Abstract
Purpose
Although preconception reproductive genetic carrier screening (RGCS) is preferred to screening during pregnancy, population-wide preconception screening is not routinely performed in the U.S. We explored the multilevel barriers to the widespread adoption of preconception RGCS in the U.S. via key informant interviews.
Methods
Semi-structured virtual video interviews were conducted with 29 informants with a breadth of professional expertise between May and October 2022. Data collection and qualitative analyses were guided by the Consolidated Framework for Implementation Research and socioecological model. Analysis focused on identifying barriers to delivering preconception RGCS at and across different levels of healthcare and exploring potential facilitators of preconception RGCS delivery.
Results
Barriers to preconception RGCS were identified at the levels of test characteristics, patients and couples, clinicians and care teams, and the external healthcare and policy environments. Across the different levels of care delivery three themes of barriers emerged: I. Fragmentation and inconsistencies hinder care delivery, II. Gaps in knowledge, misconceptions, and uncertainties about RGCS are pervasive, and III. Expanding preconception RGCS in the diverse U.S. population presents unique implementation challenges. Potential solutions were detailed by informants.
Conclusion
Identifying individual and thematic barriers to preconception RGCS delivery may help to define strategies to alleviate obstacles.
Keywords: qualitative data, carrier screening, healthcare delivery, implementation science, CFIR
Introduction
Reproductive genetic carrier screening (RCGS) is used to inform prospective parents’ risk of having a child affected by an autosomal recessive or X-linked disease. Although carriers that are heterozygous for a pathogenic variant typically do not exhibit overt clinical symptoms, their children may be at risk of the disease.1 Identifying biological parents at-risk for having an affected child allows them to seek genetic counseling and can inform reproductive decisionmaking. Both the American College of Obstetrics and Gynecology (ACOG) and American College of Medical Genetics (ACMG) endorse offering RGCS to pregnant patients, and acknowledge that performing screening preconception is preferable to prenatal screening,2–4
However, access to preconception RGCS is currently limited and often concentrated amongst individuals who seek out screening or who are undergoing fertility treatments.5,6 The delivery of preconception RGCS is complicated, and requires identifying patients prior to pregnancy, coordinating their testing and possibly that of a reproductive partner, delivering the results, and incorporating these results into their clinical care plan.7,8 Unlike RGCS programs instituted either outside of the U.S. or in relatively ancestrally homogenous U.S. communities,9–12 the geographic, socioeconomic, religious, political, and ethnic diversity of the U.S. as well as the structure of the healthcare system present distinct challenges for delivering population-wide preconception RGCS.
To better understand the barriers to broader implementation of preconception RGCS in the U.S. and to identify potential solutions that could help to guide implementation of preconception RGCS programs, we interviewed key informants with diverse professional and geographic backgrounds. We sought out informants with insights into patient care, underserved communities, healthcare industry, and the U.S. policy environment(s) that would influence if and how preconception RGCS could be offered more broadly to the public.
Materials and Methods
The study was deemed exempt by the Mass General Brigham (MGB) Institutional Review Board. Reporting of qualitative research was informed by the Consolidated criteria for reporting qualitative studies (COREQ): 32-item checklist,13 available in the Supplement.
Conceptual Framework & Interview Guide Development
The social ecological model of healthcare provided the overarching framework that we used to conceptualize the multiple levels at which barriers to preconception RGCS could exist. In the social ecological model, complex interactions between individuals, communities, care teams, the healthcare system, and external environmental context such as state and national programs and policies, may influence whether a public health screening program is acceptable to the interested parties involved.14
Given our emphasis on understanding informants’ perceptions of multilevel barriers and facilitators to preconception RGCS, the Consolidated Framework for Implementation Research15 informed interview guide development, in which we emphasized questions about the characteristics of individuals, the inner setting in which an intervention is implemented, and the outer setting in which the intervention exists.16 The interview guide was iteratively reviewed and piloted amongst the research team. A copy is available in the Supplement.
Study Population & Recruitment
An initial group of key informants was identified based on the prespecified goal to recruit informants with diverse expertise from different U.S. geographic regions. We drew upon internet-based searches (purposive sampling), informants known to the research team (convenience sampling), and recommendations from participants (snowballing approach)17 to identify additional informants. Interviews were conducted until both the research team was satisfied with the geographic and professional diversity of the informants and thematic saturation was achieved.18 Informants were recruited via emails that included a Study Fact Sheet, describing the goals of the study and the risks and benefits of participation, available in the Supplement. Informants who completed an interview were offered a $50 Amazon gift card as remuneration.
Data Collection
Of 99 informants approached, 29 agreed to participate (29%). Interviews were conducted between May and October 2022. Participants were scheduled for a single 30-minute semi-structured virtual video interview conducted using Microsoft® Teams, which allows for recording of the video interview and provides live transcription. Verbal consent to participate was obtained at the start of the interview. Informants were also asked to describe their own professional roles, which were noted by the study team. Interviews were conducted by L.E.H., a general internist and health services researcher (female), assisted by K.F., a clinical research coordinator with training in qualitative methods (female), who took field notes. At the conclusion of each interview, K.F. reviewed the video and cleaned the transcripts, making corrections to the automated transcription and removing any identifiers in preparation for data analysis.
Data Analysis
Qualitative data analysis began concurrently with data collection. Transcripts were coded with the assistance of NVivo v1.6.1 (QSR International). A hybrid inductive-deductive approach to coding was used in which CFIR constructs provided a baseline set of codes, and additional descriptive, simultaneous, versus, and structural codes were generated by the study team directly from analysis of the transcripts.19 L.E.H. and K.F. independently reviewed and coded the transcripts, meeting serially to compare results, discuss discrepancies, and update the code book, until all transcripts were coded; subsequent coding cycles further refined the initial coding and organized codes by the level of healthcare delivery (e.g. patient, provider, healthcare industry level) to which the coded text referred where possible. Analysis of codes across levels led to the recognition of emergent themes that spanned multiple levels of care delivery and were noted by informants of distinct and differing backgrounds.19 The manuscript was sent to participants and they were asked to review any quotations and descriptions attributed to them for accuracy, prior to submission for peer review.
Results
Characteristics of Key Informants
The 29 key informants who participated in the interviews had a broad range of professional expertise (Table 1). The majority (18/29, 62%) practiced clinical medicine or genetic counseling either at present or in the past, with clinical roles spanning adult and family medicine, obstetrics and gynecology, pediatrics and adolescent medicine, medical genetics, and genetic counseling. Additional areas of expertise included healthcare policy, bioethics, data and privacy systems, the insurance industry, patient and community advocacy work, non-profit leadership, genetic testing industry, community and population health, and contributions to the development of clinical guidelines. Participants were from 15 states across all four census regions.
Table 1.
Characteristics of Key Informants (N=29)
| Characteristic | N | % |
|---|---|---|
| Highest Educational Degree Completed | ||
| MD or MD/PhD | 12 | 41% |
| PhD | 6 | 21% |
| JD or JD/PhD | 3 | 10% |
| Master’s Level | 7 | 24% |
| Clinician (Past or Present) | ||
| Yes | 18 | 62% |
| No | 11 | 38% |
| Clinical Field (if relevant) | ||
| Clinical Genetics or Genetic Counseling | 8 | 28% |
| Internal or Family Medicine | 4 | 14% |
| Obstetrics & Gynecology (includes subspecialties) | 3 | 10% |
| Pediatrics (includes subspecialties) | 3 | 10% |
| Census Region of Residence | ||
| North | 4 | 14% |
| South | 12 | 41% |
| Midwest | 5 | 17% |
| West | 8 | 28% |
| Additional Role(s) or Expertise | ||
| Bioethics | 4 | 14% |
| Genetic Test Industry Experience | 3 | 10% |
| Healthcare Delivery/Insurance Industry | 3 | 10% |
| Patient Advocacy/Non-Profit Work | 6 | 21% |
| Health information technology and/or data privacy | 2 | 7% |
Missing: Degree (1)
Key Informants’ Current Priorities and Perceptions of Preconception RGCS
Key informants were asked about their priorities for reproductive care and/or genetics care. Access to reproductive healthcare was a high priority for most informants, who specifically expressed concerns about how inequitable access to reproductive health services across state lines, different levels of insurance coverage for reproductive care, accessibility of RGCS, other reproductive genetic testing, and other reproductive services. Additional priority areas included providing excellent education and counseling about reproductive genetic testing, improving reproductive healthcare quality, improving health equity, and ensuring the security of patient data and privacy. For some informants, RGCS was a priority of their work, whereas for others it was of lower relative priority compared to other aspects of health care. However, when comparing preconception versus prenatal RGCS, key informants overwhelmingly cited the relative advantages of performing reproductive RGCS prior to pregnancy:
“I think it’s a great option for women to be offered… I don’t think everybody should say yes, but I certainly think everybody should be offered it. I can’t think of a reason why you wouldn’t.”
– P20, Health behavior science researcher with expertise in genetic counseling
“We really do encourage it to happen prior to pregnancy as you’re planning your family. However, … when half of the births in the US are unplanned, it’s hard to plan when it may not be something that you thought about. So, while we encourage them to do it prior, if you are currently pregnant, it is still useful and valuable information.”
-P15, Non-profit leader supporting women’s health research and reproductive care
Barriers to Preconception RGCS in the U.S.
Despite their relative support for RGCS being offered prior to pregnancy rather than during pregnancy, key informants recognized a multitude of barriers to providing preconception RGCS on a population level (Figure 1). These barriers were organized by the level (test characteristics, patient and/or couple, clinician and clinical care team, the healthcare and insurance industry, the state, and the federal/national level) at which they would impact care delivery. Three specific themes emerged highlighting barriers to preconception RGCS that transcended different levels of care, including: I. Fragmentation and inconsistencies hinder delivery of care, II. Gaps in knowledge, misconceptions, and uncertainties about RGCS are pervasive, and III. Expanding preconception RGCS in the diverse U.S. population presents unique implementation challenges. Figure 1 highlights the different cross-level themes that are supported by individual barriers to preconception RGCS. A discussion of these barriers is organized by theme below.
Figure 1. Barriers to preconception RGCS in the U.S. are observed at all levels impacting healthcare delivery.
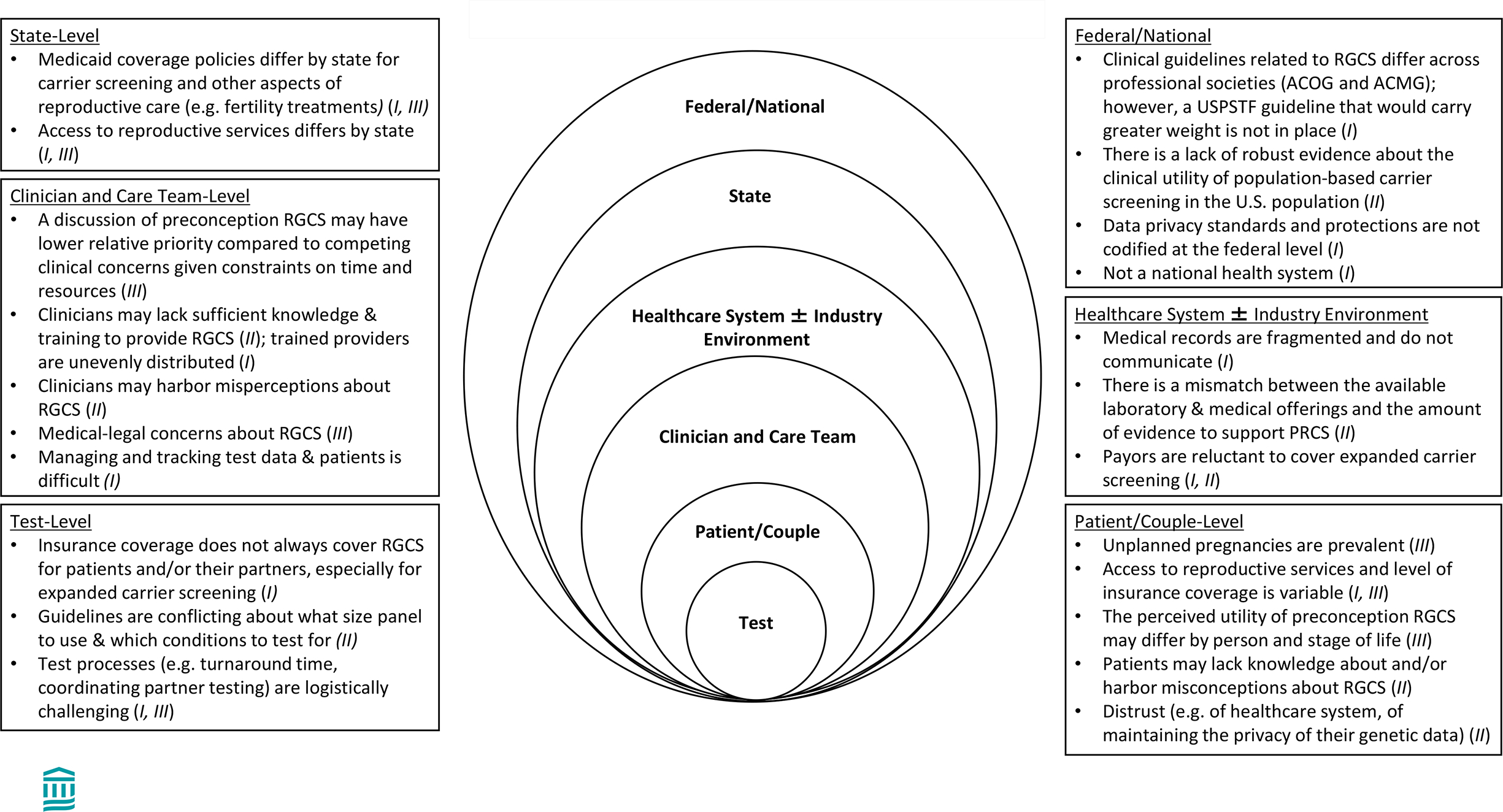
Key informants reported barriers to widespread use of preconception RGCS in the distinct U.S. environment. Barriers were organized by level. The cross-level theme(s) to which the barriers correspond are denoted by Roman Numerals in the parentheses. These cross-level themes include the following: I. Fragmentation and inconsistencies hinder delivery of care. II. Gaps in knowledge, misconceptions, and uncertainties about reproductive genetic RGCS are pervasive, and III. Expanding preconception RGCS in the diverse U.S. population presents unique implementation challenges.
Abbreviations: ACOG = American College of Obstetricians and Gynecologists; ACMG = American College of Medical Genetics; RGCS = reproductive genetic carrier screening; USPSTF = United States Preventive Services Taskforce
Theme I: Fragmentation and inconsistencies hinder delivery of care.
Fragmentation, inconsistencies, and resulting inequities in access to preconception RGCS and other reproductive care was a major theme unifying several barriers across levels. At the national level, informants cited fragmentation of the U.S. healthcare system in the absence of a national system and integrated electronic medical records, a lack of federal protections on data privacy, and discrepancies between professional medical societies regarding the number of conditions that should be included on a RGCS panel as specific barriers to preconception RGCS (Figure 1, Theme I). For example, informants cited inconsistent guidelines regarding the number of conditions to include as part of RGCS, from expanded RGCS, or screening for a large number of recessive conditions simultaneously,20 to targeted screening for a set of core conditions, as a barrier:
“So, the fact that ACOG and ACMG now say something different about when carrier screening should be offered and to what extent … does not help access. A payer will say … ACOG still doesn’t recommend expanded carrier screening… So there has to be better consistency across those guidelines.”
-P11, Researcher working in the genetic testing industry
Inconsistencies across states were also emphasized as barriers to reproductive care, generally, and preconception RGCS, because of different access to medical and support services across states. Informants also emphasized inequities across states, such as Medicaid eligibility and generosity of benefits across states, as a barrier to preconception RGCS. They also emphasized concerns that not all individuals or couples who are able to obtain preconception RGCS (including those with private insurance) who are found to be at-risk for having an affected child will necessarily be able to pursue advanced reproductive options like in vitro fertilization with preimplantation genetic diagnosis due to insufficient insurance coverage and/or financial limitations.
“The first issue is who is [preconception RGCS] being offered to and I think that’s largely an equity issue and an issue around sort of fairness and justice. The next issue… is how is it being paid for, which again raises issues around access and equity. “
– P21, Bioethicist with focus on the ethical, legal, and social implications of genetics
“We know by getting carrier screening out to the masses there are going to be people that find out that they have a risk who aren’t going to have the resources to take advantage of options to help them have a healthy family. So we say, oh, this is amazing, we can tell you have a risk for child with Tay Sachs disease, with sickle cell disease… and you could do in vitro fertilization [and] you could get a gamete donor… and then people in a situation where there’s no resources to be able to take advantage of these options… [W]e don’t want to put people in situations where they can’t use the information in a positive way… I think that giving people access to next steps is really important.”
– P1, Genetic counselor working in both academia and a non-profit
Additionally, some participants cited the overturn of Roe v. Wade and how that might limit the options available to pregnant patients found to be carrying a fetus with a severe heritable disease in different states:
“In terms of genetic screening, there’s a lot of parts of the United States where women don’t have access to genetic screening, but if they do genetic screening and they find out they are carrying a fetus with some sort of fatal mutation or other very life altering condition that if they don’t have access to abortion, the whole thing kind of falls apart.”
– Participant(P)12, Obstetrician and healthcare policy researcher
One informant emphasized that offering RGCS without considering access to support acting on the results would be irresponsible:
“You have to solve for the damage of knowing the risk…. [Y]ou just can’t tell me that my child is likely to have sickle cell because I and my partner, my wife or husband have sickle cell. You’ve now burdened me with information that I probably don’t want to know. So if you burden me with the information, you’ve hopefully already solved for my hesitancy around that. [And you] need to make sure that I have resources once I’ve been burdened with that information. What are my resources [such] that if I decide to manage that risk, to accept that risk, have that child with that partner, potentially with sickle cell, what are the back end resources after that child is born, either in the immediacy or over time?… that’s insurance, that’s wrap around services, that might be mental health support, groups with other sickle cell parents… But, to just burden me with the risk without resources is neglectful in my opinion.”
-P4, Attorney and community health advocacy leader
Concerns about fragmentation of healthcare were not limited to RGCS. Several informants noted that one might learn that they are heterozygous for pathogenic variant(s) implicated in several severe conditions via newborn screening programs, but interstate differences in newborn screening programs and follow-up protocols limit the ability to access and use newborn screening data to later inform one’s reproductive care:
“We have a lot of young adults that were tested at birth for sickle cell trait and disease, but there’s not uniformity with how states follow up with that… also information that may get lost along the way for patients…”
- P24, Expert in disease education with focus on sickle cell disease and hemoglobinopathies
Theme II: Gaps in knowledge, misconceptions, and uncertainties about reproductive genetic RGCS are pervasive.
Key informants also described a lack of knowledge, misconceptions, and uncertainties about RGCS at both the patient/couple and clinician levels (Figure 1, Theme II). For patients, these ranged from a lack of awareness about RGCS, to not understanding their personal risk of disease in the absence of symptoms, to concerns about eugenics.
“[I’m] hearing all the time, I’m not worried about this, we don’t have anyone in our family with a genetic disease. [T]here’s really not a good understanding in the general public that when you’re a carrier of something that you don’t have symptoms and that you wouldn’t know you are a carrier… unless you had an affected child or got tested. ”
-P1, Genetic counselor working in both academia and a non-profit
Clinicians were also noted to have uneven knowledge and comfort about RGCS, different understandings of the clinical utility of screening, and to hold certain misperceptions about RGCS.
“I’ve [worked traveling across] about 25% of the country, both large and small communities, and there seems to be a significant lack of understanding as to why preconception carrier screening would be important, how that information could potentially be used, and also a lack of understanding about the financial coverage for preconception [RGCS]. There’s a very common misconception by providers that you have to be pregnant first, before insurers will cover the cost of carrier screening.”
-P8, Genetic counselor working in the genetic testing industry
“So I hear a lot from families and also from providers that when they were pregnant, their doctors would say to them if you don’t want to have carrier screening, don’t worry, you’ll have newborn screening. And that makes me cringe because I definitely think that there is a place for both, and I think our medical community often blurs them….”
-P18, Genetic counselor with experience in the genetic testing industry and non-profits
At the national level, some of the uncertainty around screening was felt to result from conflicting and nonspecific professional society guidelines about RGCS, as well as the absence of a guideline from the US Preventive Services Taskforce (Figure 1, Theme II).
“I hear frequently that that these organizations come out with these ambiguous wishy washy guidelines, where it doesn’t tell clinicians what to do and when.”
-P13, Health economist
Theme 3: Expanding preconception RGCS in the diverse U.S. population presents unique implementation challenges.
Given the diversity of ways that preconception RGCS could be offered, and the variety of factors that could impact acceptance of screening, key informants endorsed the need for multiple ways of offering screening to meet the diverse motivations, goals, and needs of patients, clinicians, and the healthcare system (Figure 1, Theme III).
“I actually think… in order for this to be done in the system that we have today, it really would require multiple places and multiple modalities.”
-P18, Genetic counselor working in testing industry
“I think it could be offered in a number of different ways concurrently. … it would be a mistake to assume that everyone’s comfortable with one approach.”
-P25, Attorney with specialty in genetics and the law
Specifically, informants catalogued the diversity of patient needs based on whether they are planning a pregnancy, have access to reproductive care, trust in the healthcare system, and perceive the utility of RGCS for themselves and their family planning. Additionally, tailored approaches could meet the needs of clinicians and care teams who may have different relative priorities for offering preconception RGCS and specific concerns about delivery of this care.
Potential strategies and solutions suggested by key informants
Key informants also noted several strategies that could be used to inform preconception RGCS implementation efforts, which are detailed by relevant theme in Table 2. To address fragmentation and inconsistencies in care delivery (Theme I), informants suggested clinical processes and policies that could promote equitable access to care and reconcile inconsistencies in clinical guidelines. Informants suggested that building the evidence basis to support population-based preconception RGCS, while considering the impact of advances in medicine in technology, could help to reduce uncertainties about RGCS (Theme II). Educational interventions for clinicians, care teams, communities, and their leaders, could help to address gaps in knowledge (Theme II). Finally, to design and tailor preconception RGCS programs for diverse communities (Theme III), informants suggested directly engaging communities and interested parties, as well as applying lessons from the successes and failures of relevant examples.
Table 2. Potential solutions suggested by key informants to guide implementation of preconception reproductive carrier screening (RGCS) in the U.S.
Potential solutions are organized to address the three thematic barriers to preconception RGCS that cut across levels of healthcare delivery.
| Address the fragmentation and inconsistencies that hinder access to preconception RGCS (Theme I). | |
|
Implement and support policies promoting equitable access to reproductive care. • Use technology, virtual care, and automated processes as able to increase access to care. • Provide care in settings that would reach patients prior to pregnancy (e.g. adolescent medicine, primary care, community health programs) • Engage advanced practice providers. • Improve genetic counseling reimbursement. • Resolve issues around protecting data privacy. • Mandate insurance coverage for preconception RGCS. Reconcile inconsistencies. • Reconcile ACOG and ACMG RGCS guidelines. |
“To have good preconception screening… it would have to come out of primary care because people don’t usually go to obstetrical care until they’re actually pregnant. This is just like any other public health thing that you want people to do before they get pregnant… so this is it would really need to be integrated into primary care and part of regular well person care.” -P19, Medical geneticist with expertise in newborn screening “I believe that policies need to be changed, laws need to be set in place where […] everybody who is having children should be able to access [carrier] screening and have it covered by their health care insurance.” -P6, Patient advocacy leader for carrier screening |
| Reduce gaps in knowledge, misconceptions, and uncertainties about RGCS (Theme II). | |
|
Gather data to address uncertainties. • Investigate how advances in medicine and technology (e.g. gene therapies) impact how RGCS results could be interpreted and used. • Evaluate the implementation, outcomes, and cost-effectiveness of preconception RGCS programs. • Seek a USPSTF guideline on use of RGCS to inform primary care clinician practice. Educate interested parties about RGCS. • Educate clinicians, care teams, patient influencers, and community members about preconception RGCS. • Enhance quality of care by helping clinicians improve family health history data collection, by creating scripts to discuss testing with patients, and by facilitating high quality counseling when results are returned. |
“Finally, from the provider perspective, there are many competing priorities, for what to talk about depending on the circumstances of the visit. Certainly, having an organization like the US Preventive Services Task Force recommend something would put it on the radar screen of a lot of primary care providers.” – P12, Obstetrician and healthcare policy researcher “Making sure that nurse practitioners, as well as women’s health nurse practitioners as well as midwives, who really are part of the delivery of services in many clinics throughout the country are educated on what they ought to be saying in a scripted type format, at least.” -P16, Academic maternal fetal medicine specialist “Maybe you can educate clergy like rabbis who are doing premarital counseling. I believe that patient advocacy groups can help to get this information out.” -P6, Patient advocacy leader for carrier screening |
| Develop multiple approaches to deliver preconception RGCS to the diverse U.S. population (Theme III). | |
|
Engage diverse communities. • Address sources of healthcare mistrust and patient fears. • Market preconception RGCS to the target population. • Engage influencers and trusted sources in the community to garner support for preconception RGCS (e.g. doctors, parents, clergy). • Harness the power of coalitions and advocacy groups. Apply lessons from relevant examples. • Examine the impact of California laws for mandated prenatal screening. • Compare RGCS with other preventive genetic tests (e.g. multi-cancer early detection tests/liquid biopsies; non-invasive prenatal testing and trials of rapid exome sequencing in neonatal intensive care units). • Learn from the success and failures of other carrier screening programs (Dor Yeshorim,36 examples of SCT testing programs37 (e.g. Black Panthers, NCAA screening, marriage requirements). • Examine newborn screening programs. • Compare RGCS with at home molecular diagnostic testing (e.g. COVID-19 antigen tests, 23andMe) and cancer screening programs (e.g. acculturation of routine pap smears as part of well woman care). • Preconception programs (e.g. preconception folic acid supplementation) |
“It is truly a multi-level, multi-tiered approach that involves a lot of really different groups… So really get a good sense of who are those stakeholders and involve them early in the process of implementation.” – P18, Genetic counselor working in the genetic testing industry “It’s going to have to be at least to a significant extent patient driven. So what does that mean? It means you’re going to have to take some time before you get this off the ground to understand what the patients want from this program, not just what we want as researchers and clinicians, right, but what they want, which could well be at odds… with what we are thinking of providing, right. And… you might have to make concessions in terms of the research that could be done or the clinical applications that could be done in order to respect and embrace what the public is saying.” – P27, Bioethicist with a focus on ESLI research related to genetics “So [Tay Sachs] is a really interesting case study about how to do this in a way that really involved the high-risk community and actually made it a cultural issue. And I think the whole success behind Tay Sachs carrier screening comes from just a remarkably interesting way that it was done… there were a lot of worries about bias and discrimination and being treated differently. I think we’ve seen the same thing in looking at sickle cell. That has not gone as well as Tay Sachs… Those are two kinds of really interesting case studies to look at. “ -P9, Family medicine physician with expertise in preventive care |
Abbreviations: ACMG = American College of Medical Genetics; ACOG = American College of Obstetricians and Gynecologists; ELSI = ethnical, social, and legal implications; NCAA = National Collegiate Athletic Association; preconception RGCS = Preconception reproductive carrier screening; SCT = Sickle cell trait; USPSTF = United States Preventive Services Taskforce
Discussion
Interviews with 29 key informants with expertise and experience in clinical care, healthcare delivery, genetics and genomics, community advocacy and engagement, industry, policy, and bioethics, data privacy, and law highlighted the complexity and multilevel barriers to broader implementation of preconception RGCS in the uniquely diverse and federalist U.S. setting. The themes identified that cut across the different levels impacting care delivery provide some insights into the structural challenges to providing this testing, as well as other reproductive and/or preventive genetics care.
One of the most highly cited barriers to care delivery was concerns about inequitable access to healthcare, especially reproductive healthcare. Many informants shared that this subject was top of mind for them, especially as the interviews were conducted concurrently with the Supreme Court decision that overturned Roe vs. Wade. Specifically, the interviews began in May 2022, the same month a draft of the Dobbs vs. Jackson’s Womens Health decision was leaked to the public,21 and continued while the final decision was announced in June 2022, holding that the U.S. Constitution does not confer a right to abortion.22 The decision opened a path for individual states to ban, restrict, or protect access to abortion.23 In response to this decision, the American Society of Human Genetics, ACMG, and National Society of Genetic Counselors issued a statement condemning the ruling and specifically raising concerns about how legislation restricting access to abortion denies patients the ability to make informed decisions about their healthcare based on prenatal genetic diagnoses, exacerbating inequities in access to healthcare.24 The concerns cited by these societies mirrored those raised by our key informants. Additionally, given that RGCS, unlike other prenatal genetic tests, can be performed prior to conception, many informants emphasized greater urgency and increased relative clinical utility of performing screening preconception considering potential restrictions on how the results could inform care if performed during pregnancy.
In addition to inconsistent access to reproductive healthcare offerings across state lines,25 informants also described inconsistent and weak clinical guidelines as another major barrier to preconception RGCS delivery. Informants highlighted that differences in the number of conditions recommended to be screened for in guidelines issued by the ACMG and ACOG create confusion for ordering providers, industry, and payors. Additionally, several cited the lack of a US Preventive Services Task Force guideline recommending RGCS as a core preventive care offering, also citing insufficient evidence to issue such a recommendation at a population level. Indeed, a recently updated Cochrane review did not find any clear randomized control trial (RCT) or quasi-RCT studies comparing the outcomes of prenatal vs. preconception RGCS.26 Furthermore, a systematic review of measured outcomes in studies of RGCS highlighted the diversity of endpoints that could be assessed when trying to determines the clinical impact of preconception RGCS programs.27 Identifying which endpoints should be assessed to weight the impact of RGCS on population health and generating high quality data to answer these questions will be necessary to inform clinical guidelines.
Of course, even if there were strong clinical recommendations issued for population-based preconception RGCS, improving patient and provider knowledge and understanding of RGCS would be necessary to promote responsible care delivery. Fortunately, when preconception RGCS programs have been implemented, increasing non-genetics providers experience with RGCS has also improved their comfort with it.28 Developing tools to further facilitate providing high quality post-test counseling will be necessary to avoid leaving patients feeling unsupported and with insufficient understanding of their results.29
While our study was not designed to elicit diverse patient perspectives on how preconception RGCS is delivered to patients, informants emphasized that it is unlikely that a single approach would meet the needs of diverse patients and communities. While studies have looked at specific preferences for RGCS delivery, many of these have been performed in distinct communities or populations,10,30–33 and do not necessarily reflect the healthcare delivery system and preferences of U.S. patients. A systematic review of conjoint analyses of participant preferences for genetic testing found only a single study that included an assessment of why people choose to participate in RGCS.34,35 Future work could seek to elicit patient preferences for preconception RGCS delivery to inform the delivery of this screening test.
Limitations
Our study has several limitations to acknowledge. First, while we attempted to recruit a broad array of informants, as with any qualitative study our findings remain limited in their generalizability. Our use of snowball sampling to identify additional informants could also result in the amplification of related viewpoints. For example, our informants also included several individuals enthusiastic about expanded use of RGCS. Therefore, our findings should be used for exploratory and hypothesis-generating purposes only. Finally, a degree of subjectivity is both expected and unavoidable in qualitative inquiries and analysis. To minimize this, we drew upon theory and frameworks, as well as independent coding and reconciliation processes to try to mitigate the risk of overlaying our own biases on the interpretation and presentation of the data (reflexivity) and by using validated checklists to provide transparency regarding our processes.13
Conclusions
Qualitative analysis of interviews with 29 key informants from across the U.S. provided insights into multiple levels of barriers to widespread adoption of preconception RGCS. Enacting state and federal policies increasing and unifying support for reproductive healthcare services and issuing clear clinical guidelines would facilitate access to preconception RGCS. However, even with such changes, at the point of care delivery considering diverse and novel models for delivering this care will be necessary to engage the heterogeneous U.S. population and healthcare infrastructure. Further work understanding patient preferences for delivery of preconception RGCS and probing the impacts of population-based preconception RGCS on patients, communities, clinicians, healthcare systems, and society will be necessary to start to design effective delivery systems.
Supplementary Material
Acknowledgements
We thank Dr. Lara Traeger, PhD who provided consultation on qualitative methodologies through the Massachusetts General Hospital’s Division of Clinical Research. We also thank the study participants for donating their time and expertise to this research.
Financial Support:
Dr. Hull is supported by the National Human Genome Research Institute of the National Institutes of Health (K08HG012221). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest Disclosure:
The authors declare no conflicts of interest.
Ethics Declaration
All study procedures were reviewed by the Mass General Brigham (MGB) Institutional Review Board, which deemed the study exempt. Verbal informed consent was obtained from all participants prior to the start of each interview; transcripts were reviewed and personal identifiers were removed.
Prior Presentations:
“Key informant perspectives on implementation of preconception carrier screening programs in the U.S.” December 13th, 2022.15th Annual Conference on the Science of dissemination and Implementation in Health. Walter E. Washington Convention Center, Washington, DC. Poster Presentation.
Note to the Reader:
We recognize that there is a broad range of family structures with a desire to have and raise children. For the purposes of this paper, we use the phrasing ‘couple’ to refer to the genetic parents of the pregnancy or intended pregnancy.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability
Partial redacted transcripts will be made available upon request. Given the sensitive nature of qualitative data and to protect the identity of our informants, only the relevant, unpublished segments of interview transcripts will be made available for the purposes of verifying or contextualizing our conclusions.
References
- 1.Antonarakis SE. Carrier screening for recessive disorders. Nat Rev Genet. 2019;20(9):549–561. doi: 10.1038/s41576-019-0134-2 [DOI] [PubMed] [Google Scholar]
- 2.ACOG committee opinion no. 762 summary: Prepregnancy counseling. Obstet Gynecol. 2019;133(1):228–230. doi: 10.1097/AOG.0000000000003014 [DOI] [PubMed] [Google Scholar]
- 3.Committee opinion no. 691. Obstet Gynecol. 2017;129(3):e41–e55. doi: 10.1097/aog.0000000000001952 [DOI] [PubMed] [Google Scholar]
- 4.Gregg AR, Aarabi M, Klugman S, et al. Screening for autosomal recessive and X-linked conditions during pregnancy and preconception: a practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med. Published online July 20, 2021. doi: 10.1038/s41436-021-01203-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hull LE, Cheng D, Hallman MH, Rieu-Werden ML, Haas JS. Association of patient and site-of-care characteristics with reproductive carrier screening timing in a large integrated health system. JAMA Netw Open. 2022;5(11):e2240829. doi: 10.1001/jamanetworkopen.2022.40829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansen Taber KA, Beauchamp KA, Lazarin GA, Muzzey D, Arjunan A, Goldberg JD. Clinical utility of expanded carrier screening: results-guided actionability and outcomes. Genet Med. 2019;21(5):1041–1048. doi: 10.1038/s41436-018-0321-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rowe CA, Wright CF. Expanded universal carrier screening and its implementation within a publicly funded healthcare service. J Community Genet. 2020;11(1):21–38. doi: 10.1007/s12687-019-00443-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krier JB, Kalia SS, Green RC. Genomic sequencing in clinical practice: applications, challenges, and opportunities. Dialogues Clin Neurosci. 2016;18(3):299–312. doi: 10.31887/dcns.2016.18.3/jkrier [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathijssen IB, Holtkamp KCA, Ottenheim CPE, et al. Preconception carrier screening for multiple disorders: evaluation of a screening offer in a Dutch founder population. Eur J Hum Genet. 2018;26(2):166–175. doi: 10.1038/s41431-017-0056-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nesbit CB, Pollack CC, Mascia NS, et al. Interest in and uptake of genetic counseling for preconception carrier screening when offered to predominantly white reproductive-age persons seeking gynecologic care at a single U.S. academic medical center. J Genet Couns. 2022;31(1):109–119. doi: 10.1002/jgc4.1457 [DOI] [PubMed] [Google Scholar]
- 11.Castellani C, Picci L, Tamanini A, Girardi P, Rizzotti P, Assael BM. Association between carrier screening and incidence of cystic fibrosis. JAMA. 2009;302(23):2573–2579. doi: 10.1001/jama.2009.1758 [DOI] [PubMed] [Google Scholar]
- 12.Singer A, Sagi-Dain L. Impact of a national genetic carrier-screening program for reproductive purposes. Acta Obstet Gynecol Scand. 2020;99(6):802–808. doi: 10.1111/aogs.13858 [DOI] [PubMed] [Google Scholar]
- 13.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–357. doi: 10.1093/intqhc/mzm042 [DOI] [PubMed] [Google Scholar]
- 14.Zapka J, Taplin SH, Ganz P, Grunfeld E, Sterba K. Multilevel factors affecting quality: examples from the cancer care continuum. J Natl Cancer Inst Monogr. 2012;2012(44):11–19. doi: 10.1093/jncimonographs/lgs005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4(1):50. doi: 10.1186/1748-5908-4-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CFIR booklet. Accessed February 22, 2023. https://cfirguide.org/guide/app/#/guide
- 17.Maxwell JA. Qualitative Research Design. 3rd ed. SAGE Publications; 2013. [Google Scholar]
- 18.Guest G, Namey E, Chen M. A simple method to assess and report thematic saturation in qualitative research. PLoS One. 2020;15(5):e0232076. doi: 10.1371/journal.pone.0232076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saldana JM. The Coding Manual for Qualitative Researchers. 3rd ed. SAGE Publications; 2015. [Google Scholar]
- 20.Committee opinion no. 690 summary: Carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129(3):595–596. doi: 10.1097/aog.0000000000001947 [DOI] [PubMed] [Google Scholar]
- 21.Gerstein J, Ward A. Exclusive: Supreme Court has voted to overturn abortion rights, draft opinion shows. POLITICO. Accessed March 6, 2023. https://www.politico.com/news/2022/05/02/supreme-court-abortion-draft-opinion-00029473 [Google Scholar]
- 22.Opinions of the Court - 2021. Published December 31, 1600. Accessed March 6, 2023. https://www.supremecourt.gov/opinions/slipopinion/21
- 23.The. Tracking the states where abortion is now banned. The New York times. https://www.nytimes.com/interactive/2022/us/abortion-laws-roe-v-wade.html. Published May 24, 2022. Accessed March 6, 2023. [Google Scholar]
- 24. [Accessed March 6, 2023.]. https://www.acmg.net/PDFLibrary/Dobbs.
- 25.Weigel G, Ranji U, Long M, Salganicoff A. Coverage and use of fertility services in the U.s. KFF. Published September 15, 2020. Accessed March 6, 2023. https://www.kff.org/womens-health-policy/issue-brief/coverage-and-use-of-fertility-services-in-the-u-s/ [Google Scholar]
- 26.Hussein N, Henneman L, Kai J, Qureshi N. Preconception risk assessment for thalassaemia, sickle cell disease, cystic fibrosis and Tay-Sachs disease. Cochrane Database Syst Rev. 2021;10(10):CD010849. doi: 10.1002/14651858.CD010849.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson E, McEwen A, Newton-John T, Crook A, Jacobs C. Systematic review of outcomes in studies of reproductive genetic carrier screening: Towards development of a core outcome set. Genet Med. 2022;24(1):1–14. doi: 10.1016/j.gim.2021.08.005 [DOI] [PubMed] [Google Scholar]
- 28.Best S, Long JC, Fehlberg Z, et al. The more you do it, the easier it gets: using behaviour change theory to support health care professionals offering reproductive genetic carrier screening. Eur J Hum Genet. Published online November 24, 2022. doi: 10.1038/s41431-022-01224-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayo-Gamble TL, Schlundt D, Cunningham-Erves J, et al. Sickle cell carriers’ unmet information needs: Beyond knowing trait status. J Genet Couns. 2019;28(4):812–821. doi: 10.1002/jgc4.1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Archibald AD, Hickerton CL, Wake SA, Jaques AM, Cohen J, Metcalfe SA. “It gives them more options”: preferences for preconception genetic carrier screening for fragile X syndrome in primary healthcare. J Community Genet. 2016;7(2):159–171. doi: 10.1007/s12687-016-0262-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plantinga M, Birnie E, Abbott KM, et al. Population-based preconception carrier screening: how potential users from the general population view a test for 50 serious diseases. Eur J Hum Genet. 2016;24(10):1417–1423. doi: 10.1038/ejhg.2016.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Steijvoort E, Demuynck R, Peeters H, et al. Reasons affecting the uptake of reproductive genetic carrier screening among nonpregnant reproductive-aged women in Flanders (Belgium). J Genet Couns. 2022;31(5):1043–1053. doi: 10.1002/jgc4.1575 [DOI] [PubMed] [Google Scholar]
- 33.Housten AJ, Abel RA, Lindsey T, King AA. Feasibility of a community-based sickle cell trait testing and counseling program. J Health Dispar Res Pract. 2016;9(3). https://www.ncbi.nlm.nih.gov/pubmed/27774352 [PMC free article] [PubMed] [Google Scholar]
- 34.Hall J, Fiebig DG, King MT, Hossain I, Louviere JJ. What influences participation in genetic carrier testing? Results from a discrete choice experiment. J Health Econ. 2006;25(3):520–537. doi: 10.1016/j.jhealeco.2005.09.002 [DOI] [PubMed] [Google Scholar]
- 35.Ozdemir S, Lee JJ, Chaudhry I, Ocampo RRQ. A systematic review of discrete choice experiments and conjoint analysis on genetic testing. Patient. 2022;15(1):39–54. doi: 10.1007/s40271-021-00531-1 [DOI] [PubMed] [Google Scholar]
- 36.Jewish genetic screening. Dor Yeshorim. Published May 27, 2022. Accessed March 28, 2023. https://doryeshorim.org/
- 37.National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Population Health and Public Health Practice, Committee on Addressing Sickle Cell Disease: A Strategic Plan and Blueprint for Action. Addressing Sickle Cell Disease. (Martinez RM, Osei-Anto HA, McCormick M, eds.). National Academies Press; 2021. doi: 10.17226/25632 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Partial redacted transcripts will be made available upon request. Given the sensitive nature of qualitative data and to protect the identity of our informants, only the relevant, unpublished segments of interview transcripts will be made available for the purposes of verifying or contextualizing our conclusions.


