Abstract
Mice immunized with two different cryptococcal antigen preparations, one a soluble culture filtrate antigen (CneF) in complete Freund’s adjuvant (CFA) and the other heat-killed Cryptococcus neoformans cells (HKC), develop two different profiles of activated T cells. CneF-CFA induces CD4+ T cells responsible for delayed-type hypersensitivity (DTH) reactivity and for amplification of the anticryptococcal DTH response, whereas HKC induce CD4+ and CD8+ T cells involved in anticryptococcal DTH reactivity and activated T cells which directly kill C. neoformans cells. The main purpose of this study was to assess the level of protection afforded by each of the two different T-cell profiles against challenge with viable C. neoformans cells, thereby identifying which activated T-cell profile provides better protection. CBA/J mice immunized with CneF-CFA had significantly better protective responses, based on better clearance of C. neoformans from tissues, on longer survival times, and on fewer and smaller lesions in the brain, than HKC-immunized mice or control mice similarly infected with C. neoformans. Both immunization protocols induced an anticryptococcal DTH response, but neither induced serum antibodies to glucuronoxylmannan, so the protection observed in the CneF-CFA immunized mice was due to the activated T-cell profile induced by that protocol. HKC-immunized mice, which displayed no greater protection than controls, did not have the amplifier cells. Based on our findings, we propose that the protective anticryptococcal T cells are the CD4+ T cells which have been shown to be responsible for DTH reactivity and/or the CD4+ T cells which amplify the DTH response and which have been previously shown to produce high levels of gamma interferon and interleukin 2. Our results imply that there are protective and nonprotective cell-mediated immune responses and highlight the complexity of the immune response to C. neoformans antigens.
Cryptococcosis is a disease in which immunomodulatory or immunoreplacement therapy could be of great value. Cryptococcosis occurs more frequently in individuals with reduced T-lymphocyte function such as patients with AIDS or malignancies or those on immunosuppressive drugs (26). Furthermore, in immunodeficient individuals, cryptococcosis is often life threatening even when antifungal drugs are administered (41, 43). These findings stress the importance of T-cell functions in protection against Cryptococcus neoformans. At the present time, the component or combination of components of the T-cell-dependent host immune responses necessary for protection against C. neoformans are not sufficiently understood to develop immunotherapies.
Cell-mediated immune (CMI) responses against C. neoformans have been induced in mice by infecting the animals with the organism or by subcutaneous (s.c.) immunization with nonreplicating immunogens such as a soluble cryptococcal culture filtrate antigen (CneF) in complete Freund’s adjuvant (CFA) and heat-killed C. neoformans cells (HKC) (3, 22, 27, 32–34, 36, 38, 40). The T-cell responses to the two protocols using the nonreplicating antigens have been the focus of several investigations, and it is clear that different T-cell populations are induced by the two different immunization protocols (3, 22, 27, 32–34, 36, 38, 40). Immunization with CneF in CFA induces two functionally different CD4+ T-cell populations (3, 13, 14, 22, 27, 32–34, 36, 38, 40). One CD4+ population will transfer anticryptococcal delayed-type hypersensitivity (DTH) reactivity to naive recipient mice and is referred to as the TDH cell population (13, 14, 22). The other population is designated the Tamp cell population because it amplifies the anticryptococcal DTH response when transferred to mice at the time of their immunization with CneF-CFA (13, 14). Two additional T-cell populations known to be upregulated by other immunization protocols are CD8+ T cells that are involved in the anticryptococcal DTH response and the unconventional (major histocompatibility complex-nonrestricted) cytotoxic T cells, which can directly kill C. neoformans (27, 40). Neither of the last two T-cell populations is induced by immunization with the soluble cryptococcal antigen CneF (27, 40). When stimulated with cryptococcal antigen(s), activated CD4+ T cells induced by CneF-CFA display a predominant Th1 lymphokine profile (interleukin 2 [IL-2] and gamma interferon [IFN-γ]), and T-cell populations containing the Tamp cells produce significantly more of these two cytokines than do T-cell populations that lack Tamp cells (33). In contrast to immunization with CneF-CFA, s.c. immunization with HKC induces both CD4+ and CD8+ T cells, which are involved in the anticryptococcal DTH response (27). In addition, the direct anticryptococcal activity of an unconventional cytotoxic T cell is augmented by immunization with HKC either alone or in CFA (27, 39, 40).
In the present study, we assessed the abilities of these two immunization protocols, which induce different cellular components, to protect mice from infection with C. neoformans. After finding that CneF-CFA immunization afforded a significant level of protection, whereas HKC immunization did not, we analyzed the ability of the HKC immunization to induce Tamp cells, which are responsible for augmented IFN-γ production (5, 33). We found that HKC immunization did not induce Tamp cells, suggesting that Tamp cells may be important in the mechanism of clearance of C. neoformans.
MATERIALS AND METHODS
Mice.
Inbred, female CBA/J mice (H-2k) were purchased from Jackson Laboratory, Bar Harbor, Maine. The mice were maintained in the animal facility of the University of Oklahoma Health Sciences Center from 5 weeks of age until they were used at 8 to 12 weeks of age.
Organisms.
Serotype A C. neoformans strain 184A was used for immunization and infection. The organism was maintained and grown on modified Sabouraud dextrose agar. HKC for immunization were prepared by heating C. neoformans 184A cells for 1 h at 60°C (39). Viable C. neoformans cells for infection were collected from a 3-day culture, washed three times in endotoxin-free sterile physiological saline solution (SPSS), counted, and diluted to the appropriate concentration in SPSS. The numbers of viable C. neoformans cells in the challenge preparations were confirmed by diluting and plating the cryptococci on Sabouraud dextrose agar.
Maintenance of endotoxin-free conditions.
Endotoxin-free experimental conditions were maintained by using commercial endotoxin-free plasticware and heating all glassware for 3 h at 180°C. All reagents used in the experiments contained less than 8 pg of endotoxin/ml (minimal detectable level) when tested with the Limulus assay (Whittaker Bioproducts, Inc., Walkersville, Md.).
Preparation of CneF-CFA or CneF in IFA.
CneF was prepared as previously described (3). Briefly, a defined medium was inoculated with 109 C. neoformans cells/liter and incubated for 5 days at 30°C before the cryptococci were killed with 2% Formalin. At 24 h after the addition of Formalin, the culture supernatant was removed with a OM-141 Pellicon tangential-flow system and a 0.45-μm-pore-size cassette (Millipore, Bedford, Mass.). The culture supernatant was washed extensively with SPSS and concentrated 10-fold with a 30,000-molecular-weight cutoff cassette in the Pellicon system. The concentrated CneF was filter sterilized and stored at −20°C until used. The CneF preparation used here had a protein concentration of 0.268 mg/ml as determined by the bicinchoninic acid assay (BCA protein assay; Pierce Chemical Co., Rockford, Ill.) and a carbohydrate concentration of 5.8 mg/ml as determined by the phenol-sulfuric acid assay (12). CneF-CFA was prepared by emulsifying 1 part CneF with 1 part CFA (Difco Laboratories, Detroit, Mich.) (vol/vol) (36), and CneF-incomplete Freund’s adjuvant (IFA) was prepared similarly by using IFA (Sigma Chemical Co., St. Louis, Mo.) in place of CFA.
Immunization with HKC, HKC-CFA, CneF-CFA, or CneF-IFA.
Two different sources of C. neoformans antigens were used for immunization, one the heat-killed (1 h at 60°C) whole organism in saline or CFA and the other a soluble culture filtrate antigen (CneF) in either CFA or IFA. These two different immunization protocols were selected for this study because the T-cell profile that each induces has been well characterized. Immunization with HKC or HKC-CFA was done as previously described by injecting mice s.c. at two sites on the lower abdomen with 107 184A HKC suspended in SPSS or CFA (40). Controls for HKC or HKC-CFA immunization were mice injected at similar sites with the same volume of SPSS or SPSS-CFA, respectively.
Immunization with CneF-CFA or CneF-IFA was done as previously described (3). Briefly, mice were injected s.c. at two sites at the base of the tail with 0.1 ml of CneF-CFA or CneF-IFA at each site. Controls for the CneF-CFA or CneF-IFA immunization protocol were mice injected at two sites at the base of the tail with 0.1 ml of SPSS-CFA or SPSS-IFA, respectively, at each site (3).
Eight days after immunization, a time when the activated T cells are present (27, 40), five mice from each group were challenged by footpad injection to demonstrate the efficacy of immunization (3), and the remaining mice were infected with viable C. neoformans 184A cells. In each experiment at 24 h after CneF injection into the footpad, the mean increase in footpad thickness in mice immunized with CneF-CFA or CneF-IFA was approximately twice that of HKC- or HKC-CFA-immunized mice. These results demonstrate that the mice were responding immunologically as expected (40).
Infection with C. neoformans.
Mice were injected intravenously with 104, 105, or 106 viable C. neoformans cells in 0.2 ml of SPSS. At designated times after inoculation, mice were killed and lungs, livers, spleens, and brains were removed for cryptococcal CFU determinations (10). Five mice per group were used for the CFU studies, and 10 mice per group were used for the survival studies.
Histology.
Mice were treated as indicated above with SPSS-CFA, CneF-CFA, HKC-CFA, HKC, or saline, and 8 days later they were infected with 105 C. neoformans cells intravenously (i.v.). On day 7 of infection, brains were collected and fixed in 10% neutral buffered Formalin. Each brain was sectioned coronally at three levels: (i) at the olfactory tubercle showing the caudate nucleus and the nonolfactory telencephalic neocortex; (ii) at the posterior to the optic chiasm showing the olfactory cortex including the hippocampus, the pyriform cortex, and the entorhinal cortex, as well as the basal ganglia and thalamus (diencephalon); and (iii) at the flocculonodular lobe of the cerebellum and the brain stem. All sections were stained with hematoxylin and eosin. The total number of cryptococcal lesions seen in all fields of four sections from the different regions of the brain (the telencephalic neocortex, the diencephalon, the diencephalon/metencephalon, and the metencephalon) were counted and summed. The sum of the lesions from each brain of seven SPSS-CFA-treated, eight CneF-CFA-immunized, three HKC-CFA-immunized, four HKC-immunized, and four SPSS-treated mice were used to determine the mean number of lesions for each group.
Detection of Tamp cells.
Spleen cells were harvested at 8 days after treatment of the mice with SPSS-CFA, CneF-CFA, HKC-CFA, HKC, or SPSS. Spleen cells (108 per mouse) from mice in the designated treatment group were transferred to five naive mice, and then the mice were immediately immunized with CneF-CFA. Six days after transfer of the spleen cells and immunization with CneF-CFA, the mice were tested for DTH reactivity by footpad injection (13, 14).
Assay for anticryptococcal antibody.
Serum immunoglobulin M (IgM) and IgG antibody levels to glucuronoxylmannan (GXM) were measured by enzyme-linked immunosorbent assay (ELISA) as described previously (8). Briefly, ELISA plates were prepared by applying 50 μl of a 1.0-μg/ml solution of strain 184A GXM in phosphate-buffered saline (PBS; pH 7.6) to each well of the 96-well flat-bottom polystyrene ELISA plates (no. 25801, Corning Glass Works, Corning, N.Y.), incubating the plates overnight at room temperature, and blocking the contents of each well for nonspecific binding with 1% bovine serum albumin in PBS. Preliminary experiments revealed that strain 184A GXM bound to polystyrene. Mouse sera were serially diluted across ELISA plates, and the presence of antibody binding was detected by incubation with alkaline phosphatase-labeled goat anti-mouse IgM or IgG (Southern Biotechnology Associates Inc., Birmingham, Ala.) followed by the addition of the phosphatase substrate, p-nitrophenyl phosphate (1 mg/ml in 1.0 mM MgCl2–50 mM Na2CO3 [pH 9.8]). Plates were washed three to five times at each step with a solution of 0.05% Tween-20 (polyoxyethylenesorbitan monolaurate) in PBS with a Titertek Microplate washer 120 (Flow Laboratories). The ρ-nitrophenyl substrate was added prior to reading the optical density of each well at 405 nm on a Ceres 900 microtiter ELISA reader (Bio-Tek Instruments, Inc., Wisnooski, Vt.). IgM levels relative to those of IgM murine monoclonal antibody (MAb) 2D10 were calculated (6).
Detection of serum antigen levels.
Serum GXM levels were determined by antigen capture ELISA as described previously (7, 31). The antigen capture ELISA used MAb 2D10 (IgM) for capture and MAb 2H1 (IgG1) for detection of GXM. Prior to the assay, serum samples were diluted in PBS, incubated with 100 μg of proteinase K (Boehringer Mannheim) per ml overnight, and then boiled for 30 min. After the color in the ELISA developed, the optical density of each well was measured on a Ceres 900 microtiter ELISA reader at 405 nm. GXM levels were measured relative to those for strain 184A GXM standards, which were also treated with proteinase and heat as described above.
Statistical analysis.
Means, standard errors of the means (SEM), and unpaired Student’s t test results were used to analyze the DTH, CFU, ELISA, and brain lesion data. When comparing two groups, we used a P value of 0.05 or less to define a significant difference. Survival data were analyzed with Kaplan-Meier survival plots followed by the log-rank test (JMP Software, SAS Institute, Cary, N.C.) on an Apple Macintosh computer. To analyze the serum GXM data, the Kruskal-Wallis procedure was used, followed by the Newman-Keuls method for multiple comparisons.
RESULTS
Clearance of C. neoformans from tissues of immunized mice after an i.v. infection with 104 viable C. neoformans cells.
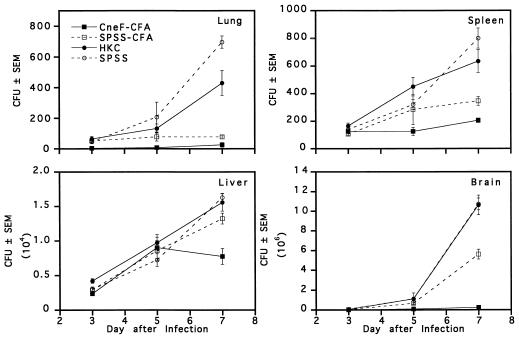
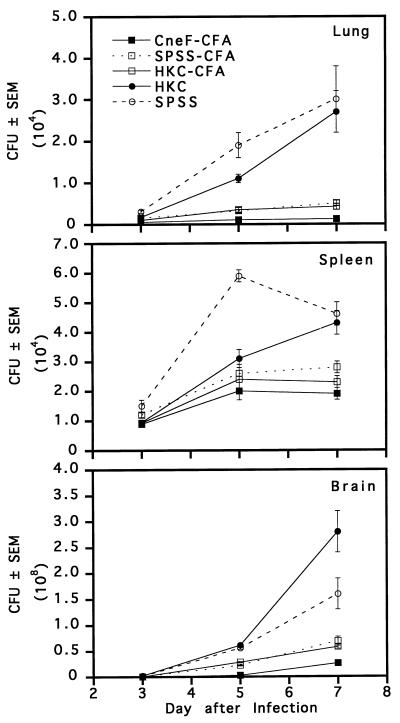
In an initial experiment, we compared the levels of clearance of C. neoformans from lungs, livers, spleens, and brains on days 3, 5, and 7 after an i.v. infection with 104 viable organisms in mice immunized with either HKC or CneF-CFA and in their respective controls (Fig. 1). When the data for the 3-day time period after infection were compared, significant differences among the groups were noted. For instance at 3 days, mice immunized with CneF-CFA had significantly fewer cryptococcal CFU in lungs (2.5 ± 2.5) and brains (3,300 ± 559) than mice immunized with HKC (65 ± 1.5 [P < 0.001] and 55,250 ± 3,677 [P < 0.0001], respectively). The fungal burden in lungs of HKC-immunized mice was comparable to that in the lungs of SPSS-CFA- (51 ± 9 CFU) and SPSS-treated (45 ± 10 CFU) control mice. In the brains of HKC-immunized mice at day 3 of infection the fungal load was similar to that in the brains of mice given SPSS (60,100 ± 4,975 CFU) but surprisingly higher than the burden in brains of mice treated with SPSS-CFA (23,312 ± 1,567 CFU; P < 0.0001). A comparison of the numbers of CFU recovered from the spleens at 3 days did not reveal significant differences among the four groups. Livers from HKC-immunized mice had significantly greater numbers of CFU than livers from CneF-CFA-immunized mice or from mice treated with SPSS-CFA or SPSS and infected 3 days before CFU determinations (P < 0.02).
FIG. 1.
Mean numbers of cryptococcal CFU in lungs, spleens, livers, and brains from immune and control mice at 3, 5, and 7 days after i.v. challenge with 104 viable C. neoformans cells. Mice were treated on day 0 with the designated preparation and were infected on day 8. Error bars represent the standard error of the mean (SEM). The data are from one of two experiments which produced similar results.
From day 3 to day 7 of infection, the numbers of cryptococcal CFU increased in all tissues of the HKC- and SPSS-treated groups (Fig. 1). In contrast, mice immunized with CneF-CFA did not show an increase in CFU in any tissues assessed except liver tissues until day 5, after which the CneF-CFA-immunized mice gained control of the infection in the livers as well (Fig. 1). The generalized protection provided by CneF-CFA was not evident in the HKC-immunized group, which showed only limited protection in the lungs at day 7 after infection (Fig. 1; compare day 7 lungs of HKC-treated and SPSS-treated mice; P = 0.015). Each tissue from the CneF-CFA-immunized mice at day 7 after infection had significantly fewer CFU than the corresponding control tissue from the SPSS-CFA-treated mice (P < 0.01), indicating that CneF-CFA immunization provided a greater overall level of protection than the CFA control. It is worth noting, however, that CFA emulsified with saline afforded a measurable level of protection in lungs, spleens, and brains compared to SPSS and HKC (Fig. 1; P < 0.02).
A comparison of the levels of protection against C. neoformans afforded by immunization protocols that induce two different activated T-cell profiles.
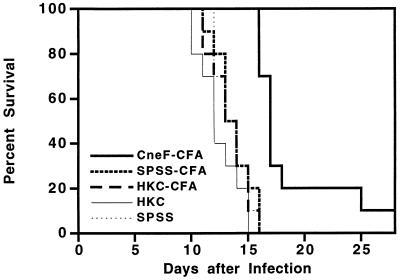
Having observed that CneF-CFA provided significant protection and that SPSS-CFA induced some protection, as measured by tissue CFU counts of C. neoformans, we were interested in whether or not the protective effects would also be evident in survival studies. Even though in previous studies we had shown that CFA added to HKC did not alter the immune responses induced (40), we were interested in whether or not CFA added to HKC altered protection. Consequently, in the next series of experiments, an HKC-CFA-immunized group was included. Mice immunized with CneF-CFA, HKC-CFA, or HKC and mice treated with SPSS-CFA or SPSS alone were infected i.v. with 105 viable C. neoformans cells on day 8 after immunization, and their survival was monitored over a 30-day period (Fig. 2). CneF-CFA-immunized mice survived significantly longer (mean survival time = 18.7 ± 1.3 days) than all other groups of mice (SPSS-CFA- and SPSS-treated group mean survival times = 13.7 ± 0.5 and 13.6 ± 0.5 days, respectively; HKC- and HKC-CFA-treated group mean survival times = 12.4 ± 0.6 and 13.4 ± 0.5 days, respectively; P < 0.0001). Mice immunized with HKC either alone or with CFA were not protected against challenge.
FIG. 2.
Percent survival of CneF-CFA-, HKC-CFA-, and HKC-immunized and control mice after challenging the mice with 105 viable C. neoformans cells on day 8 after treatment. The data are from one of two experiments which produced similar results.
Histological studies of brains from immunized and control mice infected with 105 C. neoformans cells.
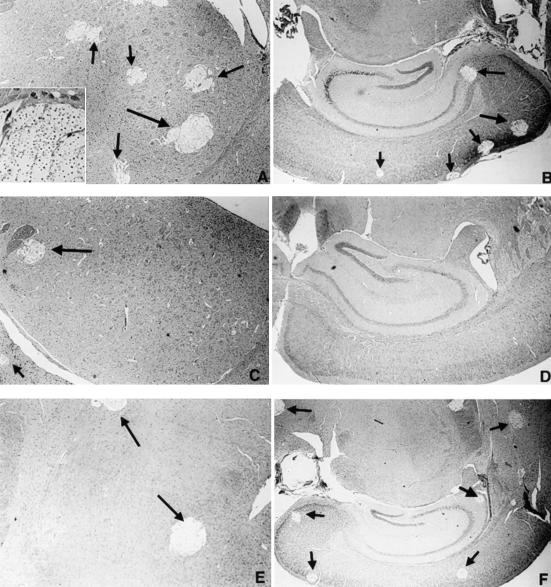
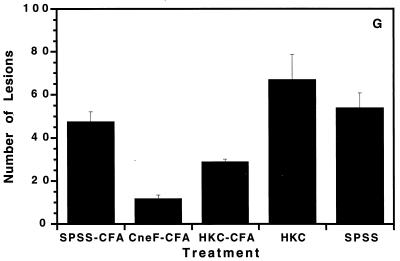
At 7 days into the infection, brains from mice immunized with CneF-CFA had fewer numbers of cryptococcal lesions and smaller lesions (Fig. 3C and D) than brains from mice treated with SPSS-CFA or HKC-CFA (Fig. 3A and B and 3E and F, respectively). The appearance of the lesions in brains from mice treated with HKC or saline was similar to that in brains from mice treated with SPSS-CFA (data not shown). When the total numbers of lesions were determined and summed for representative sections from different regions of the brain including the nonolfactory telencephalic neocortex, olfactory cortex (including the hippocampus, pyriform cortex, and entorhinal cortex), and the diencephalon (including basal ganglia and thalamus), as well as the cerebellum and brain stem, it was evident that significantly fewer cryptococcal lesions were observed in brains of CneF-CFA-immunized mice than in brains from SPSS-CFA-immunized (P = 0.00004), HKC-CFA-immunized (P = 0.0006), HKC-immunized (P = 0.0003), and SPSS-treated (P = 0.00009) mice (Fig. 3G).
FIG. 3.
Cryptococcal lesions in the brains of infected mice. Representative sections illustrate the frequency of lesions (arrows) in the basal ganglia (A, C, and E) and olfactory cortices (including the hippocampi) (B, D, and F) of mice treated with SPSS-CFA (A and B), CneF-CFA (C and D), and HKC-CFA (E and F). Note the relative paucity of cystic lesions in the brains from CneF-CFA-treated mice. The inset in panel A shows cryptococcal organisms within one of the cystic lesions. The relative differences in the total numbers of lesions seen in the brains of mice from each of the treatment groups are illustrated in the bar graph (G). Magnifications, ×28 (A to E) and ×14 (F). Each panel (A to F) shows a brain section from one mouse which is representative of similar sections from seven or eight mice in the SPSS-CFA and CneF-CFA treatment groups and three mice in the HKC-CFA group. Brain sections from four mice in each of the HKC and SPSS treatment groups appeared similar to brain sections shown in panels A and B. The means ± SEM of the summed lesions from four different sections of the brains are shown in panel G. See Materials and Methods for experimental details.
Tissue burdens of C. neoformans after infecting immunized and control mice with a higher dose (106 cells) of viable C. neoformans cells.
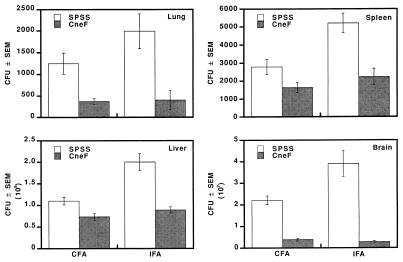
To determine whether or not the protective effects of CFA were evident by CFU counts after challenging mice with a higher number of C. neoformans cells, mice were immunized with CneF-CFA, HKC-CFA, or HKC or were treated with SPSS-CFA or SPSS and then infected with 106 viable cryptococci on day 8 after treatment. As with the two lower challenge doses (104 and 105 cells), mice immunized with CneF-CFA displayed more protection than mice in the other groups (Fig. 4). The numbers of CFU cultured from lungs, spleens, or brains of CneF-CFA-immunized mice at 7 days after infection were significantly lower than the numbers of CFU cultured from the respective tissues from SPSS-CFA-treated mice (Fig. 4; P < 0.004). The numbers of cryptococcal CFU in the livers were not significantly different among the groups infected with 106 cryptococci (data not shown). In contrast, HKC-immunized mice displayed greater numbers of CFU in the brains at 7 days after infection than any of the other groups including the SPSS controls, suggesting that immunization with HKC alone may down-regulate some host protective mechanism (Fig. 4). The protective value of CFA was evident again with this high challenge dose of 106 C. neoformans cells by the lower numbers of cryptococcal CFU in tissues of SPSS-CFA-treated and HKC-CFA-immunized mice compared to the numbers of CFU in the respective tissue of mice treated with SPSS or HKC (Fig. 4). These data confirm that CFA induces some protection against C. neoformans during the first week of infection irrespective of the presence or absence of the intact antigen or organism.
FIG. 4.
Mean numbers of cryptococcal CFU in lungs, spleens, and brains from immune and control mice at 3, 5, and 7 days after an i.v. challenge with 106 viable C. neoformans cells. Mice were treated on day 0 with the designated preparation and were infected on day 8. Error bars represent the SEM. The data are from one of two experiments which produced similar results.
Contribution of CFA to the protection induced by CneF-CFA.
We postulated that the Mycobacterium sp. in the CFA was responsible for the limited protection that was evident in the early CFU counts but was not sufficient to change significantly the survival times of the animals. To test the protective role of Mycobacterium in the adjuvant, we immunized mice with CneF-CFA, which contains Mycobacterium, or CneF-IFA, which contains no Mycobacterium, and included the appropriate controls (SPSS-CFA or SPSS-IFA). On day 7 after immunization, footpad testing was done, and the DTH responses, measured as footpad swelling, were the following: CneF-CFA immunized mice had responses of (18.8 ± 1.2) × 10−3 in., SPSS-CFA-injected mice had responses of (0.8 ± 0.2) × 10−3 in., CneF-IFA-immunized mice had responses of (16.4 ± 1.0) × 10−3 in., and SPSS-IFA-injected mice had responses of (0.8 ± 0.2) × 10−3 in. There was no significant difference between the two groups of mice treated with CneF. For similarly treated mice challenged i.v. with 105 C. neoformans cells on day 7 after treatment, we compared the numbers of CFU in lungs, livers, spleens, and brains with those in their respective controls (Fig. 5). Comparable levels of protection were induced by CneF-CFA and CneF-IFA immunization. Irrespective of whether or not Mycobacterium was in the adjuvant used with CneF, the numbers of organisms in the immunized mouse tissues were similar for the two immunized groups and significantly lower than those in the respective control tissue (Fig. 5, P < 0.02 in lungs and < 0.009 in other organs). These results indicate that CFA-induced protection was not apparent by CFU counts in mice with an ongoing anticryptococcal CMI response (Fig. 5; compare CneF in CFA to CneF in IFA). In contrast, the protective effect of Mycobacterium in the adjuvant was seen in all tissues except the lung tissue when the antigen was absent (Fig. 5; compare SPSS-CFA to SPSS-IFA; P = 0.114 [not significant] for lungs, 0.001 for spleen, 0.00004 for liver, and 0.009 for brain).
FIG. 5.
Mean numbers of cryptococcal CFU in lungs, spleens, livers, and brains from CneF-CFA- and CneF-IFA-immunized mice and their respective SPSS controls at day 7 after i.v. infection with 105 viable C. neoformans cells. Error bars show the SEM. The data are from one of two experiments which produced similar results.
Assessment of anticryptococcal Tamp cells after immunization with HKC or HKC-CFA.
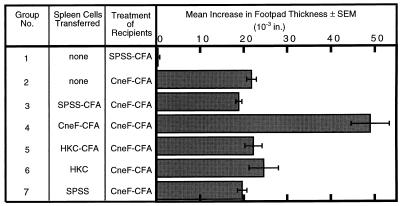
From previous studies we know that CneF-CFA induces not only the CD4+ T cells responsible for DTH reactivity but also a CD4+ cell population that amplifies the DTH response (13, 14). Cell populations containing Tamp cells make elevated levels of IL-2 and IFN-γ in comparison to spleen cell populations, which contain only T cells responsible for DTH reactivity (TDH cells) (33). Since the Th1 lymphokine, IFN-γ, has been associated with protection induced during infection with C. neoformans (1), we thought that Tamp cells, which have been shown to have an augmented capacity to produce IFN-γ (and IL-2) (5, 33), may be contributing to the differences in protection seen in mice immunized with CneF-CFA versus mice immunized with HKC. Since the presence of Tamp cells in HKC- and HKC-CFA-immunized mice had not been previously assessed, we did the following experiment. Spleen cells from mice immunized with HKC, HKC-CFA, or CneF-CFA or appropriate control spleen cells were transferred to naive recipient mice at the time of immunization. As expected, spleen cells from CneF-CFA-immunized mice contained Tamp cells as shown by the significantly amplified DTH response in the recipients of those spleen cells as compared to mice that had received spleen cells from SPSS-CFA- or SPSS-treated mice (Fig. 6, compare group 4 to groups 3 and 7; P = 0.0003). In contrast, the spleen cell populations from HKC-CFA- and HKC-immunized mice showed no evidence of Tamp cells because the recipients of those spleen cells had DTH responses equivalent to those of the mice which had been given spleen cells from SPSS-CFA- or SPSS-treated mice (Fig. 6; compare groups 5 and 6 to groups 3 and 7).
FIG. 6.
Detection of the presence of Tamp cells. Mean increases in footpad thickness after footpad challenge with CneF at 6 days after the mice were given the designated spleen cell population and were immunized with CneF-CFA are shown. Error bars indicate the SEM. The data are from one of two experiments which produced similar results.
Levels of anticryptococcal antibody in mice at 8 days after immunization (the time of infection) and at 12 days after being given 105 viable C. neoformans cells.
Some antibodies to GXM have been shown to be protective in mice (11, 29), and we were interested in whether or not anti-GXM antibodies were present in mice immunized with either CneF-CFA or HKC. For this assessment, sera were obtained from the mice on the day of infection and 12 days after infection. A preliminary experiment utilizing alkaline phosphatase-labeled antisera to mouse IgM and IgG revealed no significant increases in anti-GXM antibody titers in immunized mice at day 8 or 20 after immunization (day 0 or 12 after infection) relative to the control mice (data not shown). A second experiment was then performed to measure the anticryptococcal antibody content of sera from immune and control mice more precisely. The amount of antibody was quantified relative to IgM MAb 2D10 by ELISA. This antibody was chosen because it has the highest level of affinity of the available set of anti-Cryptococcus IgM MAbs (6). The use of a high-affinity-level antibody as a standard would tend to overestimate the amount of IgM in serum. Nevertheless, this was deliberately done to identify the possible upper-end concentrations of antibody in the serum samples. The levels of anticryptococcal IgM at 8 days after immunization (day 0 after infection) and at 20 days after immunization (day 12 after infection) in sera from mice treated with CneF-CFA, SPSS-CFA, or HKC were not different than the levels in sera from naive CBA/J mice.
Concentrations of cryptococcal GXM in sera from immunized and control infected mice.
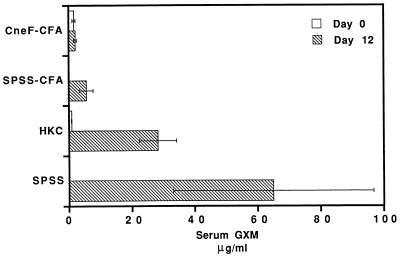
Patients with disseminated cryptococcosis typically have cryptococcal polysaccharide in their body fluids, including serum and spinal fluid (9). Furthermore, the higher the cryptococcal antigen titer at the onset of therapy, the less likely the patient will respond positively to therapy (9). Consequently, it was of interest in this study to correlate the levels of cryptococcal antigen in serum of infected mice with the severity of disease. The group of mice immunized with CneF-CFA and infected had no rise in serum GXM at 12 days after infection with C. neoformans, whereas mice immunized with HKC displayed significant increases in GXM concentrations in their sera after 12 days of infection compared to the CneF-CFA-immunized mice (P < 0.05) (Fig. 7). In fact, at 12 days into the infection, serum GXM levels in HKC-immunized, infected mice were elevated and were not significantly different from the GXM levels in serum from SPSS-treated mice infected with C. neoformans (Fig. 7). In contrast, mice given SPSS-CFA had significantly lower concentrations of cryptococcal GXM in serum than HKC- or SPSS-treated, infected mice (P < 0.05).
FIG. 7.
Serum GXM levels of CneF-CFA- and HKC-immunized and control mice at the time of infection (day 0) with 105 viable C. neoformans cells or at day 12 of the infection. Mice were immunized with CneF-CFA or HKC or were treated with SPSS-CFA or SPSS 8 days prior to infecting the animals. Error bars indicate the standard deviation.
DISCUSSION
Our objective was to determine the protective value of two previously defined immunization protocols which induce anticryptococcal CMI responses with different activated T-cell profiles. By comparing the activated T-cell profile of the CMI response induced by each procedure and relating the profile to the protective value of each protocol, it is possible to begin to establish which activated T-cell component(s) of the CMI response contributes to protection. The results of these studies clearly establish that a single s.c. injection with a soluble culture filtrate antigen of C. neoformans in either CFA or IFA induces a significantly better protective immune response against C. neoformans than does a single s.c. injection of HKC either alone or in CFA. The protective mechanism(s) against C. neoformans induced by CneF-CFA was evident by reduced CFU counts in most tissues examined after infecting mice with three different doses ranging from 104 to 106 C. neoformans cells. Of the tissues in which CFU were determined, protection was most evident in the brains from mice immunized with CneF-CFA or CneF-IFA. The significantly reduced numbers of C. neoformans CFU in brains of CneF-immunized mice could have resulted from (i) fewer organisms in extracerebral tissues thereby limiting the numbers of organisms entering the bloodstream and the brain and/or (ii) enhanced protective mechanisms in the brains of the CneF-immunized mice. Protection was visible in CneF-CFA-immunized mice not only by CFU counts but also by the significantly extended mean survival time compared to controls. Our observation that serum GXM concentrations were significantly lower in the CneF-CFA-immunized, infected mice than in infected mice previously immunized with HKC or treated with saline is in keeping with the observation that GXM concentrations tend to correlate with organ CFU counts (30). When one considers that control mice infected with C. neoformans 184A will mount an anticryptococcal CMI response that offers some protection, then the significantly extended survival time we obtained for the CneF-CFA-immunized mice over that for the controls is impressive and meaningful. Together, the reduced CFU counts and increased survival of the CneF-immunized mice show that the culture filtrate from C. neoformans 184A, which we refer to as CneF, contains antigens which induce a protective CMI response or an appropriate combination of antigens to induce protective components of the CMI response.
The protection that we observed is not likely to have been mediated by anticryptococcal antibodies, because the two different nonreplicating immunogens, CneF-CFA and HKC, that we used do not induce antibody responses to the major surface component of cryptococci, namely, GXM. Both immunogens do induce anticryptococcal CMI responses; however, the activated T-cell profiles that the immunogens induce are different. Consequently, the protection that we observed in the CneF-CFA-immunized mice is due to one or more of the anticryptococcal CMI components induced by CneF. The distinct differences in protection afforded by these two protocols, along with the differences in activated T-cell profiles, make these systems excellent models for sorting the protective CMI mechanisms from the nonprotective or potentially exacerbating CMI responses to C. neoformans.
The CMI profile induced by CneF-CFA and associated with protection is characterized by activated CD4+ T cells which upon restimulation with cryptococcal antigen make the Th1 cytokines, IL-2 and IFN-γ, along with the Th2 cytokine IL-5 (5, 33). The activated CD4+ cells can be separated into two functionally different pools, the CD4+ cells that transfer DTH reactivity (TDH cells) and the Tamp cells which amplify the CMI response and make an abundance of IFN-γ and IL-2 (5, 13, 14, 33). DTH responses induced by CneF-CFA are typically more intense (approximately twofold) than DTH reactions induced by viable or dead C. neoformans cells alone or in CFA (40). The activated CD4+ cells in the CneF-CFA-immunized mice are the most likely components responsible for the protection observed. All things considered, it is probable that both of the activated CD4+ T cells, i.e., the TDH and the Tamp cells, contribute to the protection.
Theoretically, both the TDH and the Tamp cells have the ability to contribute to protection. TDH cells recruit leukocytes into the site of antigen deposition (4), and increased leukocyte infiltrates into sites of infection would be expected to enhance clearance of the organism. Tamp cells also could enhance protection but in a manner different than that of the TDH cells. Tamp cells do not significantly increase the numbers of leukocytes that infiltrate sites of antigen deposition, but they do cause more IFN-γ and IL-2 to be produced at the DTH reaction site (5). Consequently, we propose that the Tamp cells aid in protection by producing large amounts of Th1 lymphokines at the infection site. The Th1 lymphokines can activate macrophages and possibly other natural effector cells to more aggressively kill C. neoformans (15, 19, 24, 25, 28, 35, 42). This explanation is in accordance with the findings that cryptococcal infections are exacerbated when mice are treated with an antibody that neutralizes IFN-γ (1). Thus, one would predict that animals which have small numbers of TDH cells but which lack Tamp cells, such as mice immunized with HKC, would not be protected to the same degree as mice with both cell populations. Indeed, we showed that this is the case; the DTH response to CneF injected into the footpads of HKC-immunized mice is half that of mice immunized with CneF-CFA, and the former mice lack Tamp cells and display little protection against C. neoformans.
Mice immunized with HKC with or without CFA have populations of T cells not found in the mice immunized with CneF-CFA. These are CD8+ cells which contribute to anticryptococcal DTH reactivity and unconventional cytotoxic T cells that directly kill C. neoformans (27, 39, 40). Since HKC immunization induces little to no protection in mice, these two components do not appear to be playing a role in protection. This is not to say that these two components may not have protective capabilities under appropriate conditions; however, if they provide protection, it was not sufficient in the HKC-immunized mice to be detectable.
Another observation was that CFA but not IFA introduces a protective component(s) that can be detected by early CFU counts but not in survival experiments. The protection afforded by CFA did not nullify the protection induced by CneF-CFA, because the protection observed on the basis of either CFU counts or survival time determinations was significantly greater in mice immunized with CneF-CFA than in mice immunized with SPSS-CFA. Survival results after infecting the HKC- and HKC-CFA-immunized mice with C. neoformans cells did not indicate there was a difference in the protection afforded by HKC alone compared to that afforded by HKC in CFA. CFA added to HKC boosts the protective response of the host in the same way that the CFA in saline boosts the response, because the clearance data from those two groups are similar. Our observation that CFA in the HKC immunization did not increase the survival times of the infected mice is consistent with our other findings that the addition of CFA to HKC does not induce Tamp cells or augment the anticryptococcal DTH response or the level of direct T-cell cytotoxicity for C. neoformans over that induced by HKC alone (39, 40). The early protection observed in the mice treated with saline in CFA as compared to that observed in mice treated with saline-IFA or saline alone is most likely due to the Mycobacterium in the CFA inducing an anti-Mycobacterium DTH response (37) which results in activated macrophages that can eliminate the cryptococci. In contrast, mice treated with CneF-CFA have induced both anti-Mycobacterium and anticryptococcal CMI responses (37). After the infection of mice with C. neoformans, the anti-cryptococcal CMI response is boosted and continues to protect the mice, whereas the anti-Mycobacterium CMI response is not restimulated and its effectiveness in clearance fades. This speculation is reasonable since other intracellular pathogens that induce CMI responses which activate macrophages have been shown to enhance the clearance of C. neoformans (2, 17).
Our findings that HKC immunization did not induce significant protection against C. neoformans in CBA/J mice are not altogether surprising. A number of other workers (16, 18, 20, 21, 23) have immunized mice with HKC; they used different strains of mice, different isolates of C. neoformans, different routes of administration, and different numbers of HKC, and then they challenged the mice with viable cryptococci. In some studies, little to no protection or even toxic effects were observed (16, 20, 23), whereas in a study in which HKC were given intratracheally protection was reported (21). In most of the studies, the immune parameters of the immunized mice were not fully characterized. In this and previous studies we and others (27, 40) have shown that s.c. immunization with HKC induces a different profile of activated T cells than that induced by the soluble CneF in adjuvant. As mentioned earlier the lack of protection in HKC-immunized mice is likely due, at least in part, to the lack of Tamp cells and thus insufficient levels of lymphokines such as IFN-γ and IL-2.
In summary, our results indicate that not all immunization protocols induce protective CMI responses. CneF-CFA immunization induces an anticryptococcal CMI response profile that is protective. This protective response is associated with CD4+ TDH cells and Tamp cells that make IFN-γ and IL-2. In contrast, HKC immunization does not offer protection to mice against a challenge infection with C. neoformans even though it induces potentially protective anticryptococcal CMI components (low levels of CD4+ and CD8+ TDH cells and unconventional T cells with elevated direct anticryptococcal killing ability). However, the lack of Tamp cell induction by HKC, either alone or along with the reduced level of TDH-cell induction, may account for the absence of enhanced protection. These findings emphasize that at this time one cannot predict on the basis of DTH reactivity whether or not an immunization protocol will influence clearance of C. neoformans from tissues. Furthermore, the results demonstrate the need for continued investigations to dissect and identify the various mechanisms of protection and exacerbation in cryptococcosis.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI-15716 and AI-18895.
We thank Ronald Greenfield for his help in statistical analyses of data and Rebecca Blackstock for her review of the manuscript.
REFERENCES
- 1.Aguirre K, Havell E A, Gibson G W, Johnson L L. Role of tumor necrosis factor and gamma interferon in acquired resistance to Cryptococcus neoformans in the central nervous system of mice. Infect Immun. 1995;63:1725–1731. doi: 10.1128/iai.63.5.1725-1731.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguirre K M, Sayles P C, Gibson G W, Johnson L L. Resistance to Cryptococcus neoformans is associated with an inflammatory response to Toxoplasma gondii in the central nervous system of mice. Infect Immun. 1996;64:77–82. doi: 10.1128/iai.64.1.77-82.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchanan K L, Murphy J W. Characterization of cellular infiltrates and cytokine production during the expression phase of the anticryptococcal delayed-type hypersensitivity response. Infect Immun. 1993;61:2854–2865. doi: 10.1128/iai.61.7.2854-2865.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchanan K L, Murphy J W. Kinetics of cellular infiltration and cytokine production during the efferent phase of a delayed-type hypersensitivity reaction. Immunology. 1997;90:189–197. doi: 10.1046/j.1365-2567.1997.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchanan K L, Murphy J W. Regulation of cytokine production during the expression phase of the anticryptococcal delayed-type hypersensitivity response. Infect Immun. 1994;62:2930–2939. doi: 10.1128/iai.62.7.2930-2939.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casadevall A, Mukherjee J, Devi S J N, Schneerson R, Robbins J B, Scharff M D. Antibodies elicited by a Cryptococcus neoformans glucuronoxylomannan-tetanus toxoid conjugate vaccine have the same specificity as those elicited in infection. J Infect Dis. 1992;65:1086–1093. doi: 10.1093/infdis/165.6.1086. [DOI] [PubMed] [Google Scholar]
- 7.Casadevall A, Mukherjee J, Scharff M D. Monoclonal antibody ELISAs for cryptococcal polysaccharide. J Immunol Methods. 1992;154:27–35. doi: 10.1016/0022-1759(92)90209-c. [DOI] [PubMed] [Google Scholar]
- 8.Casadevall A, Scharff M D. The mouse antibody response to infection with Cryptococcus neoformans: VH and VL usage in polysaccharide binding antibodies. J Exp Med. 1991;174:151–160. doi: 10.1084/jem.174.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diamond R D, Bennett J E. Prognostic factors in cryptococcal meningitis. A study in 111 cases. Ann Intern Med. 1974;80:176–181. doi: 10.7326/0003-4819-80-2-176. [DOI] [PubMed] [Google Scholar]
- 10.Doyle H A, Murphy J W. MIP-1α contributes to the anticryptococcal delayed-type hypersensitivity reaction and protection against Cryptococcus neoformans. J Leukocyte Biol. 1997;61:147–155. doi: 10.1002/jlb.61.2.147. [DOI] [PubMed] [Google Scholar]
- 11.Dromer F, Charreire J, Contrepois A, Carbon C, Yeni P. Protection of mice against experimental cryptococcosis by anti-Cryptococcus neoformans monoclonal antibody. Infect Immun. 1987;55:749–752. doi: 10.1128/iai.55.3.749-752.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubois M, Gilles K A, Hamilton J K, Rebers P A, Smith F. Colorimetric method for the determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- 13.Fidel P L, Jr, Murphy J W. Characterization of a cell population which amplifies the anticryptococcal delayed-type hypersensitivity response. Infect Immun. 1990;58:393–398. doi: 10.1128/iai.58.2.393-398.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fidel P L, Jr, Murphy J W. Effects of cyclosporin A on the cells responsible for the anticryptococcal cell-mediated immune response and its regulation. Infect Immun. 1989;57:1158–1164. doi: 10.1128/iai.57.4.1158-1164.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flesch I E, Schwamberger G, Kaufmann S H. Fungicidal activity of IFN-γ-activated macrophages. Extracellular killing of Cryptococcus neoformans. J Immunol. 1989;142:3219–3224. [PubMed] [Google Scholar]
- 16.Gadebusch H H. Active immunization against Cryptococcus neoformans. J Infect Dis. 1958;102:219–226. doi: 10.1093/infdis/102.3.219. [DOI] [PubMed] [Google Scholar]
- 17.Gentry L O, Remington J S. Resistance against Cryptococcus conferred by intracellular bacteria and protozoa. J Infect Dis. 1971;123:22–31. doi: 10.1093/infdis/123.1.22. [DOI] [PubMed] [Google Scholar]
- 18.Graybill J R, Taylor R L. Host defense in cryptococcosis. I. An in vivo model for evaluating immune responses. Int Arch Allergy Appl Immunol. 1978;57:101–108. [PubMed] [Google Scholar]
- 19.Hidore M R, Nabavi N, Sonleitner F, Murphy J W. Murine natural killer cells are fungicidal to Cryptococcus neoformans. Infect Immun. 1991;59:1747–1754. doi: 10.1128/iai.59.5.1747-1754.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoff C L. Immunity studies of Cryptococcus hominis (Torula histolytica) in mice. J Lab Clin Med. 1942;27:751–754. [Google Scholar]
- 21.Kawakami K, Kohno S, Kadota J I, Tohyama M, Teruya K, Kudeken N, Saito A, Hara K. T cell-dependent activation of macrophages and enhancement of their phagocytic activity in the lungs of mice inoculated with heat-killed Cryptococcus neoformans: involvement of IFN-γ and its protective effect against cryptococcal infection. Microbiol Immunol. 1995;39:135–143. doi: 10.1111/j.1348-0421.1995.tb02180.x. [DOI] [PubMed] [Google Scholar]
- 22.Khakpour F R, Murphy J W. Characterization of a third-order suppressor T cell (Ts3) induced by cryptococcal antigen(s) Infect Immun. 1987;55:1657–1662. doi: 10.1128/iai.55.7.1657-1662.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kligman A M. Studies of the capsular substance of Torula histolytica and the immunologic properties of torula cells. J Immunol. 1947;57:395–401. [PubMed] [Google Scholar]
- 24.Levitz S M, DiBenedetto D J. Differential stimulation of murine resident peritoneal cells by selectively opsonized encapsulated and acapsular Cryptococcus neoformans. Infect Immun. 1988;56:2544–2551. doi: 10.1128/iai.56.10.2544-2551.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levitz S M, Dupont M P. Phenotypic and functional characterization of human lymphocytes activated by interleukin-2 to directly inhibit growth of Cryptococcus neoformans in vitro. J Clin Invest. 1993;91:1490–1498. doi: 10.1172/JCI116354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell T G, Perfect J R. Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev. 1995;8:515–548. doi: 10.1128/cmr.8.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mody C H, Paine III R, Jackson C, Chen G H, Toews G B. CD8 cells play a critical role in delayed type hypersensitivity to intact Cryptococcus neoformans. J Immunol. 1994;152:3970–3979. [PubMed] [Google Scholar]
- 28.Mody C H, Tyler C L, Sitrin R G, Jackson C, Toews G B. Interferon-γ activates rat alveolar macrophages for anticryptococcal activity. Am J Respir Cell Mol Biol. 1991;5:19–26. doi: 10.1165/ajrcmb/5.1.19. [DOI] [PubMed] [Google Scholar]
- 29.Mukherjee J, Scharff M D, Casadevall A. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect Immun. 1992;60:4534–4541. doi: 10.1128/iai.60.11.4534-4541.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mukherjee J, Scharff M D, Casadevall A. Variable efficacy of passive antibody administration against diverse Cryptococcus neoformans strains. Infect Immun. 1995;63:3353–3359. doi: 10.1128/iai.63.9.3353-3359.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukherjee J, Zuckier L, Scharff M D, Casadevall A. Therapeutic efficacy of monoclonal antibodies to Cryptococcus neoformans glucuronoxylomannan alone and in combination with amphotericin B. Antimicrob Agents Chemother. 1994;38:580–587. doi: 10.1128/aac.38.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy J W. Clearance of Cryptococcus neoformans from immunologically suppressed mice. Infect Immun. 1989;57:1946–1952. doi: 10.1128/iai.57.7.1946-1952.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy J W. Cytokine profiles associated with induction of the anticryptococcal cell-mediated immune response. Infect Immun. 1993;61:4750–4759. doi: 10.1128/iai.61.11.4750-4759.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy J W, Gregory J A, Larsh H W. Skin testing of guinea pigs and footpad testing of mice with a new antigen for detecting delayed hypersensitivity to Cryptococcus neoformans. Infect Immun. 1974;9:404–409. doi: 10.1128/iai.9.2.404-409.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy J W, Hidore M R, Wong S C. Direct interactions of human lymphocytes with the yeast-like organism, Cryptococcus neoformans. J Clin Invest. 1993;91:1553–1566. doi: 10.1172/JCI116361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy J W, Moorhead J W. Regulation of cell-mediated immunity in cryptococcosis. I. Induction of specific afferent T suppressor cells by cryptococcal antigen. J Immunol. 1982;128:276–283. [PubMed] [Google Scholar]
- 37.Murphy J W, Mosley R L. Regulation of cell-mediated immunity in cryptococcosis. III. Characterization of second-order T suppressor cells (Ts2) J Immunol. 1985;134:577–584. [PubMed] [Google Scholar]
- 38.Murphy J W, Pahlavan N. Cryptococcal culture filtrate antigen for detection of delayed-type hypersensitivity in cryptococcosis. Infect Immun. 1979;25:284–292. doi: 10.1128/iai.25.1.284-292.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muth S M, Murphy J W. Direct anticryptococcal activity of lymphocytes from Cryptococcus neoformans-immunized mice. Infect Immun. 1995;63:1637–1644. doi: 10.1128/iai.63.5.1637-1644.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muth S M, Murphy J W. Effects of immunization with Cryptococcus neoformans cells or cryptococcal culture filtrate antigen on the direct anticryptococcal activities of murine T lymphocytes. Infect Immun. 1995;63:1645–1651. doi: 10.1128/iai.63.5.1645-1651.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Powderly W G. Therapy for cryptococcal meningitis in patients with AIDS. Clin Infect Dis. 1992;14:S54–S59. doi: 10.1093/clinids/14.supplement_1.s54. [DOI] [PubMed] [Google Scholar]
- 42.Salkowski C, Balish E. A monoclonal antibody to gamma interferon blocks augmentation of natural killer cell activity induced during systemic cryptococcosis. Infect Immun. 1991;59:486–493. doi: 10.1128/iai.59.2.486-493.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spitzer E D, Spitzer S G, Freundlich L F, Casadevall A. Persistence of the initial infection in recurrent cryptococcal meningitis. Lancet. 1993;341:505–596. doi: 10.1016/0140-6736(93)90354-j. [DOI] [PubMed] [Google Scholar]