Abstract
Fatty acid compositions in growing and resting cells of several strains of Pseudomonas putida (P8, NCTC 10936, and KT 2440) were studied, with a focus on alterations of the saturation degree, cis-trans isomerization, and cyclopropane formation. The fatty acid compositions of the strains were very similar under comparable growth conditions, but surprisingly, and contrary to earlier reports, trans fatty acids were not found in either exponentially growing cells or stationary-phase cells. During the transition from growth to the starvation state, cyclopropane fatty acids were preferentially formed, an increase in the saturation degree of fatty acids was observed, and larger amounts of hydroxy fatty acids were detected. A lowered saturation degree and concomitant higher membrane fluidity seemed to be optimal for substrate uptake and growth. The incubation of cells under nongrowth conditions rapidly led to the formation of trans fatty acids. We show that harvesting and sample preparation for analysis could provoke the enzyme-catalyzed formation of trans fatty acids. Freeze-thawing of resting cells and increased temperatures accelerated the formation of trans fatty acids. We demonstrate that cis-trans isomerization only occurred in cells that were subjected to an abrupt disturbance without having the possibility of adapting to the changed conditions by the de novo synthesis of fatty acids. The cis-trans isomerization reaction was in competition with the cis-to-cyclopropane fatty acid conversion. The potential for the formation of trans fatty acids depended on the cyclopropane content that was already present.
The bacterial cytoplasmic membrane is an important target and/or receptor for stress factors. As a response to permanent physical and chemical changes in the cell environment, several protective mechanisms and metabolic adaptation reactions have evolved (10, 52, 56). At the level of membrane lipids and fatty acids, these processes are often referred to as homeoviscous adaptation (57). Cells control the fluidity of their membranes by altering the lipid composition to compensate for changes in fluidity induced by certain environmental factors, such as temperature or the presence of toxic, membrane-active compounds. In most cases, however, microorganisms are not able to compensate for externally induced fluidity changes with 100% efficacy (7, 37, 38). They can tolerate and maybe even need a wider range of different lipid compositions to establish homeostasis, especially during growth. Lipids can coexist in physically separated microdomains with more or less fluid- or gel-phase behavior, and membrane functions are locally influenced by many factors other than fluidity (20, 43). These are the reasons for attempts to enlarge the term homeoviscous adaptation (to maintain membrane fluidity) by use of the term homeophasic adaptation (to adjust membrane fluidity).
At the level of lipid membrane composition, the predominant response of many bacteria to environmental perturbations is the alteration of lipid acyl chain structures by changing the ratio of saturated to unsaturated fatty acids during growth (8, 15, 19, 26, 42, 53). Several other mechanisms have been found in some bacteria to adjust fluidity, such as shortening or growth-dependent elongation of fatty acid chain lengths (6, 8, 14, 53), growth-dependent changes in the ratio of terminally branched iso and anteiso fatty acids (28, 30), growth-dependent methylation of cis unsaturated fatty acids to methyl branched (31) or cyclopropane saturated ones (3, 29, 33, 42), and isomerization of cis to trans double bonds (11, 16, 18, 22, 25, 40, 47).
Pseudomonas putida is a ubiquitous gram-negative bacterium and a potent pollutant degrader. Strains of this species are of ecophysiological interest because most of them are able to use at least the following three adaptation mechanisms at the level of lipids to respond to physical and chemical stresses: (i) changes in the overall degree of saturation of fatty acids (22, 35, 37), (ii) the formation of cyclopropane fatty acids (35), and (iii) cis-trans isomerization with a double bond configuration (11, 22, 35, 36, 37, 46, 59). The kind and degree of the response determine the stability and resilience of the cells and decide their fate. Commonly, the different adaptation mechanisms of P. putida have been investigated independently. The purpose of this study was the integral examination of these three adaptation responses of P. putida and their dependence on different growth phases. To gain insights into strain-dependent differences and to verify the measured effects, we chose three well-known strains of this species for use in this study.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Three strains of P. putida were used for this study. P. putida P8 was kindly supplied by H. J. Heipieper (UFZ Centre for Environmental Research Leipzig-Halle, Leipzig, Germany), and P. putida NCTC 10936 and KT 2440 were obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (Braunschweig, Germany). All three strains were maintained on Trypticase soy agar slants at 4°C and cultivated at 30°C. Shaking flasks containing 150 ml of growth medium were inoculated with cells from an overnight preculture (10% [vol/vol]). Standard mineral medium, designed for the development of 2 g of biomass/liter, was composed of the following reagents (in milligrams per liter): NH4Cl, 761; KH2PO4, 681; K2HPO4, 871; CaCl · 6H2O, 5.5; MgSO4 · 7H2O, 71.2; ZnSO4 · 7H2O, 0.4; MnSO4 · 4H2O, 0.8; CuSO4 · 5H2O, 0.8; Na2MoO4 · 2H2O, 0.3; and FeSO4 · 7H2O, 5.0. Bacteria were grown in batches on sodium succinate (2 g/liter) as the sole carbon and energy source. For the study of growth-phase-dependent changes in the fatty acid composition, samples were harvested between the early and late exponential growth phases (3 to 8 h), during a transient phase of deprivation (24 h), and during stationary phase (48 h), as monitored by measuring the optical densities of the cultures at 700 nm (data not shown).
Sample preparation.
During different growth phases, the cells were rapidly harvested without temperature changes by centrifugation at 4,000 × g for 5 min, washed in imidazole buffer (2 mM, pH 7), and recollected. This procedure was adhered to as far as possible with respect to the short centrifugation time and the constancy of temperature. From the data presented here, it seems that a brief spin at 4°C would be acceptable because it would minimize preparation artifacts. The pellets from different batches were processed in parallel in three different ways: (i) pellets were directly saponified to liberate the fatty acids without any changes in their physiological status quo, (ii) the pellets were incubated for 1 to 3 h at 30°C and analyzed immediately after incubation, and (iii) the biomass was put in a deep freezer at −20°C, stored for several days at this temperature, thawed for 1 or 15 min at 30°C, and then analyzed either immediately or after 1, 3, or 5 h of incubation in a shaking water bath at 30°C. To explore the effect of freezing and thawing, we modified the third procedure further. In some experiments, the cells were frozen twice, and in other experiments, the thawing temperature during the 2-h incubation period was set to 10, 20, 30, or 40°C prior to fatty acid analysis. Typically, two or more independent batches were pooled to collect samples for the analysis. Each experiment was performed at least twice, sometimes after a variation of the cultivation times to confirm observed trends of earlier results.
Fatty acid determination.
Fatty acids were determined as methyl esters from whole-cell hydrolysates according to the procedure of the SHERLOCK microbial identification system (MIDI Inc., Newark, Del.). Samples of about 5 to 10 mg of cell dry mass were subjected to derivatization in Teflon screw-cap test tubes. For the preparation of esters, cells were saponified at 100°C for 30 min with 1 ml of reagent I (45 g of sodium hydroxide, 150 ml of methanol, 150 ml of water), methylated at 80°C for 10 min with reagent II (325 ml of 6 M hydrochloric acid, 275 ml of methanol), extracted with 1.25 ml of reagent III (200 ml of n-hexane, 200 ml of methyl tert-butyl ether), and base washed with 3 ml of reagent IV (10.8 g of sodium hydroxide, 40 g of sodium chloride, 900 ml of water). Methylation following saponification was preferred for total fatty acid analysis because transesterification procedures are suspected to cause underestimations of cyclopropane acids and are less effective at releasing cellular hydroxyl fatty acids (45). The separation and determination of the fatty acid methyl esters were performed with a 6890 GC gas chromatography system (Agilent, Palo Alto, Calif.) equipped with a flame ionization detector (GC-FID). An automatic sample inlet was used (split injection, split ratio of 100:1, 250°C), and the separation was carried out in an Ultra2 nonpolar capillary column (length, 25 m; inside diameter, 0.20 mm; film thickness, 0.33 μm; Agilent), with hydrogen as the carrier gas (9 lb of constant pressure/in2). The temperature program was as follows: 170 to 260°C at 5 K min−1, 260 to 310°C at 40 K min−1, and 310°C for 1.5 min. Data acquisition and data analysis were controlled by ChemStation software (version 10.01; Agilent) and the Sherlock software package (version 4.5; MIDI). Fatty acid assignments were checked by comparisons of the retention times with those of known standards measured on columns with different polarities and were analyzed further after common derivatization procedures, including hydrogenation and silylation. The position of the double bond was determined by derivatization with pyrrolidine followed by an analysis of electron impact fragmentation by GC-mass spectrometry (GC-MS) (1). The cis-trans geometry of the double bonds was confirmed by comparisons of chromatographic data (retention times) and mass spectrometric data (double bond positions). The fatty acid composition was calculated from fractions of individual peaks of the total peak area. Replicate determinations indicated reproducible fatty acid profiles with relative errors of 2 to 5%, calculated as standard errors of the means (SEM).
The following fatty acid nomenclature was applied: the number preceding the colon indicates the total number of carbon atoms, the number following the colon indicates the number of double bonds, and the suffix designates the position of the double bonds (from the methyl end of the molecule, cis or trans indicates their configuration) or the position of hydroxyl groups (from the carboxyl end of the molecule). It was helpful for the sake of discussion to combine single fatty acids into groups with common structural characteristics. Such groups of interest are straight-chain saturated fatty acids (n:0), straight-chain unsaturated fatty acids with a cis (n:1 cis) or trans (n:1 trans) configuration, cyclopropane fatty acids (n:0 cyclo), and ratios of these groups. The degree of saturation, S, was defined as the ratio of straight-chain saturated fatty acids to the total amount of fatty acids. The average carbon chain length of fatty acids, ACCL, was expressed as Σ (FA × C)/100, where FA is the percentage of each fatty acid and C is the number of carbon atoms in the straight chain.
RESULTS AND DISCUSSION
Fatty acid compositions of individual strains are very similar under comparable growth conditions.
The fatty acid compositions of P. putida P8, NCTC 10936, and KT 2440 grown in batch cultures at 30°C on succinate as the sole source of carbon and energy were determined. Table 1 shows data obtained with exponentially grown cells (harvested after 6 h of cultivation) and cells from stationary phase (48 h). When the organisms were harvested during the same growth phase, their basic fatty acid profiles were qualitatively and quantitatively very similar and comprised saturated, cis (mono)unsaturated, hydroxyl, and cyclopropane fatty acids, mainly in the range of 10- to 18-carbon-atom chain lengths. Differences were found between the growth phases. At 6 and 48 h, saturated straight-chain fatty acids were in the ranges of 33 to 34% and 40 to 43%, respectively, whereas fatty acids originating from cis unsaturated ones (sum of cis, cyclo, and trans fatty acids) were in the ranges of 47 to 53% and 46 to 50%, respectively. The observed fatty acid patterns are typical for many gram-negative bacteria (34). Palmitic acid (16:0), palmitoleic acid (16:1ω7c), and vaccenic acid (18:1ω7c) were always the dominant fatty acid components of the cells. The occurrence of vaccenic acid indicated the presence of the anaerobic pathway of fatty acid biosynthesis and the absence of a fatty acid synthesis-independent desaturase system found in organisms possessing the aerobic pathway (e.g., Acinetobacter calcoaceticus [19]). Indeed, P. putida is known to be incapable of changing the saturation degree of existing fatty acids and rather relies on de novo biosynthesis of fatty acids (11).
TABLE 1.
Total fatty acid compositions (wt/wt) of P. putida strains grown on 0.2% succinate at 30°C
| Fatty acid(s) | % of totala
|
|||||
|---|---|---|---|---|---|---|
| P8
|
NCTC 10936
|
KT 2440
|
||||
| 6 h | 48 h | 6 h | 48 h | 6 h | 48 h | |
| 10:0 | 1.3 | 0b | 0.5 | 0.2 | 0.1 | 0.2 |
| 10:0 3OH | 8.3 | 3.3 | 6.7 | 3.9 | 5.5 | 2.5 |
| 12:0 | 3.8 | 4.0 | 7.4 | 9.4 | 6.5 | 10.1 |
| 12:0 2OH | 4.8 | 4.7 | 3.4 | 2.5 | 3.0 | 1.4 |
| 12:0 3OH | 4.9 | 4.2 | 4.6 | 4.5 | 4.1 | 2.3 |
| 14:0 | 0.3 | 0.7 | 0.3 | 0.7 | 0.2 | 0.7 |
| 16:1ω7c | 28.8 | 5.1 | 30.9 | 14.0 | 28.5 | 4.1 |
| 16:1ω7t | 0b | 0b | 0b | 0b | 0b | 0b |
| 16:0 | 27.3 | 36.1 | 25.4 | 28.7 | 26.8 | 31.4 |
| 17:0 cyclo | 4.5 | 29.2 | 2.5 | 17.0 | 4.3 | 25.4 |
| 18:1ω7c | 13.5 | 11.5 | 17.5 | 17.0 | 19.8 | 16.8 |
| 18:1ω7t | 0b | 0b | 0b | 0b | 0b | 0b |
| 18:0 | 0.6 | 0.6 | 0.8 | 1.0 | 0.7 | 0.8 |
| 19:0 cyclo | 0b | 0.4 | 0b | 0.8 | 0.4 | 4.0 |
| Total | 98.1 | 99.8 | 100.0 | 99.7 | 99.9 | 99.7 |
| Sum n:0c | 33.3 | 41.4 | 34.4 | 40.0 | 34.3 | 43.2 |
| Sum n:0 OH | 18.0 | 12.2 | 14.7 | 10.9 | 12.6 | 6.2 |
| Sum n:1 cis | 42.3 | 16.6 | 48.8 | 31.0 | 48.3 | 20.9 |
| Sum n:0 cyclo | 4.5 | 29.6 | 2.5 | 17.8 | 4.7 | 29.4 |
| Sum n:1 trans | 0b | 0b | 0b | 0b | 0b | 0b |
| ACCL | 15.1 | 15.5 | 15.3 | 15.5 | 15.5 | 15.7 |
| S | 52 | 54 | 49 | 51 | 47 | 50 |
| trans/cis ratio | 0b | 0b | 0b | 0b | 0b | 0b |
| cyclo/cis ratio | 0.11 | 1.79 | 0.05 | 0.57 | 0.10 | 1.40 |
Samples were taken after 6 h (exponential growth phase) and 48 h (stationary growth phase) and were analyzed immediately. Comparable results were obtained in separate analyses on three occasions (typically with <5% SEM).
Below limits of detection.
n represents each carbon atom that was found.
No trans fatty acids are formed in exponentially growing cells or stationary-phase cells.
P. putida is known for possessing trans unsaturated fatty acids in its outer membrane. Surprisingly, and in contrast to earlier reports with the same strain, P. putida P8 (11, 35), and related strains of P. putida (46, 51), trans unsaturated fatty acids were not found in this study, regardless of the time of sampling. As shown in Table 1, trans fatty acids were not formed during exponential growth or stationary phase by any of the strains, provided that the cells were analyzed immediately after sampling. This was all the more surprising since trans acids were also absent from cells of P. putida P8, which were grown in the presence of the inhibitor phenol at a concentration leading to 50% growth inhibition on succinate (data not shown). Only in long-term starved cells were small amounts of trans fatty acids detectable, as seen in an 11-day sample of P. putida P8 (2% 16:1ω7t). This phenomenon, already observed in cells of Vibrio cholerae that were starved for 30 days (25), will not be discussed here further because these trans fatty acids appeared in cells with a very undefined physiological state.
To exclude the possibility that the composition of the growth medium prevented the formation of trans fatty acids during growth, we alternatively cultivated strain P8 in a complex nutrient medium. The results were the same as those for minimal medium (Table 2). Moreover, growth experiments with P. putida P8 were repeated for media described by others (22, 24) that contained succinate as the sole carbon and energy source. In the past, such media had been chosen to investigate the impact of organic solvents on cis-trans isomerization. We performed our experiments under exactly the same growth conditions used by these laboratories, including the centrifugation, lipid extraction, and transesterification steps. In contrast to previous findings, we never found trans fatty acids in cells which had not been incubated with the toxicant if they were analyzed immediately (Table 2).
TABLE 2.
Percentage of trans fatty acids in P. putida P8 grown on complex medium or minimal medium
| Growth conditions | % trans fatty acids of total fatty acidsa
|
||
|---|---|---|---|
| A | B | C | |
| Growth on Trypticase soy broth (10 g/liter) for 4 h (exponential growth) at 30°C; sample processing as described in Materials and Methods | 0 | 15 | |
| Growth on minimal medium with 4 g of sodium succinate/liter for 8 h (late exponential phase) at 30°C; sample processing as described by Heipieper et al. (22) | 12 | 0 | 9 |
| Growth on minimal medium with 0.2% succinate for 4 h (exponential phase) at 30°C; sample processing as described by Holtwick et al. (24) | 6 | 0 | 12 |
A, values cited from the literature; B, samples analyzed immediately; C, samples analyzed after freeze-thawing and a 3-h incubation in buffer at 30°C.
Cyclopropane fatty acids are predominantly formed during the transition from the exponential to the stationary growth phase.
Cyclopropane fatty acids are typical for many gram-negative bacteria using the anaerobic pathway of fatty acid biosynthesis. Usually, only minor amounts of cyclopropane fatty acids are observed during exponential growth, and increasing amounts are detected during the transition to a starvation state, when growth ceases (3, 29, 33, 42). We were able to confirm these findings. As shown in Table 1 and in contrast to our findings for trans fatty acids, cyclopropane fatty acids were always present, although only in small amounts during the exponential growth phase. In stationary-phase cells, the amount of cyclopropane acids was up to 12 times higher than that during exponential growth. The cyclo/cis ratio was lower in P. putida NCTC 10936 (0.05 and 0.57 for 6 and 48 h, respectively) than in both P. putida KT 2440 (0.10 and 1.40 for 6 and 48 h, respectively) and P. putida P8 (0.11 and 1.79 for 6 and 48 h, respectively). Note that the increase in n:0 cyclo occurred at the expense of n:1 cis. It is known that the energy- and S-adenosyl-l-methionine-dependent cyclopropane synthase acts on phospholipid-esterified cis unsaturated fatty acids (32, 61). Although the molecular mechanisms controlling the formation of cyclopropane fatty acids as a function of the growth phase have been well established (5, 13, 58), the role of these fatty acids is not fully understood yet. Obviously, cyclopropane fatty acids stabilize lipids against turnover and degradation, so the energetic investment might be worthwhile because it minimizes membrane alterations that may lead to life-threatening changes in membrane fluidity during starvation (17, 33). There are reports that the presence of cyclopropane fatty acids seems to improve survival rates of acid-stressed cells (4), and it was established that especially large amounts of cyclopropane acids accumulated when starvation took place at high temperatures (9). Moreover, in comparison to cis unsaturated fatty acids, the presence of cyclopropane fatty acids should make the membrane somewhat more rigid because of their higher lipid melting points. This effect is small but significant (39) and may also contribute to stabilization of the lipid bilayer during the subsequent transition to starvation without much affecting the cells' ability to resume growth. The formation of cyclopropane fatty acids at the expense of cis unsaturated fatty acids toward the end of growth is favored and appears to represent an adaptation to starvation conditions.
Fatty acid chain length and hydroxy fatty acid content are only marginally affected by the growth phase.
As shown in Table 1, the calculated ACCLs were very similar for all three strains. ACCLs ranged from 15.1 to 15.5 and 15.5 to 15.7 for 6 and 48 h of growth, respectively. The increasing chain lengths indicate a somewhat higher rigidity of membranes towards the end of exponential growth. However, the increase was only marginal compared with values found for Micrococcus cryophilus (53) and Pseudomonas oleovorans (6) and does not justify discussion in terms of adaptive strategies in P. putida. The proportion of hydroxy fatty acids was slightly decreased towards the beginning of stationary phase. The sum of hydroxy fatty acids ranged between 13 and 18% and 6 and 12% after 6 and 48 h of growth, respectively. The purpose of this decrease is unclear.
The saturation degree increases during transition from exponential to stationary growth phase.
Growth-linked changes of the saturation degree (S) are the most frequently observed membrane adaptation to altering environmental conditions. The calculated saturation degrees were similar for all three strains provided that growth occurred under the same cultivation conditions (Table 1). For 6 and 48 h of growth, S ranged from 47 to 52% and 50 to 54%, respectively. This increase with prolonged cultivation and depletion of the growth substrate confirms earlier data obtained with P. putida P8 (35) and resembles data obtained with chemostat cultures of Escherichia coli at different dilution rates (2, 55). Increased contents of saturated fatty acids enhancing the rigidity of the cytoplasmic membrane are a well-known mechanism of adaptation to strong membrane-fluidizing factors such as a rise in temperature, growth on or in the presence of lipophilic organic solvents, etc. (26, 54). As shown here, an increase in saturation can also be the result of a transition to the starvation state. The variability of S along the growth curve was closely linked to the changing substrate supply, indicating that adaptive responses adjust rather than maintain membrane fluidity. Less saturation leading to higher membrane fluidity seemed to be optimal for growth. This may be explained by the role of membrane fluidity in the activity of membrane proteins involved in substrate uptake (8).
Reincubation of cells under nongrowth conditions leads to sudden formation of trans fatty acids.
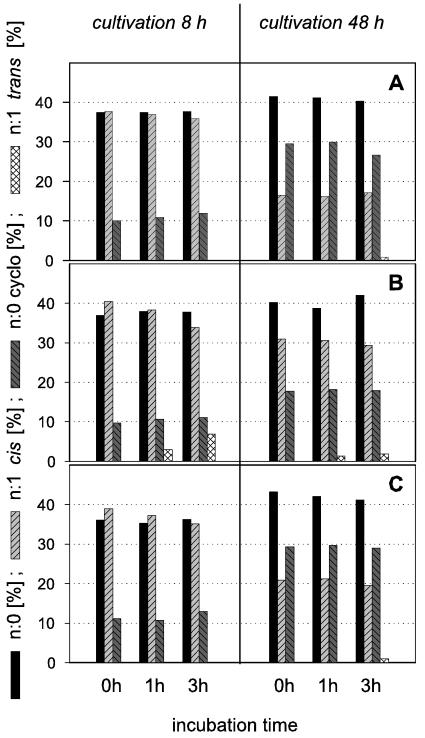
The extent to which the capacity of an organism to adapt is realized depends on its physiological state. We were interested in determining the possible changes in fatty acid composition in cells of P. putida under nongrowth conditions. To gain insights into the behavior of resting cells, we investigated variations in the fatty acid patterns of the strains after different treatments. The washed biomass was incubated in buffer (without growth substrates) for 1 to 3 h at 30°C before fatty acid analysis. Figure 1 shows the development of selected groups of fatty acids. Various combinations of cultivation times and incubation times as resting cells were studied. Whereas P. putida P8 and KT 2440 (8-h values) showed only marginal changes upon incubation as resting cells, a small percentage of palmitelaidic acid (16:1ω7t) appeared in resting cells of P. putida NCTC 10936 (grown for 8 h). The longer the incubation in buffer solution was, the more trans fatty acids formed at the expense of cis acids were found, whereas no further formation of cyclopropane acids was observed (Fig. 1). A similar behavior, but delayed and occurring to a somewhat lesser extent, was found for cells that had been harvested from stationary phase. This was consistent with a higher content of cyclopropane acids in stationary-phase cells, as it may be explained by the smaller amounts of cis acids available for cis-trans isomerization and the fact that the cells were already sufficiently adapted (via cis-cyclo conversion) at the time of incubation in buffer.
FIG. 1.
Fatty acid compositions (wt/wt) of P. putida strains (P8 [A], NCTC 10936 [B], and KT 2440 [C]) grown on 0.2% succinate at 30°C. Groups of straight-chain saturated, cyclopropane, cis, and trans fatty acids are shown. Samples were taken after 8 h (exponential growth phase) and 48 h (stationary growth phase) and processed in different ways. One sample (control, 0 h) was analyzed immediately, and the other samples were incubated for 1 or 3 h in a nutrient-free buffer at 30°C before analysis. Representative results are shown. At least two independent experiments were performed (SEM, <5%).
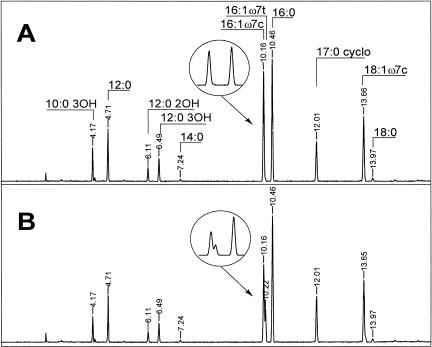
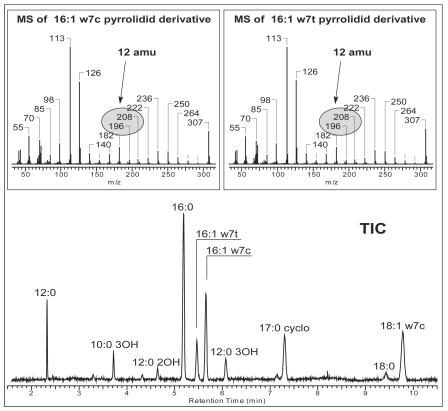
For verification of the analytical data, two original gas chromatograms of fatty acid methyl esters from exponentially growing cells of P. putida NCTC 10936 are shown as examples (Fig. 2). Palmitelaidic acid was only detectable in the sample representing resting cells (Fig. 2B). To prove that the peak at 10.22 min was really the trans compound and not an artifact or a cis fatty acid with another double bond position, we confirmed our findings by GC-MS, using a column with more polarity. For this experiment, the sample giving the chromatogram shown in Fig. 2B was analyzed in a polar column (DB 23 instead of HP Ultra2). Figure 3 shows the total ion chromatogram of the mass spectra. The elution order of fatty acid methyl esters changed as expected, and the peak interpreted as 16:1ω7t was found again and was baseline separated from 16:1ω7c. We verified the identities of both peaks by derivatization with pyrrolidine (1). Figure 3 shows mass spectra typical for 16:1 pyrrolidide compounds. They are identical, thus proving the coexistence of two chromatographically separated 16:1 compounds with double bond ω7, as seen from the unique 12-atomic-mass-unit difference between m/z 196 and 208. They thus undoubtedly represent the cis and trans isomers. Moreover, in the past, the unambiguous occurrence of these trans fatty acids (16:1ω7t and, in minor amounts, 18:1ω7t) was proven in strain P8 by infrared spectrometry (22).
FIG. 2.
GC-FID separation of fatty acid methyl esters of P. putida NCTC 10936 in a nonpolar column (HP Ultra2). Cells were grown on 0.2% succinate for 8 h. (A) The sample was analyzed immediately; (B) the sample was resuspended in imidazole buffer at 30°C for 3 h before analysis. Peaks representing 16:1 and 16:0 are enlarged.
FIG. 3.
GC-MS-total ion chromatogram (TIC) of fatty acid methyl esters of P. putida NCTC 10936 in a polar column (DB-23). The sample used was the same as that described for Fig. 2B. Mass spectra of the peaks representing 16:1ω7c and 16:1ω7t after derivatization of the fatty acid methyl esters to their corresponding pyrrolidide compounds are shown above the total ion chromatogram.
The surprising results that no trans fatty acids were formed during exponential and stationary growth but suddenly occurred during incubation in buffer may serve as a plausible explanation for the large amounts of trans acids previously found in V. cholerae (17) and in a marine bacterial isolate (41). Both organisms have in common the ability to form trans fatty acids, but possibly the trans acid contents measured were not characteristic for short-term-starved cells. For these starvation studies, the organisms were harvested and reincubated with an energy- and nutrient-free medium. Therefore, possibly the reincubation and not the short-term starvation was the cause for the large observed amounts of trans fatty acids.
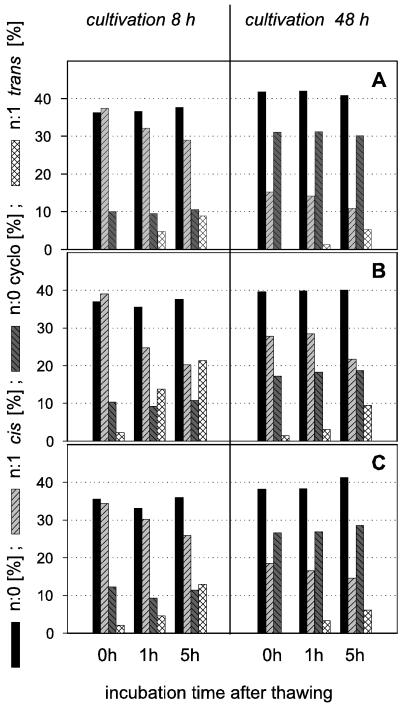
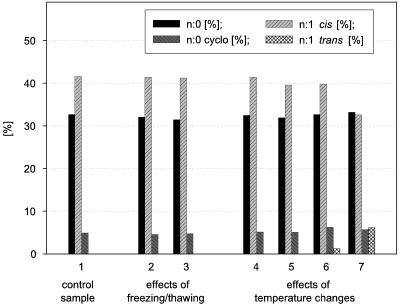
Freeze-thawing of resting cells and increasing temperatures accelerate the formation of trans fatty acids.
Figure 4 shows that the formation of trans fatty acids was promoted by a freeze-thaw procedure prior to fatty acid analysis. A combination of freeze-thawing and prolonged incubation of resting cells gave rise to the largest quantities of trans fatty acids. This observation is important because in analysis protocols from many laboratories freezing is a preferred technique for storing samples. Based on our results, this may be a source of erroneous data with regard to trans fatty acids. The occurrence of a small amount of trans fatty acids in some 0-h samples (Fig. 4) appears to be caused by the treatment prior to incubation (concentration of the biomass by centrifugation, freezing, and thawing without temperature control). To understand the influence of freeze-thawing on the formation of trans acids in more detail, we studied the effects of the temperature changes during these processes. Figure 5 shows two results obtained with P. putida KT2440. Sample freezing and thawing, even when repeated, did not lead to the formation of n:1 trans fatty acids provided that there was no increase in the temperature beyond the original growth temperature. When samples were incubated for 2 h after thawing at different temperatures, cis-trans isomerization was seen at the higher temperatures tested (30 and 40°C). At incubation temperatures that were lower than the cultivation temperature, no trans acids appeared (20 or 10°C). The strong temperature influence on the trans fatty acid formation is in accordance with earlier results (35). To verify our findings of the strong impact of sample treatment, we repeated some experiments that were done by other authors (22, 24). While no trans fatty acids could be found in samples cultivated according to the authors' specifications and analyzed immediately, the formation of trans fatty acids may be provoked by changing the sample treatment (Table 2).
FIG. 4.
Fatty acid compositions (wt/wt) of P. putida strains (P8 [A], NCTC 10936 [B], and KT 2440 [C]) grown on 0.2% succinate at 30°C. Groups of straight-chain saturated, cyclopropane, cis, and trans fatty acids are shown. Samples were harvested after 8 h (exponential growth phase) or 48 h (stationary growth phase), washed rapidly, and then frozen (−20°C). After thawing (30°C, 15 min), the samples were analyzed immediately (control, 0 h) or after an incubation time of 1 or 5 h in a nutrient-free buffer at 30°C. Representative results are shown. At least two independent experiments were performed (SEM, <5%).
FIG. 5.
Development of straight-chain saturated, cyclopropane, cis, and trans fatty acids of P. putida KT 2440 grown for 4 h on 0.2% succinate at 30°C. Samples were harvested, washed rapidly, and analyzed immediately after the following alternate treatments: 1, control sample without further treatment; 2, one freeze (−20°C)-thaw (30°C, 1 min) cycle; 3, two freeze-thaw cycles without a longer dwell time; 4 to 7, a freeze (−20°C)-thaw (10°C) cycle followed by a 2-h incubation at 10, 20, 30, and 40°C, respectively. At least two independent experiments were performed (SEM, <5%).
Conclusions.
Our results show for the first time that under conditions of undisturbed growth, no trans fatty acids are found in lipids of P. putida, regardless of the growth phase. However, upon a change in the environment of cells affecting membrane fluidity that was too abrupt to be compensated for by altered fatty acid synthesis, trans fatty acids were formed rapidly (21, 36, 59). The highest rate of trans fatty acid formation was found for cells that were harvested from the exponential growth phase and subsequently incubated for several hours in buffer at an elevated temperature. As expected, the contents of cyclopropane and saturated fatty acids remained largely constant during this treatment. The sudden appearance of trans fatty acids suggests that the cytoplasmic membrane was more fluid at the time of disturbance than was appropriate for functioning under the new conditions. Therefore, the formation of trans unsaturated fatty acids can be considered an adaptation response to increase the membrane rigidity, i.e., an emergency reaction rather than a way to fine-tune (48) the membrane fluidity during growth and multiplication (23).
The formation of trans fatty acids is catalyzed by a nonreversible cis-trans isomerase without a shift in the double bond position and without a requirement of a cofactor or energy (12, 44, 50). The enzyme has been purified (49, 50), and the encoding gene was cloned and sequenced (24, 27). It has been verified that the isomerization reaction is possible without growth (22, 36). The fact that trans fatty acids are formed rapidly in cells incubated in an energy- and nutrient-free medium suggests that the cis-trans isomerase is already present (22, 47). If that is true, then the constitutively expressed enzyme exists in a nonactive state or is spatially separated to act on the phospholipid molecules of the membrane during the exponential and stationary growth phases. There is evidence that changes in the membrane fluidity are the primary signals eliciting the isomerization reaction (26, 46). However, if a high membrane fluidity is a precondition for the cis-trans conversion, it cannot be the only one. In fact, exponentially growing cells are characterized by lower degrees of saturation but do not contain trans acids.
The extent to which trans fatty acids can be formed obviously depends on the amounts of cyclopropane fatty acids already formed from the common precursor pool of cis unsaturated fatty acids. Only the remaining cis fatty acids can be used for the formation of trans acids. The relatively stable cyclopropane acids are unavailable for conversion into trans acids. Therefore, in contrast to a former hypothesis (17) and confirmative of later reports (11), the trans/cis ratio is a less robust indicator for low nutrient levels in ecosystems than the cyclo/cis ratio (60).
Theoretically, the content of trans fatty acids can serve as a stress indicator in ecosystems because cis-trans isomerization is a way for some bacteria to respond to a temperature rise or an exposure to toxic substances (59). However, the practical use of trans fatty acids as a quantitative biomarker of specific environmental stress is questionable because of their generally low quantities, their occurrence in only a small proportion of all bacteria, the competing cyclopropane fatty acid synthesis, and the fact that they are formed only under nongrowth conditions and upon shock impacts.
Acknowledgments
We thank Hermann J. Heipieper for a critical discussion during the preparation of the manuscript and Angelika Wichmann for her technical assistance.
REFERENCES
- 1.Andersson, B. A., and R. T. Holman. 1974. Pyrrolidides for mass spectrometric determination of the position of the double bond in monounsaturated fatty acids. Lipids 9:185-190. [DOI] [PubMed] [Google Scholar]
- 2.Arneborg, N., A. S. Salskov-Iversen, and T. E. Mathiasen. 1993. The effect of growth rate and other growth conditions on the lipid composition of Escherichia coli. Appl. Microbiol. Biotechnol. 39:353-357. [Google Scholar]
- 3.Brian, B. L., and E. W. Gardner. 1968. Cyclopropane fatty acids of rugose Vibrio cholerae. J. Bacteriol. 96:2181-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang, Y. Y., and J. E. Cronan, Jr. 1999. Membrane cyclopropane fatty acid content is a major factor in acid resistance of Escherichia coli. Mol. Microbiol. 33:249-259. [DOI] [PubMed] [Google Scholar]
- 5.Chang, Y. Y., J. Eichel, and J. E. Cronan, Jr. 2000. Metabolic instability of Escherichia coli cyclopropane fatty acid synthase is due to RpoH-dependent proteolysis. J. Bacteriol. 182:4288-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, Q., A. Nijenhuis, H. Preusting, J. Dolfing, D. B. Janssen, and B. Witholt. 1995. Effects of octane on the fatty acid composition and transition temperature of Pseudomonas oleovorans membrane lipids during growth in two-liquid-phase continuous cultures. Enzyme Microb. Technol. 17:647-652. [Google Scholar]
- 7.Cossins, A. R., and M. Sinensky. 1984. Adaptation of membranes to temperature, pressure, and exogenous lipids, p. 1-20. In M. Shinitzky (ed.), Physiology of membrane fluidity, vol. II. CRC Press, Boca Raton, Fla.
- 8.Cronan, J. E., Jr., and E. P. Gelmann. 1975. Physical properties of membrane lipids: biological relevance and regulation. Bacteriol. Rev. 39:232-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cullen, J., M. C. Phillips, and G. G. Shipley. 1971. The effects of temperature on the composition and physical properties of the lipids of Pseudomonas fluorescens. Biochem. J. 125:733-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denich, T. J., L. A. Beaudette, H. Lee, and J. T. Trevors. 2003. Effect of selected environmental and physico-chemical factors on bacterial cytoplasmic membranes. J. Microbiol. Methods 52:149-182. [DOI] [PubMed] [Google Scholar]
- 11.Diefenbach, R., H. J. Heipieper, and H. Keweloh. 1992. The conversion of cis into trans unsaturated fatty acids in Pseudomonas putida P8: evidence for a role in the regulation of membrane fluidity. Appl. Microbiol. Biotechnol. 38:382-387. [Google Scholar]
- 12.Diefenbach, R., and H. Keweloh. 1994. Synthesis of trans unsaturated fatty acids in Pseudomonas putida P8 by direct isomerization of the double bond of lipids. Arch. Microbiol. 162:120-125. [DOI] [PubMed] [Google Scholar]
- 13.Eichel, J., Y. Y. Chang, D. Riesenberg, and J. E. Cronan, Jr. 1999. Effect of ppGpp on Escherichia coli cyclopropane fatty acid synthesis is mediated through the RpoS sigma factor (σS). J. Bacteriol. 181:572-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fozo, E. A., and R. G. Quivey. 2004. Shifts in the membrane fatty acid profile of Streptococcus mutans enhance survival in acidic environments. Appl. Environ. Microbiol. 70:929-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fulco, A. J. 1974. Metabolic alterations of fatty acids. Annu. Rev. Biochem. 43:215-241. [DOI] [PubMed] [Google Scholar]
- 16.Gillan, F. T., R. B. Johns, T. V. Verheyen, J. K. Volkman, and H. J. Bavor. 1981. Trans-monounsaturated acids in a marine bacterial isolate. Appl. Environ. Microbiol. 41:849-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guckert, J. B., M. A. Hood, and D. C. White. 1986. Phospholipid ester-linked fatty acid profile changes during nutrient deprivation of Vibrio cholerae: increases in the trans/cis ratio and proportions of cyclopropyl fatty acids. Appl. Environ. Microbiol. 52:794-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guckert, J. B., D. B. Ringelberg, and D. C. White. 1987. Biosynthesis of trans fatty acids from acetate in the bacterium Pseudomonas atlantica. Can. J. Microbiol. 33:748-754. [Google Scholar]
- 19.Härtig, C., N. Loffhagen, and W. Babel. 1999. Glucose stimulates a decrease of the fatty acid saturation degree in Acinetobacter calcoaceticus. Arch. Microbiol. 171:166-172. [Google Scholar]
- 20.Hazel, J. R. 1997. Thermal adaptation in biological membranes: beyond homeoviscous adaptation. Adv. Mol. Cell Biol. 19:57-101. [DOI] [PubMed] [Google Scholar]
- 21.Heipieper, H. J., and J. A. M. de Bont. 1994. Adaptation of Pseudomonas putida S12 to ethanol and toluene at the level of fatty acid composition of membranes. Appl. Environ. Microbiol. 60:4440-4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heipieper, H. J., R. Diefenbach, and H. Keweloh. 1992. Conversion of cis unsaturated fatty acids to trans, a possible mechanism for the protection of phenol-degrading Pseudomonas putida P8 from substrate toxicity. Appl. Environ. Microbiol. 58:1847-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heipieper, H. J., F. Meinhardt, and A. Segura. 2003. The cis-trans isomerase of unsaturated fatty acids in Pseudomonas and Vibrio: biochemistry, molecular biology and physiological function of a unique stress adaptive mechanism. FEMS Microbiol. Lett. 229:1-7. [DOI] [PubMed] [Google Scholar]
- 24.Holtwick, R., F. Meinhardt, and H. Keweloh. 1997. Cis-trans isomerization of unsaturated fatty acids: cloning and sequencing of the cti gene from Pseudomonas putida P8. Appl. Environ. Microbiol. 63:4292-4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hood, M. A., J. B. Guckert, D. C. White, and F. Deck. 1986. Effect of nutrient deprivation on lipid, carbohydrate, DNA, RNA, and protein levels in Vibrio cholerae. Appl. Environ. Microbiol. 52:788-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ingram, L. O. 1976. Adaptation of membrane lipids to alcohols. J. Bacteriol. 125:670-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Junker, F., and J. L. Ramos. 1999. Involvement of the cis/trans isomerase Cti in solvent resistance of Pseudomonas putida DOT-T1E. J. Bacteriol. 181:5693-5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaneda, T. 1977. Fatty acids of the genus Bacillus: an example of branched-chain preference. Bacteriol. Rev. 41:391-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kates, M., G. A. Adams, and S. M. Martin. 1964. Lipids of Serratia marcescens. J. Lipid Res. 5:132-135. [Google Scholar]
- 30.Konopasek, I., K. Strzalka, and J. Svobodova. 2000. Cold shock in Bacillus subtilis: different effects of benzyl alcohol and ethanol on the membrane organisation and cell adaptation. Biochim. Biophys. Acta 1464:18-26. [DOI] [PubMed] [Google Scholar]
- 31.Kroppenstedt, R. M. 1979. Chromatographische Identifizierung von Mikroorganismen, dargestellt am Beispiel der Actinomyceten. Kontakte 2:12-21. [Google Scholar]
- 32.Law, J. H. 1971. Biosynthesis of cyclopropane rings. Acc. Chem. Res. 4:199-203. [Google Scholar]
- 33.Law, J. H., H. Zalkin, and T. Kaneshiro. 1963. Transmethylation reactions in bacterial lipids. Biochim. Biophys. Acta 70:143-151. [Google Scholar]
- 34.Lennarz, W. J. 1966. Lipid metabolism in the bacteria. Adv. Lipid Res. 4:175-225. [DOI] [PubMed] [Google Scholar]
- 35.Loffeld, B., and H. Keweloh. 1996. Cis-trans isomerization of unsaturated fatty acids as possible control mechanism of membrane fluidity in Pseudomonas putida P8. Lipids 31:811-815. [DOI] [PubMed] [Google Scholar]
- 36.Loffhagen, N., C. Härtig, and W. Babel. 2001. Suitability of the trans/cis ratio of unsaturated fatty acids in Pseudomonas putida NCTC 10936 as an indicator of the acute toxicity of chemicals. Ecotoxicol. Environ. Saf. 50:65-71. [DOI] [PubMed] [Google Scholar]
- 37.Loffhagen, N., C. Härtig, and W. Babel. 2004. Pseudomonas putida NCTC 10936 balances membrane fluidity in response to physical and chemical stress by changing the saturation degree and the trans/cis ratio of fatty acids. Biosci. Biotechnol. Biochem. 68:317-323. [DOI] [PubMed] [Google Scholar]
- 38.Loffhagen, N., C. Härtig, D. Benndorf, and W. Babel. 2002. Effects of growth temperature and lipophilic carbon sources on the fatty acid composition and membrane lipid fluidity of Acinetobacter calcoaceticus 69V. Acta Biotechnol. 22:235-243. [Google Scholar]
- 39.MacDonald, P. M., B. D. Sykes, and R. N. McElhaney. 1985. Fluorine-19 nuclear magnetic resonance studies of lipid fatty acyl chain order and dynamics in Acholeplasma laidlawii B membranes. A direct comparison of the effects of cis and trans cyclopropane ring and double-bond substituents on orientational order. Biochemistry 24:4651-4659. [DOI] [PubMed] [Google Scholar]
- 40.Makula, R. A. 1978. Phospholipid composition of methane-utilizing bacteria. J. Bacteriol. 134:771-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malmcrona-Friberg, K., A. Tunlid, P. Marden, S. Kjelleberg, and G. Odham. 1986. Chemical changes in cell envelope and poly-β-hydroxybutyrate during short term starvation of a marine bacterial isolate. Arch. Microbiol. 144:340-345. [Google Scholar]
- 42.Marr, A. G., and J. L. Ingraham. 1962. Effect of temperature on the composition of fatty acids in Escherichia coli. J. Bacteriol. 84:1260-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McElhaney, R. N. 1984. The structure and function of the Acholeplasma laidlawii plasma membrane. Biochim. Biophys. Acta 779:1-42. [DOI] [PubMed] [Google Scholar]
- 44.Morita, N., A. Shibahara, K. Yamamoto, K. Shinkai, H. Kajimoto, and H. Okuyama. 1993. Evidence for cis-trans isomerization of a double bond in the fatty acids of the psychrophilic bacterium Vibrio sp. strain ABE-1. J. Bacteriol. 175:916-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moss, C. W., M. A. Lambert, and W. H. Merwin. 1974. Comparison of rapid methods for analysis of bacterial fatty acids. Appl. Microbiol. 28:80-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neumann, G., N. Kabelitz, and H. J. Heipieper. 2003. The regulation of the cis-trans isomerase (cti) of unsaturated fatty acids in Pseudomonas putida: correlation between cti activity and K+-uptake systems. Eur. J. Lipid Sci. Technol. 105:585-589. [Google Scholar]
- 47.Okuyama, H., N. Okajima, S. Sasaki, S. Higashi, and N. Murata. 1991. The cis/trans isomerization of the double bond of a fatty acid as a strategy for adaptation to changes in ambient temperature in the psychrophilic bacterium Vibrio sp. strain ABE-1. Biochim. Biophys. Acta 1084:13-20. [DOI] [PubMed] [Google Scholar]
- 48.Okuyama, H., S. Sasaki, S. Higashi, and N. Murata. 1990. A trans-unsaturated fatty acid in a psychrophilic bacterium, Vibrio sp. strain ABE-1. J. Bacteriol. 172:3515-3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okuyama, H., A. Ueno, D. Enari, N. Morita, and T. Kusano. 1998. Purification and characterisation of 9-hexadecenoic acid cis-trans isomerase from Pseudomonas sp. strain E-3. Arch. Microbiol. 169:29-35. [DOI] [PubMed] [Google Scholar]
- 50.Pedrotta, V., and B. Witholt. 1999. Isolation and characterization of the cis-trans-unsaturated fatty acid isomerase of Pseudomonas oleovorans Gpo12. J. Bacteriol. 181:3256-3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pinkart, H. C., J. W. Wolfram, R. Rogers, and D. C. White. 1996. Cell envelope changes in solvent-tolerant and solvent-sensitive Pseudomonas putida strains following exposure to o-xylene. Appl. Environ. Microbiol. 62:1129-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramos, J. L., E. Duque, M. T. Gallegos, P. Godoy, M. I. Ramos-Gonzalez, A. Rojas, W. Teran, and A. Segura. 2002. Mechanism of solvent tolerance in gram-negative bacteria. Annu. Rev. Microbiol. 56:743-768. [DOI] [PubMed] [Google Scholar]
- 53.Russell, N. J. 1984. Mechanisms of thermal adaptation in bacteria: blueprints for survival. Trends Biochem. Sci. 9:108-112. [Google Scholar]
- 54.Seeman, P. 1972. The membrane actions of anaesthetics and tranquillizers. Pharmacol. Rev. 24:583-655. [PubMed] [Google Scholar]
- 55.Shokri, A., A. M. Sanden, and G. Larsson. 2002. Growth rate-dependent changes in Escherichia coli membrane structure and protein leakage. Appl. Microbiol. Biotechnol. 58:386-392. [DOI] [PubMed] [Google Scholar]
- 56.Sikkema, J., J. A. M. de Bont, and B. Poolman. 1995. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 59:201-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sinensky, M. 1974. Homeoviscous adaptation: a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc. Natl. Acad. Sci. USA 71:522-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang, A. Y., and J. E. Cronan, Jr. 1994. The growth phase-dependent synthesis of cyclopropane fatty acids in Escherichia coli is the result of an RpoS(KatF)-dependent promoter plus enzyme instability. Mol. Microbiol. 11:1009-1017. [DOI] [PubMed] [Google Scholar]
- 59.Weber, F. J., S. Isken, and J. A. M. de Bont. 1994. Cis/trans isomerization of fatty acids as a defence mechanism of Pseudomonas putida strains to toxic concentrations of toluene. Microbiology 140:2013-2017. [DOI] [PubMed] [Google Scholar]
- 60.White, D. C., and D. B. Ringelberg. 1998. Signature lipid biomarker analysis, p. 255-272. In R. S. Burlage et al. (ed.), Techniques in microbial ecology. Oxford University Press, Oxford, N.Y.
- 61.Zalkin, H., J. H. Law, and H. Goldfine. 1963. J. Biol. Chem. 238:1242. [PubMed] [Google Scholar]