Abstract
The incidence of invasive Group A Streptococcus (iGAS) has varied throughout the COVID-19 pandemic. We reviewed iGAS infections in infants ≤1 year from 2012 to 2022. Twenty-five percent of cases occurred in the last quarter of 2022. Pneumonia (21.8%) was the most common presentation. Twenty-one patients (65.6%) were successfully transitioned to oral antibiotics.
Keywords: GAS, infant, invasive infection, pediatric, Streptococcus pyogenes
We reviewed cases of invasive Group A Streptococcus (iGAS) in infants <12 months of age over 11 years. Twenty-five percent of cases occurred in the final calendar quarter. While iGAS were associated with high morbidity, most were transitioned to oral antibiotics.
INTRODUCTION
Group A Streptococcus (GAS, S. pyogenes) is a common pathogen in children that may present with severe clinical phenotypes. After a period of increasing disease frequency through 2019, the incidence of invasive GAS (iGAS) in children decreased during the first 2 years of the COVID-19 pandemic followed by an increase in disease activity in the latter part of 2022 in the United States and Europe [1, 2]. iGAS infections have the highest incidence in those >65 and in infants <1 year of age [3]. Despite this recognition, there is a paucity of information regarding the clinical presentation, contemporary management, and outcomes of iGAS in very young children. Study of iGAS in infants is important given the known risk for severe disease coupled with the increasing evidence for the safety and efficacy of oral antibiotics for serious bacterial infections [4].
METHODS
Cases were identified using an IRB-approved GAS surveillance study at Texas Children’s Hospital (TCH, Houston, TX); all isolates identified by the TCH Clinical Microbiology Laboratory were captured. Cases of iGAS disease from 2012 to 2022 in children ≤12 months of age were eligible for inclusion in the primary analyses; the molecular characteristics of a subset of GAS isolates (n = 22) were included in previous studies [5, 6]. This was supplemented by review of TCH laboratory records for children with a positive polymerase chain reaction (PCR) test for GAS from a sterile site. A multiplex PCR assay capable of detecting GAS from body fluid was instituted in 2019 at TCH and utilized at provider discretion [7]. Invasive disease was defined by GAS identified by culture or PCR from a sterile site or the clinical diagnosis of streptococcal toxic shock syndrome (STSS) or necrotizing soft tissue infection regardless of the source. Medical records of the identified patients were reviewed. GAS isolates were typed using a previously described methodology [8]. While the primary focus of the study was on disease in those ≤12 months of age, the infectious diagnoses of iGAS cases in infants were compared with those in children 1–18 years old identified through the surveillance study.
We sought to correlate iGAS disease activity with interventions intended to curb the SARS-CoV-2 pandemic as well as the frequency of other major respiratory viruses. On March 24, 2020, Harris County, TX residents were required to wear a face covering in public and were strongly encouraged to limit public gatherings; face mask mandates were lifted statewide on March 10, 2021. The total number of respiratory specimens positive for respiratory syncytial virus (RSV) and/or influenza viruses during the study period were obtained from the TCH Clinical Microbiology Laboratory.
RESULTS
Demographics
During the study period, 504 cases of pediatric iGAS were captured with 32 cases identified in infants (6.3%); 8/32 cases (25%) occurred in the final quarter of 2022. The median subject age was 7.8 months interquartile ranges (IQR 1.8–9.5). Ten patients (31.3%) had premorbid conditions (Table 1). Eleven patients (34.4%) in our cohort had documented sick contacts and 5 patients (15.6%) had a household contact with GAS pharyngitis; 1 additional subject had a household member with impetigo.
Table 1.
Demographic Data, Infectious Diagnosis, and Descriptive Statistical Analysis of Cohort.
| N = 32 | |
|---|---|
| Median age, months (IQR)a | 7.8 (1.8–9.5) |
| Gender | Male: 19 (59.4%) |
| Female: 13 (40.6%) | |
| Race/ethnicity | |
| Non-Hispanic White | 7 (21.9%) |
| Black/African American | 5 (15.6%) |
| Hispanic | 18 (56.3%) |
| Asian | 2 (6.3%) |
| Medical comorbiditiesb | 10 (31.3%) |
| Cerebral palsy | 3 (9.4%) |
| Seizure disorder | 2 (6.3%) |
| Eczema | 2 (6.3%) |
| Tracheostomy dependent | 2 (6.3%) |
| Congenital heart disease | 1 (3.1%) |
| Intestinal failure | 1 (3.1%) |
| Lymphatic malformation | 1 (3.1%) |
| CHARGE syndrome | 1 (3.1%) |
| Infectious diagnosis | |
| Pneumonia/pleural empyema | 7 (21.8%) |
| Deep neck abscess | 4 (12.5%) |
| Bacteremia without a focus | 4 (12.5%) |
| Osteomyelitis | 3 (9.4%) |
| Retropharyngeal/parapharyngeal abscess | 2 (6.3%) |
| Meningitis | 2 (6.3%) |
| Pyomyositis | 2 (6.3%) |
| Mastoiditis | 2 (6.3%) |
| Epiglottitis | 1 (3.1%) |
| Septic arthritis | 1 (3.1%) |
| Tenosynovitis | 1 (3.1%) |
| Urinary tract infection | 1 (3.1%) |
| Ventriculoperitoneal shunt infection | 1 (3.1%) |
| Central-line associated bloodstream infection | 1 (3.1%) |
| Streptococcal toxic shock syndromec | 3 (9.4%) |
| emm typed | |
| 1 | 7 (25.9%) |
| 12 | 5 (18.5%) |
| 89 | 4 (14.8%) |
| 6 | 2 (7.4%) |
| 2 | 1 (3.7%) |
| 3 | 1 (3.7%) |
| 4 | 1 (3.7%) |
| 11 | 1 (3.7%) |
| 68 | 1 (3.7%) |
| 75 | 1 (3.7%) |
| 77 | 1 (3.7%) |
| 92 | 1 (3.7%) |
| Respiratory virus co-detection | 9 (28.1%) |
| RSV | 3 (9.4%) |
| RSV + Influenza A | 2 (6.3%) |
| Human metapneumovirus | 2 (6.3%) |
| Varicella zoster virus | 1 (3.1%) |
| Rhinovirus | 1 (3.1%) |
| Length of stay, days | 7.5 (5–14.5) |
| ICU admission | 19 (59.4%) |
| Mechanical ventilation | 14 (43.8%) |
| Vasopressors | 5 (15.6%) |
| Surgical source control performed | 13 (40.6%) |
| Percutaneous drainage/aspiration procedure | 7 (21.9%) |
| Duration of IV therapy, days | 7 (4.5–14.5) |
| Transition to oral antibiotics | 21 (65.6%) |
| Duration of total therapy, days | 15.5 (10–23.5) |
| Intravenous immunoglobulin administered | 1 (3.1%) |
aContinuous variables were presented as medians with interquartile ranges (IQR); categorical variables presented as n (%).
bIndividual categories of medical comorbidities are not mutually exclusive.
cStreptococcal toxic shock syndrome based on 2010 Centers for Disease Control and Prevention criteria.
dExpressed as percentage of isolates available for typing.
Diagnoses and Microbiology
The most common infectious diagnoses were pneumonia/pleural empyema (21.8%) followed by deep neck abscesses and bacteremia without a focus (12.5% each) (Table 1). A greater proportion of infant cases were due to pneumonia/pleural empyema compared with older children (8.3%, Supplementary Table 1).
In 11 cases, blood cultures were positive for GAS; other sources of GAS isolation included abscesses (n = 7), pleural (n = 4), and synovial fluid (n = 4). In 3 cases, GAS was only identified by PCR testing. Viral co-pathogens were detected in 9 patients (28.1%) of which the most common was RSV (n = 5); 7 of the cases with concomitant respiratory virus detection occurred in 2022. The correlation between iGAS in infants and RSV and influenza activity is depicted in Figure 1A.
Figure 1.
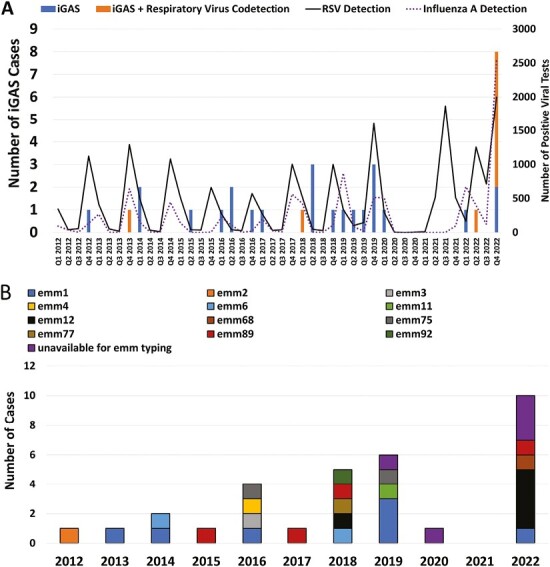
Temporal trends in infant iGAS cases. A, Quarterly number of cases of infant iGAS with and without respiratory virus codetection. The number of positive tests for RSV and influenza A at our center is also depicted. B, Trends in emm types. Cases unavailable for emm typing included those identified by molecular diagnostics with negative cultures (n = 3) and two culture-positive cases non-viable for further study.
emm typing was available for 27/29 (93.1%) culture-positive cases. emm1 was most common amongst our cohort (7/27, 25.9%) followed by emm12 (n = 5, 18.5%) and emm89 (n = 4, 14.8%); among emm12 cases, 4/5 (80%) occurred in 2022 (Figure 1B). Among isolates available for testing, 4/27 (14.8%) and 5/27 (18.5%) were resistant to clindamycin and erythromycin, respectively.
Management
Nineteen patients required admission to the intensive care unit (ICU, 59.4%). All patients received intravenous (IV) antibiotics for a median of 7 days (IQR 4.5–14.5). The most common definitive IV therapy was penicillin G (n = 12) followed by ceftriaxone (n = 8), and ampicillin (n = 8). Eight ICU patients were treated concomitantly with clindamycin and an IV β-lactam; 3/8 met criteria for STSS. The median duration of combination therapy was 2 days, followed by continuation of β-lactam monotherapy.
Twenty-one (65.6%) patients were transitioned to oral (PO) antibiotics after a median of 5 days of IV therapy. Amoxicillin was the most common therapy (n = 10), followed by amoxicillin-clavulanate (n = 4). There was no significant difference in the age of subjects with and without PO transition; the youngest subject transitioned to PO was 12 days old at hospital admission. The patients who received only IV therapy were more likely to have had positive blood cultures (81.8% vs 9.5%) and to have had a longer length of stay (average 16 days [IQR 11–22] vs 5 days [IQR 3–7]) than those transitioned to PO therapy. Transition to oral therapy was more common in infants with GAS pneumonia or deep suppurative infections. The median duration of total antibiotic therapy (IV + PO) was 15.5 days (IQR: 10–23.5 days). There were no deaths related to GAS infection.
GAS Meningitis
Two previously healthy infants were diagnosed with GAS meningitis at 6 and 9 months of age, respectively; both had magnetic resonance imaging demonstrating subdural empyema/effusion. The CSF white blood cell counts in these patients were 244 and 1188 cells/mm3, respectively. The antibiotic regimen for both patients included combination therapy of β-lactam and clindamycin and 1 subject was given immunoglobulin. These patients received a total duration of IV therapy of 42 and 21 days. One patient subsequently developed epilepsy and developmental delay.
DISCUSSION
Historically, infants <12 months of age have been identified as a high-risk group for iGAS, however, contemporary data regarding the outcomes and management are limited. We reviewed the experience at a tertiary children’s hospital of iGAS in infants during a period of rapidly changing iGAS incidence [1]. In the early SARS-COV-2 pandemic, a significant decline in iGAS was observed followed by an increase in incidence in the latter half of 2022 [1]. In our study, 25% of cases occurred in the final quarter of 2022, consistent with previous reports. A rebound in travel and social gatherings after the first years of the SARS-CoV-2 pandemic, as well as return to school and work, may have increased the spread of GAS as well as viral pathogens between individuals and households. Antecedent respiratory viral infection has been demonstrated to be important in streptococcal pathogenesis. The Centers for Disease Control and Prevention reported a substantial increase in respiratory viruses, particularly RSV, among hospitalized young children in late 2022 [9]. Given the national as well as our reported local trends, it is conceivable that the observed increase in infant iGAS, particularly in the final quarter of 2022, was a consequence of this surge in respiratory viral activity in the community.
While our isolates were diverse, emm1, emm12, and emm89 were most common in our series, albeit this is comparable to previous studies [1, 3, 6]. Consistent with recent US data [1, 6], emm12 accounted for a disproportionate number of infant iGAS cases in 2022 in our series. Notably, the proportion of infant cases due to emm12 in 2022 was comparable to that among all age groups at our center [6], however, the reason for the recent prominence of emm12 isolates remains unclear.
In our series, the majority of infants were of Hispanic ethnicity (56.3%), similar to studies of iGAS in older children in our region (48.3%) [6]. Interestingly, we found a greater proportion of iGAS in infants due to pneumonia compared with older children. This finding may be driven by the recognized young age of children hospitalized for pneumonia [10]. Nevertheless, providers caring for infants with pneumonia should consider the possibility of GAS when planning empiric therapy.
The administration of a β-lactam as well as a protein synthesis inhibiting antibiotic (eg, clindamycin) and immunoglobulin are recommended in the management of toxin-mediated GAS disease, such as STSS. In a small observational study of iGAS in children, clinical outcomes were improved in patients receiving initial treatment with a β-lactam and clindamycin combination prompting some experts to recommend this practice for all forms of iGAS [11]. While our overall sample size was small, only 8 children (25%) received combination therapy. In a study of STSS in children, only 50% received clindamycin and none received IVIG [5], highlighting that education regarding adjunctive therapies for iGAS remains an area for practice improvement. Notably, increasing clindamycin resistance among GAS has been reported in the United States and must be taken into account when considering adjunctive therapies [12].
Increasing evidence suggests that many serious bacterial infections may be safely managed with early transition to oral antibiotics once clinical improvement has been demonstrated, even among the very young [4]. Despite having serious invasive infections, most of our patients were able to be transitioned to oral antibiotics. Given the high level of acuity of many children with iGAS and the potential morbidity/mortality, additional research is needed to establish the boundaries and timing for safe utilization of oral antibiotics in iGAS.
The primary limitations of our study include its retrospective, single-center design, and small cohort size, restricting the conclusions that can be drawn regarding treatment and outcome. Additionally, the inconsistent use of molecular diagnostics likely underestimated iGAS disease as well as viral coinfection. Regardless, this work highlights the disease burden and clinical features of iGAS in infants during a multinational outbreak. Further work is needed to optimize therapeutic decision-making in these severe infections.
Supplementary Data
Supplementary materials are available at the Journal of The Pediatric Infectious Diseases Society online (http://jpids.oxfordjournals.org).
Contributor Information
Taylor Nack, Division of Infectious Diseases, Department of Pediatrics, Baylor College of Medicine and Texas Children’s Hospital, Houston, Texas, USA.
Jesus G Vallejo, Division of Infectious Diseases, Department of Pediatrics, Baylor College of Medicine and Texas Children’s Hospital, Houston, Texas, USA.
James Dunn, Department of Pathology, Baylor College of Medicine and Texas Children’s Hospital, Houston, Texas, USA.
Anthony R Flores, Division of Infectious Diseases, Department of Pediatrics, McGovern Medical School at UTHealth Houston and Children’s Memorial Hermann Hospital, Houston, Texas, USA.
J Chase McNeil, Division of Infectious Diseases, Department of Pediatrics, Baylor College of Medicine and Texas Children’s Hospital, Houston, Texas, USA.
Notes
Financial support . Dr McNeil receives funding from AHRQ (R01HS026896) for work unrelated to this manuscript. Dr Flores receives funding through NIAID (R21AI153663 and R21AI159059) which in part supported this work.
Potential conflicts of interest. Dr McNeil serves as the site investigator for a clinical trial sponsored by Nabriva Therapeutics unrelated to the work under consideration. Taylor Nack, Jesus Vallejo, and Anthony Flores have no significant relationships with commercial entities.
REFERENCES
- 1. Barnes M, Youngkin E, Zipprich J, et al. Notes from the field: increase in pediatric invasive group A streptococcus infections - Colorado and Minnesota, October-December 2022. MMWR Morb Mortal Wkly Rep 2023; 72:265–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Kempen EB, Bruijning-Verhagen PCJ, Borensztajn D, et al. Increase in invasive group a streptococcal infections in children in the Netherlands, a survey among 7 hospitals in 2022. Pediatr Infect Dis J 2023; 42:e122–4. [DOI] [PubMed] [Google Scholar]
- 3. Nelson GE, Pondo T, Toews KA, et al. Epidemiology of invasive group A streptococcal infections in the United States, 2005-2012. Clin Infect Dis 2016; 63:478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Keij FM, Kornelisse RF, Hartwig NG, et al. Efficacy and safety of switching from intravenous to oral antibiotics (amoxicillin-clavulanic acid) versus a full course of intravenous antibiotics in neonates with probable bacterial infection (RAIN): a multicentre, randomised, open-label, non-inferiority trial. Lancet Child Adolesc Health 2022; 6:799–809. [DOI] [PubMed] [Google Scholar]
- 5. Deniskin R, Shah B, Munoz FM, Flores AR.. Clinical manifestations and bacterial genomic analysis of group A streptococcus strains that cause pediatric toxic shock syndrome. J Pediatric Infect Dis Soc 2019; 8:265–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aboulhosn A, Sanson MA, Vega LA, et al. Increases in group A streptococcal infections in the pediatric population in Houston, TX, 2022. Clin Infect Dis 2023; 77:351–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnson C, Marquez C, Olson D, et al. Development and performance of a multiplex PCR assay for the detection of bacteria in sterile body fluids. Future Microbiol 2023; 18:187–95. [DOI] [PubMed] [Google Scholar]
- 8. Flores AR, Chase McNeil J, Shah B, Van Beneden C, Shelburne SA.. Capsule-negative emm types are an increasing cause of pediatric group A streptococcal infections at a large pediatric hospital in Texas. J Pediatric Infect Dis Soc 2019; 8:244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention. RSV-NET: Respiratory Syncytial Virus Hospitalization Surveillance Network, Centers for Disease Control and Prevention. https://www.cdc.gov/rsv/research/rsv-net/dashboard.html Accessed April 3, 2023.
- 10. Jain S, Williams DJ, Arnold SR, et al. ; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among US children. N Engl J Med 2015; 372:835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zimbelman J, Palmer A, Todd J.. Improved outcome of clindamycin compared with beta-lactam antibiotic treatment for invasive Streptococcus pyogenes infection. Pediatr Infect Dis J 1999; 18:1096–100. [DOI] [PubMed] [Google Scholar]
- 12. Li Y, Rivers J, Mathis S, et al. Continued increase of erythromycin nonsusceptibility and clindamycin nonsusceptibility among invasive group A streptococci driven by genomic clusters, United States, 2018-2019. Clin Infect Dis 2023; 76:e1266–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


