Abstract
Background:
Regeneration is a highly complex process that requires the coordination of numerous molecular events, and identifying the key ruler that governs is important to investigate. While it has been shown that TCTP is a multi-functional protein that regulates cell proliferation, differentiation, apoptosis, anti-apoptosis, stem cell maintenance, and immune responses, but only a few studies associated to regeneration have been reported. To investigate the multi-functional role of TCTP in regeneration, the earthworm Perionyx excavatus was chosen.
Methods:
Through pharmacological suppression of TCTP, amputation, histology, molecular docking, and western blotting, the multi-function role of TCTP involved in regeneration is revealed.
Results:
Amputational studies show that P. excavatus is a clitellum-independent regenerating earthworm resulting in two functional worms upon amputation. Arresting cell cycle at the G1/S boundary using 2 mM Thymidine confirms that P. excavatus execute both epimorphosis and morphallaxis regeneration mode. The pharmacological suppression of TCTP using buclizine results in regeneration suppression. Following the combinatorial injection of 2 mM Thymidine and buclizine, the earthworm regeneration is completely blocked, which suggests a critical functional role of TCTP in morphallaxis. The pharmacological inhibition of TCTP also suppresses the key proteins involved in regeneration: Wnt3a (stem cell marker), PCNA (cell proliferation) and YAP1 (Hippo signalling) but augments the expression of cellular stress protein p53.
Conclusion:
The collective results indicate that TCTP synchronously is involved in the process of stem cell activation, cell proliferation, morphallaxis, and organ development in the regeneration event.
Keywords: TCTP, Buclizine, Wnt3a, p53, Hippo signaling
Introduction
The homeostatic process known as ‘regeneration’ directs the recovery of damaged or lost tissues [1]. Generally, epimorphosis and morphallaxis are the main principles of regeneration [2–4]. There are differences and similarities between the two processes. After wound induction, the primary tissue injury response invites stem cells from the niche [5, 6] and will accumulate at the wound site that depends on signals for healing and regeneration [7]. The cellular mechanism of apoptosis [8] and immune responses are essential for the morphallaxis mode of regeneration, and tissue-specific stem cells are not required in this process [9] But epimorphosis requires tissue-specific stem cells, apoptosis, and immune responses [10, 11]. For example, Hydra vulgaris follows only morphallaxis [12], limb regeneration of Xenopus laevis froglet follows the epimorphosis mode of regeneration [13], and planarians follow both the regeneration principles [8]. Earthworms like Eudrilus eugeniae and Perionyx excavatus can regenerate their tails, segments, or even entire bodies from fragments that break off from the original worm. The pieces need a portion of the digestive and reproductive systems for regeneration. Earthworms can also regenerate lost tissue through the growth of new cells. This type of regeneration is more limited than fragmentation regeneration and can occur when the earthworm has minor injuries or is damaged but not fragmented [6].
During restoration, the earthworm’s body undergoes several cellular and molecular changes. The regeneration process begins with forming a blastema, a group of undifferentiated cells that can differentiate into the specific cell types required to regenerate the lost body part. The blastema grows and differentiates into the tissues needed for the new body part, such as muscles, nerves, and skin. Apoptosis is critical in regenerating body parts in E. eugeniae and other earthworms and maintaining tissue homeostasis in these organisms [14]. During the regeneration process, apoptotic cells help break down and remove damaged tissue, stimulating the proliferation of new cells in the blastema. In addition to its role in tissue remodeling during regeneration, apoptosis also plays a role in maintaining tissue homeostasis in earthworms. Studies have shown apoptotic cells in the reproductive organs of earthworms during spermatogenesis, where they help remove excess cells and maintain the proper balance of germ cells and support cells [15].
When cells die because of stress or damage, it can cause the remaining cells to divide more, a process termed “apoptosis-induced compensatory proliferation (AICP).” Stem cells must proliferate and differentiate for the compensation, but this whole process depends on various signaling molecules, such as Wnt and Hh, produced from apoptotic caspases in response to cell damage [16–19]. Apoptotic executioner caspases proceed with Apoptosis Induced Apoptosis (AIA) and AICP to compensate for lost parts [20, 21]. Those driving caspases may belong to Feeder cells [22, 23]. Collective reports indicate that most of the animal models such as Hydra, planarians, newts, Drosophila melanogaster, and mouse follow AICP for the reformation of lost parts [16, 24–28]. In earthworms, apoptotic cells were observed throughout their cell renewal period of regeneration [14]. On another side, in juvenile E. eugeniae worms, the movement of stem cells from the clitellum to the wound site was observed [5]. However, the connective link between apoptosis and stem cells is still unclear.
According to the recent report of transcriptomic analysis of E. eugeniae, while more than 3986 genes are upregulated during their regeneration [29], silencing a single protein called Translationally Controlled Tumor Protein (TCTP) influences the entire progress of regeneration of E. eugeniae regeneration [30]. TCTP has both apoptotic and anti-apoptotic functions [31, 32] and can decide the fate of cells [33]. TCTP also plays a remarkable role in regeneration [30] and regulates major cellular mechanisms like proliferation, cell homeostasis, and survival [34], maintaining the stability of p53 [32]. For example, reciprocal repression between TCTP and p53 was observed in knockout mice models [35].
Researchers have used chemicals and siRNAs for silencing TCTP, such as Buclizine and Nutlin-3a [30]. Seo and Efferth [43] confirmed that buclizine could block the TCTP protein in MCF-7 cell lines at the cellular level. The tropical earthworm, Perionyx excavatus, has a unique regeneration ability [36]. The survival capacity of P. excavatus is higher than other earthworm species [37]. P. excavatus is a regeneration animal model for the central nervous system and tissue regeneration [37, 38]. Within two weeks of the amputation, P. excavatus achieves complete regeneration, including rebuilding vital organs [37]. Recently, Bae et al. [37] observed the epimorphosis mode of head regeneration in the earthworm P. excavatus [37], but the role of morphallaxis in earthworms is vague. Martinez et al. [39] suggest that the earthworm might follow both epimorphosis and morphallaxis regeneration patterns due to high predatory injury, but clear scientific evidence is unclear [40].
We observed that P. excavatus follows both epimorphosis and morphallaxis forms of regeneration in the present study. In addition, we report that TCTP governs morphallaxis along with the epimorphosis pattern of regeneration. Also, we provide direct evidence that TCTP is a multi-functional protein that regulates many functions associated with the regeneration mechanism, especially in association with AICP.
Materials and methods
Earthworm rearing and maintenance
We reared the Earthworm, Perionyx excavatus, from the stock maintained in the Centre for Molecular and Nanomedical Sciences, International Research Centre, Sathyabama Institute of Science and Technology, Chennai, Tamilnadu, India. Earthworms were maintained according to the standard protocol [39–42]. Cow dung and leaf litter were fed to worms in equal amounts and kept in the plastic tub at the appropriate moisture condition.
Cell cycle arresting assay
To block epimorphosis, 2 mM thymidine was injected into the 24th segment of the worms (No: 10). Nuclease-free water injection was used for the control set of worms (No: 10). An injection was continued for up to 10 days. After the second day of injection, the worms were amputated on the 10th segment and observed for their regeneration kinetics with a 15 cm scale and camera.
Buclizine injection and pharmacological suppression of TCTP
In this experiment, three dose levels of Buclizine (Mankind Pharma Ltd, Chennai, India), namely 140 µg, 160 µg, and 200 µg, were injected into the post-clitellum regions (24th segment) of P. excavatus worms once per day throughout the experiments. The control worm was injected with 1X PBS. Following 2nd day of the initial injection, the worms were amputated at the 10th segment and observed for regeneration ability. The regeneration process was monitored and documented using a Canon digital camera (IXUS 285 HS).
Regeneration studies
We used the eight groups of mature P. excavatus worms in this study to understand the importance of TCTP in regeneration. Each group consisted of ten worms, and the treatment is as follows: 1. in vivo regeneration analysis, 2 and 3. Control and Buclizine treatment for TCTP silencing, 4 and 5. Control and Thymidine treatment for cell arrest analysis, and 6. Buclizine and thymidine treatment for combinatorial toxicology. 7 and 8. Control and Nutlin-3a treatment for TCTP silencing. For in vivo regeneration analysis, selected worms (group 1) were amputated in the 10th segment (anterior region-head) using a cruzine carbon steel surgical scalpel blade (size 15) and maintained in the worm bed. Every 24 h, the amputated worms were documented with the help of a canon digital camera (IXUS 285 HS).
Influence of Nutlin-3a in regeneration
To study the importance of TCTP in regeneration, mature P. excavatus worms were selected. Two batches were selected, each containing 10 worms, 10 for control and another 10 for Nutlin-3a treatment. The first batch of ten worms acted as a control and was injected with DMSO. The second batch of ten worms was injected with Nutlin-3a in the 24th segment at a dose of 5 µg/g. The control and treated worms were amputated at the 10th segment (anterior) and maintained in the soil. The worms were anesthetized by ice before amputation. The blastemal formation was carefully observed in both batches of worms with the help of a Canon digital camera (IXUS 285 HS).
Histology
Thymidine-treated 7th day regenerated worms, 5th day Nutlin-3a treated samples and their respective control samples were subjected to histological sectioning. Both the regeneration blastema of the control and treated worms were cut using sterile scalpels along with the adjacent two segments and fixed in 10% formaldehyde (Cat.F0080; Rankem, Mumbai, India) for 24 h. To remove formaldehyde, tissues were gently washed with distilled water and dehydrated with isopropanol and acetone after the tissue was cleared using xylene. Each step was performed for one hour at 50 °C. Following the xylene removal step, tissue impregnation was incubated in paraffin wax, and the 5 μm sections were made using a microtome [30]. Eosin and hematoxylin stains were used to distinguish the internal arrangements and were observed in Euromex bScope Epi-Fluorescence HBO Microscope (Catalogue Number: BS.3153-PLFi).
Western blotting
All experiment samples were quantified using Lowry’s method, and an equal volume (80 µg) of protein samples was loaded onto SDS page gel electrophoresis and applied to run at 60 volts for 2 h 30 min using Bio-Rad gel systems. The resolved proteins in the SDS-PAGE gel were transferred to the PVDF membrane. The membrane was blocked with 5% BSA in TBST buffer and then incubated with either of the following primary antibodies of Anti-TCTP (Abcam [Cambridge, UK], ab37506; dilution, 2.5:5000), Anti-p53 (Abcam, ab26; dilution, 2.5:5000) and Anti-β-actin (Abcam, ab8226; dilution, 1:5000), Anti-Wnt3a (Abcam, ab19925; dilution, 0.5:5000), Anti-PCNA (Abcam, ab18197), Anti-H3 (Abcam, ab1791; dilution, 1:5000) and Anti-YAP1 (Abcam, ab62751; dilution, 0.5:5000) for overnight at 4 °C. After washing, the secondary antibody of Anti-rabbit IgG-HRP (1:10000) or Anti-mouse IgG-HRP (1:10000) was added to protein transferred membrane. The substrate ECL was used as a developer solution. ChemiDoc XRS documented the developed membrane, Bio-Rad (Hercules, CA, USA); the band intensity was analyzed using ImageJ analysis software (NIH, Bethesda, MD, USA).
Homology modelling of TCTP protein
TCTP of Lumbricus rubellus (Humus earthworm) was selected as the target sequence for the study. The one-dimensional FASTA sequence of the target protein, TCTP, were retrieved from the UniProt protein sequence database with accession number 018477. The structural blast was performed with the TCTP sequence of L. rubellus against the PDB database to identify the template structure for homology modelling. The homology model of L. rubellus TCTP protein was generated by SWISS-MODEL homology modelling server with the crystal structure of human histamine releasing factor-translationally controlled tumor protein (HRF-TCTP) (PDB ID: 5O9M) as the template structure. The quality and reliability of the constructed model were evaluated by PROCHECK, ERRAT, and VERIFY3D servers.
Ligand preparation for docking
The structure of the ligand, buclizine, was constructed using the 2D/3D ChemBio Draw Ultra software application, version 12 (Cambridge Soft, Cambridge, MA, USA), and then copied into the ChemBio 3D Ultra software application, version 12, to create the 3D structure. Then the structure was energy minimized by the MMFF94 method using the geometry optimization function in Chem Draw 3D software, and the lower energy conformations were selected for molecular docking studies.
Molecular docking studies
A molecular docking process was carried out using Autodock vina to reveal the binding analysis of L. rubellus (closely related species of P. excavatus) TCTP protein vs. Buclizine (PubChem ID: 6729). The protein’s binding site was adjusted using a grid box and x, y, and z-axis values. The grid box (60ÅX 60Å X60Å) is centered at 4.066, 20.626, − 11.479, and the spacing is 1. All other parameters were kept as default. An exhaustiveness of 10 was assigned for docking throughout the docking process, and 10 mode numbers were assigned to achieve reliable results. Post-docking analyses were carried out using Discovery Studio.
RESULTS
Anterior regeneration and tissue growth in the earthworm, P. excavatus
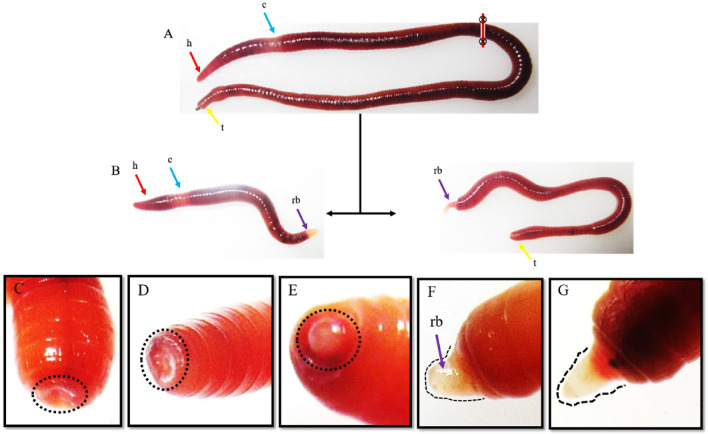
The earthworm P. excavatus is a bright brown segmented worm, and its clitellum segments are located between 13 to 17th segments, as shown in Fig. 1A. The worms were amputated at the 50th segment, i.e., away from the clitellum segments, and observed for their regeneration ability. The amputated anterior segments with head and clitellum can regenerate the tail on the 8th day of post-amputation. Similarly, the other portion of amputated posterior segments without clitellum segments form the regenerative head on the 8th day of post-amputation, as shown in the Fig. 1B. To understand the wound healing and regenerative dynamics of anterior regeneration, the earthworms are amputated at the 10th segments and observed every 24 h, as shown in Fig. 1C–G. Following 24 h of post-amputation, perfect sealing of amputation sites occurs with the formation of deep furrow (Fig. 1C), and wound healing was distinctly observed following 48 h of post-amputation (Fig. 1D). The worm starts to restore their lost tissue by forming a pre-blastemal structure following 72 h of post-amputation (Fig. 1E) and with increased size in 96 h (Fig. 1F). In proceeding days, the blastema starts to elongate further and differentiate to form the new lost segments following 120 h of post-amputation as shown in Fig. 1G.
Fig. 1.
Regeneration dynamics in clitellum independent earthworm, P. excavatus: A The earthworm, P. excavatus, appears red-brown and possesses an anterior head followed by clitellum, intestine, and anus. B amputation of earthworm at post-clitellum segments (50th segments), resulting in the development of two individual worms following eight days of post-amputation. C Following 24 h of post-amputation at the 10th segment, the wound sites were sealed by the contraction of surrounding tissues. D proliferative cells fill the deep furrow following 48 h of post-amputation E Pre-blastema structure was visibly observed on 72 h of post-amputation F Blastemal size elongated following 96 h post-amputation G Elongated blastema starts to differentiate and form visible segments following 120 h of post-amputation
P. excavatus follows both epimorphosis and morphallaxis patterns for their regeneration
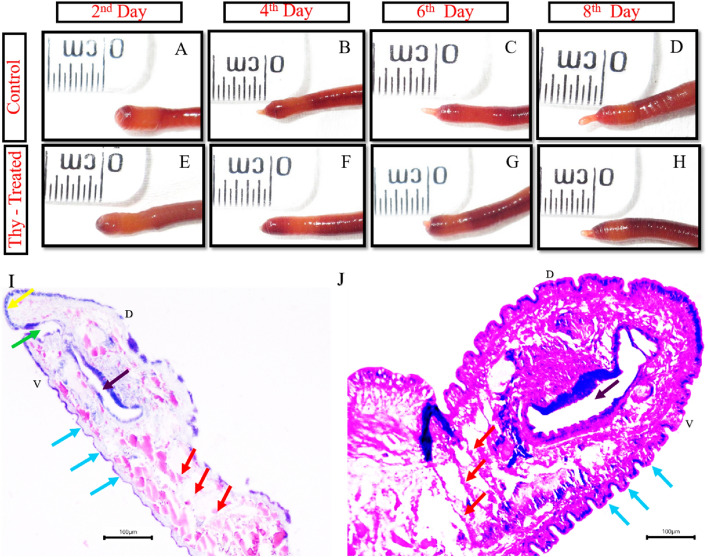
To further investigate the regeneration mechanism of clitellum-independent P. excavatus worm, the worms were injected with 2mM Thymidine to arrest the cell proliferation or synchronize the cells in the G1/S phase. The worms injected with 2mM Thymidine as described in materials and method are amputated on the 2nd day and observed for regeneration ability on the 2nd, 4th, 6th, and 8th day of post-amputation (Fig. 2A–H). The result shows that in the control worm, the blastema starts to project out on the 2nd day of post-amputation, but in the 2mM thymidine-injected worm, it is visible only with wound healing. On successive days of the 4th, 6th, and 8th days of post-amputation, the size of the regenerative bud is 1/3rd reduced in 2mM Thymidine injected worm when compared with the control worms. The delayed regeneration following cell proliferation inhibition with 2mM thymidine injection represents the worms following morphallaxis and epimorphosis for their regeneration. Comparative histological analysis of the 8th day of the regenerative bud of control and 2 mM thymidine-treated worms shows that in control worms, the bud segments are well elongated along with septum formation however this was not observed in Thymidine treated groups (Fig. 2I, J). Also, in the control worm, the functional mouth is formed along with a well-developed prostomium, but in 2 mM thymidine-treated worms, it initiates the formation of the intestine without forming a functional mouth and prostomium (Fig. 2I, J).
Fig. 2.
Regeneration following 2 mM Thymidine (cell cycle inhibitor) injection: A Control worm heals wound on 2nd day of post-amputation. B Blastema size of 0.2 mm was observed on the 4th day after post-amputation. C regenerative bud elongated to 0.3 mm on the 6th day. D blastemal size almost doubled (0.5 mm) on the 8th day upon restoration. (E) wound healing was adequately executed in Thymidine treated worm on the 2nd day of post-amputation. F blastemal growth is restricted and shows only 0.1 mm size following the 4th day of post-amputation. G unmeasurable very little blastemal growth progress was observed until the 6th day of post-amputation. H blastemal size is slightly elongated to 0.2 mm size following the 8th day of post-amputation. I histology of 8th day control blastemal tissue shows well-differentiated prostomium, mouth, septum, and elongated segments. J histology of 2 mM Thymidine injected 8th day regenerative blastema with less developed structures lacking prostomium, mouth, and with truncated regenerative body segments. Arrows (red represents septum; blue represents newly regenerated segments; yellow indicates prostomium; green represents functional mouth; purple represents intestinal tract). “D” represents the dorsal side of the earthworm, and “V” specifies the ventral surface of the earthworm
Pharmacological suppression of TCTP using Buclizine, modelling of TCTP protein, and molecular docking of TCTP and Buclizine
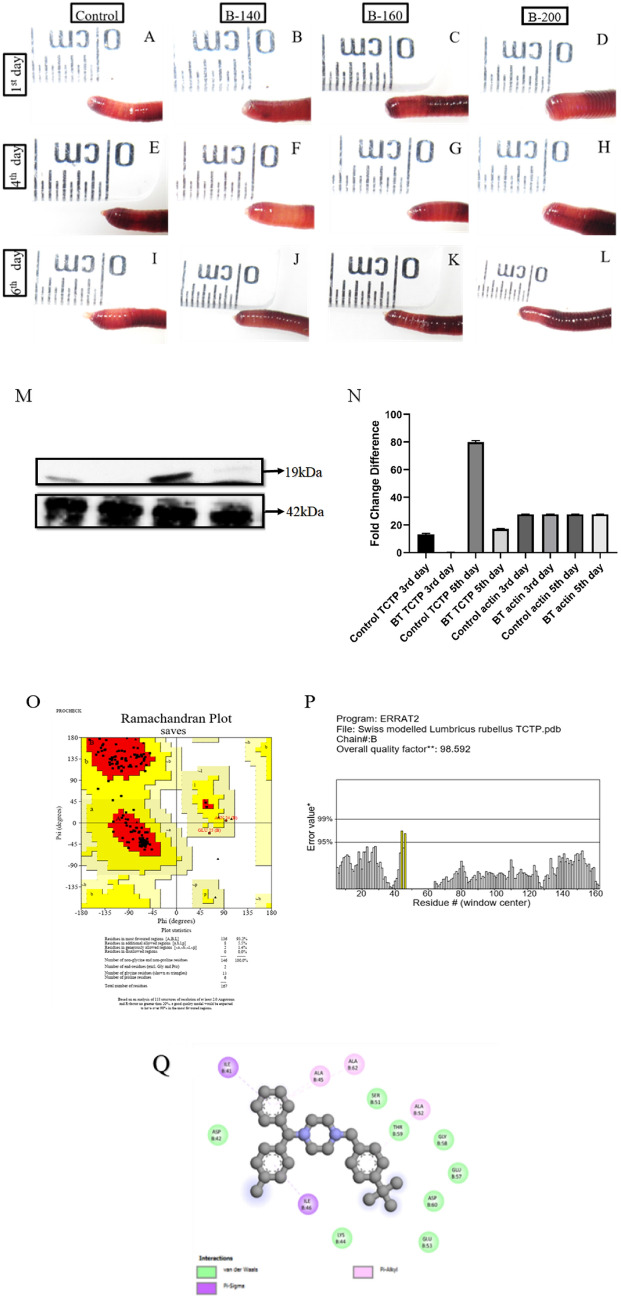
Buclizine is an antihistamine and anticholinergic compound used to prevent the symptoms caused by histamine activity. Buclizine has effectively inhibited the TCTP protein in human cells [43]. Buclizine was injected into the worm in different concentrations of 140 µg, 160 and 200 µg and observed for the regeneration ability to identify the role of TCTP in P. excavatus regeneration, as shown in Fig. 3A, L. The results show that different buclizine concentrations hinder regeneration on the 1st, 4th, and 6th day of post-amputated worms to a level of 1/4th compared to the control worms. Also, we observed no significant difference among the different concentrations of buclizine used in these experiments. Therefore, we preferred a lower concentration of 140 µg for further Western blotting investigations. Protein samples are prepared from 3rd and 5th-day control regenerating and buclizine-injected regenerated worms and subjected to Western blotting against TCTP (19 kDa) and β-actin proteins (Fig. 3M). The results indicate that in control regenerating worms, TCTP upregulated in succeeding days of the 3rd and 5th days of post-amputation. On the other hand, in the buclizine-treated worms, TCTP is completely inhibited on 3rd day of buclizine-injected regenerated worms but negligibly observed on 5th day. The β-actin is used as a positive control, and its molecular weight is 42 kDa. The graphic representation of normalized values is shown in Fig. 3N.
Fig. 3.
Pharmacological suppression of TCTP using buclizine and its effects on regeneration: A–D represents control worm (1X PBS), 140 µg buclizine, 160 µg buclizine, and 200 µg buclizine injected worms respectively following day-1 of post-amputation. E–H represents the 4th day post amputated worms correspondingly from the control worm (1X PBS), 140 µg buclizine, 160 µg buclizine, and 200 µg buclizine injected worms. In all the buclizine-injected worms, the regenerative bud size is hindered. Similarly, I-L represents the 6th day regenerative bud from the control worm, 140 µg buclizine, 160 µg buclizine, and 200 µg buclizine-injected worms, respectively. In all the buclizine-injected worms, regeneration is 1/4th reduced. M. Western blotting image represents TCTP (19 kDa) and β-actin (42 kDa) expression in 3rd day control regenerating worm, 3rd day buclizine injected regenerating worm, 5th day control regenerating worm and in 5th day buclizine injected regenerating worms respectively. N. graphical representation of Western blotting results shows the fold difference of TCTP and the expression of β-actin in control regenerating worms and the 5th day buclizine injected regenerating worms. O. Stereo chemical validation of predicted L. rubellus TCTP protein using Ramachandran plot. P. Evaluation of L. rubellus TCTP protein for unbounded atomic interactions by ERRAT server. Q. In-silico prediction of molecular-level interaction between Buclizine and TCTP protein
We performed homology modelling by generating a L. rubellus TCTP 3D protein structure. Human histamine-releasing factor-translationally controlled tumor protein (HRF-TCTP) (PDB ID: 5O9M) was selected as the template structure for protein model building. The template 5O9M exhibited 47.90% sequence identity and a GMQE score of 0.71 with L. rubellus TCTP protein. The above parameters confirm that the template 5O9M could be the best template for homology modelling. The PROCHECK server evaluated the stereochemical quality of the protein model. The results of the PROCHECK server (Fig. 3O) confirmed that 93.2% of residues were in the most favored regions, and none were placed in disallowed areas. The above result confirms that the generated 3D structure of L. rubellus is a reliable and good-quality model. In addition to the stereo-chemical assessment, the local errors of unbounded atomic interactions were assessed by the ERRAT server (Fig. 3P). The ERRAT analysis showed an overall quality factor of 98.5% for the homology-modelled 3D structure of L. rubellus. Further, the compatibility of the 3D atomic model of L. rubellus, TCTP protein with its 1D sequence was assessed by VERIFY 3D server. It is evident from the result that 99.40% of the residues with an average 3D-1D score were achieved. Therefore, all the above results confirmed the modelled 3D structure of L. rubellus TCTP protein as the quality model for further theoretical studies.
According to molecular docking results, buclizine formed hydrogen bonding and hydrophobic interactions with L. rubellus TCTP protein with a binding energy of − 6.1 kcal/mol. Two hydrogen bonds were interacting with the TCTP residues ASN49 and ALA 45 with a bond length of 3.6 Å and 3.5 Å (Fig. 3Q). Hydrophobic interactions were displayed with four residues: ALA 50, ILE 41, ALA 62, and ALA 45, as shown in Table 1. A notable feature in the docking analysis was that the residue ALA 45 exhibited hydrogen bonding and non-bonded interactions towards the ligand buclizine. The above results suggest that the residue ALA 45 and the adjacent flanking residues are the active site region of L. rubellus TCTP protein.
Table 1.
In-silico docking analysis of TCTP versus Buclizine
| S. no | Protein | Ligand | Affinity | RMSD (I. B.) |
RMSB (U. B.) |
No. of bonds | Peptide– llele interaction (non-bound) | Distances | Category |
|---|---|---|---|---|---|---|---|---|---|
| 1 | TCTP (Lumbricus terrestris) | Buclizine | − 6.1 | 4.633 | 8.898 |
2 hydrogen bonds + 6 hydrophobic bonds |
:UNK0:C - B:ASN49:O | 3.64063 | Hydrogen Bond |
| :UNK0:C - B:ALA45:O | 3.58072 | Hydrogen Bond | |||||||
| B:ALA50:CB - :UNK0 | 3.9661 | Hydrophobic | |||||||
| :UNK0:Cl - B:ILE41 | 5.40736 | Hydrophobic | |||||||
| :UNK0:Cl - B:ALA62 | 4.17849 | Hydrophobic | |||||||
| :UNK0:C - B:ALA50 | 4.28267 | Hydrophobic | |||||||
| :UNK0 - B:ALA45 | 4.19758 | Hydrophobic | |||||||
| :UNK0 - B:ALA45 | 4.92024 | Hydrophobic |
TCTP and its role in morphallaxis following combinatorial injection of buclizine and thymidine
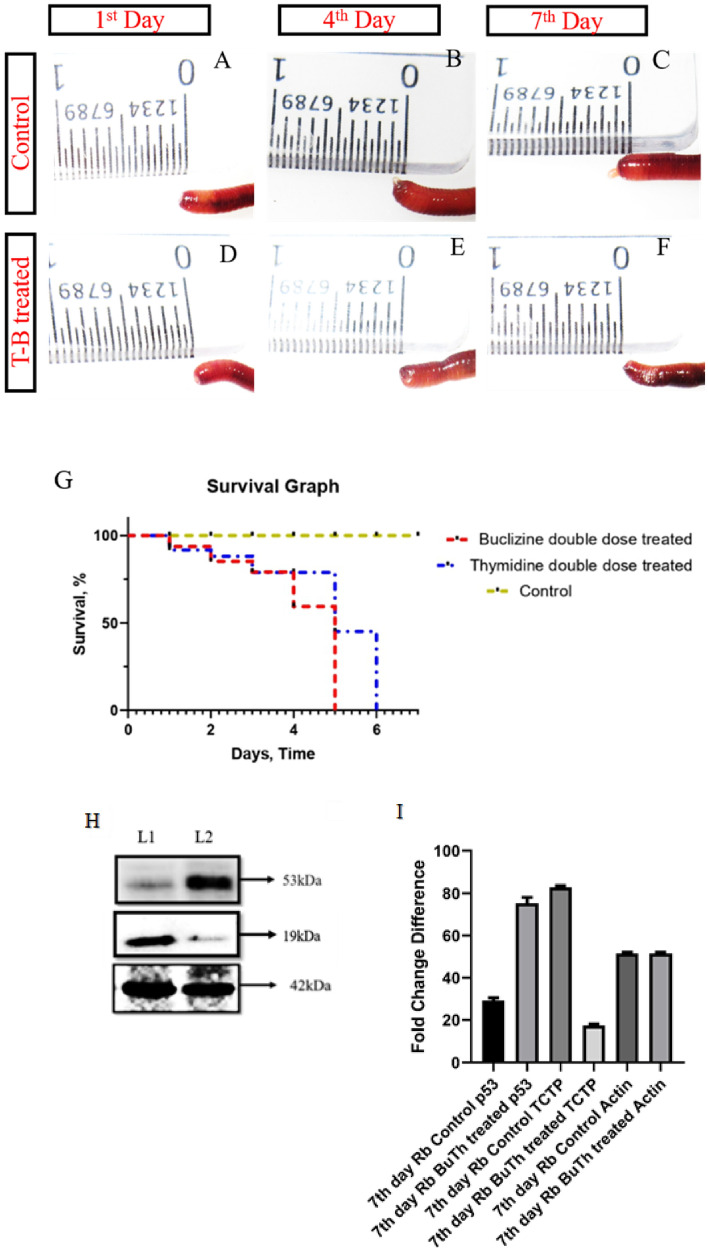
P. excavatus regenerates through epimorphosis (cellular proliferation) and morphallaxis (trans-differentiation), as confirmed by 2 mM thymidine injection. There is currently no report available in the academic literature that comprehensively covers the morphallaxis mechanism, specifically concerning trans-differentiation, within the context of TCTP. We administered a combination of 2mM Thymidine (an epimorphosis blocker) and Buclizine (a TCTP suppressor) to investigate the matter and show the results in Fig. 4A–I. Well-differentiated blastema was observed in the control group of worms on the 8th day, as depicted in Fig. 4A–C. The combinational injection of Buclizine and Thymidine completely inhibited the regeneration process for eight days post-amputation (Fig. 4D–F). However, there were no observable adverse effects on the physiological survival of the worm. The findings above suggest that TCTP governs cellular proliferation (epimorphosis) and substantially influences the morphallaxis (trans-differentiation) mechanism of regeneration, as evidenced by the total hindrance of regeneration. When we injected the worms with a double dose (two doses per day) of either 2 mM Thymidine or Buclizine, their physiological functions shut down, leading to their death within 6 days. The Mantel-Cox test was utilized to calculate and present the survival rate of worms treated with a double dose of Buclizine and Thymidine, as illustrated in Fig. 4G. The study revealed a reciprocal repression of the TCTP/p53 pattern in samples treated with buclizine/thymidine, as evidenced by the significant upregulation of p53 expression in TCTP-blocked samples (Fig. 4H–I).
Fig. 4.
Combinatorial injection of Buclizine and Thymidine: A control worm following day-1 post-amputation at 10th segment. B the amputated worms form blastema on the 4th day. C on the 7th day, the amputated worm forms elongated blastema. D Buclizine and Thymidine injected worm following 1st day of post-amputation. E complete inhibition of regeneration on 4th day of post-amputation in Buclizine and Thymidine injected worm. F Combinatorial injected worms show no sign of regeneration even after seven days of post-amputation. G Survival graph for the Control P. excavatus, buclizine, and Thymidine double dose-treated worms. After treatment, the live and dead worms on different days are counted and plotted in the survival (Kaplan–Meier) curve. Statistical analysis was accomplished using the log-rank test (Mantel-Cox), and the obtained p values < 0.05 were considered significant. H Expression of p53, TCTP, and β-actin in 8th day Control (L1) and 8th day Buclizine and Thymidine treated samples (L2). High expression of p53 and lower expression of TCTP was observed in Buclizine and Thymidine treated samples (p53–53 kDa; TCTP − 19 kDa; Actin − 42 kDa). I Band intensity of Western blotting image is quantified and represented with a bar diagram. Statistical significance is achieved when the p value is < 0.05
Inhibition of TCTP through Nutlin-3a and their counter effect on essential regenerative proteins
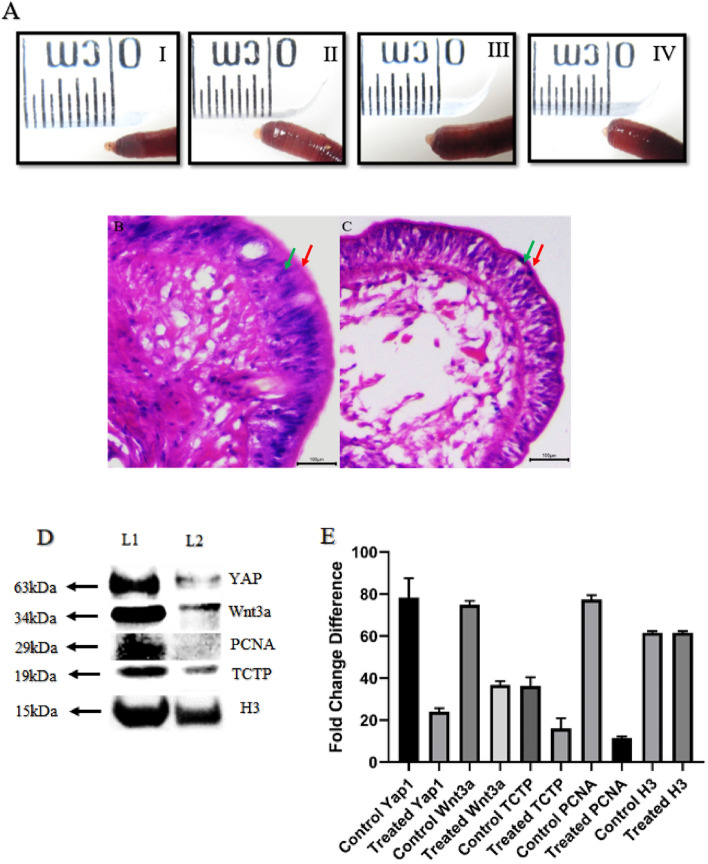
Nutlin-3a indirectly promotes TCTP degradation by activating p53. To further understand the function of TCTP in regulating the key regenerative proteins, the TCTP is pharmacologically inhibited using Nutlin-3a in three different dosages of 5, 7, and 9 µg injection. When compared with the control worms, the Nutlin-3a injected worms show an overall reduced bud size (5th day) in all the concentrations (Fig. 5A [I–IV]). It is also evident that increasing the level of Nutlin-3a gradually hinders the regeneration process in the earthworm P. excavatus. For further histological and western blotting experiments, Nutlin-3a (9 µg) injected worm samples were taken for analysis. Comparative histology of the regenerative bud tip between control (Fig. 5B) and 9 µg Nutlin-3a injected worms (Fig. 5C) shows that the outermost epithelial layer is well organized and thicker in control worm but which is lacking in Nutlin-3a treated worms. Also, the next inner layer, the outer epithelial layer, has a thickened outer covering in the control worm, which is more thinned in treated worms. The bud interior shows more compact structures in control worms but is highly diffused without any organized form observed in Nutlin-3a treated worms. Collectively, Nutlin-3a confirms that it had a direct influence on cell package and implies the role of TCTP in holding all-essential cellular functions towards regeneration. To confirm further Western blotting was performed in control and Nutlin-3a treated worms against PCNA (cell proliferation), Wnt3a (stem cell marker), and YAP1 (organ formation ruler and Hippo signaling). The results revealed that TCTP influences all the critical regeneration proteins upon inhibiting with Nutlin-3a (Fig. 5D). PCNA, Wnt3a, and YAP1 are remarkably reduced in all TCTP-inhibited worms, and it is evident that TCTP is a multi-functional protein that governs many functions associated with the regeneration mechanism. The graphical representation of the influence of Nutlin-3a in inhibiting TCTP and its associated proteins in regeneration have shown in Fig. 5E.
Fig. 5.
TCTP and its connecting link with regenerative protein: A (I). Control amputated worm with 5th day bud. II, III and IV represent progressive regeneration suppression in Nutlin-3a injected worms with 5, 7, and 9 µg, respectively. B Histology of control 5th -day regenerative blastema with well-organized tissue structures has the most thickened outer and inner epithelial layer. C In Nutlin-3a injected worms, the 5th day bud is not well organized, loosely packed with internal bud tissues and thinner layers of the outer and inner epithelium. D Western blotting image represents that when compared to the control samples (7th day regeneration—Lane 1), all frame of regenerative key proteins was notably reduced in Nutlin-3a treated samples (7th day regeneration—Lane 2). Nutlin-3a is known for TCTP silencing, and according to the result, TCTP silence influences organ formation (YAP1), stem cell activation (Wnt3a) and cell proliferation (PCNA). E. Quantification of YAP1, Wnt3a, TCTP, PCNA and H3 expression are done based on the band intensity and represented using bar diagram. The experiments were repeated in triplicate to analyze the statistical significance, representing their value as mean ± SD. p value < 0.05 was considered statistically significant data. The red arrow represents the “Outermost epithelial layer”; the Green arrow represents the “Inner layer of epithelial tissue.”
Discussion
P. excavatus is a topsoil earthworm that is habitually subjected to injury by predators, and correlating it with its enormous posterior regeneration ability represents its evolution nature to survey following an injury [36]. Regeneration studies show that the earthworm, P. excavatus, has an enormous regeneration ability. The worms amputated at the post-clitellum segment (30th segment) can regrow as an individual worm. The data confirm that P. excavatus is a clitellum-independent worm that does not requires clitellum segments for their regeneration as it is necessary for clitellum-dependent worms [5]. The pre-blastema was observed within 48 h, and within another 24 h, the blastema developed into a well-developed structure and further developed rapidly in the following hours. The data represents that anterior head regeneration is more vigorous because it needs to restore all the vital organs like mouth, tubular heart, simple brain and other organ systems to survive and perform the normal functions. Following injection of 2mM Thymidine, the regeneration potential of the earthworm was suppressed to 1/3rd level, representing the earthworm. P. excavatus can perform regeneration to a certain extent by utilizing morphallaxis when blocking epimorphosis. The epimorphosis mode of head regeneration was early reported in the earthworm P. excavatus [37], in which a high proliferative mass of cells forms the regenerative blastema. The blastema-like structure is observed in the arm regeneration of starfish, which adopts the intermediate mechanism of both morphallaxis and epimorphosis for their regeneration [44]. From histology, it confirms that the regenerative ability of the earthworm is suppressed in 2 mM Thymidine injected worms with a lack of development in their internal structures like functional mouth, septum, and segment elongation, which indirectly implies the factors or signals necessary for regeneration are not regulated correctly [45].
Following the pharmacological suppression of TCTP using an antihistamine drug, buclizine, the worm shows reduced regeneration ability which implies the critical role of TCTP in determining the regeneration ability of the worm. TCTP determines the cell fate on both ends, on a positive side through the influence of DNA damage and on a negative side as regulated by p53 [46]. The positive side of TCTP in determining the cell fate is revealed upon regeneration in that TCTP is upregulated on succeeding days of regeneration, and their pharmacological suppression hinders regeneration. TCTP also plays a crucial role in promoting the pathways related to cancer progression [47]. Therefore more studies are needed to understand their regulatory mechanism in determining the cell fate with controlled (Regeneration) and uncontrolled (Cancer) regulation upon many extracellular stimuli [48]. The modelled TCTP protein using earthworm sequences and their close interaction with buclizine and in-vivo results conclude that antihistaminics are the potent lead compounds in inhibiting the TCTP, which have a broad medicinal scope in treating cancers [48]. TCTP is a multi-functional protein that plays a crucial role in cell proliferation, cell growth, and apoptosis by interacting with many regulatory proteins [34]. TCTP’s part is also well documented in regenerative models involving epimorphosis or cell proliferation [30, 34], but its role is not revealed in the aspect of morphallaxis. The combinatorial injection of 2 mM Thymidine and Buclizine inhibit both epimorphosis and TCTP protein, respectively, resulting in complete regeneration loss, but the worms survived without any physiological stress. The data confirms that 2 mM Thymidine injections block epimorphosis and conjoined inhibition of TCTP blocks morphallaxis, which can completely block the regeneration events in the earthworm, P. excavatus. The clitellum-independent worms have a vast regeneration ability because regeneration is not restricted or dependent only on the clitellum segments, and in such worms, TCTP governs both epimorphosis and morphallaxis. There are also high possibilities with more multi-functional ability of TCTP protein with animals with more regenerative ability and animals with less regenerative ability. In these aspects, research is needed to conclude it in the near future. Surprisingly in combinatorial injected amputated worms, the p53 level increased compared to the non-injected regenerating worms. p53 also reverses the cell cycle, allowing cells to repair their DNA and inducing apoptosis in severe DNA damage [49]. In the present study, the amputated worm is subjected to double stressful conditions, notably cell cycle arrest and TCTP suppression, and in that conditions, the worm expresses abundant p53, representing that p53 promotes cell survival to repair and rescue. Several in-silico and in-vitro research have examined buclizine as an inhibitor of TCTP, but neither study reported on in-vivo models [43, 50]. Here we reported the potential in-vivo interactions of Buclizine and TCTP with visible suppression of TCTP expression and regeneration in the earthworm model. TCTP is a multi-functional protein inhibiting them with Buclizine targets TCTP and interplays with the TCTP interacting proteins [51].
Unlike buclizine, the inhibitory effect of Nutlin-3a in targeting TCTP is well documented in many in-vivo models [30, 52, 53]. The delay of posterior segment regeneration and wound closure following amputation was reported in Nutlin-3a injected clitellum dependent, Eudrilus eugeniae earthworm. Similarly, in these present studies, upon anterior regeneration, Nutlin-3a suppresses regeneration; histologically, it is evident with the improper cellular package. The data indicates that TCTP is linked with many regenerations-associated proteins, such as those involved in cell proliferation, cellular morphallaxis, cell differentiation, apoptosis, immune response, stem cell activation, and organ development. Inhibiting TCTP with Nutlin-3a suppresses the regeneration mechanism together with influences from other proteins like PCNA (cell proliferation), Wnt3a (stem cell marker), and YAP1 (organ formation ruler and Hippo signaling). Following amputation, the microenvironment at the wound site provides signals for triggering regeneration and in which DNA damage-induced responses like apoptosis [54], stem cell migration [55] play a significant role in determining the regeneration potential. TCTP determines cell fate by regulating major cellular functions like apoptosis and proliferation [32, 56, 57]. Notably, the TCTP protein is known for its anti-apoptotic role [32, 58, 59] and also act as an apoptotic protein in some context of abrogate DNA repair [60]. Like TCTP, Wnt3a is also known for its pleiotropic cellular functions regulating cell proliferation, cell renewal, cellular differentiation, apoptosis, and motility [61]. Compared to other WNTs, Wnt3a is remarkably important in determining regeneration potential in in-vitro, ex-vivo, and in-vivo conditions [62]. Following pharmacological suppression of TCTP, the Wnt3a expression decreases and directly indicates the tight regulation between TCTP and Wnt3a upon regeneration. The connective link between TCTP and β-catenin is reported in in-vitro and in-vivo cancer models [63], and in the present study the link between TCTP and Wnt3a upon regeneration is evident. The pharmacological inhibition of TCTP suppress the YAP1 signals upon regeneration and it represents the crosstalk between TCTP and YAP1. Regeneration occurs through a highly co-ordinated process and in that YAP/TAZ or Hippo pathway regulates the cell-cell interaction that determines the organ size and development [64]. YAP/TAZ complex also have a role in determining the cell fate by controlling the genes related with cell proliferation and apoptosis [65].
Apoptosis, stem cell activation, cellular proliferation, and organ development are essential for regeneration. Our studies conclude that TCTP governs both epimorphosis and morphallaxis during regeneration. Inhibiting TCTP impairs the regeneration mechanism by inhibiting all keyframes of regenerative proteins, including PCNA (proliferation), Wnt3a (Stem cell activation), and YAP1 (Hippo signaling). The cellular stress following pharmacological suppression of TCTP also initiates the p53 expression in the context of anti-apoptotic responses. Collectively, the present studies reveal the regulatory role of TCTP in connection with all critical regenerative proteins.
Acknowledgements
Authors thank ‘International Research Centre (IRC) of Sathyabama Institute of Science and Technology, Chennai’ for providing support to carry out the research work. This work was supported by the DST-SERB-INDIA (Ref. No. ECR/2016/000956). The funding was provided by DST-SERB (Grant number ECR/2016/000956).
Author contributions
KR (M-Tech, SRF, Ph.D Scholar) were involved in the writing original draft, conceptualization, data curation and figures. JDSC ( Ph.D, Associate Professor (Research)) were involved in the writing original draft, conceptualization, data curation, investigation, supervision and project administration. KSC (M.Sc, M.Phil, OVDF Fellow, Ph.D Scholar) were involved in conceptualization and data curation. KM (Ph.D, Assistant Professor) were involved in bioinformatics analysis (protein preparation). PD (M.Sc), LN (M.Sc), SG (M.Sc) were involved in minor experiments.
Data availability statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical statement
The experiments are carried out using lower invertebrate, earthworm therefore ethical statement is not needed. Necessary care is taken in experimental procedure that are intended to avoid unnecessary pain and sufering to the experimental animals.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Carlson BM. Principles of regenerative biology. Amsterdam: Elsevier; 2011. [Google Scholar]
- 2.Agata K, Saito Y, Nakajima E. Unifying principles of regeneration I: epimorphosis versus morphallaxis. Dev Growth Differ. 2007;49:73–78. doi: 10.1111/j.1440-169X.2007.00919.x. [DOI] [PubMed] [Google Scholar]
- 3.Morgan TH. Sex limited inheritance in Drosophila. Science. 1910;32:120–122. doi: 10.1126/science.32.812.120. [DOI] [PubMed] [Google Scholar]
- 4.Morgan TH. Regeneration. Stuttgart: Macmillan; 1901. [Google Scholar]
- 5.Selvan Christyraj JD, Azhagesan A, Ganesan M, Subbiah Nadar Chelladurai K, Paulraj VD, Selvan Christyraj JRS. Understanding the role of the Clitellum in the regeneration events of the earthworm Eudrilus Eugeniae. Cells Tissues Organs. 2019;208:134–41. [DOI] [PubMed]
- 6.Sivasubramaniam S. The earthworm Eudrilus Eugeniae: a model organism for regenerative biology. J Genet Genomic Sci. 2021;6:23. [Google Scholar]
- 7.Owlarn S, Klenner F, Schmidt D, et al. Generic wound signals initiate regeneration in missing-tissue contexts. Nat Commun. 2017;8:2282. doi: 10.1038/s41467-017-02338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pellettieri J. Regenerative tissue remodeling in planarians: the mysteries of morphallaxis. Seminars in cell and developmental biology. Amsterdam: Elsevier; 2019. pp. 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugiyama T, Fujisawa T. Genetic analysis of developmental mechanisms in Hydra. II. Isolation and characterization of an interstitial cell-deficient strain. J Cell Sci. 1978;29:35–52. doi: 10.1242/jcs.29.1.35. [DOI] [PubMed] [Google Scholar]
- 10.Abnave P, Ghigo E. Role of the immune system in regeneration and its dynamic interplay with adult stem cells. Semin Cell Develop Biol. 2019;87:160–168. doi: 10.1016/j.semcdb.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Bergmann A, Steller H. Apoptosis, stem cells, and tissue regeneration. Sci Signal. 2010;3:re8. doi: 10.1126/scisignal.3145re8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosch TCG. Why polyps regenerate and we don’t: towards a cellular and molecular framework for hydra regeneration. Dev Biol. 2007;303:421–433. doi: 10.1016/j.ydbio.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki M, Yakushiji N, Nakada Y, et al. Limb regeneration in Xenopus laevis froglet. ScientificWorldJournal. 2006;6:26–37. doi: 10.1100/tsw.2006.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bodó K, Kellermayer Z, László Z, et al. Injury-induced innate immune response during segment regeneration of the earthworm, Eisenia andrei. Int J Mol Sci. 2021;22:2363. doi: 10.3390/ijms22052363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu T, Wang L, Chen H, et al. Molecular and cellular mechanisms of apoptosis during dissociated spermatogenesis. Front Physiol. 2017;8:188. doi: 10.3389/fphys.2017.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan Y, Bergmann A. Apoptosis-induced compensatory proliferation. The cell is dead. Long live the cell! Trends Cell Biol. 2008;18:467–473. doi: 10.1016/j.tcb.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huh JR, Guo M, Hay BA. Compensatory proliferation induced by cell death in the Drosophila wing disc requires activity of the apical cell death caspase dronc in a nonapoptotic role. Curr Biol. 2004;14:1262–1266. doi: 10.1016/j.cub.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 18.Jung Y, Witek RP, Syn W-K, et al. Signals from dying hepatocytes trigger growth of liver progenitors. Gut. 2010;59:655–665. doi: 10.1136/gut.2009.204354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryoo HD, Gorenc T, Steller H. Apoptotic cells can induce compensatory cell proliferation through the JNK and the wingless signaling pathways. Dev Cell. 2004;7:491–501. doi: 10.1016/j.devcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 20.Pérez-Garijo A, Steller H. Spreading the word: non-autonomous effects of apoptosis during development, regeneration and disease. Development. 2015;142:3253–3262. doi: 10.1242/dev.127878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fogarty CE, Bergmann A. Killers creating new life: caspases drive apoptosis-induced proliferation in tissue repair and disease. Cell Death Differ. 2017;24:1390–1400. doi: 10.1038/cdd.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Q, Li F, Liu X, et al. Caspase 3–mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med. 2011;17:860–866. doi: 10.1038/nm.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Llames S, García-Pérez E, Meana Á, et al. Feeder layer cell actions and applications. Tissue Eng Part B Rev. 2015;21:345–353. doi: 10.1089/ten.teb.2014.0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chera S, Ghila L, Wenger Y, Galliot B. Injury-induced activation of the MAPK/CREB pathway triggers apoptosis-induced compensatory proliferation in hydra head regeneration. Dev Growth Differ. 2011;53:186–201. doi: 10.1111/j.1440-169X.2011.01250.x. [DOI] [PubMed] [Google Scholar]
- 25.Fujimoto K, Yamamoto T, Kitano T, Abé SI. Promotion of cathepsin L activity in newt spermatogonial apoptosis induced by prolactin. FEBS Lett. 2002;521:43–46. doi: 10.1016/S0014-5793(02)02817-X. [DOI] [PubMed] [Google Scholar]
- 26.Li F, Huang Q, Chen J, et al. Apoptotic cells activate the phoenix rising pathway to promote wound healing and tissue regeneration. Sci Signal. 2010;3:ra13–13. doi: 10.1126/scisignal.2000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oka T, Adati N, Shinkai T, et al. Bisphenol A induces apoptosis in central neural cells during early development of Xenopus laevis. Biochem Biophys Res Commun. 2003;312:877–882. doi: 10.1016/j.bbrc.2003.10.199. [DOI] [PubMed] [Google Scholar]
- 28.Pellettieri J, Fitzgerald P, Watanabe S, et al. Cell death and tissue remodeling in planarian regeneration. Dev Biol. 2010;338:76–85. doi: 10.1016/j.ydbio.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paul S, Balakrishnan S, Arumugaperumal A, et al. The transcriptome of anterior regeneration in earthworm Eudrilus eugeniae. Mol Biol Rep. 2021;48:259–283. doi: 10.1007/s11033-020-06044-8. [DOI] [PubMed] [Google Scholar]
- 30.Subramanian ER, Gopi Daisy N, Sudalaimani DK, et al. Function of translationally controlled tumor protein (TCTP) in Eudrilus eugeniae regeneration. PLoS One. 2017;12:e0175319. doi: 10.1371/journal.pone.0175319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koziol МJ, Gurdon JB. TCTP in development and cancer. Biochem Res Int. 2012;2012:105203. [DOI] [PMC free article] [PubMed]
- 32.Rho SB, Lee JH, Park MS, et al. Anti-apoptotic protein TCTP controls the stability of the tumor suppressor p53. FEBS Lett. 2011;585:29–35. doi: 10.1016/j.febslet.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 33.Telerman A, Amson R. The molecular programme of tumour reversion: the steps beyond malignant transformation. Nat Rev Cancer. 2009;9:206–216. doi: 10.1038/nrc2589. [DOI] [PubMed] [Google Scholar]
- 34.Chen S-H, Lu C-H, Tsai M-J. TCTP is essential for cell proliferation and survival during CNS development. Cells. 2020;9:133. doi: 10.3390/cells9010133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amson R, Pece S, Lespagnol A, et al. Reciprocal repression between P53 and TCTP. Nat Med. 2012;18:91–99. doi: 10.1038/nm.2546. [DOI] [PubMed] [Google Scholar]
- 36.Banik D, Chaudhuri PS. Regeneration ability in seventeen top soil and sub soil earthworm species. J Environ Biol. 2017;38:393. doi: 10.22438/jeb/38/3/MS-221. [DOI] [Google Scholar]
- 37.Bae YS, Kim J, Yi J, et al. Characterization of Perionyx excavatus development and its head regeneration. Biology (Basel) 2020;9:273. doi: 10.3390/biology9090273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho SJ, Lee MS, Tak ES, Lee E, Koh KS, Ahn CH, et al. Gene expression profile in the anterior regeneration of the earthworm using expressed sequence tags. Biosci Biotechnol Biochem. 2009;73:29–34. [DOI] [PubMed]
- 39.Martinez VG, Menger GJ 3rd, Zoran MJ. Regeneration and asexual reproduction share common molecular changes: upregulation of a neural glycoepitope during morphallaxis in Lumbriculus. Mech Dev. 2005;122:721–32. [DOI] [PubMed]
- 40.Chellathurai Vasantha N, Rajagopalan K, Selvan Christyraj JD, Subbiahanadar Chelladurai K, Ganesan M, Azhagesan A, et al. Heat-inactivated coelomic fluid of the earthworm Perionyx excavatus is a possible alternative source for fetal bovine serum in animal cell culture. Biotechnol Prog. 2019;35:e2817. [DOI] [PubMed]
- 41.Gopi Daisy N, Subramanian ER, Selvan Christyraj JD, Sudalai Mani DK, Selvan Christyraj JR, Ramamoorthy K, et al. Studies on regeneration of central nervous system and social ability of the earthworm Eudrilus eugeniae. Invert Neurosci. 2016;16:6. [DOI] [PubMed]
- 42.Sc JRS, Amutha K, Dinesh SM, et al. Autofluorescence in BrdU-positive cells and augmentation of regeneration kinetics by riboflavin. Stem Cells Dev. 2012;21:2071–2083. doi: 10.1089/scd.2011.0485. [DOI] [PubMed] [Google Scholar]
- 43.Seo EJ, Efferth T. Interaction of antihistaminic drugs with human translationally controlled tumor protein (TCTP) as novel approach for differentiation therapy. Oncotarget. 2016;7:16818. doi: 10.18632/oncotarget.7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yokoyama H, Ogino H, Stoick-Cooper CL, et al. Wnt/β-catenin signaling has an essential role in the initiation of limb regeneration. Dev Biol. 2007;306:170–178. doi: 10.1016/j.ydbio.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michalopoulos GK. Liver regeneration. Liver Biol Pathobiol. 2020 doi: 10.1002/9781119436812.ch45. [DOI] [Google Scholar]
- 46.Acunzo J, Baylot V, So A, Rocchi P. TCTP as therapeutic target in cancers. Cancer Treat Rev. 2014;40:760–769. doi: 10.1016/j.ctrv.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 47.Bommer UA, Kawakami T. Role of TCTP in cell biological and disease processes. Cells. 2021;10:2290. doi: 10.3390/cells10092290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seo EJ, Fischer N, Efferth T. Role of TCTP for cellular differentiation and cancer therapy. Results Probl Cell Differ. 2017;64:263–81. [DOI] [PubMed]
- 49.Feroz W, Sheikh AMA. Exploring the multiple roles of guardian of the genome: P53. Egypt J Med Hum Genet. 2020;21:1–23. doi: 10.1186/s43042-020-00089-x. [DOI] [Google Scholar]
- 50.Kumar R, Maurya R, Saran S. Identification of novel inhibitors of the translationally controlled tumor protein (TCTP): insights from molecular dynamics. Mol Biosyst. 2017;13:510–524. doi: 10.1039/C6MB00850J. [DOI] [PubMed] [Google Scholar]
- 51.Bommer U-A, Telerman A. Dysregulation of TCTP in biological processes and diseases. Cells. 2020;9:1632. doi: 10.3390/cells9071632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zuber J, Rappaport AR, Luo W, et al. An integrated approach to dissecting oncogene addiction implicates a myb-coordinated self-renewal program as essential for leukemia maintenance. Genes Dev. 2011;25:1628–1640. doi: 10.1101/gad.17269211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kang JH, Lee S-H, Lee J-S, et al. Inhibition of transglutaminase 2 but not of MDM2 has a significant therapeutic effect on renal cell carcinoma. Cells. 2020;9:1475. doi: 10.3390/cells9061475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ryoo HD, Bergmann A. The role of apoptosis-induced proliferation for regeneration and cancer. Cold Spring Harb Perspect Biol. 2012;4:a008797. doi: 10.1101/cshperspect.a008797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sahu S, Sridhar D, Abnave P, et al. Ongoing repair of migration-coupled DNA damage allows planarian adult stem cells to reach wound sites. Elife. 2021;10:e63779. doi: 10.7554/eLife.63779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hsu YC, Chern JJ, Cai Y, et al. Drosophila TCTP is essential for growth and proliferation through regulation of dRheb GTPase. Nature. 2007;445:785–788. doi: 10.1038/nature05528. [DOI] [PubMed] [Google Scholar]
- 57.Telerman A, Amson R. TCTP/tpt1-Remodeling signaling from stem cell to disease. Berlin: Springer; 2017. [Google Scholar]
- 58.Bommer UA, Thiele BJ. The translationally controlled tumour protein (TCTP) Int J Biochem Cell Biol. 2004;36:379–385. doi: 10.1016/S1357-2725(03)00213-9. [DOI] [PubMed] [Google Scholar]
- 59.Lee H-J, Song K-H, Oh SJ, et al. Targeting TCTP sensitizes tumor to T cell-mediated therapy by reversing immune-refractory phenotypes. Nat Commun. 2022;13:2127. doi: 10.1038/s41467-022-29611-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Omabe K. Translationally controlled tumor protein: a key target to abrogate DNA repair and therapeutic resistance in cancer. J Cancer Res Ther Oncol. 2022;10:1–17. [Google Scholar]
- 61.He S, Lu Y, Liu X, et al. Wnt3a: functions and implications in cancer. Chin J Cancer. 2015;34:1–9. doi: 10.1186/s40880-015-0052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang C-Y, Liang M-Z, Wu C-C, et al. WNT3A promotes neuronal regeneration upon traumatic brain injury. Int J Mol Sci. 2020;21:1463. doi: 10.3390/ijms21041463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gu X, Yao L, Ma G, et al. TCTP promotes glioma cell proliferation in vitro and in vivo via enhanced β-catenin/TCF-4 transcription. Neuro Oncol. 2014;16:217–227. doi: 10.1093/neuonc/not194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Varelas X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development. 2014;141:1614–1626. doi: 10.1242/dev.102376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.