. 1990;58:4163–4165. doi: 10.1128/iai.58.12.4163-4165.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng G, McIntosh M A. An amplifiable DNA region from the Mycoplasma hyorhinis genome. J Bacteriol. 1994;176:5929–5937. doi: 10.1128/jb.176.19.5929-5937.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denslow N D, Parten B, Nguyen H P. Analysis Renaissance. Foster City, Calif: Applied Biosystems; 1993. ProBlott: a membrane for electroblotting peptides after enzymatic digestion in gel slices; pp. 33–36. [Google Scholar]
- 17.Donelson J E. Mechanisms of antigenic variation in Borrelia hermsii and African trypanosomes. J Biol Chem. 1995;270:7783–7786. doi: 10.1074/jbc.270.14.7783. [DOI] [PubMed] [Google Scholar]
- 18.Dybvig K. DNA rearrangements and phenotypic switching in prokaryotes. Mol Microbiol. 1993;10:465–471. doi: 10.1111/j.1365-2958.1993.tb00919.x. [DOI] [PubMed] [Google Scholar]
- 19.Dybvig K, Voelker L L. Molecular biology of mycoplasmas. Annu Rev Microbiol. 1996;50:25–57. doi: 10.1146/annurev.micro.50.1.25. [DOI] [PubMed] [Google Scholar]
- 20.Golightly-Rowland L, Cole B C, Ward J R, Wiley B B. Effect of animal passage on arthritogenic and biological properties of Mycoplasma arthritidis. Infect Immun. 1970;1:538–545. doi: 10.1128/iai.1.6.538-545.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrman R. Genome structure and organization. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C: American Society for Microbiology; 1992. pp. 157–168. [Google Scholar]
- 22.Hollingshead S K, Fischetti V A, Scott J R. Size variation in group A streptococcal M protein is generated by homologous recombination between intragenic repeats. Mol Gen Genet. 1987;207:196–203. doi: 10.1007/BF00331578. [DOI] [PubMed] [Google Scholar]
- 23.Jensen L T, Ladefoged S, Birkelund S, Christiansen G. Selection of Mycoplasma hominis PG21 deletion mutants by cultivation in the presence of monoclonal antibody 552. Infect Immun. 1995;63:3336–3347. doi: 10.1128/iai.63.9.3336-3347.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jonsson A-B, Pfeifer J, Normark S. Neisseria gonorrhoeae PilC expression provides a selective mechanism for structural diversity of pili. Proc Natl Acad Sci USA. 1992;89:3204–3208. doi: 10.1073/pnas.89.8.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koomey M. Mechanisms of pilus antigenic variation in Neisseria gonorrhoeae. In: Miller V L, Kaper J B, Portnoy D A, Isberg R R, editors. Molecular genetics of bacterial pathogenesis. Washington, D.C: American Society for Microbiology; 1994. pp. 127–144. [Google Scholar]
- 26.Kotani H, McGarrity G J. Identification of mycoplasma colonies by immunobinding. J Clin Microbiol. 1986;23:783–785. doi: 10.1128/jcm.23.4.783-785.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krause D C. Mycoplasma pneumoniae cytadherence: unraveling the tie that binds. Mol Microbiol. 1996;20:247–253. doi: 10.1111/j.1365-2958.1996.tb02613.x. [DOI] [PubMed] [Google Scholar]
- 28.Ladefoged S A, Jensen L T, Brock B, Birkelund S, Christiansen G. Analysis of 0.5-kilobase-pair repeats in the Mycoplasma hominis lmp gene system and identification of gene products. J Bacteriol. 1996;178:2775–2784. doi: 10.1128/jb.178.10.2775-2784.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee C, Levin A, Branton D. Copper staining: a five-minute protein stain for sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1987;166:308–312. doi: 10.1016/0003-2697(87)90579-3. [DOI] [PubMed] [Google Scholar]
- 30.Lemcke R. The serological differentiation of Mycoplasma strains (pleuropneumonia-like organisms) from various sources. J Hyg (Cambridge) 1964;62:199–219. doi: 10.1017/s0022172400039930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levinson G, Gutman G A. Slipped-strand mispairing: a major mechanism for DNA sequence evolution. Mol Biol Evol. 1987;4:203–221. doi: 10.1093/oxfordjournals.molbev.a040442. [DOI] [PubMed] [Google Scholar]
- 32.Mernaugh, G. R., S. F. Dallo, S. C. Holt, and J. B. Baseman. 1993. Properties of adhering and nonadhering Mycoplasma genitalium. Clin. Infect. Dis. 17(Suppl. 1):S69–S78. [DOI] [PubMed]
- 33.Meyer T F. Variation of pilin and opacity-associated proteins in pathogenic Neisseria species. In: Iglewski B H, Clark V L, editors. The bacteria, a treatise on structure and function. XI. Molecular basis of bacterial pathogenesis. New York, N.Y: Academic Press; 1990. pp. 137–154. [Google Scholar]
- 34.Preston W S. Arthritis in rats caused by pleuropneumonia-like micro-organisms and the relationship of similar organisms to human rheumatism. J Infect Dis. 1942;70:180–184. [Google Scholar]
- 35.Proft T, Hilberg H, Plagens H, Herrmann R. The P200 protein of Mycoplasma pneumoniae shows common features with the cytadherence-associated proteins HMW1 and HMW3. Gene. 1996;171:79–82. doi: 10.1016/0378-1119(96)00014-5. [DOI] [PubMed] [Google Scholar]
- 36.Proft T, Hilbert H, Layh-Schmitt G, Herrmann R. The proline-rich P65 protein of Mycoplasma pneumoniae is a component of the Triton X-100-insoluble fraction and exhibits size polymorphism in the strains M129 and FH. J Bacteriol. 1995;177:337–3378. doi: 10.1128/jb.177.12.3370-3378.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robertson B D, Meyer T F. Genetic variation in pathogenic bacteria. Trends Genet. 1992;8:422–427. doi: 10.1016/0168-9525(92)90325-x. [DOI] [PubMed] [Google Scholar]
- 38.Rosengarten R, Wise K S. The Vlp system of Mycoplasma hyorhinis: combinatorial expression of distinct size variant lipoproteins generating high-frequency surface antigenic variation. J Bacteriol. 1991;173:4782–4793. doi: 10.1128/jb.173.15.4782-4793.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Runge M, Binder A, Gurr E, Fischer M, Meier J, Kirchhoff H. Epitope-sharing between Mycoplasma arthritidis and chondrocytes demonstrated by monoclonal antibodies. Zentbl Bakteriol Parasitenkd Infektionskr Hyg Abt 1 Orig. 1990;273:531–538. doi: 10.1016/s0934-8840(11)80460-3. [DOI] [PubMed] [Google Scholar]
- 40.Sarkari J, Pandit N, Moxon E R, Achtman M. Variable expression of the Opc outer membrane protein in Neisseria meningitidis is caused by size variation of a promoter containing poly-cytidine. Mol Microbiol. 1994;13:207–217. doi: 10.1111/j.1365-2958.1994.tb00416.x. [DOI] [PubMed] [Google Scholar]
- 41.Scott J R. The M protein of group A Streptococcus: evolution and regulation. In: Iglewski B H, Clark V L, editors. The bacteria, a treatise on structure and function. XI. Molecular basis of bacterial pathogenesis. New York, N.Y: Academic Press; 1990. pp. 177–204. [Google Scholar]
- 42.Seifert H S, So M. Genetic mechanisms of bacterial antigenic variation. Microbiol Rev. 1988;52:327–336. doi: 10.1128/mr.52.3.327-336.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simmons W L, Zuhua C, Glass J I, Simecka J W, Cassell G H, Watson H L. Sequence analysis of the chromosomal region around and within the V-1-encoding gene of Mycoplasma pulmonis: evidence for DNA inversion as a mechanism for V-1 variation. Infect Immun. 1996;64:472–479. doi: 10.1128/iai.64.2.472-479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 45.Stibitz S, Aaronson W, Monack D, Falkow S. Phase variation in Bordetella pertussis by frameshift mutation in a gene for a novel two-component system. Nature. 1989;338:266–269. doi: 10.1038/338266a0. [DOI] [PubMed] [Google Scholar]
- 46.Stulle K, Binder A, Sommer G, Kirchhoff H. Immunological reaction of rat tissue cells with antiserum against Mycoplasma arthritidis membranes in indirect immunofluorescence test. J Vet Med B. 1988;35:713–715. doi: 10.1111/j.1439-0450.1988.tb00549.x. [DOI] [PubMed] [Google Scholar]
- 47.Sutcliffe I C, Russell R R B. Lipoproteins of gram-positive bacteria. J Appl Bacteriol. 1995;177:1123–1128. doi: 10.1128/jb.177.5.1123-1128.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tennent J M, Hultgren S, Marklund B-I, Forsman K, Göransson M, Uhlin B E, Normark S. Genetics of adhesion expression in Escherichia coli. In: Iglewski B H, Clark V L, editors. The bacteria, a treatise on structure and function. XI. Molecular basis of bacterial pathogenesis. New York, N.Y: Academic Press; 1990. pp. 80–110. [Google Scholar]
- 49.Theiss P, Karpas A, Wise K S. Antigenic topology of the P29 surface lipoprotein of Mycoplasma fermentans: differential display of epitopes results in high-frequency phase variation. Infect Immun. 1996;64:1800–1809. doi: 10.1128/iai.64.5.1800-1809.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Voelker L L, Weaver K E, Ehle L J, Washburn L R. Association of lysogenic bacteriophage MAV1 with virulence of Mycoplasma arthritidis. Infect Immun. 1995;63:4016–4023. doi: 10.1128/iai.63.10.4016-4023.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Washburn L R. Experimental models of arthritis. In: Tully J G, Razin S, editors. Molecular and diagnostic procedures in mycoplasmology. II. New York, N.Y: Academic Press; 1996. pp. 349–359. [Google Scholar]
- 52.Washburn L R, Hirsch S. Comparison of four Mycoplasma arthritidis strains by enzyme immunoassay, metabolism inhibition, one- and two-dimensional electrophoresis, and immunoblotting. J Clin Microbiol. 1990;28:1974–1981. doi: 10.1128/jcm.28.9.1974-1981.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Washburn L R, Hirsch S, Voelker L L. Mechanisms of attachment of Mycoplasma arthritidis to host cells in vitro. Infect Immun. 1993;61:2670–2680. doi: 10.1128/iai.61.6.2670-2680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Washburn L R, Voelker L L, Ehle L J, Hirsch S, Dutenhofer C, Olson K, Beck B. Comparison of Mycoplasma arthritidis strains by enzyme-linked immunosorbent assay, immunoblotting, and DNA restriction analysis. J Clin Microbiol. 1995;33:2271–2279. doi: 10.1128/jcm.33.9.2271-2279.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Washburn L R, Weaver E J. Protection of rats against Mycoplasma arthritidis-induced arthritis by active and passive immunizations with two surface antigens. Clin Diagn Lab Immunol. 1997;4:321–327. doi: 10.1128/cdli.4.3.321-327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Willems R, Paul A, van der Heide H G, ter Avest A R, Mooi F R. Fimbrial phase variation in Bordetella pertussis: a novel mechanism for transcriptional regulation. EMBO J. 1990;9:2803–2809. doi: 10.1002/j.1460-2075.1990.tb07468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wise K S. Adaptive surface variation in mycoplasmas. Trends Microbiol. 1993;1:59–63. doi: 10.1016/0966-842X(93)90034-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wise K S, Kim M F, Theiss P M, Lo S-C. A family of strain-variant surface lipoproteins of Mycoplasma fermentans. Infect Immun. 1993;61:3327–3333. doi: 10.1128/iai.61.8.3327-3333.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wise K S, Yogev D, Rosengarten R. Antigenic variation. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C: American Society for Microbiology; 1992. pp. 473–489. [Google Scholar]
- 60.Wu H. Post-translational modification and processing of membrane proteins in bacteria. In: Inouye M, editor. Bacterial outer membranes as model systems. New York, N.Y: John Wiley & Sons, Inc.; 1987. pp. 37–71. [Google Scholar]
- 61.Yamao F, Muto A, Kawauichi Y, Iwami M, Iwagami S, Azumi Y, Osawa S. UGA is read as tryptophan in Mycoplasma capricolum. Proc Natl Acad Sci USA. 1985;82:2306–2309. doi: 10.1073/pnas.82.8.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yogev D, Rosengarten R, Watson-McKown R, Wise K S. Molecular basis of Mycoplasma surface antigenic variation: a novel set of divergent genes undergo spontaneous mutation of periodic coding regions and 5′ regulatory sequences. EMBO J. 1991;10:4069–4079. doi: 10.1002/j.1460-2075.1991.tb04983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yogev D, Watson-McKown R, Rosengarten R, Im J, Wise K S. Increased structural and combinatorial diversity in an extended family of genes encoding Vlp surface proteins of Mycoplasma hyorhinis. J Bacteriol. 1995;177:5636–5643. doi: 10.1128/jb.177.19.5636-5643.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zheng X, Teng L-J, Watson H L, Glass J I, Blanchard A, Cassell G H. Small repeating units within the Ureaplasma urealyticum MB antigen gene encode serovar specificity and are associated with antigen size variation. Infect Immun. 1995;63:891–898. doi: 10.1128/iai.63.3.891-898.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]