Abstract
PURPOSE
The phase III SKYSCRAPER-02 study determined whether the benefits of atezolizumab plus carboplatin and etoposide (CE) could be enhanced by the addition of tiragolumab in untreated extensive-stage small-cell lung cancer (ES-SCLC). We report final progression-free survival (PFS) and overall survival (OS) analyses.
METHODS
Patients received tiragolumab 600 mg/placebo, plus atezolizumab 1,200 mg and CE (four cycles), then maintenance tiragolumab/placebo plus atezolizumab. Primary end points were investigator-assessed PFS and OS in patients without history/presence of brain metastases (primary analysis set [PAS]). Additional end points included PFS and OS in all patients regardless of brain metastases status (full analysis set [FAS]), response, and safety.
RESULTS
Four hundred ninety patients were randomly assigned (FAS): 243 to tiragolumab arm and 247 to control arm. At the cutoff date (February 6, 2022; median duration of follow-up, 14.3 months [PAS] and 13.9 months [FAS]), final analysis of PFS in the PAS (n = 397) did not reach statistical significance (stratified hazard ratio [HR], 1.11; P = .3504; median, 5.4 months tiragolumab v 5.6 months control). At the cutoff date (September 6, 2022; median duration of follow-up, 21.2 months [FAS]), median OS in the PAS at final OS analysis was 13.1 months in both arms (stratified HR, 1.14; P = .2859). Median PFS and OS in the FAS were consistent with the PAS. The proportion of patients with immune-mediated adverse events (AEs) in the tiragolumab and control arms was 54.4% and 49.2%, respectively (grade 3/4: 7.9% and 7.7%). AEs leading to treatment withdrawal occurred in 8.4% and 9.3% of tiragolumab- and control-treated patients, respectively.
CONCLUSION
Tiragolumab did not provide additional benefit over atezolizumab and CE in untreated ES-SCLC. The combination was well tolerated with no new safety signals.
In SKY-02, tiragolumab + atezolizumab + chemo did not show improved PFS or OS compared with the placebo combo in patients with ES-SCLC.
INTRODUCTION
Current first-line treatment options for extensive-stage small-cell lung cancer (ES-SCLC) are atezolizumab in combination with carboplatin and etoposide (CE), on the basis of significant overall survival (OS) and progression-free survival (PFS) improvements versus CE in the phase III IMpower133 study (ClinicalTrials.gov identifier: NCT02763579)1,2 or durvalumab plus chemotherapy, on the basis of results of the phase III CASPIAN study (ClinicalTrials.gov identifier: NCT03043872).3 Significant OS improvement was also reported with serplulimab plus chemotherapy versus chemotherapy alone in the phase III ASTRUM-005 study (ClinicalTrials.gov identifier: NCT04063163).4 Although these regimens have improved outcomes in ES-SCLC, most patients experience disease progression (PD), and median survival remains at approximately 12 months.1,5 Therefore, there is a significant unmet need for new therapeutic options to improve long-term outcomes in ES-SCLC.
CONTEXT
Key Objective
Does the addition of anti–T-cell immunoreceptor with Ig and ITIM domains (TIGIT) tiragolumab to atezolizumab plus carboplatin and etoposide (CE) improve survival for extensive-stage small-cell lung cancer (SCLC)?
Knowledge Generated
The phase III SKYSCRAPER-02 trial did not meet its coprimary end points of overall survival (OS) and progression-free survival (PFS): there was no additional benefit with the tiragolumab plus atezolizumab and CE regimen versus placebo plus atezolizumab and CE. The addition of tiragolumab to the regimen was not associated with any new safety events. OS and PFS outcomes were similar between treatment arms irrespective of any history or presence of brain metastases at baseline.
Relevance (T.E. Stinchcombe)
-
The addition of tiragolumab to standard therapy did not improve clinical outcomes in extensive-stage SCLC. Agents targeting the TIGIT pathway are being investigated in non–small-cell lung cancer, and we await the results of those trials.*
*Relevance section written by JCO Associate Editor Thomas E. Stinchcombe, MD.
T-cell immunoreceptor with Ig and ITIM domains (TIGIT) is a novel inhibitory immune checkpoint receptor that is highly expressed on multiple immune cell types across different cancers, including small-cell lung cancer (SCLC).6,7 In mouse tumor models, TIGIT expression highly correlated with PD-L1 expression, and coinhibition of TIGIT and PD-L1 resulted in CD8+ T-cell–mediated tumor rejection.6 Activation of TIGIT prevents T-cell proliferation, effector cytokine production, and killing of target tumor cells, thereby limiting antitumor immune responses.6,8-11 Preventing TIGIT signaling with anti-TIGIT antibodies could help to restore this response.8,9
Tiragolumab is a fully human monoclonal antibody that binds TIGIT and prevents interaction with its ligand, polio virus receptor (PVR).6,8,9,11,12 In the phase Ia/Ib GO30103 study, tiragolumab plus atezolizumab showed preliminary antitumor activity in a number of cancers, with objective responses in non–small-cell lung cancer (NSCLC) and head and neck squamous cell carcinoma.12 Tiragolumab plus atezolizumab was also evaluated in the phase II CITYSCAPE study (ClinicalTrials.gov identifier: NCT03563716) in patients with chemotherapy-naïve, PD-L1–positive NSCLC. The combination was well tolerated and showed clinically meaningful improvements in antitumor response, PFS, and OS versus atezolizumab alone, with a greater magnitude of improvement seen in patients with high PD-L1 expression on tumor cells.13 Furthermore, the TIGIT ligand PVR is broadly expressed in SCLC cell lines and tumor samples, and is associated with poor prognosis.7,14
The randomized, placebo-controlled phase III SKYSCRAPER-02 study (ClinicalTrials.gov identifier: NCT04256421) was initiated to determine whether the antitumor effect and survival benefits of the existing standard of care (SoC), atezolizumab plus CE, could be enhanced by the addition of tiragolumab in patients with untreated ES-SCLC. Comprehensive biomarker analyses in IMpower133 and CASPIAN revealed low PD-L1 expression on tumor cells in SCLC and did not show an association between conventional immune markers (PD-L1 and tumor mutational burden) and treatment efficacy.5,15 Therefore, SKYSCRAPER-02 was planned in a biomarker unselected SCLC population with or without history/presence of brain metastases at baseline. Here, we report final PFS and OS analyses from SKYSCRAPER-02.
METHODS
Patients
Eligible patients were age 18 years and older with treatment-naïve, histologically or cytologically confirmed ES-SCLC (per modified Veterans Administration Lung Study Group staging system), measurable disease according to RECIST version 1.1, and Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0 or 1. Patients with treated or untreated brain metastases were permitted, provided the metastases were asymptomatic and measurable disease was present outside the central nervous system. Full eligibility criteria are provided in the Data Supplement (online only).
Study Design
Patients were randomly assigned 1:1 to receive four 21-day cycles of intravenous (IV) tiragolumab (600 mg once on day 1 of each cycle) or IV placebo (once on day 1 of each cycle), in combination with IV atezolizumab (1,200 mg once on day 1 of each cycle) plus IV carboplatin (area under the curve: 5 mg/mL/minute once on day 1 of each cycle for 4 cycles) and IV etoposide (100 mg/m2 once on days 1,2, and 3 of each cycle for 4 cycles). This was followed by maintenance tiragolumab or placebo plus atezolizumab in 21-day cycles until radiographic PD per RECIST version 1.1, or for as long as patients experienced clinical benefit without unacceptable toxicity as assessed by the investigator. Complete criteria for treatment beyond PD are provided in the Data Supplement. Randomization was stratified by ECOG PS (0 v 1), presence/history of brain metastases (yes v no), and lactate dehydrogenase (LDH) levels (≤upper limit of normal [ULN] v >ULN).
No tiragolumab or atezolizumab dose modifications were permitted, and no crossover was allowed between treatment arms. A double-blind, randomized safety run-in phase was conducted in 24 patients (12 in each arm), with a safety evaluation performed by an independent data monitoring committee after ≥two cycles of treatment. Patients included in the safety run-in remained double-blinded throughout and were included in the evaluation of the phase III study objectives.
The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice Guidelines. The Protocol (online only) and amendments were approved by the institutional review board or ethics committee at each site. All patients provided written informed consent.
End Points and Assessments
The primary efficacy end points were PFS and OS in all randomly assigned patients without history/presence of brain metastases at baseline (primary analysis set [PAS]). PFS was defined as the time from random assignment to the first occurrence of PD as determined by the investigator, or death from any cause. OS was defined as the time from random assignment to death from any cause. Secondary end points were PFS and OS in all randomly assigned patients, irrespective of brain metastases status at baseline (full analysis set [FAS]); confirmed objective response rate (ORR), defined as the proportion of patients with a complete response (CR) or partial response (PR) on two consecutive occasions ≥4 weeks apart; duration of response (DOR) for patients with confirmed ORR; PFS rate at 6 and 12 months; and OS rate at 12 and 24 months.
Adverse events (AEs) were evaluated in all randomly assigned patients who received at least one dose of study treatment, with severity assessed according to National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0. Tumor assessments were conducted at baseline and every 6 weeks for 48 weeks, then every 9 weeks until PD per RECIST version 1.1, withdrawal of consent, study termination, or death. Patients treated beyond PD underwent tumor assessments until discontinuation of study treatment.
Pre- and on-treatment samples of tumor tissue, serum, plasma, and blood were collected as part of the exploratory biomarker objective to identify potential biomarkers of treatment response, including the TIGIT pathway, prognosis, acquired resistance, pharmacodynamics, and safety. Exploratory biomarker analyses will be published at a later date.
Statistical Analysis
Survival analyses were performed in the PAS and FAS, with patients grouped according to assigned treatment. Safety analyses were performed according to treatment received. To control the overall type 1 error rate at 0.05 (two-sided), end points were tested hierarchically. A two-sided α of .001 and .049 was allocated to PFS and OS in the PAS, respectively. If PFS in the PAS was statistically significant at the two-sided α level of .001, OS in the PAS was tested at a two-sided α level of .05. If the OS benefit in the PAS was statistically significant, PFS and OS were tested in the FAS, using the same α-allocation ratio (1:49) and an α-recycle strategy.
A sample size of approximately 400 patients was targeted for the PAS, assuming a 15% prevalence of presence or history of brain metastases at baseline. It was estimated that approximately 470 patients would be randomly assigned within the study.
The primary analysis of the primary efficacy end point, PFS in the PAS, was planned at the time of the OS efficacy interim analysis when approximately 202 deaths had been observed in the PAS. At the time of the primary analysis of PFS, it was estimated that approximately 300 PFS events would have been observed to provide 96% power for a target PFS hazard ratio (HR) of 0.56 at a two-sided significance level of .001. This would assume a median PFS of 5.2 months in the placebo plus atezolizumab and CE arm and 9.2 months in the tiragolumab plus atezolizumab and CE arm. There was no planned interim analysis for PFS.
The final analysis of the primary end point, OS in the PAS, was planned for when approximately 288 deaths had been observed in the PAS. This would provide 85% power to detect a target OS HR of 0.70 at a two-sided significance level of .049, assuming a median OS of 12.3 months in the placebo plus atezolizumab and CE arm and 17.6 months in the tiragolumab plus atezolizumab and CE arm.
One efficacy interim and one final analysis of OS was planned. To control the type I error, stopping boundaries of these analyses were computed with the Lan-DeMets approximation to the O'Brien-Fleming.16 The stopping boundaries for the efficacy interim and final OS analyses are provided in the Data Supplement. The stratified log-rank test was used to compare PFS and OS between treatment arms; the HR for PFS and OS was estimated using a stratified Cox proportional hazards model. Kaplan-Meier methodology was used to estimate median PFS and OS, and the Brookmeyer-Crowley method was used to construct 95% CIs.
RESULTS
Patients
Overall, 490 patients (FAS) were enrolled between February 2020 and March 2021 at 121 sites across 23 countries. A total of 243 patients were randomly assigned to receive tiragolumab plus atezolizumab and CE (tiragolumab arm), and 247 were randomly assigned to receive placebo plus atezolizumab and CE (control arm; Fig 1). No safety issues were observed during the safety run-in phase. The PAS included 397 patients without history/presence of brain metastases at baseline (196 tiragolumab, 201 control).
FIG 1.
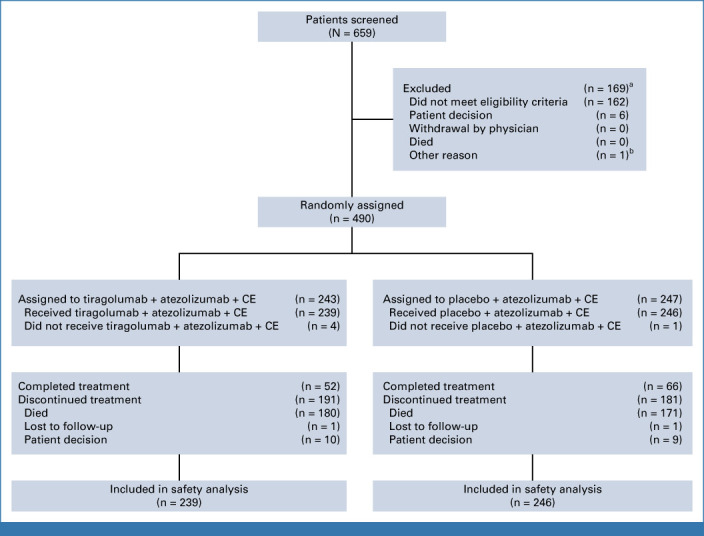
CONSORT diagram. aIncludes two patients who screen-failed, rescreened, and later enrolled. bEntered in error. CE, carboplatin plus etoposide.
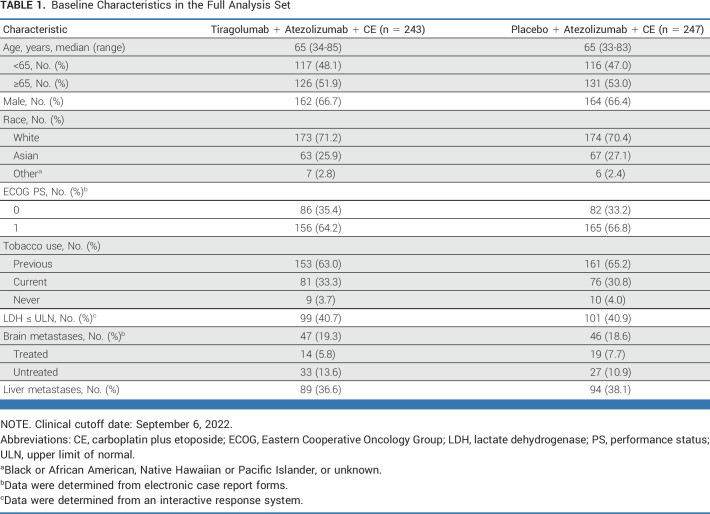
Baseline characteristics were balanced across treatment arms in the FAS (Table 1) and the PAS (Data Supplement, Table A1). In the FAS, across both arms, a higher proportion of patients with untreated brain metastases (13.6% tiragolumab, 10.9% control) were enrolled versus patients with treated brain metastases (5.8% tiragolumab, 7.7% control). Overall, 199 patients in the tiragolumab arm and 209 patients in the control arm received maintenance treatment, of whom 69 and 73 patients, respectively, received treatment beyond PD. The proportion of patients who received at least one subsequent therapy was similar in the tiragolumab and control arms in both the PAS (54.1% v 54.2%, respectively) and the FAS (53.1% v 54.7%, respectively). The most common subsequent treatment was chemotherapy (nonanthracycline) in both the tiragolumab arm and control arm in the PAS (42.9% v 42.3%, respectively) and the FAS (43.2% v 42.9%, respectively; Data Supplement, Table A2).
TABLE 1.
Baseline Characteristics in the Full Analysis Set
Progression-Free Survival
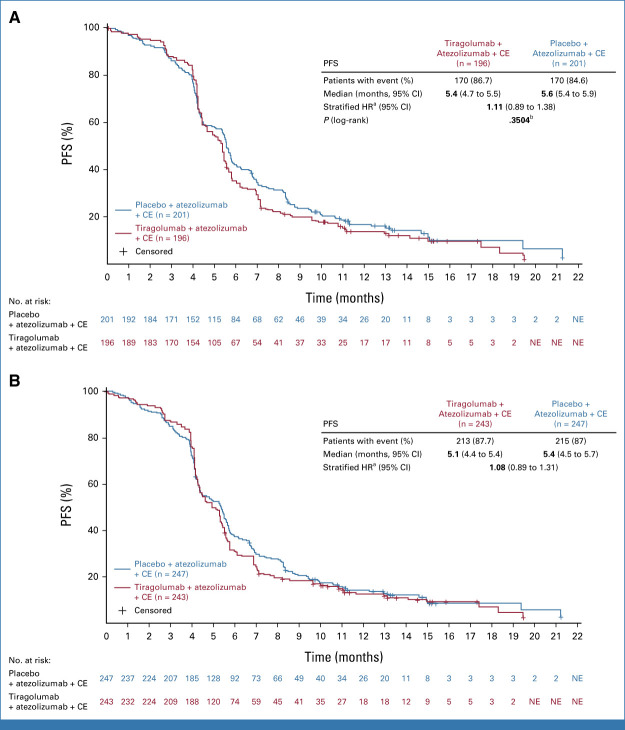
Final analysis of PFS in the PAS was planned at the time of OS interim analysis, when 212 deaths had occurred. At the clinical cutoff date (CCOD) for the final PFS analysis (February 6, 2022), PFS events were experienced by 170 (86.7%) and 170 (84.6%) patients in the PAS in the tiragolumab and control arms, respectively (Fig 2A). In the FAS, 213 (87.7%) tiragolumab patients and 215 (87.0%) control patients experienced a PFS event (Fig 2B). Median duration of follow-up was 13.9 months for the FAS and 14.3 months for the PAS. At this time point, 30 patients receiving tiragolumab and 35 receiving control remained on treatment in the FAS. At the final analysis of PFS, the primary end point of PFS in the PAS did not reach statistical significance (stratified HR, 1.11; 95% CI, 0.89 to 1.38; P = .3504; median PFS 5.4 months tiragolumab v 5.6 months control; Fig 2A). Median PFS in the FAS was similar to the PAS: 5.1 months tiragolumab and 5.4 months control (stratified HR, 1.08; 95% CI, 0.89 to 1.31; Fig 2B). In the PAS, PFS rates at 6 and 12 months were 35.2% and 14.2% with tiragolumab and 42.4% and 17.3% with control, respectively. Corresponding PFS rates in the FAS were 31.3% and 12.3% with tiragolumab and 38.0% and 14.1% with control.
FIG 2.
PFS in (A) the primary analysis set and (B) the full analysis set. Clinical cutoff date: February 6, 2022. CE, carboplatin plus etoposide; HR, hazard ratio; NE, not evaluable; PFS, progression-free survival. aStratification factors are Eastern Cooperative Oncology Group and lactate dehydrogenase. bStatistical boundary: 0.001.
No significant difference in median PFS was seen across patient subgroups defined by demographic and baseline prognostic characteristics in the PAS or FAS (data not shown).
Overall Survival
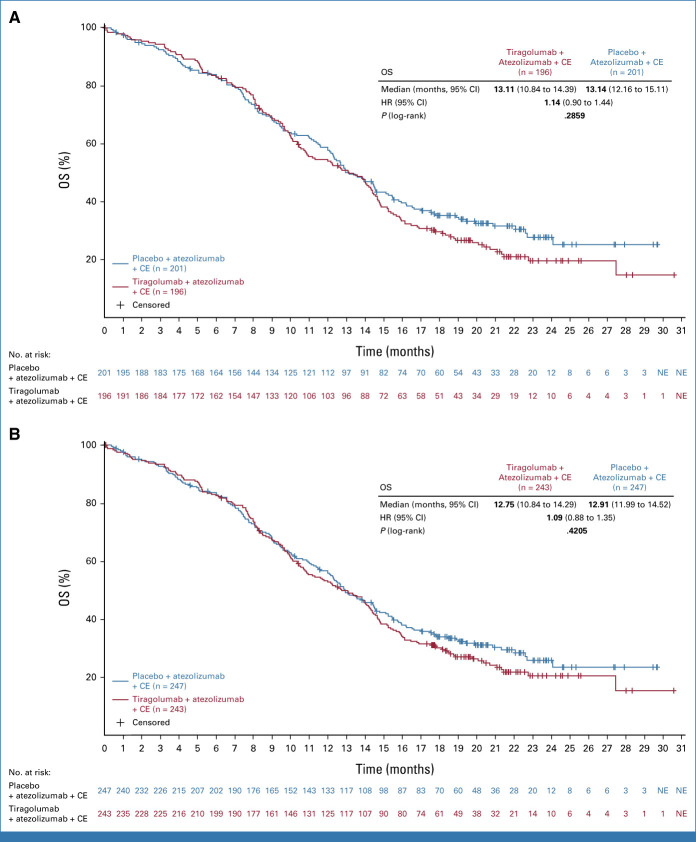
At the time of the interim OS analysis, 212 and 264 deaths had occurred in the PAS and FAS, respectively. OS data in the PAS were immature (HR, 1.04; 95% CI, 0.79 to 1.36; P = .7963; median OS, 13.6 months both treatment arms).
The final OS analysis was completed when 284 and 350 OS events had been observed in the PAS and FAS, respectively (CCOD: September 6, 2022). At this time point, 19 (7.8%) patients in the tiragolumab arm and 24 (9.7%) patients in the control arm remained on treatment in the FAS; median duration of follow-up was 21.2 months. Median OS at final OS analysis between the treatment arms was the same in the PAS (13.1 months both arms; stratified HR, 1.14; 95% CI, 0.90 to 1.44; P = .2859; Fig 3A), and similar in the FAS (12.8 months tiragolumab v 12.9 months control; stratified HR, 1.09; 95% CI, 0.88 to 1.35; P = .4205; Fig 3B). OS rates at 24 months were similar in the tiragolumab and control arms in both the PAS (20% v 28%, respectively) and the FAS (21% v 26%, respectively).
FIG 3.
OS in (A) the primary analysis set and (B) the full analysis set. Clinical cutoff date: September 6, 2022. CE, carboplatin plus etoposide; HR, hazard ratio; NE, not evaluable; OS, overall survival.
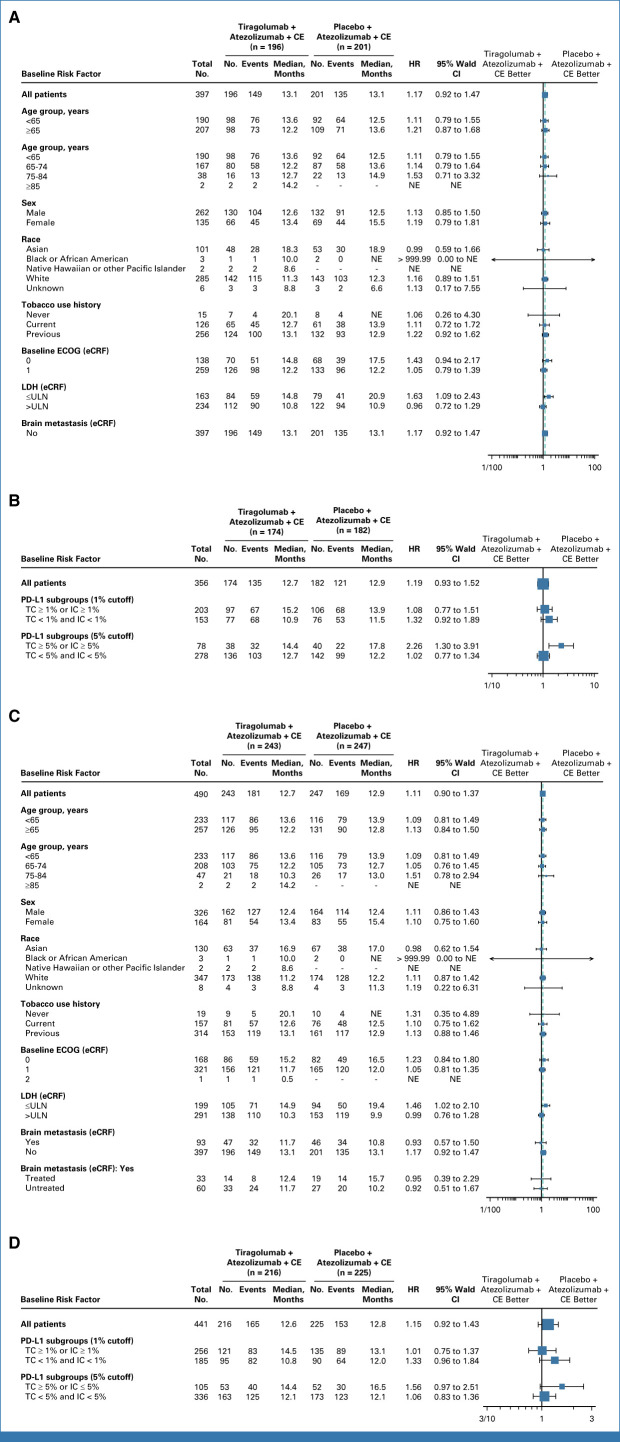
No significant difference in median OS was seen across most patient subgroups in the PAS or the FAS (Fig 4). However, in patients with LDH ≤ ULN, longer OS was observed in the control arm versus the tiragolumab arm in the FAS (HR, 1.46; 95% CI, 1.02 to 2.10; median OS, 19.4 v 14.9 months, respectively). In patients with history/presence of brain metastases at baseline in the FAS, median OS was 11.7 months with tiragolumab (n = 47) and 10.8 months with control (n = 46; unstratified HR, 0.93; 95% CI, 0.57 to 1.50; P = .7523; Fig 4B; Data Supplement, Fig A1). Within this subgroup, in patients with treated brain metastases, median OS was 12.4 months with tiragolumab (n = 14) and 15.7 with control (n = 19; unstratified HR, 0.95; 95% CI, 0.39 to 2.29). In patients with untreated brain metastases, median OS was 11.7 months (n = 33) and 10.2 months (n = 27) in the tiragolumab and control arms, respectively (unstratified HR, 0.92; 95% CI, 0.51 to 1.67; Fig 4C). In patients whose tumors expressed PD-L1 (PD-L1 ≥1% tumor cell or immune cell [SP263 VENTANA assay]), median OS was 14.5 months with tiragolumab and 13.1 months with control in the FAS (HR, 1.01; 95% CI, 0.75 to 1.37; Fig 4D). As these subgroup analyses were exploratory and conducted in small samples, the results should be interpreted with caution.
FIG 4.
Overall survival in key patient subgroups in (A) PAS, (B) PAS BEP, (C) FAS, and (D) FAS BEP. Clinical cutoff date: September 6, 2022. BEP, biomarker-evaluable; CE, carboplatin plus etoposide; ECOG, Eastern Cooperative Oncology Group; eCRF, electronic case report form; FAS, full analysis set; HR, hazard ratio; IC, immune cell score; LDH, lactate dehydrogenase; NE, not evaluable; PAS, primary analysis set; TC, tumor cell; ULN, upper limit of normal.
Tumor Response
In the PAS (CCOD: September 6, 2022), confirmed investigator-assessed ORR was 73.5% with tiragolumab and 66.7% with control (Data Supplement, Table A3). CRs were observed in 1.5% of patients in each of the tiragolumab and control arms, with PRs in 71.9% and 65.2%, respectively. Median DOR among patients with confirmed objective response in the PAS was 4.2 months with tiragolumab and 5.6 months with control. ORR in the FAS was similar to the PAS (70.8% and 65.6% with tiragolumab and control, respectively), and median DOR was 4.2 and 5.1 months, respectively.
Safety
Safety analyses were based on a CCOD of September 6, 2022. The safety population included 239 patients in the tiragolumab arm and 246 patients in the control arm. Median duration of tiragolumab or placebo treatment was similar in the tiragolumab arm and in the control arm (4.9 v 5.0 months, respectively), as was the median duration of atezolizumab treatment (4.9 v 5.0 months, respectively). Median duration of chemotherapy treatment was also similar across the treatment arms (Data Supplement, Table A4).
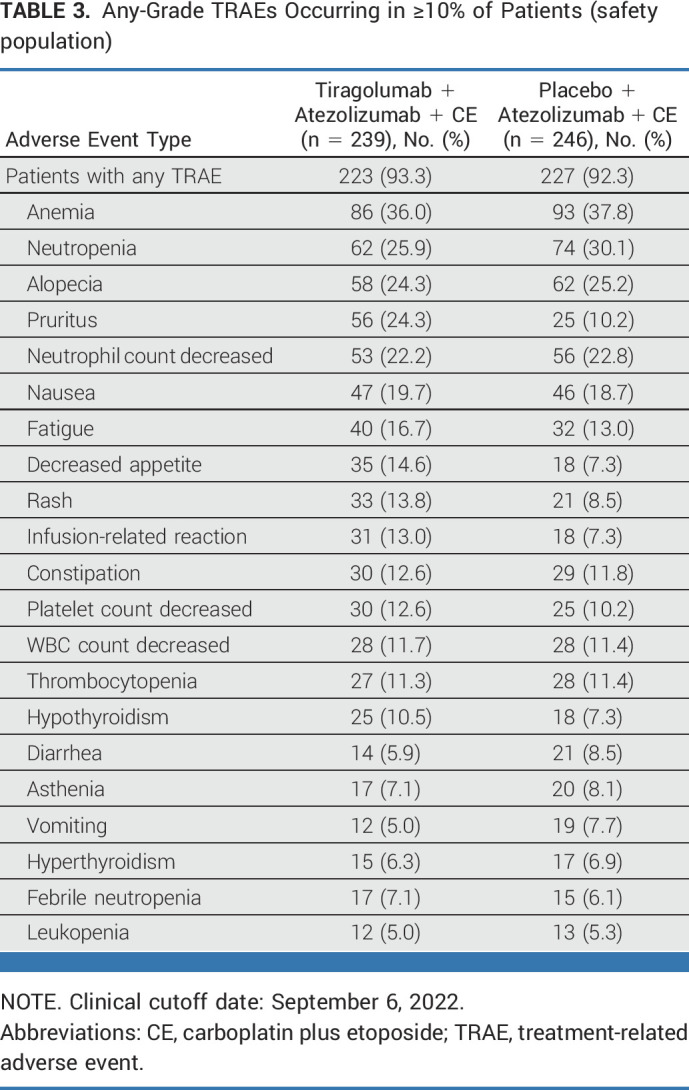
The addition of tiragolumab to atezolizumab plus CE demonstrated a similar safety profile to the control arm. In total, 238 (99.6%) patients in the tiragolumab arm and 245 (99.6%) patients in the control arm experienced ≥1 AE (Table 2), with grade 3/4 AEs in 153 (64.0%) and 157 (63.8%) patients, respectively. Treatment-related AEs (TRAEs) were reported in 223 (93.3%) and 227 (92.3%) patients in the tiragolumab and control arms, respectively (Table 2). Grade 3/4 TRAEs occurred in 126 patients (52.7%) in the tiragolumab arm and 137 (55.7%) in the control arm and were most commonly anemia and neutropenia (Table 3). The incidence of serious AEs was similar across the treatment arms (45.2% tiragolumab, 42.3% control), with febrile neutropenia and pneumonia most frequent (Data Supplement, Table A5). Grade 5 AEs were reported in 15 (6.3%) patients in the tiragolumab arm and 16 (6.5%) in the control arm. Grade 5 TRAEs occurred in one (0.4%) patient in the tiragolumab arm and five (2.0%) patients in the control arm (Table 2). AEs leading to withdrawal of any study treatment occurred in 20 patients (8.4%) in the tiragolumab arm and 23 (9.3%) in the control arm.
TABLE 2.
Safety Summary (safety population)
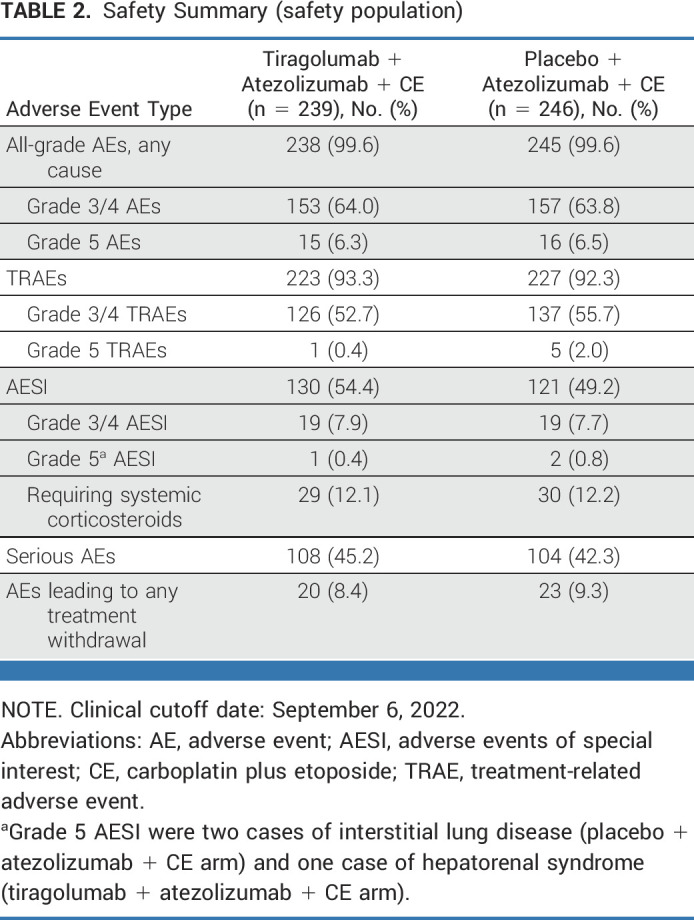
TABLE 3.
Any-Grade TRAEs Occurring in ≥10% of Patients (safety population)
Immune-mediated AEs (AEs of special interest [AESIs]) occurred in 130 (54.4%) patients in the tiragolumab arm and 121 (49.2%) patients in the control arm, with rash and hypothyroidism reported most frequently (Data Supplement, Table A6). Grade 3/4 AESIs were noted in 19 patients in each of the tiragolumab (7.9%) and control (7.7%) arms. Three grade 5 AESIs were reported: one case of hepatorenal syndrome with tiragolumab, and two cases of interstitial lung disease with control. AESIs requiring the use of systemic corticosteroids occurred in 29 patients (12.1%) in the tiragolumab arm and 30 patients (12.2%) in the control arm.
DISCUSSION
The addition of tiragolumab to atezolizumab plus CE did not provide a PFS or OS benefit compared with atezolizumab and CE in this phase III study in patients with untreated ES-SCLC. PFS rates at 6 and 12 months were lower in the tiragolumab arm than in the control arm in both the PAS and the FAS, while OS rates at 24 months were similar in both arms.
Results from the control arm of this study were consistent with data from the phase III IMpower133 study in a similar patient population. In IMpower133, median OS was 12.3 months with atezolizumab plus CE versus 10.3 months with placebo plus CE (HR, 0.70; 95% CI, 0.54 to 0.91; P = .007), with a median PFS of 5.2 and 4.3 months, respectively (HR, 0.77; 95% CI, 0.62 to 0.96; P = .02).1 We observed that survival rates were maintained in patients with brain metastases (both treated and untreated asymptomatic brain metastases) or without history/presence of brain metastases (in the FAS), consistent with data in patients with treated brain metastases in IMpower133.1 This suggests that patients with asymptomatic untreated brain metastases may also derive clinical benefit with atezolizumab plus CE. Including patients with both treated and untreated brain metastases may represent a population more reflective of real-world clinical practice and may be of interest to explore a longer follow-up in these patients. However, it must be noted that the sample size of patients with brain metastases was small in this study, and therefore, further research is required to validate these findings.
The addition of tiragolumab to atezolizumab plus CE was well tolerated, demonstrating a similar safety profile and duration of treatment to the control arm. The incidence of immune-mediated AESIs was similar across the treatment arms, with rash and hypothyroidism the most frequently reported events. A comparable proportion of patients across the treatment arms experienced grade 3/4 AESIs, with a similar proportion in each arm requiring systemic corticosteroids for AESIs. Overall, no new safety concerns were noted, and tiragolumab did not affect the safety or tolerability of atezolizumab plus CE.
In contrast to results of SKYSCRAPER-02, the combination of tiragolumab plus atezolizumab in the phase II CITYSCAPE study in chemotherapy-naïve, PD-L1–positive NSCLC showed clinically meaningful improvements in antitumor response, PFS, and OS versus atezolizumab alone, and was well tolerated.13 However, there are important biologic differences between the patient populations enrolled in CITYSCAPE and SKYSCRAPER-02. Furthermore, SCLC tumors are considered immunologic deserts with low major histocompatibility complex expression, and relatively lower PD-L1 expression on tumor cells compared with NSCLC.5,17-20 The variation in efficacy with TIGIT inhibition across tumor types highlights the need for further research into TIGIT expression and its potential prognostic and predictive impact. Although high expression of TIGIT was shown to be associated with OS and PFS in a recent meta-analysis of solid tumor data,21 targeted analyses have thus far failed to show a significant relationship between TIGIT expression and survival in patients with SCLC. By contrast, high expression of TIGIT ligand PVR (CD155) has been associated with shorter survival in SCLC, particularly alongside high expression of PD-L1.22 Clarifying mechanisms of immune regulation within SCLC and its subtypes may improve the identification of patients who may derive benefit from TIGIT inhibition as part of an immunotherapy combination.23
To our knowledge, this is the first randomized, placebo-controlled phase III study to show long-term data when combining an anti-TIGIT antibody with an anti–PD-L1 antibody for patients with untreated ES-SCLC. Although tiragolumab did not add additional benefit to atezolizumab plus chemotherapy, the control arm further confirmed the combination of atezolizumab and chemotherapy as SoC for ES-SCLC. Additional research studies, including biomarker analyses, are ongoing to elucidate differential outcomes to immune checkpoint blockade in SCLC and its subtypes.
In conclusion, tiragolumab plus atezolizumab and CE did not improve survival in patients with treatment-naïve ES-SCLC, compared with the current SoC. Despite this, data from the control arm confirmed the outcomes observed with atezolizumab plus CE in IMpower133,1 and generated evidence in a patient population with untreated brain metastases, thereby filling a data gap in IMpower133.
ACKNOWLEDGMENT
The authors thank the patients, study investigators, and clinical site staff who participated in and supported SKYSCRAPER-02. Third-party medical writing assistance, under the direction of the authors, was provided by Beth de Klerk of Ashfield MedComms, an Inizio company, and was funded by F. Hoffmann-La Roche Ltd.
Charles M. Rudin
Consulting or Advisory Role: Harpoon Therapeutics, Genentech/Roche, AstraZeneca, Bridge Medicines, Amgen, Jazz Pharmaceuticals, Earli, AbbVie, Daiichi Sankyo/UCB Japan, Kowa, Merck, D2G Oncology, Auron Therapeutics, DISCO
Research Funding: Merck (Inst), Roche/Genentech (Inst), Daiichi Sankyo (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/111056
Stephen V. Liu
Consulting or Advisory Role: Genentech, Bristol Myers Squibb, AstraZeneca, Takeda, Regeneron, Guardant Health, Janssen Oncology, MSD Oncology, Jazz Pharmaceuticals, Daiichi Sankyo/UCB Japan, Turning Point Therapeutics, Elevation Oncology, Novartis, Eisai, Gilead Sciences, Sanofi, Catalyst Pharmaceuticals, Candel Therapeutics, Merus, AbbVie, Amgen, Boehringer Ingelheim, Mirati Therapeutics
Research Funding: Genentech/Roche (Inst), Merck (Inst), Alkermes (Inst), Turning Point Therapeutics (Inst), RAPT Therapeutics (Inst), Merus (Inst), Elevation Oncology (Inst), Nuvalent Inc (Inst), Gilead Sciences (Inst), AbbVie (Inst), Ellipses Pharma (Inst)
Travel, Accommodations, Expenses: Caris Life Sciences
Ross A. Soo
Honoraria: AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Lilly, Novartis, Pfizer, Roche/Genentech, Takeda, Yuhan, Amgen, Bayer, Merck, Merck Serono, Puma Biotechnology, J INTS BIO
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Novartis, Pfizer, Roche/Genentech, Taiho Pharmaceutical, Yuhan, Takeda, Amgen, Lilly, Merck, Janssen, Puma Biotechnology, Merck Serono, Bayer, Thermo Fisher Scientific, J INTS BIO
Research Funding: AstraZeneca, Boehringer Ingelheim
Shun Lu
Consulting or Advisory Role: AstraZeneca, Pfizer, Boehringer Ingelheim, Hutchison MediPharma, Simcere, Zai Lab, GenomiCare, Yuhan, Roche, Menarini, InventisBio Co Ltd
Speakers' Bureau: AstraZeneca, Roche, Hansoh Pharma, Hengrui Therapeutics
Research Funding: AstraZeneca (Inst), Hutchison MediPharma (Inst), BMS (Inst), Hengrui Therapeutics (Inst), BeiGene (Inst), Roche (Inst), Hansoh (Inst), Lilly Suzhou Pharmaceutical Co (Inst)
Min Hee Hong
Stock and Other Ownership Interests: GI Cell, GI Biome
Honoraria: AstraZeneca, Merck, Roche
Consulting or Advisory Role: AstraZeneca, Merck, Roche, Yuhan
Research Funding: Yuhan
Maciej Bryl
Honoraria: Boehringer Ingelheim, Roche/Genentech, MSD, Bristol Myers Squibb, AstraZeneca, Takeda, Novartis
Consulting or Advisory Role: Boehringer Ingelheim, MSD, Bristol Myers Squibb, AstraZeneca, Roche/Genentech
Travel, Accommodations, Expenses: Roche/Genentech, Pfizer, Bristol Myers Squibb, Boehringer Ingelheim, MSD, AstraZeneca, Takeda, Sanofi
Daphne W. Dumoulin
Consulting or Advisory Role: Bristol Myers Squibb (Inst), Roche (Inst), MSD (Inst), AstraZeneca (Inst), Amgen (Inst)
Achim Rittmeyer
Consulting or Advisory Role: Lilly, Roche/Genentech, Boehringer Ingelheim, MSD, Bristol Myers Squibb, AstraZeneca/MedImmune, Pfizer, AbbVie, Novartis, GlaxoSmithKline
Speakers' Bureau: Roche/Genentech, Lilly, Bristol Myers Squibb, MSD, Novartis
Chao-Hua Chiu
Honoraria: AstraZeneca/MedImmune, Boehringer Ingelheim, Roche, Pfizer, Novartis, Chugai Pharma, Bristol Myers Squibb, Ono Pharmaceutical, MSD, Lilly, Amgen, Janssen, Merck KGaA, Takeda, Shionogi, Daiichi-Sankyo
Consulting or Advisory Role: Bristol Myers Squibb, Novartis, Lilly, Janssen, Merck KGaA
Melissa Johnson
Consulting or Advisory Role: Genentech/Roche (Inst), AstraZeneca (Inst), Calithera Biosciences (Inst), Merck (Inst), Sanofi (Inst), Mirati Therapeutics (Inst), AbbVie (Inst), GlaxoSmithKline (Inst), Gritstone Bio (Inst), Janssen Oncology (Inst), Amgen (Inst), Daiichi Sankyo (Inst), EcoR1 Capital (Inst), Genmab (Inst), IDEAYA Biosciences (Inst), Regeneron (Inst), Astellas Pharma (Inst), Genocea Biosciences (Inst), Molecular Axiom (Inst), Novartis (Inst), Revolution Medicines (Inst), Takeda (Inst), VBL Therapeutics (Inst), ArriVent Biopharma (Inst), Pyramid Biosciences (Inst), SeaGen (Inst), Arcus Biosciences (Inst), Boehringer Ingelheim (Inst), Bristol Myers Squibb (Inst), D3 Bio (Inst), Fate Therapeutics (Inst), Gilead Sciences (Inst), Immunocore (Inst), Jazz Pharmaceuticals (Inst), Normunity (Inst), Pfizer (Inst), Synthekine (Inst)
Research Funding: EMD Serono (Inst), Kadmon (Inst), Janssen (Inst), Mirati Therapeutics (Inst), Genmab (Inst), Pfizer (Inst), AstraZeneca (Inst), Stem CentRx (Inst), Novartis (Inst), Array BioPharma (Inst), Regeneron (Inst), Merck (Inst), Hengrui Pharmaceutical (Inst), Lycera (Inst), BeiGene (Inst), Tarveda Therapeutics (Inst), Loxo (Inst), AbbVie (Inst), Boehringer Ingelheim (Inst), Guardant Health (Inst), Daiichi Sankyo (Inst), Sanofi (Inst), CytomX Therapeutics (Inst), Dynavax Technologies (Inst), Corvus Pharmaceuticals (Inst), Incyte (Inst), Genocea Biosciences (Inst), Gritstone Bio (Inst), Amgen (Inst), Genentech/Roche (Inst), Adaptimmune (Inst), Syndax (Inst), Neovia Oncology (Inst), Acerta Pharma (Inst), Takeda (Inst), Shattuck Labs (Inst), GlaxoSmithKline (Inst), Apexigen (Inst), Atreca (Inst), OncoMed (Inst), Lilly (Inst), Immunocore (Inst), University of Michigan (Inst), TCR2 Therapeutics (Inst), Arcus Biosciences (Inst), Ribon Therapeutics (Inst), BerGenBio (Inst), Calithera Biosciences (Inst), Tmunity Therapeutics Inc (Inst), Seven and Eight Biopharmaceuticals (Inst), Rubius Therapeutics (Inst), Curis (Inst), Silicon Therapeutics (Inst), Dracen (Inst), PMV Pharma (Inst), Artios (Inst), BioAtla (Inst), Elicio Therapeutics (Inst), Erasca Inc (Inst), Harpoon (Inst), Helsinn Healthcare (Inst), Hutchison MediPharma (Inst), IDEAYA Biosciences (Inst), IGM Biosciences (Inst), Memorial Sloan-Kettering Cancer Center (Inst), NeoImmuneTech (Inst), Numab (Inst), Relay Therapeutics (Inst), Revolution Medicines (Inst), Tempest Therapeutics (Inst), Tizona Therapeutics Inc (Inst), Turning Point Therapeutics (Inst), Vyriad (Inst), Y-mAbs Therapeutics (Inst), Exelixis (Inst), Fate Therapeutics (Inst), Merus (Inst), Black Diamond Therapeutics (Inst), Kartos Therapeutics (Inst), Carisma Therapeutics (Inst), Rain Therapeutics (Inst), Nuvalent Inc (Inst), Palleon Pharmaceuticals (Inst), EQRx (Inst), Immunitas (Inst), ArriVent Biopharma (Inst), Bristol Myers Squibb (Inst), Checkpoint Therapeutics (Inst), City of Hope (Inst), Jounce Therapeutics (Inst), LockBody Therapeutics (Inst), Mythic Therapeutics (Inst), RasCal (Inst), Taiho Oncology (Inst), WindMIL (Inst)
Travel, Accommodations, Expenses: AbbVie, AstraZeneca, Genentech, Incyte, Merck, Pfizer, Sanofi
Alejandro Navarro
Consulting or Advisory Role: Boehringer Ingelheim, Bristol Myers Squibb Foundation, Pfizer, Amgen, Takeda
Speakers' Bureau: Roche, AstraZeneca Spain
Expert Testimony: Oryzon Genomics, Medsir, Hengenix
Travel, Accommodations, Expenses: Boehringer Ingelheim, Pfizer, Roche
Silvia Novello
Consulting or Advisory Role: Sanofi
Speakers' Bureau: AstraZeneca, MSD, Bristol Myers Squibb, Roche, Pfizer, Lilly, Takeda, AbbVie, Boehringer Ingelheim, Bayer, Amgen, Beigene, Novartis, Janssen
Yuichi Ozawa
Honoraria: AstraZeneca, Chugai Pharma, MSD, Ono Pharmaceutical, Taiho Pharmaceutical, Nippon Kayaku, Novartis
Consulting or Advisory Role: AstraZeneca
Sammi Hiu Tam
Employment: Genentech/Roche
Stock and Other Ownership Interests: Roche/Genentech
Namrata S. Patil
Employment: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Xiaohui Wen
Employment: Roche/Genentech
Stock and Other Ownership Interests: Roche
Patents, Royalties, Other Intellectual Property: No money compensation
Travel, Accommodations, Expenses: Roche/Genentech
Meilin Huang
Employment: Roche/Genentech
Stock and Other Ownership Interests: Roche/Genentech
Tien Hoang
Employment: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Raymond Meng
Employment: Roche/Genentech
Stock and Other Ownership Interests: Roche/Genentech
Martin Reck
Consulting or Advisory Role: Lilly, MSD Oncology, Merck Serono, Bristol Myers Squibb, AstraZeneca, Boehringer Ingelheim, Pfizer, Novartis, Roche/Genentech, AbbVie, Amgen, Mirati Therapeutics, Samsung Bioepis, Sanofi/Regeneron, Daiichi Sankyo Europe GmbH
Speakers' Bureau: Roche/Genentech, Lilly, MSD Oncology, Merck Serono, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Pfizer, Novartis, Amgen, Mirati Therapeutics, Sanofi/Aventis
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the 2022 ASCO Annual Meeting (abstr LBA8507), Chicago, IL, June 3-7, 2022.
SUPPORT
Supported by Genentech Inc and F. Hoffmann-La Roche Ltd. C.M.D.'s work on small cell lung cancer is supported by NIH R35CA263816, U24CA13274, and P30CA008748.
CLINICAL TRIAL INFORMATION
NCT04256421 (SKYSCRAPER-02)
DATA SHARING STATEMENT
For up-to-date details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://go.roche.com/data_sharing. Anonymized records for individual patients across more than one data source external to Roche cannot, and should not, be linked because of a potential increase in risk of patient reidentification.
AUTHOR CONTRIBUTIONS
Conception and design: Stephen V. Liu, Ross A. Soo, Min Hee Hong, Sammi Hiu Tam, Namrata S. Patil, Xiaohui Wen, Tien Hoang, Raymond Meng, Martin Reck
Administrative support: Achim Rittmeyer
Provision of study materials or patients: Stephen V. Liu, Ross A. Soo, Shun Lu, Achim Rittmeyer, Chao-Hua Chiu, Melissa Johnson, Alejandro Navarro, Silvia Novello, Yuichi Ozawa, Martin Reck
Collection and assembly of data: Charles M. Rudin, Stephen V. Liu, Ross A. Soo, Shun Lu, Min Hee Hong, Jong-Seok Lee, Maciej Bryl, Achim Rittmeyer, Ozgur Ozyilkan, Melissa Johnson, Alejandro Navarro, Yuichi Ozawa, Sammi Hiu Tam, Namrata S. Patil, Xiaohui Wen, Tien Hoang, Raymond Meng, Martin Reck
Data analysis and interpretation: Charles M. Rudin, Stephen V. Liu, Ross A. Soo, Shun Lu, Maciej Bryl, Daphne W. Dumoulin, Achim Rittmeyer, Chao-Hua Chiu, Melissa Johnson, Alejandro Navarro, Silvia Novello, Sammi Hiu Tam, Namrata S. Patil, Xiaohui Wen, Meilin Huang, Tien Hoang, Raymond Meng, Martin Reck
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
SKYSCRAPER-02: Tiragolumab in Combination With Atezolizumab Plus Chemotherapy in Untreated Extensive-Stage Small-Cell Lung Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Charles M. Rudin
Consulting or Advisory Role: Harpoon Therapeutics, Genentech/Roche, AstraZeneca, Bridge Medicines, Amgen, Jazz Pharmaceuticals, Earli, AbbVie, Daiichi Sankyo/UCB Japan, Kowa, Merck, D2G Oncology, Auron Therapeutics, DISCO
Research Funding: Merck (Inst), Roche/Genentech (Inst), Daiichi Sankyo (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/111056
Stephen V. Liu
Consulting or Advisory Role: Genentech, Bristol Myers Squibb, AstraZeneca, Takeda, Regeneron, Guardant Health, Janssen Oncology, MSD Oncology, Jazz Pharmaceuticals, Daiichi Sankyo/UCB Japan, Turning Point Therapeutics, Elevation Oncology, Novartis, Eisai, Gilead Sciences, Sanofi, Catalyst Pharmaceuticals, Candel Therapeutics, Merus, AbbVie, Amgen, Boehringer Ingelheim, Mirati Therapeutics
Research Funding: Genentech/Roche (Inst), Merck (Inst), Alkermes (Inst), Turning Point Therapeutics (Inst), RAPT Therapeutics (Inst), Merus (Inst), Elevation Oncology (Inst), Nuvalent Inc (Inst), Gilead Sciences (Inst), AbbVie (Inst), Ellipses Pharma (Inst)
Travel, Accommodations, Expenses: Caris Life Sciences
Ross A. Soo
Honoraria: AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Lilly, Novartis, Pfizer, Roche/Genentech, Takeda, Yuhan, Amgen, Bayer, Merck, Merck Serono, Puma Biotechnology, J INTS BIO
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Novartis, Pfizer, Roche/Genentech, Taiho Pharmaceutical, Yuhan, Takeda, Amgen, Lilly, Merck, Janssen, Puma Biotechnology, Merck Serono, Bayer, Thermo Fisher Scientific, J INTS BIO
Research Funding: AstraZeneca, Boehringer Ingelheim
Shun Lu
Consulting or Advisory Role: AstraZeneca, Pfizer, Boehringer Ingelheim, Hutchison MediPharma, Simcere, Zai Lab, GenomiCare, Yuhan, Roche, Menarini, InventisBio Co Ltd
Speakers' Bureau: AstraZeneca, Roche, Hansoh Pharma, Hengrui Therapeutics
Research Funding: AstraZeneca (Inst), Hutchison MediPharma (Inst), BMS (Inst), Hengrui Therapeutics (Inst), BeiGene (Inst), Roche (Inst), Hansoh (Inst), Lilly Suzhou Pharmaceutical Co (Inst)
Min Hee Hong
Stock and Other Ownership Interests: GI Cell, GI Biome
Honoraria: AstraZeneca, Merck, Roche
Consulting or Advisory Role: AstraZeneca, Merck, Roche, Yuhan
Research Funding: Yuhan
Maciej Bryl
Honoraria: Boehringer Ingelheim, Roche/Genentech, MSD, Bristol Myers Squibb, AstraZeneca, Takeda, Novartis
Consulting or Advisory Role: Boehringer Ingelheim, MSD, Bristol Myers Squibb, AstraZeneca, Roche/Genentech
Travel, Accommodations, Expenses: Roche/Genentech, Pfizer, Bristol Myers Squibb, Boehringer Ingelheim, MSD, AstraZeneca, Takeda, Sanofi
Daphne W. Dumoulin
Consulting or Advisory Role: Bristol Myers Squibb (Inst), Roche (Inst), MSD (Inst), AstraZeneca (Inst), Amgen (Inst)
Achim Rittmeyer
Consulting or Advisory Role: Lilly, Roche/Genentech, Boehringer Ingelheim, MSD, Bristol Myers Squibb, AstraZeneca/MedImmune, Pfizer, AbbVie, Novartis, GlaxoSmithKline
Speakers' Bureau: Roche/Genentech, Lilly, Bristol Myers Squibb, MSD, Novartis
Chao-Hua Chiu
Honoraria: AstraZeneca/MedImmune, Boehringer Ingelheim, Roche, Pfizer, Novartis, Chugai Pharma, Bristol Myers Squibb, Ono Pharmaceutical, MSD, Lilly, Amgen, Janssen, Merck KGaA, Takeda, Shionogi, Daiichi-Sankyo
Consulting or Advisory Role: Bristol Myers Squibb, Novartis, Lilly, Janssen, Merck KGaA
Melissa Johnson
Consulting or Advisory Role: Genentech/Roche (Inst), AstraZeneca (Inst), Calithera Biosciences (Inst), Merck (Inst), Sanofi (Inst), Mirati Therapeutics (Inst), AbbVie (Inst), GlaxoSmithKline (Inst), Gritstone Bio (Inst), Janssen Oncology (Inst), Amgen (Inst), Daiichi Sankyo (Inst), EcoR1 Capital (Inst), Genmab (Inst), IDEAYA Biosciences (Inst), Regeneron (Inst), Astellas Pharma (Inst), Genocea Biosciences (Inst), Molecular Axiom (Inst), Novartis (Inst), Revolution Medicines (Inst), Takeda (Inst), VBL Therapeutics (Inst), ArriVent Biopharma (Inst), Pyramid Biosciences (Inst), SeaGen (Inst), Arcus Biosciences (Inst), Boehringer Ingelheim (Inst), Bristol Myers Squibb (Inst), D3 Bio (Inst), Fate Therapeutics (Inst), Gilead Sciences (Inst), Immunocore (Inst), Jazz Pharmaceuticals (Inst), Normunity (Inst), Pfizer (Inst), Synthekine (Inst)
Research Funding: EMD Serono (Inst), Kadmon (Inst), Janssen (Inst), Mirati Therapeutics (Inst), Genmab (Inst), Pfizer (Inst), AstraZeneca (Inst), Stem CentRx (Inst), Novartis (Inst), Array BioPharma (Inst), Regeneron (Inst), Merck (Inst), Hengrui Pharmaceutical (Inst), Lycera (Inst), BeiGene (Inst), Tarveda Therapeutics (Inst), Loxo (Inst), AbbVie (Inst), Boehringer Ingelheim (Inst), Guardant Health (Inst), Daiichi Sankyo (Inst), Sanofi (Inst), CytomX Therapeutics (Inst), Dynavax Technologies (Inst), Corvus Pharmaceuticals (Inst), Incyte (Inst), Genocea Biosciences (Inst), Gritstone Bio (Inst), Amgen (Inst), Genentech/Roche (Inst), Adaptimmune (Inst), Syndax (Inst), Neovia Oncology (Inst), Acerta Pharma (Inst), Takeda (Inst), Shattuck Labs (Inst), GlaxoSmithKline (Inst), Apexigen (Inst), Atreca (Inst), OncoMed (Inst), Lilly (Inst), Immunocore (Inst), University of Michigan (Inst), TCR2 Therapeutics (Inst), Arcus Biosciences (Inst), Ribon Therapeutics (Inst), BerGenBio (Inst), Calithera Biosciences (Inst), Tmunity Therapeutics Inc (Inst), Seven and Eight Biopharmaceuticals (Inst), Rubius Therapeutics (Inst), Curis (Inst), Silicon Therapeutics (Inst), Dracen (Inst), PMV Pharma (Inst), Artios (Inst), BioAtla (Inst), Elicio Therapeutics (Inst), Erasca Inc (Inst), Harpoon (Inst), Helsinn Healthcare (Inst), Hutchison MediPharma (Inst), IDEAYA Biosciences (Inst), IGM Biosciences (Inst), Memorial Sloan-Kettering Cancer Center (Inst), NeoImmuneTech (Inst), Numab (Inst), Relay Therapeutics (Inst), Revolution Medicines (Inst), Tempest Therapeutics (Inst), Tizona Therapeutics Inc (Inst), Turning Point Therapeutics (Inst), Vyriad (Inst), Y-mAbs Therapeutics (Inst), Exelixis (Inst), Fate Therapeutics (Inst), Merus (Inst), Black Diamond Therapeutics (Inst), Kartos Therapeutics (Inst), Carisma Therapeutics (Inst), Rain Therapeutics (Inst), Nuvalent Inc (Inst), Palleon Pharmaceuticals (Inst), EQRx (Inst), Immunitas (Inst), ArriVent Biopharma (Inst), Bristol Myers Squibb (Inst), Checkpoint Therapeutics (Inst), City of Hope (Inst), Jounce Therapeutics (Inst), LockBody Therapeutics (Inst), Mythic Therapeutics (Inst), RasCal (Inst), Taiho Oncology (Inst), WindMIL (Inst)
Travel, Accommodations, Expenses: AbbVie, AstraZeneca, Genentech, Incyte, Merck, Pfizer, Sanofi
Alejandro Navarro
Consulting or Advisory Role: Boehringer Ingelheim, Bristol Myers Squibb Foundation, Pfizer, Amgen, Takeda
Speakers' Bureau: Roche, AstraZeneca Spain
Expert Testimony: Oryzon Genomics, Medsir, Hengenix
Travel, Accommodations, Expenses: Boehringer Ingelheim, Pfizer, Roche
Silvia Novello
Consulting or Advisory Role: Sanofi
Speakers' Bureau: AstraZeneca, MSD, Bristol Myers Squibb, Roche, Pfizer, Lilly, Takeda, AbbVie, Boehringer Ingelheim, Bayer, Amgen, Beigene, Novartis, Janssen
Yuichi Ozawa
Honoraria: AstraZeneca, Chugai Pharma, MSD, Ono Pharmaceutical, Taiho Pharmaceutical, Nippon Kayaku, Novartis
Consulting or Advisory Role: AstraZeneca
Sammi Hiu Tam
Employment: Genentech/Roche
Stock and Other Ownership Interests: Roche/Genentech
Namrata S. Patil
Employment: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Xiaohui Wen
Employment: Roche/Genentech
Stock and Other Ownership Interests: Roche
Patents, Royalties, Other Intellectual Property: No money compensation
Travel, Accommodations, Expenses: Roche/Genentech
Meilin Huang
Employment: Roche/Genentech
Stock and Other Ownership Interests: Roche/Genentech
Tien Hoang
Employment: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Raymond Meng
Employment: Roche/Genentech
Stock and Other Ownership Interests: Roche/Genentech
Martin Reck
Consulting or Advisory Role: Lilly, MSD Oncology, Merck Serono, Bristol Myers Squibb, AstraZeneca, Boehringer Ingelheim, Pfizer, Novartis, Roche/Genentech, AbbVie, Amgen, Mirati Therapeutics, Samsung Bioepis, Sanofi/Regeneron, Daiichi Sankyo Europe GmbH
Speakers' Bureau: Roche/Genentech, Lilly, MSD Oncology, Merck Serono, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Pfizer, Novartis, Amgen, Mirati Therapeutics, Sanofi/Aventis
No other potential conflicts of interest were reported.
REFERENCES
- 1.Horn L, Mansfield AS, Szczęsna A, et al. : First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 379:2220-2229, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Clinical Practice Guidelines in Oncology: Small Cell Lung Cancer. 2022. http://NCCN.org [Google Scholar]
- 3.Paz-Ares L, Dvorkin M, Chen Y, et al. : Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet 394:1929-1939, 2019 [DOI] [PubMed] [Google Scholar]
- 4.Cheng YC, Han L, Wu L, et al. : Effect of first-line serplulimab vs placebo added to chemotherapy on survival in patients with extensive-stage small cell lung cancer. JAMA 328:1223-1232, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu SV, Reck M, Mansfield AS, et al. : Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J Clin Oncol 39:619-630, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnston RJ, Comps-Agrar L, Hackney J, et al. : The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell 26:923-937, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Yu H, Koczara C, Lohinai Z, et al. : Expression of the immune checkpoint axis-PVR/TIGIT in small cell lung cancer. J Thorac Oncol 13:S974-S975, 2018. (10 suppl; abstr P3.12-13) [Google Scholar]
- 8.Manieri NA, Chiang EY, Grogan JL: TIGIT: A key inhibitor of the cancer immunity cycle. Trends Immunol 38:20-28, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Chiang EY, Mellman I. TIGIT-CD226-PVR axis: Advancing immune checkpoint blockade for cancer immunotherapy. J Immunother Cancer 10:e004711, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu X, Harden K, Gonzalez LC, et al. : The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol 10:48-57, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Kurtulus S, Sakuishi K, Ngiow S-F, et al. : TIGIT predominantly regulates the immune response via regulatory T cells. J Clin Invest 125:4053-4062, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Bendell JC, Bedard P, Bang Y-J, et al. : Phase Ia/Ib dose-escalation study of the anti-TIGIT antibody tiragolumab as a single agent and in combination with atezolizumab in patients with advanced solid tumors. Cancer Res 80, 2020. (16 suppl; abstr CT302) [Google Scholar]
- 13.Cho BC, Rodriguez Abreu D, Hussein M, et al. : Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): Primary and follow-up analyses of a randomised, double-blind, phase 2 study. Lancet Oncol 23:781-792, 2022 [DOI] [PubMed] [Google Scholar]
- 14.Lee JB, Hong MH, Park SY, et al. : Overexpression of PVR and PD-L1 and its association with prognosis in surgically resected squamous cell lung carcinoma. Sci Rep 11:8551, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reinmuth N, Goldman JW, Garassino MC, et al. : Durvalumab (D) ± tremelimumab (T) + platinum–etoposide (EP) in 1L extensive-stage (ES) SCLC: Characteristics of long-term survivors in the CASPIAN study. Ann Oncol 33:S97-S98, 2022. (suppl 2; abstr 141O) [Google Scholar]
- 16.DeMets DL, Lan KK. Interim analysis: The alpha spending function approach. Stat Med 13:1341-1352, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Gay C, Stewart A, Park EM, et al. : Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell 39:346-360.e7, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas A, Vilimas R, Trindade C, et al. : Durvalumab in combination with olaparib in patients with relapsed SCLC: Results from a phase II study. J Thorac Oncol 14:1447-1457, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mansfield AS, Każarnowicz A, Karaseva N, et al. : Safety and patient-reported outcomes of atezolizumab, carboplatin, and etoposide in extensive-stage small-cell lung cancer (Impower133): A randomized phase I/III trial. Ann Oncol 31:310-317, 2020 [DOI] [PubMed] [Google Scholar]
- 20.Zimmermann S, Peters S, Owinokoko T, et al. : Immune checkpoint inhibitors in the management of lung cancer. Am Soc Clin Oncol Educ Book 38:682-695, 2018 [DOI] [PubMed] [Google Scholar]
- 21.Xiao K, Xiao K, Li K, et al. : Prognostic Role of TIGIT Expression in Patients with Solid Tumors: A Meta-Analysis. J Immunol Res 2021:5440572, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Y, Cui G, Jiang Z, et al. : Survival analysis with regard to PD-L1 and CD155 expression in human small cell lung cancer and a comparison with associated receptors. Oncol Lett 17:2960-2968, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pescia C, Pini G, Olmeda E, et al. : TIGIT in lung cancer: Potential theranostic implications. Life (Basel) 13:1050, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
For up-to-date details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://go.roche.com/data_sharing. Anonymized records for individual patients across more than one data source external to Roche cannot, and should not, be linked because of a potential increase in risk of patient reidentification.