Abstract
BACKGROUND:
Global collaboration in cardio-oncology is needed to understand the prevalence of cancer therapy-related cardiovascular toxicity in different risk groups, practice settings, and geographic locations. There are limited data on the socioeconomic and racial/ethnic disparities that may impact access to care and outcomes. To address these gaps, we established the Global Cardio-Oncology Registry, a multinational, multicenter prospective registry.
METHODS:
We assembled cardiologists and oncologists from academic and community settings to collaborate in the first Global Cardio-Oncology Registry. Subsequently, a survey for site resources, demographics, and intention to participate was conducted. We designed an online data platform to facilitate this global initiative.
RESULTS:
A total of 119 sites responded to an online questionnaire on their practices and main goals of the registry: 49 US sites from 23 states and 70 international sites from 5 continents indicated a willingness to participate in the Global Cardio-Oncology Registry. Sites were more commonly led by cardiologists (85/119; 72%) and were more often university/teaching (81/119; 68%) than community based (38/119; 32%). The average number of cardio-oncology patients treated per month was 80 per site. The top 3 Global Cardio-Oncology Registry priorities in cardio-oncology care were breast cancer, hematologic malignancies, and patients treated with immune checkpoint inhibitors. Executive and scientific committees and specific committees were established. A pilot phase for breast cancer using Research Electronic Data Capture Cloud platform recently started patient enrollment.
CONCLUSIONS:
We present the structure for a global collaboration. Information derived from the Global Cardio-Oncology Registry will help understand the risk factors impacting cancer therapy-related cardiovascular toxicity in different geographic locations and therefore contribute to reduce access gaps in cardio-oncology care. Risk calculators will be prospectively derived and validated.
Keywords: cardiology, cardiotoxicity, cardiovascular disease, hematology, myocarditis
Cardio-oncology has evolved from its infancy toward a mature and well-established subspecialty in the last decade. Multiple collaborative initiatives have been undertaken to move this field forward; the establishment of national and international cardio-oncology societies, the development of position articles,1,2 the launch of several peer-reviewed cardio-oncology journals,3 and recent international endeavors for board certification, just to name a few. Increased awareness in the cardiology, oncology, hematology, and radiotherapy communities has sparked collaborations between different specialties to provide patient-tailored treatment options focused toward short- and long-term cardiovascular care and outcomes. Consequently, numerous academic and community hospitals have set up dedicated cardio-oncology clinics and programs to accommodate the growing need to serve this patient population.4
Cardio-oncology nevertheless remains a relatively new subspecialty addressing the cardiovascular care of cancer patients before, during, and after cancer treatment. Numerous cancer treatment modalities including cytotoxic and targeted chemotherapy, immunotherapy, and radiation treatment can result in significant cancer treatment-related cardiovascular toxicity (CTR-CVT).5,6 Historically, cardiotoxicity referred to myocardial dysfunction and heart failure caused by systemic anticancer treatment. However, CTR-CVT comprises a very broad spectrum of cardiovascular disorders including systemic and pulmonary hypertension, arrhythmias, coronary artery disease, autonomic dysfunction, valvular dysfunction, and pericardial disease.6,7 Novel treatments, such as immune checkpoint inhibitors (ICI), have revolutionized the therapeutic possibilities in patients with previously untreatable malignancies. With widespread implementation of these new drugs, previously unrecognized cardiovascular side effect such as ICI-associated myocarditis have emerged with potentially lethal outcome.8 This echoes the unanticipated reports of heart failure after anthracyclines in the 1970s9; the excessive long-term morbidity and mortality from accelerated coronary and valvular heart disease after mantle field radiation in the 1980s10; and the unexpected high risk of left ventricular dysfunction and heart failure with trastuzumab in the 2000s.11 This highlights the importance of continuous vigilance to monitor and investigate new anticancer drugs for their potential for cardiovascular toxicities, not only during the initial 3 phases of clinical drug trials but also after FDA approval when used on a larger scale. Postmarketing surveillance is particularly relevant for identification of safety signals in patients that may have been excluded from clinical trials, such as those with preexisting cardiovascular disease.
While several cardiovascular risk models are available for well-established cancer treatments, none has been thoroughly validated.12 Consequently, identifying patients at high risk for CTR-CVT is challenging, hampering the development of robust practical guidelines for referral to cardio-oncology before the initiation of anticancer treatment. Moreover, most position articles and guidelines rely heavily on expert opinion regarding risk assessment, imaging, biomarkers selection, and duration and frequency needed for follow-up assessments.13 Discrepancies between the most recent American and European guidelines have recently been addressed with the hope of providing a roadmap for clinicians to use in their daily practice.1 Also, the European Society of Cardiology, in conjunction with the Heart Failure Association and the European Association of Cardiovascular Imaging proposed practical position statements on risk assessment and follow-up using imaging and biomarkers.14,15 However, it is unclear how such guidance translates to treating CTR-CVT to different practice settings, various regions of the world, and in different socioeconomic and demographic groups.
A major limitation in various consensus opinions and guidelines is the lack of data due to a paucity of large-scale clinical trials upon which recommendations can be based in cardio-oncology. Additionally, most land-mark pharmacological or device interventional trials in cardiology excluded patients with active or recent cancer treatment and oncological trials in turn excluded patients with severe cardiovascular comorbidities.16 This mutual ostracizing of patient populations limits the translatability of cardiovascular guidelines in cancer patients and vice versa. Only recently, several trials have emerged that are focused on treatment or prevention of CTR-CVT with variable success.17-20 Currently, these trials lack sufficient power to change clinical practice due to discordant results, small sample sizes, and heterogeneity in inclusion criteria or definitions of end points.19 While awaiting more extensive prospective randomized clinical trials, real-world data obtained from our prospective multicenter, international registry will provide insight into optimal (or futile) screening and treatment strategies.21,22
To address these gaps, we established the Global Cardio-Oncology Registry (G-COR), a multinational, multicenter prospective registry including large academic medical centers and community hospitals.
AIMS AND OBJECTIVES
To establish the incidence of CTR-CVT in patients referred to Cardio-Oncology services in all participating centers (university/community based).
To identify risk factors for CTR-CVT, derive, and validate risk score models.
To evaluate geographic and regional differences in the use of biomarkers and imaging modalities, and their impact on the management of CTR-CVT.
To evaluate the impact of socioeconomic and demographic variables in access to care, surveillance strategies, treatments, and outcomes.
To describe outcomes of cancer survivors treated with different potentially cardiotoxic therapies in different geographic locations.
To provide a platform for multiple collaborations, substudies, and prospective clinical trials.
To provide a quality initiative, sites can evaluate their data and compare it with the general database to identify opportunities for improvement of local practices.
METHODS
Registry Design
G-COR is a multicenter international observational cohort study that will prospectively enroll patients referred to dedicated cardio-oncology services. Medical centers (both academic and community) with dedicated cardio-oncology programs were invited to participate in this registry.
The principal investigator from each site will be responsible for data entry, data accuracy, and follow-up of the enrolled patients. Data will be handled confidentially and coded. Patient privacy is protected by assigning a nonretraceable sequential subject number. Each participating center will have full access to its site’s data. Data will be collected in electronic case report forms on the Research Electronic Data Capture (REDCap) Cloud server. The collected data will be stored in a central database, hosted by the Cleveland Clinic C5 Research Division, which also provides IT support, platform development, testing and validation, and security and data protection. Data management, REDCap Cloud platform implementation and Quality control by the C5 Research Division, and prospective data and global coordination for the global phase to be done by the Cleveland Clinic Cardiovascular Outcomes Registries and Research team.
This electronic data capture platform is a Cloud-based platform. It allows to store patient data per regional guidelines within multiple locations around the globe. Being a cloud-based platform, it allows the participating sites anywhere in the world to enter their data. The Registry is hosted and monitored by the Cleveland Clinic C5 Research Division and the Cardiovascular Outcomes Registries and Research division. The Data Management group oversees database training, database-designed operating data dictionary, site activation, and queries for incomplete or out-of-range data. The REDCap Cloud is a secure validated platform, which was tested and validated for G-COR by the Cleveland Clinic Data Management team before the initiation of the Pilot phase. Before site activation, each site must obtain local Institutional Review Board (IRB) approval, execute a Data User Agreement, and must undergo protocol training monitored by the Executive Committee, and database training monitored by the Data Management team. After completion of training, database access is provided to trained healthcare providers as sites are activated.
The initial blueprint of the REDCap entry log was provided by the ONCOR registry investigators, and adjusted and modified to meet the unique research questions of G-COR.23 These platforms are designed to allow for data collection and storage in compliance with international regulations. This study was approved by the Cleveland Clinic Heart and Vascular Institute Research Committee and subsequently submitted and approved by the Cleveland Clinic IRB.
The REDCap Cloud platform will be automated to detect incomplete or out-of-range data, and investigators will be notified. The pilot phase with breast cancer (BC) patients started enrollment late in 2022 with up to 25 US centers projected to participate. This pilot phase is necessary to ensure that all the recruitment, legal agreements, Data User Agreement approvals, local IRB approvals, site activations, and data entry are functioning well. In this phase, we are testing the sites’ activation procedures, investigators’ training, and the workflow of data entry into the electronic case report forms. The pilot phase has started with 1 pillar, BC, the most common condition at most surveyed institutions, and will confirm that all processes are in place. The G-COR pilot phase has successfully started, and all the stated goals are being successfully achieved. Our goal is to have an efficient system in place to roll out the global phase. Twelve sites have already been activated and started recruitment at the time of this writing. Subsequently, with the lessons learned from the initial recruitment in the pilot phase, the G-COR international global phase will be launched subsequently.
Participating sites will prospectively enroll suitable patients. To avoid patient selection bias, sites will be instructed to enroll the first 2 eligible cardio-oncology consults every week in each pillar and subsequently consecutive patients. Each center will be allowed to establish an equivalent random mechanism that better fits their clinic schedule and patients’ demographics. Data will be entered into electronic case report forms from the patient interview and electronic health records of the clinical visits, according to the current practice standards. Uniform data collection will be achieved by using a specially designed operating data dictionary provided to each site at the time of activation, which provides definitions of medical terms, definitions of specific parameters, and (ab)normal values. Each participating center is encouraged to comply with existing national/international recommendations, although patients will be managed at the discretion of the treating physician according to their usual standard of care and no intervention or change of treatment will occur related to G-COR.
Patient Population and Data Collection
Patient recruitment and data collection will initially focus on BC in the pilot phase of the study, followed by the 3 main pillars of this global registry based on the clinical priorities determined from a survey taken among the participating centers: (1) cardio oncology in BC patients; (2) hematologic malignancies (HM) referred to cardio-oncology clinic; and (3) ICI with associated cardiovascular comorbidities or complications. All variables collected will comply with the General Data Protection Regulation (GDPR) guidelines.
Adult patients (18 years and older) with BC and HM, and patients treated with ICI who present for their initial cardio-oncology consultation at participating sites will be eligible for enrollment and follow-up provided that they are willing and able to provide written informed consent or conform to the local regulatory bodies guidelines for study participation enrollment.
Patients are referred either before initiation of treatment (preexisting cardiovascular disease, high cardiovascular risk-multiple risk factors, or high potential for cardiotoxicity cancer treatment), during cancer treatment (abnormal test results, symptoms, or cardiovascular events during treatment), or after cancer treatment (cardiovascular sequelae, coexisting cardiovascular disease, signs, symptoms, abnormal testing, or high cardiovascular complication risk after treatment). These 3 timing categories (before, during, or after cancer treatment) and the reason for the cardio-oncology referral (high risk, symptoms, abnormal test results, or multiple causes) are captured in the initial visit electronic case report form and will be evaluated as registry entry points.
Patients will have proposed clinical evaluations at baseline and at 3, 6, 9, and 12 months after enrollment, or as clinically necessary. Patients will have follow-up for 18 months after enrollment. The participating sites will have flexibility to follow their routine visits according to their clinical practice even if they do not strictly conform to the proposed visits timeline.
Data Collection
At baseline, detailed information will be collected regarding the following: (1) demographic and socioeconomic variables; (2) cardiovascular risk factors and history including previous events, interventions, and use of cardiovascular medications; (3) cancer information (eg, TNM classification, receptor status in BC patients, clinical-stage, etc); (4) cancer treatment information (eg, type, cumulative dose of chemotherapy, targeted therapy, immunotherapy, and radiotherapy); (5) functional status (ECOG and the Karnofsky performance status)24; and (6) weekly exercise habits to evaluate its impact on cancer outcomes in different groups: exercise by time (<30 minutes, 30–60, and >60) and times per week (0–1, 2–3, >4 times/wk).
New cardiovascular events or changes in cardiovascular medications, changes in cancer course or cancer treatment, along with vital signs, laboratory/biomarkers, and echocardiographic imaging data will be prospectively collected during follow-up visits.
G-COR will also collect results of additional nonroutine, noninvasive testing such as cardiovascular magnetic resonance, CT coronary angiography, CT coronary calcium score, Holter/event monitors, stress testing, SPECT, or MUGA scan, and invasive testing such as left and right heart catheterization and endomyocardial biopsy.
Demographic data with age, sex, ethnicity, race, health insurance, employment status, geographic area (urban, suburban, and rural), transportation, access to the internet and cell phone, and education history are collected. These social determinants of health are obtainable without IRBs and regulatory international entities (such as GDPR) objections. Other variables, such as Zip code (used for Social Vulnerability Index) are not collected due to conflict with de-identified data.
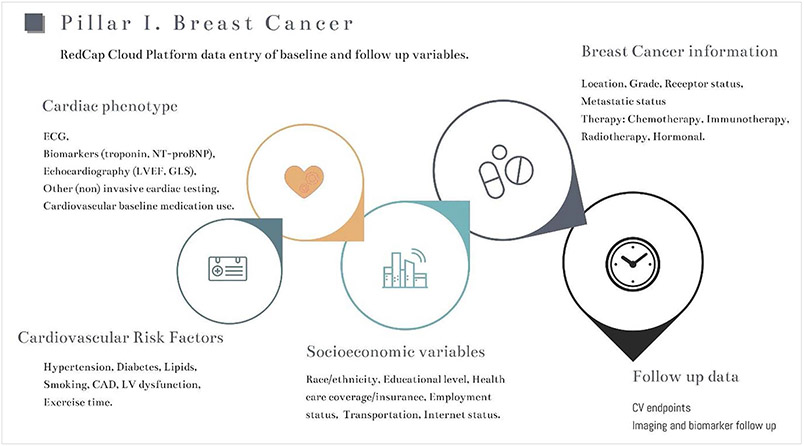
The primary outcome will be the occurrence of CTR-CVT and overall outcome including cardiovascular and oncological death.7 This will be correlated to (known and new) risk factors and risk groups, and the impact of the occurrence of cardiotoxicity on anticancer treatment and outcome. Furthermore, the multinational, multicenter design of this registry offers the opportunity to study the prevalence and management of CTR-CVT in different practice settings and geographic locations as well as racial/ethnic disparities. Finally, socioeconomic determinants of access to care and outcomes will be assessed. The data collection workflow of the G-COR pillar 1 (BC) is shown in Figure 1.
Figure 1. Data collection design for pillar I: breast cancer.
Data collection and entry of baseline and follow-up variables are shown. Specific data collection is modeled to each pillar to ensure both complete and relevant data collection. CAD indicates coronary artery disease; CV, cardiovascular; GLS, global longitudinal strain; LV, left ventricle; and LVEF, left ventricular ejection fraction.
Governance and Committees
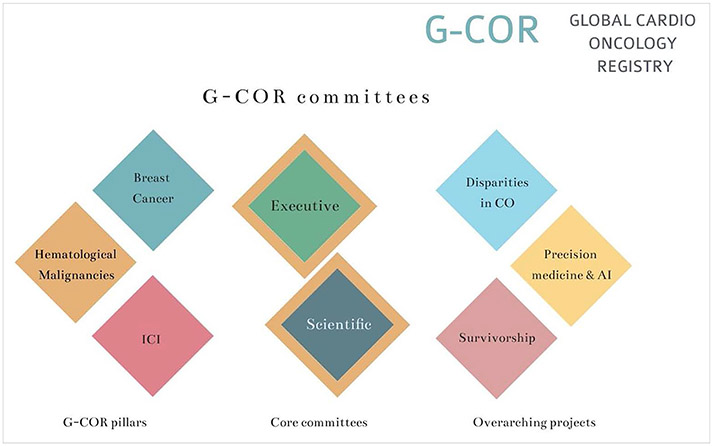
The governance of the G-COR will be performed by the designated committees. These committees have been established with investigators from a variety of settings to represent the diverse nature of the participating institutions and the patients they serve, to be as inclusive as possible. The committees include leading experts in cardio-oncology, leaders from professional medical societies, cardio-oncology leaders in different geographic areas, and practicing cardio-oncologists from both academic centers and community hospitals. Sites will have access to their own data, but statistical analysis will be done centrally and guided by the scientific and executive committees. All the data analysis, article writing, and review will be under the umbrella and approval of the scientific committees. Proposed substudies with scientific merit will be presented to and approved by the scientific committee. Each new substudy will require a new IRB submission and approval. The executive committee will oversee the overall execution of the registry. The different committees are depicted in Figure 2 and described in detail (function and committee members) in Appendix A.
Figure 2. Global Cardio-Oncology Registry (G-COR) committees.
See Appendix A for detailed information on committee members. AI indicates artificial intelligence; CO, cardio-oncology; and ICI, immune check point inhibitors.
Sites will have access to their own data, but statistical analysis will be done to G-COR data as guided by the scientific and executive committee. Furthermore, data will be available on request from the author. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Overview of Participating Centers
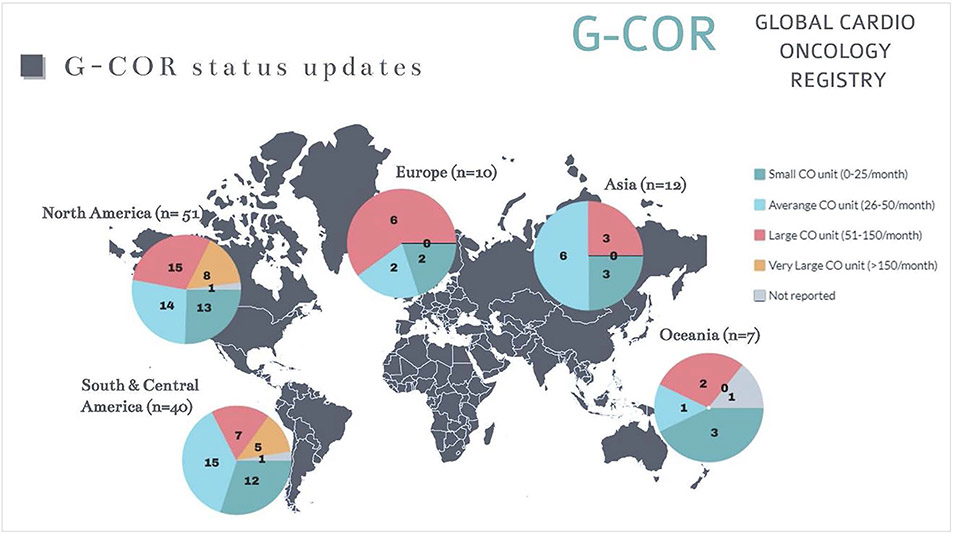
At the time of writing, a total of 119 centers (81 universities 68%, and 38 community hospitals 32%) in 21 different countries across 5 continents indicated intention and willingness to participate in this registry. Typically, these clinics were led jointly by cardiologists and hematologist/oncologist (85/119; 72%) or by cardiologists alone (34/119; 27%).
These centers completed a preregistry demographics site survey to facilitate the design and launching of the G-COR (response to specific queries; n-response). Overall, a total of 80.5±65.3 (median 42.5/mo) cardio-oncology (CO) patients are seen each month with a relatively large spread. Centers could be defined as having a small CO unit (0–25 patients/mo) n=32, average CO unit (26–50 patients/mo) n=39, large CO units (51–150 patients/mo) n=35, and very large CO units (>150 patients/mo) n=13. Details on the geographic distribution are provided in Figure 3.
Figure 3. World map of participating centers.
Information and geographic distribution of the Global Cardio-Oncology Registry (G-COR) across the 5 continents is shown. N indicates the number of participating centers in each continent. The pie charts show the details of the size of the participating centers. Color-legend is depicted on the top right. CO indicates cardio oncology.
Estimated number in the specific patient categories were as follows: mean new BC patients 17.5±12.5 month (median 10.0/mo; n-response=110); mean new HM patients 15.6±11.0/mo (median 10.0/mo; n-response=109); and a mean number of new ICI patients 6.5±6.1/mo (median 4.0/mo; n-response=98). It should be noted that the relatively low center-specific response rate for new ICI patients could very well result in an overall overestimation of average patients’ numbers. A total of 36 of 49 North American sites reported substantial racial/ethnic minorities or disadvantaged patient populations versus a total of 19 of 62 international sites.
Regarding the facilities for screening CO patients, 107 sites routinely apply advanced echocardiography including strain imaging and 93 sites have access to cardiac MRI for CO patients. Regarding the use of biomarkers, 23 primarily use troponin T, 34 troponin I, and 51 routinely use high-sensitive troponin to detect cardiotoxicity.
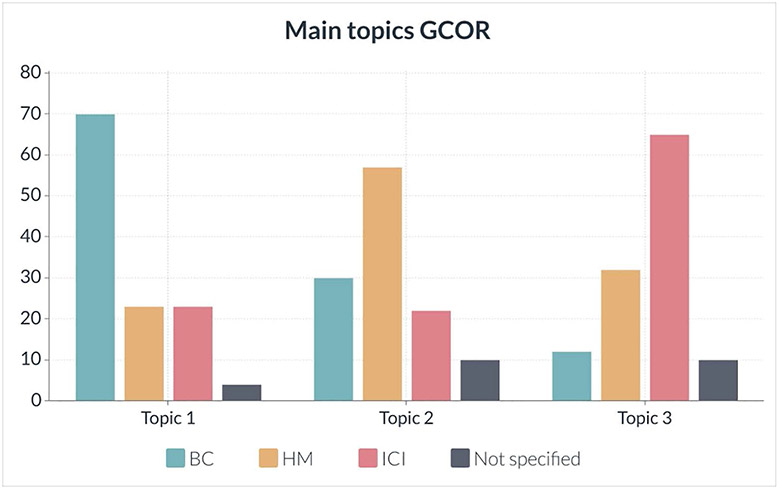
Three areas were identified as the main topics of interest at participating sites that will impact their practices (as indicated in the Methods section, see Figure 4); most centers indicated the importance of cardiotoxicity in BC, followed by HM and finally ICI-related cardiotoxicity. These subsequently formed the 3 pillars of G-COR.
Figure 4. Survey results.
This shows feedback on the main areas of research interest (sites could prioritize the different research topics). Most centers indicated BC as the primary topic of research, HM was most often mentioned as the second topic, and followed by ICI. The absolute number of responses is indicated on the vertical axis. BC indicates breast cancer; G-COR, Global Cardio-Oncology Registry; HM, hematologic malignancies; and ICI, immune checkpoint inhibitors.
DISCUSSION
In this design article, we describe the establishment and launch of the first global multicenter cardio-oncology registry (G-COR), which aims to facilitate multiple research questions and quality improvement initiatives in a real-world clinical setting. Our survey in over 100 collaborating centers identified 3 distinct research topics based on their clinical volume and needs. This led to initiation of 3 pillars of primary research focus: (1) CO in BC patients; (2) CO in hematologic malignancies; and (3) ICI-associated cardiovascular complications. Our registry will provide unique opportunities to study disparities in cardio-oncology practice and outcomes, validate risk calculators, address the implementation and adherence to national and international recommendation/guidelines, while providing a database of comprehensive clinical variables equipped for in-depth research questions, which will be determined in the future. Indeed, clinical registries are particularly well-suited for studying broader populations in a real-world practice setting and for obtaining more representative outcomes regarding the incidence, prognosis, and outcomes of CTR-CVT across various populations, the implementation and clinical yield of screening recommendations, association of cardiovascular management for prevention of cardiotoxicity, and treatment of cardiovascular complications during and after cancer treatment.25
The increasing number of cancer survivors over the last decade has reached >16 million and is projected to be over 20 million over the next decade.26 The association between cardiovascular disease and cancer includes the existence of shared risk factors, the increased incidence of cardiovascular disease in cancer patients compared with noncancer patients, and the now well-established evidence of CTR-CVT as a significant factor in the overall prognosis of cancer survivors.27 This highlights the need for a collaborative effort by the cardiology community to address the often-chronic cardiovascular healthcare needs of cancer patients and survivors.4 Based on these observations, a new algorithm for classification of cardio-oncology syndromes has recently been proposed.27 This classification explores and characterizes the complex multifaceted relationship that exists between cancer and cardiovascular disease ranging from the interplay between cancer biology and cardiovascular disease including the (in)direct effects of anticancer treatment. This will further enhance our understanding of the different interactions between cancer biology, anticancer treatment, predisposing cardiovascular conditions, and (in)direct cardiotoxicity. Larger cohorts will most likely shed more light on the complex interplay between these 2 disease entities and allow further exploration on the impact of common risk factors (eg, smoking, obesity, and diabetes) and bidirectional pathophysiological pathways (eg, chronic inflammation and genetic predisposition).22 Finally, research in cardio-oncology has been hampered by heterogeneity in patients with respect to underlying disease, disease state, anticancer treatment, and their cardiovascular baseline risk. Extracting meaningful data from a cardio-oncology registry by identifying homogenous patients’ groups can only be possible with a substantial amount of patient data.28 By enrolling high numbers of patients around the globe, we will use this unique feature of the G-COR registry design for this specific purpose, something that cannot be achieved by a single-center undertaking.25
While efforts are pursued to minimize cardiovascular damage in the cancer patient without attenuating anticancer treatment per se, clinical practice often relies on case-based decision-making due to a lack of data from large clinical trials.29 Novel treatment strategies that have been explored in small clinical trials could readily be tested and evaluated in the G-COR registry once implemented in the clinical realm. For example, the SCHOLAR30 and the SAFE-HEaRt31 studies have both shown safety and feasibility of continuing adjuvant treatment with trastuzumab in the treatment of HER2+ BC in patients with mild left ventricular dysfunction (between 40% and 50%). Until recently, an left ventricular ejection fraction of <50% in this setting dictated withholding trastuzumab therapy,1 which in return increased the risk of BC recurrence.32 Exploring these innovations in cardio-oncology within a large-scale registry will facilitate the evaluation of the reproducibility of such a treatment strategy, the feasibility in a real-world clinical setting, the rate of adoption of novel treatment recommendations, and the long-term impact of cardiac and oncological prognosis/outcomes, to name a few.
Another main advantage of our large multicenter registry design is that it offers a platform where the relatively rare complications, such as ICI-induced myocarditis, can be collected across multiple centers in a standardized manner. This allows us to study a large patient cohort within a relatively short period. We will also evaluate other nonmyocarditis ICI complications, including accelerated atherosclerosis and ischemic cardiovascular events.33,34 This is of particular importance because the indications for use of ICI in multiple cancer types are growing and are expected to rise considerably in the near future.35 Valuable knowledge on this specific topic regarding screening, identifying high-risk individuals, and treatment will equip us with the necessary tools to accommodate the expected high volume of patients presenting with very complex cardiovascular disorders in the setting of active concomitant noncardiac disease.36
An often-neglected topic in cardiovascular medicine is the impact of socioeconomic determinants of healthcare access, adherence to medical therapy, and outcomes. A recent review highlighted that racial/ethnic minorities (eg, black versus white patients), underserved communities (those with a lower socioeconomic status), and female patients (sex disparities) experience disproportionately high rates of fatal cancer and cardiovascular disease.37 The literature and research evidence of inequalities in cardio-oncology is still limited and warrants further prospective investigation.29 It should also be noted that patients with low socioeconomic status or minorities are those at the highest risk to develop both cancer and cardiovascular disease.38 At the same time, they are underrepresented in clinical trials.37,38 This registry will provide a unique opportunity to investigate the global impact of risk assessment, the current screening methods, and the effectiveness of therapeutic interventions derived from clinical trials as they are implemented in real-world practice.39
Study Limitations
G-COR has started the pilot phase and will need to secure additional funding for the global phase to provide long-term sustainability. At the present time, funding applications are in progress to meet this need.
Potential legal barriers for data sharing agreements at the international level. To address this issue, our legal team is working on solving compatibility of data sharing with different countries and compliance with GDPR. This global project includes >100 institutions from 23 countries on 5 continents. The Data User Agreements needed for each country/region are being evaluated and drafted by the Cleveland Clinic Legal Department.
The compliance with GDPR for the European sites is addressed at multiple levels:
All privacy issues and de-identified data are compliant with GDPR privacy protection rules.
Compensations and liabilities are not in play because G-COR does not include any deviation from standard clinical care.
GDPR has a provision allowing data sharing when it is in important public interest.
G-COR data are stored in the Cloud, not physically at any location.
The Legal Department at the Cleveland Clinic is working on the requirements that will be in place for the Data User Agreements (Data User Agreements) to be used with each specific country.
Ensuring accuracy of data entry by different centers, the data management group will document training of each site before activation; has set up training videos, designed operating data dictionary, and online G-COR instruction and procedures manuals, and has alarms for out-of-range values and will review data in conjunction with executive committee to minimize/eliminate this challenge.
Patients are referred to cardio oncology clinics for either cardiotoxicity (CTR-CVT) management, due to high risk of CTR-CVT, or for surveillance after exposure to cardiotoxic treatments. There is an inherent bias because patients at low risk for CTR-CVT are not referred to cardio-oncology services. It is, however, clinically relevant for the practice of cardio-oncology to establish the risk for cardiovascular events in this high-risk population, because patients with no preexistent cardiovascular disease, no risk factors, and not exposed to large doses of cardiotoxic agents have a low incidence of cardiovascular events and therefore they are not the target population that is evaluated and treated in the cardio-oncology space.
To have a comparator group, we will establish a control group population with oncology patients from the same 3 pillars (BC, hematologic malignancies, and immune checkpoint inhibitors-treated patients) not referred to cardio-oncology services, who have no risk factors and no preexistent cardiovascular disease. We will target the enrollment of 5 to 10 cancer clinic patients from each sites’ general cancer patient pool. This will yield ≈1000 patients without known cardiotoxicity or risk factors as a control group.
Future Perspectives
With the launch of G-COR, we initially set out to identify the most important topics among our participating centers. These 3 pillars (BC, HM, and ICI) provided the framework for the initial registry and determined the sequence for deploying different aspects within our registry. Our aim was to design G-COR in such a way that it can be adapted to the ever-changing clinical and preclinical needs within this field. To facilitate this process, the executive and scientific committees will oversee the roll-out of the 3 pillars and will plan the future steps based on the data, preliminary analysis, and lessons obtained from the pilot phase with up to 25 centers being launched at this time.
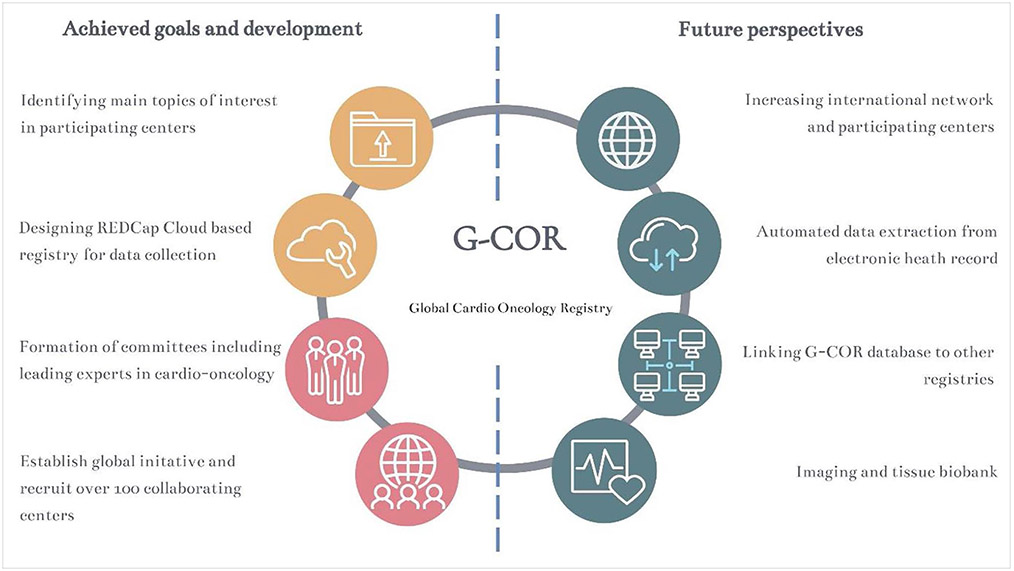
As depicted in Figure 5, we have identified several future goals that we will strive for after the initial launch of the global registry to ensure sustainability, offer opportunities to expand research potential, and enrich our registry with unique patient data.
Figure 5. Description of Global Cardio-Oncology Registry (G-COR) future goals and landmarks.
Because there have been other initiatives to create local or regional cardio-oncology registries, we will explore the possibilities to link the our data and outcomes to other cardio-oncology, primary care, and oncology registries with more in-depth demographic and socioeconomic data, clinical/imaging/biomarkers information, regional treatments variability and outcomes by geographic regions.
To make the data entry more efficient and less time-consuming, we will explore the use of automated data extraction from electronic healthcare systems into G-COR.40 The precision medicine/artificial intelligence committee will explore collaborations and substudies in this area; currently, data entry is performed manually. Automated data extraction will facilitate a larger accumulation of data while significantly reducing the time spent on data entry.40 This will allow us to extract meaningful clinical information from big data analysis.
As a long-term goal after additional structure and funding are secured, we will explore the possibilities of linking a tissue or imaging biobank to G-COR. This will allow for determining patient-specific phenotypes in great detail beyond the mere traditional cutoff values of health and disease (eg, left ventricular ejection fraction).
Because this is the starting point of an ongoing multicenter international registry, we will invite and welcome other cardio-oncology centers, particularly from geographic areas and countries that are underrepresented, to join this collaboration. This will strengthen the universal validity of our findings, result in more robust and diverse data availability, and will help to further build our cardio-oncology community with the active input from numerous cardiologists and oncologists, leaders, and practicing clinicians in this field.
Supplementary Material
WHAT IS KNOWN
Chemo-therapy-related cardiovascular toxicities impact immediate and long-term survival in cancer survivors.
Cancer therapy-related cardiovascular toxicity could result in an interruption of cancer therapy, negatively affecting the oncological outcome.
Real-world data on cancer therapy-related cardiovascular toxicity is necessary to estimate the impact of cancer therapy-related cardiovascular toxicity outside of the clinical trial setting.
Risk calculators and baseline risk assessment is largely based on the expert opinion lacking validation in the clinical field.
Little is known about the impact of socioeconomic determinants of healthcare access, adherence to medical therapy, and outcomes in cardio-oncology.
WHAT THE STUDY ADDS
We present the framework and objectives for a prospective multicenter, multinational cardio-oncology registry.
The registry will provide opportunities to study disparities in cardio-oncology practice and outcomes.
The Global Cardio-Oncology Registry will study the differences and similarities in diagnostic modalities, treatment protocols, and outcomes in cancer patients from different countries and different regions and in academic and community hospital settings.
Collected data will be suitable to validate recommended risk calculators and address the implementation and adherence to national and international recommendations/guidelines.
The registry is designed to also serve as a database of comprehensive clinical variables equipped for in-depth research questions.
Disclosures
Dr Teske has received speaker fees from Philips and Abbott; consulting from Nordic Pharma. Dr Neilan reports consulting from BMS, Abbvie, Sanofi, Genentech, Roche, and C4-Therapeutics; grant funding from BMS and AZ. Dr López-Fernández has received speaker fees from Philips, Janssen, and Incyte. Dr Szmit has received speaker fees from Amgen, Angelini, Astra Zeneca, Bayer, Bristol-Myers Squibb, Gilead, and Pfizer. Dr Guha is supported by American Heart Association-Strategically Focused Research Network Grant in Disparities in Cardio-Oncology (847740 and 863620). Dr Iakobishvili has received speaker fees from AstraZeneca, Boehringer Ingelheim, Novartis, Pfizer, Novo-Nordisk, and Bayer. Dr Sverdlov is supported by the National Heart Foundation of Australia Future Leader Fellowship (Award ID 106025) and reports research grants from the Medical Research Future Fund (Australia), NSW Health (Australia), Cancer Institute NSW (Australia), Hunter Medical Research Institute (Australia), Biotronik, RACE Oncology, Bristol Myer Squibb, Roche Diagnostics, and Vifor; and personal speaker fees from Novartis, Bayer, Bristol Myer Squibb, AstraZeneca, and Boehringer Ingelheim. Dr. Herrmann is supported by the National Cancer Institute (CA 233610) and the Miami Heart Institute. The other authors report no conflicts.
Footnotes
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCOUTCOMES.123.009905.
Contributor Information
Arco J. Teske, Department of Cardiology, University Medical Centre Utrecht, The Netherlands..
Rohit Moudgil, Department of Cardiovascular Medicine, Heart and Vascular Institute, Cleveland Clinic Foundation, OH..
Teresa López-Fernández, Hospital Universitario La Paz, Madrid, Spain..
Ana Barac, Medstar Heart Institute, Georgetown University, WA, DC..
Sherry Ann Brown, Medical College of Wisconsin, Milwaukee..
Anita Deswal, MD Anderson Cancer Center, Houston, TX..
Tomas G. Neilan, Massachusetts General Hospital, Harvard Medical School, Boston..
Sarju Ganatra, Lahey Hospital and Medical Center, Beth Israel Lahey Health, Burlington, MA..
Husam Abdel Qadir, University of Toronto, Canada..
Venu Menon, Department of Cardiovascular Medicine, Heart and Vascular Institute, Cleveland Clinic Foundation, OH..
Aaron L. Sverdlov, Newcastle Centre of Excellence in Cardio-Oncology, Calvary Mater Newcastle, Hunter Medical Research Institute, John Hunter Hospital, University of Newcastle, NSW, Australia..
Richard K. Cheng, University of Washington Medical Center, Seattle..
Silvia Makhoul, Hospital Juan A Fernández/Hospital Británico de Buenos Aires Buenos Aires, Argentina..
Arjun K. Ghosh, Barts Heart Centre, St Bartholomew’s Hospital, London, United Kingdom.; University College London Hospital, London, United Kingdom.; Hatter Cardiovascular Institute, London, United Kingdom..
Sebastian Szmit, Centre of Postgraduate Medical Education, Warsaw, Poland..
Vlad Zaha, UT Southwestern Medical Center, Dallas, TX.
Daniel Addison, Ohio State University, Columbus..
Lili Zhang, Montefiore Medical Center/Albert Einstein College of Medicine, NY..
Joerg Herrmann, Mayo Clinic, Rochester, MN..
Jun H. Chong, National Heart Centre, Singapore.
Vivek Agarwala, Narayana Superspeciality Hospital and Cancer Institute and RN Tagore Cancer Center, Kolkata, India..
Zaza Iakobishvili, Department of Cardiology, Tel Aviv Jaffa District Clalit Health Services, Tel Aviv, Israel..
Patricia Guerrero, AdventHealth, Orlando, FL..
Eric H. Yang, University of California Los Angeles..
Monika Leja, University of Michigan Medical Center, Ann Arbor..
Nausheen Akhter, Northwestern University Feinberg School of Medicine, Chicago, IL..
Avirup Guha, Cardio-Oncology Program, Department of Medicine, Georgia Cancer Center, Medical College of Georgia at Augusta University, GA..
Tochukwu M. Okwuosa, Rush University Medical Center, Chicago, IL..
Carolina Carvalho Silva, Rede D’Or Hospital Group, Sao Paulo, Brazil..
Patrick Collier, Department of Cardiovascular Medicine, Heart and Vascular Institute, Cleveland Clinic Foundation, OH..
Jeanne DeCara, University of Chicago School of Medicine, IL..
Brenton Bauer, COR Healthcare Associates/Torrance Memorial Medical Center, CA..
Carrie E. Lenneman, University of Alabama at Birmingham..
Diego Sadler, Cleveland Clinic Florida, Weston..
REFERENCES
- 1.Alexandre J, Cautela J, Ederhy S, Damaj GL, Salem J, Barlesi F, Farnault L, Charbonnier A, Mirabel M, Champiat S, et al. Cardiovascular toxicity related to cancer treatment: a pragmatic approach to the American and European Cardio-Oncology Guidelines. J Am Heart Assoc. 2020;9:e018403. doi: 10.1161/JAHA.120.018403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herrmann J, López-Fernández T, Lyon AR. The year in cardiovascular medicine 2021: cardio-oncology. Eur Heart J. 2022;43:857–862. doi: 10.193/eurheartj/ehab891 [DOI] [PubMed] [Google Scholar]
- 3.Ky B. JACC: CardioOncology: poised to serve a maturing, collaborative field. JACC CardioOncol. 2019;1:131–132. doi: 10.1016/j.jaccao.2019.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lancellotti P, Suter TM, López-Fernández T, Galderisi M, Lyon AR, van der Meer P, Cohen Solal A, Zamorano J-L, Jerusalem G, Moonen M, et al. Cardio-Oncology Services: rationale, organization, and implementation: a report from the ESC Cardio-Oncology council. Eur Heart J. 2019;40:1756–1763. doi: 10.1093/eurheartj/ehy453 [DOI] [PubMed] [Google Scholar]
- 5.Teske AJ, Linschoten M, Kamphuis JAM, Naaktgeboren WR, Leiner T, van der Wall E, Kuball J, van Rhenen A, Doevendans PA, Cramer MJ, et al. Cardio-oncology: an overview on outpatient management and future developments. Netherlands Heart Journal. 2018;26:521–532. doi: 10.1007/s12471-018-1148-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung R, Ghosh AK, Banerjee A. Cardiotoxicity: precision medicine with imprecise definitions. Open Heart. 2018;5:e000774–e000774. doi: 10.1136/openhrt-2018-000774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herrmann J, Lenihan D, Armenian S, Barac A, Blaes A, Cardinale D, Carver J, Dent S, Ky B, Lyon AR, et al. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur Heart J. 2022;43:280–299. doi: 10.1093/eurheartj/ehab674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lehmann LH, Cautela J, Palaskas N, Baik AH, Meijers WC, Allenbach Y, Alexandre J, Rassaf T, Müller OJ, Aras M, et al. Clinical strategy for the diagnosis and treatment of immune checkpoint inhibitor–associated myocarditis: a narrative review. JAMA Cardiol. 2021;6:1329–1337. doi: 10.1001/jamacardio.2021.2241 [DOI] [PubMed] [Google Scholar]
- 9.Ainger LE, Bushore J, Johnson WW, Ito J. Daunomycin: a cardiotoxic agent. J Natl Med Assoc. 1971;63:261–267. [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart JR, Fajardo LF. Radiation-induced heart disease: an update. Prog Cardiovasc Dis. 1984;27:173–194. doi: 10.1016/0033-0620(84)90003-3 [DOI] [PubMed] [Google Scholar]
- 11.Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, Murphy M, Stewart SJ, Keefe D. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–1221. doi: 10.1200/JCO.2002.20.5.1215 [DOI] [PubMed] [Google Scholar]
- 12.Lyon AR, Dent S, Stanway S, Earl H, Brezden-Masley C, Cohen-Solal A, Tocchetti CG, Moslehi JJ, Groarke JD, Bergler-Klein J, et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies. Eur J Heart Fail. 2020;22:1945–1960. doi: 10.1002/ejhf.1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, Boriani G, Cardinale D, Cordoba R, Cosyns B, et al. 2022 ESC Guidelines on cardio-oncology. Eur Heart J. 2022;43:4229–4361. doi: 10.1093/eurheartj/ehac244 [DOI] [PubMed] [Google Scholar]
- 14.Pudil R, Mueller C, Čelutkienė J, Henriksen PA, Lenihan D, Dent S, Barac A, Stanway S, Moslehi J, Suter TM, et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: a position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur J Heart Fail. 2020;22:1966–1983. doi: 10.1002/ejhf.2017 [DOI] [PubMed] [Google Scholar]
- 15.Čelutkienė J, Pudil R, López-Fernández T, Grapsa J, Nihoyannopoulos P, Bergler-Klein J, Cohen-Solal A, Farmakis D, Tocchetti CG, von Haehling S, et al. Role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: a position statement on behalf of the Heart Failure Association (HFA), the European Association of Cardiovascular Imaging (EACVI) and the Cardio-Oncology Council of the European Society of Cardiology (ESC). Eur J Heart Fail. 2020;22:1504–1524. doi: 10.1002/ejhf.1957 [DOI] [PubMed] [Google Scholar]
- 16.He J, Morales DR, Guthrie B. Exclusion rates in randomized controlled trials of treatments for physical conditions: a systematic review. Trials. 2020;21:228. doi: 10.1186/s13063-020-4139-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregorietti V, Fernandez TL, Costa D, Chahla EO, Daniele AJ. Use of Sacubitril/valsartan in patients with cardio toxicity and heart failure due to chemotherapy. Cardio-Oncology. 2020;6:24. doi: 10.1186/s40959-020-00078-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777 [DOI] [PubMed] [Google Scholar]
- 19.Torbjørn O, Lagethon HS, Geeta G. The role of cardioprotection in cancer therapy cardiotoxicity. JACC CardioOncol. 2022;4:19–37. doi: 10.1016/j.jaccao.2022.01.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thavendiranathan P, Negishi T, Somerset E, Negishi K, Penicka M, Lemieux J, Aakhus S, Miyazaki S, Shirazi M, Galderisi M, et al. ; SUCCOUR Investigators. Strain-guided management of potentially cardiotoxic cancer therapy. J Am Coll Cardiol. 2021;77:392–401. doi: 10.1016/j.jacc.2020.11.020 [DOI] [PubMed] [Google Scholar]
- 21.Weldy CS, Ashley EA. Towards precision medicine in heart failure. Nat Rev Cardiol. 2021;18:745–762. doi: 10.1038/s41569-021-00566-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandawat A, Williams AE, Francis SA. Cardio-oncology: the role of big data. Heart Fail Clin. 2017;13:403–408. doi: 10.1016/j.hfc.2016.12.010 [DOI] [PubMed] [Google Scholar]
- 23.Kamphuis JAM, Linschoten M, Cramer MJ, Alsemgeest F, van Kessel DJW, Urgel K, Post MC, Manintveld OC, Hassing HC, Liesting C, et al. ONCOR: design of the Dutch cardio-oncology registry. Netherlands Heart Journal. 2021;29:288–294. doi: 10.1007/s12471-020-01517-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conill C, Verger E, Salamero M. Performance status assessment in cancer patients. Cancer. 1990;65:1864–1866. doi: [DOI] [PubMed] [Google Scholar]
- 25.Gliklich R, Leavy M, Dreyer N. Registry Design. In: Registries for Evaluating Patient Outcomes: A User’s Guide. 4th ed. 2020;19–29. [Google Scholar]
- 26.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 27.de Boer RA, Aboumsallem JP, Bracun V, Leedy D, Cheng R, Patel S, Rayan D, Zaharova S, Rymer J, Kwan JM, et al. A new classification of cardio-oncology syndromes. Cardiooncology. 2021;7:24. doi: 10.1186/s40959-021-00110-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viswanathan T, Lang CC, Petty RD, Baxter MA. Cardiotoxicity and chemotherapy-the role of precision medicine. Diseases. 2021;9:90. doi: 10.3390/diseases9040090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohman RE, Yang EH, Abel ML. Inequity in Cardio-Oncology: identifying disparities in cardiotoxicity and links to cardiac and cancer outcomes. J Am Heart Assoc. 2021;10:e023852. doi: 10.1161/JAHA.121.023852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leaong DP, Cosman T, Alhussein MM, Kumar Tyagi N, Karampatos S, Barron CC, Wright D, Tandon V, Magloire P, Joseph P, et al. Safety of continuing trastuzumab despite mild cardiotoxicity. JACC CardioOncol. 2019;1:1–10. doi: 10.1016/j.jaccao.2019.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lynce F, Barac A, Geng X, Dang C, Yu AF, Smith KL, Gallagher C, Pohlmann PR, Nunes R, Herbolsheimer P, et al. Prospective evaluation of the cardiac safety of HER2-targeted therapies in patients with HER2-positive breast cancer and compromised heart function: the SAFE-HEaRt study. Breast Cancer Res Treat. 2019;175:595–603. doi: 10.1007/s10549-019-05191-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rushton M, Lima I, Tuna M, Johnson C, Ivars J, Pritchard K, Hawken S, Dent S. Impact of stopping trastuzumab in early breast cancer: a population-based study in Ontario, Canada. J National Cancer Institute. 2020;112:1222–1230. doi: 10.1093/jnci/djaa054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vuong JT, Stein-Merlob AF, Nayeri A, Sallam T, Neilan TG, Yang EH. Immune checkpoint therapies and atherosclerosis: mechanisms and clinical implications: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79:577–593. doi: 10.1016/j.jacc.2021.11.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drobni ZD, Alvi RM, Taron J, Zafar A, Murphy SP, Rambarat PK, Mosarla RC, Lee C, Zlotoff DA, Raghu VK, et al. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation. 2020;142:2299–2311. doi: 10.1161/CIRCULATIONAHA.120.049981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang J, Yu JX, Hubbard-Lucey VM, Neftelinov ST, Hodge JP, Lin Y. The clinical trial landscape for PD1/PDL1 immune checkpoint inhibitors. Nat Rev Drug Discov. 2018;17:854–855. doi: 10.1038/nrd.2018.210 [DOI] [PubMed] [Google Scholar]
- 36.Jing Y, Yang J, Johnson DB, Moslehi JJ, Han L. Harnessing big data to characterize immune-related adverse events. Nat Rev Clin Oncol. 2022;19:269–280. doi: 10.1038/s41571-021-00597-8 [DOI] [PubMed] [Google Scholar]
- 37.Prasad P, Branch M, Asemota D, Elsayed R, Addison D, Brown SA. Cardio-Oncology preventive care: racial and ethnic disparities. Curr Cardiovasc Risk Rep. 2020;14:18. doi: 10.1007/s12170-020-00650 [DOI] [Google Scholar]
- 38.Fazal M, Malisa J, Rhee J-W, Witteles RM, Rodriguez F. Racial and ethnic disparities in cardio-oncology. JACC CardioOncol. 2021;3:201–204. doi: 10.1016/j.jaccao.2021.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sherman RE, Anderson SA, Dal Pan GJ, Gray GW, Gross T, Hunter NL, LaVange L, Marinac-Dabic D, Marks PW, Robb MA, et al. Real-world evidence – what is it and what can it tell us? N Eng J Med. 2016;375:2293–2297. doi: 10.1056/NEJMsb1609216 [DOI] [PubMed] [Google Scholar]
- 40.Kilic A. Artificial intelligence and machine learning in cardiovascular health care. Ann Thorac Surg. 2020;109:1323–1329. doi: 10.1016/j.athoracsur.2019.09.042 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.