Abstract
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.
The MyPathway multiple-basket study (ClinicalTrials.gov identifier: NCT02091141) is evaluating targeted therapies in nonindicated tumors with relevant molecular alterations. We assessed pertuzumab + trastuzumab in a tissue-agnostic cohort of adult patients with human epidermal growth factor receptor 2 (HER2)–amplified and/or –overexpressed and/or –mutated solid tumors. The primary end point was objective response rate (ORR); secondary end points included survival and safety. At data cutoff (March 2022), 346 patients with HER2 amplification and/or overexpression with/without HER2 mutations (n = 263), or HER2 mutations alone (n = 83) had been treated. Patients with HER2 amplification and/or overexpression had an ORR of 25.9% (68/263, 95% CI, 20.7 to 31.6), including five complete responses (urothelial [n = 2], salivary gland [n = 2], and colon [n = 1] cancers). Activity was higher in those with wild-type (ORR, 28.1%) versus mutated KRAS (ORR, 7.1%). Among patients with HER2 amplification, ORR was numerically higher in patients with immunohistochemistry (IHC) 3+ (41.0%; 32/78) or 2+ (21.9%; 7/32), versus 1+ (8.3%; 1/12) or no expression (0%; 0/20). In patients with HER2 mutations alone, ORR was 6.0% (5/83, 95% CI, 2.0 to 13.5). Pertuzumab + trastuzumab showed activity in various HER2-amplified and/or -overexpressed tumors with wild-type KRAS, with the range of activity dependent on tumor type, but had limited activity in the context of KRAS mutations, HER2 mutations alone, or 0-1+ HER2 expression.
INTRODUCTION
Human epidermal growth factor receptor 2 (HER2/ERBB2) amplification and/or overexpression is observed in 2%-3% of all solid tumors.1,2 HER2-targeted therapies are approved for HER2-positive metastatic breast, gastric, gastroesophageal, and colorectal cancers (CRC),3-6 but have also shown benefit in HER2-mutant non–small-cell lung cancer (NSCLC).7-9
The MyPathway multiple-basket study is evaluating established targeted therapies in patients with advanced solid tumors and potentially actionable mutations. Previous data suggested the chemotherapy-free combination of pertuzumab + trastuzumab (P + T) has activity in multiple cancer types not indicated for HER2-targeted treatment,10-13 and led to updated NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for HER2-positive colon, salivary, and biliary cancers.14-16 However, activating mutations in genes associated with resistance to EGFR-targeted therapy (eg, KRAS and PIK3CA)17 may influence HER2 as a driver,18-20 meaning analyses in larger populations and other tumor types are needed.
Here, we report the efficacy and safety of P + T in the overall MyPathway HER2 basket.
METHODS
MyPathway (ClinicalTrials.gov identifier: NCT02091141) is an open-label, nonrandomized, multicenter, multiple-basket, US-based, tumor-agnostic phase IIa study (Data Supplement, Fig S1 [online only]). Patients in the HER2 basket were age 18 years and older, and had tumors with HER2 amplification and/or overexpression and/or activating mutations. In cases of discordant local versus central results for HER2 amplification, overexpression, or mutation status, local results took precedence (Data Supplement, Table S1). Additional methods are provided in the Data Supplement.
RESULTS
Patients
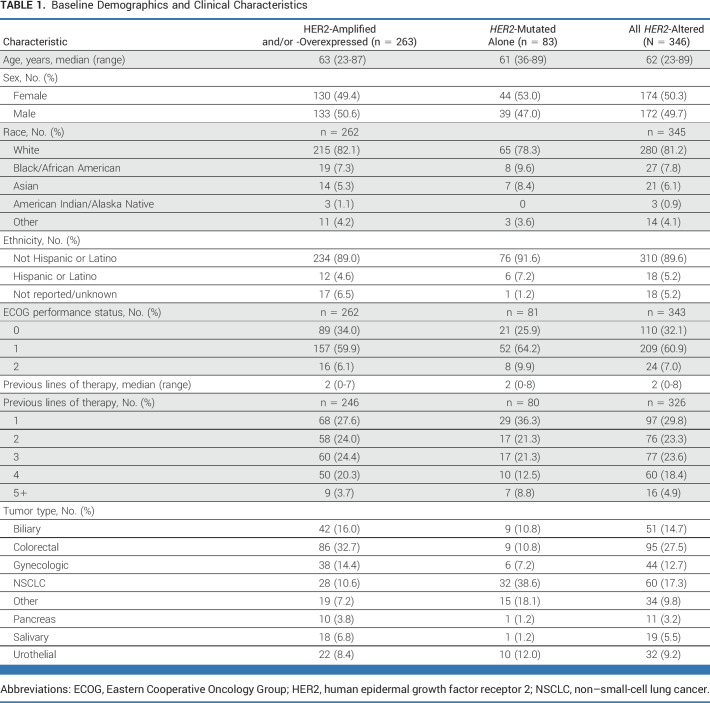
Enrollment completed between April 8, 2014, and June 3, 2019, for 346 patients, including 263 patients with HER2 amplification and/or overexpression (with or without HER2 mutations) and 83 with their sole HER2 alteration being mutation (Data Supplement, Fig S2). Baseline characteristics are provided in Table 1. Median time on treatment at data cutoff (March 24, 2022) for all patients was 2.14 months (range, 0-67.2).
TABLE 1.
Baseline Demographics and Clinical Characteristics
Outcomes Overall and by Biomarker Status
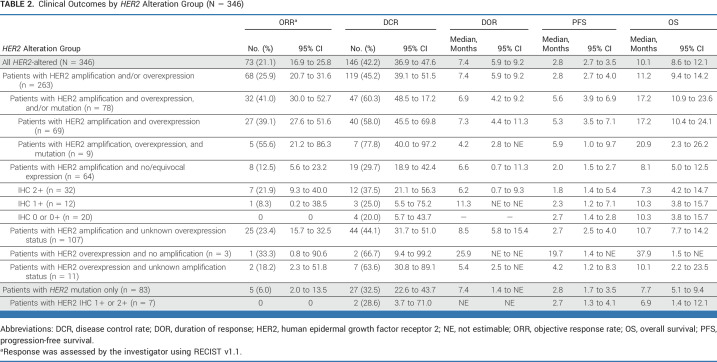
In the entire HER2 patient cohort, objective response rate (ORR) was 21.1% and disease control rate was 42.2% (including five complete responses [CRs], 62 partial responses [PRs], 79 stable disease >4 months; Table 2). Median progression-free survival (PFS) and overall survival (OS) were 2.8 and 10.1 months, respectively (Data Supplement, Fig S3). Concordance between HER2 testing methodologies for the HER2 amplification and/or overexpression cohort (n = 263) is provided in the Data Supplement (Table S2). There was a gradient of response among patients with HER2 amplification; ORR was numerically higher in patients with immunohistochemistry (IHC) 3+ (41.0%) or 2+ expression (21.9%), versus 1+ (8.3%) or no expression (0%; Table 2).
TABLE 2.
Clinical Outcomes by HER2 Alteration Group (N = 346)
Among patients with HER2 amplification and/or overexpression, P + T produced an ORR of 25.9% (Table 2), including five CRs (urothelial, n = 2; salivary gland, n = 2; and colon, n = 1). Median PFS and OS were 2.8 and 11.2 months, respectively (Data Supplement, Fig S3). Within this group, 28 patients also had HER2 mutations, and had similar outcomes to the other 235 patients in the group (Data Supplement [Fig S4 and Table S3]). By contrast, patients with HER2 mutations without known HER2 amplification or overexpression had an ORR of 6.0% (all PRs; Table 2). P + T activity in patients with amplification/overexpression versus HER2 mutation alone is contrasted in Table 2; PFS and OS are compared in the Data Supplement (Fig S3).
HER2 overexpression (IHC 3+) correlated with higher HER2 copy number (Data Supplement, Fig S5A). We observed a significant association between increasing HER2 copy-number cutoff and ORR (Data Supplement, Fig S5B). ORR in all patients with IHC 3+ was 41.0% (32/78; 95% CI, 30.0 to 52.7) and 26.1% (65/249; 95% CI, 20.8 to 32.0) in all patients with HER2 amplification. ORR was low (12.5%) in the 64 patients who had amplification with no or equivocal overexpression (Table 2).
Among patients with HER2 amplification and/or overexpression, 203 had KRAS wild-type, 28 had KRAS mutations, and 32 had unknown KRAS status (Data Supplement, Table S4). P + T activity was higher in patients with wild-type (ORR 28.1%) versus mutated (ORR 7.1%) KRAS (Fig 1A; Data Supplement [Table S5]); PFS and OS were also longer in KRAS wild-type tumors (Figs 1B and 1C). In patients with HER2 mutations only, 73/83 had wild-type KRAS (Data Supplement, Table S4). Of the 27 patients with disease control in the HER2-mutated group, none had KRAS-mutated tumors (Data Supplement, Table S5). There was no clinically significant difference between the ORRs of patients with HER2 amplification and/or overexpression with (21.4%) versus without (27.2%) PI3K pathway alterations (Data Supplement [Fig S6 and Table S6]). Clinical outcomes by PI3K/PIK3CA status are provided in the Data Supplement.
FIG 1.
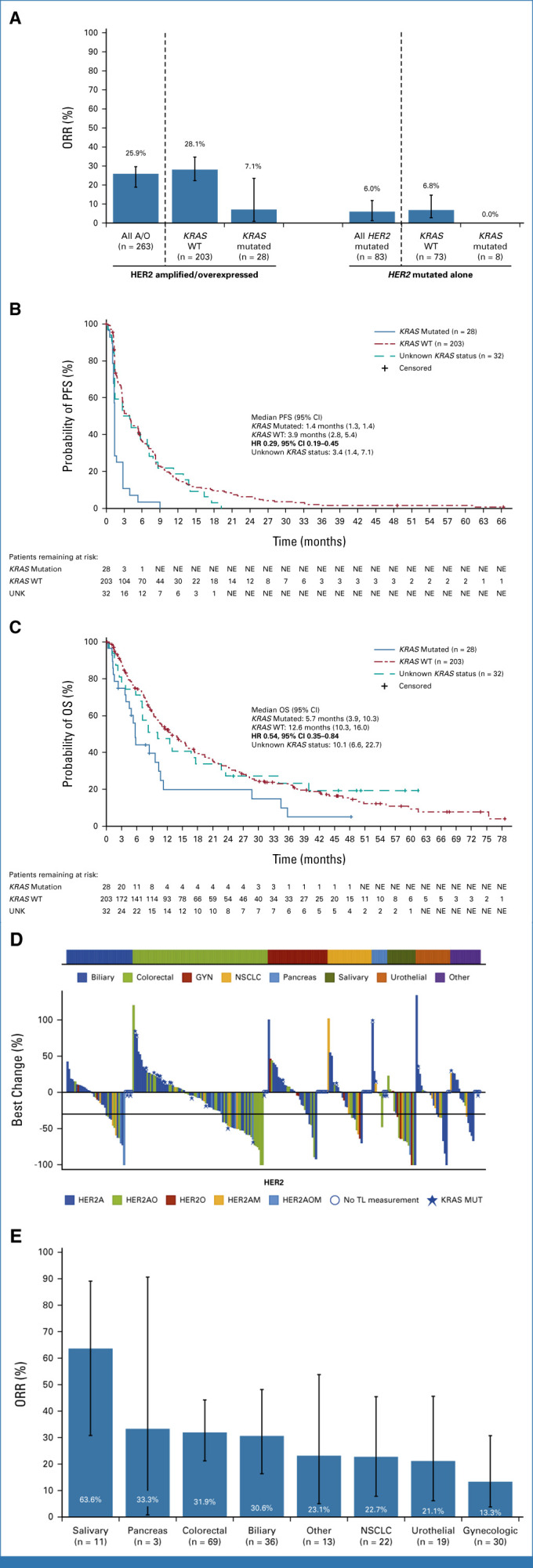
(A) ORR,a (B) PFS,b and (C) OS in patients with HER2-amplified and/or -overexpressed tumors by KRAS status. (D) Best percentage change in sum of target lesions in patients with HER2 amplification and/or overexpression by tumor group (n = 263); the horizontal line represents the 30% decrease in the sum of diameters of target lesions, from baseline. (E) ORR in patients with HER2-amplified and/or -overexpressed + KRAS wild-type tumors by tumor group (n = 202). aResponse was assessed by the investigator using RECIST v1.1. bData for patients without disease progression or death were censored at the date of the last tumor assessment (or, if no tumor assessments were performed, after the baseline visit, at the date of first treatment). Kaplan-Meier curves are for descriptive purposes only. Bar graph whiskers represent 95% CI. A/O, amplified and/or overexpressed; GYN, gynecologic; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; KRAS, Kirsten rat sarcoma viral oncogene homolog; NE, not estimable; NSCLC, non–small-cell lung cancer; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; TL, target lesion; UNK, unknown; WT, wild-type.
Responses were observed in all tumor groups of patients with HER2 amplification and/or overexpression (Fig 1D). Among the 203 patients with KRAS wild-type tumors, ORR was 63.6% (7/11) for salivary cancer (including two CRs; one ongoing at data cutoff); 31.9% (22/69) for CRC, including one CR; 30.6% (11/36) for biliary cancer; 22.7% (5/22) for NSCLC; and 21.1% (4/19) for urothelial cancer (Fig 1E; Data Supplement [Table S7]). Of three patients with pancreatic cancer, one had a PR. Of 28 patients with HER2 amplification and/or overexpression and KRAS mutations, responses were observed only in patients with CRC (Data Supplement, Table S8).
Safety
Among all 346 patients, 325 (93.9%) experienced treatment-emergent adverse events (TEAEs), with treatment-related adverse events (TRAEs) reported in 251 (72.5%), mostly diarrhea (Data Supplement, Table S9). Serious TRAEs were observed in 17 (4.9%) patients and grade ≥3 TRAEs in 42 (12.1%). Fourteen (4.0%) patients died due to TEAEs, of which two events were related to treatment (pneumonitis and sepsis). No new safety signals were observed.
DISCUSSION
P + T showed activity in various KRAS wild-type HER2-amplified and/or -overexpressed advanced solid tumors, ranging from 5.9% in uterine cancer to 63.6% in salivary gland tumors, suggesting that tumor origin is important. However, P + T had limited activity in patients with HER2-amplified and/or -overexpressed tumors carrying KRAS mutations and patients with HER2-activating mutations without HER2 amplification/overexpression. Safety was consistent with previously reported profiles for pertuzumab and trastuzumab.
In patients with HER2 amplification, ORRs were higher among those with confirmed HER2 overexpression (39.1%) versus no or equivocal HER2 overexpression (12.5%). Higher HER2 copy number was associated with higher likelihood of HER2 overexpression, and response rate significantly increased with higher copy-number cutoff. Thus, it may be informative to perform IHC testing even in patients with confirmed amplifications on next-generation sequencing.
Conflicting data have been reported concerning the predictive value of HER2-activating mutations.8,19,21-27 In our data set, patients with HER2 amplification or overexpression and concomitant HER2 mutations had similar ORR to the overall HER2 amplification and/or overexpression group (35.7% v 25.9%). Although HER2 mutations alone were not associated with response in the overall population, the therapeutic relevance of the few mutations associated with response should be further investigated.
We observed meaningful objective responses in patients with a variety of refractory solid tumors (Data Supplement, Table S7). Earlier published ORRs for the salivary (60%; 9/15), colorectal (32%; 18/57), and biliary cancer (23%; 9/39) subgroups were confirmed in this updated analysis.11-13 The NSCLC and urothelial cancer cohorts had ORRs of 22.7% and 21.1%, respectively. Responses were also seen in patients with pancreatic, cervical, and unknown primary tumors, but the numbers in these cohorts were too small to allow an estimate of the response rates.
Limitations include potential bias toward recruiting patients with tumor types known to respond to P + T, as well as risk in aggregating tumor response rates across different tumor types. Furthermore, as many of these analyses were not prespecified, and as this was a single-arm study, the results are purely exploratory and hypothesis-generating.
HER2-targeted therapy may have utility in a variety of KRAS wild-type, HER2-amplified and -overexpressed solid tumors. Substantial activity was seen in patients with refractory salivary gland, colorectal, biliary, NSCLC, and urothelial cancers.
ACKNOWLEDGMENT
The authors are grateful to the patients, families, and study teams who participated in MyPathway, and to Jessica Grindheim (Genentech) for rendering mutation data in OncoPrint format. Medical writing support was provided by Sabrina Hom, PhD, and John Bett, PhD, of Ashfield MedComms, an Inizio Company, and was funded by F. Hoffmann-La Roche/Genentech.
Christopher J. Sweeney
Stock and Other Ownership Interests: Leuchemix
Consulting or Advisory Role: Sanofi, Janssen Biotech, Astellas Pharma, Bayer, Genentech/Roche, AstraZeneca, Pfizer, Amgen, Lilly, POINT Biopharma, Cadence Pharma
Research Funding: Janssen Biotech (Inst), Astellas Pharma (Inst), Sanofi (Inst), Bayer (Inst), Dendreon, Pfizer (Inst)
Patents, Royalties, Other Intellectual Property: Leuchemix, Parthenolide, Dimethylaminoparthenolide. Exelixis: Abiraterone plus cabozantinib combination
John D. Hainsworth
Consulting or Advisory Role: Roche/Genentech (Inst)
Research Funding: Genentech (Inst)
Ron Bose
Consulting or Advisory Role: Genentech
Research Funding: Puma Biotechnology (Inst)
Howard A. Burris
Employment: HCA Healthcare
Leadership: HCA Healthcare
Stock and Other Ownership Interests: HCA Healthcare
Consulting or Advisory Role: GRAIL (Inst), Roche (Inst), Vincerx Pharma (Inst)
Research Funding: Roche/Genentech (Inst), Bristol Myers Squibb (Inst), Incyte (Inst), AstraZeneca (Inst), MedImmune (Inst), Macrogenics (Inst), Novartis (Inst), Boehringer Ingelheim (Inst), Lilly (Inst), Seagen (Inst), Merck (Inst), Agios (Inst), Jounce Therapeutics (Inst), Moderna Therapeutics (Inst), GlaxoSmithKline (Inst), Verastem (Inst), Tesaro (Inst), BioMed Valley Discoveries (Inst), TG Therapeutics (Inst), Vertex (Inst), eFFECTOR Therapeutics (Inst), Janssen (Inst), BioAtla (Inst), CicloMed (Inst), Harpoon therapeutics (Inst), Archer (Inst), Arvinas (Inst), Revolution Medicines (Inst), Array BioPharma (Inst), Bayer (Inst), Kymab (Inst), Pfizer (Inst), Takeda/Millennium (Inst), Foundation Medicine (Inst), EMD Serono (Inst), ARMO BioSciences (Inst), CALGB (Inst), Hengrui Therapeutics (Inst), XBiotech (Inst), Zymeworks (Inst), Coordination Pharmaceuticals (Inst), NGM Biopharmaceuticals (Inst), Gossamer Bio (Inst), Ryvu Therapeutics (Inst), BioTheryX (Inst), AbbVie (Inst), BeiGene (Inst), Celgene (Inst), Vertex (Inst)
Uncompensated Relationships: Bayer (Inst), Novartis (Inst), Incyte (Inst), Bristol Myers Squibb (Inst), Boehringer Ingelheim (Inst), TG Therapeutics (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/201030/
Razelle Kurzrock
Leadership: CureMatch, CureMetrix
Stock and Other Ownership Interests: CureMatch, IDbyDNA, CureMetrix
Honoraria: Roche, EUSA Pharma, NeoGenomics Laboratories, Biocom, NeoMed, LEK, AACR, Chugai Pharma USA, Wiley, Merck, Pfizer, Foundation Medicine, Turning Point Therapeutics, Bicara Therapeutics
Consulting or Advisory Role: Actuate Therapeutics, Loxo, XBiotech, NeoMed, Roche, Gaido, Soluventis, Pfizer, Merck, Turning Point Therapeutics, TD2/Volastra, Bicara Therapeutics, AstraZeneca, Biological Dynamics, Daiichi Sankyo, Eisai, EOM Pharmaceuticals, Iylon, Prosperdtx, Caris Life Sciences, Datar Genomics, NeoGenomics Laboratories, Regeneron
Speakers' Bureau: Roche, NeoGenomics Laboratories
Research Funding: Guardant Health (Inst), Sequenom (Inst), Merck Serono (Inst), Genentech (Inst), Pfizer (Inst), Foundation Medicine (Inst), Incyte (Inst), Konica Minolta (Inst), Grifols (Inst), OmniSeq (Inst), Debiopharm Group (Inst), Boehringer Ingelheim (Inst), Top Alliance BioScience (Inst), Takeda (Inst), MedImmune (Inst), Biological Dynamics (Inst)
Travel, Accommodations, Expenses: TargetCancer Foundation, NCI SWOG
Charles Swanton
Stock and Other Ownership Interests: Epic Sciences, Apogen Biotechnologies, GRAIL, Achilles Therapeutics, Bicycle Therapeutics
Honoraria: Roche, Boehringer Ingelheim, GlaxoSmithKline, Lilly, Celgene, Ono Pharmaceutical, Pfizer, Bristol Myers Squibb, Novartis, AstraZeneca, Illumina, MSD Oncology, Amgen, Roche/Genentech
Consulting or Advisory Role: Genentech/Roche, Sarah Cannon Research Institute, Medicxi, Bicycle Therapeutics, Metabomed, Roche, GRAIL, AstraZeneca, Amgen, Pfizer, Novartis, GlaxoSmithKline, MSD, Bristol Myers Squibb, Illumina, Roche, Medicxi, Achilles Therapeutics
Research Funding: Boehringer Ingelheim, BMS, Roche, AstraZeneca, Ono Pharmaceutical, Archer, Pfizer, Personalis
Patents, Royalties, Other Intellectual Property: Founder of Achilles Therapeutics: a biotechnology company funded by Syncona/Wellcome Trust to target clonal Neoantigens through vaccine and cell therapy approaches, Method for treating cancer based on identification of clonal neo-antigens (PCT/EP2016/059401), Immune checkpoint intervention in cancer (PCT/EP2016/071471), Methods for lung cancer detection (PCT/US2017/028013), Method of detecting tumour recurrence (PCT/GB2017/053289), Method for treating cancer (PCT/EP2016/059401), Method of identifying insertion/deletion mutation targets (PCT/GB2018/051892), Method for determining whether an HLA allele is lost in a tumour (PCT/GB2018/052004), Method for identifying responders to cancer treatment (PCT/GB2018/051912), Method of predicting survival rates for cancer patients (PCT/GB2020/050221)
Uncompensated Relationships: AstraZeneca (Inst)
Claire F. Friedman
Honoraria: Aptitude Health, GlaxoSmithKline
Consulting or Advisory Role: Arch Oncology, Bristol Myers Squibb, AADi
Research Funding: Bristol Myers Squibb (Inst), Genentech (Inst), Merck (Inst), Daiichi Sankyo/UCB Japan (Inst), Seagen (Inst), AstraZeneca (Inst), Hotspot Therapeutics (Inst), Marengo Therapeutics (Inst)
Travel, Accommodations, Expenses: Puma Biotechnology
Uncompensated Relationships: Genentech, Merck
Open Payments Link: https://openpaymentsdata.cms.gov/physician/477023/
David R. Spigel
Leadership: ASCO (Inst)
Consulting or Advisory Role: Genentech/Roche (Inst), Novartis (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), GlaxoSmithKline (Inst), Molecular Templates (Inst), Jazz Pharmaceuticals (Inst), Sanofi/Aventis (Inst), Regeneron (Inst), Lilly (Inst), BeiGene (Inst), Ipsen (Inst), Monte Rosa Therapeutics (Inst), AbbVie (Inst), Lyell Immunopharma (Inst), Novocure (Inst)
Research Funding: Genentech/Roche (Inst), Novartis (Inst), Celgene (Inst), Bristol Myers Squibb (Inst), Lilly (Inst), AstraZeneca (Inst), University of Texas Southwestern Medical Center - Simmons Cancer Center (Inst), Merck (Inst), G1 Therapeutics (Inst), Neon Therapeutics (Inst), Nektar (Inst), Celldex (Inst), Clovis Oncology (Inst), Daiichi Sankyo (Inst), Astellas Pharma (Inst), GRAIL (Inst), Transgene (Inst), Aeglea Biotherapeutics (Inst), Ipsen (Inst), BIND Therapeutics (Inst), Eisai (Inst), ImClone Systems (Inst), Janssen Oncology (Inst), MedImmune (Inst), Agios (Inst), GlaxoSmithKline (Inst), Tesaro (Inst), cyteir (Inst), Novocure (Inst), Elevation Oncology (Inst), Calithera Biosciences (Inst), Arcus Biosciences (Inst), Arrys Therapeutics (Inst), Bayer (Inst), BeiGene (Inst), Blueprint Medicines (Inst), Boehringer Ingelheim (Inst), Hutchison MediPharma (Inst), Incyte (Inst), Kronos Bio (Inst), Loxo (Inst), Macrogenics (Inst), Molecular Templates (Inst), PureTech (Inst), Razor Genomics (Inst), Repare Therapeutics (Inst), Rgenix (Inst), Tizona Therapeutics, Inc (Inst), Verastem (Inst), BioNTech (Inst), AbbVie (Inst), Amgen (Inst), Anheart Therapeutics (Inst), Ascendis Pharma (Inst), Endeavor BioMedicines (Inst), Erasca, Inc (Inst), Faeth Therapeutics (Inst), Fujifilm (Inst), Gilead Sciences (Inst), Jazz Pharmaceuticals (Inst), Lyell Immunopharma (Inst), Millennium (Inst), Moderna Therapeutics (Inst), Monte Rosa Therapeutics (Inst), Peloton Therapeutics (Inst), Shenzhen Chipscreen Biosciences (Inst), Stemline Therapeutics (Inst), Synthekine (Inst), Taiho Oncology (Inst), Tango Therapeutics (Inst), Tarveda Therapeutics (Inst), Zai Lab (Inst), Apollomics (Inst), Strata Oncology (Inst), Asher Biotherapeutics (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Genentech, Novartis
Tania Szado
Employment: Genentech
Stock and Other Ownership Interests: Genentech/Roche, Moderna Therapeutics, VIR Biotechnology
Katja Schulze
Employment: Genentech
Stock and Other Ownership Interests: Roche/Genentech
Research Funding: Genentech
Richard Price
Employment: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Patents, Royalties, Other Intellectual Property: Healthcare technology patent
Julia Malato
Employment: Genentech/Roche, Merck, Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche, Merck, Genentech/Roche
Travel, Accommodations, Expenses: Genentech/Roche
Amy A. Lo
Employment: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Jonathan Levy
Employment: Pharvaris
Stock and Other Ownership Interests: Pharvaris
Yong Wang
Employment: Roche/Genentech
Stock and Other Ownership Interests: Roche/Genentech
Wei Yu
Employment: Genentech/Roche
Stock and Other Ownership Interests: Roche/Genentech
Funda Meric-Bernstam
Employment: MD Anderson Cancer Center
Consulting or Advisory Role: Roche, Zymeworks, Infinity Pharmaceuticals, AbbVie, Black Diamond Therapeutics, Eisai, OnCusp Therapeutics, Lengo Therapeutics, Tallac Therapeutics, Karyopharm Therapeutics, Biovica, AstraZeneca, Seagen, Loxo, PACT Pharmaceuticals, Apeiron Biologics, EcoR1 Capital, Menarini Group, Theratechnologies, Calibr, LegoChem Biosciences, Protai
Research Funding: Novartis (Inst), AstraZeneca (Inst), Taiho Pharmaceutical (Inst), Genentech (Inst), Calithera Biosciences (Inst), Debiopharm Group (Inst), Bayer (Inst), Aileron Therapeutics (Inst), PUMA Biotechnology (Inst), CytomX Therapeutics (Inst), Jounce Therapeutics (Inst), Zymeworks (Inst), Curis (Inst), Pfizer (Inst), eFFECTOR Therapeutics (Inst), AbbVie (Inst), Boehringer Ingelheim (Inst), Guardant Health (Inst), Daiichi Sankyo (Inst), GlaxoSmithKline (Inst), Seagen (Inst), Taiho Pharmaceutical (Inst), Klus Pharma (Inst), Takeda (Inst)
Travel, Accommodations, Expenses: European Organisation for Research and Treatment of Cancer (EORTC), ESMO, Cholangiocarcinoma Foundation
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the 2021 ASCO Annual Meeting, Chicago, IL, June 4-8, 2021.
SUPPORT
Supported by F. Hoffmann-La Roche/Genentech. This work was also supported by The University of Texas MD Anderson Cancer Center Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer, a NIH Clinical Translational Science Award (1UL1TR003167), and The University of Texas MD Anderson Cancer Center Support Grant (P30 CA016672).
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
AUTHOR CONTRIBUTIONS
Conception and design: Christopher J. Sweeney, John D. Hainsworth, Ron Bose, Howard A. Burris, Razell Kurzrock, Charles Swanton, Claire F. Friedman, David R. Spigel, Tania Szado, Katja Schulze, Funda Meric-Bernstam
Provision of study materials or patients: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
MyPathway Human Epidermal Growth Factor Receptor 2 Basket Study: Pertuzumab + Trastuzumab Treatment of a Tissue-Agnostic Cohort of Patients With Human Epidermal Growth Factor Receptor 2–Altered Advanced Solid Tumors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Christopher J. Sweeney
Stock and Other Ownership Interests: Leuchemix
Consulting or Advisory Role: Sanofi, Janssen Biotech, Astellas Pharma, Bayer, Genentech/Roche, AstraZeneca, Pfizer, Amgen, Lilly, POINT Biopharma, Cadence Pharma
Research Funding: Janssen Biotech (Inst), Astellas Pharma (Inst), Sanofi (Inst), Bayer (Inst), Dendreon, Pfizer (Inst)
Patents, Royalties, Other Intellectual Property: Leuchemix, Parthenolide, Dimethylaminoparthenolide. Exelixis: Abiraterone plus cabozantinib combination
John D. Hainsworth
Consulting or Advisory Role: Roche/Genentech (Inst)
Research Funding: Genentech (Inst)
Ron Bose
Consulting or Advisory Role: Genentech
Research Funding: Puma Biotechnology (Inst)
Howard A. Burris
Employment: HCA Healthcare
Leadership: HCA Healthcare
Stock and Other Ownership Interests: HCA Healthcare
Consulting or Advisory Role: GRAIL (Inst), Roche (Inst), Vincerx Pharma (Inst)
Research Funding: Roche/Genentech (Inst), Bristol Myers Squibb (Inst), Incyte (Inst), AstraZeneca (Inst), MedImmune (Inst), Macrogenics (Inst), Novartis (Inst), Boehringer Ingelheim (Inst), Lilly (Inst), Seagen (Inst), Merck (Inst), Agios (Inst), Jounce Therapeutics (Inst), Moderna Therapeutics (Inst), GlaxoSmithKline (Inst), Verastem (Inst), Tesaro (Inst), BioMed Valley Discoveries (Inst), TG Therapeutics (Inst), Vertex (Inst), eFFECTOR Therapeutics (Inst), Janssen (Inst), BioAtla (Inst), CicloMed (Inst), Harpoon therapeutics (Inst), Archer (Inst), Arvinas (Inst), Revolution Medicines (Inst), Array BioPharma (Inst), Bayer (Inst), Kymab (Inst), Pfizer (Inst), Takeda/Millennium (Inst), Foundation Medicine (Inst), EMD Serono (Inst), ARMO BioSciences (Inst), CALGB (Inst), Hengrui Therapeutics (Inst), XBiotech (Inst), Zymeworks (Inst), Coordination Pharmaceuticals (Inst), NGM Biopharmaceuticals (Inst), Gossamer Bio (Inst), Ryvu Therapeutics (Inst), BioTheryX (Inst), AbbVie (Inst), BeiGene (Inst), Celgene (Inst), Vertex (Inst)
Uncompensated Relationships: Bayer (Inst), Novartis (Inst), Incyte (Inst), Bristol Myers Squibb (Inst), Boehringer Ingelheim (Inst), TG Therapeutics (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/201030/
Razelle Kurzrock
Leadership: CureMatch, CureMetrix
Stock and Other Ownership Interests: CureMatch, IDbyDNA, CureMetrix
Honoraria: Roche, EUSA Pharma, NeoGenomics Laboratories, Biocom, NeoMed, LEK, AACR, Chugai Pharma USA, Wiley, Merck, Pfizer, Foundation Medicine, Turning Point Therapeutics, Bicara Therapeutics
Consulting or Advisory Role: Actuate Therapeutics, Loxo, XBiotech, NeoMed, Roche, Gaido, Soluventis, Pfizer, Merck, Turning Point Therapeutics, TD2/Volastra, Bicara Therapeutics, AstraZeneca, Biological Dynamics, Daiichi Sankyo, Eisai, EOM Pharmaceuticals, Iylon, Prosperdtx, Caris Life Sciences, Datar Genomics, NeoGenomics Laboratories, Regeneron
Speakers' Bureau: Roche, NeoGenomics Laboratories
Research Funding: Guardant Health (Inst), Sequenom (Inst), Merck Serono (Inst), Genentech (Inst), Pfizer (Inst), Foundation Medicine (Inst), Incyte (Inst), Konica Minolta (Inst), Grifols (Inst), OmniSeq (Inst), Debiopharm Group (Inst), Boehringer Ingelheim (Inst), Top Alliance BioScience (Inst), Takeda (Inst), MedImmune (Inst), Biological Dynamics (Inst)
Travel, Accommodations, Expenses: TargetCancer Foundation, NCI SWOG
Charles Swanton
Stock and Other Ownership Interests: Epic Sciences, Apogen Biotechnologies, GRAIL, Achilles Therapeutics, Bicycle Therapeutics
Honoraria: Roche, Boehringer Ingelheim, GlaxoSmithKline, Lilly, Celgene, Ono Pharmaceutical, Pfizer, Bristol Myers Squibb, Novartis, AstraZeneca, Illumina, MSD Oncology, Amgen, Roche/Genentech
Consulting or Advisory Role: Genentech/Roche, Sarah Cannon Research Institute, Medicxi, Bicycle Therapeutics, Metabomed, Roche, GRAIL, AstraZeneca, Amgen, Pfizer, Novartis, GlaxoSmithKline, MSD, Bristol Myers Squibb, Illumina, Roche, Medicxi, Achilles Therapeutics
Research Funding: Boehringer Ingelheim, BMS, Roche, AstraZeneca, Ono Pharmaceutical, Archer, Pfizer, Personalis
Patents, Royalties, Other Intellectual Property: Founder of Achilles Therapeutics: a biotechnology company funded by Syncona/Wellcome Trust to target clonal Neoantigens through vaccine and cell therapy approaches, Method for treating cancer based on identification of clonal neo-antigens (PCT/EP2016/059401), Immune checkpoint intervention in cancer (PCT/EP2016/071471), Methods for lung cancer detection (PCT/US2017/028013), Method of detecting tumour recurrence (PCT/GB2017/053289), Method for treating cancer (PCT/EP2016/059401), Method of identifying insertion/deletion mutation targets (PCT/GB2018/051892), Method for determining whether an HLA allele is lost in a tumour (PCT/GB2018/052004), Method for identifying responders to cancer treatment (PCT/GB2018/051912), Method of predicting survival rates for cancer patients (PCT/GB2020/050221)
Uncompensated Relationships: AstraZeneca (Inst)
Claire F. Friedman
Honoraria: Aptitude Health, GlaxoSmithKline
Consulting or Advisory Role: Arch Oncology, Bristol Myers Squibb, AADi
Research Funding: Bristol Myers Squibb (Inst), Genentech (Inst), Merck (Inst), Daiichi Sankyo/UCB Japan (Inst), Seagen (Inst), AstraZeneca (Inst), Hotspot Therapeutics (Inst), Marengo Therapeutics (Inst)
Travel, Accommodations, Expenses: Puma Biotechnology
Uncompensated Relationships: Genentech, Merck
Open Payments Link: https://openpaymentsdata.cms.gov/physician/477023/
David R. Spigel
Leadership: ASCO (Inst)
Consulting or Advisory Role: Genentech/Roche (Inst), Novartis (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), GlaxoSmithKline (Inst), Molecular Templates (Inst), Jazz Pharmaceuticals (Inst), Sanofi/Aventis (Inst), Regeneron (Inst), Lilly (Inst), BeiGene (Inst), Ipsen (Inst), Monte Rosa Therapeutics (Inst), AbbVie (Inst), Lyell Immunopharma (Inst), Novocure (Inst)
Research Funding: Genentech/Roche (Inst), Novartis (Inst), Celgene (Inst), Bristol Myers Squibb (Inst), Lilly (Inst), AstraZeneca (Inst), University of Texas Southwestern Medical Center - Simmons Cancer Center (Inst), Merck (Inst), G1 Therapeutics (Inst), Neon Therapeutics (Inst), Nektar (Inst), Celldex (Inst), Clovis Oncology (Inst), Daiichi Sankyo (Inst), Astellas Pharma (Inst), GRAIL (Inst), Transgene (Inst), Aeglea Biotherapeutics (Inst), Ipsen (Inst), BIND Therapeutics (Inst), Eisai (Inst), ImClone Systems (Inst), Janssen Oncology (Inst), MedImmune (Inst), Agios (Inst), GlaxoSmithKline (Inst), Tesaro (Inst), cyteir (Inst), Novocure (Inst), Elevation Oncology (Inst), Calithera Biosciences (Inst), Arcus Biosciences (Inst), Arrys Therapeutics (Inst), Bayer (Inst), BeiGene (Inst), Blueprint Medicines (Inst), Boehringer Ingelheim (Inst), Hutchison MediPharma (Inst), Incyte (Inst), Kronos Bio (Inst), Loxo (Inst), Macrogenics (Inst), Molecular Templates (Inst), PureTech (Inst), Razor Genomics (Inst), Repare Therapeutics (Inst), Rgenix (Inst), Tizona Therapeutics, Inc (Inst), Verastem (Inst), BioNTech (Inst), AbbVie (Inst), Amgen (Inst), Anheart Therapeutics (Inst), Ascendis Pharma (Inst), Endeavor BioMedicines (Inst), Erasca, Inc (Inst), Faeth Therapeutics (Inst), Fujifilm (Inst), Gilead Sciences (Inst), Jazz Pharmaceuticals (Inst), Lyell Immunopharma (Inst), Millennium (Inst), Moderna Therapeutics (Inst), Monte Rosa Therapeutics (Inst), Peloton Therapeutics (Inst), Shenzhen Chipscreen Biosciences (Inst), Stemline Therapeutics (Inst), Synthekine (Inst), Taiho Oncology (Inst), Tango Therapeutics (Inst), Tarveda Therapeutics (Inst), Zai Lab (Inst), Apollomics (Inst), Strata Oncology (Inst), Asher Biotherapeutics (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Genentech, Novartis
Tania Szado
Employment: Genentech
Stock and Other Ownership Interests: Genentech/Roche, Moderna Therapeutics, VIR Biotechnology
Katja Schulze
Employment: Genentech
Stock and Other Ownership Interests: Roche/Genentech
Research Funding: Genentech
Richard Price
Employment: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Patents, Royalties, Other Intellectual Property: Healthcare technology patent
Julia Malato
Employment: Genentech/Roche, Merck, Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche, Merck, Genentech/Roche
Travel, Accommodations, Expenses: Genentech/Roche
Amy A. Lo
Employment: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Jonathan Levy
Employment: Pharvaris
Stock and Other Ownership Interests: Pharvaris
Yong Wang
Employment: Roche/Genentech
Stock and Other Ownership Interests: Roche/Genentech
Wei Yu
Employment: Genentech/Roche
Stock and Other Ownership Interests: Roche/Genentech
Funda Meric-Bernstam
Employment: MD Anderson Cancer Center
Consulting or Advisory Role: Roche, Zymeworks, Infinity Pharmaceuticals, AbbVie, Black Diamond Therapeutics, Eisai, OnCusp Therapeutics, Lengo Therapeutics, Tallac Therapeutics, Karyopharm Therapeutics, Biovica, AstraZeneca, Seagen, Loxo, PACT Pharmaceuticals, Apeiron Biologics, EcoR1 Capital, Menarini Group, Theratechnologies, Calibr, LegoChem Biosciences, Protai
Research Funding: Novartis (Inst), AstraZeneca (Inst), Taiho Pharmaceutical (Inst), Genentech (Inst), Calithera Biosciences (Inst), Debiopharm Group (Inst), Bayer (Inst), Aileron Therapeutics (Inst), PUMA Biotechnology (Inst), CytomX Therapeutics (Inst), Jounce Therapeutics (Inst), Zymeworks (Inst), Curis (Inst), Pfizer (Inst), eFFECTOR Therapeutics (Inst), AbbVie (Inst), Boehringer Ingelheim (Inst), Guardant Health (Inst), Daiichi Sankyo (Inst), GlaxoSmithKline (Inst), Seagen (Inst), Taiho Pharmaceutical (Inst), Klus Pharma (Inst), Takeda (Inst)
Travel, Accommodations, Expenses: European Organisation for Research and Treatment of Cancer (EORTC), ESMO, Cholangiocarcinoma Foundation
No other potential conflicts of interest were reported.
REFERENCES
- 1.Dumbrava EEI, Balaji K, Raghav K, et al. : Targeting ERBB2 (HER2) amplification identified by next-generation sequencing in patients with advanced or metastatic solid tumors beyond conventional indications. JCO Precis Oncol 10.1200/PO.18.00345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan M, Schwaederle M, Arguello D, et al. : HER2 expression status in diverse cancers: Review of results from 37,992 patients. Cancer Metastasis Rev 34:157-164, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baselga J, Gelmon KA, Verma S, et al. : Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol 28:1138-1144, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slamon DJ, Leyland-Jones B, Shak S, et al. : Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783-792, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Bang YJ, Van Cutsem E, Feyereislova A, et al. : Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 376:687-697, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Strickler JH, Cercek A, Siena S, et al. : MOUNTAINEER: Open-label, phase 2 study of tucatinib in combination with trastuzumab for HER2-positive metastatic colorectal cancer. Presented at the ESMO World Congress on Gastrointestinal Cancers Barcelona, July 2, 2022
- 7.Mazieres J, Barlesi F, Filleron T, et al. : Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targeted drugs: Results from the European EUHER2 cohort. Ann Oncol 27:281-286, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Li BT, Smit EF, Goto Y, et al. : Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N Engl J Med 386:241-251, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper AJ, Gainor JF: Human epidermal growth factor receptor 2-mutant non-small-cell lung cancer: Continued progress but challenges remain. J Clin Oncol 40:693-697, 2022 [DOI] [PubMed] [Google Scholar]
- 10.Hainsworth JD, Meric-Bernstam F, Swanton C, et al. : Targeted therapy for advanced solid tumors on the basis of molecular profiles: Results from MyPathway, an open-label, phase IIa multiple basket study. J Clin Oncol 36:536-542, 2018 [DOI] [PubMed] [Google Scholar]
- 11.Meric-Bernstam F, Hurwitz H, Raghav KPS, et al. : Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): An updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol 20:518-530, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurzrock R, Bowles DW, Kang H, et al. : Targeted therapy for advanced salivary gland carcinoma based on molecular profiling: Results from MyPathway, a phase IIa multiple basket study. Ann Oncol 31:412-421, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Javle M, Borad MJ, Azad NS, et al. : Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): A multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol 22:1290-1300, 2021 [DOI] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in oncology (NCCN Guidelines): Colon cancer V.2.2023. 2023. http://NCCN.org
- 15.National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in oncology (NCCN Guidelines): Head and Neck Cancers V.2.2023. 2023. http://NCCN.org
- 16.National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in oncology (NCCN Guidelines): Biliary Tract Cancers V.2.2023. 2023. http://NCCN.org
- 17.Zhao B, Wang L, Qiu H, et al. : Mechanisms of resistance to anti-EGFR therapy in colorectal cancer. Oncotarget 8:3980-4000, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berns K, Horlings HM, Hennessy BT, et al. : A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 12:395-402, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Smith AE, Ferraro E, Safonov A, et al. : HER2 + breast cancers evade anti-HER2 therapy via a switch in driver pathway. Nat Commun 12:6667, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Majewski IJ, Nuciforo P, Mittempergher L, et al. : PIK3CA mutations are associated with decreased benefit to neoadjuvant human epidermal growth factor receptor 2-targeted therapies in breast cancer. J Clin Oncol 33:1334-1339, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bose R, Kavuri SM, Searleman AC, et al. : Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov 3:224-237, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hyman DM, Piha-Paul SA, Won H, et al. : HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 554:189-194, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li BT, Shen R, Buonocore D, et al. : Ado-trastuzumab emtansine for patients with HER2-mutant lung cancers: Results from a phase II basket trial. J Clin Oncol 36:2532-2537, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smyth LM, Piha-Paul SA, Won HH, et al. : Efficacy and determinants of response to HER kinase inhibition in HER2-mutant metastatic breast cancer. Cancer Discov 10:198-213, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu X, De Angelis C, Burke KA, et al. : HER2 reactivation through acquisition of the HER2 L755S mutation as a mechanism of acquired resistance to HER2-targeted therapy in HER2(+) breast cancer. Clin Cancer Res 23:5123-5134, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Wu S, Zhuang X, et al. : Identification of an activating mutation in the extracellular domain of HER2 conferring resistance to pertuzumab. Onco Targets Ther 12:11597-11608, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meric-Bernstam F, Johnson AM, Dumbrava EEI, et al. : Advances in HER2-targeted therapy: Novel agents and opportunities beyond breast and gastric cancer. Clin Cancer Res 25:2033-2041, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).