Abstract
Loop diuretics are essential in the treatment of patients with heart failure (HF) who develop congestion. Furosemide is the most commonly used diuretic; however, some randomized controlled trials (RCTs) have shown varying results associated with torsemide and furosemide in terms of hospitalizations and mortality. We performed an updated meta-analysis of currently available RCTs comparing furosemide and torsemide to see if there is any difference in clinical outcomes in patients treated with these loop diuretics. PubMed, MEDLINE, Cochrane, and Embase databases were searched for RCTs comparing the outcomes in patients with HF treated with furosemide versus torsemide. The primary end points included all-cause mortality, all-cause hospitalizations, cardiovascular-related hospitalizations, and HF-related hospitalizations. A random-effects meta-analysis was performed to estimate the risk ratio (RR) with a 95% confidence interval (CI). A total of 10 RCTs with 4,127 patients (2,088 in the furosemide group and 2,039 in the torsemide group) were included in this analysis. A total of 56% of the patients were men and the mean age was 68 years. No significant difference was noted in all-cause mortality between the furosemide and torsemide groups (RR 1.02, 95% CI 0.91 to 1.15, p = 0.70); however, patients treated with furosemide compared with torsemide had higher risks of cardiovascular hospitalizations (RR 1.36, 95% CI 1.13 to 1.65, p = 0.001), HF-related hospitalizations (RR 1.65, 95% CI 1.21 to 2.24, p = 0.001), and all-cause hospitalizations (RR 1.06, 95% CI 1.01 to 1.11, p = 0.02). In conclusion, patients with HF treated with torsemide have a reduced risk of hospitalizations compared with those treated with furosemide, without any difference in mortality. These data indicate that torsemide may be a better choice to treat patients with HF.
Keywords: furosemide, torsemide, heart failure
Heart failure (HF) continues to be a major clinical and public health problem worldwide.1 According to the 2017 to 2020 National Health and Nutrition Examination Survey, around 6.7 million people in the United States aged >20 years had HF, a 10% increase from 6 million in 2015 to 2018.2 The average health care cost of HF in the United States is $31 billion every year, which is projected to increase to $50 billion in 2030, with an increase in the aging population.3,4
Loop diuretics, such as bumetanide, furosemide, and torsemide, are the preferred diuretic/decongesting agents in most patients with HF.5–7 Furosemide is the most commonly used loop diuretic for HF treatment, although bumetanide and torsemide have a higher oral bioavailability than furosemide.8–10 Torsemide may improve left ventricular diastolic function and myocardial fibrosis, but there was no significant difference observed in mortality compared with furosemide in patients with HF.11–13 Meta-analyses of randomized controlled trials (RCTs) have shown conflicting results in terms of mortality and readmissions.14,15 The recently published TRANSFORM-HF (Torsemide Comparison With Furosemide for Management of Heart Failure) is the largest trial to date comparing furosemide and torsemide.16 It showed no difference in the outcomes of patients after being admitted for HF. We performed an updated meta-analysis of the currently available RCTs comparing furosemide and torsemide to assess the clinical outcomes, including the TRANSFORM-HF trial.
Methods
We searched the currently available RCTs that were published until January 30, 2023, in the PubMed, MEDLINE, Cochrane, and Embase databases using search terms such as “furosemide,” “torsemide,” “diuretics,” and “heart failure” in various combinations. Only the RCTs comparing furosemide and torsemide in adult patients with HF and reporting at least 1 clinical outcome of interest were included. The main exclusion criteria were studies with a nonrandomized design and postmarketing analysis of previous RCTs.
The study reports were screened for eligibility, the risk of bias was assessed, and data were collected independently by 2 reviewers (SD and SG). Differences between the reviewers were resolved after a discussion with the third reviewer (SS). Baseline characteristics of the eligible RCTs and the patients were collected: type of HF, number (n) of patients, age, male percentage, follow-up duration, dose and route of furosemide/torsemide, major inclusion/exclusion criteria, baseline New York Heart Association class, co-morbidities such as hypertension, diabetes mellitus, coronary artery disease, myocardial infarction, and previous HF admissions. The primary end points were all-cause mortality, all-cause hospitalizations, cardiovascular (CV) hospitalizations and HF-related hospitalizations. In addition, brain natriuretic peptide/N-terminal pro-brain natriuretic peptide (pg/ml) levels were compared at follow-up.
We conducted this analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.17 The meta-analysis was performed using the Cochrane Review Manager, version 5.4 (Cochrane, London, United Kingdom).18 Pooled risk ratios (RRs) for dichotomous variables and standardized mean difference for continuous variables, along with 95% confidence intervals (CIs) were calculated for different clinical end points using the random-effects model with the Mantel-Haenszel method. A p <0.05 was considered statistically significant. Heterogeneity between the studies was calculated using the I-squared statistic (considered significant if I2 >50%). Forest plots were generated for individual clinical end points to depict the differences between the furosemide and torsemide arms. A sensitivity analysis was performed for all the outcomes by excluding each trial from the analysis.
Results
The initial search revealed 149 studies, of which 10 RCTs fulfilling the inclusion criteria were included in the meta-analysis.9,11,16,19–25 The search strategy is described in the PRISMA flow diagram (Figure 1). A total of 4,127 patients, with 2,088 in the furosemide group and 2,039 in the torsemide group, were included (Table 1). The duration of the follow-up varied from 3 to 18 months. A total of 7 studies had patients with chronic HF, and 3 studies had patients with acute on chronic HF. A total of 56% of the patients were men, and the mean age was 68 years (Table 2). For the studies that reported co-morbidities, 55% of the patients had hypertension, 56% had diabetes mellitus, 42% had previous coronary artery disease, 30% had previous myocardial infarction, and 35% had previous HF admissions. No significant publication bias was observed for the outcomes using funnel plots and Egger and Beggs analyses (Supplementary Figure 1).
Figure 1.
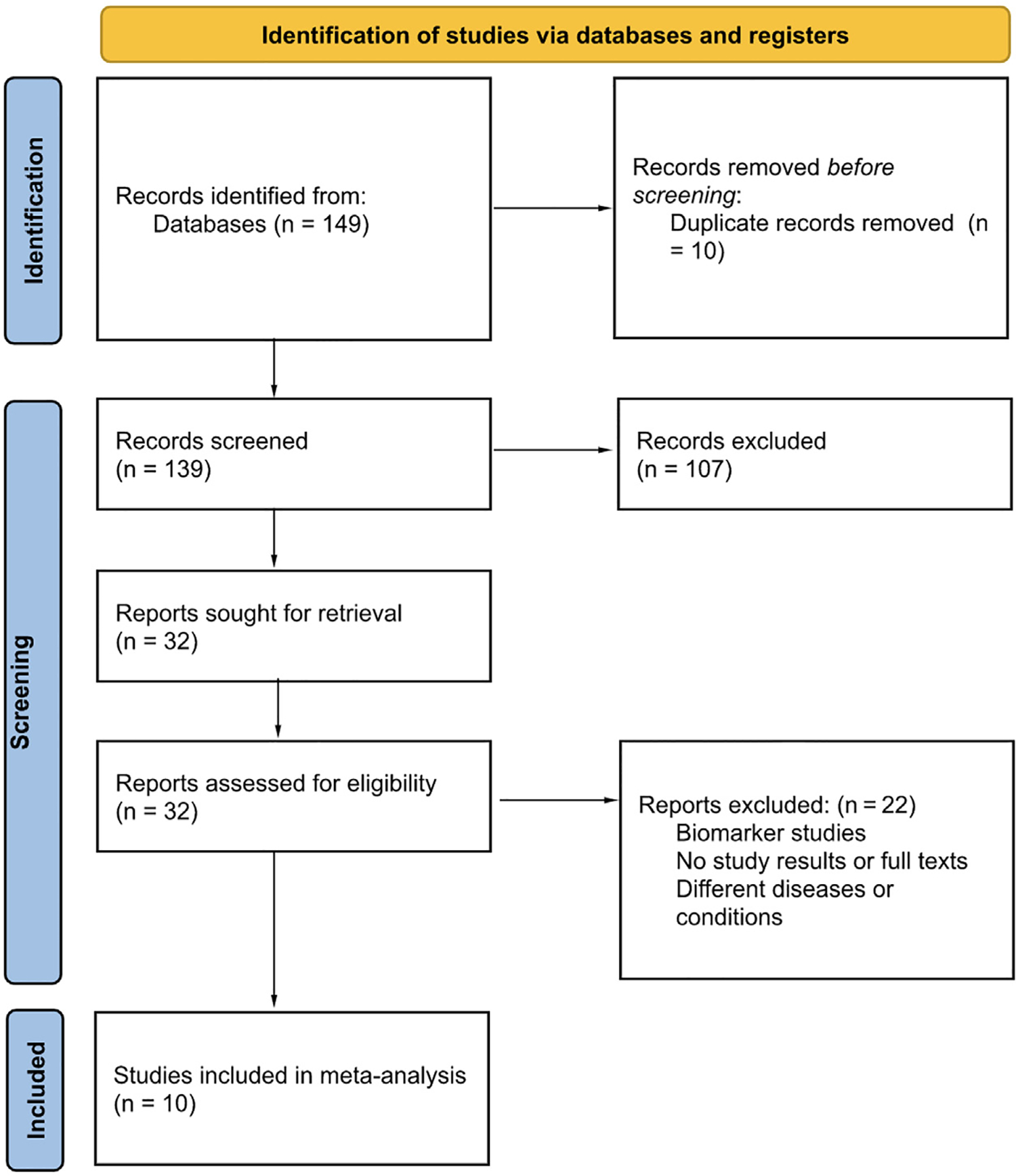
PRISMA 2020 flow diagram depicting the search strategy.
Table 1.
Baseline characteristics of the studies
| Study | Type of HF | N | Follow up (months) | Dose (mg) | Route | Inclusion criteria | Exclusion criteria |
|---|---|---|---|---|---|---|---|
| Noe et al. 1999 | Chronic | F- 137 T- 103 |
6 | F- 133* T - 59 |
F - PO† T-PO |
NYHA II/III | Contraindication to F/T |
| Stroupe et al. 2000 | Acute on chronic | F- 100 T - 93 |
12 | NA | NA | LV systolic dysfunction | Allergy to T |
| Murray et al. 2001 | Chronic | F- 121 T- 113 |
12 | F- 136* T - 72 |
NA | LV systolic dysfunction | Allergy to T |
| Muller et al. 2003 | Chronic | F- 115 T- 122 |
9 | F - 40‡ T- 10 |
F - PO† T-PO |
NYHA II–IV | − |
| Yamato et al. 2003 | Chronic | F - 25 T - 25 |
6 | F - 20–40 T - 4–8 |
F-PO T-PO |
NYHA II/III, LVDd ≥ 60mm, LV EF ≤ 45% | Na < 135 nmol/L), K < 3.5 nmol/L, Cr > 2.5 mg/dl, > mild MR |
| Paterna et al. 2005 | Acute on chronic | F - 42 T - 42 |
6–18 | F - 500 BID T - 200 BID Both later changed to PO F 250–500 mg BID |
F - IV T - IV |
Uncompensated refractory HF, LV EF <35% | − |
| TORAFIC 2011 | Chronic | F - 78 T - 77 |
8 | F - 40 T- 10 |
F-PO T-PO |
NYHA II-IV, HTN | AS, HOCM, recent ischemic CVD |
| Trippel et al. 2018 | Chronic | F- 18 T- 17 |
9 | F - 20 T - 5 |
F-PO T-PO |
T2DM, diastolic dysfunction and PIP ≥110 ng/mL | Cardiac valve disease with regurgitation grade ≥ 2, dialysis |
| Balsam et al. 2019 | Acute on chronic | F - 24 T- 16 |
3 | F- 100 T - 70 |
F-PO T-PO |
NYHA II-IV | ACS, HOCM |
| Mentz et al. 2023 | Chronic | F - 1428 T- 1431 |
12 | At treating physician’s discretion | F-PO T-PO |
LV EF < 40% | Dialysis, heart transplant |
ACS = acute coronary syndrome; AS = aortic stenosis; BID = twice daily; Cr = creatinine; CVD = cardiovascular disease; EF = ejection fraction; F = furosemide; HF = heart failure; HOCM = hypertrophic cardiomyopathy; HTN = hypertension; IV = intravenous; K = potassium; LV = left ventricle; LVDd = LV end-diastolic diameter; mg = milligrams; mg/dl = milligrams per decilitre; mm = millimetre; MR = mitral regurgitation; N = number of patients; NA = not available; Na = sodium; ng/mL = nanograms per millilitre; nmol/L = nanomoles per litre; NYHA = New York Heart Association; PIP = C-terminal propeptide of procollagen type I; PO = per oral; T = torsemide; T2DM = type 2 diabetes mellitus; TORAFIC = torasemide prolonged release versus furosemide in patients with chronic heart failure.
Mean overall daily dose.
Not specifically mentioned but patients given prescription of the medications.
Starting dose, adjusted by the treating physician.
Table 2.
Baseline characteristics of the patients
| Study | Mean age (y) | Male % | Baseline NYHA class | HTN% | DM% | CAD% | MI% | Prior HF admission % |
|---|---|---|---|---|---|---|---|---|
| Noe et al. 1999 | F - 75.1 T - 75.1 |
F - 54 T - 57.3 |
F- 2.36 T- 2.34 |
F - 59.1 T - 63.1 |
F - 33.6 T - 44.7 |
NA | F - 38.0 T - 45.6 |
NA |
| Stroupe et al. 2000 | F - 63 T - 63 |
F - 35 T - 38 |
NA | F - 47 T - 53 |
F - 43 T - 40 |
NA | F - 3 T - 7 |
NA |
| Murray et al. 2001 | F - 64.1 T - 64.1 |
F - 46 T - 49 |
F - 2.6 T - 2.8 |
F - 58 T - 61 |
F - 53 T - 46 |
F - 41 T - 35 |
F - 5 T - 12 |
F - 11 T - 25 |
| Muller et al. 2003 | F - 73.2 T - 74.4 |
F - 40.9 T - 45.1 |
F - 2.37 T - 2.47 |
F - 31.3 T - 27.1 |
F - 78.3 T - 72.1 |
NA | F - 51.3 T - 41.8 |
F - 7.8 T - 13.1 |
| Yamato et al. 2003 | F - 64.9 T - 64.7 |
F - 60 T - 56 |
F - 2.6 T - 2.7 |
F - 24 T - 28 |
NA | NA | F - 48 T - 52 |
NA |
| Paterna et al. 2005 | F - 74.3 T- 73.5 |
F - 67 T - 64 |
NA | F - 21 T - 29 |
NA | F - 52 T - 50 |
NA | NA |
| TORAFIC 2011 | F - 69.3 T - 68.1 |
F - 62 T - 55 |
NYHA II F - 89.7 % T - 96.1 % | F - 100 T - 100 |
NA | NA | NA | NA |
| Trippel et al. 2018 | F - 69.3 T - 68 |
F - 39 T - 76 |
NYHA II F - 33 % T - 59 % | F - 94 T - 100 |
F - 100 T - 100 |
F - 39 T - 53 |
NA | F - 28 T - 76 |
| Balsam et al. 2019 | F - 65 T - 74 |
F - 83.3 T - 68.8 |
F - 2 T - 2 |
F - 58.3 T - 50 |
F - 50 T - 37.5 |
F - 45.8 T - 50 |
NA | F - 67.7 T - 56.3 |
| Mentz et al. 2023 | F - 65 T - 64 |
F - 61 T - 65.2 |
NA | NA | F - 47.3 T - 48.1 |
F - 26.7 T - 29.8 |
NA | F - 33.7 T - 37 |
CAD = coronary artery disease; DM = diabetes mellitus; F = furosemide; HF = heart failure; HTN = hypertension; MI = myocardial infarction; NA = not available; NYHA = New York Heart Association; T = torsemide; TORAFIC = torasemide prolonged release versus furosemide in patients with chronic heart failure; y = years.
A total of 9 RCTs reported data on all-cause mortality, with 444 events in the furosemide group (n = 2,064) and 429 events in the torsemide group (n = 2,023). There was no significant difference in the all-cause mortality between the 2 arms (RR 1.02, 95% CI 0.91 to 1.15, p = 0.70). There was no significant heterogeneity among the studies (I2 = 0%) (Figure 2).
Figure 2.
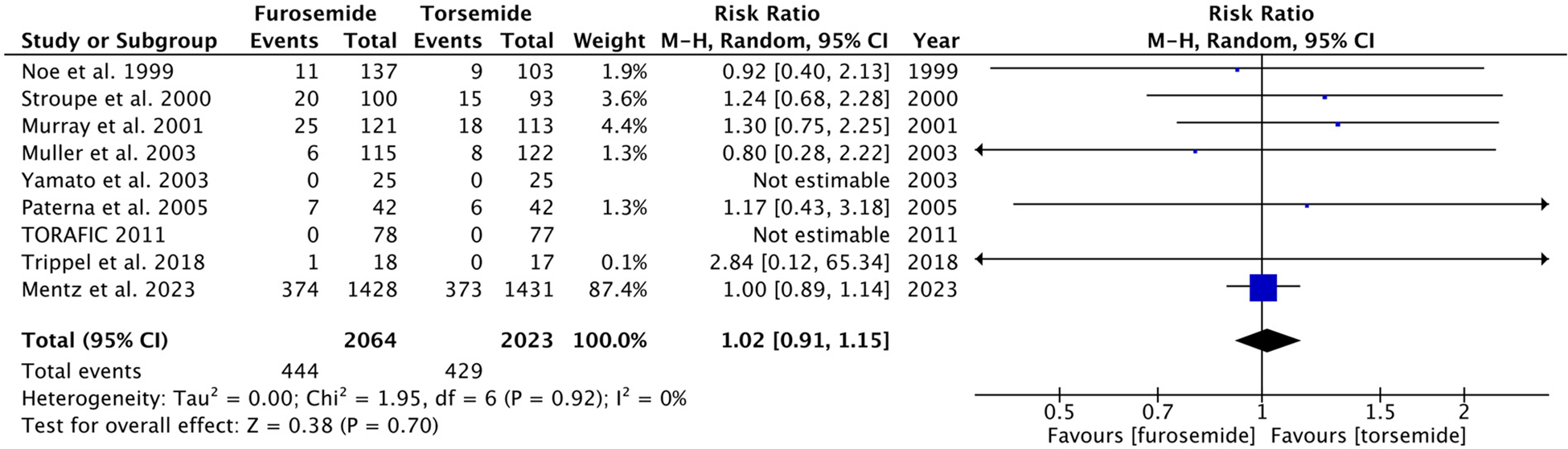
Forest plot showing all-cause mortality in furosemide and torsemide groups. M-H = Mantel-Haenszel.
The data for hospitalizations were divided into 3 categories: all-cause hospitalizations, CV hospitalizations, and HF-related hospitalizations. Of the 1,951 patients in the furosemide arm and 1,936 in the torsemide arm, a total of 1,188 and 1,117 all-cause hospitalizations were reported, respectively. The furosemide group had a marginally higher risk of hospitalizations (RR 1.06, 95% CI 1.01 to 1.11, p = 0.02) (Figure 3). The CV hospitalizations (RR 1.36, 95% CI 1.13 to 1.65, p = 0.001, I2 = 0%) and HF-related hospitalizations (RR 1.65, 95% CI 1.21 to 2.24, p = 0.001, I2 = 0%) were significantly higher in the patients treated with furosemide (Figure 3). There was no significant study heterogeneity observed for any outcome.
Figure 3.
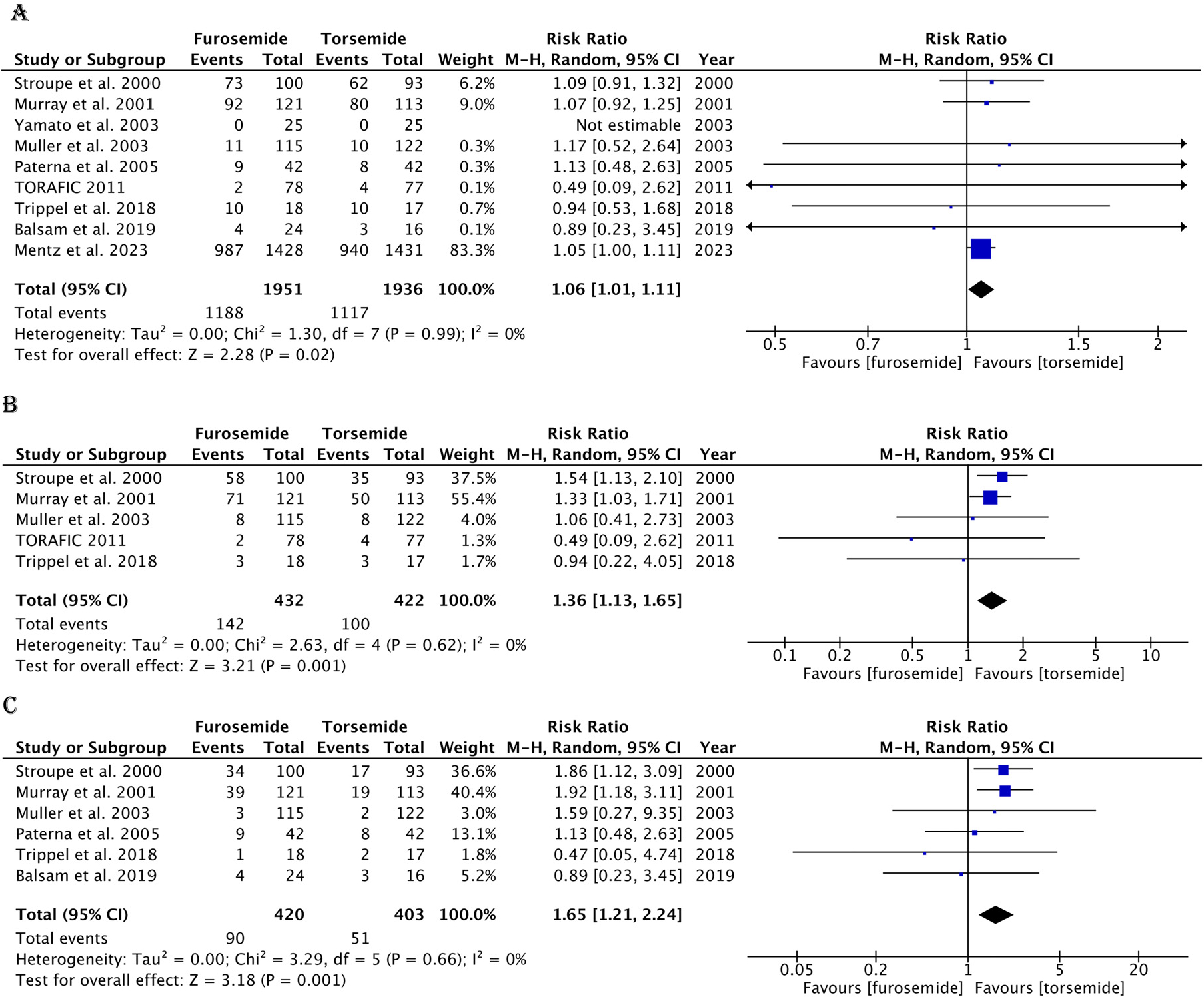
Forest plot showing hospitalizations in the furosemide and torsemide groups. (A) All-cause hospitalizations. (B) CV-related hospitalizations. (C) HF-related hospitalizations. M-H = Mantel-Haenszel.
No significant difference was found with respect to brain natriuretic peptide/N-terminal pro-brain natriuretic peptide levels in the furosemide and torsemide groups at follow-up (standardized mean difference 0.34, 95% CI −0.07 to 0.76, p = 0.10, I2 = 44%) (Supplementary Figure 2). The sensitivity analysis for primary end points by excluding each trial is listed in Table 3 and risk of bias summary is shown in Figure 4. The number needed to treat for torsemide with respect to HF hospitalizations was found to be 12.
Table 3.
Sensitivity analysis
| Trials | All-cause mortality | All-cause hospitalizations | CV related hospitalizations | HF related hospitalizations |
|---|---|---|---|---|
| Final outcome | 1.02 [0.91, 1.15] | 1.06 [1.01, 1.11] | 1.36 [1.13, 1.65] | 1.65 [1.21, 2.24] |
| Trials excluded | ||||
| Noe et al. 1999 | 1.02 [0.91, 1.15] | − | − | − |
| Stroupe et al. 2000 | 1.02 [0.90, 1.14] | 1.05 [1.00, 1.11] | 1.27 [1.00, 1.61] | 1.54 [1.04, 2.26] |
| Murray et al. 2001 | 1.01 [0.90, 1.14] | 1.05 [1.00, 1.11] | 1.41 [1.06, 1.88] | 1.49 [1.00, 2.22] |
| Muller et al. 2003 | 1.03 [0.91, 1.15] | 1.06 [1.01, 1.11] | 1.38 [1.14, 1.67] | 1.65 [1.21, 2.26] |
| Yamato et al. 2003 | 1.02 [0.91, 1.15] | 1.06 [1.01, 1.11] | − | − |
| Paterna et al. 2005 | 1.02 [0.91, 1.15] | 1.06 [1.01, 1.11] | − | 1.75 [1.25, 2.43] |
| TORAFIC 2011 | 1.02 [0.91, 1.15] | 1.06 [1.01, 1.11] | 1.38 [1.14, 1.67] | − |
| Trippel et al. 2018 | 1.02 [0.91, 1.15] | 1.06 [1.01, 1.11] | 1.37 [1.13, 1.66] | 1.69 [1.24, 2.30] |
| Balsam et al. 2019 | − | 1.06 [1.01, 1.11] | − | 1.70 [1.24, 2.34] |
| Mentz et al. 2023 | 1.16 [0.84, 1.60] | 1.07 [0.96, 1.20] | − | − |
Values in risk ratio (RR) [95% confidence interval (CI)].
CV = cardiovascular; HF = heart failure; TORAFIC = torsemide prolonged release versus furosemide in patients with chronic heart failure.
Figure 4.
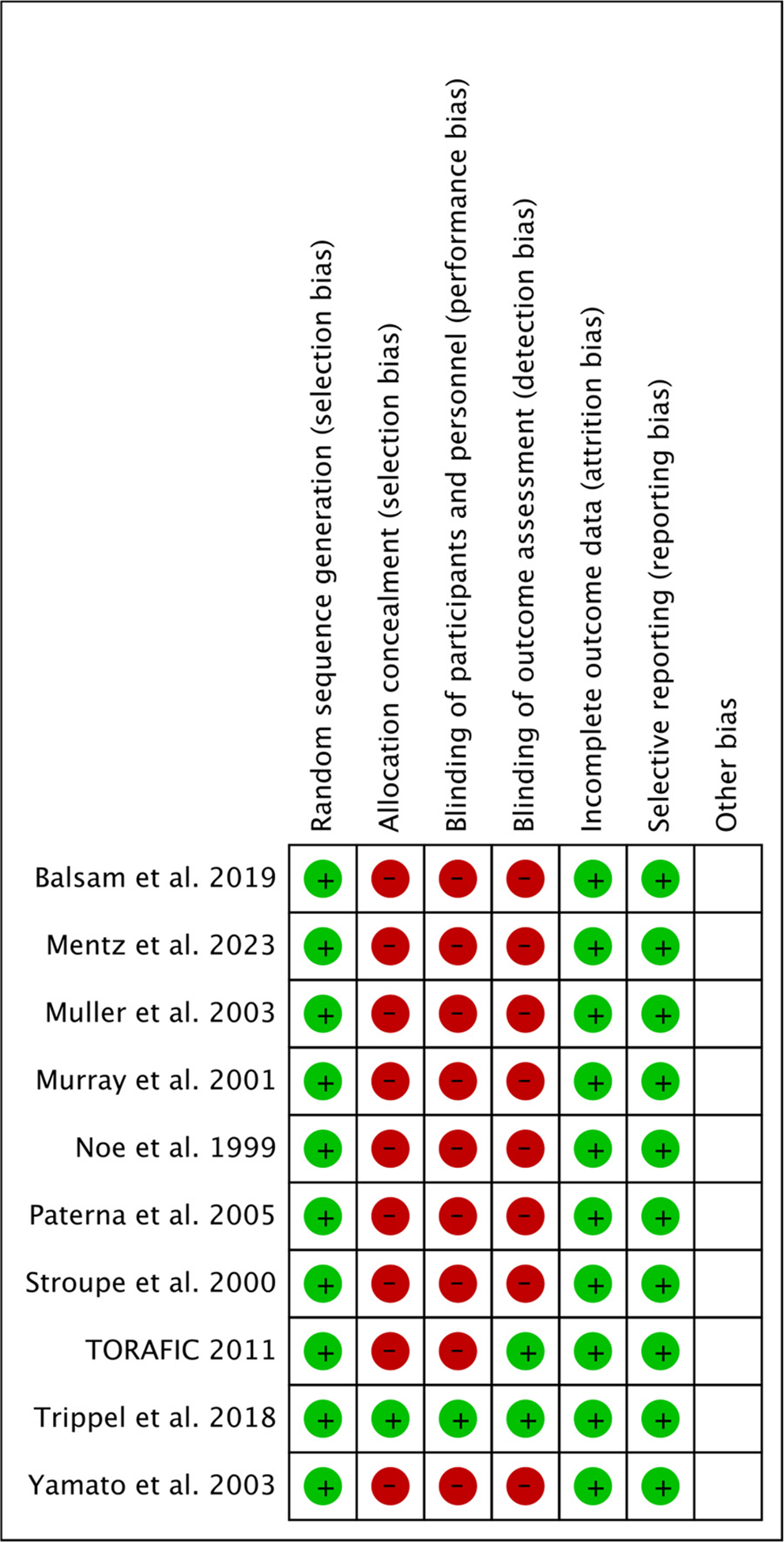
Risk of bias summary. M-H = Mantel-Haenszel.
Discussion
Our meta-analysis of 4,127 patients with HF from 10 RCTS showed that the torsemide group had a significantly lower number of hospitalizations (all-cause, CV-related, and HF-related hospitalizations) than the furosemide group, with no difference in all-cause mortality.
The current guidelines recommend loop diuretics for the management of congestion in patients with HF.5 Furosemide has long been the drug of choice, but comparisons with torsemide started as early as 1986.26 Torsemide was shown to be better in natriuresis, ameliorating cardiac sympathetic nerve activity and left ventricular remodeling, and reversing myocardial fibrosis.27–29 However, no difference was found between the 2 drugs with respect to all-cause mortality (8.7% vs 8%, p = 0.845) or CV mortality (4.9% vs 8%, p = 0.331) in an RCT by Noe et al.19 Stroupe et al20 subsequently reported reduced hospitalizations with torsemide versus furosemide related to HF (18.3% vs 34) and all CV causes (37.6% vs 58%). Similarly, another study showed improvements in HF hospitalizations (17% vs 32%, p = 0.01) and CV hospitalizations (59% vs 44%, p = 0.03) with torsemide compared with furosemide.9 However, these studies suffered from a small sample size and lack of power. On the contrary, the post hoc analyses from 2 large scale trials showed no differences in 30-day mortality or hospitalizations between the furosemide and torsemide groups; patients in the torsemide arm had greater severity of HF.13,30 In the recent largest clinical trial to compare the 2 medications by Mentz et al16 (TRANSFORM-HF), 2,859 patients were randomly allocated to the furosemide and torsemide groups. No significant difference was observed in all-cause mortality (hazard ratio [HR] 1.02, 0.89 to 1.18), total hospitalizations (RR 0.94, 0.84 to 1.07), and the composite outcomes of all-cause mortality or all-cause hospitalizations at 30 days (HR 0.94, 0.75 to 1.18) and 12 months (HR 0.92, 0.83 to 1.02).
Our results are similar to the results of TRANSFORM-HF and other trials in terms of mortality; however, we found an improvement in hospitalizations with torsemide use compared with furosemide. This could be explained by the higher variability in absorption and natriuretic activity of furosemide as opposed to torsemide.9 This is in contrast with the meta-analysis by Kido et al31 that reported no difference in rehospitalizations or mortality (the studies had heterogeneity ranging from I2 = 40% to 79%). Furthermore, the recent meta-analysis by Eid et al15 also failed to show any clinical benefit with torsemide compared with furosemide in HF. The difference in the observed results may be explained by the larger sample size and no heterogeneity among the studies in our analysis.
There are a few limitations to our meta-analysis. First, because this is a trial-level analysis, it is susceptible to the biases as the individual trials. Second, 1 of the studies initially randomly allocated patients to the 2 medications but later changed both groups to furosemide, which could have affected the overall clinical outcomes during the follow-up.22 Third, the studies included combined populations of acute and chronic HF, with substantial crossover during rehospitalizations, making it challenging to determine the benefits of torsemide in an acute scenario. Fourth, because most of the studies had combined patient populations with preserved and reduced ejection fractions, we were unable to assess the impact of baseline ejection fraction on the rate of hospitalizations. Similarly, a subanalysis to evaluate the effect of additional classes of diuretics, such as metolazone, could not be performed owing to the lack of patient-level data. An ongoing active trial (TRANSFORM-HF Ancillary Mechanistic Study) may help to understand the mechanisms of the diuretic’s benefits by examining blood and urine proteomic protein clusters.32
In conclusion, our meta-analysis showed that treatment with torsemide in patients with HF is associated with a lower prevalence of hospitalizations (all-cause, CV-related, and HF-related) than furosemide, without any difference in mortality. Although 12 patients need to be treated with torsemide (needed to treat) to prevent 1 additional HF hospitalization compared with furosemide, switching the patients to torsemide would still be beneficial in reducing health care costs by decreasing the total HF readmissions because the medications are formulary and not expensive.
Supplementary Material
Footnotes
Declaration of Competing Interest
The authors have no competing interests to declare.
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.amjcard.2023.08.079.
References
- 1.Roger VL. Epidemiology of heart failure: a contemporary perspective. Circ Res 2021;128:1421–1434. [DOI] [PubMed] [Google Scholar]
- 2.Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker-Smith CM, Beaton AZ, Boehme AK, Buxton AE, Commo-dore-Mensah Y, Elkind MSV, Evenson KR, Eze-Nliam C, Fugar S, Generoso G, Heard DG, Hiremath S, Ho JE, Kalani R, Kazi DS, Ko D, Levine DA, Liu J, Ma J, Magnani JW, Michos ED, Mussolino ME, Navaneethan SD, Parikh NI, Poudel R, Rezk-Hanna M, Roth GA, Shah NS, St-Onge MP, Thacker EL, Virani SS, Voeks JH, Wang NY, Wong ND, Wong SS, Yaffe K, Martin SS, American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2023 update: a report from the American Heart Association [published correction appears in Circulation 2023;147:e622] [published correction appears in Circulation 2023;148:e4]. Circulation 2023;147: e93–e621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Piña IL, Trogdon JG, American Heart Association Advocacy Coordinating Committee, Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiovascular Radiology and Intervention, Council on Clinical Cardiology, Council on Epidemiology and Prevention, Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013;6:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics–2012 update: a report from the American Heart Association [published correction appears in Circulation 2012;125:e1001]. Circulation 2012;125:188–197. [DOI] [PubMed] [Google Scholar]
- 5.Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines [published correction appears in Circulation 2022;145:e1033] [published correction appears in Circulation 2022;146:e185] [published correction appears in Circulation 2023;147:e674]. Circulation 2022;145:e895–e1032. [DOI] [PubMed] [Google Scholar]
- 6.Ellison DH, Felker GM. Diuretic treatment in heart failure [published correction appears in N Engl J Med 2018;378:492]. N Engl J Med 2017;377:1964–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cody RJ, Kubo SH, Pickworth KK. Diuretic treatment for the sodium retention of congestive heart failure. Arch Intern Med 1994;154:1905–1914. [PubMed] [Google Scholar]
- 8.Vargo DL, Kramer WG, Black PK, Smith WB, Serpas T, Brater DC. Bioavailability, pharmacokinetics, and pharmacodynamics of torsemide and furosemide in patients with congestive heart failure. Clin Pharmacol Ther 1995;57:601–609. [DOI] [PubMed] [Google Scholar]
- 9.Murray MD, Deer MM, Ferguson JA, Dexter PR, Bennett SJ, Perkins SM, Smith FE, Lane KA, Adams LD, Tierney WM, Brater DC. Open-label randomized trial of torsemide compared with furosemide therapy for patients with heart failure. Am J Med 2001;111:513–520. [DOI] [PubMed] [Google Scholar]
- 10.Cosín J, Díez J, TORIC Investigators. Torasemide in chronic heart failure: results of the TORIC study. Eur J Heart Fail 2002;4:507–513. [DOI] [PubMed] [Google Scholar]
- 11.Yamato M, Sasaki T, Honda K, Fukuda M, Akutagawa O, Okamoto M, Hayashi T. Effects of torasemide on left ventricular function and neurohumoral factors in patients with chronic heart failure. Circ J 2003;67:384–390. [DOI] [PubMed] [Google Scholar]
- 12.López B, Gonz alez A, Beaumont J, Querejeta R, Larman M, Díez J. Identification of a potential cardiac antifibrotic mechanism of torasemide in patients with chronic heart failure. J Am Coll Cardiol 2007;50:859–867. [DOI] [PubMed] [Google Scholar]
- 13.Mentz RJ, Hasselblad V, DeVore AD, Metra M, Voors AA, Arm-strong PW, Ezekowitz JA, Tang WH, Schulte PJ, Anstrom KJ, Hernandez AF, Velazquez EJ, O’Connor CM. Torsemide versus furosemide in patients with Acute Heart Failure (from the ASCEND-HF Trial). Am J Cardiol 2016;117:404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah P, Patel H, Mithawala P, Doshi R. Torsemide versus furosemide in heart failure patients: a meta-analysis of randomized controlled trials. Eur J Intern Med 2018;57:e38–e40. [DOI] [PubMed] [Google Scholar]
- 15.Eid PS, Ibrahim DA, Zayan AH, Elrahman MMA, Shehata MAA, Kandil H, Abouibrahim MA, Duy LM, Shinkar A, Elfaituri MK, Minh LHN, Fahmy MM, Tam DNH, Vuong NL, Shah J, Do VBD, Hirayama K, Huy NT. Comparative effects of furosemide and other diuretics in the treatment of heart failure: a systematic review and combined meta-analysis of randomized controlled trials. Heart Fail Rev 2021;26:127–136. [DOI] [PubMed] [Google Scholar]
- 16.Mentz RJ, Anstrom KJ, Eisenstein EL, Sapp S, Greene SJ, Morgan S, Testani JM, Harrington AH, Sachdev V, Ketema F, Kim DY, Desvigne-Nickens P, Pitt B, Velazquez EJ, TRANSFORM-HF Investigators. Effect of torsemide vs furosemide after discharge on all-cause mortality in patients hospitalized with heart failure: the TRANSFORM-HF randomized clinical trial. JAMA 2023;329:214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hr objartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Review Manager. Vol. 5 (RevMan 5) [Computer program]. Version 5.4 Copenhagen, Denmark: The Cochrane Collaboration; 2020. [Google Scholar]
- 19.Noe LL, Vreeland MG, Pezzella SM, Trotter JP. A pharmacoeconomic assessment of torsemide and furosemide in the treatment of patients with congestive heart failure. Clin Ther 1999;21:854–866. [DOI] [PubMed] [Google Scholar]
- 20.Stroupe KT, Forthofer MM, Brater DC, Murray MD. Healthcare costs of patients with heart failure treated with torasemide or furosemide. Pharmacoeconomics 2000;17:429–440. [DOI] [PubMed] [Google Scholar]
- 21.Müller K, Gamba G, Jaquet F, Hess B. Torasemide vs. furosemide in primary care patients with chronic heart failure NYHA II to IV–efficacy and quality of life. Eur J Heart Fail 2003;5:793–801. [DOI] [PubMed] [Google Scholar]
- 22.Paterna S, Fasullo S, Di Pasquale P. High-dose torasemide is equivalent to high-dose furosemide with hypertonic saline in the treatment of refractory congestive heart failure. Clin Drug Investig 2005;25:165–173. [DOI] [PubMed] [Google Scholar]
- 23.TORAFIC Investigators Group. Effects of prolonged-release torasemide versus furosemide on myocardial fibrosis in hypertensive patients with chronic heart failure: a randomized, blinded-end point, active-controlled study. Clin Ther 2011;33:1204–1213. .e3. [DOI] [PubMed] [Google Scholar]
- 24.Trippel TD, Van Linthout S, Westermann D, Lindhorst R, Sandek A, Ernst S, Bobenko A, Kasner M, Spillmann F, Gonz alez A, López B, Ravassa S, Pieske B, Paulus WJ, Díez J, Edelmann F, Tschöpe C. Investigating a biomarker-driven approach to target collagen turnover in diabetic heart failure with preserved ejection fraction patients. Effect of torasemide versus furosemide on serum C-terminal propeptide of procollagen type I (DROP-PIP trial). Eur J Heart Fail 2018;20:460–470. [DOI] [PubMed] [Google Scholar]
- 25.Balsam P, Ozierański K, Marchel M, Gawa»ko M, Niedziela Ł, Tymińska A, Sieradzki B, Sieradzki M, Fojt A, Bakuła E, Głowczyńska R, Peller M, Markulis M, Bednarski J, Kowalik R, Cacko A, Niewiński G, Filipiak KJ, Opolski G, Grabowski M. Comparative effectiveness of torasemide versus furosemide in symptomatic therapy in heart failure patients: preliminary results from the randomized TORNADO trial. Cardiol J 2019;26:661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Broekhuysen J, Deger F, Douchamps J, Ducarne H, Herchuelz A. Tor-asemide, a new potent diuretic. Double-blind comparison with furosemide. Eur J Clin Pharmacol 1986;31(suppl):29–34. [DOI] [PubMed] [Google Scholar]
- 27.Ballester MR, Roig E, Gich I, Puntes M, Delgadillo J, Santos B, Antonijoan RM. Randomized, open-label, blinded-endpoint, crossover, single-dose study to compare the pharmacodynamics of torasemide-PR 10 mg, torasemide-IR 10 mg, and furosemide-IR 40 mg, in patients with chronic heart failure. Drug Des Devel Ther 2015;9:4291–4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kasama S, Toyama T, Hatori T, Sumino H, Kumakura H, Takayama Y, Ichikawa S, Suzuki T, Kurabayashi M. Effects of torasemide on cardiac sympathetic nerve activity and left ventricular remodelling in patients with congestive heart failure. Heart 2006;92:1434–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.López B, Querejeta R, González A, Sánchez E, Larman M, Díez J. Effects of loop diuretics on myocardial fibrosis and collagen type I turnover in chronic heart failure. J Am Coll Cardiol 2004;43:2028–2035. [DOI] [PubMed] [Google Scholar]
- 30.Mentz RJ, Velazquez EJ, Metra M, McKendry C, Chiswell K, Fiuzat M, Givertz MM, Voors AA, Teerlink JR, O’Connor CM. Comparative effectiveness of torsemide versus furosemide in heart failure patients: insights from the PROTECT trial. Future Cardiol 2015;11:585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kido K, Shimizu M, Hashiguchi M. Comparing torsemide versus furosemide in patients with heart failure: a meta-analysis. J Am Pharm Assoc (2003) 2019;59:432–438. [DOI] [PubMed] [Google Scholar]
- 32.Cooper L Mechanistic insights from longitudinal changes in blood and urine proteins to explain efficacy and safety of torsemide vs furosemide after a heart failure hospitalization. Identifier: NCT04702958. Available at: ClinicalTrials.gov. Accessed on March 10, 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


