Abstract
PURPOSE
Immunotherapy and chemotherapy combinations have shown activity in endometrial cancer, with greater benefit in mismatch repair (MMR)–deficient (dMMR) than MMR-proficient (pMMR) disease. Adding a poly(ADP-ribose) polymerase inhibitor may improve outcomes, especially in pMMR disease.
METHODS
This phase III, global, double-blind, placebo-controlled trial randomly assigned eligible patients with newly diagnosed advanced or recurrent endometrial cancer 1:1:1 to: carboplatin/paclitaxel plus durvalumab placebo followed by placebo maintenance (control arm); carboplatin/paclitaxel plus durvalumab followed by maintenance durvalumab plus olaparib placebo (durvalumab arm); or carboplatin/paclitaxel plus durvalumab followed by maintenance durvalumab plus olaparib (durvalumab + olaparib arm). The primary end points were progression-free survival (PFS) in the durvalumab arm versus control and the durvalumab + olaparib arm versus control.
RESULTS
Seven hundred eighteen patients were randomly assigned. In the intention-to-treat population, statistically significant PFS benefit was observed in the durvalumab (hazard ratio [HR], 0.71 [95% CI, 0.57 to 0.89]; P = .003) and durvalumab + olaparib arms (HR, 0.55 [95% CI, 0.43 to 0.69]; P < .0001) versus control. Prespecified, exploratory subgroup analyses showed PFS benefit in dMMR (HR [durvalumab v control], 0.42 [95% CI, 0.22 to 0.80]; HR [durvalumab + olaparib v control], 0.41 [95% CI, 0.21 to 0.75]) and pMMR subgroups (HR [durvalumab v control], 0.77 [95% CI, 0.60 to 0.97]; HR [durvalumab + olaparib v control] 0.57; [95% CI, 0.44 to 0.73]); and in PD-L1–positive subgroups (HR [durvalumab v control], 0.63 [95% CI, 0.48 to 0.83]; HR [durvalumab + olaparib v control], 0.42 [95% CI, 0.31 to 0.57]). Interim overall survival results (maturity approximately 28%) were supportive of the primary outcomes (durvalumab v control: HR, 0.77 [95% CI, 0.56 to 1.07]; P = .120; durvalumab + olaparib v control: HR, 0.59 [95% CI, 0.42 to 0.83]; P = .003). The safety profiles of the experimental arms were generally consistent with individual agents.
CONCLUSION
Carboplatin/paclitaxel plus durvalumab followed by maintenance durvalumab with or without olaparib demonstrated a statistically significant and clinically meaningful PFS benefit in patients with advanced or recurrent endometrial cancer.
INTRODUCTION
Endometrial cancer is one of the most common cancers among women worldwide, and the incidence is rising.1 Standard of care for newly diagnosed advanced or recurrent endometrial cancer includes platinum-based chemotherapy with carboplatin plus paclitaxel.2,3 Although patients often demonstrate initial sensitivity to platinum-based chemotherapy, most subsequently experience disease progression and require additional lines of chemotherapy.4-6
CONTEXT
Key Objective
Does the addition of the anti–PD-L1 antibody durvalumab to first-line platinum-based chemotherapy followed by maintenance durvalumab with or without the poly(ADP-ribose) polymerase (PARP) inhibitor olaparib improve outcomes in newly diagnosed and recurrent endometrial cancer compared with chemotherapy alone?
Knowledge Generated
DUO-E met its primary end points, with results indicating a statistically significant and clinically meaningful progression-free survival (PFS) benefit with the addition of durvalumab to standard first-line platinum-based chemotherapy followed by maintenance durvalumab with or without olaparib. The safety profiles observed in the experimental arms were generally consistent with individual agents.
Relevance (G. Fleming)
-
These data confirm a benefit of immune checkpoint inhibitor use in the front-line treatment of advanced or metastatic endometrial cancer, with PFS improvement seen, as in other trials, in the setting of both mismatch repair-deficient and mismatch repair-proficient disease. While reports of PARP inhibitor treatment in endometrial cancer have previously been lackluster, the DUO-E results open up promising avenues for exploration of PARP inhibitor maintenance therapy in some subsets of the disease.*
*Relevance section written by JCO Associate Editor Gini Fleming, MD.
The RUBY and NRG-GY018 trials recently demonstrated efficacy with immune checkpoint inhibitors in combination with chemotherapy as first-line therapy in patients with primary advanced or recurrent endometrial cancer,7,8 building on existing evidence for immunotherapy in endometrial cancer.9-12 The results from RUBY led to approval of dostarlimab in combination with carboplatin and paclitaxel followed by dostarlimab alone for the treatment of mismatch repair (MMR)-deficient (dMMR) or microsatellite instability-high, primary advanced or recurrent endometrial cancer in the United States.13 A high unmet need for new therapies remains, especially in patients with MMR-proficient (pMMR) tumors, who comprise approximately 75% of patients with endometrial cancer.14
We hypothesized that combining pharmacologic inhibition of poly(ADP-ribose) polymerase (PARP) with an immune checkpoint inhibitor may improve outcomes in endometrial cancer, including in patients with pMMR tumors.15-18
DUO-E/GOG-3041/ENGOT-EN10 investigated whether the addition of the anti–PD-L1 antibody durvalumab to carboplatin plus paclitaxel, followed by maintenance durvalumab with or without the addition of the PARP inhibitor olaparib, improved outcomes in newly diagnosed advanced or recurrent endometrial cancer.
METHODS
Trial Design and Patients
The DUO-E/GOG-3041/ENGOT-EN10 trial (ClinicalTrials.gov identifier: NCT04269200) was a randomized, double-blind, placebo-controlled multicenter phase III trial conducted in 22 countries. Eligible patients were age 18 years and older with newly diagnosed advanced (International Federation of Gynecology and Obstetrics [FIGO] measurable stage III/newly diagnosed stage IV [2009 staging system]) or recurrent endometrial cancer of epithelial histology (excluding sarcomas). For recurrent disease, the potential for cure by surgery was poor, and previous systemic anticancer treatment was allowed only if administered in the adjuvant setting and there was ≥12 months between last dose and subsequent relapse. Patients were required to have known MMR status (determined before random assignment; Data Supplement, online only). Full eligibility criteria are provided in the Data Supplement.
Random Assignment and Study Treatment
Patients were randomly assigned 1:1:1 to three treatment arms (Data Supplement, Fig S1), stratified by MMR status (proficient v deficient), disease status (newly diagnosed v recurrent), and geographic region (Asia v non-Asia). Patients received platinum-based chemotherapy (carboplatin: area under the curve, 5 or 6 mg/mL/min once every 3 weeks for 6 cycles; paclitaxel: 175 mg/m2 once every 3 weeks for 6 cycles) plus durvalumab placebo intravenously once every 3 weeks for six cycles, followed by maintenance durvalumab placebo intravenously once every 4 weeks plus olaparib placebo tablets twice daily (control arm); platinum-based chemotherapy plus durvalumab 1,120 mg intravenously once every 3 weeks for six cycles, followed by maintenance durvalumab 1,500 mg intravenously once every 4 weeks plus olaparib placebo tablets twice daily (durvalumab arm); or platinum-based chemotherapy plus durvalumab 1,120 mg intravenously once every 3 weeks for six cycles, followed by maintenance durvalumab 1,500 mg intravenously once every 4 weeks plus olaparib 300 mg tablets twice daily (durvalumab + olaparib arm).
Treatment continued until radiologic disease progression (RECIST v1.1, investigator-assessed), unacceptable toxicity, or other discontinuation criteria were met. Patients without objective disease progression during the chemotherapy phase who met other prespecified requirements (Data Supplement) were permitted to start maintenance therapy.
Study End Points
The dual primary end points were investigator-assessed progression-free survival (PFS), defined as the time from random assignment to objective disease progression (RECIST v1.1) or death, for both the durvalumab arm versus control and the durvalumab + olaparib arm versus control. Prespecified subgroup and sensitivity analyses of PFS were conducted (Data Supplement). A prespecified, exploratory analysis of PFS in the durvalumab + olaparib versus durvalumab arms was conducted.
Secondary end points included overall survival (OS), patient-reported outcomes (using the European Organisation for Research and Treatment of Cancer [EORTC] Core Quality of Life Questionnaire [QLQ-C30]), and safety.
Assessments
Tumor assessments were performed at baseline, every 9 weeks (±1 week) for 18 weeks, and every 12 weeks (±1 week) thereafter until objective radiologic disease progression (RECIST v1.1). After disease progression, patients were assessed every 12 weeks for second progression and every 2 months for survival.
Adverse events (AEs) were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE; v5.0) and monitored throughout treatment and for 30 days after the last dose of olaparib and 90 days after the last dose of durvalumab, whichever was later. Events of myelodysplastic syndrome (MDS), AML, and new primary malignancies were reported throughout the study and during survival follow-up.
Trial Oversight
The trial adhered to the Declaration of Helsinki, Good Clinical Practice Guidelines, and the AstraZeneca policy of bioethics.19 All patients provided written informed consent. The trial was designed and sponsored by AstraZeneca in collaboration with the authors and academic groups under the GOG Foundation and the European Network of Gynecological Oncological Trial (ENGOT) groups. AstraZeneca was responsible for overseeing the collection, analysis, and interpretation of data. Authors had full access to the data, wrote the manuscript, and attest to the accuracy and completeness of data and the fidelity of the trial to the protocol. Medical writing assistance was funded by AstraZeneca.
Statistical Methods
The planned sample size was approximately 699 patients. The primary analysis of PFS was planned when both criteria were met: 64% maturity (approximately 299 events) for the durvalumab versus control comparison and 60% maturity (approximately 281 events) for durvalumab + olaparib versus control. Assuming a median PFS of 12 months for the control and an average true PFS hazard ratio (HR) of 0.70 for durvalumab versus control and 0.55 for durvalumab + olaparib versus control, the study had 80% and >99% power to demonstrate a statistically significant difference at the overall two-sided significance level of 2.5% for each comparison, respectively. The first interim analysis of OS was performed at the time of the primary PFS analysis.
A multiple testing procedure with gatekeeping strategy was used to strongly control the type I error at 5% (two-sided) across the key efficacy end points (PFS and OS) for the durvalumab + olaparib and durvalumab versus control comparisons (Data Supplement, Fig S2).
Efficacy data were summarized and analyzed in the intention-to-treat population and safety data in the safety analysis set (all randomly assigned patients who received at least one dose of investigational treatment: durvalumab/placebo or olaparib/placebo); for the maintenance phase, safety data were summarized in patients who received at least one dose of olaparib/placebo maintenance treatment.
The primary PFS analysis for each comparison was performed separately using a stratified log-rank test for generation of P values, with HRs and 95% CIs estimated using a stratified Cox proportional hazards model. Kaplan-Meier plots were presented by treatment arm and used to estimate the median PFS and the proportion of patients alive and progression-free at landmark time points (6, 12, and 18 months). The proportional hazards assumption was tested by fitting a Cox model with a treatment-by-time interaction (Data Supplement). Analyses of secondary time-to-event end points used similar methods.
RESULTS
Patients
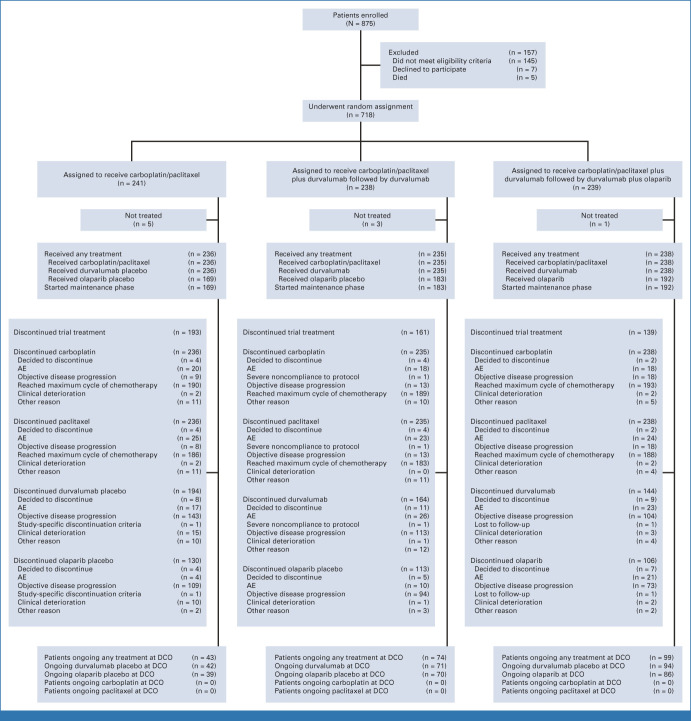
From June 2, 2020, through April 20, 2022, 718 patients were randomly assigned: 241, 238, and 239 to the control, durvalumab, and durvalumab + olaparib arms, respectively. Of those randomly assigned, 236 patients (97.9%) in the control arm, 235 (98.7%) in the durvalumab arm, and 238 (99.6%) in the durvalumab + olaparib arm received any study treatment (safety analysis set), and 169 (70.1%), 183 (76.9%), and 192 (80.3%), respectively, received olaparib/placebo maintenance (Fig 1).
FIG 1.
CONSORT diagram of patients. AE, adverse event; DCO, data cutoff.
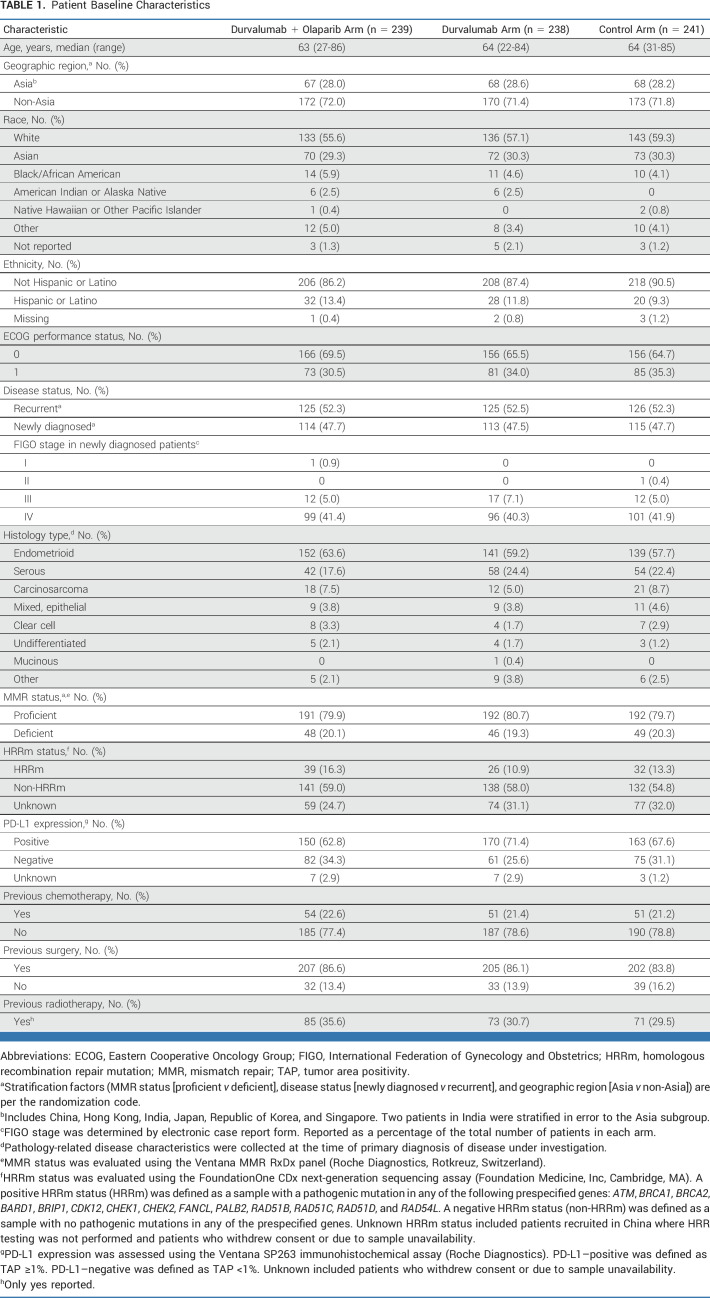
Baseline characteristics were generally balanced across treatment arms (Table 1) and representative of patients with newly diagnosed advanced or recurrent endometrial cancer (Data Supplement, Table S1). In the control, durvalumab, and durvalumab + olaparib arms, 80%, 81%, and 80% of patients, respectively, had pMMR tumors, 28%, 29%, and 28%, respectively, were from Asia, and 48%, 47%, and 48%, respectively, had newly diagnosed disease.
TABLE 1.
Patient Baseline Characteristics
Efficacy
At the data cutoff date (April 12, 2023), there were 312 PFS events (65% maturity) for the durvalumab versus control comparison and 299 PFS events (62% maturity) for the durvalumab + olaparib versus control comparison. The median (range) duration of follow-up in patients censored for PFS was 12.6 months (0.0-31.6) in the control arm, 15.4 months (0.0-29.1) in the durvalumab arm, and 15.4 months (0.0-31.7) in the durvalumab + olaparib arm.
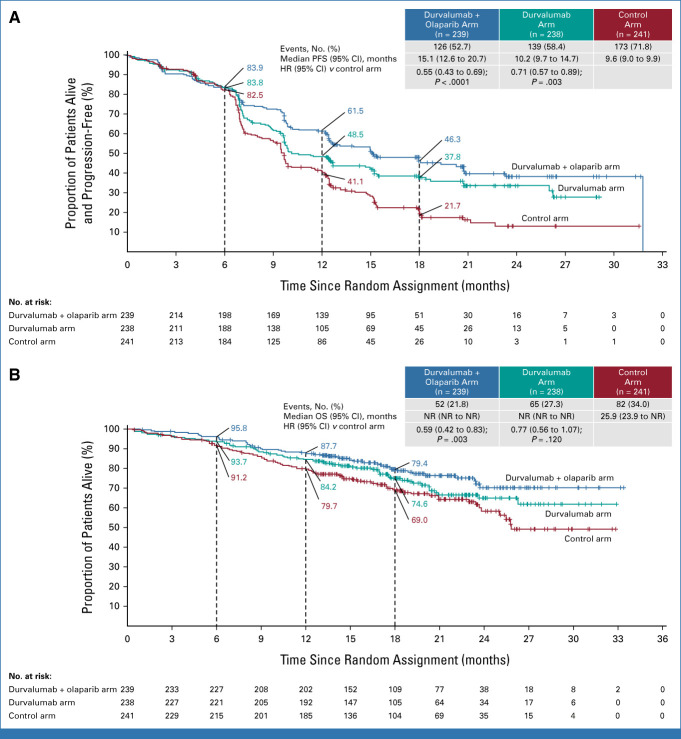
In the intention-to-treat population, the durvalumab arm had a statistically significant 29% lower risk of disease progression or death versus control (HR, 0.71 [95% CI, 0.57 to 0.89]; P = .003; median PFS 10.2 v 9.6 months; Fig 2A; Table 2). The durvalumab + olaparib arm had a statistically significant 45% lower risk of disease progression or death versus control (HR, 0.55 [95% CI, 0.43 to 0.69]; P < .0001; median PFS 15.1 v 9.6 months; Fig 2A; Table 2). The PFS Kaplan-Meier curves overlap until approximately 6 months, after which time there is a clear and sustained separation that favors both investigational treatment arms compared with control (Fig 2A). This delayed separation was expected because of the known delayed treatment effect for durvalumab, as well as the fact that olaparib maintenance therapy only started after completion of chemotherapy. The delay in separation of the curves suggested nonproportionality (P = .018 and P = .03 for the durvalumab v control and durvalumab + olaparib v control comparisons, respectively). In the presence of nonproportional hazards, the overall PFS HR is to be interpreted as an average estimate of the observed benefit. Rates at specific time points and the median PFS values are shown in Table 2. A sensitivity analysis of PFS by blinded independent central review was consistent with the results by investigator assessment for both the durvalumab versus control (HR, 0.74 [95% CI, 0.58 to 0.94]) and durvalumab + olaparib versus control (HR, 0.55 [95% CI, 0.42 to 0.70]) comparisons (Data Supplement, Fig S3). In a predefined, exploratory analysis of investigator-assessed PFS in the durvalumab + olaparib versus durvalumab arms, the HR was 0.78 (95% CI, 0.61 to 0.99; median PFS 15.1 v 10.2 months; Table 2). The first interim analysis of OS was conducted at the time of the primary PFS analysis, at which point 199 (28%) deaths had occurred in the intention-to-treat population. The median (range) duration of follow-up in patients censored for OS was 18.6 months (0.5-32.9) in the control arm, 18.4 months (2.1-33.0) in the durvalumab arm, and 18.7 months (1.1-33.4) in the durvalumab + olaparib arm. The HRs for both comparisons favored the investigational arms; however, neither comparison reached statistical significance at this first interim analysis of OS (durvalumab v control: HR, 0.77 [95% CI, 0.56 to 1.07]; P = .120; durvalumab + olaparib v control: HR, 0.59 [95% CI, 0.42 to 0.83]; P = .003, Fig 2B).
FIG 2.
Intention-to-treat analyses of (A) PFS, as assessed by the investigator according to RECIST v1.1, and (B) OS. For the PFS analysis, the HRs and CIs were estimated from a Cox proportional hazards model stratified by MMR and disease status. For the OS analysis, the HRs and CIs were estimated from an unstratified Cox proportional hazards model. P values were calculated using a stratified log-rank test. Tick marks indicate a censored observation. Patients without an event were censored at the latest evaluable RECIST assessment. HR, hazard ratio; MMR, mismatch repair; NR, not reached; OS, overall survival; PFS, progression-free survival.
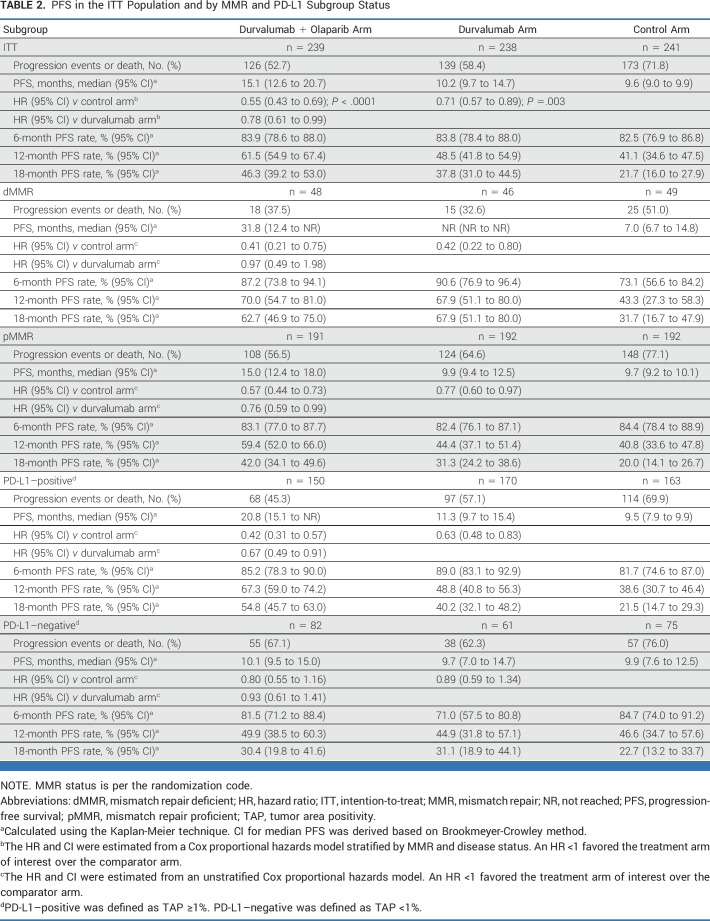
TABLE 2.
PFS in the ITT Population and by MMR and PD-L1 Subgroup Status
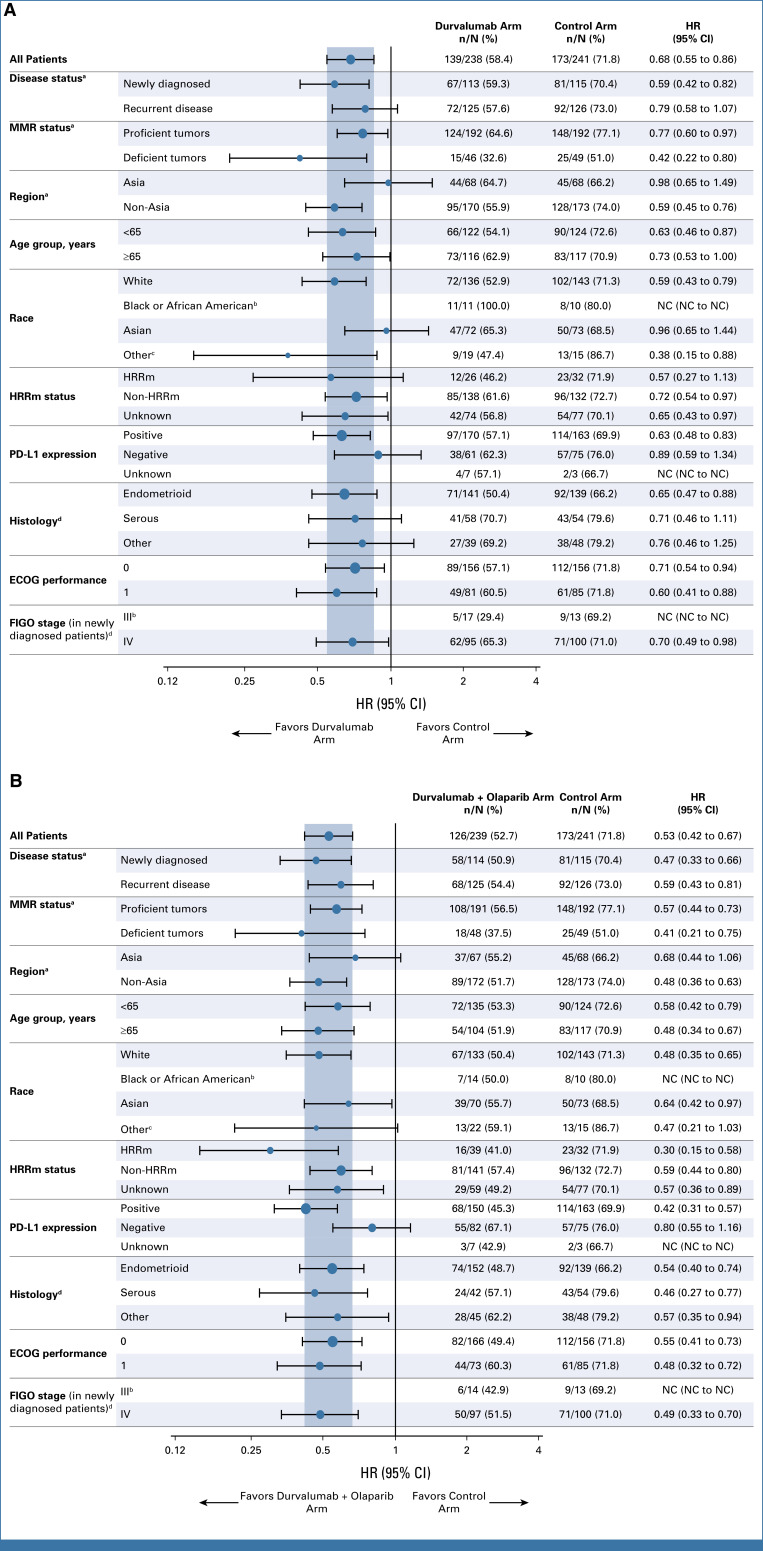
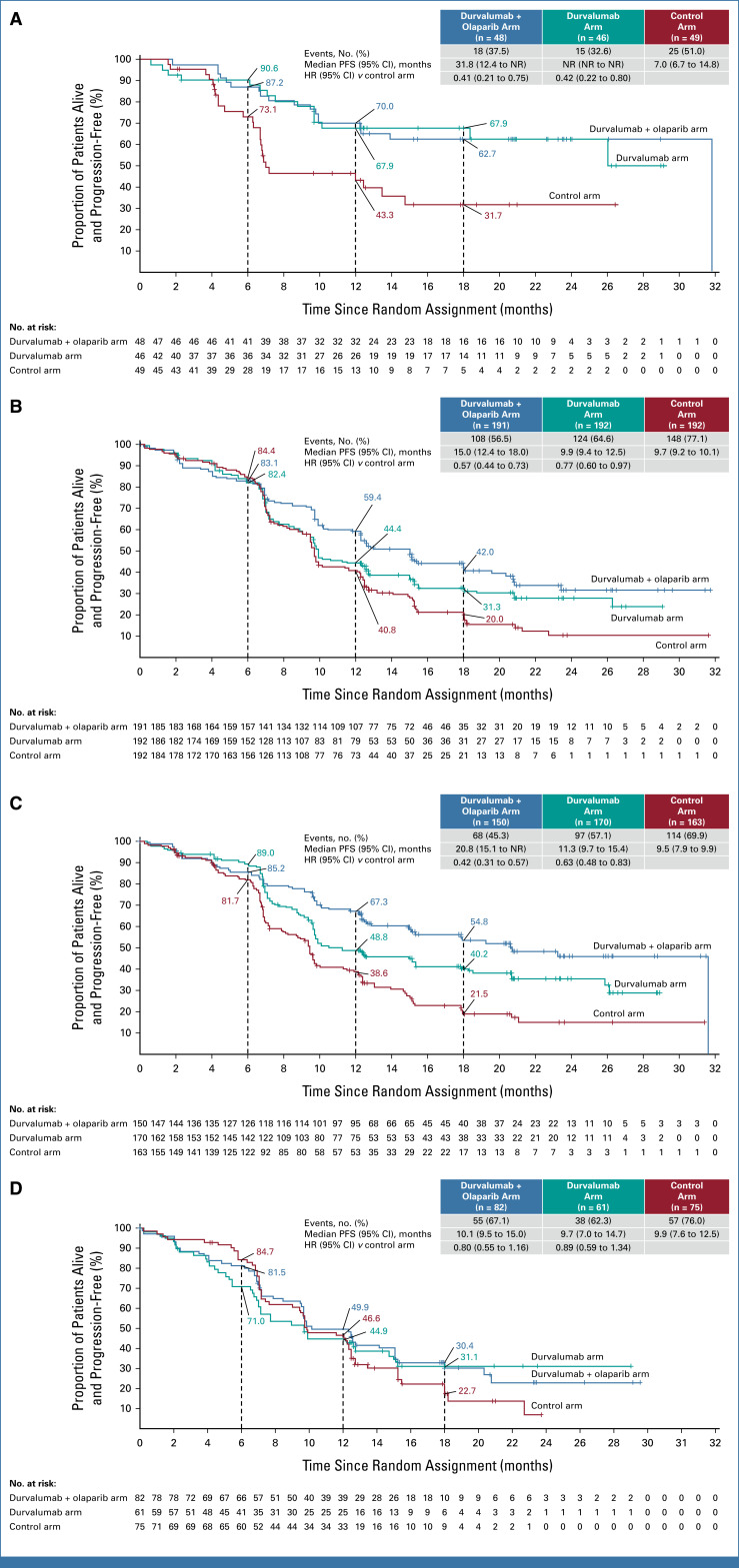
In prespecified exploratory subgroup analyses of PFS, all observed HR point estimates favored the durvalumab and durvalumab + olaparib arms versus control (Figs 3A and 3B). In the dMMR subgroup, the HRs for PFS were 0.42 (95% CI, 0.22 to 0.80, median PFS not reached [NR] v 7.0 months) for durvalumab versus control and 0.41 (95% CI, 0.21 to 0.75, median PFS 31.8 v 7.0 months) for durvalumab + olaparib versus control (Table 2 and Fig 4A). In the pMMR subgroup, the HRs for PFS were 0.77 (95% CI, 0.60 to 0.97, median PFS 9.9 v 9.7 months) for durvalumab versus control and 0.57 (95% CI, 0.44 to 0.73, 15.0 v 9.7 months) for durvalumab + olaparib versus control (Table 2 and Fig 4B). Comparison of durvalumab + olaparib versus durvalumab by MMR status is reported in Table 2. Subgroup analyses by PD-L1 status suggested a PFS benefit for both the durvalumab and durvalumab + olaparib arms compared with control in the PD-L1–positive subgroup (defined as tumor area positivity [TAP] ≥1%); the HRs for PFS were 0.63 (95% CI, 0.48 to 0.83) with median PFS 11.3 versus 9.5 months for the durvalumab arm versus control and 0.42 (95% CI, 0.31 to 0.57), with median PFS 20.8 versus 9.5 months for the durvalumab + olaparib arm versus control (Fig 4C). In the PD-L1–negative subgroup (TAP <1%), the HRs for PFS were 0.89 (95% CI, 0.59 to 1.34), median PFS 9.7 versus 9.9 for durvalumab versus control, and 0.80 (95% CI, 0.55 to 1.16), median PFS 10.1 versus 9.9 for durvalumab + olaparib versus control (Fig 4D).
FIG 3.
Subgroup analysis of PFS in (A) the durvalumab arm and (B) the durvalumab + olaparib arm. The HR and CI were estimated from an unstratified Cox proportional hazards model. An HR <1 favored the treatment arm of interest over the control arm. aStratification factors are per the randomization code. bHRs are not calculated because of the small number of patients. cIncludes patients with race not reported. dAs determined at the time of initial diagnosis of disease under investigation. ECOG, Eastern Cooperative Oncology Group; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; HRRm, homologous recombination repair mutation; MMR, mismatch repair; NC, not calculated.
FIG 4.
Exploratory PFS analyses, as assessed by the investigator according to RECIST v1.1, in (A) dMMR, (B) pMMR, (C) PD-L1–positive, and (D) PD-L1–negative subgroups. For dMMR and pMMR subgroup analyses, MMR status is as per the randomization code. The HR and 95% CI were estimated from an unstratified Cox proportional hazards model. Tick marks indicate a censored observation. Patients without an event were censored at the latest evaluable RECIST assessment. PD-L1 expression was assessed using the Ventana SP263 immunohistochemical assay (Roche Diagnostics). PD-L1–positive was defined as TAP ≥1%. PD-L1–negative was defined as TAP <1%. Unknown included patients who withdrew consent or due to sample unavailability. dMMR, mismatch repair-deficient; HR, hazard ratio; MMR, mismatch repair; NR, not reached; PFS, progression-free survival; pMMR, mismatch repair-proficient; TAP, tumor area positivity.
Safety
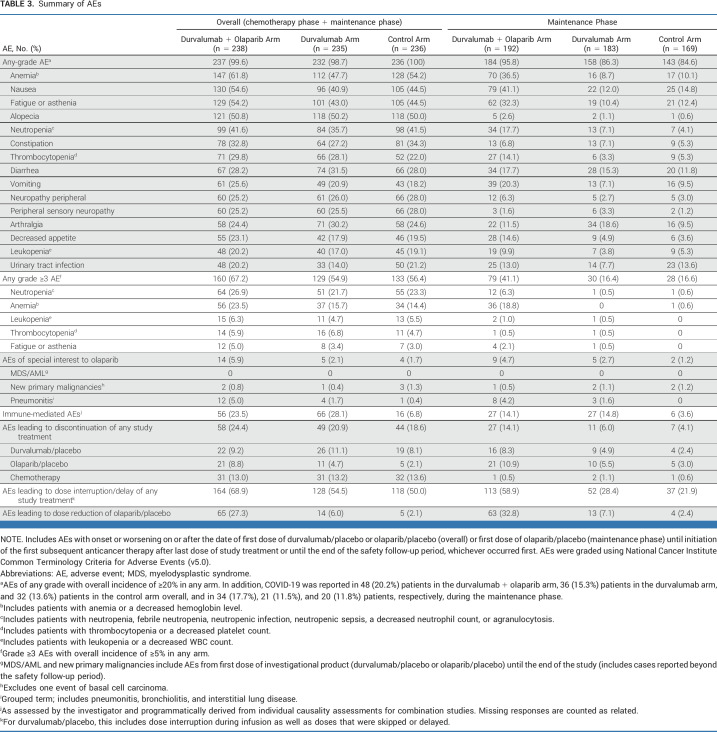
Duration of study treatment is detailed in the Data Supplement (Table S2). Across treatment arms, the most commonly reported AEs of any grade included anemia, nausea, fatigue or asthenia, and alopecia throughout the study period, and nausea, anemia, and fatigue or asthenia during the maintenance phase alone (Table 3). Most AEs occurring during the maintenance phase were low grade.
TABLE 3.
Summary of AEs
In the control, durvalumab, and durvalumab + olaparib arms, the overall incidence of grade 3 or higher treatment-emergent AEs was 56.4%, 54.9%, and 67.2%, respectively, and the incidence in the maintenance phase was 16.6%, 16.4%, and 41.1%, respectively. The overall incidence of grade 3 or higher neutropenia was 23.3%, 21.7%, and 26.0%, respectively, and the overall incidence of grade 3 or higher anemia was 14.4%, 15.7%, and 23.5%, respectively. Serious AEs occurred overall in 30.9%, 31.1%, and 35.7%, of patients in the control, durvalumab, and durvalumab + olaparib arms, respectively (Data Supplement, Table S3), and fatal events occurred in 3.4%, 1.7%, and 2.1%, respectively (Data Supplement, Table S4).
There were no cases of MDS or AML. New primary malignancies occurred in three (1.3%), one (<1%), and two (<1%) patients and pneumonitis of any grade in one (<1%), four (1.7%), and 12 (5.0%) patients in the control, durvalumab, and durvalumab + olaparib arms, respectively; most pneumonitis events occurred during the maintenance phase. There were three (1.6%) cases of pure red cell aplasia reported in the durvalumab + olaparib arm during the maintenance phase or in the follow-up period, all CTCAE grade 3, with one leading to discontinuation of study treatment; no cases were reported in the durvalumab or control arms. There were three cases of autoimmune hemolytic anemia (one [<1%] in the durvalumab arm and two [<1%] in the durvalumab + olaparib arm); all were grade 3.
Throughout the study period, immune-mediated AEs occurred in 6.8%, 28.1%, and 23.5% of patients in the control, durvalumab, and durvalumab + olaparib arms, respectively (Table 3; Data Supplement [Table S5]).
AEs were usually managed by dose modification rather than discontinuation (Table 3). AEs leading to discontinuation of any study treatment during the chemotherapy and maintenance phases occurred in 44 (18.6%), 49 (20.9%), and 58 (24.4%) patients overall in the control, durvalumab, and durvalumab + olaparib arms, respectively. The most common AEs leading to discontinuation of each agent are shown in the Data Supplement (Table S6).
Patient-Reported Outcomes
Analyses of patient-reported outcomes are ongoing.
DISCUSSION
The phase III DUO-E trial demonstrated that durvalumab in combination with first-line carboplatin and paclitaxel followed by maintenance durvalumab with or without olaparib resulted in significantly lower risk of disease progression or death than chemotherapy alone (with an average 45% risk reduction in the durvalumab + olaparib arm and 29% in the durvalumab arm compared with control) for patients with newly diagnosed advanced or recurrent endometrial cancer. These data confirm the clinical benefit of integrating immunotherapy into first-line chemotherapy, and to our knowledge, are the first to indicate that the addition of a PARP inhibitor may offer further benefit in this setting.
In prespecified exploratory subgroup analyses of PFS, all observed HR point estimates favored the durvalumab and durvalumab + olaparib arms versus control. Analyses by MMR status showed that in the dMMR subgroup, similar clinically meaningful benefit was observed in the durvalumab arm versus control (HR, 0.42 [95% CI, 0.22 to 0.80]) and in the durvalumab + olaparib arm versus control (HR, 0.41 [95% CI, 0.21 to 0.75]). In the pMMR subgroup, clinically meaningful benefit was observed in the durvalumab arm versus control (HR, 0.77 [95% CI, 0.60 to 0.97]) and the addition of maintenance olaparib to durvalumab suggested further benefit (HR v control, 0.57 [95% CI, 0.44 to 0.73]). Prespecified, exploratory analysis of the durvalumab + olaparib versus durvalumab arms suggested the contribution of olaparib was in the pMMR subgroup (HR in pMMR subgroup, 0.76 [95% CI, 0.59 to 0.99]; HR in dMMR subgroup, 0.97 [95% CI, 0.49 to 1.98]). Exploratory analyses by PD-L1 status suggested a benefit was observed for the PD-L1–positive subgroup, with a clinically meaningful improvement in PFS (HR, 0.63 [95% CI, 0.48 to 0.83] for the durvalumab arm v control and HR 0.42 [95% CI, 0.31 to 0.57] for the durvalumab + olaparib arm v control), whereas a smaller magnitude of improvement was observed for the PD-L1–negative subgroup (HR, 0.89 [95% CI, 0.59 to 1.34] and 0.80 [95% CI, 0.55 to 1.16] for durvalumab and durvalumab + olaparib arms v control, respectively). Additional biomarker analyses are ongoing.
A clinical benefit has also recently been reported for combination therapy with an immune checkpoint inhibitor and standard chemotherapy in endometrial cancer in the RUBY and NRG-GY018 trials. Caution is needed when comparing outcomes between DUO-E, RUBY, and NRG-GY018 because of differences in patient populations, including in the number of patients with newly diagnosed stage III disease, inclusion of patients with carcinosarcoma in DUO-E and RUBY but not NRG-GY018, and differences in the duration of follow-up and data maturity between trials. Both trials reported a PFS benefit in endometrial cancer (RUBY with dostarlimab and platinum-based chemotherapy versus platinum-based chemotherapy alone; NRG-GY018 with pembrolizumab and platinum-based chemotherapy versus platinum-based chemotherapy alone), with a particular benefit in dMMR subgroups of patients.7,8 Similarly, in DUO-E, a PFS benefit was observed for the durvalumab arm versus control irrespective of MMR status, although the greatest benefit was seen in the dMMR subgroups.
In support of the primary end points, the first interim analysis of OS favored both the durvalumab and durvalumab + olaparib arms compared with control, with generally consistent improvements in PFS and OS observed in the intention-to-treat population. Patients continue to be monitored for safety and efficacy, and updated OS analyses will be reported with longer follow-up.
The safety profiles of the experimental arms were generally consistent with the known profiles of the individual components of the regimens. The delivery of chemotherapy was not compromised by other treatments, and although the frequency of AEs leading to discontinuation of any treatment was numerically highest with durvalumab + olaparib, it remained similar across arms. AEs of special or potential interest related to durvalumab were consistent with the known safety profile of durvalumab. For AEs related to olaparib, there were no cases of MDS or AML, and the incidence of new primary malignancies was low. Events of pneumonitis were consistent with the known safety profile of olaparib and durvalumab. In line with the known safety profile of maintenance olaparib, events of anemia contributed to a higher rate of grade 3 or higher AEs in the durvalumab + olaparib arm.
The DUO-E trial enrolled patients globally, including approximately 28% of patients randomly assigned in the Asia region (compared with 3% of Asian race/ethnicity in RUBY and 5% of Asian race/ethnicity in NRG-GY018). In DUO-E, the small proportion of patients with stage III disease enrolled was likely because of the requirement for measurable disease.
In conclusion, to our knowledge, DUO-E is the first phase III trial to examine the combination of immunotherapy and PARP inhibition in endometrial cancer. The addition of durvalumab to standard first-line platinum-based chemotherapy followed by maintenance durvalumab with or without olaparib significantly improved PFS outcomes for patients with first-line advanced or recurrent endometrial cancer, confirming the clinical benefit of integrating immunotherapy into first-line chemotherapy and demonstrating a potential role for PARP inhibition in this setting. Predefined, exploratory subgroup analyses suggest the addition of maintenance olaparib to the combination of durvalumab plus chemotherapy may improve outcomes in the pMMR and PD-L1–positive patient populations. Although there was a higher rate of grade 3 or higher AEs in the durvalumab + olaparib arm, the safety profiles of each arm were generally consistent with the known profiles of individual components of the regimen.
ACKNOWLEDGMENT
The authors thank all the volunteers who participated in this study, their families, and the investigators.
Appendix Table A1 (online only) lists the principal investigator for each site that participated in the study.
APPENDIX
TABLE A1.
DUO-E Investigators
Shannon N. Westin
This author is a Consultant Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Roche, AstraZeneca, Genentech, Medscape, Clovis Oncology, Gerson Lehrman Group, Vaniam Group, Merck, BioAscent, OncLive, Targeted Oncology, Curio Science, GlaxoSmithKline, Eisai, Zentalis, EQRX, Lilly, Vincerx Pharma, Mereo BioPharma, Immunogen, Mersana, NGM Biopharmaceuticals, Caris Life Sciences, Nuvectis Pharma, Seagen, Immunocore, ZielBio, Verastem, Gilead Sciences, Mersana
Research Funding: AstraZeneca (Inst), Novartis (Inst), Bayer (Inst), Clovis Oncology (Inst), Roche/Genentech (Inst), GOG Foundation (Inst), Mereo BioPharma (Inst), Bio-Path Holdings, Inc (Inst), GlaxoSmithKline (Inst), Zentalis (Inst), Avenge Bio (Inst), Jazz Pharmaceuticals (Inst)
Kathleen Moore
Leadership: GOG Partners, NRG Oncology (Inst)
Honoraria: Research To Practice, Prime Oncology, Physicans' Education Resource, Great Debates and Updates
Consulting or Advisory Role: Genentech/Roche, Immunogen, AstraZeneca, VBL Therapeutics, Merck, Eisai, Mersana (Inst), Myriad Genetics, Alkermes (Inst), Blueprint Medicines (Inst), GlaxoSmithKline/Tesaro (Inst), OncXerna Therapeutics, Onconova Therapeutics, Mereo BioPharma, Novartis, Verastem/Pharmacyclics, AADi, Clovis Oncology, Caris Life Sciences, Hengrui Pharmaceutical, Novartis/Pfizer, Iovance Biotherapeutics, aadi, Duality Biologics (Inst), Janssen Oncology, Regeneron, zentalis
Research Funding: PTC Therapeutics (Inst), Lilly (Inst), Merck (Inst), Tesaro (Inst), Genentech (Inst), Clovis Oncology (Inst), Lilly Foundation (Inst), Regeneron (Inst), Bristol Myers Squibb (Inst), Verastem (Inst), Novartis Pharmaceuticals UK Ltd (Inst), AstraZeneca (Inst), Agenus (Inst), Takeda (Inst), Immunogen (Inst), Novogen (Inst), artios (Inst), Bolt Biotherapeutics (Inst), Amgen (Inst), Daiichi Sankyo/Lilly (Inst), cyteir (Inst), Immunocore (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Travel, Accommodations, Expenses: GlaxoSmithKline, AstraZeneca
Other Relationship: GOG Partners (Inst)
Hye Sook Chon
Honoraria: Curio Science, Envision Communications, MJH Healthcare Holdings, LLC, Guidepoint Global
Consulting or Advisory Role: Envision Communications, Eisai
Speakers' Bureau: Clinical Care Options
Travel, Accommodations, Expenses: Agenus
Jung-Yun Lee
Consulting or Advisory Role: AstraZeneca, MSD, Roche, Takeda
Research Funding: Clovis Oncology (Inst), Immunogen (Inst), Janssen Oncology (Inst), Merck (Inst), MSD (Inst), Synthon (Inst), MSD (Inst), Eisai (Inst), Mersana (Inst), Ascendis Pharma (Inst), AstraZeneca (Inst), Novartis (Inst), OncoQuest Pharmaceuticals (Inst), Roche (Inst), Seagen (Inst), Takeda (Inst)
Jessica Thomes Pepin
Consulting or Advisory Role: Seagen
Michael Sundborg
Employment: GlaxoSmithKline
Honoraria: GlaxoSmithKline
Speakers' Bureau: GlaxoSmithKline
Ayelet Shai
Consulting or Advisory Role: Stemline Therapeutics, Gilead Sciences
Speakers' Bureau: Novartis Biociencias
Travel, Accommodations, Expenses: Pfizer
Joseph de la Garza
Consulting or Advisory Role: AstraZeneca
Speakers' Bureau: Merck, Seagen, AstraZeneca
Other Relationship: Intuitive Surgical
Michael A. Gold
Travel, Accommodations, Expenses: ASCCP
Todd D. Tillmanns
Employment: West Clinic
Leadership: Regional One Health, St Francid
Honoraria: AstraZeneca, Intuitive Surgical, GlaxoSmithKline, Eisai, Myriad Genetics
Speakers' Bureau: Eisai, GlaxoSmithKline, AstraZeneca
Stephanie V. Blank
Stock and Other Ownership Interests: Johnson & Johnson
Research Funding: Merck (Inst), Seagen (Inst), AstraZeneca (Inst), Aravive (Inst), F. Hoffmann LaRoche (Inst), GlaxoSmithKline (Inst)
Uncompensated Relationships: AstraZeneca
Michael McCollum
Employment: Virginia Oncology Associates
Research Funding: GlaxoSmithKline (Inst), Seagen (Inst), Pfizer (Inst), Genentech (Inst)
Fernando Contreras Mejia
Honoraria: Amgen, AstraZeneca/Columbia, Bristol Myers Squibb Colombia, Lilly, Janssen, MSD Oncology, Novartis Colombia
Consulting or Advisory Role: GlaxoSmithKline, Bristol Myers Squibb Colombia
Tadaaki Nishikawa
Honoraria: Eisai, Takeda, MSD, Sanofi
Consulting or Advisory Role: Eisai
Speakers' Bureau: AstraZeneca, Eisai, Chugai/Roche, Takeda, MSD, Taiho Pharmaceutical, Sanofi, Genmab
Research Funding: AstraZeneca/Daiichi Sankyo (Inst)
Zoltan Novak
Stock and Other Ownership Interests: Richter Gedeon
Honoraria: AstraZeneca, Sofmedica, Richter Gedeon
Speakers' Bureau: MSD Oncology, Richter Gedeon
Travel, Accommodations, Expenses: GlaxoSmithKline
Andreia Cristina De Melo
Honoraria: MSD Oncology, Novartis, BMS, GlaxoSmithKline, AstraZeneca, Sanofi, Roche
Consulting or Advisory Role: MSD, Novartis, GlaxoSmithKline, AstraZeneca
Research Funding: Roche (Inst), MSD Oncology (Inst), BMS Brazil (Inst), Novartis (Inst), Clovis Oncology (Inst), AstraZeneca (Inst)
Travel, Accommodations, Expenses: MSD Oncology
Jalid Sehouli
Honoraria: AstraZeneca, Eisai, Clovis Oncology, Olympus Medical Systems, Johnson & Johnson, PharmaMar, Pfizer, Teva, Tesaro, MSD Oncology, GlaxoSmithKline, Bayer
Consulting or Advisory Role: AstraZeneca, Clovis Oncology, PharmaMar, Merck, Pfizer, Tesaro, MSD Oncology, Lilly, Novocure, Johnson & Johnson, Roche, Ingress Health, Riemser, Sobi, GlaxoSmithKline, Novartis, Alkermes
Research Funding: AstraZeneca (Inst), Clovis Oncology (Inst), Merck (Inst), Bayer (Inst), PharmaMar (Inst), Pfizer (Inst), Tesaro (Inst), MSD Oncology (Inst), Roche (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Clovis Oncology, PharmaMar, Roche Pharma AG, Tesaro, Tesaro, MSD Oncology, Olympus
Dagmara Klasa-Mazurkiewicz
Travel, Accommodations, Expenses: AstraZeneca
Christos Papadimitriou
Honoraria: Novartis, AstraZeneca, Genesis Therapeutics, MSD Oncology
Consulting or Advisory Role: Amgen Astellas BioPharma, Roche
Research Funding: Roche, WinMedica
Marta Gil-Martin
Consulting or Advisory Role: AstraZeneca
Travel, Accommodations, Expenses: MSD Oncology, GlaxoSmithKline
Birute Brasiuniene
Honoraria: Novartis, Merck Serono, Lilly, Sandoz, Swiss Pharma
Consulting or Advisory Role: AstraZeneca, MSD, Merck Serono, Swiss Pharma, Novartis, Lilly, SERVIER, Ipsen
Speakers' Bureau: AstraZeneca, MSD, Merck Serono, Swiss Pharma, Novartis, Lilly, SERVIER, Ipsen
Expert Testimony: Novartis, Merck Serono, Lilly, Sandoz, Swiss Pharma
Travel, Accommodations, Expenses: AstraZeneca, MSD, Swiss Pharma, Novartis, Ipsen, Janssen
Other Relationship: ESMO, European Organisation for Research and Treatment of Cancer (EORTC), Nordic Society of Gynecological Oncology (NSGO)
Conor Donnelly
Employment: AstraZeneca, Exploristics
Paula Michelle del Rosario
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca
Xiaochun Liu
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca
Els Van Nieuwenhuysen
Consulting or Advisory Role: Regeneron (Inst), Oncoinvent, AstraZeneca (Inst)
Research Funding: AstraZeneca (Inst), Lilly (Inst), Merck (Inst), Seagen (Inst), Roche (Inst), Novartis (Inst), Regeneron (Inst), Oncoinvent (Inst)
Travel, Accommodations, Expenses: Regeneron (Inst)
No other potential conflicts of interest were reported.
Listen to the podcast by Dr Schapira, Dr Westin, and Dr Eskander at jcopodcast.libsyn.com
SUPPORT
Supported by AstraZeneca. Medical writing assistance was provided by Rachel Dodd, PhD from Cence, funded by AstraZeneca.
CLINICAL TRIAL INFORMATION
NCT04269200 (DUO-E)
S.N.W. and K.M. contributed equally to this work.
Contributor Information
Collaborators: Sophia Frentzas, Kichendasse Ganessan, Bo Gao, Tarek Meniawy, Linda Mileshkin, Gary Richardson, Felicia Roncolato, Jean-Francois Baurain, Maryam Bourhaba, Eveline De Cuypere, Philip Debruyne, Hannelore Denys, Frederic Forget, Brigitte Honhon, Eric Joosens, Els Van Nieuwenhuysen, Vanessa da Costa Miranda, Andreia Cristina De Melo, Joao Daniel Guedes, Charles Andree Joseph de Padua, Nicolas Lazaretti, Carolina Martins Vieira, Andre Mattar, Daniela Neves Palmeiro, Christina Pimentel Oppermann Kussler, Pedro Emanuel Rubini Liedke, Joao Soares Nunes, Katsuki Arima Tiscoski, Allan Covens, Lara De Guerke, Prafull Ghatage, Lucy Gilbert, Susie Lau, Amit Oza, Diane Provencher, Omar Touhami, Li Congzhu, Wang Danbo, Lou Ge, Zhu Gen-Hai, Li Guiling, Shi Hong, Zheng Hong, Wen Hongwu, Liu Ji-Hong, Wang Jing, Wang Ke, Jiang Kui, Li Li, Wang Li, Hao Min, Zhou Qi, Gao Qinglei, Liao Sihai, Zhang Songling, Zhao Weidong, Xiaohua Wu, Wang Wuliang, Rutie Yin, Cheng Ying, Zhang Yu, Liang Zhiqing, Fernando Contreras Mejia, Angel Luis Hernandez, Carolina Ortiz Lopez, Carlos Javier Pacheco, Pedro Luis Ramos Guette, Jaime Rendon Pereira, Julian Rivera Diaz, Tomas Sanchez Villegas, Juan Pablo Suso, Marco Antonio Torregroza Otero, Karin Grisan, Elen Vettus, Bahriye Aktas, Eva Egger, Petra Krabisch, Andreas Muller, Joachim Rom, Jalid Sehouli, Antje Sperfeld, Pauline Wimberger, Andreas Zorr, George Fountzilas, Sofia Karageorgopoulou, Christos Papadimitriou, Amanda Psyrri, Florai Zagour, Karen Kar Loen Chan, Wing Ming Ho, Tibor Csoszi, Laszlo Landherr, Zoltan Novak, Zsuzsanna Papai, Robert Poka, Istvan Sipocz, Paul Devinder, Lovenish Goyal, Sudeep Gupta, Radheshyam Naik, Tushar Patil, S.P. Somashekhar, Ilan Bruchim, Svetlana Kovel, Anca Leibovici, Ora Rosengarten, Tamar Safra, Wataru Yamagami, Junzo Hamanishi, Yuichi Imai, Nobuhiro Kado, Shoji Kamiura, Hienori Kato, Eiji Kondo, Wataru Kudaka, Takashi Matsumoto, Masahiko Mori, Tadaaki Nishikawa, Shin Nishio, Aikou Okamoto, Mayu Yunokawa, Masayuki Sekine, Toshiyuki Sumi, Hirokuni Takano, Kazuhiro Takehara, Birute Brasiuniene, Arturas Inciura, Goda Jonuskiene, Jesus Elvis Cabrera Luviano, Adriana Dominguez-Andrade, Raquel Gerson-Cwilich, Carlos Hernandez Hernandez, Jesus Lopez Hernandez, Jose Luis Martinez Lira, Jaime Esteban Navarrete Aleman, Jessica Reyes Contreras, Vanessa Rosas Camargo, Wieslawa Bednarek, Dagmara Klasa-Mazurkiewicz, Tomasz Kubiatowski, Piotr Potemski, Magdalena Sikorska, Suk-Joon Chang, Sook Hee Hong, Sokbom Kang, Byoung-Gie Kim, Jan-Weon Kim, Yong Man Kim, Jung-Yun Lee, Sang Young Ryu, Yong Jung Song, Dmitriy Kirtbaya, Julya Kreynina, Alla Lisyanskaya, Yulia Makarova, Rashida Orlova, Albert Pirmagomedov, Valeria Saevets, Sufia Safina, Pavel Skopin, Alexandra Tyulyandina, Sheow Lei Lim, Lynette Ngo Su Mien, David Tan Shao Peng, Lay Tin Soh, Jesus Alarcon Company, Pilar Barretina, Purificacion Estevez-Garcia, Isaura Fernandez Perez, Fernando Galvez, Yolanda Garcia, Marta Gil-Martin, Jeronimo Martinez, Andres Redondo-Sanchez, Charles Anderson, Tara Berman, Stephanie Blank, William Bradley, James Burke, Fabio Cappuccini, Michael Carney, Setsuko Chambers, Lee-May Chen, Hye Sook Chon, Joseph de la Garza, Stephen Depasquale, Paul DiSilvestro, Babak Edraki, Evelyn Fleming, Jenny Fox, Michael Gold, Mary Gordinier, Michael Guy, Ellen Hartenbach, Chisten Haygood, Scott Jordan, Larry Kilgore, Young Kim, Joseph Lucci, Michael McCollum, Michael Mchale, Kristi McIntyre, Mark Messing, Eirwen Miller, Kathleen Moore, John Moroney, Michaela Onstad, Taylor Ortiz, Sobia Ozair, Kathryn Pennington, Krista Pfaendler, Anna Priebe, Terri Pustilnik, Kimberly Resnick, Peter Rose, Erin Salinas, Sudarshan Sharma, Urszula Sobol, Pamela Soliman, David Starks, Michael Sundborg, Eleonora Teplinsky, Jessica Thomes-Pepin, Todd Tillmanns, David Warshal, Shannon N. Westin, and Thomas Woliver
DATA SHARING STATEMENT
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Data for studies directly listed on Vivli can be requested through Vivli at https://vivli.org/. Data for studies not listed on Vivli could be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. AstraZeneca Vivli member page is also available outlining further details: https://vivli.org/ourmember/astrazeneca/.
AUTHOR CONTRIBUTIONS
Conception and design: Shannon N. Westin, Kathleen Moore, Jung-Yun Lee, Todd D. Tillmanns, Michael McCollum, Fernando Contreras Mejia, Tadaaki Nishikawa, Conor Donnelly, Xiaochun Liu
Administrative support: Kristi McIntyre, Todd D. Tillmanns, Michael McCollum
Provision of study materials or patients: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Durvalumab Plus Carboplatin/Paclitaxel Followed by Maintenance Durvalumab With or Without Olaparib as First-Line Treatment for Advanced Endometrial Cancer: The Phase III DUO-E Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Shannon N. Westin
This author is a Consultant Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Roche, AstraZeneca, Genentech, Medscape, Clovis Oncology, Gerson Lehrman Group, Vaniam Group, Merck, BioAscent, OncLive, Targeted Oncology, Curio Science, GlaxoSmithKline, Eisai, Zentalis, EQRX, Lilly, Vincerx Pharma, Mereo BioPharma, Immunogen, Mersana, NGM Biopharmaceuticals, Caris Life Sciences, Nuvectis Pharma, Seagen, Immunocore, ZielBio, Verastem, Gilead Sciences, Mersana
Research Funding: AstraZeneca (Inst), Novartis (Inst), Bayer (Inst), Clovis Oncology (Inst), Roche/Genentech (Inst), GOG Foundation (Inst), Mereo BioPharma (Inst), Bio-Path Holdings, Inc (Inst), GlaxoSmithKline (Inst), Zentalis (Inst), Avenge Bio (Inst), Jazz Pharmaceuticals (Inst)
Kathleen Moore
Leadership: GOG Partners, NRG Oncology (Inst)
Honoraria: Research To Practice, Prime Oncology, Physicans' Education Resource, Great Debates and Updates
Consulting or Advisory Role: Genentech/Roche, Immunogen, AstraZeneca, VBL Therapeutics, Merck, Eisai, Mersana (Inst), Myriad Genetics, Alkermes (Inst), Blueprint Medicines (Inst), GlaxoSmithKline/Tesaro (Inst), OncXerna Therapeutics, Onconova Therapeutics, Mereo BioPharma, Novartis, Verastem/Pharmacyclics, AADi, Clovis Oncology, Caris Life Sciences, Hengrui Pharmaceutical, Novartis/Pfizer, Iovance Biotherapeutics, aadi, Duality Biologics (Inst), Janssen Oncology, Regeneron, zentalis
Research Funding: PTC Therapeutics (Inst), Lilly (Inst), Merck (Inst), Tesaro (Inst), Genentech (Inst), Clovis Oncology (Inst), Lilly Foundation (Inst), Regeneron (Inst), Bristol Myers Squibb (Inst), Verastem (Inst), Novartis Pharmaceuticals UK Ltd (Inst), AstraZeneca (Inst), Agenus (Inst), Takeda (Inst), Immunogen (Inst), Novogen (Inst), artios (Inst), Bolt Biotherapeutics (Inst), Amgen (Inst), Daiichi Sankyo/Lilly (Inst), cyteir (Inst), Immunocore (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Travel, Accommodations, Expenses: GlaxoSmithKline, AstraZeneca
Other Relationship: GOG Partners (Inst)
Hye Sook Chon
Honoraria: Curio Science, Envision Communications, MJH Healthcare Holdings, LLC, Guidepoint Global
Consulting or Advisory Role: Envision Communications, Eisai
Speakers' Bureau: Clinical Care Options
Travel, Accommodations, Expenses: Agenus
Jung-Yun Lee
Consulting or Advisory Role: AstraZeneca, MSD, Roche, Takeda
Research Funding: Clovis Oncology (Inst), Immunogen (Inst), Janssen Oncology (Inst), Merck (Inst), MSD (Inst), Synthon (Inst), MSD (Inst), Eisai (Inst), Mersana (Inst), Ascendis Pharma (Inst), AstraZeneca (Inst), Novartis (Inst), OncoQuest Pharmaceuticals (Inst), Roche (Inst), Seagen (Inst), Takeda (Inst)
Jessica Thomes Pepin
Consulting or Advisory Role: Seagen
Michael Sundborg
Employment: GlaxoSmithKline
Honoraria: GlaxoSmithKline
Speakers' Bureau: GlaxoSmithKline
Ayelet Shai
Consulting or Advisory Role: Stemline Therapeutics, Gilead Sciences
Speakers' Bureau: Novartis Biociencias
Travel, Accommodations, Expenses: Pfizer
Joseph de la Garza
Consulting or Advisory Role: AstraZeneca
Speakers' Bureau: Merck, Seagen, AstraZeneca
Other Relationship: Intuitive Surgical
Michael A. Gold
Travel, Accommodations, Expenses: ASCCP
Todd D. Tillmanns
Employment: West Clinic
Leadership: Regional One Health, St Francid
Honoraria: AstraZeneca, Intuitive Surgical, GlaxoSmithKline, Eisai, Myriad Genetics
Speakers' Bureau: Eisai, GlaxoSmithKline, AstraZeneca
Stephanie V. Blank
Stock and Other Ownership Interests: Johnson & Johnson
Research Funding: Merck (Inst), Seagen (Inst), AstraZeneca (Inst), Aravive (Inst), F. Hoffmann LaRoche (Inst), GlaxoSmithKline (Inst)
Uncompensated Relationships: AstraZeneca
Michael McCollum
Employment: Virginia Oncology Associates
Research Funding: GlaxoSmithKline (Inst), Seagen (Inst), Pfizer (Inst), Genentech (Inst)
Fernando Contreras Mejia
Honoraria: Amgen, AstraZeneca/Columbia, Bristol Myers Squibb Colombia, Lilly, Janssen, MSD Oncology, Novartis Colombia
Consulting or Advisory Role: GlaxoSmithKline, Bristol Myers Squibb Colombia
Tadaaki Nishikawa
Honoraria: Eisai, Takeda, MSD, Sanofi
Consulting or Advisory Role: Eisai
Speakers' Bureau: AstraZeneca, Eisai, Chugai/Roche, Takeda, MSD, Taiho Pharmaceutical, Sanofi, Genmab
Research Funding: AstraZeneca/Daiichi Sankyo (Inst)
Zoltan Novak
Stock and Other Ownership Interests: Richter Gedeon
Honoraria: AstraZeneca, Sofmedica, Richter Gedeon
Speakers' Bureau: MSD Oncology, Richter Gedeon
Travel, Accommodations, Expenses: GlaxoSmithKline
Andreia Cristina De Melo
Honoraria: MSD Oncology, Novartis, BMS, GlaxoSmithKline, AstraZeneca, Sanofi, Roche
Consulting or Advisory Role: MSD, Novartis, GlaxoSmithKline, AstraZeneca
Research Funding: Roche (Inst), MSD Oncology (Inst), BMS Brazil (Inst), Novartis (Inst), Clovis Oncology (Inst), AstraZeneca (Inst)
Travel, Accommodations, Expenses: MSD Oncology
Jalid Sehouli
Honoraria: AstraZeneca, Eisai, Clovis Oncology, Olympus Medical Systems, Johnson & Johnson, PharmaMar, Pfizer, Teva, Tesaro, MSD Oncology, GlaxoSmithKline, Bayer
Consulting or Advisory Role: AstraZeneca, Clovis Oncology, PharmaMar, Merck, Pfizer, Tesaro, MSD Oncology, Lilly, Novocure, Johnson & Johnson, Roche, Ingress Health, Riemser, Sobi, GlaxoSmithKline, Novartis, Alkermes
Research Funding: AstraZeneca (Inst), Clovis Oncology (Inst), Merck (Inst), Bayer (Inst), PharmaMar (Inst), Pfizer (Inst), Tesaro (Inst), MSD Oncology (Inst), Roche (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Clovis Oncology, PharmaMar, Roche Pharma AG, Tesaro, Tesaro, MSD Oncology, Olympus
Dagmara Klasa-Mazurkiewicz
Travel, Accommodations, Expenses: AstraZeneca
Christos Papadimitriou
Honoraria: Novartis, AstraZeneca, Genesis Therapeutics, MSD Oncology
Consulting or Advisory Role: Amgen Astellas BioPharma, Roche
Research Funding: Roche, WinMedica
Marta Gil-Martin
Consulting or Advisory Role: AstraZeneca
Travel, Accommodations, Expenses: MSD Oncology, GlaxoSmithKline
Birute Brasiuniene
Honoraria: Novartis, Merck Serono, Lilly, Sandoz, Swiss Pharma
Consulting or Advisory Role: AstraZeneca, MSD, Merck Serono, Swiss Pharma, Novartis, Lilly, SERVIER, Ipsen
Speakers' Bureau: AstraZeneca, MSD, Merck Serono, Swiss Pharma, Novartis, Lilly, SERVIER, Ipsen
Expert Testimony: Novartis, Merck Serono, Lilly, Sandoz, Swiss Pharma
Travel, Accommodations, Expenses: AstraZeneca, MSD, Swiss Pharma, Novartis, Ipsen, Janssen
Other Relationship: ESMO, European Organisation for Research and Treatment of Cancer (EORTC), Nordic Society of Gynecological Oncology (NSGO)
Conor Donnelly
Employment: AstraZeneca, Exploristics
Paula Michelle del Rosario
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca
Xiaochun Liu
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca
Els Van Nieuwenhuysen
Consulting or Advisory Role: Regeneron (Inst), Oncoinvent, AstraZeneca (Inst)
Research Funding: AstraZeneca (Inst), Lilly (Inst), Merck (Inst), Seagen (Inst), Roche (Inst), Novartis (Inst), Regeneron (Inst), Oncoinvent (Inst)
Travel, Accommodations, Expenses: Regeneron (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Siegel RL, Miller KD, Fuchs HE, et al. : Cancer statistics, 2022. CA Cancer J Clin 72:7-33, 2022 [DOI] [PubMed] [Google Scholar]
- 2.Oaknin A, Bosse TJ, Creutzberg CL, et al. : Endometrial cancer: ESMO clinical Practice guideline for diagnosis, treatment and follow-up. Ann Oncol 33:860-877, 2022 [DOI] [PubMed] [Google Scholar]
- 3.Miller DS, Filiaci VL, Mannel RS, et al. : Carboplatin and paclitaxel for advanced endometrial cancer: Final overall survival and adverse event analysis of a phase III trial (NRG Oncology/GOG0209). J Clin Oncol 38:3841-3850, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aghajanian C, Filiaci V, Dizon DS, et al. : A phase II study of frontline paclitaxel/carboplatin/bevacizumab, paclitaxel/carboplatin/temsirolimus, or ixabepilone/carboplatin/bevacizumab in advanced/recurrent endometrial cancer. Gynecol Oncol 150:274-281, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller D, Filiaci V, Fleming G, et al. : Late-Breaking Abstract 1: Randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol 125:771, 2012 [Google Scholar]
- 6.Pectasides D, Xiros N, Papaxoinis G, et al. : Carboplatin and paclitaxel in advanced or metastatic endometrial cancer. Gynecol Oncol 109:250-254, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Mirza MR, Chase DM, Slomovitz BM, et al. : Dostarlimab for primary advanced or recurrent endometrial cancer. N Engl J Med 388:2145-2158, 2023 [DOI] [PubMed] [Google Scholar]
- 8.Eskander RN, Sill MW, Beffa L, et al. : Pembrolizumab plus chemotherapy in advanced endometrial cancer. N Engl J Med 388:2159-2170, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.André T, Berton-Rigaud D, Curigliano G, et al. : 549P Progression-free survival (PFS) and overall survival (OS) in patients (pts) with mismatch repair deficient (dMMR) solid tumors treated with dostarlimab in the GARNET study. Ann Oncol 33:S799-S800, 2022 [Google Scholar]
- 10.Antill Y, Kok PS, Robledo K, et al. : Clinical activity of durvalumab for patients with advanced mismatch repair-deficient and repair-proficient endometrial cancer. A nonrandomized phase 2 clinical trial. J Immunother Cancer 9:e002255, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makker V, Colombo N, Casado Herraez A, et al. : Lenvatinib plus pembrolizumab for advanced endometrial cancer. N Engl J Med 386:437-448, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marabelle A, Le DT, Ascierto PA, et al. : Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: Results from the phase II KEYNOTE-158 study. J Clin Oncol 38:1-10, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.FDA : Jemperli prescribing information, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761174s006lbl.pdf [Google Scholar]
- 14.Pina A, Wolber R, McAlpine JN, et al. : Endometrial cancer presentation and outcomes based on mismatch repair protein expression from a population-based study. Int J Gynecol Cancer 28:1624-1630, 2018 [DOI] [PubMed] [Google Scholar]
- 15.Lee EK, Konstantinopoulos PA: Combined PARP and immune checkpoint inhibition in ovarian cancer. Trends Cancer 5:524-528, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Li A, Yi M, Qin S, et al. : Prospects for combining immune checkpoint blockade with PARP inhibition. J Hematol Oncol 12:98, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart RA, Pilié PG, Yap TA: Development of PARP and immune-checkpoint inhibitor combinations. Cancer Res 78:6717-6725, 2018 [DOI] [PubMed] [Google Scholar]
- 18.Wanderley CWS, Correa TS, Scaranti M, et al. : Targeting PARP1 to enhance anticancer checkpoint immunotherapy response: Rationale and clinical implications. Front Immunol 13:816642, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.AstraZeneca : Global Policy: Bioethics, 2019. https://www.astrazeneca.com/content/dam/az/PDF/2019/Bioethics%20Policy%20final.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Data for studies directly listed on Vivli can be requested through Vivli at https://vivli.org/. Data for studies not listed on Vivli could be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. AstraZeneca Vivli member page is also available outlining further details: https://vivli.org/ourmember/astrazeneca/.