Abstract
Borrelia burgdorferi, the causative agent of Lyme disease, can contain multiple genes encoding different members of the Erp lipoprotein family. Some arthropod-borne bacteria increase the synthesis of proteins required for transmission or mammalian infection when cultures are shifted from cool, ambient air temperature to a warmer, blood temperature. We found that all of the erp genes known to be encoded by infectious isolate B31 were differentially expressed in culture after a change in temperature, with greater amounts of message being produced by bacteria shifted from 23 to 35°C than in those maintained at 23°C. Mice infected with B31 by tick bite produced antibodies that recognized each of the Erp proteins within 4 weeks of infection, suggesting that the Erp proteins are produced by the bacteria during the early stages of mammalian infection and may play roles in transmission from ticks to mammals. Several of the B31 Erp proteins were also recognized by antibodies from patients with Lyme disease and may prove to be useful antigens for diagnostic testing or as components of a protective vaccine.
Borrelia burgdorferi, the causative agent of Lyme disease, is spread to humans and other mammals through the bites of infected Ixodes ticks (8). In unfed ticks, the bacteria are primarily restricted to the midgut, and as ticks feed, bacteria migrate from the midgut to the salivary glands and are transmitted into the bite wound with the saliva (5, 19, 28, 30, 50). This mode of transmission undoubtedly requires that B. burgdorferi recognize when the host tick is feeding on a warm-blooded animal and then synthesize proteins required for transmission and the subsequent establishment of infection in the mammalian host. We are seeking to identify the proteins that are important in B. burgdorferi transmission and, ultimately, the factors that regulate their synthesis.
Many bacteria utilize environmental temperature as a signal to determine their location, synthesizing vector-specific proteins at the cooler temperatures experienced within an arthropod and mammal-specific proteins at warmer, blood temperatures (6, 20, 26, 38, 39). Differences in temperature may also change bacterial growth rates, which in turn may provide internal signals affecting the production of vector- or mammal-specific proteins. We have previously reported that B. burgdorferi increases the synthesis of certain proteins during tick feeding or after a shift in culture temperature from 23 to 35°C (34, 42, 45). This temperature change also results in a marked increase in bacterial growth rate (approximately three to four times greater in bacteria shifted from 23 to 35°C than in those maintained at 23°C) (42), and B. burgdorferi in infected ticks also undergoes a dramatic increase in growth rate during tick feeding (14, 28).
The B. burgdorferi proteins that we reported as being differentially synthesized as a result of temperature shift were recognized by sera from infected animals (34, 42), indicating that they are normally synthesized by the bacteria during mammalian infection. At least one of these proteins, OspC, is not synthesized by B. burgdorferi within the midgut of unfed ticks (16, 34), whereas bacteria within the midgut and salivary glands of ticks that have engorged with blood produce OspC (12, 15, 34), suggesting that this protein is involved with bacterial transmission or the early stages of mammalian infection.
We also found that the OspE and OspF proteins of B. burgdorferi N40 (23) were differentially synthesized after a shift in culture temperature from 23 to 35°C (42). Analyses of B. burgdorferi B31 indicated that this isolate can carry many members of a family of genes that are homologous to, and apparently allelic with, the N40 ospEF locus, which we have designated erp (OspEF-related proteins) (11, 43). We previously characterized the four erp loci present in a noninfectious B31 culture that has been passage repeatedly in laboratory culture medium and have found that each erp locus is carried on one of four homologous 32-kb circular plasmids (cp32-1 through cp32-4) (11, 43). However, infectious, low-passage-number B31 bacteria may contain at least seven 32-kb circular plasmids (cp32-1 through cp32-7), each containing an erp locus (11). Infectious B31 can also carry a related linear plasmid (lp56) (11, 48, 49) that was not previously known to contain an erp locus. Since this large number of different genes and proteins could allow for a wide range of expression patterns, characterizing all of the erp genes and their proteins within a particular isolate is an essential step toward understanding the roles that these proteins may play in B. burgdorferi transmission and the establishment of Lyme disease infections. Due to the extensive sequence similarities of the cp32 plasmids, they could not be confidently assembled by the B. burgdorferi B31 genome sequencing project of The Institute for Genomic Research (17), and their complete sequences have not yet been published. Homologs of the B31 Erp proteins have been identified in other isolates of B. burgdorferi, although no more than three loci have been described from any isolate other than B31 (1, 23, 25, 44, 46). Immune responses directed against Erp homologs have also been described, but again, these analyses are incomplete, as only one or two proteins from any isolate have been examined (13, 27, 46). In this report we describe all of the erp genes known to be carried by an infectious culture of B31 and show that all of the erp genes tested can be expressed by cultured bacteria by changing the temperature from 23 to 35°C. We also found that tick-infected laboratory animals produced antibodies that recognized all of the known B31 Erp proteins, as did many human Lyme disease patients.
MATERIALS AND METHODS
Bacteria.
B. burgdorferi B31 was originally isolated from an infected Ixodes scapularis tick collected on Shelter Island, New York (8). These bacteria have been maintained in the laboratory via an infectious cycle between I. scapularis ticks and mice (34). Clone B31-4a was derived from a single colony of infectious B31 plated on solid Barbour-Stoener-Kelly (BSK) medium (22, 31) and is also infectious in laboratory mice (11).
All B. burgdorferi isolates were cultured in BSK-H broth (Sigma, St. Louis, Mo.) supplemented with 6% rabbit serum (Sigma). Cultures used to determine temperature shift differential synthesis of B. burgdorferi proteins, and mRNAs were grown at 23 or 35°C as previously described (34, 42). Briefly, 100-ml cultures were grown at 23°C until the culture reached a density of approximately 107 bacteria per ml (approximately 3 weeks); 1 ml of this culture was diluted into 100 ml of fresh medium and grown at 35°C to a similar density (approximately 4 to 6 days). Both cultures were harvested by centrifugation.
Cloning and sequence analysis of erp genes.
Plasmid DNAs from B31-4a were purified with a Qiagen (Chatsworth, Calif.) midi-purification kit from a 100-ml culture grown at 35°C. The erpAB2 and erpX loci were PCR amplified by using oligonucleotides specific to the orf3 gene found on cp32-1 and lp56, respectively (11, 40, 49) and a conserved DNA sequence that is located approximately 1.5 kb 3′ of every erp locus (2, 11, 41) (Table 1). For PCR performed with an Expand Long Template PCR kit (Boehringer Mannheim, Indianapolis, Ind.), reaction conditions consisted of 94°C for 30 s, 50°C for 30 s, and 68°C for 8 min, followed by 20 cycles of the same conditions but with successive elongation steps increasing by 20 s each cycle (41).
TABLE 1.
Oligonucleotides used in this work
| Use | Our desig- nation | Sequence (5′-3′) |
|---|---|---|
| cp32-1 (erpAB2) cloning | ORFD-1 | ACGATAGGGTAATATCAAAAAAGG |
| CP-0 | GAAAAAGATAACATGCAAGATACG | |
| lp56 (erpX) cloning | ORFD-5 | TTACCAAAGAGGAGATATTTGCTC |
| CP-0 | GAAAAAGATAACATGCAAGATACG | |
| Gene-specific probes | ||
| erpA | E-101 | GTGCTGTTTTTATAC |
| E-104 | CAGTTATTAATTTTATCTCC | |
| erpB2 | E-115 | GAAAGTGAAAAAAGTAGAAGAATC |
| E-136 | CTTTAGCTTCTTTTTCTGCCTTAG | |
| erpG | E-33 | TGCAAGATTGATGCG |
| E-34 | ATTTTGAGGCTCTGC | |
| bapA | E-25 | AGAAACTAAAAGAGC |
| E-58 | AGTCTAATCATATCCTCAGACAGG | |
| erpK | E-505 | GAGAAGTCGGATCCTAAAAGTG |
| E-510 | TCCAATTGCAGATTCAAC | |
| erpL | E-702 | CAACACCACCTCGGTTTTTAGACC |
| E-703 | AGGACTTTTAGAAATTCTAGAGAC | |
| erpM | E-718 | TTCTTTTTCTGCTTTAACCCTAGC |
| E-721 | AATCTAAAGATAAAGTTGAGGAAG | |
| erpX | E-904 | TCTAAATCTTCTTTAATTTCGCTG |
| E-909 | GCAGTTATAGATAAAATTACGGGG | |
| flaB | FL6 | TTCAGGGTCTCAAGCGTCTTGGACT |
| FL7 | GCATTTTCAATTTTAGCAAGTGATG |
The complete sequences of the B31-4a erpIJ and erpLM loci were determined from previously described DNA fragments (11). The erpAB2 and erpIJ loci were sequenced from uncloned PCR fragments. The erpLM and erpX loci were each sequenced from two separate PCR amplification products that had been cloned into the TA vector pCR2.1 (Invitrogen, San Diego, Calif.). All DNA sequencing was performed with a model 370A Stretch automated DNA sequencer (Applied Biosystems, Foster City, Calif.).
Analysis of mRNA.
Total RNA was extracted from B. burgdorferi B31-4a grown at 23°C or shifted to 35°C, using an Ultraspec RNA isolation system (Biotecx, Houston, Tex.) according to the manufacturer’s instructions. The RNA was denatured with glyoxal and dimethyl sulfoxide, separated by agarose gel electrophoresis in 10 mM sodium phosphate buffer (pH 7.0) (32), and transferred to nylon membranes (Micron Separations, Westborough, Mass.). Probes specific for the B31 erp, bapA, and flaB (flagellin) genes were generated by PCR from recombinant plasmids carrying the appropriate loci, using the oligonucleotides listed in Table 1, as previously described (43). DNA fragments were radiolabeled with [α-32P]dATP (Du Pont, Boston, Mass.) by random priming (Life Technologies, Gaithersburg, Md.). Filters were hybridized with each radiolabeled probe at 55°C in 1% (wt/vol) bovine serum albumin–7% (wt/vol) sodium dodecyl sulfate (SDS)–0.5 M sodium phosphate (pH 7.0)–1 mM EDTA and washed at 55°C in 0.2× SSC (1× SSC is 0.15 M NaCl and 0.015 M sodium citrate)–0.1% (wt/vol) SDS as previously described (7).
Protein purification and analysis.
B. burgdorferi cultures were pelleted by centrifugation, washed twice with phosphate-buffered saline, resuspended in sample buffer (32), and lysed by boiling for 5 min.
DNA fragments encoding erpA, erpB2, erpC, erpD, erpG, erpK, erpL, erpM, and erpX were individually cloned into pProEX-1 (Life Technologies), so that each gene was in the correct reading frame to encode a fusion protein with the plasmid-encoded polyhistidine polypeptide. Recombinant plasmids were transformed into Escherichia coli DH5α (Life Technologies). A single colony from each transformation was grown at 37°C to early log phase in 100 ml of LB broth (24), induced with 100 μg of isopropyl thiogalactoside per ml, and grown for an additional 2 h before harvesting by centrifugation. The bacteria were lysed by sonication, and the fusion proteins were purified by using His-Bind Resin column chromatography kits (Novagen, Madison, Wis.) as recommended by the manufacturer.
Proteins were separated by SDS-polyacrylamide gel electrophoresis (21) and visualized by staining with Coomassie brilliant blue. Alternatively, proteins were transferred to nitrocellulose membranes (Life Technologies) and immunoblotted as previously described (45), using horseradish peroxidase-linked protein A (Amersham, Arlington Heights, Ill.) and SuperSignal chemiluminescent substrate (Pierce, Rockford, Ill.).
Antisera.
Uninfected larval I. scapularis ticks, hatched and reared at our facility, were fed on white-footed mice (Peromyscus leucopus) that had previously been infected with B. burgdorferi B31. After molting to the nymphal stage, ticks were placed on uninfected white-footed mice and allowed to feed to repletion. Approximately 15 to 25 ticks were fed on each mouse. Sera were collected 4 weeks (two mice) or 8 weeks (three mice) after tick attachment. Infection was determined by immunoreactivity to the B. burgdorferi BmpA (P39) protein, an antigen diagnostic of active infection (35, 37). Sera were also collected from two mice that were not infected with B. burgdorferi.
Sera from three of the mice infected for 8 weeks were pooled and preadsorbed with individual recombinant B31 Erp proteins to remove specific antibodies in subsequent immunoblot studies. Sera were diluted 1:500 in TBS (Tris-buffered saline)-Tween (20 mM Tris [pH 7.5], 150 mM NaCl, 0.05% [vol/vol] Tween 20) (45) and incubated at 37°C for 2 h with approximately 10 μg of each recombinant B31 Erp protein.
Sera from 10 Lyme disease patients with unknown stages of infection from Southampton, Long Island, N.Y. (37), were used in immunoblotting assays to determine reactivity to recombinant B31 Erp proteins. Additionally, sera from five noninfected humans from Montana (where Lyme disease is not endemic) were also used in these immunoblotting experiments. All human sera were diluted 1:200 in TBS-Tween for immunoblotting.
Nucleotide sequence accession numbers.
The complete sequences of the B31 erpAB2, erpIJ, erpLM, and erpX loci have been deposited in GenBank and given accession no. U78764, U72996, U72998, and AF020657, respectively. The previously described B31 erpAB1, erpCD, erpG, erpH, and erpK loci have the GenBank accession no. U44912, U44914, U42598, U44913, and U72997, respectively (11, 43).
RESULTS
erp gene complement of an infectious B. burgdorferi B31 culture.
To aid in our elucidation of Erp protein expression and function, we cloned and sequenced the erp loci carried on the known cp32 plasmid family members in an infectious culture of isolate B31. The infectious B. burgdorferi clone B31-4a carries lp56 and all of the known cp32 plasmids except cp32-2 (11). The B31 Erp proteins were found to vary significantly in sequence, with aligned pairs sharing between 100 and 17% amino acid residues (40). Since all of these plasmids are homologous throughout most of their lengths (11, 29, 41, 43, 49), we believe that all members of the erp gene family are actually alleles of one another. However, for the sake of clarity in discussing each gene and its protein, we have chosen to retain the designations of these genes as erpA, erpB, etc. (Table 2).
TABLE 2.
Designations of the B31 erp loci and characteristics of their proteins
| Plasmid | Gene | Molecular mass of native protein (kDa)
|
Apparent molecular mass of recombinant protein (kDa) | |
|---|---|---|---|---|
| Predicted | Apparent | |||
| cp32-1 | erpA | 19.6 | 19 | 25 |
| erpB2 | 43.6 | 60 | 60 | |
| cp32-2 | erpC | 20.2 | NDa | 26 |
| erpD | 38.9 | ND | 55 | |
| cp32-3 | erpG | 22.0 | ND | 30 |
| cp32-4 | erpH | 3.8 | ND | NAb |
| cp32-5 | erpI | 19.6 | 19 | 25 |
| erpJ | 43.6 | 60 | 60 | |
| cp32-6 | erpK | 28.9 | 40(?)c | 38 |
| cp32-7 | erpL | 26.1 | ND | 37 |
| erpM | 41.8 | ND | 52 | |
| lp56 | erpX | 39.8 | ND | 48 |
ND, native form of protein not detected in this work.
NA, not applicable.
Preadsorption of infected mouse sera with recombinant ErpK caused an apparent reduction in immunoblot signal strength from an antigen of approximately 40 kDa.
The erpB gene (located on cp32-1) of the infectious B. burgdorferi B31 is slightly different from the gene that we found in a high-passage-number noninfectious culture of B. burgdorferi B31 (43). We have designated the low-passage-number form of erpB as erpB2 and the high-passage-number form as erpB1. These two genes are identical except for the sequence at codon 219, which is TAG (stop) in erpB1 and GAG (glutamic acid) in erpB2. As a result, erpB2 encodes a longer, 378-amino-acid protein with a predicted molecular mass of 43.6 kDa. The erpA genes of both the low- and high-passage-number B31 bacteria were found to be identical in sequence.
The majority of bacteria in our infectious B31 culture lack cp32-2 and the erpCD locus (11), and we have not been able to isolate an infectious clone of B31 that contains this plasmid. We were therefore unable to characterize the expression of the erpCD locus during this work. The bacteria in this culture do carry cp32-3 and cp32-4 (11), which contain erpG and erpH, respectively (43). The erpG gene is followed by bapA (43, 46), which is not homologous to the erp genes and is not a member of this gene family. erpH encodes a small protein that, if processed by cleavage of the signal polypeptide, would be only 15 amino acids in length (11, 43). Since such a protein would probably be nonfunctional, we have not characterized the expression of erpH in this work.
Plasmid cp32-5 contains a bicistronic locus, erpIJ, the coding regions of which are identical to those of erpAB2. We are confident that these genes comprise two separate loci since the 5′ noncoding regions of erpAB and erpIJ are distinct (11). Additionally, we have linked the erpAB and erpIJ loci to the distinct orfC and orf3 alleles that are found only on cp32-1 and cp32-5, respectively (11, 40, 49). Finally, a probe derived from erpB hybridized to restriction endonuclease fragments originating from both cp32-1 and cp32-5 in our previous mapping experiments (11). Since we cannot differentiate between ErpA and ErpI or between ErpB2 and ErpJ mRNAs or proteins, we will refer to them collectively as ErpA/I and ErpB2/J.
Plasmid cp32-6 contains the erpK gene, apparently a monocistronic locus with no indication of another open reading frame located within 200 bp 3′ of erpK.
Plasmid cp32-7 contains a bicistronic locus, erpLM. The initial 452 nucleotides of the erpL gene are identical to those of the bbk2.11 locus of B. burgdorferi isolate 297 (1), resulting in identical predicted amino acid sequences for over half the length of these two proteins (40). The remainder of the two proteins are predicted to share only 43% identical amino acids (40). Previous partial sequencing of the 5′ end of erpL and its similarity with the monocistronic 297 bbk2.11 locus led us to erroneously predict earlier that erpL is also monocistronic (11).
Linear plasmid lp56 contains erpX, which appears to be monocistronic since there are no identifiable erp-related genes located 3′ of this gene. Furthermore, the DNA located immediately 3′ of erpX is almost identical to the sequences located directly 3′ of erpAB2, erpCD, and erpLM.
Transcriptional regulation of the erp genes.
The regions of DNA located immediately 5′ of all the B31 erp loci, which presumably include the promoters and any cis regulatory sequences, are nearly identical to that found 5′ of the N40 ospEF locus (11, 23, 41, 43), suggesting that all of these loci may be regulated similarly. The N40 OspE and OspF proteins can be differentially synthesized in culture by shifting the growth temperature from 23 to 35°C, as can a B31 protein that is antigenically similar to the N40 OspE protein (42). We therefore examined the expression of the B31-4a erp loci to determine whether they are also similarly regulated in culture.
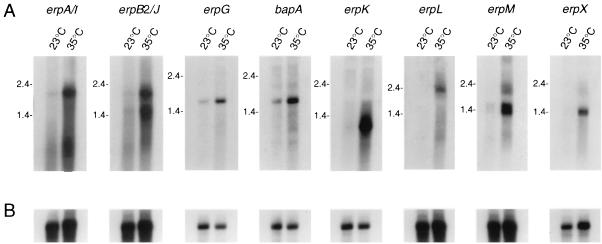
Northern blot analyses with probes specific for each erp gene indicated that significantly greater levels of the erp transcripts were present in bacteria that were shifted to 35°C relative to those maintained at 23°C (Fig. 1A). We also examined the expression of the bapA gene located 3′ of erpG on cp32-3 and found that it, too, was expressed at higher levels in the bacteria shifted to 35°C (Fig. 1A). Rehybridizations of the same RNA blots with a probe specific for the constitutively expressed flaB (flagellin) gene (7) indicated that there were comparable amounts of RNA in all samples (Fig. 1B). The slight variation seen on some flaB-probed filters is insufficient to account for the differences seen when the same filter was hybridized with erp-specific probes (compare Fig. 1A with Fig. 1B). These results lead us to predict that similar expression patterns will be observed for other homologous loci, such as the B31 erpCD and the erp homologs found in other B. burgdorferi isolates.
FIG. 1.
(A) Northern blot analysis of erp transcripts. Isolate B31 was grown in culture medium at either a constant temperature of 23°C (labeled 23°C) or shifted from 23°C to 35°C (labeled 35°C). Filters were individually incubated with radiolabeled probes specific for the B31 gene indicated above each panel. (B) Each filter was rehybridized with a probe specific for the constitutively expressed flaB gene (7). RNA molecular size markers (in kilobases) are indicated to the left of each panel.
The Northern blotting experiments also provided data indicating that the bicistronic erp loci constitute operons. The erpA/I and erpB2/J probes each hybridized to an approximately 2-kb mRNA transcript (Fig. 1), which is large enough to include both genes (1,685 bp required). Similar results were seen in assays using the erpL and erpM probes (the erpLM coding sequences are 1,832 bp in length). Both the erpB2/J and erpM probes also hybridized with RNAs having approximate sizes of 1.4 kb that did not hybridize with the erpA/I and erpL probes, respectively (Fig. 1), suggesting that these bicistronic operons may contain internal promoters for transcription of the second genes. Alternatively, the smaller transcripts may be due to degradation of the 5′ ends of these transcripts.
The erpG and bapA probes each hybridized to a transcript of approximately 1.8-kb, a size sufficient to encode both proteins (1,211 bp required), suggesting that these two genes also constitute a bicistronic operon. The erpK probe hybridized with an mRNA having an approximate size of 1 kb, a size sufficient to encode the ErpK protein (756 bp required). The erpX probe hybridized with a transcript of sufficient size to encode its protein (approximately 1.4 kb; 1,037 bp required).
Recognition of Erp proteins by animals infected with B31.
Proteins produced by B. burgdorferi during transmission from tick to mammal might be antigenic and provoke an early immune response. Such is the case with the B. burgdorferi OspC protein (18). Since the ospC gene exhibits an in vitro pattern of temperature-inducible expression similar to that of the erp genes (34, 45), we examined whether laboratory animals infected by tick bite with isolate B31 also produced antibodies that recognized the Erp proteins.
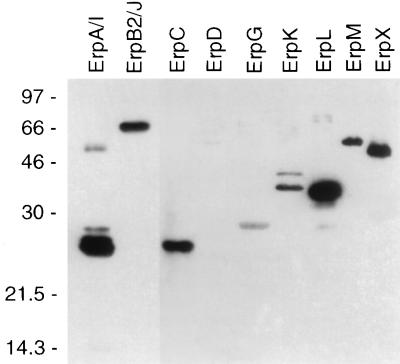
Recombinant Erp proteins were purified, separated by SDS-polyacrylamide gel electrophoresis, and transferred to nitrocellulose membranes. All of the recombinant Erp proteins migrated with apparent molecular masses that were greater than those predicted from their gene sequences (Table 2). The filters were then immunoblotted with sera from each of five mice that had been infected with B31 by tick bite. We observed that every mouse produced antibodies that reacted with all of the recombinant Erp proteins, except ErpD, within 4 weeks of tick feeding (example shown in Fig. 2). Immunoblot signals were stronger from the ErpA/I and ErpB2/J recombinant proteins (Fig. 2), suggesting that B31 may produce more of these two proteins than the other Erp proteins. As noted above, very few of the bacteria in the infectious B31 culture contain cp32-2 (which encodes ErpD), and the lack of ErpD-specific antibodies correlates with this observation. Sera from uninfected mice did not contain antibodies that recognized any Erp protein (data not shown). These data are consistent with production of the Erp proteins by B. burgdorferi during early stages of mammalian infection.
FIG. 2.
Immunoblot analysis of recombinant B31 Erp proteins. The filter was incubated with a 1:200 dilution of serum from a mouse infected with B31 by tick bite. The immunoblot signal strengths were greater from the recombinant ErpA/I and ErpB2/J proteins than from the other proteins, and the exposure time of ErpA/I and ErpB2/J in this figure was approximately 1/10 of that of the other Erp proteins. All of the recombinant Erp proteins migrated with apparent molecular masses that were greater than predicted from their sequences (Table 2). Some of the recombinant protein preparations contained probable degradation products or multimeric proteins, resulting in the presence of multiple bands. Molecular masses (in kilodaltons) are indicated to the left.
We cannot at this time rule out antibody cross-reactivity in the preceding experiment. For example, most of the bacteria in the infectious B31 culture also lack the erpC gene (encoded on cp32-2), yet sera from infected mice recognized the recombinant ErpC protein (Fig. 2), probably due to cross-reactive antibodies elicited by ErpA/I. These two proteins share extensive sequence identity (>83% identical amino acids) (43), and antibodies that recognize one protein may recognize the other, since antibodies raised against a recombinant N40 OspE protein (27) recognized both the recombinant ErpA/I and ErpC proteins (data not shown).
Differential synthesis of Erp antigens.
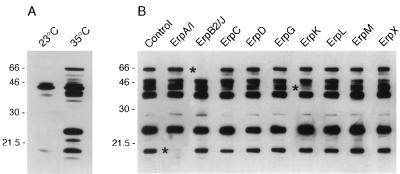
We have previously reported that raising the culture temperature from 23 to 35°C resulted in the increased production of several antigenic B31 proteins (42). Knowing that expression of the erp genes can be temperature induced and that their proteins are antigenic, we performed experiments to determine whether any of the previously detected temperature-induced B31 antigens were Erp proteins. Due to the limited amount of serum available from a single mouse, sera from three of the infected mice described above were pooled for use in these experiments. The pooled sera were preadsorbed with each recombinant Erp protein and used in immunoblot analyses with a B31 lysate that had been grown at 23°C and shifted to 35°C. The absence of a signal with an Erp preadsorbed serum would indicate that a particular immunoblot band corresponded with that Erp protein.
These experiments indicated that at least two of the major B31 antigens detectable on immunoblots are Erp proteins. Preadsorption of the sera with recombinant ErpA/I protein inhibited binding to a protein with an approximate molecular mass of 19 kDa (Fig. 3), indicating that this differentially expressed antigen was ErpA/I. Binding to ErpA/I was not blocked by preincubation with recombinant ErpC, which shares 83% identical amino acids with ErpA/I (43), indicating that the infected animals also produced antibodies against epitopes unique to ErpA/I. Preadsorption with recombinant ErpB2/J blocked antibody binding to an approximately 60-kDa differentially expressed protein, indicating that this antigen is ErpB2/J. The ErpB2/J protein is predicted to have a molecular mass of 43.6 kDa but, as noted above, the recombinant ErpB2/J protein also migrated with a larger apparent molecular mass in polyacrylamide gel electrophoresis (Fig. 2).
FIG. 3.
Identification of differentially synthesized B31 antigens. (A) Immunoblot of B31 lysates grown at a constant 23°C or shifted from 23 to 35°C. The filter was incubated with a 1:500 dilution of serum from a mouse that was infected with B31 by tick bite. (B) Immunoblots of a B31 lysate shifted from 23 to 35°C. A 1:500 dilution of pooled sera from three mice infected with B31 by tick bite was preadsorbed with each recombinant B31 Erp protein before incubation with the filter strip. Asterisks indicate the positions of bands that were absent or diminished on immunoblots prepared with sera preadsorbed with recombinant ErpA/I, ErpB2/J, or ErpK. Molecular masses (in kilodaltons) are indicated to the left of each panel.
Even though the sera used in these experiments contained antibodies that recognized the remaining Erp proteins (see above), no other recombinant Erp protein completely blocked antibody binding to a protein in the B. burgdorferi lysate. Binding to a protein with an approximate molecular mass of 40 kDa was reduced by preadsorption with recombinant ErpK (Fig. 3). All of the erp genes examined were expressed by the bacteria that were shifted to 35°C (Fig. 1), but we cannot determine from these experiments whether they were also translated. The failure to detect antibody blocking may be due to the large number of antigens that migrated with apparent molecular masses of 35 to 50 kDa (Fig. 3), and the absence of a single band in this area might be obscured by other comigrating proteins. Alternatively, since the sera apparently contained greater levels of ErpA/I- and ErpB2/J-directed antibodies (see above), other Erp protein bands may be fainter and difficult to discern. It is also possible that there were conformational differences between the recombinant and native Erp proteins such that antibodies did not recognize the recombinant forms, or that the sera were not preincubated with enough antigen to absorb all the antibodies directed against that protein.
Recognition of B31 Erp proteins by human Lyme disease patient sera.
We next analyzed sera from 10 humans with Lyme disease to determine whether they produced antibodies that recognized the B31 Erp proteins. All 10 patients contained antibodies that recognized the B. burgdorferi BmpA (P39) protein, a characteristic marker of B. burgdorferi infection (35, 37) (data not shown). All of the sera contained antibodies that bound both ErpA/I and ErpC (Table 3). Eight of the patients contained antibodies that recognized ErpB2, seven recognized ErpM, and six recognized ErpL. The remaining Erp proteins were bound by antibodies found in half or fewer of the patients. Sera from humans without Lyme disease and residing in an area where Lyme disease is not endemic lacked antibodies that recognized any of the recombinant B31 Erp proteins (data not shown). These data suggest that production of antigens similar to ErpA/I and ErpC may be common in Lyme disease spirochetes, while fewer bacteria produce antigens similar to the other B31 Erp proteins.
TABLE 3.
Reactivities of human Lyme disease patient antisera (all diluted 1:200) against recombinant B31 Erp proteins
| Patient | Reactivitya
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| ErpA/I | ErpB2/J | ErpC | ErpD | ErpG | ErpK | ErpL | ErpM | ErpX | |
| NY62 | + | + | + | − | + | + | − | − | + |
| NY66 | + | + | + | − | − | − | + | − | − |
| NY68 | + | + | + | − | − | − | + | + | − |
| NY70 | + | − | + | − | − | − | − | − | − |
| NY106 | + | + | + | − | − | − | + | + | + |
| NY113 | + | + | + | − | + | − | + | + | − |
| NY115 | + | + | + | − | − | − | + | + | + |
| NY116 | + | + | + | − | + | − | + | + | + |
| NY137 | + | − | + | + | + | − | − | + | − |
| NY170 | + | + | + | + | + | + | − | + | + |
Sera were scored for production of a detectable immunoblot signal (+) or failure to produce a signal (−).
DISCUSSION
We have found that B. burgdorferi B31 contains a large repertoire of erp genes and proteins. Expression from the B31 erp loci tested increased in response to a culture temperature shift that mimicked the environmental change within the feeding tick. Additionally, animals infected by tick bite produced antibodies that recognized the Erp proteins encoded by infectious B31. These data are consistent with production of the Erp proteins during the transmission of B. burgdorferi from ticks to mammals and suggest that they may play roles in transmission or the establishment of mammalian infection. Further studies will determine the pattern of Erp protein synthesis within unfed and fed ticks and in infected mammals.
It has been proposed that each of the numerous erp genes may be expressed at different times during mammalian infection (13, 25, 43), perhaps as a method of avoiding immune clearance similar to the presumed function of Vmp protein variation in the relapsing fever agent, Borrelia hermsii (4). Our data suggest that all of the B31 Erp proteins examined are synthesized at approximately the same time during infection, as they were recognized by antisera from tick-infected mice within the first 4 weeks of infection. It may be argued that only a subset of the erp genes were expressed during the infection times studied, and we actually detected antibodies that cross-reacted with the other Erp proteins. Yet such cross-reactivity could negate any value in sequentially producing the Erp proteins, since protective antibodies that recognize the later-appearing proteins might already be present. The identity of the B31 erpAB2 and erpIJ genes also argues against the theory of sequential expression of the erp loci. Additionally, the conserved 5′ noncoding regions of these regulons (1, 11, 23, 25, 41, 43, 44, 46) indicate that the same regulatory factors probably interact with all of their promoters, suggesting that expression of these loci would not be individually controlled.
The in vitro expression of the B31 erp genes that we have observed stands in contrast with reports that Erp homologs of other B. burgdorferi isolates were not expressed in cultured bacteria (1, 13, 44, 46). The promoter regions of all reported erp homologs are nearly identical, which, as noted above, indicates that they are all probably regulated by the same cis and trans factors. The apparently contradictory in vitro expression patterns may be a consequence of the culture conditions used in different experiments, since the other reports studied protein synthesis in bacteria grown continuously at 35°C but not in cultures shifted from 23 to 35°C. It is important, however, that regulated expression of the B. burgdorferi erp genes can be observed in the laboratory, as the bacterial factors responsible might now be detected and studied in detail.
Not all of the Erp proteins are essential for mammalian infection, as bacteria lacking cp32-2 (which encodes ErpC and ErpD) are apparently infectious and transmitted between ticks and mammals. Most, if not all, of the Erp proteins are dispensable for growth of B. burgdorferi in culture, since a high-passage-number clone of B31 contains only cp32-1, cp32-3, and cp32-4, with a mutated erpB gene (11, 43). The truncated erpB1 allele found in the high-passage-number bacteria (43) also demonstrates that these bacteria can acquire small mutations during cultivation in addition to the previously described loss of plasmids (3, 9, 10, 33, 36, 47), any of which may contribute to the concurrent loss of infectivity.
The ErpA/I and ErpB2/J proteins elicited a strong immune response in animals infected with B31. The ErpA/I and ErpC proteins were also recognized as antigens by sera from 10 of 10 Lyme disease patients from Long Island, while 8 patients’ sera also recognized the ErpB2/J protein. These data suggest that structural features of at least some of the B31 Erp proteins may be conserved among different B. burgdorferi bacteria and may be involved in essential functions. Epitope conservation also suggests that some B31 Erp proteins could serve as useful Lyme disease diagnostic antigens or components of a protective vaccine. Analysis of sera from Lyme disease patients from other geographic locations will indicate whether the production of antigens similar to the B31 Erp proteins is common in other infectious B. burgdorferi bacteria.
ACKNOWLEDGMENTS
We thank Martine Bos, Alan MacDonald, and Erol Fikrig for providing sera, Kit Tilly, Joseph Hinnebusch, Stephen Porcella, and Abdallah Elias for constructive comments on the manuscript, Gary Hettrick and Robert Evans for artwork, and Kelly Matteson and Carole Smaus for secretarial assistance.
REFERENCES
- 1.Akins D R, Porcella S F, Popova T G, Shevchenko D, Baker S I, Li M, Norgard M V, Radolf J D. Evidence for in vivo but not in vitro expression of a Borrelia burgdorferi outer surface protein F (OspF) homologue. Mol Microbiol. 1995;18:507–520. doi: 10.1111/j.1365-2958.1995.mmi_18030507.x. [DOI] [PubMed] [Google Scholar]
- 2.Amouriaux P, Assous M, Margarita D, Baranton G, Saint Girons I. Polymerase chain reaction with the 30-kb circular plasmid of Borrelia burgdorferi B31 as a target for detection of the Lyme borreliosis agents in cerebrospinal fluid. Res Microbiol. 1993;144:211–219. doi: 10.1016/0923-2508(93)90046-5. [DOI] [PubMed] [Google Scholar]
- 3.Barbour A G. Plasmid analysis of Borrelia burgdorferi, the Lyme disease agent. J Clin Microbiol. 1988;26:475–478. doi: 10.1128/jcm.26.3.475-478.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbour A G, Tessier S L, Stoenner H G. Variable major proteins of Borrelia hermsii. J Exp Med. 1982;156:1312–1324. doi: 10.1084/jem.156.5.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benach J L, Coleman J L, Skinner R A, Bosler E M. Adult Ixodes dammini on rabbits: a hypothesis for the development and transmission of Borrelia burgdorferi. J Infect Dis. 1987;155:1300–1306. doi: 10.1093/infdis/155.6.1300. [DOI] [PubMed] [Google Scholar]
- 6.Bölin I, Portnoy D A, Wolf-Watz H. Expression of the temperature-inducible outer membrane proteins of yersiniae. Infect Immun. 1985;48:234–240. doi: 10.1128/iai.48.1.234-240.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bono J L, Tilly K, Stevenson B, Hogan D, Rosa P. Oligopeptide permease in Borrelia burgdorferi: putative peptide-binding components encoded by both chromosomal and plasmid loci. Microbiology. 1998;144:1033–1044. doi: 10.1099/00221287-144-4-1033. [DOI] [PubMed] [Google Scholar]
- 8.Burgdorfer W, Barbour A G, Hayes S F, Benach J L, Grunwaldt E, Davis J P. Lyme disease—a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 9.Busch U, Will G, Hizo-Teufel C, Wilske B, Preac-Mursic V. Long term in vitro cultivation of Borrelia burgdorferi sensu lato strains: influence on plasmid patterns, genome stability and expression of proteins. Res Microbiol. 1997;148:109–118. doi: 10.1016/S0923-2508(97)87642-5. [DOI] [PubMed] [Google Scholar]
- 10.Carroll J A, Gherardini F C. Membrane protein variations associated with in vitro passage of Borrelia burgdorferi. Infect Immun. 1996;64:392–398. doi: 10.1128/iai.64.2.392-398.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casjens S, van Vugt R, Tilly K, Rosa P A, Stevenson B. Homology throughout the multiple 32-kilobase circular plasmids present in Lyme disease spirochetes. J Bacteriol. 1997;179:217–227. doi: 10.1128/jb.179.1.217-227.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coleman J L, Gebbia J A, Piesman J, Degen J L, Bugge T H, Benach J L. Plasminogen is required for efficient dissemination of B. burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell. 1997;89:1111–1119. doi: 10.1016/s0092-8674(00)80298-6. [DOI] [PubMed] [Google Scholar]
- 13.Das S, Barthold S W, Stocker Giles S, Montgomery R R, Telford S R, Fikrig E. Temporal pattern of Borrelia burgdorferi p21 expression in ticks and the mammalian host. J Clin Invest. 1997;99:987–995. doi: 10.1172/JCI119264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Silva A M, Fikrig E. Growth and migration of Borrelia burgdorferi in Ixodes ticks during blood feeding. Am J Trop Med Hyg. 1995;53:397–404. doi: 10.4269/ajtmh.1995.53.397. [DOI] [PubMed] [Google Scholar]
- 15.de Silva A M, Telford III S R, Brunet L R, Barthold S W, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fingerle V, Hauser U, Liegl G, Petko B, Preac-Mursic V, Wilske B. Expression of outer surface proteins A and C of Borrelia burgdorferi in Ixodes ricinus. J Clin Microbiol. 1995;33:1867–1869. doi: 10.1128/jcm.33.7.1867-1869.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J-F, Fleischmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams M D, Gocayne J, Weidmann J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fujii C, Cotton M D, Horst K, Roberts K, Hatch B, Smith H O, Venter J C. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs R, Jauris S, Lottspeich F, Preac-Mursic V, Wilske B, Soutschek E. Molecular analysis and expression of a Borrelia burgdorferi gene encoding a 22kDa protein (pC) in Escherichia coli. Mol Microbiol. 1992;6:503–509. doi: 10.1111/j.1365-2958.1992.tb01495.x. [DOI] [PubMed] [Google Scholar]
- 19.Gern L, Zhu Z, Aeschlimann A. Development of Borrelia burgdorferi in Ixodes ricinus females during blood feeding. Ann Parasitol Hum Comp. 1990;65:89–93. [Google Scholar]
- 20.Hinnebusch B J, Perry R D, Schwan T G. Role of the Yersinia pestis hemin storage (hms) locus in the transmission of plague by fleas. Science. 1996;273:367–370. doi: 10.1126/science.273.5273.367. [DOI] [PubMed] [Google Scholar]
- 21.King G J, Swanson J. Studies on gonococcus infection. XV. Identification of surface proteins of Neisseria gonorrhoeae correlated with leukocyte association. Infect Immun. 1978;21:575–584. doi: 10.1128/iai.21.2.575-584.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurtti T J, Munderloh U G, Johnson R C, Ahlstrand G G. Colony formation and morphology in Borrelia burgdorferi. J Clin Microbiol. 1987;25:2054–2058. doi: 10.1128/jcm.25.11.2054-2058.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lam T T, Nguyen T-P K, Montgomery R R, Kantor F S, Fikrig E, Flavell R A. Outer surface proteins E and F of Borrelia burgdorferi, the agent of Lyme disease. Infect Immun. 1994;62:290–298. doi: 10.1128/iai.62.1.290-298.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- 25.Marconi R T, Sung S Y, Norton Hughes C A, Carlyon J A. Molecular and evolutionary analyses of a variable series of genes in Borrelia burgdorferi that are related to ospE and ospF, constitute a gene family, and share a common upstream homology box. J Bacteriol. 1996;178:5615–5626. doi: 10.1128/jb.178.19.5615-5626.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller J F, Mekalanos J J, Falkow S. Coordinate regulation and sensory transduction in the control of bacterial virulence. Science. 1989;243:916–922. doi: 10.1126/science.2537530. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen T-P K, Lam T T, Barthold S W, Telford III S R, Flavell R A, Fikrig E. Partial destruction of Borrelia burgdorferi within ticks that engorged on OspE- or OspF-immunized mice. Infect Immun. 1994;62:2079–2084. doi: 10.1128/iai.62.5.2079-2084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piesman J, Oliver J R, Sinsky R J. Growth kinetics of the Lyme disease spirochete (Borrelia burgdorferi) in vector ticks (Ixodes dammini) Am J Trop Med Hyg. 1990;42:352–357. doi: 10.4269/ajtmh.1990.42.352. [DOI] [PubMed] [Google Scholar]
- 29.Porcella S F, Popova T G, Akins D R, Li M, Radolf J D, Norgard M V. Borrelia burgdorferi supercoiled plasmids encode multicopy tandem open reading frames and a lipoprotein gene family. J Bacteriol. 1996;178:3293–3307. doi: 10.1128/jb.178.11.3293-3307.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ribeiro J M C, Mather T N, Piesman J, Spielman A. Dissemination and salivary delivery of Lyme disease spirochetes in vector ticks (Acari: Ixodidae) J Med Entomol. 1987;24:201–205. doi: 10.1093/jmedent/24.2.201. [DOI] [PubMed] [Google Scholar]
- 31.Rosa P, Samuels D S, Hogan D, Stevenson B, Casjens S, Tilly K. Directed insertion of a selectable marker into a circular plasmid of Borrelia burgdorferi. J Bacteriol. 1996;178:5946–5953. doi: 10.1128/jb.178.20.5946-5953.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 33.Schwan T G, Burgdorfer W, Garon C F. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun. 1988;56:1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simpson W J, Burgdorfer W, Schrumpf M E, Karstens R H, Schwan T G. Antibody to a 39-kilodalton Borrelia burgdorferi antigen (P39) as a marker for infection in experimentally and naturally inoculated animals. J Clin Microbiol. 1991;29:236–243. doi: 10.1128/jcm.29.2.236-243.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simpson W J, Garon C F, Schwan T G. Analysis of supercoiled circular plasmids in infectious and non-infectious Borrelia burgdorferi. Microb Pathog. 1990;8:109–118. doi: 10.1016/0882-4010(90)90075-2. [DOI] [PubMed] [Google Scholar]
- 37.Simpson W J, Schrumpf M E, Schwan T G. Reactivity of human Lyme borreliosis sera with a 39-kilodalton antigen specific to Borrelia burgdorferi. J Clin Microbiol. 1990;28:1329–1337. doi: 10.1128/jcm.28.6.1329-1337.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spencer R R, Parker R R. Rocky mountain spotted fever: infectivity of fasting and recently fed ticks. Public Health Rep. 1923;38:333–339. [Google Scholar]
- 39.Spencer R R, Parker R R. Rocky mountain spotted fever: experimental studies on tick virus. Public Health Rep. 1924;39:3027–3040. [Google Scholar]
- 40.Stevenson, B., S. Casjens, and P. Rosa. Evidence of past recombination events among the genes encoding the Erp antigens of Borrelia burgdorferi. Microbiology, in press. [DOI] [PubMed]
- 41.Stevenson B, Casjens S, van Vugt R, Porcella S F, Tilly K, Bono J L, Rosa P. Characterization of cp18, a naturally truncated member of the cp32 family of Borrelia burgdorferi plasmids. J Bacteriol. 1997;179:4285–4291. doi: 10.1128/jb.179.13.4285-4291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stevenson B, Schwan T G, Rosa P A. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:4535–4539. doi: 10.1128/iai.63.11.4535-4539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stevenson B, Tilly K, Rosa P A. A family of genes located on four separate 32-kilobase circular plasmids in Borrelia burgdorferi B31. J Bacteriol. 1996;178:3508–3516. doi: 10.1128/jb.178.12.3508-3516.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suk K, Das S, Sun W, Jwang B, Barthold S W, Flavell R A, Fikrig E. Borrelia burgdorferi genes selectively expressed in the infected host. Proc Natl Acad Sci USA. 1995;92:4269–4273. doi: 10.1073/pnas.92.10.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tilly K, Casjens S, Stevenson B, Bono J L, Samuels D S, Hogan D, Rosa P. The Borrelia burgdorferi circular plasmid cp26: conservation of plasmid structure and targeted inactivation of the ospC gene. Mol Microbiol. 1997;25:361–373. doi: 10.1046/j.1365-2958.1997.4711838.x. [DOI] [PubMed] [Google Scholar]
- 46.Wallich R, Brenner C, Kramer M D, Simon M M. Molecular cloning and immunological characterization of a novel linear-plasmid-encoded gene, pG, of Borrelia burgdorferi expressed only in vivo. Infect Immun. 1995;63:3327–3335. doi: 10.1128/iai.63.9.3327-3335.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu Y, Kodner C, Coleman L, Johnson R C. Correlation of plasmids with infectivity of Borrelia burgdorferi sensu stricto type strain B31. Infect Immun. 1996;64:3870–3876. doi: 10.1128/iai.64.9.3870-3876.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zückert W R, Filipuzzi-Jenny E, Meister-Turner J, Stålhammar-Carlemalm M, Meyer J. Repeated DNA sequences on circular and linear plasmids of Borrelia burgdorferi sensu lato. In: Axford J S, Rees D H E, editors. Lyme borreliosis. New York, N.Y: Plenum Press; 1994. pp. 253–260. [Google Scholar]
- 49.Zückert W R, Meyer J. Circular and linear plasmids of Lyme disease spirochetes have extensive homology: characterization of a repeated DNA element. J Bacteriol. 1996;178:2287–2298. doi: 10.1128/jb.178.8.2287-2298.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zung J L, Lewengrub S, Rudzinska M A, Spielman A, Telford S R, Piesman J. Fine structural evidence for the penetration of the Lyme disease spirochete Borrelia burgdorferi through the gut and salivary tissues of Ixodes dammini. Can J Zool. 1989;67:1737–1748. [Google Scholar]