Abstract
The extracellular capsule polysaccharide (CPS) of Vibrio vulnificus is a primary virulence factor which allows survival of the bacteria in the human host. To study the genes involved in expression of the capsule, we generated mutants that lost the ability to produce CPS following the insertion of a minitransposon into the genome of an encapsulated, clinical strain of V. vulnificus. A genomic region, from one nonencapsulated mutant, containing the transposon and flanking V. vulnificus DNA was cloned, and a probe complementary to the chromosomal DNA immediately adjacent to the transposon was used to locate this fragment in the genome of the encapsulated parent strain. The fragment, which contained a putative capsule gene, was cloned and, when supplied in trans, complemented the mutation in the nonencapsulated mutant to restore capsule production. In addition, virulence studies, using the 50% lethal dose assay, showed that the restoration of capsule production also restored the virulence of the organism. Sequence analysis of the gene disrupted by the transposon revealed that it matched a nucleotide-sugar epimerase of Vibrio cholerae O139, with 75 and 85% identities at the nucleotide and amino acid levels, respectively. In addition, computer analysis recognized epimerases of various organisms as highly similar to the putative epimerase of V. vulnificus. Finally, a combination of PCR amplification and Southern blotting showed that this epimerase is common to at least 10 strains of V. vulnificus that each express a serologically distinct CPS. Our results indicate that the epimerase gene is essential for capsule expression in V. vulnificus.
Vibrio vulnificus is well documented as the causative agent of septicemia and infectious disease following ingestion of raw seafood (8, 9). This gram-negative bacillus also causes wound infections from exposure of an open wound to contaminated seawater and may account for more than 50% of all vibrio-associated extraintestinal illnesses in the United States (9).
Assorted virulence factors which advance the pathophysiology of V. vulnificus, including the extracellular capsular polysaccharide (CPS), have been described (2, 30, 36, 41, 49, 57), and those isolates which do not express the CPS are considered avirulent (47, 58, 59). The capsule permits V. vulnificus to evade nonspecific host defense mechanisms such as activation of the alternative pathway of the complement cascade and complement-mediated opsonophagocytosis (34, 39, 55).
An opaque (designated by the suffix “O”) colony morphology on an agar surface is indicative of an encapsulated strain that is lethal to mice and capable of survival in normal human serum, as opposed to the nonencapsulated, translucent (T) strains (58, 59) which arise randomly during routine bacteriological manipulation of opaque strains (47). Once a strain is nonencapsulated, the incidence of reversion back to the encapsulated state, although rare, has been reported (58, 59), suggesting that the loss of CPS production may be reversible. The instance of reversion is also true for capsule expression in Pseudomonas atlantica (4) and Neisseria meningitidis (24).
The capsule gene locus of V. vulnificus has not been identified, leaving the gene arrangement to be speculated upon with examples of well-defined systems of Escherichia coli, Haemophilus influenzae, and Klebsiella pneumoniae as possibilities. Concurrent with the extensive serological identification of E. coli capsules (37), studies involving V. vulnificus capsules have focused solely on capsule types, resulting in the identification of at least 13 distinct serological CPS types (44) and 15 CPS chemotypes identified by high-performance anion-exchange chromatography and nuclear magnetic resonance spectroscopy (23).
Our laboratory set out to identify genes involved in capsule expression of V. vulnificus. Utilizing transposon mutagenesis, we generated mutants that no longer expressed a discernible polysaccharide capsule. DNA sequence of the cloned chromosomal region surrounding one transposon insertion revealed that the transposon had disrupted an open reading frame (ORF) that was found highly similar to the nucleotide-sugar epimerase gene of Vibrio cholerae O139.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The characteristics of selected bacterial strains and plasmids are shown in Table 1 for simplification. Additional strains and plasmids used in this study are briefly described herein. The encapsulated V. vulnificus clinical strain 1003(O) was used for transposon mutagenesis, from which the nonencapsulated transposon mutant ABZ1(T) was derived. The plasmid vector pEIS (constructed by Richard Cooper, Department of Veterinary Science, Louisiana State University) consists of pGP704 (32, 53) with a mini-Tn10/kan transposable element (26) cloned into its multiple cloning site (MCS) and was used for transposon delivery into V. vulnificus. E. coli SM10λpir (32, 43) was used as the donor strain for conjugation experiments introducing the pEIS vector into V. vulnificus. The plasmid pBluescript SK− (Stratagene, La Jolla, Calif.) and the mobilizable vector pBBR1MCS (27) were used to clone V. vulnificus chromosomal DNA, and each vector was propagated in E. coli DH5α MCR (New England Biolabs Inc., Beverly, Mass.). E. coli MC1061 cells (54) and the transfer-proficient helper plasmid pRK2013 (17), which encodes all of the transfer genes necessary for successful mobilization of a plasmid, were used in triparental matings. Ten strains of V. vulnificus, each with a different capsular serotype (44), were examined for the epimerase gene. V. cholerae O139 was used in Southern hybridizations and was also examined for the epimerase gene.
TABLE 1.
Bacterial strains and plasmids used
| Strain or plasmid | Descriptiona | Reference or source |
|---|---|---|
| V. vulnificus strains | ||
| 1003(O) | Encapsulated; Colr | 45 |
| 1003(T) | Nonencapsulated; spontaneously derived from 1003(O) | 44 |
| ABZ1(T) | Nonencapsulated; epimerase::mini-Tn10 Kmr; derived from 1003(O) | This study |
| ABZ1(O) | Encapsulated; derived from ABZ1(T); contains pEpiBBR; Clr | This study |
| Plasmids | ||
| pBluescript SK− | Cloning vector; Apr | Stratagene |
| pBBR1MCS | Cloning vector; Mob+ Clr | 27 |
| pPCG1 | Mini-Tn10 and flanking DNA of ABZ1(T) cloned into pBluescript with SacI | This study |
| pEpiBS | Epimerase gene of 1003(O) cloned into pBluescript with EcoRI | This study |
| pEpiBBR | Insert from pEpiBS excised by XbaI and HindIII and cloned into pBBR1MCS | This study |
Colr, colistin resistance; Kmr, kanamycin resistance; Clr, chloramphenicol resistance; Apr, ampicillin resistance.
Growth conditions.
V. vulnificus and E. coli cells were propagated in heart infusion (HI) broth (Difco, Detroit, Mich.) supplemented with 2% NaCl for 18 h at 37°C with shaking at 200 rpm, unless otherwise stated. Colistin (118 μg/ml), kanamycin (50 μg/ml), ampicillin (50 μg/ml), and chloramphenicol (30 μg/ml) were used when appropriate.
Transposon mutagenesis.
To disrupt genes essential for capsule synthesis in V. vulnificus, transposon mutagenesis was performed in the following manner. Suspensions (1.5 ml of each) of V. vulnificus 1003(O) and E. coli SM10λpir cells were separately pelleted, washed twice in HI broth, and resuspended in 15 μl of broth and then combined. Isopropyl-β-d-thiogalactopyranoside (Sigma Chemical Co., St. Louis, Mo.) was added to a final concentration of 1 mM to induce transposition (26). The bacteria were then spotted onto a nitrocellulose filter (Schleicher & Schuell, Keene, N.H.) placed on the surface of an HI agar plate and incubated at 30°C for 16 h. After incubation, the filter was submerged into 10 ml of HI broth to remove all cells, and 100-μl aliquots were spread onto HI-kanamycin-colistin agar and incubated at 37°C. Nonencapsulated, translucent colony phenotypes were selected and streaked onto HI-kanamycin-colistin plates. Stable, translucent colonies were selected and stored at −85°C in HI broth containing 15% glycerol.
DNA isolation.
Genomic DNA was isolated by the method of Chan and Goodwin (13). This protocol was modified for nonencapsulated strains by omitting the addition of cetyltrimethylammonium bromide. Plasmid DNA was isolated by using a Perfect Prep plasmid isolation kit (5 Prime 3 Prime, Inc., Boulder, Colo.) according to the manufacturer’s instructions.
Probe synthesis.
To locate the transposon insertion in the chromosome of each nonencapsulated mutant, a 980-bp digoxigenin (dig)-labeled probe specific for the kanamycin resistance gene (35) of the mini-Tn10 was generated using a PCR DIG probe synthesis kit (Boehringer Mannheim, Indianapolis, Ind.). The primers were designed by using the Primer Designer program (Scientific and Educational Software) and have the sequences 5′ CAACAAAGCCACGTTGTGTCTCAAAATCTC 3′ for the coding strand and 5′ TCAAGTCAGCGTAATGCTCTGCCAGTG 3′ for the complementary strand.
To detect the putative epimerase gene in V. vulnificus 1003(O) and V. cholerae O139, a dig-labeled probe (epimerase-probe A) specific for 139 bases of the sequenced region of pPCG1 was prepared using the PCR DIG probe synthesis kit as described above. The primers were designed as described above and have the sequences 5′ ATAGTGTTGACCATCCGGTG 3′ for the coding strand and 5′ GGACGCCCCCAAGAGCCATA 3′ for the noncoding strand.
We synthesized a second probe specific for the putative epimerase gene of V. vulnificus (epimerase-probe B) which was specific for 83 bp internal to that of epimerase-probe A. The probe was synthesized by using the PCR DIG probe synthesis kit (Boehringer Mannheim). Sequences of the primers used were 5′ACGAACTTATGGCTCATAGC3′ for the coding strand and 5′CCAAGAGCCATACACCGTAA3′ for the noncoding strand.
Gel electrophoresis and Southern blotting.
To detect the DNA fragment into which the transposon had inserted, the genomic DNA of each transposon mutant was digested individually with ApaI, EcoRI, KpnI, SalI, SacI, SacII, and XbaI (New England Biolabs) according to the manufacturer’s protocol. Following electrophoresis on a 0.8% agarose gel, the DNA was transferred to a nitrocellulose filter (42). The kanamycin probe was allowed to hybridize at 55°C, and the blot was developed according to the instructions for the Genius 3 kit (Boehringer Mannheim).
The genomic DNA of V. cholerae O139 and V. vulnificus 1003(O) was isolated, digested with EcoRI, and electrophoresed as described above. The DNA was blotted onto nitrocellulose, and the epimerase-probe A was allowed to hybridize at 55°C and developed by using the Genius 3 kit (Boehringer Mannheim).
Following PCR amplification, Southern blotting was used to verify that the region amplified in all 10 strains of V. vulnificus was indeed a region of the putative capsule gene and not an artifact. The PCR products were electrophoresed on 8% nondenaturing polyacrylamide gels and were electroblotted onto charged nylon Zeta-Probe blotting membranes (Bio-Rad, Hercules, Calif.) in 0.5× TBE buffer (50 mM boric acid, 50 mM Tris, 1 mM EDTA) at 80 V for 1 h, as specified by the manufacturer. The DNA was then denatured, fixed onto the membrane in 0.4 M sodium hydroxide for 10 min, rinsed with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), and air dried. To avoid false hybridization, the epimerase-probe B was allowed to hybridize at 55°C and the blot was developed according to the instructions for the Genius 3 kit (Boehringer Mannheim).
Cloning and nucleotide sequencing.
To identify the gene disrupted by the transposon insertion, chromosomal DNA of one nonencapsulated mutant, ABZ1(T), was digested with SacI (New England Biolabs) and ligated into similarly digested pBluescript SK− DNA, using a Fast-Link DNA ligation and screening kit (Epicentre Technologies, Madison, Wis.). The resulting plasmid, pPCG1 (putative capsule gene 1), was then used to transform E. coli DH5α MCR cells in a Gene Pulser (Bio-Rad) according to the manufacturer’s instructions. Plasmid DNA from kanamycin- and ampicillin-resistant transformants was verified by Southern blotting with the kanamycin probe described above. Relevant clones were sequenced by using the T3 and T7 primers of pBluescript according to the protocol for the CircumVent sequencing kit (New England Biolabs).
To further analyze the putative capsule gene disrupted by the transposon, the gene was cloned from the wild-type encapsulated strain 1003(O), from which ABZ1(T) was derived. The genomic DNA of V. vulnificus 1003(O) was isolated, digested with EcoRI, and electrophoresed in a 0.8% agarose gel. The 6.5-kb fragment, previously recognized by epimerase-probe A, was excised from the gel and purified by using a QIAquick Gel extraction kit (Qiagen Inc., Chatsworth, Calif.). The fragment was ligated into pBluescript previously digested with EcoRI and treated with calf intestinal alkaline phosphatase (Gibco BRL, Gaithersburg, Md.), and the resulting plasmid was designated pEpiBS. Electroporation was used to transform E. coli DH5α MCR cells, and colonies were blotted onto nitrocellulose filters and hybridized at 68°C with the dig-labeled epimerase-probe A according to the suggested procedure for the Genius 3 kit (Boehringer Mannheim). The plasmid DNA that contained an insert of the putative gene of V. vulnificus was then used for nucleotide sequencing with an ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction kit (Perkin-Elmer, Foster City, Calif.). Centrisep spin columns (Princeton Separations, Adelphia, N.J.) were used to purify PCR fragments, and a Perkin-Elmer ABI 310 automated sequencer was utilized to sequence the template.
The 6.5-kb insert from pEpiBS was cloned into the mobilizable vector, pBBR1MCS, to allow the broad-host-range plasmid to be introduced into V. vulnificus by conjugation. Because the MCS of pBBR1MCS does not contain an EcoRI site, a double digest, using the enzymes XbaI and HindIII, was required to remove the 6.5-kb insert from pEpiBS, excising the complete insert from the pBluescript vector. The resulting vector was designated pEpiBBR.
Complementation.
To complement the disrupted capsule gene of mutant ABZ1(T), the mobilizable vector pEpiBBR was introduced into E. coli MC1061 (54). Because MC1061 does not possess the transfer (tra) genes which are necessary for conjugation, a helper plasmid, pRK2013, was required. By means of a triparental mating, whereby 1-ml aliquots of overnight cultures of (i) an E. coli strain containing pRK2013, (ii) E. coli MC1061 containing pEpiBBR, and (iii) V. vulnificus ABZ1(T) were mixed and washed in HI broth. The resulting cell pellet was resuspended in 30 μl of HI broth and spotted onto a nitrocellulose filter on the surface of an HI agar plate and incubated overnight at 30°C. The filter was added to 5 ml of HI broth and vortexed to remove all discernible cells. Onto HI-chloramphenicol-colistin plates, 100 μl of the cell suspension was added, and plates were incubated overnight at 30°C, selecting for only V. vulnificus cells containing the pEpiBBR vector.
LD50.
The protocol used to determine the 50% lethal doses (LD50s) of V. vulnificus strains was that of Wright et al. (57), involving intraperitoneal injections of V. vulnificus cells immediately followed by intraperitoneal injections of ferric ammonium citrate into 6- to 8-week-old male mice. Following a 48-h period, mortalities were totaled and the method of Reed and Muench (38) was used to calculate the LD50 for each strain tested.
PCR amplification.
To determine the distribution of the V. vulnificus putative epimerase gene among 10 serologically diverse capsular types, a PCR amplification approach was used for detection. The same primers as used to synthesize the epimerase-probe A (see above) were used to amplify the target region. Chromosomal DNA was prepared from 1.5-ml broth cultures of each capsular serotype. Pelleted cells were washed once with 1 M NaCl and once with 0.5 M NaCl, resuspended in 500 μl of water, and heated at 100°C for 15 min. Cell debris was removed by centrifugation, and the supernatant fluid, containing the chromosomal DNA, was used as the template. Ready-To-Go PCR beads (Pharmacia Biotech Inc., Piscataway, N.J.) were used, with the additions of 2.5 μl of DNA template, 1 μl of each 10 μM primer solution, and 20.5 μl of sterile, deionized water to each tube. Amplification was performed in a Perkin-Elmer 480 Thermal Cycler, using the parameters suggested for the Ready-To-Go PCR beads (Pharmacia) with a modification of the annealing temperature to 60°C. PCR products were electrophoresed on 8% polyacrylamide gels, stained with ethidium bromide, and visualized by using an Eagle Eye II (Stratagene).
Computer analysis.
All nucleotide sequences obtained were entered into the Basic Localization Alignment Search Tool (BLAST) (1) for comparison to various similar nucleotide sequences. Translation from nucleotide sequence was performed by using the Clone program (Science and Educational Software), and the determined amino acid sequence was also entered into BLAST and matched with proteins of similar sequence. Protein secondary structure was predicted by using the SSP program (48).
Nucleotide sequence accession number.
The sequence of the epimerase gene of V. vulnificus has been submitted to GenBank and can be located at accession no. AF059755.
RESULTS
Transposon mutagenesis and Southern blotting.
Transposon mutagenesis was used to disrupt genes involved in the capsule synthesis of V. vulnificus. Using the encapsulated V. vulnificus strain 1003(O) as the parent, successful insertion of the transposon in a target gene gave 23 nonencapsulated transposon mutants from eight separate experiments. The chromosomal DNA from each of the 23 nonencapsulated mutants was digested with seven restriction enzymes, chosen because their recognition sites are not present in the transposon and digestion yields the transposon flanked by the chromosomal DNA of the gene disrupted. Through Southern blot analysis, each mutant was examined for the presence of the transposon by hybridization with the kanamycin probe. Insertions were mapped to seven distinct chromosomal regions according to the fragment size recognized by the kanamycin probe, using all restriction enzymes listed above. Of interest in this study is one mutant, ABZ1(T), that contained a unique site of transposon insertion compared to the additional 22 mutants.
Identification of a putative capsule gene.
To identify a gene disrupted by the transposon, the genomic region containing the transposon and flanking chromosomal DNA of one nonencapsulated mutant, ABZ1(T), was cloned into pBluescript. The insert, containing the 1.8-kb transposon, was 2.3 kb in size. Sequence analysis revealed 246 bp of V. vulnificus chromosomal DNA before the transposon was located. The derived nucleotide sequence was entered into BLAST (1) and found to be similar to 246 bases of a nucleotide-sugar epimerase gene, ORF9 of Vibrio cholerae O139 (14).
Cloning and sequencing of the epimerase gene.
Southern blot analysis, using the dig-labeled epimerase-probe A, revealed the size of the EcoRI-digested fragments from V. vulnificus 1003(O) and V. cholerae O139 that contained the epimerase gene (Fig. 1). In V. cholerae O139, the probe recognized a fragment which was previously cloned by Comstock et al. and shown to contain genes responsible for capsule production of O139 that were not detected in O1 strains of V. cholerae (14). The probe also recognized a 6.5-kb fragment of V. vulnificus 1003(O) (Fig. 1), as well as 1003(T) (data not shown). The fragment from 1003(O) was extracted from an agarose gel and cloned into pBluescript, resulting in pEpiBS. The insert was partially sequenced, and the epimerase gene was located immediately adjacent to the T3 region of pBluescript, as shown in Fig. 2. Analysis of the nucleotide sequence showed that the epimerase of V. vulnificus matched that of V. cholerae O139, with 75 and 85% identity at the nucleotide and amino acid levels, respectively.
FIG. 1.
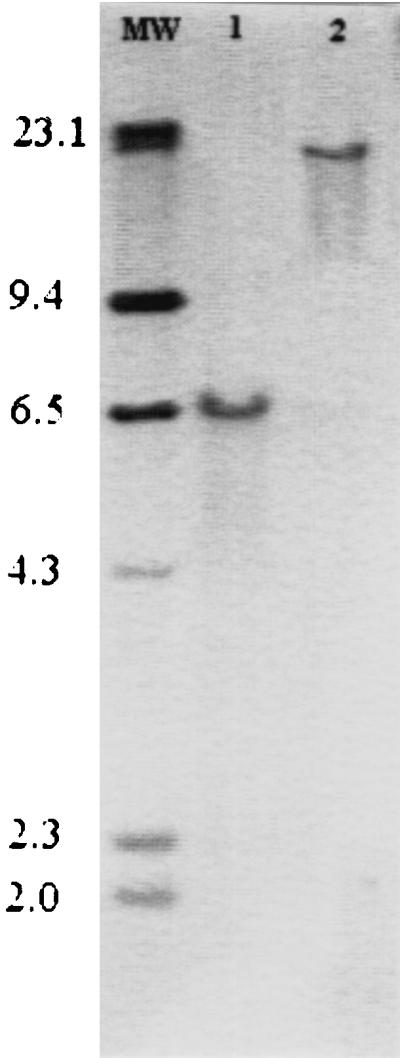
Southern blot of epimerase-probe A hybridized to a 6.5-kb fragment of V. vulnificus 1003 (lane 1) and a 21-kb fragment of V. cholerae O139 (lane 2), both digested with EcoRI. Lane MW contains molecular weight markers, sizes of which are shown in kilobases at the left.
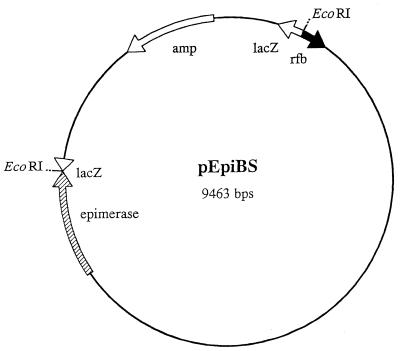
FIG. 2.
Map of the pEpiBS vector, which consists of a 6.5-kb insert of V. vulnificus 1003(O) chromosomal DNA cloned into pBluescript at EcoRI restriction enzyme sites. Genes originating from the pBluescript vector itself are designated by open arrows. The positions and orientations of the epimerase gene and rfbQRS are shown by the striped and solid arrows, respectively.
In addition to location of the epimerase gene, sequence analysis of the pEpiBS insert showed that immediately adjacent to the T7 region of pBluescript and approximately 5 kb upstream from the epimerase gene (Fig. 2) is a region of at least 250 bp that is 96% homologous to the rfbQRS sequence of V. cholerae O139 (7), indicating further homology between these two Vibrio species.
Complementation.
Following several triparental matings, the pEpiBBR vector was successfully introduced into V. vulnificus mutant ABZ1(T), supplying the epimerase gene in trans. Capsule production, in the once-nonencapsulated strain, was restored, resulting in the encapsulated strain designated ABZ1(O).
LD50.
The LD50s of V. vulnificus 1003(O), 1003(T), ABZ1(T), and ABZ1(O) were determined by using the “iron mouse” model to ascertain the role of capsule in virulence. Results are summarized on Table 2. The encapsulated strain 1003(O), from which the transposon mutants were derived, gave an LD50 of 0.87 cells. The spontaneously derived, nonencapsulated strain 1003(T) gave an LD50 of >6.5 × 107 cells, the greatest inoculum tested. The nonencapsulated mutant ABZ1(T) also resulted in a high LD50, determined as >4.9 × 107 cells, the greatest inoculum tested. The strain ABZ1(O), which acquired its ability to synthesize CPS through complementation, has proven to be highly virulent, with an LD50 of 9.7 cells.
TABLE 2.
LD50s of encapsulated and nonencapsulated strains of V. vulnificus
| Strain | Phenotype | LD50 (cells) |
|---|---|---|
| 1003(O) | Opaque | 0.87 |
| 1003(T) | Translucent | >6.50 × 107a |
| ABZ1(T) | Translucent | >4.90 × 107a |
| ABZ1(O) | Opaque | 9.70 |
Highest amount of cells used to challenge mice. No deaths resulted from injections at this level.
Detection of the epimerase gene in 10 V. vulnificus capsular serotypes.
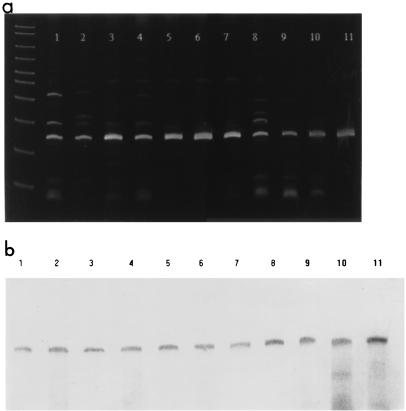
The epimerase gene was shown to be present in strains 1003(O) and 1003(T), but its dissemination among additional encapsulated strains of V. vulnificus remained to be determined. PCR was used to demonstrate the presence of the epimerase gene in the genome of 10 V. vulnificus strains, each with a distinct CPS serotype. As shown in Fig. 3, all of the 10 V. vulnificus capsular serotypes and V. vulnificus 1003(O) show amplification of the 139-bp target region and were hybridized by epimerase-probe B that is specific for 83 bp of the epimerase gene. PCR amplification coupled with Southern blot analysis verified the presence of an epimerase gene in the chromosome of each strain.
FIG. 3.
(a) PCR amplification of the 139-bp region of the putative epimerase gene in V. vulnificus C7184 (lane 1), 1010 (lane 2), 1014 (lane 3), 1007 (lane 4), 1657 (lane 5), 1866 (lane 6), 1002 (lane 7), 549 (lane 8), 938 (lane 9), 1456 (lane 10), and 1003 (lane 11). (b) Southern blot of the PCR products (see above) hybridized by an 83-bp probe (epimerase-probe B) specific for a region of the epimerase gene of V. vulnificus.
DISCUSSION
V. vulnificus expresses a negatively charged, extracellular CPS which serves to protect this pathogen in hostile environments of the human host. Capsule expression has been directly associated with virulence by mouse lethality studies (11, 47, 57) and transferrin-bound iron utilization (12, 33, 46). Kreger et al. (28) reported that the acidic surface polysaccharide was a major protective antigen by showing that mice vaccinated with surface antigen preparations were protected from overt infection when challenged with live encapsulated V. vulnificus cells. These findings suggest that protection from disease caused by V. vulnificus may be prominently humoral immunity targeting the capsular antigen and not cell-mediated immunity.
The direct association of capsule expression with virulence in assorted gram-negative pathogens has stimulated interest in the events leading to capsule expression and regulation. E. coli (15, 40), H. influenzae (29), N. meningitidis (20), Salmonella typhi (22), and K. pneumoniae (3) represent pathogens whose capsule genes are confined to a single chromosomal locus. This arrangement may permit a simple regulation of a large number of genes (39). The group II capsules of E. coli exemplify this arrangement, where three functional regions are collectively responsible for the assembly, translocation, and expression of the complete polysaccharide capsule (10). Because the capsule gene locus of V. vulnificus is not known, the gene arrangement can only be speculated upon when drawn from known systems such as those mentioned.
Our strategy for locating the gene locus was to disrupt a gene essential for capsule expression in V. vulnificus by transposon mutagenesis and then to identify that gene through sequence analysis. This preliminary analysis may lead to the identification of additional genes involved in CPS production.
In this study, sequence analysis has shown that one gene disrupted in the nonencapsulated mutant ABZ1(T) was very similar to a small segment of a 12-kb region believed to encode the surface polysaccharide of V. cholerae O139. Specifically, the V. vulnificus putative capsule gene product was found to be similar to the ORF9 protein of V. cholerae O139, which has been identified as a putative nucleotide-sugar epimerase (14). The BLAST (1) similarity search, using the amino acid sequence of the putative epimerase of V. vulnificus 1003(O), has shown it to also be highly similar to epimerases of several organisms in addition to V. cholerae O139. Those exhibiting the greatest identity to the putative epimerase of V. vulnificus are identified on Table 3. Orf2 of E. coli O111 has been described by Bastin et al. (5) and found to be homologous to epimerases of various organisms. This organism produces an O-antigen capsule so called because the sugar compositions of the O-antigen lipopolysaccharide (LPS) side chain and the capsule are identical (21). CapI of Staphylococcus aureus M is encoded by part of a capsule gene locus and has also been shown to be highly homologous with various epimerases. CapI has been described by Lin et al. (31) as the NAD-dependent enzyme involved in the synthesis of N-acetylgalactosaminouronic acid due to its high homology to the VipB protein of Salmonella typhi, whose Vi polysaccharide is a homopolymer of N-acetylgalactosaminouronic acid. The putative glucose epimerase of Bacillus thuringiensis was identified by Dunn and Ellar (16) as part of a virulence locus consisting of PK1 and capsule genes.
TABLE 3.
Comparison of amino acid sequences found to be highly similar to the putative epimerase of V. vulnificus determined by BLAST
| Organism | Homologous protein | Amino acid identity (%) | Smallest sum probability (P) |
|---|---|---|---|
| V. cholerae O139 | Nucleotide-sugar epimerase | 85 | 1.3 × 10−199 |
| E. coli O111 | Orf2 (hypothetical protein) | 63.5 | 8.5 × 10−149 |
| S. aureus M | CapI | 56 | 1.5 × 10−128 |
| B. thuringiensis | Glucose epimerase | 53.7 | 1.4 × 10−119 |
Each of the epimerases identified possesses one common feature, the dependence on NAD as a cofactor for the formation of a ketose intermediate during enzymatic function (18). Wierenga et al. (56) have defined an amino acid “fingerprint” in addition to a βαβ fold that identifies the NAD binding domain of a protein. The epimerase of V. vulnificus, described in this study, contains the consensus amino acid sequence and the βαβ fold (Table 4), required for NAD binding, in its N terminus, as defined by computer analysis (48). The C-terminus domain of epimerases is generally responsible for binding to the substrate (52), with the nucleotide portion serving as the binding anchor (19). While various amino acid sequences have been suggested to be involved in substrate binding (52), a consensus sequence has not been identified for substrate specificity.
TABLE 4.
Aligned NAD binding domains showing the fingerprint region and βαβ fold in the N termini of putative epimerasesa
| Organism | Sequence
|
|---|---|
| β α β | |
| Vv 1003 | K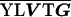 AAGFIG AAGFIG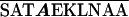 GH GH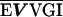 D D |
| Vc O139 | KYLVTGAAGFIGSATVKKLTEQGHHVVGID |
| Ec O111 | KYLVTGAAGFIGFHVSKRLLEAGHQVVGID |
| Sa M | KILITGTAGFIGSHLAKKLIKQGHYVIGVD |
| Bt | KILVTGAAGFIGFHLTKRLLAQNFHVIGVD |
Amino acid sequences shown: V. vulnificus (Vv) 1003 residues 2 to 31; V. cholerae (Vc) O139 residues 2 to 31; E. coli (Ec) O111 residues 2 to 31; S. aureus (Sa) M residues 2 to 31; and B. thuringiensis (Bt) residues 5 to 34. Residues identical in all sequences are in boldface.
In addition to identifying the putative epimerase of V. vulnificus by nucleotide and amino acid sequence analysis, complementation was used to illustrate its role in capsule production. When introduced into the nonencapsulated ABZ1(T), pEpiBBR supplied the epimerase gene in trans and resulted in the restoration of capsule production. The presence of the epimerase gene allowed the once-nonencapsulated mutant to express a polysaccharide capsule, indicating its involvement in capsule production. In addition, complementation resulted in a lowering of the LD50 of the nonencapsulated mutant from >4.9 × 107 to 9.7 cells once capsule production was reestablished. The newfound ability of ABZ1(O) to express a polysaccharide capsule restored the virulence of the organism and indicates the requisite of CPS for virulence of V. vulnificus.
A region of V. vulnificus DNA upstream of the epimerase gene was found highly similar to rfbQRS of V. cholerae O139, which has been reported to be an insertion sequence that may be involved in DNA rearrangement (6). Insertion sequences, similar to the one mentioned here, that are linked to genes involved in polysaccharide synthesis are not unusual in Vibrio species (50), suggesting its position relative to the epimerase may indicate the presence of other neighboring genes involved in CPS or even LPS production. But because the epimerase and rfbQRS are separated by approximately 5 kb, they may not be labeled as necessarily linked until the adjoining DNA is identified as part of a capsule locus.
In summary, epimerases mediate for the interconversion of epimers, such as glucose and galactose, which differ in configuration at a single asymmetric center. Epimerization of activated monosaccharides generally occurs in the early stages, or precursor synthesis, of capsule formation (51). As shown in this study, the loss of a functional epimerase causes the disruption of capsule production, which may be due to the depletion of precursors required for capsule synthesis. An epimerase may be common to organisms that utilize the same precursors for capsule synthesis or quite possibly even express a common sugar in their capsules. Because the putative nucleotide epimerase described in this study was found to be common to all encapsulated V. vulnificus strains tested, it is most likely a gene involved in the synthesis of CPS conserved among strains to provide an essential element for the expression of all capsule types.
Further analysis of the capsule gene locus of V. vulnificus is imperative, but this study has proven to be a worthwhile endeavor of identification of a capsule gene and possibly the location of the capsule gene locus.
ACKNOWLEDGMENTS
This research was supported by the Louisiana Sea Grant College Program, a part of the National College Program maintained by NOAA, U.S. Department of Commerce. The Louisiana Program is also supported by the State of Louisiana.
Without the constant intuition and involvement of V. R. Srinivasan, this work would not have been possible. We are grateful for the continuous assistance of Richard Cooper, who provided the pEIS vector and the use of his automated sequencer. We also thank Eric Achberger and Gregg Pettis for support throughout the study. We thank Jim Oliver and Debi Linkous, University of North Carolina at Charlotte, for their generosity in allowing the use of their facilities. Without their expertise and guidance, the virulence studies would not have been performed.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Amaro C, Biosca E G, Fouz B, Toranso A E, Garay E. Role of iron, capsule, and toxins in the pathogenicity of Vibrio vulnificus biotype 2 for mice. Infect Immun. 1994;62:759–763. doi: 10.1128/iai.62.2.759-763.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arakawa Y, Wacharotayankun R, Nagatsuka T, Ito H, Kato N, Ohta M. Genomic organization of the Klebsiella pneumoniae CPS region responsible for serotype K2 capsular polysaccharide synthesis in the virulent strain Chedid. J Bacteriol. 1995;177:1788–1796. doi: 10.1128/jb.177.7.1788-1796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartlett D H, Silverman M. Nucleotide sequence of IS492, a novel insertion sequence causing variation in extracellular polysaccharide production in the marine bacterium Pseudomonas atlantica. J Bacteriol. 1989;171:1763–1766. doi: 10.1128/jb.171.3.1763-1766.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bastin D A, Stevenson G, Brown P K, Haase A, Reeves P R. Repeat unit polysaccharides of bacteria: a model for polymerization resembling that of ribosomes and fatty acid synthetase, with a novel mechanism for determining chain length. Mol Microbiol. 1993;7:725–734. doi: 10.1111/j.1365-2958.1993.tb01163.x. [DOI] [PubMed] [Google Scholar]
- 6.Bik E M, Bunschoten A E, Gouw R D, Mooi F R. Genesis of the novel epidemic Vibrio cholerae O139 strain: evidence for horizontal transfer of genes involved in polysaccharide synthesis. EMBO J. 1995;14:209–216. doi: 10.1002/j.1460-2075.1995.tb06993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bik E M, Bunschoten A E, Willems R J, Chang A C, Mooi F R. Genetic organization and functional analysis of the otn DNA essential for cell-wall polysaccharide synthesis in Vibrio cholerae O139. Mol Microbiol. 1996;20:799–811. doi: 10.1111/j.1365-2958.1996.tb02518.x. [DOI] [PubMed] [Google Scholar]
- 8.Blake P A, Merson M H, Weaver R E, Hollis D G, Heublin P C. Disease caused by a marine vibrio: clinical characteristics and epidemiology. N Engl J Med. 1979;300:1–5. doi: 10.1056/NEJM197901043000101. [DOI] [PubMed] [Google Scholar]
- 9.Bonner J R, Coker A S, Berryman C R, Pollock H M. Spectrum of Vibrio infections in a gulf coast community. Ann Intern Med. 1983;99:464–469. doi: 10.7326/0003-4819-99-4-464. [DOI] [PubMed] [Google Scholar]
- 10.Boulnois G, Drake R, Pearce R, Roberts I. Genome diversity at the serA-linked capsule locus in Escherichia coli. FEMS Microbiol Lett. 1992;100:121–124. doi: 10.1111/j.1574-6968.1992.tb14029.x. [DOI] [PubMed] [Google Scholar]
- 11.Brennaman B, Soucy D, Howard R J. Effect of iron and liver injury on the pathogenesis of Vibrio vulnificus. J Surg Res. 1987;43:527–531. doi: 10.1016/0022-4804(87)90126-0. [DOI] [PubMed] [Google Scholar]
- 12.Bullen J J, Spalding P B, Ward C G, Gutteridge J M C. Hemochromatosis, iron, and septicemia caused by Vibrio vulnificus. Arch Intern Med. 1991;151:1606–1609. [PubMed] [Google Scholar]
- 13.Chan J W Y F, Goodwin P H. Extraction of genomic DNA from extracellular polysaccharide-synthesizing Gram-negative bacteria. BioTechniques. 1995;18:418–422. [PubMed] [Google Scholar]
- 14.Comstock L E, Johnson J A, Michalski J M, Morris J G, Jr, Kaper J B. Cloning and sequence of a region encoding a surface polysaccharide of Vibrio cholerae O139 and characterization of the insertion site in the chromosome of Vibrio cholerae O1. Mol Microbiol. 1996;19:815–826. doi: 10.1046/j.1365-2958.1996.407928.x. [DOI] [PubMed] [Google Scholar]
- 15.Drake C R, Boulnois G J, Roberts I S. The Escherichia coli serA-linked capsule locus and its flanking sequences are polymorphic, genetic evidence for the existence of more than two groups of capsule gene clusters. J Gen Microbiol. 1993;139:1707–1714. doi: 10.1099/00221287-139-8-1707. [DOI] [PubMed] [Google Scholar]
- 16.Dunn, M. G., and D. J. Ellar. Molecular genetic identification of a virulence locus in Bacillus thuringiensis. Submitted for publication.
- 17.Figurski D H, Helinski D R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frey P A. Complex pyridine nucleotide-dependent transformations. In: Dolphin D, Poulson R, Avramovic O, editors. Pyridine nucleotide coenzymes: chemical, biochemical, and medical aspects. New York, N.Y: John Wiley & Sons, Inc.; 1987. pp. 461–511. [Google Scholar]
- 19.Frey P A. The Leloir pathway: a mechanistic imperative for three enzymes to change the stereochemical configuration of a single carbon in galactose. FASEB J. 1996;10:461–470. [PubMed] [Google Scholar]
- 20.Frosch M, Weisgerber C, Meyer T. Molecular characterization and expression in Escherichia coli of the gene complex encoding the polysaccharide capsule of Neisseria meningitidis group B. Proc Natl Acad Sci USA. 1989;86:1669–1673. doi: 10.1073/pnas.86.5.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldman R C, White D, Orskov F, Orskov I, Rick P D, Lewis M S, Bhattacharjee A K, Leive L. A surface polysaccharide of Escherichia coli O111 contains O-antigen and inhibits agglutination of cells by O-antiserum. J Bacteriol. 1982;151:1210–1221. doi: 10.1128/jb.151.3.1210-1221.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashimoto Y, Li N, Yokoyama H, Ezaki T. Complete nucleotide sequencing and molecular characterization of ViaB region encoding Vi antigen in Salmonella typhi. J Bacteriol. 1993;175:4456–4465. doi: 10.1128/jb.175.14.4456-4465.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayat U, Reddy G P, Bush C A, Johnson J A, Wright A C, Morris J G., Jr Capsular types of Vibrio vulnificus: an analysis of strains from clinical and environmental sources. J Infect Dis. 1993;168:758–762. doi: 10.1093/infdis/168.3.758. [DOI] [PubMed] [Google Scholar]
- 24.Hilse R, Hammerschmidt S, Bautsch W, Frosch M. Site-specific insertion of IS1301 and distribution in Neisseria meningitidis strains. J Bacteriol. 1996;178:2527–2532. doi: 10.1128/jb.178.9.2527-2532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnston J M, Andes A, Glasser G. Vibrio vulnificus: a gastronomic hazard. JAMA. 1983;249:1756–1757. [PubMed] [Google Scholar]
- 26.Kleckner N, Bender J, Gottesman S. Uses of transposons with emphasis on Tn10. Methods Enzymol. 1991;204:139–180. doi: 10.1016/0076-6879(91)04009-d. [DOI] [PubMed] [Google Scholar]
- 27.Kovach M E, Phillips R W, Elzer P H, Roop R M, Peterson K M. PBBR1MCS: a broad-host-range cloning vector. BioTechniques. 1993;16:800–802. [PubMed] [Google Scholar]
- 28.Kreger A S, Gray L D, Testa J. Protection of mice against Vibrio vulnificus disease by vaccination with surface antigen preparations and anti-surface antigen antisera. Infect Immun. 1984;45:537–543. doi: 10.1128/iai.45.3.537-543.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kroll S, Zamze S, Loynds B, Moxon E. Common organization of chromosomal loci for production of different capsular polysaccharides in Haemophilus influenzae. J Bacteriol. 1989;171:3343–3347. doi: 10.1128/jb.171.6.3343-3347.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krovacek K, Baloda S B, Dumontet S, Mansson I. Detection of potential virulence markers of Vibrio vulnificus strains isolated from fish in Sweden. Comp Immunol Microbiol Infect Dis. 1994;17:63–70. doi: 10.1016/0147-9571(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 31.Lin W S, Cunneen T, Lee C Y. Sequence analysis and molecular characterization of genes required for the biosynthesis of type 1 capsular polysaccharide in Staphylococcus aureus. J Bacteriol. 1994;176:7005–7016. doi: 10.1128/jb.176.22.7005-7016.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris Jr J G, Wright A C, Simpson L M, Wood P K, Johnson D E, Oliver J D. Virulence of Vibrio vulnificus: association with utilization of transferrin-bound iron, and lack of correlation with levels of cytotoxin or protease production. FEMS Microbiol Lett. 1987;40:55–59. [Google Scholar]
- 34.Moxon E R, Kroll J S. The role of bacterial polysaccharide capsules as virulence factors. Curr Top Microbiol Immunol. 1990;150:65–83. doi: 10.1007/978-3-642-74694-9_4. [DOI] [PubMed] [Google Scholar]
- 35.Oka A, Sugisaki H, Takanami M. Nucleotide sequence of the kanamycin resistance transposon Tn903. J Mol Biol. 1981;147:217–226. doi: 10.1016/0022-2836(81)90438-1. [DOI] [PubMed] [Google Scholar]
- 36.Oliver J D, Wear J E, Thomas M B, Warner M, Linder K. Production of extracellular enzymes and cytotoxicity by Vibrio vulnificus. Diagn Microbiol Infect Dis. 1986;5:99–111. doi: 10.1016/0732-8893(86)90112-4. [DOI] [PubMed] [Google Scholar]
- 37.Orskov F, Orskow I. Escherichia coli serotyping and disease in man and animals. Can J Microbiol. 1992;38:699–704. [PubMed] [Google Scholar]
- 38.Reed L J, Muench H. A simple method of estimating fifty percent end points. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 39.Roberts I S. The biochemistry and genetics of capsular polysaccharide production in bacteria. Annu Rev Microbiol. 1996;50:285–315. doi: 10.1146/annurev.micro.50.1.285. [DOI] [PubMed] [Google Scholar]
- 40.Roberts I S, Mountford R, High N, Bitter-Suermann D, Jann K. Molecular cloning and analysis of the genes for the production of the K5, K7, K12, and K92 capsular polysaccharides in Escherichia coli. J Bacteriol. 1986;168:1228–1233. doi: 10.1128/jb.168.3.1228-1233.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodrigues D P, Ribeiro R V, Hofer E. Analysis of some virulence factors of Vibrio vulnificus isolated from Rio de Janeiro, Brazil. Epidemiol Infect. 1992;108:463–467. doi: 10.1017/s0950268800049979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 43.Simon R, Priefer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 44.Simonson J G, Danieu P, Siebeling R J. Abstracts of the 94th General Meeting of the American Society for Microbiology 1994. Washington, D.C: American Society for Microbiology; 1994. Distribution of Vibrio vulnificus capsular polysaccharide and lipopolysaccharide serotypes, abstr. Q-223; p. 427. [Google Scholar]
- 45.Simonson J G, Siebeling R J. Immunogenicity of Vibrio vulnificus capsular polysaccharides and polysaccharide-protein conjugates. Infect Immun. 1993;61:2053–2058. doi: 10.1128/iai.61.5.2053-2058.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simpson L M, Oliver J D. Ability of Vibrio vulnificus to obtain iron from transferrin and other iron-binding proteins. Curr Microbiol. 1987;15:155–157. [Google Scholar]
- 47.Simpson L M, White V K, Zane S F, Oliver J D. Correlation between virulence and colony morphology in Vibrio vulnificus. Infect Immun. 1987;55:269–272. doi: 10.1128/iai.55.1.269-272.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Solovyev V V, Salamov A A. Predicting a-helix and b-strand segments of globular proteins. CABIOS. 1994;10:661–669. doi: 10.1093/bioinformatics/10.6.661. [DOI] [PubMed] [Google Scholar]
- 49.Stelma G N, Jr, Reyes A L, Peeler J T, Johnson C H, Spaulding P L. Virulence characteristics of clinical and environmental isolates of Vibrio vulnificus. Appl Environ Microbiol. 1992;58:2776–2782. doi: 10.1128/aem.58.9.2776-2782.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stroeher U H, Parasivam G, Dredge B K, Manning P A. Novel Vibrio cholerae O139 genes involved in lipopolysaccharide biosynthesis. J Bacteriol. 1997;179:2740–2747. doi: 10.1128/jb.179.8.2740-2747.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sutherland I W. Biotechnology of microbial exopolysaccharides. In: Baddiley J, Carey N H, Higgins I J, Potter W G, editors. Cambridge studies in biotechnology 9. Cambridge, England: Cambridge University Press; 1990. pp. 54–59. [Google Scholar]
- 52.Thoden J B, Frey P A, Holden H M. Molecular structure of the NADH/UDP-glucose abortive complex of UDP-galactose 4-epimerase from Escherichia coli: implications for the catalytic mechanism. Biochemistry. 1996;35:5137–5144. doi: 10.1021/bi9601114. [DOI] [PubMed] [Google Scholar]
- 53.Timmis K N, DeLorenzo V, Herrero M, Jakubzik U. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing and chromosomal insertion of cloned DNA in gram-negative eubacteria. J Bacteriol. 1990;172:6568–6572. doi: 10.1128/jb.172.11.6568-6572.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wertman K F, Wyman A R, Botstein D. Host/vector interaction which affect the viability of recombinant phage lambda clones. Gene. 1986;49:253–262. doi: 10.1016/0378-1119(86)90286-6. [DOI] [PubMed] [Google Scholar]
- 55.Whitfield C. Bacterial extracellular polysaccharides. Can J Microbiol. 1988;34:415–420. doi: 10.1139/m88-073. [DOI] [PubMed] [Google Scholar]
- 56.Wierenga R K, Terpstra P, Hol W G J. Prediction of the occurrence of the ADP-binding βαβ-fold in proteins, using an amino acid sequence fingerprint. J Mol Biol. 1986;187:101–107. doi: 10.1016/0022-2836(86)90409-2. [DOI] [PubMed] [Google Scholar]
- 57.Wright A C, Simpson L M, Oliver J D. Role of iron in the pathogenesis of Vibrio vulnificus infections. Infect Immun. 1981;34:503–507. doi: 10.1128/iai.34.2.503-507.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wright A C, Simpson L M, Oliver J D, Morris J G., Jr Phenotypic evaluation of acapsular transposon mutants of Vibrio vulnificus. Infect Immun. 1990;58:1769–1773. doi: 10.1128/iai.58.6.1769-1773.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoshida S I, Ogawa M, Mizuguchi Y. Relation of capsular materials and colony opacity to virulence of Vibrio vulnificus. Infect Immun. 1985;47:446–451. doi: 10.1128/iai.47.2.446-451.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]