Highlights
-
•
11 volatile compounds shape fruity, cereal, and herbal aromas in Hakka rice wine.
-
•
Undecanal contributing to cereal aroma in Hakka rice wine.
-
•
Kodamaea ohmeri is the predominant fungus during fermentation.
-
•
Pediococcus, W. paramesenteroides and Enterobacter are main bacteria in brewing.
-
•
Bacillus and Staphylococcus increase gradually in post-fermentation process.
Keywords: Hakka Huangjiu, Key odorants, Gas chromatography–mass spectrometry (GC–MS), Microbial community, Non-Saccharomyces
Abstract
Hakka rice wine is produced from grains by co-fermentation with abundant microbes in an open fermentation environment. Indigenous microbiota and enzymes convert the nutrients in grains into flavor compounds through enzymatic biochemical reactions and microbial metabolism. High-throughput sequencing technology revealed that non-Saccharomyces yeasts dominated the traditional fermentation process, with genera such as Kodamaea ohmeri, Candida orthopsilosis, and Trichosporon asteroides forming a dynamic community that highly correlated with the evolution of 80 volatile compounds in Hakka rice wine. Among the 104 volatile compounds detected by GC–MS, 22 aroma-active compounds with relative odor activity values (ROAV) > 1 were quantified, 11 of which made significant contributions (P < 0.05) to the overall aroma and were responsible for the sweet, grainy, and herbal aromas of Hakka rice wine.
Introduction
Rice wine is a traditional Chinese alcoholic beverage appreciated by consumers for its full-bodied aroma, soft taste, and high nutritional value dating back to ancient times. Typically, Chinese rice wine fermentation uses grain as feedstock and Qu as a starter for saccharification (Chen & Xu, 2010). Hakka rice wine, an essential component of Chinese rice wine, has been endemic to the Lingnan area for thousands of years. Owing to its unique brewing process and environment, it possesses a distinctive aroma, which separates it from other aromatic Chinese rice wine, and is widely appreciated by consumers (Zhao et al., 2023). Several researchers have studied the attractive aroma of Chinese rice wine and attributed it to its raw materials, fermentation, and enzymatic chemical reactions (Yang et al., 2020). Among these factors, the aroma produced during the fermentation process is the most critical volatile flavor in Chinese rice wine and is primarily produced by various microorganisms in Qu and the wine-making environment (Chen et al., 2021). Therefore, the functional studies on microorganisms plays a crucial role in analyzing flavor components and mechanisms of flavor formation.
Traditional Hakka rice wine is made from brown sweet rice through natural mixed fermentation with sweet distiller yeast. Fermentation decomposes starch and increases the concentration of low-molecular-weight polysaccharides, mainly composed of glucose. These sugars serve as nutritional sources for fermenting microorganisms that metabolize the sugars to produce different amounts of higher alcohols, esters, alcohols, aldehydes, and organic acids. These compounds are responsible for the distinct aroma and flavor profiles of Hakka rice wine (Yuan et al., 2023).
The Chinese rice wine fermentation process involves a gradual introduction of filamentous fungi, yeasts, and bacteria to the wine mash from the surrounding environment, leading to the formation of a multifunctional microbial community that gives the wine a distinct and unique flavor (Wang et al., 2014). During fermentation, Aspergillus, as a filamentous fungus, is able to generate amylase and protease enzymes, promoting the degradation of starch and protein, thereby improving wine flavor (Zhang et al., 2019). Saccharomyces is a facultative anaerobe commonly found in starter cultures. It proliferates along with Rhizopus and Aspergillus species throughout the wine-making process (Xiao et al., 2022). As fermentation progresses and oxygen content in the wine mash decreases, Saccharomyces metabolizes glucose and amino acids, producing large quantities of alcohol and various flavor compounds (Zhao et al., 2020). The bacteria in wine mash also plays vital roles in enzyme and aroma production. Among them, Lactobacillus promotes the Maillard reaction and maintains the brewing microbial community. Its primary metabolite, lactic acid, is an essential substance for forming of ethyl lactate and other aromatic components (Benito, 2019). Bacillus can hydrolyze proteins and starch, which contributes to the formation of Maillard reaction products and enhances aroma and flavor (Wu et al., 2021). During fermentation, coexisting microorganisms in wine mash interact to form a specific microbial community that contributes to the flavor characteristics of rice wine (Chen et al., 2013). During Hakka rice wine brewing, numerous microorganisms with complex metabolic vitality are produced due to the open form of fermentation. Under the combined action of various microorganisms, a unique flavors can be produced. Currently, research on the flavor of Hakka rice wine mainly focuses on detecting flavor compounds. However, research on the flavor formation mechanism of Hakka rice wine remains inadequate. For instance, the analysis of microbial community composition and masking, metabolite changes, and flavor quality during the fermentation process of Hakka rice wine has not been reported and requires further study. To understand the evolution of flavor compounds during Hakka rice wine fermentation, the current study used high-throughput sequencing with MiSeq and headspace solid phase microextraction-gas chromatography-mass spectrometry to analyze the dynamic changes in flavor compounds. The study aimed to determine the role of microorganisms in producing flavor compounds during fermentation and to explore the aroma generation mechanism in Hakka rice wine brewing. The findings are essential for enriching the science of Hakka rice wine brewing, and guiding its production and quality control.
Materials and methods
Materials and chemicals
Materials: Fermented wash was obtained from the Mei Ling Quan Winery (from China). Starters were obtained from Yulin City Magic Raw Ingredient Non-Steaming JiuQu Factory (from China).
Chemical standards for identification: Palmitic acid ethyl ester (98 %), ethyl myristate (98 %), isoamyl acetate (99.5 %), 2-ethylhexanol (99.5 %), 1-octanol (99 %), phenethyl alcohol (99 %), linalool (99 %), 2-methyl-1-propanol (99 %), cedarwood oil (99 %), 4-ethyl-2-methoxyphenol (98 %), 1-nonanal (96 %), octanal (99 %), undecanal (98 %), and 2-octanol (98 %) were purchased from Maclean Technology Ltd.
Fermentation procedure
In brief, 25 kg of brown glutinous rice was washed and soaked in water overnight in water at 25℃. After steaming, the rice was cooled to room temperature, it was mixed with the fermentation starters (250 g) and transferred to wine jars for fermentation. The fermentation period is 32 days. Fermented mash collected during the saccharifying time (1 and 2 d) and brewing time (1, 3, 5, 7, 14, 21, and 30 d) were put into a sterile tube, sealed, and stored at − 80℃ until further analysis.
DNA extraction, PCR amplification, and Illumina MiSeq sequencing
Extracted DNA was measured using agarose gel electrophoresis, and analyzing the taxonomic composition of the bacterial and fungal communities, primers 338F (5′-GTGCCAGCM GCCGCGGTAA-3′) and 806R (5′-CCGTCAATTCMTTTRAGTTT −3′) were used for PCR amplification of the bacterial 16S rRNA gene. The fungal 18S rRNA gene was amplified by a two-step amplification procedure using the primers ITS1F (5′-CTTGGTCATTTAGAG GAAGTAA-3′) and ITS2R (5′-GCTGCGTTCTTCATCGATGC-3′). The gene was amplified using a two-step amplification procedure. Specific DNA was extracted, PCR was performed, and Illumina MiSeq sequencing (2 × 150 bp) was performed using the Illumina MiSeq platform. Sequencing data were processed using the QIME2 amplicon analysis software. Amplified sequence variants were obtained using denoising, quality control, splicing and mosaic programs and were analyzed using denoising paired QIME DADA2 (Zhang, 2015).
Gas chromatography–mass spectrometry (GC–MS) analysis
Gas chromatography (GC) conditions: high purity helium as carrier gas, flow rate: 1 mL/min; DB-Wax column (30 m × 0.25 mm × 0.25 μm), no split injection, inlet temperature 250 °C; start temperature 40 °C, hold for 5 min, ramp up to 120 °C at 5 °C/min, then ramp up to 240 °C at 10 °C/min, hold for 5 min. Mass spectrometry (MS) conditions: interface temperature: 280 °C, connecting rod temperature: 150 °C; EI ionization source, electron energy of 70 eV, ion source temperature of 230 °C, mass scan range 35–450 (m/z); ACQ mode Scan (Jiang et al., 2020).
Qualitative and quantitative methods for aromatic compounds
Qualitative method: The various chemical components in the aroma of the detected Hakka rice wine were determined by comparing the detected substance spectra with the standard spectra in NIST11 and comparison with the retention indices reported in the relevant literature. (When using the headspace solid-phase microextraction method of extraction, the analysis of the samples required the deduction of siloxane-type impurity peaks generated by the extraction head in the profiles to identify the volatile components in Hakka rice wine accurately.) Quantification method, 2-octanol (0.1 mg/L) was used as an internal standard for the analysis and the concentration of the various volatile components in Hakka rice wine (Gao et al., 2020).
Aroma reconstruction experiments
Considering wine mash, which fermented for 30 days as an example, its artificial matrix was prepared as follows: first, 200 mL of Huangjiu was subjected to Solvent Assisted Flavor Evaporation (SAFE), and the non-volatile fraction was collected and dissolved in 200 mL of 14 % (v/v) ethanol/water solution. Then, the volatile fraction of the solution was then separated from the nonvolatile fraction by SAFE. This process was repeated thrice. Finally, the artificial matrix was obtained by dissolving the amber-colored residue in 200 mL of 14 % (v/v) ethanol/water solution. The alcohol contents of the artificial matrices were identical to those of the respective samples.
To validate the importance and contribution of the odorants to the Hakka rice wine, odorants with high odor activity values (ROAVs, ≥1) were dissolved in an artificial flavorless matrix based on their detected concentrations. The odor activity value (OAV) calculated based on the relative concentration of the internal standard is defined as the relative odor activity value (ROAV). The specific calculation formula is ROAVs = Ci/OTi, where Ci represents the relative content of the compound by internal standard and OTi represents its odor threshold in water (Table S1). When ROAVs ≥ 1, it indicates a significant contribution of the compound to the flavor of the sample (Zheng et al., 2023). The control samples were evaluated as described in Section “Aroma reconstruction and omission experiments to analyze the key flavor compounds in Hakka rice wine”.
Electronic nose analysis
Ten milliliters of the sample were absorbed in 20 mL septum-sealed screw cap bottles and equilibrated for 30 mins at 25 °C before testing. Afterward, the aroma headspace was introduced into the electronic nose at a speed of 300 mL/min, with a purge time of 60 s and detection time of 100 s, and sampling was performed every 1 s (Hong et al., 2015).
Sensory evaluation
The sensory panel consisted of 10 healthy, non-smoking panelists (4 men and 6 women) aged between 20 and 30 years, selected from the Guangdong Provincial Key Lab of Food Safety and Quality at South China Agricultural University. All panelists had at least 2 years of experience conducting sensory evaluations of Chinese rice wine. The study was reviewed and approved by the South China Agricultural University and informed consent was obtained from each subject prior to their participation in the study. After obtaining a preliminary understanding of the aromatic profiles of Hakka rice wine, the panelists selected eight aromas: sourness, soy sauce-like aroma, alcoholic, sweet, honey, herbal, cooked grain, Qu-aroma. The intensity of each aroma in Hakka rice wine infusions was scored on a scale from 0 (not perceptible) to 7 (strong intensity). The sensory aroma profile was measured using the average scores, and all sensory experiments were conducted more than three times (Wang et al., 2022).
Omission experiments
Omission tests were conducted to confirm the key odorants in Hakka rice wine. The omission models were prepared by omitting one or more odorants from the complete recombination models. The omission models and Hakka rice wine were evaluated using triangle tests. Two complete aroma model samples and one omission model sample were randomly numbered and out of order, and the sensory evaluator selected the sample with the greatest differences among three samples. Three replicate tests were performed for each group (Wang et al., 2023).
Statistical analysis
The relevant graphs were plotted using OriginPro 13.0. All analyses for significant differences (p ≤ 0.05) (Duncan’s tests) were performed using SPSS Partial least square regression (PLSR) analysis conducted using XLSTAT 2016 (Addinsoft, New York, NY). Similarities between the original samples and their corresponding recombination models were analyzed using Pearson’s two-tailed test. The heat map was plotted by first normalizing and then drawing by cluster analysis of the matrix data on the BMKCloud platform.
Results
Analysis of flavor components in Hakka rice wine
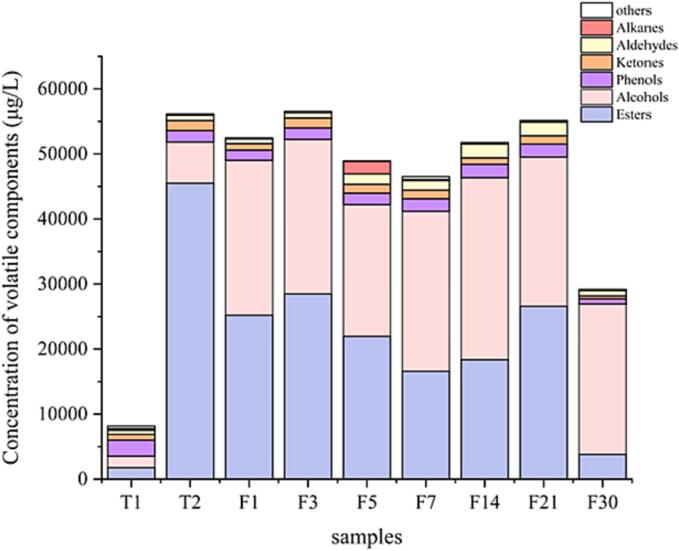
As shown in Fig. 1, the fermentation of Hakka rice wine produced dominant volatile compounds, such as alcohols, esters, aldehydes, and ketones. In total, 44, 56, 57, 58, 59, 63, 61, 55, and 42 compounds were identified during saccharification (days 1 and 2) and fermentation (days 1, 3, 5, 7, 14, 21, and 30), respectively. In addition, the concentration of volatile compounds increased significantly on the second day of saccharification. This phenomenon may be attributed to the commencement of yeast fermentation, leading to the generation of a wide range of esters and higher alcohols.
Fig. 1.
Volatile profiles in Hakka rice wine during fermentation.
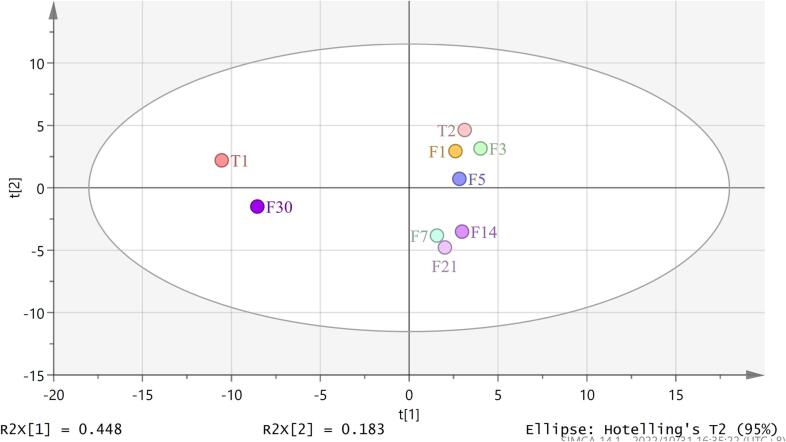
According to the PCA results (Fig. 2), the Hakka rice wine samples partially overlapped at different fermentation times but displayed a distinct separation trend. This suggests that large differences were present in the composition of the aroma compounds at different wine fermentation times.
Fig. 2.
PCA of samples during different fermentation time periods, T1/T2 for the first and second day of saccharification, F1/F3/F5/F7/F14/F21/F30 for fermentation days 1 ∼ 30.
A heat map was used to plot the data from samples at different fermentation times to analyze the changes in volatile compounds during fermentation. The results are shown in Fig. S1, which shows the presence of 104 volatile compounds classified into six categories based on their chemical structural characteristics: 40 esters, 25 alcohols, 4 phenols, 6 ketones, 11 aldehydes, 11 alkanes, and 7 others. Alcohols were the most abundant compounds in Hakka rice wine, with concentrations that gradually increased from 21.78 % to 45.30 % during fermentation. Esters were the second most abundant compound, increasing in the same pattern as alcohols and accounting for 43.96 % of the total aroma content at the end of fermentation. Aldehydes were present in lower amounts than the other types of compounds, comprising only 2.93 % of the total aroma compounds. The primary aldehydes identified in this study were 1-nonanal, decyl aldehyde, undecanal, and benzaldehyde, which increased in concentration during fermentation. Phenols, ketones, and alkanes accounted for 4.20 % of the total aromatic compound content. The phenol content gradually increased over time. Acetoin is the primary ketone in Hakka rice wine and is primarily produced during saccharification. The highest content of 3-methyl-1-butanol (19.1 ug/mL) was observed at the end of fermentation, followed by 2-methyl-1-propanol (2.4 ug/mL), palmitic acid ethyl ester (1.85 ug/mL), phenethyl alcohol (0.742 ug/mL), 2,4-di-tert-butylphenol (0.657 ug/mL), and 2-ethylhexanol (0.503 ug/mL).
Analysis of the essential flavor compounds in Hakka rice wine
Table 1 lists 22 aromatic compounds with ROAVs > 1 that were identified in Hakka rice wine during fermentation. These compounds include esters, alcohols, aldehydes, phenols, and ketones. The compounds detected from day 1 of saccharification to day 30 of fermentation are shown in the table, with 7, 15, 15, 16, 14, 19, 17, 16, and 13 aroma compounds with ROAVs > 1, respectively. Saccharification was characterized mainly by esters, accounting for 31.82 % of the compounds with ROAVs > 1, followed by alcohols accounting for 22.73 %. Prominent ROAVs were observed for compounds such as isoamyl acetate (ROAV = 192.6), ethyl myristate (ROAV = 16.9), palmitic acid ethyl ester (ROAV = 15.2), 2-ethylhexanol (ROAV = 52.8), and phenethyl alcohol (ROAV = 4620558.8). During the post-fermentation stage, the ROAVs of some alcohols, aldehydes, ketones, and phenols gradually increased, among which the ROAVs of 1-octanol, 4-ethyl-2-methoxyphenol, ionone, 1-nonanal, undecanal, and octanal exceeded those of the saccharification stage by more than 1.5 times.
Table 1.
Aroma compounds with ROAVs ≥ 1 during fermentation.
| No. | Compounds | ROAVs |
Odor description | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | F1 | F3 | F5 | F7 | F14 | F21 | F30 | |||
| Esters A1 |
Ethyl palmitate | 0.9 | 15.2 | 11.4 | 15.2 | 12.4 | 0.1 | 0.0 | 0.0 | 2.2 | Faint waxy, creamy aroma. |
| A2 | Ethyl hexanoate | 0.3 | 1.0 | 1.2 | 1.0 | 0.8 | 0.6 | 1.6 | 0.7 | 0.3 | Fruit aroma |
| A3 | Ethyl tetradecanoate | 0.6 | 16.9 | 24.6 | 16.9 | 11.0 | 7.5 | 6.5 | 12.0 | 1.2 | Coconut and iris like aroma |
| A4 | Isoamyl acetate | 0.0 | 192.6 | 8.9 | 7.3 | 6.7 | 4.4 | 11.3 | 3.7 | 1.1 | Banana aroma |
| A5 | Ethyl caprylate | 0.0 | 3.8 | 7.4 | 3.8 | 3.0 | 1.6 | 7.8 | 1.9 | 0.4 | Brandy aroma |
| A6 | Phenethyl acetate | 0.0 | 1.8 | 2.2 | 1.8 | 1.7 | 1.4 | 2.9 | 1.5 | 0.4 | Sweet aroma |
| A7 | Ethyl laurate | 0.0 | 1.25 | 4.33 | 1.25 | 0.49 | 0.36 | 0.62 | 0.0 | 0.0 | Faint fruity, oily aroma |
| Alcohols B1 |
Menthol | 0.98 | 0.0 | 0.0 | 0.0 | 0.0 | 2.47 | 0.0 | 1.98 | 0.75 | Peppermint Aroma |
| B2 | 2-ethyl-1-hexanol | 61.4 | 52.8 | 65.3 | 52.8 | 53.8 | 68.8 | 65.7 | 73.3 | 50.3 | Flower aroma |
| B3 | Octanol | 2.0 | 5.1 | 6.4 | 5.1 | 4.8 | 4.2 | 9.4 | 5.2 | 2.4 | Oily and citrusy aroma |
| B4 | Phenylethyl alcohol | 155148.3 | 4620558.8 | 4429262.8 | 4620558.8 | 4583116.1 | 4726012.6 | 5285961.8 | 4978599.4 | 1484192.3 | Rose aroma |
| B5 | Linalool | 3.4 | 6.1 | 8.5 | 6.1 | 7.0 | 5.9 | 12.7 | 6.2 | 3.2 | Lemon aroma |
| B6 | Isobutanol | 0.0 | 3.3 | 3.3 | 3.3 | 0.0 | 3.5 | 4.2 | 3.2 | 4.0 | Alcoholic scent |
| B7 | Cedarwood oil | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 52.8 | 62.6 | 25.0 | 25.0 | Characteristic odour |
| Phenols C1 |
2,4-Di-t-butylphenol | 1.5 | 0.9 | 0.8 | 0.9 | 0.9 | 1.0 | 1.1 | 0.9 | 0.4 | |
| C2 | 4-Ethyl-2-methoxyphenol | 0.0 | 7.4 | 5.8 | 7.4 | 7.2 | 9.2 | 7.7 | 11.1 | 1.7 | Spice and herbal aroma |
| Aldehydes and Ketones D1 |
Ionone | 0.0 | 0.0 | 48.5 | 105.9 | 96.3 | 86.7 | 74.2 | 79.7 | 0.0 | Sweet woody and fruity aroma |
| D2 | Nonanal | 3.0 | 4.1 | 4.1 | 4.1 | 5.1 | 5.2 | 5.4 | 5.0 | 2.6 | Beeswax flower Scent |
| D3 | Octanal | 33.6 | 0.0 | 0.0 | 0.0 | 52.5 | 63.1 | 72.6 | 70.5 | 34.2 | Fruity jasmine aroma |
| D4 | Lauryl aldehyde | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 98.8 | 0.0 | 0.0 | 0.0 | Pine leaf oil and orange oil aroma |
| D5 | Undecanal | 0.0 | 18.1 | 0.0 | 18.1 | 32.8 | 13.0 | 41.1 | 47.1 | 11.0 | Rose and woody orange peel aroma |
| D6 | Hexanal | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.20 | 0.0 | 0.0 | 0.0 | Oily, grassy and apple aroma |
Aroma reconstruction and omission experiments to analyze the key flavor compounds in Hakka rice wine
Table 2 shows the results of the analysis of aromatic compounds in 30-d fermented Hakka rice wine based on ROAVs. Isoamyl acetate (ROAV = 1.12, with fruity and banana aroma), ethyl myristate (ROAV = 1.15, with fruity and coconut aroma), 1-Octanol (ROAV = 2.36, with fruity and citrus aroma), 2-ethylhexanol (ROAV = 50.33, with sweet and floral aroma), and phenethyl alcohol (ROAV = 1484192.32, with floral and rose aroma) were identified as the primary sources of sweet and fruity aromas in Hakka rice wine. By contrast, 1-nonanal (ROAV = 2.62, with floral and beeswax aroma), octanal (ROAV = 5.16, with floral and waxy aroma), and undecanal (ROAV = 10.99, with rose and waxy aroma) were identified as the main sources of cereal aroma in Hakka rice wine. Finally, 4-ethyl-2-methoxyphenol (ROAV = 1.70, with a herbaceous and unfair aroma) was considered the main source of herbaceous aromas in Hakka rice wine.
Table 2.
Key aroma compounds with roavs > 1 in the 30d fermentation sample, “*” is a significant difference (P<0.05) in flavor when the compound is omitted; “**” is a highly significant difference (P<0.01) when the compound is omitted.
| No. | Compounds | CAS | Thresholds (ug/L) | Relative content of compounds (ug/mL) | ROAV | Odor description |
|---|---|---|---|---|---|---|
| Esters A1 |
Ethyl palmitate* | 628-97-7 | 850 | 1.85 | 2.17 | Faint waxy, creamy aroma. |
| A2 | Ethyl tetradecanoate* | 124-06-1 | 180 | 0.208 | 1.15 | Coconut and iris like aroma |
| A3 | Isoamyl acetate* | 123-92-2 | 93.93 | 0.105 | 1.12 | Banana aroma |
| Alcohols B1 |
2-Ethyl-1-hexanol* | 104-76-7 | 10 | 0.503 | 50.33 | Flower aroma |
| B2 | Octanol* | 111-87-5 | 40 | 0.0943 | 2.36 | Oily and citrusy aroma |
| B3 | Phenylethyl alcohol** | 60-12-8 | 0.0005 | 0.742 | 1484192.32 | Rose aroma |
| B4 | Linalool* | 78-70-6 | 6 | 0.0191 | 3.19 | Lemon aroma |
| B5 | Isobutanol* | 78-83-1 | 597 | 2.38 | 3.99 | Alcoholic scent |
| B6 | Cedarwood oil | 8000-27-9 | 0.50 | 0.0125 | 25.01 | Characteristic odour |
| Phenols C1 |
4-Ethyl-2-methoxyphenol* | 2785-89-9 | 33.00 | 0.0560 | 1.70 | Spice and herbal aroma |
| Aldehydes D1 |
Nonanal* | 124-19-6 | 122.45 | 0.321 | 2.62 | Beeswax flower Scent |
| D2 | Octanal | 124-13-0 | 22.5 | 0.116 | 5.16 | Fruity jasmine aroma |
| D3 | Undecanal* | 112-44-7 | 5 | 0.0549 | 10.99 | Rose and woody orange peel aroma |
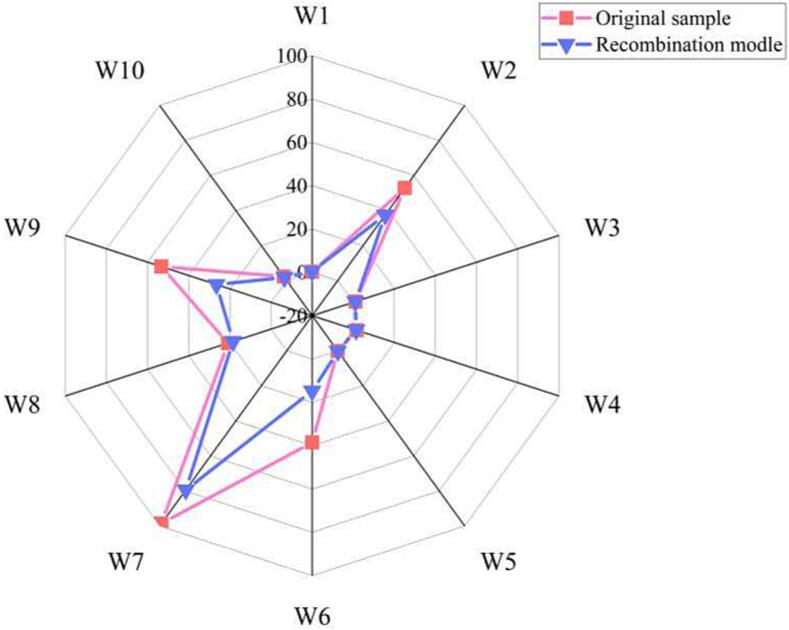
The contribution of the volatile aroma compounds with high ROAV to the overall aroma of Hakka rice wine was evaluated through aroma recombination. A radar plot of the electronic nose odor characteristics (Fig. 3) showed that the aroma characteristics of the original samples and their corresponding reconstituted compounds had an excellent similarity of 86 %. This result confirmed the successful identification and quantification of aromatic compounds in Hakka rice wine. Sensory evaluation of the original and recombinant samples revealed that the alcoholic, sweet, cooked grain and honey-like aromas in the recombinant sample were consistent with those in the original samples. However, sourness, soy sauce-like aromas, and herbal flavors were weaker in the reconstituted samples.
Fig. 3.
Flavor evaluation of 30-d fermentation sample with reconstruction sample by electronic nose, W1 ∼ W10 are electronic nose aromatic composition, benzene sensor; hydroxide sensor; aromatic composition, ammonia sensor; hydrogen compounds sensor; short-chain alkane aromatic composition sensor; methyl compounds sensor; sulfide sensor; alcohols, aldehydes and ketones compounds sensor; aromatic composition, organic sulfide sensor; long-chain alkane compounds sensor.
Thirteen omission models were created through omission experiments to investigate the contribution of aroma compounds to the overall odor of Hakka rice wine. As indicated in Table 2, one model had a highly significant difference (P < 0.01), ten omission models were significantly different (P < 0.05), and two omission models were not significantly different (P > 0.05). After omitting each ester, significant differences in the aroma of Hakka rice wine were observed (P < 0.05), confirming that sweet, fruity, and honey-like aromas were the primary components of the wine-fermented fragrance. However, no significant differences were noted upon omitting octanal. Significantly different grain aromas were observed between the reconstituted and omitted models, in which 1-nonanal, octanal, and undecanal were omitted, indicating that these compounds play a crucial role in the grain aroma of Hakka rice wine. Finally, the omission of 4-ethyl-2-methoxyphenol from the reconstituted model resulted in sensory panelists detecting differences in herbal odors between the reconstituted and omitted models. Thus, 4-ethyl-2-methoxyphenol was identified as a key odorant that contributed significantly (p < 0.05) to the herbal aroma of Hakka rice wine.
Microbial composition of starter and wine mash during fermentation
Fig. S2 and Fig. S3 shows the structure of fungal and bacterial communities in the starter culture of Hakka rice wine. At the phylum level, Ascomycota and Basidiomycota were detected as fungal phyla, whereas six bacterial phyla were identified: Proteobacteria, Actinobacteria, Firmicutes, Cyanobacteria, Bacteroidetes, and Deinococcus. At the genus level, fungi were represented by seven genera: Aspergillus, Didymellaceae, Xeromyces, Nectriaceae, Xerochrysium, Pleosporales, and Wallemia, whereas there were ten bacterial genera: Methylobacterium, Pantoea, Microbacterium, Lactobacillus, Curtobacterium, Saccharopolyspora, Microbacteriaceae, Rhizobium, Pseudomonas, and Aureimonas. Aspergillus was the predominant fungal genus detected in the starter, making up 98.65 % of relative abundance. Among the bacterial microorganisms, Methylobacterium, Pantoea, Microbacterium, Lactobacillus, and Curtobacterium were the most dominant genera.
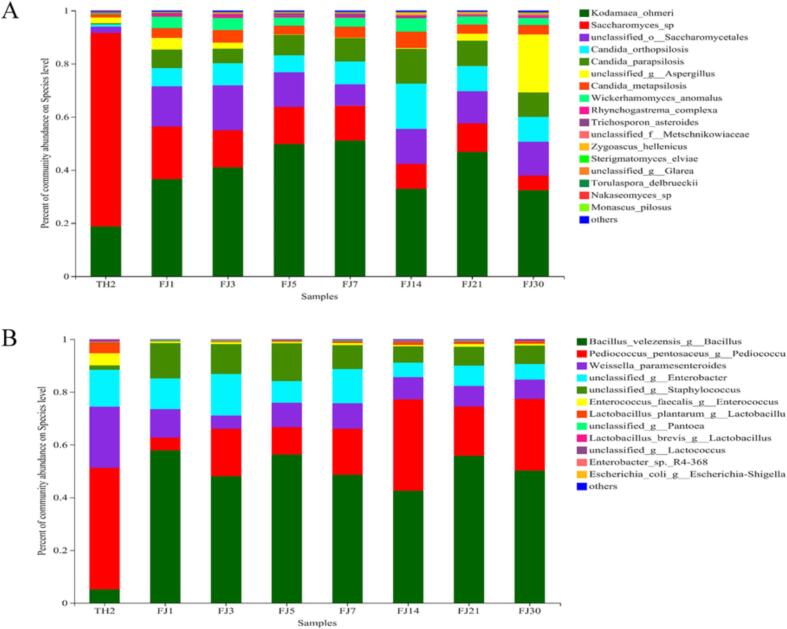
The microbial community structure in the wine mash was observed during fermentation, as shown in Fig. S4 and Fig. 4. Two fungal (Ascomycota and Basidiomycota) and two bacterial phyla (Firmicutes and Proteobacteria) were identified. A total of 18 fungal and 12 bacterial species were identified. The dominant fungal species in the pre-saccharification stage was Saccharomyces cerevisiae, with a relative abundance of 72.83 %.
Fig. 4.
The composition of fungal communities (A) and bacterial communities (B) in wine mash at the genus level.
During the post-fermentation phase, the relative abundance of non-Saccharomyces yeast and Aspergillus gradually increased and became dominant in wine mash. The dominant fungal genus was Kodamaea ohmeri, accounting for 41.5 % of the total fungal population, followed by Saccharomycetales, Candida orthopsilosis, Wickerhamomyces anomalus, and Aspergillus, which accounted for 43.5 % of the total fungal population. In addition, other non-Saccharomyces yeasts were identified, comprising only 1.1 % of all fungal species, such as Zygoascus hellenicus, Metschnikowiaceae, Trichosporon asteroides, and Issatchenkia orientalis.
The species and relative abundances of bacteria changed over time during fermentation. According to the sequencing results, the most significant bacterial species present during fermentation were Bacillus velezensis, Pediococcus, Weissella paramesenteroides, Enterobacter, Lactobacillus plantarum, Enterococcus faecalis, and Staphylococcus. Among them, the significant bacteria during saccharification and fermentation were Pediococcus, Weissella paramesenteroides, and Enterobacter, accounting for 46.7 %, 23.2 %, and 14.02 %, respectively. During post-fermentation, Bacillus and Staphylococcus gradually became more abundant, accounting for 42 %–58 % and 6 %–15 % of the samples, respectively. In addition, the bacterial flora underwent considerable post fermentation changes.
Correlation between microflora and aroma compounds during the fermentation of Hakka rice wine
This study explored the influence of microbial communities on the production of volatile compounds during brewing. Pearson’s correlation coefficients were calculated to assess the correlation between the microbial flora and volatile compounds in maiden wine. Network diagrams were constructed to determine the correlation between the microbial species and major flavor compounds (Fig. S5). Pearson’s correlation analysis revealed correlations among 19 fungal genera, 13 bacterial genera, and 80 volatile compounds. The major fungal genera included Candida, Torulaspora delbrueckii, Kodamaea ohmeri, Ascomycota, Metschnikowiaceae, Zygoascus hellenicus, and Aspergillus, and the major bacterial genera were Pediococcus, Weissella paramesenteroides, Enterobacter, Lactobacillus plantarum, Pantoea, and Escherichia-Shigella. Escherichia-Shigella is positively correlated with aroma development in Hakka rice wine. The yeasts promoted the production of 35 volatile organic compounds, including seven alcohols, 14 esters, four aldehydes, five ketones, and four alkane analogs.
During fermentation, the presence of specific microorganisms positively correlated with the production of various volatile aromatic compounds. Candida yielded 13 volatile aromatic compounds, including citronellol for the rose aroma, benzaldehyde for the bitter almond aroma, and octanal and 3-octanol for the fruity jasmine aroma. Saccharomyces cerevisiae was responsible for the production of isoamyl acetate for banana aroma, 6-methyl-5-hepten-2-one for fruit aroma, propanoic acid, 2-methyl propanoic acid 3-hydroxy-2,4,4-trimethylpentyl ester and 6,10-dimethyl-5,9-undecadien-2-one. Moreover, Kodamaea ohmeri produced 3-methyl-1-butanol with an apple brandy aroma and ionone with a rose aroma. Finally, Aspergillus was positively correlated with higher relative concentrations of certain aroma compounds, such as hexyl trifluoroacetate, 1-decanol, carbonic acid, and butyl 2-pentyl ester.
During fermentation, bacterial communities were dominated by Bacillus velezensis, Pediococcus pentosaceus, Lactococcus, and Lactobacillus. Bacillus velezensis yielded 3-methyl-1-butanol with an apple brandy aroma, whereas Pediococcus pentosaceus and Lactobacillus plantarum were responsible for the production of isoamyl acetate with a banana aroma, 6-methyl-5-hepten-2-one with a fruit aroma, 2-methyl propanoic acid 3-hydroxy-2,4,4-trimethylpentyl ester, and 6,10-Dimethyl-5,9-undecadien-2-one.
Discussion
Traditional Hakka rice wine is brewed from brown sweet rice as the primary ingredient using an open fermentation process with sweet distiller yeast. During fermentation, volatile flavor compounds produced by the metabolic activities of microorganisms within the starter and brewing environments constitute crucial aromatic constituents of wine (Liu et al., 2015). This study employed multiomics technology to study the effects of bacteria and fungi present in wine mash at various fermentation stages on the flavor characteristics of traditional Hakka rice wine.
During fermentation, 104 volatile organic compounds (VOCs) were detected in the samples at nine different time points. Among them, 11 VOCs contributed significantly to developing the Hakka rice wine flavor, imparting fruity, cereal, and herbal aromas. The dominant microorganism during fermentation was Kodamaea ohmeri, which combined with key yeasts, such as Candida orthopsilosis, Metschnikowiaceae, Trichosporon asteroides, and Torulaspora delbrueckii. The spontaneous formation of a non-Saccharomyces community significantly influences the production of alcohols, esters, and aldehydes during fermentation, resulting in a unique aroma profile for Hakka rice wine. The aroma of Hakka rice wine is determined by the content of volatile compounds and their odor activity values (ROAVs). Compounds with a ROAV > 1 significantly contributed to wine aroma. During fermentation, 22 aromatic compounds with ROAVs > 1 were detected, including alcohols, esters, aldehydes, and phenolics, which are the major volatile compounds significantly contribute to the overall aroma in fermented products (Zhang et al., 2022). Alcohols are mainly formed by glucose metabolism and decarboxylation of amino acids, and are produced in large quantities during alcoholic fermentation. Furthermore, higher alcohols have a high proportion and impart floral and fruity aroma characteristics to wines (Yun et al., 2021). Esters, accounting for 43.96 % of the total aroma compound content, are the second most abundant aroma compounds and primarily originate from the esterification of alcohols with acids produced by microorganisms during sugar fermentation or lipolysis (Mo et al., 2009). Ethyl esters gradually increase during fermentation and contribute to the unique flavors of fermented products via fruity and floral smells (Fan & Qian, 2006). Aldehydes constitute 2.93 % of the total aroma compound content, and microbial metabolism and auto-oxidation of unsaturated fatty acids are crucial for their formation. The key odorant in Hakka rice wine is undecanal, which imparts a grainy aroma. Ketones, phenols, and alkanes, accounting for 4.2 % of the total aromatic compound content, play a minor role in the overall aroma of the wine. Phenols were produced by the decarboxylation of hydroxycinnamic acid and p-coumaric acid (Shen et al., 2017) and showed a gradual increase in fermentation time. The dominant phenol compound in the Hakka rice wine was 4-ethyl-2-methoxyphenol, exhibiting a herbal smell.
Flavor development in Hakka rice wine is intricately linked to complex processes of microbial metabolism. The initial group of microorganisms involved in the fermentation was primarily sourced from the starter culture. Due to the open brewing process, the microbial community and volatile compounds in the liquor undergo dynamic changes. During saccharification (T1/T2), the dominant microorganisms in the liquor were Saccharomyces cerevisiae (72.83 %) and Pediococcus pentosaceus (46.7 %), which significantly contributed to the formation of key flavor compounds in the liquor, including undecanal, phenylethyl alcohol, ethyl palmitate, and isoamyl acetate. Phenylethyl alcohol, a prominent higher alcohol, is one of the most abundant organoleptic compounds in Hakka rice wine (Lin et al., 2023). The rich nutrient environment encourages microorganisms' rapid growth and metabolic activities during saccharification. Saccharomyces cerevisiae absorbs amino acids from wine mash and then incorporates amino groups into their structures. The remaining-keto acids undergo an irreversible chain reaction, which eventually leads to the formation of higher alcohols. In addition, carboxylic acid hydrolase family enzymes, lipases, esterases, and cutinases were frequently reported in wine mash to catalyze ester formation through an enzyme-catalyzed esterification reaction during fermentation (Levisson et al., 2009). In the alcoholic fermentation stage (F1-F30), adding white wine significantly altered the microbial community structure in the liquor compared to that in the saccharification stage. This stage is characterized by the production of large quantities of alcohol, and microorganisms associated with alcohol production, such as Candida orthopsilosis, Wickerhamomyces anomalous, and Bacillus velezensiss, have increased. As fermentation proceeds, higher alcohol concentrations and acidity reduce microbial activity in the mash. However, owing to the accumulation and interaction of preflavor compounds, the content of flavor substances in the liquor increases. During the brewing of Hakka rice wine, multiple microorganisms can be associated with the same aroma compounds. Moreover, a particular microorganism may highly correlate with multiple volatile compounds. The microorganisms Kodamaea ohmeri, Candida orthopsilosis, Trichosporon asteroides, Wickerhamomyces anomalus, Metschnikowiaceae, and non-Saccharomyces were screened as functionally significant microbiota with an essential effect on the formation of key aromatic compounds, such as isoamyl acetate (banana aroma), ethyl caprylate (brandy aroma), cedarwood oil (cypress aroma), ionone (sweet wood aroma), and octanal (jasmine and fruity aroma). These compounds contribute significantly to the flavor of the wine. Kodamaea ohmeri (41.5 %) was identified as the dominant fungus during the fermentation process. It is a non-saccharomyces yeast that exhibits excellent saccharification ability (Bi et al., 2016). The proteins, lipases, and β-galactosidases secreted by this microorganism make significant contributions to the flavor (Cardoso et al., 2015). During fermentation, Kodamaea ohmeri was significantly correlated with ionone and 3-methyl-1-butanol, contributing to the brandy fruit aroma of the wine. Metschnikowiaceae synergistically produce more fatty acids, esters, and alcohols when cocultivated with Saccharomyces cerevisiae (Oro et al., 2018). In addition, it produces a variety of extracellular enzymes during fermentation that releases aroma compounds (Gobbi et al., 2013). In this study, although their relative abundance only accounted for 1.1 % of the total, certain non-Saccharomyces were positively correlated with aroma production, such as Zygoascus hellenicus, Metschnikowiaceae, Trichosporon asteroides, Issatchenkia orientalis, and Torulaspora delbrueckii. These microorganisms, including ethyl caprylate, isoamyl acetate, ethyl laurate, and ethyl hexanoate, were positively correlated with ester production during fermentation. These compounds impart characteristic flavors to fruits and bananas and are beneficial for forming a pleasant flavor in the final product. Yeasts play a key role in aroma development during fermentation, and compared to fermentation using S. cerevisiae exclusively, co-fermentation of non-Saccharomyces yeasts with S. cerevisiae results in enhanced flavor profiles (Hu et al., 2020). Each yeast species encodes different concentrations of extracellular enzymes that exert distinct effects on the aromatic profile of wine (Borren & Tian, 2021).
During fermentation, the bacteria in wine mash play an essential role in producing flavor compounds. Bacillus velezensis was identified as a functional microorganism positively correlated with the formation of 3-methyl-1-butanol and imparts the rose aroma to Hakka rice wine. It was suggested that Bacillus velezensis has a strong enzyme production capacity and secretes numerous hydrolytic enzymes, including amylase, acidic protease, and fibrinolytic enzymes, to promote the formation of flavor compounds. Lactobacillus is the key bacterium in the flavor formation of several fermented foods and transforms fermentable sugars in dairy products into lactic acid (Jung et al., 2012). After fermentation with Lactobacillus, the enzymes, and metabolites produced, including lactic acid, aromatic compounds, acetoin, free amino acids, organic acids, and extracellular polysaccharides, play essential roles in the acidity, taste, aroma, and texture of the final products (Sakandar et al., 2020). The findings of the current study reveal that the abundance of Lactobacillus gradually increased post-fermentation (F14-F30) and was positively correlated with the formation of isoamyl acetate and ionone with rose and fruit aromas. Pediococcus pentosaceus improves the flavor and nutrition of products and facilitate storage (Jiang et al., 2021). It is also an important functional flora with various probiotic effects and is usually found in large numbers in different starter types. In addition, Pediococcus pentosaceus generates anti-Listeria monocytogenes, and lactococci produce lactic acid, which may help maintain a beneficial microbial community composition in the sap (Porto et al., 2017). In this study, Pediococcus pentosaceus was significantly produced during saccharification (T2) and was positively associated with the production of 3-methyl-1-butanol and Ionone. The primary source of Pediococcus pentosaceu was the starter. Lactococcus is a homotypic bacterium capable of fermenting sugars and releasing lactic acid during fermentation (Yang et al., 2023), which improves the overall flavor of the wine. Non-Saccharomyces communities present in wine mash significantly contribute to forming esters, alcohols, and aldehydes. In addition, non-Saccharomyces strains are viable alternatives for commercial wine fermentation because their fermentative characteristics are similar to Saccharomyces. For instance, Schizosaccharomyces pombe is suitable for cofermentation with Lanchancea thermotolerans to produce wine with lower levels of acetic acid, no malic acid, and higher levels of total esters than commercial S. cerevisiae strains (Del Fresno et al., 2017). The results of this study showed that the non-Saccharomyces community was significantly more abundant in wine mash during the post-fermentation stage and made a notable contribution to the production of aroma compounds compared with S. cerevisiae.
This study investigated the composition of essential aroma compounds in Hakka rice wine and analyzed the dynamic processes and mechanisms of aroma formation during fermentation by microorganisms. These results unequivocally demonstrate that the non-Saccharomyces yeast community, spontaneously formed during fermentation, is positively correlated with the formation of 35 VOCs that contribute to the aroma profile of Hakka rice wine with grain, fruity, and herbal aromas. This study provides new insights into the role of microbiota in developing Hakka rice wine. The research findings can offer a theoretical basis for the development and optimization of fermentation processes in Hakka rice wine production. Future studies should focus on investigating the assembled yeast community to improve flavor quality and increase the international status of Hakka rice wine.
CRediT authorship contribution statement
Junyi Wang: Investigation, Resources, Writing – review & editing. Ziyi Wang: Methodology, Resources, Writing – original draft. Fangqing He: Investigation, Software, Validation. Zhuangguang Pan: Project administration, Resources. Yixuan Du: Data curation, Investigation. Zhiying Chen: Funding acquisition, Supervision. Yuxin He: Data curation, Formal analysis. Yuanming Sun: Conceptualization, Supervision. Meiying Li: Funding acquisition, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by the Guangdong Key Area R&D Program (2022B0202020004) and the Heyuan Science and Technology Program (230510171473339). We express our gratitude to Meilingquan Company for providing the samples and assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101121.
Contributor Information
Junyi Wang, Email: wangjunyi_0913@stu.scau.edu.cn.
Ziyi Wang, Email: wangziyi@stu.scau.edu.cn.
Fangqing He, Email: hfq1999@stu.scau.edu.cn.
Zhuangguang Pan, Email: hn20213141055@stu.scau.edu.cn.
Yixuan Du, Email: duyixuan@stu.scau.edu.cn.
Zhiying Chen, Email: chenzhiying@stu.scau.edu.cn.
Yuxin He, Email: 1415639689@stu.scau.edu.cn.
Yuanming Sun, Email: ymsun@scau.edu.cn.
Meiying Li, Email: lmy1982@scau.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Benito S. The impacts of Schizosaccharomyces on winemaking. Applied Microbiology and Biotechnology. 2019;103(11):4291–4312. doi: 10.1007/s00253-019-09827-7. [DOI] [PubMed] [Google Scholar]
- Bi C.Y.T., N'Guessan F.K., Kouakou C.A., Jacques N., Casaregola S., Djè M.K. Identification of yeasts isolated from raffia wine (Raphia hookeri) produced in Cote d'Ivoire and genotyping of Saccharomyces cerevisiae strains by PCR inter-delta. World Journal of Microbiology & Biotechnology. 2016;32(8):125-+. doi: 10.1007/s11274-016-2095-3. [DOI] [PubMed] [Google Scholar]
- Borren E., Tian B. The Important Contribution of Non-Saccharomyces Yeasts to the Aroma Complexity of Wine: A Review. Foods. 2021;10(1):13. doi: 10.3390/foods10010013. Article 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso V.M., Borelli B.M., Lara C.A., Soares M.A., Pataro C., Bodevan E.C., Rosa C.A. The influence of seasons and ripening time on yeast communities of a traditional Brazilian cheese. Food Research International. 2015;69:331–340. doi: 10.1016/j.foodres.2014.12.040. [DOI] [Google Scholar]
- Chen G.M., Huang Z.R., Wu L., Wu Q., Guo W.L., Zhao W.H.…Sun B.G. Microbial diversity and flavor of Chinese rice wine (Huangjiu): An overview of current research and future prospects. Current Opinion in Food Science. 2021;42:37–50. doi: 10.1016/j.cofs.2021.02.017. [DOI] [Google Scholar]
- Chen S., Xu Y., Qian M.C. Aroma Characterization of Chinese Rice Wine by Gas Chromatography-Olfactometry, Chemical Quantitative Analysis, and Aroma Reconstitution. Journal of Agricultural and Food Chemistry. 2013;61(47):11295–11302. doi: 10.1021/jf4030536. [DOI] [PubMed] [Google Scholar]
- Chen S.A., Xu Y. The Influence of Yeast Strains on the Volatile Flavour Compounds of Chinese Rice Wine. Journal of the Institute of Brewing. 2010;116(2):190–196. doi: 10.1002/j.2050-0416.2010.tb00417.x. [DOI] [Google Scholar]
- Del Fresno J.M., Morata A., Loira I., Bañuelos M.A., Escott C., Benito S.…Suárez-Lepe J.A. Use of non-Saccharomyces in single-culture, mixed and sequential fermentation to improve red wine quality. European Food Research and Technology. 2017;243(12):2175–2185. doi: 10.1007/s00217-017-2920-4. [DOI] [Google Scholar]
- Fan W.L., Qian M.C. Characterization of aroma compounds of Chinese “Wuliangye” and “Jiannanchun” liquors by aroma extract dilution analysis. Journal of Agricultural and Food Chemistry. 2006;54(7):2695–2704. doi: 10.1021/jf052635t. [DOI] [PubMed] [Google Scholar]
- Gao P., Jiang Q.X., Xu Y.S., Yang F., Yu P.P., Xia W.S. Aroma profiles of commercial Chinese traditional fermented fish (Suan yu) in Western Hunan: GC-MS, odor activity value and sensory evaluation by partial least squares regression. International Journal of Food Properties. 2020;23(1):213–226. doi: 10.1080/10942912.2020.1716790. [DOI] [Google Scholar]
- Gobbi M., Comitini F., Domizio P., Romani C., Lencioni L., Mannazzu I., Ciani M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiology. 2013;33(2):271–281. doi: 10.1016/j.fm.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Hong X.Z., Wang J., Qi G.D. E-nose combined with chemometrics to trace tomato-juice quality. Journal of Food Engineering. 2015;149:38–43. doi: 10.1016/j.jfoodeng.2014.10.003. [DOI] [Google Scholar]
- Hu L.L., Liu R., Wang X.H., Zhang X.Y. The Sensory Quality Improvement of Citrus Wine through Co-Fermentations with Selected Non-Saccharomyces Yeast Strains and Saccharomyces cerevisiae. Microorganisms. 2020;8(3):16. doi: 10.3390/microorganisms8030323. Article 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Mu Y.C., Wei S., Mu Y., Zhao C. Study on the dynamic changes and formation pathways of metabolites during the fermentation of black waxy rice wine. Food Science & Nutrition. 2020;8(5):2288–2298. doi: 10.1002/fsn3.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S.M., Cai L.Z., Lv L.X., Li L.J. Pediococcus pentosaceus, a future additive or probiotic candidate. Microbial Cell Factories. 2021;20(1):14. doi: 10.1186/s12934-021-01537-y. Article 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M.J., Nam Y.D., Roh S.W., Bae J.W. Unexpected convergence of fungal and bacterial communities during fermentation of traditional Korean alcoholic beverages inoculated with various natural starters. Food Microbiology. 2012;30(1):112–123. doi: 10.1016/j.fm.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Levisson M., van der Oost J., Kengen S.W.M. Carboxylic ester hydrolases from hyperthermophiles. Extremophiles. 2009;13(4):567–581. doi: 10.1007/s00792-009-0260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.D., Yang H.X., Jiang L., Zhang Y.J., Li J., Zhu X.X. Analysis of the flavour components, total phenolic content and antioxidant capacity between rice wine from two raw rice and starters [Article; Early Access] International Journal of Food Science and Technology. 2023;13 doi: 10.1111/ijfs.16783. [DOI] [Google Scholar]
- Liu S.P., Mao J., Liu Y.Y., Meng X.Y., Ji Z.W., Zhou Z.L., Ai-lati A. Bacterial succession and the dynamics of volatile compounds during the fermentation of Chinese rice wine from Shaoxing region. World Journal of Microbiology & Biotechnology. 2015;31(12):1907–1921. doi: 10.1007/s11274-015-1931-1. [DOI] [PubMed] [Google Scholar]
- Mo X.L., Fan W.L., Xu Y. Changes in Volatile Compounds of Chinese Rice Wine Wheat Qu During Fermentation and Storage. Journal of the Institute of Brewing. 2009;115(4):300–307. doi: 10.1002/j.2050-0416.2009.tb00385.x. [DOI] [Google Scholar]
- Oro L., Feliziani E., Ciani M., Romanazzi G., Comitini F. Volatile organic compounds from Wickerhamomyces anomalus, Metschnikowia pulcherrima and Saccharomyces cerevisiae inhibit growth of decay causing fungi and control postharvest diseases of strawberries. International Journal of Food Microbiology. 2018;265:18–22. doi: 10.1016/j.ijfoodmicro.2017.10.027. [DOI] [PubMed] [Google Scholar]
- Porto M.C.W., Kuniyoshi T.M., Azevedo P.O.S., Vitolo M., Oliveira R.P.S. Pediococcus spp.: An important genus of lactic acid bacteria and pediocin producers. Biotechnology Advances. 2017;35(3):361–374. doi: 10.1016/j.biotechadv.2017.03.004. [DOI] [PubMed] [Google Scholar]
- Sakandar H.A., Hussain R., Khan Q.F., Zhang H.P. Functional microbiota in Chinese traditional Baijiu and Mijiu Qu (starters): A review. Food Research International. 2020;138(10) doi: 10.1016/j.foodres.2020.109830. [DOI] [PubMed] [Google Scholar]
- Shen J.X., Rana M.M., Liu G.F., Ling T.J., Gruber M.Y., Wei S. Differential Contribution of Jasmine Floral Volatiles to the Aroma of Scented Green Tea. Journal of Food Quality. 2017;10 doi: 10.1155/2017/5849501. [DOI] [Google Scholar]
- Wang J., Zhang B., Wu Q., Jiang X.Y., Liu H.J., Wang C.Z.…Yu Y.G. Sensomics-assisted flavor decoding of coarse cereal Huangjiu. Food Chemistry. 2022;381(15) doi: 10.1016/j.foodchem.2022.132296. [DOI] [PubMed] [Google Scholar]
- Wang P.P., Kan Q.X., Yang L.X., Huang W.T., Wen L.F., Fu J.Y.…Cao Y. Characterization of the key aroma compounds in soy sauce by gas chromatography-mass spectrometry-olfactometry, headspace-gas chromatography-ion mobility spectrometry, odor activity value, and aroma recombination and omission analysis. Food Chemistry. 2023;419(10) doi: 10.1016/j.foodchem.2023.135995. [DOI] [PubMed] [Google Scholar]
- Wang P.X., Mao J., Meng X.Y., Li X.Z., Liu Y.Y., Feng H. Changes in flavour characteristics and bacterial diversity during traditional fermentation of Chinese rice wines from Shaoxing region. Food Control. 2014;44:58–63. doi: 10.1016/j.foodcont.2014.03.018. [DOI] [Google Scholar]
- Wu X.Y., Zhu P.C., Li D.L., Zheng T.F., Cai W., Li J.H.…Du G.C. Bioaugmentation of Bacillus amyloliquefaciens-Bacillus kochii co-cultivation to improve sensory quality of flue-cured tobacco. Archives of Microbiology. 2021;203(9):5723–5733. doi: 10.1007/s00203-021-02556-4. [DOI] [PubMed] [Google Scholar]
- Xiao R., Chen S.Q., Wang X.Q., Chen K.Q., Hu J., Wei K.…Lu F.G. Microbial community starters affect the profiles of volatile compounds in traditional Chinese Xiaoqu rice wine: Assement via high-throughput sequencing and gas chromatography-ion mobility spectrometry. Lwt-Food Science and Technology. 2022;170(12) doi: 10.1016/j.lwt.2022.114000. [DOI] [Google Scholar]
- Yang S.J., Bai M., Kwok L.Y., Zhong Z., Sun Z.H. The intricate symbiotic relationship between lactic acid bacterial starters in the milk fermentation ecosystem [Review; Early Access] Critical Reviews in Food Science and Nutrition. 2023;18 doi: 10.1080/10408398.2023.2280706. [DOI] [PubMed] [Google Scholar]
- Yang Y.J., Hu W.Y., Xia Y.J., Mu Z.Y., Tao L.R., Song X.…Ai L.Z. Flavor Formation in Chinese Rice Wine (Huangjiu): Impacts of the Flavor-Active Microorganisms, Raw Materials, and Fermentation Technology. Frontiers in Microbiology. 2020;11(14) doi: 10.3389/fmicb.2020.580247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H.W., Zhang C., Chen S.Y., Zhao Y., Tie Y., Yin L.G.…Zuo Y. Effect of different moulds on oenological properties and flavor characteristics in rice wine. Lwt-Food Science and Technology. 2023;173(8) doi: 10.1016/j.lwt.2022.114201. [DOI] [Google Scholar]
- Yun J., Cui C.J., Zhang S.H., Zhu J.J., Peng C.Y., Cai H.M.…Hou R.Y. Use of headspace GC/MS combined with chemometric analysis to identify the geographic origins of black tea. Food Chemistry. 2021;360(9) doi: 10.1016/j.foodchem.2021.130033. [DOI] [PubMed] [Google Scholar]
- Zhang K.Z., Li Q., Wu W.C., Yang J.G., Zou W. Wheat Qu and Its Production Technology, Microbiota, Flavor, and Metabolites. Journal of Food Science. 2019;84(9):2373–2386. doi: 10.1111/1750-3841.14768. [DOI] [PubMed] [Google Scholar]
- Zhang X. Biodiversity of the symbiotic bacteria associated with toxic marine dinoflagellate Alexandrium tamarense. Journal of Biosciences and Medicines. 2015;3(06):23. [Google Scholar]
- Zhang X.J., Gao P., Xia W.S., Jiang Q.X., Liu S.Q., Xu Y.S. Characterization of key aroma compounds in low-salt fermented sour fish by gas chromatography-mass spectrometry, odor activity values, aroma recombination and omission experiments. Food Chemistry. 2022;397(8) doi: 10.1016/j.foodchem.2022.133773. [DOI] [PubMed] [Google Scholar]
- Zhao W.H., Ruan F.X., Qian M., Huang X.Y., Li X.L., Li Y.X.…Dong H. Comparing the differences of physicochemical properties and volatiles in semi-dry Hakka rice wine and traditional sweet rice wine via HPLC, GC-MS and E-tongue analysis. Food Chemistry-X. 2023;20(6) doi: 10.1016/j.fochx.2023.100898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X.X., Wang Y.R., Cai W.C., Yang M.J., Zhong X.D., Guo Z., Shan C.H. High-throughput Sequencing-based Analysis of Microbial Diversity in Rice Wine Koji from Different Areas. Current Microbiology. 2020;77(5):882–889. doi: 10.1007/s00284-020-01877-9. [DOI] [PubMed] [Google Scholar]
- Zheng Y.R., Zhang C.H., Ren D.B., Bai R.X., Li W.T., Wang J.T.…Yi L.Z. Headspace solid-phase microextraction coupled with gas chromatography-mass spectrometry (HS-SPME-GC-MS) and odor activity value (OAV) to reveal the flavor characteristics of ripened Pu-erh tea by co-fermentation. Frontiers. Nutrition. 2023;10:11. doi: 10.3389/fnut.2023.1138783. Article 1138783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.