Abstract
Since Doxil's first clinical approval in 1995, lipid nanoparticles have garnered great interest and shown exceptional therapeutic efficacy. It is clear from the licensure of two RNA treatments and the mRNA-COVID-19 vaccination that lipid nanoparticles have immense potential for delivering nucleic acids. The review begins with a list of lipid nanoparticle types, such as liposomes and solid lipid nanoparticles. Then it moves on to the earliest lipid nanoparticle forms, outlining how lipid is used in a variety of industries and how it is used as a versatile nanocarrier platform. Lipid nanoparticles must then be functionally modified. Various approaches have been proposed for the synthesis of lipid nanoparticles, such as High-Pressure Homogenization (HPH), microemulsion methods, solvent-based emulsification techniques, solvent injection, phase reversal, and membrane contractors. High-pressure homogenization is the most commonly used method. All of the methods listed above follow four basic steps, as depicted in the flowchart below. Out of these four steps, the process of dispersing lipids in an aqueous medium to produce liposomes is the most unpredictable step. A short outline of the characterization of lipid nanoparticles follows discussions of applications for the trapping and transporting of various small molecules. It highlights the use of rapamycin-coated lipid nanoparticles in glioblastoma and how lipid nanoparticles function as a conjugator in the delivery of anticancer-targeting nucleic acids. High biocompatibility, ease of production, scalability, non-toxicity, and tailored distribution are just a meager of the enticing allowances of using lipid nanoparticles as drug delivery vehicles. Due to the present constraints in drug delivery, more research is required to utterly realize the potential of lipid nanoparticles for possible clinical and therapeutic purposes.
Keywords: Rapamycin-coated lipid, Lipid nanoparticles, Nucleic acid medications, Biocompatibility, Nontoxicity, Targeted delivery
Introduction
To conserve active components, improve performance, and manage site-specific pharmaceutical distribution, a variety of drug methods for dispersion have been created (Mitchell et al. 2021). For many years (Mitchell et al. 2021; Gordillo-Galeano and Mora-Huertas 2018; Patra et al. 2018), a great deal of research has been done on nanoparticles for medication delivery. Clinical trials have effectively administered both hydrophobic and hydrophilic medications using lipid-based nanoparticles such liposomes, solid lipid nanoparticles (SLNs), and nanostructured lipid carriers (NLCs) (Yingchoncharoen et al. 2016). Lipid nanoparticles, such as those that contain nucleic acids, are aqueous capsules that are submicron in size. Others include those having an oily, solid, or amorphous core with lipid coats encasing and stabilizing them, collectively known as lipid nanoparticles (LNPs). Over the last 30 years, they have conducted in-depth research to develop pharmaceutical formulations, which has resulted in the approval of 23 drugs in the United States and Europe (Bobo et al. 2016; Bulbake et al. 2017; Anselmo and Mitragotri 2019). The American FDA received 63% of the liposome formulations for cancer therapy and 84% for intravenous delivery (Kapoor et al. 2017). For the approval of Doxil®, a liposome formulation of doxorubicin, created first by Sequus and authorized for subsequent treatment of Kaposi's sarcoma (Barenholz 2012), was arguably one of the most significant achievements in nanomedicine. Higher therapeutic indices were obtained using these liposomes, which were mostly filled with small-molecule chemotherapeutic medicines. These liposomes greatly enhanced their pharmacokinetic profile and decreased unintended negative effects (Fan and Zhang 2013). Epaxal® and Inflexal V®, lipid-based vaccines, as well as Definity® and SonoVue®, lipid vesicles used as ultrasound contrast agents, were all developed around this period. The first decade of the twenty-first century saw a relatively slow development of liposome formulations in the pharmaceutical industry due to the success of targeted therapies, such as small molecules and antibodies, which outperformed traditional chemotherapeutics in terms of therapeutic indices. Phospholipids, which include lipids with diverse hydrocarbon structures, such as phosphatidic acid (PA), phosphatidylglycerol (PG), phosphatidylethanolamine (PE), phosphatidylcholine (PC), and phosphatidylserine (PS), typically include a set of polar heads and two hydrophobic alkyl tails. Different head groups provide lipids negative (PA, PS, and PG) or neutral (PC and PE) charges when the pH is physiological. Alkenes in lipid tails promote the formation of liposomes at room temperature by lowering the temperature at which these unsaturated lipids transform from solid to liquid.
Lipid-based nanoparticles (LNPs) are real particles with a diameter of approximately 100 nm that are assembled from different types of lipids and other chemical components that work together to overcome biological barriers, or bio-barriers, so that LNPs selectively develop in or around disease-target cells to functionally deliver therapeutic agents for treatment or imaging agents for diagnosis. Lipid nanoparticles have a wide range of capabilities, and adding to this being at the nanoscale doubles its applications by addressing the diverse range of functional requirements. LNPs are considered appropriate vehicles to provide an integrated, personalized approach for cancer diagnosis and therapy in future cancer disease management.
LNPs have various properties that can be used in a plethora of medical applications. One of these properties is the accumulation of LNPs around the diseased cell as previously mentioned, which helps in detecting cancerous cells. Identifying cancer disease-specific biomarkers in vivo is crucial for personalized medicine to truly take off. Drug-loaded barcoded lipid nanoparticles have been used to measure tumor sensitivity to different drugs (Sidow and Spies 2015; Sottoriva et al. 2015).
Phospholipids are more biocompatible than polymeric and inorganic substances because of their potential for self-replication into liposomes, which have an aqueous core encased in lipid layers. As a result, medications like vincristine or amphotericin B that are both water-soluble and water-insoluble can be effectively encapsulated in the lipid layers and core, respectively. Lipid-based forms of drug delivery may be categorized as lipid nanodiscs, lipoplexes with counter ion complexes, large unilamellar vesicles, giant unilamellar vesicles, multilamellar vesicles, multivesicular vesicles, and lipid nanoparticles depending on the particle size and lamellarity (Akbarzadeh et al. 2013; Waghule et al. 2022). The smallest nanodisc, which typically has a diameter of 10 nm (Denisov et al. 2004; Bayburt et al. 2002), is made up of a single lipid bilayer that is kept in place by lipoproteins and encircled by them. After hydration, lipid films could self-assemble into multilamellar vesicles—MLVs. These MLVs can then be homogenized using extrusion or sonication to create unilamellar SUVs or LUVs with diameters of 30 nm or 100 nm. While MLVs could offer the contents of liposomes more protection from the environment, LUVs are frequently employed in liposome formulations (Moon et al. 2011). Vyxeos®, a liposomal formulation containing cytarabine and daunorubicin in a constant molar ratio, has a unique bilayer/compartment structure since a hyperosmotic external buffer was utilized (Dicko et al. 2010). The anesthetic bupivacaine (Exparel®) may be dispersed and dispensed by MVs of micron widths for local analgesia, whereas GUVs can be used as cell-mimicking model systems (Fenz and Sengupta 2012). Despite the numerous advantages lipid nanoparticles offer for consistent and efficient drug administration, they are challenging to quantify due to their complex physicochemical makeup and specialized manufacturing methods. Both the final medicinal product's nanoparticles and the characteristics of the lipid species present in these formulations need to be studied.
Cancer cells are located and eliminated by the immune system through immunotherapy. Biological therapy is another type of immunotherapy. Different applications of immunotherapy include attacking cancer cells by stimulating the immune system and preventing cancer recurrence (Guevara et al. 2020).
Lipid nanoparticles increase the efficiency of immunotherapy. mRNA-lipid nanoparticle predominantly expressed in secondary lymphoid organs that are injected intravenously than in mRNA-dendritic cells (Firdessa-Fite and Creusot 2020).
The different applications of lipid nanoparticles in immunotherapy include the following:
-
(i)
Delivery of immunotherapeutic agents to target cells.
-
(ii)
Protects immunotherapeutic agents from degradation.
-
(iii)
Enhancement of cellular uptake of immunotherapeutic agents.
Gene Therapy: Gene therapy uses genes to treat or prevent various diseases. Genes are the instructions that tell cells how to make proteins. If a gene is missing or defective, it can cause disease. Gene therapy works by delivering a normal gene to cells that require it. Lipid nanoparticles (LNPs) are tiny spheres composed of lipids (fats) that can be used to deliver genes to cells. LNPs are biocompatible and nontoxic, making them a safe and effective way to deliver genes to the body (Zhao and Huang 2014).
Gene therapy combined with LNPs has the potential to treat a wide range of diseases (Amer 2014).
-
(i)
Genetic disorders, such as cystic fibrosis and sickle cell anemia.
-
(ii)
Cancers.
-
(iii)
Infectious diseases, such as HIV/AIDS and hepatitis C.
-
(iv)
Neurological disorders, such as Parkinson's disease and Alzheimer's disease.
LNPs have been used to deliver genes to cells in the liver, lungs, muscle, and other tissues. They have also been used to deliver genes to immune cells, which can help boost the immune system’s ability to fight against cancer and other diseases. LNPs are a promising delivery system for nucleic acid-based therapies, such as mRNA vaccines and siRNAs. The four main components of lipid nanoparticles for vaccines and gene therapy are as follows (Hald Albertsen et al. 2022).
Structural lipids: These lipids form the backbone of LNP and provide stability.
Functional lipids: These lipids play a role in the interaction of LNPs with cells and tissues.
Helper lipids: These lipids help improve the delivery efficiency of LNPs.
PEGylated lipids: These lipids help reduce LNP immunogenicity and increase its circulation time.
Lipid nanoparticles (LNPs) are presently employed to deliver functional mRNAs, similar to those found in COVID-19 mRNA vaccines. There is significant interest in utilizing lipid nanoparticles (LNPs) for the in vivo mRNA delivery of base editors (Jiang et al. 2020; Song et al. 2020). In contrast, current studies predominantly employ viral vectors for in vivo base editing or direct gene engineering of zygotes (Rees and Liu 2018; Levy et al. 2020).One example is the prevailing belief that viruses exploit the endocytic machinery of host cells (Davey et al. 2011; Lin and Guttman 2010; Hassan et al. 2021; Sicari et al. 2020; Zeltzer et al. 2018; Staring et al. 2018). Following replication within the host cell, viral proteins and RNA fragments are encapsulated into extracellular vesicles (EVs) and released through exocytosis, utilizing exosomal release pathways to disseminate to neighboring cells (Nguyen et al. 2003; Sadeghipour and Mathias 2017; Badierah et al. 2021; Meckes 2015). Lipid nanoparticles (LNPs), approved for clinical use, serve as vehicles for transporting mRNA and have recently been employed as a delivery platform for LNP-mRNA vaccines targeting COVID-19, developed by Pfizer-BioNTech and Moderna (Baden et al. 2021a; Wang 2021). Exploration of applications beyond mRNA vaccines is underway for lipid nanoparticles (LNPs) (Kulkarni et al. 2021; Huang et al. 2022). LNPs have been utilized for co-delivering siRNA and mRNA (Ball et al. 2018), as well as for delivering therapeutic mRNA in various in vivo disease models (Nabhan et al. 2016; An et al. 2017). This involves clinical trials assessing immunogenicity to provide protection against Zika and influenza viruses (Richner et al. 2017; Bahl et al. 2017).
Combination therapy using solid lipid nanoparticles and stem cells has been used in various therapeutic applications. The former regulates mesenchymal stem cells, promotes neural differentiation in pluripotent stem cells, and exhibits anticancer activity against tumors. Their various applications include gene translation efficiency, bone regeneration and osteogenic differentiation (Kamarehei 2022).
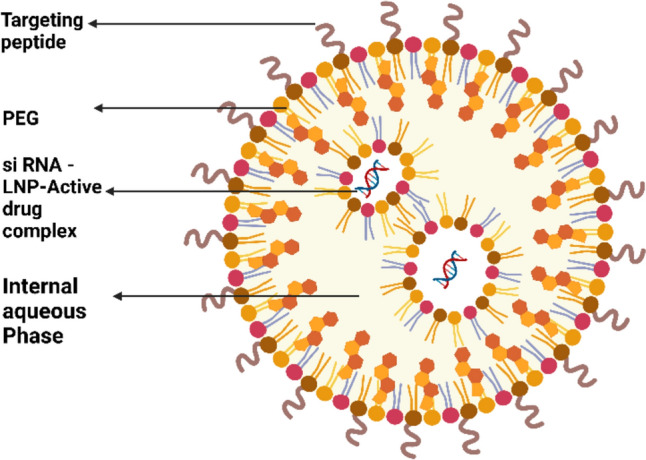
Lipid nanoparticles (LNPs) have emerged as promising platforms for delivering small interfering RNA (siRNA) therapeutics to target the genetic causes of various diseases. siRNA, a double-stranded RNA molecule, can specifically silence the expression of disease-causing genes through a process called RNA interference (RNAi). However, the inherent instability and poor cellular uptake of siRNAs pose significant challenges for their clinical translation. LNP technology addresses these challenges by encapsulating siRNA within a lipid bilayer, protecting it from degradation, and facilitating its delivery into target cells.
Various LNP formulations with distinct characteristics and advantages have been developed to optimize siRNA delivery. Some commonly used LNP formulations include.
Ionizable cationic lipids: These lipids have a net positive charge at physiological pH, enabling them to electrostatically interact with negatively charged siRNA and form stable complexes. Upon cellular uptake, the lipids become protonated and destabilize the LNP, releasing siRNA into the cytoplasm.
Polymeric nanoparticles: These nanoparticles are composed of synthetic polymers, offering greater stability and versatility than liposomes. They can be tailored to incorporate specific targeting ligands or release mechanisms to enhance siRNA delivery.
Hybrid nanoparticles: These nanoparticles combine the features of ionizable cationic lipids and polymers, thereby leveraging the advantages of each component. They can provide a balance between stability, targeting efficiency, and controlled release.
Combination Therapy with LNPs LNPs hold promise for combination therapy, in which multiple therapeutic agents are co-delivered to synergistically enhance treatment efficacy. This approach can potentially overcome the resistance to single-agent therapies and broaden the therapeutic window.
siRNA and small-molecule drugs: LNPs can simultaneously deliver siRNA to silence disease-causing genes and small-molecule drugs to target specific protein pathways. This combination can effectively modulate both gene expression and protein activity, thereby increasing overall therapeutic impact.
siRNA and antibodies: LNPs can co-deliver siRNA to target specific mRNA molecules and antibodies to neutralize specific proteins. This combination can simultaneously silence gene expression and inhibit protein function, thereby providing a more comprehensive therapeutic strategy.
siRNA and mRNA: LNPs can co-deliver siRNA to silence disease-causing genes and mRNA to encode therapeutic proteins. This combination can simultaneously knock down harmful genes and introduce beneficial proteins, thereby offering a novel approach to gene therapy (Kulkarni et al. 2019).
According to the studies performed by Clayton et al. (2014), the combinatorial effect of increased polyethylene glycol (PEG) density along with LNP-shielded LNP surface charge also reduced the hemolytic activity, immunostimulatory potential, and association with apolipoprotein E (ApoE). APOE is a target ligand for hepatocytes. The hindrance caused by high-density PEG could be solved by incorporating an exogenous targeting ligand, such as siRNA, into highly shielded LNPs.
However, the adverse properties of nucleic acid therapies make their use in vivo difficult. The clinical translation of gene therapies has been made possible by the revolutionary development of lipid nanoparticle (LNP) delivery technology. The latest LNP technology for hepatic gene therapy, including formulation design parameters, production methods, preclinical development, and clinical translation, can be used to treat liver disorders by silencing pathogenic genes, expressing therapeutic proteins, or correcting genetic defects. LNPs can deliver siRNA, mRNA, DNA, or gene editing complexes (Trucillo et al. 2020).
Nucleic acid medications, also known as oligonucleotide therapeutics or gene-silencing drugs, represent a novel class of drugs that directly target the genetic cause of diseases. Unlike traditional small-molecule drugs that target proteins, nucleic acid medications interact with genetic materials, DNA, or RNA to modulate gene expression. This approach holds promise for revolutionizing the treatment of a wide range of diseases including cancer, infectious diseases, and genetic disorders (Ransohoff et al. 2018; Dü 2005).
Several types of nucleic acid medication exist, each with a distinct mechanism of action. Antisense oligonucleotides (ASOs) are short synthetic DNA strands that bind to complementary RNA sequences and prevent their translation into proteins. Small interfering RNAs (siRNAs) are small double-stranded RNA molecules that trigger the degradation of complementary mRNA molecules. RNA interference (RNAi) is a biological process in which siRNAs or other small RNAs trigger the degradation of mRNA molecules. Gene therapy uses nucleic acids to introduce new genetic material into cells to correct genetic defects or to treat diseases (Kulkarni et al. 2019).
Nucleic acid medications offer several advantages over traditional small molecule drugs. They can target specific genes or RNA molecules with high specificity to minimize off-target effects. Their effects can be long lasting, as they can alter the expression of genes or RNA molecules for extended periods. In addition, nucleic acid medications have the potential to treat a wide range of diseases.
Despite their promise, several challenges remain to be addressed in the widespread clinical application of nucleic acid medications. Efficient delivery to target cells, without degradation or unwanted side effects, is a major challenge. Ensuring safety is crucial because these drugs can potentially trigger immune responses or alter the expression of non-target genes. Demonstrating clinical efficacy in large-scale clinical trials is essential for widespread adoption. As research progresses, these challenges are being actively addressed, and nucleic acid medications have the potential to revolutionize the treatment of a wide range of diseases (Ransohoff et al. 2018).
Lipid nanoparticles (LNPs) are known for their minimal side effects, excellent biocompatibility, and high physical stability. The lipid-based internal structure of LNPs makes them an effective carrier for lipophilic drugs. Chitosan, a natural cationic polysaccharide derived from chitin, is a representative biopolymer used to modify the surfaces of LNPs. Chitosan has low cytotoxicity and excellent biocompatibility and biodegradability (Fonte et al. 2012). In the realm of delivery systems, lipid-based nanoparticles offer numerous advantages, including formulation simplicity, self-assembly, biocompatibility, high bioavailability, capacity to carry substantial payloads, and ability to control various physicochemical properties to modulate biological characteristics (Fonseca-Santos et al. 2015; Sercombe et al. 2015).
Inorganic nanoparticles possessing magnetic, radioactive, or plasmonic properties are particularly well suited for applications in diagnostics, imaging, and photothermal therapies. These nanoparticles generally exhibit favorable biocompatibility and stability, catering to specialized applications that require properties that are not achievable with organic materials. Nevertheless, their clinical utilization is constrained by challenges such as low solubility and concerns about toxicity, particularly in formulations incorporating heavy metals (Arias et al. 2018; Manshian et al. 2017).
Inorganic nanoparticles with magnetic, radioactive, or plasmonic properties are uniquely suited for applications in diagnostics, imaging, and photothermal therapies. These nanoparticles generally exhibit good biocompatibility and stability, filling specific roles that require properties unattainable by organic materials. Near-infrared light, which serves as another exogenous trigger, is characterized by low absorption by natural tissues, ensuring excellent biocompatibility (Riley et al. 2018; Dariva et al. 2019).
Overall, polymeric NPs are ideal candidates for drug delivery because they are biodegradable, water-soluble, biocompatible, biomimetic, and stable during storage. These nanoparticles can be formulated to allow precise control of multiple features, making them effective delivery vehicles owing to their biocompatibility and straightforward formulation parameters.
The active pharmaceutical ingredients (APIs) should also be examined. At the beginning of this review study, we will define these three different LNPs. This review will focus on lipid nanoparticles for targeted drug administration since there have been so many outstanding research on liposomes.
Liposomes: the earliest generation of lipid nanoparticles
Alec D. Bangham (1921–2010) was a British biophysicist who made significant contributions to the field of liposome research. He is best known for his pioneering work on the development of liposomes as a drug delivery system. Bangham's early work on liposomes focused on understanding their physical properties and how they interact with cells. He discovered that liposomes could fuse with cell membranes, allowing them to deliver their contents into cells. This discovery had important implications for the development of liposomes as a drug delivery system (Dü 2005).
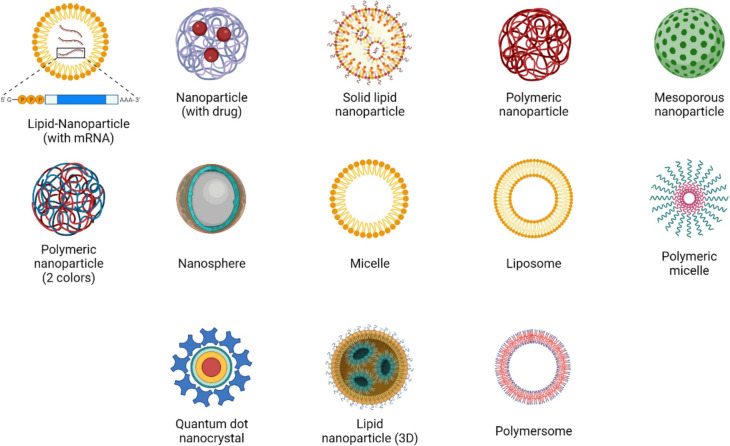
Lipid nanoparticles (LNPs) have grown in prominence as prospective medication delivery systems in the pharmaceutical industry. Currently, LNPs are used in a variety of industries, including those that deal with food and drink, cosmetics, medical imaging, and other cutting-edge domains like nanoreactors (Bi et al. 2023). Examples of hydrophilic and hydrophobic substances that may be carried by liposomes, an early kind of LNP, include proteins, small compounds, and nucleic acids. As a consequence, liposomes are a very flexible nanocarrier platform. Actually, the primary one nanomedicine delivery technology to cross the bridge from the lab to the clinic was liposomes. The use of several liposomal drugs in clinical settings has been approved and is effective. A lipophilic lipid bilayer is sandwiched between two hydrophilic lipid layers to form the spherical lipid-based vesicular structures known as liposomes. In the peer-reviewed scientific literature (Pinheiro et al. 2020; Soni et al. 2016; Liu et al. 2009; Hua et al. 2018; Chowdhury et al. 2017; Mateos-Maroto et al. 2023), the flexibility and benefits of liposomes as a therapeutic delivery vehicle for small compounds, peptides, genes, and monoclonal antibodies are well established and recognized. Liposomes, a kind of nanomedicine, are used to treat neurological problems, cancer, diabetes, and inflammation. Liposomes are essential in many different facets of healthcare systems. In contrast to oral medication administration, parenteral drug delivery has advantages, such as avoiding first-pass metabolism, reduced gastrointestinal permeability, and gastrointestinal side effects (Petrenko et al. 2023). Parenteral dosing also provides the opportunity for customized medication delivery, increasing bioavailability and decreasing unintended adverse effects. The use of liposomes for the parenteral administration of different chemicals and genes has been discussed extensively in numerous of studies (You et al. 2018) through (Pinedo and Smorenburg 2006) and (Liu et al. 2022) through (Bergers and Benjamin 2003). Since phospholipids are biodegradable, biocompatible, and similar to the lipids found in biological membranes, they are being carefully studied for both their potential for drug administration and their ability to organize the structure. Liposomes' ability to self-organize gives them a thermodynamic edge. They have been used to treat cancer patients, enhance tumor response, and lessen off-target effects in cancer therapy (examples: AmBisome®, Doxil®). Although the worldwide market for liposomal doxorubicin was estimated to be worth $814.6 million in 2015 alone, many patients may not be able to access them due to the high cost of these innovative medicines (Ferrara and Kerbel 2005). DaunoXome®, Myocet®, DepoCyt®, Marqibo®, and Onivyde® are only a few examples of the cancer nanomedicines based on lipid-based nanotechnology that have been commercialized. The first RNAi treatment FDA15 authorized, Onpattro®, was recently released. The nanomedicine industry has seen tremendous growth over the previous five years and is now thought of as a high-risk, high-reward venture. Khosravi-Darani (Smith and Petrenko 1997) and Mozafari (Pasqualini and Ruoslahti 1996) assert that biomembranes and cells are precisely replicated in liposomes. Due to their similarities to biological membranes, they are considered as a "ideal model" for studying the appearance, functionality, and evolution of early cell membranes (Chang et al. 2009; Katanasaka et al. 2010). Additionally, they are used as carrier systems in the food, drug, agricultural, and cosmetic industries. These materials include genetic material, pharmaceuticals, and nutraceuticals. The phospholipid molecules used in the vesicle structure of lipids make up the majority of these naturally occurring bilayers. The key property shared by molecules that form bilayers is amphiphilicity. It's also important to comprehend that not all phospholipid-based nanostructures are liposomes. There have also been reports of lipid or phospholipid molecules arranged in hexagonal, lamellar, micellar, or cubic phases to generate non-liposomal forms (Murase et al. 2010). The constantly sealed vesicular structures known as liposomes, on the other hand, are primarily composed of phospholipid bilayers in an aqueous media (Pasqualini and Ruoslahti 1996). Since liposomes were initially presented to the scientific community 35 years ago, significant advancements have been achieved in the engineering approaches used to enhance liposomal composition (Pasqualini et al. 2000). These innovations have led to an extended half-life of liposomes in circulation, the elimination of hazardous solvents from their production process, and deft cell and tissue targeting strategies (Pasqualini and Ruoslahti 1996). Compared to liposomes, nanoliposomes have a higher surface area, which may increase the bioavailability, solubility, and controlled release of the encapsulated contents while enabling more accurate targeting. Using safe components from natural sources, such as soy, milk, or egg, liposomes may be made (Oku et al. 2002). They could be granted the necessary permission for use in foods as a result of this. According to a recent research (Zhang et al. 2010a; He et al. 2010), even breast milk, the world's first natural meal, includes lipid vesicles. According to Sonali (Morisco et al. 2011), the phospholipid components of liposomes and liposomes have a number of advantageous impacts on human health, including memory enhancement and liver protection (Accardo et al. 2010; Falciani et al. 2011). The ability of lipid vesicles to be selectively accessed is very useful. In order to create a sufficient concentration of bioactive at the target site, it is crucial to transport bioactive molecules to the area where their action is required (Zhao et al. 2009). Liposomes may be utilized for the encapsulation, distribution, and release of lipid-soluble and amphiphilic chemicals, medicines, and biological components like peptides or genes since they include both hydrous phases and lipids in their structure. Because of these distinct characteristics, liposomes have a broad variety of applications in the food industry, the delivery of modern pharmaceuticals, and gene therapy (see Fig. 1).
Fig. 1.
Various lipid nanoparticles as liposomes
The advances in liposomal drug delivery formulations using doxorubicin and amphotericin B
Liposomal drug delivery formulations have emerged as a promising approach to enhance the therapeutic efficacy and reduce the toxicity of conventional drugs. Doxorubicin and amphotericin B are two widely used drugs that have been extensively studied in liposomal formulations.
Doxorubicin is a potent anticancer drug, but its use is limited by its dose-dependent cardiotoxicity. Liposomal formulations of doxorubicin have been shown to reduce cardiotoxicity while maintaining efficacy. For example, Doxil, a PEGylated liposomal doxorubicin, has been approved for the treatment of ovarian cancer, Kaposi's sarcoma, and multiple myeloma (Zhao et al. 2018).
Amphotericin B is a potent antifungal drug, but its use is limited by its severe side effects, including nephrotoxicity. Liposomal formulations of amphotericin B have been shown to reduce nephrotoxicity while maintaining efficacy. For example, AmBisome, a liposomal amphotericin B, has been approved for the treatment of severe systemic fungal infections (Edward and Goldman 1998).The development of liposomal drug delivery formulations has been a significant advance in the treatment of cancer and fungal infections. Liposomal formulations can improve the therapeutic index of drugs by reducing toxicity and increasing efficacy. As research continues, liposomal drug delivery formulations are likely to play an increasingly important role in the treatment of a wide range of diseases.
Here is a (Table 1) summarizing the key differences between liposomal doxorubicin and amphotericin B (Zhao et al. 2018).
| Feature | Liposomal doxorubicin | Liposomal amphotericin B |
|---|---|---|
| Disease | Cancer | Fungal infections |
| Mechanism of action | Intercalates DNA, inhibiting DNA replication and transcription | Disrupts fungal cell membranes |
| Toxicity | Cardiotoxicity | Nephrotoxicity |
| Benefits of liposomal formulation | Reduced cardiotoxicity, increased efficacy | Reduced nephrotoxicity, increased efficacy |
| Examples | Doxil, Daunoxome | AmBisome |
Table 1.
Lipid nanoparticles functionalized with peptide used for in vitro studies
| Peptide | Cancer type | Molecular target | Nanoparticle size(nm) | Encapsulant | References |
|---|---|---|---|---|---|
| Tumor Vasculature | |||||
| WIFPWIQL | Colon | BiP/GRP78 | 120 | DOX | Katanasaka et al. (2010) |
| NGR, APRPG, and GNGRG | Colon | Unsure, APN | 110–150 | DOX | Murase et al. (2010) |
| Tumor Cells | |||||
| FCFWKTCT | Lung, Breast, Pancreas | Somatostatin Receptor | 100 | DOX, 111 In | Zhang et al. (2010a), Helbok et al. (2012) |
| Pyr-HWSYGLRPG | Breast | LHRH Receptor | 120–150 | Mitoxantrone | He et al. (2010) |
| TAT (AYGRKKRRQRRR) | Colon, Liver | None | 100 | DiD, Calcein | Kuai et al. (2011), Kuai et al. (2010) |
| Pep-1 (KETWWETWWTEWSQPKKRKVC) | Breast, Skin | None | 160 | Au | Kang et al. 2011) |
| Poly-R, NGR (CYGGRGNG) | Skin | None, APN | 85–103 | Rhodamine | Takara et al. (2010) |
| DMPGTVLP | Breast | Unknown | 90–100 | DOX, Rhodamine | Wang et al. (2010a, b) |
| CSNIDARAC | Lung | Unknown | 200 | DOX, Cy7.5 | He et al. (2011) |
| And Tumor Vasculature Cells | |||||
| RGD peptides | Ovary, Breast, Sarcoma | α v Integrins | 90–250 | Paclitaxel, DOX, CA-4, Qd, Gd | Zhao et al. (2009), Jiang et al. (2010), Du et al. (2011) Mulder et al. (2009) |
| Cyclic RGD peptides | Skin, Breast, Colon, Lung | α v Integrins | 35–100 | Matrine, Gd, DiD |
Jiang et al. (2010) |
| ARYCRGDCFDATWLPPR | Lung | α v Integrins, Neuropilin-1 | 65–75 | Paclitaxel | Meng et al. (2010) |
| LARLLT | Lung | EGF Receptor | 110–150 | Rhodamine, Cy5.5 | Song et al. (2009) |
Liposome's: trap and transport medications
Taxane liposomes have demonstrated a prolonged elimination time, increased antitumor efficacy against various murine and human tumors, and reduced systemic toxicity compared to Taxol® (Manshian et al. 2017). Additionally, they exhibit antitumor effects in Taxol-resistant tumor models (Lunnoo et al. 2019). Abraxane®, the sole non-liposomal paclitaxel (PTX) preparation (albumin nanoparticle-based PTX preparation), and Lipusu® (a liposomal PTX approved by the State FDA of China) have entered the realm of clinical applications. LEP-ETU (NeoPharm) and EndoTAG®-1 (Medigene) have advanced to phase II in clinical trials. In general, liposomes and protein nanoparticles offer a promising avenue for optimizing PTX delivery, with their commercialization poised to enter the modern drug delivery market. Lipid nanoparticles (LNPs) have been utilized for co-delivering siRNA and mRNA (Ball et al. 2018), as well as delivering therapeutic mRNA in various in vivo disease models (Nabhan et al. 2016; An et al. 2017), including clinical trials focused on immunogenicity for protection against Zika and influenza viruses (Richner et al. 2017; Bahl et al. 2017). Notably, recent clinical trials have investigated the application of naked VEGF-A mRNA in a citrate saline solution, without LNPs as RNA carriers, in patients with type 2 diabetes (Gan et al. 2019) and individuals undergoing coronary artery bypass grafting (CABG) (Anttila et al. 2020, 2022; Collén et al. 2022). Given the advancement of LNP-based mRNA therapeutics in human clinical trials, the study data also suggests a similar process may occur in humans. Specifically, there is an indication that a portion of LNP-mRNA distribution between cells or organs might occur via extracellular vesicles (EVs), emphasizing the need for further investigations.
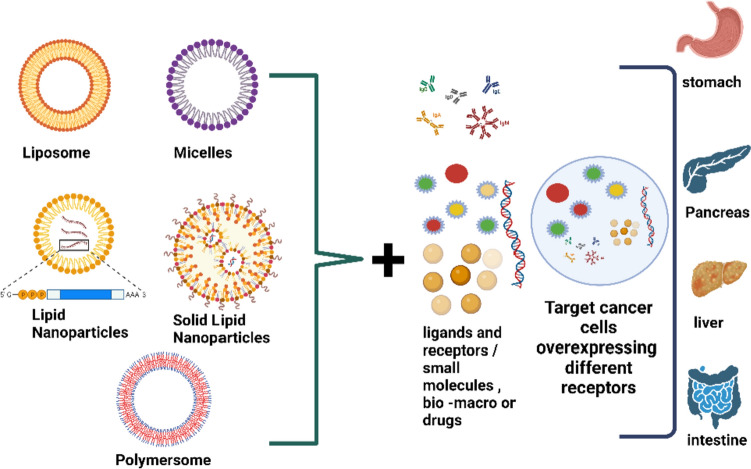
Ligands and receptors tested as LNP: targeting agents in cancer therapies
Nanocarriers are nanoparticles that encapsulate pharmaceuticals. A few of the various materials that have been developed for nanocarriers include lipids (liposomes), bio-compatible polymers (like polymeric nanoparticles), and surfactants (micelles) (Zhang et al. 2010b; Jiang et al. 2010; Du et al. 2011; Chen et al. 2017; Moody and Korman 1988; Tu et al. 2020; Cao et al. 2023). According to studies by Bonacucina (Loi et al. 2013) and Mishra (Negussie et al. 2010), drugs may be dissolved in hydrophilic or hydrophobic components, enclosed in vesicles, or integrated in matrixes. Self-assembling vesicles known as liposomes include an aqueous compartment within lipid bilayers (Apolinario et al. 2021). According to Torchilin (Du et al. 2011) and Abu Lila (Helbok et al. 2012), lipophilic medicines are soluble in the lipid bilayer whereas hydrophilic pharmaceuticals may be easily contained in the aqueous core. Since the middle of 1990s, the FDA has approved the use of liposomes that include chemotherapy medications including doxorubicin (Doxil) and daunorubicin (DaunoXome). Micelles are self-assembling nano-aggregates formed by surfactants, such as surfactant-based amphiphilic block copolymers (5–50 nm). Clinical studies have used two different doxorubicin micelle formulations, SP1049C (which includes pluronics) and NK911 (which comprises a polyethylene glycol-poly (aspartic acid) block copolymer). The branching macromolecules that make up dendrimers have a high degree of molecular regularity and negligible polydispersity. Dendrimers may trap hydrophobic drugs, assisting in the solubilization process. The surfaces of dendrimers may potentially chemically conjugate medicines, according to Duncan and Izzo (Kuai et al. 2011) and Nanjwade (Kuai et al. 2010). Drugs may either be chemically or physically conjugated to polymeric nanoparticles by interacting with or forging a covalent link with the polymer. Polymeric nanoparticles are most often nanoscale polymeric matrix matrices. A number of polymeric macromolecular conjugates, including Oncaspar (PEGL asparaginase), PEG-INTRON (PEG -a-interferon 2b), and Zinostatin (Styrene Maleic Anhydride), have been given clinical clearance for the treatment of a number of malignancies as an alternative to polymeric nanocarriers. The target receptor could have tissue- or tumor-specific characteristics. Receptors that are more frequent in the vascular system in connection with malignancies are also included in the list of tumor-specific ligands. It might not be very feasible to identify specific receptors that are solely expressed on tumor cells and not on normal ones. Galactose derivatives target the asialoglycoprotein receptor (ASGPR), rituximab (Kang et al. 2011) targets CD20 (expressed on normal and malignant B cells), and transferrin (Tf) targets the Tf receptor. The degree of receptor expression on tumors, ability to internalize and rate, binding affinity of the receptor, size of the ligand, immunogenicity of the substance. The transport efficiency of nanocarriers with internalizing antibodies is typically greater when compared to those without internalizing antibodies. In contrast, a non-internalizing antibody solely binds to the cell's surface, which may be advantageous for boosting collateral effects and enabling immunological processes like antibody-dependent cellular cytotoxicity (ADCC) (Takara et al. 2010). Some examples of possible targeting ligands include transferrin, folate, antibodies, and peptides. The folate receptor (FR) is not expressed in the majority of normal tissues but is often elevated in a variety of human cancers, including ovarian, colorectal, and breast cancers (Wang et al. 2010a, b). FR is a very accurate tumor marker as a result. Folic acid has been shown to promote the transport of certain anticancer drugs to tumors via polymers or liposomes. Like other small molecule ligands, folic acid benefits from being reasonably priced, non-immunogenic, and having simple conjugation chemistry. Many tumor cells have extremely high levels of the transferrin receptor (TfR) because they need more iron. Tf or anti-TfR antibodies or antibody fragments may be chemically bonded to nanocarriers to aid TfR-mediated dispersion. To make unique nanocarriers, Fab and scFv antibody fragments may also be employed in lieu of entire antibodies (Allen 2002). Immunogenicity, high cost, and the hefty mass of the ligands are all potential drawbacks. Peptides have attracted a lot of interest as ligand-targeting agents due to their advantages over antibodies, including being smaller, less immunogenic, more stable, and simpler to make. The arginine–glycine–aspartic acid (RGD) peptide is being used as a targeting ligand for tumor vasculature because it interacts with the avb3 integrin receptor. Avb3 integrin is widely expressed in the tumor vasculature as well as in a variety of metastatic cancer cells, according to Ruoslah (He et al. 2011) and Desgrosellier and Cheresh (Lowery et al. 2011). Using modern screening techniques like phage display (Mulder et al. 2009; Li et al. 2011), new peptides with strong binding affinities and specificities for certain cells, tissues, and organs have been found.
Solid lipid nanoparticles and nano-structured lipid carriers
Solid lipid nanoparticles (SLNs) and nano-structured lipid carriers (NLCs) have been suggested as potential marketable and alluring substitutes because of their all-natural composition (Goutayer et al. 2010). Since 1990, SLNs and NLCs have been recognized as acceptable carriers in substitute of liposomes, emulsions, and polymeric nanoparticles (Meng et al. 2010). These spherical LNPs have a size range of 40–1000 nm (Song et al. 2009). Surfactants and solid phase lipids make up their composition (McClements 2021). The emulsifier is a surfactant, while the dispersed phase is a solid lipid. The lipid components of SLNs are solid at body and ambient temperatures (Mai et al. 2009; Wang et al. 2022) and can exist as waxes, very pure triglycerides, complex glyceride mixtures, or even complex glyceride combinations. Surfactants are used to increase stability when they are present in concentrations of between 0.5 and 5%. The wise choice of lipids and surfactants, among other physicochemical features and traits, may have an impact on particle size and drug loading (Yan et al. 2011, 2012). They are safer than polymeric carriers since no organic solvents are used in their production, and they are more effective than liposomes in terms of drug stability and prolonged release. They are unaffected by large-scale manufacture as well. Despite the fact that the solid lipid has a crystalline structure, SLNs usually show erratic gelation tendencies and have poor integration rates (Park et al. 2010; Grange et al. 2010). In order to address possible issues with SLNs, NLCs were created as the following wave of SLNs in the late 1990s. NLCs enhance pharmaceutical release, stability, and capacity loading while in storage. It may be identified from SLNs by looking at the rigid matrix's structure. When NLCs are at room temperature, both liquid and solid lipids can be found there (Moos and Morgan 2000). The three categories of NLCs examined in the paper are formless, imperfect, and numerous types, which are discussed in the section that follows (Li et al. 2016). Some of the procedures used to make LNPs include high-pressure homogenization (HPH), solvent emulsification/evaporation, supercritical fluid extraction of emulsions (SFEE), ultrasonication or high-speed homogenization (Kuo and Chou 2014; Kuo and Wang 2014; Neves et al. 2016), and spray-drying. There are two different HPH procedures: cold and hot. The medication gets dissolved or solubilized in the lipid and melted at a temperature that is 5–10 °C over its melting point in these two fundamental production processes. SLNs and NLCs are advantageous for parenteral, cutaneous, pulmonary, and topical drug administration due to a remarkable spectrum of properties. These products were created to lessen the negative effects of the potent medications they contain while increasing the therapeutic efficiency. In the fields of food, cosmetics, and gene transfer, they have also demonstrated a great deal of promise. Due to the limitations and difficulties mentioned above, there are still a finite number of items available on the market. The shortcomings of traditional colloidal carriers, such as emulsions, liposomes, and polymeric nanoparticles, have been resolved by the development of SLNs. These carriers provide benefits such a favorable release profile, targeted drug administration, and great physical stability. Next-generation lipid nanoparticles (NGLNPs) are SLNs that have undergone modifications to improve stability and capacity loading. These LNPs can be applied in therapeutic treatment, research, and the provision of healthcare.
Functional modifications of lipid nanoparticles
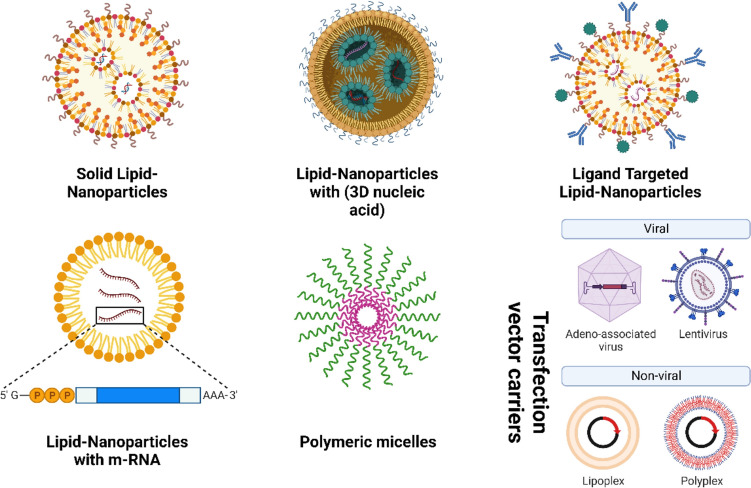
Unaltered LNP drug delivery systems have substantial disadvantages despite their advantages, such as the necessity for target selectivity, a short blood circulation period, and variable in vivo efficacy. The development of improved LNP formulations addresses each of these problems (see Fig. 2).
Fig. 2.
Functional modifications of lipid nanoparticle
Targeted liposomes
For the purpose of locating and attaching to certain cell receptors (such as the folate receptor), targeted liposomes with ligands on the surface have been created. To create targeted liposomes, small molecule ligands, peptides, or monoclonal antibodies are frequently added to the surface of LNPs.
Peptides or monoclonal antibodies
Using nicotinic cholinergic receptors to improve neuronal absorption, these nanosystems made of lipid nanoparticles (LNPs) had been integrated with RVG29 peptide, a 29-amino-acid cell-penetrating peptide (Pinheiro et al. 2020). These RVG29 lipid nanoparticles were demonstrated to be an effective way to release flavonoids and an intriguing approach for prospective therapies of Alzheimer's disease since they simultaneously target the blood–brain barrier and promote neuronal protection (Pinheiro et al. 2020). Nanoparticles are the sole delivery systems in the brain, and they may be functionalized with certain ligands to target particular cells or react to stimuli at the area of target (Soni et al. 2016; Chowdhury et al. 2017). One element that has the ability to connect to these receptors is the RVG29 peptide (Liu et al. 2009; Hua et al. 2018). For brain delivery, a variety of methods including lipid nanoparticles functionalized with RVG29 have been proposed (You et al. 2018; Oswald et al. 2017; Pearce et al. 2012). Nevertheless, no methods for delivering quercetin to the brain were identified despite the active RVG29 targeting. To take advantage of quercetin's neuroprotective capabilities, it is advantageous to use the created lipid nanoparticles functionalized with RVG29 peptide and stocked with quercetin. A potential outcome was seen with the RVG29 nanoparticles, which at the time were thought to have great promise for treating Alzheimer's disease. The RVG29 peptide's functionalization of the nanoparticles was proven by infrared spectroscopy. The N–H stretching and bending vibrations (3302 cm−1, 1558 cm−1) and C=O stretching vibrations (1660 cm−1) that define peptide interactions with amino acids may be found as bands in the RVG29 peptide spectra. RVG29-functionalized lipid nanoparticles exhibit these bands in their FTIR spectra as well, demonstrating that they were effectively created (Hua et al. 2018). The total cytotoxicity performance for all types of RVG29-nanoparticles is less than 16%, even at the maximum concentration assessed. Furthermore, the dosage used in the permeability studies had no discernible negative effects, indicating that this concentration range is safe to use and that higher doses might be used to increase quercetin's positive effects on the brain. The noteworthy outcomes also demonstrated the ability of all the nanoparticles (LNPs) modified with RVG29 and loaded with quercetin to prevent the aggregation of amyloid-beta. This implies that these nanosystems have the capacity to improve permeability in order to prevent amyloid-beta fibrillation, underlining their enormous potential as a possible nursing for Alzheimer's disease in future (Liu et al. 2009; Chowdhury et al. 2017). Doxil and Doxorubicin, two clinically significant anti-cancer drugs, are often used as treatments whose pharmacokinetic and pharmacodynamic characteristics have been effectively adjusted by embedding them in lipid nanoparticles (Pinedo and Smorenburg 2006). To effectively treat cancer, these the subsequent generations nanoparticles must overcome a number of challenges. They must travel via the mononuclear phagocyte system (MPS) before passively collecting in the tumor by the EPR effect. Then, they need to penetrate the cancer cell membrane while still within the tumor tissue. In order for the drugs, they have collected to have the greatest effect on the cancer cells, they must finally ensure that they reach those cells. Careful examination of these difficulties is helpful when discussing nanoparticle-mediated drug delivery, and it requires more explanation (Pinedo and Smorenburg 2006). Due of the first-generation lipid nanoparticles' effectiveness in the clinic, lipids are a common ingredient for the production of second-generation targeted nanoparticles. Peptides have become the most popular targeting ligands due to their high affinity for a variety of different cellular targets, ability to be fabricate at a comparatively inexpensively and highly reliability, and ability to connect to nanoparticles missing losing their affinity for binding (Liu et al. 2022). Many lipid-based nanoparticle forms that have arisen, such as liposomes, nanocapsules, lipid polymer hybrid nanoparticles, nanoemulsions, and solid lipid nanoparticles, are shown in the figure (Fig. 3).
Fig. 3.
Lipid-based nanoparticle for Target delivery
Lipid nanoparticles with peptide activity have been extensively developed for cancer treatment but have not yet transitioned from animal research to clinical application. In order to forward the discussion of a prospective strategy that may result in further advancement in the treatment of cancer, we largely concentrate on the methods that solve the three problems mentioned previously. Further investigators are developing novel peptide-functionalized lipid nanoparticles and targeted nanoparticles using non-lipid components including polymers, carbon nanotubes, and silica in addition to other non-peptide targeting ligands such antibodies and proteins (Pangburn et al. 2009; Blanco et al. 2011). NPs that bind to peptides and target the blood arteries in the tumor anti-vascular therapy may be able to stop tumor growth and make it go dormant, according to current studies on the function of tumor vasculature in the genesis and progression of solid tumors (Bergers and Benjamin 2003). These new vasculatures are used by cancer cells to grow and metastasize as the illness progresses. Fast angiogenesis is essential for cancer cells to get the nutrients and oxygen they need for fast growth. The clinical success of anti-vascular therapies in restricting blood supply to the tumor, depriving it of the nutrients needed for rapid spread, and blocking a route for metastasis is what drove the development of vascular-targeted nanoparticles as anti-vascular agent routes of administration to tumor-associated endothelial cells (Ferrara and Kerbel 2005). Virus displays a common technique for locating and isolating peptides associated to proteins produced by tumor-related endothelial cells is called "biopanning." (Smith and Petrenko 1997; Pasqualini and Ruoslahti 1996). When phage display biopanning is used on complex systems like cancer cells and tissues, the bulk of the molecules to which the separated peptides attach is unknown. The peptides PIVO-24 (YPHYSLPGSSTL) and PIVO-8 (SNPFSKPYGLTV) were found to bind not only to the oral cancer tumor mass but also to tumor specimens taken from human breast, liver, lung, pancreas, and colon tumors during the study of in vivo phage presentation biopanning targeted at human oral cancer xenografts in mice. Additionally, in vitro research has shown that the peptides PIVO-24 and PIVO-8 may facilitate receptor-mediated endocytosis in endothelial cells, which allows the nanoparticles to pass through their membranes and aggregate inside the cells (Chang et al. 2009). When correlated to non-targeted PEGylated liposomes, in vivo experiments with the PEGylated peptide-functionalized liposomes unveiled about a two-fold accumulation in subcutaneous murine lung cancer tumors of mice.
The tumor also included non-targeted liposomes, but in considerably lower amounts. The instances of PIVO-8 and PIVO-24 functionalized liposomes were considerably reduced when administered to mice with lung, colon (HCT116), liver (Mahlavu), and pancreatic (H460 PaCa-2) cancers. Tumor volume and angiogenesis decreased across all tumor types, demonstrating the improved and adaptable therapeutic credibility attained by clearly focusing on both the tumor vasculature and tumor cells straight away.
Liposomes functionalized with HSPA5 targeting peptides (WIFPWIQL) accumulated four times more in VEGF-stimulated endothelial cells than in DU145 prostate cells or C26 colon cancer cells, proving their potential to efficiently and selectively target neovascular cells (Katanasaka et al. 2010). When contrasted with animals that received non-targeted liposomes, the tumor volume in the animals receiving the doxorubicin-containing peptide-functionalized liposomes had decreased by around 58% after 26 days (Katanasaka et al. 2010). Liposomes that have been functionalized with APRPG NGR GNGRG are a different target. These peptides have the power to target a variety of compounds found in endothelial cells that are linked to cancer (Murase et al. 2010). The NGR peptide has been identified as a target by the membrane-associated enzyme ERAP1 N(APN), a cell surface protein assumed to be involved in chemokine release and tumor invasion (Staring et al. 2018). Nevertheless, the molecular target of the APRPG peptide remains unknown.
According to in vivo research, dual targeting liposomes are able to significantly reduce drug accretion in the friable in relation to single-targeting liposomes and non-targeting liposomes, even if they do not demonstrate any difference in drug accretion in the kidneys, lungs, heart, or liver. In contrast to single liposomes and non-targeting liposomes, the twin-targeting liposomes did not promote tumor accumulation when connected. It was intriguing to see the twin targeting liposomes and, to a lesser extent, the single targeting liposome in tumor tissue that was excised four hours after liposome injections. The angiogenic arteries only absorbed the targeted liposomes; they did not do so with the non-targeted liposomes. The dual-targeting liposomes showed the most anti-tumor impact among the three liposomal formulations, despite there being no variations in total drug concentration in the tumor.
SK-OV-3, a human ovarian cancer cell line using epithelial-like morphology, is one example of a cancer cell that may be addressed using liposomes functioning with RGD peptides. A pharmacological contrast substance targeting MCF7/A human breast cancer, S-180 murine sarcoma, B16F10 ATCC® CCL-6475TM is a murine melanoma cell line generated from a C57BL/6 J mouse murine melanoma (Zhao et al. 2009; Zhang et al. 2010b; Jiang et al. 2010; Du et al. 2011). It is interesting to note that such a pharmacological combination is more effective when delivered in non-targeted liposomes than when delivered in peptide-functionalized liposomes with just DOX or CA-4 (Zhang et al. 2010a).
Surprisingly, targeted nanoparticles demonstrated a considerably greater distribution efficiency toward vitronectin-expressing cells in avb3 and were far more lethal than managing liposomes. When utilized to treat gliomas, co-functionalized doxorubicin-loaded liposomes containing c(RGDfK) and peptide-22 were shown to increase tumor localization in vivo with a decrease in IC50 in vitro (Chen et al. 2017). The Gastrin-releasing Peptide Receptor (GRPR), also known as BB2, is significantly overexpressed in a range of cancers, including gliomas, pancreatic cancer, and lung cancer. This inherent ligand binds to this G protein-coupled receptor. Despite the large number of peptides discovered for the GRPR receptor, such as 105 GB-6,105, AN-215,106, and BBN7-14, there are few studies on the functionalization of liposomes for the limited delivery of drugs to this receptor. To prepare liposomes, C-amino acid statin (Sta), the D-enantiomer of the GRPR antagonist peptide, was combined with DSPE-PEG2000 lipids. A549 cells with enhanced GRPR in vitro demonstrated improved nanoparticle localization. The lipid nanoparticles functionalized with peptides are listed in Table 1.
In today’s fast-paced world, there is an urgent need for early detection and diagnosis of diseases. This important requirement has led to the development of advancements in medical imaging and diagnosis. Current challenges in this field are precision and sensitivity. These challenges are met by advanced applications in medical imaging and diagnostics, such as.
Artificial Intelligence: AI algorithms can analyze medical images, such as X-rays, CT scans, and MRIs, to identify and classify tumors with high accuracy. This can help doctors diagnose cancer at an early stage, when treatment is most effective.
Nanoparticles: Nanoparticles can be used to deliver drugs directly to diseased tissues, without affecting healthy cells. This can improve the effectiveness of chemotherapy and other cancer treatments while reducing side effects.
3D printing: 3D-printed surgical guides can be used to help surgeons plan and perform complex surgeries more accurately. These guides can be customized to the patient's individual anatomy, reducing the risk of complications.
Virtual reality (VR): VR simulations can be used to train surgeons and other medical professionals in a safe and realistic environment. This can help them improve their skills and reduce the risk of errors during real-life procedures.
Augmented reality (AR): AR overlays medical images onto the patient's body, helping surgeons see tumors and other abnormalities more clearly during surgery. This can improve the accuracy of procedures and reduce the risk of complications.
The above listed advancements nanoparticles have emerged as promising tools in biomedical imaging, offering enhanced sensitivity, specificity, and spatiotemporal resolution compared to conventional imaging agents. Their unique properties, including their small size, tunable surface chemistry, and ability to encapsulate various imaging probes, make them versatile platforms for diverse imaging modalities.
In the context of fluorescence imaging, nanoparticles provide bright and stable emission signals, enabling real-time monitoring of cellular processes and targeted drug delivery. Magnetic nanoparticles, on the other hand, are widely employed in magnetic resonance imaging (MRI) due to their ability to generate contrast signals and enhance image resolution. Additionally, nanoparticles can be functionalized with radionuclides for positron emission tomography (PET) and single-photon emission computed tomography (SPECT), facilitating molecular imaging and disease diagnosis.
Furthermore, nanoparticles can be engineered to combine imaging and therapeutic capabilities, resulting in theranostic agents. These multifunctional nanoparticles simultaneously enable disease detection and drug delivery, offering a promising approach for personalized medicine.
Overall, nanoparticles have revolutionized the field of biomedical imaging, providing valuable tools for early disease diagnosis, drug development, and personalized treatment strategies. Their continued development holds immense potential for advancing medical imaging and improving patient care (Han et al. 2019).
Specific receptors
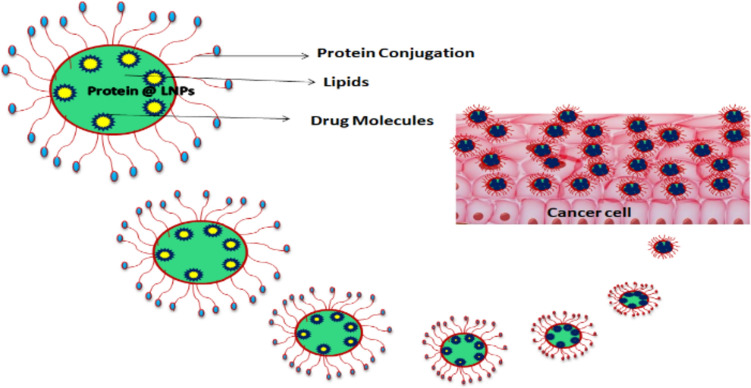
Lipid nanoparticles can be an imperative approach to overcome the following problem of drugs that is increasing the compound's bioavailability and ability to target certain organs and tissues. One of the many pathways presently available to transport medications into the brain involves the transferrin receptor. Liver, spleen, bone marrow, and blood–brain barrier (BBB) cells are among the tissues in which they are highly expressed (Moos and Morgan 2000; Li et al. 2016; Kuo and Wang 2014). Study that delivered a nerve growth factor to several tissues in vitro using liposomes functionalized with transferrin (Kuo and Chou 2014). However, no published strategies for utilizing transferrin-functionalized nanoparticles to transport quercetin to specific tissues and organs were found (Fig. 4).
Fig. 4.
Conjugation drug molecules to an Equilibrated Nanoparticle Protein Enables cancer Cell Targeting
Since they have been used for a variety of medications, lipid nanoparticles are a helpful alternative for drug delivery systems (Neves et al. 2016; Han et al. 2019; Loureiro et al. 2017). Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) are the two basic categories into which these nanoparticles can be categorized (Frias et al. 2016).
At typical room and body temperatures, SLN creates an environment with more cavities than NLC, which is entirely composed of liquid and solid lipids. This improves consistency and loading capacity and prevents drug exclusion during storage (Neves et al. 2013). The lipids utilized to make these nanoparticles are perfect for biology-related uses and precise targeting since they are biodegradable, biocompatible, and well-tolerated by living things (Naseri et al. 2015).
Drugs are protected from proteolytic breakdown by solid lipid nanoparticles (SLNs), which also enable precise intracellular targeting. Theoretically, the drug that was trapped is picked up by a unique uptake process known as "nanocitose" and penetrates target cells, in contrast to similar efflux-transporters like P-gp. In MDA-MB-436 cells, the effectiveness of P-gp in lowering the permeability of the anticancer medicines was investigated. The experimentally confirmed enhancement in the pharmacological effectiveness of the medicine incorporated in SLN as compared to the free drug is evidence that the system safeguards the medication.
The normal dispersion of sensitized antibodies is prevented by hydrostatic pressure, a tumor, and a damaged vascular. For ligand–SLN systems, however, simple diffusion with improved penetration into the tumor partition has been shown (Naseri et al. 2015). A crucial biological process known as multidrug resistance (MDR) relies on the P-gp of the plasma membrane functioning correctly. Combination anti-CD44v6 enhanced the targeting of CD44 + cancer cells, reduced P-gp expression, and safeguarded the medication against cell efflux events. However, compared to SLN drug molecules, the target moiety did not boost pharmacological efficacy, showing that receptor-mediated endocytosis has its drawbacks. The negative clinical outcomes associated with MDR activities of malignancies, which mostly involve P-gp hyperfunction and often arise in late stages (Mudshinge et al. 2011), may be combated by more potent nano-integrated therapeutic agents.
Numerous tactics have been employed used to improve target medicine distribution. In this instance, hCMEC/D3 cell monolayer testing was conducted using solid lipid nanoparticles (SLNs) functionalized with APOE-apolipoprotein E. These nanosystems are excellent for target delivery because to their stated length of 160 nm, a negative charge of -14 mV, and unique lipophilicity. Since clathrin-mediated endocytosis, the preferred internalization mechanism in charge of cellular uptake, was hampered, the nanoparticles mainly utilized the transcellular route to cross cell walls. For targeted medication delivery, taking into account the procedures involved in moving these nanosystems across blood–brain cells may be useful (Cavaco et al. 2017).
Lipid nanoparticles functionalization with folate
Lipid composition coupled with nucleic acid/lipid nanoparticle production using microfluidics enhances control over the self-association process. We expanded our research to include mNALPs functionalized with folate (FolA) ligands in order to more precisely examine the absorption of mNALPs. The folate receptor (FR), which is preferentially taken up by functionalized nanoparticles, is markedly overexpressed in many human malignancies (Neves et al. 2017). mNALPs functionalized with the folate receptor (FolA-mNALPs) were created by substituting folate-conjugated DSPE-PEG (2000) for 18.4% of the lipid formulation previously used. Fluorescence correlation spectroscopy measurements show that FolA-mNALPs are identical to non-functionalized mNALPs in terms of size and sample class. The stability of FolA-mNALP in plasma and blood serum has also been studied. FCS experiments were carried out in buffers containing progressively higher volume fractions of serum and plasma to PBS, ranging from 20 to 80% (v/v) and from 30 to 90% (v/v), respectively. FolA-mNALPs were amplified ten times in serum/PBS or plasma/PBS and then diluted to the Cy3-dsDNA initial concentration of cDNA = 45 nM. Separately, it was shown that sample volume decrease (solvent evaporation) up to a 36-fold concentration did not result in mNALP aggregation or other material losses. The stability of FolA-mNALPs was determined by comparing the FCS diffusion time in plasma/PBS and serum/PBS to the Cy3-dsDNA diffusion time over time in the same medium. This idea illustrates how reasonable lipid composition suggestions combined with microfluidic synthesis of nucleic acid/lipid nanoparticles increase control over the self-congregation process. The majority of nanoparticles typically include a single 21 bp dsDNA or siRNA molecule wrapped in a single, very curved lipid bilayer in order to maximize their physicochemical features. When adopting the solvent exchange technique, mNALPs produced by hydrodynamic focusing on microfluidic chips show signs of improved colloidal properties. In particular, they exhibit a 20% improvement in entrapment efficiency, a much smaller size division, a negligible number of big aggregates. The mechanical and scalable manufacture of nanoparticles using the continuous-flow microfluidic approach is possible without compromising product quality. The quantity of siRNA material that is entrapped every hour increases predictably by an N-fold factor when the channels are parallelized, where N is the sum of all the channels in the flow. Small and sparsely distributed mNALPs are necessary for possible therapeutic uses in the therapies of solid malignancies.
Functionalized with small molecule ligand for enhanced intracellular activity LNP formulation of siRNA
The therapeutic method of silencing disease-related genes using short interfering RNA (siRNA) is substantial and promising (Paulos et al. 2004; Davidson and McCray 2011; Dorsett and Tuschl 2004). On the other hand, to fully realize the promise of siRNA therapy, sophisticated ways to deliver siRNA into target cells in vivo must be developed. This is due to the fact that "naked" siRNA molecules degrade rapidly in biological fluids, aggregate in target tissues, and disintegrate rapidly in biological fluids, making it impossible for them to translocate across target cell membranes to function intracellularly.
Lipid nanoparticle (LNP) siRNA formulations have a good chance of solving these problems with siRNA dispersal to hepatocytes after intravenous (i.v.) administration (Fig. 5). The newly invented cationic lipid component of LNP siRNA systems efficiently silenced genes in hepatocytes in animal models at dosage levels as low as 0.08 g siRNA/kg body weight (Pecot et al. 2011).
Fig. 5.

Intracellular activity of LNP formulation of siRNA
Apolipoprotein E (ApoE), which is found in the LNP, facilitates uptake into hepatocytes through the LDL and forager receptors on hepatocytes. Furthermore, ApoE renders mice unconscious due to its restricted capacity to silence genes. The requirement for Apo E highlights the crucial role of an element on the surface of the LNP that tracks and initiates uptake into target cells and suggests that minimal gene silencing activity is to be expected unless an LNP is capable of linking a serum protein that fosters ingestion into the cell that is relevant or has ligands on the LNP surface that facilitate uptake. Because of this, an intriguing possibility emerges. When a specific siRNA enters the cell cytoplasm, it is precisely engineered to silence a single gene, thus more absorption into tissue that is not the target, which might not articulate that gene, may have only a minimal effect (Semple et al. 2010; Yan et al. 2005; Akinc et al. 2010).
Target ligands, including antibodies, peptides, and antibody fragments, are directed toward certain cell surface receptors to drive liposomes to particular cells (Sakurai et al. 2023; Sapra and Allen 2003; Cressman et al. 2009; Paolo et al. 2011a, b). Condensed immunogenicity and much enhanced simplicity of ligand manufacture and compounding into LNP are two important potential benefits of small molecule targeting ligands trapped in lipid anchors in LNP. The greatest example, anisamide, has been shown to improve LNP transport to prostate and lung cancer cells that overstate sigma receptors, a PEG-lipid that may easily become LNP by joining an anisamide (Anthiya et al. 2023; Banerjee et al. 2004a; Li and Huang 2006; Chen et al. 2009). It has been discovered that a subclass of tiny substances called cardiovascular glycosides makes it easier for different cell types to absorb LNP. In vitro studies have demonstrated that the absorption and gene silencing of LNP siRNA systems may be improved by the insertion of a cardiac glycoside (strophanthidin) via a PEG-lipid bridge. Additionally, it has been shown that the ubiquitous Na + /K + ATPase cell surface receptor must be expressed for this weak LNP system to be taken up. Target identification and therapeutic applications may benefit from the effectiveness of small molecule Cellomics screening for target ligands discovery and the possibility of strophanthidin-focused LNP siRNA systems.
It demonstrates the outstanding efficacy of cutting-edge screening techniques for identifying compounds that enhance LNP absorption into target cells. Since it is familiar with the protein target, binding affinities, and relationships between structure and activity, it is attentively following our screening work on small compounds that are well-known medications. Further testing is necessarily necessary since many substances that increase absorption may also interact with intracellular receptors, making them unsuitable for use as extracellular targeting ligands. Since it is widely known that cardiac glycosides bind to an extracellular region of the Na + /K + ATPase, which is ubiquitously articulated on all mammalian cells, secondary screening was easy in this situation (Li et al. 2008; Paula et al. 2005).
Cancer-targeted nucleic acid delivery and quantum dot imaging using receptor aptamer conjugator lipid nanoparticles
Due to their high loading capacity and versatility in adjusting their physical, chemical, and biological character, liposomal delivery approaches have attracted extensive research as carriers for a variety of therapies (Akbarzadeh et al. 2013). In contrast to other transportable vehicles, liposomes do have certain benefits and drawbacks. There are many quantum dots. A measurable sum of fluorescence photons may be found at the target sites when the lipid component, targeting ligands, vesicle size, surface charge, and other parameters are precisely optimized.
It is believed that nanomedicines that can deliver treatments to a predetermined illness site and track the progression of the disease combine therapeutics and diagnostics. Integrative research in this area, which uses theranostic to detect and cure cancer, has grown significantly in recent years. The use of a variety of materials that were previously employed in nanomedicine in clinical and research settings is now authorized (Banerjee et al. 2004b). On the other hand, it is still important in nanomedicine to integrate a variety of potential materials into a multifunctional stand.
In this study, two distinct payload molecules—siRNA and Q-dots—are investigated in liposomal formulations. The PEGylation of the vesicles for longer transmission in the blood was effectively performed by avoiding the reticulo-endothelial system (RES) (Peer et al. 2007). Additionally, to enable the vehicle to target tumors, aptamer molecules attached to the liposomal surface were aligned with the EGF receptor. Aptamers, which are nucleic acid molecules with great specificity and resemblance to their necessary target proteins, have previously been shown to have uniqueness like that of antibodies, although being somewhat smaller and less immunogenic (Jokerst et al. 2011). Aptamers are impressive in the diagnostic and therapeutic domains due to all of their functional characteristics (Ni et al. 2011; Hong et al. 2011; Jayasena 1999).
In this study, tumor-targeted liposomes with Q-dots and siRNA molecules were combined with anti-EGFR aptamers. In mouse tumor xenografts, theranostic liposomes will be examined using imaging and cancer-targeted siRNA transfection. Theranostic liposomal system for the administration of diagnostic and therapeutic siRNA and Q-dots is provided as a consequence of this study's emphasis on the importance of an innovative theranostic delivery system for the treatment of cancer (Hong et al. 2011; Jayasena 1999).
Solid lipid nanoparticles as a vehicle for brain-targeted drug delivery: (two new strategies of functionalization with apolipoprotein E)
Only tiny, lipid-soluble particles with masses of around 450 Daltons may pass across the BBB by passive diffusion. The central nervous system (CNS) can presently only be reached by fewer than 2% of all potentially effective medication options (Lassalle et al. 2012). Blending solid fat nanoparticles with apolipoprotein E produced a unique route for brain penetration since endothelial cells along the blood–brain barrier express low-density lipoprotein receptors, aims to strengthen the bond. Apolipoprotein E was used to successfully create solid lipid nanoparticles in two different methods. Solid lipid nanoparticle (SLN)-based drug delivery to the brain is known to be highly effective. These spherical particles are made of byproducts that are biocompatible and biodegradable and built of solid lipids with melting temperatures exceeding body temperature; they stay solid after delivery (Pardridge 2002). SLNs are naturally able to cross the blood–brain barrier because of their tiny size and lipid-borne (lipolysis) makeup. In brain endothelial cells, they may also readily evade the P-GB excretion process (particularly when coated with polysorbate 80). Their lowest cytotoxicity has been demonstrated in vitro (Blasi et al. 2007; Muller et al. 1997; Chattopadhyay et al. 2008). Giving polyethylene glycol (PEG) and polysorbate hydrophilic coatings improves blood flow efficiency and increases the likelihood that the brain will inhale them (Kreuter 2005; Kaur et al. 2008). Nanoparticles may also be created using polysorbate 80. Increased cerebral delivery caused by superficial plasma apolipoprotein absorption is linked to LDL receptor recognition and endothelial cell compliance via brain microtubules (Mirchandani et al. 2021; Goppert and Muller 2005). Apo E-linked nanoparticles can disguise lipoprotein particles (such as LDL) that are endocytosed in the BBB endothelium and transcytosis into the brain via the BBB endothelium (Prabhakar et al. 2013). This strategy makes even the drug-release transporters unstable enough that the complete nano-carrier and loaded drug can pass across the BBB, delicately delivering the medication into the brain (Hoffmann et al. 2001). SLNs are the most dependable distribution method because they can incorporate both lipophilic and hydrophilic compounds and allow for numerous management strategies (Kreuter 2005). With the aid of these nanoparticles, anti-cancer, analgesic, anti-aging, and antibacterial drugs may all be transported on to the brain more effectively, which also improves their pharmacokinetic profiles and permits larger concentrations in the brain (Goppert and Muller 2005). In the present work, a unique kind of structure was produced with the aid of SLNs and a specific interaction with Apo E molecules. It took advantage of the substantial connections between avidin and biotin. Two Apo E-related approaches are employed to create these novel systems, SLN-DSPE-Apo E and SLN-palmitate-Apo E, which result in very potent drug carriers (Chattopadhyay et al. 2008; Mirchandani et al. 2021).
They have been successfully employed to create SLN with Apo E and hold a lot of potential for the delivery of drugs into the brain. In light of the fact that Apo E-activated SLNs imitate lipoprotein particles that penetrate the BBB endothelium and enter the brain, these novel systems may provide a potential approach for brain targeting. The schematic representation of SLN as a vehicle for targeted drug delivery is shown in the figure (Fig. 6).
Fig. 6.
Solid lipid nanoparticles (SLN) as a vehicle for targeted drug delivery
Rapamycin lipid nanocapsules in glioblastoma
In order to create innovative treatments that selectively target cancer cells and the tumor microenvironment, it is critically required that the molecular events that cause glioblastoma's malignancy be taken into consideration. Protein kinase B (PKB) and phosphatidylinocytol 3-kinase (PI3K) intracellular kinetic target of Akt/rapamycin (mTOR) and effective signaling pathway regulation the growth, development, differentiation, and survival of cells are crucial (Mirchandani et al. 2021; Hoffmann et al. 2001; Michaelis et al. 2006). PI3K, Akt, and mTOR are amplified when a cytokine, growth factor, or receptor tyrosine kinase (RTK) that activates this pathway is present. Two distinct multi-protein complexes, mTORC1 and mTORC2, are used by the mTOR cell to control growth and survival (McClements 2021).
The upsurge and inactivation of members of the AkT (Ak strain transforming) family, the tumor-suppressing properties of PTEN (homologous phosphatase and tensin), or the subtle Wnt (Wingless-related integration sit) pathway can all activate this pathway (Bjornsti and Houghton 2004; Jiang and Liu 2009). Radiation may also make glioblastoma cell lines and vascular endothelium display the mTOR signal (Saxton and Sabatini 2017).
A macrolide antibiotic called rapamycin (Cirolimus) interacts to the FK506-binding protein 12 (FKBP12), which is a naturally occurring protein. Streptomyces hygroscopicus materials found on Easter Island were the source of its first isolation. The rapamycin–FKBP12 complex prevents proteins important in the control of transcription, translation, and cell cycle from being phosphorylated (Eshleman et al. 2002). Data from three PTEN-null GB cell lines have shown that radiation suppresses the inhibition of the surviving apoptosis protein (IAP) family protein when combined with phospho-actin suppression. As a consequence, rapamycin increased radiation sensitivity by targeting Akt via mTOR (Heimberger et al. 2005). Pre-clinical studies have unequivocally demonstrated that PTEN-deficient tumors and the PI3K hypersensitivity that goes along with them are extremely susceptible to the drug rapamycin (Sonoda et al. 2001). These discoveries provide a strong foundation for the development of therapeutic latent tumor-selective mTOR inhibitors. p70S6 kinase (p70S6K) and eukaryotic initiation factor 4Ebinding protein 1 (prior to 4E-BP-phase), two smaller molecules, are phosphorylated less often by drugs such rapamycin and its variants, CCI-779, and RAD001, which inhibit cell motility. Growing data from preliminary and early clinical research shows that these mTOR inhibitors, whether used alone or in combination, may be directly and indirectly beneficial in preventing retardation of growth in a variety of malignancies, embracing GB (Anandharaj et al. 2011; Mecca et al. 2018; Hsu et al. 2020).
Rapamycin performed well in preclinical studies, but its advancement in clinical trials was delayed by the absence of suitable formulations. With the exception of low-dose treatments like immunosuppressive, tablet formulation is not feasible owing to the poor oral bioavailability (15%) (Wanigasooriya et al. 2020). Intravenous (i.v.) formulation is challenging due to rapamycin's low solubility in water (2.6 g/mL) and frequent excipients (Trepanier et al. 1998; Simamora et al. 2001). Additionally, pharmacokinetic studies revealed that Rapamycin strongly inhibited erythrocyte growth. Solid tumors might not be able to get it right away (Wanigasooriya et al. 2020). This centered on developing ester derivatives, such as Temsirolimus and CCI-779, which were simpler to manufacture. Intravenous ethanol formulations may still produce hemolysis even if CCI-779 may decrease mTOR. Phase I research revealed that the CCI-779 prodrug was rapidly hydrolyzed back into rapamycin in the plasma, allowing the likely panel to reabsorb into erythrocytes and preventing the development of cancer. There has been an increase in treatment-related toxicities as a consequence of recent advancements with the Everolimus derivative in phase II. Rapamycin's bioavailability has been increased through the use of nanovectorization technology. They offer physical defense and help you solve solubility issues. As novel nanocarriers for the treatment of GB, lipid nanoparticles loaded with rapamycin (LNCrapa) were created in this work. Although LNC-rapa was more cytotoxic than rapamycin and effectively inhibited mTOR phosphorylation, it had no synergistic effects when combined with 8 Gy radiation. Binding rapamycin has been shown to have its biological effect. The activation of phosphorylated Akt with the mTOR block and its dependency on the oxidative state are evidence of the intricacy of the PI3k/Akt/mTOR in the GP, which may be used to explain this outcome (Simamora et al. 2001).
Glioblastoma is the most aggressive type of brain cancer. It is characterized by rapid growth and invasion into surrounding healthy tissue. Despite advances in treatment, the prognosis for glioblastoma remains poor, with a median survival of less than 15 months.
Rapamycin is a natural product that has been shown to have anti-cancer properties. It works by inhibiting the mTOR signaling pathway, which is involved in cell growth and proliferation. However, rapamycin has limited solubility and bioavailability, which make it difficult to deliver to tumors at effective doses.
Lipid nanocapsules are a type of nanoparticle that can be used to deliver drugs to tumors more effectively. They are made of a lipid bilayer that surrounds a liquid core. The drug can be incorporated into the lipid bilayer or the liquid core. Lipid nanocapsules are able to target tumors by taking advantage of the "enhanced permeability and retention" (EPR) effect. This effect is due to the fact that tumors have leaky blood vessels that allow nanoparticles to enter the tumor more easily than they can enter healthy tissue.
Rapamycin lipid nanocapsules combine the anti-cancer properties of rapamycin with the improved delivery efficiency of lipid nanocapsules. This makes them a promising new approach for the treatment of glioblastoma (Chinnaiyan et al. 2018).
There are two main methods used for loading rapamycin into lipid nanocapsules: solvent evaporation and thin-film hydration.
Solvent evaporation In the solvent evaporation method, rapamycin and lipids are dissolved in a water-immiscible organic solvent, such as ethanol or chloroform. The organic solvent is then evaporated, leaving behind a thin film of lipids and rapamycin. The film is then hydrated with water, causing the lipids to form vesicles. The rapamycin is then trapped inside the vesicles.
Thin-film hydration In the thin-film hydration method, rapamycin and lipids are dissolved in a mixture of water and organic solvent. The mixture is then spread onto a solid surface, such as glass, and allowed to dry. This forms a thin film of lipids and rapamycin. The film is then hydrated with water, causing the lipids to form vesicles. The rapamycin is then trapped inside the vesicles.
The best method for loading rapamycin into lipid nano-capsules will depend on the specific application.
NPs have the potential to improve the stability and solubility of encapsulated cargos, promote transport across membranes and prolong circulation times to increase safety and efficacy (Mazuryk et al. 2016; Kou et al. 2018). For these reasons, NP research has been widespread, generating promising results in vitro and in small animal models (Blanco et al. 2015). For example, a LNP siRNA drug termed Onpattro (patisiran) was recently approved by the FDA for the treatment of amyloidosis (Mitragotri et al. 2017). In the context of genome editing, NPs have the potential to be less toxic and immunogenic than viral vectors, which have a history of safety concerns (Riley et al. 2019; Calvo et al. 2000). Looking to the future, NPs have the potential to improve genome editing by exerting more precise control and reducing safety concerns. Several companies, including CRISPR Therapeutics, Intellia Therapeutics and Editas Medicine, are currently developing CRISPR-Cas9 therapeutics. Intellia Therapeutics is currently developing LNPs for in vivo delivery to treat several liver diseases, including amyloidosis, α1-antitrypsin deficiency and hepatitis B virus infection. With precision NP design, gene editing holds promise to cure diseases and significantly improve patient lives. In recent research, they have found.
However, the intersection of nanotechnology and venom-based therapies is an area of active research that holds promising potential for cancer treatment. Researchers have been exploring the use of nanotechnology to deliver venom components selectively to cancer cells, enhancing the specificity and effectiveness of these compounds. This approach involves encapsulating venom-derived toxins in nanoparticles that can target cancer cells while minimizing damage to healthy tissues. For example, a study published in the journal Biomaterials in 2015 (Abina et al. 2003) described the use of nanocapsules to deliver venom from the Brazilian social wasp Polybia paulista to cancer cells. The researchers demonstrated that the nanocapsules could enhance the selective cytotoxicity of the venom against cancer cells. Similarly, there are studies investigating the combination of scorpion venom and nanotechnology for cancer therapy. Another study published in the Journal of Controlled Release in 2017 explored the use of nanoparticles to deliver a peptide from scorpion venom to target cancer cells. It is important to note that the field of nanotechnology in cancer treatment is dynamic, and new research findings may have emerged.
Microscopy Microscopy is a powerful tool for visualizing lipid nanoparticles and characterizing their morphology, size, and distribution. Several microscopy techniques are commonly used for this purpose, including:
Transmission electron microscopy (TEM): TEM provides high-resolution images of lipid nanoparticles, allowing for detailed examination of their structure and surface properties.
Scanning electron microscopy (SEM): SEM offers a wider field of view than TEM and is useful for visualizing the overall morphology and distribution of lipid nanoparticles.
Atomic force microscopy (AFM): AFM provides information about the surface topography and mechanical properties of lipid nanoparticles.
Particle size analysis
Particle size analysis is crucial for determining the size distribution of lipid nanoparticles as this parameter has a significant impact on their biological properties. Common particle size analysis techniques include:
Dynamic light scattering (DLS): DLS measures the diffusion of light scattered by lipid nanoparticles, providing information about their hydrodynamic diameter.
Non-invasive backscatter (NIBS): NIBS is a non-destructive technique that measures the size of lipid nanoparticles by analyzing the scattered light pattern.
Analytical ultracentrifugation (AUC): AUC measures the sedimentation rate of lipid nanoparticles in an ultracentrifuge, providing information about their size and density.
Spectroscopy Spectroscopy techniques provide insights into the chemical composition and structure of lipid nanoparticles. Common spectroscopic techniques include (Marcelo et al. 2000):
Fourier-transform infrared spectroscopy (FT-IR): FT-IR measures the vibrational absorption spectrum of lipid nanoparticles, allowing for identification of their constituent lipids.
Nuclear magnetic resonance (NMR): NMR provides information about the molecular structure of lipid nanoparticles, including the arrangement and conformation of their lipids.
Ultraviolet–visible (UV–vis) spectroscopy: UV–vis spectroscopy measures the light absorption spectrum of lipid nanoparticles, which can be used to determine their concentration and purity (Brubach et al. 2007).
In addition to these general techniques, specific analytical methods may be employed depending on the properties and intended use of the lipid nanoparticles. For instance, zeta potential measurements are used to assess the surface charge of lipid nanoparticles, which influences their stability and interactions with biological systems. Drug loading and release studies are conducted to evaluate the efficiency with which lipid nanoparticles encapsulate and release therapeutic agents (Kamberi and Tran 2012).
Functional hybrid nano-emulsions for sumatriptan intranasal delivery
It seems that advanced technologies—nano-hybrids managed as drugs have proven to be very strategic. Tryptones and commercially available intranasal sumatriptan (SMT) are some medications used to treat painful migraine symptoms. Nevertheless, we can see that its high hydrophilicity and poor mucosation define the SMT performance of the intranasal pathway. More than 20% of people worldwide suffer from migraine, a painful and manageable condition that often manifests itself by sporadic headache spells linked to digestive issues (Brubach et al. 2007). Serotonin (5-hydroxytryptamine), a potential migraine headache trigger, is known to cause migraines even if the disease's exact cause is unknown. We discovered in the late 1980s that a class of agonists called serotonin tryptones was created to treat migraine, (Yeh et al. 2018). At least two insoluble medicines are dynamically stabilized in a nanoemulsion (NE), a drug delivery system (DDS). They have a great loading capacity for hydrophobic molecules since they are predominantly made of watery and oily elements. Many different types of drug release profiles can be prolonged by them, increasing their viability. Furthermore, it is guaranteed that NE will accurately traverse the BBB with particles smaller than 200 nm. A great DDS for the continuous release of medications with a neurological impact has been shown to be NE (Muzzi et al. 2020).
Lipid nanoparticles (LNPs) have undergone extensive investigation in advanced clinical trials, showcasing their versatility in delivering genetic material and addressing various medical challenges. One prominent avenue of research involves the co-delivery of small interfering RNA (siRNA) and messenger RNA (mRNA). LNPs have proven effective in delivering therapeutic mRNA in diverse in vivo disease models. Clinical trials have particularly focused on assessing LNPs' immunogenicity for safeguarding against viral infections, such as Zika and influenza. Recent clinical trials have explored the use of naked vascular endothelial growth factor A (VEGF-A) mRNA in a citrate saline solution, where LNPs as RNA carriers. This unconventional approach has been tested in patients with type 2 diabetes and individuals undergoing coronary artery bypass grafting (CABG). LNPs have been extensively studied for drug delivery in various medical fields. They are commonly used in the delivery of nucleic acid-based therapeutics, such as RNA interference (RNAi) and messenger RNA (mRNA) vaccines. LNPs have gained significant attention due to their ability to encapsulate and protect therapeutic payloads, facilitated cellular uptake, and enhanced bioavailability. Notable examples include the use of LNPs in the development of COVID-19 vaccines, such as the Pfizer-BioNTech and Moderna mRNA vaccines. Clinical trials involving LNPs are likely to cover a wide range of therapeutic areas, including cancer, genetic disorders, and infectious diseases. The progress of LNPs in human clinical trials signals their pivotal role in genetic material delivery. The data from these trials also suggest a potential mechanism where part of the LNP-mRNA distribution among cells or organs may occur through extracellular vesicles (EVs). This intriguing avenue warrants further investigation to elucidate the intricacies of LNPs' interaction with biological systems in humans.
In summary, advanced clinical trials with LNPs underscore their significance in genetic medicine, presenting promising avenues for therapeutic development and prompting further exploration into their intricate mechanisms of action in the human body (see Table 2).
Table 2.
Lipid nanoparticles in the commercial market and clinical trials
| LNP/subgroup | Active compound | Disease/Applications | Out Come -Product | References |
|---|---|---|---|---|
| Solid lipid nanoparticle | Mitoxantrone | Hepatocarcinoma | Mitoxantrone-loaded polybutylcyanacrylate nanoparticles (DHADPBCA-NPs) (phase II clinical trial) | Nirale et al. (2020) |
| Doxorubicin | Hepatocarcinoma | Doxorubicin Transdrug (DT) (Phase III clinical trial) | Zhou et al. (2009) | |
| siRNA targeting transthyretin gene | amyloidosis) | Onpattro (Patisiran) | Holtze (2013) | |
| Liposomes | Doxorubicin/daunorubicin | Cancer | Doxil, Myocet, Vixeos, DaunoXome, Transdrug | Akinc et al. (2019), Ostrosky-Zeichner et al. (2003) |
| Paclitaxel | Cancer | Abraxane | Guo et al. (2013), Gradishar et al. (2005), Yardley (2013) | |
| Amphotericin B | Visceral leishmaniasis | Albecet, Ambisome, Amphotec | Ran et al. (2017), Adedoyin et al. (1997), Stone et al. (2016) | |
| Verteporfin |
Age-related macular Degeneration |
Visudyne | Clemons and Stevens (1998) | |
| Lipid polymer hybrid nanoparticle | Docetaxel | Pancreatic cancer | Docetaxel polymeric nanoparticle (phase I clinical trial) | Cheng et al. (2021), Song et al. (2016) |
| Docetaxel | Lung cancer with KRAS mutation | BIND-014 (Docetaxel Nanoparticles for Injectable Suspension) (phase II clinical trial) | Vetten et al. (2014) | |
| Docetaxel | Prostate cancer |
BIND-014 (Docetaxel Nanoparticles for Injectable Suspension) (phase II clinical trial) |
Packer et al. (2021) | |
| Nanostructured lipid carriers | Self-amplifying RNA | COVID-19 | THEMBA II T-CELL Vaccine (phase I/II clinical trial) | Autio et al. (2018), El-Salamouni et al. (2015) |
| mRNA-1273 | COVID-19 | Spikevax | Hadinoto et al. (2013) |
Conclusions and future outlook
LNPs are widely used in preclinical and clinical research for drug delivery. Several LNPs have received approval for clinical use and have demonstrated their advantages over other drug delivery methods. To highlight LNPs as a drug delivery method, this study focuses on three different lipid-based systems: SLNs, NLCs, and hybrid liposome-polymer nanoparticles. Static mixers, microfluidics (chip-based microfluidics, capillary-based microfluidics), and more traditional methods (solvent-based emulsification, non-solvent-based emulsification, bulk nanoprecipitation) have been developed for the preparation of these LNPs. Since hydrophobic lipids and hydrophobic drugs have corresponding hydrophobicity, it is easier to encapsulate hydrophobic drugs in LNPs than hydrophilic ones. However, new solutions have been developed to solve the problem of encapsulating hydrophilic drugs. For example, the efficacy of LNPs for RNA delivery is based on the creation of ionizable lipids. The development of novel lipids and the improvement of LNP composition will open new opportunities for drug delivery. Targeted drug delivery is another major difficulty. The development of novel targeted delivery systems is underway, but clinical reality is still far from being achieved. LNP formulation of siRNA functionalized with strophanthidin for targeted delivery of nucleic acids. There is also a brief explanation of receptor–aptamer conjugator lipid nanoparticle quantum dot imaging and small molecule ligands for enhanced intracellular activity in cancer cells. There is also interest in using solid lipid nanoparticles, such as Rapamycin Lipid Nanocapsules in Glioblastoma, to deliver drugs to specific areas of the brain. Understanding the relationship between lipid chemistry/structure and function will certainly aid future development of more effective LNPs for drug delivery. Future LNP developers will also have access to powerful tools thanks to state-of-the-art technologies such as machine learning or meta-data analysis of research published in the literature.
Lipid nanoparticles (LNPs) have undergone extensive investigation in advanced clinical trials, showcasing their versatility in delivering genetic material and addressing various medical challenges. One prominent avenue of research involves the co-delivery of small interfering RNA (siRNA) and messenger RNA (mRNA). LNPs have proven effective in delivering therapeutic mRNA in diverse in vivo disease models. Clinical trials have particularly focused on assessing LNPs' immunogenicity for safeguarding against viral infections, such as Zika and influenza. Recent clinical trials have explored the use of naked vascular endothelial growth factor A (VEGF-A) mRNA in a citrate saline solution, where LNPs as RNA carriers. This unconventional approach has been tested in patients with type 2 diabetes and individuals undergoing coronary artery bypass grafting (CABG). LNPs have been extensively studied for drug delivery in various medical fields. They are commonly used in the delivery of nucleic acid-based therapeutics, such as RNA interference (RNAi) and messenger RNA (mRNA) vaccines. LNPs have gained significant attention due to their ability to encapsulate and protect therapeutic payloads, facilitated cellular uptake, and enhanced bioavailability. Notable examples include the use of LNPs in the development of COVID-19 vaccines, such as the Pfizer-BioNTech and Moderna mRNA vaccines. Clinical trials involving LNPs are likely to cover a wide range of therapeutic areas, including cancer, genetic disorders, and infectious diseases. The progress of LNPs in human clinical trials signals their pivotal role in genetic material delivery. The data from these trials also suggest a potential mechanism where part of the LNP-mRNA distribution among cells or organs may occur through extracellular vesicles (EVs). This intriguing avenue warrants further investigation to elucidate the intricacies of LNPs' interaction with biological systems in humans.
In summary, advanced clinical trials with LNPs underscore their significance in genetic medicine, presenting promising avenues for therapeutic development and prompting further exploration into their intricate mechanisms of action in the human body.
Drug delivery: Lipid-based nanoparticulate drug delivery systems have shown promising effects for targeting drugs in lymphatic systems, brain tissues, lungs, and skin.
Chemotherapy: New generations of solid lipid nanoparticles (SLNs), such as nanostructured lipid carriers (NLC), lipid drug conjugates (LDC), polymeric lipid hybrid nanoparticles (PLN), and long-circulating SLNs, have improved the role of SLNs as versatile drug carriers for various types of chemotherapy.
Vaccine delivery: The recent development of lipid nanoparticle-based mRNA vaccines for combating COVID-19 has highlighted the emerging role of these nanocarriers as potential vehicles for the delivery of a wide variety of therapeutics, including antigens.
Other emerging trends include the following
Anti-aging and anti-wrinkle effects: Nourishment and hydration to the skin: Treating hyperpigmentation: Cleansing: Repairing, restoring, and conditioning hair.
The current development and efficacy of LNP@Drugs for RNA delivery is not yet mature. The delivery system platform is still in its infancy, including nucleic acid modification technology and sequence design. And, more importantly, there are many questions about LNPs: How does the internal structure of LNPs prevent nucleic acid degradation? How do nucleic acids interact with ionizable lipids? What factors affect the amount of RNA in each LNP? What is the localization of each lipid fraction in LNPs? Do the lipids dissociate during long-term storage? These questions suggest that the clinical application of LNP@mRNA still has a long way to go. There are still some challenges and issues that remain unresolved. Despite the challenges in developing site-specific mRNA therapeutics, we are optimistic that advances in chemical synthesis and nanotechnology will lead to the introduction of increasingly effective therapies. This is a promising avenue to improve health outcomes and expand access to effective treatments to a broader population. It is hoped that LNP@drugs and mRNA formulations will find a wide range of applications in biopharmaceutical fields and lead to breakthroughs in future.
Acknowledgements
We want to thank all lab members.
Author contributions
Conceptualization, Formal analysis, Investigation, Writing—original draft was performed by MD, WW, SUMR, RAD, JS, SSI, AS. Supervision was conducted by SM and JG. Writing—review & editing is performed by SM and RH.
Funding
The authors declare that no funds, grants, or other support was received during the preparation of this manuscript.
Data availability
No data were used for the research described in the article.
Code availability
No code is used here.
Declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Manikandan Dhayalan, Wei Wang have contributed equally to this work.
Contributor Information
Manikandan Dhayalan, Email: manikandandhayalan88@gmail.com.
Antony Stalin, Email: a.staanlin@gamil.com, Email: antonystalin@uestc.edu.cn.
Saurav Mallik, Email: sauravmtech2@gmail.com, Email: smallik@hsps.harvard.edu, Email: smallik@arizona.edu.
References
- Abina SHB, Kalle CV, Schmidt M, Cormack MPM, Wulffraat N, Leboulch P, Lim A, Osborne CS, Pawliuk R, Morillon E, Sorensen R, Forster A, Fraser P, Cohen JI, Basile GDS, Alexander I, Wintergerst U, Frebourg T, Aurias A, Lyonnet DS, Romana S, Weiss IR, Gross F, Valensi F, Delabesse E, Macintyre E, Sigaux F, Soulier J, Leiva LE, Wissler M, Prinz C, Rabbitts TH, Deist FL, Fischer A, Calvo MC. LMO2-associated clonal T cell proliferation in two patients after gene therapy for. SCID-X1. Science. 2003;302:1181–1185. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- Accardo A, Mansi R, Morisco A, Mangiapia G, Paduano L, Tesauro D, Radulescu A, Aurilio M, Aloj L, Arra C, Morelli G. Peptide modified nanocarriers for selective targeting of bombesin receptors. Mol Biosyst. 2010;6:878–887. doi: 10.1039/b923147a. [DOI] [PubMed] [Google Scholar]
- Adedoyin A, Bernardo JF, Swenson CE, Bolsack LE, Horwith G, DeWit S, Kelly E, Klasterksy J, Sculier JP, DeValeriola D, Anaissie E, Berestein GL, Cuentas AL. Pharmacokinetic profile of ABELCET (amphotericin B lipid complex injection): combined experience from phase I and phase II studies. Antimicrob Agents Chemother. 1997;41(10):2201–2208. doi: 10.1128/AAC.41.10.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, Samiei M, Kouhi M, Nejati-Koshki K. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8:102. doi: 10.1186/1556-276X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinc A, Querbes W, De S, Qin J, Frank-Kamenetsky M, Jayaprakash KN, Jayaraman M, Rajeev KG, Cantley WL, Dorkin JR, Butler JS, Qin L, Racie T, Sprague A, Fava E, Zeigerer A, Hope MJ, Zerial M, Sah DW, Fitzgerald K, Tracy MA, Manoharan M, Koteliansky V, Fougerolles A, Maier MA. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol Ther. 2010;18:1357–1364. doi: 10.1038/mt.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinc A, Maier MA, Manoharan M, Fitzgerald K, Jayaraman M, Barros S, Ansell S, Du X, Hope J, Madden TD, Mui BL, Semple SC, Tam YK, Ciufolini M, Witzigmann D, Kulkarni JA, Meel RVD, Cullis PR. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat Nanotechnol. 2019;14(12):1084–1087. doi: 10.1038/s41565-019-0591-y. [DOI] [PubMed] [Google Scholar]
- Amer MH. Gene therapy for cancer: present status and future perspective. Mol Cell Ther. 2014;2(1):27. doi: 10.1186/2052-8426-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An D, Schneller JL, Frassetto A, Liang S, Zhu X, Park J-S, Theisen M, Hong S-J, Zhou J, Rajendran R, Levy B, Howell R, Besin G, Presnyak V, Sabnis S, Murphy-Benenato KE, Kumarasinghe ES, Salerno T, Mihai C, Lukacs CM, Chandler RJ, Guey LT, Venditti CP, Martini PGV. Systemic messenger RNA therapy as a treatment for methylmalonic acidemia. Cell Rep. 2017;21(12):3548–3558. doi: 10.1016/j.celrep.2017.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anandharaj A, Cinghu S, Park WY. Rapamycin-mediated mTOR inhibition attenuates survivin and sensitizes glioblastoma cells to radiation therapy. Acta Biochim Biophys Sin (shanghai) 2011;43:292–300. doi: 10.1093/abbs/gmr012. [DOI] [PubMed] [Google Scholar]
- Anselmo AC, Mitragotri S. Nanoparticles in the clinic: an update. Bioeng Transl Med. 2019;4:e10143. doi: 10.1002/btm2.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthiya S, Ozturk SC, Yanik H, Tavukcuoglu E, Sahin A, Datta D, Charisse K, Alvarez DM, Loza MI, Calvo A, Sulheim E, Loevenich S, Klinkenberg G, Schmid R, Manoharan M, Esendagli G, Alonso MJ. Targeted siRNA lipid nanoparticles for the treatment of KRAS-mutant tumors. J Control Release. 2023;357:67–83. doi: 10.1016/j.jconrel.2023.03.016. [DOI] [PubMed] [Google Scholar]
- Anttila V, Saraste A, Knuuti J, Jaakkola P, Hedman M, Svedlund S, Lagerström-Fermér M, Kjaer M, Jeppsson A, Gan L-M. Synthetic mRNA encoding VEGF-A in patients undergoing coronary artery bypass grafting: design of a phase 2a clinical trial. Mol Therapy Methods Clin Dev. 2020;18:464–472. doi: 10.1016/j.omtm.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anttila V, Saraste A, Knuuti J, Hedman M, Jaakkola P, Laugwitz K, Krane M, Jeppsson A, Sillanmäki S, Rosenmeier J, Zingmark P, Rudvik A, Garkaviy P, Watson C, Pangalos MN, Chien KR, Danielson RF, Collén A, Gan LM. Direct intramyocardial injection of VEGF mRNA in patients undergoing coronary artery bypass grafting. Mol Ther. 2022;31(3):866–874. doi: 10.1016/j.ymthe.2022.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apolinario AC, Hauschke L, Nunes JR, Lopes LB. Lipid nanovesicles for biomedical applications: 'What is in a name'? Prog Lipid Res. 2021;82:101096. doi: 10.1016/j.plipres.2021.101096. [DOI] [PubMed] [Google Scholar]
- Arias LS, Pessan JP, Vieira APM, Lima TMTD, Delbem ACB, Monteiro DR. Iron oxide nanoparticles for biomedical applications: a perspective on synthesis, drugs, antimicrobial activity, and toxicity. Antibiotics. 2018;7(2):46. doi: 10.3390/antibiotics7020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autio KA, Dreicer R, Anderson J, Garcia JA, Alva A, Hart LL, Milowsky MI, Posadas EM, Ryan CJ, Graf RP, Dittamore R, Schreiber NA, Summa JM, Youssoufian H, Morris MJ, Scher HI. Safety and efficacy of BIND-014, a docetaxel nanoparticle targeting prostate-specific membrane antigen for patients with metastatic castration-resistant prostate cancer: a phase 2 clinical trial. JAMA Oncol. 2018;4(10):1344–1351. doi: 10.1001/jamaoncol.2018.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, Mcgettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T. COVE study groupet. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badierah RA, Uversky VN, Redwan EM. Dancing with Trojan horses: an interplay between the extracellular vesicles and viruses. J Biomol Struct Dyn. 2021;39(8):3034. doi: 10.1080/07391102.2020.1756409. [DOI] [PubMed] [Google Scholar]
- Bahl K, Senn JJ, Yuzhakov O, Bulychev A, Brito LA, Has Sett KJ, Laska ME, Smith M, Almarsson Ö, Thompson J, Ribeiro AM, Watson M, Zaks T, Ciaramella G. Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against H10N8 and H7N9 influenza viruses. Mol Ther. 2017;25(6):1316–1327. doi: 10.1016/j.ymthe.2017.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball RL, Hajj KA, Vizelman J, Bajaj P, Whitehead KA. Lipid nanoparticle formulations for enhanced co-delivery of siRNA and mRNA. Nano Lett. 2018;18:3814–3822. doi: 10.1021/acs.nanolett.8b01101. [DOI] [PubMed] [Google Scholar]
- Banerjee R, Tyagi P, Li S, Huang L. Anisamide-targeted stealth liposomes: a potent carrier for targeting doxorubicin to human prostate cancer cells. Int J Cancer. 2004;112:693–700. doi: 10.1002/ijc.20452. [DOI] [PubMed] [Google Scholar]
- Barenholz Y. Doxil(R)–the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- Bayburt TH, Grinkova YV, Sligar SG. Self-assembly of discoidal phospholipid bilayer nanoparticles with membrane scaffold proteins. Nano Lett. 2002;2:853–856. doi: 10.1021/nl025623k. [DOI] [Google Scholar]
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- Bi D, Unthan DM, Hu L, Bussmann J, Remaut K, Barz M, Zhang H. Polysarcosine-based lipid formulations for intracranial delivery of mRNA. J Control Release. 2023;356:1–13. doi: 10.1016/j.jconrel.2023.02.021. [DOI] [PubMed] [Google Scholar]
- Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- Blanco E, Hsiao A, Mann AP, Landry MG, Meric-Bernstam F, Ferrari M. Nanomedicine in cancer therapy: innovative trends and prospects. Cancer Sci. 2011;102:1247–1252. doi: 10.1111/j.1349-7006.2011.01941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi P, Giovagnoli S, Schoubben A, Ricci M, Rossi C. Solid lipid nanoparticles for targeted brain drug delivery. Adv Drug Deliv Rev. 2007;59:454–477. doi: 10.1016/j.addr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res. 2016;33:2373–2387. doi: 10.1007/s11095-016-1958-5. [DOI] [PubMed] [Google Scholar]
- Brubach JB, Jannin V, Mahler B, Bourgaux C, Lessieur P, Roy P, Ollivon M. Structural and thermal characterization of glyceryl behenate by X-ray diffraction coupled to differential calorimetry and infrared spectroscopy. Int J Pharm. 2007;336(2):248–256. doi: 10.1016/j.ijpharm.2006.11.057. [DOI] [PubMed] [Google Scholar]
- Bulbake U, Doppalapudi S, Kommineni N, Khan W (2017) Liposomal formulations in clinical use: an updated review. Pharmaceutics 9 [DOI] [PMC free article] [PubMed]
- Calvo MC, Bey SH, Basile GDS, Gross F, Yvon E, Nusbaum P, Selz F, Hue C, Certain S, Casanova JL, Bousso P, Deist FL, Fischer A. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288(5466):669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- Cao S, Zhang W, Pan H, Huang Z, Guo M, Zhang L, Xu X, Saw PE. Bioactive lipid-nanoparticles with inherent self-therapeutic and anti-angiogenic properties for cancer therapy. Acta Biomater. 2023;157:500–510. doi: 10.1016/j.actbio.2022.12.022. [DOI] [PubMed] [Google Scholar]
- Cavaco MC, Pereira C, Kreutzer B, Gouveia LF, Silva-Lima B, Brito AM, Videira M. Evading P-glycoprotein mediated-efflux chemoresistance using Solid Lipid Nanoparticles. Eur J Pharm Biopharm. 2017;110:76–84. doi: 10.1016/j.ejpb.2016.10.024. [DOI] [PubMed] [Google Scholar]
- Chang DK, Chiu CY, Kuo SY, Lin WC, Lo A, Wang YP, Li PC, Wu HC. Antiangiogenic targeting liposomes increase therapeutic efficacy for solid tumors. J Biol Chem. 2009;284:12905–12916. doi: 10.1074/jbc.M900280200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay N, Zastre J, Wong HL, Wu XY, Bendayan R. Solid lipid nanoparticles enhance the delivery of the HIV protease inhibitor, atazanavir, by a human brain endothelial cell line. Pharm Res. 2008;25:2262–2271. doi: 10.1007/s11095-008-9615-2. [DOI] [PubMed] [Google Scholar]
- Chen Y, Sen J, Bathula SR, Yang Q, Fittipaldi R, Huang L. Novel cationic lipid that delivers siRNA and enhances therapeutic effect in lung cancer cells. Mol Pharm. 2009;6:696–705. doi: 10.1021/mp800136v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Duan Z, Yuan Y, Li R, Pang L, Liang J, Xu X, Wang J. Peptide-22 and cyclic RGD functionalized liposomes for glioma targeting drug delivery overcoming BBB and BBTB. ACS Appl Mater Interfaces. 2017;9:5864–5873. doi: 10.1021/acsami.6b15831. [DOI] [PubMed] [Google Scholar]
- Cheng X, Gao J, Ding Y, Lu Y, Wei Q, Cui D, Fan J, Li X, Zhu E, Lu Y, Wu Q, Li L, Huang W. Multi-functional liposome: a powerful theranostic nano-platform enhancing photodynamic therapy. Adv Sci (weinh) 2021;8(16):e2100876. doi: 10.1002/advs.202100876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnaiyan P, Won M, Wen PY, Rojiani AM, Werner-Wasik M, Shih HA, Ashby LS, Michael Yu HH, Stieber VW, Malone SC, Fiveash JB, Mohile NA, Ahluwalia MS, Wendland MM, Stella PJ, Kee AY, Mehta MP. A randomized phase II study of everolimus in combination with chemoradiation in newly diagnosed glioblastoma: results of NRG Oncology RTOG 0913. Neuro Oncol. 2018;20:666–673. doi: 10.1093/neuonc/nox209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury A, Kunjiappan S, Panneerselvam T, Somasundaram B, Bhattacharjee C. Nanotechnology and nanocarrier-based approaches on treatment of degenerative diseases, International. Nano Lett. 2017;7:91–122. doi: 10.1007/s40089-017-0208-0. [DOI] [Google Scholar]
- Clayton P, Nelson CA, Weeden T, Taylor KM, Moreland RJ, Scheule RK, Phillips L, Leger AJ, Cheng SH, Wentworth BM. Antisense oligonucleotide-mediated suppression of muscle glycogen synthase 1 synthesis as an approach for substrate reduction therapy of pompe disease. Mol Ther Nucleic Acids. 2014;3:e206. doi: 10.1038/mtna.2014.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemons KV, Stevens DA. Comparison of fungizone, Amphotec, Am Bisome, and Abelcet for treatment of systemic murine cryptococcosis. Antimicrob Agents Chemother. 1998;42(4):899–902. doi: 10.1128/AAC.42.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collén A, Bergenhem N, Carlsson L, Chien KR, Hoge S, Gan L-M, Fritsche-Danielson R. VEGFA mRNA for regenerative treatment of heart failure. Nat Rev Drug Discov. 2022;21:79–80. doi: 10.1038/s41573-021-00355-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressman S, Dobson I, Lee JB, Tam YY, Cullis PR. Synthesis of a labeled RGD-lipid, its incorporation into liposomal nanoparticles, and their trafficking in cultured endothelial cells. Bioconjug Chem. 2009;20:1404–1411. doi: 10.1021/bc900041f. [DOI] [PubMed] [Google Scholar]
- Dariva CG, Coelho JFJ, Serra AC. Near infrared light-triggered nanoparticles using singlet oxygen photocleavage for drug delivery systems. J Control Rel. 2019;294:337–354. doi: 10.1016/j.jconrel.2018.12.042. [DOI] [PubMed] [Google Scholar]
- Davey NE, Travé G, Gibson TJ. How viruses hijack cell regulation. Trends Biochem Sci. 2011;36:159. doi: 10.1016/j.tibs.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Davidson BL, McCray PB., Jr Current prospects for RNA interference-based therapies. Nat Rev Genet. 2011;12:329–340. doi: 10.1038/nrg2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denisov IG, Grinkova YV, Lazarides AA, Sligar SG. Directed self-assembly of monodisperse phospholipid bilayer Nanodiscs with controlled size. J Am Chem Soc. 2004;126:3477–3487. doi: 10.1021/ja0393574. [DOI] [PubMed] [Google Scholar]
- Di Paolo D, Ambrogio C, Pastorino F, Brignole C, Martinengo C, Carosio R, Loi M, Pagnan G, Emionite L, Cilli M, Ribatti D, Allen TM, Chiarle R, Ponzoni M, Perri P. Selective therapeutic targeting of the anaplastic lymphoma kinase with liposomal siRNA induces apoptosis and inhibits angiogenesis in neuroblastoma. Mol Ther. 2011;19:2201–2212. doi: 10.1038/mt.2011.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo D, Brignole C, Pastorino F, Carosio R, Zorzoli A, Rossi M, Loi M, Pagnan G, Emionite L, Cilli M, Bruno S, Chiarle R, Allen TM, Ponzoni M, Perri P. Neuroblastoma-targeted nanoparticles entrapping siRNA specifically knockdown ALK. Mol Ther. 2011;19:1131–1140. doi: 10.1038/mt.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicko A, Kwak S, Frazier AA, Mayer LD, Liboiron BD. Biophysical characterization of a liposomal formulation of cytarabine and daunorubicin. Int J Pharm. 2010;391:248–259. doi: 10.1016/j.ijpharm.2010.02.014. [DOI] [PubMed] [Google Scholar]
- Dorsett Y, Tuschl T. siRNAs: applications in functional genomics and potential as therapeutics. Nat Rev Drug Discov. 2004;3:318–329. doi: 10.1038/nrd1345. [DOI] [PubMed] [Google Scholar]
- Dü G. Gregoriadis, introduction: the origins of liposomes: Alec Bangham at Babraham. Methods Enzymol. 2005;391:1–3. doi: 10.1016/S0076-6879(05)91029-X. [DOI] [Google Scholar]
- Du H, Cui C, Wang L, Liu H, Cui G. Novel tetrapeptide, RGDF, mediated tumor specific liposomal doxorubicin (DOX) preparations. Mol Pharm. 2011;8:1224–1232. doi: 10.1021/mp200039s. [DOI] [PubMed] [Google Scholar]
- Edward J, Goldman M. Lipid formulations of amphotericin B. Clevel Clin J Med. 1998;65(8):423–427. doi: 10.3949/ccjm.65.8.423. [DOI] [PubMed] [Google Scholar]
- El-Salamouni NS, Farid RM, El-Kamel AH, El-Gamal SS. Effect of sterilization on the physical stability of brimonidine loaded solid lipid nanoparticles and nanostructured lipid carriers. Int J Pharm. 2015;496(2):976–983. doi: 10.1016/j.ijpharm.2015.10.043. [DOI] [PubMed] [Google Scholar]
- Eshleman JS, Carlson BL, Mladek AC, Kastner BD, Shide KL, Sarkaria JN. Inhibition of the mammalian target of rapamycin sensitizes U87 xenografts to fractionated radiation therapy. Cancer Res. 2002;62:7291–7297. [PubMed] [Google Scholar]
- Falciani C, Accardo A, Brunetti J, Tesauro D, Lelli B, Pini A, Bracci L, Morelli G. Target-selective drug delivery through liposomes labeled with oligobranched neurotensin peptides. ChemMedChem. 2011;6:678–685. doi: 10.1002/cmdc.201000463. [DOI] [PubMed] [Google Scholar]
- Fan Y, Zhang Q. Development of liposomal formulations: from concept to clinical investigations. Asian J Pharm Sci. 2013;8:81–87. doi: 10.1016/j.ajps.2013.07.010. [DOI] [Google Scholar]
- Fenz SF, Sengupta K. Giant vesicles as cell models. Integr Biol (camb) 2012;4:982–995. doi: 10.1039/c2ib00188h. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- Firdessa-Fite R, Creusot RJ. Nanoparticles versus dendritic cells as vehicles to deliver mRNA encoding multiple epitopes for immunotherapy. Mol Ther Methods and Clin Dev. 2020;16:50–62. doi: 10.1016/j.omtm.2019.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca-Santos B, Gremião MPD, Chorilli M. Nanotechnology-based drug delivery systems for the treatment of Alzheimer’s disease. Int J Nanomed. 2015;10:4981–5003. doi: 10.2147/IJN.S87148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonte P, Andrade F, Araújo F, Andrade C, J. das Neves, B. Sarmento, Chitosan-coated solid lipid nanoparticles for insulin delivery. Methods Enzymol. 2012;508:295–314. doi: 10.1016/B978-0-12-391860-4.00015-X. [DOI] [PubMed] [Google Scholar]
- Frias I, Neves AR, Pinheiro M, Reis S. Design, development, and characterization of lipid nanocarriers-based epigallocatechin gallate delivery system for preventive and therapeutic supplementation. Drug Des Devel Ther. 2016;10:3519–3528. doi: 10.2147/DDDT.S109589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan L-M, Lagerström-Fermér M, Carlsson LG, Arfvidsson C, Egnell A-C, Rudvik A, Kjaer M, Collén A, Thompson JD, Joyal J, Chialda L, Koernicke T, Fuhr R, Chien KR, Fritsche-Danielson R. Intradermal delivery of modified mRNA encoding VEGF-A in patients with type 2 diabetes. Nat Commun. 2019;10(1):871. doi: 10.1038/s41467-019-08852-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goppert TM, Muller RH. Polysorbate-stabilized solid lipid nanoparticles as colloidal carriers for intravenous targeting of drugs to the brain: comparison of plasma protein adsorption patterns. J Drug Target. 2005;13:179–187. doi: 10.1080/10611860500071292. [DOI] [PubMed] [Google Scholar]
- Gordillo-Galeano A, Mora-Huertas CE. Solid lipid nanoparticles and nanostructured lipid carriers: a review emphasizing on particle structure and drug release. Eur J Pharm Biopharm. 2018;133:285–308. doi: 10.1016/j.ejpb.2018.10.017. [DOI] [PubMed] [Google Scholar]
- Goutayer M, Dufort S, Josserand V, Royere A, Heinrich E, Vinet F, Bibette J, Coll JL, Texier I. Tumor targeting of functionalized lipid nanoparticles: assessment by in vivo fluorescence imaging. Eur J Pharm Biopharm. 2010;75:137–147. doi: 10.1016/j.ejpb.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, Hawkins M, O’Shaughnessy J. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- Grange C, Geninatti-Crich S, Esposito G, Alberti D, Tei L, Bussolati B, Aime S, Camussi G. Combined delivery and magnetic resonance imaging of neural cell adhesion molecule-targeted doxorubicin-containing liposomes in experimentally induced Kaposi's sarcoma. Cancer Res. 2010;70:2180–2190. doi: 10.1158/0008-5472.CAN-09-2821. [DOI] [PubMed] [Google Scholar]
- Guevara ML, Persano F, Persano S. Advances in lipid nanoparticles for mRNA-based cancer immunotherapy. Front Chem. 2020;8:589959. doi: 10.3389/fchem.2020.589959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P, Hsu TM, Zhao Y, Martin CR, Zare RN. Preparing amorphous hydrophobic drug nanoparticles by nanoporous membrane extrusion. Nanomedicine (lond) 2013;8(3):333–341. doi: 10.2217/nnm.12.119. [DOI] [PubMed] [Google Scholar]
- Hadinoto K, Sundaresan A, Cheow WS. Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review. Eur J Pharm Biopharm. 2013;85(3):427–443. doi: 10.1016/j.ejpb.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Hald Albertsen C, Kulkarni JA, Witzigmann D, M. Lind,K. Petersson, J.B Simonsen, The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv Drug Deliv Rev. 2022;188:114416. doi: 10.1016/j.addr.2022.114416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Xu K, Taratula Q, Farsad K. Applications of nanoparticles in biomedical imaging. In Nanoscale. 2019;11(3):799–819. doi: 10.1039/C8NR07769J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan Z, Kumar ND, Reggiori F, Khan G. How viruses hijack and modify the secretory transport pathway. Cells. 2021;10(10):2535. doi: 10.3390/cells10102535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Zhang L, Song C. Luteinizing hormone-releasing hormone receptor-mediated delivery of mitoxantrone using LHRH analogs modified with PEGylated liposomes. Int J Nanomed. 2010;5:697–705. doi: 10.2147/ijn.s12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Na MH, Kim JS, Lee GY, Park JY, Hoffman AS, Nam JO, Han SE, Sim GY, Oh YK, Kim IS, Lee BH. A novel peptide probe for imaging and targeted delivery of liposomal doxorubicin to lung tumor. Mol Pharm. 2011;8:430–438. doi: 10.1021/mp100266g. [DOI] [PubMed] [Google Scholar]
- Heimberger AB, Wang E, McGary EC, Hess KR, Henry VK, Shono T, Cohen Z, Gumin J, Sawaya R, Conrad CA, Lang FF. Mechanisms of action of rapamycin in gliomas. Neuro Oncol. 2005;7:1–11. doi: 10.1215/S1152851704000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbok A, Rangger C, von Guggenberg E, Saba-Lepek M, Radolf T, Thurner G, Andreae F, Prassl R, Decristoforo C. Targeting properties of peptide-modified radiolabeled liposomal nanoparticles. Nanomedicine. 2012;8:112–118. doi: 10.1016/j.nano.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Hoffmann MM, Scharnagl H, Panagiotou E, Banghard WT, Wieland H, Marz W. Diminished LDL receptor and high heparin binding of apolipoprotein E2 Sendai associated with lipoprotein glomerulopathy. J Am Soc Nephrol. 2001;12:524–530. doi: 10.1681/ASN.V123524. [DOI] [PubMed] [Google Scholar]
- Holtze C. Large-scale droplet production in microfluidic devices-an industrial perspective. J Phys D Appl Phys. 2013;46(11):114008. doi: 10.1088/0022-3727/46/11/114008. [DOI] [Google Scholar]
- Hong H, Goel S, Zhang Y, Cai W. Molecular imaging with nucleic acid aptamers. Curr Med Chem. 2011;18:4195–4205. doi: 10.2174/092986711797189691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SPC, Chen YC, Chiang HC, Huang YC, Huang CC, Wang HE, Wang YS, Chi KH. Rapamycin and hydroxychloroquine combination alters macrophage polarization and sensitizes glioblastoma to immune checkpoint inhibitors. J Neurooncol. 2020;146:417–426. doi: 10.1007/s11060-019-03360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua H, Zhang X, Mu H, Meng Q, Jiang Y, Wang Y, Lu X, Wang A, Liu S, Zhang Y, Wan Z, Sun K. RVG29-modified docetaxel-loaded nanoparticles for brain-targeted glioma therapy. Int J Pharm. 2018;543:179–189. doi: 10.1016/j.ijpharm.2018.03.028. [DOI] [PubMed] [Google Scholar]
- Huang X, Kong N, Zhang X, Cao Y, Langer R, Tao W. The landscape of mRNA nanomedicines. Nat Med. 2022;28:2273–2287. doi: 10.1038/s41591-022-02061-1. [DOI] [PubMed] [Google Scholar]
- Jayasena SD. Aptamers: an emerging class of molecules that rival antibodies in diagnostics. Clin Chem. 1999;45:1628–1650. doi: 10.1093/clinchem/45.9.1628. [DOI] [PubMed] [Google Scholar]
- Jiang BH, Liu LZ. PI3K/PTEN signaling in angiogenesis and tumorigenesis. Adv Cancer Res. 2009;102:19–65. doi: 10.1016/S0065-230X(09)02002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Yang SJ, Wang JC, Yang LJ, Xu ZZ, Yang T, Liu XY, Zhang Q. Sequential treatment of drug-resistant tumors with RGD-modified liposomes containing siRNA or doxorubicin. Eur J Pharm Biopharm. 2010;76:170–178. doi: 10.1016/j.ejpb.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Jiang T, Henderson JM, Coote K, Cheng Y, Valley HC, Zhang X-O, Wang Q, Rhym LH, Cao Y, Newby GA, Bihler H, Mense M, Weng Z, Anderson DG, McCaffrey AP, Liu DR, Xue W. Chemical modifications of adenine base editor mRNA and guide RNA expand its application scope. Nat Commun. 2020;11:1979. doi: 10.1038/s41467-020-15892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. Nanoparticle PEGylation for imaging and therapy. Nanomedicine (lond) 2011;6:715–728. doi: 10.2217/nnm.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarehei F. The effects of combination therapy by solid lipid nanoparticle and dental stem cells on different degenerative diseases. In Am J Transl Res. 2022;14(5):3327–3343. [PMC free article] [PubMed] [Google Scholar]
- Kamberi M, Tran TN. UV-visible spectroscopy as an alternative to liquid chromatography for determination of everolimus in surfactant-containing dissolution media: A useful approach based on solid-phase extraction. J Pharm Biomed Anal. 2012;70:94–100. doi: 10.1016/j.jpba.2012.05.038. [DOI] [PubMed] [Google Scholar]
- Kang MJ, Lee S, Kim BK, Eum JY, Park SH, Kang MH, Oh CH, Choo J, Choi YW. Pep-1 Peptide-modified liposomal carriers for intracellular delivery of gold nanoparticles. Chem Pharm Bull (tokyo) 2011;59:109–112. doi: 10.1248/cpb.59.109. [DOI] [PubMed] [Google Scholar]
- Kapoor M, Lee SL, Tyner KM. Liposomal drug product development and quality: current US experience and perspective. AAPS J. 2017;19:632–641. doi: 10.1208/s12248-017-0049-9. [DOI] [PubMed] [Google Scholar]
- Katanasaka Y, Ishii T, Asai T, Naitou H, Maeda N, Koizumi F, Miyagawa S, Ohashi N, Oku N. Cancer antineovascular therapy with liposome drug delivery systems targeted to BiP/GRP78. Int J Cancer. 2010;127:2685–2698. doi: 10.1002/ijc.25276. [DOI] [PubMed] [Google Scholar]
- Kaur IP, Bhandari R, Bhandari S, Kakkar V. Potential of solid lipid nanoparticles in brain targeting. J Control Release. 2008;127:97–109. doi: 10.1016/j.jconrel.2007.12.018. [DOI] [PubMed] [Google Scholar]
- Kou L, Bhutia YD, Yao Q, He Z, Sun J, Ganapathy V. Transporter-guided delivery of nanoparticles to improve drug permeation across cellular barriers and drug exposure to selective cell types. Front Pharmacol. 2018;9:1–16. doi: 10.3389/fphar.2018.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuter J. Application of nanoparticles for the delivery of drugs to the brain. Int Congr Ser. 2005;1277:85–94. doi: 10.1016/j.ics.2005.02.014. [DOI] [Google Scholar]
- Kuai R, Yuan W, Qin Y, Chen H, Tang J, Yuan M, Zhang Z, He Q. Efficient delivery of payload into tumor cells in a controlled manner by TAT and thiolytic cleavable PEG co-modified liposomes. Mol Pharm. 2010;7:1816–1826. doi: 10.1021/mp100171c. [DOI] [PubMed] [Google Scholar]
- Kuai R, Yuan W, Li W, Qin Y, Tang J, Yuan M, Fu L, Ran R, Zhang Z, He Q. Targeted delivery of cargoes into a murine solid tumor by a cell-penetrating peptide and cleavable poly(ethylene glycol) comodified liposomal delivery system via systemic administration. Mol Pharm. 2011;8:2151–2161. doi: 10.1021/mp200100f. [DOI] [PubMed] [Google Scholar]
- Kulkarni JA, Witzigmann D, Chen S, Cullis PR, Van Der Meel R. Lipid nanoparticle technology for clinical translation of siRNA therapeutics. Acc Chem Res. 2019;52(9):2435–2444. doi: 10.1021/acs.accounts.9b00368. [DOI] [PubMed] [Google Scholar]
- Kulkarni JA, Witzigmann D, Thomson SB, Chen S, Leavitt BR, Cullis PR, Van Der Meel R. The current landscape of nucleic acid therapeutics Nat. Nanotechnol. 2021;16:630–643. doi: 10.1038/s41565-021-00898-0. [DOI] [PubMed] [Google Scholar]
- Kuo YC, Chou PR. Neuroprotection against degeneration of sk-N-mc cells using neuron growth factor-encapsulated liposomes with surface cereport and transferrin. J Pharm Sci. 2014;103:2484–2497. doi: 10.1002/jps.24081. [DOI] [PubMed] [Google Scholar]
- Kuo Y-C, Wang L-J. Transferrin-grafted catanionic solid lipid nanoparticles for targeting delivery of saquinavir to the brain. J Taiwan Inst Chem Eng. 2014;45:755–763. doi: 10.1016/j.jtice.2013.09.024. [DOI] [Google Scholar]
- Lassalle HP, Marchal S, Guillemin F, Reinhard A, Bezdetnaya L. Aptamers as remarkable diagnostic and therapeutic agents in cancer treatment. Curr Drug Metab. 2012;13:1130–1144. doi: 10.2174/138920012802850038. [DOI] [PubMed] [Google Scholar]
- Levy JM, Yeh WH, Pendse N, Davis JR, Hennessey E, Butcher R, Koblan LW, Comander J, Liu Q, Liu DR. Cytosine and adenine base editing of the brain, liver, retina, heart and skeletal muscle of mice via adeno-associated viruses. Nat Biomed Eng. 2020;4:97–110. doi: 10.1038/s41551-019-0501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SD, Huang L. Targeted delivery of antisense oligodeoxynucleotide and small interference RNA into lung cancer cells. Mol Pharm. 2006;3:579–588. doi: 10.1021/mp060039w. [DOI] [PubMed] [Google Scholar]
- Li SD, Chono S, Huang L. Efficient oncogene silencing and metastasis inhibition via systemic delivery of siRNA. Mol Ther. 2008;16:942–946. doi: 10.1038/mt.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Su B, Meng S, Ju L, Yan L, Ding Y, Song Y, Zhou W, Li H, Tang L, Zhao Y, Zhou C. RGD-targeted paramagnetic liposomes for early detection of tumor: in vitro and in vivo studies. Eur J Radiol. 2011;80:598–606. doi: 10.1016/j.ejrad.2011.01.051. [DOI] [PubMed] [Google Scholar]
- Li S, Peng Z, Dallman J, Baker J, Othman AM, Blackwelder PL, Leblanc RM. Crossing the blood-brain-barrier with transferrin conjugated carbon dots: a zebrafish model study. Colloids Surf B Biointerfaces. 2016;145:251–256. doi: 10.1016/j.colsurfb.2016.05.007. [DOI] [PubMed] [Google Scholar]
- Lin AEJ, Guttman JA. Hijacking the endocytic machinery by microbial pathogens. Protoplasma. 2010;244:75–90. doi: 10.1007/s00709-010-0164-2. [DOI] [PubMed] [Google Scholar]
- Liu Y, Huang R, Han L, Ke W, Shao K, Ye L, Lou J, Jiang C. Brain-targeting gene delivery and cellular internalization mechanisms for modified rabies virus glycoprotein RVG29 nanoparticles. Biomaterials. 2009;30:4195–4202. doi: 10.1016/j.biomaterials.2009.02.051. [DOI] [PubMed] [Google Scholar]
- Liu P, Chen G, Zhang J. A review of liposomes as a drug delivery system: current status of approved products, regulatory environments, and future perspectives. Molecules. 2022;27(4):1372. doi: 10.3390/molecules27041372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi M, Di Paolo D, Soster M, Brignole C, Bartolini A, Emionite L, Sun J, Becherini P, Curnis F, Petretto A, Sani M, Gori A, Milanese M, Gambini C, Longhi R, Cilli M, Allen TM, Bussolino F, Arap W, Pasqualini R, Corti A, Ponzoni M, Marchio S, Pastorino F. Novel phage display-derived neuroblastoma-targeting peptides potentiate the effect of drug nanocarriers in preclinical settings. J Control Release. 2013;170:233–241. doi: 10.1016/j.jconrel.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loureiro JA, Andrade S, Duarte A, Neves AR, Queiroz JF, Nunes C, Sevin E, Fenart L, Gosselet F, Coelho MA, Pereira MC. Resveratrol and grape extract-loaded solid lipid nanoparticles for the treatment of Alzheimer's disease. Molecules. 2017;22:277. doi: 10.3390/molecules22020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery A, Onishko H, Hallahan DE, Han Z. Tumor-targeted delivery of liposome-encapsulated doxorubicin by use of a peptide that selectively binds to irradiated tumors. J Control Release. 2011;150:117–124. doi: 10.1016/j.jconrel.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunnoo T, Assawakhajornsak J, Puangmali T. In silico study of gold nanoparticle uptake into a mammalian cell: interplay of size, shape, surface charge, and aggregation. J Phys Chem C. 2019;123:3801–3810. doi: 10.1021/acs.jpcc.8b07616. [DOI] [Google Scholar]
- Mai J, Song S, Rui M, Liu D, Ding Q, Peng J, Xu Y. A synthetic peptide mediated active targeting of cisplatin liposomes to Tie2 expressing cells. J Control Release. 2009;139:174–181. doi: 10.1016/j.jconrel.2009.06.024. [DOI] [PubMed] [Google Scholar]
- Manshian BB, Jiménez J, Himmelreich U, Soenen SJ. Personalized medicine and follow-up of therapeutic delivery through exploitation of quantum dot toxicity. Biomaterials. 2017;127:1–12. doi: 10.1016/j.biomaterials.2017.02.039. [DOI] [PubMed] [Google Scholar]
- Marcelo DTT, Adriana FS, Gislaine PA, Cibele NP, Giselle C, Anderson OR, Vani XO, Nunez CDLF. The wasp venom antimicrobial peptide polybia-CP and its synthetic derivatives display antiplasmodial and anticancer properties. Bioeng Transl Med. 2000;5(3):e10167. doi: 10.1002/btm2.10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos-Maroto A, Gai M, Brückner M, da Costa Marques R, Harley I, Simon J, Mailänder V, Morsbach S, Landfester K. Systematic modulation of the lipid composition enables the tuning of liposome cellular uptake. Acta Biomater. 2023;158:463–474. doi: 10.1016/j.actbio.2022.12.058. [DOI] [PubMed] [Google Scholar]
- Mazuryk J, Deptuła T, Polchi A, Gapiński J, Giovagnoli S, Magini A, Emiliani C, Kohlbrecher J, Patkowski A. Rapamycin-loaded solid lipid nanoparticles: morphology and impact of the drug loading on the phase transition between lipid polymorphs. Colloids Surf, A. 2016;502:54–65. doi: 10.1016/j.colsurfa.2016.05.017. [DOI] [Google Scholar]
- McClements DJ. Advances in edible nanoemulsions: digestion, bioavailability, and potential toxicity. Prog Lipid Res. 2021;81:101081. doi: 10.1016/j.plipres.2020.101081. [DOI] [PubMed] [Google Scholar]
- Mecca C, Giambanco I, Donato R, Arcuri C. Targeting mTOR in glioblastoma: rationale and preclinical/clinical evidence. Dis Markers. 2018;2018:9230479. doi: 10.1155/2018/9230479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meckes DG. Exosomal communication goes viral. J Virol. 2015;89(10):5200–5203. doi: 10.1128/JVI.02470-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng S, Su B, Li W, Ding Y, Tang L, Zhou W, Song Y, Li H, Zhou C. Enhanced antitumor effect of novel dual-targeted paclitaxel liposomes. Nanotechnology. 2010;21:415103. doi: 10.1088/0957-4484/21/41/415103. [DOI] [PubMed] [Google Scholar]
- Michaelis K, Hoffmann MM, Dreis S, Herbert E, Alyautdin RN, Michaelis M, Kreuter J, Langer K. Covalent linkage of apolipoprotein e to albumin nanoparticles strongly enhances drug transport into the brain. J Pharmacol Exp Ther. 2006;317:1246–1253. doi: 10.1124/jpet.105.097139. [DOI] [PubMed] [Google Scholar]
- Mirchandani Y, Patravale VB, Brijesh S. Solid lipid nanoparticles for hydrophilic drugs. J Control Release. 2021;335:457–464. doi: 10.1016/j.jconrel.2021.05.032. [DOI] [PubMed] [Google Scholar]
- Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20:101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitragotri S, Lammers T, Bae YH, Schwendeman S, Smedt SD, Leroux JC, Peer D, Kwon IC, Harashima H, Kikuchi A, Oh YK, Torchilin V, Hennink W, Hanes J, Park K. Drug delivery research for the future: expanding the nano horizons and beyond. J Control Release. 2017;246:183–184. doi: 10.1016/j.jconrel.2017.01.011. [DOI] [PubMed] [Google Scholar]
- Moody TW, Korman LY. The release of bombesin-like peptides from small cell lung cancer cells. Ann N Y Acad Sci. 1988;547:351–359. doi: 10.1111/j.1749-6632.1988.tb23902.x. [DOI] [PubMed] [Google Scholar]
- Moon JJ, Suh H, Bershteyn A, Stephan MT, Liu H, Huang B, Sohail M, Luo S, Um SH, Khant H, Goodwin JT, Ramos J, Chiu W, Irvine DJ. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat Mater. 2011;10:243–251. doi: 10.1038/nmat2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos T, Morgan EH. Transferrin and transferrin receptor function in brain barrier systems. Cell Mol Neurobiol. 2000;20:77–95. doi: 10.1023/A:1006948027674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisco A, Accardo A, Tesauro D, Palumbo R, Benedetti E, Morelli G. Peptide-labeled supramolecular aggregates as selective doxorubicin carriers for delivery to tumor cells. Biopolymers. 2011;96:88–96. doi: 10.1002/bip.21491. [DOI] [PubMed] [Google Scholar]
- Mudshinge SR, Deore AB, Patil S, Bhalgat CM. Nanoparticles: emerging carriers for drug delivery. Saudi Pharm J. 2011;19:129–141. doi: 10.1016/j.jsps.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder WJ, Castermans K, van Beijnum JR, Oude Egbrink MG, Chin PT, Fayad ZA, Lowik CW, Kaijzel EL, Que I, Storm G, Strijkers GJ, Griffioen AW, Nicolay K. Molecular imaging of tumor angiogenesis using alphavbeta3-integrin targeted multimodal quantum dots. Angiogenesis. 2009;12:17–24. doi: 10.1007/s10456-008-9124-2. [DOI] [PubMed] [Google Scholar]
- Muller RH, Ruhl D, Runge S, Schulze-Forster K, Mehnert W. Cytotoxicity of solid lipid nanoparticles as a function of the lipid matrix and the surfactant. Pharm Res. 1997;14:458–462. doi: 10.1023/A:1012043315093. [DOI] [PubMed] [Google Scholar]
- Murase Y, Asai T, Katanasaka Y, Sugiyama T, Shimizu K, Maeda N, Oku N. A novel DDS strategy, "dual-targeting", and its application for antineovascular therapy. Cancer Lett. 2010;287:165–171. doi: 10.1016/j.canlet.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Muzzi M, Zecchi R, Ranieri G, Urru M, Tofani L, De Cesaris F, Panconesi A, Chiarugi A. Ultra-rapid brain uptake of subcutaneous sumatriptan in the rat: implication for cluster headache treatment. Cephalalgia. 2020;40:330–336. doi: 10.1177/0333102419896370. [DOI] [PubMed] [Google Scholar]
- Nabhan JF, Wood KM, Rao VP, Morin J, Bhamidipaty S, LaBranche TP, Gooch RL, Bozal F, Bulawa CE, Guild BC. Intrathecal delivery of frataxin mRNA encapsulated in lipid nanoparticles to dorsal root ganglia as a potential therapeutic for Friedreich's ataxia. Sci Rep. 2016;6:20019. doi: 10.1038/srep20019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseri N, Valizadeh H, Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application. Adv Pharm Bull. 2015;5:305–313. doi: 10.15171/apb.2015.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negussie AH, Miller JL, Reddy G, Drake SK, Wood BJ, Dreher MR. Synthesis and in vitro evaluation of cyclic NGR peptide targeted thermally sensitive liposome. J Control Release. 2010;143:265–273. doi: 10.1016/j.jconrel.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves AR, Lucio M, Martins S, Lima JL, Reis S. Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. Int J Nanomed. 2013;8:177–187. doi: 10.2147/IJN.S37840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves AR, Queiroz JF, Reis S. Brain-targeted delivery of resveratrol using solid lipid nanoparticles functionalized with apolipoprotein E. J Nanobiotechnol. 2016;14:27. doi: 10.1186/s12951-016-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves AR, Queiroz JF, Lima SAC, Reis S. Apo E-functionalization of solid lipid nanoparticles enhances brain drug delivery: uptake mechanism and transport pathways. Bioconjug Chem. 2017;28:995–1004. doi: 10.1021/acs.bioconjchem.6b00705. [DOI] [PubMed] [Google Scholar]
- Nguyen DG, Booth A, Gould SJ, Hildreth JEK. Evidence that HIV budding in primary macrophages occurs through the exosome release pathway. J Biol Chem. 2003;278:52347–52354. doi: 10.1074/jbc.M309009200. [DOI] [PubMed] [Google Scholar]
- Ni X, Castanares M, Mukherjee A, Lupold SE. Nucleic acid aptamers: clinical applications and promising new horizons. Curr Med Chem. 2011;18:4206–4214. doi: 10.2174/092986711797189600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirale P, Paul A, Yadav KS. Nanoemulsions for targeting the neurodegenerative diseases: Alzheimer's Parkinson's and Prion's. Life Sci. 2020;245:117394. doi: 10.1016/j.lfs.2020.117394. [DOI] [PubMed] [Google Scholar]
- Oku N, Asai T, Watanabe K, Kuromi K, Nagatsuka M, Kurohane K, Kikkawa H, Ogino K, Tanaka M, Ishikawa D, Tsukada H, Momose M, Nakayama J, Taki T. Anti-neovascular therapy using novel peptides homing to angiogenic vessels. Oncogene. 2002;21:2662–2669. doi: 10.1038/sj.onc.1205347. [DOI] [PubMed] [Google Scholar]
- Ostrosky-Zeichner L, Marr KA, Rex JH, Cohen SH. Amphotericin B: time for a new ″Gold Standard″. Clin Infect Dis. 2003;37(3):415. doi: 10.1086/376634. [DOI] [PubMed] [Google Scholar]
- Oswald M, Geissler S, Goepferich A. Targeting the central nervous system (CNS): a review of rabies virus-targeting strategies. Mol Pharm. 2017;14:2177–2196. doi: 10.1021/acs.molpharmaceut.7b00158. [DOI] [PubMed] [Google Scholar]
- Packer M, Gyawali D, Yerabolu R, Schariter J, White P. A novel mechanism for the loss of mRNA activity in lipid nanoparticle delivery systems. Nat Commun. 2021;12(1):6777. doi: 10.1038/s41467-021-26926-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn TO, Petersen MA, Waybrant B, Adil MM, Kokkoli E. Peptide- and aptamer-functionalized nanovectors for targeted delivery of therapeutics. J Biomech Eng. 2009;131:074005. doi: 10.1115/1.3160763. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Why is the global CNS pharmaceutical market so under-penetrated? Drug Discov Today. 2002;7:5–7. doi: 10.1016/S1359-6446(01)02082-7. [DOI] [PubMed] [Google Scholar]
- Park JH, von Maltzahn G, Xu MJ, Fogal V, Kotamraju VR, Ruoslahti E, Bhatia SN, Sailor MJ. Cooperative nanomaterial system to sensitize, target, and treat tumors. Proc Natl Acad Sci U S A. 2010;107:981–986. doi: 10.1073/pnas.0909565107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualini R, Ruoslahti E. Organ targeting in vivo using phage display peptide libraries. Nature. 1996;380:364–366. doi: 10.1038/380364a0. [DOI] [PubMed] [Google Scholar]
- Pasqualini R, Koivunen E, Kain R, Lahdenranta J, Sakamoto M, Stryhn A, Ashmun RA, Shapiro LH, Arap W, Ruoslahti E. Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res. 2000;60:722–727. [PMC free article] [PubMed] [Google Scholar]
- Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Swamy MK, Sharma S, Habtemariam S, Shin HS. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16:71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paula S, Tabet MR, Ball WJ., Jr Interactions between cardiac glycosides and sodium/potassium-ATPase: three-dimensional structure-activity relationship models for ligand binding to the E2-Pi form of the enzyme versus activity inhibition. Biochemistry. 2005;44:498–510. doi: 10.1021/bi048680w. [DOI] [PubMed] [Google Scholar]
- Paulos CM, Reddy JA, Leamon CP, Turk MJ, Low PS. Ligand binding and kinetics of folate receptor recycling in vivo: impact on receptor-mediated drug delivery. Mol Pharmacol. 2004;66:1406–1414. doi: 10.1124/mol.104.003723. [DOI] [PubMed] [Google Scholar]
- Pearce TR, Shroff K, Kokkoli E. Peptide targeted lipid nanoparticles for anticancer drug delivery. Adv Mater. 2012;24:3803–3822. doi: 10.1002/adma.201200832. [DOI] [PubMed] [Google Scholar]
- Pecot CV, Calin GA, Coleman RL, Lopez-Berestein G, Sood AK. RNA interference in the clinic: challenges and future directions. Nat Rev Cancer. 2011;11:59–67. doi: 10.1038/nrc2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- Petrenko V, Sinturel F, Riezman H, Dibner C. Lipid metabolism around the body clocks. Prog Lipid Res. 2023;91:101235. doi: 10.1016/j.plipres.2023.101235. [DOI] [PubMed] [Google Scholar]
- Pinedo HM, Smorenburg CH. Drugs affecting growth of tumours. Birkhauser. 2006;14:1–247. [Google Scholar]
- Pinheiro RGR, Granja A, Loureiro JA, Pereira MC, Pinheiro M, Neves AR, Reis S. RVG29-functionalized lipid nanoparticles for quercetin brain delivery and Alzheimer's disease. Pharm Res. 2020;37:139. doi: 10.1007/s11095-020-02865-1. [DOI] [PubMed] [Google Scholar]
- Prabhakar K, Afzal SM, Surender G, Kishan V. Tween 80 containing lipid nanoemulsions for delivery of indinavir to brain. Acta Pharm Sin B. 2013;3:345–353. doi: 10.1016/j.apsb.2013.08.001. [DOI] [Google Scholar]
- Ran R, Sun Q, Baby T, Wibowo D, Middelberg AP, Zhao CXS. Multiphase microfluidic synthesis of micro-and nanostructures for pharmaceutical applications. Chem Eng Sci. 2017;169:78–96. doi: 10.1016/j.ces.2017.01.008. [DOI] [Google Scholar]
- Ransohoff JD, Wei Y, Khavari PA. The functions and unique features of long intergenic non-coding RNA. Nat Rev Mol Cell Biol. 2018;19(3):143–157. doi: 10.1038/nrm.2017.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees HA, Liu DR. Base editing: precision chemistry on the genome and transcriptome of living cells. Nat Rev Genet. 2018;19:770–788. doi: 10.1038/s41576-018-0059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richner JM, Himansu S, Dowd KA, Butler SL, Salazar V, Fox JM, Julander JG, Tang WW, Shresta S, Pierson TC, Ciaramella G, Diamond MS. Modified mRNA vaccines protect against Zika virus infection. Cell. 2017;168(6):1114. doi: 10.1016/j.cell.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley RS, Dang MN, Billingsley MM, Abraham B, Gundlach L, Day ES. Evaluating the mechanisms of light-triggered siRNA release from nanoshells for temporal control over gene regulation. Nano Lett. 2018;18:3565–3570. doi: 10.1021/acs.nanolett.8b00681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley RS, June CH, Langer Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18:175–196. doi: 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghipour S, Mathias RA. Herpesviruses hijack host exosomes for viral pathogenesis. Semin Cell Dev Biol. 2017;67:91–100. doi: 10.1016/j.semcdb.2017.03.005. [DOI] [PubMed] [Google Scholar]
- Sakurai Y, Yoshikawa K, Arai K, Kazaoka A, Aoki S, Ito K, Nakai Y, Tange K, Furihata T, Tanaka H, Akita H. siRNA delivery to lymphatic endothelial cells via ApoE-mediated uptake by lipid nanoparticles. J Control Release. 2023;353:125–133. doi: 10.1016/j.jconrel.2022.11.036. [DOI] [PubMed] [Google Scholar]
- Sapra P, Allen TM. Ligand-targeted liposomal anticancer drugs. Prog Lipid Res. 2003;42:439–462. doi: 10.1016/S0163-7827(03)00032-8. [DOI] [PubMed] [Google Scholar]
- Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple SC, Akinc A, Chen J, Sandhu AP, Mui BL, Cho CK, Sah DW, Stebbing D, Crosley EJ, Yaworski E, Hafez IM, Dorkin JR, Qin J, Lam K, Rajeev KG, Wong KF, Jeffs LB, Nechev L, Eisenhardt ML, Jayaraman M, Kazem M, Maier MA, Srinivasulu M, Weinstein MJ, Chen Q, Alvarez R, Barros SA, De S, Klimuk SK, Borland T, Kosovrasti V, Cantley WL, Tam YK, Manoharan M, Ciufolini MA, Tracy MA, de Fougerolles A, MacLachlan I, Cullis PR, Madden TD, Hope MJ. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol. 2010;28:172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:286. doi: 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicari D, Chatziioannou A, Koutsandreas T, Sitia R, Chevet E. Role of the early secretory pathway in SARS-CoV-2 infection. J Cell Biol. 2020;219(9):202006005. doi: 10.1083/jcb.202006005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidow A, Spies N. Concepts in solid tumor evolution. Trends Genet. 2015;31(4):208–214. doi: 10.1016/j.tig.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simamora P, Alvarez JM, Yalkowsky SH. Solubilization of rapamycin. Int J Pharm. 2001;213:25–29. doi: 10.1016/S0378-5173(00)00617-7. [DOI] [PubMed] [Google Scholar]
- Smith GP, Petrenko VA. Phage Display. Chem Rev. 1997;97:391–410. doi: 10.1021/cr960065d. [DOI] [PubMed] [Google Scholar]
- Song S, Liu D, Peng J, Deng H, Guo Y, Xu LX, Miller AD, Xu Y. Novel peptide ligand directs liposomes toward EGF-R high-expressing cancer cells in vitro and in vivo. FASEB J. 2009;23:1396–1404. doi: 10.1096/fj.08-117002. [DOI] [PubMed] [Google Scholar]
- Song SY, Kim KP, Jeong SY, Park J, Park J, Jung J, Chung HK, Lee SW, Seo MH, Lee JS, Jung KH, Choi EK. Polymeric nanoparticle-docetaxel for the treatment of advanced solid tumors: phase I clinical trial and preclinical data from an orthotopic pancreatic cancer model. Oncotarget. 2016;7(47):77348–77357. doi: 10.18632/oncotarget.12668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song CQ, Jiang T, Richter M, Rhym LH, Koblan LW, Zafra MP, Schatoff EM, Doman JL, Cao Y, Dow LE, Zhu LJ, Anderson DG, Liu DR, Yin H, Xue W. Adenine base editing in an adult mouse model of tyrosinaemia. Nat Biomed Eng. 2020;4:125–130. doi: 10.1038/s41551-019-0357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni S, Ruhela RK, Medhi B. Nanomedicine in central nervous system (CNS) disorders: a present and future prospective. Adv Pharm Bull. 2016;6:319–335. doi: 10.15171/apb.2016.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda Y, Ozawa T, Aldape KD, Deen DF, Berger MS, Pieper RO. Akt pathway activation converts anaplastic astrocytoma to glioblastoma multiforme in a human astrocyte model of glioma. Cancer Res. 2001;61:6674–6678. [PubMed] [Google Scholar]
- Sottoriva A, Kang H, Ma Z, Graham TA, Salomon MP, Zhao J, Marjoram P, Siegmund K, Press MF, Shibata D, Curtis C. A big bang model of human colorectal tumor growth. Nat Genet. 2015;47(3):209–216. doi: 10.1038/ng.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staring J, Raaben M, Brummelkamp TR. Viral escape from endosomes and host detection at a glance. J Cell Sci. 2018;131(15):jcs216259. doi: 10.1242/jcs.216259. [DOI] [PubMed] [Google Scholar]
- Stone NR, Bicanic T, Salim R, Hope W. Liposomal amphotericin B (AmBisome((R): a review of the pharmacokinetics, pharmacodynamics, clinical experience and future directions. Drugs. 2016;76(4):485–500. doi: 10.1007/s40265-016-0538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takara K, Hatakeyama H, Ohga N, Hida K, Harashima H. Design of a dual-ligand system using a specific ligand and cell penetrating peptide, resulting in a synergistic effect on selectivity and cellular uptake. Int J Pharm. 2010;396:143–148. doi: 10.1016/j.ijpharm.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Trepanier DJ, Gallant H, Legatt DF, Yatscoff RW. Rapamycin: distribution, pharmacokinetics and therapeutic range investigations: an update. Clin Biochem. 1998;31:345–351. doi: 10.1016/S0009-9120(98)00048-4. [DOI] [PubMed] [Google Scholar]
- Trucillo P, Campardelli R, Reverchon E. Liposomes: From bangham to supercritical fluids. Processes. 2020;8(9):1022. doi: 10.3390/pr8091022. [DOI] [Google Scholar]
- Tu Y, Tao J, Wang F, Liu P, Han Z, Li Z, Ma Y, Gu Y. A novel peptide targeting gastrin releasing peptide receptor for pancreatic neoplasm detection. Biomater Sci. 2020;8:2682–2693. doi: 10.1039/D0BM00162G. [DOI] [PubMed] [Google Scholar]
- Vetten MA, Yah CS, Singh T, Gulumian M. Challenges facing sterilization and depyrogenation of nanoparticles: effects on structural stability and biomedical applications. Nanomedicine. 2014;10(7):1391–1399. doi: 10.1016/j.nano.2014.03.017. [DOI] [PubMed] [Google Scholar]
- Waghule T, Saha RN, Alexander A, Singhvi G. Tailoring the multi-functional properties of phospholipids for simple to complex self-assemblies. J Control Release. 2022;349:460–474. doi: 10.1016/j.jconrel.2022.07.014. [DOI] [PubMed] [Google Scholar]
- Wang X. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2021;384:1576–1578. doi: 10.1056/NEJMc2036242. [DOI] [PubMed] [Google Scholar]
- Wang T, D'Souza GG, Bedi D, Fagbohun OA, Potturi LP, Papahadjopoulos-Sternberg B, Petrenko VA, Torchilin VP. Enhanced binding and killing of target tumor cells by drug-loaded liposomes modified with tumor-specific phage fusion coat protein. Nanomedicine (lond) 2010;5:563–574. doi: 10.2217/nnm.10.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Yang S, Petrenko VA, Torchilin VP. Cytoplasmic delivery of liposomes into MCF-7 breast cancer cells mediated by cell-specific phage fusion coat protein. Mol Pharm. 2010;7:1149–1158. doi: 10.1021/mp1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H-Q, Xie X-P, Yang W, Zhang L. Identifying Biomarkers of Cisplatin Sensitivity in Non-Small Cell Lung Cancer via Comprehensive Integrative Analysis. Curr Bioinform. 2022;17:498–509. doi: 10.2174/1574893617666220407105905. [DOI] [Google Scholar]
- Wanigasooriya K, Tyler R, Barros-Silva JD, Sinha Y, Ismail T, Beggs AD. Radiosensitising cancer using phosphatidylinositol-3-kinase (PI3K), protein kinase B (AKT) or mammalian target of rapamycin (mTOR) inhibitors. Cancers (basel) 2020;12:1278. doi: 10.3390/cancers12051278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Kuipers F, Havekes LM, Havinga R, Dontje B, Poelstra K, Scherphof GL, Kamps JA. The role of apolipoprotein E in the elimination of liposomes from blood by hepatocytes in the mouse. Biochem Biophys Res Commun. 2005;328:57–62. doi: 10.1016/j.bbrc.2004.12.137. [DOI] [PubMed] [Google Scholar]
- Yan Z, Zhan C, Wen Z, Feng L, Wang F, Liu Y, Yang X, Dong Q, Liu M, Lu W. LyP-1-conjugated doxorubicin-loaded liposomes suppress lymphatic metastasis by inhibiting lymph node metastases and destroying tumor lymphatics. Nanotechnology. 2011;22:415103. doi: 10.1088/0957-4484/22/41/415103. [DOI] [PubMed] [Google Scholar]
- Yan Z, Wang F, Wen Z, Zhan C, Feng L, Liu Y, Wei X, Xie C, Lu W. LyP-1-conjugated PEGylated liposomes: a carrier system for targeted therapy of lymphatic metastatic tumor. J Control Release. 2012;157:118–125. doi: 10.1016/j.jconrel.2011.07.034. [DOI] [PubMed] [Google Scholar]
- Yardley DA. nab-Paclitaxel mechanisms of action and delivery. J Control Release. 2013;170(3):365–372. doi: 10.1016/j.jconrel.2013.05.041. [DOI] [PubMed] [Google Scholar]
- Yeh WZ, Blizzard L, Taylor BV. What is the actual prevalence of migraine? Brain Behav. 2018;8:e00950. doi: 10.1002/brb3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yingchoncharoen P, Kalinowski DS, Richardson DR. Lipid-based drug delivery systems in cancer therapy: what is available and what is yet to come. Pharmacol Rev. 2016;68:701–787. doi: 10.1124/pr.115.012070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You L, Wang J, Liu T, Zhang Y, Han X, Wang T, Guo S, Dong T, Xu J, Anderson GJ, Liu Q, Chang YZ, Lou X, Nie G. Targeted brain delivery of rabies virus glycoprotein 29-modified deferoxamine-loaded nanoparticles reverses functional deficits in parkinsonian mice. ACS Nano. 2018;12:4123–4139. doi: 10.1021/acsnano.7b08172. [DOI] [PubMed] [Google Scholar]
- Zeltzer S, Zeltzer CA, Igarashi S, Wilson J, Donaldson JG, Goodrum F. Virus control of trafficking from sorting endosomes. Mbio. 2018;9(4):e00683. doi: 10.1128/mBio.00683-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Jin W, Wang X, Wang J, Zhang X, Zhang Q. A novel octreotide modified lipid vesicle improved the anticancer efficacy of doxorubicin in somatostatin receptor 2 positive tumor models. Mol Pharm. 2010;7:1159–1168. doi: 10.1021/mp1000235. [DOI] [PubMed] [Google Scholar]
- Zhang YF, Wang JC, Bian DY, Zhang X, Zhang Q. Targeted delivery of RGD-modified liposomes encapsulating both combretastatin A-4 and doxorubicin for tumor therapy: in vitro and in vivo studies. Eur J Pharm Biopharm. 2010;74:467–473. doi: 10.1016/j.ejpb.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Zhao L, Huang L. Lipid nanoparticles for gene delivery. Adv Genet. 2014;88:13–36. doi: 10.1016/B978-0-12-800148-6.00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Wang JC, Sun QS, Luo CL, Zhang Q. RGD-based strategies for improving antitumor activity of paclitaxel-loaded liposomes in nude mice xenografted with human ovarian cancer. J Drug Target. 2009;17:10–18. doi: 10.1080/10611860802368966. [DOI] [PubMed] [Google Scholar]
- Zhao, Woodle M, Mixson AJ. Advances in delivery systems for doxorubicin. J Nanomed Nanotechnol. 2018;09(05):519. doi: 10.4172/2157-7439.1000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Sun X, Zeng L, Liu J, Zhang Z. A randomized multicenter phase II clinical trial of mitoxantrone-loaded nanoparticles in the treatment of 108 patients with unresected hepatocellular carcinoma. Nanomedicine. 2009;5(4):419–423. doi: 10.1016/j.nano.2009.01.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used for the research described in the article.
No code is used here.