Abstract
A State of the Art lecture titled “Immunothrombosis in Neurovascular Diseases” was presented at the International Society on Thrombosis and Haemostasis Congress in 2023. Despite significant clinical advancements in stroke therapy, stroke remains a prominent contributor to both mortality and disability worldwide. Brain injury resulting from an ischemic stroke is a dynamic process that unfolds over time. Initially, an infarct core forms due to the abrupt and substantial blockage of blood flow. In the subsequent hours to days, the surrounding tissue undergoes gradual deterioration, primarily driven by sustained hypoperfusion, programmed cell death, and inflammation. While anti-inflammatory strategies have proven highly effective in experimental models of stroke, their successful translation to clinical use has proven challenging. To overcome this translational hurdle, a better understanding of the distinct immune response driving ischemic stroke brain injury is needed. In this review article, we give an overview of current knowledge regarding the immune response in ischemic stroke and the contribution of immunothrombosis to this process. We discuss therapeutic approaches to overcome detrimental immunothrombosis in ischemic stroke and how these can be extrapolated to other neurovascular diseases, such as Alzheimer’s disease and multiple sclerosis. Finally, we summarize relevant new data on this topic presented during the 2023 International Society on Thrombosis and Haemostasis Congress.
Keywords: neutrophil extracellular traps, neutrophils, platelets, stroke, thrombosis
1. Introduction
Stroke is one of the leading causes of death and disability in the world [1]. Ischemic stroke accounts for approximately 70% of all strokes and occurs when a blood clot obstructs blood flow to the brain [2]. Since neurons are very sensitive to even transient periods of ischemia, the mainstay of ischemic stroke therapy is focused on rapid removal of the occlusive thrombus [3]. Depending on the location and size of the thrombus, patients who experience stroke and are eligible for therapy either receive thrombolytic therapy or undergo an endovascular procedure to restore blood flow to the brain. Unfortunately, a majority of patients who experience stroke are ineligible for reperfusion therapy [3]. Furthermore, stroke survivors are at a high risk for recurrent stroke and have higher chances of developing dementia [4].
The brain injury associated with ischemic stroke is dynamic and evolves over time [5]. Initially, an infarct core will form, caused by the direct and substantial blockade of blood flow. In the next hours to days, the surrounding tissue will gradually deteriorate as a consequence of continued hypoperfusion, programmed cell death, and inflammation. Importantly, this process can also occur in patients who initially achieve full restoration of blood flow (reperfusion). Indeed, extensive preclinical and clinical evidence has reported that inflammation increases stroke risk and aggravates stroke outcomes irrespective of reperfusion therapy [6]. In animal models of stroke, blocking excessive inflammation has been shown to be very effective [7,8]. However, anti-inflammatory approaches have been hard to translate to the clinic. Approximately 30% of patients who experience stroke develop an infection in the first days after stroke, and this is associated with a higher risk of poor outcome or death [[9], [10], [11]]. It is, therefore, not inconceivable that therapeutic interventions targeting the immune system come with a risk that is overlooked in preclinical stroke models using young and healthy rodents. To overcome this translational hurdle, a better understanding of the distinct immune response driving ischemic stroke brain injury is needed.
In this review article, we summarize the state of the art on the immune response contributing to ischemic stroke pathophysiology with a focus on immunothrombosis, the intricate interplay between inflammation and thrombus formation.
2. Brain Neutrophil Recruitment in the Acute Phase of Stroke
It is well recognized that brain ischemia induces a strong inflammatory phenotype that is associated with secretion of a variety of inflammatory proteins attracting and activating leukocytes [12]. This process happens very quickly, already in the early moments of ischemia and changes over time. Early on, the inflammatory response is neutrophil-driven and associated with worsened outcomes (Figure 1) [[13], [14], [15]]. As time passes, the predominant infiltrating immune cells change to monocytes and macrophages, which assume a reparative phenotype [16,17]. This phenotype is strongly driven by microglial cells, the brain resident macrophages. Reactive microglia engulf neutrophils within the ischemic lesion, serving as a defense mechanism against the damaging effects of neutrophils in the ischemic brain [18,19].
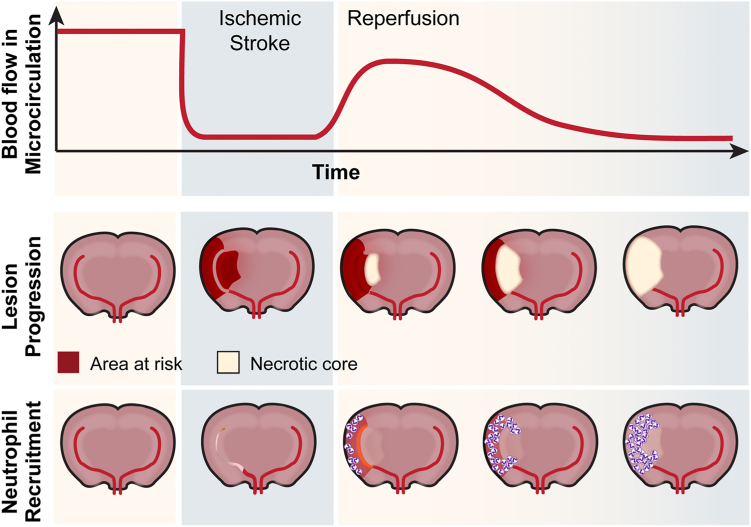
Figure 1.
Neutrophil recruitment during cerebral ischemia-reperfusion injury leads to reduced blood flow in the microcirculation and increased lesion progression. Before an ischemic stroke, the brain has healthy blood flow with few neutrophils in the tissue. Upon ischemic stroke brain injury, blood flow ceases to the infarct area, leaving a region of the brain at risk for tissue death. Upon reperfusion, blood flow is restored to the area, but neutrophils begin to infiltrate immediately. Over time, neutrophil recruitment results in decreased blood flow in the microcirculation and increased tissue damage.
Several clinical studies have employed innovative imaging techniques to visualize neutrophil recruitment to the brain in the acute setting [20]. Using single-photon emission computed tomography, Akopov et al. [15] were the first to report that brain neutrophil recruitment correlated strongly with the severity of brain tissue damage and subsequent poor neurologic outcome. Taking advantage of recent advancements in endovascular procedures, several groups have been able to validate these early studies by sampling blood directly from the affected brain territory [21]. These studies confirmed a neutrophil-predominant response that is initiated before reperfusion is achieved [22,23] and that most likely occurs through pial collateral channels. Critically, for studying therapeutic interventions, a very similar inflammatory response occurs in models of experimental ischemic stroke (Figure 1) [24]. Additionally, the abundance of infiltrated immune cells correlates with the extent of brain injury. In the first 24 hours after murine stroke, neutrophils are mainly recruited to the cortical penumbral tissue, actively contributing to brain damage [25]. In support of this, a multitude of preclinical studies have targeted neutrophils and seen drastic improvement in ischemic stroke outcomes (reviewed in the study by Jickling et al. [14]). However, translation to human studies has largely failed. It is important to note that these clinical studies were all focused on preventing transendothelial migration of neutrophils to the brain parenchyma [26,27]. As no study, so far, has found a transendothelial migration mechanism that is unique to the brain, these strategies are associated with an infection risk, which might be particularly dangerous to the immunosuppressed patient who experienced stroke. Besides fighting infections, cytoprotective neutrophils have also been reported to be involved in poststroke recovery [28]. Therefore, targeting general neutrophil function, or blocking overall neutrophil recruitment might not be the best therapeutic approach.
Over the last decade, it has become clear that neutrophils do not necessarily need to extravasate to exert neurotoxicity. Several histologic studies have found neutrophils particularly within the vasculature or perivascular space of cerebral vessels [[29], [30], [31], [32]]. This phenomenon is independent of stroke severity in animal models and has been validated by histopathologic studies in patients who experienced ischemic stroke [29,32,33]. In particular, at early time points after stroke onset, neutrophils are confined to the vascular compartment. Within the vasculature, neutrophils aggravate stroke outcomes by impairing microvascular blood flow downstream of the original occlusion site [[34], [35], [36], [37]]. Of translational relevance, a recent study found that this phenomenon increases with age [38]. Aged mice and elderly patients who experience stroke have dysregulated granulopoiesis after stroke, resulting in distinct neutrophils that are associated with worse reperfusion and outcome [38]. This phenotype was linked to a prothrombotic response in the cerebral microvasculature.
3. Immunothrombosis as a Driver of Ischemic Stroke Brain Injury
Insights into how neutrophils drive ischemic stroke brain injury came from an unexpected ally to the neutrophil: the platelet. Platelets play a critical role in the formation of obstructive thrombi causing stroke [39] and antiplatelet agents are part of standard of care for the prevention and treatment of ischemic stroke [40]. However, platelets also play a role in ischemic stroke downstream of the occlusive thrombus. Moreover, the mechanisms by which platelets do this depend on their interaction with neutrophils.
The first hint at a role for platelets in stroke beyond their classic function came from the seminal publication from Kleinschnitz et al. [41], who found that blocking platelet adhesion or activation but not platelet–platelet aggregation ameliorated murine stroke outcomes. These findings were later reproduced by many groups with the use of many different genetic and/or therapeutic approaches [42]. These studies imply a heterotypic interaction between platelets and immune cells drives stroke progression downstream of the original occlusive thrombus. Mechanistically, hypoxia induced by the occlusive thrombus will initiate release of endothelial von Willebrand factor, which upon exposure to blood will capture platelets via their glycoprotein (GP) Ib receptor (Figure 2) [43,44]. Platelets subsequently become strongly activated by signaling through their collagen receptor GPVI in combination with locally generated thrombin [45]. This induces a procoagulant platelet phenotype [46,47], which subsequently generates fibrin and recruits neutrophils through a plethora of receptors present on the activated platelet surface [[48], [49], [50]]. Once recruited, neutrophils get activated by platelet released damage-associated molecular patterns such as HMGB1, inducing the formation of neutrophil extracellular traps (NETs) (Figure 2) [[51], [52], [53]]. Although the primary function of NETs is to trap and kill pathogens to help fight infection [50], they are prothrombotic and neurotoxic when formed in the brain [50,54,55]. Importantly, preventing or degrading NETs protects mice from ischemic stroke brain injury [33,[56], [57], [58]]. When we blocked NET formation with a natural NET inhibitor, brain neutrophil recruitment was unaffected and the specific acute inhibition of NETs was associated with greatly improving long-term stroke outcomes [33]. From a translational point of view, NET inhibition strategies will likely improve stroke outcomes also independent of reperfusion injury by aiding in thrombolysis [[57], [58], [59], [60]]. Important for clinical translation, most targets of this pathway have been validated across different laboratories using different stroke models, including comorbidities and coadministration of tissue plasminogen activator. In fact, comorbidities such as hyperglycemia [[61], [62], [63]], hyperlipidemia [64,65], and older age [[66], [67], [68], [69]], which are common in patients who experience stroke, have been reported to be associated with increased platelet–leukocyte interactions and an increased propensity to form NETs.
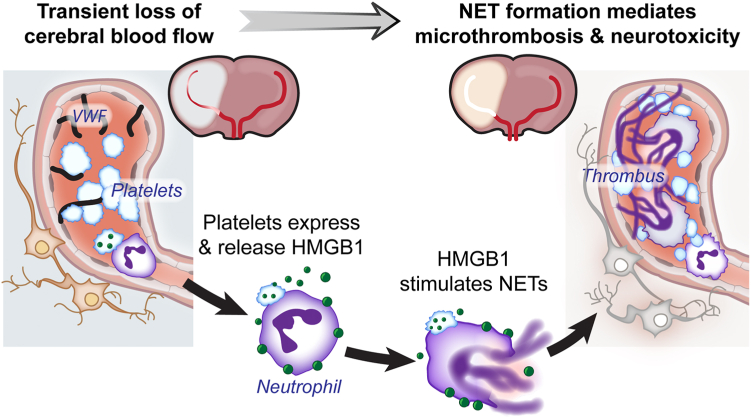
Figure 2.
Platelet-dependent neutrophil extracellular trap (NET) release induces microthrombosis and neurotoxicity during ischemic stroke brain injury. Hypoxic injury results in release of von Willebrand factor (VWF) from cerebral endothelial cells, leading to platelet recruitment and activation. Platelet activation results in expression of platelet P-selectin and phosphatidylserine allowing for increased neutrophil-platelet interactions. Activated platelets can then release high mobility group box-1 (HMGB1), which can bind to neutrophils, resulting in NET release. Intravascular NETs trap additional neutrophils, platelets, and other immune cells to form a thrombus.
In support of these experimental studies, clinical studies have found strong associations with several components of the immunothrombosis pathway and ischemic stroke outcomes. Patients who experience ischemic stroke and have dysregulated von Willebrand factor levels or increased platelet surface GPVI expression are more likely to experience a stroke or have worse outcomes after stroke [70,71]. Likewise, increased in vitro procoagulant platelet responses are associated with stroke severity and outcomes and have been shown to predict stroke recurrence [[72], [73], [74]]. Lastly, increased NET biomarkers levels [[75], [76], [77], [78], [79]] or a reduced ability to degrade NETs is also associated with worse ischemic stroke outcomes [80].
4. Translation to the Clinic: A New Horizon
As mentioned in the introduction, several drugs that target the immune system, which were effective in preclinical stroke models, have failed in clinical trials. Part of this translational problem has long been the difference in how stroke is modeled in a laboratory compared to a real-life patient who experienced stroke. In particular, the most commonly used preclinical ischemic stroke model is the transient middle cerebral artery occlusion model. In this model, cerebral blood flow is blocked for a predefined time after which reperfusion is initiated. In contrast, most clinical trials mainly included patients who experienced stroke and for whom reperfusion therapy was either not available or not successful due to the low efficacy of thrombolytics. It is more than likely that for any therapeutic strategy to work in patients who experienced stroke, at least partial reperfusion is needed [81]. Additionally, it has become evident that not all inflammation is bad in stroke. Not only do neutrophils contribute to immunothrombosis but there is also a subtype of neutrophils that exerts an anti-inflammatory phenotype (N2 neutrophils), a phenotype that is modulated by toll-like receptor 4 signaling [82,83]. Furthermore, neutrophils ameliorate long-term stroke outcomes by releasing cathelicidin antimicrobial peptide, which promotes endothelial cell proliferation and angiogenesis after stroke [84]. These studies highlight the versatility of neutrophils in the acute phase of stroke and beyond and argue against any broad inhibition of neutrophil function in stroke.
In light of the successful thrombectomy trials, interest has reemerged in neuroprotective and/or anti-inflammatory drugs to further improve outcomes in patients who experience stroke and achieve reperfusion [85]. Trials in this patient population will be the first to really evaluate the translational potential of preclinical stroke research and will be the critical first step in the evaluation of a new generation of anti-inflammatory drugs for the broader stroke patient population. Of interest to the topic of this review article, 2 drugs have recently successfully undergone the first clinical trials to assess safety and efficacy in patients who experienced ischemic stroke.
ApTOLL is a DNA aptamer that acts as an antagonist to toll-like receptor 4. ApTOLL administration to healthy adult volunteers was previously found to be safe with a favorable pharmacokinetic profile [86]. In patients who experienced ischemic stroke, 0.2 mg/kg of ApTOLL administered within 6 hours of onset in combination with thrombectomy was safe and associated with significant clinical benefit. ApTOLL significantly reduced infarct volume and stroke severity measured at 72 hours after stroke and reduced mortality and disability at 90 days compared with placebo [87]. A unique feature of this trial was the innovative selection of eligible patients who experienced stroke. For this trial, patients with very small strokes or very big strokes were excluded. This strategy was recently optimized to identify the ideal target population for neuroprotective drugs in patients who experienced stroke and were undergoing thrombectomy [88].
Glenzocimab is a Fab fragment of a humanized anti-GPVI monoclonal antibody [89]. In healthy volunteers, glenzocimab inhibited collagen-induced platelet aggregation in a dose-dependent manner without alter bleeding time, platelet counts, or GPVI expression levels [90]. While not yet published, the results of the Acute Ischemic Strole Interventional Study (ACTIMIS) trial (NCT03803007) were recently presented at several international conferences [91]. The ACTIMIS trial was a phase 1b/2a clinical trial in patients who experienced ischemic stroke where glenzocimab was included as an add-on therapy to standard of care. The trial was very positive, with both a significant reduction in mortality and an improvement in neurologic outcomes specifically in patients undergoing thrombectomy. The most remarkable result from this trial was the reduction in intracranial hemorrhages, both symptomatic and asymptomatic. Importantly, this effect persisted in patients treated with tissue plasminogen activator, aspirin, and glenzocimab [92]. While counterintuitive, this reduction in bleeding is in line with preclinical studies where glenzocimab was shown to not affect inflammatory bleeding, such as that present in the ischemic stroke brain [93].
5. Platelets and Neutrophils in the Brain Beyond Stroke
In addition to their role in stroke, platelets and neutrophils are known to drive other forms of neurovascular diseases either separately or in combination with each other.
Alzheimer’s disease (AD) is a progressive brain disorder resulting in dementia and is caused by the accumulation of extracellular neurofibrillary tangles composed of tau proteins and amyloid-β (Aβ) plaques [94,95]. The formation of tau neurofibrillary tangles is due to the alternative splicing of the microtubule-associated protein tau gene [96], resulting in the formation of a soluble protein, while Aβ plaques are generated from the cleavage of amyloid precursor protein (APP) by β-secretase and γ-secretase [97]. This results in the formation of Aβ oligomers, which are detrimental to the surrounding neurons. Interestingly, platelets are one of the most abundant sources of APP in blood due to their release upon platelet activation [98]. However, whether platelets play a causative role in the development of AD remains unclear. Human studies have demonstrated increased platelet activation in patients with AD [98]. In addition, patients with AD have increased platelet β-secretase activity, which increases cleavage of APP [99]. In vitro studies as well as murine models of AD have suggested a potential role for platelets in the development of AD. In vitro, antiplatelet drugs inhibit the formation of Aβ aggregates, while in murine models, antiplatelet therapies limit the amount of Aβ aggregates deposited in blood vessels [100,101]. Furthermore, platelets isolated from mice with AD induce blood vessel damage and neuroinflammation in non-AD mice brains, thus providing evidence for a direct role for platelets in the pathogenesis of AD [102].
In addition to platelets, neutrophil infiltration and activation are associated with the development of AD. Neutrophil-associated myeloperoxidase is increased in the brains of patients with AD as well as in murine models of AD [103,104]. Interestingly, neutrophil localization appears to associate with small vessels within the brain with the occasional neutrophil near Aβ plaques [105]. Furthermore, increased NET released is observed in human and murine models of AD with NETs, potentially contributing to blood–brain barrier (BBB) breakdown and neuronal damage [106]. Importantly, the increase in neutrophil adhesion in the brain during AD is associated with alterations in cerebral blood flow, reducing perfusion to areas of the brain and increasing memory deficits in murine models [105,107]. However, blocking neutrophil adhesion through genetic deletion of neutrophil specific receptors or depletion of neutrophils reduces tau deposits and improves cognitive function [104,106]. As platelets are key regulators of neutrophil adhesion and activation, whether platelets facilitate these interactions during AD is unknown and a potential area of future research.
Multiple sclerosis (MS) is characterized by the development self-reactive T cells against myelin proteins in the central nervous system [108]. The autoimmune nature of the MS results in inflammation, leading to significant demyelination and axonal damage. A majority of patients with MS go through disease cycles where initial neurologic symptoms are present, followed by a period of clinical stability, which is interrupted with recurring episodes of clinical neurologic symptoms [108]. While there are significant roles for T cells in the development of MS, platelets and neutrophils are also believed to play roles in the pathogenesis of the disease. Previous studies have documented enhanced platelet activation in patients with MS, while more recent studies have demonstrated platelet deposition in plaques from a patient with MS [[109], [110], [111]]. Furthermore, platelets are commonly found in the brains of mice in a model of experimental-induced autoimmune encephalomyelitis (EAE), an experimental MS model in mice [111]. Interestingly, platelet depletion blunts the progression of EAE, resulting in decreased inflammation and leukocyte infiltration [112]. Platelet-mediated EAE disease progression occurs through multiple processes, including integrin αIIb and GPIb, as well as through the release of serotonin and platelet activating factor [111,113,114]. Genetic deletion of the platelet activating factor or serotonin receptor results in improvement of EAE in mice, while serotonin uptake inhibitors have reduced clinical disease burden in human patients [113,115].
Previous studies have demonstrated a prominent role for neutrophils in the initial formation of lesions along with that prior to disease relapse [116]. In animal models, depletion of neutrophils before disease onset and relapse significantly reduces disease burden [117]. Neutrophil and T-cell recruitment to the central nervous system in MS is dependent on the breakdown of the BBB [118]. Importantly, increased BBB permeability is associated with early recruitment of neutrophils, while neutrophil depletion preserves BBB function [117]. Furthermore, while deletion of neutrophils preserves the BBB, inflammatory cells still entered into the space adjacent to the BBB but are unable to traffic to the brain, indicating neutrophils are key regulators of BBB integrity and recruitment of other immune cells [117,119]. Neutrophil-dependent breakdown on the BBB is believed to occur through release of matrix metalloproteinase and reactive oxygen species [118]. While NETs are known to be increased in sera of some patients with MS, mainly males, they do not correlate with disease severity, and it is unknown whether they play a role in the breakdown of the BBB in MS; therefore, additional studies are needed to define the role of NETs in the development and progression of MS [26,120].
While there are prominent roles for platelets and neutrophils in MS disease progression, reciprocal interaction between the 2 cells in propagating MS has not been definitely shown. However, given the close interaction between the 2 cells in driving inflammation and cellular recruitment, more studies are warranted to clearly define if platelets and neutrophils synergistically work together to influence the development of MS.
6. International Society on Thrombosis and Haemostasis Congress Report
At the International Society on Thrombosis and Haemostasis 2023 conference, there was a particular focus on immunothrombosis, and several interesting new studies were presented. Here, we highlight some that covered cerebrovascular disorders.
Since the US Food and Drug Administration/European Medicines Agency approval of thrombectomy for ischemic stroke, retrieved stroke clots have provided a wealthy source of information [121]. However, classic histologic procedures are limited by the number of antibodies you can use simultaneously, and therefore, complex colocalization studies are difficult. Roberts et al. [122] presented work from her group showing a novel way to analyze ischemic stroke thrombi by using imaging mass cytometry, allowing the use of 20 antibodies at the same time. While limited in the number of thrombi analyzed so far, they found an association between increased leukocyte content and cardioembolic etiology [122].
Although classically regarded as 2 very different diseases, venous thromboembolism (VTE) is observed in approximately 10% of patients who experience ischemic stroke despite VTE thromboprophylaxis [123]. New work presented by Dhanesha et al. [123] at this year’s conference hints at a role for neutrophils and immunothrombosis in the pathophysiology of poststroke VTE [124]. Dhanesha et al. [123] found a hyperreactive neutrophil phenotype that persisted after stroke. Through a mechanism involving the interaction with neutrophil integrin α9 and VCAM-1, poststroke neutrophils induced greater VTE thrombi, stabilized by NETs.
While not mentioned earlier in this review article, the contact pathway also contributes to immunothrombosis through the generation of bradykinin and fibrin [125]. Targeting the contact pathway through inhibition of factor XI has recently gained a lot of attention because of its high efficacy in preventing pathologic clot formation without inducing bleeding [126]. Several articles have previously demonstrated the efficacy of targeting the contact pathway in stroke [127,128], and data were presented at the 2023 International Society on Thrombosis and Haemostasis conference that this could also be the case for EAE and experimental cerebral malaria. In an experimental model of EAE, pharmacologic blockade of factor XI reduced the clinical severity of EAE, which was attributed to reduced BBB disruption, decreased axonal damage, and fibrin(ogen) accumulation in the spinal cord [124]. In a model of experimental cerebral malaria, Pinheiro et al. [129] found a specific role for the inflammatory axis of the contact pathway where they found that high molecular kininogen influenced vasogenic edema and neurologic integrity in experimental cerebral malaria.
7. Future Directions
Over the last 10 years, our knowledge of the immune players involved in ischemic stroke brain injury has evolved. While platelets play critical roles in the initiation and progression of ischemic stroke, there is strong evidence that other immune cells, including neutrophils, are directly involved in driving the pathophysiology of stroke. As antiplatelet therapies are not beneficial in all populations, continuing to develop novel therapeutic targets against noncanonical platelet receptors or activation pathways as well as immune cells may provide significant benefit in primary and recurrent stroke. Previous clinical trials targeting classical neutrophil function such as adhesion demonstrated no clinical benefit and, in some cases, worsen stroke outcomes due to infection. On the other hand, the development of new traditional antiplatelet drugs, have slightly improved outcomes, but with the risk of increased bleeding. To significantly improve ischemic stroke outcomes, development of therapies that specifically target immunothrombosis while leaving platelet hemostatic function and neutrophil immune function intact is needed. Recent clinical trials have demonstrated some success, specifically with GPVI inhibition. However, therapies against additional pathways and receptors are warranted, including protease activator receptor 4, which was recently shown to play a significant role in racial disparities associated with incident stroke and stroke outcomes [130]. In addition to targeting platelets, development of drugs against neutrophil functions, which promote thrombosis, such as NET formation, has the potential to significantly improve morbidity and mortality based on preclinical studies. These findings now must be translated into larger animal models and in clinical studies. While the field has advanced and our understanding of the role immunothrombosis plays in ischemic stroke brain injury has improved, there is still much unknown, which limits our ability to bring advancements on the clinical front.
Acknowledgments
The authors would like to thank Diana Lim for her assistance with the figures.
Funding
This work was funded by an American Heart Association Postdoctoral Fellowship (21POST830138, to F.D.); the National Heart, Lung, and Blood Institute (R01HL163019, to R.A.C.); and an American Society of Hematology Graduate Award (to A.A.).
Author contributions
F.D., A.A., and R.A.C. wrote the manuscript.
Relationship disclosure
There are no competing interests to disclose.
Footnotes
Handling Editor: Dr Neil Zakai
References
- 1.Feigin V.L., Nguyen G., Cercy K., Johnson C.O., Alam T., Parmar P.G., et al. GBD 2016 Lifetime Risk of Stroke Collaborators. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. 2018;379:2429–2437. doi: 10.1056/NEJMoa1804492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsao C.W., Aday A.W., Almarzooq Z.I., Anderson C.A.M., Arora P., Avery C.L., et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2023 update: a report from the American Heart Association. Circulation. 2023;147:e93–e621. doi: 10.1161/CIR.0000000000001123. [DOI] [PubMed] [Google Scholar]
- 3.Tawil S.E., Muir K.W. Thrombolysis and thrombectomy for acute ischaemic stroke. Clin Med (Lond) 2017;17:161–165. doi: 10.7861/clinmedicine.17-2-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalaria R.N., Akinyemi R., Ihara M. Stroke injury, cognitive impairment and vascular dementia. Biochim Biophys Acta. 2016;1862:915–925. doi: 10.1016/j.bbadis.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donnan G.A., Fisher M., Macleod M., Davis S.M. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Y., He Y., Yan S., Chen L., Zhang R., Xu J., et al. Reperfusion injury is associated with poor outcome in patients with recanalization after thrombectomy. Stroke. 2023;54:96–104. doi: 10.1161/STROKEAHA.122.039337. [DOI] [PubMed] [Google Scholar]
- 7.Kim J.Y., Kawabori M., Yenari M.A. Innate inflammatory responses in stroke: mechanisms and potential therapeutic targets. Curr Med Chem. 2014;21:2076–2097. doi: 10.2174/0929867321666131228205146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jayaraj R.L., Azimullah S., Beiram R., Jalal F.Y., Rosenberg G.A. Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation. 2019;16:142. doi: 10.1186/s12974-019-1516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ingeman A., Andersen G., Hundborg H.H., Svendsen M.L., Johnsen S.P. In-hospital medical complications, length of stay, and mortality among stroke unit patients. Stroke. 2011;42:3214–3218. doi: 10.1161/STROKEAHA.110.610881. [DOI] [PubMed] [Google Scholar]
- 10.Finlayson O., Kapral M., Hall R., Asllani E., Selchen D., Saposnik G. Canadian Stroke Network; Stroke Outcome Research Canada (SORCan) Working Group. Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology. 2011;77:1338–1345. doi: 10.1212/WNL.0b013e31823152b1. [DOI] [PubMed] [Google Scholar]
- 11.de Jonge J.C., van de Beek D., Lyden P., Brady M.C., Bath P.M., van der Worp H.B. Temporal profile of pneumonia after stroke. Stroke. 2022;53:53–60. doi: 10.1161/STROKEAHA.120.032787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westendorp W.F., Dames C., Nederkoorn P.J., Meisel A. Immunodepression, infections, and functional outcome in ischemic stroke. Stroke. 2022;53:1438–1448. doi: 10.1161/STROKEAHA.122.038867. [DOI] [PubMed] [Google Scholar]
- 13.Cai W., Liu S., Hu M., Huang F., Zhu Q., Qiu W., et al. Functional dynamics of neutrophils after ischemic stroke. Transl Stroke Res. 2020;11:108–121. doi: 10.1007/s12975-019-00694-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jickling G.C., Liu D., Ander B.P., Stamova B., Zhan X., Sharp F.R. Targeting neutrophils in ischemic stroke: translational insights from experimental studies. J Cereb Blood Flow Metab. 2015;35:888–901. doi: 10.1038/jcbfm.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akopov S.E., Simonian N.A., Grigorian G.S. Dynamics of polymorphonuclear leukocyte accumulation in acute cerebral infarction and their correlation with brain tissue damage. Stroke. 1996;27:1739–1743. doi: 10.1161/01.str.27.10.1739. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W., Zhao J., Wang R., Jiang M., Ye Q., Smith A.D., et al. Macrophages reprogram after ischemic stroke and promote efferocytosis and inflammation resolution in the mouse brain. CNS Neurosci Ther. 2019;25:1329–1342. doi: 10.1111/cns.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gliem M., Schwaninger M., Jander S. Protective features of peripheral monocytes/macrophages in stroke. Biochim Biophys Acta. 2016;1862:329–338. doi: 10.1016/j.bbadis.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Otxoa-de-Amezaga A., Miró-Mur F., Pedragosa J., Gallizioli M., Justicia C., Gaja-Capdevila N., et al. Microglial cell loss after ischemic stroke favors brain neutrophil accumulation. Acta Neuropathol. 2019;137:321–341. doi: 10.1007/s00401-018-1954-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L., Cheng S.Q., Sun Y.Q., Yu J.B., Huang X.X., Dong Y.F., et al. Resolvin D1 reprograms energy metabolism to promote microglia to phagocytize neutrophils after ischemic stroke. Cell Rep. 2023;42 doi: 10.1016/j.celrep.2023.112617. [DOI] [PubMed] [Google Scholar]
- 20.Yilmaz G., Granger D.N. Leukocyte recruitment and ischemic brain injury. Neuromolecular Med. 2010;12:193–204. doi: 10.1007/s12017-009-8074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoll G., Schuhmann M.K., Nieswandt B., Kollikowski A.M., Pham M. An intravascular perspective on hyper-acute neutrophil, T-cell and platelet responses: similarities between human and experimental stroke. J Cereb Blood Flow Metab. 2022;42:1561–1567. doi: 10.1177/0271678X221105764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou P., Li T., Jin J., Liu Y., Li B., Sun Q., et al. Interactions between neutrophil extracellular traps and activated platelets enhance procoagulant activity in acute stroke patients with ICA occlusion. EBioMedicine. 2020;53 doi: 10.1016/j.ebiom.2020.102671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kollikowski A.M., Schuhmann M.K., Nieswandt B., Müllges W., Stoll G., Pham M. Local leukocyte invasion during hyperacute human ischemic stroke. Ann Neurol. 2020;87:466–479. doi: 10.1002/ana.25665. [DOI] [PubMed] [Google Scholar]
- 24.Beuker C., Strecker J.K., Rawal R., Schmidt-Pogoda A., Ruck T., Wiendl H., et al. Immune cell infiltration into the brain after ischemic stroke in humans compared to mice and rats: a systematic review and meta-analysis. Transl Stroke Res. 2021;12:976–990. doi: 10.1007/s12975-021-00887-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nadkarni N.A., Arias E., Fang R., Haynes M.E., Zhang H.F., Muller W.A., et al. Platelet endothelial cell adhesion molecule (PECAM/CD31) blockade modulates neutrophil recruitment patterns and reduces infarct size in experimental ischemic stroke. Am J Pathol. 2022;192:1619–1632. doi: 10.1016/j.ajpath.2022.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tillack K., Naegele M., Haueis C., Schippling S., Wandinger K.P., Martin R., et al. Gender differences in circulating levels of neutrophil extracellular traps in serum of multiple sclerosis patients. J Neuroimmunol. 2013;261:108–119. doi: 10.1016/j.jneuroim.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Enlimomab Acute Stroke Trial Investigators Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology. 2001;57:1428–1434. doi: 10.1212/wnl.57.8.1428. [DOI] [PubMed] [Google Scholar]
- 28.Wanrooy B.J., Wen S.W., Wong C.H. Dynamic roles of neutrophils in post-stroke neuroinflammation. Immunol Cell Biol. 2021;99:924–935. doi: 10.1111/imcb.12463. [DOI] [PubMed] [Google Scholar]
- 29.Perez-de-Puig I., Miró-Mur F., Ferrer-Ferrer M., Gelpi E., Pedragosa J., Justicia C., et al. Neutrophil recruitment to the brain in mouse and human ischemic stroke. Acta Neuropathol. 2015;129:239–257. doi: 10.1007/s00401-014-1381-0. [DOI] [PubMed] [Google Scholar]
- 30.Otxoa-de-Amezaga A., Gallizioli M., Pedragosa J., Justicia C., Miró-Mur F., Salas-Perdomo A., et al. Location of neutrophils in different compartments of the damaged mouse brain after severe ischemia/reperfusion. Stroke. 2019;50:1548–1557. doi: 10.1161/STROKEAHA.118.023837. [DOI] [PubMed] [Google Scholar]
- 31.Neumann J., Riek-Burchardt M., Herz J., Doeppner T.R., König R., Hütten H., et al. Very-late-antigen-4 (VLA-4)-mediated brain invasion by neutrophils leads to interactions with microglia, increased ischemic injury and impaired behavior in experimental stroke. Acta Neuropathol. 2015;129:259–277. doi: 10.1007/s00401-014-1355-2. [DOI] [PubMed] [Google Scholar]
- 32.Enzmann G., Mysiorek C., Gorina R., Cheng Y.J., Ghavampour S., Hannocks M.J., et al. The neurovascular unit as a selective barrier to polymorphonuclear granulocyte (PMN) infiltration into the brain after ischemic injury. Acta Neuropathol. 2013;125:395–412. doi: 10.1007/s00401-012-1076-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denorme F., Portier I., Rustad J.L., Cody M.J., de Araujo C.V., Hoki C., et al. Neutrophil extracellular traps regulate ischemic stroke brain injury. J Clin Invest. 2022;132 doi: 10.1172/JCI154225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rolfes L., Riek-Burchardt M., Pawlitzki M., Minnerup J., Bock S., Schmidt M., et al. Neutrophil granulocytes promote flow stagnation due to dynamic capillary stalls following experimental stroke. Brain Behav Immun. 2021;93:322–330. doi: 10.1016/j.bbi.2021.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Erdener Ş.E., Tang J., Kılıç K., Postnov D., Giblin J.T., Kura S., et al. Dynamic capillary stalls in reperfused ischemic penumbra contribute to injury: a hyperacute role for neutrophils in persistent traffic jams. J Cereb Blood Flow Metab. 2021;41:236–252. doi: 10.1177/0271678X20914179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El Amki M., Glück C., Binder N., Middleham W., Wyss M.T., Weiss T., et al. Neutrophils obstructing brain capillaries are a major cause of no-reflow in ischemic stroke. Cell Rep. 2020;33 doi: 10.1016/j.celrep.2020.108260. [DOI] [PubMed] [Google Scholar]
- 37.Denorme F., Manne B.K., Portier I., Eustes A.S., Kosaka Y., Kile B.T., et al. Platelet necrosis mediates ischemic stroke outcome in mice. Blood. 2020;135:429–440. doi: 10.1182/blood.2019002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gullotta G.S., De Feo D., Friebel E., Semerano A., Scotti G.M., Bergamaschi A., et al. Age-induced alterations of granulopoiesis generate atypical neutrophils that aggravate stroke pathology. Nat Immunol. 2023;24:925–940. doi: 10.1038/s41590-023-01505-1. [DOI] [PubMed] [Google Scholar]
- 39.Shaik N.F., Regan R.F., Naik U.P. Platelets as drivers of ischemia/reperfusion injury after stroke. Blood Adv. 2021;5:1576–1584. doi: 10.1182/bloodadvances.2020002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mendelson S.J., Prabhakaran S. Diagnosis and management of transient ischemic attack and acute ischemic stroke: a review. JAMA. 2021;325:1088–1098. doi: 10.1001/jama.2020.26867. [DOI] [PubMed] [Google Scholar]
- 41.Kleinschnitz C., Pozgajova M., Pham M., Bendszus M., Nieswandt B., Stoll G. Targeting platelets in acute experimental stroke: impact of glycoprotein Ib, VI, and IIb/IIIa blockade on infarct size, functional outcome, and intracranial bleeding. Circulation. 2007;115:2323–2330. doi: 10.1161/CIRCULATIONAHA.107.691279. [DOI] [PubMed] [Google Scholar]
- 42.De Meyer S.F., Langhauser F., Haupeltshofer S., Kleinschnitz C., Casas A.I. Thromboinflammation in brain ischemia: recent updates and future perspectives. Stroke. 2022;53:1487–1499. doi: 10.1161/STROKEAHA.122.038733. [DOI] [PubMed] [Google Scholar]
- 43.Yau J.W., Teoh H., Verma S. Endothelial cell control of thrombosis. BMC Cardiovasc Disord. 2015;15:130. doi: 10.1186/s12872-015-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruggeri Z.M. The role of von Willebrand factor in thrombus formation. Thromb Res. 2007;120(Suppl 1):S5–S9. doi: 10.1016/j.thromres.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rayes J., Watson S.P., Nieswandt B. Functional significance of the platelet immune receptors GPVI and CLEC-2. J Clin Invest. 2019;129:12–23. doi: 10.1172/JCI122955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Denorme F., Campbell R.A. Procoagulant platelets: novel players in thromboinflammation. Am J Physiol Cell Physiol. 2022;323:C951–C958. doi: 10.1152/ajpcell.00252.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ajanel A., Campbell R.A., Denorme F. Platelet mitochondria: the mighty few. Curr Opin Hematol. 2023;30:167–174. doi: 10.1097/MOH.0000000000000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hua V.M., Abeynaike L., Glaros E., Campbell H., Pasalic L., Hogg P.J., et al. Necrotic platelets provide a procoagulant surface during thrombosis. Blood. 2015;126:2852–2862. doi: 10.1182/blood-2015-08-663005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan Y., Alwis I., Wu M.C.L., Kaplan Z., Ashworth K., Bark D., Jr., et al. Neutrophil macroaggregates promote widespread pulmonary thrombosis after gut ischemia. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aam5861. [DOI] [PubMed] [Google Scholar]
- 50.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 51.Pir G.J., Parray A., Ayadathil R., Pananchikkal S.V., Mir F.A., Muhammad I., et al. Platelet-neutrophil association in NETs-rich areas in the retrieved AIS patient thrombi. Int J Mol Sci. 2022;23 doi: 10.3390/ijms232214477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kollikowski A.M., Pham M., März A.G., Papp L., Nieswandt B., Stoll G., et al. Platelet activation and chemokine release are related to local neutrophil-dominant inflammation during hyperacute human stroke. Transl Stroke Res. 2022;13:364–369. doi: 10.1007/s12975-021-00938-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Essig F., Babilon L., Vollmuth C., Kollikowski A.M., Pham M., Solymosi L., et al. High mobility group box 1 protein in cerebral thromboemboli. Int J Mol Sci. 2021;22 doi: 10.3390/ijms222011276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manda-Handzlik A., Demkow U. The brain entangled: the contribution of neutrophil extracellular traps to the diseases of the central nervous system. Cells. 2019;8:1477. doi: 10.3390/cells8121477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allen C., Thornton P., Denes A., McColl B.W., Pierozynski A., Monestier M., et al. Neutrophil cerebrovascular transmigration triggers rapid neurotoxicity through release of proteases associated with decondensed DNA. J Immunol. 2012;189:381–392. doi: 10.4049/jimmunol.1200409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Meyer S.F., Suidan G.L., Fuchs T.A., Monestier M., Wagner D.D. Extracellular chromatin is an important mediator of ischemic stroke in mice. Arterioscler Thromb Vasc Biol. 2012;32:1884–1891. doi: 10.1161/ATVBAHA.112.250993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peña-Martínez C., Durán-Laforet V., García-Culebras A., Ostos F., Hernández-Jiménez M., Bravo-Ferrer I., et al. Pharmacological modulation of neutrophil extracellular traps reverses thrombotic stroke tPA (tissue-type plasminogen activator) resistance. Stroke. 2019;50:3228–3237. doi: 10.1161/STROKEAHA.119.026848. [DOI] [PubMed] [Google Scholar]
- 58.Peña-Martínez C., Durán-Laforet V., García-Culebras A., Cuartero M.I., Moro M.Á., Lizasoain I. Neutrophil extracellular trap targeting protects against ischemic damage after fibrin-rich thrombotic stroke despite non-reperfusion. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.790002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laridan E., Denorme F., Desender L., François O., Andersson T., Deckmyn H., et al. Neutrophil extracellular traps in ischemic stroke thrombi. Ann Neurol. 2017;82:223–232. doi: 10.1002/ana.24993. [DOI] [PubMed] [Google Scholar]
- 60.Ducroux C., Di Meglio L., Loyau S., Delbosc S., Boisseau W., Deschildre C., et al. Thrombus neutrophil extracellular traps content impair tPA-induced thrombolysis in acute ischemic stroke. Stroke. 2018;49:754–757. doi: 10.1161/STROKEAHA.117.019896. [DOI] [PubMed] [Google Scholar]
- 61.Gauer J.S., Ajjan R.A., Ariëns R.A.S. Platelet-neutrophil interaction and thromboinflammation in diabetes: considerations for novel therapeutic approaches. J Am Heart Assoc. 2022;11 doi: 10.1161/JAHA.122.027071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Denorme F., Portier I., Kosaka Y., Campbell R.A. Hyperglycemia exacerbates ischemic stroke outcome independent of platelet glucose uptake. J Thromb Haemost. 2021;19:536–546. doi: 10.1111/jth.15154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wong S.L., Demers M., Martinod K., Gallant M., Wang Y., Goldfine A.B., et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med. 2015;21:815–819. doi: 10.1038/nm.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mehta J.L., Nicolini F.A., Donnelly W.H., Nichols W.W. Platelet-leukocyte-endothelial interactions in coronary artery disease. Am J Cardiol. 1992;69:8B. doi: 10.1016/0002-9149(92)91343-3. –13B. [DOI] [PubMed] [Google Scholar]
- 65.Boulaftali Y., Owens A.P., III, Beale A., Piatt R., Casari C., Lee R.H., et al. CalDAG-GEFI deficiency reduces atherosclerotic lesion development in mice. Arterioscler Thromb Vasc Biol. 2016;36:792–799. doi: 10.1161/ATVBAHA.115.306347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martinod K., Witsch T., Erpenbeck L., Savchenko A., Hayashi H., Cherpokova D., et al. Peptidylarginine deiminase 4 promotes age-related organ fibrosis. J Exp Med. 2017;214:439–458. doi: 10.1084/jem.20160530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Denorme F., Andrianova I., Cody M.J., Kosaka Y., Campbell R.A. Age-specific impact of type I interferons on cerebral thrombosis and inflammation. Blood Adv. 2023;7:6672–6675. doi: 10.1182/bloodadvances.2023010495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davizon-Castillo P., McMahon B., Aguila S., Bark D., Ashworth K., Allawzi A., et al. TNF-alpha-driven inflammation and mitochondrial dysfunction define the platelet hyperreactivity of aging. Blood. 2019;134:727–740. doi: 10.1182/blood.2019000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Campbell R.A., Franks Z., Bhatnagar A., Rowley J.W., Manne B.K., Supiano M.A., et al. Granzyme A in human platelets regulates the synthesis of proinflammatory cytokines by monocytes in aging. J Immunol. 2018;200:295–304. doi: 10.4049/jimmunol.1700885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Induruwa I., McKinney H., Kempster C., Thomas P., Batista J., Malcor J.D., et al. Platelet surface receptor glycoprotein VI-dimer is overexpressed in stroke: the glycoprotein VI in Stroke (GYPSIE) study results. PLoS One. 2022;17 doi: 10.1371/journal.pone.0262695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carter A.M., Catto A.J., Mansfield M.W., Bamford J.M., Grant P.J. Predictive variables for mortality after acute ischemic stroke. Stroke. 2007;38:1873–1880. doi: 10.1161/STROKEAHA.106.474569. [DOI] [PubMed] [Google Scholar]
- 72.Prodan C.I., Stoner J.A., Cowan L.D., Dale G.L. Higher coated-platelet levels are associated with stroke recurrence following nonlacunar brain infarction. J Cereb Blood Flow Metab. 2013;33:287–292. doi: 10.1038/jcbfm.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Prodan C.I., Joseph P.M., Vincent A.S., Dale G.L. Coated-platelets in ischemic stroke: differences between lacunar and cortical stroke. J Thromb Haemost. 2008;6:609–614. doi: 10.1111/j.1538-7836.2008.02890.x. [DOI] [PubMed] [Google Scholar]
- 74.Kirkpatrick A.C., Vincent A.S., Dale G.L., Prodan C.I. Increased platelet procoagulant potential predicts recurrent stroke and TIA after lacunar infarction. J Thromb Haemost. 2020;18:660–668. doi: 10.1111/jth.14714. [DOI] [PubMed] [Google Scholar]
- 75.Tsai N.W., Lin T.K., Chen S.D., Chang W.N., Wang H.C., Yang T.M., et al. The value of serial plasma nuclear and mitochondrial DNA levels in patients with acute ischemic stroke. Clin Chim Acta. 2011;412:476–479. doi: 10.1016/j.cca.2010.11.036. [DOI] [PubMed] [Google Scholar]
- 76.Rainer T.H., Wong L.K., Lam W., Yuen E., Lam N.Y., Metreweli C., et al. Prognostic use of circulating plasma nucleic acid concentrations in patients with acute stroke. Clin Chem. 2003;49:562–569. doi: 10.1373/49.4.562. [DOI] [PubMed] [Google Scholar]
- 77.Lam N.Y., Rainer T.H., Wong L.K., Lam W., Lo Y.M. Plasma DNA as a prognostic marker for stroke patients with negative neuroimaging within the first 24 h of symptom onset. Resuscitation. 2006;68:71–78. doi: 10.1016/j.resuscitation.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 78.Maïer B, Di Meglio L, Desilles JP, Solo Nomenjanahary M, Delvoye F, Kyheng M, et al NEUTROSTROKE Investigators. Neutrophil activation in patients treated with endovascular therapy is associated with unfavorable outcomes and mitigated by intravenous thrombolysis. J Neurointerv Surg. Published online April 17, 2023. 10.1136/jnis-2022-020020 [DOI] [PubMed]
- 79.Datsi A., Piotrowski L., Markou M., Köster T., Kohtz I., Lang K., et al. Stroke-derived neutrophils demonstrate higher formation potential and impaired resolution of CD66b + driven neutrophil extracellular traps. BMC Neurol. 2022;22:186. doi: 10.1186/s12883-022-02707-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grosse G.M., Blume N., Abu-Fares O., Götz F., Ernst J., Leotescu A., et al. Endogenous deoxyribonuclease activity and cell-free deoxyribonucleic acid in acute ischemic stroke: a cohort study. Stroke. 2022;53:1235–1244. doi: 10.1161/STROKEAHA.121.036299. [DOI] [PubMed] [Google Scholar]
- 81.Fisher M., Savitz S.I. Pharmacological brain cytoprotection in acute ischaemic stroke - renewed hope in the reperfusion era. Nat Rev Neurol. 2022;18:193–202. doi: 10.1038/s41582-021-00605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Clemente-Moragón A., Oliver E., Calle D., Cussó L., Gómez M., Pradillo J.M., et al. Neutrophil β1 adrenoceptor blockade blunts stroke-associated neuroinflammation. Br J Pharmacol. 2023;180:459–478. doi: 10.1111/bph.15963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.García-Culebras A., Durán-Laforet V., Peña-Martínez C., Moraga A., Ballesteros I., Cuartero M.I., et al. Role of TLR4 (toll-like receptor 4) in N1/N2 neutrophil programming after stroke. Stroke. 2019;50:2922–2932. doi: 10.1161/STROKEAHA.119.025085. [DOI] [PubMed] [Google Scholar]
- 84.Xie W., Huang T., Guo Y., Zhang Y., Chen W., Li Y., et al. Neutrophil-derived cathelicidin promotes cerebral angiogenesis after ischemic stroke. J Cereb Blood Flow Metab. 2023;43:1503–1518. doi: 10.1177/0271678X231175190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lyden P.D., Diniz M.A., Bosetti F., Lamb J., Nagarkatti K.A., Rogatko A., et al. A multi-laboratory preclinical trial in rodents to assess treatment candidates for acute ischemic stroke. Sci Transl Med. 2023;15 doi: 10.1126/scitranslmed.adg8656. [DOI] [PubMed] [Google Scholar]
- 86.Hernández-Jiménez M., Martín-Vílchez S., Ochoa D., Mejía-Abril G., Román M., Camargo-Mamani P., et al. First-in-human phase I clinical trial of a TLR4-binding DNA aptamer, ApTOLL: safety and pharmacokinetics in healthy volunteers. Mol Ther Nucleic Acids. 2022;28:124–135. doi: 10.1016/j.omtn.2022.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hernández-Jiménez M., Abad-Santos F., Cotgreave I., Gallego J., Jilma B., Flores A., et al. Safety and efficacy of ApTOLL in patients with ischemic stroke undergoing endovascular treatment: a phase 1/2 randomized clinical trial. JAMA Neurol. 2023;80:779–788. doi: 10.1001/jamaneurol.2023.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Olivé-Gadea M., Requena M., Campos D., Garcia-Tornel A., Deck M., Muchada M., et al. Defining a target population to effectively test a neuroprotective drug. Stroke. 2021;52:505–510. doi: 10.1161/STROKEAHA.120.032025. [DOI] [PubMed] [Google Scholar]
- 89.Wichaiyo S., Parichatikanond W., Rattanavipanon W. Glenzocimab: a GPVI (glycoprotein VI)-targeted potential antiplatelet agent for the treatment of acute ischemic stroke. Stroke. 2022;53:3506–3513. doi: 10.1161/STROKEAHA.122.039790. [DOI] [PubMed] [Google Scholar]
- 90.Voors-Pette C., Lebozec K., Dogterom P., Jullien L., Billiald P., Ferlan P., et al. Safety and tolerability, pharmacokinetics, and pharmacodynamics of ACT017, an antiplatelet GPVI (glycoprotein VI) Fab. Arterioscler Thromb Vasc Biol. 2019;39:956–964. doi: 10.1161/ATVBAHA.118.312314. [DOI] [PubMed] [Google Scholar]
- 91.Mazighi M., Peeters A., Richard S., Molina C., Lemmens R., Toni D., et al. ACTIMIS trial: safety interim analysis data of glenzocimab, a novel antiplatelet agent on top of acute ischemic stroke standard of care [abstract] Res Pract Thromb Haemost. 2021;5:LPB0051. [Google Scholar]
- 92.Jandrot-Perrus M., Pottecher J., Toledano E., Commenducci A., Meilhoc A., Gharakhanian S., et al. Glycoprotein VI-targeted antiplatelet therapy with glenzocimab is safe in patients exposed to current antithrombotic and fibrinolytic drugs [abstract] Res Pract Thromb Haemost. 2023;7:PB0364. [Google Scholar]
- 93.Jadoui S., Le Chapelain O., Ollivier V., Mostefa-Kara A., Di Meglio L., Dupont S., et al. Glenzocimab does not impact glycoprotein VI-dependent inflammatory haemostasis. Haematologica. 2021;106:2000. doi: 10.3324/haematol.2020.270439. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hardy J.A., Higgins G.A. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 95.Frost B., Jacks R.L., Diamond M.I. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem. 2009;284:12845–12852. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Trabzuni D., Wray S., Vandrovcova J., Ramasamy A., Walker R., Smith C., et al. MAPT expression and splicing is differentially regulated by brain region: relation to genotype and implication for tauopathies. Hum Mol Genet. 2012;21:4094–4103. doi: 10.1093/hmg/dds238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Golde T.E., Cai X.D., Shoji M., Younkin S.G. Production of amyloid beta protein from normal amyloid beta-protein precursor (beta APP) and the mutated beta APPS linked to familial Alzheimer’s disease. Ann N Y Acad Sci. 1993;695:103–108. doi: 10.1111/j.1749-6632.1993.tb23036.x. [DOI] [PubMed] [Google Scholar]
- 98.Sevush S., Jy W., Horstman L.L., Mao W.W., Kolodny L., Ahn Y.S. Platelet activation in Alzheimer disease. Arch Neurol. 1998;55:530–536. doi: 10.1001/archneur.55.4.530. [DOI] [PubMed] [Google Scholar]
- 99.Johnston J.A., Liu W.W., Coulson D.T., Todd S., Murphy S., Brennan S., et al. Platelet beta-secretase activity is increased in Alzheimer’s disease. Neurobiol Aging. 2008;29:661–668. doi: 10.1016/j.neurobiolaging.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 100.Alexander P., Visagan S., Jawhar S., Kare A., Issa N., Issa R., et al. Antiplatelets and vascular dementia: a systematic review. J Aging Res. 2022;2022 doi: 10.1155/2022/9780067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Donner L., Fälker K., Gremer L., Klinker S., Pagani G., Ljungberg L.U., et al. Platelets contribute to amyloid-β aggregation in cerebral vessels through integrin αIIbβ3-induced outside-in signaling and clusterin release. Sci Signal. 2016;9 doi: 10.1126/scisignal.aaf6240. [DOI] [PubMed] [Google Scholar]
- 102.Kniewallner K.M., Foidl B.M., Humpel C. Platelets isolated from an Alzheimer mouse damage healthy cortical vessels and cause inflammation in an organotypic ex vivo brain slice model. Sci Rep. 2018;8 doi: 10.1038/s41598-018-33768-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reynolds W.F., Rhees J., Maciejewski D., Paladino T., Sieburg H., Maki R.A., et al. Myeloperoxidase polymorphism is associated with gender specific risk for Alzheimer’s disease. Exp Neurol. 1999;155:31–41. doi: 10.1006/exnr.1998.6977. [DOI] [PubMed] [Google Scholar]
- 104.Volkman R., Ben-Zur T., Kahana A., Garty B.Z., Offen D. Myeloperoxidase deficiency inhibits cognitive decline in the 5XFAD mouse model of Alzheimer’s disease. Front Neurosci. 2019;13:990. doi: 10.3389/fnins.2019.00990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Baik S.H., Cha M.Y., Hyun Y.M., Cho H., Hamza B., Kim D.K., et al. Migration of neutrophils targeting amyloid plaques in Alzheimer’s disease mouse model. Neurobiol Aging. 2014;35:1286–1292. doi: 10.1016/j.neurobiolaging.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zenaro E., Pietronigro E., Della Bianca V., Piacentino G., Marongiu L., Budui S., et al. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat Med. 2015;21:880–886. doi: 10.1038/nm.3913. [DOI] [PubMed] [Google Scholar]
- 107.Cruz Hernández J.C., Bracko O., Kersbergen C.J., Muse V., Haft-Javaherian M., Berg M., et al. Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer’s disease mouse models. Nat Neurosci. 2019;22:413–420. doi: 10.1038/s41593-018-0329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fletcher J.M., Lalor S.J., Sweeney C.M., Tubridy N., Mills K.H. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2010;162:1–11. doi: 10.1111/j.1365-2249.2010.04143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sheremata W.A., Jy W., Horstman L.L., Ahn Y.S., Alexander J.S., Minagar A. Evidence of platelet activation in multiple sclerosis. J Neuroinflammation. 2008;5:27. doi: 10.1186/1742-2094-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dziedzic A., Miller E., Saluk-Bijak J., Niwald M., Bijak M. The molecular aspects of disturbed platelet activation through ADP/P2Y12 pathway in multiple sclerosis. Int J Mol Sci. 2021;22:6572. doi: 10.3390/ijms22126572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Langer H.F., Choi E.Y., Zhou H., Schleicher R., Chung K.J., Tang Z., et al. Platelets contribute to the pathogenesis of experimental autoimmune encephalomyelitis. Circ Res. 2012;110:1202–1210. doi: 10.1161/CIRCRESAHA.111.256370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kocovski P., Jiang X., D’Souza C.S., Li Z., Dang P.T., Wang X., et al. Platelet depletion is effective in ameliorating anxiety-like behavior and reducing the pro-inflammatory environment in the hippocampus in murine experimental autoimmune encephalomyelitis. J Clin Med. 2019;8:162. doi: 10.3390/jcm8020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Edwards L.J., Constantinescu C.S. Platelet activating factor/platelet activating factor receptor pathway as a potential therapeutic target in autoimmune diseases. Inflamm Allergy Drug Targets. 2009;8:182–190. doi: 10.2174/187152809788681010. [DOI] [PubMed] [Google Scholar]
- 114.Starossom S.C., Veremeyko T., Yung A.W., Dukhinova M., Au C., Lau A.Y., et al. Platelets play differential role during the initiation and progression of autoimmune neuroinflammation. Circ Res. 2015;117:779–792. doi: 10.1161/CIRCRESAHA.115.306847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ghareghani M., Zibara K., Sadeghi H., Dokoohaki S., Sadeghi H., Aryanpour R., et al. Fluvoxamine stimulates oligodendrogenesis of cultured neural stem cells and attenuates inflammation and demyelination in an animal model of multiple sclerosis. Sci Rep. 2017;7:4923. doi: 10.1038/s41598-017-04968-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Woodberry T., Bouffler S.E., Wilson A.S., Buckland R.L., Brüstle A. The emerging role of neutrophil granulocytes in multiple sclerosis. J Clin Med. 2018;7:511. doi: 10.3390/jcm7120511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Aubé B., Lévesque S.A., Paré A., Chamma É., Kébir H., Gorina R., et al. Neutrophils mediate blood-spinal cord barrier disruption in demyelinating neuroinflammatory diseases. J Immunol. 2014;193:2438–2454. doi: 10.4049/jimmunol.1400401. [DOI] [PubMed] [Google Scholar]
- 118.Pierson E.R., Wagner C.A., Goverman J.M. The contribution of neutrophils to CNS autoimmunity. Clin Immunol. 2018;189:23–28. doi: 10.1016/j.clim.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Carlson T., Kroenke M., Rao P., Lane T.E., Segal B. The Th17-ELR+ CXC chemokine pathway is essential for the development of central nervous system autoimmune disease. J Exp Med. 2008;205:811–823. doi: 10.1084/jem.20072404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Naegele M., Tillack K., Reinhardt S., Schippling S., Martin R., Sospedra M. Neutrophils in multiple sclerosis are characterized by a primed phenotype. J Neuroimmunol. 2012;242:60–71. doi: 10.1016/j.jneuroim.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 121.Desilles J.P., Di Meglio L., Delvoye F., Maïer B., Piotin M., Ho-Tin-Noé B., et al. Composition and organization of acute ischemic stroke thrombus: a wealth of information for future thrombolytic strategies. Front Neurol. 2022;13 doi: 10.3389/fneur.2022.870331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Roberts L., Haley M., South K., Smith C., Allan S. Detailed assessment of the microenvironment of stroke thrombi using imaging mass cytometry. Res Pract Thromb Haemost. 2023:7. doi: 10.1016/j.rpth.2023.100311. [DOI] [Google Scholar]
- 123.Dhanesha N., Ansari J., Pandey N., Kaur H., Virk C., Stokes K.Y. Poststroke venous thromboembolism and neutrophil activation: an illustrated review. Res Pract Thromb Haemost. 2023;7 doi: 10.1016/j.rpth.2023.100170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kohs T., Fallon M., Oseas E., Healy L., Lorentz C., Tucker E., et al. Pharmacological targeting of the contact pathway attenuates experimental autoimmune encephalomyelitis in mice. Res Pract Thromb Haemost. 2023;7 doi: 10.1016/j.rpth.2023.100482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Renné T., Stavrou E.X. Roles of factor XII in innate immunity. Front Immunol. 2019;10:2011. doi: 10.3389/fimmu.2019.02011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Verhamme P., Yi B.A., Segers A., Salter J., Bloomfield D., Büller H.R., et al. Abelacimab for prevention of venous thromboembolism. N Engl J Med. 2021;385:609–617. doi: 10.1056/NEJMoa2105872. [DOI] [PubMed] [Google Scholar]
- 127.Kleinschnitz C., Stoll G., Bendszus M., Schuh K., Pauer H.U., Burfeind P., et al. Targeting coagulation factor XII provides protection from pathological thrombosis in cerebral ischemia without interfering with hemostasis. J Exp Med. 2006;203:513–518. doi: 10.1084/jem.20052458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hagedorn I., Schmidbauer S., Pleines I., Kleinschnitz C., Kronthaler U., Stoll G., et al. Factor XIIa inhibitor recombinant human albumin Infestin-4 abolishes occlusive arterial thrombus formation without affecting bleeding. Circulation. 2010;121:1510–1517. doi: 10.1161/CIRCULATIONAHA.109.924761. [DOI] [PubMed] [Google Scholar]
- 129.Pinheiro A., Silbak S., Merkulova A., Skomorovska-Prokvolit Y., McCrae K., Midem D., et al. High molecular kininogen cleavage in cerebral malaria. Res Pract Thromb Haemost. 2023;7 doi: 10.1016/j.rpth.2023.100325. [DOI] [Google Scholar]
- 130.Denorme F., Armstrong N.D., Stoller M.L., Portier I., Tugolukova E.A., Tanner R.M., et al. The predominant PAR4 variant in individuals of African ancestry worsens murine and human stroke outcomes. J Clin Invest. 2023;133 doi: 10.1172/JCI169608. [DOI] [PMC free article] [PubMed] [Google Scholar]