Abstract
PURPOSE
In patients with peritoneal metastasis (PM) from gastric cancer (GC), chemotherapy is the treatment of choice. Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) are still being debated. This randomized, controlled, open-label, multicenter phase III trial (EudraCT 2006-006088-22; ClinicalTrials.gov identifier: NCT02158988) explored the impact on overall survival (OS) of HIPEC after CRS.
PATIENTS AND METHODS
Adult patients with GC and histologically proven PM were randomly assigned (1:1) to perioperative chemotherapy and CRS alone (CRS-A) or CRS plus HIPEC (CRS + H). HIPEC comprised mitomycin C 15 mg/m2 and cisplatin 75 mg/m2 in 5 L of saline perfused for 60 minutes at 42°C. The primary end point was OS; secondary endpoints included progression-free survival (PFS), other distant metastasis-free survival (MFS), and safety. Analyses followed the intention-to-treat principle.
RESULTS
Between March 2014 and June 2018, 105 patients were randomly assigned (53 patients to CRS-A and 52 patients to CRS + H). The trial stopped prematurely because of slow recruitment. In 55 patients, treatment stopped before CRS mainly due to disease progression/death. Median OS was the same for both groups (CRS + H, 14.9 [97.2% CI, 8.7 to 17.7] months v CRS-A, 14.9 [97.2% CI, 7.0 to 19.4] months; P = .1647). The PFS was 3.5 months (95% CI, 3.0 to 7.0) in the CRS-A group and 7.1 months (95% CI, 3.7 to 10.5; P = .047) in the CRS + H group. The CRS + H group showed better MFS (10.2 months [95% CI, 7.7 to 14.7] v CRS-A, 9.2 months [95% CI, 6.8 to 11.5]; P = .0286). The incidence of grade ≥3 adverse events (AEs) was similar between groups (CRS-A, 38.1% v CRS + H, 43.6%; P = .79).
CONCLUSION
This study showed no OS difference between CRS + H and CRS-A. PFS and MFS were significantly better in the CRS + H group, which needs further exploration. HIPEC did not increase AEs.
INTRODUCTION
In locally advanced gastric cancer (GC), laparoscopy reveals about 30% of patients with previously undetected peritoneal metastases (PMs). Very few trials address treatment of synchronous isolated PMs. Cytoreductive surgery (CRS) was developed as a promising treatment strategy for PMs in GC.1 CRS involves en bloc resection of macroscopically detectable lesions and oncological resection of the primary cancer including lymphadenectomy and peritonectomy. Hyperthermic intraperitoneal chemotherapy (HIPEC) can be used to eliminate micrometastases after CRS. In HIPEC, chemotherapy agents are heated to 41°C-43°C to intensify their effect and enhance tissue penetration. The effect of cisplatin and mitomycin C is enhanced by hyperthermia, so these agents are most commonly used for HIPEC.2 Retrospective observational studies revealed an increased median overall survival (OS) of up to 27.7 months after the combination of CRS and HIPEC in patients with peritoneal metastatic gastric cancer (pmGC).3 However, because of the poor prognosis and complex characteristics of GC subtypes, the clinical value of CRS and HIPEC remains controversial.
CONTEXT
Key Objective
The GASTRIPEC-I trial analyzed the additional benefit on hyperthermic intraperitoneal chemotherapy (HIPEC) after cytoreductive surgery (CRS) in peritoneal metastatic gastric cancer (pmGC).
Knowledge Generated
HIPEC after CRS in pmGC did not affect overall survival (OS), but progression-free survival and other distant metastasis-free survival were significantly prolonged, and addition of HIPEC did not increase surgical complications. When complete CRS is achieved, additional HIPEC significantly improves OS.
Relevance (E.M. O'Reilly)
-
Hyperthermic intraperitoneal chemotherapy has a debated role in advanced gastrointestinal cancers. The data from this randomized study inform that hyperthermic intraperitoneal chemotherapy following debulking surgery, confers a palliative benefit in patients with metastatic GC with peritoneal metastases with a subset having more durable benefit. The debate continues.*
*Relevance section written by JCO Associate Editor Eileen M. O'Reilly, MD.
PATIENTS AND METHODS
Study Design
The GASTRIPEC-I trial was a prospective, randomized, parallel-group, open-label, controlled, multicenter phase III study of CRS with versus without HIPEC after preoperative chemotherapy for GC including adenocarcinoma of the esophagogastric junction (AEG) with histologically proven primary PMs (ClinicalTrials.gov identifier: NCT02158988; clinicaltrialsregister.eu: 2006-006088-22).4 Center selection is described in the Data Supplement (Tables S1 and S2, online only).
The trial was conducted in accordance with the Declaration of Helsinki, approved by the Federal Institute for Drugs and Medical Devices (BfArM) and by all involved ethics committees.
Participants
Patients age 18-75 years, presenting with GC (including AEG) and biopsy-confirmed PMs without further distant metastases except Krukenberg tumors not pretreated with chemotherapy/radiotherapy were eligible (for details see the Data Supplement, Table S3).
Random Assignment and Masking
Patients were randomly assigned (1:1) to CRS plus HIPEC (CRS + H) or CRS alone (CRS-A) using a modified Pocock algorithm.5 Random assignment was stratified by center, human epidermal growth factor receptor 2 (HER2) status (positive v negative/unknown), and Peritoneal Cancer Index (PCI) score (≤6, 7-13, >13; Data Supplement, Methods).
Procedures
After initial staging, including computed tomography of the chest, abdomen, and pelvis, laparoscopic evaluation of PCI, and histological proof of PMs, all patients received preoperative and postoperative chemotherapy according to their HER2 status. The aim of CRS was complete cytoreduction.6 For details see the Data Supplement (Methods). Patients were followed up for 30 months after random assignment or until death. For details see the Data Supplement (Table S4).
Adverse events (AEs) were assessed according to National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0).
Outcomes
The primary end point was OS, defined as the time from random assignment to death from any cause. If a patient stopped study treatment before CRS, they were censored (sensitivity analysis without these censorings). Secondary end points included progression-free survival (PFS; defined as interval between random assignment and disease progression or death from any cause) and other distant metastasis-free survival (MFS; defined as the time from random assignment to occurrence of new distant metastases, excluding PMs and preexisting Krukenberg tumor or death from any cause). A patient was censored for PFS and MFS in case of premature termination of trial treatment.
Further secondary end points included secondary surgical procedures within 2.5 years after CRS directly caused by tumor progression; length of hospital stay (calculated as number of days in hospital per 100 days in the study); 30-day complication rate after CRS counting death from any cause, severe sepsis, septic shock, or renal failure as events to monitor safety concerns over HIPEC; and frequency of AEs and serious AEs with special attention to expected toxicities and predefined AEs (Data Supplement, Table S5).
Statistical Analysis
The primary end point was the effect on OS of adding HIPEC to CRS and chemotherapy. We aimed to identify a hazard ratio of 0.65 for OS using the Fleming-Harrington test with a two-sided global significance level of 5%, power of 80%, observation time of 2.5 years, OS rate of 75%-80% 4 months after random assignment (the expected time point of CRS and HIPEC), and 15% censoring during the whole trial, thus requiring 180 patients in total (Data Supplement, Methods). We aimed to enroll these patients within 36 months.
Analyses followed the intention-to-treat principle and were based on the full analysis set. We expected the treatment effect to be reflected in later events, so the primary (OS) and main secondary end points (PFS and MFS) were analyzed using the Fleming-Harrington test with P = 0 and q = 1, that is, higher weights on later events, and estimated by Kaplan-Meier methodology. The secondary safety end point—30-day rate of complications after CRS—was examined using a noninferiority test according to Farrington/Manning7 with the null hypothesis rate in the group CRS + H – CRS-A ≤5%.
Safety analyses were performed in the safety analysis set, which comprised all patients who received any investigational medicinal product or started CRS. Notably, safety analyses of CRS or HIPEC also considered patients who started but did not complete CRS (Data Supplement, Table S6).
Role of the Funding Source
The funders of this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study after termination of the study and had final responsibility for the decision to submit for publication.
RESULTS
Recruitment for the GASTRIPEC-I trial started in February 2014, with first patient in on March 4. By June 2018, 281 patients at 23 centers had been screened for eligibility, and 176 were ineligible most commonly because of selection criteria not being met (135 [77%]; Fig 1). In total, 105 patients were randomly assigned to treatment, 52 to CRS + H and 53 patients to CRS-A (Fig 1). Recruitment was stopped prematurely on July 13, 2018, because of slow recruitment. Treatment and follow-up were completed according to study protocol for all remaining patients. The details of participants' baseline characteristics are provided in Table 1.
FIG 1.
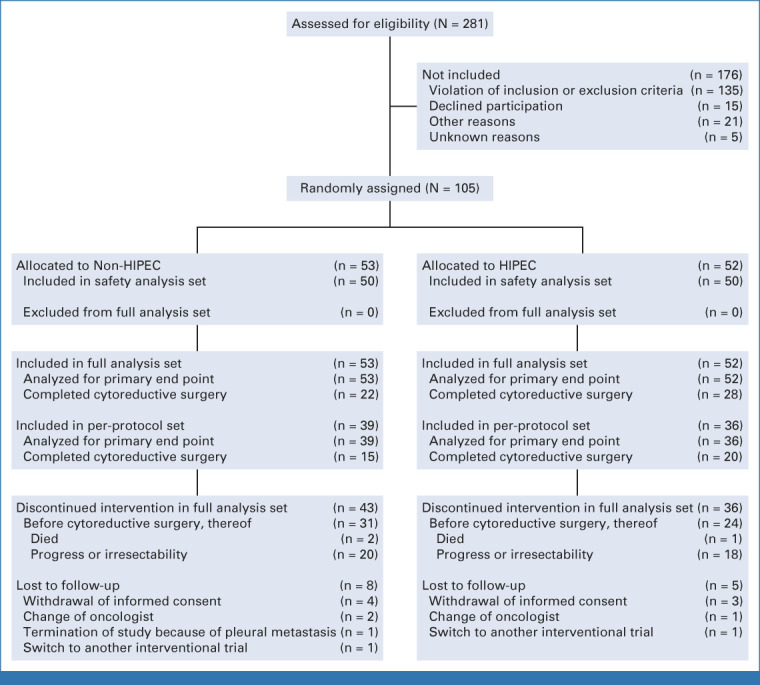
CONSORT diagram. HIPEC, hyperthermic intraperitoneal chemotherapy.
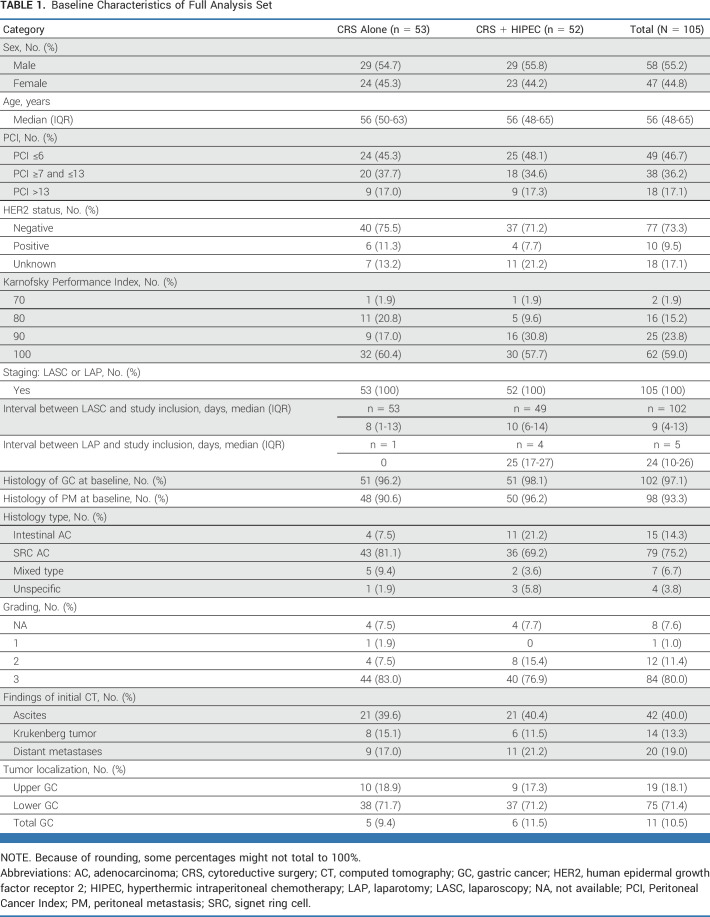
TABLE 1.
Baseline Characteristics of Full Analysis Set
Preoperative chemotherapy was started in 100 of 105 patients. Owing to progression under treatment or other reasons (Data Supplement, Table S7), only 22 of 53 patients (41%) in the CRS-A group and 28 of 52 patients (54%) in the CRS + H group received all three cycles of preoperative chemotherapy and underwent complete CRS (Data Supplement, Table S8).
The timing of CRS was nearly the same in both groups. Owing to progressive disease, 13 of 53 patients (24.5%) in the CRS-A group and 11 of 52 patients (21.2%) in the CRS + H group had unresectable tumors. CRS with the aim of complete cytoreduction was possible in 22 of 53 patients (41.5%) in the CRS-A group and in 28 of 52 patients (53.8%) in the CRS + H group (Data Supplement, Table S9).
HIPEC was administered to all patients (n = 28) in the CRS + H group who underwent surgery. According to the study design, another 11 patients in the CRS + H group received HIPEC although resection was not possible (Data Supplement, Table S9). Most patients (24 of 28 [85.7%]) had synchronous HIPEC. More details are provided in the Data Supplement (Table S9).
Pathologic findings are described in the Data Supplement (Table S10) and did not differ between groups.
From the 50 patients who underwent complete cytoreduction, 36 patients (72%) received the first postoperative chemotherapy according to the protocol (cycle 4). The median interval from CRS and start of the 4th cycle was 53 days (IQR, 46-69 days). Thirty-two patients (64%) received the second postoperative cycle (cycle 5) and 26 patients (52%) the third postoperative cycle (cycle 6). Chemotherapy other than mandated in the study protocol (eg, fluorouracil, folinic acid, oxaliplatin, and docetaxel [FLOT]) was administered to 11 of 53 patients (20.8%) in the CRS-A group and 13 of 52 patients (25.0%) in the CRS + H group (Data Supplement, Table S8). At least one protocol violation was documented in 14 of 53 patients (26.4%) in the CRS-A group and 16 of 52 patients (30.8%) in the CRS + H group, so the per-protocol set comprised 39 patients (73.6%) in the CRS-A group and 36 patients (69.2%) in the CRS + H group.
The median observation time for the primary end point was 3.6 (range, 0.2-65.4) months; most patients were censored for premature termination of study treatment before CRS (CRS-A: 31 patients, CRS + H: 24 patients). Patients with complete CRS were observed for a median of 13.9 (range, 3.0-65.4) months.
The median OS for both groups was 14.9 months (CRS-A, 14.9 months [97.2% CI, 7.0 to 19.4], 22 events, v CRS + H, 14.9 months [97.2% CI, 8.7 to 17.7], 27 events), and the upper quartiles were 23.7 months for CRS-A (97.2% CI, 14.9 to 26.4) and 25.4 months for CRS-H (97.2% CI, 15.4 to 62.5; Fleming-Harrington P = .1647; Fig 2; Data Supplement, Fig S2). One-, 2-, and 3-year OS rates were 60.5% (95% CI, 37.8 to 77.1), 15.4% (95% CI, 3.9 to 33.9), and 0.0% in the CRS-A group and 58.2% (95% CI, 38.0 to 73.8), 25.5% (95% CI, 11.3 to 42.4), and 13.6% (95% CI, 3.9 to 29.5) in the CRS- + H group, respectively. Cox regression analysis adjusting for stratification factors revealed a hazard ratio of 0.72 (95% CI, 0.39 to 1.32) for use of HIPEC. Therefore, we did not observe a statistically significant effect for HIPEC on OS nor for the stratification factors, HER2 status and PCI (Data Supplement, Table S11).
FIG 2.
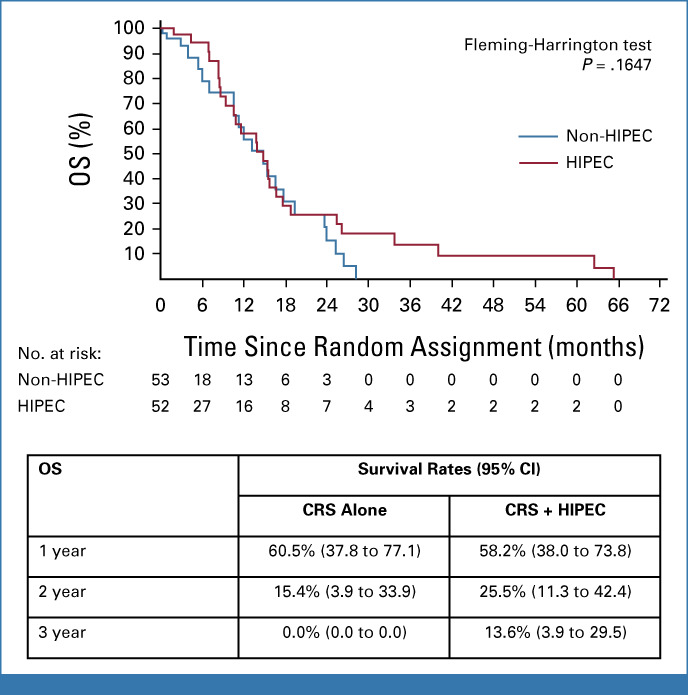
Kaplan-Meier estimation of OS. CRS, cytoreductive surgery; HIPEC, hyperthermic intraperitoneal chemotherapy; OS, overall survival.
The median PFS was statistically significantly improved by the addition of HIPEC, from 3.5 months (95% CI, 3.0 to 7.0) in the CRS-A group to 7.1 months (95% CI, 3.7 to 10.5; P = .0472) in the CRS + H group (Fig 3). It should be noted that, over the longer term, both OS and PFS were apparently influenced by two patients observed as long-term survivors.
FIG 3.
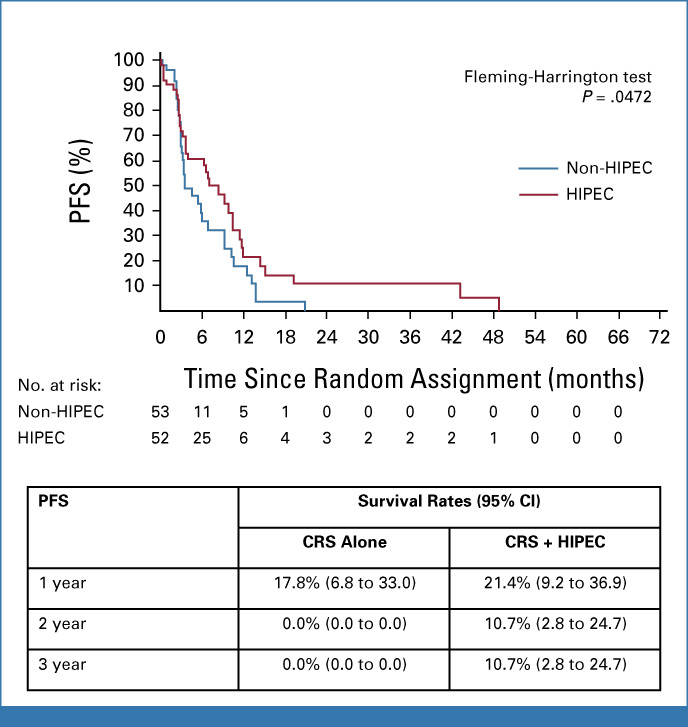
Kaplan-Meier estimation of PFS. CRS, cytoreductive surgery; HIPEC, hyperthermic intraperitoneal chemotherapy; PFS, progression-free survival.
The median time to occurrence of other distant metastases was statistically significantly longer in the CRS + H group at 10.2 (95% CI, 7.7 to 14.7) months versus 9.2 (95% CI, 6.8 to 11.5) months in the CRS-A group (upper quartile: CRS-A 13.3 months [95% CI, 9.3 to 16.3], CRS + H 15.1 months [95% CI, 12.8 to 43.2]; Fig 4, Fleming-Harrington P = .0286). The need for secondary surgical interventions after CRS did not differ significantly between groups (4 of 22 patients [18.2%] in the CRS-A group and 5 of 28 patients [17.9%] in the CRS + H group; Fisher exact test P = .92). The median duration of hospitalization was similar between groups (CRS-A, 15.1 [IQR, 8.4-26.4] days per 100 days in the study; CRS + H, 13.5 [IQR, 7.0-29.1] days per 100 days in the study; Mann-Whitney U test P = .992).
FIG 4.
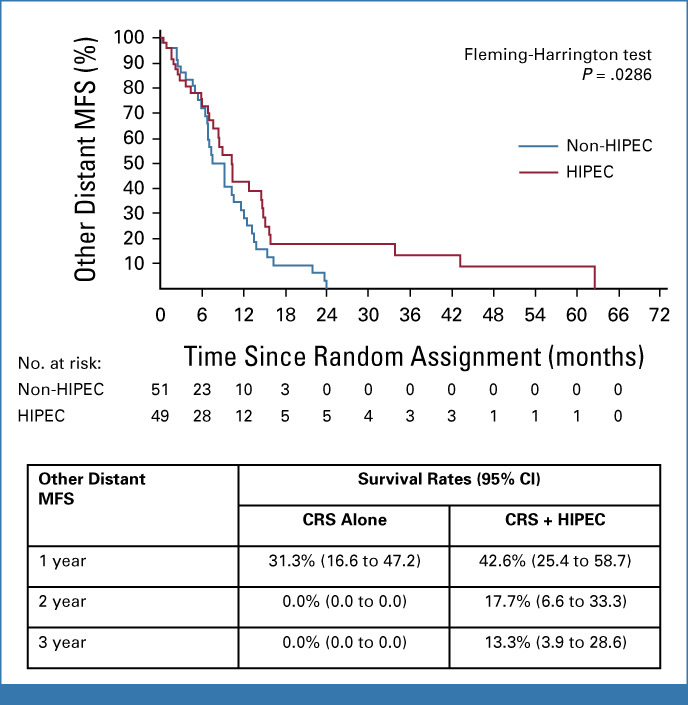
Kaplan-Meier estimation of other distant MFS (full analysis set). CRS, cytoreductive surgery; HIPEC, hyperthermic intraperitoneal chemotherapy; MFS, metastasis-free survival.
Quality-of-life analyses using the QLQ-C30 and QLQ-STO-22 instruments did not reveal any treatment effect (group effect in random-effects model for QLQ-C30 global health status P = .102).
Analyses of primary and secondary end points in the per-protocol set confirmed the full analysis set results (Table 2).
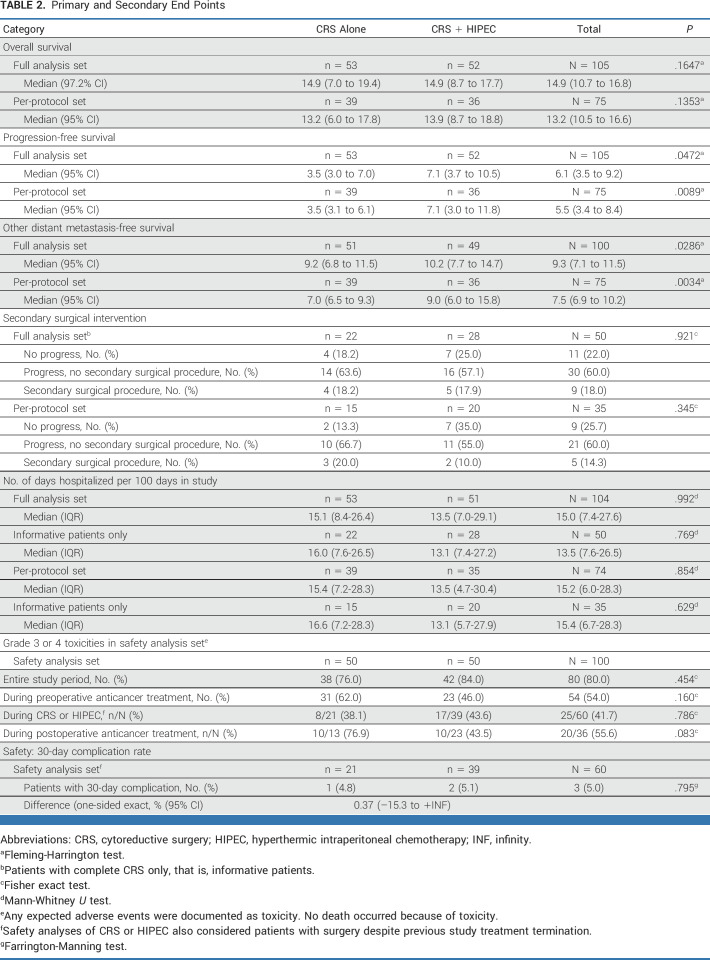
TABLE 2.
Primary and Secondary End Points
The safety analysis set comprised 50 patients in the CRS-A group and 50 patients in the CRS + H group (Fig 1). The frequency of toxicities and AEs did not differ between groups. Rates of grade ≥3 toxicity during preoperative, perioperative, and postoperative chemotherapy were 46.0%, 46.3%, and 43.5% in the CRS + H group and 62.0%, 38.1%, and 76.9% in CRS-A group (P = .160, P = .79, and P = .08, respectively; Data Supplement, Table S12). Postoperative grade 3 or 4 toxicities and further AEs over the whole study period are listed in the Data Supplement (Tables S12 and S13). No critical increase of the complication rate was observed after HIPEC. Observed events were one septic shock in each group and renal failure, followed by death in the CRS + H group.
In the planned subgroup analysis of 35 patients with CCR0 after completed CRS (CRS-A, 16 patients; CRS + H, 19 patients), a statistically significant improvement of OS and other distant MFS was observed after treatment with HIPEC. The effect disappeared in the subgroup of patients with CCR ≥1 (Data Supplement, Table S14). Further unplanned subgroup analyses did not show any treatment effect of HIPEC on OS (Data Supplement, Table S15).
DISCUSSION
The prognosis for patients with pmGC is very poor. The GASTRIPEC-I trial gives the best available data to date on adding HIPEC to gastrectomy and CRS. To our knowledge, it is the first trial to investigate the specific role of HIPEC in patients with histologically proven synchronous isolated PMs in primary GC with a measurement of peritoneal tumor burden by PCI and including preoperative chemotherapy.
The GASTRIPEC-I-trial did not show any increase in the complication rate but failed to demonstrate a significant OS benefit from the addition of HIPEC after CRS versus CRS-A. OS in the full analysis set of both groups was 14.9 months. Possible factors contributing to this result include that GASTRIPEC-I included 44% of patients with a PCI ≥7 and 40% had ascites, a known factor for poor prognosis after CRS.8 More than half of patients did not undergo CRS because of inoperable disease after neoadjuvant chemotherapy.
PFS—a secondary end point—was significantly improved by adding HIPEC to CRS. This forms a strong argument for the efficacy of the HIPEC regimen9 we used. HIPEC, as used in this setting, also seems to have a systemic effect reflected in the frequency and pattern of new distant metastases as assessed by the other distant MFS end point (full analysis set P = .0286; per-protocol set P = .0034). However, OS and PFS were influenced by two long-term survivors, but demonstrated significance in PFS and MFS in favor of the HIPEC arm because of the Fleming-Harrington test, which evaluates long-term events.
In a prespecified subgroup analysis of those patients undergoing complete CRS and achieving CCR0, OS was significantly improved by HIPEC (Data Supplement, Table S15; Fleming P = .0428). This finding suggests that the completeness of CRS in combination with HIPEC is the most important factor determining survival after surgical treatment of pmGC. This result might serve as a basis for future research into the role of HIPEC in patients with pmGC in whom CCR0 can be achieved.
Randomized studies of palliative chemotherapy in patients with advanced, recurrent, or metastatic GC report a median OS of 5.3-12.5 months.10 With the integration of checkpoint inhibitors, the latest trials report a median OS of 14.4 months in selected patients (PD-L1 combined positive score ≥5).11 PMs are a known poor prognostic factor compared with other metastatic sites. Specific data for isolated PMs are lacking because randomized clinical trials do not include patients with isolated PMs as they are usually not measurable by RECIST, and a laparoscopic evaluation is not performed.
In the GASTRIPEC-I-trial, epirubicin, oxaliplatin, plus capecitabine (EOX) was used as perioperative chemotherapy. At the time of study initiation, EOX was one of the best investigated regimens for metastatic GC.10 Since then, FLOT has been proven to be superior to epirubicin and cisplatin plus either fluorouracil or capecitabine in the perioperative curative setting for patients without PM.12 This might be the reason why some investigators used FLOT instead of the recommended chemotherapy (EOX) in the GASTRIPEC-I trial. The chemotherapy backbone of the GASTRIPEC-I trial was not amended because it still is unclear whether FLOT is superior to EOX for PMs. Additionally, taxanes in palliative first-line chemotherapy compared with a platinum plus fluorouracil backbone could not prove a benefit.13
CRS in this trial was performed in 52 patients (49.5%) in total. Disease progressed in 53 of 105 patients (50.5%). CCR0 was achieved in only 36 of 52 patients (69.2%), although only experienced surgeons, who performed at least 20 cytoreductive procedures annually, were allowed to treat patients in this trial. This is a relatively low complete resection rate but demonstrates the aggressiveness of pmGC and the responsibility of the treating surgeon. The complete resection rate in our study compares well with published series in which CCR0/1 is reported in 59%-84% of pmGC cases.1,3 However, in these studies, 54% of patients had only cytological positive lavage1 and 25% of the patients had metachronous PMs.3
The frequency of surgically necessary secondary interventions was similar between groups and was not negatively affected by HIPEC in our trial. Between 30 days after CRS, one patient (2.6%) in the CRS + H group and nobody in the CRS-A group died. Cause of death was renal insufficiency, and the death was judged to be related to the trial treatment.
The frequencies of toxicities and grade 3-4 AEs due to chemotherapy and surgery (with and without HIPEC) were similar in both groups. Postoperative chemotherapy started after a median interval of 53 days in 72% of patients who underwent complete CRS.
In the FLOT4 trial, 52%-60% of patients started postoperative chemotherapy and 37%-46% completed the postoperative chemotherapy in the ECF/ECX and FLOT arms, respectively.12 Therefore, our data compare well with the literature.
Overall, HIPEC did not increase postoperative complications in this study. Our complication rate compares well with published data for gastrectomy and CRS with or without HIPEC. Bonnot et al1 observed severe surgical complications, such as anastomotic leakage, in 21.3% of patients, and 30-day mortality was 3.2%. In summary, the safety profiles of the treatment regimens in our trial were as expected and not significantly different between groups.
When comparing the efficacy parameters of GASTRIPEC-I with existing data, it should be remembered that our trial investigated a well-defined study population in a randomized manner. To our knowledge, to date, only three prospective randomized phase III trials have reported the effect of HIPEC in pmGC.3,14,15 A Chinese phase III trial (reported only in Chinese) randomly assigned 85 patients to chemotherapy with versus without HIPEC and reported a significant improvement of 1- and 3-year survival rates.15 Another randomized trial included 16 evaluable patients and suggested an additional benefit of CRS plus HIPEC and fluorouracil/folinic acid, oxaliplatin, and irinotecan (FOLFOXIRI) versus FOLFOXIRI alone.14 Yang et al3 randomly assigned 68 chemo-naïve patients to CRS versus CRS plus HIPEC; 25% of patients had metachronous metastases. In this heterogeneous population, a benefit of additional HIPEC was suggested—the median OS improved from 6.5 months to 11.0 months. In the CYTO-CHIP study, data from 277 patients with pmGC were retrospectively analyzed. The median OS was improved to 18.8 months in the 180 patients who underwent CRS + HIPEC versus 12.1 months in 97 patients with CRS-A. However, 54% of the patients were included on the basis of cytological lavage only.1
All these trials lead to the conclusion that HIPEC may be beneficial in a highly selected patient population.
Further research is necessary to identify patients suitable to receive CCR0 resection. Laparoscopic evaluation of PCI is accepted as the gold standard for staging GC. Unfortunately, owing to early termination of the GASTRIPEC-I trial, patient numbers were too small to allow for meaningful analyses of PCI subgroups.
Data Supplement (Fig S1) presents an unplanned forest plot analysis of a subgroup of patients with PCI score ≥7 who seemed to gain additional benefit from HIPEC in addition to CRS. Similar benefit was shown in the PRODIGE 7 trial for a subgroup of patients with PCI score ranging from 7 to 13.16 This seems to contradict the recommendation to do CRS + HIPEC only in patients with PCI ≤7.1 A subgroup analysis of patients with long-term survival in those receiving CCR0 plus additional (adjuvant) HIPEC showed statistical significance when analyzed with the Fleming-Harrington test looking for late events. In the forest plot analysis on the basis of pooled data, patients with a high tumor burden (PCI >7) seem to benefit from palliative HIPEC but do not have an increased chance of long-term survival.
In the ongoing Dutch PERISCOPE II trial (ClinicalTrials.gov identifier: NCT03348150) investigating CRS and HIPEC versus chemotherapy alone, a PCI <7 is an inclusion criterion. In addition to PERISCOPE II in the Netherlands, there are currently ongoing randomized controlled trials exploring the efficacy of HIPEC in patients with pmGC in France (GASTRICHIP: ClinicalTrials.gov identifier: NCT01882933) and China (HIPEC-01: ClinicalTrials.gov identifier: NCT02356276; WuhanU_HIPEC: ClinicalTrials.gov identifier: NCT02528110). However, after the successful results of integrating immune checkpoint inhibition and other novel targets into the GC treatment algorithm,17 anticancer treatment both with and without CRS and HIPEC should be included in future trials. Multiplex profiling of PMs from GC might be another advance in this direction.18 However, the results from the GASTRIPEC-I and PRODIGE7 trials16 suggest that patients with higher PCI scores should not be excluded from future trials testing the benefit of HIPEC.
The hypothesis we used to generate the sample size might be criticized for three main reasons. First, at the time of the trial design, no survival data were available in the literature for patients treated with CRS-A. Therefore, the most relevant limitation of the GASTRIPEC-I-trial was that we underestimated the rate of disease progression in patients with solitary PM despite chemotherapy (50.5%). In a future trial, it would be better to randomize intraoperatively. Second, the postulated increase in median OS from 5 to 6 months in the CRS + H group was probably an overestimate. Third, OS is the most robust end point but might be influenced by new effective subsequent therapies, such as checkpoint inhibitors. Perhaps PFS might be a more appropriate end point.
In conclusion, GASTRIPEC-I showed that HIPEC does not add morbidity or mortality to CRS and gastrectomy. The addition of HIPEC significantly prolonged PFS and MFS but only showed an OS benefit in the subgroup of patients in whom a complete CRS could be performed. HIPEC shows benefit in a highly selected subgroup. Future trials should aim to further identify this subgroup.
ACKNOWLEDGMENT
We thank the German Cancer Aid to support this trial. We also thank the study participants and their families, who gave their consent and took part in the trial. Additionally, we thank Dr Petra Neuhaus for study and data management as well as help with all regulatory aspects of the study. Thanks also to S.W. for assistance with the main study.
Alfred Koenigsrainer
Research Funding: Heparegenix (Inst), German Cancer Aid (Inst)
Travel, Accommodations, Expenses: Heparegenix
Ines Gockel
Honoraria: Johnson & Johnson/Janssen, Falk Foundation, Onkowissen
Daniel Reim
Research Funding: Mölnlycke (Inst)
Roger Wahba
Consulting or Advisory Role: Sirtex Medical
Research Funding: Olympus
Travel, Accommodations, Expenses: Sirtex Medical, Olympus
Tobias Keck
Speakers' Bureau: AstraZeneca
Arved Weimann
Speakers' Bureau: Abbott Nutrition, Baxter (Inst), Fresenius Kabi (Inst), Falk Foundation, B.Braun
Research Funding: Mucos (Inst), B.Braun (Inst)
Travel, Accommodations, Expenses: Abbott Nutrition, Baxter, Fresenius KAbi, Falk Foundation
Uncompensated Relationships: ESPEN—European Society for Clinical Nutrition and Metabolism, German Society for Nutritional Medicine—DGEM
Johann Pratschke
Employment: Charité University Medicine Berlin
Honoraria: Baxter, Johnson & Johnson/MedTech, Medtronic, Merck Serono, Intuitive Surgical, Chiesi GmbH
Sandra Wegel
Employment: Charité University Medicine Berlin
Nicolas Moosmann
Travel, Accommodations, Expenses: Amgen
Volker Heinemann
Honoraria: Roche, Amgen, Sanofi, Merck, Servier, Pfizer, Pierre Fabre, AstraZeneca, MSD, Seagen
Consulting or Advisory Role: Merck, Amgen, Roche, MSD, Bristol-Myers Squibb, Novartis, Pierre Fabre, Terumo, GlaxoSmithKline, Servier/Pfizer, AstraZeneca, Oncosil, Nordic Bioscience
Research Funding: Merck (Inst), Amgen (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Merck
Peter Thuss-Patience
Consulting or Advisory Role: Bristol-Meyers-Squibb, Merck Serono, Lilly, Novartis, Pfizer, Roche, AstraZeneca, Daiichi Sankyo, Astellas Pharma, MSD, Servier
Research Funding: Merck/Pfizer (Inst)
Travel, Accommodations, Expenses: Merck Serono
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the 2015 ASCO annual meeting, Chicago, IL, May 29-June 02, 2015; and the European Society for Medical Oncology, Paris, France (FNP 13760), September 16-21, 2021.
SUPPORT
Supported by German Cancer Aid (trial number 108891) and promoted by the Oncology Working Group of the German Society for Visceral Surgery (CAO-V).
CLINICAL TRIAL INFORMATION
NCT02158988 (GASTRIPEC)
DATA SHARING STATEMENT
Summary statistics that go beyond the scope of published material will be made available to researchers for meta-analysis upon reasonable request and if the necessary data analysis is not unduly time-consuming. Individual patient data that underlie published results will not be shared since patients can no longer be asked for consent.
AUTHOR CONTRIBUTIONS
Conception and design: Beate Rau, Alfred Koenigsrainer, Volker Heinemann, Evelyn Trips, Peter Michael Schlag, Peter Thuss-Patience
Administrative support: Alfred Koenigsrainer, Johann Pratschke, Peter Thuss-Patience
Provision of study materials or patients: Beate Rau, Hauke Lang, Alfred Koenigsrainer, Ines Gockel, Horst-Guenter Rau, Hendrik Seeliger, Christian Lerchenmueller, Daniel Reim, Martin Angele, Tobias Keck, Pompiliu Piso, Silke Schuele, Johann Pratschke, Alexander Rehders, Nicolas Moosmann, Jochen Gaedcke, Volker Heinemann, Peter Thuss-Patience
Collection and assembly of data: Beate Rau, Hauke Lang, Alfred Koenigsrainer, Ines Gockel, Horst-Guenter Rau, Hendrik Seeliger, Christian Lerchenmueller, Daniel Reim, Roger Wahba, Martin Angele, Steffen Heeg, Tobias Keck, Arved Weimann, Stefan Topp, Andreas Brandl, Silke Schuele, Peter Jo, Sandra Wegel, Alexander Rehders, Nicolas Moosmann, Jochen Gaedcke, Volker Heinemann, Markus Loeffler, Peter Thuss-Patience
Data analysis and interpretation: Beate Rau, Alfred Koenigsrainer, Horst-Guenter Rau, Hendrik Seeliger, Tobias Keck, Pompiliu Piso, Johann Pratschke, Evelyn Trips, Peter Thuss-Patience
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Effect of Hyperthermic Intraperitoneal Chemotherapy on Cytoreductive Surgery in Gastric Cancer With Synchronous Peritoneal Metastases: The Phase III GASTRIPEC-I Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Alfred Koenigsrainer
Research Funding: Heparegenix (Inst), German Cancer Aid (Inst)
Travel, Accommodations, Expenses: Heparegenix
Ines Gockel
Honoraria: Johnson & Johnson/Janssen, Falk Foundation, Onkowissen
Daniel Reim
Research Funding: Mölnlycke (Inst)
Roger Wahba
Consulting or Advisory Role: Sirtex Medical
Research Funding: Olympus
Travel, Accommodations, Expenses: Sirtex Medical, Olympus
Tobias Keck
Speakers' Bureau: AstraZeneca
Arved Weimann
Speakers' Bureau: Abbott Nutrition, Baxter (Inst), Fresenius Kabi (Inst), Falk Foundation, B.Braun
Research Funding: Mucos (Inst), B.Braun (Inst)
Travel, Accommodations, Expenses: Abbott Nutrition, Baxter, Fresenius KAbi, Falk Foundation
Uncompensated Relationships: ESPEN—European Society for Clinical Nutrition and Metabolism, German Society for Nutritional Medicine—DGEM
Johann Pratschke
Employment: Charité University Medicine Berlin
Honoraria: Baxter, Johnson & Johnson/MedTech, Medtronic, Merck Serono, Intuitive Surgical, Chiesi GmbH
Sandra Wegel
Employment: Charité University Medicine Berlin
Nicolas Moosmann
Travel, Accommodations, Expenses: Amgen
Volker Heinemann
Honoraria: Roche, Amgen, Sanofi, Merck, Servier, Pfizer, Pierre Fabre, AstraZeneca, MSD, Seagen
Consulting or Advisory Role: Merck, Amgen, Roche, MSD, Bristol-Myers Squibb, Novartis, Pierre Fabre, Terumo, GlaxoSmithKline, Servier/Pfizer, AstraZeneca, Oncosil, Nordic Bioscience
Research Funding: Merck (Inst), Amgen (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Merck
Peter Thuss-Patience
Consulting or Advisory Role: Bristol-Meyers-Squibb, Merck Serono, Lilly, Novartis, Pfizer, Roche, AstraZeneca, Daiichi Sankyo, Astellas Pharma, MSD, Servier
Research Funding: Merck/Pfizer (Inst)
Travel, Accommodations, Expenses: Merck Serono
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bonnot PE, Piessen G, Kepenekian V, et al. : Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastases (CYTO-CHIP study): A propensity score analysis. J Clin Oncol 37:2028-2040, 2019 [DOI] [PubMed] [Google Scholar]
- 2.van Stein RM, Aalbers AGJ, Sonke GS, et al. : Hyperthermic intraperitoneal chemotherapy for ovarian and colorectal cancer: A review. JAMA Oncol 7:1231-1238, 2021 [DOI] [PubMed] [Google Scholar]
- 3.Yang XJ, Huang CQ, Suo T, et al. : Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: Final results of a phase III randomized clinical trial. Ann Surg Oncol 18:1575-1581, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rau B, Loeffler M, Rau H-G, et al. : Perioperative chemotherapy and cytoreductive surgery with versus without HIPEC in gastric cancer with limited peritoneal metastases: A randomized phase III study (GASTRIPEC). J Clin Oncol 33, 2015. (suppl 15; abstr TPS4132) [Google Scholar]
- 5.Pocock SJ: Clinical Trials—A Practical Approach. Chichester, New York, Brisbane, Toronto, Singapore, John Wiley & Sons, 1983, p 265 [Google Scholar]
- 6.Jacquet P, Sugarbaker PH: Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 82:359-374, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Farrington CP, Manning G: Test statistics and sample size formulae for comparative binomial trials with null hypothesis of non-zero risk difference or non-unity relative risk. Stat Med 9:1447-1454, 1990 [DOI] [PubMed] [Google Scholar]
- 8.White MG, Kothari A, Ikoma N, et al. : Factors associated with resection and survival after laparoscopic HIPEC for peritoneal gastric cancer metastasis. Ann Surg Oncol 27:4963-4969, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gronau F, Feldbruegge L, Oberwittler F, et al. : HIPEC in peritoneal metastasis of gastric origin: A systematic review of regimens and techniques. J Clin Med 11:1456, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham D, Starling N, Rao S, et al. : Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 358:36-46, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Janjigian YY, Shitara K, Moehler M, et al. : First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 398:27-40, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Batran SE, Homann N, Pauligk C, et al. : Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 393:1948-1957, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Yamada Y, Boku N, Mizusawa J, et al. : Docetaxel plus cisplatin and S-1 versus cisplatin and S-1 in patients with advanced gastric cancer (JCOG1013): An open-label, phase 3, randomised controlled trial. Lancet Gastroenterol Hepatol 4:501-510, 2019 [DOI] [PubMed] [Google Scholar]
- 14.Rudloff U, Langan RC, Mullinax JE, et al. : Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: Results of the GYMSSA trial. J Surg Oncol 110:275-284, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng HJ, Wei ZG, Zhen L, et al. : Clinical application of perioperative continuous hyperthermic peritoneal perfusion chemotherapy for gastric cancer. Nan Fang Yi Ke Da Xue Xue Bao 29:295-297, 2009 [PubMed] [Google Scholar]
- 16.Quénet F, Elias D, Roca L, et al. : Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 22:256-266, 2021 [DOI] [PubMed] [Google Scholar]
- 17.Chen K, Wang X, Yang L, et al. : The anti-PD-1/PD-L1 immunotherapy for gastric esophageal cancer: A systematic review and meta-analysis and literature review. Cancer Control 28:107327482199743, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang R, Song S, Harada K, et al. : Multiplex profiling of peritoneal metastases from gastric adenocarcinoma identified novel targets and molecular subtypes that predict treatment response. Gut 69:18-31, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Summary statistics that go beyond the scope of published material will be made available to researchers for meta-analysis upon reasonable request and if the necessary data analysis is not unduly time-consuming. Individual patient data that underlie published results will not be shared since patients can no longer be asked for consent.