Abstract
Background:
The addition of growth factiors is commonly applied to improve the osteogenic differentiation of stem cells. However, for human pluripotent stem cells (hPSCs), their complex differentiation processes result in the unknown effect at different stages. In this study, we focused on the widely used bone forming peptide-1 (BFP-1) and investigated the effect and mechanisms of its addition on the osteogenic induction of hPSCs as a function of the supplementation period.
Methods:
Monolayer-cultured hPSCs were cultured in osteogenic induction medium for 28 days, and the effect of BFP-1 peptide addition at varying weeks was examined. After differentiation for varying days (0, 7, 14, 21 and 28), the differentiation efficiency was determined by RT–PCR, flow cytometry, immunofluorescence, and alizarin red staining assays. Moreover, the expression of marker genes related to germ layers and epithelial-mesenchymal transition (EMT) was investigated at day 7.
Results:
Peptide treatment during the first week promoted the generation of mesoderm cells and mesenchymal-like cells from hiPSCs. Then, the upregulated expression of osteogenesis marker genes/proteins was detected in both hESCs and hiPSCs during subsequent inductions with BFP-1 peptide treatment. Fortunately, further experimental design confirmed that treating the BFP-1 peptide during 7–21 days showed even better performance for hESCs but was ineffective for hiPSCs.
Conclusion:
The differentiation efficiency of cells could be improved by determining the optimal treatment period. Our study has great value in maximizing the differentiation of hPSCs by adding osteogenesis peptides based on the revealed mechanisms and promoting the application of hPSCs in bone tissue regeneration.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13770-023-00597-y.
Keywords: Human pluripotent stem cells, Osteogenic differentiation, Bone forming peptide, Supplement period, Epithelial-mesenchymal transition
Introduction
To solve the shortcomings of current therapies, such as limited autogenous sources, secondary surgery and immunogenicity, bone tissue engineering technology has been developed to treat significant bone defects in the clinic [1, 2]. However, how to obtain enough functional seed cells of osteoblasts still needs to be solved. Human mesenchymal stem cells (hMSCs) and human pluripotent stem cells (hPSCs) are commonly applied to induce osteoblast-like cells in vitro. The latter exhibit better abilities in both long-term self-renewal and specialized differentiation, becoming an outstanding cell source in the field of regenerative medicine [3, 4].
It is well known that hPSCs must be differentiated toward osteoprogenitor cells or osteoblast-like cells before transplantation to avoid tumorigenicity. To date, many studies have been performed to achieve the osteogenic differentiation of hPSCs using the embryoid body method or monolayer induction method [5, 6]. Unfortunately, the differentiation efficiencies in most published reports are relatively low [5]. In this regard, many kinds of growth factors have been applied to establish robust and efficient induction methods for the derivation of osteoblast-like cells from hPSCs [7, 8]. However, they are mainly added throughout the whole induction process. Notably, during in vivo embryonic development, mesoderm and ectoderm cells differentiate toward mesenchymal cells, which further differentiate into osteogenic precursor cells and osteoblasts [9, 10]. There is no doubt that each growth factor should be supplemented at a specific stage to treat corresponding cell types [11]. For example, it has been reported that enamel matrix derivatives (EMD) added at the early stage promote the differentiation of hPSCs into osteoblasts, but they inhibit cell maturation and mineralization at the late stage [12]. Therefore, hPSCs treated with growth factors at specific stages of osteogenic differentiation are critical to establishing a stepwise osteogenic induction system.
Bone morphogenetic proteins (BMPs), a member of the TGF-β superfamily, play important roles in processes such as human embryonic development, bone formation and cartilage formation [13]. Moreover, many researchers believe BMPs are the most potent osteoinductive growth factors available [14]. Nevertheless, the use of BMPs has some drawbacks that should not be neglected. For example, they are produced as recombinant proteins in heterologous expression systems at high costs. Since high dose requirements are used in clinical therapies, BMP-based treatments are not only very costly for patients but also cause new problems, including instability, immune response and real safety applications [15–17]. Adverse effects such as heterotopic bone formation, pseudoarthrosis and local inflammation have been reported after treatment with BMP-2 in nonspinal orthopedic interventions. BMPs can trigger problematic immune responses in some cases [18]. Therefore, there is a clear need for alternative approaches to either completely avoid or, at least, significantly reduce the amount of BMPs needed in BTE strategies to promote tissue healing with high efficiency and fewer side effects. To solve these problems, cost-effective peptides have recently received much attention [19]. Peptides can be easily produced in vitro using liquid- or solid-phase synthesis procedures in a cost-effective manner and, due to their small size, are more unlikely to trigger immune responses [20]. It is worth noting that peptides, with their short amino acid sequences, exhibit superior stability due to their robust secondary and tertiary structures, resistance to enzymatic degradation, and chemical stability [21]. Furthermore, their size also supports more molecules to be grafted on the biomaterial, exposing accelerated densities of active sites to the target cells. Finally, different peptides can be involved in biomaterial to promote the cell adhesion, proliferation, differentiation of stem cells [19]. In 2012, an osteogenesis bone forming peptide-1 (BFP-1; GQGFSYPYKAVFSTQ sequence) was derived from the immature form of bone morphogenetic protein-7 (BMP-7) with enhanced osteogenesis performance [22]. Compared to the BMP-7 protein, BFP-1 avoids folding and self-assembly and reduces steric hindrance during the modification process, showing excellent prospects in tissue engineering [23]. To date, the BFP-1 peptide has been proven to promote the aggregation and osteodifferentiation of hPSCs [24, 25]. However, BFP-1-containing induction medium was applied constantly [22, 26], and it is necessary to clarify the specific supplementation time period to maximize its effect on osteogenic differentiation.
In this study, monolayer-cultured hESCs and hiPSCs were cultured in osteogenic induction medium for 28 days, and the effect of BFP-1 addition at varying weeks was serially assessed. After differentiation for varying days (0, 7, 14, 21 and 28), RT–PCR and immunofluorescence were used to study the expression of related gene/protein markers in cell samples, and the differentiation efficiency was also determined by alizarin red staining assay. Based on these results using peptide treatment throughout the induction process or not as controls, the impact of peptide addition on the differentiation process of hPSCs was discussed to confirm the optimal addition times for hESCs and hiPSCs. Moreover, the expression of marker genes related to germ layers and epithelial-mesenchymal transition (EMT) was investigated at day 7 to study the impact of the BFP-1 peptide on the lineage differentiation and osteogenic induction process of hESCs and hiPSCs. Our work has great value in improving the in vitro osteogenic differentiation efficiency of hPSCs by adding functional osteogenesis compounds at specific stages and promoting the fundamental and clinical applications of osteoblast-like cells.
Materials and methods
Cell culture
The hESCs lines H9 was provided by WiCell Research Institute (Madison, WI, USA) [27]. The hiPSCs lines f-hiPSCs derived via Sendai virus (Invitrogen, Carlsbad, CA, USA) were supplied by Guangzhou Institutes of Biomedicine and Health (Guangzhou, China) as gifts and the f-hiPSCs was generated from human skin fibroblasts. Both cell lines were cultured in E8 medium on Matrigel-coated cell culture plates. After growing to approximately 90% confluence, the cells were passaged at a split ratio of 1:6 by exposure to 0.5 mM EDTA for 4–5 min at 37 °C. All cells were cultured under standard culture conditions (37 °C, 100% humidity and 5%CO2) in an incubator (PHCbi, Tokyo, Japan).
Osteogenic differentiation of hPSCs
After growing to approximately 70% confluence, the medium of hPSCs was changed to osteogenic induction medium (OM; DMEM containing 15% fetal bovine serum, 1% nonessential amino acids, 0.1 mM β-mercaptoethanol, 1% penicillin/streptavidin, 5 µg mL−1 vitamin C, 10 mM sodium glycerophosphate and 10–8 M dexamethasone). To find an optimal concentration, after culturing for 2 days, BFP-1 peptide was added into the OM at varying concentrations (0 µg mL−1, 1 µg mL−1, 5 µg mL−1 and 20 µg mL−1) for another 12 days. The induction efficiency was analyzed by alizarin red staining at day 14.
Then, using a concentration of 20 µg mL−1 for the BFP-1 peptide, 28 days of osteogenic induction of both H9 hESCs and f-hiPSCs was performed. The peptide-containing OM was applied each week. Moreover, osteogenic differentiation in OM with or without peptide addition throughout the 28 days of induction was used as a control. Cell samples were obtained for the assays at the desired time intervals (0 days, 7 days, 14 days, 21 days and 28 days). The viability of cells was investigated using a cell counting kit-8 reagent as we recently reported [28]. In addition, the morphology of these cell samples was photographed using an inverted microscope with a CCD.
Finally, based on previous experimental settings, hPSCs were also treated with BFP-1 peptide for specific periods of 7–21 days with the same control group settings.
Quantitative real-time polymerase chain reaction (RT–PCR)
To assess the effect of BFP-1 peptide addition at varying weeks on gene transcription, total RNA was obtained using TRIzol reagent for hPSCs. Then, mRNA was extracted from the samples through a chloroform-isopropanol precipitation method. cDNA was obtained by reverse transcription of mRNA using SuperQuick RT MasterMix. Using ACTB as an endogenous reference gene, the gene expression of OCT-4, ALP, CD73, RUNX2, COL1A1 and OPN was detected by RT–PCR utilizing a SYBR®PremixEx Taq™ Kit. In addition, germ layer marker genes related to endoderm (AFP, GATA4), mesoderm (T, MEOX1, MIXL1) and ectoderm (PAX6, SOX1, FOXD3) were measured in hPSCs after 7 days of induction. The epithelial-mesenchymal transition (EMT) marker genes Snail, Twist, Slug, E-cadherin, β-catenin, ZO-1, N-cadherin, Vimentin and α-SMA were also investigated. Three parallel samples were set for each sample, and the comparative CT (2−ΔΔCT) method was employed to evaluate fold gene expression differences between groups. Primer sequences are shown in Supplementary Table S1.
Immunofluorescence analyses
Immunofluorescence staining was applied to detect the expression of the osteogenesis-related marker proteins RUNX2, COL1A1 and OPN during the osteogenic differentiation of hPSCs over 28 days. Cells on the cell culture plates were fixed with 4% (v/v) paraformaldehyde for 30 min and then treated with 0. 2% Triton-100 for 30 min. After blocking in 3% BSA solution for 2 h, the cells were incubated overnight with primary antibodies diluted at a ratio of 1:100. Then, the cells were incubated with the corresponding secondary antibodies for 1 h. Finally, the cell nuclei were stained with DAPI for 5 min. All staining steps were followed by washing in DPBS buffer 3 times. The stained signals in the cells were photographed using a confocal fluorescence microscope (Axiovert 200 M; Carl Zeiss Jena, Oberkochen, Germany). The wavelengths of DAPI, green fluorescent labeled antibody and red fluorescent labeled antibody are 405 nm, 488 nm, and 594 nm, respectively. Information on these antibodies is shown in Table S2.
Moreover, the expression level of each of these proteins in cell samples was quantitatively measured as determined by the fluorescence of pristine pictures using ImageJ software.
Flow cytometry study
After incubation for up to 28 days, H9 hESCs were digested into single cells and then fixed with 1% paraformaldehyde. Cells on ice were permeated in 200 μL precooled 90% methanol solution for 30 min. Subsequently, the samples were washed twice with flow buffer (DPBS containing 2% FBS) and incubated with a rabbit anti-RUNX2 antibody at a dilution rate of 1:100 in flow buffer for 30 min at 37 °C. This was followed by incubation with a secondary antibody of Fluor 488-labeled goat anti-rabbit IgG at a dilution rate of 1:500 in DPBS. Finally, all cell samples were analyzed by a BD FACS Calibur System (BD, USA) and FlowJo software. Information on these antibodies is shown in Table S3.
For hiPSCs, we constructed a RUNX2-GFP hiPSCs line from f-hiPSCs using CRISPR–Cas9 gene editing technology, and the endogenous promoter of the RUNX2 gene drove the expression of GFP. Purified RUNX2-GFP hiPSCs were acquired through further puromycin screening and cell subcloning culture methods. As described above, RUNX2-GFP hiPSCs were cultured in OM with or without BFP-1 peptide and subjected to flow cytometry analysis after induction for 7 days for 28 days.
Alizarin red staining assay
Calcium deposits in cell samples were quantified using alizarin red (AS) staining. Briefly, fixed cells were stained with 2% (w/v) alizarin red solution (0.01 M Tris buffer, pH = 4.2) for 20 min. Then, the cells were washed with distilled water to remove excess AS. Finally, cells were observed under an inverted microscope with a CCD, and the whole plate was recorded using a mobile phone. To quantify the orange–red coloration of AS, the substrates were immersed in 1% (m/v) hexadecylpyridinium chloride solution overnight to dissolve the deposited calcium mineral. Then, 100 μL of supernatant was collected into new 96-well cell culture plates, and the OD value at 490 nm wavelength was detected using a microplate reader (Bio-Rad; USA).
Statistical analysis
All data were statistically analyzed using Student's t-test and expressed as the mean ± standard deviation. The difference was considered significant when p < 0.05. Each data point was obtained by performing three independent replicates.
Results and discussion
Survival of hPSCs in BFP-1 peptide-containing osteogenic induction medium
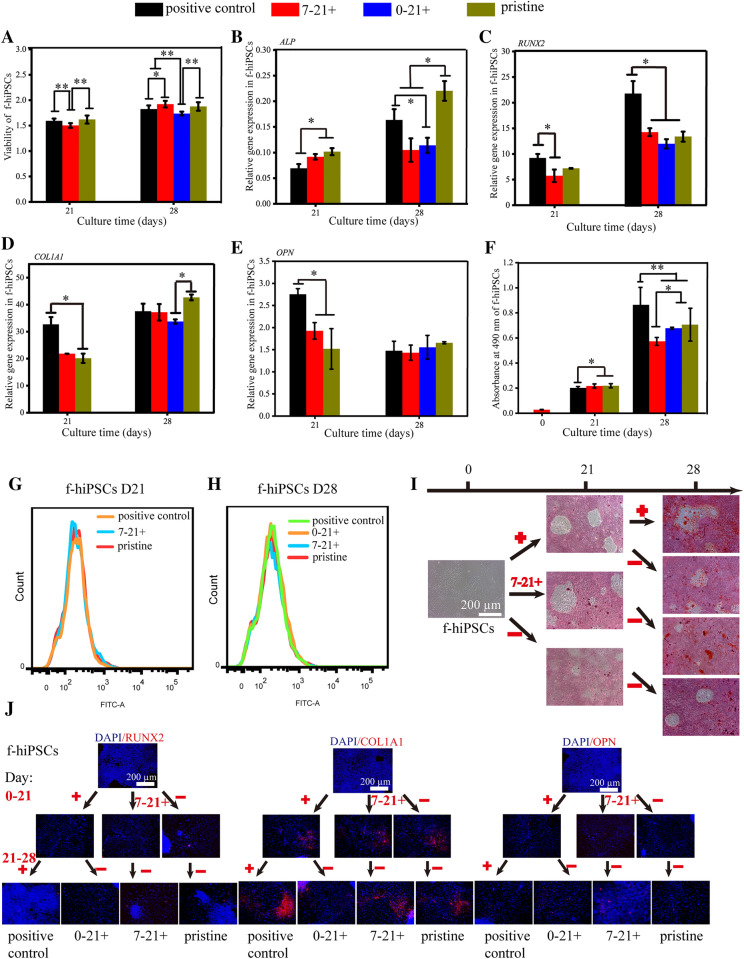
Prior to osteogenic induction, we performed a simple cell experiment to select the optimal concentration for BFP-1 peptide supplementation. After treatment with peptide at varying concentrations (0 µg mL−1, 1 µg mL−1, 5 µg mL−1 and 20 µg mL−1) for 14 days, alizarin red (AS) staining results showed that BFP-1 accelerated calcium deposition in f-hiPSC samples (Fig. 1B, Fig. S1). Moreover, a significant difference was found only in the 20 µg mL−1 group in comparison to the pristine control. Therefore, BFP-1 peptide at 20 µg mL−1 was applied for the following studies.
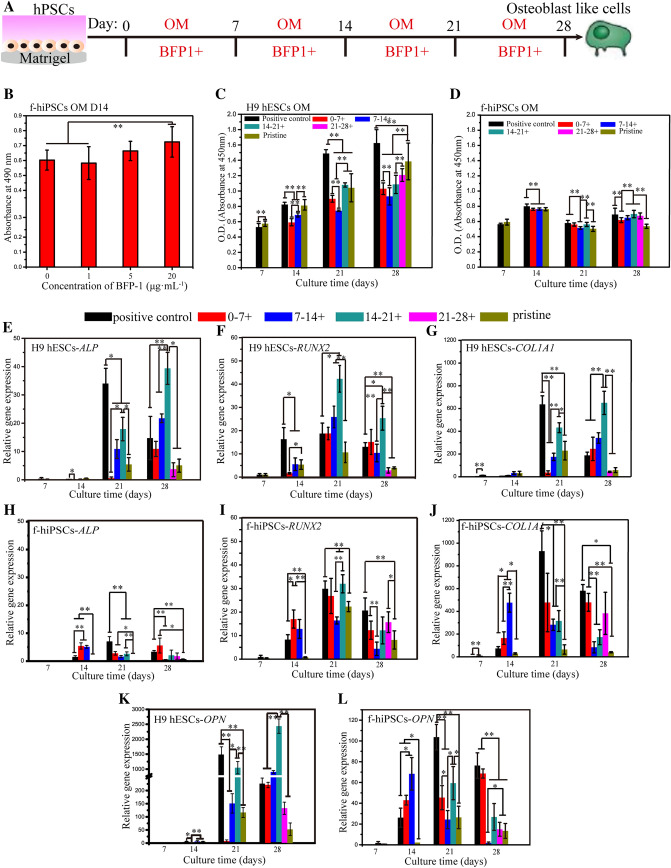
Fig. 1.
Osteogenic induction of hPSCs with BFP-1 peptide treatment. An Illustrated image of the addition of BFP-1 peptide in osteogenic induction medium (OM) at various periods for monolayer cultured hPSCs. In addition, the whole induction process without or with BFP-1 treatment was conducted as pristine and positive controls, respectively. B The osteogenic differentiation of f-hiPSCs as a function of BFP-1 peptide concentration at day 14 was evaluated by alizarin red staining. C-D For BFP-1 peptide-treated hPSCs, the viabilities of H9 hESCs (C) and f-hiPSCs (D) during osteogenic induction were detected at the desired intervals (0, 7, 14, 21, and 28 days) using a CCK8 reagent. E-L After culturing for 7 days, the gene expression of RUNX2, ALP, COL1A1, and OPN in cell samples was measured by RT–PCR. The expression of these genes in undifferentiated hPSCs was standardized to 1. + indicates OM supplemented with peptide. Scale bar, 100 μm. n = 3. * represents p < 0.05, ** represents p < 0.01
Then, the impact of one week of supplementation with the BFP-1 peptide on the osteogenic differentiation of both H9 hESCs and f-hiPSCs was studied, as shown in Fig. 1A. In addition, peptides containing OM applied during the whole induction were used as a positive control. Before differentiation, hPSCs presented undifferentiated cell morphology with defined edges and large nuclei-to-cytoplasm ratios (Figs. S2, S3)). After induction for 7 or 14 days, many cobblestone- or spindle-shaped cells appeared in all groups. For cell viability, BFP-1 peptide treatment showed a slightly negative effect on both cell lines at day 7 (Fig. 1C, D). However, after induction for more than 14 days, the viability of H9 hESCs treated with peptide for 0–7 days was remarkably lower than that of both pristine and positive controls. Interestingly, this result was not found for f-hiPSCs. For both H9 hESCs and f-hiPSCs at days 14, 21 and 28, it is worth noting that the positive control group exhibited higher cell viabilities than the pristine group. In addition, peptide addition for 7–14 days only reduced the viability of H9 hESCs but not f-hiPSCs. After differentiation for another 14 days, both cell types secreted much collagen and were arranged in multiple layers with inapparent cell morphology (Figs. S2, S3). Consistently, after culturing for 28 days, a cobblestone morphology was found due to the increased extracellular matrix. Similarly, it was found that peptide treatment for 7–21 days promoted the viability of f-hiPSCs, but contrary results were detected for H9 hESCs (p < 0.01). In addition, for both cell types, peptide treatment throughout the whole induction process accelerated cell survival except for the first 7 days.
The impact of BFP-1 peptide supplementation on marker expression in hPSCs
Dynamic changes in the expression of markers related to osteogenesis are commonly used to monitor the osteogenic differentiation of hPSCs [9]. In this study, ALP, RUNX2, COL1A1 and OPN were analyzed using both RT–PCR and immunofluorescence technology.
Alkaline phosphatase (ALP) is considered a marker of early differentiation and may be involved in the calcification of the bone matrix [29]. For both H9 hESCs and f-hiPSCs incubated in pristine OM, cells expressed the ALP gene at quite low levels during the 14 days of induction, and upregulation was measured at day 21 for H9 hESCs and day 28 for f-hiPSCs (Fig. 1E, H). For the positive control group with peptide treatment throughout the induction, apparently enhanced gene expression was found for H9 hESCs after differentiation for more than 21 days, and the time point was day 14 for f-hiPSCs (p < 0.01). Then, for the peptide addition at each period, quite different results were measured between H9 hESCs and f-hiPSCs. It was found that peptide addition during days 0–7 only promoted gene expression in H9 hESCs at day 28, but much higher results were found from day 7 for f-hiPSCs (p < 0.05). Then, peptide addition during 7–14 days enhanced the gene expression in H9 hESCs from day 21. However, a positive result, a high expression level resembling that of the 0–7 days treatment group, was found only at day 14 for f-hiPSCs. After 14 days of induction, peptide treatment also upregulated gene expression in both cell lines. Notably, a very high gene expression level was measured in H9 hESCs at day 28. Finally, when the supplementation period was 21–28 days, positive results were observed only in f-hiPSCs. In summary, for ALP expression, BFP-1 should be supplemented during 7–21 days for H9 hESCs and for 7–28 days for f-hiPSCs.
RUNX2 is an early critical marker for the osteoblastic differentiation of stem cells, and its expression can upregulate markers associated with mineralization, such as COL1A1, OCN, and bone sialoprotein (BSP) [30]. For both cell lines, the gene expression of RUNX2 peaked at day 21. Then, the application of BFP-1 peptide throughout the 28 days of differentiation did not change the tendency (Fig. 1F, I). Notably, after induction for more than 14 days, the positive control group exhibited a much higher gene expression level for RUNX2 at each time point for both cell lines (p < 0.05). This indicated that the addition of the BFP-1 peptide could promote the expression of the critical RUNX2 gene in hPSCs. Then, peptide supplementation for 0–7 days remarkably upregulated gene expression in f-hiPSCs after induction foAr 7 days, but contrasting results were observed in H9 hESCs. Fortunately, consistent positive results were found for both cell lines during the following inductions. When the addition period was 7–14 days, the expression of the RUNX2 gene in H9 hESCs at days 21 and 28 was higher than that in the pristine control (p < 0.05), but reduced results were detected for f-hiPSCs. For both cell lines after 14 days of induction in pristine OM, it was found that the gene expression levels were apparently promoted with one week of peptide treatment, even higher than the positive groups at day 21. It is surprising that the 14–21 days group exhibited the highest gene expression levels at day 21 for both cell lines, which proved that this time period is highly recommended in terms of critical RUNX2 expression. Finally, peptide addition for 21–28 days only upregulated gene expression in H9 hESCs at day 28.
COL1A1 is a matrix-mineralizing protein that regulates bone formation through the control of morphology, differentiation, and other biological functions of cells [30]. In total, regardless of the values, the gene expression pattern of COL1A1 in both H9 hESCs and f-hiPSCs resembled that of the ALP gene after induction for more than 21 days (Fig. 1G, J). For f-hiPSCs, peptide addition during the period of 0–7 days, especially 7–14 days, could remarkably upregulate the gene expression at day 14, both of which were significantly higher than the positive control groups (p < 0.05). However, the promoting effect was found only at day 28 in H9 hESCs with peptide treatment during days 0–14. In addition, it is worth noting that the highest gene expression levels were measured for f-hiPSCs in positive groups after culturing for more than 21 days. This suggests that peptide treatment during 14–21 days is also crucial for the expression of OCN.
OPN is a noncollagenous, phosphorylated glycoprotein localized in bone and dentin [31]. Osteoblasts can secrete OPN, and thus, it is regarded as a marker of osteogenesis during the induction of hPSCs [32]. For the pristine groups, OPN expression in both cell lines increased with increasing induction time and reached peak values at day 21 (Fig. 1K, L). For positive controls, apparent upregulation was detected at day 21 and 28. Then, peptide supplementation during 0–7 days only upregulated gene expression in H9 hESCs at day 28, but upregulation was found in f-hiPSCs after induction for more than 7 days. Changing the time period to 14–21 days, peptide addition significantly promoted the expression of the OPN gene in H9 hESCs at day 28 and in f-hiPSCs at day 14. Surprisingly, peptide treatment for 14–21 days resulted in the highest expression levels at day 21 and day 28. However, no such huge accelerated results were found for f-hiPSCs. Finally, peptide treatment for 21–28 days was not effective for f-hiPSCs.
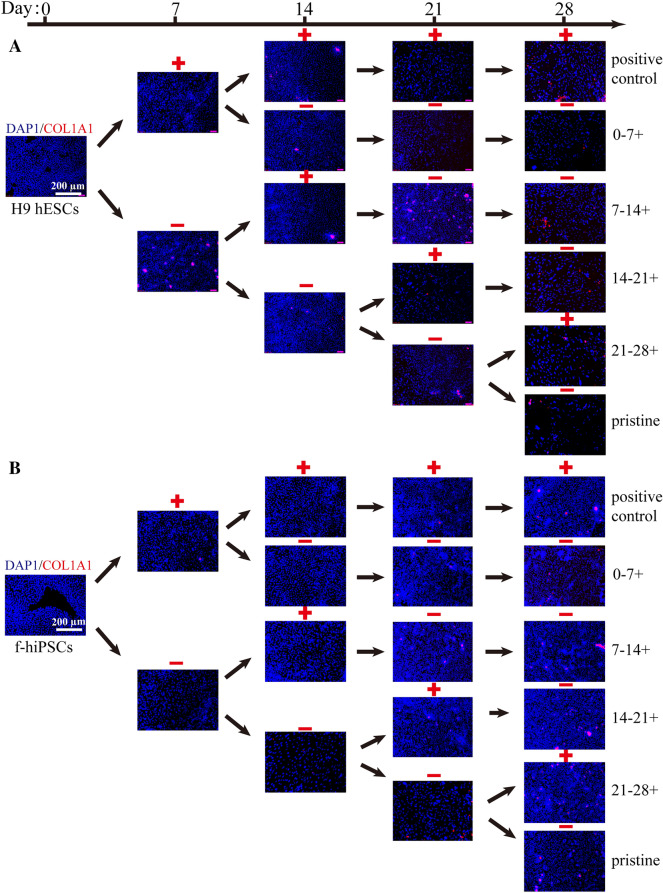
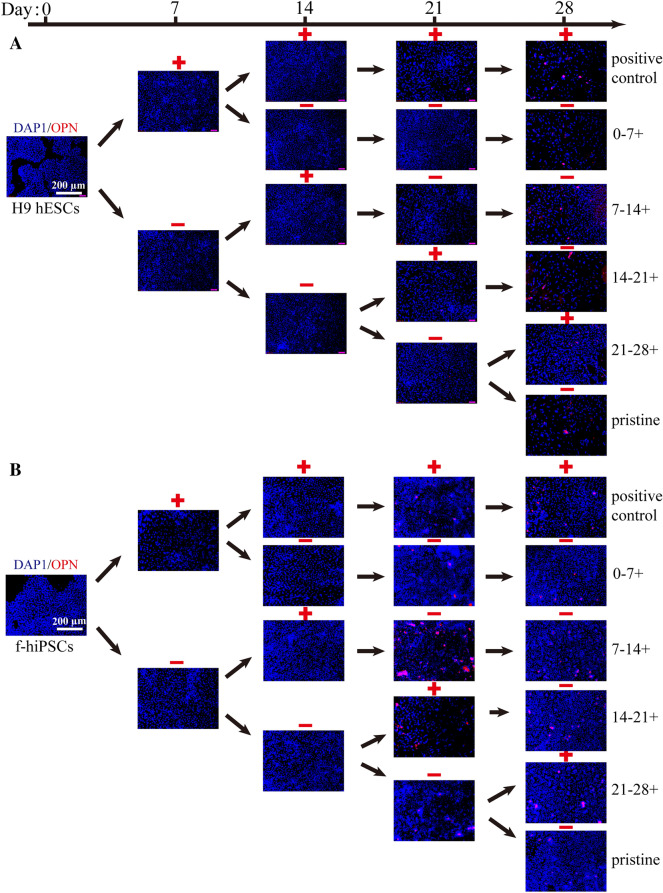
Meanwhile, the protein expression levels of RUNX2, COL1A1 and OPN in H9 hESCs and f-hiPSCs were also detected by immunofluorescence analyses (Figs. 2, 3, 4). Consistent with the RT–PCR results, BFP-1 peptide addition at each designated period could promote the expression of RUNX2 in hPSCs (Fig. 2). Moreover, similar expression patterns were found for the RUNX2 protein. Then, cells treated with peptide for 14–21 days showed the best protein expression in H9 hESCs both at days 14 and 21. For f-hiPSCs, the promoting effect was not as apparent for all groups at day 14. Fortunately, in contrast to the gene expression results, we found that peptide treatment for 7–14 days could promote the expression of RUNX2 in f-hiPSCs after induction for more than 21 days. For the COL1A1 protein, quite different results were found for H9 hESCs at day 28 and f-hiPSCs throughout the whole process (Fig. 3). This should be because COL1A1 is a kind of extracellular matrix protein that is secreted from the induced cells. However, it is consistent to find that peptide addition during 14–21 days or whole 21 days could promote the protein expression in H9 hESCs at day 21. Then, in comparison to both the pristine and positive groups, the addition of peptide during 7–14 days significantly increased the expression of COL1A1 in f-hiPSCs at day 21 (p < 0.05), the values of which were almost the same as those in cells treated with peptide for 14–21 days. Finally, consistent with the gene expression of OPN, peptide treatment for 0–7 days or throughout the 28 days of culturing accelerated the expression of OPN protein in both H9 hESCs and f-hiPSCs (Fig. 4). Then, unlike the markedly increased gene expression result, no positive results were detected for the 14–21 days treatment group in H9 hESCs. However, consistent positive results were measured for f-hiPSCs. Interestingly, more OPN protein was detected in H9 hESCs treated with peptide for 7–14 days at day 21, and the same result was also found in f-hiPSCs. Notably, these immunofluorescence results demonstrated that only a small number of induced cells positively expressed all three protein markers (Figs. S4-S10), proving that the differentiation efficiency needs to be improved.
Fig. 2.
The expression of marker proteins in hPSCs during the 28 days of osteogenic differentiation. The protein expression of RUNX2 (red) in cells was measured every 7 days by immunofluorescence. Cell nuclei were stained blue with DAPI. Scale bars, 200 µm
Fig. 3.
The expression of marker proteins in hPSCs during 28 days of osteogenic differentiation. The protein expression of COL1A1 (red) in cells was measured every 7 days by immunofluorescence. Cell nuclei were stained blue with DAPI. Scale bars, 200 µm
Fig. 4.
The expression of marker proteins in hPSCs during 28 days of osteogenic differentiation. The protein expression of OPN (red) in cells was measured every 7 days by immunofluorescence. Cell nuclei were stained blue with DAPI. Scale bars, 200 µm
BFP-1 peptide promotes calcium deposition in hPSCs
To investigate the effect of BFP-1 peptide supplementation on the osteogenic differentiation efficiency of hPSCs, AS staining was conducted every 7 days for 28 days. As shown in Fig. 5A and B, typical calcium nodules were found after 21 days of induction for both H9 hESCs and f-hiPSCs in the pristine group. However, at day 14, some typical calcium nodules were found in both cell lines in the positive group. Quantitative results confirmed that the application of the BFP-1 peptide throughout the induction process could remarkably promote calcium deposition in hPSCs (Fig. 5C, D).
Fig. 5.
Analyses of alizarin red staining for induced hPSCs. A-B Images of alizarin red-stained H9 hESCs (A) and f-hiPSCs (B) on different days (0, 7, 14, 21, and 28). C, D Cetylpyridinium bromide solution was applied to dissolve deposited alizarin red, and the absorbance at 490 nm was measured. Scale bars, 100 µm. n = 3. * represents p < 0.05, ** represents p < 0.01
Then, peptide treatment during 0–7 days significantly promoted the deposition of calcium in H9 hESCs after induction for 14 days (Fig. 5). However, calcium depositions resembling the pristine control were found after induction for more than 21 days, which were much lower than the positive control. Moreover, it is surprising to find that this treatment exhibited nearly no impact on calcium deposition in f-hiPSCs. These results proved that BPF-1 peptide supplementation for 0–7 days is unnecessary in terms of calcium deposition in hPSCs. Changing the peptide treatment period to 7–14 days or 14–21 days remarkably promoted calcium deposition in hPSCs in the following differentiation process (p < 0.01), which suggested that the addition of peptide during the time period of 7–21 days benefited calcium deposition. Finally, the absorbance of alizarin red-stained cells at day 28 was not changed by peptide addition during days 21–28 for f-hiPSCs, and that of H9 hESCs was significantly lower than that of the positive control.
BFP-1 peptide applied at a defined stage promoted the osteogenic induction of hPSCs
As described previously, the impact of the supplementation period of the osteogenesis BFP-1 peptide on the osteogenic induction of hPSCs was investigated in detail. It is certain that the addition of BFP-1 peptide can apparently change the induction process of cells. Consistent with reported results, the BFP-1 peptide harbors good cytocompatibility, and the changes in viability of differentiated cells should be due to the selective killing effect from the OM (Fig. 1C, D) [33]. Then, using pristine OM as a control, the critical results of marker expression and calcium nodule formation were discussed for H9 hESCs and f-hiPSCs, respectively.
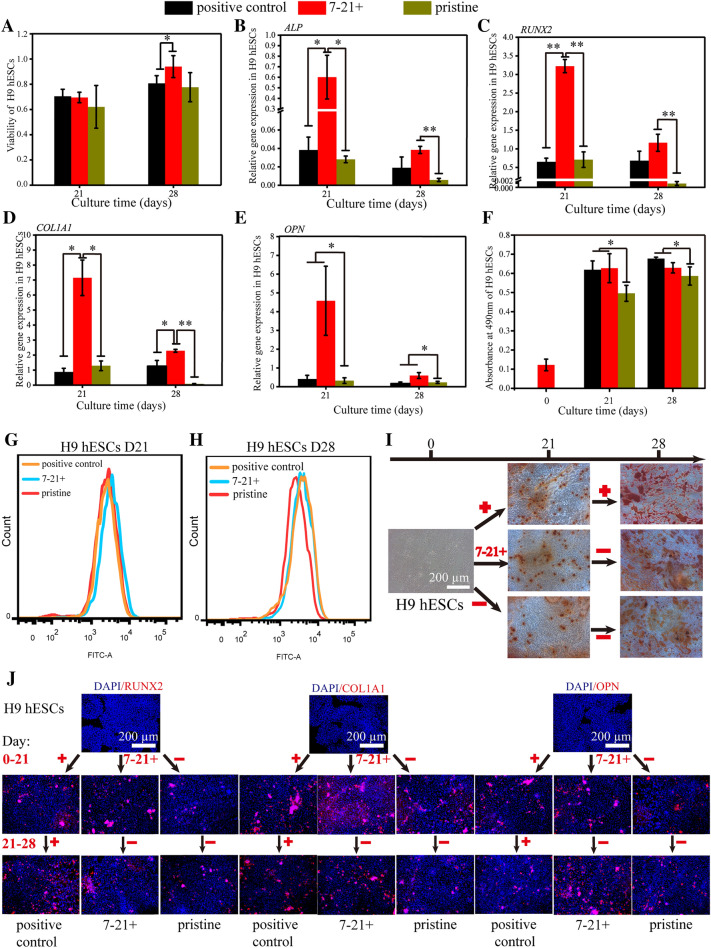
For H9 hESCs, peptide treatment during the first week mainly upregulated the gene expression of RUNX2, ALP, COL1A1, and OPN at day 28 (Fig. 1E–G and K). However, remarkable downregulation was also detected during days 14–21 of culture. Moreover, this treatment failed to significantly enhance the protein expression of RUNX2 and COL1A1 during 28 days of induction. Although much more deposited calcium was detected in the 0–7 days group after 14 days of induction, the values were much less than those of the positive control at days 21 and 28 (Fig. 5C). Then, both RT–PCR and immunofluorescence analyses showed that peptide addition for 7–14 days upregulated the expression of early markers of ALP and RUNX2 in H9 hESCs at day 21 and promoted the expression of latter markers of OPN at day 28. More importantly, we found that calcium deposition in cell samples was greatly enhanced and showed comparable performance to the positive control. Afterwards, much better promoting effects on the expression of all 4 gene markers were found at day 21 for cells with peptide supplementation during days 14–21 in comparison to the 7–14 days group. Furthermore, cell samples exhibited the highest gene expression among all groups for these 4 markers at day 28. Unfortunately, the time point for RUNX2 protein was day 28, and poor OPN expression results were not obtained. These results demonstrated that the period of 7–21 days is highly recommended for H9 hESCs. Finally, the addition of BFP-1 peptide during 21–28 days only accelerated the gene expression of OPN with enhanced calcium deposition, but the levels of both were lower than those in other peptide treatment groups [34, 35].
For f-hiPSCs, it is surprising to find that the addition of BFP-1 peptide during days 0–7 significantly promoted all marker expression after induction for more than 7 days (Fig. 1H–J, L). Unfortunately, the treatment had no impact on critical calcium deposition in cells throughout the 28 days of induction (Fig. 5D). Then, peptide supplementation during 7–14 days also significantly upregulated the expression of all marker genes at day 14, and upregulated gene expression was found for ALP and COL1A1 in the following induction process. Then, enhanced protein expression was found for RUNX2, COL1A1 and OPN at day 21. Fortunately, the calcium deposition in cells was remarkably accelerated and harbored the highest values at final day 28. Changing the addition period to 14–21 days, the expression of all 4 markers in cells was enhanced after induction for more than 21 days, especially for the expression of RUNX2 at day 21. Moreover, better calcium deposition results were also measured. Finally, peptide treatment during 21–28 days was found to upregulate the gene expression of RUNX2 and COL1A1, as well as the protein expression of RUNX2 and OPN, in f-hiPSCs. Notably, these results proved that BFP-1 peptide treatment at each week could promote the osteogenic induction of f-hiPSCs, but the period of 7–21 days seems more efficient.
The above results demonstrated that the BFP-1 peptide should not be added throughout the osteogenic induction process of H9 hESCs, and the supplementation period exhibited a great influence on the differentiation efficiency of both H9 hESCs and f-hiPSCs. More importantly, the difference among various types of hPSCs is nonnegligible. It was previously shown that adding BFP-1 peptide during days 7–14 and days 14–21 could significantly promote the efficiency of osteogenic differentiation of hPSCs, especially in the H9 hESCs line. To further confirm this result, BFP-1 peptide was added at 7–21 days in the following section.
Treatment with the BFP-1 peptide during the period of 7–21 days significantly promoted the viability of H9 hESCs after induction for 28 days, the values of which were even higher than those of the positive control group (Fig. 6A, Fig. S11). Then, for each investigated osteogenesis-related gene, ALP, RUNX2, COL1A1 and OPN, at day 21, remarkably higher gene expression levels were detected in cells with peptide addition at the designated period in comparison to both the negative and positive groups (Fig. 6B–E). Such a promoting effect remained apparent for the genes RUNX2, COL1A1 and OPN at day 28. Similar results were also found for the expression of the protein markers COL1A1 and OPN in cell samples by immunofluorescence (Fig. 6J). However, after induction for both 21 and 28 days, no apparent difference was found for the expression of RUNX2 in cells with peptide addition during 7–21 or 0–28 days, although lower protein expression was observed in the negative control groups. Moreover, flow cytometry analyses showed that target peptide treatment for 7–21 days accelerated the generation of RUNX2 + cells after 21 days of differentiation in comparison to the other two control groups (Fig. 6G, H, Fig. S14). At day 28, almost the same expression rates were measured for the peptide-treated groups, and both were higher than those of the negative group. Finally, after culturing for more than 21 days, more typical calcium nodules were found in H9 hESCs treated with peptide than in the pristine control (Fig. 6I, Fig. S12). Quantitative results confirmed that peptide treatment during days 7–21 and days 0–21 significantly promoted calcium deposition in H9 hESCs after 21 days of differentiation (Fig. 6F). These results proved that the addition of BFP-1 during the period of 7–21 days showed excellent performance in cell survival and osteogenesis marker expression for H9 hESCs, even better than the group with peptide addition throughout the whole induction.
Fig. 6.
The osteogenic differentiation of H9 hESCs with peptide addition during 7–21 days of induction. A After 7 days of induction, H9 hESCs were treated with BFP-1 peptide for 14 days. After culturing for 21 days and 28 days, the viability of the cells was measured using a CCK8 reagent. B-E The gene expression of ALP (B), RUNX2 (C), COL1A1 (D) and OPN (E) in cell samples was measured by RT–PCR. The expression of these genes in undifferentiated H9 hESCs was standardized to 1. G–H The positive expression of RUNX2 protein in induced cell samples at days 21 (G) and 28 (H) was measured by flow cytometry. F-I Cells were stained with alizarin red. Images of cell samples (I) are shown. Then, the deposited calcium was quantitatively detected at a wavelength of 490 nm (F). J The protein expression of RUNX2, COL1A1 and OPN in cells was studied by immunofluorescence. Scale bars, 200 µm. n = 3. * represents p < 0.05, ** represents p < 0.01
Unfortunately, when the cell type was changed to f-hiPSCs, except for similar cell viability results, no upregulation was found for the expression of any of the 4 gene markers in cells treated with peptide for 7–21 days (Fig. 7A–E). Almost the same number of RUNX2 + cells was measured among all groups after differentiation for both 21 and 28 days, as confirmed by flow cytometry analyses (Fig. 7G, H, Fig. S17). Except for remarkably accelerated OPN protein expression at day 28, similar results were found in the immunofluorescence study (Fig. 7J). Moreover, less deposited calcium in cells was also measured at day 28 for cells with peptide addition during days 7–21 (Fig. 7F, I). Fortunately, consistent with previously mentioned results, better results were found in the positive group for marker gene/protein expression and calcium deposition.
Fig. 7.
The osteogenic differentiation of f-hiPSCs treated with peptide addition at specific periods. A After 7 days of induction, f-hiPSCs were treated with BFP-1 peptide for 14 days. Then, the effect of peptide supplementation on the differentiation of cells was also studied during the period of 21–28 days. The viability of cells was measured using a CCK8 reagent. B-E The gene expression of ALP (b), RUNX2 (c), COL1A1 (d) and OPN (E) in cell samples was measured by RT–PCR. G-H The expression of RUNX2 protein in cells after induction for 21 (G) or 28 (H) days as measured by flow cytometry. F-I Cells were stained with alizarin red. Images of cell samples (I) are shown. Then, the deposited calcium was quantitatively detected at a wavelength of 490 nm (F). J The protein expression of RUNX2, COL1A1 and OPN in cells was studied by immunofluorescence. Scale bars, 200 µm. n = 3. * represents p < 0.05, ** represents p < 0.01
These results indicated that 14 days of BFP-1 peptide addition should be conducted after induction for 7 days for H9 hESCs, but a simple whole addition throughout the 28 days of induction is recommended for f-hiPSCs. It is well known that the osteogenic differentiation of hPSCs undergoes various stages, such as proliferation, differentiation, matrix deposition and mineralization, so that the expression of osteogenic-related genes is dynamically changed during the differentiation process [36]. In 2021, we summarized this intrinsic regulatory mechanism in detail [28]. Our results suggested that mesenchymal-like cells were obtained at day 7. With prolonged osteogenic culture time, cells began to express the osteogenic gene RUNX2 at a high level for 14–21 days. Then, preosteoblasts positively expressing RUNX2 stimulate the expression of the late osteogenic differentiation marker genes COL1A1 and OPN, which further promotes the formation of mature osteoblasts [28]. We speculated that mesoderm, mesenchymal and preosteoblasts were obtained after culturing for 7 days, 14 days and 21 days, respectively. Consistent with published results, an apparent difference was found between the expression patterns of osteogenesis-related markers in H9 hESCs and f-hiPSCs [37, 38]. We recently reported that H9 hESCs and f-hiPSCs undergo similar expression changes for markers related to pluripotency and osteogenic differentiation, but different expression changes were found for extracellular matrix protein markers [28]. Notably, H9 hESCs exhibited much better performance than f-hiPSCs in extracellular matrix synthesis. Therefore, the inherent difference among hPSC types can be the reason why different regulatory effects were detected for BFP-1 addition on the osteogenic induction of H9 hESCs and f-hiPSCs.
Up to now, many kinds of peptide decorated hydrogels or self-assembling peptide hydrogels have developed for the osteogenic induction of MSCs [39–41]. Consistently, the definition of optimal supplemental stages of osteogenesis peptide could accelerate the design of peptide-related hydrogels for the osteogenic induction of hPSCs in three dimensions. It is well known that both potent osteogenic factors and appropriate vehicles are essential for bone regeneration. The smart sustained-release system combines both organs, which not only supports and controls the sustained release of osteogenic factors but also provides good biocompatibility and osteoinduction(P24) [35, 42, 43]. Thus, in future study, hPSCs will undergo a corresponding induction process based on the targeted cell types of osteogenesis peptides before cell seeding, or the release of peptides will be controlled using intelligent sustained-release systems.
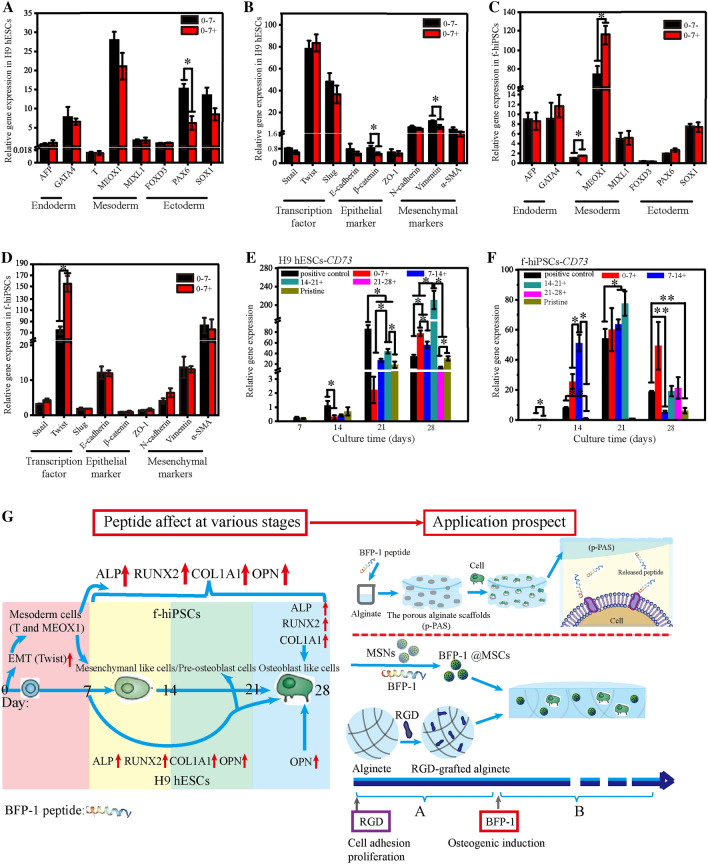
It has been reported that the enhanced osteogenic differentiation of hPSCs by BFP-1 peptide treatment may be due to the upregulation of critical RUNX2 expression [22, 44]. Our results showed that peptide addition for 7–21 days could significantly promote the efficiency of osteogenic differentiation of hPSCs, especially in the H9 hESC line. Mesenchymal cells derived from mesoderm and ectoderm cells differentiate into osteogenic precursor cells and osteoblasts, and we preliminarily concluded that BFP-1 peptide treatment promoted the osteoblastic differentiation of mesenchymal cells by activating the RUNX2 pathway [9, 10]. After 21 days in culture, peptide addition may promote the differentiation of preosteoblasts into osteoblasts. To verify these conclusions, as well as obtain more knowledge about the impact of the BFP-1 peptide on the osteogenic differentiation process of hPSCs, the expression of marker genes related to germ layers and epithelial-mesenchymal transition (EMT) was studied in detail at day 7. As shown in Fig. 8A and B, peptide addition in the first week had no or a negative impact on the generation of mesoderm and ectoderm cells in H9 hESCs, resulting in reduced expression of the mesenchymal marker CD73 at days 14 and 21 (Fig. 8E). These results can explain why peptide addition during the first week could not promote the osteogenic induction of H9 hESCs. However, treatment in the first week significantly upregulated the gene expression of the mesoderm markers T and MEOX1 as well as the transcription factor Twist in f-hiPSCs (p < 0.05; Fig. 8C, D), suggesting that BFP-1 peptide addition could promote the EMT process and the generation of mesoderm cells. This may be because human fibroblast-derived hiPSCs are more conducive to the transition from an epithelial phenotype to a mesenchymal phenotype. It has been reported that mouse dermal fibroblasts reflect a propensity to differentiate toward a mesenchymal lineage [37]. In future studies, other skin-derived hiPSC lines will be applied to confirm this phenomenon, and much effort will be made to investigate the mechanisms. Consistently, the expression of CD73 was markedly increased during the following induction process (Fig. 8F). In addition, peptide addition at the fourth week is workless in terms of CD73 gene expression. However, peptide addition at the other three weeks promoted the expression of CD73 genes, suggesting that the BFP-1 peptide should be added throughout the osteogenic induction process, as mentioned above. These results suggested a large difference between BFP-1 peptide-induced spontaneous differentiation of H9 hESCs and f-hiPSCs. Based on these results, the effect of peptide addition each week on the osteogenic differentiation of H9 hESCs and f-hiPSCs is summarized in Fig. 8G.
Fig. 8.
The mechanism underlying the effect of BFP-1 peptide supplementation on the osteogenic differentiation of hPSCs. A-C The impact of BFP-1 peptide addition on the differentiation process of both H9 hESCs (A) and f-hiPSCs (C) was investigated after 7 days of induction. The relative expression of marker genes related to the germ layer (endoderm: AFP, GATA4; mesoderm: T, MEOX1 and MIXL1; ectoderm: PAX6, SOX1 and FOXD3) was measured using RT–PCR. B-D The expression of gene markers related to epithelial-mesenchymal transition (EMT), including Snail, Twist, Slug, E-cadherin, β-catenin, ZO-1, N-cadherin, Vimentin and α-SMA, was also investigated. E, F The gene expression of the mesenchymal marker CD73 was detected in hESCs (E) and hiPSCs (F) with peptide treatment at varying periods. The expression of these genes in hPSCs before differentiation was standardized to 1. G A dynamic map summarizing the impact of BFP-1 peptide addition on the osteogenic differentiation of hPSCs during various weeks of induction over 28 days. n = 3. * indicates p < 0.05, and ** indicates p < 0.01
Conclusion
In the present study, hPSCs were induced to differentiate into osteoblast-like cells in common FBS-containing osteogenic induction medium using a monolayer induction method. Simultaneously, 7 days of osteogenic BFP-1 peptide supplementation was conducted, and the induction process was monitored in detail. BFP-1 peptide treatment remarkably upregulated the expression of all investigated genes/protein markers in hPSCs, but a large difference was found between H9 hESCs and f-hiPSCs. Moreover, the supplementation period is quite important to maximize the performance of peptides in accelerating the osteogenic induction of hPSCs because peptides added throughout the 28 days of osteogenic induction showed no good results in H9 hESCs. Specifically, the epithelial-mesenchymal transition and subsequent mesoderm cell generation processes were significantly improved in f-hiPSCs with peptide addition during the period of 0–7 days but not in H9 hESCs. Then, the treatment of BFP-1 peptide during the period of 7–21 days showed even better performance than the positive control group for H9 hESCs, but this period is workless for f-hiPSCs. In contrast, peptide should be added during 21–28 days for f-hiPSCs rather than H9 hESCs. We have clarified the function of BFP-1 in the osteogenic induction of hPSCs at each stage for the first time and will design corresponding BFP-1 smart drug release systems in the future.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was funded by the Foundation for the Talents of Innovative and Entrepreneurial of Lanzhou (NO. 2021-RC-127), the Open Subject Foundation of Key Laboratory of Dental Maxillofacial Reconstruction and Biological Intelligence Manufacturing, Fundamental Research Funds for the Central Universities (NO. lzujbky-2021-kb05 and lzujbky-2021-ey14), the Lanzhou University Hospital of Stomatology Research Support Fund, the Natural Science Foundation of Gansu Province (NO. 21JR7RA863, NO. 20JR5RA150 and 22JR5RA499), and the China Postdoctoral Science Foundation (NO.2022M721443).
Authors contributions
PZ, YLY and BL contributed to designing the study and critically revised the manuscript. PZ, YMS, HJL, LJ and JMS contributed to drafting and reviewing the manuscript. YMS, LW, ZXW, LZL, SQM, SZL and YZ performed all the experimental work. YMS contributed to performing the statistical analysis and interpreting the results. All authors have read and approved the article and have due diligence to ensure the integrity of the manuscript. Neither the entire manuscript nor any part of its content has been published or accepted elsewhere.
Data availability
The data presented in this study are available on request from all the authors.
Declarations
Conflict of interest
The authors declare no competing financial interest.
Ethical statement
No animal experiments were carried out for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yameng Song and Hongjiao Li have contributed equally.
Contributor Information
Bin Liu, Email: liubkq@lzu.edu.cn.
Yaling Yang, Email: yangyaling2011@163.com.
Ping Zhou, Email: zhoup@lzu.edu.cn.
References
- 1.Oryan A, Alidadi S, Moshiri A, Maffulli N. Bone regenerative medicine: classic options, novel strategies, and future directions. J Ortho Surg Res. 2014;9:18. doi: 10.1186/1749-799X-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu W, Huang Y, Liu D, Zeng T, Wang J, Li A, et al. Human Umbilical mesenchymal stem cells and nanohydroxyapatite/polyamide 66 promotes angiogenesis and bone regeneration in large bone defect. Tissue Eng Regen Med. 2022;19:1321–1336. doi: 10.1007/s13770-022-00471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Karp JM, Ferreira LS, Khademhosseini A, Kwon AH, Yeh J, Langer RS. Cultivation of human embryonic stem cells without the embryoid body step enhances osteogenesis in vitro. Stem Cells. 2006;24:835–843. doi: 10.1634/stemcells.2005-0383. [DOI] [PubMed] [Google Scholar]
- 6.Teng S, Liu C, Krettek C, Jagodzinski M. The application of induced pluripotent stem cells for bone regeneration: current progress and prospects. Tissue Eng Part B Rev. 2014;20:328–339. doi: 10.1089/ten.teb.2013.0301. [DOI] [PubMed] [Google Scholar]
- 7.Maia FR, Bidarra SJ, Granja PL, Barrias CC. Functionalization of biomaterials with small osteoinductive moieties. Acta Biomater. 2013;9:8773–8789. doi: 10.1016/j.actbio.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Shi R, Huang Y, Ma C, Wu C, Tian W. Current advances for bone regeneration based on tissue engineering strategies. Front Med. 2019;13:160–188. doi: 10.1007/s11684-018-0629-9. [DOI] [PubMed] [Google Scholar]
- 9.Long F. Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol. 2011;13:27–38. doi: 10.1038/nrm3254. [DOI] [PubMed] [Google Scholar]
- 10.Matsushita Y, Ono W, Ono N. Growth plate skeletal stem cells and their transition from cartilage to bone. Bone. 2020;136:115359. doi: 10.1016/j.bone.2020.115359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J, Hollinger JO. Effects of dual delivery of rhPDGF-BB and rhBMP-2 on osteogenic differentiation of human mesenchymal stem cells. Tissue Eng Regen Med. 2014;11:143–148. doi: 10.1007/s13770-013-1118-5. [DOI] [Google Scholar]
- 12.Levi B, Hyun JS, Montoro DT, Lo DD, Chan CK, Hu S, et al. In vivo directed differentiation of pluripotent stem cells for skeletal regeneration. Proc Natl Acad Sci USA. 2012;109:20379–20384. doi: 10.1073/pnas.1218052109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamai N, Myoui A, Hirao M, Kaito T, Ochi T, Tanaka J, et al. A new biotechnology for articular cartilage repair: subchondral implantation of a composite of interconnected porous hydroxyapatite, synthetic polymer (PLA-PEG), and bone morphogenetic protein-2 (rhBMP-2) Osteoarthr Cartil. 2005;13:405–417. doi: 10.1016/j.joca.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Ducy P, Karsenty G. The family of bone morphogenetic proteins. Kidney Int. 2000;57:2207–2214. doi: 10.1046/j.1523-1755.2000.00081.x. [DOI] [PubMed] [Google Scholar]
- 15.Lo KW, Ulery BD, Ashe KM, Laurencin CT. Studies of bone morphogenetic protein-based surgical repair. Adv Drug Deliv Rev. 2012;64:1277–1291. doi: 10.1016/j.addr.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chrastil J, Low JB, Whang PG, Patel AA. Complications associated with the use of the recombinant human bone morphogenetic proteins for posterior interbody fusions of the lumbar spine. Spine (Phila Pa 1976) 2013;38:E1020–E1027. doi: 10.1097/BRS.0b013e3182982f8e. [DOI] [PubMed] [Google Scholar]
- 17.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors (Chur, Switzerland) 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 18.Hwang CJ, Vaccaro AR, Lawrence JP, Hong J, Schellekens H, Alaoui-Ismaili MH, et al. Immunogenicity of bone morphogenetic proteins. J Neurosurg Spine. 2009;10:443–451. doi: 10.3171/2009.1.SPINE08473. [DOI] [PubMed] [Google Scholar]
- 19.Visser R, Rico-Llanos GA, Pulkkinen H, Becerra J. Peptides for bone tissue engineering. J Control Release. 2016;244:122–135. doi: 10.1016/j.jconrel.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 20.Tang S, Zhao J, Xu S, Li J, Teng Y, Quan D, et al. Bone induction through controlled release of novel BMP-2-related peptide from PTMC11-F127-PTMC11 hydrogels. Biomed Mater (Bristol, England) 2012;7:015008. doi: 10.1088/1748-6041/7/1/015008. [DOI] [PubMed] [Google Scholar]
- 21.Jain A, Jain A, Gulbake A, Shilpi S, Hurkat P, Jain SK. Peptide and protein delivery using new drug delivery systems. Crit Rev Ther Drug. 2013;30:293–329. doi: 10.1615/CritRevTherDrugCarrierSyst.2013006955. [DOI] [PubMed] [Google Scholar]
- 22.Kim HK, Kim JH, Park DS, Park KS, Kang SS, Lee JS, et al. Osteogenesis induced by a bone forming peptide from the prodomain region of BMP-7. Biomaterials. 2012;33:7057–7063. doi: 10.1016/j.biomaterials.2012.06.036. [DOI] [PubMed] [Google Scholar]
- 23.Ko E, Yang K, Shin J, Cho SW. Polydopamine-assisted osteoinductive peptide immobilization of polymer scaffolds for enhanced bone regeneration by human adipose-derived stem cells. Biomacromol. 2013;14:3202–3213. doi: 10.1021/bm4008343. [DOI] [PubMed] [Google Scholar]
- 24.Wang M, Deng Y, Zhou P, Luo Z, Li Q, Xie B, et al. In vitro culture and directed osteogenic differentiation of human pluripotent stem cells on peptides-decorated two-dimensional microenvironment. ACS Appl Mater Interfaces. 2015;7:4560–4572. doi: 10.1021/acsami.5b00188. [DOI] [PubMed] [Google Scholar]
- 25.Yang Y, Luo Z, Zhao Y. Osteostimulation scaffolds of stem cells: BMP-7-derived peptide-decorated alginate porous scaffolds promote the aggregation and osteo-differentiation of human mesenchymal stem cells. Biopolymers. 2018;109:e23223. doi: 10.1002/bip.23223. [DOI] [PubMed] [Google Scholar]
- 26.Li W, Zheng Y, Zhao X, Ge Y, Chen T, Liu Y, et al. Osteoinductive effects of free and immobilized bone forming peptide-1 on human adipose-derived stem cells. PLoS ONE. 2016;11:e0150294. doi: 10.1371/journal.pone.0150294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 28.Zhou P, Shi JM, Song JE, Han Y, Li HJ, Song YM, et al. Establishing a deeper understanding of the osteogenic differentiation of monolayer cultured human pluripotent stem cells using novel and detailed analyses. Stem Cell Res Ther. 2021;12:41. doi: 10.1186/s13287-020-02085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Draper JS, Pigott C, Thomson JA, Andrews PW. Surface antigens of human embryonic stem cells: changes upon differentiation in culture. J Anat. 2002;200:249–258. doi: 10.1046/j.1469-7580.2002.00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mao SH, Chen CH, Chen CT. Osteogenic potential of induced pluripotent stem cells from human adipose-derived stem cells. Stem Cell Res Ther. 2019;10:303. doi: 10.1186/s13287-019-1402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forsprecher J, Wang Z, Goldberg HA, Kaartinen MT. Transglutaminase-mediated oligomerization promotes osteoblast adhesive properties of osteopontin and bone sialoprotein. Cell Adh Migr. 2011;5:65–72. doi: 10.4161/cam.5.1.13369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carvalho MS, Silva JC, Hoff CM, Cabral JMS, Linhardt RJ, da Silva CL, et al. Loss and rescue of osteocalcin and osteopontin modulate osteogenic and angiogenic features of mesenchymal stem/stromal cells. J Cell Physiol. 2020;235:7496–7515. doi: 10.1002/jcp.29653. [DOI] [PubMed] [Google Scholar]
- 33.Jing X, Xie B, Li X, Dai Y, Nie L, Li C. Peptide decorated demineralized dentin matrix with enhanced bioactivity, osteogenic differentiation via carboxymethyl chitosan. Dent Mater. 2021;37:19–29. doi: 10.1016/j.dental.2020.09.019. [DOI] [PubMed] [Google Scholar]
- 34.Senta H, Park H, Bergeron E, Drevelle O, Fong D, Leblanc E, et al. Cell responses to bone morphogenetic proteins and peptides derived from them: biomedical applications and limitations. Cytokine Growth Factor Rev. 2009;20:213–222. doi: 10.1016/j.cytogfr.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Luo Z, Zhang S, Pan J, Shi R, Liu H, Lyu Y, et al. Time-responsive osteogenic niche of stem cells: A sequentially triggered, dual-peptide loaded, alginate hybrid system for promoting cell activity and osteo-differentiation. Biomaterials. 2018;163:25–42. doi: 10.1016/j.biomaterials.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 36.Karner E, Backesjo CM, Cedervall J, Sugars RV, Ahrlund-Richter L, Wendel M. Dynamics of gene expression during bone matrix formation in osteogenic cultures derived from human embryonic stem cells in vitro. Biochim Biophys Acta. 2009;1790:110–118. doi: 10.1016/j.bbagen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barruet E, Hsiao EC. Using human induced pluripotent stem cells to model skeletal diseases. Methods Mol Biol. 2016;1353:101–118. doi: 10.1007/7651_2014_171. [DOI] [PubMed] [Google Scholar]
- 39.Qiu M, Wang D, Liang W, Liu L, Zhang Y, Chen X, et al. Novel concept of the smart NIR-light-controlled drug release of black phosphorus nanostructure for cancer therapy. Proc Natl Acad Sci USA. 2018;115:501–506. doi: 10.1073/pnas.1714421115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alshehri S, Susapto HH, Hauser CAE. Scaffolds from self-assembling tetrapeptides support 3D spreading, osteogenic differentiation, and angiogenesis of mesenchymal stem cells. Biomacromol. 2021;22:2094–2106. doi: 10.1021/acs.biomac.1c00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Q, Zhang H, Pan J, Teng B, Zeng Z, Chen Y, et al. Tripeptide-based macroporous hydrogel improves the osteogenic microenvironment of stem cells. J Mater Chem B. 2021;9:6056–6067. doi: 10.1039/D1TB01175H. [DOI] [PubMed] [Google Scholar]
- 42.Ji Y, Wang M, Liu W, Chen C, Cui W, Sun T, et al. Chitosan/nHAC/PLGA microsphere vehicle for sustained release of rhBMP-2 and its derived synthetic oligopeptide for bone regeneration. J Biomed Mater Res A. 2017;105:1593–1606. doi: 10.1002/jbm.a.35962. [DOI] [PubMed] [Google Scholar]
- 43.Li J, Wei J, Li A, Liu H, Sun J, Qiao H. A dual peptide sustained-release system based on nanohydroxyapatite/polyamide 66 scaffold for synergistic-enhancing diabetic rats' fracture healing in osteogenesis and angiogenesis. Front Bioeng Biotechnol. 2021;9:657699. doi: 10.3389/fbioe.2021.657699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee SJ, Won JE, Han C, Yin XY, Kim HK, Nah H, et al. Development of a three-dimensionally printed scaffold grafted with bone forming peptide-1 for enhanced bone regeneration with in vitro and in vivo evaluations. J Colloid Interface Sci. 2019;539:468–480. doi: 10.1016/j.jcis.2018.12.097. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from all the authors.