Abstract
Though life expectancy of people living with HIV (PLHIV) is now comparable to that of HIV-negative persons, their health-related quality of life (HRQoL) lags behind. Lower HRQoL among PLHIV may vary meaningfully, shaped in part by social factors, including stigma. Using data from Positive Voices, a national cross-sectional probability survey of adults ≥ 18 years living with HIV and accessing HIV care services in England and Wales (N = 4,422), we conducted latent class analysis on responses to a HRQoL measure (problems with mobility, usual activities, self-care, pain/discomfort, anxiety/depression) to identify HRQoL patterns, followed by multinomial logistic regression to examine relationships between HRQoL classes and a 4-item measure of HIV-related stigma and discrimination in health care. Four classes emerged: All Problems (18% prevalence); Pain and Distress (18%); Pain and Mobility (9%); No Problems (55%). Scale scores of HIV-related stigma and discrimination in health care were positively, significantly associated with membership in the All Problems (adjusted odds ratio [aOR] = 2.05; 95% confidence interval [CI] = 1.85, 2.28), Pain and Distress (aOR = 1.56; CI = 1.41, 1.73), and Pain and Mobility classes (aOR = 1.33; CI = 1.16, 1.52) compared to the No Problems class. A similar trend was observed for individual stigma and discrimination items. HRQoL among PLHIV in England and Wales varies and may be underpinned or exacerbated by HIV-related stigma and discrimination in health care. Ensuring stigma-mitigation interventions reach all health care systems/providers and emotional support services reach all PLHIV may improve HRQoL for PLHIV.
Keywords: health-related quality of life, people living with HIV, stigma, latent class analysis
In contexts where treatment is readily available and accessible, antiretroviral therapy (ART) has transformed HIV into a manageable chronic illness (WHO, 2017). Life expectancy among people living with HIV (PLHIV) who are ART-adherent now differs little from HIV-negative persons (Nakagawa et al., 2013; WHO, 2017). However, research from high-income contexts, such as the United Kingdom (UK), demonstrates that health-related quality of life (HRQoL) remains lower among PLHIV than HIV-negative persons, regardless of viral suppression status (Bing et al., 2000; Miners et al., 2014). HRQoL is a multidimensional construct that reflects how health (and health care) affects, and is affected by, quality of life; indicates how well a person functions in their life; and characterizes their perceived mental and physical wellbeing (Karimi & Brazier, 2016).
While HRQoL among PLHIV has long been used as an indicator in the monitoring and evaluation of HIV treatments and interventions (Cooper et al., 2017), there have been few targeted efforts focused on improving HRQoL, as much attention and resources have been devoted to expanded testing, ART availability, and ART-adherence in accordance with the UNAIDS 90–90-90 targets (Joint United Nations Program on HIV/AIDS (UNAIDS), 2014). Recognizing this oversight, Lazarus et al. (2016) recently called for adding good HRQoL to current targets, noting the needs of PLHIV beyond the treatment cascade.
In 2018, Public Health England (PHE) announced that as of 2017, the UK had achieved the UNAIDS 90–90-90 targets ahead of the 2020 deadline, with 87% of all PLHIV in the UK being virally suppressed (Nash et al., 2018). Understanding HRQoL in the wake of achieving the 90–90-90 targets can reveal domains in need of attention that are distinct from but potentially consequential to progress along the HIV treatment cascade (Lazarus et al., 2016; Safreed-Harmon et al., 2019) and can ultimately contribute to improving the HRQoL of PLHIV overall, which remains a focus of England’s National Health Service Outcomes Framework and the British HIV Association Standards of Care (BHIVA, 2018). For PLHIV in the UK who are virally suppressed, improving HRQoL could support sustained viral suppression, keeping people well for longer; for those who are not virally suppressed, improving HRQoL could reduce barriers to their successful progression along the HIV treatment cascade. For both groups, improving HRQoL could support not only physical health, but also mental and social health, and the overt focus on HRQoL could effectively communicate to PLHIV that their overall wellbeing – not just their viral load – matters.
Ensuring optimal HRQoL requires cross-cutting action, but a focus on stigma and discrimination is paramount given that these experiences are closely intertwined with HRQoL (Marsicano et al., 2014; Nöstlinger et al., 2014; Stigma Index UK, 2016). Stigma and discrimination related to HIV status in particular have been linked to HRQoL among PLHIV in multiple high-income countries (Nobre et al., 2018; Reinius et al., 2018; Rydström et al., 2016). Moreover, a recent meta-analysis found that HIV-related stigma and discrimination were associated with greater depression and anxiety, lower social support, lower access to and use of health and social services, poorer physical health, and lower ART-adherence (Rueda et al., 2016). HIV-related stigma and discrimination in the health care setting may be especially consequential, given the extent to which PLHIV interact with and rely on health care systems for survival and the management of multiple physical and mental health comorbidities (Guaraldi et al., 2019; Safreed-Harmon et al., 2019). Such experiences may discourage PLHIV from staying engaged in HIV treatment or from accessing other types of necessary care, which may hasten HIV disease progression and leave other conditions untreated (Rueda et al., 2016; Sweeney & Vanable, 2016). Furthermore, the cumulative effect of stigma and discrimination can negatively impact physical and mental health, which may further complicate HIV care and HRQoL (Hatzenbuehler et al., 2009; Lick et al., 2013; Logie & Gadalla, 2009).
The above literature aligns with the HIV Stigma Framework, which posits that the stigma of HIV can impact health outcomes of PLHIV through multiple stigma mechanisms, including enacted and anticipated stigma (Earnshaw & Chaudoir, 2009). Enacted stigma refers to acts of prejudice or discrimination that one believes are due to a stigmatized identity/attribute (Herek, 2007), while anticipated stigma refers to the degree to which one expects to be stigmatized by others (i.e., experience prejudice, discrimination, and stereotyping) due to a particular stigmatized identity/attribute (Earnshaw & Quinn, 2012; Quinn & Chaudoir, 2009). Anticipated stigma may include affective (e.g., fear) as well as behavioral (e.g., avoidance) responses to stigma (Furukawa et al., 2020; Maksut et al., 2020; Reisner et al., 2015; Ricci & Dixon, 2015). Enacted and anticipated HIV-related stigma have both been linked to mental distress, such as depression and anxiety (Algarin et al., 2020a; Peltzer & Pengpid, 2019; Rice et al., 2019), and physical health and functioning (Algarin et al., 2020b; Parcesepe et al., 2020; Peltzer & Pengpid, 2019; Reinius et al., 2018).
To meet new goals of improving the quality of life and psychosocial wellness of PLHIV, a better understanding of the nature of HRQoL and the factors which shape it are needed. Because HRQoL encompasses multiple physical and mental health domains, diverse experiences of HRQoL may exist, requiring a more nuanced approach to identifying HRQoL types and how these relate to stigma and discrimination (Karimi & Brazier, 2016). Summary scores and other traditional approaches to analyzing HRQoL cannot capture how patterns of diverse combinations of HRQoL problems may be experienced or heterogeneous subgroups of experiences of HRQoL that may be differentially related to stigma and discrimination (e.g., Biraguma et al., 2018; Emuren et al., 2018; Lédo et al., 2018; McGowan et al., 2017). The purpose of these analyses was to identify patterns of HRQoL among PLHIV in England and Wales and determine the extent to which these patterns were associated with HIV-related health care stigma and discrimination. We hypothesized that multiple, distinct patterns of HRQoL among PLHIV in this sample would emerge, reflecting lifestyle, physical, and mental health needs that differ according to each unique HRQoL pattern. Further, we hypothesized that HIV-related stigma and discrimination would be positively associated with any patterns that were indicative of compromised HRQoL, with the strength of the associations differing according to stigma type (i.e., greater associations for enacted versus anticipated stigma) and each unique HRQoL pattern.
Method
Study Sample
Data were drawn from Positive Voices, a national cross-sectional probability survey of adults 18 years and older living with HIV and accessing HIV care services in England and Wales. Recruited through random sampling, 4,422 PLHIV (5.3% of diagnosed PLHIV in England and Wales) from 73 HIV clinics were invited to complete the anonymous, self-administered survey on paper or online. The survey included sections related to HIV diagnostic setting and treatment; quality of life, health, and wellbeing; experience with health systems, care, and services; sex and relationships; lifestyle risk behaviors; stigma and discrimination; housing, work, and finances; and met and unmet needs (Kall et al., 2020). CD4-count data were obtained through data linkage with PHE surveillance records. Detailed methods have been described elsewhere (Kall et al., 2020). Positive Voices was approved by the Health Research Authority and the London Harrow National Health Services Research Ethics Committees. The current secondary analysis of deidentified data was considered exempt from review by the Johns Hopkins University Institutional Review Board.
Measures
The EuroQol-5D-5L (EQ-5D-5L), a five-item non-HIV-specific scale that assesses physical and mental health, was used to measure HRQoL: mobility (walking), self-care (washing and dressing), usual activities (work, study, housework, family or leisure activities), pain or discomfort, and anxiety or depression (Buchholz et al., 2018; EuroQol Group, 1990; Janssen et al., 2018). Using a 5-point Likert response option (0–4), participants rated the amount of difficulty with each of the domains. Higher values were indicative of greater difficulty or symptom severity. Responses for each item were dichotomized as no problems with the activity/condition (coded 0) versus any problem with the activity/condition (a response of 1–4 recoded as 1) to improve interpretability and estimability, as numerous parameter estimates were indeterminable and set at boundary values when indicators were modeled with all five response options (likely due to small values for some response options).
Four items were selected from the HIV Stigma Index to assess stigma and discrimination related to HIV in the health care setting (Stigma Index UK, 2016). The question – “Because of your HIV status, have you experienced any of the following in a health care setting?” – was applied to four different scenarios: (a) “been worried that you would be treated differently than other patients,” (b) “avoided seeking health care when you needed it,” (c) “been treated differently from other patients,” (d) “felt that you were refused health care or delayed a treatment or medical procedure.” Scenarios (a) and (b) reflect anticipated stigma in the form of fear and avoidance of health care, while scenarios (c) and (d) reflect enacted stigma in the form of discrimination. Response options included “No,” “Yes, in the past year,” and “Yes, more than a year ago.” Because we were interested in links between HRQoL and stigma regardless of recency of exposure, we dichotomized each stigma variable by collapsing both affirmative responses into ever (coded 1) versus never having had the experience (coded 0). We also summed responses across items to arrive at a composite score ranging from 0–4, to reflect changes in the likelihood of HRQoL class membership per unit increase in experience of stigma and discrimination.
Statistical Analysis
Missingness was assessed, and participants with > 40% missing on the HRQoL or stigma and discrimination scales were excluded. Missing responses among remaining participants were imputed with simple mean imputation. Descriptive statistics were calculated for sociodemographic characteristics, reported health care stigma and discrimination, and reported HRQoL. Cronbach’s alpha was calculated for each scale, and an alpha > 0.70 was considered indicative of adequate internal reliability.
We used latent class analysis with the 5 HRQoL indicators to detect patterns of HRQoL. Latent class analysis is a statistical method for identifying patterns (or classes) by grouping individuals according to their responses to a given set of items; patterns in responses to observed indicators reflect underlying latent classes by which alike participants may be expected to cluster together (Nylund-Gibson & Choi, 2018). While traditional regression approaches would be useful for determining independent associations between each HRQoL dimension and HIV-related stigma and discrimination, such approaches may mask underlying patterns of HRQoL that are differentially related to stigma and discrimination. Latent class analysis is the most appropriate method for detecting such patterns, after which regression can be performed to assess associations between HIV-related stigma and discrimination and each HRQoL pattern identified by the latent class analysis.
To select the best-fitting latent class model, we considered parsimony and several fit statistics for models with 2–5 classes, including Akaike’s Information Criterion (AIC), Bayesian Information Criterion (BIC), entropy statistic of class delineation, Lo-Mendell-Rubin (LMR) likelihood ratio test, and bootstrapped likelihood ratio test (BLRT) (Akaike, 1987; Lo et al., 2001; McLachlan & Peel, 2000; Nylund et al., 2007; Sclove, 1987). After model selection, we assessed the conditional independence assumption (i.e., controlling for class, indicators within that class are independent of one another) by comparing the observed and expected standardized bivariate residuals from the model (Magidson & Vermunt, 2005). We assessed estimability of the selected model by considering class-specific sample sizes, as relatively small class sizes (e.g., < 9%) can pose problems with estimating parameters, though debate remains with regard to what constitutes “small” (Nylund-Gibson & Choi, 2018). We also checked if any confidence intervals of conditional probabilities overlapped, and if any parameters had been set to .0 or 1.0, another possible indication of estimability issues. Using the T-rule, we determined that a model with five or fewer classes would be potentially identifiable, and we then further assessed identifiability by varying the starting values of selected models.
Next, we fit latent class regression of each quality of life class from the selected model on HIV-related health care stigma and discrimination, following the Three-Step Maximum Likelihood method developed by Vermunt (2010). Each HRQoL class was regressed on the full 4-item HIV-related health care stigma and discrimination scale and separately on each of the 4 individual items from the scale. Wald tests, with statistical significance set at p < .05, and 95% confidence intervals (CI) were calculated and examined. Covariates included sociodemographic characteristics and HIV care and treatment variables that were associated with HRQoL class membership and HIV-related health care stigma and discrimination. Descriptive statistics were calculated in Stata Version 15 (StataCorp, 2015), and latent class analysis and regression were conducted in Mplus Version 8 (Muthen & Muthen, 1996–2018).
Results
Sample Characteristics
Of the 4,422 participants who completed the survey, 4 (.1%) were excluded due to missing key demographic data. Other excluded participants included 70 (1.6%) who did not complete any, plus 14 who completed < 40%, of the HRQoL scale; and 129 (2.9%) who did not complete any, plus 111 who completed < 40%, of the stigma and discrimination scale. After accounting for overlap, a total of 281 (6.4%) participants were excluded on account of missing data. These individuals were significantly more likely to report being a cisgender woman compared to a cisgender man; Black African or other ethnic minority compared to White British or Irish; straight or heterosexual, asexual, or other sexual identity compared to gay or homosexual; educated at the primary or less, upper secondary, or technical/vocational level compared to Bachelor’s or postgraduate level; and unemployed or economically inactive compared to employed. In addition, participants with missing scale data were significantly more likely to report not taking ART and to report being virally suppressed but detectable compared to suppressed and undetectable. Of the remaining 4,137 participants, 215 participant responses (5.2%) were subjected to simple mean imputation: 110 participants (2.7%) for the HRQoL scale and 105 participants (2.5%) for the stigma and discrimination scale, which totaled to 206 (5.0%) after accounting for overlap. These participants were more likely to be Black or African, unemployed, heterosexual, and cisgender female. Given the low overall level of missingness, we did not expect for simple mean imputation to bias our findings; however, we performed a complete-case sensitivity analysis for comparison.
Summary statistics for sociodemographic characteristics, HRQoL, and HIV-related health care stigma and discrimination are presented in Table 1. The average age was 48 years (range = 18–85), with over a third of participants being 45–54 years. More than two thirds identified as a cisgender man. Roughly 43% (1,782/4,137) were cisgender, gay, white British or Irish men, and about 14% (592/4,137) were cisgender, heterosexual, Black African women. Over a third had completed upper secondary education, and nearly two thirds were employed (full- or part-time). Ninety-eight percent reported currently taking ART. Of those with linked viral load and CD4 information, 80% reported an undetectable viral load and 70% had a CD4 count > 500 cell/mm3 (43% overall, with 39% missing).
Table 1.
Sociodemographic characteristics, reported HRQoL and HIV-related health care stigma and discrimination among PLHIV in England and Wales, 2017 (N = 4,137).
| Continuous variables | Mean (SD) |
| Age in years | 48.1 (11.1) |
| Health-related quality of life (full scale) | .5 (.8) (range=0–4) |
| HIV-related health care stigma and discrimination (full scale) | .8 (1.2) (range=0–4) |
| Categorical variables | n (%) |
| Age in years | |
| 18–34 | 460 (11.1) |
| 35–44 | 1,053 (25.5) |
| 45–54 | 1,451 (35.1) |
| ≥55 | 1,088 (26.3) |
| Missing/Unknown | 85 (2.1) |
| Ethnicity | |
| White, British or Irish | 2,113 (51.1) |
| White, other | 403 (9.7) |
| Black, African | 1,037 (25.1) |
| Other minorities | 530 (12.8) |
| Missing/Unknown | 54 (1.3) |
| Education | |
| Primary or less | 237 (5.7) |
| Upper secondary | 1,515 (36.6) |
| Technical/vocational | 388 (9.4) |
| Bachelor’s or equivalent | 1,128 (27.3) |
| Postgraduate | 644 (15.6) |
| Missing/Unknown | 225 (5.4) |
| Employment | |
| Employed (full- or part-time) | 2,618 (63.3) |
| Unemployed | 389 (9.4) |
| Economically inactive | 986 (23.8) |
| Missing/Unknown | 144 (3.5) |
| Sexual identity | |
| Gay or lesbian/homosexual | 2,084 (50.4) |
| Straight/heterosexual | 1,616 (39.1) |
| Bisexual | 173 (4.2) |
| Asexual, Other | 55 (1.3) |
| Missing/Unknown | 209 (5.1) |
| Gender | |
| Cisgender male | 2,945 (71.2) |
| Cisgender female | 1,053 (25.5) |
| Transgender, Non-binary, Other | 38 (.9) |
| Missing/Unknown | 101 (2.4) |
| HIV care and treatment | |
| Taking ART | 4,034 (97.5) |
| Not taking ART | 84 (2.0) |
| Missing/Unknown | 19 (.5) |
| Last CD4 count ≥500 cell/mm3 | 1,776 (42.9) |
| Last CD4 count <500 cell/mm3 | 757 (18.3) |
| Missing/Unknown | 1,604 (38.8) |
| Undetectable viral load (≤50 copies/ml) | 3,312 (80.1) |
| Suppressed, detectable viral load (>50≤200 copies/ml) | 51 (1.2) |
| Detectable viral load (>200 copies/ml) | 58 (1.4) |
| Don’t know | 499 (12.1) |
| Missing/Unknown | 217 (5.3) |
| Health-related quality of life (HRQoL) | |
| Problems with mobility | 1,108 (26.8) |
| Problems with self-care | 559 (13.5) |
| Problems with usual activities | 1,173 (28.4) |
| Problems with pain or discomfort | 1,881 (45.5) |
| Problems with anxiety or depression | 2,046 (49.5) |
| HIV-related health care stigma and discrimination | |
| Worried would be treated differently | 1,445 (34.9) |
| Avoided seeking needed care | 727 (17.6) |
| Treated differently from other patients | 787 (19.0) |
| Felt care or treatment was refused or delayed | 465 (11.2) |
Regarding the HRQoL scale, roughly two thirds of participants (2,665/4,137) reported problems in at least one domain, and over 40% (1,717/4,137) reported problems in at least two domains. Nearly half of all participants reported problems with pain or discomfort (1,881/4,137) and anxiety or depression (2,046/4,137). Over a quarter reported problems with mobility (1,108/4,137) and carrying out usual activities (1,173/4,137). More than 1 in 7 reported problems with self-care (559/4,137). Internal consistency of the scale was adequate (α = .82).
Regarding the HIV-related health care stigma and discrimination scale, over 40% of participants (1,662/4,137) endorsed at least one item, and nearly a quarter (990/4,137) endorsed at least two items. Over a third reported worry that they would be treated differently by providers due to their HIV status (1,445/4,137), including 15% in the past year (626/4,137). More than 15% avoided seeking needed care in the past (727/4,137), including 9% in the past year (378/4,137). Almost one in five felt they had been treated differently compared to other patients as a result of their HIV status (787/4,137), including 7% (297/4,137) in the past year. One in nine individuals indicated that they had been refused care or treatment, or that it had been delayed, because of their HIV status (465/4,137), including 4.5% in the past year (187/4,137). Internal consistency of the scale was adequate (α = .77).
HRQoL Classes
Table 2 displays fit statistics for models with 2–5 classes. The 5-class model produced the lowest AIC value (18,172.01), followed by the 4-class model (18,193.53). The 4-class model produced the lowest BIC value (18,339.07), which increased with the 5-class model (18,355.51). Classification accuracy was high for the 4-class model (entropy = .80; lowest classification probability = .71) and mixed for the 5-class model (entropy = .84; lowest classification probability = .40). Two of the classes in the 5-class model had relatively low prevalence (5% and 8%), compared to one class in the 4-class model (9%). The LMR and BLRT indicated that the 4-class model fit the data better than the 3-class model (LMR: LL = −9130.54, p < .001; BLRT: LL = −9130.54, p < .001), and that the 5-class model fit better than the 4-class model (LMR: LL = −9073.76, p < .05; BLRT: LL = −9,073.76, p < .001). LMR and BLRT log-likelihood values indicated a marginal gain in information with the addition of class 5 (+56.78) compared to the addition of class 4 (+211.78).
Table 2.
Latent class analysis fit statistics for 2–5 class models of quality of life among people living with HIV in England and Wales, 2017 (N=4,137).
| Class # | Parameters | AIC | BIC | Entropy | Lowest classification probability | Lowest class prevalence (%) | LMR LRT Log-likelihood (p-value) | BLRT Log-likelihood (p-value) |
|---|---|---|---|---|---|---|---|---|
| 2 | 11 | 18,706.63 | 18,776.24 | .90 | .94 | 29 | −12,226.82 (p < .001) | −12,226.82 (p < .001) |
| 3 | 17 | 18,295.08 | 18,402.65 | .75 | .69 | 20 | −9,342.32 (p < .001) | −9,342.32 (p < .001) |
| 4 | 23 | 18,193.53 | 18,339.07 | .80 | .71 | 9 | −9,130.54 (p < .001) | −9,130.54 (p < .001) |
| 5 | 29 | 18,172.01 | 18,355.51 | .84 | .40 | 5 | −9,073.76 (p = .017) | −9,073.76 (p < .001) |
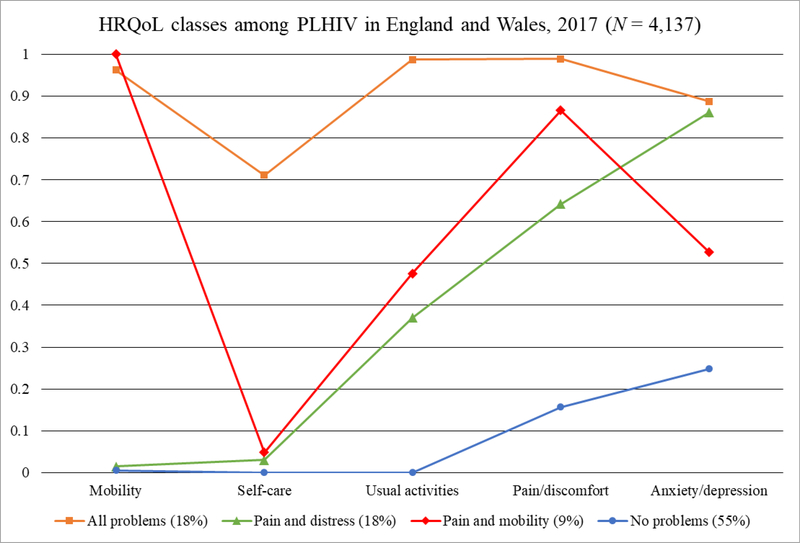
Taken together, the more parsimonious 4-class model was selected (Figure 1). Observed and expected standardized bivariate residuals from the 4-class model were within acceptable limits (< 1.96), suggesting that the conditional independence assumption was met. Class-specific sample sizes ranged from n = 384 to n = 2,294. The conditional probability of problems with mobility for class 3 was set at 1.0. Parameter estimates did not change after running the model numerous times with different starting values.
Figure 1.
Conditional probabilities (y-axis) of HRQoL indicators (x-axis) by HRQoL class among PLHIV in England and Wales, 2017 (N=4,137)
Table 3 shows the conditional probabilities of endorsing each HRQoL indicator separately by class. The first class was labeled “All Problems” (18% prevalence; n = 729), as it featured a high probability of endorsing all HRQoL indicators (.71 - .99). Class 2 was named “Pain and Distress” (18% prevalence; n = 730), as it featured a high probability of endorsing problems with pain or discomfort (.64) and anxiety or depression (.86), but a low probability of endorsing problems with the other HRQoL indicators (≤ 0.37). Class 3 was named “Pain and Mobility” (9% prevalence; n = 384), as it featured a high probability of endorsing problems with mobility (1.00) and pain or discomfort (.87), a low probability of endorsing problems with self-care (.05), and a relatively equal probability of endorsing problems with usual activities (.48) and problems with anxiety or depression (.53). Class 4 was labeled “No Problems” (55% prevalence; n = 2,294), as it included a low probability of endorsing problems across any of the HRQoL indicators (≤ .25).
Table 3.
Conditional probabilities (CP) and 95% confidence intervals (CI) of endorsing problems across five HRQoL domains, given membership in HRQoL latent classes among a sample of PLHIV in England and Wales, 2017 (N=4,137).
| All Problems n = 729 (18%) |
Pain and Distress n = 730 (18%) |
Pain and Mobility n = 384 (9%) |
No Problems n = 2,294 (55%) |
|
|---|---|---|---|---|
| HRQoL Domain | CP (CI) | CP (CI) | CP (CI) | CP (CI) |
| Mobility | .96 (.94, .98) | .02 (.00, .27) | 1.00 (1.00, 1.00)* | .01 (.00, .01) |
| Self-care | .71 (.61, .81) | .03 (.01, .05) | .05 (.00, .11) | .00 (.00, .01) |
| Usual activities | .99 (.97, 1.00) | .37 (.29, .45) | .48 (.34, .61) | .00 (.00, .01) |
| Pain or discomfort | .99 (.97, 1.00) | .64 (.58, .71) | .87 (.75, .98) | .16 (.13, .18) |
| Anxiety or depression | .89 (.86, .92) | .86 (.80, .92) | .53 (.28, .78) | .25 (.21, .28) |
Set by Mplus
HRQoL Classes and HIV-Related Health Care Stigma and Discrimination
Bivariate associations between each class and HIV-related stigma and discrimination in health care are presented in Table 4. Modeling the full 4-item scale as the exposure found a one-unit increase in stigma and discrimination to be significantly associated with higher odds of membership in the All Problems class (OR = 1.71; CI = 1.58, 1.85) and the Pain and Distress class (OR = 1.48; CI = 1.35, 1.62) relative to the No Problems class. However, stigma and discrimination were not associated with membership in the Pain and Mobility class relative to the No Problems class (OR = 1.12; CI = 0.98, 1.29). All individual scale items were also associated with higher odds of membership in the All Problems class compared to the No Problems class and the Pain and Distress class compared to the No Problems class at the p < .001 significance level. Two of the four individual scale items were associated with higher odds of membership in the Pain and Mobility class compared to the No Problems class at the p < .01 significance level. Overall, associations with the strongest magnitude were those related to having been treated differently from other patients and to feeling that care or treatment was refused or delayed due to one’s HIV status.
Table 4.
Unadjusted and adjusted odds ratios (OR) and 95% confidence intervals (CI) of latent class regression models of HRQoL class on HIV-related health care stigma and discrimination among people living with HIV in England and Wales, 2017 (N = 4,137).
| All Problems n = 729 (18%) |
Pain and Distress n = 730 (18%) |
Pain and Mobility n = 384 (9%) |
No Problems n = 2,294 (55%) |
||||
|---|---|---|---|---|---|---|---|
| Unadjusted OR (CI) | Adjusted OR (CI) | Unadjusted OR (CI) | Adjusted OR (CI) | Unadjusted OR (CI) | Adjusted OR (CI) | Reference | |
| Worried would be treated differently | 2.60*** (2.15, 3.17) | 3.48***a (2.82, 4.30) | 2.44*** (1.92, 3.11) | 2.51***a (1.96, 3.22) | 1.12 (.83, 1.52) | 1.55**a (1.13, 2.14) | 1.00 |
| Avoided seeking needed care | 3.64*** (2.89, 4.57) | 4.73***b (3.70, 6.04) | 2.72*** (2.02, 3.66) | 2.80***b (2.07, 3.78) | 1.13 (.73, 1.74) | 1.61*b (1.02, 2.52) | 1.00 |
| Treated differently from other patients | 3.90*** (3.11, 4.88) | 5.45***c (4.09, 7.27) | 2.76*** (2.06, 3.70) | 2.90***c (2.13, 3.95) | 1.66** (1.15, 2.39) | 2.23***c (1.54, 3.22) | 1.00 |
| Felt care or treatment was refused/delayed | 4.65*** (3.56, 6.07) | 5.92***d (4.32, 8.12) | 2.03*** (1.38, 2.99) | 2.07***d (1.40, 3.04) | 1.84** (1.17, 2.90) | 2.01**d (1.28, 3.15) | 1.00 |
| Full scale (continuous) | 1.71*** (1.58, 1.85) | 2.05***e (1.85, 2.28) | 1.48*** (1.35, 1.62) | 1.56***e (1.41, 1.73) | 1.12 (.98, 1.29) | 1.33***e (1.16, 1.52) | 1.00 |
p < 0.05
p < 0.01
p < 0.001
Controlling for age and education
Controlling for age, education, and viral suppression status
Controlling for age, ethnicity, education, and employment
Controlling for employment, education, and viral suppression status
Controlling for age, education, and employment
Multivariable associations between each class and HIV-related stigma and discrimination in health care are presented in Table 4. All significant bivariate associations retained statistical significance when controlling for possible confounders, and all parameter estimates increased in magnitude. Modeling the full 4-item scale as the exposure found a one-unit increase in stigma and discrimination to be significantly associated with higher odds of membership in the All Problems (aOR = 2.05; CI = 1.85, 2.28), Pain and Distress (aOR = 1.56; CI = 1.41, 1.73), and Pain and Mobility classes (aOR = 1.33; CI = 1.16, 1.52) compared to the No Problems class. All individual scale items were also significantly associated with higher odds of membership in the All Problems (aORs = 3.48–5.92), Pain and Distress (aORs = 2.07–2.90), and Pain and Mobility classes (aORs = 1.55–2.23) relative to the No Problems class at various significance levels. For the Pain and Mobility class, HRQoL as a continuous exposure and two of the individual scale items that were not significant in the unadjusted models became significant in the adjusted models. Overall, associations with the strongest magnitude were those related to having been treated differently from other patients and to feeling that care or treatment was refused or delayed due to one’s HIV status.
In our complete-case sensitivity analysis, a 4-class solution also emerged as the best-fitting model, with each class resembling its counterpart in the main analysis, with the exception of slight differences in expected class prevalences. Regarding the adjusted parameter estimates in the regression model, 13/15 (87%) were comparable to those found in the main analysis, with no change in inference, while 2/15 (13%) notably changed: Having worried that one would be treated differently due to one’s HIV status was marginally rather than strongly associated with membership in the Pain and Distress class (aOR = 1.42; CI = 0.99, 2.04; p = 0.055) relative to the No Problems class, and having avoided seeking needed health care was not associated (rather than associated) with membership in the Pain and Mobility class (aOR = 1.34; CI = 0.73, 2.45) relative to the No Problems class (not displayed).
Discussion
In this study, we explored HRQoL patterns among PLHIV and their relationship with HIV-related stigma and discrimination in the health care setting. We identified four latent classes of HRQoL among PLHIV in England and Wales. Compromised HRQoL was common, with nearly half of our sample expected to fall in a class with some level of HRQoL problems. Problems across all physical and mental domains were featured in class 1, and associations with HIV-related stigma and discrimination were of strongest magnitude in this class relative to the other classes. Mental health and mobility problems distinguished classes 2 and 3 from one another, as both classes featured problems with pain or discomfort. Associations with HIV-related health care stigma and discrimination were of stronger magnitude in the Pain and Distress class compared to the Pain and Mobility class.
Compared to the general population in England, our sample reported lower HRQoL overall and across each HRQoL domain (Kall et al., 2020), confirming prior research indicating lower HRQoL among PLHIV than HIV-negative persons (Bing et al., 2000; Miners et al., 2014). Our findings also reveal heterogeneity with regard to HRQoL among PLHIV. Though HRQoL is a multidimensional concept and is commonly assessed as such, HRQoL is often reduced to one dimension through the methods used to analyze it or through the terms used to describe it (e.g., descriptors such as high/low) (Cooper et al., 2017). A prior study that measured HRQoL among PLHIV in the UK approached the construct in two ways – the presence of any problems in a HRQoL domain being collapsed into one category of ‘functional problems’ and modeling individual HRQoL indicators as outcomes – neither of which considered how problems across multiple HRQoL domains may cluster together (McGowan et al., 2017). Here, more nuanced patterns of HRQoL emerged among PLHIV. PLHIV more often suffer from more multiple morbidities than HIV-negative persons, including a variety of physical and mental ailments (Brandt et al., 2017; Farahani et al., 2017; Guaraldi et al., 2019; Maciel et al., 2018). Since PLHIV represent a heterogenous population, they are likely to have different illness experiences with regard to these morbidities, resulting in different HRQoL patterns. These results suggest that there may be value in taking a more nuanced approach to HRQoL assessment, one in which the findings are used to describe the complexity of PLHIV’s experiences and inform tailored strategies to reduce stigma and discrimination in the health care setting. Moreover, health systems could consider setting HRQoL targets, collecting HRQoL data, devising protocols to craft HRQoL patient-profiles, and collaborating with patients to create individualized plans for improving HRQoL (Guaraldi et al., 2019; Lazarus et al., 2016; Safreed-Harmon et al., 2019).
By demonstrating differential associations with stigma and discrimination by HRQoL class, our findings extend prior research that has documented an association between HRQoL and HIV-related stigma and discrimination in the health care setting (Reinius et al., 2018; Rydström et al., 2016). Importantly, our study adds the extent to which dissimilar patterns of HRQoL may be differentially related to such stigma and discrimination. We show that, despite all HRQoL classes being associated with stigma and discrimination, the strength of the association differed greatly by class. For the All Problems class, which by far had the strongest association with HIV-related health care stigma and discrimination, this may be partially explained by the number of problems reported. PLHIV with a wider variety of health issues, including HIV-related and non-HIV-related health issues – as was likely the case for those in the All Problems class – interact with the health system much more extensively and frequently than those with fewer health issues (Guaraldi et al., 2019; Kendall et al., 2014; Maciel et al., 2018; Schouten et al., 2014). This higher frequency of interactions with a wider range of providers yields more opportunities to be exposed to stigma and discrimination, especially if stigma-mitigation efforts have not reached all health care and service providers, as may be the case for non-HIV specialist providers, such as general practitioners and dentists (Okala et al., 2018; Stigma Index UK, 2016). In addition to targeting general practitioners and dentists, stigma-mitigation interventions could be extended to other non-HIV specialist providers who practice in areas that may be frequented by PLHIV, including hepatology, endocrinology, orthopedics, psychology, and physiatry. Finally, our results underscore the potential impact of concerted efforts to support PLHIV with multiple morbidities and prior experiences of stigma and discrimination. Our recommendations align with those put forth by the British HIV Association Standards of Care, which encourage training all health care (including HIV-specialist and non-HIV specialist providers) and social service providers on treating PLHIV, identifying and addressing stigma faced by PLHIV, and making recourse for reporting stigma available to PLHIV (BHIVA, 2018). Current patient experience surveys, which are used to assess patient (PLHIV) satisfaction with providers, could be enhanced by including HRQoL and stigma measures (BHIVA, 2018).
Notably, the All Problems class and Pain and Distress class were more strongly linked to HIV-related health care stigma and discrimination than the Pain and Mobility class. This could be explained by the presence of mental health problems in both classes. Research has shown how stigma leads to increased risk of mental distress for people with a minority status in multiple high-income contexts, including the UK (Hatzenbuehler, 2009; Hatzenbuehler & Pachankis, 2016; Kneale et al., 2020; Meyer, 2003; Schulman & Erickson-Schroth, 2019; White et al., 2019). Many participants in our sample must manage not only the stigmatized status of being HIV positive, but also that of being a sexual, ethnic, and/or other minority. Intersecting stigmas may increase risk for mental distress even more (English et al., 2018; Jackson-Best & Edwards, 2018; Price et al., 2019; Shangani et al., 2020). Therefore, the featuring of mental health problems in the All Problems class and Pain and Distress class may reflect having had more frequent or impactful experiences with HIV-related health care stigma and discrimination in the past. Future research may consider exploring the extent to which HRQoL patterns among PLHIV differ across other characteristics, such as gender identity, sexuality, and ethnicity.
These findings should be interpreted in light of several limitations. The EQ-5D-5L is a brief, generic measure, and while it yielded some diversity in HRQoL patterns, a longer or HIV-specific instrument designed for PLHIV that assesses more domains may reveal more nuance and diversity in HRQoL patterns. Relatedly, two of the items were double-barreled, asking participants if they experienced “pain or discomfort” or had felt “anxious or depressed.” Participants endorsing one or the other or both may vary from one another in ways that could not be captured, though anxiety and depression are common comorbidities (Brown et al., 2001; Lamers et al., 2011). Only four items were used to assess stigma and discrimination experiences in health care settings. Increasing the number of items and the diversity of stigma experiences including anticipated, perceived, and intersectional stigmas could provide additional insight into the relationships examined here. Further, we summed these responses to arrive at a composite score of stigma and discrimination; however, summing stigma and discrimination experiences in such a manner, particularly ones that differ in type (enacted versus anticipated), may not accurately capture their operative properties. Causality between HRQoL class membership and HIV-related health care stigma and discrimination cannot be established because the data are cross-sectional. While the identifiability of the 4-class model cannot be guaranteed, the fact that the same parameter estimates emerged with varying starting values suggests the model is identifiable. Several confidence intervals of the conditional probabilities overlapped, indicating potential concerns with estimability, though the majority were fairly narrow, indicating precision. Moreover, though the conditional probability of problems with mobility for class 3 was set at the boundary value of 1.0, this was in the expected direction. Finally, though the original sample was representative of PLHIV receiving HIV care in England and Wales, >5% were excluded due to missing data, and data were imputed at the item level for a small subset of remaining participants. Both groups of participants significantly differed from retained participants across multiple sociodemographic characteristics, possibility resulting in the sample’s no longer being representative of PLHIV receiving HIV care in England and Wales, reducing the generalizability of the findings. Despite these limitations, our study utilized a large population of PLHIV engaged in HIV care in England and Wales to extend prior research with PLHIV by documenting different patterns of HRQoL that warrant further investigation and unique solutions.
The UK is a world leader in the HIV response. Despite this achievement, PLHIV in England and Wales continue to experience compromised HRQoL, which varies across physical and mental domains. HIV-related health care stigma and discrimination may underpin or exacerbate HRQoL problems and could threaten HIV treatment progress made thus far. These findings call for greater consideration of the diverse needs of PLHIV and tailored interventions to improve quality of life. Future research is needed to explore the extent to which these and other patterns of HRQoL among PLHIV exist, determine other possible contributing factors to the emergence of such patterns, and develop and implement interventions to address them. Through systematic, nuanced monitoring of HRQoL, stigma-mitigation across HIV- and non-HIV care institutions, and increased support for PLHIV with multiple morbidities, HRQoL among PLHIV can be improved.
Acknowledgments
This study was funded by the National Institutes of Health (R01MH110358). Jessica L. Maksut received research support from the National Institutes of Allergy and Infectious Diseases (T32AI102623). The Positive Voices 2017 survey was supported by funding from Public Health England and research grants from Gilead Sciences, Inc. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
The authors have no conflicts of interest to report.
References
- Akaike H (1987). Factor analysis and AIC. Psychometrika, 52(3), 317–332. [Google Scholar]
- Algarin AB, Sheehan DM, Varas-Diaz N, Fennie K, Zhou Z, Spencer EC, Cook CL, Cook RL, & Ibanez GE (2020a). Enacted HIV-related stigma’s association with anxiety & depression among people living with HIV (PLWH) in Florida. AIDS and Behavior, doi: 10.1007/s10461-020-02948-5 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algarin AB, Sheehan DM, Varas-Diaz N, Fennie KP, Zhou Z, Spencer EC, Cook RL, Morano JP, & Ibanez GE (2020b). Health care-specific enacted HIV-related stigma’s association with antiretroviral therapy adherence and viral suppression among people living with HIV in Florida. AIDS Patient Care and STDs, 34(7), 316–326. doi: 10.1089/apc.2020.0031 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing EG, Hays RD, Jacobson LP, Chen B, Gange SJ, Kass NE, Chmiel JS, Zucconi SL (2000). Health-related quality of life among people with HIV disease: Results from the multicenter AIDS cohort study. Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation, 9(1), 55–63. doi: 10.1023/a:1008919227665 [doi] [DOI] [PubMed] [Google Scholar]
- Biraguma J, Mutimura E, & Frantz JM (2018). Health-related quality of life and associated factors in adults living with HIV in Rwanda. SAHARA J: Journal of Social Aspects of HIV/AIDS Research Alliance, 15(1), 110–120. doi: 10.1080/17290376.2018.1520144 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt C, Zvolensky MJ, Woods SP, Gonzalez A, Safren SA, & O’Cleirigh CM (2017). Anxiety symptoms and disorders among adults living with HIV and AIDS: A critical review and integrative synthesis of the empirical literature. Clinical Psychology Review, 51, 164–184. doi:S0272-7358(16)30060-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- British HIV Association (BHIVA). (2018). Standards of care for people living with HIV ().London: Mediscript Ltd. [Google Scholar]
- Brown TA, Campbell LA, Lehman CL, Grisham JR, & Mancill RB (2001). Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. Journal of Abnormal Psychology, 110(4), 585–599. doi: 10.1037//0021-843x.110.4.585 [doi] [DOI] [PubMed] [Google Scholar]
- Buchholz I, Janssen MF, Kohlmann T, & Feng YS (2018). A systematic review of studies comparing the measurement properties of the three-level and five-level versions of the EQ-5D. PharmacoEconomics, 36(6), 645–661. doi: 10.1007/s40273-018-0642-5 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper V, Clatworthy J, Harding R, Whetham J, & Emerge Consortium. (2017). Measuring quality of life among people living with HIV: A systematic review of reviews. Health and Quality of Life Outcomes, 15(1), 220–017-0778–6. doi: 10.1186/s12955-017-0778-6 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw VA, & Chaudoir SR (2009). From conceptualizing to measuring HIV stigma: A review of HIV stigma mechanism measures. AIDS and Behavior, 13(6), 1160–1177. doi: 10.1007/s10461-009-9593-3 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw VA, & Quinn DM (2012). The impact of stigma in healthcare on people living with chronic illnesses. Journal of Health Psychology, 17(2), 157–168. doi: 10.1177/1359105311414952 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emuren L, Welles S, Polansky M, Evans AA, Macalino G, Agan BK, & Infectious Disease Clinical Research Program HIV Working Group. (2018). Lower health-related quality of life predicts all-cause hospitalization among HIV-infected individuals. Health and Quality of Life Outcomes, 16(1), 107–018-0931-x. doi: 10.1186/s12955-018-0931-x [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- English D, Rendina HJ, & Parsons JT (2018). The effects of intersecting stigma: A longitudinal examination of minority stress, mental health, and substance use among black, latino, and multiracial gay and bisexual men. Psychology of Violence, 8(6), 669–679. doi: 10.1037/vio0000218 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- EuroQol Group. (1990). EuroQol--a new facility for the measurement of health-related quality of life. Health Policy (Amsterdam, Netherlands), 16(3), 199–208. doi:0168-8510(90)90421-9 [pii] [DOI] [PubMed] [Google Scholar]
- Farahani M, Mulinder H, Farahani A, & Marlink R (2017). Prevalence and distribution of non-AIDS causes of death among HIV-infected individuals receiving antiretroviral therapy: A systematic review and meta-analysis. International Journal of STD & AIDS, 28(7), 636–650. doi: 10.1177/0956462416632428 [doi] [DOI] [PubMed] [Google Scholar]
- Furukawa NW, Maksut JL, Zlotorzynska M, Sanchez TH, Smith DK, & Baral SD (2020). Sexuality disclosure in U.S. gay, bisexual, and other men who have sex with men: Impact on healthcare-related stigmas and HIV pre-exposure prophylaxis denial. American Journal of Preventive Medicine, doi:S0749-3797(20)30125-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaraldi G, Arends J, Buhk T, Cascio M, Curran A, Teofilo E, Van Den Berk G, Verger C (2019). “Moving fourth”: A vision toward achieving healthy living with HIV beyond viral suppression. AIDS Reviews, 21(3), 135–142. doi: 10.24875/AIDSRev.19000088 [doi] [DOI] [PubMed] [Google Scholar]
- Hatzenbuehler ML (2009). How does sexual minority stigma “get under the skin”? A psychological mediation framework. Psychological Bulletin, 135(5), 707–730. doi: 10.1037/a0016441 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzenbuehler ML, Nolen-Hoeksema S, & Dovidio J (2009). How does stigma “get under the skin”?: The mediating role of emotion regulation. Psychological Science, 20(10), 1282–1289. doi: 10.1111/j.1467-9280.2009.02441.x [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzenbuehler ML, & Pachankis JE (2016). Stigma and minority stress as social determinants of health among lesbian, gay, bisexual, and transgender youth: Research evidence and clinical implications. Pediatric Clinics of North America, 63(6), 985–997. doi:S0031-3955(16)41055-2 [pii] [DOI] [PubMed] [Google Scholar]
- Herek GM (2007). Confronting sexual stigma and prejudice: Theory and practice. Journal of Social Issues, 63(4), 905–925. doi: 10.1111/j.1540-4560.2007.00544.x [DOI] [Google Scholar]
- Jackson-Best F, & Edwards N (2018). Stigma and intersectionality: A systematic review of systematic reviews across HIV/AIDS, mental illness, and physical disability. BMC Public Health, 18(1), 919–018-5861–3. doi: 10.1186/s12889-018-5861-3 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen MF, Bonsel GJ, & Luo N (2018). Is EQ-5D-5L better than EQ-5D-3L? A head-to-head comparison of descriptive systems and value sets from seven countries. PharmacoEconomics, 36(6), 675–697. doi: 10.1007/s40273-018-0623-8 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joint United Nations Program on HIV/AIDS (UNAIDS). (2014). 90–90-90: An ambition treatment target to help end the AIDS epidemic. UNAIDS. Retrieved from https://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf [Google Scholar]
- Kall M, Auzenbergs M, & Delpech V (2020). Positive voices: The national survey of people living with HIV - findings from the 2017 survey. London: Public Health England. Retrieved from https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/857922/PHE_positive_voices_report_2019.pdf [Google Scholar]
- Karimi M, & Brazier J (2016). Health, health-related quality of life, and quality of life: What is the difference? PharmacoEconomics, 34(7), 645–649. doi: 10.1007/s40273-016-0389-9 [doi] [DOI] [PubMed] [Google Scholar]
- Kendall CE, Wong J, Taljaard M, Glazier RH, Hogg W, Younger J, & Manuel DG (2014). A cross-sectional, population-based study measuring comorbidity among people living with HIV in Ontario. BMC Public Health, 14, 161–2458-14–161. doi: 10.1186/1471-2458-14-161 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneale D, Thomas J, & French R (2020). Inequalities in health and care among lesbian, gay and bisexual people aged 50 and over in the United Kingdom: A systematic review and meta-analysis of sources of individual participant data. The Journals of Gerontology, Series B, Psychological Sciences and Social Sciences, doi:gbaa071 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers F, van Oppen P, Comijs HC, Smit JH, Spinhoven P, van Balkom AJ, Nolen WA, Zitman FG, Beekman ATF, Penninx BW (2011). Comorbidity patterns of anxiety and depressive disorders in a large cohort study: The Netherlands study of depression and anxiety (NESDA). The Journal of Clinical Psychiatry, 72(3), 341–348. doi: 10.4088/JCP.10m06176blu [doi] [DOI] [PubMed] [Google Scholar]
- Lazarus JV, Safreed-Harmon K, Barton SE, Costagliola D, Dedes N, Del Amo Valero J, Gatell JM, Baptistia-Leite R, Mendao L, Porter K, Vella S, Rockstroh JK (2016). Beyond viral suppression of HIV - the new quality of life frontier. BMC Medicine, 14(1), 94–016-0640–4. doi: 10.1186/s12916-016-0640-4 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lédo AP, Rodriguez-Prieto I, Lins L, Neto MG, & Brites C (2018). Association between health-related quality of life and physical functioning in antiretroviral-naive HIV-infected patients. The Open AIDS Journal, 12, 117–125. doi: 10.2174/1874613601812010117 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lick DJ, Durso LE, & Johnson KL (2013). Minority stress and physical health among sexual minorities. Perspectives on Psychological Science: A Journal of the Association for Psychological Science, 8(5), 521–548. doi: 10.1177/1745691613497965 [doi] [DOI] [PubMed] [Google Scholar]
- Lo Y, Mendell N, & Rubin D (2001). Testing the number of components in a normal mixture. Biometrika, 88(3), 767–778. [Google Scholar]
- Logie C, & Gadalla TM (2009). Meta-analysis of health and demographic correlates of stigma towards people living with HIV. AIDS Care, 21(6), 742–753. doi: 10.1080/09540120802511877 [doi] [DOI] [PubMed] [Google Scholar]
- Maciel RA, Klück HM, Durand M, & Sprinz E (2018). Comorbidity is more common and occurs earlier in persons living with HIV than in HIV-uninfected matched controls, aged 50 years and older: A cross-sectional study. International Journal of Infectious Diseases: IJID: Official Publication of the International Society for Infectious Diseases, 70, 30–35. doi:S1201-9712(18)30045-6 [pii] [DOI] [PubMed] [Google Scholar]
- Magidson J, & Vermunt J (2005). Latent class models. In Kaplan D (Ed.), The sage handbook of quantitative methodology for the social sciences (pp. 175–198). Thousand Oaks, CA: Sage. [Google Scholar]
- Maksut JL, Sanchez TH, Wiginton JM, Scheim AI, Logie CH, Zlotorzynska M, Lyons C, Baral SD (2020). Gender identity and sexual behavior stigmas, severe psychological distress, and suicidality in an online sample of transgender women in the united states. Annals of Epidemiology, 52, 15–22. doi:S1047-2797(20)30280-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano E, Dray-Spira R, Lert F, Aubrière C, Spire B, Hamelin C, & ANRS-Vespa2 study group. (2014). Multiple discriminations experienced by people living with HIV in France: Results from the ANRS-Vespa2 study. AIDS Care, 26 Suppl 1, S97–S106. doi: 10.1080/09540121.2014.907385 [doi] [DOI] [PubMed] [Google Scholar]
- McGowan JA, Sherr L, Rodger AJ, Fisher M, Miners A, Anderson J, Johnson MA, Elford J, Collins S, Hart G, Phillips AN, Speakman A, & Lampe FC for the Antiretrovirals, Sexual Transmission Risk and Attitudes (ASTRA) Study Group. (2017). Age, time living with diagnosed HIV infection, and self-rated health. HIV Medicine, 18(2), 89–103. doi: 10.1111/hiv.12398 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan G, & Peel D (2000). Finite mixture models. New York: Wiley. [Google Scholar]
- Meyer IH (2003). Prejudice, social stress, and mental health in lesbian, gay, and bisexual populations: Conceptual issues and research evidence. Psychological Bulletin, 129(5), 674–697. doi: 10.1037/0033-2909.129.5.674 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miners A, Phillips A, Kreif N, Rodger A, Speakman A, Fisher M, Anderson J, Collins S, Hart G, Sherr L, & Lampe FC for the ASTRA (Antiretrovirals, Sexual Transmission and Attitudes) Study. (2014). Health-related quality-of-life of people with HIV in the era of combination antiretroviral treatment: A cross-sectional comparison with the general population. The Lancet HIV, 1(1), e32–40. doi:S2352-3018(14)70018-9 [pii] [DOI] [PubMed] [Google Scholar]
- Nakagawa F, May M, & Phillips A (2013). Life expectancy living with HIV: Recent estimates and future implications. Current Opinion in Infectious Diseases, 26(1), 17–25. doi: 10.1097/QCO.0b013e32835ba6b1 [doi] [DOI] [PubMed] [Google Scholar]
- Nash S, Desai S, Croxford S, Guerra L, Lowndes C, Connor N, & Gill O (2018). Progress towards ending the HIV epidemic in the United Kingdom: 2018 report. London: Public Health England. Retrieved from https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/821273/Progress_towards_ending_the_HIV_epidemic_in_the_UK.pdf [Google Scholar]
- Nobre N, Pereira M, Roine RP, Sutinen J, & Sintonen H (2018). HIV-related self-stigma and health-related quality of life of people living with HIV in Finland. The Journal of the Association of Nurses in AIDS Care: JANAC, 29(2), 254–265. doi:S1055-3290(17)30201-7 [pii] [DOI] [PubMed] [Google Scholar]
- Nöstlinger C, Rojas Castro D, Platteau T, Dias S, & Le Gall J (2014). HIV-related discrimination in European health care settings. AIDS Patient Care and STDs, 28(3), 155–161. doi: 10.1089/apc.2013.0247 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylund KL, Asparouhov T, & Muthen BO (2007). Deciding on the number of classes in latent class analysis and growth mixture modeling: A monte carlo simulation study. Structural Equation Modeling, 14(4), 535–569. [Google Scholar]
- Nylund-Gibson K, & Choi AY (2018). Ten frequently asked questions about latent class analysis. Translational Issues in Psychological Science, 4(4), 440–461. doi: 10.1037/tps0000176 [DOI] [Google Scholar]
- Okala S, Doughty J, Watt RG, Santella AJ, Conway DI, Crenna-Jennings W, bewe R, Morton J, Lut I, Thorley L, Benton L, Hibbert M, Jeffries JMC, Kunda C, Morris S, Osborne K, Patterson H, Sharp L, Valiotis G, Hudson A, Delpech V (2018). The people living with HIV STIGMA Survey UK 2015: Stigmatising experiences and dental care. British Dental Journal, 225(2), 143–150. doi: 10.1038/sj.bdj.2018.530 [doi] [DOI] [PubMed] [Google Scholar]
- Parcesepe AM, Nash D, Tymejczyk O, Reidy W, Kulkarni SG, & Elul B (2020). Gender, HIV-related stigma, and health-related quality of life among adults enrolling in HIV care in Tanzania. AIDS and Behavior, 24(1), 142–150. doi: 10.1007/s10461-019-02480-1 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltzer K, & Pengpid S (2019). Prevalence and associated factors of enacted, internalized and anticipated stigma among people living with HIV in South Africa: Results of the first national survey. HIV/AIDS (Auckland, N.Z.), 11, 275–285. doi: 10.2147/HIV.S229285 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price M, Polk W, Hill NE, Liang B, & Perella J (2019). The intersectionality of identity-based victimization in adolescence: A person-centered examination of mental health and academic achievement in a U.S. high school. Journal of Adolescence, 76, 185–196. doi:S0140-1971(19)30153-8 [pii] [DOI] [PubMed] [Google Scholar]
- Quinn DM, & Chaudoir SR (2009). Living with a concealable stigmatized identity: The impact of anticipated stigma, centrality, salience, and cultural stigma on psychological distress and health. Journal of Personality and Social Psychology, 97(4), 634–651. doi: 10.1037/a0015815 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinius M, Wiklander M, Wettergren L, Svedhem V, & Eriksson LE (2018). The relationship between stigma and health-related quality of life in people living with HIV who have full access to antiretroviral treatment: An assessment of Earnshaw and Chaudoir’s HIV stigma framework using empirical data. AIDS and Behavior, 22(12), 3795–3806. doi: 10.1007/s10461-018-2041-5 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisner SL, Pardo ST, Gamarel KE, White Hughto JM, Pardee DJ, & Keo-Meier CL (2015). Substance use to cope with stigma in healthcare among U.S. female-to-male trans masculine adults. LGBT Health, 2(4), 324–332. doi: 10.1089/lgbt.2015.0001 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci B, & Dixon L (2015). What can we do about stigma? Psychiatric Services (Washington, D.C.), 66(10), 1009. doi: 10.1176/appi.ps.661004 [doi] [DOI] [PubMed] [Google Scholar]
- Rice WS, Turan B, Fletcher FE, Nápoles TM, Walcott M, Batchelder A, Kempf MD, Konkle-Parker DJ, Wilson TE, Tien PE, Wingood GM, Neilands TB, Johnson MO, Weiser SD, & Turan JM (2019). A mixed methods study of anticipated and experienced stigma in health care settings among women living with HIV in the United States. AIDS Patient Care and STDs, 33(4), 184–195. doi: 10.1089/apc.2018.0282 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda S, Mitra S, Chen S, Gogolishvili D, Globerman J, Chambers L,L, Wilson M, Logie CH, Shi Q, Morassaei S, Rourke SB (2016). Examining the associations between HIV-related stigma and health outcomes in people living with HIV/AIDS: A series of meta-analyses. BMJ Open, 6(7), e011453–2016-011453. doi: 10.1136/bmjopen-2016-011453 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydström LL, Wiklander M, Navér L, Ygge BM, & Eriksson LE (2016). HIV-related stigma and health-related quality of life among children living with HIV in Sweden. AIDS Care, 28(5), 665–671. doi: 10.1080/09540121.2015.1120267 [doi] [DOI] [PubMed] [Google Scholar]
- Safreed-Harmon K, Anderson J, Azzopardi-Muscat N, Behrens GMN, d’Arminio Monforte A, Davidovich U, del Amo J, Kall M, Noori T, Porter K, Lazarus JV (2019). Reorienting health systems to care for people with HIV beyond viral suppression. The Lancet HIV, 6(12), e869–e877. doi:S2352-3018(19)30334-0 [pii] [DOI] [PubMed] [Google Scholar]
- Schouten J, Wit FW, Stolte IG, Kootstra NA, van der Valk M, Geerlings SE, Prins M, & Reiss P for the AGEhIV Cohort Study Group. (2014). Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: The AGEhIV cohort study. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 59(12), 1787–1797. doi: 10.1093/cid/ciu701 [doi] [DOI] [PubMed] [Google Scholar]
- Schulman JK, & Erickson-Schroth L (2019). Mental health in sexual minority and transgender women. The Medical Clinics of North America, 103(4), 723–733. doi:S0025-7125(19)30008-2 [pii] [DOI] [PubMed] [Google Scholar]
- Sclove S (1987). Application of model-selection criteria to some problems in multivariate analysis. Psychometrika, 52(3), 333–343. [Google Scholar]
- Shangani S, Gamarel KE, Ogunbajo A, Cai J, & Operario D (2020). Intersectional minority stress disparities among sexual minority adults in the USA: The role of race/ethnicity and socioeconomic status. Culture, Health & Sexuality, 22(4), 398–412. doi: 10.1080/13691058.2019.1604994 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stigma Index UK. (2016). The people living with HIV stigma survey UK 2015: National findings. London: Stigma Index UK. Retrieved from http://www.stigmaindexuk.org/reports/2016/NationalReport.pdf [Google Scholar]
- Sweeney SM, & Vanable PA (2016). The association of HIV-related stigma to HIV medication adherence: A systematic review and synthesis of the literature. AIDS and Behavior, 20(1), 29–50. doi: 10.1007/s10461-015-1164-1 [doi] [DOI] [PubMed] [Google Scholar]
- White LC, Cooper M, & Lawrence D (2019). Mental illness and resilience among sexual and gender minority refugees and asylum seekers. The British Journal of General Practice: The Journal of the Royal College of General Practitioners, 69(678), 10–11. doi: 10.3399/bjgp19X700349 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO). (2017). Ten years in public health, 2007–2017: Report by Dr. Margaret Chan, Director-General Geneva: WHO. Retrieved from https://apps.who.int/iris/bitstream/handle/10665/255355/9789241512442-eng.pdf;jsessionid=8919079FD5F34BAD9F6DA8D5BDE8A071?sequence=1 [Google Scholar]