ABSTRACT
Gut microbiota plays an essential role in the progression of nonalcoholic fatty liver disease (NAFLD), making the gut-liver axis a potential therapeutic strategy. Bacteroides genus, the enriched gut symbionts, has shown promise in treating fatty liver. However, further investigation is needed to identify specific beneficial Bacteroides strains for metabolic disorders in NAFLD and elucidate their underlying mechanisms. In this study, we observed a positive correlation between the abundance of Bacteroides thetaiotaomicron (B. theta) and the alleviation of metabolic syndrome in the early and end stages of NAFLD. Administration of B. theta to HFD-fed mice for 12 weeks reduced body weight and fat accumulation, decreased hyperlipidemia and insulin resistance, and prevented hepatic steatohepatitis and liver injury. Notably, B. theta did not affect these indicators in low-fat diet (LFD)-fed mice and exhibited good safety. Mechanistically, B. theta regulated gut microbial composition, characterized by a decreased Firmicutes/Bacteroidetes ratio in HFD-Fed mice. It also increased gut-liver folate levels and hepatic metabolites, alleviating metabolic dysfunction. Additionally, treatment with B. theta increased the proportion of polyunsaturated fatty acid in the mouse liver, offering a widely reported benefit for NAFLD improvement. In conclusion, this study provides evidence that B. theta ameliorates NAFLD by regulating gut microbial composition, enhancing gut-liver folate and unsaturated fatty acid metabolism, highlighting the therapeutic role of B. theta as a potential probiotic for NAFLD.
KEYWORDS: Bacteroides thetaiotaomicron, nonalcoholic fatty liver disease, gut microbiota, folate metabolism, unsaturated fatty acids
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a common liver disorder that affects approximately a quarter of the world’s population. It encompasses a disease spectrum from mild steatosis with or without inflammation to nonalcoholic steatohepatitis (NASH), which can progress to fibrosis, cirrhosis and even hepatocellular carcinoma.1 NAFLD is closely associated with metabolic syndromes such as obesity, type 2 diabetes, insulin resistance, and cardiovascular disease, leading to its alternative name, metabolic-associated fatty liver disease.2 Currently, the traditional drug development strategies for NAFLD are usually toward metabolic targets, inflammatory pathways, gut-liver axis, and antifibrotic targets.3 However, there are no approved drugs for treating NAFLD to date, and the primary clinical recommendation is weight loss through dietary changes and exercise. Therefore, there is an urgent need to discover effective therapeutic agents for the prevention and treatment of NAFLD.
The emerging field of intestinal microecology has shed light on the potential of regulating the gut-liver axis as a treatment for NAFLD.4 Numerous recent studies have identified a link between gut microbiome changes and NAFLD’s pathogenesis. Certain bacterial strains, such as Lactobacillus, Bifidobacterium, Akkermansia muciniphila, and Bacteroides uniformis, have been found to have protective effects against NAFLD in animal models.5–10 We previously discovered that the abundance of the Bacteroidaceae family and Bacteroides genus was associated with the alleviation of western diet (WD)/CCl4-induced early NASH mice model.11 Among these bacteria, Bacteroides thetaiotaomicron (B. theta), a Gram-negative anaerobe in the intestinal microflora of humans and mice, has shown various beneficial effects, including reinforcement of the host mucosal barrier, maintenance of immune response homeostasis, and modulation of nutrient metabolism.12 B. theta-treated mice displayed lower hepatic steatosis and triglyceride content, restored mucosal barrier function, and reduced lipopolysaccharides (LPS) translocation in experimental alcohol-related liver disease.13 Additionally, B. theta was reported to reduce diet-induced body-weight gain and adiposity in mice.14 However, the protective effects of B. theta against NAFLD, particularly against metabolic disorders in the liver, have yet to be extensively studied. Dietary fiber and gut microbial-derived metabolites exert multiple effects on the host energy metabolism by changing the intestinal environment and directly affecting various host peripheral tissues.15 Hence, in this study, we aimed to investigate the effect of B. theta and its potential mechanisms, focusing on gut microbial composition and hepatic metabolite regulation, in protecting against NAFLD-related metabolic dysfunctions induced by a high-fat diet in mice.
Results
B. theta abundance is positively correlated with the improvement of lipid metabolism dysfunction in NAFLD mice
To identify the specific gut microbiota that correlated with the improvement of advanced NAFLD, which was characterized by hepatic lipid metabolism dysfunction, fibrosis, and even HCC, the mice were pre-induced by a Western diet combined with carbon tetrachloride (WD/CCl4) for 4 weeks. Then, we treated the mice with berberine (BBR) for 20 weeks in a WD/CCl4 plus diethylnitrosamine (WD/CCl4/DEN)−induced mouse model (Figure 1a, up) or for 8 weeks in a WD/CCl4−induced mouse model (Figure 1a, down) to alleviate the advanced NAFLD. As expected, BBR treatment decreased the WD/CCl4/DEN-induced intrahepatic TG (Figure 1b) and CHO levels (Figure 1c), ameliorated severe intrahepatic steatosis, ballooning, and inflammation (Figure 1d). However, the WD/CCl4/DEN-induced fibrosis was not significantly alleviated by BBR (Figure 1d). These effects were confirmed by the decreased NAFLD activity score (NAS) but not the fibrosis score (Figure 1e,f), which suggested that BBR might be more effective against metabolic abnormalities in NAFLD and less effective for the long-term and severe chemical-induced fibrosis in this experimental condition. BBR might protect against NAFLD partially through regulating intestinal microbiota.11,16 Intestinal microbiota sequencing analysis showed that the increased ratio of Bacteroidaceae (family) and Bacteroides (genus) was positively correlated with the improvement of advanced NAFLD (Figure 1g,h). Among the changed Bacteroides genus, Bacteroides thetaiotaomicron (B. theta) strains accounted for the highest proportion and were significantly increased by BBR (Figure 1i). Metabolic prediction through the KEGG databases indicated that these changes were associated with lipid metabolism, biosynthesis of unsaturated fatty acids, insulin signaling, and folate metabolism (Figure 1j). These results were similar to our previous report on the 8-week treatment of BBR in the early stage of NAFLD induced by WD/CCl4 (Figure 1a, down),11 which also showed an increased abundance of B. theta at the species level (Figure 1k) and significant alleviation of lipid metabolism dysfunction. These results suggested that a high abundance of B. theta might positively correlate with improving lipid metabolism dysfunction, and therapy with B. theta might be an effective strategy for preventing and treating hepatic lipid metabolism dysfunction in NAFLD.
Figure 1.
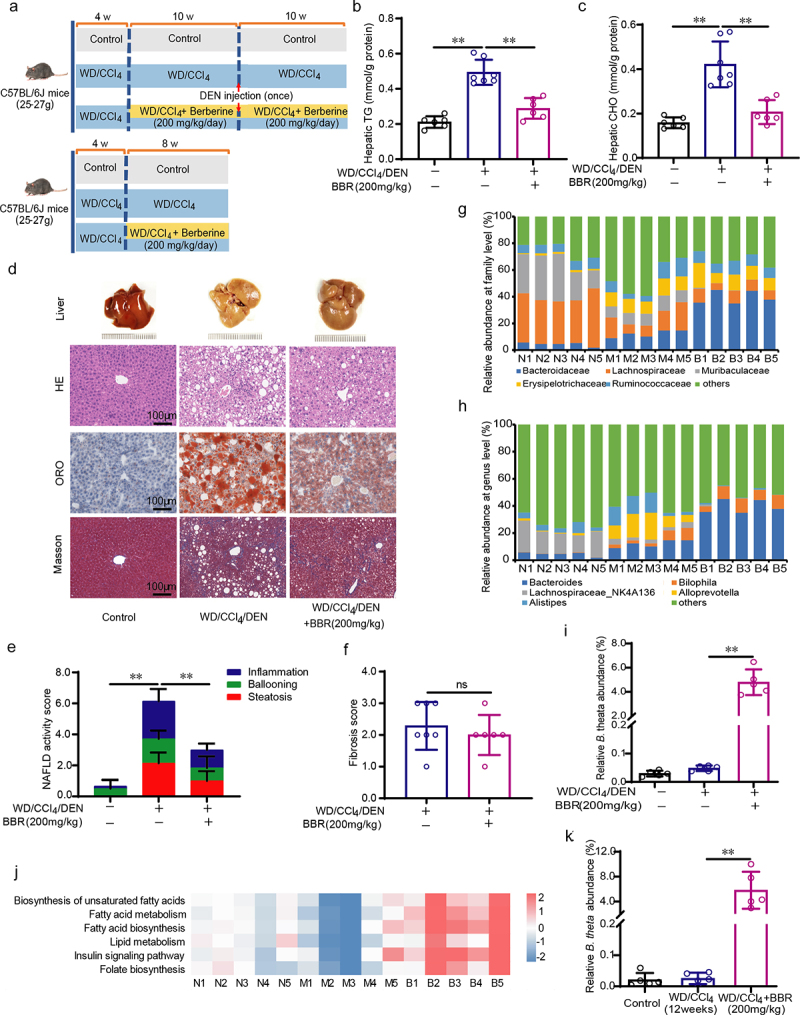
B.Theta abundance is positively correlated with the improvement of lipid metabolism dysfunction in NAFLD mice. (a) Schematic of the experimental design. (b, c) triglyceride (TG) (b) and cholesterol (CHO)(c) in the mouse liver (n = 6 ~ 7). (d) Representative liver histology visualized by H&E staining, oil red O (ORO) staining, and Masson’s trichrome staining. (e,f) NAFLD activity score (NAS) (e) and fibrosis score (f) of mouse liver (n = 6 ~ 7). (g-h) the abundance of the most prevalent gut microbiota at the family (g) and genus (h) levels analyzed by 16S rRNA gene sequencing with fecal samples (n = 5). (i) B. theta abundance at the species level (n = 5). (j) Predicted lipid metabolism-related microbiome function based on the KEGG database. (k) B. theta abundance at the species level in mice with WD/CCl4 treatment for 12 weeks. Data were presented as means with SD or represented figures. *p < .05 and **p < .01 vs. model or control group. ns, not significant. N, Control; M, WD/CCl4/DEN; B, WD/CCl4/DEN+BBR (200 mg/kg).
B. theta alleviates hyperlipidemia and insulin resistance in NAFLD mice
To examine the causality between lipid metabolism dysfunction in NAFLD and the abundance of B. theta, we utilized the HFD-induced obesity mice model to further validate the role of B. theta. Male mice were initially treated with a low-fat diet (LFD) or HFD for 4 weeks, followed by continuous treatment with LFD/HFD alone or with B. theta gavage three times per week for 12 weeks (Figure 2a). From the first week of treatment, the mice in the B. theta-treated HFD group showed a reduced rate of body weight gain, which was significantly lower than that of the HFD group after 12 weeks of treatment (Figure 2b). This result is consistent with previous reports that B. theta could reduce body weight in obese mice.14 Additionally, B. theta treatment led to a slight decrease in body fat percentage in both LFD and HFD-treated mice (Figure 2c). No significant differences were observed in the food intake in both LFD and HFD groups after B. theta treatment (Figure 2d), indicating its safety for long-term oral consumption. Moreover, the HFD elevated serum TG and CHO, while supplementation with B. theta remarkably reduced them in the HFD but not LFD-treated mice (Figure 2e,f). Furthermore, the HFD increased non-fasting blood glucose (Figure 2g) and fasting insulin (Figure 2h) levels, and the calculated HOMA2-IR index (Figure 2i), whereas B. theta effectively reversed these parameters specifically in the HFD-treated group without affecting the LFD-treated group (Figure 2g–i). These results highlight the alleviation of HFD-induced weight gain, hyperlipidemia, and insulin resistance in mice through oral intake of B. theta strains without any discernible side effects.
Figure 2.
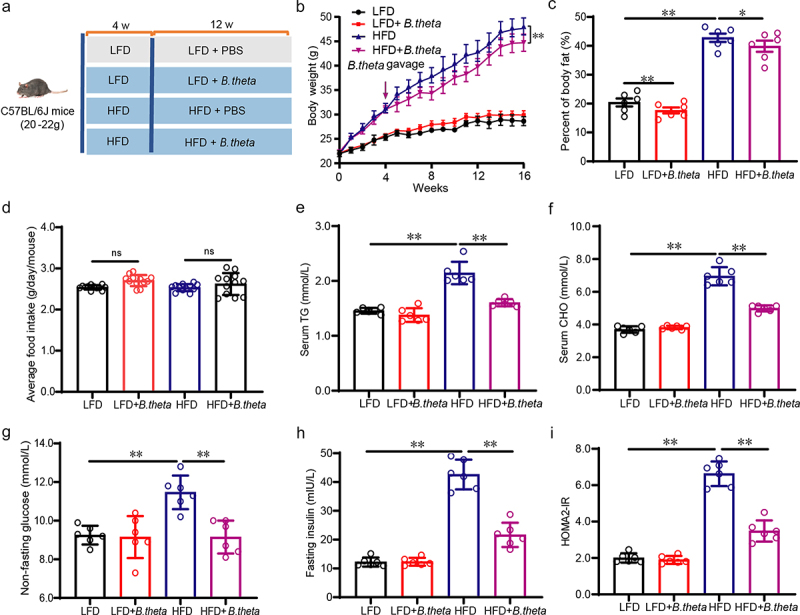
B. theta alleviates hyperlipidemia and insulin resistance in NAFLD mice. (a) Animal experimental schedule. (b) Body weight. (c) Body fat. (d) Average food intake per week. (e) Serum TG. (f) Serum CHO. (g) Non-fasting blood glucose. (h) Fasting insulin. (i) HOMA2-IR index. Data are presented as means with SD. n = 6, *p < .05 and **p < .01 vs. model or control group. ns, not significant.
B. theta treatment prevents hepatic steatohepatitis and liver injury in NAFLD mice
Excessive dietary fat deposition in the liver leads to hepatic steatosis, which, in turn, triggers oxidative stress and inflammatory response in hepatocytes.17,18 HFD significantly increased the levels of serum ALT (Figure 3a) and AST (Figure 3b) in mice, suggesting liver injury, whereas treatment with B. theta effectively reversed the liver injury. HFD also induced fat accumulation (Figure 3c, H&E and ORO staining) and increased liver weight (Figure 3d), while treatment with B. theta alleviated these phenotypes, as well as reducing the liver index (Figure 3e) in the HFD-treated mice, with no significant effect for LFD-fed mice (Figure 3c–e). The liver histopathology of the HFD-fed mice exhibited distorted hepatic lobules, with intracytoplasmic lipid droplets accounting for more than 50% of the field of view, accompanied by inflammatory cell infiltration and ballooning (Figure 3c,f). Remarkably, administration of B. theta showed significant improvement in the disrupted hepatic histopathology, evidenced by the quantification of the NAFLD activity score and a reduction in the number of F4/80-positive cells in mice fed with HFD (Figure 3c,f). The lipid-lowering effect of B. theta was further demonstrated by decreased hepatic TG (Figure 3g) and CHO (Figure 3h) levels in the HFD-fed mice following treatment, while no such effect was observed in the LFD group. These findings further suggested that B. theta treatment might effectively prevent hepatic steatohepatitis and liver injury in NAFLD mice without causing significant toxicity.
Figure 3.
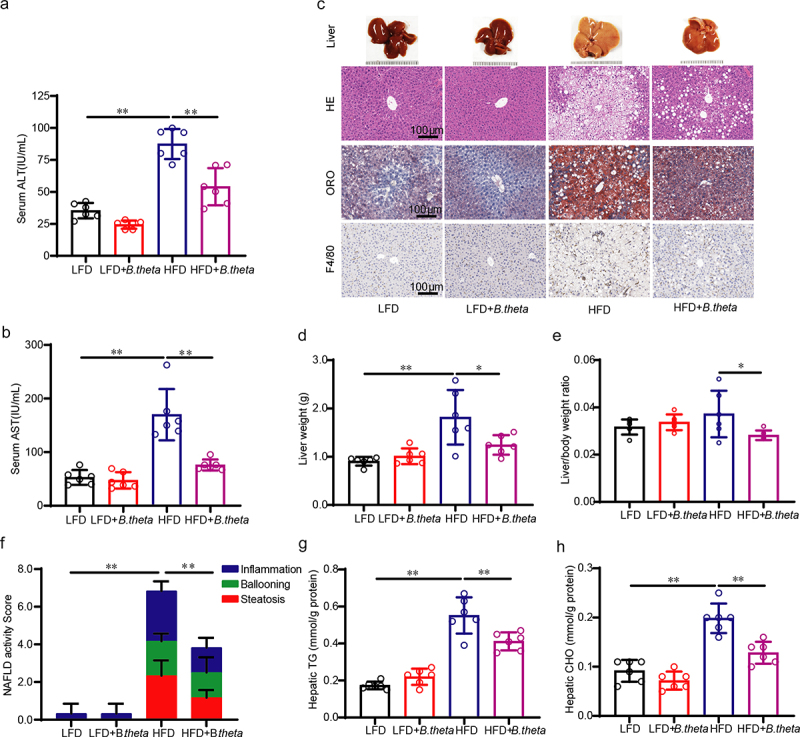
B. theta treatment prevents hepatic steatohepatitis and liver injury in NAFLD mice. (a) Serum ALT. (b) Serum AST. (c) Representative liver appearance, H&E staining, oil red O (ORO) staining, and F4/80 immunohistochemical stains of liver sections. (d) Liver weight. (e) Liver index; (f) quantification of NAFLD activity score (NAS), including steatosis, ballooning, and inflammation grade. (g) Liver TG. (h) Liver CHO. The data are presented as means with SD; n = 6, *p < .05 and **p < .01 vs. model or control group. ns, not significant.
B. theta treatment modulates the gut microbial composition in NAFLD mice
To elucidate the underlying mechanisms of B. theta in improving hepatic steatohepatitis, we investigated its effects on the gut microbial composition in mice using 16s-RNA-amplicon sequencing. Although the differences in operational taxonomic units (OTUs) were not statistically significant (Figure 4a), B. theta treatment significantly increased microbiota diversity, as indicated by higher Simpson’s index of diversity and Shannon index in both LFD and HFD-fed mice (Figure 4b). The ratio of Firmicutes to Bacteroidetes (F/B ratio) is widely accepted to have an important influence on intestinal homeostasis, with an increased F/B ratio often associated with obesity.19 At the phylum level, Firmicutes and Bacteroidetes were found to be the dominant bacteria (Figure 4c), and the B. theta treatment exhibited a decreased F/B ratio in HFD but not LFD-fed mice (Figure 4c,d), suggesting the potential roles of B. theta in affecting intestinal homeostasis and obesity.19 KEGG pathway analysis revealed an upregulation in the biosynthesis of folate and unsaturated fatty acids (Figure 4e), which might potentially contribute to the alleviation of obesity, fatty acid metabolism dysfunction, and insulin resistance associated with HFD-induced hepatic steatohepatitis. These results indicated that B. theta administration induces changes in gut microbial composition, which may have beneficial effects on alleviating HFD-induced hepatic steatohepatitis.
Figure 4.
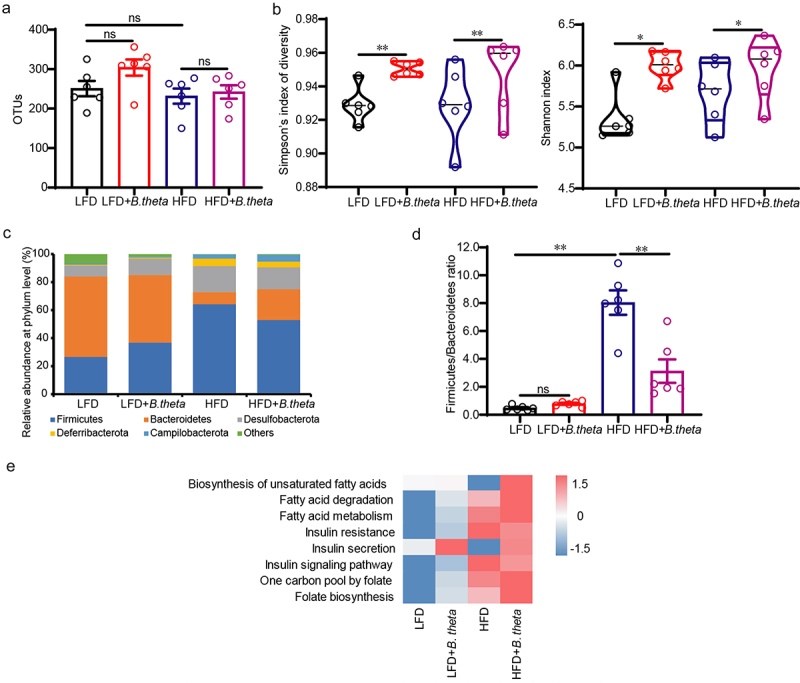
B. theta treatment modulates the gut microbial composition in NAFLD mice. The gut microbial composition was obtained through 16s-RNA-amplicon sequencing. (a) Plots of operational taxonomic units (OTUs). (b) Changes in microbiota diversity presented by the Simpson’s index of diversity and Shannon index. (c) Relative abundance of bacteria at the phylum level. (d) The ratio of firmicutes to Bacteroidetes. (e) Prediction of lipid metabolism-related microbiome function based on KEGG database. The data are presented as means with SD; n = 6, *p < .05 and **p < .01 vs. model or control group. ns, not significant.
B. theta treatment enhances gut folate biosynthesis and modulates hepatic folate metabolism in NAFLD mice
Folate and folate-mediated one-carbon metabolism (Figure 5a) were widely reported to reduce metabolic abnormalities associated with NAFLD.20,21 Based on the enhanced folate biosynthesis and one carbon pool by B. theta treatment using KEGG pathways analysis, we thus investigate whether the mechanism and causative effects of the enriched B. theta for hepatic steatohepatitis are related to folate metabolism. B. theta significantly increased total folate levels in mouse fecal (Figure 5b) and liver (Figure 5c), indicating an enhancement of folate biosynthesis. B. theta treatment increased the levels of S-adenosylmethionine (SAM) (Figure 5d), glutathione (GSH) (Figure 5e), and phosphatidylcholine (PC) (Figure 5f), implying activation of folate-mediated one-carbon metabolism in the liver.21 The relationship was further confirmed by the higher mRNA levels of folate-mediated one-carbon metabolism-related genes, including Pemt, Aldh1l1, Dhfr, and Mat1a (Figure 5g). These findings demonstrated that B. theta supplementation might enhance gut folate biosynthesis and modulate hepatic folate metabolism in HFD-fed mice, thereby alleviating metabolic dysfunction associated with NAFLD.
Figure 5.
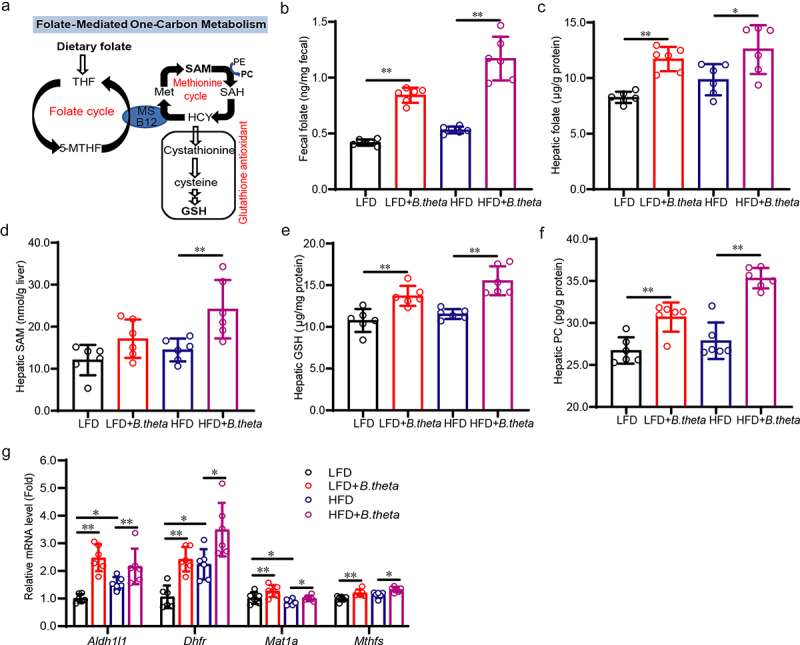
B. theta treatment enhances gut folate biosynthesis and modulates hepatic folate metabolism in NAFLD mice. (a) Schematic illustrates a network of folate-mediated one-carbon metabolism. (b,c) total folate level in mouse fecal (b) and liver (c). (d-f) SAM (d), GSH (e), and PC (f) in the mouse liver. (g) mRNAs related to folate-mediated one-carbon metabolism. The data are presented as means with SD; n = 6, *p < .05 and **p < .01 vs. model or control group. ns, not significant. GSH, glutathione; met, methionine; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine; HCY, homocysteine; PE, phosphatidylethanolamine; PC, phosphatidylcholine; MS, vitamin B12-dependent methionine synthase; 5-MTHF, 5-methyltetrahydrofolate.
B. theta regulates the unsaturated fatty acid in NAFLD mice
Multiple studies have suggested that saturated fatty acids (SFAs) and monounsaturated fatty acids (MUFAs) are positively correlated with the severity of liver steatosis, while polyunsaturated fatty acids (PUFAs) and the PUFA/MUFA ratio are negatively correlated with it.22–25 In light of the evidence by gut microbial sequencing indicating enhanced biosynthesis of unsaturated fatty acids by B. theta, we further investigated the fatty acid composition in the liver (Figure 6a) using the LC-MS/MS method and evaluated the specific fatty acids contributing to the protective role of B. theta in NAFLD mice. The results indicated that HFD increased the composition of total SFAs compared with that in the low-fat diet (LFD)-treated group (Figure 6b), which aligned with previous studies.25 However, treatment with B. theta did not have any impact on total SFAs either in LFD and HFD mice (Figure 6b), suggesting that its effects are independent of SFAs. The total MUFAs were decreased after B. theta treatment in both LFD and HFD-fed mice (Figure 6c), primarily due to the decreased levels of palmitoleic/7-hexadecenoic acid (C16:1) (Figure 6d), which suggested the C16:1 might participant the progression of hepatic steatosis and the therapeutic effect by B. theta. Importantly, we detected a slight decrease in total PUFAs in HFD-fed model mice and a significant increase after B. theta treatment (Figure 6e), resulting in an increased PUFA/MUFA ratio (Figure 6f), two crucial indicators for alleviating NAFLD.25,26 The increased composition of PUFAs induced by B. theta mainly consisted of C18:2, C18:3, C18:4, C20:4, C20:5, and C22:6, making up more than 80% of the PUFAs (Figure 6g–h). Among them, ω-3 PUFAs (18:3, 20:5, 22:6), which have shown beneficial effects on glycolipid metabolism dysfunction in animal experiments and clinics, are expected to become a new target drug for treating fatty liver.22,26 In parallel, B. theta did not affect the activity of stearoyl-CoA desaturase 1 (SCD1) (Figure 6i) but significantly increased the activity of fatty acid desaturase 1 (FADS1) (Figure 6j) and FADS2 (Figure 6k), which is consistent with the increased levels of C20:4 and C18:2 after B. theta treatment (Figure 6g,h). The multifaceted modulation of PUFAs in the liver of mice mediated by B. theta indicates that this is a key mechanism underlying the alleviation of NAFLD. Hence, these findings align with the predictions made by gut microbial sequencing and emphasize the relevance of fatty acid composition changes, specifically the elevated levels of PUFAs induced by B. theta, in contributing to its therapeutic role in NAFLD.
Figure 6.
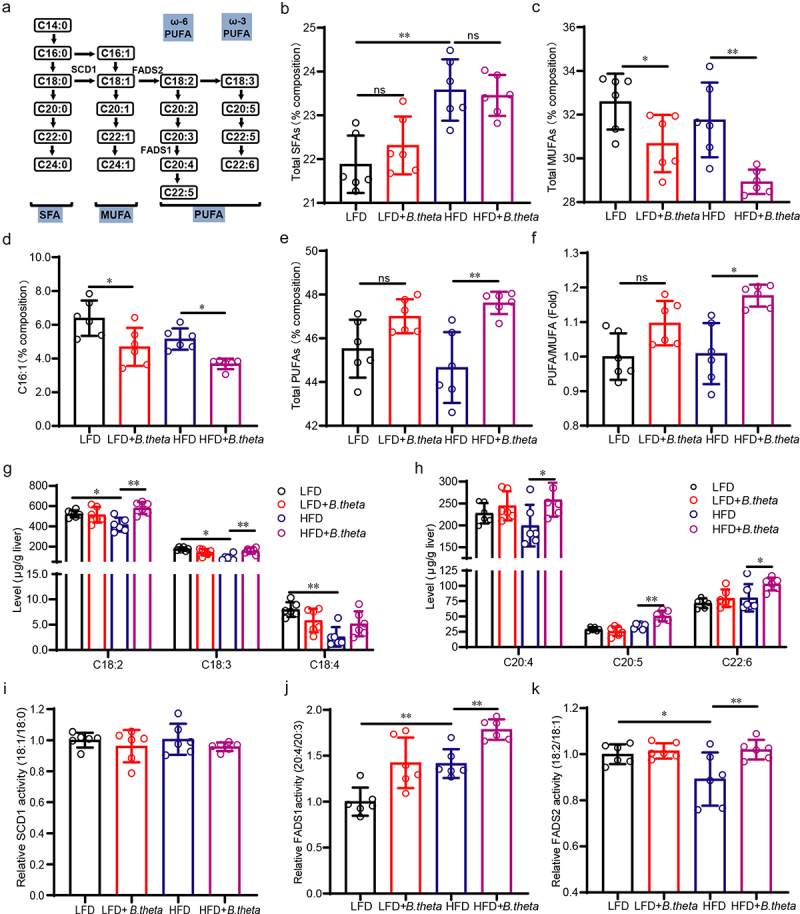
B. theta regulates the unsaturated fatty acid in NAFLD mice. (a) Fatty acid composition and the elongation and desaturation process. (b-e) percent composition of total SFAs (b), total MUFAs (c), C16:1 fatty acid (d), and total PUFAs (e). (f) PUFA/MUFA ratio. (g,h) the contents of C18:2, C18:3, C18:4, C20:4, C20:5, and C22:6 PUFAs; (i-k) desaturase activity of stearoyl-CoA desaturase 1 (SCD1), fatty acid desaturase 1 (FADS1) and FADS2. The data are presented as means with SD; n = 6, *p < .05 and **p < .01 vs. model or control group. ns, not significant.
Discussion
As was widely discussed, the alterations of specific intestinal microbiota could either drive or obstruct the progression of NAFLD.27,28 Therefore, there is a need to characterize the gut microbiota in metabolic dysfunction and identify potential microbial targets for therapeutic interventions. In this study, we observed a positive correlation between the abundance of B. theta and the improvement of lipid metabolism dysfunction in the early and end-stage of NAFLD. Further study elucidated that B. theta administration decreased hyperlipidemia and insulin resistance, while protecting against hepatic steatohepatitis and liver injury in mice with NAFLD. Mechanistically, B. theta modulated gut microbiota composition, promoted gut-liver folate metabolism, and regulated hepatic unsaturated fatty acids in NAFLD mice, collectively contributing to the alleviation of hepatic metabolic dysfunction (Figure 7). The findings highlight the importance of gut microbiota modulation, specifically using B. theta, as a promising avenue for treating NAFLD and related metabolic disorders.
Figure 7.
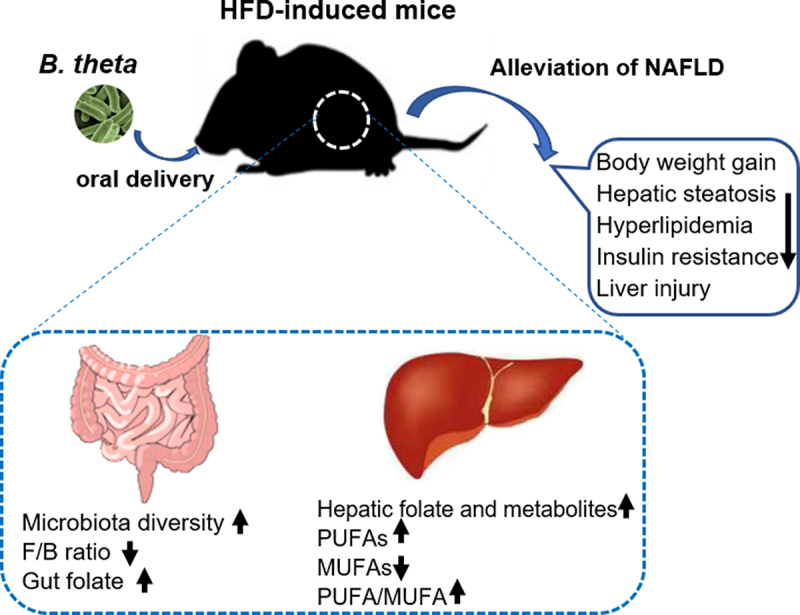
Bacteroides thetaiotaomicron (B. theta) administration alleviates nonalcoholic fatty liver disease (NAFLD) in HFD-induced mice through multiple mechanisms. A high-fat diet-induced fatty liver in mice accompanied by dysbiosis of gut microbiota and its metabolites. B. theta administration resulted in several beneficial effects, as indicated by the following changes: 1) increase in microbiota diversity and decrease in Firmicutes/Bacteroidetes (F/B) ratio; 2) enhancement of gut folate biosynthesis and hepatic folate metabolism; 3) increase in hepatic polyunsaturated fatty acids (PUFAs) and decrease in monounsaturated fatty acids (MUFAs) levels, with decreased PUFA/MUFA ratio. These multiple mechanisms collectively contribute to the therapeutic effects of B. theta on the development of NAFLD.
Bacteroides thetaiotaomicron, a representative species of Bacteroides, is commonly found in the intestines of humans and rodents, with a remarkable ability to acquire and degrade plant polysaccharides and regulate immunity and energy metabolism.12,29,30 We previously reported that berberine might alleviate NASH partially by regulating gut microbiota.11 This study identified B. theta as a potential probiotic for treating NAFLD induced by a WD/CCl4/DEN combination treatment. This finding was consistent with our deep analysis results in an early NASH mouse model induced by a WD/CCl4 treatment.11 Though several studies have also reported the role of BBR in regulating gut microbiota,31,32 the upregulation for the B. theta in different stages of NAFLD upon berberine treatment was first observed in the current work. However, we found that B. theta was only associated with improving metabolic disorders but not fibrosis. Therefore, we further investigated the role of B. theta in the HFD-induced obese mice, which exhibit metabolic syndromes without fibrosis and are known to have altered microbial communities.33,34 Previous studies have shown that gavage with B. theta can protect mice against adiposity.14,35 Similarly, we found that B. theta decreased hyperlipidemia and insulin resistance and prevented hepatic steatohepatitis and liver injury in HFD-fed mice. Importantly, we observed that B. theta did not affect food intake, body weight, and hepatic histopathology in LFD-fed mice, which indicated its safety. However, it is worth noting that B. theta has also been reported to promote diet-induced obesity in antibiotic-treated mice.27 We hypothesize that the intestinal microenvironment may be disrupted in mice treated with antibiotics or those that are germ-free. This disruption could potentially impact the effects of B. theta, as its anti-obesity properties may be influenced by the presence of a specific intestinal microenvironment or its interaction with other microbial species or bacteria-host interplay.12 As evidence, we found that gavage with B. theta resulted in increased microbiota diversity in both low-fat and high-fat diet-fed mice (Figure 4b), as well as a decreased Firmicutes/Bacteroidetes (F/B) ratio in HFD-fed mice (Figure 4c,d), which is typically associated with obesity. Moreover, some bacteria have been reported to interact with or enhance the effects of B. theta, while B. theta itself may also influence their abundance.5 For example, A. muciniphila, which has been reported to counteract adiposity in HFD mice,36 was found to be increased after B. theta gavage in our study (Data not shown). Further studies are warranted to elucidate how B. theta, in conjunction with other microbial species, modulates metabolism.
Previous studies have highlighted various mechanisms through which B. theta improves fatty liver disease. For instance, B. theta has been shown to decrease body weight gain and adiposity and regulate lipid metabolism-related gene expression in white adipose tissue of mice fed with high-fat diets.14 Additionally, B. theta could ameliorate hepatic steatosis in experimental alcohol-related liver disease through a restored mucosal barrier and reduced LPS translocation.13 Moreover, B. theta-derived sphingolipids have been observed to be transferred from the microbiome to the colon and liver, improving diet-induced hepatic steatosis.30 In line with these previous findings, we further investigated the new functions and mechanisms of B. theta in our study. We demonstrated that B. theta regulated gut microbiota composition, increased gut folate biosynthesis, and modulated liver folate metabolism. Folate supplementation in rodents has been widely reported to reduce metabolic abnormalities associated with NAFLD, including steatosis, inflammation, oxidative stress, insulin resistance, and hyperglycemia.20,21,37 We also examined the effect of B. theta on hepatic S-adenosylmethionine (SAM) levels and downstream metabolites, such as phosphatidylcholine (PC) and glutathione (GSH) synthesis. SAM is involved in key metabolic pathways, including transmethylation and transsulfuration, and is crucial for the synthesis of PC, an essential component for very low-density lipoprotein assembly and lipid export. SAM also participates in the synthesis of cysteine, a precursor for taurine and GSH, which play roles in countering hepatic oxidative stress.21 Remarkably, our study has made the novel discovery that B. theta supplementation in HFD-fed obese mice resulted in a decrease in MUFAs and an increase in PUFAs in mice liver, while SFAs remained unaffected. The potential benefits of these changes by B. theta are consistent with previous conclusions indicating that increased levels of SFAs and MUFAs accelerated the severity of liver steatosis, whereas higher levels of PUFAs and a higher PUFA/MUFA ratio are negatively correlated with the NAFLD progression.22–25 Hence, these results further supported the intricate mechanisms by which B. theta may treat NAFLD.
In summary, our study expands on the knowledge of B. theta‘s functions and mechanisms in the context of fatty liver disease. By regulating the gut microbiota structure, increasing gut folate synthesis, modulating gut-liver folate metabolism, and enhancing hepatic unsaturated fatty acid levels, B. theta might exhibit promising therapeutic potential for NAFLD. Our discovery also provides potential avenues for future research and intervention strategies targeting the gut microbiota in treating the metabolic dysfunction in NAFLD.
Materials and methods
Preparation of bacteria strains
The Bacteroides thetaiotaomicron (B. theta) strain ATCC 29,148 was derived from ATCC and cultured on blood agar at 37°C under strictly anaerobic conditions. Cultures were centrifuged at 2500 g for 10 minutes, washed twice with sterile phosphate-buffered saline (PBS), and re-suspended in sterile PBS containing 10% glycerol to achieve a concentration of 1 × 109 colony-forming units (CFUs) per milliliter. The bacterial suspension was stored at −80°C for subsequent use. A viability confirmation was performed by culture to guarantee that B. theta grew well. For in vivo experiments, live B. theta was administered to C57BL/6J mice via oral gavage three times per week for a total duration of 12 weeks. Each dose consisted of 1 × 108 CFUs of B. theta suspended in 0.1 mL of PBS per mouse.
Animal experiments
Male C57BL/6J mice were obtained from SPF (Beijing) Biotechnology Co., Ltd. and were housed in a 12-hour light/dark light cycle with ad libitum access to water and food. As previously described,38 the mice were subjected to a western diet/carbon tetrachloride/dimethylnitrosamine (WD/CCl4/DEN)-induced model (Figure 1a, up). Briefly, the mice (25.0–27.0 g) were fed a western diet (TP26300122, Trophic Animal Feed High-Tech Co., Ltd. China) along with high-sugar drinking water containing 23.1 g/L d-fructose and 18.9 g/L d-glucose. They were intraperitoneally injected with 0.2 mL/kg CCl4 in corn oil once a week. After 4 weeks of induction, the mice were continuously exposed to the WD/CCl4 regimen and treated with or without berberine (BBR) in food (equivalently 200 mg/kg/day by gavage) for an additional 20 weeks. At the 10-week mark of BBR treatment, a single intraperitoneal injection of 100 mg/kg DEN was administered, and the dose of CCl4 was increased from 0.2 mL/kg to 0.5 mL/kg. Similarly, WD/CCl4-induced mouse model with a 4-week pretreatment of western diet/carbon tetrachloride (WD/CCl4) and subsequent 8 weeks therapy of 200 mg/kg/day BBR in food (Figure 1a, down), was also utilized as we previously reported.11 For the animal experiments involving B. theta treatment, 24 mice (20.0–22.0 g) were randomly assigned to four groups: LFD+PBS (10% low-fat diet with 100 µL PBS gavage per mouse), LFD+B. theta (10% low-fat diet with 100 µL B. theta gavage per mouse), HFD+PBS (60% high-fat diet with PBS gavage), and HFD+B. theta (60% high-fat diet with B. theta gavage). The mice were treated with LFD or HFD for 4 weeks, followed by B. theta or PBS gavage for 12 weeks. Food intake and body weight were recorded weekly. The mouse fat mass was measured using an NMR body composition analyzer (QMR06-090 H, NIUMAG, China) one day before the end of the experiment. Fasting and non-fasting blood glucose levels were measured via the tail vein using a blood glucose meter (ACCU-CHECK Performa, Roche, Switzerland). At the end of the experiment, the mice were sacrificed, and blood samples were collected. Intralobular pieces of the liver were quickly frozen in liquid nitrogen for liver biochemistry and subsequent mechanism studies. For histological analyses, liver slices were fixed with 4% paraformaldehyde (Servicebio, #G1101). This study was approved by the Institutional Animal Care and Use Committee of the Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences (SYXK(Jing) 2017–0023), and animal experiments were conducted following the National Guidelines for Housing and Care of Laboratory Animals.
Analysis of biochemical parameters
The collected blood samples were centrifuged at 2500 g for 10 minutes, and the serum was collected. Commercial assay kits from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) were used to measure the serum levels of ALT (C009-2-1), AST (C010-2-1), TG (A110-1-1-TG), and CHO (A111-1-1). Serum insulin was measured using a mouse insulin ELISA kit (SEKM-0141, Solarbio). Insulin resistance was calculated using a homeostasis model assessment‐2 (HOMA2) index through an online-based calculator on the Diabetes Trials Unit of the University of Oxford website (https://www.dtu.ox.ac.uk/homacalculator/). Liver biochemistry analysis was performed by homogenizing the mouse liver, and the levels of hepatic TG and CHO were measured using assay kits according to the manufacturer’s instructions. Commercial kits were also used to measure folate (FA, E-EL-0009c, Elabscience), S-adenosylmethionine (SAM, FY-EU12032, Wuhan Feiyue Biotechnology Co., Ltd), glutathione (GSH, BC1175, Solarbio), and phosphatidylcholine (PC, KS13966, Shanghai Keshun Science and Technology Co., Ltd.). The liver homogenates were extracted using the corresponding lysis buffer with protease inhibitor cocktail (C0001, Targetmol) and quantified using the BCA protein assay kit (23225, Thermo Scientific, New York, NY, USA).
Histological analysis
The fixed liver tissues were stained with hematoxylin and eosin (H&E) staining to evaluate liver steatosis, ballooning, and inflammation. The NAFLD classifications were assessed by two experts blinded to the experimental groups according to the NAFLD activity score (NAS) criteria, which incorporates scores for steatosis (0–3), hepatocellular ballooning (0–2), and lobular inflammation (0–3).39 Similarly, the Masson trichrome staining was conducted to evaluate the fibrosis stage (0–4). Steatosis was further confirmed by Oil Red O (ORO) staining in frozen sections. Immunohistochemical (IHC) staining of F4/80 (GB113373, Servicebio, China), a macrophage lineage marker,40 was performed to further evaluate the inflammation in the liver.
Intestinal microbiological analysis
Five or six mice in each group were selected to perform gut microbiological analysis using 16S rRNA gene sequencing by Oebiotech (Shanghai, China). Briefly, genomic DNA was isolated from samples using the DNeasy PowerSoil Kit (QIAGEN) and amplified using primers specific for the V3-V4 region of 16S rRNA (343F–5ʹ TACGGRAGGCAGCAG 3ʹ and 798 R–5ʹAGGGTATCTAATCCT3ʹ). The amplification products were checked by 1% agarose gel electrophoresis, purified using AMPure XP beads (Agencourt), and subjected to another round of PCR, as described earlier.11 The final amplicon was quantified using the Qubit quantification system (Life Technologies) and purified again. Equal amounts of purified amplicons were pooled for subsequent sequencing using the Illumina MiSeq System (Illumina Inc., San Diego, CA, USA). The raw FASTQ sequencing files were preprocessed for bioinformatics analysis using QIIME software (version 1.8.0). The clean reads were processed to remove primer sequences and cluster them into operational taxonomic units (OTUs) using V search software (version 2.4.2) with a 97% similarity cutoff. Based on the OTU counts, the community structure and alpha diversity (presented as the Simpson’s index of diversity and Shannon index) were analyzed to assess the composition of the sample community. Functional inference associated with metabolic regulation was analyzed using the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) quantitative analysis of long-chain fatty acid
Freshly collected liver samples were frozen in liquid nitrogen and stored at −80°C until further analysis. The determination of long-chain fatty acids, including saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids (PUFAs), were conducted by the Beijing Bio-Tech Pack Technology Company Ltd. In brief, a 50 mg liver sample was suspended in 1 mL Isopropyl alcohol: acetonitrile (1:1) and then vortexed and centrifugation at 12,000 g for 10 minutes. The concentrations of fatty acids in supernatants were detected by LC-MS/MS, with fatty acids (19:0) as the internal standard. Chromatography was performed on Waters UPLC BEH C8 column (2.1 × 100 mm, 1.7 µm) with a column temperature of 55°C. Phase A (acetonitrile: water = 1:10, 0.1% acetic acid, 1 mM ammonium acetate) and phase B (isopropyl alcohol: acetonitrile = 1:1) were used as the mobile phase for gradient elution, with a flow rate of 0.26 mL/minute and a sample loading volume of 5 μL. The Waters XEVO TQ-S Micro mass spectrometry system was used for mass spectrometry analysis in negative ion mode ESI. Other parameters were as follows: Ion source voltage −2 kV, temperature 150°C, desolvation temperature 500°C, desolvation gas flow rate 1000 L/hour; The micro-hole voltage 10.0 V and the gas flow rate 150 L/hour. The peak area of targeted data was calculated using TargetLynx quantitative software.
Activity index of desaturase
The activity of stearoyl-CoA desaturase 1 (SCD1), fatty acid desaturase 1 (FADS1), and FADS2 was estimated by assessing the product-to-precursor ratios, specifically C18:1/C18:0 for SCD1, C20:4/C20:3 for FADS1, and C18:2/C18:1 for FADS2. This approach has been extensively utilized in prior studies.25
RNA extraction and real-time quantitative PCR
Total RNA in liver tissues was extracted using RaPure total RNA kit (R4011, Magen) according to the manufacturer’s protocol. The mRNA levels of Pemt, Aldh1l1, Dhfr, and Mat1a were amplified with specific primers (Table 1) and quantified with one-step real-time quantitative reverse transcript PCR (qRT-PCR) using HiScript II One Step qRT-PCR SYBR Green Kit (Q221–01, Vazyme) as previously used.38 The relative mRNA amounts were calculated by the comparative Ct method after normalizing against the amount of the internal control mRNA level of glyceraldehyde 3-phosphate dehydrogenase (Gapdh).
Table 1.
Sequence of primers.
| Genes | Forward primer (5’→3’) | Reverse primer (5’→3’) |
|---|---|---|
| Aldh1l1 | CAGGAGGTTTACTGCCAGCTA | CACGTTGAGTTCTGCACCCA |
| Dhfr | CGCTCAGGAACGAGTTCAAGT | TGCCAATTCCGGTTGTTCAATA |
| Mat1a | GTGCTGGATGCTCACCTCAAG | CCACCCGCTGGTAATCAACC |
| Mthfs | ACCAATTCCAGAGCAATCACAT | CTCCTCCCGAACATCTCCCT |
Statistical analyses
The data were presented as mean ± standard deviation (SD) or representative figures. The data were analyzed by analysis of variance (ANOVA) followed by Student-Newman-Keuls (SNK) post hoc tests using GraphPad Prism 8 software. Kruskal-Wallis H test or Mann-Whitney U test were used for the nonparametric test. The threshold for statistical significance was set at *p < 0.05 or ** p < 0.01.
Funding Statement
This work was supported by CAMS Innovation Fund for Medical Sciences (CIFMS 2021-I2M-1-028).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available in the article or from the corresponding author upon reasonable request.
Ethics approval
The animal experiment was approved by the Institutional Animal Care and Use Committee of the Institute of Medicinal Biotechnology & Chinese Academy of Medical Sciences (SYXK(Jing) 2017–0023).
References
- 1.Powell EE, Wong VW, Rinella M.. Non-alcoholic fatty liver disease. Lancet. 2021;397(10290):2212–16. doi: 10.1016/S0140-6736(20)32511-3. [DOI] [PubMed] [Google Scholar]
- 2.Eslam M, Sanyal AJ, George J, Sanyal A, Neuschwander-Tetri B, Tiribelli C, Kleiner DE, Brunt E, Bugianesi E, Yki-Järvinen H. International consensus panel. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 3.Negi CK, Babica P, Bajard L, Bienertova-Vasku J, Tarantino G. Insights into the molecular targets and emerging pharmacotherapeutic interventions for nonalcoholic fatty liver disease. Metabolism. 2022;126:154925. doi: 10.1016/j.metabol.2021.154925. [DOI] [PubMed] [Google Scholar]
- 4.Guo K, Xu S, Zeng Z. “Liver-gut” axis: a target of traditional Chinese medicine for the treatment of non-alcoholic fatty liver disease. Front Endocrinol (Lausanne). 2022;13:1050709. doi: 10.3389/fendo.2022.1050709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gauffin Cano P, Santacruz A, Moya Á, Sanz Y. Bacteroides uniformis CECT 7771 ameliorates metabolic and immunological dysfunction in mice with high-fat-diet induced obesity. PloS ONE. 2012;7(7):e41079. doi: 10.1371/journal.pone.0041079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim S, Lee Y, Kim Y, Seo Y, Lee H, Ha J, Lee J, Choi Y, Oh H, Yoon Y. et al. Akkermansia muciniphila prevents fatty liver disease, decreases serum triglycerides, and maintains gut homeostasis. Appl Environ Microbiol. 2020;86(7):e03004–19. doi: 10.1128/AEM.03004-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee HB, Do MH, Jhun H, Ha SK, Song HS, Roh SW, Chung WH, Nam YD, Park HY. Amelioration of hepatic steatosis in mice through bacteroides uniformis CBA7346-mediated regulation of high-fat diet-induced insulin resistance and Lipogenesis. Nutrients. 2021;13(9):2989. doi: 10.3390/nu13092989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meroni M, Longo M, Dongiovanni P. The role of probiotics in nonalcoholic fatty liver disease: a new insight into therapeutic strategies. Nutrients. 2019;11(11):2642. doi: 10.3390/nu11112642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang Y, Huang J, Zhang WY, Qin S, Yang YX, Ren H, Yang QB, Hu H. Effects of probiotics on nonalcoholic fatty liver disease: a systematic review and meta-analysis. Therap Adv Gastroenterol. 2019;12:1756284819878046. doi: 10.1177/1756284819878046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Z, Chen L, Zhao Y, Wang C, Duan C, Yang G, Niu C, Li S. Lactobacillus plantarum NA136 ameliorates nonalcoholic fatty liver disease by modulating gut microbiota, improving intestinal barrier integrity, and attenuating inflammation. Appl Microbiol Biotechnol. 2020;104(12):5273–5282. doi: 10.1007/s00253-020-10633-9. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Liu NN, Li JR, Dong B, Wang MX, Tan JL, Wang XK, Jiang J, Lei L, Li HY. et al. Combined use of bicyclol and berberine alleviates mouse nonalcoholic fatty liver disease. Front Pharmacol. 2022;13:843872. doi: 10.3389/fphar.2022.843872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zocco MA, Ainora ME, Gasbarrini G, Gasbarrini A. Bacteroides thetaiotaomicron in the gut: molecular aspects of their interaction. Dig Liver Dis. 2007;39(8):707–12. doi: 10.1016/j.dld.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Sangineto M, Grander C, Grabherr F, Mayr L, Enrich B, Schwarzler J, Dallio M, Bukke VN, Moola A, Moschetta A. et al. Recovery of bacteroides thetaiotaomicron ameliorates hepatic steatosis in experimental alcohol-related liver disease. Gut Microbes. 2022;14(1):2089006. doi: 10.1080/19490976.2022.2089006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu R, Hong J, Xu X, Feng Q, Zhang D, Gu Y, Shi J, Zhao S, Liu W, Wang X. et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat Med. 2017;23(7):859–868. doi: 10.1038/nm.4358. [DOI] [PubMed] [Google Scholar]
- 15.Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. 2015;7(4):2839–49. doi: 10.3390/nu7042839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao Y, Pan Q, Cai W, Shen F, Chen GY, Xu LM, Fan JG. Modulation of gut microbiota by Berberine improves steatohepatitis in high-fat diet-fed BALB/C mice. Arch Iran Med. 2016;19:197–203. [PubMed] [Google Scholar]
- 17.Huang M, Kim HG, Zhong X, Dong C, Zhang B, Fang Z, Zhang Y, Lu X, Saxena R, Liu Y. et al. Sestrin 3 protects against diet-induced nonalcoholic steatohepatitis in mice through suppression of transforming growth factor β signal transduction. Hepatology. 2020;71(1):76–92. doi: 10.1002/hep.30820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang M, Li J, Li H, Dong B, Jiang J, Liu N, Tan J, Wang X, Lei L, Li H. et al. Down-regulating the high level of 17-beta-hydroxysteroid dehydrogenase 13 plays a therapeutic role for non-alcoholic fatty liver disease. Int J Mol Sci. 2022;23(10):5544. doi: 10.3390/ijms23105544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stojanov S, Berlec A, Štrukelj B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms. 2020;8(11):1715. doi: 10.3390/microorganisms8111715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sid V, Siow YL, K O. Role of folate in nonalcoholic fatty liver disease. Can J Physiol Pharmacol. 2017;95(10):1141–1148. doi: 10.1139/cjpp-2016-0681. [DOI] [PubMed] [Google Scholar]
- 21.Qiao S, Bao L, Wang K, Sun S, Liao M, Liu C, Zhou N, Ma K, Zhang Y, Chen Y. et al. Activation of a specific gut bacteroides-folate-liver axis benefits for the alleviation of nonalcoholic hepatic steatosis. Cell Rep. 2020;32(6):108005. doi: 10.1016/j.celrep.2020.108005. [DOI] [PubMed] [Google Scholar]
- 22.de Castro GS, Calder PC, de Castro GS. Non-alcoholic fatty liver disease and its treatment with n-3 polyunsaturated fatty acids. Clin Nutr. 2018;37(1):37–55. doi: 10.1016/j.clnu.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Zhou H, Urso CJ, Jadeja V. Saturated fatty acids in obesity-associated inflammation. J Inflamm Res. 2020;13:1–14. doi: 10.2147/JIR.S229691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hliwa A, Ramos-Molina B, Laski D, Mika A, Sledzinski T. The role of fatty acids in non-alcoholic fatty liver disease progression: an update. Int J Mol Sci. 2021;22(13):22. doi: 10.3390/ijms22136900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagumalli SK, Willett RA, de Conti A, Tryndyak VP, Avigan MI, da Costa GG, Beland FA, Rusyn I, Pogribny IP, da Costa GG. Lipidomic profiling of the hepatic esterified fatty acid composition in diet-induced nonalcoholic fatty liver disease in genetically diverse Collaborative Cross mice. J Nutr Biochem. 2022;109:109108. doi: 10.1016/j.jnutbio.2022.109108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spooner MH, Jump DB. Nonalcoholic fatty liver disease and omega-3 fatty acids: mechanisms and clinical use. Annu Rev Nutr. 2023;43(1):199–223. doi: 10.1146/annurev-nutr-061021-030223. [DOI] [PubMed] [Google Scholar]
- 27.Cho SH, Cho YJ, Park JH. The human symbiont bacteroides thetaiotaomicron promotes diet-induced obesity by regulating host lipid metabolism. J Microbiol. 2022;60(1):118–127. doi: 10.1007/s12275-022-1614-1. [DOI] [PubMed] [Google Scholar]
- 28.Hu W, Gao W, Liu Z, Fang Z, Wang H, Zhao J, Zhang H, Lu W, Chen W. Specific strains of faecalibacterium prausnitzii ameliorate nonalcoholic fatty liver disease in mice in association with gut microbiota regulation. Nutrients. 2022;14(14):2945. doi: 10.3390/nu14142945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zafar H, Saier MH. Gut bacteroides species in health and disease. Gut Microbes. 2021;13(1):1–20. doi: 10.1080/19490976.2020.1848158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le HH, Lee MT, Besler KR, Johnson EL. Host hepatic metabolism is modulated by gut microbiota-derived sphingolipids. Cell Host & Microbe. 2022;30(6):798–808.e7. doi: 10.1016/j.chom.2022.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Shou JW, Li XY, Zhao ZX, Fu J, He CY, Feng R, Ma C, Wen BY, Guo F. et al. Berberine-induced bioactive metabolites of the gut microbiota improve energy metabolism. Metabolism. 2017;70:72–84. doi: 10.1016/j.metabol.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Su C, Jiang Z, Yang Y, Zhang Y, Yang M, Zhang X, Du Y, Zhang J, Wang L. et al. Berberine attenuates choline-induced atherosclerosis by inhibiting trimethylamine and trimethylamine-N-oxide production via manipulating the gut microbiome. NPJ Biofilms Microbio. 2021;7(1):36. doi: 10.1038/s41522-021-00205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thaiss CA, Itav S, Rothschild D, Meijer MT, Levy M, Moresi C, Dohnalová L, Braverman S, Rozin S, Malitsky S. et al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature. 2016;540(7634):544–551. doi: 10.1038/nature20796. [DOI] [PubMed] [Google Scholar]
- 34.Guirro M, Costa A, Gual-Grau A, Herrero P, Torrell H, Canela N, Arola L, Nerurkar PV. Effects from diet-induced gut microbiota dysbiosis and obesity can be ameliorated by fecal microbiota transplantation: a multiomics approach. PloS ONE. 2019;14(9):e0218143. doi: 10.1371/journal.pone.0218143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR. et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Sci. 2013;341(6150):1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM. et al. Cross-talk between akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110(22):9066–71. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.da Silva RP, Kelly KB, Al Rajabi A, Jacobs RL, da Silva RP. Novel insights on interactions between folate and lipid metabolism. Biofactors. 2014;40(3):277–283. doi: 10.1002/biof.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li H, Liu NN, Li JR, Wang MX, Tan JL, Dong B, Lan P, Zhao LM, Peng ZG, Jiang JD. Bicyclol ameliorates advanced liver diseases in murine models via inhibiting the IL-6/STAT3 signaling pathway. Biomed Pharmacother. 2022;150:113083. doi: 10.1016/j.biopha.2022.113083. [DOI] [PubMed] [Google Scholar]
- 39.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 40.Dos Anjos Cassado A. F4/80 as a major Macrophage Marker: the case of the peritoneum and spleen. Results Probl Cell Differ. 2017;62:161–179. doi: 10.1007/978-3-319-54090-0_7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available in the article or from the corresponding author upon reasonable request.


