Abstract
Interleukin-15 (IL-15) is a recently discovered cytokine produced by a wide range of different cell types including fibroblasts, keratinocytes, endothelial cells, and macrophages in response to lipopolysaccharide or microbial infection. This suggests that IL-15 may play a crucial role in the activation of phagocytic cells against pathogens. We studied polymorphonuclear leukocyte (PMN) activation by IL-15, evaluated as enhancement of PMN anti-Candida activity as well as IL-8 production, following stimulation with the cytokine. The PMN response to IL-15 depends on binding to the IL-15 receptor. Our experiments show that binding of a biotinylated human IL-15–immunoglobulin G2b IgG2b fusion protein was competed by the addition of human recombinant IL-15 (rIL-15) or of human rIL-2, suggesting that IL-15 binding to PMN might involve the IL-2Rβ and IL-2Rγ chains, which have been shown to be constitutively expressed by PMN. In addition, we show by reverse transcription-PCR and by flow cytometry with a specific anti-IL-15Rα chain monoclonal antibody that PMN express the IL-15Rα chain at the mRNA and protein levels. Incubation with IL-15 activated PMN to secrete the chemotactic factor IL-8, and the amount secreted was increased by costimulation with heat-inactivated Candida albicans. In addition, IL-15 primed the metabolic burst of PMN in response to formyl-methionyl-leucyl-phenylalanine but was not sufficient to trigger the respiratory burst or to increase the production of superoxide in PMN exposed to C. albicans. IL-15 also increased the ability of PMN to phagocytose heat-killed C. albicans organisms in a dose-dependent manner, without opsonization by antibodies or complement-derived products. In the same concentration range, IL-15 was as effective as gamma interferon (IFN-γ) and IL-2 in increasing the C. albicans growth-inhibitory activity of PMN. Taken together, these results suggest that IL-15 is a potent stimulant of both proinflammatory and antifungal activities of PMN, activating several antimicrobial functions of PMN involved in the cellular response against C. albicans.
Interleukin-15 (IL-15) is a recently discovered cytokine that shares many biological activities with IL-2 and requires both β and γ chains of the IL-2 receptor (IL-2R) for binding and signaling (1, 22). However, the IL-15R complex includes a specific α subunit (IL-15Rα), distinct from the IL-2Rα chain (11, 13). IL-15 stimulates the growth of activated T, B, and NK cells and tumor-infiltrating lymphocytes (2, 23, 24), acts as a chemoattractant for T lymphocytes (40), induces lymphokine-activated killer activity in NK cells, and induces the generation of cytolytic effector cells (6, 11). There is increasing evidence that IL-15 can also affect phagocytic cells. We have recently shown that IL-15 acts as a proinflammatory cytokine that induces monocytes to secrete IL-8 and monocyte chemotactic protein 1 (4), while other investigators have shown that it induces morphological changes and delays apoptosis in polymorphonuclear leukocytes (PMN) (20). One major difference between IL-2 and IL-15 is their cellular source. Whereas IL-2 is produced principally by T cells, IL-15 mRNA is present in macrophages and many nonlymphoid tissues including placenta, skeletal muscle, and epithelial and fibroblast cell lines (23). IL-15 expression is induced in macrophages by microbial activators such as lipopolysaccharide, mycobacteria, or Toxoplasma gondii (17, 30). These observations suggest that IL-15 may play a role in the activation of the immune response to infection. PMN form the first line of defense in the inflammatory response against invading pathogens. Neutropenia or PMN dysfunctions result in severe infections, including systemic candidiasis (33). Inflammatory reactions result in the production of cytokines such as tumor necrosis factor alpha, granulocyte colony-stimulating factor, IL-2, gamma interferon (IFN-γ), and IL-8, which further attract and activate incoming PMN (15, 16, 32). The main function of activated PMN is to phagocytose and kill microbial pathogens. However, there is evidence that they can also behave as a secondary source of cytokines (IL-8, IL-12, and TNF-α) which can have important autocrine and paracrine effects (12). In this study, we investigated the expression of the α chain of the IL-15R on PMN and the effect of IL-15 on IL-8 secretion, superoxide anion release, phagocytosis, and candidacidal activity. These studies are important to clarify the role of IL-15 in the early steps of the innate immune response to invading pathogens.
MATERIALS AND METHODS
PMN isolation.
Whole blood obtained from healthy donors after informed consent was diluted 1:2 with saline (0.9% NaCl), layered on Lymphoprep (Nycomed; Pharma AS, Oslo, Norway), and centrifuged at 400 × g for 30 min at room temperature. The PMN layer, on the surface of the erythrocyte cell pellet, was collected, and contaminating erythrocytes were lysed by hypotonic shock in sterile distilled water for 30 s at room temperature. The cells were washed twice in phosphate-buffered saline (PBS) before being adjusted to the desired concentration. All cell suspensions contained less than 1% monocytes as determined by monoesterase staining. Cell viability was greater than 95% by trypan blue exclusion immediately after isolation and after 6 and 18 h of incubation. The cells were cultured in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum with 2 mM l-glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 5 mM HEPES buffer (GIBCO Laboratories, Grand Island, N.Y.); this is referred to as complete medium. Therefore, unless otherwise indicated, all the experiments were performed with few or no opsonins present.
Cytokines and reagents.
Recombinant human IL-15 was kindly provided by Tony Troutt (Immunex Corp., Seattle, Wash.). To obtain human IL-15–mouse immunoglobulin G2b (IgG2b) fusion protein, cDNA encoding IL-15 was fused to genomic DNA encoding for the Fc portion of mouse IgG2b. Biotinylation of human IL-15–mouse IgG2b fusion protein resulted in higher stability of the cytokine without reduction of its biological activity as determined by the CTLL proliferation assay (10). Highly purified human IL-2 was kindly provided by Cetus Corp. (Emeryville, Calif.); recombinant human IL-8 and IFN-γ were from Peprotech (Rocky Hill, N.J.). fMLP (formyl-methionyl-leucyl-phenylalanine) was from Sigma Chemical Co. (St. Louis, Mo.). All reagents and media were shown to be free of endotoxin by using a standard Limulus amebocyte lysate LAL assay (BioWhittaker, Walkersville, Md.).
Flow cytometry analysis.
PMN were preincubated for 30 min at 4°C in PBS containing 2% goat serum plus 0.2% sodium azide, washed twice with 1% bovine serum albumin (BSA) in PBS, and incubated (2 × 105 cells in 50 μl of 1% BSA in PBS) for 60 min at 4°C with the biotinylated IL-15–IgG2b fusion protein (1.3 μg per sample) or with isotype-matched biotin-conjugated IgG (Pharmingen, San Diego, Calif.) with or without the addition of unlabeled IL-15 or IL-2. The cells were then washed twice with 1% BSA in PBS and further incubated with fluorescein isothiocyanate (FITC)-conjugated avidin (Sigma) for 20 min at 4°C. IL-15 binding was assessed by flow cytometry. To determine the expression of the α chains of the IL-15R and IL-2R, PMN were indirectly labeled for 20 min at 4°C with anti-IL-15Rα (clone M160; a kind gift of Tony Troutt, Immunex Corp., Seattle, Wash.) or anti-CD25 (Pharmingen) monoclonal antibodies (MAb) followed by washing and incubation with FITC-conjugated goat anti-mouse Ig. R 1-30 (anti-β2-microglobulin) (26) was used as a positive control. Labeled PMN were analyzed by flow cytometric analysis with a FACScan (Becton Dickinson, Immunocytometry System, San Jose, Calif.).
RT-PCR analysis.
RNA extraction and reverse transcription-PCR (RT-PCR) analysis were performed as previously described (9). Briefly, total RNA was purified with TRIzol (GIBCO/BRL) as specified by the manufacturer. cDNA synthesis was performed with 2 μg of RNA in a total volume of 20 μl containing 50 mM Tris-HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 0.1 M dithiothreitol, 40 U of RNase OUT RNase inhibitor (Life Technologies), 0.5 μg of oligo(dT), and 200 U of Superscript II reverse transcriptase (Life Technologies). The reaction mixture was incubated at 42°C for 50 min, and the reaction was stopped by heating at 90°C for 5 min. A 2-μl aliquot of the cDNA obtained was amplified in a 50-μl reaction mixture containing 500 mM KCl, 100 mM Tris-HCl (pH 8.8), 25 mM MgCl2, 2 mM each deoxynucleoside triphosphate, 2 mg of BSA (Pharmacia) per ml, 200 nM each primer, and 5 U of Taq DNA polymerase (Life Technologies). The mixture was capped with 50 μl of sterile mineral oil. To ensure that equivalent amounts of cDNA were used in each reaction, PCR was also performed for β-actin from each sample and the cDNA was adjusted to equivalent levels. The following oligonucleotides were used in the PCR: IL-15R sense (5′-GCCAGCGCCACCCTCCACAGTAA-3′) and IL-15R antisense (5′-GCCAGCGGGGGAGTTTGCCTTGAC-3′), with cycling conditions of 1 min at 94°C, 1 min at 80°C, and 2 min at 72°C for 35 cycles; and β-actin sense (5′-GAGCGGGAAATCGTGCGTGACATT-3′) and β-actin antisense (5′-GAAGGTAGTTTCGTGGATGCC-3′), with cycling conditions of 1 min at 94°C, 90 s at 62°C, and 2 min at 72°C for 27 cycles. A sample (15 μl) of each PCR mixture was electrophoresed through a 2% agarose gel and visualized with ethidium bromide.
Culture of C. albicans.
Candida albicans CA2 was kindly supplied by A. Cassone (Istituto Superiore di Sanità, Rome, Italy) and was grown by weekly transfer onto fresh Sabouraud dextrose agar (Biolife, Milan, Italy). CA2 is an agerminative strain and grows as a pure yeast form in vitro at 28 or 37°C in conventional mycologic media (27). For activation by microbial antigen, C. albicans was washed in PBS, pelleted by centrifugation at 400 × g for 10 min, and killed by heating in boiling water for 10 min.
Measurement of IL-8 protein production.
IL-8 in culture supernatants was determined by using a commercially available enzyme-linked immunosorbent assay (Amersham, Little Chalfont, England) that allows the detection of IL-8 above 30 pg/ml.
Superoxide anion production.
Determination of superoxide anion release by PMN was based on a sensitive assay that utilizes dihydrorhodamine 123 (DHR-123) and was performed essentially as described previously (35). Briefly, PMN (2 × 105 cells in 0.2 ml of medium) were preincubated at 37°C for 15 min with the fluorescent probe DHR-123 (Molecular Probes, Inc., Princeton, N.J.) and with catalase (Sigma) before being subjected to stimulation as described in Results. The fluorescence of DHR-123-loaded cells did not substantially increase because of the intrinsic fluorescence of heat-inactivated Candida cells; Candida particles have a spontaneous fluorescence much lower than that of DHR-123-loaded PMN (31).
After 30 min, the cells were analyzed with a FACScan. At least 5,000 events were measured, and on the basis of forward and side scatter, the window for neutrophil-gated cells was set. Although this assay does not provide quantitative data, the extent of superoxide anion production can be estimated on the basis of the increase in green fluorescence intensity of stimulated PMN in comparison to resting cells (36). To calculate the percentage of cells that converted DHR-123, a cutoff level was settled on the basis of the intrinsic fluorescence of resting PMN.
Phagocytosis.
Heat-killed C. albicans was fluorescein labeled as previously described (31). Briefly, yeasts were resuspended in carbonate buffer (pH 10) containing 1 mg of FITC per ml and incubated at room temperature for 2 h. FITC-labeled Candida cells were separated from free fluorescein by extensive washing with PBS. PMN (106 cells/ml) were incubated (10:1) with FITC-labeled Candida cells for 30 min at 37°C, washed twice, and kept at 4°C in PBS until examined by flow cytometry (FACScan). When necessary, C. albicans cells opsonized by incubation in 50% AB human serum at 37°C for 30 min and washed with PBS. Cells kept at 4°C with FITC-labeled C. albicans were used as a negative control because they do not phagocytose the organisms, as confirmed in parallel examinations by fluorescence microscopy. PMN were gated on the basis of their light-scattering properties; at least 10,000 events were acquired. To differentiate uningested or cell surface-adherent organisms from ingested organisms, ethidium bromide was added at 50 μg/ml. In fact, ingested particles of FITC-C. albicans maintained their green fluorescence whereas particles bound to the cell surface but not internalized were quenched by addition of the red fluorochrome (18). The extent of phagocytosis was assessed as the increase of the mean channel fluorescence of total PMN. An aliquot of each sample was cytocentrifuged and stained with Giemsa for a visual check of phagocytosis under a light microscope.
Growth inhibition of C. albicans.
To perform growth inhibition experiments, PMN were resuspended in complete medium at 2 × 106 cells/ml, dispensed in triplicate (100 μl/well) in 96-well microtiter tissue culture plates, and treated with increasing amounts (10 to 1,000 ng/ml) of human recombinant IL-15 or IL-2 (1,000 U/ml). IFN-γ (500 U/ml) was used as a positive control. After a 3-h incubation, C. albicans yeast was added to achieve the effector-to-target ratio of 10:1. Preliminary experiments performed in our laboratory showed that optimal sensitivity was achieved with an effector-to-target ratio of 10:1 (data not shown). C. albicans was also added to six wells without effector cells to serve as controls. After a 3-h incubation at 37°C, Triton X-100 (Sigma) (final concentration, 0.1%) was added to the wells. Serial dilutions from each well were then made in distilled water and plated (quadruplicate samples) on Sabouraud dextrose agar. After a 24-h incubation at 37°C, the colonies were counted, and the results were expressed as percent CFU inhibition, according to the formula (1 - CFU in samples/CFU without PMN) × 100.
Statistical analysis.
Comparison among treatments was performed by Student’s t test or by analysis of variance as appropriate. When a difference among multiple treatments was found, the Newman-Keuls multiple-comparison test was used to identify which of the means were significantly different from the others at the 0.05 significance level.
RESULTS
IL-15 binding to PMN.
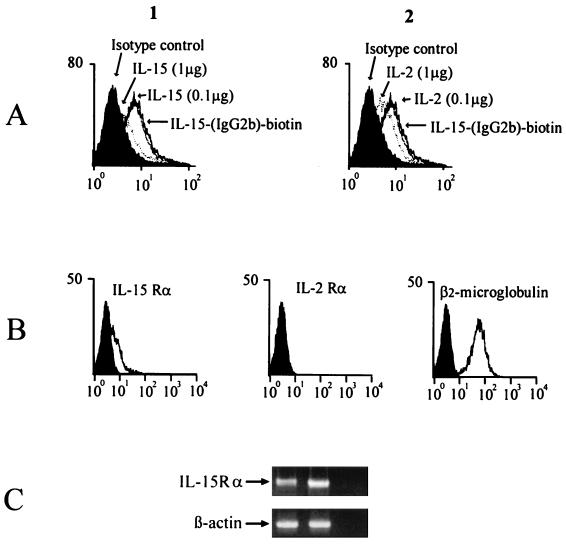
To assess IL-15 binding, PMN were incubated with biotinylated human IL-15–IgG2b fusion protein, alone or in the presence of increasing concentrations of human recombinant IL-15 (rIL-15) or human rIL-2 (Fig. 1A). PMN incubated with IL-15–IgG2b fusion protein showed a significant increase in green fluorescence compared to cells incubated with control IgG (P < 0.05). Both IL-15 and IL-2 can inhibit the binding of IL-15–IgG2b fusion protein to PMN. Addition of a two fold excess (1 μg) of unlabeled recombinant IL-15 was approximately twice as effective as addition of an equal amount of IL-2 in blocking the fusion protein binding (24 and 12% differences, respectively, in median channel value shift to the left). These results suggest that unstimulated PMN express IL-15-binding sites and that both IL-2Rβ and IL-2Rγ may form part of the IL-15R complex. Besides β and γ chains, IL-15R includes a distinct and specific IL-15Rα chain which has been described recently (22). We studied PMN expression of the IL-15Rα subunit at both RNA and protein levels (Fig. 1B and C). By RT-PCR, IL-15Rα mRNA was detected in unstimulated PMN. By FACS analysis with a specific anti-IL-15Rα chain MAb, the IL-15Rα chain was expressed at a detectable level by ≈20% of unstimulated PMN.
FIG. 1.
PMN expression of the IL-15 receptor. (A) Binding of IL-15 IgG 2b fusion protein to PMN. PMN were incubated with biotin-conjugated IL-15 IgG2b fusion protein or with an equal amount of isotype-matched biotinylated IgG (dark area) followed by staining with streptavidin-FITC. Different amounts of IL-15 (panel 1) or IL-2 (panel 2) were added to PMN incubated with biotin-conjugated IL-15 IgG2b fusion protein. The x axis represents the intensity of green fluorescence expressed in a log scale as mean channel, and the y axis represents the number of cells per channel. (B) IL-15Rα expression determined by FACS analysis. PMN were stained with MAb M160 (anti-IL-15Rα) anti-CD25, or anti-β2-microglobulin. (C) IL-15Rα mRNA expression. cDNA derived from PMN (left lane) or mitogen-activated PBL (middle lane) were amplified with primers specific for the IL-15Rα chain or β-actin, used as positive control. The right lane represents the negative control.
Effects of IL-15 on IL-8 production by PMN.
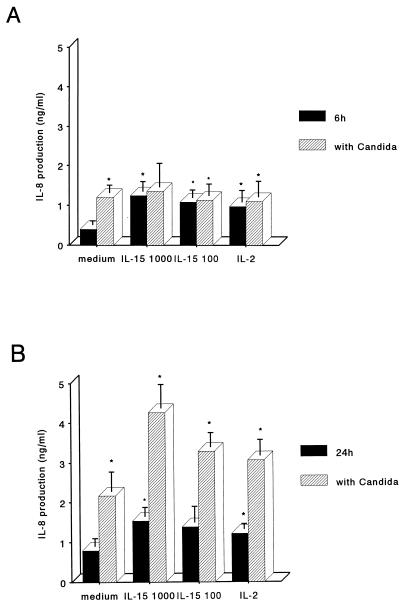
PMN activation in response to IL-15 was evaluated by measuring IL-8 production by PMN. As shown in Fig. 2, a basal level of IL-8 was detectable in supernatants of PMN cultured in complete medium. The addition of IL-15 at concentrations as low as 100 ng/ml led to increased IL-8 release after 6 h of stimulation, and greater amounts were detectable after 24 h. We then studied the effects of C. albicans on IL-8 release following PMN activation by IL-15. Heat-inactivated C. albicans alone was as potent as 1,000 ng of IL-15 per ml. PMN treated with IL-15 and heat-killed C. albicans released significantly greater amounts of IL-8. These results indicate that IL-15 synergizes with inactivated C. albicans in inducing IL-8 release by PMN.
FIG. 2.
IL-8 production by IL-15-stimulated PMN. Cells were stimulated with different concentrations of IL-15 (100 or 1,000 ng/ml), with IL-2 (50 or 1,000 U/ml), or with medium alone with or without the addition of heat-inactivated Candida cells. Supernatants were collected after 6 h (A) or 24 h (B) and assayed for IL-8 by enzyme-linked immunosorbent assay. IL-8 concentrations are presented on the y axis as the mean ± SE of values from three experiments. Asterisks indicate a significant increase of IL-8 production (P < 0.05).
Effects of IL-15 on superoxide anion generation by PMN.
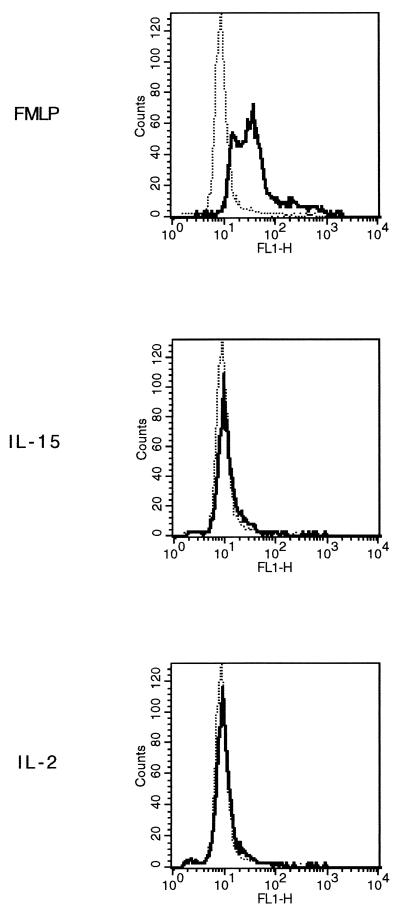
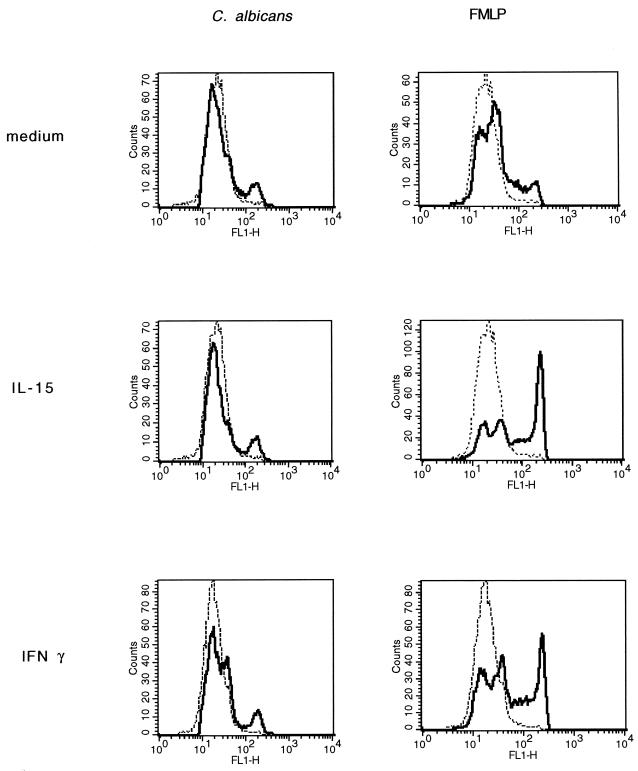
Cytokines stimulate PMN by inducing the secretion of proinflammatory cytokines and by activating their antimicrobial oxidative pathway (3, 33). To determine whether IL-15 stimulates superoxide anion production by PMN, we used a fluorescent probe (DHR-123) that increases its green fluorescence when exposed to reactive oxygen (35). PMN were preincubated with DHR-123 and then stimulated with IL-15 (1,000 ng/ml), IL-2 (1,000 U/ml), fMLP (10 nM), or medium alone. After 30 min, the fluorescence intensity of resting and stimulated PMN was assessed by FACS. As shown in Fig. 3, the percentages (mean ± standard error [SE]) of positive PMN from four independent donors increased from 3% ±2% to 89% ±4% upon fMLP stimulation (P < 0.05), while neither IL-15 nor IL-2 induced superoxide anion production. These results were confirmed by the cytochrome c reduction method (data not shown). In addition, to examine if IL-15 synergized with either fMLP or C. albicans in superoxide anion release, PMN were incubated for 3 h with IL-15 (1,000 ng/ml), IFN-γ (500 U/ml), or medium alone. The cells were then loaded with DHR-123 and stimulated with heat-killed C. albicans or 10 nM fMLP for 30 min. As shown in Fig. 4, fMLP induced superoxide anion production in PMN preincubated with medium. The percentages (mean ± SE) of positive cells from three separate donors increased from 2% ±1% to 25% ±7% upon fMLP stimulation (P < 0.05). C. albicans alone could also induce detectable levels of superoxide, but to a lower extent (2% ± 1% and 8% ± 4%, respectively). Priming of PMN with IL-15 significantly augmented the PMN oxidative burst in response to fMLP (50% ± 5% versus 25% ± 7%; P < 0.05) but not to C. albicans (11% ± 3% versus 8% ± 4). IFN-γ, used as control, was able to prime PMN for additional induction of the oxidative metabolite with either fMLP (53% ± 11% versus 25% ± 7%; P < 0.05) or C. albicans, although to a lower extent (19% ± 3% versus 8% ± 4%; P < 0.05).
FIG. 3.
Induction of the respiratory burst in IL-15-stimulated PMN. PMN preincubated with DHR-123 were stimulated at 37°C with fMLP (10 nM), IL-15 (1,000 ng/ml), IL-2 (1,000 U/ml), or medium alone (dotted lines) for 30 min, and green fluorescence was assessed by FACS. The x axis represents the intensity of green fluorescence expressed in a log scale as mean channel, and the y axis represents the number of cells per channel.
FIG. 4.
Priming of the respiratory burst by IL-15. PMN were preincubated at 37°C with IL-15 (1,000 ng/ml), IFN-γ (500 U/ml), or medium alone for 3 h. They were then loaded with DHR-123 and stimulated with heat-killed Candida (PMN-to-Candida ratio, 10:1), fMLP (10 nM), or medium alone. After 30 min, superoxide anion production was evaluated by flow cytometry as an increase in green fluorescence intensity. The x axis represents the intensity of green fluorescence expressed in a log scale as mean channel, and the y axis represents the number of cells per channel.
Enhancement of C. albicans phagocytosis by IL-15.
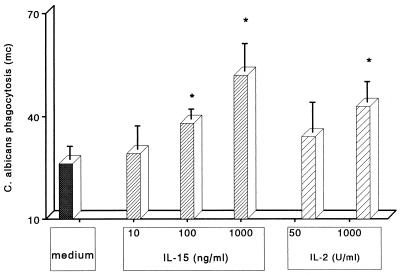
We next investigated whether IL-15 stimulation of PMN increased the rate of phagocytosis of C. albicans. PMN were preincubated with increasing concentrations of IL-15 (10 to 1,000 ng/ml), IL-2 (50 or 1,000 U/ml), or medium alone; after 30 min, heat-inactivated FITC-conjugated C. albicans was added. As shown in Fig. 5, IL-15 induced a dose-dependent increase in phagocytosis by PMN, as indicated by the increase in the green fluorescence mean channel from three independent experiments. As previously reported, IL-2 did not induce a significant increase in PMN phagocytosis at 50 U/ml (19) but was active at 1,000 U/ml. The percentage (mean ± SE) of PMN that phagocytosed C. albicans increased from 45% ± 3% to 66% ± 4% upon IL-15 stimulation or to 56% ±3% upon IL-2 stimulation. These results indicate that IL-15 significantly increased the number of phagocytosing cells (P < 0.05) as well as the rate of phagocytosis. In the presence of opsonins, basal phagocytosis was high in resting cells (82% ± 4%), but it increased in IL-15-stimulated PMN (94% ± 1%) (P < 0.05); the mean channel fluorescence increased from 60 ± 6 to 180 ± 10 (P < 0.05).
FIG. 5.
Phagocytosis of heat-inactivated Candida by PMN stimulated with IL-15. PMN were preincubated with different concentrations of IL-15 (from 10 to 1,000 ng/ml), IL-2 (50 or 1,000 U/ml), or medium alone for 3 h. Heat-inactivated FITC-labeled Candida cells were then added to the cultures at a PMN-to-Candida ratio of 10:1, and the cultures were further incubated for 30 min at 37°C before the extent of phagocytosis was assessed by flow cytometry in the presence of ethidium bromide. The extent of phagocytosis is represented on the y axis as the mean ± SE of green fluorescence mean channel from three independent experiments. Asterisks indicate a significant increase in phagocytosis (P < 0.05).
Enhancement of PMN candidacidal activity by IL-15.
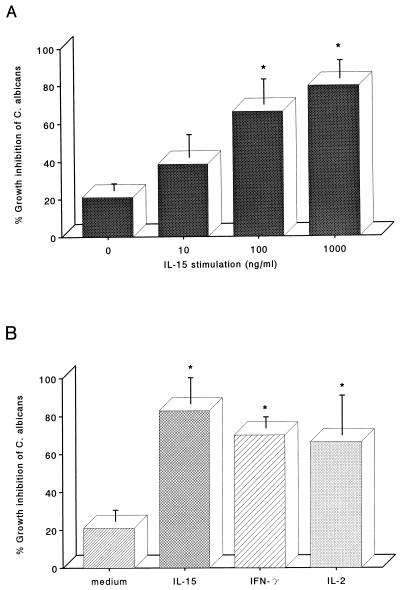
To assess the effect of IL-15 on the antifungal activity of PMN, cells were incubated for 3 h with increasing concentrations of IL-15 (10 to 1,000 ng/ml) before the addition of C. albicans. Untreated PMN showed low levels of antifungal activity, which were increased by IL-15 treatment (Fig. 6A). PMN activation by IL-15 was dose dependent; concentrations as low as 100 ng/ml were sufficient to increase the killing of yeasts by PMN. The effect of IL-15 on the antifungal activity of PMN was compared with the effect of other PMN stimulants such as IFN-γ and IL-2. PMN were preincubated with IFN-γ (500 U/ml), IL-2 (1,000 U/ml), or IL-15 (1,000 ng/ml), of concentrations known to induce maximal PMN activation. The PMN were then incubated with C. albicans for assessment of function. Figure 6B shows that IL-15 had similar potency to IFN-γ and IL-2.
FIG. 6.
Enhancement of PMN anti-Candida activity by IL-15. (A) PMN were incubated with increasing concentrations of IL-15 (from 10 to 1,000 ng/ml) for 3 h, exposed to C. albicans, and assayed for anti-Candida activity as described in the text. (B) PMN were stimulated for 3 h with IFN-γ (500 U/ml), IL-2 (1,000 U/ml), IL-15 (1,000 ng/ml), or medium alone before their anti-Candida activity was assessed. Results represent mean percent growth inhibition ± SE for three independent experiments. Asterisks indicate a significant increase in the anti-Candida activity (P < 0.05).
DISCUSSION
In this paper, we demonstrate that IL-15 enhances the response of PMN to the yeast C. albicans. Our binding experiments have shown that both IL-15 and IL-2 partially inhibited the binding of IL-15–IgG2b fusion protein to PMN, suggesting that the receptor complexes of both of these cytokines involve the IL-2Rβ and IL-2Rγ chains, as previously reported (21). However, IL-2 and IL-15 utilize different α-chain receptor subunits (11, 22). By using specific MAbs, we showed that, unlike the IL-2Rα chain, the IL-15Rα chain is detectable on unstimulated PMN, although at a low level (15, 25). Taken together, these results can account for the fact that IL-2 competed less than IL-15 in our binding experiments. They also corroborate the hypothesis of Girard et al., which proposes that PMN express a specific IL-15Rα chain, as suggested by the different effects of the two cytokines in the induction of morphological alterations and in the delay of apoptosis in PMN (20).
PMN microbicidal action involves both oxidative and nonoxidative pathways. Reactive oxygen radicals are released when PMN interact with microbial pathogens or are stimulated via G-protein-coupled receptors (e.g., fMLP receptor) (3, 33). Cytokines such as IFN-γ or G-CSF alone do not induce the respiratory burst, but preincubation of PMN with IFN-γ or G-CSF primes cells for the induction of oxygen radicals by fMLP or by C. albicans. IL-15 was also effective in enhancing the oxidative respiratory burst in response to fMLP, although it failed to induce superoxide anions by itself and to prime PMN for Candida induction of the oxygen radicals. This suggests that either the IL-15 enhancement of PMN anti-Candida activity is mediated through a nonoxidative pathway or the priming effect is not detectable under our experimental conditions. IL-2 is equally effective in the activation of antifungal functions of PMN (15). As reported by Djeu et al. (15), IL-2-mediated anti-Candida activity is not related to activation of the metabolic burst because IL-2 does not display any priming activity on the respiratory burst elicited by fMLP. These results indicate that IL-2 and IL-15, although sharing activating properties on PMN, might utilize distinct stimulatory pathways, probably because these two cytokines may activate common receptor components such as the β and γ chains of the IL-2R or distinct receptor subunits like the IL-15Rα chain.
Phagocytosis is considered to be a required step for the intracellular killing of the vegetative form of Candida. We have shown that IL-15 induces a dose-dependent increase of phagocytosis of C. albicans at the same concentration range at which this cytokine induces PMN candidacidal activity. This result suggests that IL-15 may stimulate the phagocytosis and the consequent intracellular killing of yeast involving nonoxidative mechanisms such as lysosomal antimicrobial peptides and enzymes. We have indirect evidence that the activation of PMN by IL-15 may be associated with the release of antimicrobial peptides (e.g., defensins, CAP37/azurocidin, and lactoferrin) (8). In fact, we observed that PMN activated by IL-15 display an increased expression of CD11b, a marker of specific granules, suggesting that IL-15 probably induces neutrophil degranulation (29a). Another possible mechanism that might be involved in PMN activation by IL-15 is dependent on the expression of the inducible enzyme nitric oxide synthase (iNOS). Although resting PMN do not express iNOS, IL-15 may be capable of inducing its expression. However, in other cell types, the kinetics of iNOS expression are very different from those observed for the anti-Candida activity of IL-15 (28, 38, 39, 41). Besides its antimicrobial activities, IL-15 has proinflammatory properties that may enhance the extent of its anti-Candida activity in vivo. We showed that IL-15 induces the secretion of the neutrophil chemotactic factor IL-8 within 6 h of stimulation. IL-8 is secreted mostly by monocytes and endothelial cells, but it is also expressed, in smaller amounts, by PMN (7, 37). We recently reported that IL-15 stimulates monocytes to express IL-8 at both the mRNA and protein levels in the same concentration range that is effective in neutrophil activation (4). This suggests that the production of IL-15 in the early steps of the inflammatory response to pathogens might increase the amount of PMN infiltrating the tissues. Besides being a chemotactic factor for PMN, IL-8 itself stimulates antimicrobial functions by enhancing degranulation and anti-Candida activity of PMN (5, 16). Since IL-15 induced IL-8 secretion, we tested the possibility that IL-8 mediates the enhancement of anti-Candida activity induced by IL-15; however, we could not detect any change of IL-15 activity on PMN when IL-8 neutralizing antibodies were added to the culture (data not shown). These results suggest that under our experimental conditions the antifungal activity of IL-15 did not depend on IL-8 secretion. This does not contradict the observed antifungal activity of IL-8, because the concentrations of IL-8 that we detected after 6 h of stimulation with IL-15 are not optimal for activation of PMN anti-Candida activity (16). Upon longer stimulation in vitro or in vivo, IL-8 secretion might be important for the anti-Candida effect of IL-15, but it is likely that in our experimental setting, IL-15 directly activated PMN against C. albicans. Unlike IL-2, IL-15 expression is induced in macrophages by microbial activators such as LPS, mycobacteria, or Toxoplasma gondii (17). We are currently investigating the mechanisms that regulate IL-15 expression by monocytes upon infection with fungi or other microbial pathogens. We found that C. albicans up-regulates IL-15 mRNA expression in human monocytes (29b). Taken as a whole, these results suggest that IL-15 may be expressed in the early steps of the aspecific immune response to bacteria and yeasts. IL-15 produced by activated tissue-resident macrophages or by fibroblasts may activate PMN recruited to the site of infection to kill pathogens and determine an additional infiltration of PMN through IL-8 release. In addition, IL-15, which is chemotactic for T cells, may induce T-cell infiltration and maintain their proliferative response to Candida antigens. However, in vivo, IL-15 anti-Candida activity may be biased by other factors such as cytokines, Igs, or complement factors that could affect the response to IL-15; indeed, Vazquez et al. have recently reported that IL-15 enhances monocyte activity against opsonized Candida cells (34). Our results suggest that IL-15 may play a significant role in human innate immunity against C. albicans and may offer an adjunct for use in the prevention and treatment of fungal infections in immunocompromised patients with chronic granulomatous disease (CGD), a genetically inherited disease characterized by increased susceptibility to fungal infections (14). This is dependent on the lack of superoxide anion production by phagocytic cells because of mutations of genes encoding the subunits of NADPH oxidase. In patients with CGD, IFN-γ is currently used for prophylaxis of infections. On the basis of our results, IL-15 might potentiate the antimicrobial functions of PMN by a nonoxidative pathway in CGD patients. It will be possible to test this hypothesis since animal models of CGD have recently become available (29).
ACKNOWLEDGMENTS
We thank Antonio Cassone for helpful discussions.
This work was partly supported by a grant from the First National Project on Tuberculosis, (contract 783, Istituto Superiore di Sanità, Rome, Italy) and by the Italian Ministry of University and Scientific Research (60% grant). R.B. was supported by a fellowship from Telethon, (Rome, Italy).
REFERENCES
- 1.Anderson D M, Kumaki S, Ahdieh M, Bertles J, Tometsko M, Loomis A, Giri J, Copeland N G, Gilbert D J, Jenkins N A, et al. Functional characterization of the human interleukin-15 receptor alpha chain and close linkage of IL15RA and IL2RA genes. J Biol Chem. 1995;270:29862–29869. doi: 10.1074/jbc.270.50.29862. [DOI] [PubMed] [Google Scholar]
- 2.Armitage R J, Macduff B M, Eisenman J, Paxton R, Grabstein K H. IL-15 has stimulatory activity for the induction of B cell proliferation and differentiation. J Immunol. 1995;154:483–490. [PubMed] [Google Scholar]
- 3.Babior B M. Phagocytic defects. Hematol Oncol Clin North Am. 1988;2:201–237. [PubMed] [Google Scholar]
- 4.Badolato R, Negro Ponzi A, Millesimo M, Notarangelo L D, Musso T. Interleukin-15 (IL-15) induces IL-8 and monocyte chemotactic protein 1 production in human monocytes. Blood. 1997;90:2804–2809. [PubMed] [Google Scholar]
- 5.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 6.Bamford R N, Grant A J, Burton J D, Peters C, Kurys G, Goldman C K, Brennan J, Roessler E, Waldmann T A. The interleukin (IL) 2 receptor beta chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci USA. 1994;91:4940–4944. doi: 10.1073/pnas.91.11.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bazzoni F, Cassatella M A, Rossi F, Ceska M, Dewald B, Baggiolini M. Phagocytosing neutrophils produce and release high amounts of the neutrophil-activating peptide 1/interleukin 8. J Exp Med. 1991;173:771–774. doi: 10.1084/jem.173.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borregaard N, Cowland J B. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89:3503–3521. [PubMed] [Google Scholar]
- 9.Bulfone-Paus S, Durkop H, Paus R, Krause H, Pohl T, Onu A. Differential regulation of human T lymphoblast functions by IL-2 and IL-15. Cytokine. 1997;9:507–513. doi: 10.1006/cyto.1996.0194. [DOI] [PubMed] [Google Scholar]
- 10.Bulfone-Paus S, Ungureanu D, Pohl T, Lindner G, Krause H, Paus R, Kunzendorf U. Interleukin-15 protects from lethal apoptotic cell death in mice. Nat Med. 1997;3:1124–1128. doi: 10.1038/nm1097-1124. [DOI] [PubMed] [Google Scholar]
- 11.Carson W E, Giri J G, Lindemann M J, Linett M L, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, Caligiuri M A. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cassatella M A. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–26. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 13.Chae D W, Nosaka Y, Strom T B, Maslinski W. Distribution of IL-15 receptor α chains on human peripheral blood mononuclear cells and effect of immunosuppressive drugs on receptor expression. J Immunol. 1996;157:2819. [PubMed] [Google Scholar]
- 14.Cohen M S, Isturiz R E, Malech H L, Root R K, Wilfert C M, Gutman L, Buckley R H. Fungal infection in chronic granulomatous disease. The importance of the phagocyte in defence against fungi. Am J Med. 1981;71:59–66. doi: 10.1016/0002-9343(81)90259-x. [DOI] [PubMed] [Google Scholar]
- 15.Djeu J Y, Liu J H, Wei S, Rui H, Pearson C A, Leonard W J, Blanchard D K. Function associated with IL-2 receptor-β on human neutrophils. J Immunol. 1993;150:960–970. [PubMed] [Google Scholar]
- 16.Djeu J Y, Matsushima K, Oppenheim J J, Shiotsuki K, Blanchard D K. Functional activation of human neutrophils by recombinant monocyte-derived neutrophil chemotactic factor/IL-8. J Immunol. 1990;144:2205–2210. [PubMed] [Google Scholar]
- 17.Doherty T M, Seder R A, Sher A. Induction and regulation of IL-15 expression in murine macrophages. J Immunol. 1996;156:735–741. [PubMed] [Google Scholar]
- 18.Fattorossi A, Nisini R, Pizzolo J G, D’Amelio R. New, simple flow cytometry technique to discriminate between internalized and membrane-bound particles in phagocytosis. Cytometry. 1989;10:320–325. doi: 10.1002/cyto.990100311. [DOI] [PubMed] [Google Scholar]
- 19.Girard D, Gosselin J, Heitz D, Paquin R, Beaulieu A D. Effects of interleukin-2 on gene expression in human neutrophils. Blood. 1995;88:1170–1176. [PubMed] [Google Scholar]
- 20.Girard D, Paquet M, Paquin R, Beaulieu A D. Differential effects of interleukin-15 (IL-15) and IL-2 on human neutrophils: modulation of phagocytosis, cytoskeleton rearrangement, gene expression, and apoptosis by IL-15. Blood. 1996;88:3176–3184. [PubMed] [Google Scholar]
- 21.Giri J G, Ahdieh M, Eisenman J, Shanebeck K, Grabstein K, Kumaki S, Namen A, Park L S, Cosman D, Anderson D. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 1994;13:2822–2830. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giri J G, Kumaki S, Ahdieh M, Friend D J, Loomis A, Shanebeck K, DuBose R, Cosman D, Park L S, Anderson D M. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J. 1995;14:3654–3663. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grabstein K H, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn M A, Ahdieh M, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 24.Lewko W M, Smith T L, Bowman D J, Good R W, Oldham R K. Interleukin-15 and the growth of tumor-derived activated T-cells. Cancer Biother. 1995;10:13–20. doi: 10.1089/cbr.1995.10.13. [DOI] [PubMed] [Google Scholar]
- 25.Liu J H, Wei S, Ussery D, Epling-Burnette P K, Leonard W J, Djeu J Y. Expression of interleukin-2 receptor γ chain on human neutrophils. Blood. 1994;84:3870–3875. [PubMed] [Google Scholar]
- 26.Malavasi F, Calligaris-Cappio F, Dellabona P, Richiardi P, Carbonara A O. Characterization of a murine monoclonal antibody specific for human early lymphohemopoietic cells. Hum Immunol. 1984;9:9. doi: 10.1016/0198-8859(84)90003-x. [DOI] [PubMed] [Google Scholar]
- 27.Mattia E, Carruba G, Angiolella L, Cassone A. Induction of germ tube formation by N-acetyl-d-glucosamine in Candida albicans: uptake of inducer and germinative response. J Bacteriol. 1982;152:555–562. doi: 10.1128/jb.152.2.555-562.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melillo G, Taylor L S, Brooks A, Cox G W, Varesio L. Regulation of inducible nitric oxide synthase expression in IFN-γ-treated murine macrophages cultured under hypoxic conditions. J Immunol. 1996;157:2638–2644. [PubMed] [Google Scholar]
- 29.Morgesten D E, Gifford M A, Doerschuk C M, Dinauer M C. Absence of respiratory burst in X-linked chronic granulomatous disease mice leads to abnormalities in both host defense and inflammatory response to Aspergillus fumigatus. J Exp Med. 1997;185:207–218. doi: 10.1084/jem.185.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29a.Musso, T., et al. Unpublished data.
- 29b.Musso, T., et al. Unpublished data.
- 30.Nishimura H, Hiromatsu K, Kobayashi N, Grabstein K H, Paxton R, Sugamura K, Bluestone J A, Yoshikai Y. IL-15 is a novel growth factor for murine gamma delta T cells induced by Salmonella infection. J Immunol. 1996;156:663–669. [PubMed] [Google Scholar]
- 31.Perticari S, Presani G, Mangiarotti M G, Banfi E. Simultaneous flow cytometric method to measure phagocytosis and oxidative products by neutrophils. Cytometry. 1991;12:687–691. doi: 10.1002/cyto.990120713. [DOI] [PubMed] [Google Scholar]
- 32.Peveri P, Waltz A, Dewal B, Baggiolini M. A novel neutrophil activating factor produced by human mononuclear phagocytes. J Exp Med. 1988;167:1883–1885. doi: 10.1084/jem.167.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas E L, Lehrer R I, Rest R F. Human neutrophil antimicrobial activity. Rev Infect Dis. 1988;10:S450–451. doi: 10.1093/cid/10.supplement_2.s450. [DOI] [PubMed] [Google Scholar]
- 34.Vazquez N, Walsh T J, Friedman D, Chanock S J, Lyman C A. Interleukin-15 augments superoxide production and microbicidal activity of human monocytes against Candida albicans. Infect Immun. 1998;66:145–150. doi: 10.1128/iai.66.1.145-150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vowells S J, Sekhsaria S, Malech H L, Shalit M, Fleisher T A. Flow cytometric analysis of the granulocyte respiratory burst: a comparison study of fluorescent probes. J Immunol Methods. 1995;178:89–97. doi: 10.1016/0022-1759(94)00247-t. [DOI] [PubMed] [Google Scholar]
- 36.Waddel T K, Fialkow L, Chan C K, Kishimoto T K, Downey G P. Potentiation of the oxidative burst of human neutrophils. A signaling role for L-selectin. J Biol Chem. 1994;269:18485–18491. [PubMed] [Google Scholar]
- 37.Wei S, Liu J D, Blanchard D K, Djeu J Y. Induction of IL-8 gene expression in human polymorphonuclear neutrophils by recombinant IL-2. J Immunol. 1994;152:3630–3636. [PubMed] [Google Scholar]
- 38.Weinberg J B, Misukonis M A, Shami P J, Mason S N, Sauls D L, Dittman W A, Wood E R, Smith G K, McDonald B, Bachus K E, Haney A F, Granger D L. Human mononuclear phagocyte inducible nitric oxide synthase (iNOS): analysis of iNOS mRNA, iNOS protein, biopterin, and nitric oxide production by blood monocytes and peritoneal macrophages. Blood. 1995;86:1184–1195. [PubMed] [Google Scholar]
- 39.Wheeler M A, Shannon D S, Garcia-Cardena G, Nathan C F, Weiss R M, Sessa W C. Bacterial infection induces nitric oxide synthase in human neutrophils. J Clin Invest. 1997;99:110–116. doi: 10.1172/JCI119121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilkinson P C, Liew F Y. Chemoattraction of human blood T lymphocytes by interleukin-15. J Exp Med. 1995;181:1255–1259. doi: 10.1084/jem.181.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan L, Vandivier W R, Suffredini A F, Danner R L. Human polymorphonuclear leukocytes lack detectable nitric oxide synthase activity. J Immunol. 1994;153:1825–1834. [PubMed] [Google Scholar]