Abstract
Gastroesophageal reflux disease (GERD) is caused by the reflux of gastric contents into the esophagus due to a decline in esophageal clearance and anti-reflux barrier mechanisms. Mucosal injury is caused by a combination of gastric juice directly damaging the esophageal mucosa and the immune and inflammatory mechanism in which inflammatory cytokines released from the esophageal mucosal epithelium cause neutrophil migration, triggering inflammation. Gastric secretion inhibitors are the first-line treatment for GERD, but they can be combined with prokinetic agents and Chinese herbal remedies. However, pharmacotherapy cannot improve anatomical problems or prevent physical causes of GERD, such as reflux of non-acidic contents. Therefore, surgery can be warranted, depending on the pathology. Intraluminal endoscopic therapy, which is both less invasive and more effective than surgery, was recently developed and applied in Europe and the United States. In Japan, intraluminal endoscopic therapies, such as anti-reflux mucosectomy, anti-reflux mucosal ablation, and endoscopic submucosal dissection, for GERD have been independently developed.
Keywords: gastroesophageal reflux disease (GERD), potassium-competitive acid blocker (P-CAB), proton pump inhibitor (PPI), intraluminal endoscopic therapy, anti-reflux mucosal ablation (ARMA), endoscopic submucosal dissection for GERD (EGD-G)
Introduction
Gastroesophageal reflux disease (GERD) is a disease entity in which troublesome symptoms and complications are caused by the reflux of the stomach contents, mainly gastric acid, into the esophagus (1). There has been a high prevalence of GERD in Europe and the United States (US) for a long time; however, in the late 1990s, it started to increase in Japan as well with the decline in Helicobacter pylori infection rates, westernization of the Japanese diet, increase in obesity rates, and aging of the population.
Currently, approximately 15% of the Japanese population experiences reflux symptoms weekly, making it among the most common upper gastrointestinal tract diseases in routine clinical practice. However, the pathology of GERD is complex, with interactions of chemical, mechanical, psychogenic, and neurological mechanisms causing esophageal mucosal injury and various symptoms. Therefore, it is commonly refractory to treatment.
New concepts and diagnostic tools to elucidate its pathology have recently emerged, and research and the development of treatments suited to each pathology have advanced.
1. GERD Causes and Pathology
1) Esophageal mucosal injury
The reflux of stomach contents, comprising substances such as acid, bile, pepsin, food content, and gastrointestinal microorganisms, is a major factor that triggers esophageal mucosal injury in GERD. These substances were conventionally believed to act directly on the esophageal mucosa, triggering inflammation and mucosal injury, followed by the entry of nociceptive stimulants, such as acid and pepsin from the injury site being dispersed within the esophageal mucosa and causing direct activation of the nociceptors deep within the esophageal mucosa. This results in signals being transmitted to the central nervous system that are perceived as symptoms (2). In fact, an experimental esophagitis study in rats reported a reduced expression in the functional fractionation of tight junction protein, which regulates esophageal mucosal permeability, and pathological examinations of esophageal biopsy samples with a transmission electron microscope revealed that the dilated intercellular spaces (DISs) were significantly more dilated in patients with GERD than controls (3). However, in an experimentally created rat reflux esophagitis model, the DISs were conversely located in the deep layer of the esophageal mucosa, and there were no dilated spaces on the surface of the esophagus, which would be necessary to allow acid to infiltrate the deep layer (4).
Souza et al. created a rat acid reflux model and reported that the infiltration of immunocompetent cells, such as lymphocytes and macrophages, induced by reflux started from the deep layers of the mucosa and then moved to the superficial layers before esophageal mucosal injury occurred (5). Furthermore, stopping the oral intake of a proton pump inhibitor (PPI), which is used to treat patients with reflux esophagitis, reportedly causes changes such as the infiltration of inflammatory cells to the deep mucosal layer and dilation of intercellular spaces before esophageal mucosal injury occurs (6). These findings demonstrate a new concept that, rather than acid and pepsin directly injuring the esophageal mucosal epithelium, these stimuli cause mucosal injury through an autoimmune mechanism mediated by the release of cytotoxic substances by inflammatory cells, such as neutrophils, which are induced to migrate through the secretion of inflammatory cytokines from the esophageal mucosal epithelium, such as interleukin (IL)-8 and IL-1β (2,6). Prostaglandins and inflammatory mediators secreted by the epithelium depolarize nociceptors on nerve terminals, and nociceptive stimuli are transmitted to the central nervous system, causing reflux symptoms such as heartburn (7).
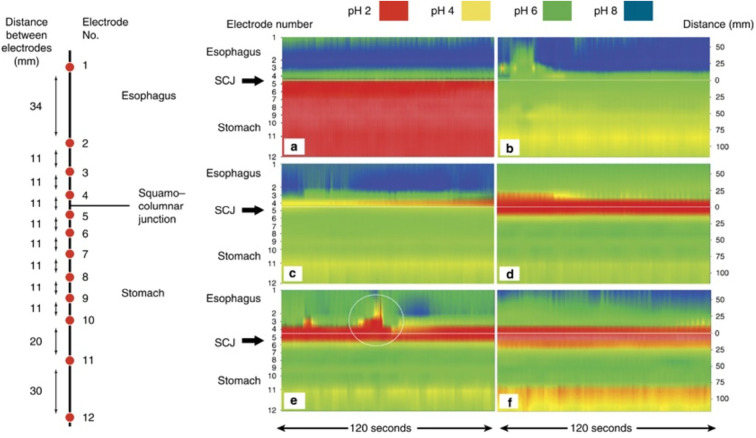
The presence of a gastric acid pockets has recently been considered an important source of gastric acid in postprandial reflux. Immediately after a meal, the food and gastric acid mix, neutralizing the inside of the stomach. However, over time, an acid pocket appears atop the food layer (Fig. 1) (8). This phenomenon is also observed in healthy individuals; however, the acid pocket is large in patients with GERD, inducing excess acid reflux. Furthermore, the presence of an esophageal hiatal hernia causes longer retention of the acid pocket that may increase the intra-esophageal acid exposure time. PPI administration reduces the size of the acid pocket (8).
Figure 1.
Characterization of acidity in the distal esophagus and proximal stomach under fasting conditions and in response to a large meal using high-resolution pH-metry in healthy subjects (8). The left panel shows the position of the 12-electrode catheter relative to that of the squamocolumnar junction (SCJ). The catheter was clipped to the SCJ with an endoclip using a loop tied between electrodes 4 and 5, 10.5 cm proximal to electrode 12, at the tip of the catheter. The right panel illustrates the pH contour plots (120 s duration each) of the high-resolution 12-electrode pH-meter (a) in the fasting state, showing marked intragastric acidity; (b) 3 min after the meal, showing intragastric buffering by food; (c) 17 min after the meal, showing emergence of the acid pocket at the esophagogastric junction; (d) 43.5 min after the meal, showing acid pocket enlargement; (e) 47.5 min after the meal, showing an acid reflux episode from the acid pocket (circled) despite simultaneous distal intragastric buffering; and (f) 73.5 min after the meal, simultaneously recording the proximal acid pocket and distal gastric acidity. This figure is from reference 8, and its use has been permitted by the publisher.
2) Esophageal perception
In a crossover study, physiological saline adjusted to various pH levels of 2-7.5, and capsaicin, a transient receptor potential channel, vanilloid subtype 1 (TRPV1) stimulatory ligand, were injected into the esophageal submucosa of healthy adults with no history of heartburn or acid reflux symptoms. Symptom intensity was investigated using a visual analog scale. The symptoms appeared only when capsaicin was injected and did not appear when acids with a pH of 2 were injected (9). These findings suggest that the cause of the symptoms is not the direct stimulus of acid but rather perceptual stimuli mediated by TRPV1. Furthermore, TRPV1-positive fibers, which have TRPV1 receptors, are reportedly expressed in the human esophagus, and TRPV1 expression is increased in patients with esophagitis and non-erosive reflux disease (NERD) (10).
Another study reported that the infusion of acid into the esophagus of healthy adults increases the production of prostaglandin E2 (PGE2) from the esophageal mucosal epithelium, and the amount of PGE2 produced is significantly correlated with heartburn symptom severity (7). When PGE2 production was inhibited with premedication of non-steroidal anti-inflammatory drugs, only heartburn symptoms were significantly reduced. Furthermore, heartburn symptoms were significantly improved by the administration of an EP1 receptor antagonist, which binds to PGE2 receptors (11).
3) Esophageal clearance mechanism
Esophageal clearance is a mechanism involving esophageal motility and salivation in which the stomach contents, including gastric acid, that have refluxed into the esophagus are expelled back into the stomach.
Esophageal peristaltic action is an important factor involved in expelling solids and liquids from the esophagus into the stomach. Primary peristalsis occurs due to swallowing stimuli, while secondary peristalsis occurs due to local stimulation of the esophageal walls unrelated to swallowing. The lower esophageal sphincter (LES) relaxes in response to swallowing action, and when primary peristaltic waves appear in the esophagus, relaxation of the LES ends once the food bolus is transferred into the stomach. When acid reflux occurs, the reflux liquid is expelled into the stomach by peristaltic waves, and the remaining gastric acid is neutralized by bicarbonate present in the saliva. The pH in the esophagus normally returns to the pre-acid reflux pH of 5-7 after 7-10 swallows (12,13). A decline in the clearance function due to reduced esophageal motility prolongs the acid exposure time associated with gastroesophageal reflux, making the individual prone to esophageal mucosal injury.
In terms of the height of contraction waves on conventional manometry, a contraction wave height of ≥30 mmHg is important for emptying the esophagus in the supine position (14). The appearance of peristaltic waves with wave heights of 20 mmHg or more is important for esophageal clearance during the measurement of esophageal pressure using recently developed high-resolution manometry (HRM) approaches (15). The absence of peristaltic waves along the entire length of the esophagus or the presence of non-propulsive peristaltic waves induced by some peristaltic waves, can cause impaired acid expulsion and/or difficulty swallowing. Peristalsis is evaluated based on peristaltic velocity, and peristaltic velocity is evaluated by an increase in contraction waves. The normal peristaltic velocity is reportedly approximately 3.0 cm/s in the proximal esophagus and approximately 3.5 cm/s in the distal esophagus. When the peristaltic velocity is high (≥6 cm/s), the contraction waves are almost synchronous, which makes it difficult to expel food and reflux liquid toward the anus (12).
During salivation, chemoreceptors in the esophagus are activated owing to the presence of acid, and salivation is activated by the esophago-salivary reflex, which stimulates the salivary glands. Gastric acid in the esophagus is neutralized by bicarbonate present in the saliva and expelled into the stomach by increased clearance. Salivation is significantly decreased during sleep; therefore, increased reflux events during the night extend the intra-esophageal acid exposure time (16). Furthermore, an impaired acid clearance capacity due to factors such as chronic diseases that reduce salivation, pharmaceuticals, and aging prolongs the intra-esophageal acid exposure time.
4) Anti-reflux barrier mechanism
The LES is a high-pressure zone that encompasses 3-4 cm of the lower esophagus and works as an anti-reflux barrier mechanism that separates the negative-pressure thoracic cavity from the positive-pressure abdominal wall. This high-pressure zone normally comprises two components: the LES, a smooth muscle internal sphincter; and the crural diaphragm (CD), which acts as an external sphincter from around the esophagus. Normally, the LES, CD, and phrenoesophageal membrane comprise a complex three-dimensional structure that forms the esophagogastric junction (EGJ).
The effects of the LES and CD on the motor function of the EGJ must be considered. The resting EGJ pressure varies depending on the measurement method and respiratory movement; however, it maintains an average pressure of approximately 20 mmHg relative to the gastric pressure (17). The LES relaxes within 1-2.5 s of swallowing, and the EGJ pressure decreases; however, when the peristaltic contractions reach the LES, the LES contracts, and the EGJ pressure increases. The EGJ pressure is high at the level of the diaphragm and increases during inspiration. The factors that affect EGJ pressure include an elevated abdominal pressure, body position, gastric motility during fasting, food intake, smoking, pregnancy, psychological stress, and certain pharmaceuticals (anticholinergic drugs, nitrites, and calcium antagonists).
Transient LES relaxation (TLESR) is LES relaxation that occurs without swallowing. However, TLESR is a physiological rather than a pathological condition because it occurs during belching due to the reflux of excess air in the stomach. TLESR is vagally mediated in response to distension of the gastric fundus after eating (18). It is characteristically longer than LES relaxation time after swallowing, lasting 10 s or longer (mean, 15-20 s) compared to 5-8 s for LES relaxation time, and CD movement is suppressed during LES relaxation. Gastric acid may also reflux with air, which also occurs in healthy individuals, and almost all acid reflux in patients with NERD and mild reflux esophagitis occur during TLESR (19).
2. Treatment of GERD
1) Pharmacotherapy
The aim of GERD treatment is to control bothersome symptoms, improve the quality of life (QOL), and prevent complications, such as bleeding and stenosis. According to the GERD diagnosis and treatment flowchart in the Evidence-based Clinical Practice Guidelines for Gastroesophageal Reflux Disease 2021 (20), PPIs should be administered for two to four weeks to patients who have not undergone an endoscopic examination, and their condition should be evaluated for symptom improvement. When conducting an endoscopic examination, the condition is classified as severe reflux esophagitis (Los Angeles classification Grade C or D), mild reflux esophagitis (Grade A or B), or NERD based on the severity of esophageal mucosal injury on endoscopy, and the treatment strategy is formulated accordingly.
A meta-analysis of the initial treatment of severe reflux esophagitis with the non-healing rate of reflux esophagitis as the outcome demonstrated that the non-healing rate after 4 and 8 weeks was significantly lower with potassium-competitive acid blockers (P-CAB) than with PPIs; therefore, the administration of P-CAB (vonoprazan 20 mg/day) for 4 weeks was proposed. Treatment may also be extended to eight weeks if the effect is insufficient. In the long-term treatment of severe reflux esophagitis, P-CAB is also associated with a lower recurrence rate on endoscopy than PPIs, and the guidelines propose proactive maintenance therapy with P-CAB to prevent complications.
A standardized systematic review of the initial treatment of mild reflux esophagitis was conducted, and a meta-analysis with the endoscopic mucosal non-healing rate as the outcome showed no significant difference in the non-healing rate after four and eight weeks of PPI or P-CAB administration. Therefore, a PPI or P-CAB is recommended as the first-line drug for the initial treatment of mild reflux esophagitis. The guidelines recommend using a PPI for long-term maintenance based on substantial evidence gathered thus far. In contrast, while P-CAB is also expected to have a maintenance effect that is similar to or better than that of a PPI, insufficient information exists on the safety of its long-term administration; therefore, the guidelines suggest the use of P-CAB.
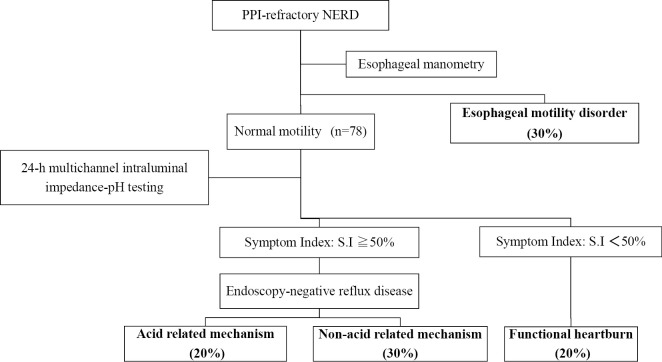
A clinical evaluation is conducted after four weeks of PPI administration for the initial treatment of NERD; if the condition has improved, the PPI is continued as maintenance therapy. If no improvement is observed, a detailed pathological assessment is conducted for PPI-refractory NERD. Specifically, esophageal manometry is performed to exclude esophageal motility disorders, and 24-h multichannel intraluminal impedance-pH (MII-pH) monitoring is conducted. Cases are classified into three groups: acid reflux-related mechanism, non-acid reflux-related mechanism, and functional heartburn unrelated to gastroesophageal reflux (Fig. 2) (21).
Figure 2.
Pathophysiological classification of PPI-refractory NERD. Based on the results obtained from intra-esophageal manometry and 24-h-long intra-esophageal pH/impedance monitoring, the subjects were classified into three groups according to the Rome III criteria: acid reflux-related mechanism, non-acid reflux-related mechanism, and functional heartburn. This figure is original from the present authors.
Patients with persistent symptoms or those whose mucosal injuries have not healed on an endoscopic examination despite normal doses of a PPI for eight weeks are treated as having PPI-refractory GERD. Several pathologies can be considered to underlie PPI-refractory GERD, similar to PPI-refractory NERD (21). Doubling the dose of the PPI to be received twice daily or switching to vonoprazan is recommended for patients with insufficient inhibition of acid secretion. However, if the pathology is suspected to be caused by factors other than the reflux of excess gastric acid into the esophagus, such as esophageal motility disorders, the concomitant use of prokinetic agents or Chinese herbal remedies is also recommended.
We examined the improvement in functional dyspepsia (FD) with GERD symptoms in a randomized double-blind placebo-controlled crossover study using the prokinetic agent acotiamide (100 mg) and a placebo in 16 patients with PPI-refractory NERD with FD who were diagnosed with mild esophageal motility disorders on esophageal manometry using HRM. An evaluation of FD symptoms revealed that the acotiamide group had a significantly reduced feeling of fullness compared with the placebo group; however, there was no marked difference in early satiety, epigastric pain, or epigastric burning sensation. A study reported that, upon an evaluation of GERD symptoms using the revised F scale, there was a significant reduction in the total score, GERD-related score, and FD-related score in the acotiamide versus placebo group (22). The concomitant use of mosapride (23,24) and rikkunshito (25) is reportedly effective in improving symptoms.
Baclofen, a GABA-B receptor agonist, and its derivatives have mainly been developed as anti-reflux drugs in Europe and the US (26,27); however, their efficacy is limited, and they may have adverse reactions in the central nervous system (28). Therefore, these drugs are not widely used in clinical practice. The use of tricyclic antidepressants and selective serotonin re-uptake inhibitors is recommended to improve hyperesthesia (29,30); however, these drugs have limited therapeutic effects.
2) Surgical treatment
Anatomical problems, such as esophageal hiatal hernia and LES relaxation, cannot be improved with pharmacotherapy. Furthermore, physical causes, such as esophageal reflux of non-acidic contents, cannot be prevented by pharmacotherapy. Therefore, treatments other than pharmacotherapy have also been investigated.
Surgical treatment of esophageal hiatal hernias involves anatomical repair and anti-reflux surgery. Anatomical repair includes reduction of the hernia, resection of the hernial sac, and suturing of the hiatus, while anti-reflux surgery includes the Nissen and Toupet procedures as fundoplication. Anti-reflux surgery is formally performed using laparotomy; however, these procedures are almost always performed laparoscopically, which has both an improved safety and efficacy compared with laparotomy (31-34). Surgical procedures have a high efficacy and patient satisfaction (35), and their efficacy is especially high when the procedures are performed by experienced surgeons (36,37). However, there are also reports of surgical complications and the onset of symptoms, such as difficulty swallowing, abdominal distension, and flatulence (38); problems with maintaining long-term effects; and cost-effectiveness (39). In addition, the surgical mortality rate is 0.1-0.2% (40), suggesting problems with the surgical treatment of benign diseases. Against this background, the possibility of intraluminal endoscopic therapy as a less invasive and effective treatment has been explored in Europe and the US since 1990.
3) Intraluminal endoscopic therapy
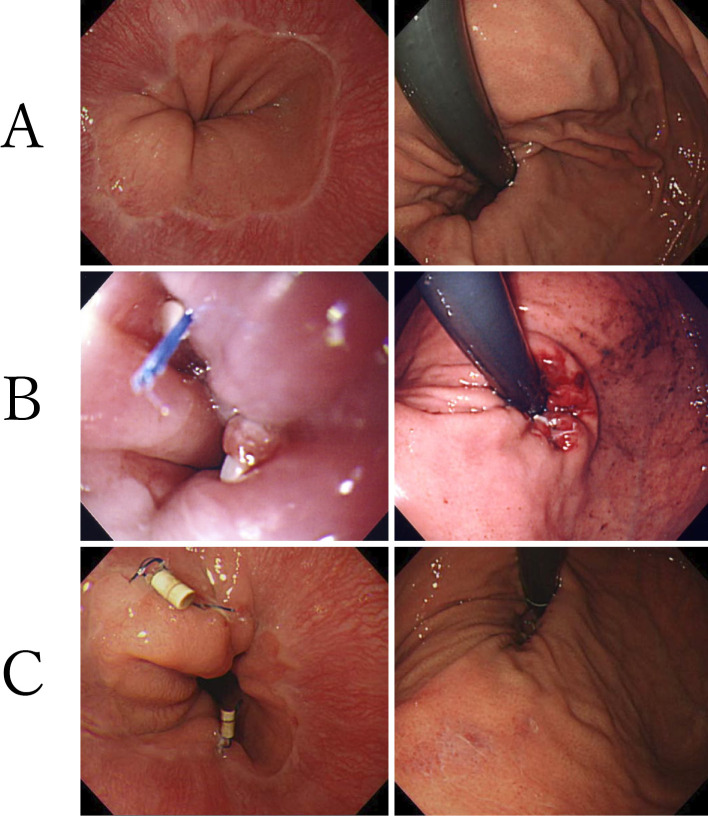
In 2000, three intraluminal endoscopic therapy procedures for GERD based on different principles were developed and applied in clinical practice: plicating the gastric fold, thermal tissue remodeling/neurolysis, and bulking injection of the LES zone (41). In our facility, we introduced and investigated endoluminal gastroplication (ELGP) as intraluminal endoscopic therapy. Nineteen patients with PPI-refractory NERD who showed no improvement in symptoms after receiving normal doses of PPI for eight weeks underwent ELGP. An average of 2.4 plications were made, and follow-up was conducted for 12 months (Fig. 3). The mean value of the frequency scale for the symptoms of GERD (FSSG), a scale for evaluating GERD symptoms, improved significantly from 19.1±10.5 preoperatively to 9.3±9.9 at 12 months postoperatively. In addition, the PPI usage rate decreased significantly from 100% to 1.6±1.2%, while the number of plications decreased significantly from 2.4±0.8 to 0.8±1.0. The symptom sensitivity index, an index of esophageal sensitivity, decreased significantly from 16.1±12.9 to 3.9±8.3 on 24-h MII-pH monitoring. This suggests a reduced esophageal sensitivity to acid. This procedure had no serious complications and is considered a viable treatment option for PPI-refractory NERD (42).
Figure 3.
Actual ELGP for patients with PPI-refractory NERD. A: Preoperative image (GERD with mild esophageal hiatal hernia). B: Immediately after ELGP (plication performed in three locations). C: Three months after ELGP (plication remains in two locations). This figure is original from the present authors. ELGP: endoluminal gastroplication, GERD: gastroesophageal reflux disease, NERD: non-erosive reflux disease, PPI: proton pump inhibitor
ELGP came to be covered by insurance in Japan in April 2006 as an endoscopic esophageal cardiac plication surgery (K667-3: 12,000 points). However, the procedure cannot currently be performed because of device supply issues.
Several reports have demonstrated the efficacy of these intraluminal endoscopic therapies for GERD symptoms; however, safety and long-term data have shown limited efficacy and indications, and several therapies have been withdrawn from the market (41). Therefore, only methods involving full-thickness suturing of the stomach wall, such as transoral incisionless fundoplication (TIF), medigus ultrasonic surgical endostapler (MUSEⓇ, Medigus, Omer, Israel), and StrettaⓇ(Mederi Therapeutics, Greenwich, USA), which thermally denatures the muscle layer in the LES region using radio waves, are performed in Europe and the US.
4) Current situation in Europe and the US
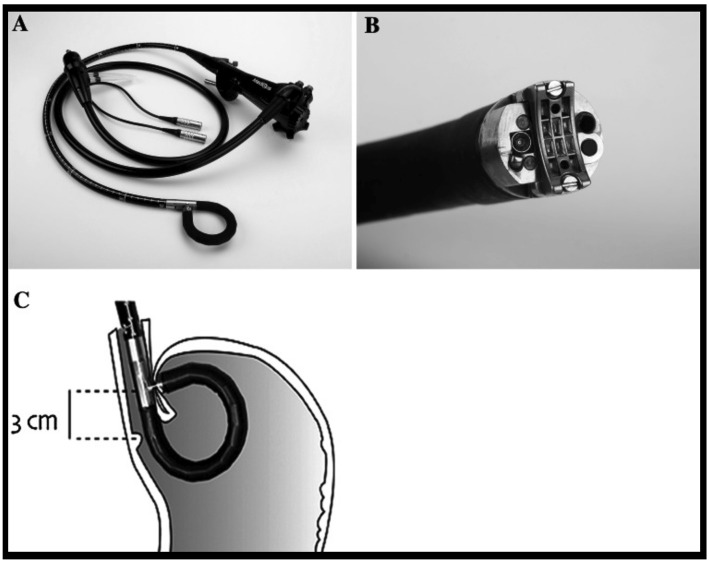
The TIF procedure involves the insertion of an EsophyXⓇ (EndoGastric Solutions, Redwood City, USA) device into the stomach through the endoscope, wherein the full thickness of the fornix is pulled into the gastric lumen under inverted endoscopic observation, and an H-fastener is passed through the esophageal wall and stomach wall to suture the full thickness at the esophagogastric junction line. This procedure is repeated several times within 200-270° from the greater curvature of the stomach to form an esophagogastric junction valve. Five randomized controlled trials reported improvements in the health-related QOL (HRQOL) and heartburn symptoms as well as a reduction in the PPI usage rate and esophageal acid reflux. The long-term efficacy and safety have also been reported by a few meta-analyses (43). MUSEⓇ uses a special endoscope to perform ultrasound-guided full-thickness suturing of the anterior wall of the cardia using a stapler (Fig. 4). There have been no reports of randomized controlled trials for this technique; however, a number of small-scale studies have reported an improvement in the GERD-related HRQOL and reduced PPI use (44). In the future, it will be necessary to conduct long-term investigations in a large number of randomized cases.
Figure 4.
Medigus Ultrasonic Surgical Endostapler (MUSE®) (44). A: Endoscopic tool. B: Tip part (15.5-mm diameter). C: The cartridge was positioned 3 cm proximal to the esophagogastric junction, and plication was performed. This figure is from reference 44, and its use was permitted by the publisher.
The StrettaⓇ procedure inserts a needle electrode into the lower esophagus close to the cardia and narrows the lumen by heating and denaturing the smooth muscle tissue with radio waves to prevent reflux. A meta-analysis reported that the procedure effectively improved the HRQOL and heartburn symptoms and reduced the PPI usage rate and esophageal acid reflux (45). A large number of intraluminal endoscopic therapies, mainly based on these three types of procedures, are performed in Europe and the US. However, these devices have not been approved by pharmaceutical regulatory authorities in Japan, nor are these procedures yet covered by Japanese national health insurance.
5) Current situation in Japan
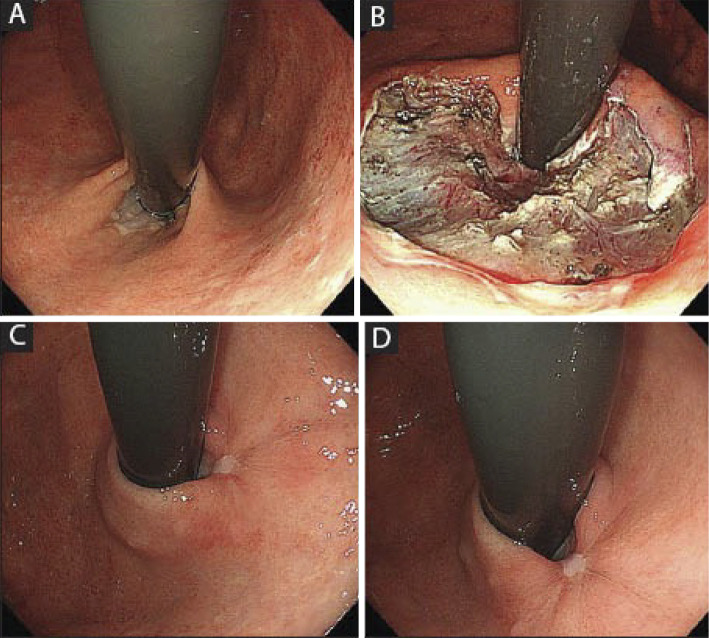
Intraluminal endoscopic therapies based on new concepts have been researched and developed in Japan over the past few years. Anti-reflux mucosectomy (ARMS) is a procedure developed by Inoue et al. (46) in which a subtotal incision is made endoscopically in the cardia, and the dilated cardia is reduced by cicatricial stenosis to reduce reflux (Fig. 5). A previous study retrospectively investigated the outcomes, such as the symptom score and PPI usage rate, in 109 patients with PPI-refractory GERD treated with ARMS (47). The inclusion criteria for that study were typical reflux symptoms, abnormal esophageal acid exposure, cases with a positive symptom index (SI) or symptom association probability (SAP), sliding hiatal hernia ≤3 cm, and no esophageal motor dysfunction. The primary outcome (symptom score by GerdQ and Frequency Scale for the Symptoms of GERD) showed significant improvement after the ARMS procedure, and 40-50% of patients were able to stop taking PPIs. The acid exposure time and DeMeester score also improved significantly in the 24-h MII-pH test. No serious complications were observed.
Figure 5.
Anti-reflux mucosectomy (ARMS) (46). A: Before ARMS. B: Immediately after ARMS, the lesser curvature remained, and an ulcer accounting for two-thirds of the circumference was found. C, D: Two months after ARMS, the cardia lip was reshaped. This figure is from reference 46, and its use was permitted by the publisher.
Anti-reflux mucosal ablation (ARMA), also developed by Inoue et al., improved ARMS mucosectomy to a simpler mucosal ablation. ARMA procedure is an endoscopic subtotal mucosal ablation in the cardia instead of mucosectomy and the dilated cardia is reduced by cicatricial stenosis to reduce reflux. The criteria for performing ARMA in their study were the presence of at least 1 typical reflux symptom more than twice a week despite double-dose PPI treatment for at least 6 months and pathologic esophageal acid exposure, defined by a DeMeester score >14.7 or acid exposure time (AET) >4.2%. The exclusion criteria were age <20 years old, primary esophageal motility disorders, sliding hiatal hernia >3 cm on gastroscopy, a grade IV Hill's flap valve, and pregnancy. The primary outcome (symptom improvement evaluated by the GERD-Health Related Quality of Life Questionnaire and the Frequency Scale for the Symptoms of GERD) showed significant improvement after the ARMA procedure (48).
According to a recent meta-analysis of ARMS and ARMA, the 1- and 3-year clinical success rates were 78% and 73%, respectively. In addition, the PPI discontinuation rate at 1 year significantly improved by 64%. Adverse reactions to dysphagia were observed in 11% of cases, while 7% of cases required dilatation (49).
In contrast, endoscopic submucosal dissection for GERD (ESD-G), developed by Ota et al., is a method in which the mucosa on the esophageal side of the EGJ is resected, and reflux is reduced by cicatricial stenosis (50). The criteria for performing ESD-G in their study were patients with endoscopic findings suggesting the presence of GERD, such as reflux esophagitis, cloudiness of the lower esophageal mucosa, or presence of Barrett's epithelium without esophageal motor dysfunction and the presence of GERD-related symptoms that persisted despite PPI therapy for eight weeks. Significant improvement was noted in symptoms and endoscopic mucosal injury in 35 patients with PPI-refractory GERD treated with this procedure, and the efficacy of gastric secretion inhibitors was significantly reduced. However, no marked improvement was observed in reflux episodes in the objective index of impedance monitoring (51).
Intraluminal endoscopic therapies, a type of treatment for GERD being researched and developed in Japan, do not require special devices and are relatively simple to perform; therefore, they can be performed safely at a low cost. It may also be possible to expand the indications for this procedure to include postoperative cases, such as reflux esophagitis after gastrectomy and weight loss surgery. Furthermore, EGD-G and ARMS are covered by Japanese health insurance as endoscopic anti-reflux mucosectomy (K653-6) as of the FY2022 revision of the medical fees. However, ARMA is not yet covered by insurance. Thus, further deployment and dissemination of this procedure are expected in the future.
Conclusion
Although GERD is a benign disease, it significantly reduces the QOL of patients with persistent and bothersome symptoms. However, the QOL improves when symptoms are controlled with treatment and can be improved to the same level as in healthy individuals if the symptoms are eradicated (52).
When gastric acid reflux is significantly involved in GERD pathology, potent acid secretion inhibitors, such as vonoprazan, can be effective. However, when the condition is refractory to these treatments, more complex pathologies other than acid reflux are assumed to be involved, and standard treatment approaches are limited. Recently, therapeutic agents designed to suit various pathologies have been developed, and non-drug treatments, such as intraluminal endoscopic therapy, have progressed. Appropriate treatments to suit individual pathologies will be possible with the further clarification of GERD pathology and the development of minimally invasive and highly effective diagnostic and treatment methods, which may help improve the QOL of patients with GERD.
Author's disclosure of potential Conflicts of Interest (COI).
Kunio Kasugai: Honoraria, Sanwa Kagaku Kenkyusho, EA Pharma, Takeda Pharmaceutical and Otsuka Pharmaceutical.
References
- 1. Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 101: 1900-1920; quiz 1943, 2006. [DOI] [PubMed] [Google Scholar]
- 2. Smout AJ, Bredenoord AJ. GERD: a challenge to our view of reflux oesophagitis pathogenesis. Nat Rev Gastroenterol Hepatol 13: 504-505, 2016. [DOI] [PubMed] [Google Scholar]
- 3. Tobey NA, Carson JL, Alkiek RA, Orlando RC. Dilated intercellular spaces: a morphological feature of acid reflux - damaged human esophageal epithelium. Gastroenterology 111: 1200-1205, 1996. [DOI] [PubMed] [Google Scholar]
- 4. Miwa H, Kondo T, Oshima T. Gastroesophageal reflux disease-related and functional heartburn: pathophysiology and treatment. Curr Opin Gastroenterol 32: 344-352, 2016. [DOI] [PubMed] [Google Scholar]
- 5. Souza RF, Huo X, Mittal V, et al. Gastroesophageal reflux might cause esophagitis through a cytokine-mediated mechanism rather than caustic acid injury. Gastroenterology 137: 1776-1784, 2009. [DOI] [PubMed] [Google Scholar]
- 6. Dunbar KB, Agoston AT, Odze RD, et al. Association of acute gastroesophageal reflux disease with esophageal histologic changes. JAMA 315: 2104-2112, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kondo T, Oshima T, Tomita T, et al. The nonsteroidal anti-inflammatory drug diclofenac reduces acid-induced heartburn symptoms in healthy volunteers. Clin Gastroenterol Hepatol 13: 1249-1255.e1, 2015. [DOI] [PubMed] [Google Scholar]
- 8. Kahrilas PJ, McColl K, Fox M, et al. The acid pocket: a target for treatment in reflux disease? Am J Gastroenterol 108: 1058-1064, 2013. [DOI] [PubMed] [Google Scholar]
- 9. Lee RH, Korsapati H, Bhalla V, Varki N, Mittal RK. Esophageal submucosal injection of capsaicin but not acid induces symptoms in normal subjects. J Neurogastroenterol Motil 22: 436-443, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bhat YM, Bielefeldt K. Capsaicin receptor (TRPV1) and non-erosive reflux disease. Eur J Gastroenterol Hepatol 18: 263-270, 2006. [DOI] [PubMed] [Google Scholar]
- 11. Kondo T, Sei H, Yamasaki T, et al. A novel prostanoid EP1 receptor antagonist, ONO-8539, reduces acid-induced heartburn symptoms in healthy male volunteers: a randomized clinical trial. J Gastroenterol 52: 1081-1089, 2017. [DOI] [PubMed] [Google Scholar]
- 12. Helm JF, Dodds WJ, Riedel DR, Teeter BC, Hogan WJ, Arndorfer RC. Determinants of esophageal acid clearance in normal subjects. Gastroenterology 85: 607-612, 1983. [PubMed] [Google Scholar]
- 13. Helm JF, Dodds WJ, Pelc LR, Palmer DW, Hogan WJ, Teeter BC. Effect of esophageal emptying and saliva on clearance of acid from the esophagus. N Engl J Med 310: 284-288, 1984. [DOI] [PubMed] [Google Scholar]
- 14. Kahrilas PJ, Dodds WJ, Hogan WJ. Effect of peristaltic dysfunction on esophageal volume clearance. Gastroenterology 94: 73-80, 1988. [DOI] [PubMed] [Google Scholar]
- 15. Kahrilas PJ, Bredenoord AJ, Fox M, et al.; International High Resolution Manometry Working Group. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 27: 160-174, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Helm JF. Role of saliva in esophageal function and disease. Dysphagia 4: 76-84, 1989. [DOI] [PubMed] [Google Scholar]
- 17. Pandolfino JE, Kahrilas PJ; American Gastroenterological Association. AGA technical review on the clinical use of esophageal manometry. Gastroenterology 128: 209-224, 2005. [DOI] [PubMed] [Google Scholar]
- 18. Mittal RK, Holloway RH, Penagini R, Blackshaw LA, Dent J. Transient lower esophageal sphincter relaxation. Gastroenterology 109: 601-610, 1995. [DOI] [PubMed] [Google Scholar]
- 19. Hayashi Y, Iwakiri K, Kotoyori M, Sakamoto C. Mechanisms of acid gastroesophageal reflux in the Japanese population. Dig Dis Sci 53: 1-6, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Evidence-based Clinical Practice Guidelines for Gastroesophageal Reflux Disease (GERD) 2021 (revised 3rd edition). The Japanese Society of Gastroenterology , Ed. Nankodo, Tokyo, 2021. [Google Scholar]
- 21. Tamura Y, Funaki Y, Izawa S, et al. Pathophysiology of functional heartburn based on Rome III criteria in Japanese patients. World J Gastroenterol 21: 5009-5016, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Funaki Y, Ogasawara N, Kawamura Y, et al. Effects of acotiamide on functional dyspepsia patients with heartburn who failed proton pump inhibitor treatment in Japanese patients: a randomized, double-blind, placebo-controlled crossover study. Neurogastroenterol Motil 32: e13749, 2020. [DOI] [PubMed] [Google Scholar]
- 23. Futagami S, Iwakiri K, Shindo T, et al. The prokinetic effect of mosapride citrate combined with omeprazole therapy improves clinical symptoms and gastric emptying in PPI-resistant NERD patients with delayed gastric emptying. J Gastroenterol 45: 413-421, 2010. [DOI] [PubMed] [Google Scholar]
- 24. Miyamoto M, Manabe N, Haruma K. Efficacy of the addition of prokinetics for proton pump inhibitor (PPI) resistant non-erosive reflux disease (NERD) patients: significance of frequency scale for the symptom of GERD (FSSG) on decision of treatment strategy. Intern Med 49: 1469-1476, 2010. [DOI] [PubMed] [Google Scholar]
- 25. Tominaga K, Kato M, Takeda H, et al.; G-PRIDE Study Group. A randomized, placebo-controlled, double-blind clinical trial of rikkunshito for patients with non-erosive reflux disease refractory to proton-pump inhibitor: the G-PRIDE study. J Gastroenterol 49: 1392-1405, 2014. [DOI] [PubMed] [Google Scholar]
- 26. Grossi L, Spezzaferro M, Sacco LF, Marzio L. Effect of baclofen on oesophageal motility and transient lower oesophageal sphincter relaxations in GORD patients: a 48-h manometric study. Neurogastroenterol Motil 20: 760-766, 2008. [DOI] [PubMed] [Google Scholar]
- 27. Li S, Shi S, Chen F, Lin J. The effects of baclofen for the treatment of gastroesophageal reflux disease: a meta-analysis of randomized controlled trials. Gastroenterol Res Pract 2014: 307805, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Falk GW. Inhibition of transient lower esophageal sphincter relaxation in GERD: will lesogaberan advance the field? Gastroenterology 139: 377-379, 2010. [DOI] [PubMed] [Google Scholar]
- 29. Broekaert D, Fischler B, Sifrim D, Janssens J, Tack J. Influence of citalopram, a selective serotonin reuptake inhibitor, on oesophageal hypersensitivity: a double-blind, placebo-controlled study. Aliment Pharmacol Ther 23: 365-370, 2006. [DOI] [PubMed] [Google Scholar]
- 30. Ostovaneh MR, Saeidi B, Hajifathalian K, et al. Comparing omeprazole with fluoxetine for treatment of patients with heartburn and normal endoscopy who failed once daily proton pump inhibitors: double-blind placebo-controlled trial. Neurogastroenterol Motil 26: 670-678, 2014. [DOI] [PubMed] [Google Scholar]
- 31. Kornmo TS, Ruud TE. Long-term results of laparoscopic Nissen fundoplication due to gastroesophageal reflux disease. A ten year follow-up in a low volume center. Scand J Surg 97: 227-230, 2008. [DOI] [PubMed] [Google Scholar]
- 32. Broeders JA, Rijnhart-de Jong HG, Draaisma WA, Bredenoord AJ, Smout AJ, Gooszen HG. Ten-year outcome of laparoscopic and conventional Nissen fundoplication: randomized clinical trial. Ann Surg 250: 698-706, 2009. [DOI] [PubMed] [Google Scholar]
- 33. Bona D, Aiolfi A, Asti E, Bonavina L. Laparoscopic Toupet fundoplication for gastroesophageal reflux disease and hiatus hernia: proposal for standardization using the “critical view” concept. Updates Surg 72: 555-558, 2020. [DOI] [PubMed] [Google Scholar]
- 34. Erenoglu C, Miller A, Schirmer B. Laparoscopic Toupet versus Nissen fundoplication for the treatment of gastroesophageal reflux disease. Int Surg 88: 219-225, 2003. [PubMed] [Google Scholar]
- 35. Peters JH, DeMeester TR. Indications, benefits and outcome of laparoscopic Nissen fundoplication. Dig Dis 14: 169-179, 1996. [DOI] [PubMed] [Google Scholar]
- 36. Salminen P, Hiekkanen H, Laine S, Ovaska J. Surgeons' experience with laparoscopic fundoplication after the early personal experience: does it have an impact on the outcome? Surg Endosc 21: 1377-1382, 2007. [DOI] [PubMed] [Google Scholar]
- 37. Brown CN, Smith LT, Watson DI, Devitt PG, Thompson SK, Jamieson GG. Outcomes for trainees vs experienced surgeons undertaking laparoscopic anti-reflux surgery - is equipoise achieved? J Gastrointest Surg 17: 1173-1180, 2013. [DOI] [PubMed] [Google Scholar]
- 38. Garg SK, Gurusamy KS. Laparoscopic fundoplication surgery versus medical management for gastro-oesophageal reflux disease (GORD) in adults. Cochrane Database Syst Rev 2015: CD003243, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sandhu DS, Fass R. Current trends in the management of gastroesophageal reflux disease. Gut Liver 12: 7-16, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yadlapati R, Hungness ES, Pandolfino JE. Complications of anti-reflux surgery. Am J Gastroenterol 113: 1137-1147, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kasugai K, Tokudome K, Funaki Y, Yoneda M. Endoscopic therapy for gastroesophageal reflux disease. Gastroenterol Endosc 51: 1269-1283, 2009(in Japanese). [Google Scholar]
- 42. Tokudome K, Funaki Y, Sasaki M, et al. Efficacy of endoluminal gastroplication in Japanese patients with proton pump inhibitor-resistant, non-erosive esophagitis. World J Gastroenterol 18: 5940-5947, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Richter JE, Kumar A, Lipka S, Miladinovic B, Velanovich V. Efficacy of laparoscopic Nissen fundoplication vs. transoral incisionless fundoplication or proton pump inhibitors in patients with gastroesophageal reflux disease: a systematic review and network meta-analysis. Gastroenterology 154: 1298-1308.e7, 2018. [DOI] [PubMed] [Google Scholar]
- 44. Zacherl J, Roy-Shapira A, Bonavina L, et al. Endoscopic anterior fundoplication with the Medigus Ultrasonic Surgical Endostapler (MUSE) for gastroesophageal reflux disease: 6-month results from a multi-center prospective trial. Surg Endosc 29: 220-229, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fass R, Cahn F, Scotti DJ, Gregory DA. Systematic review and meta-analysis of controlled and prospective cohort efficacy studies of endoscopic radiofrequency for treatment of gastroesophageal reflux disease. Surg Endosc 31: 4865-4882, 2017. [DOI] [PubMed] [Google Scholar]
- 46. Inoue H, Ito H, Ikeda H, et al. Anti-reflux mucosectomy for gastroesophageal reflux disease in the absence of hiatus hernia: a pilot study. Ann Gastroenterol 27: 346-351, 2014. [PMC free article] [PubMed] [Google Scholar]
- 47. Sumi K, Inoue H, Kobayashi Y, et al. Endoscopic treatment of proton pump inhibitor-refractory gastroesophageal reflux disease with anti-reflux mucosectomy: experience of 109 cases. Dig Endosc 33: 347-354, 2020. [DOI] [PubMed] [Google Scholar]
- 48. Inoue H, Tanabe M, de Santiago ER, et al. Anti-reflux mucosal ablation (ARMA) as a new treatment for gastroesophageal reflux refractory to proton pump inhibitors: a pilot study. Endosc Int Open 8: E133-E138, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rodriguez de Santiago E, Sanchez-Vegazo CT, Penas B, et al. Anti-reflux mucosectomy (ARMS) and anti-reflux mucosal ablation (ARMA) for gastroesophageal reflux disease: a systematic review and meta-analysis. Endosc Int Open 9: E1740-E1751, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ota K, Takeuchi T, Harada S, et al. A novel endoscopic submucosal dissection technique for proton pump inhibitor-refractory gastroesophageal reflux disease. Scand J Gastroenterol 49: 1409-1413, 2014. [DOI] [PubMed] [Google Scholar]
- 51. Ota K, Takeuchi T, Kojima Y, et al. Outcomes of endoscopic submucosal dissection for gastroesophageal reflux disease (ESD-G) for medication-refractory gastroesophageal reflux disease: 35 cases underwent ESD-G including 15 cases followed more than 5 years. BMC Gastroenterol 21: 432, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bytzer P. Goals of therapy and guidelines for treatment success in symptomatic gastroesophageal reflux disease patients. Am J Gastroenterol 98: S31-S39, 2003. [DOI] [PubMed] [Google Scholar]