Abstract
The endosymbiotic bacteria Wolbachia can invade insect populations by modifying host reproduction through cytoplasmic incompatibility (CI), an effect that results in embryonic lethality when Wolbachia-carrying males mate with Wolbachia-free females. Here we describe a transgenic system for recreating CI in the major arbovirus vector Aedes aegypti using CI factor (cif) genes from wAlbB, a Wolbachia strain currently being deployed to reduce dengue transmission. CI-like sterility is induced when cifA and cifB are co-expressed in testes; this sterility is rescued by maternal cifA expression, thereby reproducing the pattern of Wolbachia-induced CI. Expression of cifB alone is associated with extensive DNA damage and disrupted spermatogenesis. The strength of rescue by maternal cifA expression is dependent on the comparative levels of cifA/cifB expression in males. These findings are consistent with CifB acting as a toxin and CifA as an antitoxin, with CifA attenuating CifB toxicity in both the male germline and in developing embryos. These findings provide important insights into the interactions between cif genes and their mechanism of activity and provide a foundation for the building of a cif gene-based drive system in Ae. aegypti.
Subject terms: Applied microbiology, Gene expression, Bacterial genes
The Wolbachia cifA and cifB genes generate cytoplasmic incompatibility (CI) in insect hosts but the role of cifA is still debated. Here, the authors report the transgenic recapitulation of CI in the major arbovirus vector Aedes aegypti and provide evidence for cifA inhibiting cifB toxicity in the male germline.
Introduction
Many strains of the maternally transmitted intracellular bacteria Wolbachia can invade arthropod populations and remain at high frequency through modifications of host reproduction. Most commonly this occurs via a mechanism known as cytoplasmic incompatibility (CI), whereby modifications of the paternal chromatin result in embryonic lethality when Wolbachia-carrying males mate with Wolbachia-free females. If the females carry a compatible Wolbachia strain, CI-induced lethality is rescued, which provides Wolbachia-carrying females with a relative fitness advantage. The genetic basis for incompatibility stems from two syntenic and co-diverging genes which are broadly referred to as the CI factors, cifA and cifB1,2. These cif gene sequences can be highly divergent and homologues are sorted into five phylogenetic groups (Types I-V)3. Wolbachia strains can possess multiple pairs of similar or divergent cif gene types, which can result in complex patterns of mating incompatibility between strains4,5.
CI has been recapitulated using transgenic systems expressing the cif genes and CI induction and rescue have been attributed to CifB and CifA respectively2,6–14. However, for some host species and/or cif homologue combinations, the simultaneous co-expression of both cifA and cifB was found to be necessary for CI induction1,6,9. This finding led to a proposed “two-by-one” genetic model of CI, as both CifA and CifB were needed for CI induction while only CifA was required for rescue9 – although there are a minority of cases where cifB expression alone has been able to induce rescuable CI10,11. Despite the frequent requirement for cifA co-expression in achieving CI induction, its function in generating rescuable sterility is unknown.
There are currently two competing theories for how CI functions: the toxin-antidote (TA)15 and host-modification (HM)16 models. A key difference between these models concerns at which stage the modification(s) associated with CI induction occur. The TA model predicts that sperm-deposited CifB modifies the paternal chromatin in fertilised embryos unless inhibited by maternally-inherited CifA present in the zygote8,12,15,17. The CifA “antidote” is expected to neutralise CifB toxicity through the specific binding of cognate Cif pairs, which explains why incompatibility arises between Wolbachia strains encoding different Cif homologues. As the TA model predicts that for rescuable CI induction the CifB modification(s) should occur in the embryo, a potential role of CifA in CI induction is the prevention of premature modifications during spermatogenesis. Alternatively, the HM model predicts that CifB-mediated modification(s) occur during spermatogenesis16,18, with CifA acting as a co-factor. This model does not necessitate the packaging of either Cif peptide into sperm, and posits that rescue is attributed to either CifA-mediated reversal of the paternal chromatin modifications or compensatory modifications of the maternal chromatin. However, the HM model provides a less parsimonious explanation for the complex and diverse patterns of compatibility between Wolbachia strains observed in nature, as this would require a large number of host targets19.
Despite the high prevalence of Wolbachia infections in insect host species, a native strain of the bacterium has not been found in the major arbovirus mosquito vector Aedes aegypti20. Wolbachia strains introduced into Ae. aegypti from other insect host species have resulted in the generation of Ae. aegypti lines that exhibit strong CI21–24 and block the transmission of some positive-strand RNA viruses of significant public health concern25, such as dengue virus. Release of Wolbachia-carrying mosquitoes of both sexes can result in the spread and stable fixation of the bacterium in a population. The “replacement” strategy aims to spread Wolbachia strains that block the transmission of arboviruses and has so far focused on two strains wMel (native to Drosophila melanogaster) and wAlbB (native to Ae. albopictus). Releases of wMel-carrying Ae. aegypti in several countries by the World Mosquito Program (WMP)26–28 and releases of wAlbB-carrying Ae. aegypti in areas of Greater Kuala Lumpur, Malaysia29, have both led to significant decreases in dengue transmission. Alternatively, inundative male-only releases of wAlbB-carriers has successfully reduced vector population sizes by reducing reproductive capacity through incompatible mating30–33. Wolbachia-induced CI is fundamental to each of these approaches, but is as yet incompletely understood.
The wAlbB genome encodes two sets of cognate cif genes of different types (Type III and IV)34. Despite significant sequence divergence, both sets possess the same putative protein domains and are assumed to be active since they do not contain predicted loss-of-function mutations; however, to date there have been no functional studies on them. CI has been successfully recreated using another Type IV cif gene set (from the wPip strain, derived from the mosquito Culex pipiens) in transgenic D. melanogaster7,10. To build a transgenic system to synthetically recapitulate CI induction and rescue in a major arbovirus vector, we generated an Ae. aegypti germline expression system utilising the Type IV cif genes from wAlbB (cifAwAlbB(TIV) and cifBwAlbB(TIV)). The findings of this study provide insights into the role of CifA in the male germline and lays the groundwork for the construction of a transgenic system capable of inheritance at greater than Mendelian ratios; such a synthetic cif gene drive system could be used for the delivery of antiviral effectors in Ae. aegypti.
Results
cifAwAlbB(TIV)/cifBwAlbB(TIV) co-expression in testes is required for CI induction
A piggyBac-based transgene construct comprising cifBwAlbB(TIV) transcriptionally regulated by the testis-specific Beta-2-tubulin (β2t) promoter35 (Fig. 1a) was generated and microinjected into pre-blastoderm Ae. aegypti embryos, resulting in two independent genomic insertion lines (B1 and B2). Crosses between β2t-cifBwAlbB(TIV) males and wild-type females resulted in complete sterility, and this was not rescuable by wAlbB Wolbachia-carrying females (Fig. 1b). Although mating was visually confirmed, dissection and microscopic analysis of the female spermathecae (sperm storage organs) revealed no sperm transfer (Fig. 1b). Dissection of the testes and seminal vesicles from β2t-cifBwAlbB(TIV) males showed a disruption in the production of mature spermatozoa (Supplementary Movie 1).
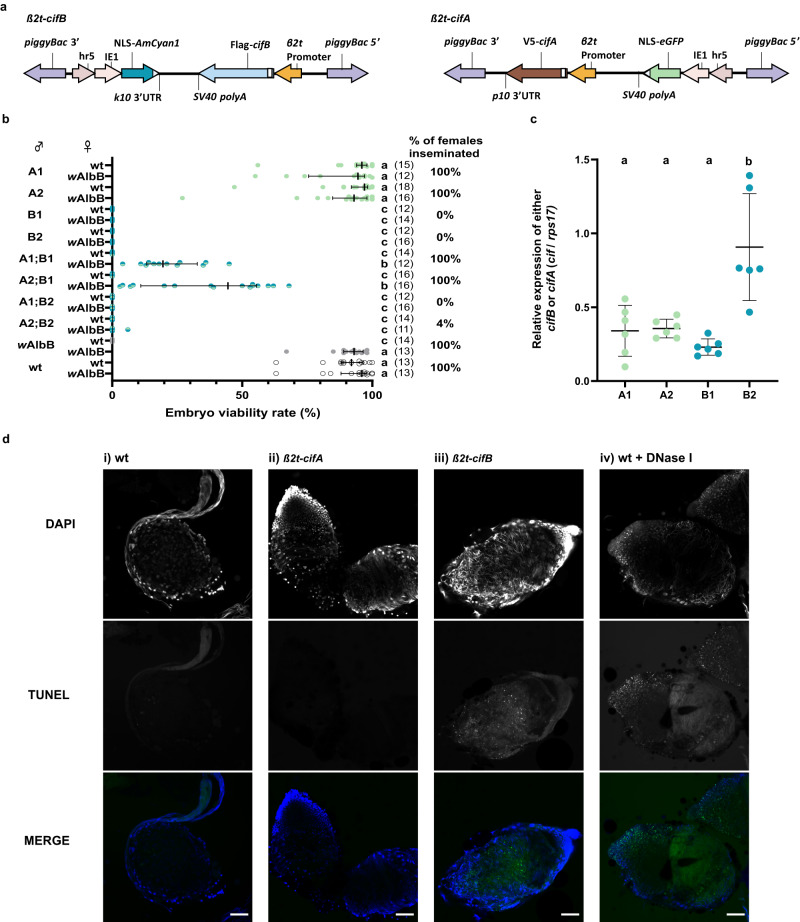
Fig. 1. CifA attenuates the toxicity of CifB during spermatogenesis resulting in rescuable CI.
a Construct design of β2t-cifB and β2t-cifA. b Expression of cifB alone from either genomic insertion site (B1 or B2) impaired sperm production (indicated by the percentage of females inseminated in each cross) rendering males infertile, while expression of cifA from either genomic insertion site (A1 or A2) did not affect male fertility. Depending on the combination of genomic insertion sites, co-expression of cifA;cifB could rescue sperm production and induce sterility when crossed with wild-type (wt) females, which was rescued in crosses with wAlbB-carrying females. Lines denote median, error bars interquartile ranges, and numbers in parentheses the n. Letters indicate significant differences with an α = 0.05 calculated by a Kruskal–Wallis test: H = 261.7, P < 0.0001, d.f. = 19 followed by a two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli to correct for multiple comparisons, individual P-values are listed in the Source Data file. c The relative expression of either cifA or cifB relative to housekeeping gene rps17 in pooled adult testes (mean and s.d. are shown, n = 5 for each group). Letters indicate significant differences with an α = 0.05 calculated by a one-way ANOVA and Tukey’s post-hoc multiple pairwise comparisons test. d TUNEL staining on testes squashes from >5 day old males. DNA breaks labelled by TUNEL staining were not observed in i) wt or ii) β2t-cifA testes, whereas an abundance of DNA breaks was observed in iii) β2t-cifB and iv) wt testes treated with DNase I. DNA was labelled with DAPI stain. Scale bar, 50 µm. Source data are provided as a Source Data file.
We hypothesised that testis-specific expression of cifAwAlbB(TIV) might restrict the toxicity of CifBwAlbB(TIV), resulting in restored spermatogenesis. A piggyBac-based transgene construct comprising the β2t promoter driving cifAwAlbB(TIV) expression (Fig. 1a) was generated and microinjected into Ae. aegypti embryos, resulting in two independent genomic β2t-cifAwAlbB(TIV) insertion lines (A1 and A2). Heterozygous males from each line were crossed with either wild-type or wAlbB Wolbachia-carrying females, and effects on embryo viability were assessed. No reduction in median viability was observed, indicating no effect of β2t-expressed cifAwAlbB(TIV) on male fertility (Fig. 1b).
To test whether cifAwAlbB(TIV) attenuates cifBwAlbB(TIV) toxicity in testes, sub-lines heterozygous for both β2t-cifAwAlbB(TIV) and β2t-cifBwAlbB(TIV) were generated and males from each sub-line were crossed with either wild-type or wAlbB Wolbachia-carrying females. These females were bloodfed and allowed to oviposit, after which spermathecae were dissected and the frequency of sperm transfer was assessed (Fig. 1b). Co-expression of β2t-cifAwAlbB(TIV) was found to rescue the β2t-cifBwAlbB(TIV) mediated disruption of spermatogenesis, although this rescue was dependent on the comparative expression levels of cifAwAlbB(TIV) and cifBwAlbB(TIV). RT-qPCR targeting cifBwAlbB(TIV) in testes from the β2t-cifBwAlbB(TIV) insertion lines showed significant between-line variation in expression – likely due to positional effects of the transgene in the different genomic loci (Fig. 1c). When the mean relative expression of cifAwAlbB(TIV) was comparable to that of cifBwAlbB(TIV) in the testes (A1/A2;B1), all mated female spermathecae contained sperm (Fig. 1b). However, when the mean relative expression of cifAwAlbB(TIV) was lower than that of cifBwAlbB(TIV) in the testes (A1/A2;B2), the majority of mated female spermathecae contained no sperm (Fig. 1b). Despite the successful production and transfer of sperm to wild-type females by males combining cifBwAlbB(TIV) B1 with either the cifAwAlbB(TIV) A1 or A2 insertions, all the crosses resulted in inviable embryos (Fig. 1b). However, if the females carried wAlbB Wolbachia there was a significant increase in the viability rate of the offspring (median of 19.5% and 44.5% when crossed with A1;B1 and A2;B1 males respectively), indicating that at least part of the sterility observed for β2t-cifAwAlbB(TIV);cifBwAlbB(TIV) males was a result of the same cif gene mechanism that occurs in canonical CI induction (Fig. 1b).
As cifBwAlbB(TIV) encodes two PD-(D/E)XK nuclease domains that are predicted to be functional due to the presence of known catalytic residues3, it was hypothesised that the spermless phenotype might be the result of arrested sperm development due to DNA damage arising from CifB nuclease activity. TUNEL (terminal deoxynucleotidyl transferase dUTP nick-end labelling) assays of testes squashes from β2t-cifBwAlbB(TIV) males revealed considerable DNA damage in comparison to wild-type or β2t-cifAwAlbB(TIV) testes (Fig. 1d). As DNA breaks also occur during apoptosis, this assay does not discriminate whether the DNA damage observed is the direct result of CifBwAlbB(TIV) nuclease activity or whether CifBwAlbB(TIV) induces apoptosis through another mechanism.
Paternal Cif dosage affects maternal rescue
Placement of a T2A self-cleaving peptide sequence between two adjacent protein coding sequences allows their transcription as a single mRNA but their translation as two independent peptides at near equimolar quantities through ribosomal skipping36. As comparable cifAwAlbB(TIV)/cifBwAlbB(TIV) expression levels resulted in rescuable CI induction when the genes were transcribed from different genomic loci, we generated β2t-cifAwAlbB(TIV)-T2A-cifBwAlbB(TIV) (hereafter β2t-cifAwAlbB(TIV)-cifBwAlbB(TIV)) lines to test the effect of expressing the cif genes from the same locus (Fig. 2a). Males carrying a single β2t-cifAwAlbB(TIV)-cifBwAlbB(TIV) insertion were found to produce mature sperm and induce complete sterility when crossed with wild-type females (median of 0% viable embryos) (Fig. 2b). However, the level of rescue was low when these males were crossed with wAlbB Wolbachia-carrying females (median of 6% viable embryos) (Fig. 2b). This result was similar for all four independent β2t-cifAwAlbB(TIV)-cifBwAlbB(TIV) genomic insertion lines generated (Supplementary Fig. 1).
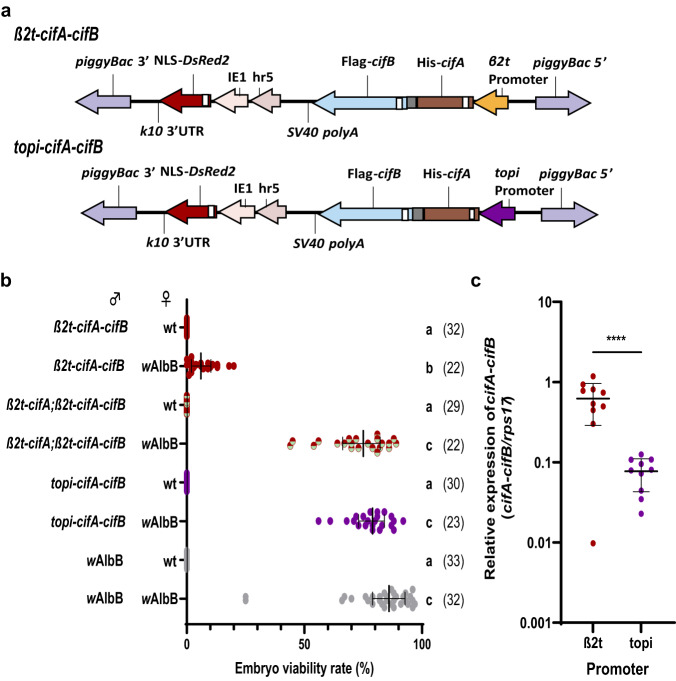
Fig. 2. The paternal dosage of Cifs affects the capacity of maternal CifA to rescue incompatibility.
a Construct design of β2t-cifA-cifB and topi-cifA-cifB. b Increasing the relative expression of cifA in comparison to cifB (β2t-cifA;β2t-cifA-cifB) or lowering the expression of both cif genes (topi-cifA-cifB) did not affect CI penetrance when males were crossed with wild-type (wt) females. However, it did improve the capacity of wAlbB-carrying females to rescue CI. Lines denote median and error bars interquartile ranges, numbers in parentheses denote the n. Letters indicate significant differences with an α = 0.05 calculated by a Kruskal–Wallis test: H = 209.7, P < 0.0001, d.f. = 7, followed by a two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli to correct for multiple comparisons, individual P-values are listed in the Source Data file. c The relative expression of the cifA-cifB transcript was significantly lower when the expression was regulated by the topi promoter (two-tailed unpaired t-test p < 0.0001, mean and s.d. are shown, n = 10 for both groups). Source data are provided as a Source Data file.
As the comparative expression levels of the cif genes was seen to affect the viability of sperm (Fig. 1b), it was hypothesised that increasing the expression of cifAwAlbB(TIV) relative to that of cifBwAlbB(TIV) would further inhibit sperm damage and improve the rescue capacity of wAlbB-carrying females. To increase the paternal dosage of CifA the previously described β2t-cifAwAlbB(TIV) line (A2) was crossed into the β2t-cifAwAlbB(TIV)-cifBwAlbB(TIV) line. The penetrance of sterility induced by heterozygous β2t-cifAwAlbB(TIV);β2t-cifAwAlbB(TIV)-cifBwAlbB(TIV) males was not attenuated when crossed with wild-type females (median of 0% viable embryos)(Fig. 2b). However, the higher dosage of CifAwAlbB(TIV) was found to significantly increase the rescue capability of wAlbB Wolbachia-carrying females to a level comparable to that of wAlbB Wolbachia-carrying male and female control crosses (median of 75% and 86% viable embryos respectively)(Fig. 2b). To investigate the effect that lowering the overall levels of the Cif peptides in the testes had on CI penetrance and rescue capacity, a transgene construct was generated that swapped the β2t promoter with the testes-specific matotopetli (topi)(AAEL023352) promoter (Fig. 2a), which is a weaker promoter than β2t (Fig. 2c). In contrast to β2t-mediated cifAwAlbB(TIV)-cifBwAlbB(TIV) expression, topi-driven cifAwAlbB(TIV)-cifBwAlbB(TIV) expression resulted in full rescue by wAlbB Wolbachia-carrying females (Fig. 2b, c and Supplementary Fig. 1).
The toxicity of CifB peptides encoding functional nuclease domains (Types II-V) is likely to be dependent on nuclease catalytic activity; mutation of key catalytic residues in these domains ablates toxicity in transgenic yeast1,7,10 and reduces CI penetrance in Drosophila7. However, it is not known if this activity occurs in the male reproductive tissues. To determine whether cifA/BwAlbB(TIV) co-expression resulted in DNA breaks in cells undergoing spermatogenesis, TUNEL assays were performed on testes dissected from β2t-cifAwAlbB(TIV)-cifBwAlbB(TIV) males. Despite the production of mature spermatozoa, DNA breaks were still observed in β2t-cifAwAlbB(TIV)-cifBwAlbB(TIV) testes (Fig. 3). However, no DNA breaks were observed in testes dissected from either the β2t-cifAwAlbB(TIV);β2t-cifAwAlbB(TIV)-cifBwAlbB(TIV) or topi-cifAwAlbB(TIV)-cifBwAlbB(TIV) lines (Fig. 3). As males from both these lines can induce complete CI (Fig. 2b), it suggests that inducing DNA breaks during spermatogenesis is not required for the induction of CI. Furthermore, no DNA breaks were observed in wAlbB Wolbachia-carrying testes despite wAlbB encoding two Types of CifB peptide with expected nuclease activity (Fig. 3).
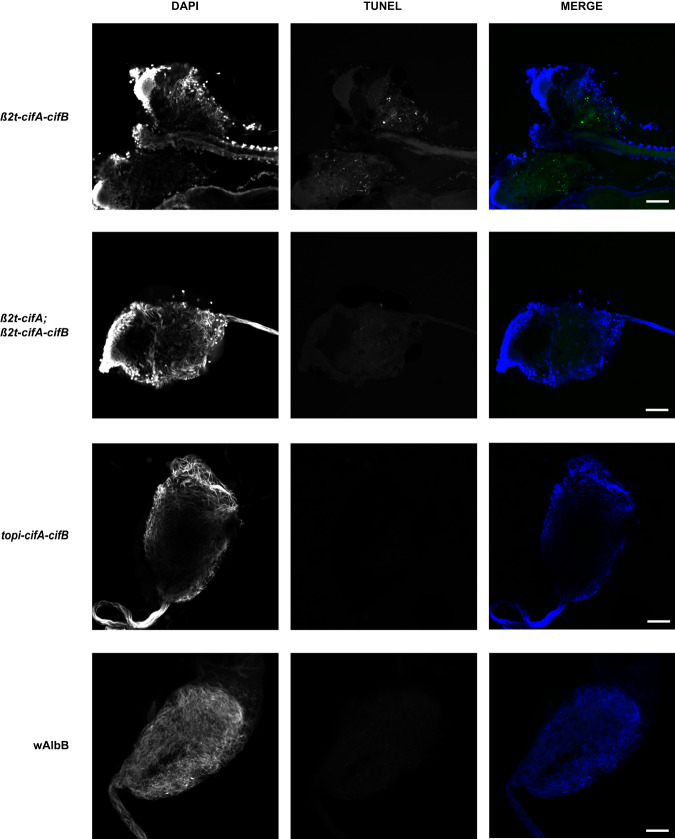
Fig. 3. CifB-induced DNA damage is inhibited during spermatogenesis.
TUNEL assays on 5-day old Ae. aegypti dissected reproductive tissues revealed DNA breaks occurring in β2t-cifA-cifB testes which was inhibited by additional expression of cifA (β2t-cifA;β2t-cifA-cifB) whilst lowering the overall dosage of the Cifs (topi-cifA-cifB) also resulted in a lack of DNA breaks. Although wAlbB is predicted to produce two sets of nuclease CifB peptides no DNA damage was not observed in wAlbB-carrying testes. Scale bar, 50 µm.
Maternal transgenic expression of cifAwAlbB(TIV) rescues transgenic CifBwAlbB(TIV) -induced sterility
Now that CI induction through transgenic expression of cifA/BwAlbB(TIV) had been demonstrated, a line that expressed cifAwAlbB(TIV) in the female germline was generated to determine whether both CI induction and rescue could be achieved in a transgenic Ae. aegypti system. The Ae. aegypti exuperantia (exu) promoter was selected to drive cifAwAlbB(TIV) (Fig. 4a) as it promotes high expression in the ovaries following a bloodmeal and deposition of the gene product in oocytes37. Because wAlbB encodes two-sets of incompatible cif gene pairs it was not expected that maternal transgenic expression of cifAwAlbB(TIV) would rescue CI induced by Wolbachia carrying males. As expected, when heterozygous females from the resulting exu-cifAwAlbB(TIV) line were crossed with wAlbB Wolbachia-carrying males the median embryo viability rate was not increased from 0% (Fig. 4b). However, when crossed with β2t-cifAwAlbB(TIV)-cifBwAlbB(TIV) males, the embryo viability rate was significantly increased (median of 1.5% viable embryos) compared with wild-type females, indicating partial rescue (Fig. 4b). Similar to wAlbB Wolbachia-carrying females the rescue capacity of exu-cifAwAlbB(TIV) females was increased when crossed with either β2t-cifAwAlbB(TIV);β2t-cifAwAlbB(TIV)-cifBwAlbB(TIV) or β2t-cifAwAlbB(TIV);topi-cifAwAlbB(TIV)-cifBwAlbB(TIV) males. However, the rescue provided by maternal cifAwAlbB(TIV) expression was lower (median of 28% and 24% viable embryos respectively) (Fig. 4b) than provided by the presence of wAlbB Wolbachia (median of 75% and 79% viable embryos respectively) (Fig. 2b). As the expression of cifAwAlbB(TIV) does not affect the fertility of females when crossed with wild-type males (Fig. 4b), the lower embryo viability rate in these crosses is likely due to the incomplete rescue of CifBwAlbB(TIV)-mediated inviability, rather than any CifAwAlbB(TIV)-mediated decrease in fertility.
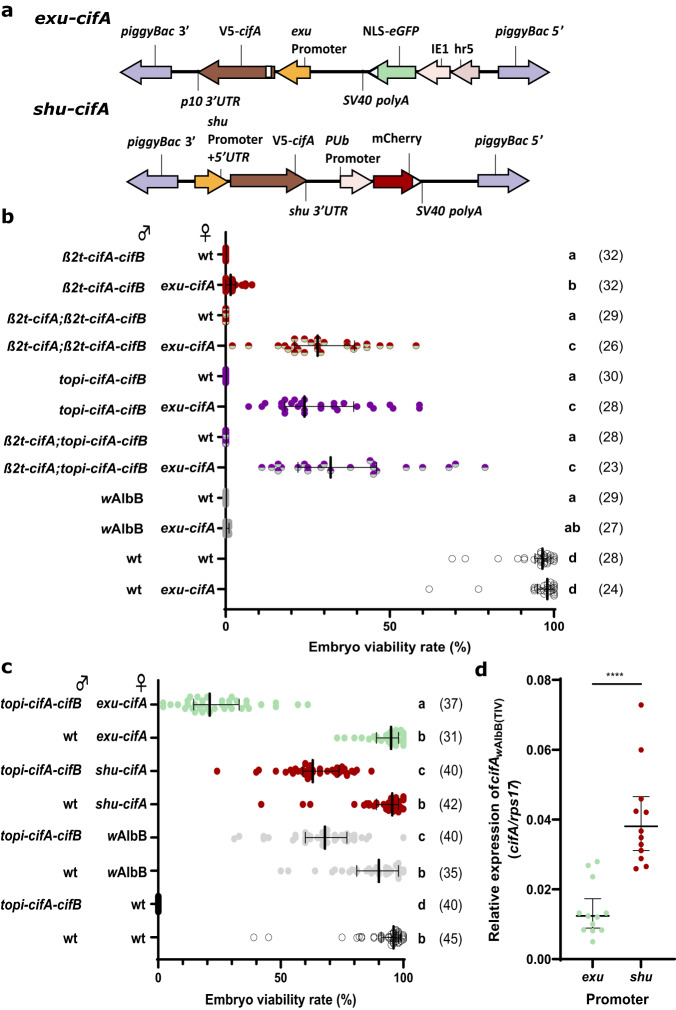
Fig. 4. Maternal cifAwAlbB(TIV) expression rescues CI induced by paternal cifA/BwAlbB(TIV) expression.
a Construct design of exu-cifA and shu-cifA. b Manipulating the paternal Cif dosage in transgenic males improves the capacity of exu-cifA females to rescue incompatibility. Expression of cifA in females does not affect their fertility when crossed with wild-type (wt) males. c Rescue capacity of transgenic females is increased when cifA expression is regulated by the shu promoter. In both b and c, Lines denote median and error bars interquartile ranges, numbers in parentheses denote the n. Letters indicate significant differences with an α = 0.05 calculated by a Kruskal–Wallis test: (b H = 311.0, P < 0.0001, d.f. = 11)(c H = 243.2, P < 0.0001, d.f. = 7) followed by a two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli to correct for multiple comparisons, individual P-values are listed in Source Data File. d The expression of cifA relative to rps17 was higher in gravid ovaries taken from shu-cifA compared to exu-cifA females (two-tailed Mann–Whitney U test P < 0.0001, mean and s.d are shown, n = 12 for both groups). Source data are provided as a Source Data file.
We have shown that CifB-mediated toxicity is inhibited by CifA during spermatogenesis, consequently toxicity is likely to occur after CifB is deposited into oocytes upon fertilisation. Therefore, if rescue is dependent on CifA inhibition of CifB toxicity (through binding) then the levels of maternally deposited CifA must match or exceed that of paternally deposited CifB. It is probable that the levels of maternal CifA deposition (under the exu promoter) are not sufficient to prevent lethality in every embryo whereas maternally-inherited wAlbB Wolbachia likely produce CifA constantly which ensures CI rescue. Consequently, to improve the rescue capacity of the transgenic females an equal or greater abundance of CifA relative to CifB must be achieved, which may be generated through the lowering of paternal CifB or the increasing of maternal CifA deposition. An overexpression of cifAwPip(TI) in transgenic Anopheles gambiae males was found to reduce the penetrance of CI induced by cifA/BwPip(TI) co-expression11, suggesting that significantly higher levels of cifA expression reduces the deposition of unbound CifB into fertilised oocytes. To test whether an overexpression of cifAwAlbB(TIV) in males might reduce the deposition of unbound CifBwAlbB(TIV), and therefore improve the rescue capability of transgenic females, males heterozygous for both the β2t-cifAwAlbB(TIV) (A2) and topi-cifAwAlbB(TIV)-cifBwAlbB(TIV) insertions were crossed with exu-cifAwAlbB(TIV) females. A higher expression of cifAwAlbB(TIV) comparative to cifBwAlbB(TIV) in β2t-cifAwAlbB(TIV);topi-cifAwAlbB(TIV)-cifBwAlbB(TIV) males did not significantly increase rescue capability of either wild-type or exu-cifAwAlbB(TIV) females (Fig. 4b). This suggests that the increase in embryo viability rate observed for β2t-cifAwAlbB(TIV);β2t-cifAwAlbB(TIV)-cifBwAlbB(TIV) in comparison to β2t-cifAwAlbB(TIV)-cifBwAlbB(TIV) crosses (Figs. 2b and 4b) is the result of an inhibition of CifBwAlbB(TIV)-induced toxicity in the male germline, and not a reduction in deposition of the toxin.
The promoter/3’UTR sequences of the Ae. aegypti shut-down (shu) gene were expected to improve deposition into developing oocytes38,39. Therefore, a shu-cifAwAlbB(TIV) line (Fig. 4a) was generated and heterozygous females were crossed with topi-cifAwAlbB(TIV)-cifBwAlbB(TIV) males (Fig. 4c). Changing the regulatory sequences resulted in a 42% increase in embryo viability in comparison to exu-cifAwAlbB(TIV) females when mated with transgenic males, with shu-cifAwAlbB(TIV) females being able to rescue incompatibility to a level comparable to those carrying wAlbB Wolbachia (median of 63% and 68% viable embryos respectively) (Fig. 4c). This increase coincided with a significantly higher cifAwAlbB(TIV) expression in blood-fed shu-cifAwAlbB(TIV) ovaries (Fig. 4d). However, different expression patterns between the promoters might also result in different levels of CifA deposition.
Discussion
The capacity to mediate cytoplasmic incompatibility is central to the remarkable ecological success of Wolbachia and is key to the deployment of Wolbachia as a vector control tool. A variety of artificial Wolbachia transinfections using different strains have now been generated in Ae. aegypti, and all - with the exception of wAu, which does not encode an active cif gene pair – induce highly penetrant CI21–24,40. Ae. aegypti is an ideal model organism for investigating cif gene function given its very high CI penetrance. This may be due in part to the fact that Ae. aegypti is not a native Wolbachia carrier, whereas in D. melanogaster CI has incomplete penetrance probably due to compensatory host adaptations aimed at suppressing CI and thereby minimising associated fitness costs41,42. Furthermore, the severe and growing public health burden of Ae. aegypti, its tractability in the laboratory, and the potential for building cif gene-based drive systems that require relatively high transgene population frequencies before drive is achieved (high-threshold drive)43, makes studies on cif gene function in this species particularly timely and relevant. Although considerable research on the effects of cif gene expression using transgenic tools has been conducted in D. melanogaster1,2,6–10,12, the synthetic recapitulation of CI using the cif genes in Aedes mosquitoes has not yet been reported. Here we demonstrate transgenic CI induction and rescue in this species using a previously untested Type IV cif gene pair from the wAlbB Wolbachia strain.
As both cifAwAlbB(TIV) and cifBwAlbB(TIV) expression was necessary for CI induction whilst only cifAwAlbB(TIV) was required for rescue, our results conform to the previously described two-by-one model of CI9. However, this model does not elucidate the role(s) of paternal cifA expression in the induction mechanism. Here, we provide clear evidence for the role of CifA in the attenuation of CifB-mediated toxicity in the male germline. CifA-mediated inhibition of CifB toxicity has been well documented in both transgenic yeast and insect cell studies and is largely attributed to the formation of CifA-CifB heterodimers, which prevents CifB from directly interacting with unknown chromatin targets1,7,8,12,17. The requirement for the inactivation of the CifBwAlbB(TIV) toxin in the testes to generate rescuable sterility in our study indicates that the modification/toxicity associated with sterility induction does not occur in the male reproductive tissues, but rather suggests it occurs in the fertilised embryo where Wolbachia-derived or maternally deposited CifAwAlbB(TIV) is required to prevent sperm-bound CifBwAlbB(TIV) toxicity (Supplementary Fig. 2). When binding is prevented through the mutation of residues in the CifA-CifB binding interface, transgenic D. melanogaster females which express the mutant cifA are unable to rescue CI induced by cifA-cifB expressing males12, suggesting that the same mode of toxin inhibition is maintained both during spermatogenesis and after fertilisation in compatible Wolbachia crosses.
Although we demonstrated the importance of CifA-mediated inhibition of CifB toxicity during spermatogenesis in this study, there have been reports of CI induction through the expression of cifB alone10,11, which suggests that paternal CifA is not always essential. It is probable that differences in i) cifB expression levels, ii) host sensitivity to toxicity, and iii) differences in catalytic activity between cifB homologues contribute to variations between studies. Interestingly, several studies have also demonstrated that cifB expression does not affect male fertility unless cifA is also expressed2,6,9. One explanation for this phenomenon is that in addition to its role in toxin inhibition there is evidence to suggest that CifA:CifB complex formation might suppress the premature degradation of CifB10,17. Therefore, CifA might be necessary to both inhibit CifB toxicity and ensure the correct deposition of CifB into sperm nuclei. Alternatively, another study has shown that paternal cifBwMel(TI) expression resulted in spermatid DNA damage, yet the fertility of these males was unaffected18 – which might suggest that cifB expression could select for undamaged sperm that receive lower levels of CifB.
The transgenic recapitulation of CI induction and rescue in our study opens the potential for the generation of gene drive systems capable of spreading broad-action anti-pathogen effectors (linked to the cif drive elements) through targeted Ae. aegypti populations. A simple way to build a cif drive system using promoter sequences already outlined in this study would be to have cifA and cifB in the same transgene construct and introduced into a single genomic locus. The drive system could be constructed with the topi promoter driving male-specific germline expression of cifA and cifB and shu driving female germline expression of cifA. Provided that a critical population threshold frequency is surpassed, the advantage of carrying the cif drive allele for females (conferring CI rescue in matings with cif drive allele carrying males) will outweigh the cost incurred by carrying the cif drive allele for males (causing incompatibility in matings with wild-type females), and the cif drive system would be expected to spread and persist. Mathematical modelling of drive systems based on the cif genes in various design configurations suggests that they will possess high invasion-threshold frequencies, and thus are likely to be inherently more confinable to target populations than analogous homing drive systems; by splitting the induction and rescue components the drive could be further confined temporally as well as spatially43. Although requiring higher population frequencies before drive is achieved and therefore necessitating a greater volume of initial mosquito releases, these drive designs are expected to be more easily controlled and regulated from a biosafety and geopolitical perspective. While Wolbachia virus inhibition and density have so far remained stable in field populations of Ae. aegypti over several years, it is possible that in the longer term both will be ameliorated by natural selection, and likewise it is possible that “escape” mutations will arise in dengue or other arboviruses that reduce the effectiveness of transmission blocking. In this context it is important to continue research aiming to develop transgenic approaches for spreading antiviral effectors in this species. In addition, not all disease-transmitting insect species are likely to be able to support Wolbachia transinfections. Meanwhile, the sterility generated by cifB-expressing transgenic males provides additional options for innunaditive sterile male-only release strategies, aimed at population suppression of Ae. aegypti or other pest insects. Moreover, as we have shown that the presence of CifB can cause cell damage, it is possible that some of the fitness costs associated with the presence of some Wolbachia strains (e.g. reduced lifespan, fecundity, egg hatch rates) may be in part due to cumulative DNA damage resulting from unbound CifB. If this is the case, transgenic expression of the cognate CifA under a non-specific promoter may mitigate this damage and could result in Wolbachia transinfections with improved fitness profiles.
Methods
Generation of constructs
The coding sequences of the Type IV wAlbB cifA (QBB83746.1) and cifB (QBB83745.1) genes were codon optimised for expression in Ae. aegypti, synthesised, and cloned into pUC-GW-Amp plasmids using the GENEWIZ PriorityGENE service (Azenta Life Sciences). Constructs were engineered to express the cif genes either together (linked with a T2A peptide sequence) or independently under the control of the germline-specific promoters β2t, topi, exu, or shu. Fluorescent marker genes were regulated by either the Hr5/ie1 promoter/enhancer or PUb promoter sequences. The complete sequences of the β2t-cifBwAlbB(TIV), β2t-cifAwAlbB(TIV), β2t-cifAwAlbB(TIV)-cifBwAlbB(TIV), topi-cifAwAlbB(TIV)-cifBwAlbB(TIV), exu-cifAwAlbB(TIV), and shu-cifAwAlbB(TIV) plasmids have been deposited in GenBank with the accession numbers OR961086, OR961087, OR961088, OR961089, OR961090, and OR961091 respectively.
Mosquito maintenance and experimental procedures
Mosquito rearing
All mosquito colonies were maintained at 27 °C and 70% humidity with 12-h light/dark cycles. Larvae were fed tropical fish pellets (Tetra) whilst adults had access to 5% sucrose solution ad libitum. Blood meals were provided using a Hemotek artificial blood-feeding system (Hemotek Ltd) and human blood (Scottish National Blood Bank). Damp Grade 1 filter-paper (Whatman plc) was provided as an oviposition source for egg collection. Eggs were desiccated for 5–10 days before hatching in water containing 1 g/l bovine liver powder (MP Biomedicals). The wAlbB-carrying Ae. aegypti line used in this study was generated previously through embryo microinjection40.
Generation of transgenic lines
The posterior pole of freshly laid pre-blastoderm Ae. aegypti embryos (<2 h old) were microinjected44 using a Nikon Eclipse TS100 microscope and air pump (Jun-Air). The injection mix consisted of a final concentration of 500 ng/μl donor plasmid and 300 ng/μl helper plasmid [PUb hyperactive piggyBac transposase (AGG1245)]38 in 1x injection buffer. Microinjection survivors (G0) were screened for marker gene expression at the 4th instar larval stage using the Leica M165FC fluorescent microscope and appropriate filter setting, those transiently expressing the marker gene were individualised and mated with 3 wild-type mosquitoes. To identify transgenic lines the resultant G1 generation from these crosses were likewise screened at the 4th instar. Lines were maintained by mating virgin transgenic females with an excess of wild type males, offspring were screened at 4th instar stage.
Embryo viability assays
Virgin females were mated with an excess of males in small (15 × 15 × 15 cm) Bugdorm cages (Megaview Science Co.). After blood-feeding, non-bloodfed females were removed and remaining individuals were left 3 days to become gravid. Females were individualised onto small damp Grade 1 filter-paper disks (Whatman plc). Females were left for 2 days to lay eggs, after which the females were removed and spermathecae were dissected to confirm mating. Egg cones were left for 5–10 days before counting the percentage viability of embryos on each cone. The viability (ie. the ability to hatch) of Ae. aegypti embryos can be observed easily due to phenotypic differences using a light microscope - inviable embryos display egg collapse which signals an early arrest in development, whilst viable embryos remain turgid indicating embryo development. To ensure the percentage viability count was reliable selected cones were floated, and after 2 days the egg cones were removed and dried, and the percentage hatch rate was calculated.
RNA extraction and reverse transcription quantitative PCR (RT-qPCR) analyses
Male reproductive tissues were dissected in pools of 3, homogenesised in TRIzol reagent (Thermo Fisher Scientific) and stored at −80 °C. Ovaries were dissected from individual females 72 h after blood-feeding. RNA extractions were conducted as instructed by the manufacturer’s instructions and the pelleted RNA was resuspended in RNase free water. The purity /concentration of RNA was quantified using the NanoDrop One spectrophotometer (Thermo Fisher Scientific) and up to 2 µg of RNA was used per 20 µl cDNA synthesis reaction (High-Capacity cDNA Reverse Transcription Kit, Thermo Fisher Scientific). 10 µl RT-qPCR reactions consisting of 2 µl cDNA, 5 µl Fast SYBR™ Green Master Mix (Thermo Fisher Scientific), 2 µl distilled water, and 0.5 µl of both forward and reverse primers (5 µM) were run on the QuantStudio™ 3 Real-Time PCR System (Thermo Fisher Scientific). The primers used in this study were: cifAF, 5’ GCGAACGATACACCACCTTC 3’; cifAR, 5’ TTCCCACACGTTCATCATGC 3’; cifBF, 5’ AAGATCGCCATCCTGACCAA 3’; cifBR, 5’ GCGATTTTCTCCAGCTCTCC 3’; rps17F, 5’ CACTCCCAGGTCCGTGGTAT 3’; rps17R, 5’ GGACACTTCCGGCACGTAGT 3’. The primers targeting cifB could be used to determine the relative expression of either cifB alone or the bicistronic cifA-cifB sequence. The 2-ΔCt method was used to determine the expression of the cif genes relative to the housekeeping reference gene rps17.
Microscopy
Brightfield microscopy
The reproductive tissues (testes, seminal vesicle and accessory glands) from 5-day old males were dissected in PBS and mounted onto a glass slide. The coverslip was lightly pressed to break the tissue and release sperm (if any was produced). A series of brightfield images were taken using the Leica DMi8 (Leica Microsystems) and 10× objective lens, successive images were used to create time-lapse videos.
TUNEL assays
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling (TUNEL) assays were used to detect single- and double-stranded DNA nicks and fragmentation via the ApopTag® Fluorescein Direct In Situ Apoptosis Detection Kit (Merck Group). Testes dissected from 5-day old males were fixed in 4% formaldehyde, permeabilised in PBST, and treated with components from the kit. For the positive control, a selection of wild-type testes were treated with DNase I (Thermo Fisher Scientific) for 20 min before fixation. Tissues were mounted in Fluoroshield™ with DAPI, histology mounting medium (Sigma-Aldrich) and imaged using a Zeiss LSM 880 confocal microscope (Zeiss) with a 20x objective. Fluorescein-labelled DNA breaks and DAPI-stained nuclei were imaged using a 488 nm and 405 nm laser respectively with GaAsP detectors, settings were kept constant for all images taken.
Statistics and reproducibility
Statistical analysis was performed using the GraphPad Prism software. A Shapiro–Wilk normality test was used to determine if the data was normally distributed. For multiple comparisons of embryo viability one or more of the groups were not normally distributed so non-parametric Kruskal–Wallis tests with were performed with a two-stage step-up procedure of the Benjamini, Krieger and Yekutieli method for controlling the false discovery rate. For comparisons of gene expression between two groups, when the data was normally distributed an unpaired t-test (two-tailed) was performed, if not normally distributed a Mann–Whitney U test was performed. For the TUNEL assays, a representative image of a dissected and stained teste from each group was selected from 4-10 independent tissue images. All data used for statistical analysis along with the results of the tests used are included in the Source Data file.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
The study was supported by MRC award MC_ST_CVR_2019 and Wellcome Trust Award 202888/Z/16/Z.
Author contributions
C.J.M., T.H.A., and S.P.S. conceived and designed the experiments. C.J.M. and T.H.A. collected, interpreted, and analysed the data. T.H.-S. and J.M. contributed with data interpretation. T.H.-S. and L.A. provided materials and H.W.-C. provided technical advice required for experimentation. C.J.M., T.A. and S.P.S. wrote the manuscript whilst all authors provided comments/edits and approved the final submission draft.
Peer review
Peer review information
Nature Communications thanks the anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
Datasets generated and/or analysed during the current study are available in the main manuscript or are appended as supplementary data. The source data, statistical analysis tests, and their outcomes used for figures are available to view in the Source Data file. The plasmid DNA sequences used to generate transgenic lines in this study are deposited in GenBank with the accession codes OR961086, OR961087, OR961088, OR961089, OR961090, and OR961091. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-45238-7.
References
- 1.Beckmann JF, Ronau JA, Hochstrasser M. A Wolbachia deubiquitylating enzyme induces cytoplasmic incompatibility. Nat. Microbiol. 2017;2:17007. doi: 10.1038/nmicrobiol.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LePage DP, et al. Prophage WO genes recapitulate and enhance Wolbachia-induced cytoplasmic incompatibility. Nature. 2017;543:243–247. doi: 10.1038/nature21391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez J, Klasson L, Welch JJ, Jiggins FM. Life and Death of Selfish Genes: Comparative Genomics Reveals the Dynamic Evolution of Cytoplasmic Incompatibility. Mol. Biol. Evol. 2021;38:2–15. doi: 10.1093/molbev/msaa209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sicard M, et al. Cytoplasmic Incompatibility Variations in Relation with Wolbachia cid Genes Divergence in Culex pipiens. mBio. 2021;12:e02797-20. doi: 10.1128/mBio.02797-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonneau M, et al. Culex pipiens crossing type diversity is governed by an amplified and polymorphic operon of Wolbachia. Nat. Commun. 2018;9:319. doi: 10.1038/s41467-017-02749-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shropshire JD, Rosenberg R, Bordenstein SR. The impacts of cytoplasmic incompatibility factor (cifA and cifB) genetic variation on phenotypes. Genetics. 2021;217:1–13. doi: 10.1093/genetics/iyaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H, Ronau JA, Beckmann JF, Hochstrasser M. A Wolbachia nuclease and its binding partner provide a distinct mechanism for cytoplasmic incompatibility. Proc. Natl Acad. Sci. 2019;116:22314–22321. doi: 10.1073/pnas.1914571116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horard B, et al. Paternal transmission of the Wolbachia CidB toxin underlies cytoplasmic incompatibility. Curr. Biol. 2022;32:1319–1331.e5. doi: 10.1016/j.cub.2022.01.052. [DOI] [PubMed] [Google Scholar]
- 9.Shropshire JD, Bordenstein SR. Two-By-One model of cytoplasmic incompatibility: Synthetic recapitulation by transgenic expression of cifA and cifB in Drosophila. PLoS Genet. 2019;15:e1008221. doi: 10.1371/journal.pgen.1008221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun G, Zhang M, Chen H, Hochstrasser M. The CinB Nuclease from w No Wolbachia Is Sufficient for Induction of Cytoplasmic Incompatibility in Drosophila. mBio. 2022;13:e0317721. doi: 10.1128/mbio.03177-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams KL, et al. Wolbachia cifB induces cytoplasmic incompatibility in the malaria mosquito vector. Nat. Microbiol. 2021;6:1575–1582. doi: 10.1038/s41564-021-00998-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao Y, et al. Structural and mechanistic insights into the complexes formed by Wolbachia cytoplasmic incompatibility factors. Proc. Natl Acad. Sci. 2021;118:e2107699118. doi: 10.1073/pnas.2107699118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, et al. Crystal Structures of Wolbachia CidA and CidB Reveal Determinants of Bacteria-induced Cytoplasmic Incompatibility and Rescue. Nat. Commun. 2022;13:1608. doi: 10.1038/s41467-022-29273-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beckmann JF, Van Vaerenberghe K, Akwa DE, Cooper BS. A single mutation weakens symbiont-induced reproductive manipulation through reductions in deubiquitylation efficiency. Proc. Natl Acad. Sci. 2021;118:e2113271118. doi: 10.1073/pnas.2113271118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beckmann JF, et al. The Toxin–Antidote Model of Cytoplasmic Incompatibility: Genetics and Evolutionary Implications. Trends Genet. 2019;35:175–185. doi: 10.1016/j.tig.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shropshire JD, Leigh B, Bordenstein SR. Symbiont-mediated cytoplasmic incompatibility: What have we learned in 50 years? Elife. 2020;9:e61989. doi: 10.7554/eLife.61989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terretaz K, Horard B, Weill M, Loppin B, Landmann F. Functional analysis of Wolbachia Cid effectors unravels cooperative interactions to target host chromatin during replication. PLoS Pathog. 2023;19:e1011211. doi: 10.1371/journal.ppat.1011211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaur R., Shropshire J. D., Leigh B. A., Bordenstein S. R. Nuclease proteins CifA and CifB promote spermatid DNA damage associated with symbiont-induced cytoplasmic incompatibility. bioRxiv10.1101/2022.04.04.487029 (2022).
- 19.Namias A, Sicard M, Weill M, Charlat S. From Wolbachia genomics to phenotype: molecular models of cytoplasmic incompatibility must account for the multiplicity of compatibility types. Curr. Opin. Insect Sci. 2022;49:78–84. doi: 10.1016/j.cois.2021.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Ross PA, et al. An elusive endosymbiont: Does Wolbachia occur naturally in Aedes aegypti? Ecol. Evol. 2020;10:1581–1591. doi: 10.1002/ece3.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xi Z, Khoo CCH, Dobson SL. Wolbachia Establishment and Invasion in an Aedes aegypti Laboratory Population. Science. 2005;310:326–328. doi: 10.1126/science.1117607. [DOI] [PubMed] [Google Scholar]
- 22.Fraser JE, et al. Novel Wolbachia-transinfected Aedes aegypti mosquitoes possess diverse fitness and vector competence phenotypes. PLoS Pathog. 2017;13:e1006751. doi: 10.1371/journal.ppat.1006751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMeniman CJ, et al. Stable Introduction of a Life-Shortening Wolbachia Infection into the Mosquito Aedes aegypti. Science. 2009;323:141–144. doi: 10.1126/science.1165326. [DOI] [PubMed] [Google Scholar]
- 24.Hughes GL, Rasgon JL. Transinfection: a method to investigate Wolbachia -host interactions and control arthropod-borne disease. Insect Mol. Biol. 2014;23:141–151. doi: 10.1111/imb.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffmann AA, Ross PA, Rašić G. Wolbachia strains for disease control: ecological and evolutionary considerations. Evol. Appl. 2015;8:751–768. doi: 10.1111/eva.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryan PA, et al. Establishment of wMel Wolbachia in Aedes aegypti mosquitoes and reduction of local dengue transmission in Cairns and surrounding locations in northern Queensland, Australia. Gates Open Res. 2020;3:1547. doi: 10.12688/gatesopenres.13061.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinto SB, et al. Effectiveness of Wolbachia-infected mosquito deployments in reducing the incidence of dengue and other Aedes-borne diseases in Niterói, Brazil: A quasi-experimental study. PLoS Negl. Trop. Dis. 2021;15:e0009556. doi: 10.1371/journal.pntd.0009556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Utarini A, et al. Efficacy of Wolbachia-Infected Mosquito Deployments for the Control of Dengue. N. Engl. J. Med. 2021;384:2177–2186. doi: 10.1056/NEJMoa2030243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nazni WA, et al. Establishment of Wolbachia Strain wAlbB in Malaysian Populations of Aedes aegypti for Dengue Control. Curr. Biol. 2019;29:4241–4248.e5. doi: 10.1016/j.cub.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crawford JE, et al. Efficient production of male Wolbachia-infected Aedes aegypti mosquitoes enables large-scale suppression of wild populations. Nat. Biotechnol. 2020;38:482–492. doi: 10.1038/s41587-020-0471-x. [DOI] [PubMed] [Google Scholar]
- 31.Beebe NW, et al. Releasing incompatible males drives strong suppression across populations of wild and Wolbachia -carrying Aedes aegypti in Australia. Proc. Natl Acad. Sci. 2021;118:e2106828118. doi: 10.1073/pnas.2106828118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martín-Park A, et al. Pilot trial using mass field-releases of sterile males produced with the incompatible and sterile insect techniques as part of integrated Aedes aegypti control in Mexico. PLoS Negl. Trop. Dis. 2022;16:e0010324. doi: 10.1371/journal.pntd.0010324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu W-L, et al. Lab-scale characterization and semi-field trials of Wolbachia Strain wAlbB in a Taiwan Wolbachia introgressed Ae. aegypti strain. PLoS Negl. Trop. Dis. 2022;16:e0010084. doi: 10.1371/journal.pntd.0010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinha A, Li Z, Sun L, Carlow CKS. Complete Genome Sequence of the Wolbachia w AlbB Endosymbiont of Aedes albopictus. Genome Biol. Evol. 2019;11:706–720. doi: 10.1093/gbe/evz025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith RC, Walter MF, Hice RH, O’Brochta DA, Atkinson PW. Testis-specific expression of the β2 tubulin promoter of Aedes aegypti and its application as a genetic sex-separation marker. Insect Mol. Biol. 2007;16:61–71. doi: 10.1111/j.1365-2583.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 36.Daniels RW, Rossano AJ, Macleod GT, Ganetzky B. Expression of Multiple Transgenes from a Single Construct Using Viral 2A Peptides in Drosophila. PLoS One. 2014;9:e100637. doi: 10.1371/journal.pone.0100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akbari, O. S., Papathanos, P. A., Sandler, J. E., Kennedy, K. & Hay, B. A. Identification of germline transcriptional regulatory elements in Aedes aegypti. Sci. Rep. 20154, 3954. [DOI] [PMC free article] [PubMed]
- 38.Darren H. E. Identification and characterization of germline-specific promoters for remobilization of transgenes in the mosquitoes, Aedes aegypti and Anopheles gambiae (Texas A&M University, 2007).
- 39.Anderson MAE, et al. Closing the gap to effective gene drive in Aedes aegypti by exploiting germline regulatory elements. Nat. Commun. 2023;14:338. doi: 10.1038/s41467-023-36029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ant TH, Herd CS, Geoghegan V, Hoffmann AA, Sinkins SP. The Wolbachia strain wAu provides highly efficient virus transmission blocking in Aedes aegypti. PLoS Pathog. 2018;14:e1006815. doi: 10.1371/journal.ppat.1006815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poinsot D, Bourtzis K, Markakis G, Savakis C, Merçot H. Wolbachia Transfer from Drosophila melanogaster into D. simulans: Host Effect and Cytoplasmic Incompatibility Relationships. Genetics. 1998;150:227–237. doi: 10.1093/genetics/150.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shropshire JD, Hamant E, Cooper BS. Male Age and Wolbachia Dynamics: Investigating How Fast and Why Bacterial Densities and Cytoplasmic Incompatibility Strengths Vary. mBio. 2021;12:e0299821. doi: 10.1128/mbio.02998-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li, J. & Champer, J. Harnessing Wolbachia cytoplasmic incompatibility alleles for confined gene drive: A modeling study. PLoS Genet.19, e1010591 (2023). [DOI] [PMC free article] [PubMed]
- 44.Morris AC, Eggleston P, Crampton JM. Genetic transformation of the mosquito Aedes aegypti by micro‐injection of DNA. Med Vet. Entomol. 1989;3:1–7. doi: 10.1111/j.1365-2915.1989.tb00467.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Datasets generated and/or analysed during the current study are available in the main manuscript or are appended as supplementary data. The source data, statistical analysis tests, and their outcomes used for figures are available to view in the Source Data file. The plasmid DNA sequences used to generate transgenic lines in this study are deposited in GenBank with the accession codes OR961086, OR961087, OR961088, OR961089, OR961090, and OR961091. Source data are provided with this paper.