Abstract
The ability of butyric acid, an extracellular metabolite from periodontopathic bacteria, to induce apoptosis in murine WEHI 231 cells, splenic B cells, and human RAJI cells was examined. The culture filtrate of Porphyromonas gingivalis, Prevotella loescheii, and Fusobacterium nucleatum, which contains high a percentage of butyric acid, induced DNA fragmentation in WEHI 231 cells. Volatile fatty acid, especially butyric acid, significantly suppressed B-cell viability in a concentration-dependent fashion. The DNA fragmentation assay indicated that butyric acid rapidly induced apoptosis in WEHI 231 cells (with 1.25 mM butyric acid and 6 h after treatment), splenic B cells (with 1.25 mM butyric acid), and RAJI cells (with 2.5 mM butyric acid). Incubation of WEHI 231 cells with butyric acid for 16 h resulted in the typical ladder pattern of DNA fragmentation and the apoptoic change such as chromatin condensation and hypodiploid nuclei. Cell cycle analysis implied that butyric acid arrested the cells at the G1 phase. The inhibitory assay suggested that butyric acid-induced apoptosis of WEHI 231 and splenic B cells was inhibited by W-7, a calmodulin inhibitor. These results suggest that calmodulin-dependent regulation is involved in the signal transduction pathway of butyric acid.
It is recognized that periodontal diseases are infectious and that periodontal tissue breakdown results from the interaction of specific anaerobic bacteria and host immune mechanisms. A recent study indicates that severe destructive adult periodontitis is a multibacterial infection and that certain combinations of periodontopathogens, namely, Porphyromonas, Prevotella, and Fusobacterium spp., seem to be important in the pathogenesis of the disease (43). These bacteria produce an elaborate variety of virulence factors such as proteases, lipopolysaccharides, and fimbriae (42).
The metabolism of each of these bacteria is also characterized by the production of an identifiable pattern of short-chain fatty acids, which are major by-products of anaerobic metabolism that are released into the microenvironment at the infection site (18) and can diffuse across biological membranes (40). Previous studies have demonstrated that these fatty acids exert inhibitory effects on gingival fibroblast proliferation (41), colon cancer cell growth (15), and phagocytosis (12, 37). Our previous study (23) demonstrated that short-chain fatty acids, especially volatile fatty acids present in the culture filtrates of Porphyromonas gingivalis, Prevotella loescheii, and Fusobacterium nucleatum, greatly inhibited murine T- and B-cell proliferation and cytokine production by concanavalin A-stimulated splenic T cells. Furthermore, we found that a representative volatile fatty acid, butyric acid, induced cytotoxicity and apoptosis in murine thymocytes, splenic T cells, and human Jurkat T cells (24).
Apoptosis is an active process controlled by intracellular regulatory systems. For example, it is suggested that in several apoptotic systems, intracellular signal-transducing systems, e.g., protein phosphorylation and Ca2+ signaling, are involved in the control of the induction of DNA fragmentation (16, 39). In this study, we report that culture filtrates of periodontopathic bacteria and butyric acid present in the filtrate induce apoptosis in murine WEHI 231 cells, splenic B cells, and human RAJI cells. To understand better the intracellular mechanism of the DNA fragmentation in this system, we examined the effects of several kinds of specific modulators of cell functions on DNA fragmentation.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
P. gingivalis W83 and ATCC 33277, P. loescheii ATCC 15930, Prevotella intermedia ATCC 25261 and 25611, F. nucleatum JMC 8532 and ATCC 23726, Actinobacillus actinomycetemcomitans Y4, and Capnocytophaga ochracea ATCC 33596 were used in this study. P. gingivalis W83 was kindly provided by K. Okuda, Tokyo Dental College, Tokyo, Japan. P. gingivalis, P. loescheii, and P. intermedia were separately grown in brain heart infusion (BHI) broth (Difco Laboratories, Detroit, Mich.) supplemented with 5% bovine serum, 5 μg of hemin per ml, and 0.4 μg of menadion per ml in a model 1024 anaerobic system (Forma Scientific, Marietta, Ohio) for 2 days. A. actinomycetemcomitans, C. ochracea, and F. nucleatum were grown in BHI broth supplemented with 5% bovine serum, at 37°C for 2 days in a 5% CO2 atmosphere.
Preparation of bacterial culture filtrates.
The cultures were incubated for 2 days and centrifuged at 10,000 × g for 20 min at 4°C. The pH of the 48-h P. gingivalis, P. loescheii, P. intermedia, and F. nucleatum spent media ranged from 6.5 to 6.8, while the pH of A. actinomycetemcomitans and C. ochracea spent media ranged from 6.0 to 6.3. The supernatant fluid was removed, adjusted to pH 7.0, and sterilized by filtration through a 0.22-μm-pore-size membrane filter (Millipore Corp., Bedford, Mass.). A pH-adjusted sterile BHI medium was used as control.
Short-chain fatty acid.
Highly purified butyric, propionic, and isovaleric acids were purchased from Sigma Chemical Co. (St. Louis, Mo.). Solutions of fatty acid ranging in concentration from 0.15 to 5 mM were diluted in RPMI 1640 (Gibco Laboratories, Grand Island, N.Y.) medium and adjusted to pH 7.2 with sodium hydroxide.
Mice.
C3H/HeN mice were obtained from Charles River Breeding Laboratories (Kanagawa, Japan). The mice were maintained in the Animal Facility of Nihon University School of Dentistry at Matsudo under standard care and given food and water ad libitum. Female and male mice were used at 9 to 10 weeks of age.
B-cell preparation.
Spleens were aseptically removed, and single-cell suspensions were prepared by gently teasing the cells through sterile stainless steel screens. Preparations of B cells from mouse spleens were obtained as described previously (23). Briefly, splenic cell suspensions were treated with a cocktail of monoclonal antibodies (rat anti-mouse Thy 1.2, anti-Lyt2, and anti-L3T4 antibodies) for 30 min at 4°C, followed by incubation with rabbit anti-rat immunoglobulin G and complement (Low Tox rabbit complement; Cedarlane Laboratories Ltd., Ontario, Canada) for 30 min at 37°C. This purified B-cell preparation contained less than 2% Thy-1+ cells, as determined by immunofluorescence with a FACScan fluorescence-activated cell sorter (Becton Dickinson and Co., Sunnyvale, Calif.). The B lymphoma cell lines WEHI 231 (mouse) and RAJI (human) were obtained from Japan Cancer Research Resources Bank. These cells were cultured at 37°C in a moist atmosphere of 5% CO2 in complete medium consisting of RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, 100 U of penicillin per ml, 100 μl of streptomycin per ml, and 0.05 mM 2-mercaptoethanol.
Cell proliferation assay.
As a method of assessing cellular proliferation following the addition of fatty acid, the colorimetric MTT [3-(4,5-dimethyl-2-thiazoyl)-2,5-diphenyl tetrazolium bromide; Sigma) assay was performed (21). In viable cells, the mitochondrial enzyme succinate dehydrogenase can metabolize MTT into a formazan dye that absorbs light at 550 nm. WEHI 231 and RAJI cells were seeded at a density of 2.0 × 105 cells per well in 0.1 ml of complete medium in flat-bottom 96-well plates. Butyric, propionic, and isovaleric acids in RPMI 1640 were added to a final concentration of 0.15 to 5 mM, and each concentration of fatty acid was tested in quadruplicate. After incubation for 42 h, 20 μl of MTT (5 mg/ml in phosphate-buffered saline [pH 7.2]) was added to each well. Following 6 h of incubation, the supernatants were decanted, and the formazan precipitates were solubilized by the addition of 150 μl of 100% dimethyl sulfoxide (Sigma) and placed on a plate shaker for 10 min. Absorbance at 550 nm was determined on a Corona MT32 spectrophotomeric microplate reader (Corona Electric Co., Ibaraki, Japan). The absorbance of the untreated cultures was set at 100%. The mean relative absorbance and the standard error of the mean (SE) were calculated for every concentration of fatty acid tested.
B-cell culture for apoptosis.
B cells were suspended in complete medium. Cells (4.0 × 106 per well for splenic B cells and 106 per well for WEHI 231 cells and RAJI cells) were cultured in 1 ml of medium in 24-well tissue culture plates (Falcon; Becton Dickinson Labware, Lincoln Park, N.J.) in the presence or absence of various concentrations of butyric acid or 100 μl of culture filtrates. At the times indicated in the figures, cells were harvested and centrifuged at 400 × g for 5 min and washed twice with ice-cold phosphate-buffered saline. Cells were resuspended in 400 μl of hypotonic lysis buffer (0.2% Triton X-100, 10 mM Tris, 1 mM EDTA [pH 8.0]) and centrifuged for 15 min at 13,800 × g (31). Half of the supernatant, containing small DNA fragments, was subjected to gel electrophoresis, while the other half, as well as the pellet containing large pieces of DNA and cell debris, was used for the diphenylamine (DPA) assay (see below).
Gel electrophoresis.
One-half of the supernatants was treated with an equal volume of absolute isopropyl alcohol and 0.5 M NaCl to precipitate the DNA and stored at −20°C overnight. After centrifugation at 13,800 × g for 15 min, the pellet was washed with 200 μl of 70% ethanol and allowed to dry at room temperature. The DNA was resuspended in 12 μl of TE solution (10 mM Tris-HCl, 1 mM EDTA [pH 7.4]–3 μl of loading buffer (50% glycerol, 1× Tris-acetate-EDTA, 10% saturated bromophenol blue, 1% xylene cyanol) at 37°C for 20 min and then electrophoresed on a 1.7% agarose gel containing 0.71 μg of ethidium bromide per ml for 1 h. Gels were photographed by using UV transillumination.
DNA fragmentation assay.
The DPA reaction was performed by the method of Paradones et al. (33). Perchloric acid (0.5 M) was added to the pellets containing uncut DNA (resuspended with 200 μl of hypotonic lysis buffer) and to the other half of the supernatants containing DNA fragments, and then 2 volumes of a solution containing 0.088 M DPA, 98% (vol/vol) glacial acetic acid, 1.5% (vol/vol) sulfuric acid, and 0.5% (vol/vol) 1.6% acetaldehyde solution was added. The samples were stored at 4°C for 48 h. The colorimetric reaction was quantitated spectrophotometrically at 575 nm, using a model UV-160A UV spectrophotometer (Shimazu Co. Ltd., Tokyo, Japan). The percentage of fragmentation was calculated as the ratio of DNA in the supernatants to the total DNA.
Flow cytometric analysis.
Nuclear DNA content was analyzed by flow cytometry (Becton Dickinson, Pont de Claix, France) after propidium iodide staining by the method described by Nicoletti et al. (32). Cells were pelleted, resuspended in hypotonic fluorochrome solution (50 μg of propidium iodide per ml in 0.1% sodium citrate–0.1% Triton X-100), and kept at 4°C in the dark overnight before the analysis.
Detection of morphological apoptosis.
After treatment with reagents, cells were fixed with 2% glutaraldehyde solution (TA AB Laboratory, Aldermastone, England) for 1 h and stained with 0.2 mM Hoechst 33258 to visualize the location of DNA. Cells were examined with a fluorescence microscope (BHT-RFC; Olympus, Tokyo, Japan) for determination of fragmentation of nuclei and/or condensation of chromatin.
Reagents.
Hoechst 33258 [2′-(4-hydroxyphenyl)-5-(4-methyl-1-piperazinyl)-2,5′-bi-1H-benzimidazole], saturosporine, and EGTA were purchased from Sigma. H-7 [1-(5-isoquinolinesulfonyl)-2-methylpiperazine dihydrochloride] and HA1004 (5-isoquinolinesulfonamide dihydrochloride) were from Seikagaku Kogyo (Tokyo, Japan). Genistein (4′,5,7-trihydroxyisoflavone) and herbimycin A were from Wako Pure Chemical Co. (Osaka, Japan). W-7 [N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide-HCl] was from Funakoshi Co. (Tokyo, Japan).
Statistics.
The significance of differences between groups was determined by Student’s t test.
RESULTS
Effect of culture filtrates on DNA fragmentation.
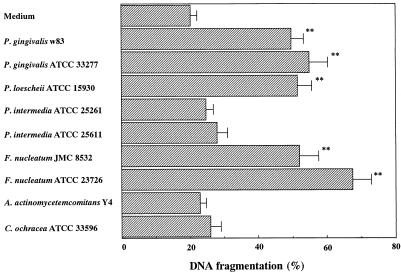
After a 48-h growth period, the pH of P. gingivalis, P. loescheii, P. intermedia, and F. nucleatum spent media regularly ranged from 6.5 to 6.8, while the pH of A. actinomycetemcomitans and C. ochracea spent media regularly ranged from 6.0 to 6.3. The induction of apoptosis by culture filtrates was indicated by the colorimetric DNA fragmentation assay. The spent media of P. gingivalis, P. loescheii, and F. nucleatum significantly increased the amount of DNA fragmentation compared with the control media, BHI, for the bacterial cultures in WEHI 231 cells (P < 0.01; Fig. 1). On the other hand, the supernatants from P. intermedia, A. actinomycetemcomitans, and C. ochracea did not affect DNA fragmentation. When we tested various dilutions of the bacterial culture filtrate, it was possible to induce apoptosis if the control levels were reduced to near zero with lower concentrations of the filtrate of P. gingivalis, P. loescheii, and F. nucleatum. The level of spontaneous apoptosis in WEHI 231 cells which are cultured only in complete medium for 21 h was 9.8% ± 1.0%. Although in our study a sterile BHI medium, after 21 h of culture, induced a slight apoptosis, the number of induced apoptotic cells was negligible compared to that seen after exposure of the cells to the culture filtrate of P. gingivalis, P. loescheii, and F. nucleatum. However, this result suggests that the trace amounts of apoptosis-inducing factor present in this preparation may contribute to DNA fragmentation.
FIG. 1.
Effect of spent medium from periodontopathic bacteria on DNA fragmentation. WEHI 231 cells were cultured for 21 h with 100 μl of bacterial spent medium. Harvested cells were assayed by the DPA assay. The results are expressed as the mean ± SE from three different experiments with triplicate cultures. The level of spontaneous apoptosis in WEHI 231 cells cultured only in complete medium for 21 h was 9.8% ± 1.0%. Values significantly different from those for the controls at P < 0.01 (∗∗) are indicated.
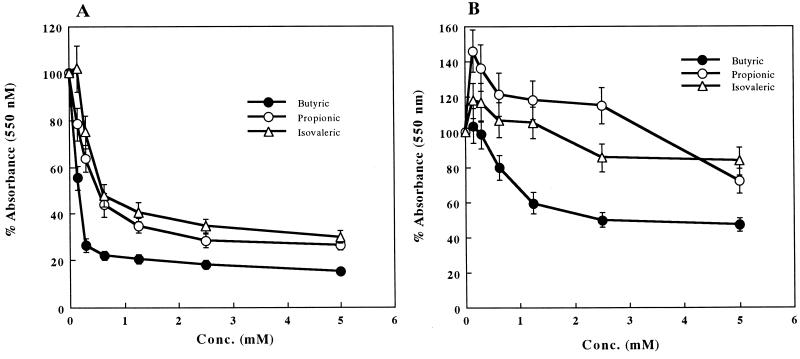
Effect of volatile fatty acids on cell proliferation.
We examined the effects of various concentrations of volatile fatty acids on the proliferative activity of mouse WEHI 231 cells and human RAJI cells. After 21 h of incubation, the volatile fatty acids caused a reduction in cell proliferative activity, as assessed by the colorimetric MTT assay. WEHI 231 cells exhibited a marked, dose-dependent response to butyric, propionic, and isovaleric acids (Fig. 2A). With 5 mM butyric, propionic, and isovaleric acids, the proliferative responses of WEHI 231 cells were significantly suppressed by 84.5, 73.5, and 69.9%, respectively. While butyric acid also exhibited a dose-dependent inhibition in RAJI cells, propionic and isovaleric acids were less effective (Fig. 2B). The decrease of both cell proliferation by butyric acid prompted us to determine the type of cell death induced by butyric acid.
FIG. 2.
Dose-dependent effects of volatile fatty acids on cell proliferation. WEHI 231 (A) and RAJI (B) cells were cultured with butyric, propionic, and isovaleric acids for 21 h. Cellular proliferation was determined by the MTT assay and expressed as percentage of the absorbance value obtained without volatile fatty acids. The results are expressed as the mean ± SE from three different experiments with triplicate cultures.
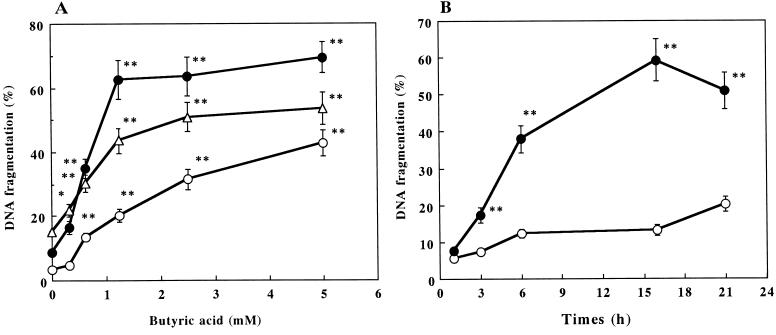
DNA fragmentation caused by butyric acid.
The induction of apoptosis by butyric acid was indicated by the colorimetric DNA fragmentation assay, electrophoresis of the fragmented DNA, nuclear morphology, and flow cytometric analysis of DNA contents. When the three types of B cells (WEHI 231, splenic B, and RAJI cells) were cultured in the presence of 0.31 to 5.0 mM butyric acid for 21 h and quantitated by the DNA fragmentation assay, a dose-dependent increase in DNA fragmentation was seen (Fig. 3A). Butyric acid induced a substantial and near-maximal (69.4% with 5 mM butyric acid) increase in DNA fragmentation (62.6%) for WEHI 231 cells at 1.25 mM (P < 0.01). For splenic B cells and RAJI cells, 2.5 mM butyric acid increased the amount of DNA fragmentation to 50.9 and 31.5%, respectively (Fig. 3A). These results indicate that different degrees of apoptosis induction by butyric acid depend on the differences in sensitivity of the cell population. In similar experiments, cells were cultured with 5 mM butyric acid and examined for DNA fragmentation at various times over a 21-h time period (Fig. 3B). Treatment of WEHI 231 cells with butyric acid for 6 h resulted in a markably increase in DNA fragmentation.
FIG. 3.
Dose-response curves and time course of butyric acid-induced apoptosis. (A) WEHI 231 (•), RAJI (○), and splenic B (▵) cells were cultured with butyric acid for 21 h. (B) WEHI 231 cells were cultured in the presence (•) or absence (○) of butyric acid (5 mM). Harvested cells were assayed by the DPA assay. The results are expressed as the mean ± SE from three different experiments with triplicate cultures. Values significantly different from those for the controls at P < 0.01 (∗∗) and P < 0.05 (∗) are indicated.
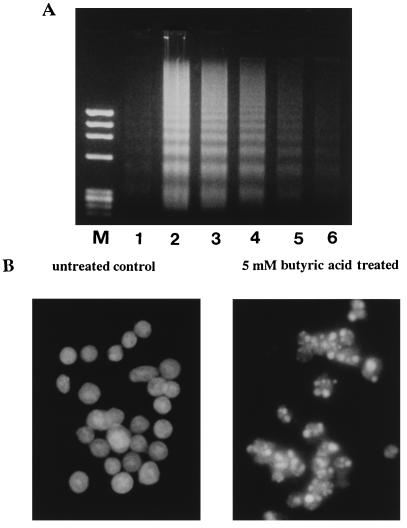
The induction of apoptosis by butyric acid in the B-cell population was further confirmed by electrophoresis of fragmented DNA (Fig. 4A). Low-molecular-weight DNA fragments extracted from WEHI 231 cells cultured with various concentrations of butyric acid for 16 h showed typical oligonucleosomal ladders in a concentration-dependent fashion (Fig. 4A). Negligible cleavage of DNA into nucleosomal fragments was seen with untreated WEHI 231 cells. When we examined the morphological changes in the nuclei of 5 mM butyric acid-treated WEHI 231 cells by DNA staining with Hoechst 33258, the characteristic features of apoptosis, including condensation and aggregation of chromatin near the nuclear membrane, were observed (Fig. 4B).
FIG. 4.
(A) Agarose gel electrophoresis of DNA extracted from WEHI 231 cells treated with butyric acid for 16 h. Lanes: M, molecular weight markers (HaeIII-digested φX174 DNA); 1, untreated control cells; 2 to 6, cells treated with 5, 2.5, 1.25, 0.62, and 0.31 mM butyric acid. (B) Fluorescence microscopy appearance of WEHI 231 cells untreated or treated with butyric acid for 16 h and stained with Hoechst 33258. Normal nuclear morphology is observed in untreated cells; in contrast, small and condensed nuclei with typical apoptotic morphology are observed in treated cells.
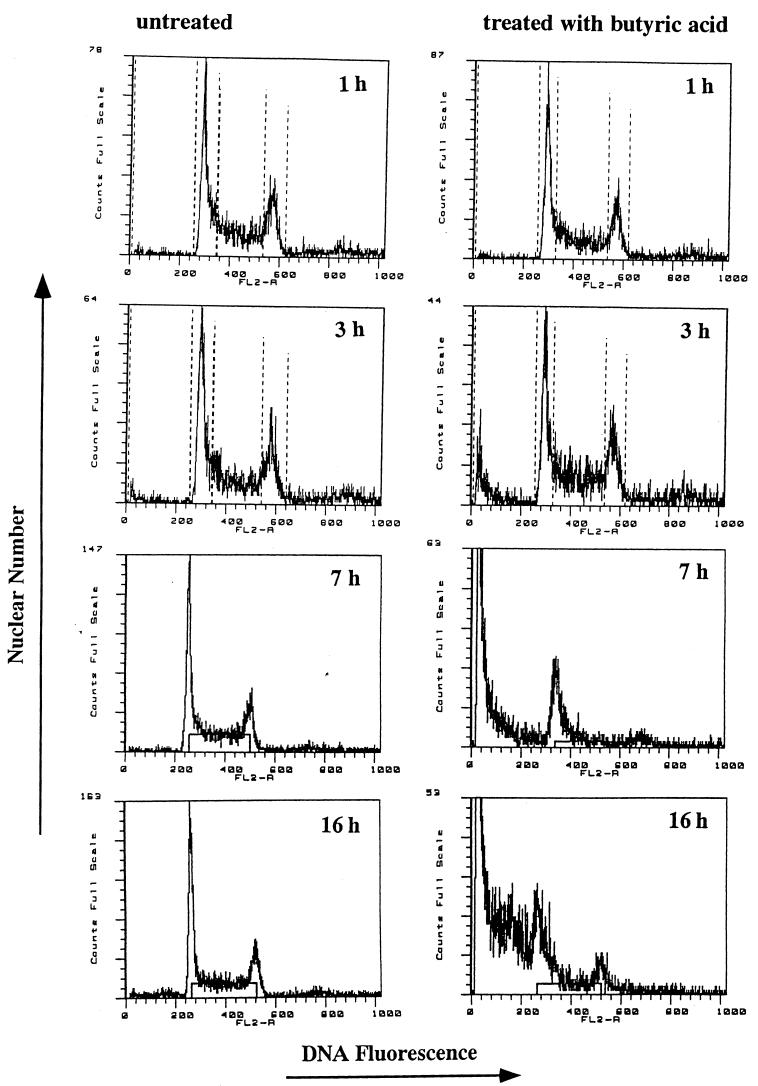
The DNA content of butyric acid-treated WEHI 231 cells, stained by propidium iodide, was also analyzed with a flow cytometer (Fig. 5). Apoptoic nuclei were distinguishable by the hypodiploid DNA contents compared with the diploid DNA contents of normal cells. A single peak of DNA, indicating diploid DNA content, characterized untreated WEHI 231 cells. In contrast, the percentage of WEHI 231 cells with hypodiploid DNA was increased by treatment with butyric acid in a time-dependent fashion. Flow cytometric analysis revealed that DNA degradation appeared to occur 3 h after butyric acid treatment.
FIG. 5.
Flow cytometric analysis of untreated (left) and 5 mM butyric acid-treated (right) WEHI 231 cells. Cells were cultured for 1, 3, 7, and 16 h. DNA content was analyzed by propidium iodine staining. Note the hypodiploid DNA peak typical of apoptosis in treated cultures.
Cell cycle distribution induced by butyric acid.
Flow cytometric analysis of WEHI 231 cells treated with butyric acid revealed that the cells were blocked at the G1/S interface of the cell cycle in time-dependent fashion (Table 1). By 7 h, control cells had progressed through the cycle to more or less the same distribution as at 1 h, whereas the butyric acid-treated cells showed both an increase in G1 and a decrease in S and G2/M cells. This arrest of the suppressed cells in vitro was found to be unresponsive to removal of butyric acid (data not shown). Furthermore, in RAJI cells, 21-h treatment with 5 mM butyric acid also caused the G1 phase arrest, after some delay compared to WEHI 231 cells (data not shown). These results suggest that butyric acid blocks transition of the cells from G1 to the S phase of the cell cycle and the thereby irreversibly halts the progression of WEHI 231 and RAJI cells to mitosis.
TABLE 1.
Effect of butyric acid on cell cycle distributiona
| Culture | Time (h) | % of cells at indicated phase of cell cycleb
|
||
|---|---|---|---|---|
| G0/G1 | S | G2/M | ||
| Untreated | 1 | 40.5 | 45.0 | 14.5 |
| 4 | 35.7 | 45.9 | 18.4 | |
| 7 | 41.1 | 42.9 | 16.0 | |
| 5 mM butyric acid treated | 1 | 38.5 | 47.8 | 17.4 |
| 4 | 41.1 | 42.2 | 16.8 | |
| 7 | 56.8 | 38.1 | 5.1 | |
WEHI 231 cells were cultured for the indicated times in the absence or presence of 5 mM butyric acid. Harvested cells were analyzed by flow cytometry.
Mean of three experiments carried out in triplicate.
Effect of modulators of intracellular signal transduction pathway.
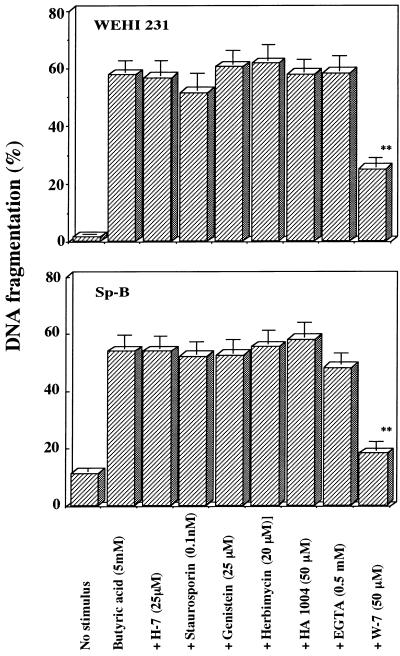
To investigate the intracellular mechanism involved in the induction of butyric acid-induced B-cell apoptosis, the involvement of known intracellular signal transduction pathways was examined by use of specific modulators of protein phosphorylation systems and Ca2+ signaling (17, 20). As shown in Fig. 6, protein kinase C inhibitors H-7 and staurosporine, tyrosine kinase inhibitors genistein and herbimycin A, protein kinase A/G inhibitor HA1004, and extracellular Ca2+ chelator EGTA showed little or no apparent effect on DNA fragmentation of butyric acid-treated WEHI 231 cells and splenic B cells. On the other hand, among the modulators of Ca2+ signaling, W-7 significantly (P < 0.01) inhibited DNA fragmentation of butyric acid-treated cells (57.8 to 25.2% for WEHI 231 cells and 53.5 to 17.9% for splenic B cells). W-7 also inhibited DNA fragmentation of human Jurkat T cells and mouse thymocytes treated with butyric acid (data not shown). Chlorpromazine, another modulator of Ca2+ signaling, also inhibited DNA fragmentation of butyric acid-treated WEHI 231 cells (data not shown). Since both W-7 and chlorpromazine have the ability to inhibit calmodulin function (17), it is suggested that calmodulin participates in the induction of butyric acid-induced apoptosis.
FIG. 6.
Effects of various inhibitors on butyric acid-induced apoptosis. WEHI 231 and splenic B cells were treated with various inhibitors in the presence of 5 mM butyric acid for 21 h. Harvested cells were assayed by the DPA assay. The results are expressed as the mean ± SE from three different experiments with triplicate cultures. Values significantly different from those for the controls at P < 0.01 (∗∗) are indicated.
DISCUSSION
The data presented here indicate that culture supernatant of P. gingivalis, P. loescheii, and F. nucleatum induced DNA fragmentation in WEHI 231 cells (Fig. 1). Since the volatile fatty acids found in the culture filtrates consisted primarily of butyric and isovaleric acids for P. gingivalis, propionic, butyric, and isovaleric acids for P. loescheii, and butyric acid for F. nucleatum, and these commercial volatile fatty acids significantly depressed mouse splenic B-cell proliferation (23), we hypothesized that volatile fatty acids in each culture filtrate have B-cell apoptosis-inducing activity. In the present study, we further demonstrated the capacity of volatile fatty acids, and butyric acid in particular, to regulate proliferation and apoptosis of B cells.
The data reported in this study indicate that different degrees of inhibition of the human and mouse B-cell proliferative responses resulted from exposure of the cells to the various volatile fatty acids (Fig. 2). Especially butyric acid, which is a virulence factor common to P. gingivalis, P. loescheii, and F. nucleatum (23), suppressed 79.3 and 40.6% of WEHI 231 cells and RAJI cell proliferation, even at a low concentration (1.25 mM). On the basis of our previous results showing that 13.3 to 26.8 mM butyric acid was detected in culture filtrates from the above three strains (23), along with a previous study showing that butyric acid concentrations in subgingival plaque from periodontitis sites could reach 14.4 to 20.0 mM (25, 30), and its concentration in periodontal pockets has been shown to correlate with the severity of periodontal disease (4), butyric acid can be recognized as an important virulent factor of these periodontopathogens. On the other hand, a very low level (0.15 mM) of propionic and isovaleric acids stimulated the proliferation of RAJI cells (Fig. 2A), and the same level of butyric acid also mediated the proliferation of human epithelial and fibroblast cell line (data not shown). Tumor necrosis factor is cytotoxic to some tumor cells but stimulates the proliferation of other cell types (2). Fas signal transduction also triggers either proliferation or apoptosis in human fibroblasts, depending the magnitude of Fas expression (1, 14). Although it is not clear on what the balance between cell proliferation and loss by death depends, our results suggest that the signal induced by butyric acid must mediate not only cytotoxic but also proliferative signals.
We have shown that in vitro, butyric acid-stimulated mouse WEHI 231 cells, splenic B cells, and human RAJI cells underwent apoptosis a specific form of programmed cell death characterized by internucleosomal DNA digestion, revealed by colorimetric DNA fragmentation assay (Fig. 3) followed by gel electrophoresis (Fig. 4A). Cell death was also associated with chromatin condensation (Fig. 4B) and flow cytometric determination of the proportion of cells with hypodiploid DNA (Fig. 5).
Flow cytometric analysis revealed that maximal DNA degradation appeared to occur 16 h after butyric acid treatment (Fig. 3B), as evidenced by the maximal appearance of DNA with low fluorescence (Fig. 5). Kinetic studies of flow cytometric cell cycle analysis of propidium iodide-stained WEHI 231 cells treated with butyric acid revealed the appearance of a “sub-G1” population below the G1 region. This extra sub-G1 peak displaying reduced fluorescence of the DNA is likely due to a reduction in cell volume and nuclear condensation characteristics of apoptoic cells (9). Cell cycle distribution also changed during incubation: after 7 h of incubation, butyric acid treatment resulted in fewer cells in the S and G2/M phases of the cell cycle, which means that butyric acid-induced inhibition of cell growth was correlated to an arrest in the G1 phase of the cell cycle (Table 1). These findings suggest that the proliferation inhibition and apoptosis induction are dependent on G1-phase accumulation. This G1-phase accumulation occurred prior to maximal DNA fragmentation observed at 16 h, as shown in Fig. 3B. Our previous study (24) showed that butyric acid arrested Jurkat cells at the G1 phase. Furthermore, similar results demonstrated that butyric acid induced a blockage in the G1 phase of the cell cycle in a human colon cancer cell line (29) and in a human myeloid leukemic cell line (6). These results suggest that G1 arrest due to butyric acid is a general property of this agent.
Eukaryotic cell cycle progression is controlled by an evolutionarily conserved mechanism, which requires the sequential activation of a series of serine/threonine protein kinases, the cyclin-dependent kinases (Cdks) (13). Cell cycle regulation by extracellular signals such as growth factors is likely to take place primarily in the G1 phase of the cell cycle, because cells become refractory to external signals once they are committed to replicate DNA (34). Several lines of evidence suggest Cdk inhibitors play an important role in growth regulation by external signals. p21CIP1/SDI1/WAF1 is involved in irradiation- and serum starvation-induced G1 arrest (11). Moreover, p27kip1 has been shown to mediate cell cycle arrest induced by cell-cell contact or transforming growth factor β in fibroblasts and epithelial cells (44) and that induced by cyclic AMP in macrophages (22). Evidence also suggests that p27kip1 is involved in the surface immunoglobulin-mediated growth arrest and in the CD40-mediated cell cycle progression of WEHI 231 cells (19). Therefore, it is possible that Cdks and Cdk inhibitors play important roles in butyric acid-induced cell proliferation and apoptosis. Further studies will be required to clarify this possibility.
Interest in the action of butyrate was greatly increased since the demonstration that the molecule exerts a potent differentiating and antiproliferative effect. The molecule affects gene expression at different levels (5), but few data are available on its intracellular targets. Recent report indicates that treatment of K562 cells with sodium butyrate increases tyrosine phosphorylation and activation of mitogen-activated protein kinase (36). Russo et al. (38) demonstrated that casein kinase II down-regulation is involved in the signal transduction pathway started by butyrate. Although some studies have shown that inhibition of DNA synthesis by butyric sodium salt is associated with a hyperacetylation of histones H3 and H4 due to an n-butyrate-induced decrease in activity of some histone deacetylase (3, 35), the mechanism of butyric acid-induced apoptosis is still unknown.
With respect to the mechanism by which butyric acid elicits apoptosis, butyric acid triggers apoptosis by means of calmodulin-dependent enzymes. Calcium and calmodulin have been implicated as participants in various apoptoic signaling pathways. Ionomycin, which allows Ca2+ entry into cells, increases apoptosis in phorbol ester-treated T cells (28), and glucocorticoid-induced T-cell apoptosis is associated with calmodulin mRNA induction (10, 39). Although there is some evidence that in some cells apoptoic degradation of DNA may occur via a non-calcium-dependent endonuclease (26), the characteristic DNA fragmentation process frequency depends on activation of a Ca2+-dependent endogenous endonuclease (7), and calmodulin inhibitors can reduce the activity of this Ca2+-dependent endonuclease (27). Recently, Cohen et al. (8) identified a novel cytoskeleton-associated cell death serine/threonine kinase whose activation by Ca2+/calmodulin may be linked to the biochemical mechanism underlying the cytoskeletal alterations that occur during cell death. Thus, calmodulin may play a direct role in regulating the enzyme responsible for the characteristic extensive chromatin and DNA damage of apoptosis.
In conclusion, we have reported here that culture filtrates of periodontopathic bacteria and butyric acid present in the filtrate induce apoptosis in murine WEHI 231 cells, splenic B cells, and human RAJI cells. This specific form of programmed cell death was characterized by DNA fragmentation assay, gel electrophoresis, chromatin condensation, and flow cytometric analysis. These data support the hypothesis that activation of apoptosis is at least one essential step in the butyric acid-induced immunosuppressive pathway and that butyric acid can modulate the immunoregulatory cell population in periodontal tissue by inducing B-cell death through apoptosis. We have tested other cell lines, including monocytes/macrophages, epithelial cells, and fibroblasts, but not T and B cells, and demonstrated that butyric acid induced apoptosis in monocytes/macrophages at similar levels as in T and B cells; however, epithelial cells and fibroblasts were not as sensitive (data not shown). Therefore, whatever the mechanism by which butyric acid induces apoptosis, it is clear that a variety of cell lines are susceptible. In fact, our data suggest that butyric acid may be an apoptosis-inducing agent in most lymphoreticular cells.
ACKNOWLEDGMENTS
This work was supported in part by grants-in-aid (08672169) for scientific research from the Ministry of Education, Science, and Culture of Japan and by a Suzuki research grant from Nihon University School of Dentistry at Matsudo.
REFERENCES
- 1.Aggarwal B B, Singh S, LaPushin R, Totpal K. Fas antigen signals proliferation of normal human diploid fibroblasts and its mechanism is different from tumor necrosis factor receptor. FEBS Lett. 1995;364:5–8. doi: 10.1016/0014-5793(95)00339-b. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal B B, Vikek J. Tumor necrosis factors: structure, function and mechanism of action. New York, N.Y: Marcel Dekker; 1992. [Google Scholar]
- 3.Boffa L C, Vidali G, Mann R S, Allfrey V G. Suppression of histone deacetylation in vivo and in vitro by sodium butyrate. J Biol Chem. 1978;253:3364–3366. [PubMed] [Google Scholar]
- 4.Botta G A, Radin L, Costa A, Schito G, Blasi G. Gas-liquid chromatography of the gingival fluid as an aid in periodontal diagnosis. J Periodontal Res. 1985;20:450–457. doi: 10.1111/j.1600-0765.1985.tb00827.x. [DOI] [PubMed] [Google Scholar]
- 5.Bugaut M, Bentejac M. Biological effects of short-chain fatty acids in nonruminant mammals. Annu Rev Nutr. 1993;13:217–241. doi: 10.1146/annurev.nu.13.070193.001245. [DOI] [PubMed] [Google Scholar]
- 6.Calabresse C, Venturin L, Ronco G, Villa P, Degos L, Belpomme D, Chomienne C. Selective induction of apoptosis in myeloid leukemic cell lines by monoacetone glucose-3 butyrate. Biochem Biophys Res Commun. 1994;201:266–283. doi: 10.1006/bbrc.1994.1698. [DOI] [PubMed] [Google Scholar]
- 7.Cohen J J, Duke R C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocytes nuclei leads to cell death. J Immunol. 1984;132:38–42. [PubMed] [Google Scholar]
- 8.Cohen O, Feinstein E, Kimchi A. DAP-kinase is a Ca2+/calmodulin-dependent, cytoskeletal-associated protein kinase, with cell death-inducing functions that depend on its catalytic activity. EMBO J. 1997;16:998–1008. doi: 10.1093/emboj/16.5.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darzinkiewicz Z, Bruno S, Bino G D, Gorczyca W, Hotz M A, Lassota P, Traganos P. Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13:795–808. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- 10.Dowd D R, MacDonald D N, Komn B S, Haussler M R, Miesfeld R. Evidence for early induction of calmodulin gene expression in lymphocyte undergoing glucocorticoid-mediated apoptosis. J Biol Chem. 1991;266:18423–18426. [PubMed] [Google Scholar]
- 11.Duttaroy A, Qian J F, Smith J S, Wang E. Up-regulated p21CIP1 expression is part of the regulation quantitatively controlling serum deprivation-induced apoptosis. J Cell Biochem. 1997;64:434–446. [PubMed] [Google Scholar]
- 12.Eftimiadi C, Tonetti M, Cavallero A, Sacco O, Rossi G A. Short chain fatty acids produced by anaerobic bacteria inhibit phagocytosis by human lung phagocytes. J Infect Dis. 1990;161:138–142. doi: 10.1093/infdis/161.1.138. [DOI] [PubMed] [Google Scholar]
- 13.Even M E, Sluss H K, Sherr C J, Matsushime H, Kato J, Livingstone D M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993;73:487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- 14.Freiberg R A, Spencer D M, Choate K A, Duh H J, Schreiber S L, Crabtree G R, Khavari P A. Fas signal transduction triggers either proliferation or apoptosis in human fibroblasts. J Invest Dermatol. 1977;108:215–219. doi: 10.1111/1523-1747.ep12334273. [DOI] [PubMed] [Google Scholar]
- 15.Gamet L, Daviaud D, Denis-Pouxviel C, Remesy C, Murat J C. Effect of short-chain fatty acids on growth and differentiation of the human colon-cancer cell line HT29. Int J Cancer. 1976;52:286–289. doi: 10.1002/ijc.2910520222. [DOI] [PubMed] [Google Scholar]
- 16.Gerschenson, L. E., and R. J. Rotello. Apoptosis: a different type of cell death. FASEB J. 6:2450–2455. [DOI] [PubMed]
- 17.Godfrained T, Miller R, Wibo M. Calcium antagonism and calcium entry blockade. Pharmacol Rev. 1986;38:321–416. [PubMed] [Google Scholar]
- 18.Gorbach S, Mayhew J, Bartlett J, Thadepalli H, Onderdonk A. Rapid diagnosis of anaerobic infections by direct gas liquid chromatography of clinical specimens. J Clin Invest. 1976;57:478–484. doi: 10.1172/JCI108300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hannon G J, Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994;371:257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- 20.Hidaka H, Kobayashi R. Pharmacology of protein kinase inhibitors. Annu Rev Pharmacol Toxicol. 1992;32:377–397. doi: 10.1146/annurev.pa.32.040192.002113. [DOI] [PubMed] [Google Scholar]
- 21.Jordan J P, Hand C M, Markowitz R S, Black P. Test for chemotherapeutic sensitivity of cerebral gliomas: use of colorimetric MTT assay. J Neurooncol. 1992;14:19–35. doi: 10.1007/BF00170942. [DOI] [PubMed] [Google Scholar]
- 22.Kato J Y, Matsuoka M, Polyak K, Massaque J, Sherr C J. Cyclic AMP-induced G1 phase arrest mediated by an inhibitor (p27Kip1) of cyclin-dependent kinase 4 activation. Cell. 1994;79:487–496. doi: 10.1016/0092-8674(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 23.Kurita-Ochiai T, Fukushima K, Ochiai K. Volatile fatty acids, metabolic by-products of periodontopathic bacteria, inhibit lymphocyte proliferation and cytokine production. J Dent Res. 1995;74:1367–1373. doi: 10.1177/00220345950740070801. [DOI] [PubMed] [Google Scholar]
- 24.Kurita-Ochiai T, Fukushima K, Ochiai K. Butyric acid-induced apoptosis of murine thymocytes, splenic T cells, and human Jurkat T cells. Infect Immun. 1997;65:35–41. doi: 10.1128/iai.65.1.35-41.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Margolis H C, Duckworth J H, Moreno E C. Composition and buffer capacity of pooled starved plaque fluid from caries-free and caries-susceptible individuals. J Dent Res. 1988;67:1476–1482. doi: 10.1177/00220345880670120701. [DOI] [PubMed] [Google Scholar]
- 26.Matsuoka K, Kubota M, Adachi S, Kuwakado K, Hirota H, Wakazono Y, Akiyama Y, Mikawa H. Different mode of cell death induced by calcium ionophore in human leukemia cell lines: possible role of constitutive endo-nuclease. Exp Cell Res. 1994;210:19–25. doi: 10.1006/excr.1994.1003. [DOI] [PubMed] [Google Scholar]
- 27.McConkey D J, Jondal M, Orrenius S. Cellular signaling in thymocyte apoptosis. Semin Immunol. 1992;4:371–377. [PubMed] [Google Scholar]
- 28.Nakajima H, Golstein P, Henkart P A. The target cell nucleus is not required for cell-mediated granzyme- or Fas-based cytotoxicity. J Exp Med. 1995;181:1905–1909. doi: 10.1084/jem.181.5.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakano K, Mizuno T, Sowa Y, Orita T, Yoshino T, Okuyama Y, Fujita T, Ohtani-Fujita N, Matsukawa Y, Tokino T, Yamagishi H, Oka T, Nomura H, Sakai T. Butyrate activates the WAF1/Cip1 gene promoter through Sp1 sites in a p53-negative human colon cancer cell line. J Biol Chem. 1977;272:22199–22206. doi: 10.1074/jbc.272.35.22199. [DOI] [PubMed] [Google Scholar]
- 30.Naleway, C., H. Chou, T. Manos, C. Goodman, P. Robinson, and R. Singer. 1989. Assessment of the potential relationship between levels of SCFA found in subgingival plaque and periodontal health. J. Dent. Res. 68(Suppl.):121. (Abstract.)
- 31.Newell M K, Haughn L J, Maroun C R, Julius M H. Death of mature T cells by separate ligation of CD4 and the T-cell receptor for antigen. Nature. 1990;347:286–289. doi: 10.1038/347286a0. [DOI] [PubMed] [Google Scholar]
- 32.Nicoletti I, Migliorati G, Pagliacci M C, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 33.Paradones C E, Illera V A, Peckham D, Stunz L L, Ashman R F. Regulation of apoptosis in vitro in mature spleen T cells. J Immunol. 1993;151:3521–3529. [PubMed] [Google Scholar]
- 34.Pardee A B. G1 events and regulation of cell proliferation. Science. 1989;246:603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- 35.Riggs M G, Whittaker R G, Neumann J R, Ingram V M. n-Butyrate causes histone modification in Hela and Friend erythroleukemia cells. Nature. 1977;268:462–464. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- 36.Rivero J A, Adunyah S E. Sodium butyrate induces tyrosine phosphorylation and activation of MAP kinase (ERK-1) in human K562 cells. Biochem Biophys Res Commun. 1996;224:796–801. doi: 10.1006/bbrc.1996.1102. [DOI] [PubMed] [Google Scholar]
- 37.Rotstein O D, Vittorini T, Kao J, McBurney M E, Nasmith P E, Grinstein S. A soluble bacteroides by-product impairs phagocytic killing of Escherichia coli by neutrophils. Infect Immun. 1989;57:745–753. doi: 10.1128/iai.57.3.745-753.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russo G L, Pietra V P, Mercurio C, Ragione F D, Marshak D R, Oliva A, Zappia V. Down-regulation of protein kinase CKII activity by sodium butyrate. Biochem Biophys Res Commun. 1977;233:673–677. doi: 10.1006/bbrc.1997.6515. [DOI] [PubMed] [Google Scholar]
- 39.Schwartzman R A, Cidlowski J A. Apoptosis: the biochemistry and molecular biology of programmed cell death. Endocrine Rev. 1993;14:133–151. doi: 10.1210/edrv-14-2-133. [DOI] [PubMed] [Google Scholar]
- 40.Siegel, I. 1977. Permeability of oral mucosa to organic acid. J. Dent. Res. 56(Suppl.):52. (Abstract.) [DOI] [PubMed]
- 41.Singer R, Bucker B. Butyrate and propionate: important components of toxic dental plaque extracts. Infect Immun. 1981;32:458–463. doi: 10.1128/iai.32.2.458-463.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Socransky S, Haffajee A. Microbiological mechanisms in the pathogenesis of destructive periodontal disease: a critical assessment. J Periodontal Res. 1991;26:195–212. doi: 10.1111/j.1600-0765.1991.tb01646.x. [DOI] [PubMed] [Google Scholar]
- 43.Soder P O, Jin L J, Soder B. DNA probe detection of periodontopathogens in advanced periodontitis. Scand J Dent Res. 1993;101:363–370. doi: 10.1111/j.1600-0722.1993.tb01133.x. [DOI] [PubMed] [Google Scholar]
- 44.Toyoshima H, Hunter T. p27, novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]