Abstract
Humans infected with Helicobacter pylori have abnormally low levels of the antioxidant vitamin C, which protects against the formation of carcinogenic nitrosamines, in gastric juice. Guinea pigs, like humans and nonhuman primates, have a dietary requirement for vitamin C. As such, these species have gastrointestinal vitamin C transport systems not found in other animals. We have developed and characterized a guinea pig model of chronic gastric H. pylori infection with the rodent-adapted Sydney strain of H. pylori. At 4 weeks postinfection, five of six animals of the infected group and zero of two animals of the control group were positive for H. pylori as determined by culture or PCR. At 15 weeks, six of six animals of the infected group and zero of two animals of the control group were positive. H. pylori-specific seroconversion was observed among infected animals. There were no histologic abnormalities in the gastric antra or fundi of control guinea pigs. In contrast, there was multifocal, mild to moderate lymphohistiocytic antral gastritis and formation of antral lymphoid follicles in H. pylori-infected animals. The lesion distribution in the gastric antra paralleled that observed in H. pylori-infected humans. The H. pylori-infected guinea pig should prove useful in modeling the interaction of helicobacter and vitamin C in gastric carcinogenesis.
Humans infected with Helicobacter pylori have in their gastric juices abnormally low levels of the antioxidant vitamin C, which theoretically protects against the formation of carcinogenic nitrosamines and reactive oxygen species (28). Importantly, risk for development of gastric adenocarcinoma is epidemiologically linked to colonization by H. pylori, which has been classified by the World Health Organization as a class I carcinogen (7).
Nonhuman primates and guinea pigs are the only laboratory animals that, like humans, have a dietary requirement for vitamin C. This is because they lack the enzyme gulonolactone oxidase. They are unable to synthesize vitamin C and must rely on daily dietary intake of this important antioxidant vitamin. Thus, guinea pigs, along with nonhuman primates and humans, have gastrointestinal vitamin C transport systems not found in other animals (30, 31). Vitamin C is concentrated approximately fivefold in the gastric juice of guinea pigs relative to that in plasma (27), which is similar to the degree of concentration reported for humans (28, 29).
In addition to its dietary requirement for vitamin C, the guinea pig model of H. pylori infection is desirable for its anatomic and immunologic features. The guinea pig stomach is considerably larger than the mouse stomach. The guinea pig stomach lacks a nonglandular region and is thus anatomically more similar to the human stomach than are the stomachs of other common rodent species. Guinea pigs also have an immunological similarity to humans that is lacking in other rodent species, namely, the secretion of a well-characterized homolog of interleukin-8 (IL-8) (35). It has been shown that a pathogenetically important subset of H. pylori isolates (i.e., CagA+ strains) induce IL-8 secretion from gastric tissue (10). IL-8 is a chemokine which acts to attract neutrophils (1) and which is believed to play a role in the development of H. pylori gastritis (12).
In this report, we describe the development and characterization of experimental H. pylori infection in guinea pigs as a preface to investigating the interaction of H. pylori and vitamin C transport and metabolism in the stomach.
MATERIALS AND METHODS
Infection protocol. (i) Animals.
Female Hartley strain guinea pigs were obtained from Hazelton Research Products (Denver, Pa.). Two age groups were used: 16 2-week-old 200-g weanling animals and 3 adult females (>1 kg). Animals were maintained in polycarbonate caging and were fed standard guinea pig chow (PMI Feeds, St. Louis, Mo.). All animal manipulations were approved by the MIT Institutional Animal Care and Use Committee.
(ii) Bacteria.
H. pylori Sydney was used (22). Cultures used for dosing guinea pigs were grown under microaerophilic conditions for 24 to 48 h in brucella broth supplemented with 5% fetal calf serum. Broth cultures were examined by phase microscopy and by Gram staining for motility and purity. Bacteria were pelleted at 13,000 × g for 20 min and the pellet was resuspended in freezer media (brucella broth plus 30% glycerol) at an optical density at 600 nm (OD600) of 1.0 (equivalent to approximately 108 CFU/ml).
(iii) Dosing scheme.
Guinea pigs were dosed orally with 1 mg of omeprazole per kg of body weight per day starting 1 day before the first H. pylori dose and continuing for 1 day after the final dose of H. pylori. Twelve weanling guinea pigs were dosed orally with 1 ml of live H. pylori suspension (108 CFU) every other day for a total of 3 doses. Four control guinea pigs were sham dosed with 1 ml of sterile freezer media. Three adult female guinea pigs were sham dosed with 1 ml of killed H. pylori antigen (see below, “Serologic evaluation”).
Microbiological evaluation. (i) Bacterial isolation from gastric tissue.
At necropsy, two 4-mm-diameter punch biopsy samples were obtained aseptically from the antra and bodies of the stomachs. These biopsy samples were homogenized in a sterile tissue grinder, and an aliquot of the resulting slurry was plated on blood agar plates (Remel, Lenexa, Kans.) or Glaxo plates (containing vancomycin, polymyxin B, bacitracin, amphotericin B, and nalidixic acid) (23) for microaerobic isolation of H. pylori. H. pylori organisms were gram negative and had characteristic morphology and motility when examined by phase microscopy. The organism was identified by strong catalase, urease, and oxidase reactions and by resistance to nalidixic acid and cephalothin. Although growth of H. pylori was generally evident within 1 week, plates were maintained for 3 weeks before a determination of no growth was made.
(ii) Bacterial isolation from feces.
Fecal samples were obtained at scheduled intervals for H. pylori culture. A single fresh guinea pig fecal pellet was suspended in 2 ml of phosphate-buffered saline (PBS). A 100-μl aliquot was used to inoculate a Glaxo plate. Plates were incubated for 3 weeks before a determination of no growth was made.
(iii) DNA isolation for PCR. (a) Gastric tissue.
Gastric tissue biopsies obtained at necropsy were ground up and prepared following the protocol for a commercially available DNA preparation kit (Boehringer Mannheim, Piscataway, N.J.). A 22.5-μl sample of the resulting DNA preparation was used for H. pylori PCR.
(b) Feces.
Half of a guinea pig fecal pellet was suspended in 2 ml of PBS. The suspension was centrifuged at 700 × g for 5 min, and 300 μl of the resulting supernatant was used in a Qiagen kit, following the directions for blood. A 100-μl sample of the resulting DNA preparation was used for H. pylori PCR.
(iv) PCR.
H. pylori PCR was performed as previously described, using the H. pylori-specific primers P3 and P4 (24) to perform PCR on DNA isolated from gastric tissue and using both the P3 and P4 primers and the all-helicobacter primers C97 and C98 (26) to perform PCR on DNA isolated from feces. Between 12 and 18 μl of DNA extract was added to a 100-μl (final volume) reaction tube containing Taq polymerase buffer (Boehringer Mannheim, La Jolla, Calif.) supplemented with 1 mM MgCl2 to a final concentration of 3.75 mM, 0.5 μM concentrations of each of the two primers, 200 μM concentrations of each deoxynucleotide, and 200 μg of bovine serum albumin per ml. Samples were heated at 94°C for 4 min, briefly centrifuged, and cooled to 60°C. Taq polymerase (3.2 U) (Pharmacia, Piscataway, N.J.) and 1.25 U of polymerase enhancer (Perfect Match; Stratagene, La Jolla, Calif.) were added, and then an overlay of 100 μl of mineral oil was added. Amplification took place in a thermal cycler under the following conditions: denaturation at 94°C for 1 min, annealing at 65°C (with P3 and P4 H. pylori-specific primers) or 59°C (with all-helicobacter primers) for 2 min, and extension at 72°C for 2 min. A total of 35 cycles were performed, followed by a 4-min extension step. A 15-μl sample was electrophoresed though a 6% Visigel separation matrix (Stratagene) and was visualized by staining with ethidium bromide and viewing by UV illumination.
Serologic evaluation. (i) Preparation of H. pylori antigen.
Cultures used for preparing H. pylori antigen were grown for 24 to 48 h in brucella broth supplemented with 5% fetal calf serum. Broth cultures were examined by phase microscopy and by Gram staining for motility and purity. The OD600 was read to estimate the number of CFU of H. pylori in the culture. Bacteria were pelleted at 13,000 × g for 20 min. Bacteria were then subjected to five cycles of sonication with alternating freeze-thaw cycles (5 min at −70°C, followed by a quick thaw at room temperature). Sonicates were examined for motility by phase microscopy to ensure that no live bacteria remained. As a final step to ensure that bacterial proteins, but no live bacteria, were present, the sonicate was filtered through a 0.2-μm-pore-size filter. The sonicate concentration was then adjusted so that each 1-ml aliquot represented the sonicated equivalent of 108 CFU.
(ii) Measurement of anti-H. pylori serum IgG by ELISA.
Standard enzyme-linked immunosorbent assay (ELISA) methods were used for serum immunoglobulin G measurement. Briefly, microtiter plate wells were incubated overnight at 4°C with 100 μl of H. pylori antigen (10 μg/ml) in carbonate buffer (pH 9.6). Sera were diluted 1:100 and applied to the wells for 1 h at 37°C. The secondary antibody, peroxidase-conjugated goat anti-guinea pig immunoglobulin G (A 7289; Sigma), diluted 1:2,000 in PBS–1% bovine serum albumin, was applied to the wells for 1 h at 37°C. OD450 was recorded after a 60-min incubation with ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid] substrate (Kirkegaard and Perry Laboratories, Gaithersburg, Md.) using an ELISA plate reader (MR7000; Dynatech Laboratories, Chantilly, Va.). The antibody response against H. pylori antigen was considered significant if the OD was greater than the mean OD plus 3 standard deviations (SD) measured for samples from sham-dosed control animals.
Histopathologic evaluation.
Sections of antrum and stomach body were fixed in neutral buffered 10% formalin. Formalin-fixed tissues were embedded in paraffin, sectioned at 5 μm, and stained with Warthin-Starry silver stain to visualize bacteria in tissues. Hematoxylin and eosin (H&E) stain was used to stain 5-μm sections of fixed tissues for assessment of histopathology.
RESULTS
Clinical and gross necropsy findings.
Guinea pigs did not exhibit clinical signs of gastritis (vomiting, loss of appetite, or weight loss). The weights of infected guinea pigs were not significantly different from the weights of control guinea pigs at either 4 or 15 weeks postinoculation. There were no grossly visible gastric lesions at necropsy in any of the guinea pigs. The pH of the gastric juice was measured at necropsy and was not significantly different for control and infected guinea pigs (1.70 ± 0.14 versus 1.68 ± 0.13 at 4 weeks or 1.92 ± 0.66 at 15 weeks).
Gastric colonization.
Colonization was assessed by gastric culture and by PCR of gastric tissue obtained at necropsy. Samples of gastric antrum and fundus were cultured separately; results are summarized in Table 1. Culture and PCR results for individual animals did not always coincide, which may have reflected uneven colonization of the mucosa. At 4 weeks postinfection, five of six animals were culture positive and three of six were PCR positive. At 15 weeks postinfection, two of six animals were culture positive and six of six were PCR positive. Later, it was found that the reduced culture recovery at 15 weeks postinfection was associated with improperly stored culture plates.
TABLE 1.
Gastric colonization by H. pyloria
| Infection and time (wk) after dosing | No. of animals positive for H. pylori infection/no. tested bya:
|
|||
|---|---|---|---|---|
| Culture or PCR | Culture (antrum biopsy) | Culture (fundus biopsy) | PCR | |
| None (control) | ||||
| 4 | 0/2 | 0/2 | 0/2 | 0/2 |
| 15 | 0/2 | 0/2 | 0/2 | 0/2 |
| H. pylori | ||||
| 4 | 5/6 | 5/6 | 5/6 | 3/6 |
| 15 | 6/6 | 1/6 | 1/6 | 6/6 |
Colonization was assessed by gastric culture and by PCR of gastric tissue obtained at necropsy. Samples of gastric antrum and fundus were cultured separately.
Serology.
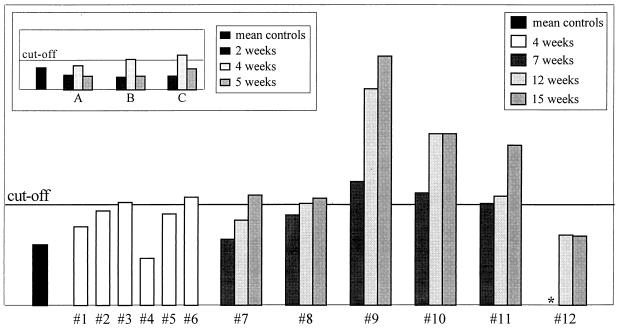
As detailed in Fig. 1, two of six guinea pigs were seropositive at 4 weeks and all but one were seropositive at 15 weeks. Titers continued to rise (Fig. 1), suggesting that seropositivity correlated with active infection. To determine whether seropositivity correlated with active infection or merely with exposure to H. pylori antigen, three adult female guinea pigs were dosed with killed H. pylori antigen, following the same dosing schedule and receiving a similar biomass as the infection group (inset to Fig. 1). One of the three guinea pigs remained seronegative for 5 weeks. The other two guinea pigs were borderline seropositive at 4 weeks but seronegative at 5 weeks postexposure (a decreasing titer), in contrast to the infected guinea pigs, who had rising titers for the duration of their infections (Fig. 1).
FIG. 1.
H. pylori serology. OD readings are plotted versus individual guinea pig serum samples analyzed in duplicate. The cutoff line is the mean plus 3 SD of the OD readings of sham-dosed control guinea pigs at 4, 7, 12, and 15 weeks postdosing. One-third of the infected guinea pigs were seropositive at 4 weeks postinfection. OD readings for each of the six 15-week infected guinea pigs at 7, 12, and 15 weeks postinfection are plotted. One animal remained seronegative for the 15-week duration of the study. (Inset) To determine whether seropositivity correlated with active infection or merely with exposure to H. pylori antigen, three adult female guinea pigs (A, B, and C) were dosed with killed H. pylori antigen, following the same dosing schedule and receiving the same quantity of antigen as the experimentally infected group. The cutoff line is the mean plus 3 SD of the OD readings from these guinea pigs prior to dosing. Two of the three guinea pigs were borderline seropositive at 4 weeks but seronegative at 5 weeks postexposure (a decreasing titer), in contrast to the H. pylori-infected guinea pigs, who had rising titers for the duration of their infection. ∗, sample unavailable.
Detection of H. pylori in feces. (i) Fecal cultures.
Fecal cultures were consistently negative, although samples spiked with approximately 104 CFU of H. pylori culture grew readily (data not shown), indicating that this negative result was not due to inhibitory factors in the feces. Thus, live H. pylori organisms were not present in the feces at levels detectable by culture.
(ii) Fecal PCR.
Fecal samples were consistently negative, although signal could be detected in fecal samples spiked with as few as 16 CFU of H. pylori.
Histopathology.
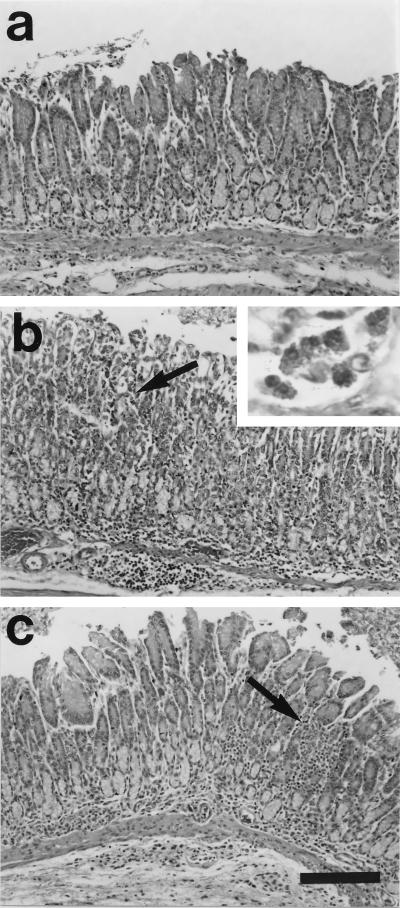
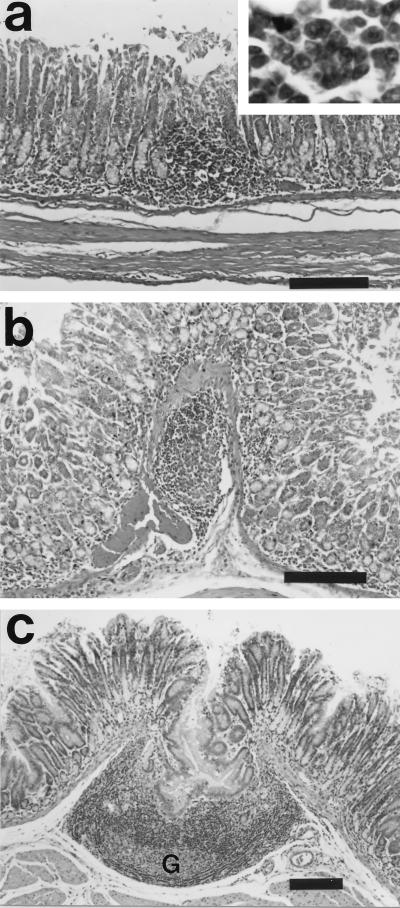
There were no histological abnormalities in any of the control guinea pigs at either 4 or 15 weeks (Fig. 2a). In contrast, moderate multifocal to diffuse antral gastritis was present in the H. pylori-infected guinea pigs at both time points (Fig. 2b and c). Lesions were frequently concentrated at the junction between antral and fundic mucosa but were otherwise not seen in fundic mucosa. Inflammation was present throughout the lamina propria, and in some instances it extended through the muscularis mucosa into the submucosa (Fig. 2b). The inflammatory infiltrate was comprised of a mixed population of mononuclear cells, eosinophils, and heterophils (polymorphonuclear phagocytes). At both 4 (Fig. 3a and b) and 15 (Fig. 3c) weeks, lymphoid aggregates and lymphoid follicular structures were present in the antrum (Fig. 3). The inflammation was in general more extensive at 15 weeks than at 4 weeks, with more prominent and more numerous lymphoid nodules and more extensive inflammatory infiltrate. Helical organisms were rarely detected by Warthin-Starry silver staining (data not shown).
FIG. 2.
Experimental H. pylori infection causes antral gastritis in the guinea pig. There were no abnormalities in the gastric antra or fundi (not shown) of the control sham-dosed guinea pigs at either 4 or 15 weeks after dosing (a). At 4 weeks (b) and 15 weeks (c) there was a moderate lymphohistiocytic, eosinophilic infiltrate in the submucosa and deep mucosa, with multifocal extensions to the superficial mucosa (arrows). The inset illustrates eosinophils. Active inflammation characterized by heterophilic infiltrate (guinea pig polymorphonuclear phagocytes) was present in the mucosa in scattered foci. At 4 weeks postinfection, the infiltrate was concentrated in the corpus-antrum junction, and at 15 weeks postinfection it extended throughout the antrum. H&E stain was used. Bar = 150 μm.
FIG. 3.
Experimental H. pylori infection induces formation of gastric mucosa-associated lymphoid tissue in the guinea pig. At 4 weeks postinfection, lymphoid infiltrates formed multifocal aggregates in the deep mucosa (a) and submucosa (b). The inset illustrates lymphocytes. At 15 weeks postinfection (c), lymphofollicular organization was prominent. The follicles were large (note scale bar), had prominent germinal centers (G), and featured hyperplastic ingrowth of the overlying mucosal epithelium. H&E stain was used. Bar = 150 μm.
DISCUSSION
These results demonstrate that guinea pigs can be readily colonized by the rodent-adapted Sydney strain of H. pylori. The H. pylori-infected guinea pigs in our study had a significant antral gastritis within 4 weeks of infection and were persistently colonized for at least 15 weeks postinfection. Gastritis was not present in the fundi of infected guinea pigs, which is consistent with our finding that gastric juice pH was not significantly altered in the infected guinea pigs. However, H. pylori organisms were apparently present in the fundi, as it was cultured from fundic biopsies obtained at necropsy (Table 1). At 15 weeks, the distribution of the gastritis was still antral, but there was additional development of gastric mucosa-associated lymphoid tissue. These lymphoid follicular structures are consistent with chronic helicobacter-induced gastritis in humans and other species. Lymphoid follicles are prominent in the stomachs of ferrets with Helicobacter mustelae (4, 13), dogs with Helicobacter felis (21), cats with H. pylori (17, 24), mice with H. felis (11), macaques with H. pylori (2, 5), gnotobiotic pigs with H. pylori (20), and H. pylori-infected humans with antral gastritis (14, 15).
The gastric inflammatory infiltrate was a mixed population of lymphocytes, macrophages, and heterophils (guinea pig polymorphonuclear phagocyte cells) with prominent eosinophils (Fig. 2b, inset). In humans, it has been observed that infection with H. pylori induces gastric secretion of IL-8 (3). IL-8 is a chemokine that attracts neutrophils (1); in guinea pigs, IL-8 also attracts eosinophils (6), so the prominent eosinophilic inflammation is consistent with IL-8 induction. Future studies will examine the cytokine profile directly. The presence of an IL-8 homolog in guinea pigs is a significant advantage over rat and mouse rodent models, which lack an IL-8 homolog (35).
Organisms were seen only rarely in Warthin-Starry silver-stained sections of infected guinea pigs. Evaluation of bacterial colonization with silver-stained histologic sections is an insensitive method for detection of H. pylori. In BALB/c mice infected with the Sydney strain of H. pylori, colonization was scored histologically as undetectable to barely detectable, although bacterial colonization was quantitated by culture as 105.8 CFU/g (22). In contrast to histological detection of bacteria in Warthin-Starry silver-stained sections, culture and PCR techniques routinely detect 100 or fewer CFU of organisms per g. We estimate that fewer than 105 organisms/g of tissue were present in these guinea pigs, but this level of colonization was nevertheless sufficient to produce significant gastritis.
It has been speculated that vitamin C plays a role in the pathogenesis of H. pylori-related disease in humans. Specifically, ascorbic acid is the component of vitamin C that has been shown to inhibit N-nitrosation in vitro and thus to lead to decreased levels of nitrosamines, many of which are potent carcinogens (33). Ascorbic acid has been measured in the gastric juice of normal individuals at levels 3 to 5 times its concentration in plasma, suggesting that there is active gastric secretion of ascorbic acid (25). Interestingly, the total vitamin C and ascorbic acid concentrations (but not plasma ascorbic acid concentration) in gastric juice are significantly lower in individuals infected with H. pylori (28, 29). Gastric juice ascorbic acid levels were shown to increase after intravenous injection of ascorbic acid in human volunteers without H. pylori gastritis, but not in individuals with gastritis (8). Thus, ascorbic acid secretion appears to be impaired in individuals with H. pylori gastritis. Because there is a significant risk factor for gastric carcinoma associated with H. pylori infection, lowered ascorbic acid levels may increase the risk for gastric carcinoma. Correa et al. (8) have hypothesized that ascorbic acid in the normal gastric microenvironment acts as an antioxidant and free-radical scavenger and thus protects against the formation of carcinogenic nitrosamines and oxidative damage to DNA by reactive oxygen species.
In addition to the link between decreased gastric juice ascorbic acid levels and increased risk of cancer, there is evidence that dietary, serum, and gastric juice ascorbic acid levels can influence gastric H. pylori colonization. A recent study examined the effect of diet in a mouse model of H. pylori. In this study, dietary supplementation with vitamin C (not a normal component of mouse diets) resulted in a 75% lower rate of recovery of H. pylori from gastric cultures of mice experimentally infected with H. pylori than that from mice fed a diet of unsupplemented mouse chow (34). Also, vitamin C directly inhibits the growth of H. pylori on culture media (16). Vitamin C supplementation studies have been performed and a correlation between vitamin C status and severity of gastritis is an active area of research (8, 9). We developed the guinea pig model specifically to study vitamin C because the guinea pig is the only small laboratory animal that lacks gulonolactone oxidase and thus has gastrointestinal vitamin C absorption mechanisms similar to those in humans. We have developed a technique for acquiring gastric juice and measuring gastric vitamin C in guinea pigs and found that guinea pigs concentrated vitamin C approximately fivefold in their gastric juices as compared to their sera (27). Guinea pig gastric juice ascorbic acid concentrations were comparable to those found for human gastric juice ascorbic acid, which ranged from 0.36 to 2.53 mg/dl (28, 30, 31). The guinea pig stomach has also been studied extensively by using in vitro physiology techniques such as measurement of fluxes in Ussing chambers that cannot be readily adapted to mouse tissues (18, 19, 32). Thus, the guinea pig should provide the ideal animal model to study the roles of H. pylori gastritis and gastric vitamin C transport in the progression of gastritis and to dissect what factors influence the severity of the inflammatory response in the gastric mucosa.
ACKNOWLEDGMENTS
This work was supported by NIH grants RR07036 and RO1A1-RR37740 and by a grant from Astra Research Center Boston.
We thank G. Perrone and R. Russell of the Tufts Human Nutrition Research Center on Aging for performing high-performance liquid chromatography assays of vitamin C content.
REFERENCES
- 1.Baggiolini M, Loetscher P, Moser B. Interleukin-8 and the chemokine family. Int J Immunopharmacol. 1995;17:103–108. doi: 10.1016/0192-0561(94)00088-6. [DOI] [PubMed] [Google Scholar]
- 2.Baskerville A, Newell D G. Naturally occurring chronic gastritis and C. pylori infection in the Rhesus monkey: a potential model for gastritis in man. Gut. 1988;29:465–472. doi: 10.1136/gut.29.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basso D, Scrigner M, Toma A, Navaglia F, DiMario F. Helicobacter pylori infection enhances mucosal interleukin-1 beta, interleukin-6, and the soluble receptor of interleukin-2. Int J Clin Lab Res. 1996;26:207–210. doi: 10.1007/BF02592984. [DOI] [PubMed] [Google Scholar]
- 4.Batchelder M, Fox J G, Hayward A, Yan L, Shames B, Murphy J C, Palley L. Natural and experimental Helicobacter mustelae reinfection following successful antimicrobial eradication in ferrets. Helicobacter. 1996;1:34–42. doi: 10.1111/j.1523-5378.1996.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 5.Bronsdon M A, Schoenknecht F D. Campylobacter pylori isolated from the stomach of the monkey, Macaca nemestrina. J Clin Microbiol. 1988;26:1725–1728. doi: 10.1128/jcm.26.9.1725-1728.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins P, Weg V, Faccioli L, Watson M, Moqbel R, Williams T. Eosinophil accumulation induced by human interleukin-8 in the guinea-pig in vivo. Immunology. 1993;79:312–318. [PMC free article] [PubMed] [Google Scholar]
- 7.Correa P. Mechanisms of gastric carcinogenesis. In: Joossens J V, Hill M J, Geboers J, editors. Diet and human carcinogenesis. Amsterdam, The Netherlands: Elsevier; 1985. pp. 109–115. [Google Scholar]
- 8.Correa P, Fontham E, Ruiz B, Malcom G, Hunter F, Zavala D. Gastric juice ascorbic acid after intravenous injection: effect of ethnicity, pH, and Helicobacter pylori infection. J Natl Cancer Inst. 1995;87:52–53. doi: 10.1093/jnci/87.1.52. [DOI] [PubMed] [Google Scholar]
- 9.Correa P, Malcolm G, Fontham E, Schmidt B, Ruiz B, Bravo J C, Bravo L E, Zarama G, Realpe J L. H. pylori and the metabolism of vitamin C. Gut. 1997;41:A48. [Google Scholar]
- 10.Crabtree J, Covacci A, Farmery S, Xiang Z, Tompkins D, Perry S, Lindley I, Rappuoli R. Helicobacter pylori induced interleukin-8 expression in gastric epithelial cells is associated with CagA positive phenotype. J Clin Pathol. 1995;48:41–45. doi: 10.1136/jcp.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enno A, O’Rourke J L, Howlett C R, Jack A, Dixon M F, Lee A. MALToma-like lesions in the murine gastric mucosa after long-term infection with Helicobacter felis. Am J Pathol. 1995;147:217–223. [PMC free article] [PubMed] [Google Scholar]
- 12.Figura, N. 1996. Helicobacter pylori exotoxins and gastroduodenal diseases associated with cytotoxic strain infection. Aliment. Pharmacol. Ther. 10(Suppl. 1):79–96. [DOI] [PubMed]
- 13.Fox J G, Cabot E B, Taylor N S, Laraway R. Gastric colonization by Campylobacter pylori subsp. mustelae in ferrets. Infect Immun. 1988;56:2994–2996. doi: 10.1128/iai.56.11.2994-2996.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox J G, Correa P, Taylor H W, Zavala D, Fontham E, Janney F, Rodriguez E, Hunter F, Diavolitsis S. Campylobacter pylori-associated gastritis and immune response in a population at increased risk of gastric carcinoma. Am J Gastroenterol. 1989;84:775–781. [PubMed] [Google Scholar]
- 15.Genta R, Hamner H. The significance of lymphoid follicles in the interpretation of gastric biopsy specimens. Arch Pathol Lab Med. 1994;118:740–743. [PubMed] [Google Scholar]
- 16.Goldie J, Jalali S, Zanten S V, Stowe C, Hunt R. Ascorbic acid inhibits the growth and urease activity of Campylobacter pylori. Gut. 1989;30:A1484. [Google Scholar]
- 17.Handt L K, Fox J G, Stalis I H, Rufo R, Lee G, Linn J, Li X, Kleanthous H. Characterization of feline Helicobacter pylori strains and associated gastritis in a colony of domestic cats. J Clin Microbiol. 1995;33:2280–2289. doi: 10.1128/jcm.33.9.2280-2289.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito S, Lacy E, Rutten M, Critchlow J, Silen W. Rapid repair of injured gastric mucosa. Scand J Gastroenterol Suppl. 1984;101:87–95. [PubMed] [Google Scholar]
- 19.Joutsi T, Paimela H, Bhowmik A, Kiviluoto T, Kivilaakso E. Role of Na(+)-H(+)-antiport in restitution of isolated guinea pig gastric epithelium after superficial injury. Dig Dis Sci. 1996;41:2187–2194. doi: 10.1007/BF02071399. [DOI] [PubMed] [Google Scholar]
- 20.Krakowka S, Morgan D R, Kraft W G, Leunk R D. Establishment of gastric Campylobacter pylori infection in the neonatal gnotobiotic piglet. Infect Immun. 1987;55:2789–2796. doi: 10.1128/iai.55.11.2789-2796.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee A, Krakowka S, Fox J G, Otto G, Eaton K A, Murphy J C. Role of Helicobacter felis in chronic canine gastritis. Vet Pathol. 1992;29:487–494. doi: 10.1177/030098589202900601. [DOI] [PubMed] [Google Scholar]
- 22.Lee A, O’Rourke J, Ungria M, Robertson R, Daskalopoulos G, Dixon M. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology. 1997;112:1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 23.McColm A A, Bagshaw J, O’Malley C, McLaren A. Development of a mouse model of gastric colonisation with Helicobacter pylori. Gut. 1995;37:A50. [Google Scholar]
- 24.Perkins S E, Yan L L, Shen Z, Hayward A, Murphy J C, Fox J G. Use of PCR and culture to detect Helicobacter pylori in naturally infected cats following triple antimicrobial therapy. Antimicrob Agents Chemother. 1996;40:1486–1490. doi: 10.1128/aac.40.6.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruiz B, Correa P, Fontham E, Rood J, Malcom G, Torrado J, Perez A, Ramakrishnan T, Hunter F. Ascorbic acid, Helicobacter pylori, and Lewis phenotype among blacks and whites in New Orleans. Cancer Lett. 1994;83:323–329. doi: 10.1016/0304-3835(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 26.Shen Z, Fox J G, Dewhirst F E, Paster B J, Foltz C J, Yan L, Shames B, Perry L. Helicobacter rodentium sp. nov., a urease-negative Helicobacter species isolated from laboratory mice. Int J Syst Bacteriol. 1997;47:627–634. doi: 10.1099/00207713-47-3-627. [DOI] [PubMed] [Google Scholar]
- 27.Shomer, N. H., and J. G. Fox. Unpublished observations.
- 28.Sobala G M, Crabtree J E, Dixon M F, Schorah C J, Taylor J D, Rathbone B J, Heatley R V, Axon A T. Acute Helicobacter pylori infection: clinical features, local and systemic immune response, gastric mucosal histology, and gastric juice ascorbic acid concentration. Gut. 1991;32:1415–1418. doi: 10.1136/gut.32.11.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sobala G M, Pignatelli B, Schorah C J, et al. Simultaneous determination of ascorbic acid nitrite, total nitrosocompounds and bile acids in fasting gastric juice, and gastric mucosal histology implications for gastric carcinogenesis. Carcinogenesis. 1991;12:193–198. doi: 10.1093/carcin/12.2.193. [DOI] [PubMed] [Google Scholar]
- 30.Stevenson N. Active transport of L-ascorbic acid in the human ileum. Gastroenterology. 1974;67:952–956. [PubMed] [Google Scholar]
- 31.Stevenson N, Brush M. Existence and characteristics of Na+-dependent active transport of ascorbic acid in guinea pig. Am J Clin Nutr. 1969;22:318–326. doi: 10.1093/ajcn/22.3.318. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki A, Kameyama J, Tsukamoto M, Kaneko K, Suzuki Y. Stimulation of C1− and HCO3− secretion by intramural cholinergic neurons in guinea pig antrum in vitro. Am J Physiol. 1993;264:G118–G125. doi: 10.1152/ajpgi.1993.264.1.G118. [DOI] [PubMed] [Google Scholar]
- 33.Tannenbaum S, Wishnok K, Leaf C. Inhibition of nitrosamine formation by ascorbic acid. Am J Clin Nutr. 1991;53:247S–250S. doi: 10.1093/ajcn/53.1.247S. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Sjunnesson H, Sturegard E, Wadstrom T, Wilden R, Alejung P. Dietary components influence the ability to recover H. pylori from infected Balb/Ca mice. Gut. 1997;41:A121. [Google Scholar]
- 35.Yoshimura T, Johnson D. cDNA cloning and expression of guinea pig neutrophil attractant protein-1 (NAP-1). NAP-1 is highly conserved in guinea pig. Immunology. 1993;151:6225–6236. [PubMed] [Google Scholar]