Iron deficiency anemia (IDA) is the commonest cause of anemia in the United States and worldwide. In the United States, it has been estimated that some 5%–11% of women and 1%–4% of men are iron deficient, and approximately 5% and 2%, respectively, have IDA.1 Although the cause of IDA may include inadequate iron intake or absorption, which are common in children and premenopausal women, IDA in adult men and postmenopausal women is often the result of chronic occult gastrointestinal bleeding.
Although iron homeostasis is complicated, a basic understanding of its biology is important in the context of IDA (see Fleming,2 Ganz and Nemeth,3 Camaschella,4 and Anderson and Frazer5 for review). In brief, non-heme iron is absorbed primarily in the proximal small intestine (the absorption of heme iron is poorly understood), although active absorption is via the divalent metal transporter-1, which is expressed in the proximal duodenum (Figure 1). It is well recognized that in some forms of gastric bypass in which the typical iron-absorbing segment of the duodenum is bypassed, iron malabsorption ensues.
Figure 1.
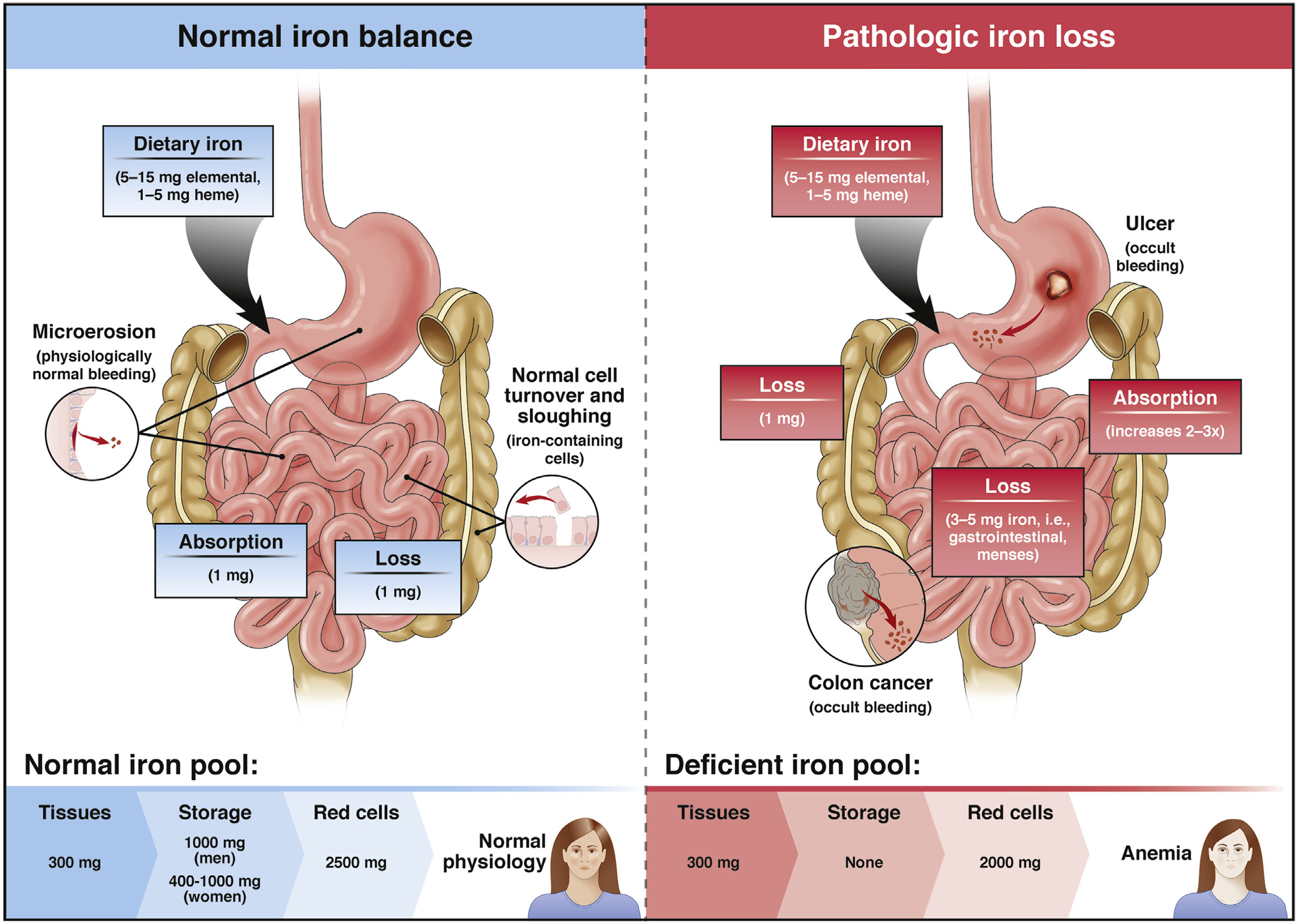
Iron homeostasis. The molecular regulation of iron homeostasis is complex, including interplay between divalent metal transporter-1 found in enterocytes in the proximal duodenum (large arrow), hepcidin, and ferroportin (see the text for details). Iron balance is tightly regulated under normal circumstances, with losses balanced by iron absorption. When iron losses through occult bleeding exceed the capacity to absorb iron, iron is depleted first from iron stores then from the red blood cell pool, ultimately leading to IDA. From Rockey,11 modified with permission.
The ferroportin/hepcidin axis is also critically important in iron homeostasis. Hepcidin, a 25-amino acid peptide produced by hepatocytes via complex regulatory mechanisms, is distributed via the circulation to its target sites, where it binds to its receptor, ferroportin. Ferroportin is highly expressed at the basolateral surface of duodenal enterocytes, where it acts as a cellular iron exporter. Increased levels of hepcidin limit membrane insertion of ferroportin, blocking iron exit, with iron-laden enterocytes sloughed during their natural cycle of epithelial renewal, serving as a primary mechanism for removal of excess iron. Therefore, when the body is iron replete, hepcidin concentrations are high and iron delivery to the circulation is reduced. In contrast, in the iron-deficiency state, hepcidin levels are low and there is active iron delivery to the circulation.
Important regulators of hepcidin, and therefore of systemic iron homeostasis, include plasma iron concentrations, body iron stores, infection and inflammation, and erythropoiesis. Disturbances in the regulation of hepcidin contribute to the pathogenesis of many iron disorders. For example, hepcidin deficiency causes iron overload in hereditary hemochromatosis and non-transfused β-thalassemia, whereas overproduction of hepcidin is associated with iron-restricted anemias seen in patients with chronic kidney disease, chronic inflammatory diseases, some cancers, and inherited iron-refractory IDA.
Under normal conditions, iron homeostasis is tightly regulated.6,7 Typical daily elemental iron loss is 0.25–0.75 mg from iron lost via sloughing of intestinal epithelial cells and microscopic gastrointestinal bleeding. With daily blood loss of 0.5–1.5 mL/d, a stool weight of 150 g, and circulating hemoglobin of 15 g/dL, stool hemoglobin concentration is 0.5–1.5 mg/g. In aggregate, the average daily iron loss is approximately 1 mg (Figure 1), which is precisely balanced by the same amount of iron absorption. Because the absorptive capacity of the small intestine for iron can increase in response to iron depletion, iron deficiency results only when iron loss exceeds the absorptive capacity of the small bowel. It is critical to emphasize that iron absorption is not only complex as highlighted above, but is limited (see Abbaspour et al8 and Camaschella9 for review), so that iron depletion only occurs when intestinal absorptive capacity of iron is outstripped by iron loss.
The degree to which blood can be “hidden” in the gastrointestinal tract is emphasized by the observation that although instillation of 50–100 mL of blood into the stomach may produce melena, patients losing 100 mL of blood per day may have grossly normal-appearing stools.10,11 This concept is consistent with the clinical observation that truly occult bleeding is a common cause of IDA.
Virtually any gastrointestinal tract lesion that causes a mucosal defect can bleed enough to lead to occult blood loss and therefore cause IDA. Indeed, the clinical spectrum of IDA is broad because many different lesions occurring in many different sites in the gastrointestinal tract are capable of bleeding in an occult manner.11,12 Endoscopic evaluation of patients with IDA has shown that nearly two-thirds of patients will have lesions identified in the gastrointestinal tract that are believed to be capable of causing occult bleeding (Figure 2).13 Although gastrointestinal tract malignancies, especially right-sided colonic cancers, have historically been considered to be the most common and important lesions identified during endoscopy, cancers have been identified in patients with IDA in all parts of the gastrointestinal tract and, furthermore, the most common causes of occult bleeding in patients with IDA are inflammatory ulcerative upper gastrointestinal tract lesions (Figure 2).13 Only a small proportion of patients will be found to have a lesion capable of occult bleeding and causing IDA in each the upper and lower gastrointestinal tract simultaneously14 (Figures 2 and 3). Notwithstanding, because of the propensity for a variety of gastrointestinal tract lesions to bleed in an occult fashion, the standard of care for postmenopausal women and men with IDA is to evaluate the gastrointestinal tract in search of a bleeding lesion.13
Figure 2.
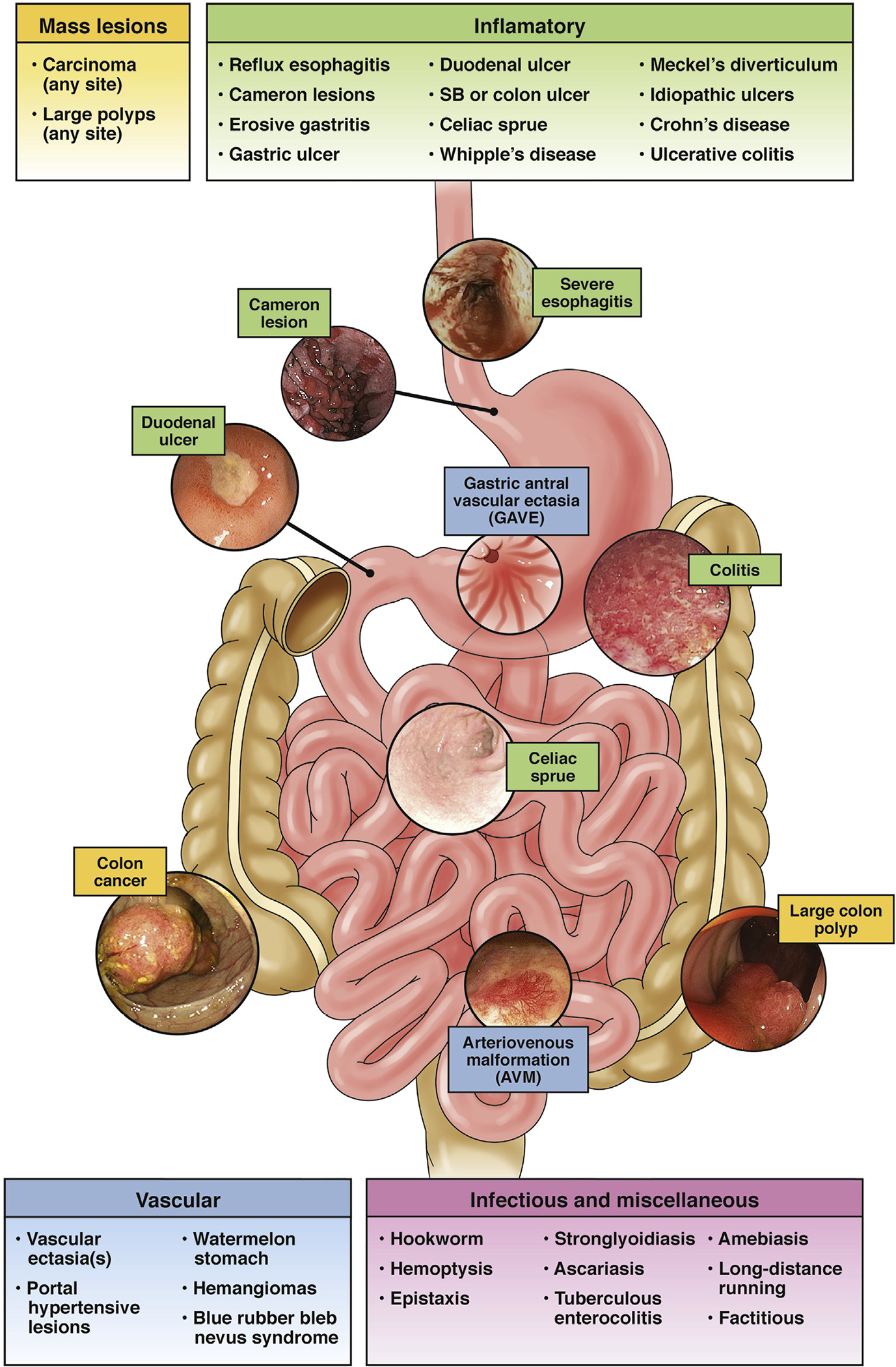
Gastrointestinal tract lesions causing IDA. Virtually any gastrointestinal tract lesion can bleed in an occult fashion. Highlighted in red are the more common causes of occult gastrointestinal bleeding that lead to IDA. SB, small bowel.
Figure 3.
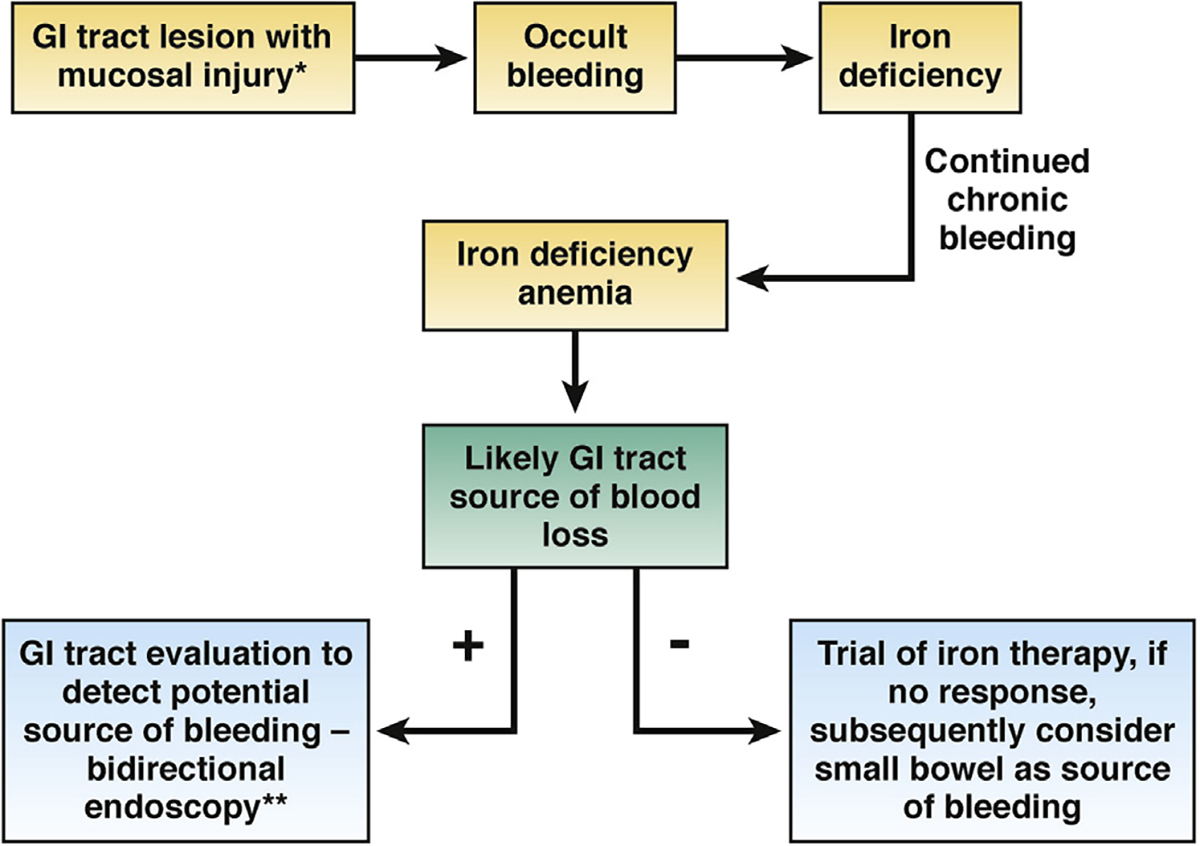
The role of endoscopy in IDA. In patients who have gastrointestinal (GI) tract lesions, occult bleeding leads to IDA, which usually should be pursued with endoscopy. In asymptomatic patients, if initial bidirectional endoscopy fails to identify a lesion, best evidence suggests that a trial of iron therapy is the most appropriate management approach. If that fails to correct IDA, further evaluation is typically indicated. *See Figure 2 for typical lesions. **Bidirectional endoscopy at the same sitting is preferred over sequential endoscopy at separate times. Note, in patients with IDA and symptoms, endoscopy should be directed first at the source of symptoms. If endoscopy in that location (ie, upper or lower tract) is negative, the portion of the gastrointestinal tract (ie, upper or lower tract) not yet investigated should be examined.
Although the effectiveness of fecal occult blood tests (FOBTs) has been well validated for use in colon cancer populations, the use of FOBTs in other populations has been more controversial. In theory, because FOBTs detect occult bleeding, it is possible that they may be useful in detection of occult bleeding in patients with IDA.15–23 In a systematic review of the use of FOBTs in patients with IDA, it was found that the sensitivity of FOBTs for presumptive causes of IDA detected at endoscopy was 0.58 (95% confidence interval [CI], 0.53–0.63), with a specificity of 0.84 (95% CI, 0.75–0.89).23 Results were similar in both guaiac-based testing and fecal immunochemical testing. Given this poor sensitivity and specificity, the Panel did not believe that the result of an FOBT would substantially influence the decision as to whether to perform endoscopy or not, and it was decided not to specifically address the use of FOBT in the evaluation of IDA. This assessment should not preclude future consideration of the use of FOBT in an algorithm in certain populations of patients with IDA.
This technical review will not discuss the details of the presentation of anemia, but rather will focus on the diagnosis and evaluation of IDA. This review will also not address patients with overt gastrointestinal bleeding. In patients with IDA, blood loss is typically chronic and occult, and therefore rarely associated with overt bleeding or hemodynamic compromise, unless the lesion responsible for chronic occult bleeding begins bleeding aggressively. Indeed, a syndrome of acute on chronic gastrointestinal bleeding, in which patients known to have IDA spontaneously develop acute bleeding, has been recognized.24 Recognition of this entity emphasizes the wide spectrum of lesions in many different locations in the gastrointestinal tract that can bleed, and that often present with highly variable clinical features.
Despite the publication of a number of observational studies focused on IDA, and the presence of several scholarly reviews, there remains a great deal of controversy about best practices in the evaluation and management of IDA. Although it is well-appreciated that occult gastrointestinal bleeding is likely to be responsible for IDA in postmenopausal woman and in men, and therefore endoscopy is warranted, best practices regarding the type of endoscopy and the appropriate evaluation for Helicobacter pylori, celiac disease, atrophic gastritis, and of the small bowel, are not well established.
Given a number of questions surrounding the most appropriate approach to the gastrointestinal evaluation of IDA, the American Gastroenterological Association Institute called for a technical review of the clinical spectrum of IDA, with a focus on optimal evaluation and management approaches. The main purpose was to critically review studies using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology and to generate summary evidence and estimates for the Guidelines Panel to develop evidence-based recommendations.
It should be noted that this technical review does not address evaluation of patients with iron deficiency without anemia. In addition, it does not specifically address the evaluation of patients with IDA and prominent gastrointestinal symptoms (eg, dysphagia, odynophagia, abdominal pain, diarrhea, change in bowel habit, and intermittent hematochezia). These patients should be evaluated as indicated based on their gastrointestinal symptoms. It should be emphasized that a careful history is fundamentally important in these patients because subtle symptoms are often present and should be sought after. The guideline addresses the gastrointestinal evaluation of IDA primarily in patients without dominant gastrointestinal tract symptomatology, who we have considered asymptomatic.
Although iron replacement therapy is an important consideration in IDA patients, the Review Panel believed that addressing the type of iron therapy and route of treatment (ie, oral vs intravenous administration) was outside the scope of this review. We look forward to future guidelines, perhaps in collaboration with hematological societies, to address this important issue.
Methods
The technical review and its accompanying guideline were conducted according to the GRADE framework.25 The American Gastroenterological Association Clinical Guideline Committee selected the members of the Technical Review and Clinical Guideline Panels who were screened to minimize any conflict of interest. The technical review collected and evaluated pertinent literature concerning the diagnosis and endoscopic evaluation of IDA, as well as appropriate investigations for H pylori, celiac disease, atrophic gastritis, and of the small bowel. Using these data, the Clinical Guideline Panel produced the final set of recommendations, as described.26
Formulation of Clinical Questions
The Technical Review and Guideline Panel formulated the clinical questions using the PICO format, which frames a clinical question by defining a specific patient population (P), intervention (I), comparator (C), and outcome(s). The Panel finalized 5 questions on the topic (Table 1).
Table 1.
PICO Questions
| Question no. | Diagnosis or intervention related question | PICO question |
|||
|---|---|---|---|---|---|
| Population | Intervention(s) | Comparator | Outcome | ||
|
| |||||
| 1 | Establishing an accurate diagnosis of IDA? | Adults with anemia defined as hemoglobin <13 g/dL in men and <12 g/dL in nonpregnant women | Ferritin | Bone marrow biopsy (gold standard) | Diagnosis of IDA |
| 2 | What is the utility of bidirectional endoscopy in patients with suspected endoscopic gastrointestinal lesion as a source of IDA? | Women aged ≥45 y and men Premenopausal women aged <45 y | Bidirectional endoscopy | Do nothing (observation only) | All-cause mortality, morbidity related to anemia (eg, cardiac events), mortality related to gastrointestinal lesions, morbidity related to gastrointestinal lesions, endoscopy-related or periprocedural morbidity, endoscopy-related or periprocedural mortality |
| 3a | Should we obtain routine gastric biopsies for H pylori in patients with IDA? | Adults with IDA undergoing endoscopic workup without endoscopic gastric pathology | Routine gastric biopsies for H pylori | Do nothing, noninvasive testing | Resolution of anemia |
| 3b | Should we obtain routine gastric biopsies for chronic autoimmune atrophic gastritis in patients with IDA? | Routine gastric biopsies | Do nothing | ||
| 4 | Should we obtain routine small bowel biopsies for celiac disease in patients with IDA? | Adults with IDA undergoing endoscopic workup without endoscopic small bowel pathology | Routine small bowel biopsies for celiac testing | Do nothing, noninvasive testing | |
| 5 | In asymptomatic IDA patients with negative bidirectional endoscopy, should small bowel investigations be pursued? | Adults with IDA without warning signs. | Any small bowel investigation (endoscopy or imaging) | Do nothing | All-cause mortality, morbidity related to anemia (eg, cardiac events), mortality related to gastrointestinal lesions, morbidity related to gastrointestinal lesions, endoscopy-related or periprocedural morbidity, endoscopy-related or periprocedural mortality. |
PICO, population, intervention, comparator, outcome.
When direct evidence to inform any of the PICO questions was not available, we identified indirect evidence. We aimed to define the prevalence of gastrointestinal neoplastic and/or malignant lesions, celiac disease and/or small intestinal villous atrophy, H pylori infection, and chronic atrophic autoimmune gastritis in patients with IDA. We aimed to define the diagnostic accuracy of ferritin cutoffs, as well as tissue transglutaminase (TTG) IgA antibodies to diagnose celiac disease in patients with IDA.
The Systematic Review Process
Before conducting any systematic review, we identified systematic reviews published on any of the PICO questions. If we could not identify any systematic review or the available systematic reviews had low methodological quality, we conducted a de novo systematic review for the PICO question. The systematic review is reported according the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) proposal.27,28 The Technical Review Panel developed a protocol to guide the systematic review a priori.
Literature Search Strategy
Under the guidance of the Technical Review Panel, an experienced medical librarian conducted a comprehensive search of the following databases from prespecified start dates to April 2019: MEDLINE Epub Ahead of Print, MEDLINE In-Process & Other Non-Indexed Citations, MEDLINE Daily, MEDLINE, EMBASE Classic, EMBASE, and Wiley’s Cochrane Library. The prespecified start dates of the date range of the search and the study designs of interest were determined by the Technical Review Panel for each PICO question separately. The search was limited to English and human studies. Controlled vocabulary and keywords were used to search for the studies. The final search strategies are available in Appendix 1. To assure comprehensiveness, the reference lists of previously published systematic reviews, clinical guidelines, and the included studies were searched to identify other relevant studies that may have been missed by the search strategy.
Eligibility Criteria
We aimed to include randomized controlled trials (RCT) and/or nonrandomized comparative studies of different diagnostic and/or intervention strategies for each of the PICO questions. When we could not identify any RCT or nonrandomized comparative studies, we tried to identify diagnostic test accuracy studies of the different diagnostic strategies. If none of the aforementioned study designs was available, we included single cohort and prevalence studies to inform rates of occurrence (ie, prevalence or incidence rates).
Except for PICO 1 (the diagnostic accuracy of ferritin for IDA), we aimed to include studies of patients with IDA without overt gastrointestinal bleeding. Due to the scarcity of data on asymptomatic patients and to account for the variability seen in clinical practice, we included studies regardless of FOBT, the severity of anemia, and the presence of symptoms. Studies that included patients with overt gastrointestinal bleeding were included only if they reported separate results for patients without overt bleeding.
For studies of celiac disease, we only included studies from the United States due to the variable prevalence of celiac disease between countries.29 Except for studies from large databases, we only included studies that diagnosed celiac disease based on biopsies.
Study Selection and Data Extraction
The references identified by the search strategy were uploaded to Rayyan, a web-based platform for the initial steps of systematic reviews.30 The title and abstract of each reference were reviewed by 2 blinded reviewers for inclusion. The full texts of eligible references were reviewed then abstracted using Microsoft Excel sheets. The outcomes of interest for each PICO question are summarized (Table 1).
Data Synthesis
When comparative studies were available, we used the DerSimonian-Liard random-effects model to pool their relative risks.31 To pool the proportions from prevalence studies, we used the double arcsine transformation with the inversevariance the fixed-effects model.32 We used this approach to allow larger studies, which are more inclusive than smaller studies and less prone to selection bias, to have an appropriately larger effect on the pooled estimates. We used the I2 statistic to quantify heterogeneity with a threshold of 50% for comparative relative effect estimates as an indicator of substantial heterogeneity.33 We assessed for publication bias using funnel plot asymmetry tests if there was a sufficient number of studies with no significant heterogeneity.34 The statistical analyses were conducted using the package meta 4.9–2 in R 3.5.3.35,36
Assessing the Quality of the Evidence
The risk of bias for the individual studies was assessed depending on the study design. RCTs and nonrandomized comparative studies were assessed using the Cochrane Collaboration’s tool for assessing risk of bias in randomized trials and the Newcastle-Ottawa Scale, respectively.37,38 For single cohort studies and studies of prevalence, we used the Joanna Briggs Institute tool for assessing risk of bias in prevalence studies.39
We used the GRADE approach to assess the certainty (quality) of evidence for the body of evidence from the systematic reviews and meta-analyses. In this approach, the evidence is graded for each outcome as very low, low, moderate, or high. Evidence derived from RCTs start at a high certainty of evidence, but then is rated down for risk of bias, inconsistency, indirectness, imprecision, and/or other factors. Evidence derived from observational studies starts at low certainty of evidence, but certainty in the evidence can be rated up for large magnitude of effect and/or the presence of dose–response relationship, where appropriate.25
Evidence to Decision Framework
As this technical review was conducted to inform clinical practice guidelines, in addition to the comprehensive critical evaluation of the available evidence on risk and benefits of the different interventions and diagnostic tests, we also considered information about patients’ preferences and values, resource utilization, and cost-effectiveness when available. Because we were unable to identify evidence to support one evaluation and management approach over another, we performed simple modeling analyses to assess the utility of different ferritin thresholds, serologic tests or biopsy for celiac disease, and noninvasive tests or biopsy for H pylori using reimbursement data from the Centers for Medicare and Medicaid Services as a surrogate for the costs to compare them (https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Provider-Charge-Data/index).
The Importance of Establishing an Accurate Diagnosis of Iron Deficiency Anemia
Quality of evidence and summary.
The certainty in the evidence with regard to the use of ferritin to make a diagnosis of iron deficiency is high, suggesting that this test be used to make the diagnosis of IDA. We used a commonly defined threshold of a hemoglobin level <13 g/dL in men and <12 g/dL in nonpregnant women for anemia. A ferritin level of 45 ng/mL was identified to have the optimal tradeoff between sensitivity and specificity for the diagnosis of IDA (Table 2).
Table 2.
Sensitivity and Specificity of Different Ferritin Cutoffs for Diagnosis of Iron Deficiency Anemia
| Test result | No. of results per 1000 patients tested (95% CI) |
No. of participants (studies) | Certainty of the Evidence (GRADE) | Comments | |||
|---|---|---|---|---|---|---|---|
| Prevalence 20%, typically seen in men and postmenopausal women |
Prevalence 60%, typically seen in premenopausal women |
||||||
| Ferritin <45 ng/mL Fer | ritin <15 ng/mL | Ferritin <45 ng/mL Feri | Ferritin <15 ng/mL | ||||
|
| |||||||
| TP | 170 (164–174) | 118 (110–124) | 510 (492–522) | 354 (330–372) | 809 (52) | ⊕⊕⊕⊕ HIGH | Detection of TP will lead to starting iron replacement therapy and likely additional investigations to assess for blood loss as the source of IDA. |
| 52 more TP in ferritin <45 ng/mL | 156 more TP in ferritin < 45 ng/mL | ||||||
| FN | 30 (26–36) | 82 (76–90) | 90(78–108) | 246 (228–270) | FN will likely have worsening of anemia with possible morbidity, or mortality, due to anemia and/or cardiopulmonary complications. They may or may not undergo anemia workup, and may or may not undergo endoscopy to evaluate for blood loss as the source of IDA. | ||
| 52 fewer FN in ferritin <45 ng/mL | 156 fewer FN in ferritin <45 ng/mL | ||||||
| TN | 736 (728–752) | 792 (712–792) | 368 (364–376) | 396 (356–396) | 1860 (52) | ⊕⊕⊕⊕ HIGH | TN will require different investigations to assess the etiology/mechanism of anemia. They may not need workup for blood loss. |
| 56 fewer TN in ferritin <45 ng/mL | 28 fewer TN in ferritin <45 ng/mL | ||||||
| FP | 64 (48–72) | 8 (8–88) | 32 (24–36) | 4 (4–44) | FP will likely undergo unnecessary investigations to assess for blood loss as the source of IDA with possible delay in diagnosis of the actual etiology of the anemia. |
||
| 56 more FP in ferritin < 45 ng/mL | 28 more FP in ferritin <45 ng/mL | ||||||
NOTE. Summary of findings: PICO (population, intervention, comparator, outcome) 1: Should ferritin <45 ng/mL vs ferritin <15 ng/mL be used to diagnose iron deficiency in patients with anemia?
Patient or population: Patients with anemia.
Setting: Outpatient.
New test: Ferritin cutoff value: 45 ng/mL and 15 ng/mL.
Reference test: Bone marrow biopsy.
Pooled sensitivity ferritin <45 ng/mL: 0.85 (95% CI, 0.82–0.87). Pooled specificity ferritin <45 ng/mL: 0.92 (95% CI, 0.91–0.94).
Pooled sensitivity ferritin <15 ng/mL: 0.59 (95% CI, 0.55–0.62). Pooled specificity ferritin <15 ng/mL: 0.99 (95% CI, 0.89–0.99).
FN, false negative; FP, false positive; TN, true negative; TP, true positive.
Because anemia is a common clinical condition and its diagnosis can lead to invasive testing, it is essential to verify the presence of anemia as well as iron deficiency. Although different societies and organizations have proposed different cutoffs for anemia, here we have defined anemia as a hemoglobin level <13 g/dL in men and <12 g/dL in nonpregnant women.40 There is also often considerable controversy about how best to make a diagnosis of IDA. The distinction between IDA and other types of anemia is important because a diagnosis of IDA often prompts further evaluation. Therefore, we aimed to define a threshold for a laboratory test, to be used to define IDA and initiate gastrointestinal tract workup. The gold standard test to make a diagnosis of IDA is bone marrow biopsy. However, this test is invasive, cumbersome, and not commonly performed to evaluate IDA. In contrast, several blood tests, including mean corpuscular volume, transferrin saturation, and ferritin, have been commonly used to diagnose IDA. Mean corpuscular volume, although obtained routinely, lacks both sensitivity and specificity for the diagnosis of IDA. Transferrin saturation is often difficult to use in clinical practice, largely because patients with chronic disease have falsely low transferrin levels and interpretation of iron saturation in this setting is imprecise. In contrast, ferritin, depending on its level, is both sensitive and specific.41
A false-negative ferritin level could label an iron-deficient anemic patient to be iron sufficient, leading to a delay in workup, including possibly missing an important gastrointestinal tract lesion. In contrast, a false-positive ferritin value would label an iron-sufficient anemic patient as having IDA and lead to unnecessary workup, which is costly and poses increased risk to the patient. We explored a ferritin threshold that minimizes false negatives without significantly increasing false positives.
We limited our search strategy to systematic reviews and meta-analyses (Appendix 1). The search identified 221 references, 217 of them were excluded based on title and abstract review, and only 1 met the inclusion criteria after reviewing the full texts.41 This systematic review included 55 studies that evaluated different diagnostic methods, including mean corpuscular volume, transferrin saturation, and serum ferritin, and compared them with bone marrow biopsy. They extracted individual patient data to develop receiver operating characteristic curves and assessed diagnostic accuracy of the different tests at different thresholds. The study had low risk of bias based on the AMSTAR 2 tool. The key finding of this study was that ferritin had the highest likelihood ratio for the diagnosis of IDA.41
We examined the evidence surrounding ferritin cutoffs and IDA. Although ferritin levels from 0 to 100 ng/mL have been examined in the setting of IDA, we focused on clinically relevant levels—15 ng/mL and 45 ng/mL. At a level of 15 ng/mL, with bone marrow biopsy being the reference standard, the likelihood ratio for having IDA is 11.141 and the sensitivity was 0.59 (95% CI, 0.55–0.62), with a specificity of 0.99 (95% CI, 0.89–0.99) (Appendix 2). A ferritin level of 45 ng/mL has a sensitivity and specificity for IDA of 0.85 (95% CI, 0.82–0.87) and 0.92 (95% CI, 0.91–0.94), respectively. Further, given the varied prevalence of IDA found across different populations within the United States, we modeled the performance of these cutoffs in typical populations of patients42 (Appendix 2, Table 3). In each prevalence setting, we found there to be substantially more false negatives when a ferritin level <15 ng/mL was used, with only a modest gain in the reduction of false positives. For example, in a scenario in which the prevalence of IDA among 1000 anemic patients was 20%, using a ferritin of cutoff of 15 ng/mL would miss 48 patients with IDA or approximately one-quarter of all patients with true IDA (Tables 2 and 3). Increasing the cutoff from 15 ng/mL to 45 ng/mL would substantially reduce the false-negative rate, but also increases the number of false positives, which will lead to unnecessary endoscopies; however, this latter increase is expected to result in only a small number of severe complications downstream (eg, <1 perforation in a population of 1 million men and premenopausal women). This is likely to be offset by a substantial reduction in the number of missed colon and gastrointestinal cancers (Table 3). The data, however, point out that optimizing ferritin cutoff levels to increase sensitivity has limitations. Nonetheless, the evidence favors a cutoff value of 45 ng/mL to make an accurate diagnosis of IDA.
Table 3.
Outcomes Associated With Different Ferritin Cutoffs in IDA
| Population | Premenopausal women | Men and postmenopausal women | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 | Prevalence of colon cancer | 0.06% | 0.67% | ||||
| 1 | Prevalence of gastroesophageal (GE) cancer | 0.01% | 0.07% | ||||
| 2 | Theoretical population count | 1,000,000 | 1,000,000 | ||||
| 3 | Estimated prevalence count of colon cancer in the theoretical population | 592 | 6,667 | ||||
| 3 | Estimated prevalence count of GE cancer in the theoretical population | 62 | 728 | ||||
| 4 | Estimated prevalence count of anemia in the theoretical population | 56,500 | 43,500 | ||||
| 5 | Estimated prevalence count of iron-deficiency anemia (IDA) in the theoretical population | 32,900 | 8,187 | ||||
| 6 | Estimated prevalence of colon cancer in patients with IDA | 0.95% | 8.88% | ||||
| 6 | Estimated prevalence of GE cancer in patients with IDA | 0.24% | 1.96% | ||||
| 7 | Estimated prevalence count of colon cancer in IDA patient from the theoretical population | 313 | 727 | ||||
| 7 | Estimated prevalence count of GE cancer in IDA patient from the theoretical population | 79 | 160 | ||||
| 8 | Ferritin Threshold | 15 | 45 | 15 | 45 | ||
|
| |||||||
| 9 | Sensitivity | 0.59 | 0.85 | 0.59 | 0.85 | ||
| 10 | Specificity | 0.99 | 0.92 | 0.99 | 0.92 | ||
| 11 | Estimated number of missed colon cancers due to false negatives (patients with IDA being considered to be iron-sufficiency) | 128 | 47 | 298 | 109 | ||
| 11 | Estimated number of missed GE cancers due to false negatives | 32 | 12 | 66 | 24 | ||
| 12 | Estimated number of unnecessary endoscopic procedures due to false positives (iron-sufficient patients mislabeled as iron-deficient) | 329 | 2632 | 82 | 655 | ||
| 13 | Estimated number of endoscopic perforations due to false positive | 0.31 | 2.48 | 0.08 | 0.62 | ||
NOTE.
1. Calculated by dividing the estimated prevalence counts of colon or GE cancer in the population (from the SEER Cancer Statistics Review) by the counts of the population (from the US Census Database).73
2. A theoretical number of people from the age/gender group.
3. Calculated by multiplying the prevalence from 1 by the theoretical population count.
4. Calculated by multiplying the estimated prevalence percentage of anemia from loannou GN et al. (2002) by the theoretical population count.42
5. Calculated by multiplying the estimated prevalence percentage of iron-deficiency anemia from loannou GN et al. (2002) by the count in line 4.42
6. Estimated from pooling the prevalence studies as detailed in PICO 2.
7. Calculated by multiplying the estimated prevalence percentage in line 6 by the estimated prevalence count in line 5.
8–10. From Guyatt GH et al. (1992).41
11. Calculated by multiplying the estimated prevalence of colon or GE cancer (line 6) by the false negatives rate (1-sensitivity from line 9).
12. Calculated by multiplying by the false positives rate (1-specificity from line 10) by the estimated count of patients with IDA (line 5).
13. Calculated by multiplying the estimated number unnecessary procedures by estimated prevalence of perforation (0.08%).
We also emphasize several caveats to the use of ferritin in clinical practice. First, patients with certain underlying conditions, particularly inflammatory diseases, may have falsely high ferritin levels because ferritin is an acute phase reactant.43 For example, this has specifically confounded evaluation of IDA in patients with inflammatory bowel disease. In fact, some experts have proposed assessment of the degree of inflammation by using C-reactive protein to assess the degree of inflammation.44 Although this approach is theoretically attractive in patients with mixed IDA and anemia of chronic disease—which can be a diagnostic dilemma, the Review Panel thought that additional testing beyond that recommended here would likely inadvertently complicate the evaluation process. Ferritin levels may also be difficult to interpret in patients with chronic kidney disease who have been often frequently transfused and may also have underlying inflammation. Additionally, ferritin levels cannot be reliably used to diagnose total iron stores in patients who have received recent blood transfusion or who are on oral or intravenous iron replacement therapy. In aggregate, it should be emphasized that before subjecting a patient to invasive procedures, the diagnosis of IDA should be as definitive as possible.
Bidirectional Endoscopy in Patients With Iron Deficiency Anemia
Quality of evidence and summary.
We identified moderate-quality indirect evidence supporting bidirectional endoscopy in patients with IDA, specifically multiple descriptive studies reporting the finding of endoscopic lesions in patients with IDA. Therefore, in patients with no obvious other source of chronic blood loss, available evidence suggests that the benefits of identifying an important lesion with bidirectional endoscopy outweighs the small risks associated with invasive testing (Table 4). Finally, in patients who have gastrointestinal symptoms, evaluation should be site-directed.
Table 4.
Bidirectional Endoscopy in Asymptomatic Men And Postmenopausal Women With Iron Deficiency Anemia
| Certainty assessment |
Summary of findings |
|
|---|---|---|
| Outcome No. of participants (studies) | Overall certainty of evidence | Impact |
|
| ||
| Detection of malignancy 12,040 (18 studies) | ⊕⊕⊕◯ MODERATEa | Although we could not identify any direct evidence from comparative studies using bidirectional endoscopy in men and postmenopausal with IDA, high-quality indirect evidence from screening trials (RCTs and nonrandomized studies) demonstrate substantial mortality reduction even in a setting with substantially lower baseline risk for colon cancer than found in IDA. This provides us with at least moderate certainty in the evidence of benefit in the endoscopic evaluation for IDA. We focused on the outcome of identifying malignancy as an outcome, which is critical for decision making in this setting. We were able to identify studies of the diagnostic yield of bidirectional endoscopy. Bidirectional endoscopy detected lower gastrointestinal malignancy in 8.9% (95% CI, 8.3–9.5) and upper gastrointestinal malignancy in 2.0% (95% CI, 1.7–2.3) of asymptomatic men and postmenopausal women with IDA. Although this estimate is likely an overestimation due to the inclusion of symptomatic patients (high risk of bias), which makes the exact baseline risk for malignancy in IDA uncertain in this risk group, we have high certainty in the evidence that it is many fold higher (approximately 100-fold) than an average risk screening population of similar age. |
Certainty in the evidence rated down due to indirectness (for the intervention).
Because the presence of IDA in postmenopausal women (for the purposes of this discussion, postmenopausal refers to the ceasing of menstruation) or men is a sine quo non for occult gastrointestinal bleeding, endoscopic evaluation is a cornerstone of the evaluation of IDA. Bidirectional endoscopy refers to esophagogastroduodenoscopy (EGD) and colonoscopy. The prevalence of gastrointestinal culprit lesions varies depending on many factors, including age, underlying risk factors, and the presence of gastrointestinal symptoms (specific symptoms, eg, unintentional weight loss, anorexia, abdominal pain, heartburn, and/or change in stool character). To guide our recommendations, we aimed to assess the benefits and harms of bidirectional endoscopy. Because we could not identify any study, randomized or nonrandomized, that compared bidirectional endoscopy with observation or oral iron therapy alone in patients with IDA in any population group, we identified the following indirect evidence to assist the Panel in making decisions: we identified systematic reviews that evaluated the benefits of screening colonoscopy to no endoscopic evaluation45,46; we identified observational cohort and cross-sectional studies to assess the frequency (or “diagnostic yield”) of finding gastrointestinal tract lesions, and most importantly malignancy, during bidirectional endoscopy in patients with IDA15–20,42,47–66; we identified studies that evaluated the rates of complications of gastrointestinal endoscopy67–72; and we used the available epidemiologic reports to model the expected benefit and harm of bidirectional endoscopy for the different age/sex groups (Table 4).73,74
Clinical Variables Important in Evaluation
Gastrointestinal symptoms in patients with IDA might15,16,51 or might not75,76 help direct gastrointestinal tract evaluation toward specific pathology. It is generally considered to be a best practice to consider gastrointestinal tract symptoms in the evaluation process—and it is essential that a careful history be taken. Endoscopy should generally be directed at the site of symptoms, which is desirable to minimize both risk and cost (see Figure 3). Because dual lesions are rare, identification of an obvious abnormality consistent with bleeding, such as a mass lesion, large ulceration, or severe inflammation that is a likely cause of the symptoms, makes further evaluation unnecessary. It should be emphasized that clinical judgment is important in assessing whether a specific lesion accounts for occult bleeding resulting in IDA. For example, it is highly unlikely that trivial gastrointestinal tract lesions bleed enough to cause IDA.12 Although the choice of sequence of procedures (colonoscopy followed by upper endoscopy or vice versa) varies based on local practice, both procedures, if necessary, should be performed on the same day. If the patient has upper gastrointestinal tract symptoms, EGD should be performed initially. In the patient in whom EGD is performed initially and clearly identifies a bleeding lesion, there is some controversy about whether colonoscopy should or should not be performed. In this scenario, whether or not to perform colonoscopic evaluation should be individualized based on the risk and benefit of the procedure, and will depend on variables such as the risk that the patient may have an underlying colorectal cancer.
History and clinical signs should be used to help direct investigation toward localization of a putative bleeding site. A history of peptic ulcer disease increases the likelihood that this may explain the IDA. A history of liver disease raises the possibility of bleeding associated with portal hypertension, including portal hypertensive gastropathy. A history of inflammatory bowel disease suggests bleeding from gastrointestinal tract ulceration. Ingestion of aspirin or other nonsteroidal anti-inflammatory drugs makes bleeding from ulceration more likely. Abdominal pain raises the possibility of an ulcerative process, other mucosal injury, or perhaps obstruction. Pain, anorexia, and/or weight loss point to malignancy. History is also critical in ascertaining whether an extra-intestinal site may be the source of gastrointestinal bleeding, especially from the nasopharynx or pulmonary system.
Physical examination may provide valuable information as to the cause of bleeding. Cutaneous signs (spider angiomata, Dupuytren’s contractures) or other evidence of liver disease (splenomegaly, ascites, caput) suggest the possibility of portal hypertension. Acanthosis nigricans may reflect underlying cancer (particularly gastric cancer); cutaneous telangiectasias of skin and/or mucous membranes and lips raises the possibility of hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu); pigmented lip lesions are seen with Peutz-Jeghers syndrome; cutaneous tumors suggest neurofibromatosis; and purpura is consistent with vascular disease (Henloch-Schönlein purpura or polyarteritis nodosa).
Evidence Supporting Bidirectional Endoscopy in Postmenopausal Women and Men
We focused on the outcome of identifying malignancy as an outcome, which is the most important clinical finding in most patients with IDA and is a critical concern in this setting. Although we could not identify any direct evidence from comparative studies using bidirectional endoscopy in men and postmenopausal with IDA, high-quality indirect evidence from screening trials (RCTs and nonrandomized studies) demonstrates substantial mortality reduction even in a setting with substantially lower baseline risk for colon cancer than found in IDA.45,46 This provides at least moderate certainty in the evidence of benefit in the endoscopic evaluation for IDA.
We searched the literature for studies that reported the prevalence of gastrointestinal tract neoplasms in patients with IDA published in or after 1990. Our search strategy identified 922 references, 772 of them were excluded by reviewing the titles and abstract, and only 24 studies met the inclusion criteria after reviewing the full texts. Sixteen studies (9632 patients) reported the diagnostic yield of bidirectional endoscopy in men and postmenopausal women with IDA (Figure 4). Bidirectional endoscopy detected lower gastrointestinal malignancy in 8.9% (95% CI, 8.3–9.5) and upper gastrointestinal malignancy in 2.0% (95% CI, 1.7–2.3) of largely men and postmenopausal women with IDA.15,20,47–49,51–56,60–62,65,66
Figure 4.
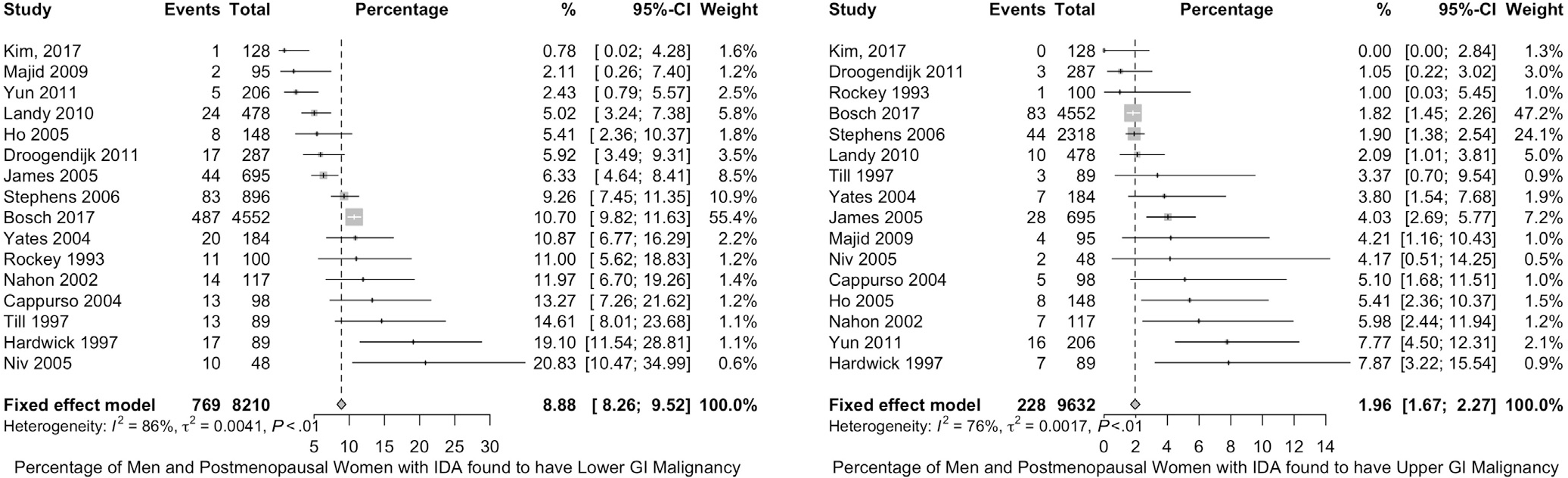
Frequency of colorectal and upper gastrointestinal (GI) tract malignancy in men and postmenopausal women with IDA. Forest plots of studies reporting the frequency of colon cancer (left) and upper gastrointestinal tract cancer (right) in men and postmenopausal women with IDA are shown.
It should be emphasized that this estimate is likely an overestimation due to the inclusion of some symptomatic patients in the reported cohorts (high risk of bias), which makes the exact baseline risk for malignancy in IDA uncertain in this risk group. Based on the available data, we have high certainty in the evidence that the risk of malignancy is many fold higher (up to 100-fold) than an average risk screening population of similar age.
In conclusion, in postmenopausal women and men with IDA, the Technical Review Panel identified evidence supporting bidirectional endoscopy over no endoscopy. This assumes that there is no other obvious source of chronic blood loss. Additionally, in patients with IDA who also have gastrointestinal symptoms, evaluation should be site-directed. For patients in whom a definitive source of bleeding is identified in either the upper or lower gastrointestinal tract during initial endoscopic evaluation (see above and Rockey13), other portions of the gastrointestinal tract need not be routinely or obligatorily evaluated.
Should Bidirectional Endoscopy Be Performed in Premenopausal Women?
Quality of evidence and summary.
We identified moderate-quality indirect evidence supporting bidirectional endoscopy in premenopausal women with IDA, including descriptive studies reporting the finding of endoscopic lesions in patients with IDA. Therefore, in patients with no obvious other source of chronic blood loss (in particular, in women without abnormal menses), available evidence suggests that the benefit of bidirectional endoscopy outweighs the risk of no endoscopy (Table 5). In patients who have gastrointestinal symptoms, evaluation should be site-directed. Because there is very little evidence in younger premenopausal women, in the judgment of the Panel, the risk of endoscopy should be considered carefully.
Table 5.
Bidirectional Endoscopy in Asymptomatic Premenopausal Women With Iron Deficiency Anemia
| Certainty assessment |
Summary of findings |
|
|---|---|---|
| Outcome No. of participants (studies) | Overall certainty of evidence | Impact |
|
| ||
| Detection of malignancy 1026 (10 studies) | ⊕⊕⊕◯ MODERATEa | Although we could not identify any direct evidence from comparative studies using bidirectional endoscopy in asymptomatic premenopausal women with IDA, high-quality indirect evidence from screening trials (RCTs and nonrandomized studies) demonstrate substantial mortality reduction, provided the baseline risk for colon cancer does not substantially fall below established thresholds (ie, 0.6/1000 for 50-year-old woman at average risk without IDA). This provides us with at least moderate certainty in the evidence of benefit in the endoscopic evaluation for IDA. We focused on the outcome of identifying malignancy as an outcome, which is critical for decision making in this setting. We were able to identify studies of the diagnostic yield of bidirectional endoscopy. Bidirectional endoscopy detected lower gastrointestinal malignancy in 0.9% (95% CI, 0.3–1.9) and upper gastrointestinal malignancy in 0.2% (95% CI, 0.0–0.9) of asymptomatic premenopausal women with IDA. Although this estimate is likely an overestimation due to the inclusion of symptomatic patients (high risk of bias), which makes the exact baseline risk for malignancy in IDA uncertain in this risk group, we are confident that it is several fold higher than the 0.6/1000 (0.06%) rate, particularly in the mid to upper age range of premenopausal women. However, the high propensity of benefit of endoscopy in IDA quickly diminishes as age declines and therefore, the harms of endoscopy will eventually outweigh the benefits. No reliable data were found that further defined this age threshold. |
Certainty in the evidence rated down due to indirectness (for the intervention).
IDA is commonly identified in premenopausal (defined as having menstruation) women. On one hand, blood loss through childbirth and menstruation may explain IDA in many patients (a careful history exploring menorrhagia and other gynecologic disorders that may be a potential source of abnormal blood loss is important). On the other hand, evidence suggests that this group of patients may harbor gastrointestinal tract lesions consistent with chronic occult bleeding leading to IDA.16–19,50,57,58 Although we could not identify any direct evidence from comparative studies using bidirectional endoscopy in asymptomatic premenopausal women with IDA, high-quality indirect evidence from screening trials (RCTs and nonrandomized studies) demonstrate substantial mortality reduction, provided that the baseline risk for colon cancer does not fall substantially below established thresholds (ie, 0.6/1000 for 50-year-old woman at average risk without IDA).45,46 This provides at least moderate certainty in the evidence of benefit in the endoscopic evaluation for IDA in premenopausal women. It should be noted that the benefit of endoscopy in IDA is likely to be diminished in younger patients and, therefore, the harms of endoscopy will likely outweigh the benefits at some age threshold.
We focused on the outcome of identifying malignancy as an outcome, which is critical for decision making in this setting. Our search strategy identified 9 studies (910 patients) that reported the diagnostic yield of bidirectional endoscopy in premenopausal women.16–19,42,49,50,57,58 Bidirectional endoscopy detected lower gastrointestinal malignancy in 0.9% (95% CI, 0.3–1.9) and upper gastrointestinal malignancy in 0.2% (95% CI, 0.0–0.9) of premenopausal women with IDA (Figure 5). This estimate is likely an overestimation due to the inclusion of symptomatic women in the reported cohorts (high risk of bias). In addition, the number of studies identifying upper gastrointestinal tract malignancies was limited; together, these factors make it difficult to determine the precise baseline risk for malignancy in IDA in this risk group. Nonetheless, we are confident that the risk is substantially higher than the 0.6/1000 (0.06%) rate of malignancy expected in a 50-year-old woman at average risk without IDA—particularly in the mid to upper age range of premenopausal women. No reliable data are available with which to further define this age threshold. Finally, in younger women with IDA, patient preferences regarding the risks and benefits of endoscopic evaluation should be considered carefully.
Figure 5.
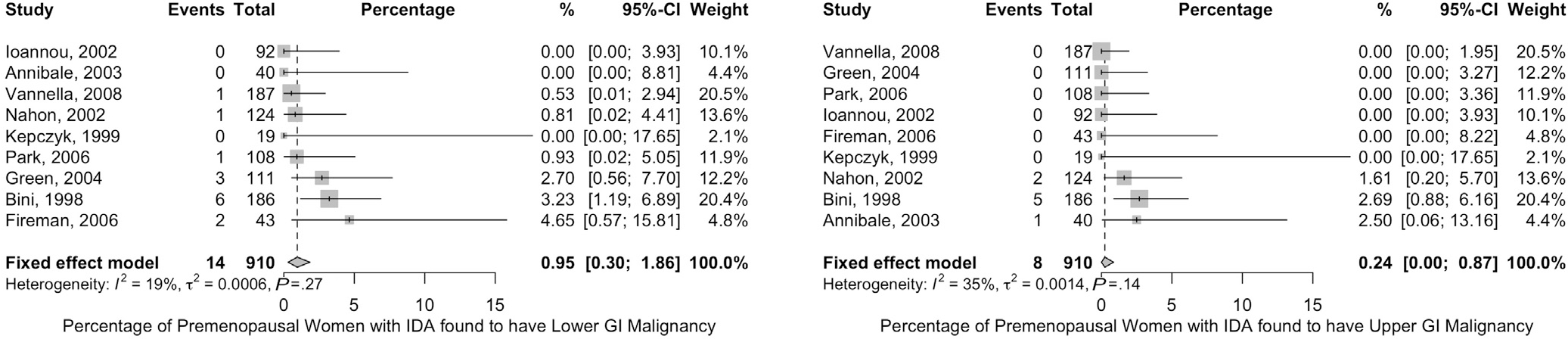
Frequency of colorectal and upper gastrointestinal (GI) tract malignancy in premenopausal women with IDA. Forest plots of studies reporting the frequency of colon cancer (left) and upper gastrointestinal tract cancer (right) in premenopausal women with IDA are shown.
In conclusion, in asymptomatic premenopausal women, the currently available evidence suggests that bidirectional endoscopy provides benefit compared with no endoscopy. This approach assumes that there is no obvious other source of chronic blood loss, which is a particularly difficult assessment in many premenopausal women. The Panel also found evidence that suggests that in patients with IDA who also have gastrointestinal symptoms, evaluation should be site-directed.
Are Routine Gastric Biopsies for Helicobacter pylori Indicated in Patients With Iron Deficiency Anemia?
Quality of evidence and summary.
We identified low-quality evidence supporting noninvasive testing for H pylori in patients with IDA. Available evidence suggests that H pylori may cause IDA in select populations, in particular in pediatric populations. However, the role of H pylori as a causal factor for IDA in the majority of adult men and postmenopausal women is unclear. Therefore, given the associated cost of gastric biopsy and weak evidence to support the effectiveness of eradicating H pylori in adult patients with IDA, the Review Panel concluded that routine gastric biopsy and histologic assessment to detect H pylori is unlikely to be cost-effective (Table 6). A strategy of noninvasive testing for patients with negative colonoscopy and EGD appeared to be associated with reasonable benefit and less cost.
Table 6.
Testing/Treating for Helicobacter pylori + Iron Replacement Compared With Iron Replacement Alone for Patients With Iron Deficiency Anemia
| Certainty assessment |
Summary of findings |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of participants (studies), follow-up | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall certainty of evidence | Number of participants |
Anticipated absolute effects |
||
| With iron replacement alone | With testing/treating for H pylori + iron replacement | Improvement with iron replacement alone | Mean difference with testing/treating for H pylori + iron replacement | |||||||
|
| ||||||||||
| Improvement of Hgb (follow-up: range 8’12 wk; assessed with: change in Hgb, g/dL) | ||||||||||
|
| ||||||||||
| 123 (3 RCTs) | Seriousa | Not serious | Not serious | Seriousb | None | ⊕⊕◯◯ LOW |
61 | 62 | The mean improvement of Hgb was 2.8 g/dL | MD 2.2 g/dL higher (1.3 higher to 3 higher) |
|
| ||||||||||
| Improvement of ferritin (follow-up: range 8–12 wk; assessed with: change in ferritin, ng/mL) | ||||||||||
|
| ||||||||||
| 123 (3 RCTs) | Seriousa | Not serious | Not serious | Seriousb | None | ⊕⊕◯◯ LOW |
61 | 62 | The mean improvement of ferritin was 0 ng/mL | MD 23.2 ng/mL higher (12.2 higher to 34.3 higher) |
Hgb, hemoglobin; MD, mean difference.
Due to lack of allocation concealment.
Due to the small sample size.
In addition to causing peptic ulcer disease and increasing the risk of gastric malignancies, H pylori causes atrophic gastritis and hypochlorhydria, which can lead to poor iron absorption and thus IDA. Observational studies have shown an association between iron deficiency and H pylori infection.77 British guidelines have previously recommended testing and treating for H pylori in patients with recurrent IDA and negative bidirectional endoscopy.78 Nonetheless, it should be emphasized that the role of H pylori as a causal agent in IDA is controversial.
We searched the literature for RCTs that evaluated the benefit of treating H pylori in patients with IDA. Our search identified 167 references, 32 of them were retrieved for full-text review based on title and abstract screening, and only 3 of them met the inclusion criteria79–81 (Figure 6). To assist the Panel in decision making, we also identified systematic reviews of the prevalence of H pylori in the United States and the diagnostic accuracy of the different noninvasive tests for H pylori compared with gastric biopsies.82–84
Figure 6.
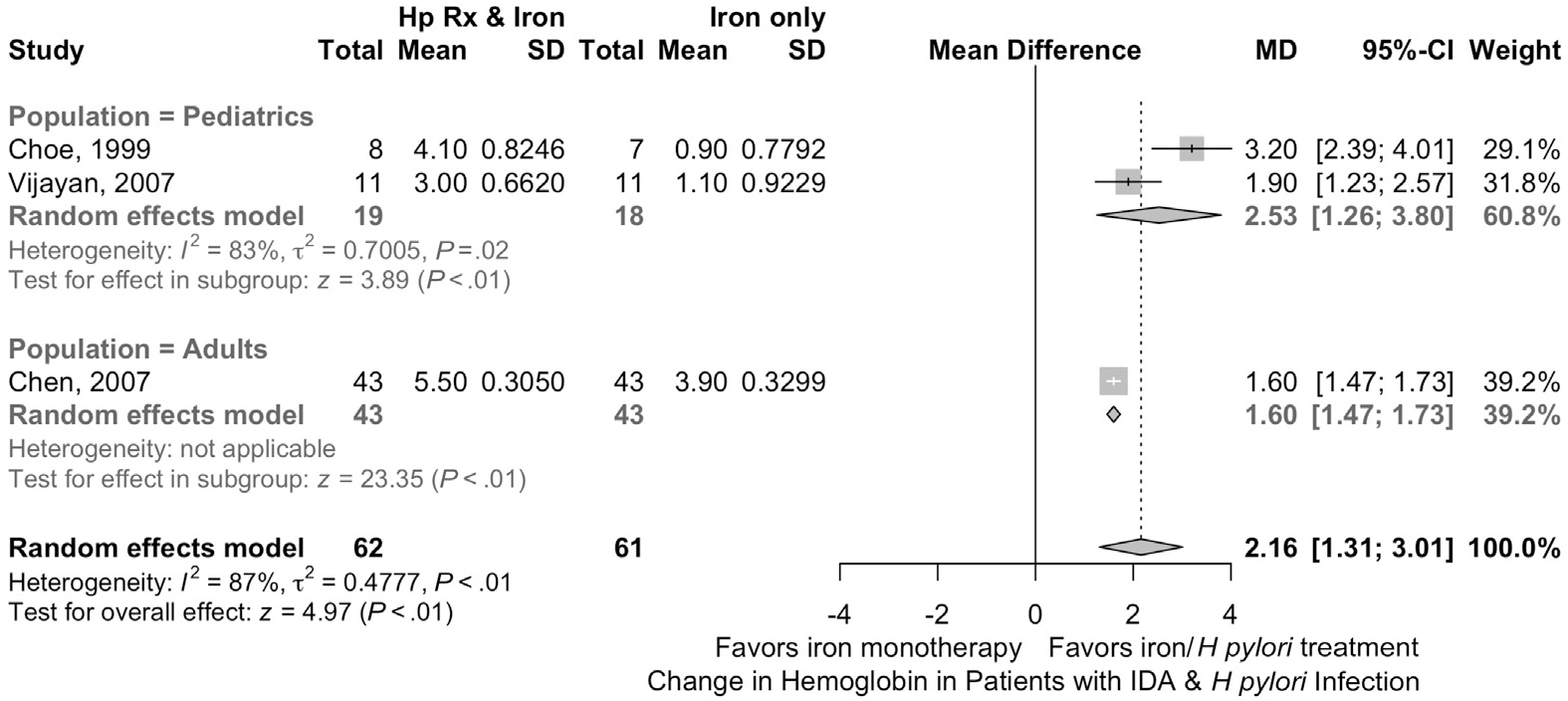
Iron treatment in patients with H pylori and IDA. Shown is a forest plot depicting the effectiveness of iron therapy in patients with H pylori and IDA. Hp Rx, H pylori treatment; MD, mean difference.
We first evaluated testing strategies to evaluate for H pylori, including the accuracy and cost of various diagnostic approaches, focused on noninvasive tests (Appendix 3). At a fixed specificity of 90%, the urea breath test 13C had the greatest sensitivity to detect active H pylori infection, followed by serologic testing and lastly stool antigen testing.83 The estimated overall prevalence of H pylori in the United States is 35.6% (95% CI, 30.0%–41.1%).82,84 In a hypothetical population of 1,000,000 adult patients, we estimated that 32,900 patients will have IDA. Of those, about 11,712 patients will have H pylori infection based on the overall prevalence of H pylori in the United States. The use of urea breath testing to diagnose H pylori instead of obtaining biopsies routinely during endoscopy will lead to the accurate diagnosis of 3418 H pylori–infected patients and 142 H pylori–infected patients will be missed. False positives will lead to the treatment of 451 noninfected patients. Hence, an approach that starts by performing bidirectional endoscopy without routine gastric biopsies for the evaluation of asymptomatic IDA then testing for H pylori using urea breath testing for patients with negative bidirectional endoscopy will lead to missing 147 cases of H pylori (of 3560 based on 35.6% prevalence). Those missed cases can be diagnosed by repeating endoscopy with biopsies if an alternative source of IDA is not identified. Such an approach would lead to a major decrease in cost compared with obtaining biopsies routinely in the first bidirectional endoscopy encounter, with negligible risk (Appendix 3). Although not reported here, the cost of using alternative noninvasive tests with comparable diagnostic accuracy, such as H pylori stool antigen testing, is less than the cost of using urea breath testing. Hence, we believe that the use of the available noninvasive tests, which have comparable diagnostic accuracy, will still lead to a major decrease in the cost compared with obtaining biopsies routinely. It is important to note that the calculated costs that we are reporting include assumptions that we reported for transparency. For example, the costs were obtained from the Centers for Medicare and Medicaid Services data and we assumed that the biopsies were placed in a single specimen container. They also did not include other assumptions that may be of importance, such as the burden on the patient from taking time off or needing transportation to perform the required procedures and tests.
Two of the 3 RCTs that we identified and pooled were in pediatric populations. Iron replacement combined with treatment of H pylori was associated with more rapid iron repletion compared with iron replacement alone. Patients who received H pylori treatment had a mean improvement in hemoglobin that was 2.2 g/dL (95% CI, 1.3–3.0) and ferritin was 23.2 ng/mL (95% CI, 12.2–34.3) more than the improvement with iron replacement alone (3 studies, 113 patients). The certainty of evidence was rated as low due to increased risk of bias (lack of allocation concealment) and imprecision (small sample size). Further, we recognize that data used in pediatric populations might not be generalizable to adults, also reducing the certainty of evidence. Finally, prior systematic reviews and meta-analyses found that the treatment of H pylori may be associated with decreased risk of gastric cancer.85,86
In summary, although the bulk of the evidence indicating that identification and eradication of H pylori leads to more rapid iron repletion, these data are largely in pediatric age groups, which might not be generalizable to adult populations and, therefore, the quality of the evidence was judged to be low. We also found that noninvasive indirect testing for H pylori has excellent diagnostic accuracy and an approach that utilizes such indirect testing is associated with minimizing the costs of testing. Therefore, in asymptomatic patients with IDA, the Technical Review Panel concluded that there was not enough evidence to support routine random gastric biopsy and testing may be considered in patients with negative bidirectional endoscopy using noninvasive testing methods for H pylori followed by treatment if positive over no testing.
The Role of Routine Gastric Biopsies for Autoimmune Atrophic Gastritis in Patients With Iron Deficiency Anemia
Quality of evidence and summary.
Although emerging evidence suggests an association between atrophic gastritis and IDA, the evidence that supports that the identification of atrophic gastritis followed by specific treatment leads to improvement of IDA is weak. Given the lack of evidence, the Review Panel concluded that there is insufficient evidence of benefit for routine gastric biopsy to diagnose atrophic gastritis, that is, the potential harms and additional cost of biopsy are likely to outweigh potential benefit (Table 7).
Table 7.
Routine Gastric Biopsies to Test for Atrophic Gastritis vs Not Testing for Atrophic Gastritis in Patients With Iron Deficiency Anemia
| Certainty assessment |
Summary of findings |
|
|---|---|---|
| Outcome No. of participants (studies) | Overall certainty of evidence | Impact |
|
| ||
| Benefits and harms of diagnosing autoimmune gastritis in the context of IDA | ||
| 567 (6 studies) | ⊕◯◯◯ VERY LOWa,b |
Neither RCTs or observational studies have directly assessed the benefits of routine gastric biopsies during EGD to assess for atrophic body gastritis in patients with IDA. The pooled diagnostic yield of routine gastric biopsies to assess for non-H pylori atrophic body gastritis in patients with IDA was 10.0% (95% CI, 7.6–12.7).a,b The pooled estimate of 10% is unlikely to reflect the true incidence of non-H pylori atrophic body gastritis in patients with IDA due to very serious risk of bias related to the patient selection methods. |
| We could not identify any study that evaluated the benefits of diagnosing non-H pylori atrophic body gastritis in patients with IDA. There were no studies that evaluated whether the use of IV iron replacement compared with oral has any additional benefits in this population, that is, by overcoming poor iron absorption. Observational studies of patients suspected to have autoimmune gastritis or pernicious anemia have shown a pooled gastric cancer incidence of 0.27% per person-year. However, there was insufficient comparative evidence to support the benefit of surveillance endoscopy.c | ||
IV, intravenous.
None of the studies was an inception cohort and they were all referred patients; many of them included only patients with negative bidirectional endoscopy (selection bias).
The point estimates of individual studies ranged from 7.4% to 19.5%.
The diagnosis in many of the included studies was based on low vitamin B-12 level only.
Autoimmune atrophic gastritis is associated with destruction of parietal cells in the gastric corpus, which leads to hypo- or achlorhydria, which can interfere with iron absorption and lead to IDA. Observational studies of patients suspected to have autoimmune gastritis or pernicious anemia have shown a pooled gastric cancer incidence of 0.27% per person-year.87 However, there was insufficient comparative evidence to support the benefit of surveillance endoscopy. Autoimmune gastritis presents as IDA in young patients and vitamin B-12 deficiency (pernicious anemia) in older patients. Making a diagnosis of autoimmune atrophic gastritis requires separate biopsies of the gastric antrum and corpus and can be supported by the presence of hypo- or achlorhydria, hypergastrinemia, anti-parietal cells antibodies, and/or anti-intrinsic factor antibodies. It is notable that autoimmune atrophic gastritis has no specific treatment. However, observational studies have raised the possibility of increased risk of gastric adenocarcinoma and carcinoid tumors in patients with atrophic gastritis.88 Guidelines published by The European Society of Gastrointestinal Endoscopy recommend considering endoscopic follow-up every 3–5 years in such patients, although the effectiveness of such an approach remains highly uncertain.89
No comparative evidence to illustrate the benefits vs harms of identifying atrophic gastritis in IDA was found. As a fall back, we identified 6 studies composed of 567 patients that reported the frequency of finding autoimmune atrophic gastritis in IDA patients. The pooled prevalence was 10.1% (95% CI, 7.6%–12.8%). Although establishing a diagnosis of autoimmune atrophic gastritis may prevent further evaluation and may direct iron repletion therapy in the patient with established atrophic gastritis, the certainty of evidence that the benefits of identifying atrophic gastritis outweighs the harms was very low due to indirectness of evidence, high risk of bias (selection bias), and inconsistency (different inclusion criteria and workup approach).51,90–94
In conclusion, in patients with IDA, the Review Panel did not find enough evidence that benefits of random gastric biopsies or noninvasive testing to diagnose atrophic body gastritis would outweigh potential harms.
What Is the Utility of Routine Small Bowel Biopsies for Celiac Disease in Patients With Iron Deficiency Anemia?
Quality of evidence and summary.
Although celiac disease is a well-known cause of IDA and it is generally accepted that making a diagnosis of celiac disease in patients presenting with IDA is likely to be important, evidence supporting routine use of small bowel biopsy during EGD is sparse. Rather, the evidence suggests that performing serologic testing as an initial approach in those with clinically suspected celiac disease is more cost-effective (Table 8).
Table 8.
Testing Strategies in Celiac Disease
| Certainty assessment |
Summary of findings |
|
|---|---|---|
| Outcome No. of participants (studies) | Overall certainty of evidence | Impact |
|
| ||
| Benefits and harms of diagnosing celiac disease in the context of IDA | ||
| 7993 (11 studies) | ⊕◯◯◯ VERY LOWa,b |
Neither RCTs or observational studies have directly compared routine small bowel biopsies during EGD in asymptomatic patients with IDA to clinically targeted workup (symptoms and signs followed by serologic testing). The pooled diagnostic yield of random duodenal biopsies to assess for celiac sprue-like histologic changes in patients with IDA in the United States was 1.15% (95% CI, 0.89–1.44). Serologic testing with TTG IgA antibodies has a pooled sensitivity of 0.93 (95% CI, 0.90–0.95) and pooled specificity of 0.98 (95% CI, 0.96–0.99). If patients with asymptomatic IDA are screened with TTG before EGD, 9 cases of 115 celiac disease cases in 10,000 patients with IDA will be missed and 208 additional patients will undergo duodenal biopsies due to false positives. As patients with IDA will be expected to undergo diagnostic EGD regardless of the serologic testing status, there are no expected additional harms from the procedural standpoint considering the comparable safety profiles of EGD with and without small bowel biopsies. |
| Cost of obtaining routine small bowel biopsies compared with obtaining biopsies based on the results of serologic testing | ||
| Not measured | — | Unless the pretest probability is high, that is, >10%, it is highly unlikely that routine small bowel biopsies in asymptomatic patients with IDA will be cost-effective. Routinely obtaining small bowel biopsies compared with determining TTG IgA status will cost $67,000 for every missed celiac disease case due to false-negative TTG IgA. If patients present for endoscopy with unknown TTG IgA status, obtaining routine small bowel biopsies will cost $48,000 to avoid missing the 1 in 1000 false-negative case of celiac disease case if diagnostic EGD is followed by serologic testing. Therefore, the balance between the expected benefits, potential harms, and cost is likely to favor serologic testing over routine small bowel biopsies unless the prevalence of celiac disease is >5% in the nontertiary referral setting. |
Some of the studies were not inception cohorts and/or did not clearly state enrolling consecutive patients (selection bias).
The point estimates of individual studies ranged from 0% to 8%.
Celiac disease, which causes injury to the small bowel, is a well-known cause of IDA. The mechanism appears to be at least 2-fold, including both occult bleeding95 and malabsorption of iron. Therefore, great emphasis has been placed on the diagnosis of celiac disease, particularly in populations at high risk for it. It is currently common practice to obtain routine “screening” small bowel biopsies during bidirectional endoscopy (in patients without an obvious source of occult gastrointestinal bleeding).
Prior published guidelines recommend routine small bowel biopsies in patients with IDA regardless of the status of celiac disease serologic tests.96 Previous studies using the Clinical Outcomes Research Initiative database emphasize the common use of small bowel biopsies; these were performed in 10%–38% of anemic patients in general and 50%–93% of patients with iron deficiency without anemia.97,98
We searched the literature for comparative studies (randomized trials or nonrandomized observational studies) that assessed the benefits of routine small bowel biopsy compared with noninvasive testing or not testing for celiac disease in asymptomatic patients with IDA. However, our search did not identify any study that met the inclusion criteria. Hence, we searched for studies that evaluated the frequency of finding celiac disease in patients with IDA, and studies of the diagnostic accuracy of noninvasive testing for celiac disease to use them as indirect evidence to assist the Clinical Guideline Panel in making a decision.
The search strategy identified 825 references. We excluded 644 references based on title and abstract review. After reviewing the full texts, we included 11 studies the reported the frequency of finding celiac disease in IDA patients in the United States, and a systematic review that reported the diagnostic accuracy of noninvasive testing for celiac disease. We focused on studies from the United States due to the variable prevalence of celiac disease between countries.29
Of the 11 studies identified, 7 assessed the prevalence of celiac disease in IDA patients based on small bowel biopsies, 2 studies based on serologic testing, and 2 studies of pathologic databases. The pooled diagnostic yield of random duodenal biopsies to assess for celiac sprue-like histologic changes in patients with IDA in the United States was low at 1.15% (95% CI, 0.89%–1.44%). The studies included data from 7993 patients and certainty of evidence was very low due to increased risk of bias (mainly selection bias) and serious imprecision16–18,76,99–105 (Figure 7).
Figure 7.
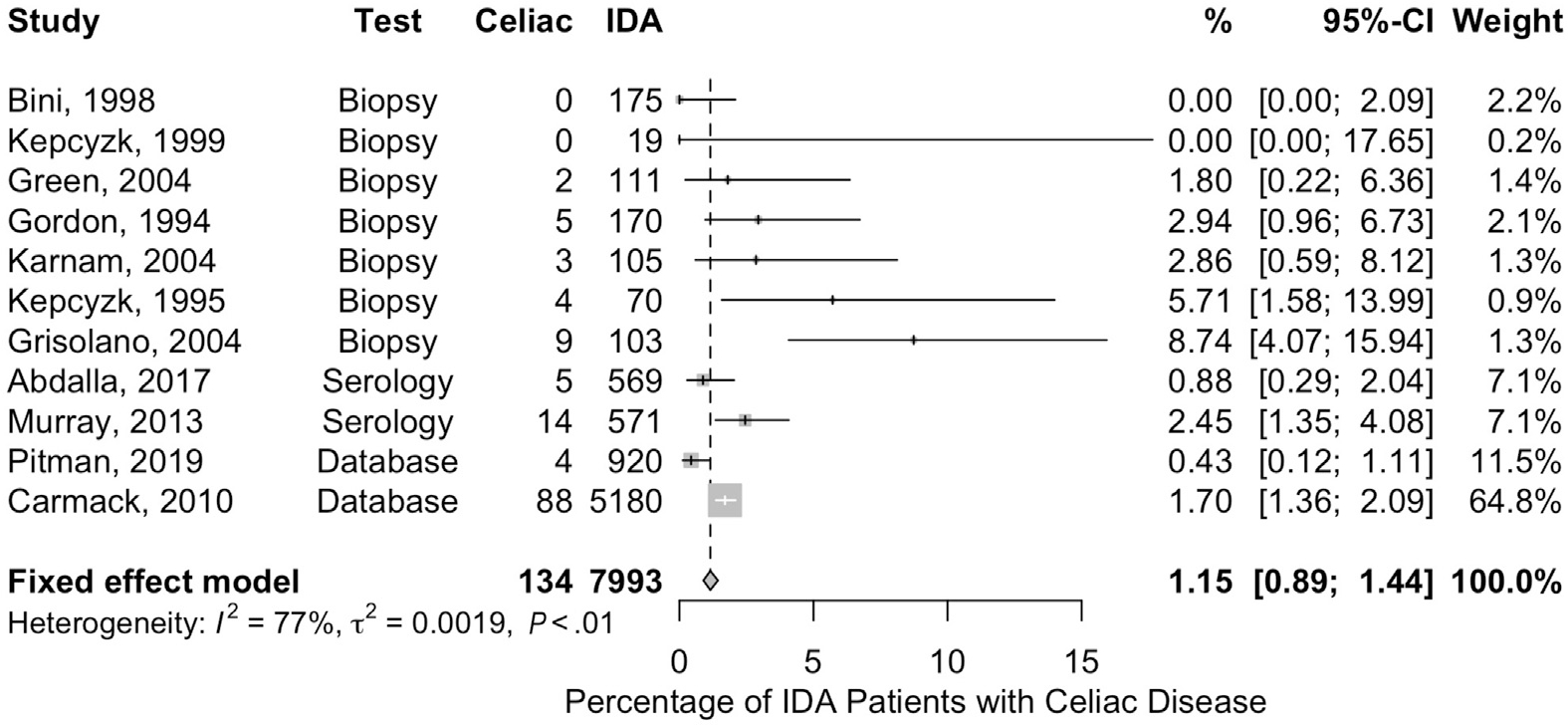
Frequency of celiac disease in patients with IDA. Shown is a forest plot depicting the frequency with which celiac disease was detected in different studies of patients with IDA.
We identified a systematic review conducted by the Southern California Evidence–based Practice Center, which assessed the diagnostic accuracy of serologic testing for celiac disease.106 Serologic testing with TTG IgA antibodies has a pooled sensitivity of 0.93 (95% CI, 0.90–0.95) and pooled specificity of 0.98 (95% CI, 0.96–0.99).
To further inform the Panel, we examined 3 strategies that utilized small bowel biopsy and serologic testing in a theoretical population of asymptomatic IDA patients. We focused on each of these approaches because they are in widespread use, and serologic assessment to detect celiac disease has gained considerable interest among experts and clinicians.107 The first strategy assumes that the endoscopist performs routine small bowel biopsies in every patient with IDA. The second strategy starts by obtaining TTG IgA in every patient, followed by obtaining small bowel biopsies for those who test positive. In this strategy, patients who test negative and have negative bidirectional endoscopy receive oral iron replacement therapy, which will be expected to fail in patients with celiac disease due to malabsorption (false-negative TTG IgA). Those patients who fail iron replacement therapy would end up undergoing repeat endoscopy with biopsies. The third strategy accounts for a common scenario found in practice in which the patient presents for diagnostic endoscopy with no prior celiac testing. In this strategy, evaluation begins with performing diagnostic bidirectional endoscopy in every patient, followed by obtaining TTG IgA for every patient. Those who test positive will end up having a second endoscopy with biopsies to confirm the diagnosis, and those who test negative will receive iron replacement therapy. Similar to the second strategy, those who fail iron replacement therapy will undergo repeat endoscopy with small bowel biopsies to assess for celiac disease. The strategy in which initial serologic testing is performed in all patients appears to be the most cost-saving, while a strategy in which routine small bowel biopsies are obtained is associated with the highest cost (Appendix 4). Similar to the calculations we reported in the case of H pylori, it is important to note that the calculations reported here also include assumptions, which we have reported for transparency, and they might miss some assumptions, such as the patient burden from missing work and transportation. The costs were derived from the Centers for Medicare and Medicaid Services publicly available data and we assumed that all of the obtained biopsies were placed in a single specimen container.
The approach of using noninvasive serologic testing is also supported by a recent study107 that showed that the cumulative incidence of celiac disease diagnosis in patients with negative celiac serologic testing followed for a mean of 8.8 years was extremely low (0.06%; 95% CI, 0.01%–0.11%).
In conclusion, based on the available evidence, in asymptomatic patients with IDA and clinically suspected celiac disease, the bulk of the evidence supports initial serologic testing (followed by small bowel biopsy only if positive) to routine small bowel biopsy. Patients who have symptoms or signs of celiac disease or who have other indicators of malabsorption should be managed based on the entirety of the clinical evidence and will likely still require small bowel biopsies due to the possibility of false-negative serologic testing.
After Negative Bidirectional Endoscopy, in Patients With Iron Deficiency Anemia, When Should Small Bowel Evaluation Be Performed?
Quality of evidence and summary.
Even though the use of capsule endoscopy to evaluate the small bowel has become commonplace in practice, there is little evidence supporting the routine use of capsule endoscopy to evaluate the small bowel in asymptomatic patients with IDA immediately after negative bidirectional endoscopy. Given the paucity of evidence in asymptomatic patients with IDA and negative bidirectional endoscopy, the Review Panel concluded that a trial of iron therapy before capsule endoscopy is the most appropriate initial approach (Table 9). Capsule endoscopy may then be pursued in patients who fail to respond to iron replacement therapy.
Table 9.
Video Capsule Endoscopy Compared With Nothing for Asymptomatic Patients With Iron Deficiency Anemia and Negative Bidirectional Endoscopy
| Certainty assessment |
Summary of findings |
|
|---|---|---|
| Outcome No. of participants (studies) | Overall certainty of evidence | Impact |
|
| ||
| Reduction of adverse outcomes 2899 (16 studies) | ⊕◯◯◯ VERY LOWa |
We could not identify any direct evidence from comparative studies that performing VCE would reduce the risk of having or developing any adverse outcomes such as death or cancers in asymptomatic patients with IDA and negative bidirectional endoscopy. We could not identify any direct evidence from diagnostic accuracy studies that informs the rate of falsely negative or falsely positive VCE. We were able to identify studies of the diagnostic yield of VCE that were limited by very serious risk of bias. Although arteriovenous malformations and other benign lesions are well known to be identified in the small bowel, we focused on the outcome of finding malignancy because it is the most serious finding. VCE detected malignancy in 1.3% (95% CI, 0.8–1.8) of patients with IDA and negative bidirectional endoscopy. This estimate is likely an overestimation due to the aforementioned risk of bias, which stems from the inclusion of symptomatic patients. The comparative efficacy of VCE in IDA remains undefined. |
VCE, video capsule endoscopy.
Most of the studies were not inception cohorts, included referred patients, and/or did not clearly state enrolling consecutive patients (selection bias).
The small bowel is a well-appreciated source of bleeding in patients with IDA. Small bowel tumors, ulcers, vascular ectasia (also arteriovenous malformation), and even Crohn’s disease have all been reported.13 Small bowel imaging (including computed tomography or magnetic resonance enterography), while ineffective at detecting angiodysplasia and superficial inflammation, is effective at detecting small bowel malignancy. Imaging may be considered initially if malignancy is suspected. Having said that, 2 major advances in small bowel investigation have begun to reshape the evaluation and management of patients with IDA; these include capsule endoscopy (CE) and balloon enteroscopy. Each has advantages and disadvantages, particularly with regard to the level of invasiveness (CE is noninvasive) and ability to administer therapy (therapy can be administered via balloon enteroscopy).
We searched the literature for studies that directly compared the small bowel investigation with iron replacement therapy alone (randomized trial or nonrandomized studies) but we were unable to identify any. To assist the Panel in making a recommendation, we identified studies that evaluated the frequency of finding small bowel neoplasia in IDA patients with negative bidirectional endoscopy and no overt gastrointestinal bleeding. We selected neoplasia as an outcome, as it is critically important as a diagnosis that should not be missed. The search strategy identified 532 references and 457 of them were excluded based on title and abstract screening. Of the remaining references, 16 studies composed of 2899 patients were included after reviewing the full-text articles. CE detected malignancy in 1.3% (95% CI, 0.8–1.8) of patients with IDA and negative bidirectional endoscopy (Figure 8). The certainty of evidence was very low due to high risk of bias (selection bias due to inclusion of symptomatic patients and patients referred to specialty centers for CE).108–123 Additionally, in a recent study that followed 93 patients with IDA for more than 5 years, no small bowel malignancies were identified.124 However, the comparative efficacy of CE in IDA remains undefined.
Figure 8.
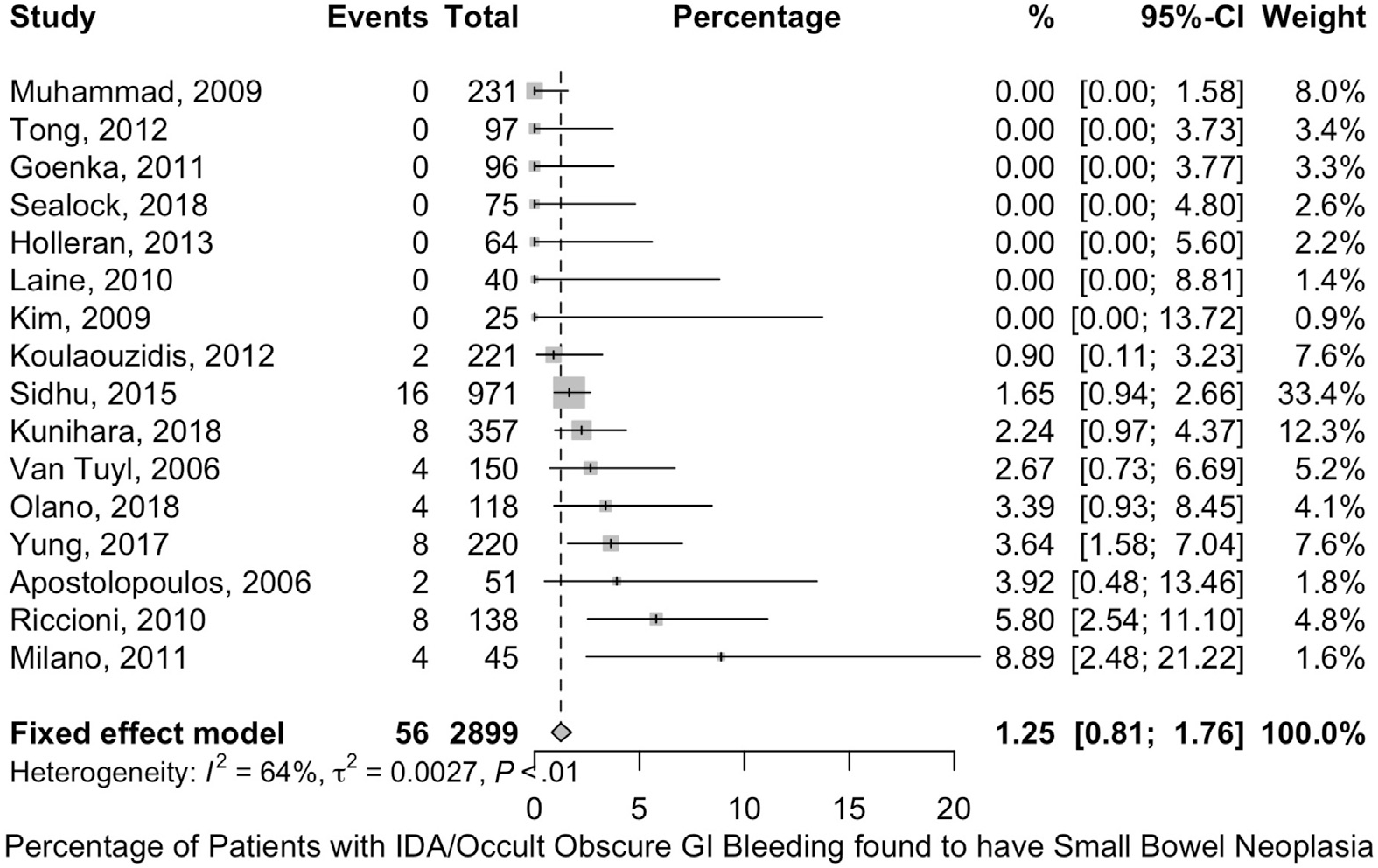
Frequency of small bowel neoplastic lesions in patients with IDA. Shown is a forest plot depicting the frequency with which small bowel neoplasia was detected in different studies of patients with IDA. GI, gastrointestinal.
Although the studies published on CE and balloon enteroscopy in patients with IDA have demonstrated that a substantial number of patients will have putative bleeding lesions identified with these 2 modalities, it is difficult to draw meaningful conclusions from these studies because the patient populations studied are extremely heterogeneous and poorly described, there are no or poor definitions of putative bleeding lesions, there is lack of consistency in technique, and outcomes are generally not meaningful. Despite drawbacks, the data suggest that abnormalities are more commonly detected with CE (CE is highly effective at identifying vascular lesions and inflammatory changes, which may cause IDA) and balloon enteroscopy than with modalities, such as push enteroscopy or small bowel imaging studies.13,116,120 Given the superior visualization ability of CE, the limited availability and the invasive nature of balloon enteroscopy, and the often incomplete evaluation of the small bowel with this examination, the Review Panel did not consider balloon enteroscopy as a viable first-line diagnostic possibility.
Push enteroscopy is widely available in clinical practice, and is often performed in patients with IDA and negative bidirectional endoscopy. However, there is a lack of data supporting its use and additionally it provides an incomplete examination of the small bowel. Therefore, push enteroscopy should be not be considered a diagnostic modality to evaluate IDA. Given these considerations, capsule endoscopy is the preferred modality to evaluate the small bowel in patients with IDA.
In conclusion, in asymptomatic patients with IDA, there is insufficient evidence to support the routine use of CE after negative bidirectional endoscopy. Instead, the Technical Review Panel believed that CE should be considered a second-line diagnostic tool, best employed after a trial of iron therapy. Because available literature does not comment on the appropriate time course for a trial of iron therapy or the types of iron replacement therapy, the type and duration of iron therapy before investigation of the small bowel should be based on clinical judgment. It should also be noted that there may be circumstances where CE could be warranted as a first-line investigative approach, such as in those requiring ongoing antiplatelet or anticoagulation medications, or those requiring blood transfusion or with refractory IDA.
Future Directions
There is a great need for further evidence in this field. Although we have come to a number of specific conclusions based on the available data, we emphasize that a number of evidence gaps exist in the field. For example, we have not addressed iron deficiency without anemia. An important area has to do with investigation of the risks and benefits of gastrointestinal evaluation in premenopausal women and other patient subgroups; further research in these areas is necessary. In asymptomatic patients with negative bidirectional endoscopy the following issues remain: 1) whether H pylori testing in patients with IDA (and subsequent treatment) is indicated; 2) what is the role of atrophic gastritis in IDA? And what is the benefit, if any, of aggressive diagnostic evaluation for this disorder in patients with IDA? 3) what is the best approach to diagnose celiac disease in patients with IDA? Formal outcome studies and cost-effectiveness analyses of serology vs biopsy to detect celiac disease are needed; 4) the timing and need for routine small bowel investigation in asymptomatic IDA patients with negative bidirectional endoscopy is not clear, better evidence of the benefit of CE is required specifically in this population; 5) a better understanding of the natural history of IDA in patients with negative bidirectional endoscopy is needed; and finally, 6) once gastrointestinal evaluation is complete, when should patients be referred to hematology for further evaluation?
Supplementary Material
Acknowledgments
The authors sincerely thank Ms Kellee Kaulback, Medical Information Officer, Health Quality Ontario, for helping in the literature search for this technical review. Author contributions: Don C. Rockey: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; supervisory activities. Osama Altayar: study concept and design; acquisition of data; systematic review protocol development; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content. Falck-Ytter: study concept and design; systematic review protocol development; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; supervisory activities. Denise Kalmaz: study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; supervisory activities.
Funding
DCR was supported by the NIDDK - P30DK123704.
Abbreviations used in this paper:
- CE
capsule endoscopy
- CI
confidence interval
- EGD
esophagogastroduodenoscopy
- FOBT
fecal occult blood test
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- IDA
iron deficiency anemia
- PICO
population, intervention, comparator, outcome
- RCT
randomized controlled trial
- TTG
tissue transglutaminase
Footnotes
Conflicts of interest
All members were required to complete a disclosure statement. These statements are maintained at the American Gastroenterological Association Institute (AGA) headquarters in Bethesda, Maryland. Technical review authors disclosed all potential conflicts of interest according to the AGA Institute policy.
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2020.06.045.
References
- 1.Looker AC, Dallman PR, Carroll MD, et al. Prevalence of iron deficiency in the United States. JAMA 1997;277:973–976. [DOI] [PubMed] [Google Scholar]
- 2.Fleming MD. The regulation of hepcidin and its effects on systemic and cellular iron metabolism. Hematology Am Soc Hematol Educ Program 2008:151–158. [DOI] [PubMed] [Google Scholar]
- 3.Ganz T, Nemeth E. Hepcidin and iron homeostasis. Biochim Biophys Acta 2012;1823:1434–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iron Camaschella C. and hepcidin: a story of recycling and balance. Hematology Am Soc Hematol Educ Program 2013;2013:1–8. [DOI] [PubMed] [Google Scholar]
- 5.Anderson GJ, Frazer DM. Current understanding of iron homeostasis. Am J Clin Nutr 2017;106:1559S–1566S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell 2004;117:285–297. [DOI] [PubMed] [Google Scholar]
- 7.Tussing-Humphreys L, Pusatcioglu C, Nemeth E, et al. Rethinking iron regulation and assessment in iron deficiency, anemia of chronic disease, and obesity: introducing hepcidin. J Acad Nutr Diet 2012;112:391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health. J Res Med Sci 2014;19:164–174. [PMC free article] [PubMed] [Google Scholar]
- 9.Camaschella C Iron deficiency. Blood 2019;133:30–39. [DOI] [PubMed] [Google Scholar]
- 10.Schiff L, Stevens RJ, Shapiro N, et al. Observations on the oral administration of citrate blood in man. Am J Med Sci 1942;203:409. [Google Scholar]
- 11.Rockey DC. Occult gastrointestinal bleeding [see comments]. N Engl J Med 1999;341:38–46. [DOI] [PubMed] [Google Scholar]
- 12.Ahlquist DA, McGill DB, Schwartz S, et al. Fecal blood levels in health and disease. A study using HemoQuant. N Engl J Med 1985;312:1422–1428. [DOI] [PubMed] [Google Scholar]
- 13.Rockey DC. Occult and obscure gastrointestinal bleeding: causes and clinical management. Nat Rev Gastroenterol Hepatol 2010;7:265–279. [DOI] [PubMed] [Google Scholar]
- 14.Rockey DC. Occult gastrointestinal bleeding. Gastroenterol Clin North Am 2005;34:699–718. [DOI] [PubMed] [Google Scholar]
- 15.Rockey DC, Cello JP. Evaluation of the gastrointestinal tract in patients with iron-deficiency anemia. N Engl J Med 1993;329:1691–1695. [DOI] [PubMed] [Google Scholar]
- 16.Bini EJ, Micale PL, Weinshel EH. Evaluation of the gastrointestinal tract in premenopausal women with iron deficiency anemia [see comments]. Am J Med 1998;105:281–286. [DOI] [PubMed] [Google Scholar]
- 17.Kepczyk T, Cremins JE, Long BD, et al. A prospective, multidisciplinary evaluation of premenopausal women with iron-deficiency anemia [see comments]. Am J Gastroenterol 1999;94:109–115. [DOI] [PubMed] [Google Scholar]
- 18.Green BT, Rockey DC. Gastrointestinal endoscopic evaluation of premenopausal women with iron deficiency anemia. J Clin Gastroenterol 2004;38:104–109. [DOI] [PubMed] [Google Scholar]
- 19.Fireman Z, Zachlka R, Abu Mouch S, et al. The role of endoscopy in the evaluation of iron deficiency anemia in premenopausal women. Isr Med Assoc J 2006;8:88–90. [PubMed] [Google Scholar]
- 20.Majid S, Salih M, Wasaya R, et al. Predictors of gastrointestinal lesions on endoscopy in iron deficiency anemia without gastrointestinal symptoms. BMC Gastroenterol 2008;8:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cilona A, Zullo A, Hassan C, et al. Is faecal-immunochemical test useful in patients with iron deficiency anaemia and without overt bleeding? Dig Liver Dis 2011;43:1022–1024. [DOI] [PubMed] [Google Scholar]
- 22.Ayling RM, Lewis SJ, Cotter F. Potential roles of artificial intelligence learning and faecal immunochemical testing for prioritisation of colonoscopy in anaemia. Br J Haematol 2019;185:311–316. [DOI] [PubMed] [Google Scholar]
- 23.Lee MW, Pourmorady JS, Laine L. Use of fecal occult blood testing as a diagnostic tool for clinical indications: a systematic review and meta-analysis. Am J Gastroenterol 2020;115:662–670. [DOI] [PubMed] [Google Scholar]
- 24.Rockey DC, Hafemeister AC, Reisch JS. Acute on chronic gastrointestinal bleeding: a unique clinical entity. J Investig Med 2017;65:892–898. [DOI] [PubMed] [Google Scholar]
- 25.Guyatt GH, Oxman AD, Schunemann HJ, et al. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 2011;64:380–382. [DOI] [PubMed] [Google Scholar]
- 26.Ko CW, Siddique SM, Patel A, et al. AGA Clinical Practice Guidelines on the Gastrointestinal Evaluation of Iron Deficiency Anemia. Gastroenterology 2020;159:1085–1094. [DOI] [PubMed] [Google Scholar]
- 27.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-Analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 29.Singh P, Arora A, Strand TA, et al. Global prevalence of celiac disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2018;16:823–836.e2. [DOI] [PubMed] [Google Scholar]
- 30.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan—a web and mobile app for systematic reviews. Syst Rev 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 32.Barendregt JJ, Doi SA, Lee YY, et al. Meta-analysis of prevalence. J Epidemiol Community Health 2013;67:974–978. [DOI] [PubMed] [Google Scholar]
- 33.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. CMAJ 2007;176. 1091–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2018. [Google Scholar]
- 36.Schwarzer G meta: {A}n {R} package for meta-analysis. R News 2007;7:40–45. [Google Scholar]
- 37.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. Volume 2018. Ottawa, Canda: Department of Epidemiology and Community Medicine, University of Ottawa, 2011. [Google Scholar]
- 39.Munn Z, Moola S, Lisy K, et al. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc 2015;13:147–153. [DOI] [PubMed] [Google Scholar]
- 40.World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization, 2011. (WHO/NMH/NHD/MNM/11.1). Available at: http://www.who.int/vmnis/indicators/haemoglobin.pdf. Accessed July 27, 2020. [Google Scholar]
- 41.Guyatt GH, Oxman AD, Ali M, et al. Laboratory diagnosis of iron-deficiency anemia: an overview. J Gen Intern Med 1992;7:145–153. [DOI] [PubMed] [Google Scholar]
- 42.Ioannou GN, Rockey DC, Bryson CL, et al. Iron deficiency and gastrointestinal malignancy: a population-based cohort study. Am J Med 2002;113:276–280. [DOI] [PubMed] [Google Scholar]
- 43.Beard JL, Murray-Kolb LE, Rosales FJ, et al. Interpretation of serum ferritin concentrations as indicators of total-body iron stores in survey populations: the role of biomarkers for the acute phase response. Am J Clin Nutr 2006;84:1498–1505. [DOI] [PubMed] [Google Scholar]
- 44.Dignass AU, Gasche C, Bettenworth D, et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis 2015;9:211–222. [DOI] [PubMed] [Google Scholar]
- 45.Fitzpatrick-Lewis D, Ali MU, Warren R, et al. Screening for colorectal cancer: a systematic review and meta-analysis. Clin Colorectal Cancer 2016;15:298–313. [DOI] [PubMed] [Google Scholar]
- 46.Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ 2014;348:g2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hardwick RH, Armstrong CP. Synchronous upper and lower gastrointestinal endoscopy is an effective method of investigating iron-deficiency anaemia. Br J Surg 1997;84:1725–1728. [PubMed] [Google Scholar]
- 48.Till SH, Grundman MJ. Prevalence of concomitant disease in patients with iron deficiency anaemia. BMJ 1997;314:206–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nahon S, Lahmek P, Lesgourgues B, et al. Predictive factors of GI lesions in 241 women with iron deficiency anemia. Am J Gastroenterol 2002;97:590–593. [DOI] [PubMed] [Google Scholar]
- 50.Annibale B, Lahner E, Chistolini A, et al. Endoscopic evaluation of the upper gastrointestinal tract is worthwhile in premenopausal women with iron-deficiency anaemia irrespective of menstrual flow. Scand J Gastroenterol 2003;38:239–245. [DOI] [PubMed] [Google Scholar]
- 51.Capurso G, Baccini F, Osborn J, et al. Can patient characteristics predict the outcome of endoscopic evaluation of iron deficiency anemia: a multiple logistic regression analysis. Gastrointest Endosc 2004;59:766–771. [DOI] [PubMed] [Google Scholar]
- 52.Yates JM, Logan EC, Stewart RM. Iron deficiency anaemia in general practice: clinical outcomes over three years and factors influencing diagnostic investigations. Postgrad Med J 2004;80:405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.James MW, Chen CM, Goddard WP, et al. Risk factors for gastrointestinal malignancy in patients with iron-deficiency anaemia. Eur J Gastroenterol Hepatol 2005; 17:1197–1203. [DOI] [PubMed] [Google Scholar]
- 54.Niv E, Elis A, Zissin R, et al. Iron deficiency anemia in patients without gastrointestinal symptoms—a prospective study. Fam Pract 2005;22:58–61. [DOI] [PubMed] [Google Scholar]
- 55.Ho CH, Chau WK, Hsu HC, et al. Predictive risk factors and prevalence of malignancy in patients with iron deficiency anemia in Taiwan. Am J Hematol 2005;78:108–112. [DOI] [PubMed] [Google Scholar]
- 56.Stephens MR, Hopper AN, White SR, et al. Colonoscopy first for iron-deficiency anaemia: a numbers needed to investigate approach. QJM 2006;99:389–395. [DOI] [PubMed] [Google Scholar]
- 57.Park DI, Ryu SH, Oh SJ, et al. Significance of endoscopy in asymptomatic premenopausal women with iron deficiency anemia. Dig Dis Sci 2006;51:2372–2376. [DOI] [PubMed] [Google Scholar]
- 58.Vannella L, Aloe Spiriti MA, Cozza G, et al. Benefit of concomitant gastrointestinal and gynaecological evaluation in premenopausal women with iron deficiency anaemia. Aliment Pharmacol Ther 2008;28:422–430. [DOI] [PubMed] [Google Scholar]
- 59.Carter D, Maor Y, Bar-Meir S, et al. Prevalence and predictive signs for gastrointestinal lesions in premenopausal women with iron deficiency anemia. Dig Dis Sci 2008;53:3138–3144. [DOI] [PubMed] [Google Scholar]
- 60.Landy J, Macfarlane B. Synchronous bidirectional endoscopy for iron deficiency anaemia: is it appropriate for patients under 50? Postgrad Med J 2010;86:338–340. [DOI] [PubMed] [Google Scholar]
- 61.Yun GW, Yang YJ, Song IC, et al. A prospective evaluation of adult men with iron-deficiency anemia in Korea. Intern Med 2011;50:1371–1375. [DOI] [PubMed] [Google Scholar]
- 62.Droogendijk J, Beukers R, Berendes PB, et al. Screening for gastrointestinal malignancy in patients with iron deficiency anemia by general practitioners: an observational study. Scand J Gastroenterol 2011;46:1105–1110. [DOI] [PubMed] [Google Scholar]
- 63.Carter D, Levi G, Tzur D, et al. Prevalence and predictive factors for gastrointestinal pathology in young men evaluated for iron deficiency anemia. Dig Dis Sci 2013;58:1299–1305. [DOI] [PubMed] [Google Scholar]
- 64.Carter D, Bardan E, Derazne E, et al. The incidence of gastrointestinal pathology and subsequent anemia in young men presenting with iron deficiency without anemia. Eur J Gastroenterol Hepatol 2016;28:1126–1129. [DOI] [PubMed] [Google Scholar]
- 65.Bosch X, Montori E, Guerra-Garcia M, et al. A comprehensive evaluation of the gastrointestinal tract in iron-deficiency anemia with predefined hemoglobin below 9mg/dL: a prospective cohort study. Dig Liver Dis 2017;49:417–426. [DOI] [PubMed] [Google Scholar]
- 66.Kim NH, Park JH, Park DI, et al. Should asymptomatic young men with iron deficiency anemia necessarily undergo endoscopy? Korean J Intern Med 2018;33:1084–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Day LW, Kwon A, Inadomi JM, et al. Adverse events in older patients undergoing colonoscopy: a systematic review and meta-analysis. Gastrointest Endosc 2011;74:885–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang L, Mannalithara A, Singh G, et al. Low rates of gastrointestinal and non-gastrointestinal complications for screening or surveillance colonoscopies in a population-based study. Gastroenterology 2018;154:540–555.e8. [DOI] [PubMed] [Google Scholar]
- 69.Quine MA, Bell GD, McCloy RF, et al. Prospective audit of perforation rates following upper gastrointestinal endoscopy in two regions of England. Br J Surg 1995;82:530–533. [DOI] [PubMed] [Google Scholar]
- 70.Merchea A, Cullinane DC, Sawyer MD, et al. Esophagogastroduodenoscopy-associated gastrointestinal perforations: a single-center experience. Surgery 2010;148:876–880; discussion 881–882. [DOI] [PubMed] [Google Scholar]
- 71.Sieg A, Hachmoeller-Eisenbach U, Eisenbach T. Prospective evaluation of complications in outpatient GI endoscopy: a survey among German gastroenterologists. Gastrointest Endosc 2001;53:620–627. [DOI] [PubMed] [Google Scholar]
- 72.Keren D, Rainis T, Stermer E, et al. A nine-year audit of open-access upper gastrointestinal endoscopic procedures: results and experience of a single centre. Can J Gastroenterol 2011;25:83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Noone A, Howlader N, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2015. Bethesda, MD: National Cancer Institute. Available at: https://seer.cancer.gov/archive/csr/1975_2015/. Updated September 10, 2018. Accessed July 27, 2020. [Google Scholar]
- 74.US Census Bureau Population Division. Annual Estimates of the Resident Population by Single Year of Age and Sex for the United States: April 1, 2010 to July 1, 2018 (NC-EST2018-AGESEX-RES). Washington, DC: US Census Bureau, 2018. [Google Scholar]
- 75.McIntyre AS, Long RG. Prospective survey of investigations in outpatients referred with iron deficiency anaemia. Gut 1993;34:1102–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kepczyk T, Kadakia SC. Prospective evaluation of gastrointestinal tract in patients with iron-deficiency anemia. Dig Dis Sci 1995;40:1283–1289. [DOI] [PubMed] [Google Scholar]
- 77.Hudak L, Jaraisy A, Haj S, et al. An updated systematic review and meta-analysis on the association between Helicobacter pylori infection and iron deficiency anemia. Helicobacter 2017;22(1). [DOI] [PubMed] [Google Scholar]
- 78.Goddard AF, McIntyre AS, Scott BB. Guidelines for the management of iron deficiency anaemia. British Society of Gastroenterology. Gut 2000;46(Suppl 3–4): IV1–IV5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen LH, Luo HS. Effects of H pylori therapy on erythrocytic and iron parameters in iron deficiency anemia patients with H pylori-positive chronic gastristis. World J Gastroenterol 2007;13:5380–5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Choe Y, Kim S, Son B, et al. Randomized placebo-controlled trial of Helicobacter pylori eradication for iron-deficiency anemia in preadolescent children and adolescents. Helicobacter 1999;4:135–139. [DOI] [PubMed] [Google Scholar]
- 81.Vijayan G, Sundaram RC, Bobby Z, et al. Increased plasma malondialdehyde and fructosamine in anemic H pylori infected patients: effect of treatment. World J Gastroenterol 2007;13:796–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology 2017;153:420–429. [DOI] [PubMed] [Google Scholar]
- 83.Best LM, Takwoingi Y, Siddique S, et al. Non-invasive diagnostic tests for Helicobacter pylori infection. Cochrane Database Syst Rev 2018;3:CD012080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ferwana M, Abdulmajeed I, Alhajiahmed A, et al. Accuracy of urea breath test in Helicobacter pylori infection: meta-analysis. World J Gastroenterol 2015;21:1305–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee YC, Chiang TH, Chou CK, et al. Association between Helicobacter pylori eradication and gastric cancer incidence: a systematic review and meta-analysis. Gastroenterology 2016;150:1113–1124.e5. [DOI] [PubMed] [Google Scholar]
- 86.Seta T, Takahashi Y, Noguchi Y, et al. Effectiveness of Helicobacter pylori eradication in the prevention of primary gastric cancer in healthy asymptomatic people: a systematic review and meta-analysis comparing risk ratio with risk difference. PLoS One 2017;12:e0183321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Song M, Latorre G, Ivanovic-Zuvic D, et al. Autoimmune diseases and gastric cancer risk: a systematic review and meta-analysis. Cancer Res Treat 2019;51:841–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vannella L, Lahner E, Osborn J, et al. Systematic review: gastric cancer incidence in pernicious anaemia. Aliment Pharmacol Ther 2013;37:375–382. [DOI] [PubMed] [Google Scholar]
- 89.Pimentel-Nunes P, Libanio D, Marcos-Pinto R, et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 2019;51:365–388. [DOI] [PubMed] [Google Scholar]
- 90.Annibale B, Capurso G, Chistolini A, et al. Gastrointestinal causes of refractory iron deficiency anemia in patients without gastrointestinal symptoms. Am J Med 2001;111:439–445. [DOI] [PubMed] [Google Scholar]
- 91.Dickey W, Kenny BD, McMillan SA, et al. Gastric as well as duodenal biopsies may be useful in the investigation of iron deficiency anaemia. Scand J Gastroenterol 1997;32:469–472. [DOI] [PubMed] [Google Scholar]
- 92.Hershko C, Hoffbrand AV, Keret D, et al. Role of autoimmune gastritis, Helicobacter pylori and celiac disease in refractory or unexplained iron deficiency anemia. Haematologica 2005;90:585–595. [PubMed] [Google Scholar]
- 93.Kaye PV, Garsed K, Ragunath K, et al. The clinical utility and diagnostic yield of routine gastric biopsies in the investigation of iron deficiency anemia: a case-control study. Am J Gastroenterol 2008;103:2883–2889. [DOI] [PubMed] [Google Scholar]
- 94.Marignani M, Delle Fave G, Mecarocci S, et al. High prevalence of atrophic body gastritis in patients with unexplained microcytic and macrocytic anemia: a prospective screening study. Am J Gastroenterol 1999;94:766–772. [DOI] [PubMed] [Google Scholar]
- 95.Mant MJ, Bain VG, Maguire CG, et al. Prevalence of occult gastrointestinal bleeding in celiac disease. Clin Gastroenterol Hepatol 2006;4:451–454. [DOI] [PubMed] [Google Scholar]
- 96.Rubio-Tapia A, Hill ID, Kelly CP, et al. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol 2013;108:656–676; quiz 677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Harewood GC, Holub JL, Lieberman DA. Variation in small bowel biopsy performance among diverse endoscopy settings: results from a national endoscopic database. Am J Gastroenterol 2004;99:1790–1794. [DOI] [PubMed] [Google Scholar]
- 98.Lebwohl B, Tennyson CA, Holub JL, et al. Sex and racial disparities in duodenal biopsy to evaluate for celiac disease. Gastrointest Endosc 2012;76:779–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Abdalla A, Saifullah SM, Osman M, et al. Prevalence of occult celiac disease in females with iron deficiency in the United States: an NHANES analysis. J Community Hosp Intern Med Perspect 2017;7:347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carmack SW, Genta RM. The diagnostic value of the duodenal biopsy: a clinico-pathologic analysis of 28,000 patients. Dig Liver Dis 2010;42:485–489. [DOI] [PubMed] [Google Scholar]
- 101.Gordon SR, Smith RE, Power GC. The role of endoscopy in the evaluation of iron deficiency anemia in patients over the age of 50. Am J Gastroenterol 1994;89:1963–1967. [PubMed] [Google Scholar]
- 102.Grisolano SW, Oxentenko AS, Murray JA, et al. The usefulness of routine small bowel biopsies in evaluation of iron deficiency anemia. J Clin Gastroenterol 2004;38:756–760. [DOI] [PubMed] [Google Scholar]
- 103.Karnam US, Felder LR, Raskin JB. Prevalence of occult celiac disease in patients with iron-deficiency anemia: a prospective study. South Med J 2004;97:30–34. [DOI] [PubMed] [Google Scholar]
- 104.Murray JA, McLachlan S, Adams PC, et al. Association between celiac disease and iron deficiency in Caucasians, but not non-Caucasians. Clin Gastroenterol Hepatol 2013;11:808–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pitman M, Sanders DS, Green PHR, et al. Rates of duodenal biopsy during upper endoscopy differ widely between providers: implications for diagnosis of celiac disease. J Clin Gastroenterol 2019;53:e61–e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Maglione MA, Okunogbe A, Ewing B, et al. Diagnosis of Celiac Disease. Rockville, MD: Agency for Healthcare Research and Quality, 2016. [PubMed] [Google Scholar]
- 107.Choung RS, Khaleghi S, Cartee AK, et al. Community-based study of celiac disease autoimmunity progression in adults. Gastroenterology 2020;158:151–159 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Apostolopoulos P, Liatsos C, Gralnek IM, et al. The role of wireless capsule endoscopy in investigating unexplained iron deficiency anemia after negative endoscopic evaluation of the upper and lower gastrointestinal tract. Endoscopy 2006;38:1127–1132. [DOI] [PubMed] [Google Scholar]
- 109.Goenka MK, Majumder S, Kumar S, et al. Single center experience of capsule endoscopy in patients with obscure gastrointestinal bleeding. World J Gastroenterol 2011;17:774–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Holleran GE, Barry SA, Thornton OJ, et al. The use of small bowel capsule endoscopy in iron deficiency anaemia: low impact on outcome in the medium term despite high diagnostic yield. Eur J Gastroenterol Hepatol 2013;25:327–332. [DOI] [PubMed] [Google Scholar]
- 111.Kim S, Kedia PS, Jaffe DL, et al. Impact of capsule endoscopy findings on patient outcomes. Dig Dis Sci 2009;54:2441–2448. [DOI] [PubMed] [Google Scholar]
- 112.Koulaouzidis A, Yung DE, Lam JH, et al. The use of small-bowel capsule endoscopy in iron-deficiency anemia alone; be aware of the young anemic patient. Scand J Gastroenterol 2012;47:1094–1100. [DOI] [PubMed] [Google Scholar]
- 113.Kunihara S, Oka S, Tanaka S, et al. Management of occult obscure gastrointestinal bleeding patients based on long-term outcomes. Therap Adv Gastroenterol 2018; 11:1756284818787408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Laine L, Sahota A, Shah A. Does capsule endoscopy improve outcomes in obscure gastrointestinal bleeding? Randomized trial versus dedicated small bowel radiography. Gastroenterology 2010;138:1673–1680.e1; quiz e11–e12. [DOI] [PubMed] [Google Scholar]
- 115.Milano A, Balatsinou C, Filippone A, et al. A prospective evaluation of iron deficiency anemia in the GI endoscopy setting: role of standard endoscopy, videocapsule endoscopy, and CT-enteroclysis. Gastrointest Endosc 2011;73:1002–1008. [DOI] [PubMed] [Google Scholar]
- 116.Muhammad A, Pitchumoni CS. Evaluation of iron deficiency anemia in older adults: the role of wireless capsule endoscopy. J Clin Gastroenterol 2009;43:627–631. [DOI] [PubMed] [Google Scholar]
- 117.Olano C, Pazos X, Avendano K, et al. Diagnostic yield and predictive factors of findings in small-bowel capsule endoscopy in the setting of iron-deficiency anemia. Endosc Int Open 2018;6:E688–E693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Riccioni ME, Urgesi R, Spada C, et al. Unexplained iron deficiency anaemia: is it worthwhile to perform capsule endoscopy? Dig Liver Dis 2010;42:560–566. [DOI] [PubMed] [Google Scholar]
- 119.Sidhu PS, McAlindon ME, Drew K, et al. The utility of capsule endoscopy in patients under 50 years of age with recurrent iron deficiency anaemia: is the juice worth the squeeze? Gastroenterol Res Pract 2015;2015:948574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tong J, Svarta S, Ou G, et al. Diagnostic yield of capsule endoscopy in the setting of iron deficiency anemia without evidence of gastrointestinal bleeding. Can J Gastroenterol 2012;26:687–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Van Tuyl SA, Van Noorden JT, Kuipers EJ, et al. Results of videocapsule endoscopy in 250 patients with suspected small bowel pathology. Dig Dis Sci 2006;51:900–905. [DOI] [PubMed] [Google Scholar]
- 122.Yung DE, Rondonotti E, Giannakou A, et al. Capsule endoscopy in young patients with iron deficiency anaemia and negative bidirectional gastrointestinal endoscopy. United European Gastroenterol J 2017;5:974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sealock RJ, Thrift AP, El-Serag HB, et al. Long-term follow up of patients with obscure gastrointestinal bleeding examined with video capsule endoscopy. Medicine (Baltimore) 2018;97:e11429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dickey S, Rockey DC. The natural history of iron deficiency anemia. Am J Med Sci 2019;358:357–362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


