Abstract
Although there is consensus on the management of Brugada Syndrome (BrS) patients with high risk for sudden cardiac arrest, asymptomatic or intermediate risk patients present clinical management challenges. This document explores the management opinions of experts throughout the world for BrS patients who do not fit guideline recommendations. Four real-world clinical scenarios were presented with commentary from small expert groups for each case. All authors voted on case-specific questions to evaluate the level of consensus among the entire group in nuanced diagnostic and management decisions relevant to each case. Points of agreement, points of controversy and gaps in knowledge are highlighted.
Introduction
Brugada syndrome (BrS) is a heritable arrhythmia syndrome associated with electrocardiogram (ECG) features of ST segment elevation in the right precordial leads followed by T-wave inversion and increased risk of sudden cardiac arrest (SCA) in patients with a structurally normal heart, though microstructural abnormalities are likely present.1 BrS patients may present with syncope or aborted SCA or be asymptomatic. Although there is consensus on the management of BrS patients with high risk for SCA, asymptomatic or intermediate risk patients present clinical management challenges. Harnessing experts from around the globe, this document explores the management opinions of clinicians for patients who do not fit guideline recommendations.
Four real-world clinical scenarios, not modified for the purpose of this publication were presented to small groups of experts, who discuss management recommendations, including points of agreement or disagreement, and compose voting questions for all authors. All authors then voted on case-specific questions to further evaluate the level of consensus among the entire group in nuanced diagnostic and management decisions relevant to each case. Finally, key points as well as gaps in knowledge are summarized (Table 1–3).
Table 1:
Points of Agreement
| Importance of high-lead (V1-V2) ECG recordings in the diagnosis of Brugada Syndrome. |
| Comprehensive evaluation of syncope to distinguish arrhythmic from non-arrhythmic syncope in Brugada Syndrome. |
| Asymptomatic relative with a normal clinical evaluation and negative cascade genetic testing can be released from clinical follow-up |
| ICD is recommended in confirmed Brugada Syndrome patients with arrhythmic syncope. |
| Focused genetic testing (SCN5A) in a cardio-genetics clinic with genetic counseling is beneficial and can help guide risk stratification when a Brugada Syndrome diagnosis is confirmed. |
Table 3:
Gaps in Knowledge
| The exact role of provocative drug testing in relatives of Brugada Syndrome patients (when and how often). |
| What is the natural history of cardiac arrest attributed to BrS phenocopy in the setting of substance abuse, and whether the risk of recurrent VF justifies ICD implantation. |
| Should relatives of Brugada Syndrome patients have longitudinal follow-up and what testing should be performed. |
| The role of asymptomatic fever-induced type 1 ECG pattern without spontaneous type 1 ECG pattern, family history of BrS or a P/LP mutation in a BrS susceptibility gene is unresolved. |
| The role of provocative sodium channel blocker drug testing to diagnose BrS syndrome. Specifically, additional research is needed to determine the indications and to better define differences in the sensitivity and specificity of different sodium channel blockers and the impact of regional differences in access to certain IV sodium channel blocker drugs. |
Case 1
A 50 year-old man with bipolar disorder (stable off medication), hypertension and obstructive sleep apnea presented to the emergency department (ED) with myalgias, nausea, headache, fatigue, and chest pain. He was febrile at 38.6 C. Serum electrolytes and cardiac enzymes were normal. ECG showed ST elevation in V1-V3 (Figure 1a). Urgent coronary angiography showed no significant obstructive coronary artery disease (CAD). However, during the procedure the patient had ventricular fibrillation (VF) requiring defibrillation. This VF did not occur during contrast injection or catheter manipulation with the catheter resting in the aorta.
Figure 1.
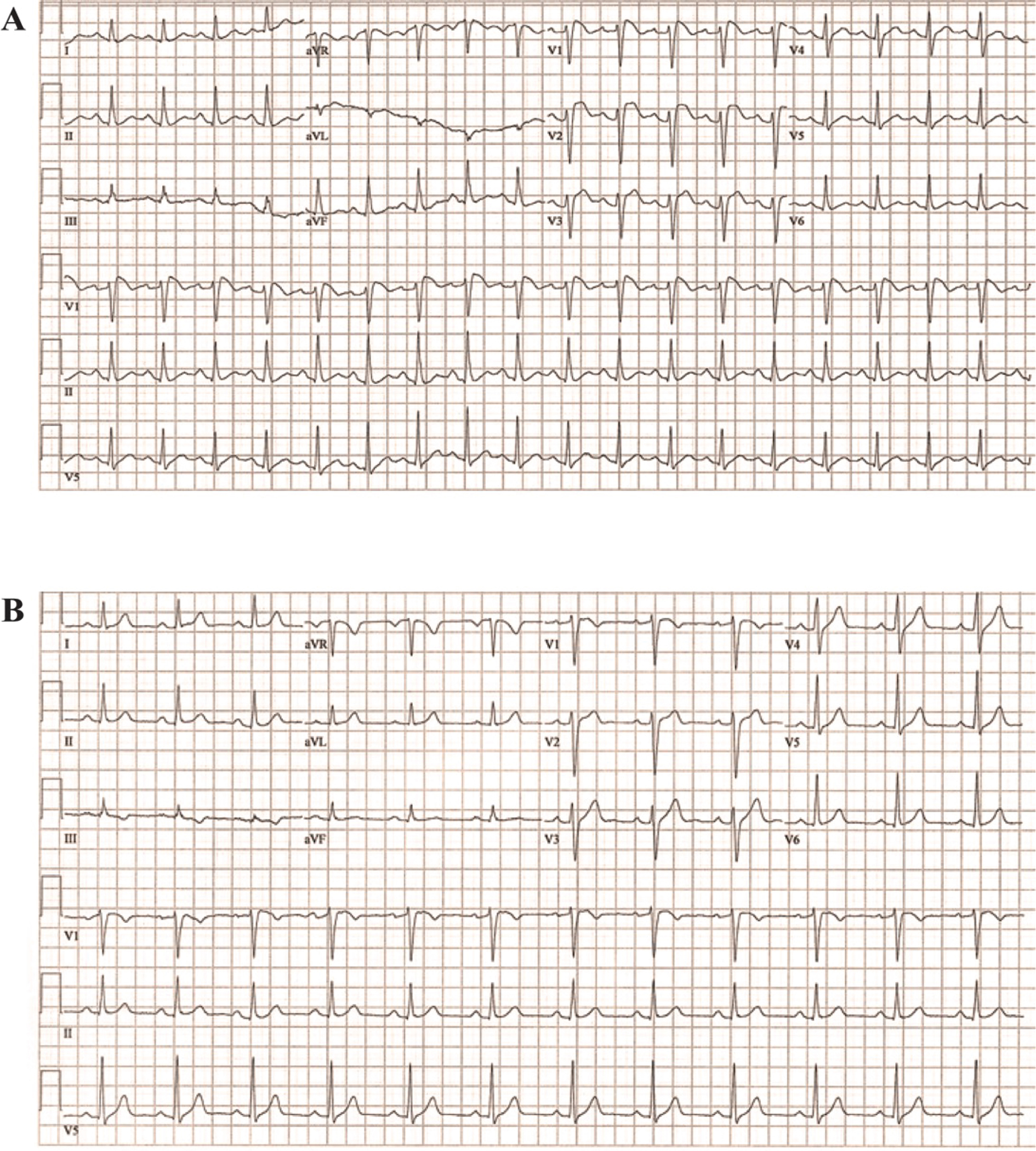
Case 1 ECGs. Panel A. Spontaneous ECG during fever. Panel B. Repeat ECG when afebrile.
Repeat ECG when the patient was afebrile showed resolution of ST elevation in V1–3 (Figure 1b). Specific and expanded genetic testing was negative for pathogenic variant. Cardiac imaging (echocardiogram and MRI) was normal. Ventricular programmed electrical stimulation (PES) using up to 3 extra-stimuli repeatedly induced self-terminating ventricular tachycardia (15–20 sec).
Key Questions
1) Would you perform a drug provocation study or do additional risk stratification? 2) Would you recommend an implantable cardioverter defibrillator (ICD)? 3) What type of screening/counseling would you recommend to family members given negative genetic testing?
Expert Panel Commentary (Cerrone [Chair], Wilde, London, Behr, Shimizu)
The majority of panelists agreed that based on the data provided, including fever-induced Type 1 ECG pattern and non-provoked VF, the diagnosis of BrS is confirmed.2 However, one panelist suggested the diagnosis could be questionable based on the Shanghai score system,3 in which this individual would reach only 3 points, 3.5 points considered diagnostic, since a Type 1 ECG during fever is not classified as “spontaneous”.3 The possibility of a false positive was also raised, based on the morphology of the ST segment elevation in V1.
All panelists agreed on the known limitations of a drug challenge and possibility of false positive results.4–6 A pharmacological challenge was considered unnecessary for the diagnosis since the patient already showed an fever-induced Type 1 pattern,3 although some experts suggested that a provocative test could validate the one-time finding of Type 1 during fever and be used as a tool for cascade screening.6,7
Most of the expert panel for Case 1 supported additional screening tools including a high-lead ECG (V1 and V2 placed in the 2nd and 3rd intercostal space) and 12-lead Holter (with the option of recording high and conventional precordial leads simultaneously). Additional components to help risk stratification and overall assessment include detailed medical and family history; reviewing past ECGs; signal-averaged ECG; treadmill exercise testing; and careful analysis of ECG characteristics such as QRS spike wave at V1-V3 leads, J wave at inferolateral leads, and QRS duration.2,8–10 All panelists agreed on the limited indication and value of the electrophysiology study (EPS).11 None of the experts favored its use in this case especially, because of a limited negative predictive value12 and it was pointed out that the induced NSVT runs with 3 extra-stimuli were non-specific and the EPS should be considered negative.11
The critical question to consider when deciding on whether to implant an ICD is whether the patient’s cardiac arrest was triggered by the angiogram or purely coincidental. If the arrest is considered a spontaneous event, the long-term risk of SCA would be sufficiently high (around 8%/year) to support consideration of an ICD.11,13 In addition, none of the currently available risk stratifications strategies have sufficient negative predictive value given a history of unprovoked SCA.13,14 The experts who leaned toward defining the VF as a non-spontaneous episode because it occurred during a medical procedure (even if not connected to high-risk interventions) were inclined to not implant an ICD.13 The experts emphasized the need for a detailed discussion with the patient regarding risks and benefit and gaps of knowledge. The possibility of long-term monitoring with an ILR should be considered if an ICD is not implanted.13,15,16
If in the future, the patient requires a therapy for Bipolar disease, with drugs that are contraindicated in the setting of BrS ( www.brugadadrugs.org), all authors agree that he should be followed closely with ECG monitoring on regular intervals. There was agreement on screening relatives with ECG, high-lead ECG and 12-lead Holter looking for spontaneous Type 1 pattern.17 Relatives should be counseled to have an ECG recorded during fever when possible and to implement modifying measures, such as prompt treatment of fevers and avoidance of agents known to be pro-arrhythmic in the setting of BrS.2,3,13 Children should repeat an ECG after puberty and all adult family members with a first negative ECG should repeat it in several years. Provocative drug challenge was not deemed necessary, but could be considered, after informed decision with the relatives, especially in the presence of a suspicious ECG pattern.
Case 1 Summary (Perez, Roden)
The experts agreed on most of the major management questions raised by this case of a 50 year-old man with a Type I Brugada pattern in the setting of a febrile illness and a cardiac arrest during coronary angiography (Figure 2 and Supplemental Table I). However, there are a few points of disagreement worth highlighting. While the 2013 expert consensus statement on inherited arrhythmias states that BrS “is diagnosed in patients with ST-segment elevation with Type 1 morphology…either spontaneously or after provocative drug test…”18, a minority of the experts here proposed that a Type 1 pattern induced by a fever should not be considered spontaneous and that there is a role for drug provocation in this case. However, there is a gap in knowledge of whether a sodium channel blocker test is more accurate than a fever-induced ECG in attesting a BrS diagnosis. Further research is needed to answer this question. The disagreement on appropriateness of EPS and ICD was based in part on whether the episode of VF was provoked by the coronary angiogram of which, most of the expert commentary panel for Case 1 felt it was unprovoked. The entire author panel was more likely to recommend an ICD. Regardless, there was agreement that if the VF episode was unprovoked, then the EPS would not add value as a negative study would not be sufficiently reassuring. Finally, there was discrepancy on the ideal strategy for family screening, possibly due to the lack of a clear guideline on routine use of provocative drug testing to screen relatives and the frequency of screening. While most experts agreed a drug challenge should be considered in family members if there is clinical suspicion, it remains controversial whether routine drug challenge should be recommended in the absence of symptoms or equivocal ECG findings.
Figure 2.
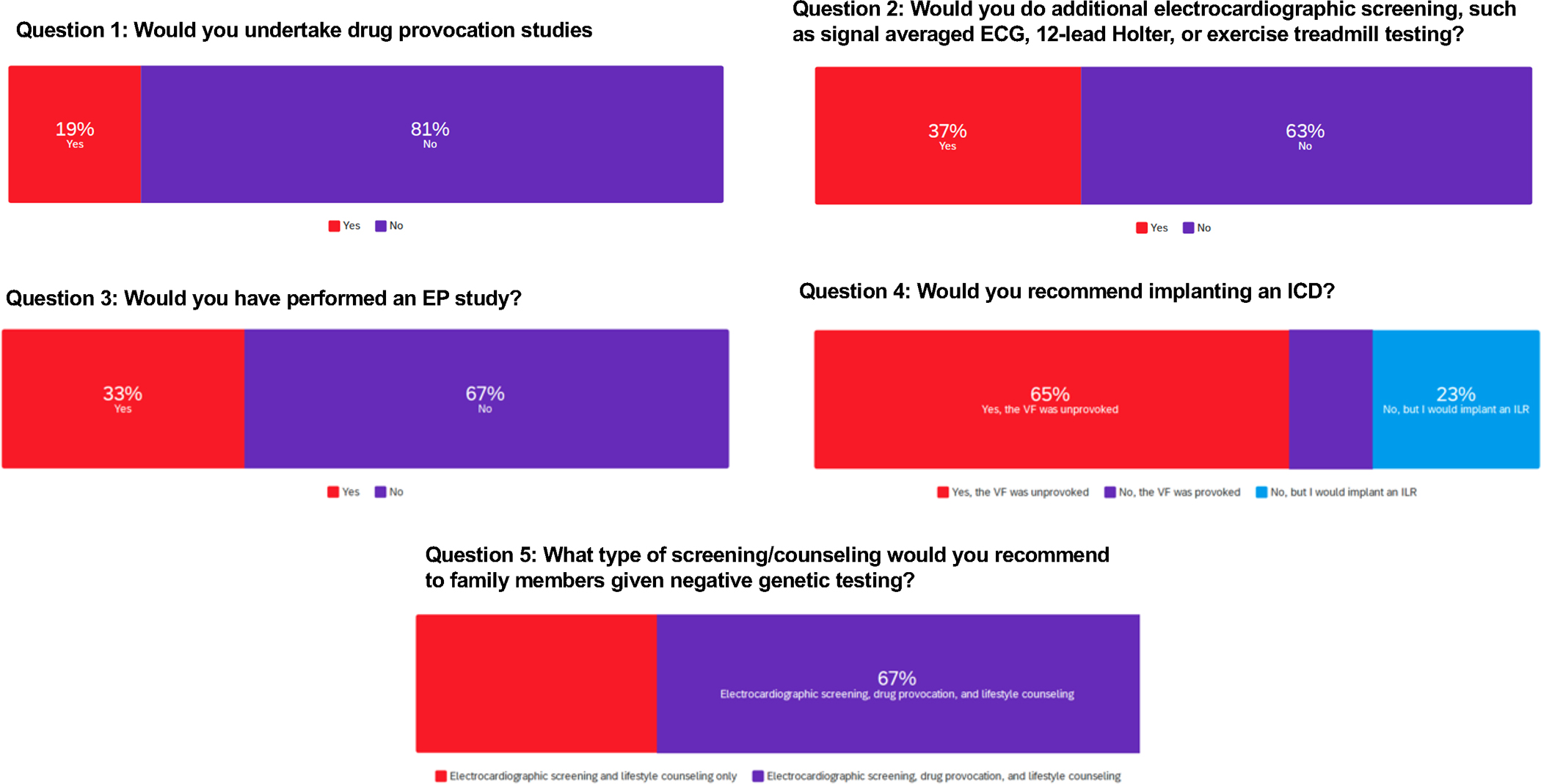
Case 1 Group Voting. Survey results from questions related to case 1.
Case 2
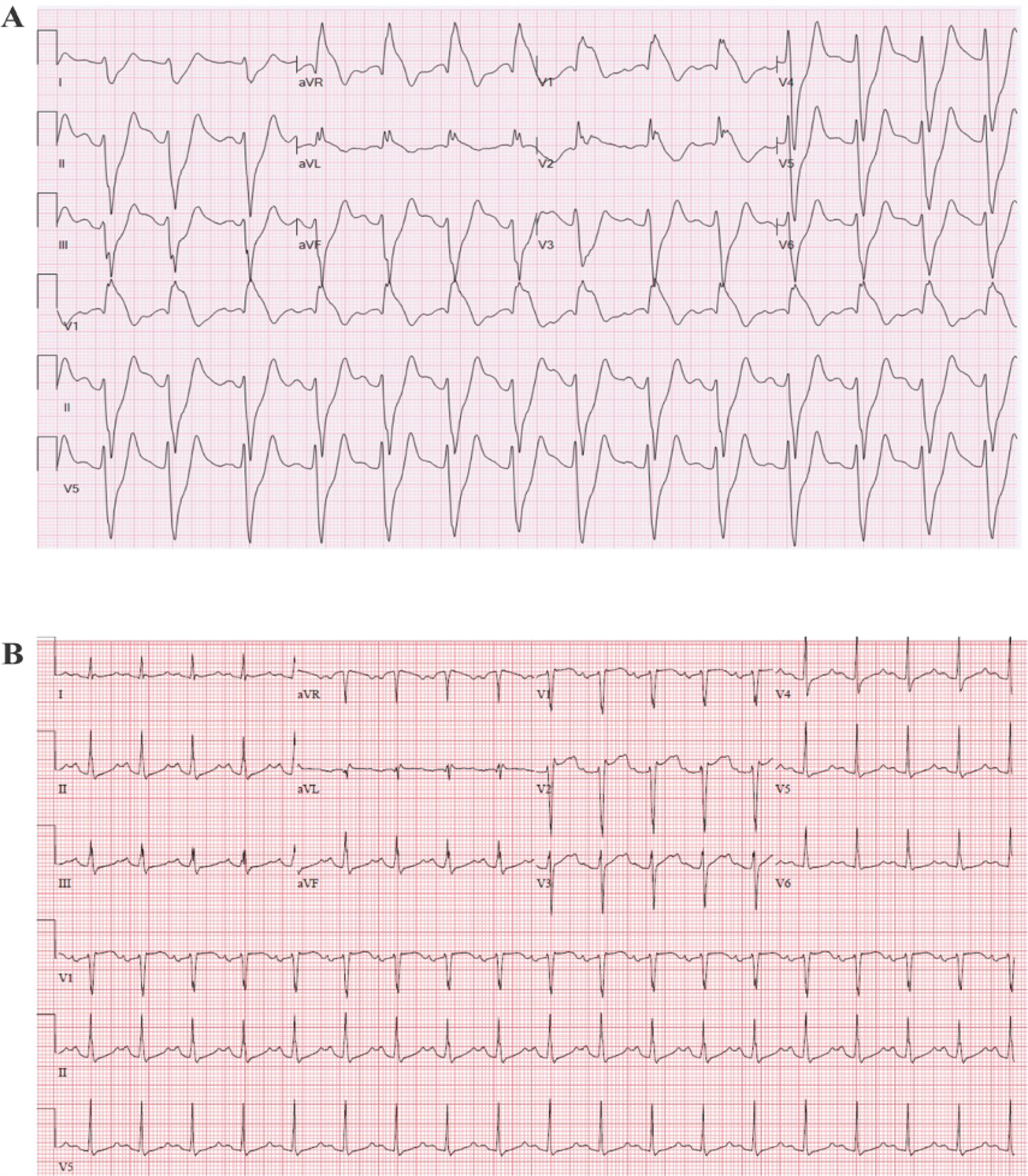
A 33 year-old female was seen in the emergency department following her first episode of syncope preceded by a prodrome of lightheadedness (ECG (Figure 3a)). She reported symptoms of an upper respiratory infection but no fever. She had no significant past medical history and her family history was positive for coronary artery disease. Genetic testing revealed a pathogenic mutation in SCN5A (c.2533delG). At the time of expert consultation, the ECG (Figure 3a) from the ED was not immediately available and an in-office ECG (Figure 3b) was obtained without a Type 1 Brugada ECG pattern. As such, she underwent procainamide challenge and developed Type 1 Brugada ECG pattern.
Figure 3.
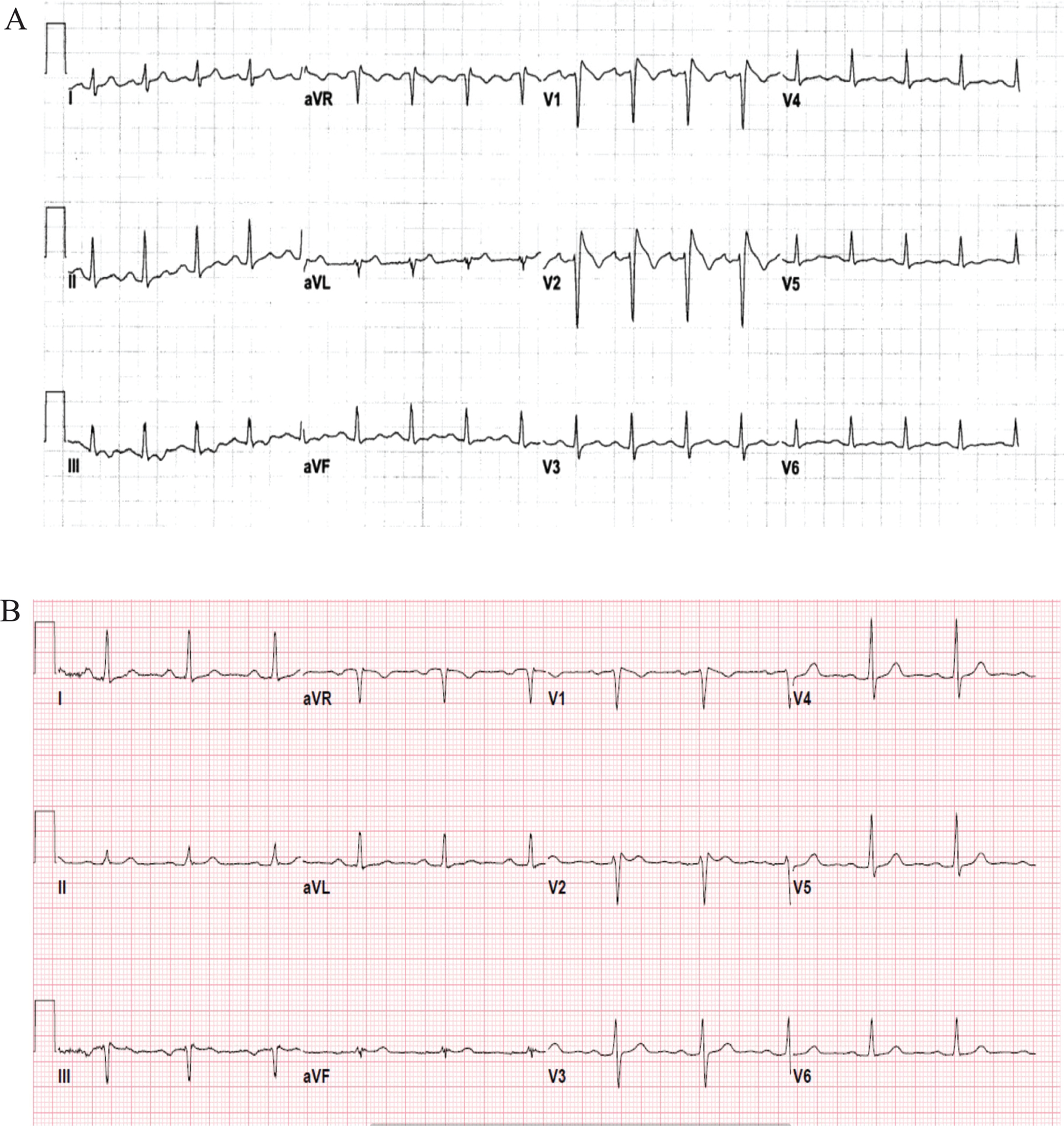
Case 2 ECG. Spontaneous ECG in the emergency department following syncope.
Key Questions
1) Would you perform a diagnostic EPS? 2) Would you recommend an ICD? 3) Should asymptomatic relatives with normal clinical evaluation and negative genetic testing be released from clinical follow-up?
Expert Panel Commentary (Sy [Chair], Deasmundis, Gollob, Krahn, Sarquella-Brugada)
Case 2 expert panelists agreed that the initial ECG from the emergency department shows a spontaneous Type 1 BrS ECG pattern. Procainamide challenge reproduced the ECG pattern, but the provocation study was considered unnecessary given the index ECG. The presence of a ‘pathogenic variant’ in SCN5A further confirms the diagnosis and may have implications in terms of arrhythmic outcomes.19,20 The panel agreed that the interpretation of genetic variants is ideally performed in a multi-disciplinary cardio-genetics service, especially in light of recent evidence that non-SCN5A variants often have limited/disputed evidence for pathogenicity in BrS.21
In terms of diagnosis, the patient has BrS based on her ECG, clinical presentation and genetic testing result.18,22 This yields a Shanghai score of at least 5.0, confirming BrS diagnosis.3
Although arrhythmic events dominate in males >40 with BrS, this younger female patient’s history of syncope is concerning and her prognosis and management hinges on the evaluation of the syncopal event. Systematic history taking is crucial in differentiating non-arrhythmic syncope and arrhythmic syncope. Specifically, the presence of prodromal symptoms (nausea, diaphoresis, etc.) or triggers (emotional distress, prolonged standing, cough, or micturition) would point towards non-arrhythmic etiology.23 Importantly, non-arrhythmic syncope occurs frequently in patients with BrS (up to 57% in one study), but is not associated with malignant outcomes.24,25 In contrast, patients with arrhythmic syncope have a ~2–3%/year risk of subsequent SCA.12,24 Clinical history should provide sufficient discrimination of the likely mechanism of syncope, and additional investigations such as tilt table testing and EPS are non-specific.
Risk stratification in BrS continues to evolve. The presence of a spontaneous type 1 Brugada ECG pattern is a consistent marker of increased risk, especially in the setting of syncope.26–28 Beyond a spontaneous type 1 Brugada ECG pattern, additional ECG markers have been reported to be associated with an increased risk of arrhythmia but these were not present in this patient. 29
The utility of a EPS for risk stratification remains contentious.11,26,30,31 In particular, the incremental value of VF inducibility is questionable in the present case if the patient is deemed to have arrhythmic syncope because a negative test would be insufficient to withhold recommending an ICD. Hence, three panellists would not recommend an EPS in this scenario. However, two panellists would recommend an EPS to further evaluate the arrhythmic risk and evaluate the HV interval and sinus node recovery time given that bradyarrhythmias may be associated with BrS, especially in patients with a pathogenic SCN5A variant.
There is complex interplay between gender and risk in BrS. Male patients with BrS are more likely to exhibit a spontaneous Type 1 BrS pattern as well as inducibility of ventricular fibrillation and have a greater risk of malignant arrhythmia.32 The present patient poses a less common clinical scenario, a female patient with a spontaneous Type 1 BrS pattern and a pathogenic mutation in SCN5A. Epidemiological data suggests that gender alone is not an independent predictor of outcomes once other variables such as the presence of a spontaneous Type 1 ECG pattern are taken into account.12,27,32 Moreover, female BrS patient with pathogenic SCN5A mutations may have increased risk of malignant arrhythmias.33 However, it is acknowledged that risk stratification in female patients with BrS is less well understood because the vast majority of patients in clinical studies, and even more so in those with clinical events, are male.34
Current guidelines would recommend consideration of a prophylactic ICD in the setting of probable arrhythmic syncope and a spontaneous Type 1 ECG pattern.18,35 However, it is critical to engage the patient in shared decision-making after a thorough discussion of the potential benefits as well as the lifetime risks of ICD implantation in young patients including infection, system revision, and inappropriate shocks. If the patient declines ICD implantation, the merits and limitations of adjuvant strategies such as quinidine therapy and/or catheter ablation can be discussed as alternatives with limited evidence from small observational studies.36,37 Lifestyle advice regarding medication avoidance (brugadadrugs.org), restraint from alcohol intoxication, and prompt fever treatment is recommended.
The experts agreed that the patient’s relatives should be offered clinical evaluation as well as cascade testing for the pathogenic SCN5A variant identified in the proband.38 Relatives who have clinical evidence of BrS and/or carriers of the SCN5A variant should be carefully screened for arrhythmic symptoms. Sodium-channel blocker challenge can be offered in selected patients based on their symptom status, ECG and preference. Asymptomatic patients should receive lifestyle advice and clinical follow-up. A diagnostic EPS is not recommended in asymptomatic relatives.
In general, asymptomatic relatives with a completely normal resting ECG (including high-lead ECG) and negative for the SCN5A variant can be released from clinical follow-up.38 However, there is increasing appreciation of the complex heritability of BrS. Of note, a patient’s genetic background (beyond SCN5A variants) may contribute to variable expressivity in families with a pathogenic variant in SCN5A.39–41
Case 2 Summary (Cutler, Huang)
This case identifies key questions: is provocative drug testing needed in a patient that presents with a spontaneous Type 1 pattern ECG? The panelists agreed that the presenting ECG displayed a Type 1 pattern and that a procainamide challenge test was not needed. Was the syncopal episode arrhythmic or non-arrhythmic? The panel was unanimous in recommending a detailed history of the syncope to distinguish arrhythmic vs. non-arrhythmic syncope as the determination of arrhythmic syncope is crucial in the decision to recommend an ICD. Finally, does an EPS add value to the risk stratification of this patient? As in the literature, whether an EPS should be performed was debated. Three of the 5 panelists would not recommend EPS implying that syncope with ECG findings was sufficient for diagnosis and prescribing treatment. In contrast, 2 panelists recommended EPS looking for ventricular arrhythmia inducibility and/or SA nodal or AV conduction pathology.
The entire author panel was divided on whether to recommend an EPS; the majority would not (Figure 4 and Supplemental Table II). While 74% of the experts would recommend an ICD implant, an additional 18.5% would recommend an ICD if an EPS was positive. There was consensus that asymptomatic relatives with negative genetic testing could be released from follow-up.
Figure 4.
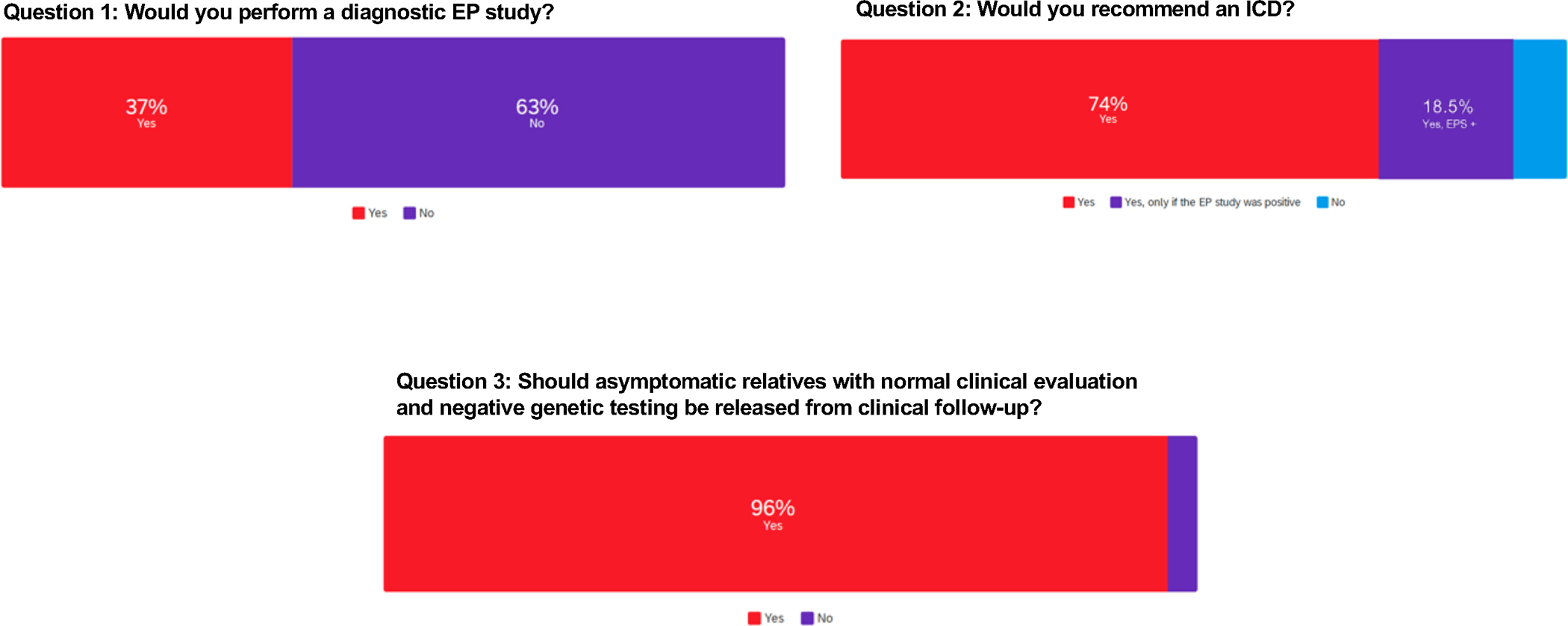
Case 2 Group Voting. Survey results from questions related to case 2.
In conclusion, case 2 highlights the importance of a thorough history to distinguish between arrhythmic and non-arrhythmic syncope. However, there are instances when all available clues still may not clearly differentiate arrhythmic vs. non-arrhythmic syncope and additional risk stratification tools, e.g., spontaneous vs. induced ECG changes, genotype details if positive, or EPS may be needed.
Case 3
A 26 year-old female became unresponsive following a period of diaphoresis, flushing, and tunnel vision after ingesting alcohol. A bystander applied an automated electrical defibrillator and no shock was advised. When emergency medical services (EMS) arrived, the patient was arousable to sternal rub. In the emergency room an ECG (Figure 5a) and cardiac imaging (echocardiogram, cardiac MRI) were normal. Family and personal history were negative for SCA, syncope or febrile seizures.
Figure 5.
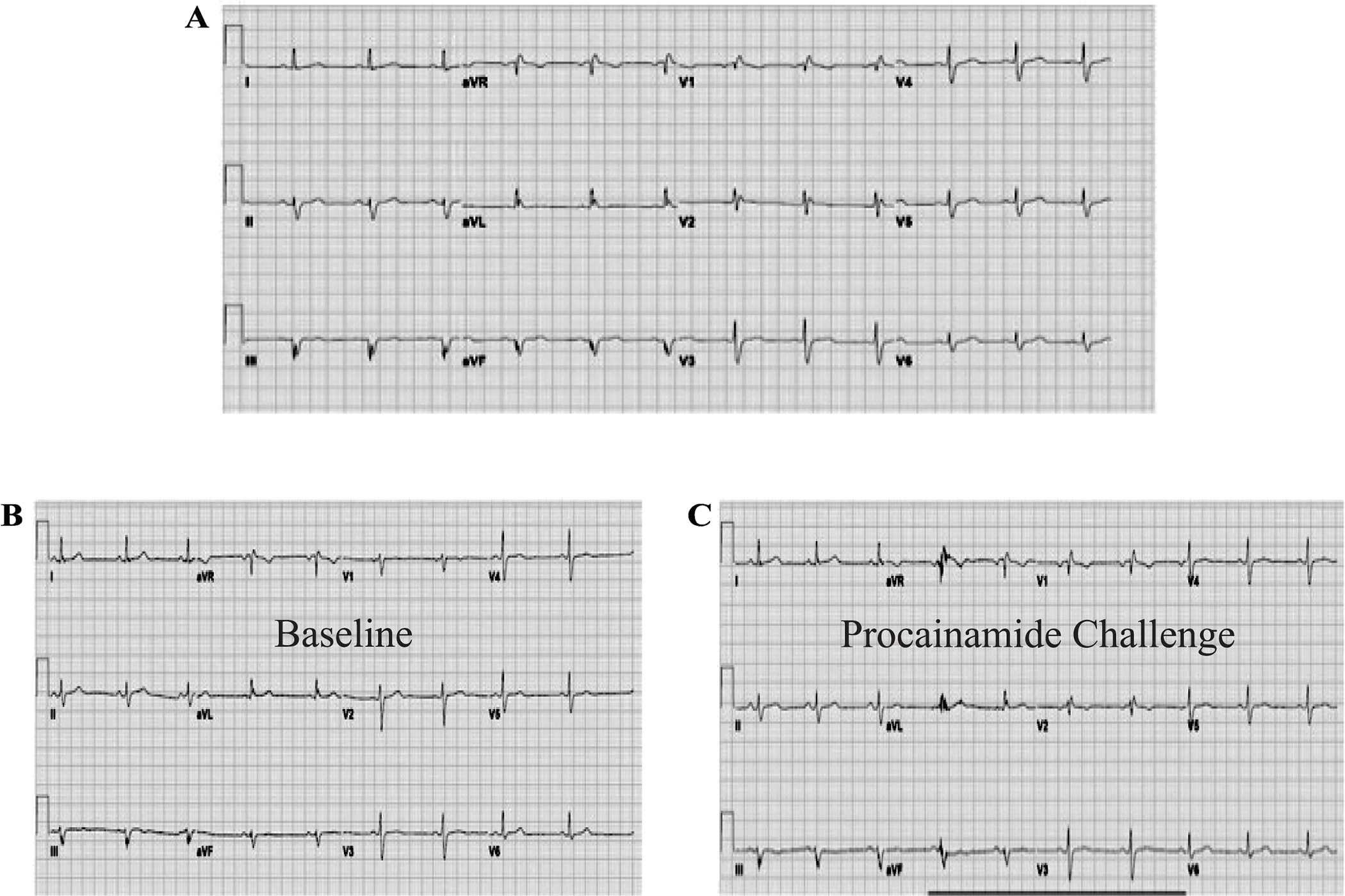
Case 3 ECGs. Panel A. Spontaneous ECG in the emergency department following syncope. Panel B. Baseline ECG during Procainamide challenge. Panel C. Repeat ECG during Procainamide challenge.
Subsequently, a procainamide challenge was performed (Figure 5b–c). She was discharged home with a life-vest and had syncope while wearing the life-vest. No ventricular arrhythmia was detected on life-vest interrogation. Genetic testing showed a likely pathogenic mutation in SCN5A (c.4978A>G). A diagnostic EPS was performed with no inducible ventricular arrhythmias.
Key Questions
1) Would you diagnosis this case as BrS? 2) Would you perform additional risk stratification and/or recommend an ICD? 3) Would you recommend ECG screening and/or genetic testing for family members?
Expert Panel Commentary (Crotti [Chair], Arbelo, Brugada, Sacher, Watanabe)
All experts agreed that the ECGs provided do not fulfill criteria for the diagnosis of BrS. However, they acknowledged that the diagnosis cannot be ruled out completely for the following reasons: 1) the absence of a high-lead ECG at baseline and during procainamide challenge and 2) use of procainamide as a drug challenge instead of ajmaline.
BrS can only be diagnosed in the presence of the Type 1 Brugada pattern characterized by J point elevation of >2 mV with coved ST elevation and T wave inversion in at least one right precordial ECG lead (i.e. V1 or V2).2,3,18,42–44 Placement of the right precordial leads in a more superior position (i.e. 2nd or 3rd intercostal spaces) increases the ECG sensitivity to identify a Type 1 Brugada ECG pattern.9,45–48 Other situations such as fever, vagal stimulation, alcohol, cocaine intoxication or electrolyte abnormalities may unmask Type 1 pattern when ECG manifestations are not apparent at baseline.49,50 The presence of other known causes of ST-segment elevation in right precordial leads (so-called phenocopies) should be excluded.51,52
When the baseline ECG does not show a typical Type 1 Brugada ECG pattern, but there is a reasonable suspicion, intravenous administration of a sodium channel blockers may convert the ECG pattern into Type 1.53–55 Unfortunately, not all drugs appear to have the same diagnostic yield for drug-induced Type I ECG patttern.2 In retrospective analysis, Ajmaline may be superior to other sodium channel blockers yet, the sensitivity and specificity of provocative drug testing remains elusive and Ajmaline is not available in all countries.56–58 As such, further research is needed to better define the role of provocative drug testing with IV sodium channel blockade in the diagnosis of BrS.
The appropriate strategy for risk stratification depends on whether the diagnosis of BrS is confirmed. If a diagnosis of BrS is not made, the work-up and management should follow the recommendations for the management of patients with syncope.59 If BrS is confirmed, a detailed evaluation of each syncopal event is warranted60 in an attempt to classify each as arrhythmic or not.25,61 as recommended arrhythmic syncope treatment in BrS is an ICD.13,18,35,62 This is particularly important given the high prevalence of vasovagal syncope in published cohorts of BrS.61 Based on the clinical information available for this case, including the absence of ventricular arrhythmia on AED and LifeVest, this patient’s syncope was likely not arrhythmic.
In BrS patients with syncope of unclear mechanism an EPS to assess inducibility of sustained (i.e. ≥30 seconds) polymorphic ventricular arrhythmias11,60 could be appropriate because the EPS has also shown to have a negative predictive value (92.4%).61 However, in this case we do not have a diagnosis of BrS and therefore, the majority of panelists would not recommend an EPS. Three experts believe that in the absence of a BrS diagnosis, without personal history of febrile seizures or palpitation, no family history of SCA, and non-arrhythmic syncope, only regular clinical follow-up is recommended.18,35,62 Two experts would recommend an implantable loop recorder and all panelists agreed that an ICD was not indicated.
According to a recent consensus document on the use of molecular screening in cardiac diseases, genetic testing should be performed only in patients with a type 1 standard or high-lead ECG occurring either spontaneously or induced by sodium-channel block, and only SCN5A should be screened in a clinical setting since it is the only gene with definite association with BrS.38,63 All panelists agreed that in the absence of a diagnosis of BrS, molecular screening should not have been performed. However, once a likely pathogenic variant on SCN5A was identified, the data should be appropriately managed.
Two experts suggested that cascade screening should not be performed unless a Type 1 pattern was identified in the proband. In contrast, two panelists recommended that genetic and complete clinical evaluation should be offered to first-degree relatives. The remaining expert recommended that variant classification should be re-evaluated in an independent laboratory with a specific expertise. Importantly, the distinction between variant of uncertain significance (VUS) and likely pathogenic can sometimes be subtle and change over time. Indeed, this variant has been re-classified as a VUS (PP3-PP5-BS2) in an independent laboratory and should not be used to support diagnosis nor for cascade screening.
Case 3 Summary (Probst, Lubitz)
In the present case, a young adult woman experienced syncope after a brief prodrome. An AED was applied, and no shock was advised. The patient was rousable without a shock, suggesting a non-arrhythmic event. Her subsequent syncopal event while wearing a LifeVest confirmed lack of tachyarrhythmia. Her ECG showed transient abnormal early repolarization in the right precordial leads, reproduced with a procainamide challenge.
Of the entire author voting group, most (70%) indicated that they would not have pursued EPS. This observation highlights the variability of opinion and practice among providers of the utility of an electrophysiology study in the diagnostic work up of unexplained syncope, particularly in the setting of confounding genetic testing results, even though such testing may not have been indicated. Nevertheless, EPS was performed and was negative (Figure 6 and Supplemental Table III). At variance to current consensus documents, slightly over half the experts would have performed genetic testing, with 41% favoring a broad genetic panel and 11% focusing on SCN5A variants only. Genetic testing identified a likely pathogenic missense variant in SCN5A (c.4978A>G), later reclassified as a variant of uncertain significance. Most (63%) of experts were in favor of implantation of a loop recorder to further assess the syncope etiology.
Figure 6.
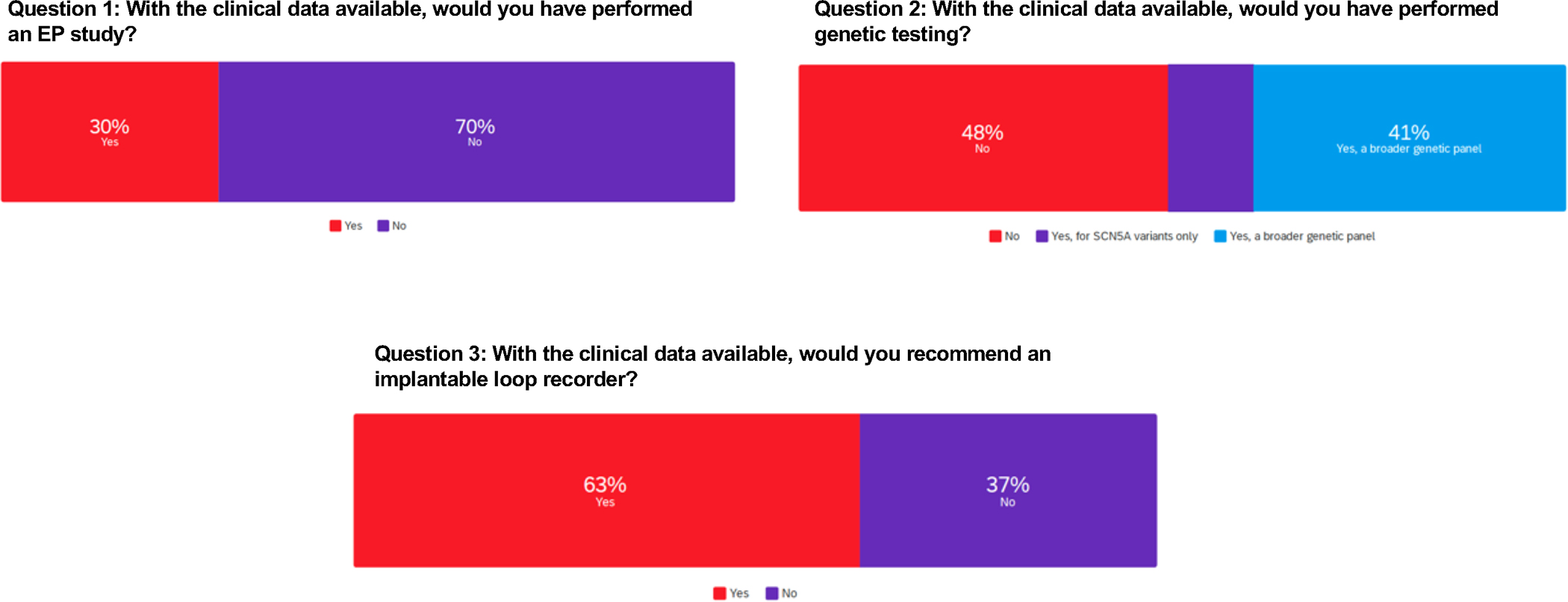
Case 3 Group Voting. Survey results from questions related to case 3.
The current case highlights the importance of understanding the etiology of syncope, diagnostic electrocardiographic criteria for BrS, and genetic variant interpretation to avoid unnecessary exams and potentially harmful treatments for patients.
Case 4
A 32 year-old man without known past medical history was arrested for operating a vehicle while intoxicated. He was found with a bag containing a “white substance” in his mouth that apparently burst. While in police custody, he had a seizure treated with midazolam and then had a VF cardiac arrest. He was hypotensive and required intubation by the emergency medical service. In the emergency department, telemetry monitoring showed marked ST elevation. ECG was performed (Figure 7a) and urgent EP consult requested for “rule out BrS”. Troponin was elevated, blood alcohol was 56 mg/dL, and toxicology screen was positive for cocaine. Within 3 hours of presentation to the hospital, his ECG (Figure 7b) normalized and respiratory status improved. He was extubated, became agitated and left the hospital against medical advice.
Figure 7.
Case 4 ECGs. Panel A. Spontaneous ECG in the emergency department. Panel B. Repeat ECG three hours after presenting to the emergency department.
Key Questions
1) If he had recurrent episodes of VF in the emergency department, how would you have managed this? 2) If his family brought him to your clinic for a follow-up visit, what further testing, if any, would you recommend? 3) Can cocaine and alcohol intoxication be considered like performing an ajmaline or procainamide challenge with respect to diagnosis of BrS?
Expert Panel Commentary (Experts: Mackall [Chair], Nademanee, Scheinman, Shoemaker)
The patient presented with VF arrest in the setting of cocaine and alcohol intoxication. In addition to VF, acute cocaine intoxication can present with acute hypertension, coronary vasospasm, and myocardial infarction/ischemia. Moreover, chronic cocaine abuse increases risk of acute coronary syndrome, cardiomyopathy and may increase risk of coronary artery disease.64 The presenting ECG shows prolonged PR interval and QRS complex and ST segment elevation with T wave inversion consistent with possible Brugada pattern or cardiac ischemia. One expert felt the ECG more likely reflected conduction block due to sodium channel intoxication than a Type 1 Brugada pattern.65
Cocaine intoxication is a recognized clinical scenario in which the Brugada pattern ECG represents a Brugada phenocopy. Brugada phenocopies have been described in other cases of overdose with medications that have sodium channel blocking effects such as tricyclic antidepressants, anti-seizure drugs, or Class IC antiarrhythmics.66 The electrophysiologic effects of cocaine are related to sodium channel blockade, manifest as prolonged PR interval and QRS widening. This patient had both cocaine and alcohol intoxication which is more toxic than cocaine alone because the metabolite coca-ethylene has a more pronounced sodium channel effect and longer half-life.67 The primary difference between drug-induced Brugada ECG and Brugada phenocopy is the presumed level of sodium channel blockade with a therapeutic dose of a sodium channel blocker compared to drug overdose.
The treatment of recurrent VF in this patient should include prompt defibrillation followed by evaluation and treatment for acute coronary vasospasm or myocardial infarction, as appropriate. Fluid resuscitation and sodium bicarbonate is recommended to treat acidosis and restore sodium channel function by promoting dissociation of cocaine from the sodium channels. Furthermore, cocaine toxicity can result in QT interval prolongation secondary to blocking of potassium channels, leading to Torsade de Pointes. In such cases, the preferred treatment would include IV magnesium and lidocaine. Importantly, isoproterenol and beta-blockers are contraindicated with recurrent VF from cocaine toxicity.
Because the ECG was not diagnostic for BrS, panelists disagreed on whether isoproterenol would be the medication of choice. Quinidine would not be recommended for treating recurrent VF because of its sodium channel blocking properties and risk for hypotension. One of the key variables missing from the patient’s summary is the body temperature on arrival to the ED. Cocaine toxicity causes hyperthermia which greatly affects Brugada substrates and could precipitate tachyarrhythmia.
Brugada phenocopies have been described in clinical settings other than drug overdose, including electrolyte disorders and inflammatory syndromes. Distinguishing phenocopy from BrS involves taking a careful family history of SCA and personal history of syncope or febrile seizure. A review of pertinent laboratory data to evaluate for electrolyte disturbances and current medications to identify drugs that potentiate sodium channel blockade (e.g. lithium or tricyclic antidepressants, and phenytoin) should be performed. On physical examination, the presence of pectus excavatum68 or pericardial rub (pericarditis)69 should be noted, if present, as both conditions may present with a Type 2 Brugada ECG pattern. A high-lead ECG should be performed, and prior ECGs should be reviewed to verify absence or presence of spontaneous Type 1 Brugada pattern. Additional imaging is suggested to rule out any structural cardiac condition or pulmonary embolism70. One expert observed that these data would be helpful in a decision regarding genetic testing.
Two panelists asserted that if the office evaluation was negative, then Brugada phenocopy in the setting of cocaine intoxication was likely. Two experts would perform a sodium channel blocker challenge as a negative drug challenge would confirm the diagnosis of Brugada phenocopy.51,71 Genetic testing would only be considered if a BrS diagnosis was possible or probable based on the Shanghai Score, acknowledging that SCN5A mutations are identified in only 20% of cases.72,73
Cocaine with alcohol intoxication cannot be considered the equivalent of an ajmaline or procainamide challenge. The levels of cocaine and alcohol and their metabolite coca-ethylene contribute to metabolic derangement, altered sympathetic and parasympathetic activity, and sodium channel blockade. ECG changes demonstrating a wide QRS complex, a Brugada pattern and ventricular arrhythmias have all been reported in cocaine intoxication due to primarily sodium channel blocking effects. In contrast, a positive drug challenge with ajmaline or procainamide results in a Type I pattern that reflects abnormal sodium channel function at doses that would not normally elicit ECG changes. While the experts agreed that a Brugada pattern evident with cocaine intoxication would not be diagnostic of BrS, one panelist felt that a Brugada pattern in the presence of alcohol intoxication with an otherwise negative toxicology screen would be equivalent to a drug challenge.
Case 4 Summary (Horie, Kaufman)
Case 4 is challenging because this young patient had a cardiac arrest in the setting of cocaine and alcohol intoxication, then left the hospital against medical advice and was not available for further evaluation. The experts agreed on the details of acute management. If the patient were available for further evaluation, the experts would focus on personal and family history, examination for pectus excavatum or pericardial rub, and additional ECG recordings. They would also consider follow-up imaging (echocardiogram or MRI), and drug challenge to distinguish BrS phenocopy from actual BrS. Genetic testing would be considered only if diagnosis of probable BrS was made. The authors agreed that BrS phenocopy induced by cocaine was not equivalent to ajmaline or procainamide drug challenge. One expert considered that a BrS pattern induced by alcohol alone would be equivalent to a drug challenge.
When the entire group of authors was polled, there were different opinions on whether to consider an ICD (Figure 8 and Supplemental Table IV). The majority (74%) said no, while the others would implant an ICD either based on the VF arrest alone (there was concern for likelihood of repeat drug exposure) or if EPS or imaging studies were abnormal. The authors were divided on whether to proceed with drug challenge to diagnose BrS and not simply BrS phenocopy, with 63% in favor. Most authors recommended imaging, (echo or MRI) to identify possible occult structural heart disease that can underlie cardiac arrest in a young person, even if a provocative event is the trigger. One important gap in knowledge is the natural history of cardiac arrest attributed to BrS phenocopy in the setting of substance abuse, and whether the risk of recurrent VF justifies ICD implantation.
Figure 8.
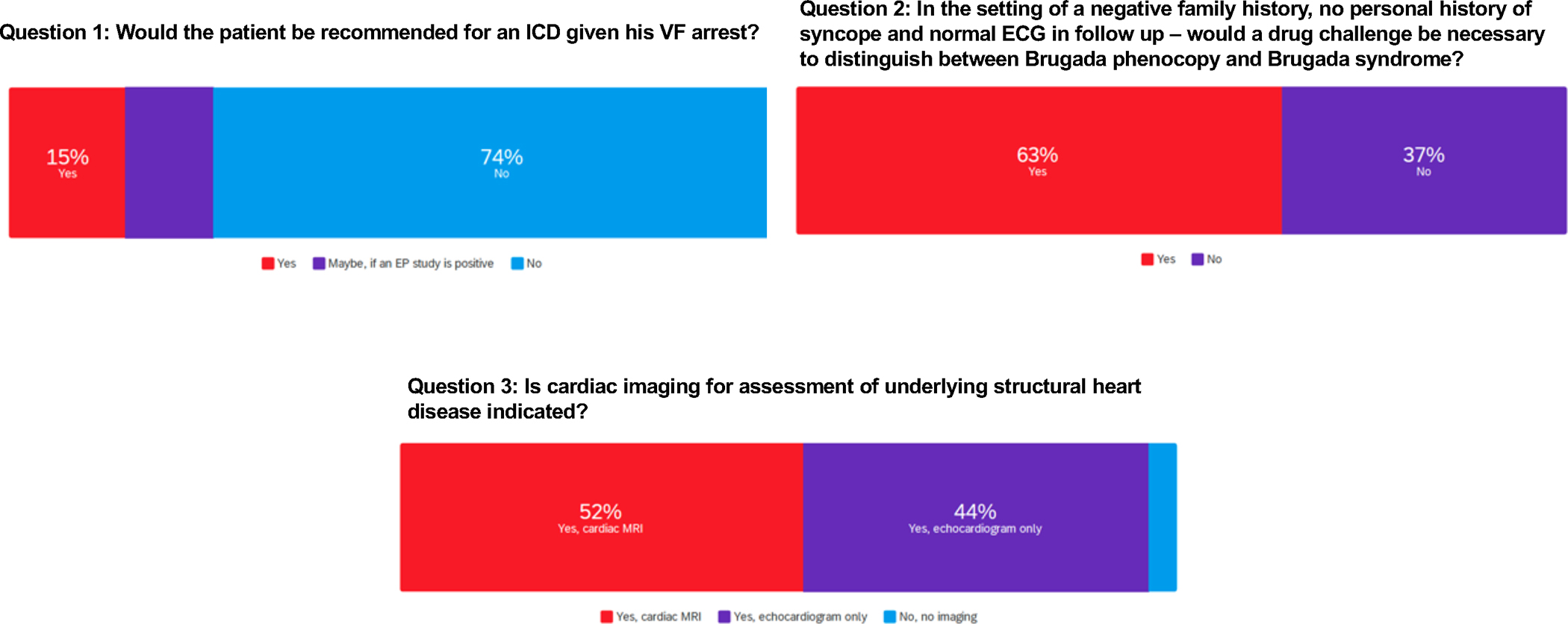
Case 4 Group Voting. Survey results from questions related to case 4.
Conclusions
Experts agree that the diagnosis of BrS requires careful evaluation of available clinical history and data to rule out Brugada phenocopy and confirm BrS. Examination of all available ECGs, including high-lead ECGs, is valuable. Once a diagnosis of BrS is confirmed risk stratification is paramount to guide when lifestyle modification is insufficient and ICD implantation, with its serious implications, should be recommended. To this end, it is crucial for the clinician to distinguish arrhythmic from non-arrhythmic syncope. The experts are divided on the best use of additional risk stratification strategies. Focused genetic testing can be appropriate for diagnosed BrS patients and facilitate cascade family screening but is best performed in a multi-disciplinary cardio-genetics center. Application of the guidelines to real patients requires a thoughtful and individualized approach.
Supplementary Material
Table 2:
Points of Controversy
| Electrophysiology study to guide risk stratification in patient with Brugada Syndrome. |
| Routine use of provocative drug testing in asymptomatic relatives of patients with Brugada Syndrome. |
| Value of genetic testing in the absence of a confirmed Brugada Syndrome diagnosis but with syncope and whether such testing, if performed, should be narrow (SCN5a only) or broad. |
| Value of provocative drug testing to help distinguish Brugada Syndrome from Brugada Phenocopy. |
Acknowledgments:
E.A., L.C., C.D., V.P., F.S., G.S.-B., and A.A.M.W. are members of the European reference Network for rare, low prevalence and complex diseases of the ERN-GUARD Heart.
Sources of Funding:
Dr. Arbelo receives support from Funddacio La Marato de TV3 (Projecte 245/U/2020). Dr. Behr receives support from The Robert Lancaster Memorial Fund. Dr. Krahn receives support from the Paul Brunes Chair in Heart Rhythm Disorders (Vancouver, BC). Dr. London receives support from National Institutes of Health R01 HL062300, R01 HL077398, R01 HL115955. Dr. Lubitz previously received support from NIH grants R01HL139731 and R01HL157635, and American Heart Association 18SFRN34250007 during this project. Dr. Roden receives support from NIH grants R01HL149826 and R01HL164675. Dr. Eckhardt receives support from NIH grants R01HL163987, R01HL141343, R01HL139738, and the Gary and Marie Weiner Professor of Cardiovascular Medicine Research.
Disclosures:
Dr. Lubitz is a full-time employee of Novartis Institutes of BioMedical Research as of July 18, 2022. Dr. Lubitz previously received sponsored research support from Bristol Myers Squibb, Pfizer, Boehringer Ingelheim, Fitbit, Medtronic, Premier, and IBM, and has consulted for Bristol Myers Squibb, Pfizer, Blackstone Life Sciences, and Invitae.
Nonstandard Abbreviations and Acronyms
- BrS
Brugada Syndrome
- ECG
Electrocardiogram
- CAD
Coronary Artery Disease
- ED
Emergency Department
- EMS
Emergency Medical Services
- EPS
Electrophysiology Study
- ICD
Implantable Cardioverter Defibrillator
- SCA
Sudden Cardiac Arrest
- VF
Ventricular Fibrillation
- VUS
Variant of Uncertain Significance
Footnotes
Supplemental Materials:
References:
- 1.Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20:1391–1396. doi: 10.1016/0735-1097(92)90253-j [DOI] [PubMed] [Google Scholar]
- 2.Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, Gussak I, LeMarec H, Nademanee K, Perez Riera AR, et al. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111:659–670. doi: 10.1161/01.CIR.0000152479.54298.51 [DOI] [PubMed] [Google Scholar]
- 3.Antzelevitch C, Yan GX, Ackerman MJ, Borggrefe M, Corrado D, Guo J, Gussak I, Hasdemir C, Horie M, Huikuri H, et al. J-Wave syndromes expert consensus conference report: Emerging concepts and gaps in knowledge. Europace. 2017;19:665–694. doi: 10.1093/europace/euw235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viskin S, Rosso R, Friedensohn L, Havakuk O, Wilde AA. Everybody has Brugada syndrome until proven otherwise? Heart Rhythm. 2015;12:1595–1598. doi: 10.1016/j.hrthm.2015.04.017 [DOI] [PubMed] [Google Scholar]
- 5.Hasdemir C, Payzin S, Kocabas U, Sahin H, Yildirim N, Alp A, Aydin M, Pfeiffer R, Burashnikov E, Wu Y, Antzelevitch C. High prevalence of concealed Brugada syndrome in patients with atrioventricular nodal reentrant tachycardia. Heart Rhythm. 2015;12:1584–1594. doi: 10.1016/j.hrthm.2015.03.015 [DOI] [PubMed] [Google Scholar]
- 6.Therasse D, Sacher F, Babuty D, Mabo P, Mansourati J, Kyndt F, Redon R, Schott JJ, Barc J, Probst V, Gourraud JB. Value of the sodium-channel blocker challenge in Brugada syndrome. Int J Cardiol. 2017;245:178–180. doi: 10.1016/j.ijcard.2017.05.099 [DOI] [PubMed] [Google Scholar]
- 7.Ueoka A, Morita H, Watanabe A, Morimoto Y, Kawada S, Tachibana M, Miyamoto M, Nakagawa K, Nishii N, Ito H. Prognostic Significance of the Sodium Channel Blocker Test in Patients With Brugada Syndrome. J Am Heart Assoc. 2018;7. doi: 10.1161/JAHA.118.008617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adler A, Rosso R, Chorin E, Havakuk O, Antzelevitch C, Viskin S. Risk stratification in Brugada syndrome: Clinical characteristics, electrocardiographic parameters, and auxiliary testing. Heart Rhythm. 2016;13:299–310. doi: 10.1016/j.hrthm.2015.08.038 [DOI] [PubMed] [Google Scholar]
- 9.Veltmann C, Papavassiliu T, Konrad T, Doesch C, Kuschyk J, Streitner F, Haghi D, Michaely HJ, Schoenberg SO, Borggrefe M, et al. Insights into the location of type I ECG in patients with Brugada syndrome: correlation of ECG and cardiovascular magnetic resonance imaging. Heart Rhythm. 2012;9:414–421. doi: 10.1016/j.hrthm.2011.10.032 [DOI] [PubMed] [Google Scholar]
- 10.Morita H, Kusano KF, Miura D, Nagase S, Nakamura K, Morita ST, Ohe T, Zipes DP, Wu J. Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation. 2008;118:1697–1704. doi: 10.1161/CIRCULATIONAHA.108.770917 [DOI] [PubMed] [Google Scholar]
- 11.Sroubek J, Probst V, Mazzanti A, Delise P, Hevia JC, Ohkubo K, Zorzi A, Champagne J, Kostopoulou A, Yin X, et al. Programmed Ventricular Stimulation for Risk Stratification in the Brugada Syndrome: A Pooled Analysis. Circulation. 2016;133:622–630. doi: 10.1161/CIRCULATIONAHA.115.017885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Probst V, Veltmann C, Eckardt L, Meregalli PG, Gaita F, Tan HL, Babuty D, Sacher F, Giustetto C, Schulze-Bahr E, et al. Long-term prognosis of patients diagnosed with Brugada syndrome: Results from the FINGER Brugada Syndrome Registry. Circulation. 2010;121:635–643. doi: 10.1161/CIRCULATIONAHA.109.887026 [DOI] [PubMed] [Google Scholar]
- 13.Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NA, Charron P, Corrado D, Dagres N, de Chillou C, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022;43:3997–4126. doi: 10.1093/eurheartj/ehac262 [DOI] [PubMed] [Google Scholar]
- 14.Probst V, Goronflot T, Anys S, Tixier R, Briand J, Berthome P, Geoffroy O, Clementy N, Mansourati J, Jesel L, et al. Robustness and relevance of predictive score in sudden cardiac death for patients with Brugada syndrome. Eur Heart J. 2021;42:1687–1695. doi: 10.1093/eurheartj/ehaa763 [DOI] [PubMed] [Google Scholar]
- 15.Scrocco C, Ben-Haim Y, Devine B, Tome-Esteban M, Papadakis M, Sharma S, Macfarlane PW, Behr ER. Role of subcutaneous implantable loop recorder for the diagnosis of arrhythmias in Brugada syndrome: A United Kingdom single-center experience. Heart Rhythm. 2022;19:70–78. doi: 10.1016/j.hrthm.2021.08.034 [DOI] [PubMed] [Google Scholar]
- 16.Dwivedi A, Joza J, Malkani K, Mendelson TB, Priori SG, Chinitz LA, Fowler SJ, Cerrone M. Implantable Loop Recorder in Inherited Arrhythmia Diseases: A Critical Tool for Symptom Diagnosis and Advanced Risk Stratification. JACC Clin Electrophysiol. 2018;4:1372–1374. doi: 10.1016/j.jacep.2018.07.008 [DOI] [PubMed] [Google Scholar]
- 17.Stiles MK, Wilde AAM, Abrams DJ, Ackerman MJ, Albert CM, Behr ER, Chugh SS, Cornel MC, Gardner K, Ingles J, et al. 2020 APHRS/HRS expert consensus statement on the investigation of decedents with sudden unexplained death and patients with sudden cardiac arrest, and of their families. Heart Rhythm. 2021;18:e1–e50. doi: 10.1016/j.hrthm.2020.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, Blom N, Brugada J, Chiang CE, Huikuri H, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–1963. doi: 10.1016/j.hrthm.2013.05.014 [DOI] [PubMed] [Google Scholar]
- 19.Yamagata K, Horie M, Aiba T, Ogawa S, Aizawa Y, Ohe T, Yamagishi M, Makita N, Sakurada H, Tanaka T, et al. Genotype-Phenotype Correlation of SCN5A Mutation for the Clinical and Electrocardiographic Characteristics of Probands With Brugada Syndrome: A Japanese Multicenter Registry. Circulation. 2017;135:2255–2270. doi: 10.1161/CIRCULATIONAHA.117.027983 [DOI] [PubMed] [Google Scholar]
- 20.Ciconte G, Monasky MM, Santinelli V, Micaglio E, Vicedomini G, Anastasia L, Negro G, Borrelli V, Giannelli L, Santini F, et al. Brugada syndrome genetics is associated with phenotype severity. Eur Heart J. 2021;42:1082–1090. doi: 10.1093/eurheartj/ehaa942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosseini SM, Kim R, Udupa S, Costain G, Jobling R, Liston E, Jamal SM, Szybowska M, Morel CF, Bowdin S, et al. Reappraisal of Reported Genes for Sudden Arrhythmic Death: Evidence-Based Evaluation of Gene Validity for Brugada Syndrome. Circulation. 2018;138:1195–1205. doi: 10.1161/CIRCULATIONAHA.118.035070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krahn AD, Behr ER, Hamilton R, Probst V, Laksman Z, Han HC. Brugada Syndrome. JACC Clin Electrophysiol. 2022;8:386–405. doi: 10.1016/j.jacep.2021.12.001 [DOI] [PubMed] [Google Scholar]
- 23.Task Force for the D, Management of S, European Society of C, European Heart Rhythm A, Heart Failure A, Heart Rhythm S, Moya A, Sutton R, Ammirati F, Blanc JJ, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30:2631–2671. doi: 10.1093/eurheartj/ehp298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olde Nordkamp LR, Vink AS, Wilde AA, de Lange FJ, de Jong JS, Wieling W, van Dijk N, Tan HL. Syncope in Brugada syndrome: prevalence, clinical significance, and clues from history taking to distinguish arrhythmic from nonarrhythmic causes. Heart Rhythm. 2015;12:367–375. doi: 10.1016/j.hrthm.2014.10.014 [DOI] [PubMed] [Google Scholar]
- 25.Sacher F, Arsac F, Wilton SB, Derval N, Denis A, de Guillebon M, Ramoul K, Bordachar P, Ritter P, Hocini M, et al. Syncope in Brugada syndrome patients: prevalence, characteristics, and outcome. Heart Rhythm. 2012;9:1272–1279. doi: 10.1016/j.hrthm.2012.04.013 [DOI] [PubMed] [Google Scholar]
- 26.Priori SG, Gasparini M, Napolitano C, Della Bella P, Ottonelli AG, Sassone B, Giordano U, Pappone C, Mascioli G, Rossetti G, et al. Risk stratification in Brugada syndrome: results of the PRELUDE (PRogrammed ELectrical stimUlation preDictive valuE) registry. J Am Coll Cardiol. 2012;59:37–45. doi: 10.1016/j.jacc.2011.08.064 [DOI] [PubMed] [Google Scholar]
- 27.Honarbakhsh S, Providencia R, Garcia-Hernandez J, Martin CA, Hunter RJ, Lim WY, Kirkby C, Graham AJ, Sharifzadehgan A, Waldmann V, et al. A Primary Prevention Clinical Risk Score Model for Patients With Brugada Syndrome (BRUGADA-RISK). JACC Clin Electrophysiol. 2021;7:210–222. doi: 10.1016/j.jacep.2020.08.032 [DOI] [PubMed] [Google Scholar]
- 28.Sieira J, Conte G, Ciconte G, Chierchia GB, Casado-Arroyo R, Baltogiannis G, Di Giovanni G, Saitoh Y, Julia J, Mugnai G, et al. A score model to predict risk of events in patients with Brugada Syndrome. Eur Heart J. 2017;38:1756–1763. doi: 10.1093/eurheartj/ehx119 [DOI] [PubMed] [Google Scholar]
- 29.Vitali F, Brieda A, Balla C, Pavasini R, Tonet E, Serenelli M, Ferrari R, Delise P, Rapezzi C, Bertini M. Standard ECG in Brugada Syndrome as a Marker of Prognosis: From Risk Stratification to Pathophysiological Insights. J Am Heart Assoc. 2021;10:e020767. doi: 10.1161/JAHA.121.020767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giustetto C, Drago S, Demarchi PG, Dalmasso P, Bianchi F, Masi AS, Carvalho P, Occhetta E, Rossetti G, Riccardi R, et al. Risk stratification of the patients with Brugada type electrocardiogram: a community-based prospective study. Europace. 2009;11:507–513. doi: 10.1093/europace/eup006 [DOI] [PubMed] [Google Scholar]
- 31.Sieira J, Conte G, Ciconte G, de Asmundis C, Chierchia GB, Baltogiannis G, Di Giovanni G, Saitoh Y, Irfan G, Casado-Arroyo R, et al. Prognostic value of programmed electrical stimulation in Brugada syndrome: 20 years experience. Circ Arrhythm Electrophysiol. 2015;8:777–784. doi: 10.1161/CIRCEP.114.002647 [DOI] [PubMed] [Google Scholar]
- 32.Benito B, Sarkozy A, Mont L, Henkens S, Berruezo A, Tamborero D, Arzamendi D, Berne P, Brugada R, Brugada P, Brugada J. Gender differences in clinical manifestations of Brugada syndrome. J Am Coll Cardiol. 2008;52:1567–1573. doi: 10.1016/j.jacc.2008.07.052 [DOI] [PubMed] [Google Scholar]
- 33.Milman A, Gourraud JB, Andorin A, Postema PG, Sacher F, Mabo P, Conte G, Giustetto C, Sarquella-Brugada G, Hochstadt A, et al. Gender differences in patients with Brugada syndrome and arrhythmic events: Data from a survey on arrhythmic events in 678 patients. Heart Rhythm. 2018;15:1457–1465. doi: 10.1016/j.hrthm.2018.06.019 [DOI] [PubMed] [Google Scholar]
- 34.Sacher F, Meregalli P, Veltmann C, Field ME, Solnon A, Bru P, Abbey S, Jais P, Tan HL, Wolpert C, et al. Are women with severely symptomatic brugada syndrome different from men? J Cardiovasc Electrophysiol. 2008;19:1181–1185. doi: 10.1111/j.1540-8167.2008.01223.x [DOI] [PubMed] [Google Scholar]
- 35.Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36:2793–2867. doi: 10.1093/eurheartj/ehv316 [DOI] [PubMed] [Google Scholar]
- 36.Belhassen B, Rahkovich M, Michowitz Y, Glick A, Viskin S. Management of Brugada Syndrome: Thirty-Three-Year Experience Using Electrophysiologically Guided Therapy With Class 1A Antiarrhythmic Drugs. Circ Arrhythm Electrophysiol. 2015;8:1393–1402. doi: 10.1161/CIRCEP.115.003109 [DOI] [PubMed] [Google Scholar]
- 37.Pappone C, Brugada J, Vicedomini G, Ciconte G, Manguso F, Saviano M, Vitale R, Cuko A, Giannelli L, Calovic Z, et al. Electrical Substrate Elimination in 135 Consecutive Patients With Brugada Syndrome. Circ Arrhythm Electrophysiol. 2017;10:e005053. doi: 10.1161/CIRCEP.117.005053 [DOI] [PubMed] [Google Scholar]
- 38.Wilde AAM, Semsarian C, Marquez MF, Shamloo AS, Ackerman MJ, Ashley EA, Sternick EB, Barajas-Martinez H, Behr ER, Bezzina CR, et al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) Expert Consensus Statement on the state of genetic testing for cardiac diseases. Europace. 2022;24:1307–1367. doi: 10.1093/europace/euac030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Probst V, Wilde AA, Barc J, Sacher F, Babuty D, Mabo P, Mansourati J, Le Scouarnec S, Kyndt F, Le Caignec C, et al. SCN5A mutations and the role of genetic background in the pathophysiology of Brugada syndrome. Circ Cardiovasc Genet. 2009;2:552–557. doi: 10.1161/CIRCGENETICS.109.853374 [DOI] [PubMed] [Google Scholar]
- 40.Wijeyeratne YD, Tanck MW, Mizusawa Y, Batchvarov V, Barc J, Crotti L, Bos JM, Tester DJ, Muir A, Veltmann C, et al. SCN5A Mutation Type and a Genetic Risk Score Associate Variably With Brugada Syndrome Phenotype in SCN5A Families. Circ Genom Precis Med. 2020;13:e002911. doi: 10.1161/CIRCGEN.120.002911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bezzina CR, Barc J, Mizusawa Y, Remme CA, Gourraud JB, Simonet F, Verkerk AO, Schwartz PJ, Crotti L, Dagradi F, et al. Common variants at SCN5A-SCN10A and HEY2 are associated with Brugada syndrome, a rare disease with high risk of sudden cardiac death. Nat Genet. 2013;45:1044–1049. doi: 10.1038/ng.2712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savastano S, Rordorf R, Vicentini A, Petracci B, Taravelli E, Castelletti S, D’Errico A, Torchio M, Dossena C, Novara P, et al. A comprehensive electrocardiographic, molecular, and echocardiographic study of Brugada syndrome: validation of the 2013 diagnostic criteria. Heart Rhythm. 2014;11:1176–1183. doi: 10.1016/j.hrthm.2014.04.010 [DOI] [PubMed] [Google Scholar]
- 43.Brugada J, Campuzano O, Arbelo E, Sarquella-Brugada G, Brugada R. Present Status of Brugada Syndrome: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72:1046–1059. doi: 10.1016/j.jacc.2018.06.037 [DOI] [PubMed] [Google Scholar]
- 44.Richter S, Sarkozy A, Paparella G, Henkens S, Boussy T, Chierchia GB, Brugada R, Brugada J, Brugada P. Number of electrocardiogram leads displaying the diagnostic coved-type pattern in Brugada syndrome: a diagnostic consensus criterion to be revised. Eur Heart J. 2010;31:1357–1364. doi: 10.1093/eurheartj/ehq049 [DOI] [PubMed] [Google Scholar]
- 45.Sangwatanaroj S, Prechawat S, Sunsaneewitayakul B, Sitthisook S, Tosukhowong P, Tungsanga K. New electrocardiographic leads and the procainamide test for the detection of the Brugada sign in sudden unexplained death syndrome survivors and their relatives. Eur Heart J. 2001;22:2290–2296. doi: 10.1053/euhj.2001.2691 [DOI] [PubMed] [Google Scholar]
- 46.Shimizu W, Matsuo K, Takagi M, Tanabe Y, Aiba T, Taguchi A, Suyama K, Kurita T, Aihara N, Kamakura S. Body surface distribution and response to drugs of ST segment elevation in Brugada syndrome: clinical implication of eighty-seven-lead body surface potential mapping and its application to twelve-lead electrocardiograms. J Cardiovasc Electrophysiol. 2000;11:396–404. doi: 10.1111/j.1540-8167.2000.tb00334.x [DOI] [PubMed] [Google Scholar]
- 47.Miyamoto K, Yokokawa M, Tanaka K, Nagai T, Okamura H, Noda T, Satomi K, Suyama K, Kurita T, Aihara N, et al. Diagnostic and prognostic value of a type 1 Brugada electrocardiogram at higher (third or second) V1 to V2 recording in men with Brugada syndrome. Am J Cardiol. 2007;99:53–57. doi: 10.1016/j.amjcard.2006.07.062 [DOI] [PubMed] [Google Scholar]
- 48.Nagase S, Hiramatsu S, Morita H, Nishii N, Murakami M, Nakamura K, Kusano KF, Ito H, Ohe T. Electroanatomical correlation of repolarization abnormalities in Brugada syndrome: detection of type 1 electrocardiogram in the right ventricular outflow tract. J Am Coll Cardiol. 2010;56:2143–2145. doi: 10.1016/j.jacc.2010.06.050 [DOI] [PubMed] [Google Scholar]
- 49.Miyazaki T, Mitamura H, Miyoshi S, Soejima K, Aizawa Y, Ogawa S. Autonomic and antiarrhythmic drug modulation of ST segment elevation in patients with Brugada syndrome. J Am Coll Cardiol. 1996;27:1061–1070. doi: 10.1016/0735-1097(95)00613-3 [DOI] [PubMed] [Google Scholar]
- 50.Antzelevitch C, Brugada R. Fever and Brugada syndrome. Pacing Clin Electrophysiol. 2002;25:1537–1539. doi: 10.1046/j.1460-9592.2002.01537.x [DOI] [PubMed] [Google Scholar]
- 51.Baranchuk A, Nguyen T, Ryu MH, Femenia F, Zareba W, Wilde AA, Shimizu W, Brugada P, Perez-Riera AR. Brugada phenocopy: new terminology and proposed classification. Ann Noninvasive Electrocardiol. 2012;17:299–314. doi: 10.1111/j.1542-474X.2012.00525.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Behr ER, Dalageorgou C, Christiansen M, Syrris P, Hughes S, Tome Esteban MT, Rowland E, Jeffery S, McKenna WJ. Sudden arrhythmic death syndrome: familial evaluation identifies inheritable heart disease in the majority of families. Eur Heart J. 2008;29:1670–1680. doi: 10.1093/eurheartj/ehn219 [DOI] [PubMed] [Google Scholar]
- 53.Serra G, Baranchuk A, Bayes-De-Luna A, Brugada J, Goldwasser D, Capulzini L, Arazo D, Boraita A, Heras ME, Garcia-Niebla J, et al. New electrocardiographic criteria to differentiate the Type-2 Brugada pattern from electrocardiogram of healthy athletes with r’-wave in leads V1/V2. Europace. 2014;16:1639–1645. doi: 10.1093/europace/euu025 [DOI] [PubMed] [Google Scholar]
- 54.Meregalli PG, Ruijter JM, Hofman N, Bezzina CR, Wilde AA, Tan HL. Diagnostic value of flecainide testing in unmasking SCN5A-related Brugada syndrome. J Cardiovasc Electrophysiol. 2006;17:857–864. doi: 10.1111/j.1540-8167.2006.00531.x [DOI] [PubMed] [Google Scholar]
- 55.Hong K, Brugada J, Oliva A, Berruezo-Sanchez A, Potenza D, Pollevick GD, Guerchicoff A, Matsuo K, Burashnikov E, Dumaine R, et al. Value of electrocardiographic parameters and ajmaline test in the diagnosis of Brugada syndrome caused by SCN5A mutations. Circulation. 2004;110:3023–3027. doi: 10.1161/01.CIR.0000144299.17008.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolpert C, Echternach C, Veltmann C, Antzelevitch C, Thomas GP, Spehl S, Streitner F, Kuschyk J, Schimpf R, Haase KK, Borggrefe M. Intravenous drug challenge using flecainide and ajmaline in patients with Brugada syndrome. Heart Rhythm. 2005;2:254–260. doi: 10.1016/j.hrthm.2004.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ensam B, Cheung CC, Almehmadi F, Gregers Winkel B, Scrocco C, Brennan P, Leong K, Muir A, Vanarva A, Tfelt-Hansen J, et al. The Utility of Sodium Channel Provocation in Unexplained Cardiac Arrest Survivors and Electrocardiographic Predictors of Ventricular Fibrillation Recurrence. Circ Arrhythm Electrophysiol. 2022;15:e011263. doi: 10.1161/CIRCEP.122.011263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilde AAM, Amin AS, Morita H, Tadros R. Use, misuse, and pitfalls of the drug challenge test in the diagnosis of the Brugada syndrome. Eur Heart J. 2023;44:2427–2439. doi: 10.1093/eurheartj/ehad295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brignole M, Moya A, de Lange FJ, Deharo JC, Elliott PM, Fanciulli A, Fedorowski A, Furlan R, Kenny RA, Martin A, et al. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J. 2018;39:1883–1948. doi: 10.1093/eurheartj/ehy037 [DOI] [PubMed] [Google Scholar]
- 60.Mascia G, Bona RD, Ameri P, Canepa M, Porto I, Parati G, Crotti L, Brignole M. Brugada syndrome and syncope: a practical approach for diagnosis and treatment. Europace. 2021;23:996–1002. doi: 10.1093/europace/euaa370 [DOI] [PubMed] [Google Scholar]
- 61.Hernandez-Ojeda J, Arbelo E, Jorda P, Borras R, Campuzano O, Sarquella-Brugada G, Iglesias A, Mont L, Brugada R, Brugada J. The role of clinical assessment and electrophysiology study in Brugada syndrome patients with syncope. Am Heart J. 2020;220:213–223. doi: 10.1016/j.ahj.2019.10.016 [DOI] [PubMed] [Google Scholar]
- 62.Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018;72:e91–e220. doi: 10.1016/j.jacc.2017.10.054 [DOI] [PubMed] [Google Scholar]
- 63.Crotti L, Marcou CA, Tester DJ, Castelletti S, Giudicessi JR, Torchio M, Medeiros-Domingo A, Simone S, Will ML, Dagradi F, et al. Spectrum and prevalence of mutations involving BrS1- through BrS12-susceptibility genes in a cohort of unrelated patients referred for Brugada syndrome genetic testing: implications for genetic testing. J Am Coll Cardiol. 2012;60:1410–1418. doi: 10.1016/j.jacc.2012.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim ST, Park T. Acute and Chronic Effects of Cocaine on Cardiovascular Health. Int J Mol Sci. 2019;20. doi: 10.3390/ijms20030584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bayes de Luna A, Brugada J, Baranchuk A, Borggrefe M, Breithardt G, Goldwasser D, Lambiase P, Riera AP, Garcia-Niebla J, Pastore C, et al. Current electrocardiographic criteria for diagnosis of Brugada pattern: a consensus report. J Electrocardiol. 2012;45:433–442. doi: 10.1016/j.jelectrocard.2012.06.004 [DOI] [PubMed] [Google Scholar]
- 66.Yap YG, Behr ER, Camm AJ. Drug-induced Brugada syndrome. Europace. 2009;11:989–994. doi: 10.1093/europace/eup114 [DOI] [PubMed] [Google Scholar]
- 67.McCance EF, Price LH, Kosten TR, Jatlow PI. Cocaethylene: pharmacology, physiology and behavioral effects in humans. J Pharmacol Exp Ther. 1995;274:215–223. [PubMed] [Google Scholar]
- 68.Awad SF, Barbosa-Barros R, Belem Lde S, Cavalcante CP, Riera AR, Garcia-Niebla J, Anselm DD, Baranchuk A. Brugada phenocopy in a patient with pectus excavatum: systematic review of the ECG manifestations associated with pectus excavatum. Ann Noninvasive Electrocardiol. 2013;18:415–420. doi: 10.1111/anec.12082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shehadeh M, O’Donoghue S. Acute Pericarditis-Induced Brugada Phenocopy: A Case Report and Review of the Literature. Cureus. 2020;12:e9761. doi: 10.7759/cureus.9761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Elikowski W, Lazowski S, Fertala N, Zawodna-Marszalek M, Szczesniewski P, Bolewski A, Zytkiewicz M. Brugada phenocopy in pulmonary embolism - clinicopathological case study and literature review. Pol Merkur Lekarski. 2022;50:378–383. [PubMed] [Google Scholar]
- 71.Dendramis G Brugada syndrome and Brugada phenocopy. The importance of a differential diagnosis. Int J Cardiol. 2016;210:25–27. doi: 10.1016/j.ijcard.2016.02.097 [DOI] [PubMed] [Google Scholar]
- 72.Kapplinger JD, Tester DJ, Alders M, Benito B, Berthet M, Brugada J, Brugada P, Fressart V, Guerchicoff A, Harris-Kerr C, et al. An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm. 2010;7:33–46. doi: 10.1016/j.hrthm.2009.09.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hu D, Barajas-Martinez H, Pfeiffer R, Dezi F, Pfeiffer J, Buch T, Betzenhauser MJ, Belardinelli L, Kahlig KM, Rajamani S, et al. Mutations in SCN10A are responsible for a large fraction of cases of Brugada syndrome. J Am Coll Cardiol. 2014;64:66–79. doi: 10.1016/j.jacc.2014.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


