Abstract
Background
Inadvertent postoperative hypothermia (a drop in core body temperature to below 36°C) occurs as an effect of surgery when anaesthetic drugs and exposure of the skin for long periods of time during surgery result in interference with normal temperature regulation. Once hypothermia has occurred, it is important that patients are rewarmed promptly to minimise potential complications. Several different interventions are available for rewarming patients.
Objectives
To estimate the effectiveness of treating inadvertent perioperative hypothermia through postoperative interventions to decrease heat loss and apply passive and active warming systems in adult patients who have undergone surgery.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 2), MEDLINE (Ovid SP) (1956 to 21 February 2014), EMBASE (Ovid SP) (1982 to 21 February 2014), the Institute for Scientific Information (ISI) Web of Science (1950 to 21 February 2014) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL), EBSCO host (1980 to 21 February 2014), as well as reference lists of articles. We also searched www.controlled‐trials.com and www.clincialtrials.gov.
Selection criteria
Randomized controlled trials of postoperative warming interventions aiming to reverse hypothermia compared with control or with each other.
Data collection and analysis
Three review authors identified studies for inclusion in this review. One review author extracted data and completed risk of bias assessments; two review authors checked the details. Meta‐analysis was conducted when appropriate by using standard methodological procedures as expected by The Cochrane Collaboration.
Main results
We included 11 trials with 699 participants. Ten trials provided data for analysis. Trials varied in the numbers and types of participants included and in the types of surgery performed. Most trials were at high or unclear risk of bias because of inappropriate or unclear randomization procedures, and because blinding of assessors and participants generally was not possible. This may have influenced results, but it is unclear how the results may have been influenced. Active warming was found to reduce the mean time taken to achieve normothermia by about 30 minutes in comparison with use of warmed cotton blankets (mean difference (MD) ‐32.13 minutes, 95% confidence interval (CI) ‐42.55 to ‐21.71; moderate‐quality evidence), but no significant difference in shivering was noted. Active warming was found to reduce mean time taken to achieve normothermia by almost an hour and a half in comparison with use of unwarmed cotton blankets (MD ‐88.86 minutes, 95% CI ‐123.49 to ‐54.23; moderate‐quality evidence), and people in the active warming group were less likely to shiver than those in the unwarmed cotton blanket group (Relative Risk=0.61 95% CI= 0.42 to 0.86; low quality evidence). There was no effect on mean temperature difference in degrees celsius at 60 minutes (MD=0.18°C, 95% CI=‐0.10 to 0.46; moderate quality evidence), and no data were available in relation to major cardiovascular complications. Forced air warming was found to reduce time taken to achieve normothermia by about one hour in comparison to circulating hot water devices (MD=‐54.21 minutes 95% CI= ‐94.95, ‐13.47). There was no statistically significant difference between thermal insulation and cotton blankets on mean time to achieve normothermia (MD =‐0.29 minutes, 95% CI=‐25.47 to 24.89; moderate quality evidence) or shivering (Relative Risk=1.36 95% CI= 0.69 to 2.67; moderate quality evidence), and no data were available for mean temperature difference or major cardiovascular complications. Insufficient evidence was available about other comparisons, adverse effects or any other secondary outcomes.
Authors' conclusions
Active warming, particularly forced air warming, appears to offer a clinically important reduction in mean time taken to achieve normothermia (normal body temperature between 36°C and 37.5°C) in patients with postoperative hypothermia. However, high‐quality evidence on other important clinical outcomes is lacking; therefore it is unclear whether active warming offers other benefits and harms. High‐quality evidence on other warming methods is also lacking; therefore it is unclear whether other rewarming methods are effective in reversing postoperative hypothermia.
Keywords: Humans, Bedding and Linens, Air, Body Temperature, Hypothermia, Hypothermia/etiology, Hypothermia/therapy, Postoperative Complications, Postoperative Complications/therapy, Randomized Controlled Trials as Topic, Rewarming, Rewarming/instrumentation, Rewarming/methods, Shivering, Time Factors
Plain language summary
Treating unintentional hypothermia after surgery
Review question We wanted to find out the effects of different methods of rewarming adult patients with unintentional hypothermia (a core body temperature below 36°C) after surgery.
Background Patients can get cold during surgery, particularly because of the drugs used as anaesthetics. This can cause potentially dangerous heart problems. Cold can also make patients shiver and feel uncomfortable after an operation. Different ways of rewarming patients after surgery have been developed, such as using thermal insulation (e.g. reflective blankets) and active warming (whereby heat is transferred directly from the device to the patient, e.g. electric blanket, heat lamp).
Study characteristics We looked at the evidence up to February 2014 and included 11 studies involving 699 participants. Ten studies provided data for analysis.The studies involved adults (over 18 years of age) who were undergoing routine or emergency surgery. We did not include studies in which patients were kept cold deliberately during the operation, were having head surgery or skin grafts or were under a local anaesthetic. We looked at studies comparing different rewarming methods versus each other or versus normal care (hospital blankets).
Key results We can be quite certain that temperature goes back to normal (between 36°C and 37.5°C) more than an hour faster when active warming methods are used to warm hypothermic patients than when hospital blankets are used, and that this result is important for people involved in the care of patients with hypothermia after surgery. Not enough evidence was found to show whether active warming methods provide other benefits or harms to patients. Some evidence suggests that forced air warming (one type of active warming) is better at rewarming patients than circulating hot water devices and radiant heaters (other types of active warming), but we do not know whether forced air warming is the best active warming method overall, as evidence on all methods of active warming was not available.
There was not enough evidence to be certain if other ways of rewarming patients (such as reflective blankets) have benefits or harms for patients. Quality of the evidence Most of the evidence was moderate to low in quality. Methods used to assign patients to treatment groups were generally unclear or inadequate, and it was not possible to keep patients or people assessing patients unaware of the treatment given. This may have biased the results, but we are not sure what influence this could have had on the overall results.
Summary of findings
for the main comparison.
| Active warming compared with control (cotton blankets) for treating postoperative hypothermia | |||||
|
Patient or population: adults with postoperative hypothermia Settings: postsurgical care Intervention: active warming Comparison: control (cotton blankets) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Cotton blanket | Warmed group | ||||
|
Time to normothermia (minutes) Active vs unwarmed cotton blankets |
Mean time taken to achieve normothermia across control groups was 272 minutes | Mean time taken to achieve normothermia in the intervention groups was 88.86 minutes lower (123.49 to 54.23 lower) | 95 (2 studies) | ⊕⊕⊕⊝ Moderatea,b | |
|
Time to normothermia (minutes) Active vs warmed cotton blankets |
Mean time taken to achieve normothermia across control groups was 101 minutes | Mean time taken to achieve normothermia in the intervention groups was 32.13 minutes lower (42.55 to 21.71 lower) | 220 (2 studies) | ⊕⊕⊕⊝ Moderatea,b | |
|
Mean temperature difference (60 minutes after warming started) Active vs warmed cotton blankets |
Mean temperature across control groups was 35.9°C |
Mean temperature difference in the intervention groups was 0.18 °C higher (0.1 lower to 0.46 higher) | 102 (2 studies) | ⊕⊕⊕⊝ Moderatea,b | |
|
Rate of rewarming (°C per hour) Active vs unwarmed cotton blankets |
Mean rate of rewarming across control groups was 0.70°C per hour |
Mean difference in rewarming rates was 0.08°C per hour higher (0.30 lower to 0.46 higher) |
24 (1 study) |
⊕⊕⊕⊝ Moderateb,c | |
| Major cardiovascular complications: not reported | See comment | See comment | Not estimable | ‐ | See comment |
|
Shivering (number of patients) Active vs warmed cotton blankets |
288 per 1000 | 274 per 1000 (150 to 502) | RR 0.95 (0.52 to 1.74) | 103 (2 studies) | ⊕⊕⊝⊝ Lowb,c,d |
|
Shivering (number of patients) Active vs unwarmed cotton blankets |
1000 per 1000 |
610 per 1000 (420 to 860) |
RR 0.61 (0.42 to 0.86) |
36 (1 study) |
⊕⊕⊝⊝ Lowb,c |
|
Length of stay: days Active vs warmed cotton blankets |
Mean length of hospital stay across control groups was 10.5 days | Mean length of stay in the intervention groups was 0.64 days lower (2.76 lower to 1.48 higher) | 130 (1 study) | ⊕⊕⊝⊝Lowa,b,c | |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; °C: degrees Celsius. | |||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aSerious risk of bias, downgraded 1 place: inadequate/unclear randomization methods, which could lead to selection bias. bBlinding was not possible, unclear what effect this has. Not downgraded. cSerious risk of imprecision, downgraded 1 place: wide confidence intervals where clinical action would differ if the extreme ends of the confidence interval were true. dNo events observed in either arm of one of the included trials. Not downgraded.
2.
| Thermal insulation compared with cotton blankets for treating postoperative hypothermia | |||||
|
Patient or population: adults with postoperative hypothermia Settings: postsurgical care Intervention: thermal insulation Comparison: cotton blanket | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Cotton blanket | Thermal insulation | ||||
|
Time to normothermia (minutes) Thermal vs warmed cotton blanket |
Mean time taken to achieve normothermia across control groups was 115.1 minutes |
Mean time taken to achieve normothermia in the intervention groups was 0.29 minutes lower (25.47 lower to 24.89 higher) |
138 (1 study) |
⊕⊕⊕⊝ Moderatea,b | |
| Mean temperature difference (60 minutes after warming started): not reported | See comment | See comment | Not estimable | ‐ | See comment |
|
Rate of rewarming: not reported |
See comment | See comment | Not estimable | ‐ | See comment |
| Major cardiovascular complications: not reported | See comment | See comment | Not estimable | ‐ | See comment |
|
Pain (number of patients) Thermal vs warmed cotton blanket |
674 per 1000 |
532 per 1000 (377 to 741) |
RR 0.79 (0.56 to 1.10) | 138 (1 study) |
⊕⊕⊕⊝ Moderatea,b |
|
Shivering (number of patients) Thermal vs warmed cotton blanket |
196 per 1000 |
266 per 1000 (135 to 741) |
RR 1.36 (0.69 to 2.67) | 138 (1 study) |
⊕⊕⊕⊝ Moderatea,b |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aBlinding was not possible, unclear what effect this has. Not downgraded. bSerious risk of imprecision, downgraded 1 place: wide confidence intervals where clinical action would differ if the extreme ends of the confidence interval were true.
Background
Description of the condition
Regulation of temperature
Body temperature is usually maintained at between 36ºC and 37.5ºC by balancing the body's heat loss and heat gain. Heat is gained as a product of metabolism, particularly that associated with muscular activity, and is lost through convection, conduction, radiation from the skin and evaporation by sweating and through the respiratory tract.
To maintain this balance, information from temperature sensors in deep tissues and the skin is processed by the brain. Heat loss is increased through sweating, increased blood flow through the skin and increased respiration. Heat loss is reduced by reducing blood flow through the skin, and increased heat production occurs mainly by inducing muscular activity (shivering) and increasing the basal metabolic rate (the background rate of energy used by a person at rest).
A useful concept in thinking about heat regulation is that the body has a central compartment comprising the major organs, where temperature is tightly regulated, and a peripheral compartment, where temperature varies widely. Typically, peripheral areas may be 2ºC to 4ºC cooler than the core compartment.
Effects of perioperative care and anaesthesia on thermal regulation
Exposure of the skin during the perioperative period can increase heat loss. Use of cool intravenous and irrigation fluids and inspired or insufflated (blown into body cavities) gases may directly cool patients. Sedatives and anaesthetic agents inhibit normal responses to cold, by which surface blood vessels are constricted, effectively resulting in greater blood flow to peripheral areas and increased heat loss. During the early stage of anaesthesia, these effects mean that core temperature decreases rapidly as a result of redistribution of heat from the central to the peripheral compartment, which is followed by a more gradual decline, reflecting ongoing heat loss.
With epidural or spinal analgesia, peripheral blockade of vasoconstriction (narrowing of the blood vessels) below the level of the nerve block results in vasodilatation (widening of the blood vessels) and greater ongoing heat loss.
Perioperative hypothermia complications
Hypothermia may result in increased morbidity by altering various systems and functions. Patients often comment on subsequent shivering upon awakening from anaesthesia as one of the most uncomfortable immediate postoperative experiences. Shivering originates as a response to cold and is the result of involuntary muscular activity with the objective of increasing metabolic heat (Sessler 2001).
Cardiac complications are the principal cause of morbidity during the postoperative phase. Prolonged ischaemia is usually associated with cellular damage. For this reason, treating factors that lead to ischaemia, such as low body temperature, seems likely to be important. Hypothermia stimulates the release of noradrenaline, which results in peripheral vasoconstriction and hypertension (Sessler 1991; Sessler 2001)—factors that favour or increase the chances of myocardial ischaemia (reduced blood supply to the heart). It appears that increased risk of cardiac complications can be reversed by maintenance of normothermia (Frank 1997).
Some studies have shown that intraoperative hypothermia accompanied by vasoconstriction constitutes an independent factor that slows wound healing and increases the risk of surgical wound infection (Kurz 1996; Melling 2001).
Even moderate hypothermia (35ºC) can alter physiological coagulation mechanisms by affecting platelet function and modifying enzymatic reactions. Decreased platelet activity produces increased bleeding and a greater need for transfusion (Rajagopalan 2008).
Moderate hypothermia can also reduce the metabolic rate, manifesting as a prolonged effect of certain drugs used during anaesthesia and some uncertainty about their effects. This is particularly significant for elderly patients (Heier 1991; Heier 2006; Leslie 1995).
For these reasons, inadvertent non‐therapeutic hypothermia is considered an adverse effect of general and regional anaesthesia (Bush 1995; Putzu 2007; Sessler 1991). Body temperature is therefore frequently monitored to aid in maintaining normothermia during surgery and to ensure timely detection of unintended hypothermia.
Postoperative rewarming after inadvertent hypothermia has occurred
The risk of inadvertent perioperative hypothermia varies widely, for example, reports from audits describe risks of 1.5% (Al‐Qahtani 2011) to 20% (Harper 2008). Patients who are most susceptible to heat loss include the elderly, patients with higher anaesthetic risk (American Society of Anesthesiologists (ASA) grade III to IV), people with cachexia (loss of body mass due to increased metabolic rate associated with cancer and other chronic conditions), burn victims, patients with hypothyroidism and those affected by corticoadrenal insufficiency.
Once hypothermia has occurred, it is important that an intervention is instituted to attempt to rewarm the patient as promptly as possible to minimize potential complications. As most patients are awake soon after their surgery is completed, it is important that any intervention is well tolerated and effective.
Description of the intervention
Once inadvertent postoperative hypothermia has occurred, it seems logical to reverse the situation. The mechanisms by which this might be achieved are similar to those used to prevent hypothermia. The objective is to minimize heat loss by reducing radiation and convection from the skin, evaporation from exposed areas and cooling caused by the introduction of cold intravenous fluids or irrigation fluids, or use of cold gases for respiration. Interventions used to maintain body temperature can be classified as follows.
Passive warming systems aimed at reducing heat loss and thus preventing hypothermia, including changes to environmental temperature, passive insulation by covering exposed body surface and closed or semiclosed anaesthesia circuits with low flows.
Active warming systems aimed at transferring heat to the patient. The effectiveness of these systems might depend on various factors such as the design of the machine, the type of heat transfer, placement of the system over the patient and the total body area covered in the heat exchange. The following systems are used for active warming: infrared lights, electric blankets, mattresses or blankets with warm water circulation, forced‐air warming or convective air warming transfer, warming of intravenous and irrigation fluids and warming and humidifying of anaesthetic air.
Interventions to decrease heat loss through distribution: pharmacological agents.
Intravenous nutrients. These have been proposed as a way of inducing increased metabolism and thus energy production.
Why it is important to do this review
The clinical effectiveness of the different types of patient warming devices that can be used has been assessed in a very extensive guideline commissioned in the UK by the National Institute for Health and Clinical Excellence (NICE) (NICE 2008). This report concludes that evidence of clinical effectiveness and cost‐effectiveness is sufficient for recommendations to be made on the use of forced air warming to prevent and treat perioperative hypothermia. Nevertheless, most of the data are derived from intermediate outcomes such as temperature. The search for evidence covers until year 2007, and so it may need updating.
This review serves as one of several reviews focused in this area. Cochrane reviews have been prepared to cover warming of gases used in minimally invasive abdominal surgery (Birch 2011) and use of warmed and humidified inspired gases in ventilated adults and patients (Kelly 2010) and a review on active warming is in preparation (Urrútia 2011). Areas remaining to be covered include the following.
Preoperative or intraoperative thermal insulation, or both.
Preoperative or intraoperative warming, or both, of intravenous and irrigation fluids.
Preoperative or intraoperative pharmacological interventions, or both, including intravenous nutrients.
Postoperative treatment of inadvertent hypothermia.
Although the interventions for treating inadvertent postoperative hypothermia are similar, they are not the same, and treating established hypothermia in an awake patient is very different from preventing its occurrence in the anaesthetised patient. Treatment of the patient in the postanaesthesia care unit (PACU) is complex, and feelings of pain, cold, nausea and shivering are all interlinked, so a clinically effective intervention is required to optimise the patient experience and to provide objective evidence of return to normothermia.
Objectives
To estimate the effectiveness of treating inadvertent perioperative hypothermia through postoperative interventions to decrease heat loss and apply passive and active warming systems in adult patients who have undergone surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) or quasi‐randomized controlled trials (such as allocation by alternation) of interventions used during the postoperative period.
Types of participants
We included adults (over 18 years of age) undergoing elective and emergency surgery (including surgery for trauma) under general or regional (central neuraxial block) anaesthesia, or both. Participants were eligible regardless of whether they were awake or were still anaesthetized, including those breathing spontaneously and those remaining ventilated.
The following groups were not included.
Patients who had been treated with therapeutic hypothermia (e.g. whilst undergoing cardiopulmonary bypass).
Patients undergoing operative procedures under local anaesthesia.
Patients with isolated severe head injuries resulting in impaired temperature control.
Interventions aimed solely at the treatment of shivering in the absence of hypothermia.
Types of interventions
This review considers any intervention aimed at restoring normal body temperature during the postoperative period compared with usual care or another intervention. Consideration is given only to interventions that were commenced in the immediate postoperative period in the PACU or in critical care and were applied until normothermia was reached.
Categories of intervention include the following.
Active warming—forced air warmers, electric mattresses and blankets, radiant heaters, warm water mattresses or blankets.
Thermal insulation or passive warming—reflective and non‐reflective blankets, suits and head covering.
Warming of Intravenous fluids by any method.
Irrigation fluids—few of these are applicable, although urology studies may utilize warmed fluid for bladder irrigation.
Warming of inspired gases—applicable only to ventilated patients, who may use a variety of humidifiers.
Pharmacological interventions—ketamine, calcium channel blockers, intravenous nutrients, opiates.
Studies reporting multiple co‐interventions (e.g. electric blanket plus warm inspired gases) were not eligible for inclusion, unless the same co‐intervention was used in both groups (e.g. electric blanket plus warm inspired gases vs electric blanket plus warm intravenous fluid).
Types of outcome measures
Primary outcomes
Time taken to achieve normothermia.
Mean temperature difference (either difference between means in the groups or the mean of within‐patient differences) after one hour of starting the intervention.
Rate of rewarming.
Major cardiovascular complications (cardiovascular death, non‐fatal myocardial infarction, non‐fatal stroke and non‐fatal cardiac arrest).
Secondary outcomes
Infection and complications of the surgical wound (wound healing and dehiscence) as defined by study authors.
Pressure ulcer.
Bleeding complications (blood loss, transfusions, coagulopathy).
Other cardiovascular complications (bradycardia, arrhythmias).
Patients' reported outcomes (i.e. anxiety, comfort in postsurgical wake‐up, etc.).
All‐cause mortality during the study period.
Length of stay (in postanaesthesia care unit, hospital).
Unplanned high dependency or intensive care admission.
Adverse effects—including temperature greater than 37.5°C, burns and feeling too hot.
Shivering.
Search methods for identification of studies
We conducted a single search across the suite of reviews on this topic (thermal insulation, warming of intravenous and irrigation fluids) with the following strategy, which was refined following a cross‐check with studies included in the NICE guideline on this topic (NICE 2008).
Electronic searches
To identify eligible randomized clinical trials, we searched the following electronic databases: the Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 1) (see Appendix 1); MEDLINE, Ovid SP (1956 to February 2014) (see Appendix 2); EMBASE, Ovid SP (1982 to February 2014) (see Appendix 3); Institute for Scientific Information (ISI) Web of Science (1950 to February 2014) (see Appendix 4); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL), EBSCO host (1980 to February 2014) (see Appendix 5). In searching these databases, we used both subject headings and free‐text terms with no language or date restrictions. We adapted our MEDLINE search strategy for searching all other databases.
Searching other resources
To identify additional published, unpublished and ongoing studies, we searched the Science Citation Index and checked the references of relevant studies and reviews. We also searched the databases of ongoing trials, such as the following.
.
Data collection and analysis
Selection of studies
For new searches, we (SW, PA and GC) independently sifted the results of literature searches to identify relevant studies such that each record was reviewed by two people. This was done once for all interventions, and the intervention was recorded on a data extraction form (see Appendix 6). If an article could not be excluded by review of the title and abstract, we retrieved a full copy of the article. Reasons for exclusion were recorded. Disagreements about inclusion or exclusion were resolved by discussion involving another review author (AS) if necessary.
Data extraction and management
We (SW, PA and GC) extracted relevant data independently onto a data extraction form (see Appendix 6), resolving disagreements by discussion or by referral to a clinical expert (AS).
SW entered data into RevMan, and PA and GC checked for transcription errors.
The following data were extracted.
General information, such as title, authors, contact address, publication source, publication year, country.
Methodological characteristics and study design.
Clinical and demographic characteristics of study participants.
Description of the intervention and the control. We collected information about type and duration of surgery, surgical team experience and prophylactic antibiotic administration when available.
Outcome measures as noted above.
Results for each study group.
Assessment of risk of bias in included studies
SW, PA and GC independently assessed risk of bias for each study (those included in the NICE guideline and newly identified studies) using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Disagreements were resolved by discussion or by involving another review author (AS).
Trials were considered as having low risk of bias when all of the following criteria were assessed as adequate. Trials were considered as having high risk of bias when one or more of the following criteria were not assessed as adequate.
Random sequence generation (checking for possible selection bias). We describe for each included study the method used to generate the allocation sequence when it is provided in sufficient detail to allow an assessment of whether it should produce comparable groups. We assess these methods as adequate (any truly random process, e.g. random number table, computer random number generator); inadequate (any non‐random process, e.g. odd or even date of birth, hospital or clinic record number); or unclear.
Allocation concealment (checking for possible selection bias). We describe for each included study the method used to conceal the allocation sequence when it is provided in sufficient detail, and we determine whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment. We assess these methods as adequate (e.g. telephone or central randomization, consecutively numbered sealed opaque envelopes); inadequate (open random allocation, unsealed or non‐opaque envelopes, alternation, date of birth); or unclear.
Blinding of participants, personnel and outcome assessors (checking for possible performance and detection bias). We describe for each included study the methods used, if any, to blind participants, personnel and outcome assessors from knowledge of which intervention a participant received. When blinding is not possible, we assess whether lack of blinding was likely to have introduced bias. Blinding is assessed separately for different outcomes or classes of outcomes. We assess these methods as adequate; inadequate; or unclear.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). We describe for each included study and for each outcome the completeness of data, including attrition and exclusions from the analysis. We state whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total number of randomized participants), reasons for attrition or exclusion when reported and whether missing data were balanced across groups or were related to outcomes. When sufficient information is reported or can be supplied by the trial authors, we reinclude missing data in the analyses that we undertake. We consider intention‐to‐treat as adequate when all dropouts or withdrawals were accounted for, and as inadequate when the number of dropouts or withdrawals was not stated, or when the reasons for dropouts or withdrawals were not stated.
Selective reporting. We report for each included study which outcomes of interest are and are not reported. We did not search for trial protocols.
Other bias. We describe for each included study any important concerns that we have about other possible sources of bias. We assess whether each study was free of other problems that could put it at risk of bias: yes; no; or unclear.
With reference to (1) to (6) above, we considered the likely magnitude and direction of the bias when interpreting the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses (see Sensitivity analysis).
Measures of treatment effect
Dichotomous data were analysed using risk ratios with 95% confidence intervals. Continuous data were analysed using mean differences and 95% confidence intervals.
Unit of analysis issues
All trials were randomized by individual, and outcome data were reported for participants.
Dealing with missing data
We analysed available data on an intention‐to‐treat (ITT) basis.
Assessment of heterogeneity
Before obtaining pooled estimates of relative effects, we carried out a statistical heterogeneity analysis by assessing the value of the I2 statistic, thereby estimating the percentage of total variance across studies that is due to heterogeneity rather than to chance (Higgins 2002). We considered a value greater than 30% as a sign of important heterogeneity, and, if present, we sought an obvious explanation for the heterogeneity by considering the design of the trials. We then proceeded to a meta‐analysis only when the direction of effect was the same for all point estimates.
Assessment of reporting biases
We recorded the number of included studies that report each outcome, but we did not use any statistical techniques to try to identify the presence of publication bias. We planned that if we identified more than 10 studies for a comparison, we would generate a funnel plot and analyse it by visual inspection.
Data synthesis
We used DerSimonian and Laird random‐effects model meta‐analyses of risk ratios in RevMan 5.2 for dichotomous data and weighted mean differences for continuous data. Pooled estimates include a 95% confidence interval.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses.
Type of anaesthesia (general or combined anaesthesia vs exclusively regional anaesthesia).
Duration of anaesthesia (less than 30 minutes, 30 to 60 minutes, longer than 60 minutes).
Participant age (older people age >80 years vs younger age < 80 years).
Pregnant women.
ASA score (ASA I and II vs ASA III and IV).
Urgency of surgery (elective vs non‐elective).
Type of surgery (body cavity surgery vs peripheral surgery).
Awake participants in PACU vs ventilated participants on critical care.
Variation in definitions of outcomes.
However, insufficient data were available for performance of planned subgroup analyses.
Sensitivity analysis
We planned to carry out sensitivity analysis according to the methodological study quality (including only trials with low risk of bias) but did not do this because variation in the risk of bias in included studies was lacking.
Summary of findings tables
We constructed 'Summary of findings' tables for the comparison between active warming and control (Table 1), and for the comparison between thermal insulation and control (Table 2). These tables contain all three primary outcomes (regardless of whether or not we found useful data) and secondary outcomes when data were available, as these comparisons were believed to yield the most useful data. All evidence included in the 'Summary of findings' tables is given a GRADE (Grading of Recommendation, Assessment, Development and Evaluation) quality rating.
'Summary of findings' tables were not constructed for comparisons of active warming versus active warming or active warming versus thermal insulation because available data were limited. No GRADE quality ratings are provided for evidence related to these comparisons.
Results
Description of studies
Results of the search
The search for this review was carried out as part of a single search for three related reviews on prevention and treatment of perioperative hypothermia (thermal insulation, warming of irrigation and intravenous fluids). Figure 1 summarizes the search results, as combined for searches conducted in June 2011, June 2012, February 2013, November 2013 and February 2014. These searches yielded a total of 4094 hits. For this review, we retrieved 17 papers for consideration and included 11. We tried to contact the authors of three studies (Bredahl 1995; Ciufo 1995; Grossman 2002) to clarify details, but we were unable to contact them or they were not able to provide further information.
1.

Study flow diagram.
Included studies
We included 11 studies (Characteristics of included studies), eight comparing active warming versus control (Alfonsi 2003; Ereth 1992; Giuffre 1991; Grossman 2002; Summers 1990; Weyland 1994a; Weyland 1994b; Yang 2012), one comparing thermal insulation versus control (Hershey 1997), one comparing active warming versus thermal insulation (Bredahl 1995) and another comparing different methods of active warming (Ciufo 1995).
Several trials comparing active warming versus control included two different active warming groups, which were averaged so that results could be entered into the meta‐analysis, and one study (Alfonsi 2003) did not report data in relation to any of the specified outcomes and therefore could not be entered into any analyses.
All studies included participants undergoing general anaesthesia for a range of procedures, but outcome reporting was poor. Time to normothermia, mean temperature difference and shivering were most commonly reported, but not all studies reported all outcomes. Some trials reported means with ranges rather than standard deviations. When this occurred, ranges were converted to standard deviations to enable entry of all data into the meta‐analysis using the formula (min‐max)/4.
Excluded studies
We excluded six studies (Characteristics of excluded studies) because the presence of hypothermia was not clearly reported, because participants were deliberately cooled, because warming was started intraoperatively or because multiple interventions were used.
Risk of bias in included studies
Summaries of risk of bias judgements are provided in Figure 2 and Figure 3.
2.
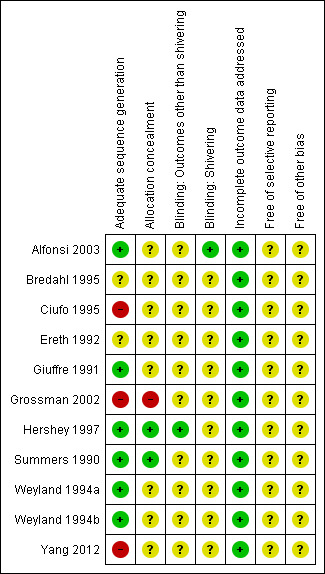
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
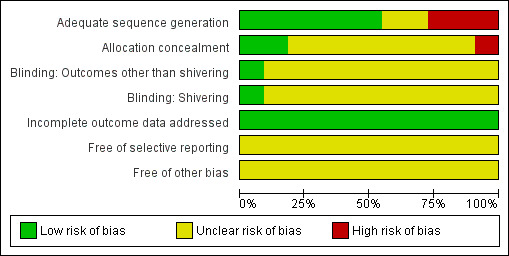
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Some trials used inappropriate randomization methods or did not report sufficient information to allow a judgement to be made, so it is unclear whether selection bias is present, and what effect this may have on the results.
Blinding
In general it was not possible to blind participants or investigators to the warming intervention; therefore potential biases in temperature recording may be present, but the direction that this effect would have is unclear.
Incomplete outcome data
Trials included in this review are relatively short in duration, and no serious issues with attrition were identified.
Selective reporting
Trial protocols were not obtained and reviewed, so no definitive reporting biases were identified. Studies often did not report the outcomes chosen for this review, but it is unclear whether these data were actually collected.
Other potential sources of bias
No other definitive sources of potential bias were identified.
Effects of interventions
Active warming compared with control
Primary outcomes
1. Time to achieve normothermia
Four trials (Giuffre 1991; Grossman 2002; Weyland 1994a; Yang 2012) compared active warming methods (forced air warming, circulating hot water devices, radiant blankets, radiant warmers, electric blankets) versus cotton blankets (see Analysis 1.1). When a single trial reported more than one active warming method in comparison with a single control, the active warming groups were combined to enable entry of the results into a single meta‐analysis. Overall, active warming resulted in a shorter time to normothermia than was reported for the control. Some heterogeneity was observed in the results, with I² of 69%. As differences in the type of control group were noted, it was decided to undertake separate analyses of active warming in comparison with unwarmed blankets, and active warming in comparison with warmed blankets. Tests for subgroup differences were statistically significant (Chi² = 9.45, df = 1, P value 0.00, I² = 89%) and fully accounted for the heterogeneity observed in the results. Subgroup analyses showed statistically significant differences between active warming and control groups in favour of active warming. As would be expected, the observed difference was greater between active and unwarmed blankets (MD ‐88.86 minutes, 95% CI ‐123.49 to ‐54.23; moderate‐quality evidence) than between active and warmed blankets (MD ‐32.13 minutes, 95% CI ‐42.55 to ‐21.71; moderate‐quality evidence).
1.1. Analysis.

Comparison 1 Active vs Control, Outcome 1 Time to achieve normothermia.
2. Mean temperature difference (after 60 minutes of warming)
Two trials (Ereth 1992; Summers 1990) compared forced air warming versus warmed blankets (see Analysis 1.2). No statistically significant differences were found between the two interventions in mean temperature after 60 minutes of warming (MD 0.18, 95% CI ‐0.10 to 0.46; moderate‐quality evidence).
1.2. Analysis.
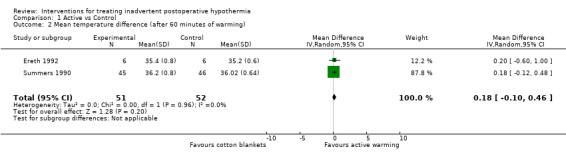
Comparison 1 Active vs Control, Outcome 2 Mean temperature difference (after 60 minutes of warming).
3. Rate of rewarming
This outcome was not prespecified in our protocol, but we included it, as we believed it measured the effect of rewarming on temperature. One trial (Weyland 1994b) compared two methods of active warming (n = 12) versus cotton blankets (n = 12) and reported rate of rewarming in terms of degrees Celsius per hour. No significant differences were noted in rate of rewarming between participants warmed with active methods (M = 0.78°C per hour, SD = 0.57) and those warmed with cotton blankets (M = 0.70°C per hour, SD = 0.52, MD = 0.08°C per hour, 95% CI ‐0.30 to 0.46).
4. Major cardiovascular complications
No trials included in the comparison between active warming and control reported this outcome.
Secondary outcomes
1. Length of stay (hospital stay)
One trial (Yang 2012) comparing radiant warming (n = 65) versus warmed blankets (n = 65) reported length of hospital stay. Mean hospital stay was 9.82 days (SD = 7.0) in the radiant warming group and 10.46 days (SD = 5.18) in the warmed blanket group, and the difference between groups was not statistically significant (MD ‐0.64 days, 95% CI ‐2.76 to 1.48; low‐quality evidence).
2. Participants' reported outcomes: comfort
One trial (Summers 1990) comparing forced air warming (n = 45) versus warmed blankets (n = 46) reported the Christop Comfort Scale, which was assessed on a 20‐cm visual analogue scale (where 0 = miserably cold, 10 = comfortable and 20 = miserably hot). At 30 minutes after admission to the PACU, participants warmed with forced air warming had a mean comfort score of 17.05 (SD = 2.94), and participants warmed with blankets had a mean comfort score of 9.20 (SD = 2.70), indicating that the blanket group was more comfortable. At discharge, results were similar, with the forced air warming group reporting scores towards uncomfortably hot (mean = 17.66, SD = 2.34) compared with the blanket group for whom scores were closer towards comfortable (mean = 10.26, SD = 1.71).
3. Shivering
Three trials (Ereth 1992; Summers 1990; Weyland 1994a) comparing active warming methods versus warmed and unwarmed blankets reported shivering (see Analysis 1.3). A statistically significant lower risk of shivering was noted in the active warming group versus the unwarmed blanket group (RR 0.61, 95% CI 0.42 to 0.86; low‐quality evidence). However, no statistically significant difference in risk of shivering was noted when active warming was compared with warmed blankets (RR 0.95, 95% CI 0.52 to 1.74; low‐quality evidence).
1.3. Analysis.
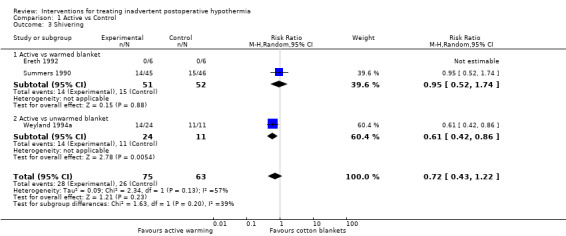
Comparison 1 Active vs Control, Outcome 3 Shivering.
4. Secondary outcomes not reported
No trials included in the comparison between active warming and control reported infection and complications of the wound, pressure ulcer, bleeding complications, other cardiovascular complications, all‐cause mortality, unplanned high dependency or intensive care admission and adverse effects.
Thermal insulation compared with control
Primary outcomes
1. Time to achieve normothermia
One trial (Hershey 1997) compared reflective coverings (n = 92) versus warmed cotton blankets (n = 46). Mean time to normothermia was 114.81 minutes (SD = 74.12) in the reflective coverings group and 115.1 minutes (SD = 69.6) in the cotton blanket group. No statistically significant differences were found between the two interventions (MD ‐0.29 minutes, 95% CI‐25.47 to 24.89; moderate‐quality evidence).
2. Mean temperature difference
No trials comparing thermal insulation versus control reported this outcome.
3. Rate of rewarming
No trials comparing thermal insulation versus control reported this outcome.
4. Major cardiovascular complications
No trials comparing thermal insulation versus control reported this outcome.
Secondary outcomes
1. Pain
One trial (Hershey 1997) reported that 59/94 participants in the reflective blanket group and 31/46 participants in the cotton blanket group required pain medication. No statistically significant differences were noted between the two groups (RR 0.79, 95% CI 0.56 to 1.10; moderate‐quality evidence).
2. Shivering
One trial (Hershey 1997) also reported that 25/94 participants in the reflective blanket group and 9/46 in the cotton blanket group shivered. No statistically significant differences in risk of shivering were noted between groups (RR 1.36, 95% CI 0.69 to 2.67; moderate‐quality evidence).
3. Secondary outcomes not reported
None of the trials included in the comparison between thermal insulation and control reported infection and complications of the wound, pressure ulcer, bleeding complications, other cardiovascular complications, participants' reported outcomes, length of stay, all‐cause mortality, unplanned high dependency or intensive care admission or adverse effects.
Active warming compared with thermal insulation
Primary outcomes
1. Time taken to achieve normothermia
No trials comparing active warming versus thermal insulation reported this outcome.
2. Mean temperature difference (after 60 minutes of warming)
One trial (Bredahl 1995) compared radiant warming (n = 15) versus reflective blankets (n = 15). This study reported that median temperature was 35.5°C for both groups, and provided interquartile ranges on a graph. The authors of the trial concluded that no statistically significant differences were evident between the groups.
3. Rate of rewarming
No trials comparing active warming versus thermal insulation reported this outcome.
4. Major cardiovascular complications
No trials comparing active warming versus thermal insulation reported this outcome.
Secondary outcomes
1. Shivering
One trial (Bredahl 1995) reported that 8/15 in the radiant warming group and 9/15 in the thermal insulation group shivered. No statistically significant differences in risk of shivering were noted between the two groups (RR 0.76, 95% CI 0.18 to 3.24).
2. Secondary outcomes not reported
None of the trials included in the comparison between active warming and thermal insulation reported infection and complications of the wound, pressure ulcer, bleeding complications, other cardiovascular complications, participants' reported outcomes, length of stay, all‐cause mortality, unplanned high dependency or intensive care admission or adverse effects.
Active warming compared with active warming
Primary outcomes
1. Time to achieve normothermia
Two trials (Ciufo 1995; Grossman 2002) compared forced air warming versus circulating hot water devices. A statistically significant difference in time to achieve normothermia favoured forced air warming (see Analysis 2.1; MD ‐54.21 minutes, 95% CI ‐94.95 to ‐13.47). Another trial (Giuffre 1991) compared forced air warming versus radiant heaters and also found a statistically significant difference in time to normothermia in favour of forced air warming (MD ‐36.50 minutes, 95% CI ‐71.36 to ‐1.64).
2.1. Analysis.
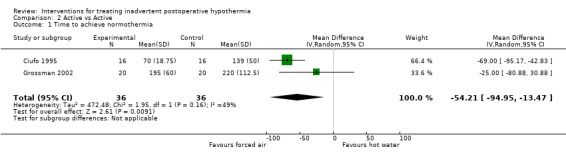
Comparison 2 Active vs Active, Outcome 1 Time to achieve normothermia.
One trial (Weyland 1994a) compared radiant heaters versus electric blankets and found statistically significant differences in time to normothermia in favour of radiant heaters (MD ‐83.00 minutes, 95% CI ‐113.37 to ‐52.63).
2. Mean temperature difference
No trials comparing different active warming methods reported this outcome.
3. Rate of rewarming
No trials comparing different active warming methods reported this outcome.
4. Major cardiovascular complications
No trials comparing different active warming methods reported this outcome.
Secondary outcomes
1. Shivering
One trial (Weyland 1994a) comparing radiant heater versus electric blanket reported that 2/12 participants in the radiant heater group and 9/12 in the electric blanket group shivered. A statistically significant difference was noted in risk of shivering, in which participants warmed with the radiant heater had a lower risk of shivering than those warmed with the electric blanket (RR 0.22, 95% CI 0.06 to 0.82).
2. Secondary outcomes not reported
None of the trials included in the comparison between different active warming methods reported infection and complications of the wound, pressure ulcer, bleeding complications, other cardiovascular complications, participants' reported outcomes, length of stay, all‐cause mortality, unplanned high dependency or intensive care admission or adverse effects (secondary outcomes).
Discussion
Summary of main results
Overall limited evidence was available. Most available evidence was related to comparisons between active warming and cotton blanket control, in which active warming was found to reduce the time taken to achieve normothermia by about one hour in comparison with warmed or unwarmed cotton blankets; this is likely to be clinically significant. A small quantity of data was available for the outcomes of mean temperature (60 minutes after warming started), rate of rewarming, shivering and length of stay, but no evidence showed an effect of active warming on these outcomes.
In comparisons of thermal insulation versus cotton blanket control, and of active warming versus thermal insulation, the small amount of available evidence suggested no effect of the interventions on any of the reported outcomes. It is unclear whether the lack of effect is due to lack of available evidence, or to the fact that no differences were observed between the various warming methods in terms of reported outcomes.
Evidence on comparisons of different active warming methods versus each other was insufficient to enable conclusions to be drawn about which active warming method is superior, although some evidence was available, suggesting that forced air warming reduced the time needed to achieve normothermia by about one hour in comparison with circulating hot water devices.
No evidence was available on the relation to any of the warming interventions for some of the primary and secondary outcomes that we wished to explore.
Thus meaningful conclusions about active warming on outcomes other than time to achieve normothermia and about thermal insulation on any outcome cannot be drawn.
Overall completeness and applicability of evidence
Evidence was limited overall, and reporting of outcomes other than those related to temperature was limited in the available evidence. These results are likely to be applicable to a range of surgical situations for a variety of patients, but subgroup analysis was not possible; therefore it is unclear whether the effects of rewarming methods are different for different subgroups of patients.
Quality of the evidence
Most of the included studies were considered to be of low quality and at risk of bias. This assessment is mainly due to unclear or inappropriate randomization methods and to the fact that it was not possible to blind participants or assessors to the warming method to which participants were randomly assigned. It is unclear what effect this has on the results. In addition, studies were generally very small; therefore it is likely that they were underpowered to detect meaningful differences in outcomes, especially rare events (such as mortality).
Potential biases in the review process
Some decisions about data analysis, particularly regarding inclusion of the outcomes 'rate of rewarming,' 'shivering' and 'pain,' and investigations of heterogeneity were undertaken after data were reviewed; this may have introduced bias. Outcomes in the 'Summary of findings' tables were chosen after results of the studies were noted.
Agreements and disagreements with other studies or reviews
The NICE guideline recommends the use of forced air warming, a method of active warming, for patients with inadvertent postoperative hypothermia (NICE 2008), and our findings do not contradict that recommendation. The guideline was based on modelling of the effects of temperature differences on important patient outcomes and an economic analysis, and we have not attempted to replicate that.
Authors' conclusions
Implications for practice.
Active warming appears to reverse the effects of postoperative hypothermia more quickly than those of standard hospital cotton blankets. It is not clear whether some active warming methods are better than others. These results do not contradict the recommendations of the NICE guideline.
Implications for research.
Currently high‐quality evidence on important clinical outcomes is lacking; therefore it is unclear whether active warming confers other benefits and harms, or whether other rewarming methods are effective in reversing postoperative hypothermia.
History
Protocol first published: Issue 6, 2012 Review first published: Issue 11, 2014
| Date | Event | Description |
|---|---|---|
| 7 August 2012 | Amended | Typo corrected |
Acknowledgements
This review builds on the work undertaken as part of the UK National Institute of Health and Care Excellence (NICE) clinical guideline on inadvertent perioperative hypothermia (NICE 2008), and we would like to acknowledge the work of this group.
We would like to thank Anna Lee (content editor); Cathal Walsh and Nathan Pace (statistical editors); Oliver Kimberger, Janneke Horn and Rainer Lenhardt (peer reviewers); and Anne Lyddiatt (consumer) for help and editorial advice provided during the preparation of this systematic review.
Appendices
Appendix 1. Search strategy for CENTRAL
#1 MeSH descriptor Rewarming explode all trees #2 (intervention* adj3 treat*):ti,ab or vasodilatat* or infrared light* or intravenous nutrient* or warming system* or ((Mattress* or blanket*) near (warm water or Electric)) or (warm* near (air or CO2 or fluid* or an?esthetic* or IV or gas* or device* or patient* or passive* or active* or skin or surg*)) or (warming or blanket*):ti,ab or pharmacological agent* or thermal insulat* or pre?warm* or re?warm* #3 (#1 OR #2) #4 MeSH descriptor Hypothermia explode all trees #5 MeSH descriptor Body Temperature Regulation explode all trees #6 MeSH descriptor Shivering explode all trees #7 hypo?therm* or normo?therm* or thermo?regulat* or shiver* or ((thermal or temperature) near (regulat* or manage* or maintain*)) or (low* near temperature*) or thermo?genesis or ((reduc* or prevent*) and temperature and (decrease or decline)) or (heat near (preserv* or loss or retention or retain* or balance)) or (core near (thermal or temperature*)) #8 (#4 OR #5 OR #6 OR #7) #9 (#3 AND #8)
Appendix 2. Search strategy for MEDLINE (Ovid SP)
1. Rewarming/ or (intervention* adj3 treat*).ti,ab. or vasodilatat*.mp. or infrared light*.mp. or intravenous nutrient*.mp. or warming system*.mp. or ((Mattress* or blanket*) adj3 (warm water or Electric)).mp. or (warm* adj3 (air or CO2 or fluid* or an?esthetic* or IV or gas* or device* or patient* or passive* or active* or skin or surg*)).mp. or (warming or blanket*).ti,ab. or pharmacological agent*.mp. or thermal insulat*.mp. or (pre?warm* or re?warm*).mp. 2. exp Hypothermia/ or exp body temperature regulation/ or exp piloerection/ or exp shivering/ or hypo?therm*.af. or normo?therm*.mp. or thermo?regulat*.mp. or shiver*.mp. or ((thermal or temperature) adj2 (regulat* or manage* or maintain*)).mp. or (low* adj2 temperature*).mp. or thermo?genesis.mp. or ((reduc* or prevent*).af. and (temperature adj3 (decrease or decline)).mp.) or (heat adj2 (preserv* or loss or retention or retain* or balance)).mp. or (core adj2 (thermal or temperature*)).mp. 3. 1 and 2 4. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 3. Search strategy for EMBASE (Ovid SP)
1. warming/ or (intervention* adj3 treat*).ti,ab. or vasodilatat*.mp. or infrared light*.mp. or intravenous nutrient*.mp. or warming system*.mp. or ((Mattress* or blanket*) adj3 (warm water or Electric)).mp. or (warm* adj3 (air or CO2 or fluid* or an?esthetic* or IV or gas* or device* or patient* or passive* or active* or skin or surg*)).mp. or (warming or blanket*).ti,ab. or pharmacological agent*.mp. or thermal insulat*.mp. or (pre?warm* or re?warm*).mp. 2. exp HYPOTHERMIA/ or exp thermoregulation/ or reflex/ or exp SHIVERING/ or hypo?therm*.af. or normo?therm*.mp. or thermo?regulat*.mp. or shiver*.mp. or ((thermal or temperature) adj2 (regulat* or manage* or maintain*)).mp. or (low* adj2 temperature*).mp. or thermo?genesis.mp. or ((reduc* or prevent*).af. and (temperature adj3 (decrease or decline)).mp.) or (heat adj2 (preserv* or loss or retention or retain* or balance)).mp. or (core adj2 (thermal or temperature*)).mp. 3. 1 and 2 4. (placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 4. Search strategy for ISI Web of Science
#1 TS=((hypo?therm* or normo?therm* or thermo?regulat* or shiver*) or ((thermal or temperature) SAME (regulat* or manage* or maintain*)) or (low* SAME temperature*) or thermo?genesis or ((reduc* or prevent*) and temperature and (decrease or decline)) or (heat SAME (preserv* or loss or retention or retain* or balance)) or (core SAME (thermal or temperature*))) #2 TS=((intervention* SAME treat*) or (vasodilatat* or infrared light* or intravenous nutrient* or warming system*) or ((Mattress* or blanket*) SAME (warm water or Electric)) or (warm* and (air or CO2 or fluid* or an?esthetic* or IV or gas* or device* or patient* or passive* or active* or skin or surg*))) or TI=(warming or blanket*) or TI=(pharmacological agent* or thermal insulat* or pre?warm* or re?warm*) #3 #1 and #2 #4 TS=(random* or (trial* SAME (control* or clinical*)) or placebo* or multicenter* or prospective* or ((blind* or mask*) SAME (single or double or triple or treble))) #5 #3 and #4
Appendix 5. Search strategy for CINAHL (EBSCO host)
S1 (MM "Warming Techniques") S2 vasodilatat* or infrared light* or intravenous nutrient* or warming system* S3 intervention* N3 treat* S4 ((Mattress* or blanket*) and (warm water or Electric)) S5 (warm* and (air or CO2 or fluid* or an?esthetic* or IV or gas* or device* or patient* or passive* or active* or skin or surg*)) S6 AB warming or blanket* S7 AB pharmacological agent* S8 TI thermal insulat* or AB (pre?warm* or re?warm*) S9 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 S10 (MM "Hypothermia") OR (MM "Body Temperature Regulation") OR (MM "Shivering") S11 hypo?therm* or normo?therm* or thermo?regulat* or shiver* S12 AB ((thermal or temperature) and (regulat* or manage* or maintain*)) S13 low* N3 temperature* S14 ( reduc* or prevent* ) and temperature and ( decrease or decline ) S15 thermogenesis S16 heat N3 (preserv* or loss or retention or retain* or balance) S17 core N3 (thermal or temperature*) S18 S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 S19 S9 and S18
Appendix 6. Data extraction form
| Cochrane Anaesthesia Review Group Study selection, quality assessment & data extraction form Interventions for treating inadvertent perioperative hypothermia |
Code of paper: |
|
| Reviewer initials: | Date: | |
| First author | Journal/Conference proceedings, etc | Year |
| |
Study eligibility
| RCT/Quasi/CCT (delete as appropriate) | Relevant participants | Relevant interventions | Relevant outcomes |
| Yes/No/Unclear |
Yes/No/Unclear |
Yes/No/Unclear |
Yes/No*/Unclear |
* Issue relates to selective reporting: When authors may have taken measurements for particular outcomes but not reported these within the paper(s). Reviewers should contact trialists for information on possible non‐reported outcomes & reasons for exclusion from publication. Study should be listed in ‘Studies awaiting assessment’ until clarified. If no clarification is received after three attempts, study should be excluded.
| Do not proceed if any of the above answers is ‘No.’ If study to be included in ‘Excluded studies’ section of the review, record below the information to be inserted into the ‘Table of excluded studies’ |
| |
| Freehand space for comments on study design and treatment: |
Methodological quality
| Allocation of intervention | ||
| State here method used to generate allocation and reasons for grading (quote) | Grade (circle) | |
| Page number | Adequate (random) | |
| Inadequate (e.g. alternate) | ||
| Unclear | ||
|
Concealment of allocation Process used to prevent foreknowledge of group assignment in an RCT, which should be seen as distinct from blinding | ||
| State here method used to conceal allocation and reasons for grading (quote) | Grade (circle) | |
| Page number | Adequate | |
| Inadequate | ||
| Unclear | ||
| Blinding | Page number | |
| Person responsible for participants' care | Yes/No | |
| Participant | Yes/No | |
| Outcome assessor | Yes/No | |
| Other (please specify) | Yes/No | |
|
Intention‐to‐treat An intention‐to‐treat analysis is one in which all participants in a trial are analysed according to the intervention to which they were allocated, whether or not they received it | ||
| Number of participants entering trial | ||
| Number excluded | ||
| % excluded (more or less than 15%) | ||
| Not analysed as ‘intention‐to‐treat’ | ||
| Unclear | ||
| Were withdrawals described? | Yes/No/Not clear | |
| Free text: | ||
Participants and trial characteristics
| Participant characteristics | ||
| Further details | Page number | |
| Age (mean, median, range, etc.) | ||
| Sex of participants (numbers/%, etc.) | ||
| Trial characteristics | ||
| Further details | Page number | |
| Single‐centre/multi‐centre | ||
| Country/countries | ||
| How was participant eligibility defined? | ||
| How many people were randomly assigned? | ||
| How many people were analysed? | ||
| Control group (size and details, e.g. 2 cotton blankets + fluid warmer + HME) | ||
| Intervention group 1 (size and details) | ||
| Intervention group 2 (size and details) | ||
| Intervention group 3 (size and details) | ||
| Time treatment applied (e.g. 30 minutes preoperatively) | ||
| Duration of treatment (mean ± SD) | ||
| Total anaesthetic time | ||
| Duration of follow‐up | ||
| Time points when measurements were taken during the study | ||
| Time points reported in the study | ||
| Time points you are using in RevMan | ||
| Trial design (e.g. parallel/cross‐over*) | ||
| Other | ||
* If cross‐over design, please refer to the Cochrane Editorial Office for further advice on how to analyse these data.
| Relevant outcomes | ||
| Reported in paper (circle) | Page number | |
| Infection and complications of surgical wound | Yes/No | |
| Major CVS complications (CVS death, MI, CVA) | Yes/No | |
| Risk of hypothermia (core temperature) | Yes/No | |
| Pressure ulcers | Yes/No | |
| Bleeding complications | Yes/No | |
| Other CVS complications (arrhythmias, hypotension) | Yes/No | |
| Participant‐reported outcomes (shivering, discomfort) | Yes/No | |
| All‐cause mortality | Yes/No | |
| Adverse effects | Yes/No | |
| Relevant subgroups | Page number | |
| Age > 80 | Yes/No | |
| Pregnancy | Yes/No | |
| ASA scores | Yes/No | |
| Urgency | Yes/No | |
Subgroups
Number of participants
| Age > 80 | Pregnant | Elective | Urgent | ASA I or II | ASA III or IV | |
| Control | ||||||
| Intervention 1 | ||||||
| Intervention 2 | ||||||
| Intervention 3 | ||||||
| | ||||||
| Free text: | ||||||
| For continuous data | ||||||||||||||||
| Code of paper |
Outcomes |
Unit of measurement |
Control group | Intervention 1 (thermal insulation) | Intervention 2 | Intervention 3 | ||||||||||
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |||||||||
| Temperature at end of surgery | Degrees C | |||||||||||||||
| Temperature at ................. | Degrees C | |||||||||||||||
| Time taken to achieve normothermia | Minutes | |||||||||||||||
| Rate of temperature change | Degrees C per minute | |||||||||||||||
| Number of units of red cells transfused | Units | |||||||||||||||
| For dichotomous data (n = number of participants) | ||||||||||||||||
| Code of paper |
Outcomes |
Control group | Intervention 1 (thermal insulation) | Intervention 2 | Intervention 3 | Free text | ||||||||||
| n | n | n | n | |||||||||||||
| Wound complications | ||||||||||||||||
| Major CVS complications (CVS death, non‐fatal MI, non‐fatal CVA and non‐fatal arrest) | ||||||||||||||||
| Bleeding complications (coagulopathy) | ||||||||||||||||
| Pressure ulcers | ||||||||||||||||
| Other CVS complications (hypotension, bradycardia, hypotension) | ||||||||||||||||
|
Other information that you feel is relevant to the results Indicate whether any data were obtained from the primary author; whether results were estimated from graphs, etc. or were calculated by you using a formula (this should be stated and the formula given). In general, if results not reported in paper(s) are obtained, this should be made clear here to be cited in the review. |
| |
| Freehand space for writing actions such as contact with study authors and changes |
References to trial
Check other references identified by searches. If there are further references to this trial, link the papers now and list below. All references to a trial should be linked under one Study ID in RevMan.
| Code each paper | Author(s) | Journal/Conference proceedings, etc. | Year |
References to other trials
| Did this report include any references to published reports of potentially eligible trials not already identified for this review? | ||
| First author | Journal/Conference | Year of publication |
| Did this report include any references to unpublished data from potentially eligible trials not already identified for this review? If yes, list contact names and details | ||
| | ||
Data and analyses
Comparison 1. Active vs Control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to achieve normothermia | 4 | 315 | Mean Difference (IV, Random, 95% CI) | ‐50.08 [‐79.71, ‐20.46] |
| 1.1 Active vs unwarmed blankets | 2 | 95 | Mean Difference (IV, Random, 95% CI) | ‐88.86 [‐123.49, ‐54.23] |
| 1.2 Active vs warmed blankets | 2 | 220 | Mean Difference (IV, Random, 95% CI) | ‐32.13 [‐42.55, ‐21.71] |
| 2 Mean temperature difference (after 60 minutes of warming) | 2 | 103 | Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.10, 0.46] |
| 3 Shivering | 3 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.43, 1.22] |
| 3.1 Active vs warmed blanket | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.52, 1.74] |
| 3.2 Active vs unwarmed blanket | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.42, 0.86] |
Comparison 2. Active vs Active.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to achieve normothermia | 2 | 72 | Mean Difference (IV, Random, 95% CI) | ‐54.21 [‐94.95, ‐13.47] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alfonsi 2003.
| Methods | Single‐centre RCT conducted in France | |
| Participants | 18 males 18 to 40 years of age, ASA I and II, undergoing knee or shoulder arthroscopy | |
| Interventions | Forced air cover (n = 9) Single cotton blanket (n = 9) |
|
| Outcomes | Tympanic and skin temperature, fingertip blood flow, oxygen consumption | |
| Notes | No usable outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Randomization was based on computer‐generated codes maintained in sequentially numbered opaque envelopes |
| Allocation concealment | Unclear risk | Not described |
| Blinding Outcomes other than shivering | Unclear risk | Not described |
| Blinding Shivering | Low risk | Investigators recording shivering were blinded to skin and core temperature |
| Incomplete outcome data addressed All outcomes | Low risk | No loss to follow‐up |
| Free of selective reporting | Unclear risk | Not assessed |
| Free of other bias | Unclear risk | No other concerns |
Bredahl 1995.
| Methods | Single‐centre randomized controlled trial | |
| Participants | 30 patients aged 50 years and over undergoing major elective thoracic, abdominal or orthopaedic surgery, ASA I and II, rectal temperature less than 35.5°C measured within 5 minutes of arrival in PACU, stable haemodynamics | |
| Interventions | Reflective blanket (n = 15) Radiant heater (n = 15) | |
| Outcomes | Rectal and skin temperature, shivering, hypoxaemia | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Not described |
| Allocation concealment | Unclear risk | Not described |
| Blinding Outcomes other than shivering | Unclear risk | Not described |
| Blinding Shivering | Unclear risk | Not described |
| Incomplete outcome data addressed All outcomes | Low risk | No loss to follow‐up |
| Free of selective reporting | Unclear risk | Not assessed |
| Free of other bias | Unclear risk | No other concerns |
Ciufo 1995.
| Methods | Single‐centre randomized controlled trial conducted in USA | |
| Participants | 2 patients admitted to PACU with core temperature less than 34.7°C | |
| Interventions | Interventions: Blanketrol coil heated water blanket (n = 16) Bair Hugger forced air warming blanket (n = 16) | |
| Outcomes | Time to normothermia with subgroup analysis for males and females | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | High risk | Participants were alternately assigned to 1 of the 2 groups |
| Allocation concealment | Unclear risk | Unclear who assigned participants to groups |
| Blinding Outcomes other than shivering | Unclear risk | Not stated |
| Blinding Shivering | Unclear risk | Not described |
| Incomplete outcome data addressed All outcomes | Low risk | No loss to follow‐up |
| Free of selective reporting | Unclear risk | Not assessed |
| Free of other bias | Unclear risk | No concerns |
Ereth 1992.
| Methods | Single‐centre randomized controlled trial conducted in USA | |
| Participants | 12 participants with a temperature less than 35°C during recovery from elective total hip arthroplasty | |
| Interventions | Forced air warming (n = 6) Warmed cotton blankets (n = 6) | |
| Outcomes | Skin and pulmonary artery temperature, surface temperature gradient, haemodynamic variables, oxygen consumption | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Not described |
| Allocation concealment | Unclear risk | Not described |
| Blinding Outcomes other than shivering | Unclear risk | Not described |
| Blinding Shivering | Unclear risk | Not described |
| Incomplete outcome data addressed All outcomes | Low risk | No loss to follow‐up |
| Free of selective reporting | Unclear risk | Not assessed |
| Free of other bias | Unclear risk | No concerns |
Giuffre 1991.
| Methods | Single‐centre randomized controlled trial conducted in USA | |
| Participants | 92 participants over the age of 18 years admitted to PACU with admission temperatures of 35°C or less. Participants were excluded if they had a planned admission to critical care, preoperative fever or sepsis, an open undressed burn, neurological problems with thermal instability, inability to co‐operate with rewarming interventions, continually tossed off covers or combativeness, or if they did not allow temperature assessment. 2 participants were inadvertently excluded from the study | |
| Interventions | Warmed cotton blanket (n = 31) Radiant lights (n = 30) Forced air warming blanket (n = 29) | |
| Outcomes | Time to normothermia, with subgroup analysis of shivering and non‐shivering | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Shuffled piles of envelopes containing equal numbers of treatments were used for random group assignment. Seperate piles were used for men and for women |
| Allocation concealment | Unclear risk | Not described |
| Blinding Outcomes other than shivering | Unclear risk | Not described |
| Blinding Shivering | Unclear risk | Not described |
| Incomplete outcome data addressed All outcomes | Low risk | No loss to follow‐up |
| Free of selective reporting | Unclear risk | Not assessed |
| Free of other bias | Unclear risk | No concerns |
Grossman 2002.
| Methods | Single‐centre RCT | |
| Participants | 60 postoperative general surgery critical care participants who were admitted from the OR to the SICU with core temperature less than 34.5°C. Participants with fever, sepsis or thermal instability or who underwent bladder irrigation were excluded | |
| Interventions | Cotton blanket (n = 20) Forced air blanket (n = 20) Circulating water blanket (n = 20) | |
| Outcomes | Time to normothermia | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | High risk | On admission to SICU, the nurse chose the next index card from the admission pile and applied the indicated intervention |
| Allocation concealment | High risk | Inadequate |
| Blinding Outcomes other than shivering | Unclear risk | Not stated |
| Blinding Shivering | Unclear risk | Not described |
| Incomplete outcome data addressed All outcomes | Low risk | No loss to follow‐up |
| Free of selective reporting | Unclear risk | Not assessed |
| Free of other bias | Unclear risk | No concerns |
Hershey 1997.
| Methods | Single‐centre RCT conducted in USA | |
| Participants | 144 adults undergoing laparotomy with general anaesthesia in a stable condition with a PACU admission temperature of 36°C or less. 4 participants were excluded from analysis because of missing data | |
| Interventions | Warmed cotton blankets (n = 46) Warmed blankets + reflective blanket (n = 46) Warmed blankets + reflective blanket + reflective head covering (n = 48) | |
| Outcomes | Time to normothermia, blood pressure, pulse rate, sinus rhythm, shivering, pain | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | A staff member in the nursing research department used a table of random numbers to assign participants prospectively to study groups |
| Allocation concealment | Low risk | As each eligible participant was admitted to the PACU, the investigator opened the envelope that contained the participant's group assignment |
| Blinding Outcomes other than shivering | Low risk | Not stated |
| Blinding Shivering | Unclear risk | Not described |
| Incomplete outcome data addressed All outcomes | Low risk | No loss to follow‐up |
| Free of selective reporting | Unclear risk | Not assessed |
| Free of other bias | Unclear risk | No concerns |
Summers 1990.
| Methods | RCT in 2 PACUs in USA | |
| Participants | 91 participants with a core temperature of 36°C or less on arrival at PACU | |
| Interventions | Warmed blankets (n = 46) Bair hugger (n = 45) | |
| Outcomes | Shivering, pain, temperature, oximetry, comfort, blood pressure | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Coin toss |
| Allocation concealment | Low risk | Coin toss |
| Blinding Outcomes other than shivering | Unclear risk | Not stated |
| Blinding Shivering | Unclear risk | Not described |
| Incomplete outcome data addressed All outcomes | Low risk | No loss to follow‐up |
| Free of selective reporting | Unclear risk | Not assessed |
| Free of other bias | Unclear risk | No concerns |
Weyland 1994a.
| Methods | Single‐centre RCT conducted in Germany | |
| Participants | ASA I and III undergoing major elective surgery by abdominal access with a postoperative temperature less than 35°C, on mechanical ventilation, body weight within ‐10% to +30% of normal, no irrigation instituted preoperatively | |
| Interventions | Standard hospital blankets (n = 11) Radiant heater (n = 12) Electric heating blanket (n = 12) | |
| Outcomes | VO2, time to normothermia, rate pressure product, thermal energy balance, shivering | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Random assignment weighted for sex |
| Allocation concealment | Unclear risk | Not stated |
| Blinding Outcomes other than shivering | Unclear risk | Not stated |
| Blinding Shivering | Unclear risk | Not described |
| Incomplete outcome data addressed All outcomes | Low risk | No loss to follow‐up |
| Free of selective reporting | Unclear risk | Not assessed |
| Free of other bias | Unclear risk | No concerns |
Weyland 1994b.
| Methods | Single‐centre RCT conducted in Germany | |
| Participants | 35 ASA I and II participants after laparoscopic hernioplastic repair with core temperature < 36°C | |
| Interventions | Radiant heater (n = 11) Forced air warming (n = 12) Normal cotton blanket (n = 12) |
|
| Outcomes | Temperature, VO2 | |
| Notes | Translated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Participants were randomly allocated to 3 groups, weighted by body weight |
| Allocation concealment | Unclear risk | Not stated |
| Blinding Outcomes other than shivering | Unclear risk | Not stated |
| Blinding Shivering | Unclear risk | Not described |
| Incomplete outcome data addressed All outcomes | Low risk | No loss to follow‐up |
| Free of selective reporting | Unclear risk | Not assessed |
| Free of other bias | Unclear risk | No concerns |
Yang 2012.
| Methods | Single‐centre quasi‐randomized controlled trial conducted in Taiwan | |
| Participants | 130 participants aged 18 years and older who underwent spinal surgery under general anaesthesia, who were conscious and able to communicate, who did not have fever 72 hours before the study and who had an operation time of 3 to 6 hours, operational wound 10 to 15 cm, tympanic membrane temperature between 34°C and 35.5°C on arrival to the PACU. Participants transferred to the ICU were excluded | |
| Interventions | Warmed cotton blankets Radiant warmer | |
| Outcomes | Time to normothermia, length of hospital stay, wound infection, warming rates | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | High risk | Randomization was based on the last digit of their chart number. Odd numbers were assigned to the blanket group and even numbers to the radiant warmer group |
| Allocation concealment | Unclear risk | Not stated |
| Blinding Outcomes other than shivering | Unclear risk | Not stated |
| Blinding Shivering | Unclear risk | Not described |
| Incomplete outcome data addressed All outcomes | Low risk | No loss to follow‐up |
| Free of selective reporting | Unclear risk | Not assessed |
| Free of other bias | Unclear risk | No concerns |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Clevenger 1994 | Cardiopulmonary bypass patients deliberately cooled |
| Inoue 2011 | Warming started intraoperatively, not postoperatively |
| Nalda 1985 | Unclear whether hypothermic |
| Rodriguez 1983 | Unclear how the 2 groups were formed |
| Smith 1999 | Multiple interventions |
| Yoo 2013 | Intraoperative reflective blanket plus active warming; subgroup analysis of people who became normothermic is not applicable as participants were not randomly assigned |
Differences between protocol and review
We made the following changes to our published protocol (Campbell 2012).
We did not include studies reporting multiple co‐interventions, unless the same co‐intervention was used in both groups, We made this change because studies using different co‐interventions cannot be meaningfully interpreted.
Our investigation of heterogeneity was hampered by inadequate description of the trials, and our investigation of subgroups was hampered by insufficient data.
Our investigation of the impact of blinding on outcomes was hampered by inadequate descriptions of blinding and insufficient data. We had planned to categorise performance and detection bias separately, but instead just looked for evidence of blinding by outcome, when possible, and reported this. Only one trial reported any blinding, and that pertained to only one outcome, so it was not possible to assess blinding by groups of outcomes.
Inclusion of some outcomes was based on post hoc decisions made after reviewing the evidence. 'Rate of rewarming' was included as we believed that this outcome was measuring the effect of rewarming on temperature. We expected the outcomes of 'shivering' and 'pain' to be included under 'patient's reported outcomes,' but after reviewing the evidence, we found that they were investigator assessed. We believe that these outcomes should still be included.
Performing sensitivity analysis of trials using different 'levels' of control groups (warms and unwarmed cotton blankets) was a post hoc decision.
Contributions of authors
Sheryl Warttig (SW), Phil Alderson (PA), Gillian Campbell (GC), Andrew F Smith (AS)
Conceiving of the review: PA, NICE
Co‐ordinating the review: SW, PA
Undertaking manual searches: not applicable
Screening search results: SW, PA, GC
Organizing retrieval of papers: SW, PA, GC
Screening retrieved papers against inclusion criteria: SW, PA, GC
Appraising quality of papers: SW, PA
Abstracting data from papers: SW
Writing to authors of papers to ask for additional information: SW
Providing additional data about papers: AS
Obtaining and screening data on unpublished studies: not applicable
Managing data for the review: SW, PA
Entering data into Review Manager (RevMan 5.2): SW, PA
Analysing RevMan statistical data: SW, PA
Performing other statistical analyses not using RevMan: not applicable
Interpreting data: SW, PA
Making statistical inferences: SW, PA
Writing the review: SW, PA
Securing funding for the review: AS
Performing previous work that served as the foundation of the present study: not applicable
Serving as guarantor for the review (one review author): AS
Taking responsibility for reading and checking the review before submission: SW, PA, GC, AS
Sources of support
Internal sources
-
Morecambe Bay University Hospitals Trust, UK.
GC is employed by the hospital trust as an anaesthetic research registrar
External sources
-
National Institute for Health Research, UK.
NIHR provided a programme grant supporting a series of reviews on perioperative care
Declarations of interest
Sheryl Warttig: none known.
Phil Alderson: none known.
Gillian Campbell: none known.
Andrew F Smith: none known.
New
References
References to studies included in this review
Alfonsi 2003 {published data only}
- Alfonsi P, Nourredine KE, Adam F, Chauvin M, Sessler DL. Effect of postoperative skin‐surface warming on oxygen consumption and the shivering threshold. Anaesthesia 2003; Vol. 58, issue 12:1228‐34. [PUBMED: 14705689] [DOI] [PMC free article] [PubMed]
Bredahl 1995 {published data only}
- Bredahl C, Lambert‐Jensen P, Freundlich M. Effect of a radiant heater on post‐operative hypothermia: comparison with a reflective blanket. European Journal of Anaesthesiology 1995; Vol. 12, issue 6:565‐9. [PUBMED: 8665878] [PubMed]
Ciufo 1995 {published data only}
- Ciufo D, Dice S, Coles C. Rewarming hypothermic postanesthesia patients: a comparison between a water coil warming blanket and a forced‐air warming blanket. Journal of Post Anesthesia Nursing 1995; Vol. 10, issue 3:155‐8. [PUBMED: 7783024] [PubMed]
Ereth 1992 {published data only}
- Ereth MH, Lennon RL, Sessler DI. Limited heat transfer between thermal compartments during rewarming in vasoconstricted patients. Aviation, Space, and Environmental Medicine 1992; Vol. 63, issue 12:1065‐9. [PUBMED: 1456917] [PubMed]
Giuffre 1991 {published data only}
- Giuffre M, Finnie J, Lynam DA, Smith D. Rewarming postoperative patients: lights, blankets, or forced warm air. Journal of Post Anesthesia Nursing 1991; Vol. 6, issue 6:387‐93. [PUBMED: 1836227] [PubMed]
Grossman 2002 {published data only}
Hershey 1997 {published data only}
Summers 1990 {published data only}
- Summers S, Dudgeon N, Byram K, Zingsheim K. The effects of two warming methods on core and surface temperatures, hemoglobin oxygen saturation, blood pressure, and perceived comfort of hypothermic postanesthesia patients. Journal of Post Anesthesia Nursing 1990; Vol. 5, issue 5:354‐64. [PUBMED: 2213631] [PubMed]
Weyland 1994a {published data only}
Weyland 1994b {published data only}
Yang 2012 {published data only}
References to studies excluded from this review
Clevenger 1994 {published data only}
Inoue 2011 {published data only}
- Inoue S, Shinjo T, Kawaguchi M, Nakajima Y, Furuya H. Amino acid infusions started after development of intraoperative core hypothermia do not affect rewarming but reduce the incidence of postoperative shivering during major abdominal surgery: a randomized trial. Journal of Anesthesia 2011; Vol. 25, issue 6:850‐4. [PUBMED: 21927856] [DOI] [PubMed]
Nalda 1985 {published data only}
- Nalda MA, Gomar C, Luis M. The effect of ketanserin on post‐anaesthetic vasoconstriction and shivering. European Journal of Anaesthesiology 1985; Vol. 2, issue 3:265‐77. [PUBMED: 2933253] [PubMed]
Rodriguez 1983 {published data only}
- Rodriguez JL, Weissman C, Damask MC, Askanazi J, Hyman AI, Kinney JM. Physiologic requirements during rewarming: suppression of the shivering response. Critical Care Medicine 1983; Vol. 11, issue 7:490‐7. [PUBMED: 6407803] [PubMed]
Smith 1999 {published data only}
Yoo 2013 {published data only}
Additional references
Al‐Qahtani 2011
- Al‐Qahtani AS, Messahel FM. Benchmarking inadvertent perioperative hypothermia guidelines with the National Institute for Health and Clinical Excellence. Saudi Medical Journal 2011;32(1):27‐31. [PUBMED: 21212912] [PubMed] [Google Scholar]
Birch 2011
- Birch DW, Manouchehri N, Shi X, Hadi G, Karmali S. Heated CO2 with or without humidification for minimally invasive abdominal surgery. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD007821.pub2] [DOI] [PubMed] [Google Scholar]
Bush 1995
- Bush HLJ, Hydo LJ, Fischer E, Fantini GA, Silane MF, Barie PS. Hypothermia during elective abdominal aortic aneurysm repair: the high price of avoidable morbidity. Journal of Vascular Surgery 1995;21(3):392‐400. [PUBMED: 7877221] [DOI] [PubMed] [Google Scholar]
Frank 1997
- Frank SM, Fleisher LA, Breslow MJ, Higgins MS, Olson KF, Kelly S, Beattie C. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA 1997;277(14):1127‐34. [PUBMED: 9087467] [PubMed] [Google Scholar]
Harper 2008
- Harper CM, Andrzejowski JC, Alexander R. NICE and warm. British Journal of Anaesthesia 2008;101(3):293‐5. [DOI: 10.1093/bja/aen233] [DOI] [PubMed] [Google Scholar]
Heier 1991
- Heier T, Caldwell JE, Sessler DI, Miller RD. Mild intraoperative hypothermia increases duration of action and spontaneous recovery of vecuronium blockade during nitrous oxide‐isoflurane anesthesia in humans. Anesthesiology 1991;74:815‐9. [PUBMED: 1673591] [DOI] [PubMed] [Google Scholar]
Heier 2006
- Heier T, Caldwell JE. Impact of hypothermia on the response to neuromuscular blocking drugs. Anesthesiology 2006;104(5):1070‐80. [PUBMED: 16645461] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Kelly 2010
- Kelly M, Gillies D, Todd DA, Lockwood C. Heated humidification versus heat and moisture exchangers for ventilated adults and children. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD004711.pub2] [DOI] [PubMed] [Google Scholar]
Kurz 1996
- Kurz A, Sessler DI, Lenhardt RA. Perioperative normothermia to reduce the incidence of surgical‐wound infection and shorten hospitalization. Study of wound infections and temperature group. New England Journal of Medicine 1996;334:1209‐15. [PUBMED: 8606715] [DOI] [PubMed] [Google Scholar]
Leslie 1995
- Leslie K, Sessler DI, Bjorksten AR, Moayeri A. Mild hypothermia alters propofol pharmacokinetics and increases the duration of action of atracurium. Anesthesia and Analgesia 1995;80(5):1007‐14. [PUBMED: 7726398] [DOI] [PubMed] [Google Scholar]
Melling 2001
- Melling AC, Ali B, Scott EM, Leaper J. Effects of preoperative warming on the incidence of wound infection after clean surgery: a randomised controlled trial. Lancet 2001;358:876‐80. [PUBMED: 11567703] [DOI] [PubMed] [Google Scholar]
NICE 2008
- The management of inadvertent perioperative hypothermia in adults: clinical guideline 65. National Institute for Health and Clinical Excellence 2008.
Putzu 2007
- Putzu M, Casati A, Berti M, Pagliarini G, Fanelli G. Clinical complications, monitoring and management of perioperative mild hypothermia: anesthesiological features. Acta Biomedica 2007;78(3):163‐9. [PUBMED: 18330074] [PubMed] [Google Scholar]
Rajagopalan 2008
- Rajagopalan S, Mascha E, Na J, Sessler DI. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology 2008;108(1):71‐7. [PUBMED: 18156884] [DOI] [PubMed] [Google Scholar]
Sessler 1991
- Sessler DI, Rubinstein EH, Moayeri A. Physiologic responses to mild perianesthetic hypothermia in humans. Anesthesiology 1991;75:594‐610. [PUBMED: 1928769] [DOI] [PubMed] [Google Scholar]
Sessler 2001
- Sessler D. Complications and treatment of mild hypothermia. Anesthesiology 2001;95:531‐43. [PUBMED: 9148354] [DOI] [PubMed] [Google Scholar]
Urrútia 2011
- Urrútia G, Roqué i Figuls M, Campos JM, Paniagua P, Cibrian Sánchez S, Maestre L, et al. Active warming systems for preventing inadvertent perioperative hypothermia in adults. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD009016] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Campbell 2012
- Campbell G, Alderson P, Smith AF, Warttig S. Interventions for treating inadvertent postoperative hypothermia. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD009892] [DOI] [PMC free article] [PubMed] [Google Scholar]


