Abstract
Background:
To enhance articular cartilage healing, microfractures (Mfx) and bone marrow aspirate concentrate (BMAC) are commonly used, and some form of scaffold is often used together to increase its efficacy. Herein, we compared the efficacy of atelocollagen scaffold to that of collagen scaffold when used with Mfx or BMAC on osteochondral defect of animal.
Methods:
This experiment was designed in two stages, and therapeutic effects of Mfx and BMAC were respectively evaluated when used with atelocollagen or collagen scaffold. Femoral condyle defects were artificially created in male New Zealand White rabbits, and in each stage, 12 rabbits were randomly allocated into three treatment groups: test group with additional atelocollagen scaffold, the positive control group with collagen scaffold, and the negative control group. Then, for 12 weeks, macroscopic and histological evaluations were performed.
Results:
At 12 weeks, defects in the test group were fully regenerated with normal cartilage-like tissue, and were well integrated with the surrounding cartilage at both stages experiment, whereas defects in the control groups were not fully filled with regenerated tissue, and the tissue appeared as fibrous tissue. Histologically, the regenerated tissue in the test group showed a statistically significant improvement compared to the positive and negative control groups, achieving a similar structure as normal articular cartilage.
Conclusion:
The results showed that implantation of the atelocollagen scaffold enhanced cartilage regeneration following osteochondral defects in rabbits. This suggests that the atelocollagen scaffold can be used with Mfx or BMAC for effective regeneration of osteochondral defects.
Keywords: Cartilage repair, Atelocollagen scaffold, BMAC, Microfracture, Osteochondral defect
Introduction
Articular cartilage refers to the hyaline cartilage that covers the joint surfaces. The articular cartilage absorbs external shocks and reduces the friction between two bones, enabling smooth and painless joint motion. Chondrocytes produce collagen, proteoglycan, and hyaluronic acid to form the extracellular matrix, which forms the basis for the mechanical composition of the cartilage. Collagen is the main component of the joint cartilage, and accounts for approximately 60% of the dry weight. Regeneration of the cartilage tissue rarely occurs following damage because of lack of cartilage tissue in blood vessel and limited regeneration ability of chondrocyte. Therefore, osteochondral lesions can cause early osteoarthritis if the damaged cartilage is left unattended [1]. The treatment method for articular cartilage differs depending on the degree of patient status and cartilage damage. Various technologies have been developed for use in cartilage repair, ranging from synthetic materials with or without cells to methods that include scaffold transplantation [2]. The optimal treatment would be an arthroscopic procedure to minimize morbidity and that provide a framework for maintaining cells in cartilage production, growth factors for matrix synthesis, cells, and protection to joint tissue [3, 4].
Microfracture (Mfx) is a method of bone marrow stimulation that induces bleeding in the perforated area by drilling the cartilage disc using an awl to treat damaged cartilage [5, 6]. It meets many of above criteria and is effective at relieving pain in the short term. However, stimulated bone marrow can be easily washed by synovial fluid, and there is a risk of deprivation and wear during the rehabilitation process following the procedure. In addition, bone marrow exposed to synovial fluid is not an environment in which stem cells in the bone marrow can differentiate into cartilage cells, and as a result, it can turn into fibrous cartilage rather than normal hyaline cartilage [7]. Bone marrow aspirate concentrate (BMAC) containing eukaryotic cells and various cytokines have been widely used to treat osteoarthritis because of the relatively low cost and low amount of required stem cells compared to cultured BMSCs [8, 9]. Bone marrow aspirate is a known source of mesenchymal stem cells for regeneration of tissues, such as cartilage [10, 11]. It is also a source of abundant growth factors, including platelet-derived growth factors (PDGF) and transforming growth factors (TGF-b) [12, 13].
Also, the use of collagen scaffold improves organizational treatment by supplementing collagen, which is naturally produced by cells during the organization treatment process. Recent studies have shown that the treatment effects of collagen scaffolds vary depending on their pore size. The most applicable collagen scaffold has very small pore size of less than 20 μm, making cells difficult to migrated into the defect. To overcome some of the current treatment limitations, new atelocollagen scaffold may be necessary. Atelocollagen is the modified collagen, in which has the same effectiveness as collagen with less antigenicity by removing telopeptides at the end of the collagen molecule. We have developed an atelocollagen scaffold and assessed its efficacy on the osteochondral defect healing when used with Mfx or BMAC. in the present study and confirmed its regeneration and repair effect through comparative tests with other products in control groups. We hypothesized that implantation of an atelocollagen scaffold would enhance the healing process, and compared its efficacy against other collagen scaffold in this experiment.
Materials and methods
Preparation of the atelocollagen and collagen scaffolds
A type I atelocollagen scaffold (CartiSeal) derived from porcine dermal skin was manufactured by Cellontech Co. Ltd. (Seoul, Korea). Type I and III collagen scaffolds (Chondro-Gide) were manufactured by Geistlich Pharma AG Co. Ltd. (Wolhusen, Switzerland).
Experimental design
There were two stages of experiment. In the first stage, 12 New Zealand White rabbits were randomized into test group (Mfx with atelocollagen scaffold), positive group (Mfx with collagen scaffold), and negative group (Mfx only). In the second stage, 12 New Zealand White rabbits were randomized into test group (BMAC with atelocollagen scaffold), positive group (BMAC with collagen scaffold), and negative group (BMAC only).
All New Zealand White rabbits (aged 30 weeks and weighing 3.6 ~ 4.2 kg) were kept in a standard rabbit cage (420 W × 500 D × 350 H) in a controlled environment with a 12 h light–dark cycle, room temperature of 22 ± 3 °C, and humidity of 50 ± 10%. Rabbits were acclimated to the study environment for 4 weeks. The animals were fed standard laboratory food pellets ad libitum. At each stage, animals were randomized into the treatment group (n = 4), and after creating the defect, the respective treatments were given. Articular cartilage defects were created bilaterally in the femur. No specific immobilization was performed after the operation; the rabbits were kept in separate cages and allowed to move freely. Animals were euthanized in a carbon dioxide euthanasia chamber after 3, 6, and 12 weeks. The entire knee was dissected, examined macroscopically, and photographed. Distal femurs were fixed in 10% buffered formalin (Sigma-Aldrich, St. Louis, MO, USA). Cartilage regeneration was evaluated histologically using hematoxylin–eosin-and Safranin O-stained sections. Immunohistochemistry with antibodies against type I and type II collagen was performed according to the polymer protocol. Histological analysis was performed using a semi-quantitative grading scoring system.
Surgical procedure
This study was approved by the Ethics Committee (Cellontech Institutional Animal Care and Use Committee [IACUC]) for animal experimentation and followed all institutional guidelines for the care and treatment of experimental animals (IACUC No. SC-IACUC-19-002/SC-IACUC-21-002). All surgeries were performed under sterile conditions. Each animal was premedicated according to its weight with an intramuscular injection of a mixture of Zoletil (Verbac, Carros, France) and Rompun (Bayer, Berlin, Germany) to ensure effective anesthesia. Subsequently, all animals in the BMAC group underwent bilateral iliac crest bone marrow aspiration using a biopsy needle. Aspirated samples were centrifuged at 1500 rpm for 15 min to allow the separation of plasma and red cells. The joint was opened with a medial parapatellar incision, and the patella was dislocated with the leg in full extension. A full-thickness defect (4 mm in diameter, 3 mm in depth) in the articular cartilage and subchondral bone was made with an electric burr applied to the femoral condyle of the femur. The Mfx group was applied from the periphery to the center of the defect. After this treatment, the wound was sutured in two layers with double 3–0 Vicryl sutures (Ethicon Inc., Somerville, NJ, USA), and the skin was sutured with a continuous 3–0 nylon suture (Ailee, Busan, Korea). Animals were divided into three groups according to the postoperative treatment, as follows: the experimental group was implanted with atelocollagen scaffold, the positive control group was implanted with collagen scaffold, and no treatment was used as a negative control. The surgical wounds were disinfected daily with potadine solution (Samil, Seoul, Korea), and skin sutures were removed 10 days after surgery. Cefazolin (Chong Kun Dang, Seoul, Korea), an antibiotic, was administered daily by intramuscular injection for 7 days.
Macroscopic analysis
The chondral defect in the articular cartilage defect group was observed 3, 6, and 12 weeks after surgery. The knee joint was approached as described above, and the distal femoral condyles were dissected. The gross appearance of defects was assessed. We considered the surface characteristics of the repair, its continuity with the host tissue, and the presence of osteoarthritic changes throughout the joint. The graft surfaces were inspected for color, integrity, contour, and smoothness.
Histological analysis
After sacrificing each specimen, the harvested tissues were fixed, decalcified, dehydrated, cleaned, embedded in paraffin, and sectioned. Then, 5 µm-thick sections of embedded tissues were cut using a microtome (Thermo Shandon, Finesse ME Microtome, Sweden) and stained with hematoxylin–eosin (KPNT, SHE-001, Cheongju, Korea). Paraffin sections were stained with Safranin O (Scytek Laboratories, SOC-1, Logan, UT, USA) to assess negatively charged matrix proteoglycans, and toluidine blue (Sigma-Aldrich) to demonstrate the distribution of glycosaminoglycans. Immunohistochemistry with antibodies for type I and type II collagen was performed according to a polymer protocol (Vector Laboratories, Burlingame, CA, USA). Antibody type I was provided by Abcam (Abcam, ab6308, Cambridge, UK) and antibody type II (Merck, CP-18, Darmstadt, Germany) was prepared according to the standard ABC protocol.
All specimens were graded using a histological scale, which is a modification of the scoring systems described by O’Driscoll et al., Pineda, and Wakitani [14–18]. The scale comprises seven categories and subjects are assigned a score ranging from to 0–23 points (Table 1). A lower score indicates better cartilage repair.
Table 1.
Histological grading scale for the cartilage defects
| Category | Points |
|---|---|
| Cell morphology | |
| Hyaline cartilage | 0 |
| Mostly hyaline cartilage | 1 |
| Mostly fibrocartilage | 2 |
| Mostly non-cartilage | 3 |
| Non-cartilage only | 4 |
| Matrix-staining | |
| Normal | 0 |
| Slightly reduced | 1 |
| Moderately reduced | 2 |
| Markedl reduced | 3 |
| None | 4 |
| Surface regularity | |
| Smooth (<3/4) | 0 |
| Slight disruption | 1 |
| Severe disruption | 2 |
| Filling of the defect | |
| 111%– | 1 |
| 91–110% | 0 |
| 76–90% | 1 |
| 51–75% | 2 |
| 26–50% | 3 |
| –25% | 4 |
| Thickness of cartilage | |
| >2/3 | 0 |
| 1/3–2/3 | 1 |
| <1/3 | 2 |
| Integration of donor with host adjacent cartilage | |
| Normal continuity and integration | 0 |
| Decreased cellularity | 1 |
| Gap (lack of continuity) on one side | 2 |
| Gap (lack of continuity) on two side | 3 |
| Percentage replacement of subchondral bone | |
| 90–100% | 0 |
| 75–89% | 1 |
| 50–74% | 2 |
| 25–49% | 3 |
| 0–24% | 4 |
| Total score | 23 |
*This table was modified from the scale described by O’ Driscoll, Pineda, and Wakitani
Statistical analysis
All data are expressed as the mean ± standard deviation. The results were compared by two-way analysis of variance using SigmaPlot 10.0 software (Systat Software Inc., San Jose, CA, USA). P-values < 0.05 were considered significant.
Results
Macroscopic observation
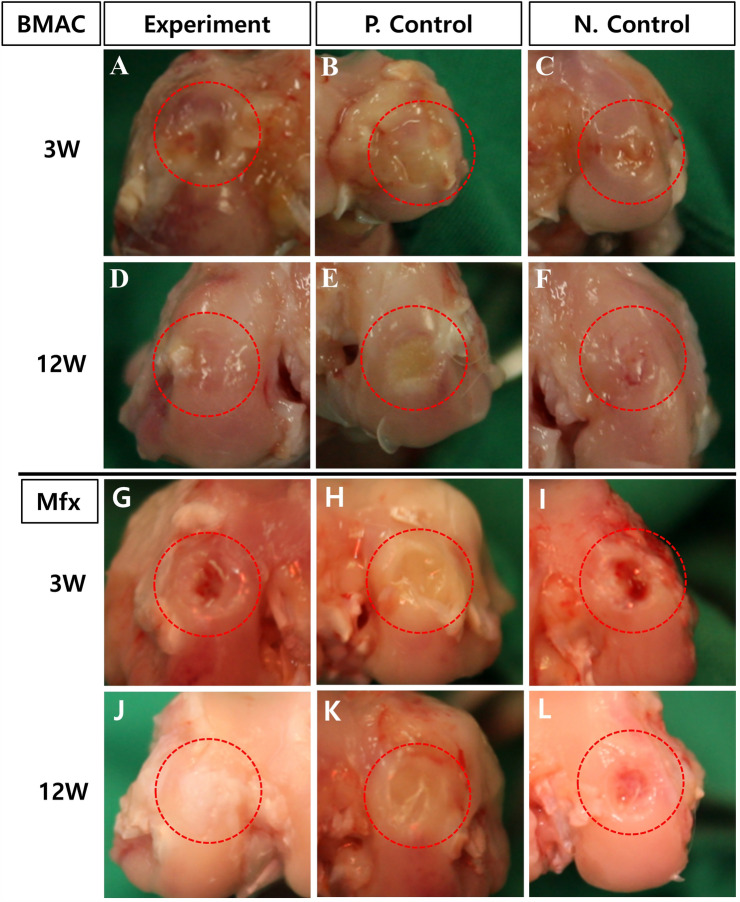
All animals survived till study completion. After implantation of the collagen scaffold, there were no cases of death, weight loss, inflammation, or abnormal reaction in any of the rabbits. Further, there were no signs of inflammation, osteoarthritic changes, or obvious synovitis in any of the rabbits. The appearance of the restored cartilage was also examined, assessing color, integrity, and smoothness. The defect conditions observed after 3 and 12 weeks later are shown in Fig. 1. After 3 weeks of treatment, macroscopic observation showed surface irregularities, with a red-whitish appearance in the test group and negative control group, while the treated groups had defects filled with irregular fibrous tissue (Fig. 1A, B, C and G, H, I). At 12 weeks, defects in the test group were fully regenerated with normal cartilage-like tissue and were well-integrated with the surrounding cartilage (Fig. 1D, J). The defect areas in the positive control group were not fully filled with regenerated tissue, and the tissue appeared fibrous (Fig. 1E, K). However, the regenerated area of the negative control group showed red, irregular tissue with depressions, and the margin of the defect was sharply differentiated from the surrounding normal cartilage (Fig. 1F, L).
Fig. 1.
Macroscopic evaluation of artificial defects along the femoral condyle of the distal femur at 3 and 12 weeks in the atelocollagen-implanted experimental group (A, D, G, and J) and the collagen-implanted positive control group (B, E, H, and K) and non-treat negative control group (C, F, I, and L): 3 weeks (A, B, C and G, H, I), 12 weeks (D, E, F and J, K, L) after surgery. Red dotted lines indicate defect sites. (BMAC: Bone Marrow Aspirate Concentrate, Mfx: Microfracture)
Histological observation
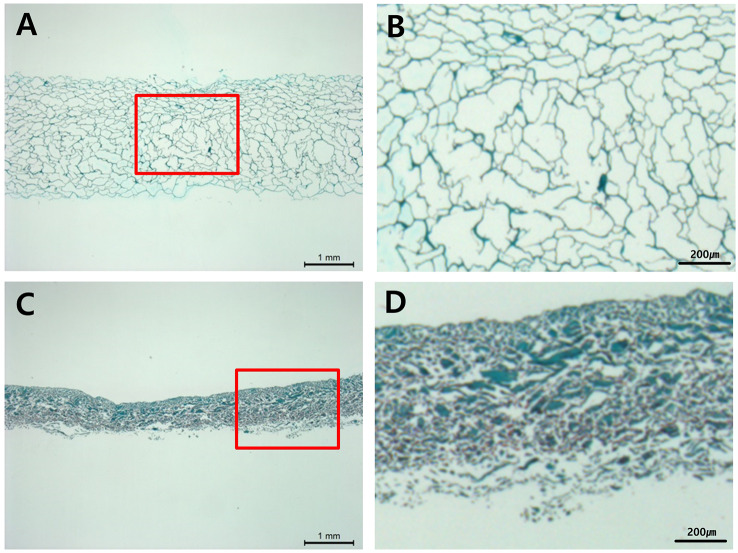
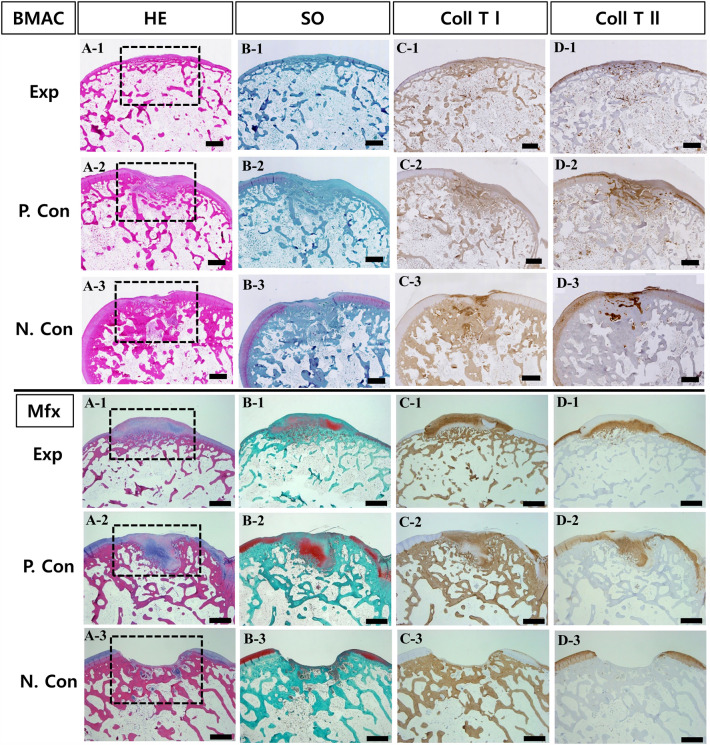
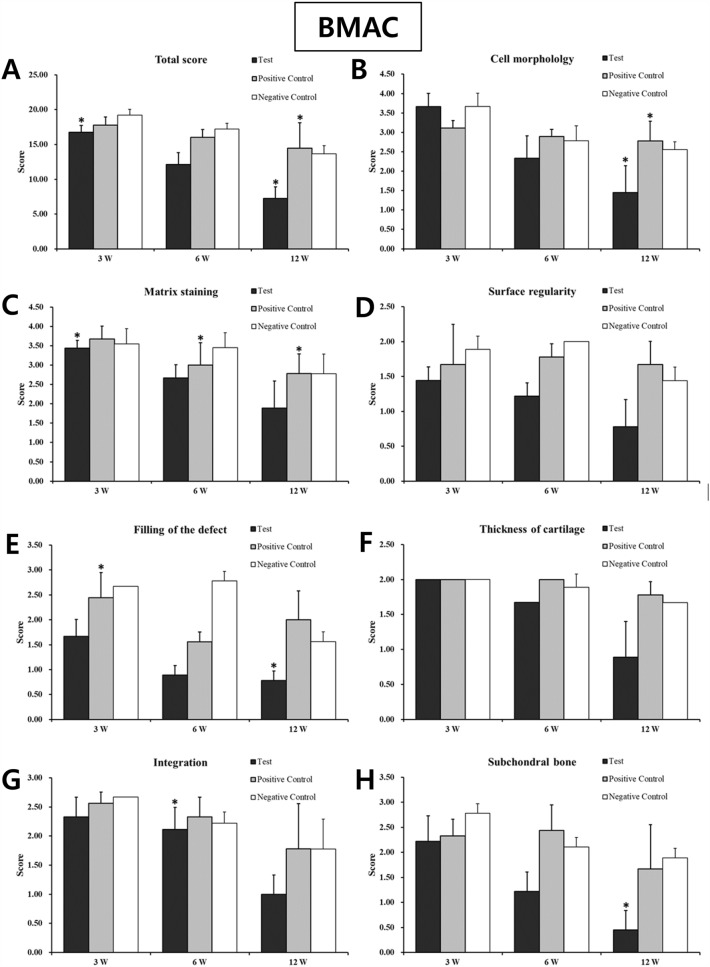
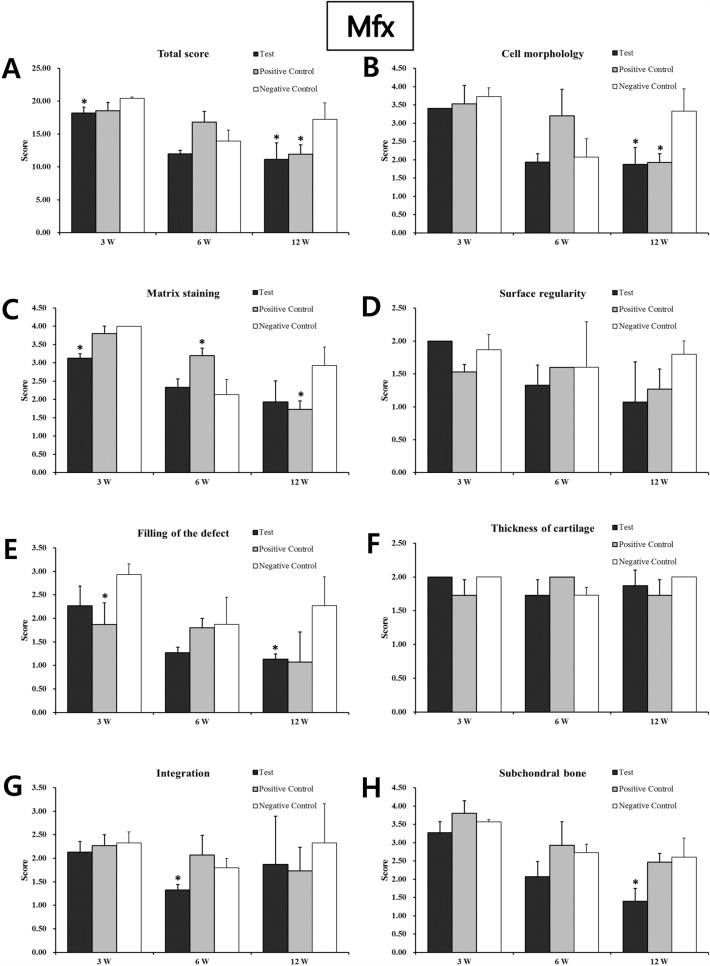
As a result of tissue fragments performed on the unimplanted product to identify the shape and voids of the implant, the Type I atelocollagen scaffold (CartiSeal) showed a uniform form of porosity with an irregular form of porosity (Fig. 2A, B). Type I and III collagen scaffolds (Chondro-Gide) in the positive control, showed a very dense structure (Fig. 2C, D). After gross morphological analysis, histological and immunohistochemical analyses were performed at 3, 6, and 12 weeks after therapeutic implantation to prove that the defective site was filled with newly repaired cartilage. Sample histological images for the BMAC- and Mfx-treated groups at 12 weeks are provided in Fig. 3. In the BMAC-treated group, cartilage defects in the test group were filled with newly regenerated tissue similar to normal cartilage, and the border between the regenerated tissue and normal cartilage was completely integrated (Fig. 3A-1). The regenerated tissue in the test group showed typical features of a hyaline-like cartilage. Safranin O staining revealed the presence of glycosaminoglycans (Fig. 3B-1). Immunohistochemical staining showed that the regenerated cartilage was predominantly composed of type II collagen layered with type I collagen (Fig. 3C-1 and D-1). In the positive and negative control groups, cartilage defects at 12 weeks were not completely filled with tissue (Fig. 3A-2 and A-3). In the Mfx-treated group, cartilage defects in the test group were overgrowth cartilage-like, filled with newly regenerated cartilage, similar to the subchondral cartilage (Fig. 3A-4). In the positive control group, cartilage defects at 12 weeks were almost completely filled with tissue (Fig. 3A-5). In contrast, in the negative control group, the surface layer was still disrupted without resurfacing, the cells were further exposed, and the tissue integrity was disorganized (Fig. 3A-6). Safranin O staining revealed the presence of glycosaminoglycans (Fig. 3B-4). Immunohistochemical staining showed that the regenerated cartilage was type II collagen layered with type I collagen in all groups but the negative control (Fig. 3C-4 and D-4). We further used a modified O’Driscoll scoring system to compare the quality of regenerated tissue in the test group with that of the positive and negative control groups (Table 2). In the BMAC-treated group, the total scores from histological evaluation in the test group at 12 weeks after implantation were 7.24 ± 1.64, whereas those in the positive and negative control groups were 14.46 ± 3.67 and 13.68 ± 3.67, respectively. The total histological evaluation scores at 12 weeks after implantation were significantly different between the test group and negative control groups (filling of defect, p = 0.008; subchondral bone, p = 0.004). In the Mfx-treated group, the total scores from histological evaluation in the test group at 12 weeks after implantation were 11.14 ± 2.54, whereas those in the positive control and negative control group were 11.93 ± 1.45 and 17.26 ± 2.47, respectively. The total histological evaluation scores at 12 weeks after implantation were significantly different between the test, positive control, and negative control groups (cell morphology, p = 0.029; filling of defect, p = 0.034; subchondral bone, p = 0.030). Finally, the test group performed significantly better than the control group (Figs. 4, 5).
Fig. 2.
Histological analysis for product to shape and pore size. Type I atelocollagen scaffold (CartiSeal), Safranin O stain: A scale bar 1 mm, B scale bar 200 µm. Type I & III collagen scaffold (Chondro-Gide), Safranin O stain: C scale bar 1 mm, D scale bar 200 µm. Red dotted lines indicate enlarge sites
Fig. 3.
Histological and immunohistochemical evaluations of articular cartilage resurfacing with atelocollagen scaffold at 12 weeks (12.5 ×). The letters represent the following: A hematoxylin and eosin staining, B safranin O staining, C type I collagen immunohistochemical staining, and D type II collagen immunohistochemical staining. The numbers represent the following: (1, 4) rabbits at 12 weeks after atelocollagen scaffold implant test group, (2, 5) rabbits at 12 weeks after collagen scaffold implant positive control group, (3, 6) rabbits at 12 weeks after non-treat negative control group. Black dotted lines indicate defect sites. (BMAC: Bone Marrow Aspirate Concentrate, Mfx: Microfracture). Scale bars: 1 mm
Table 2.
Histological scoring result for the cartilage defects
| Time after implantation | Test | Test | Group (n = 3) | |
|---|---|---|---|---|
| Found (ppm) | P. Control | N. Control | ||
| BMAC | 3W | 16.77 ± 0.97* | 17.78 ± 1.17 | 19.23 ± 0.84 |
| 6W | 12.11 ± 1.71 | 16.00 ± 1.15 | 17.23 ± 0.84 | |
| 12W | 7.24 ± 1.64* | 14.46 ± 3.67* | 13.684 ± 1.16 | |
| Mfx | 3W | 18.20 ± 0.87* | 18.53 ± 1.29 | 20.43 ± 0.21 |
| 6W | 11.99 ± 0.53 | 16.80 ± 1.64 | 13.93 ± 1.70* | |
| 12W | 11.14 ± 2.54* | 11.93 ± 1.45* | 17.26 ± 2.47 | |
Result are given as mean ± standard deviation (SD)
*Statistical difference compared to the control group (p < 0.05)
BMAC Bone marrow aspirate concentrate; Mfx microfracture
Fig. 4.
Histological evaluation scores of regenerated cartilage in the BMAC in vivo animal study. The total scores of the test group at 3, 6, and 12 weeks were significantly better than those of the control group (Student’s t-test; *p < 0.05 and **p < 0.01). *A total score; B cell morphology; C matrix staining; D surface regularity; E filling of the defect; F cartilage thickness; G integration of donor with host; H replacement of the subchondral bone. (BMAC: Bone Marrow Aspirate Concentrate)
Fig. 5.
Histological evaluation scores of regenerated cartilage in the Mfx in vivo animal study. The total scores of the experimental group at 3, 6, and 12 weeks were significantly better than those of the control group (Student’s t-test; *p < 0.05 and **p < 0.01). *A total score; B cell morphology; C matrix staining; D surface regularity; E filling of the defect; F cartilage thickness; G integration of donor with host; H replacement of the subchondral bone. (Mfx: Microfracture).
Discussion
As damaged cartilage tissue lacks the ability to regenerate, treatments such as scaffold-based bone marrow stem cell transplantation and Mfx are required to alleviate symptoms of injury and improve cartilage function. Marrow stimulation technique, such as Mfx, may be the most important treatment option since it is the first selected one [19]. It can induce the migration of mesenchymal stem cell (MSC) and other necessary growth factors to the cartilage defect by promoting the flow of blood low and formation of blood clot to allow an influx from the bone marrow. However, Mfx often displays inadequate healing capacity as it mostly promotes fibrocartilage regeneration, not hyaline-like cartilage [20]. To overcome this shortcoming, various treatment options are applied in addition to Mfx.
One of the options is using biodegradable scaffolds. In the first experiment, CartiSeal and Chondro-Gide were used after Mfx, and their results were compared. In this experiment, Mfx combined with collagen supplementation showed more effective treatment results than Mfx alone; in particular, at 12 weeks, good results were macroscopically confirmed in the repair of the defect, cartilage thickness, and cartilage surface. Also histologically, although there was no significance at 6 weeks, both CartiSeal and Chondro-Gide showed significantly better scores than the Mfx alone group at 12 weeks. When collagen supplementation was used together, it could successfully confine the blood from the bone marrow to the defect site; therefore, MSC and growth factors from bone marrow were able to be concentrated to the defect and efficiently promoted the regeneration of articular cartilage. Especially CartiSeal showed better results in histological evaluation than Mfx and Chondro-Gide. It may be due to CartiSeal having more porous structure than Chondro-Gide, so bone marrow cells flowed smoothly from the beginning. One of the interesting results was that at 6 weeks, the histologic score of Mfx alone group was improved enough to the point when there was no significant difference to CartiSeal- or Chondro-Gide-treated group. It may be due to bone marrow blood, which was drawn by Mfx, stimulated and promoted the surrounding tissues for the regeneration. However, just like its histological score dropped at 12 weeks, its therapeutic effect was not long-lasted as when collagen supplements were used.
Other option is adding MSC, growth factors, and BMAC for the treatment. Especially, BMAC contains high concentration of MSCs, various growth factors, including platelet-derived growth factor (PDGF), transforming growth factor-b (TGF-b), and vascular endothelial growth factor (VEGF) [21]. When BMAC was used for the treatment of articular cartilage defect in addition to Mfx, it resulted in significantly improved cartilage repair in animal and human patients [22].
In the second experiment, where BMAC was used in addition to CartiSeal and Chondro-Gide, after Mfx, bone marrow stem cell transplantation using collagen supplements showed more effective treatment results than those using only bone marrow stem cell transplantation. In particular, at week 12, good results were macroscopically obtained in the repair of the defect, cartilage thickness, and cartilage surface. Also, in histological evaluation, CartiSeal- and Chondro-Gide-treated group showed significantly better scores at 12 weeks. When BMAC was used in combination with collagen scaffold, it may be absorbed into the collagen scaffold and sustaining the stable release of growth factors over time to the defect site. Type I collagen scaffold has been used as carrier for BMP, and promoted bone formation as well as cartilage repair [23]. At 6 weeks, there was no significant difference between CartiSeal-treated group and BMAC-only group. It may be due to therapeutic effect of BMAC on cartilage regeneration, but it did not last until 12 weeks result. One interesting finding from this experiment was that although both CartiSeal and Chondro-Gide are similar collagen scaffold, they showed different results in terms of gross observation and histological evaluation. Just like the first experiment, it may be due to the structural difference between CartiSeal and Chondro-Gide. To support this finding, additional experiment may necessary to compare the different structure of CartiSeal and Chondro-Gide and their impact on clinical effects.
Since damaged cartilage tissue lacks the ability to regenerate, treatments such as scaffold-based BMAC stem cell implantation and Mfx are required to alleviate symptoms and improve cartilage function. In this animal study, an atelocollagen scaffold was evaluated for the treatment of osteochondral defects. Implantation of the atelocollagen scaffold resulted in morphologically and histologically enhanced regeneration of articular cartilage, ultimately displaying the regeneration of hyaline-like cartilage tissue. Overall, these results suggest that BMAC and Mfx-applied implantation of an atelocollagen scaffold can be used for effective regeneration of osteochondral defects.
Data availability
The data presented in this study are available on request from all the authors.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This study was approved by the ethics committee (Cellontech Institutional Animal Care and Use Committee [IACUC]) for animal experimentation and followed all institutional guidelines for the care and treatment of experimental animals (IACUC No. SC-IACUC-19-002/SC-IACUC-21-002).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gilbert JE. Current treatment options for the restoration of articular cartilage. Am J Knee Surg. 1998;11:42–46. [PubMed] [Google Scholar]
- 2.McNickle AG, Provencher MT, Cole BJ. Overview of existing cartilage repair technology. Sports Med Arthrosc Rev. 2008;16:196–201. doi: 10.1097/JSA.0b013e31818cdb82. [DOI] [PubMed] [Google Scholar]
- 3.Stoddart MJ, Grad S, Eglin D, Alini M. Cells and biomaterials in cartilage tissue engineering. Regen Med. 2009;4:81–98. doi: 10.2217/17460751.4.1.81. [DOI] [PubMed] [Google Scholar]
- 4.Goldring MB, Otero M, Tsuchimochi K, Ijiri K, Li Y. Defining the roles of inflammatory and anabolic cytokines in cartilage metabolism. Ann Rheum Dis. 2008;67:iii75–82. [DOI] [PMC free article] [PubMed]
- 5.Rodrigo JJ. Improvement of full-thickness chondral defect healing in the human knee after debridement and microfracture using continuous passive motion. Am J Knee Surg. 1994;7:109–116. [Google Scholar]
- 6.Knutsen G, Drogset JO, Engebretsen L, Grøntvedt T, Isaksen V, Ludvigsen TC, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture: findings at five years. J Bone Joint Surg Am. 2007;89:2105–2112. doi: 10.2106/00004623-200710000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Kreuz PC, Steinwachs MR, Erggelet C, Krause SJ, Konrad G, Uhl M, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthr Cartil. 2006;14:1119–1125. doi: 10.1016/j.joca.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Park YB, Ha CW, Rhim JH, Lee HJ. Stem cell therapy for articular cartilage repair: review of the entity of cell populations used and the result of the clinical application of each entity. Am J Sports Med. 2018;46:2540–2552. doi: 10.1177/0363546517729152. [DOI] [PubMed] [Google Scholar]
- 9.Jäger M, Jelinek EM, Wess KM, Scharfstädt A, Jacobson M, Kevy SV, et al. Bone marrow concentrate: a novel strategy for bone defect treatment. Curr Stem Cell Res Ther. 2009;4:34–43. doi: 10.2174/157488809787169039. [DOI] [PubMed] [Google Scholar]
- 10.Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18:1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- 11.Fortier LA, Nixon AJ, Williams J, Cable CS. Isolation and chondrocytic differentiation of equine bone marrow-derived mesenchymal stem cells. Am J Vet Res. 1998;59:1182–1187. doi: 10.2460/ajvr.1998.59.09.1182. [DOI] [PubMed] [Google Scholar]
- 12.Chen FH, Tuan RS. Mesenchymal stem cells in arthritic diseases. Arthritis Res Ther. 2008;10:223. doi: 10.1186/ar2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilke MM, Nydam DV, Nixon AJ. Enhanced early chondrogenesis in articular defects following arthroscopic mesenchymal stem cell implantation in an equine model. J Orthop Res. 2007;25:913–925. doi: 10.1002/jor.20382. [DOI] [PubMed] [Google Scholar]
- 14.O’Driscoll SW, Keeley FW, Salter RB. Durability of regenerated articular cartilage produced by free autogenous periosteal grafts in major full-thickness defects in joint surfaces under the influence of continuous passive motion. A follow-up report at one year. J Bone Joint Surg Am. 1988;70:595–606. doi: 10.2106/00004623-198870040-00017. [DOI] [PubMed] [Google Scholar]
- 15.O’Driscoll SW, Keeley FW, Salter RB. The chondrogenic potential of free autogenous periosteal grafts for biological resurfacing of major full-thickness defects in joint surfaces under the influence of continuous passive motion. An experimental investigation in the rabbit. J Bone Joint Surg Am. 1986;68:1017–1035. doi: 10.2106/00004623-198668070-00008. [DOI] [PubMed] [Google Scholar]
- 16.Pineda S, Pollack A, Stevenson S, Goldberg V, Caplan A. A semiquantitative scale for histologic grading of articular cartilage repair. Acta Anat. 1992;143:335–340. doi: 10.1159/000147272. [DOI] [PubMed] [Google Scholar]
- 17.Rutgers M, van Pelt MJ, Dhert WJ, Creemers LB, Saris DB. Evaluation of histological scoring systems for tissue-engineered, repaired and osteoarthritic cartilage. Osteoarthr Cartil. 2010;18:12–23. doi: 10.1016/j.joca.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, et al. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76:579–592. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Madry H, Gao L, Eichler H, Orth P, Cucchiarini M. Bone marrow aspirate concentrate-enhanced marrow stimulation of chondral defects. Stem Cells Int. 2017;2017:1609685. [DOI] [PMC free article] [PubMed]
- 20.Yang HS, et al. Hyaline cartilage regeneration by combined therapy of microfracture and long-term bone morphogenetic protein-2 delivery. Tissue Eng Part A. 2011;17(13-14):1809–1818. doi: 10.1089/ten.tea.2010.0540. [DOI] [PubMed] [Google Scholar]
- 21.Liu XN, et al. Enhanced tendon-to-bone healing of chronic rotator cuff tears by bone marrow aspirate concentrate in a rabbit model. Clin Orthop Surg. 2018;10(1):99. doi: 10.4055/cios.2018.10.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fortier LA, et al. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. JBJS. 2010;92(10):1927–1937. doi: 10.2106/JBJS.I.01284. [DOI] [PubMed] [Google Scholar]
- 23.Kuo AC, et al. Microfracture and bone morphogenetic protein 7 (BMP-7) synergistically stimulate articular cartilage repair. Osteoarthritis Cartilage. 2006;14(11):1126–1135. doi: 10.1016/j.joca.2006.04.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from all the authors.