Abstract
Water samples were taken systematically from a 100-km2 area of mainly dairy farmland in northwestern England and examined for Campylobacter spp. Pulsed-field gel electrophoresis-restriction fragment length polymorphism (PFGE-RFLP) and flaA strain typing of Campylobacter jejuni and Campylobacter coli isolates were done. Data on the water source and the adjacent environment were recorded and examined as explanatory variables. Campylobacter spp. were isolated from 40.5% (n = 119) of the water samples tested. C. jejuni was isolated from 14.3%, C. coli was isolated from 18.5%, and Campylobacter lari was isolated from 4.2% of the samples. Campylobacter hyointestinalis was not isolated from any water source. The difference in prevalence between water types (trough, running, and standing) was significant (P = 0.001). C. jejuni was the species most commonly isolated from trough-water and running-water sources, while C. coli was the most frequently isolated from standing water (P < 0.001). No association was found between the presence of Escherichia coli and that of Campylobacter spp. The final multivariable logistic regression model for Campylobacter spp. included the following variables: water source, soil type, aspect, and amount of cattle fecal material in the environment (fecal pat count). Strain typing demonstrated a diverse population of C. jejuni and the presence of a common C. coli flaA type that was widely distributed throughout the area. Most of the isolates within the common flaA type were discriminated by PFGE-RFLP. These findings suggest a possible role for environmental water in the epidemiology of Campylobacter spp. in a farming environment.
Campylobacter spp. are important causes of enteric disease in humans (1). Campylobacteriosis is usually a self-limiting but debilitating and painful disease that has enormous economic impact in terms of treatment costs, lost production, and human welfare (21). Human disease can arise from a variety of sources, and poultry (10), raw milk (19), and untreated surface water (8) are considered the most important (3). Consumption or handling of poultry is thought to be associated with a high proportion of sporadic cases. The contribution of waterborne disease to sporadic cases is less well established, although water is often implicated in outbreaks of disease. In England and Wales between 1992 and 1995, there were six outbreaks of Campylobacter enteritis associated with consumption of water from private supplies, involving a total of 128 people (8). Campylobacter spp. have been isolated from a range of different water types, including sewage outflows, river water, groundwater, and seawater (13). In temperate climates, a consistent and marked seasonality is seen in human cases of Campylobacter enteritis, with a peak in late spring and early summer and a second, smaller peak in early autumn (20). The isolation of Campylobacter spp. from sewage effluent shows a similar trend. Conversely, the seasonality seen in water other than sewage effluent does not show the same pattern and may be the reverse of that seen in human cases (14). Campylobacter spp. are found commonly in the guts of many warm-blooded animals and are not thought to replicate in the environment, so fecal contamination is considered the major source of Campylobacter spp. in water.
In this study, we estimated the prevalence of Campylobacter spp. in water samples taken systematically from a 100-km2 rural area. Samples came from a variety of water sources, including ponds, streams, ditches, and cattle water troughs. Cattle feces, wildlife feces, and soil from the ground adjacent to the water were examined for Campylobacter spp. Water samples were examined for Campylobacter spp. and Escherichia coli, and data on environmental conditions adjacent to the water were recorded. These data, and geographical data derived from a geographical information system (GIS), were examined as possible determinants of the distribution of Campylobacter spp. in water. Isolates from water were assigned to species on the basis of PCR assay results, and Campylobacter jejuni and Campylobacter coli isolates were strain typed by both flaA and pulsed-field gel electrophoresis (PFGE)-restriction fragment length polymorphism (RFLP) methods.
MATERIALS AND METHODS
Study design.
The study area was an area of farmland in Cheshire, United Kingdom, 10 by 10 km square. The predominantly rural area had a widely varying geography and contained a large number of small ponds, produced by the historical practice of digging out the deep clay layers, known as marl, to spread on the land as fertilizer. The study was undertaken between 22 May and 26 July 2000. Samples were taken from within 270 sampling squares, spread systematically over the area, each measuring 100 by 100 m. The sampling design is described in more detail elsewhere (4, 15).
Up to 500 ml of water was sampled from the surface of any water source within the sampling square and placed in a sterile, opaque container. The sampling of cattle feces, wildlife feces, and soil is described elsewhere (4). All samples were immediately placed in cool boxes in the field and stored at 4°C before processing.
Laboratory processing of samples.
Up to 500 ml of water was filtered through a 0.2-μm-pore-size filter in a Nalgene filter unit for isolation of Campylobacter spp. and through a 0.45-μm-pore-size filter for E. coli isolation. The pH of the filtrate was measured with a pH meter. For Campylobacter isolation, the filter was placed in 9 ml of Campylobacter enrichment broth (Lab M-lab135) containing 5% lysed horse blood and CVTC supplement (cefoperazone, vancomycin, trimethoprim, and cycloheximide; Lab Mx131) and incubated at 42 ± 1°C for 48 ± 4 h under microaerobic conditions. The broths were then inoculated onto Campylobacter blood-free (modified CCDA) agar (Lab M-lab112) containing CA antibiotic supplement (cefoperazone and amphotericin; Lab M-x112 x212) and incubated as before for 48 ± 4 h. Up to four colonies having the appearance of Campylobacter spp. were subcultured onto Columbia agar (Lab M-lab1) supplemented with 5% defibrinated horse blood (Columbia blood agar) and incubated for 24 to 48 h. Isolates showing small, gram-negative, curved rods that were catalase and oxidase negative and failed to grow in oxygen were identified as presumptive Campylobacter spp. Isolates thus identified were frozen in Microbank tubes (Pro-Lab Diagnostics) at −80°C.
For E. coli isolation, water filters were placed in 9 ml of buffered peptone water, vortexed, and incubated aerobically for 24 ± 4 h at 37°C. A loopful of this broth was then subcultured onto eosin methylene blue agar plates, which were incubated at 37°C for 20 to 24 h. Up to two colonies showing typical E. coli morphology were subcultured onto nutrient agar and identified as presumptive E. coli by standard methods.
Assignment to species and strain typing.
Isolates were identified as C. jejuni, C. coli, Campylobacter lari, Campylobacter hyointestinalis, or nonspecific Campylobacter spp. by single-reaction PCR with previously described primers and conditions (16, 9). flaA typing of C. jejuni and C. coli isolates was done by the method of Nachamkin et al. (17), with some modifications (15). C. lari cannot be typed by this method. PFGE typing of C. jejuni, C. coli, and C. lari isolates was done by the rapid method of Ribot et al. (18), with some modifications (15). Matching and dendrogram analyses of the banding patterns by the unweighted-pair group method with average linkages were performed with Molecular Analyst software (Bio-Rad Laboratories) by using the Dice coefficient with a 2% tolerance window. Isolates from the same sample that had the same flaA and PFGE types were considered to be one strain, and only one was included in the analysis.
Explanatory variables.
Data on the environmental conditions at the time of sampling were recorded (see Table 2). Bovine fecal pat counts were determined by enumerating the fecal pats present within a 5-m radius of 16 points evenly located within the surrounding sampling square (15). Up to 32 fecal pats were sampled, and the age of each pat was scored on a four-point scale, 1 being the youngest and 4 the oldest (4), and this was used to calculate the average age score for each sampling square. The variable “level of recent fecal contamination” was calculated by subtracting the average age score from four (to reverse the direction of age scoring and so make it an indicator of how recent the fecal contamination appeared to be) and multiplying this by the fecal pat count. Bovine fecal samples were pooled for microbiological examination, producing up to eight pools per sampling square. The variable “positive bovine fecal pats” was calculated as the total number of pats from pools positive for Campylobacter spp. in each sampling square (15).
TABLE 2.
Results of univariable analyses examining the relationship between variables recorded at the time of sampling or derived from GIS and the isolation of Campylobacter spp. from environmental water samples
| Variable and category | No. of samples | Proportion positive | P valuea |
|---|---|---|---|
| Water source | |||
| Trough | 28 | 0.11 | 0.001 |
| Running | 30 | 0.57 | |
| Standing | 61 | 0.46 | |
| Date sampled (days from start of study) | |||
| 0-14 | 27 | 0.56 | 0.071 (0.009) |
| 15-29 | 32 | 0.47 | |
| 30-44 | 28 | 0.36 | |
| 45+ | 30 | 0.23 | |
| Sun | |||
| Overcast | 57 | 0.42 | 0.62 |
| Some cloud | 42 | 0.33 | |
| Clear | 16 | 0.44 | |
| Rain | |||
| Dry | 86 | 0.35 | 0.11 |
| Rain | 29 | 0.52 | |
| Ground condition | |||
| Wet | 52 | 0.38 | 0.69 |
| Dry | 69 | 0.42 | |
| Bovine fecal pat count | |||
| 0 | 42 | 0.36 | 0.65 (0.33) |
| 1-30 | 18 | 0.33 | |
| 31-90 | 29 | 0.48 | |
| 91+ | 32 | 0.44 | |
| Avg pat age | |||
| No pats present | 39 | 0.31 | 0.28 |
| Old | 49 | 0.43 | |
| Young | 33 | 0.48 | |
| Level of recent fecal contamination | |||
| None found | 46 | 0.37 | 0.59 (0.70) |
| Low | 39 | 0.41 | |
| Moderate | 12 | 0.58 | |
| High | 24 | 0.38 | |
| Soil type | |||
| Nonclay | 26 | 0.23 | 0.04 |
| Clay | 95 | 0.45 | |
| Distance to built land (km) | |||
| 0-0.1 | 24 | 0.38 | 0.95(0.55) |
| 0.1-0.2 | 41 | 0.39 | |
| 0.2-0.3 | 35 | 0.43 | |
| 0.3-0.7 | 20 | 0.45 | |
| Distance to hedge (km) | |||
| 0-0.07 | 27 | 0.37 | 0.92(0.70) |
| 0.07-0.15 | 53 | 0.40 | |
| 0.15-0.22 | 26 | 0.46 | |
| 0.22-0.37 | 13 | 0.38 | |
| Distance to wood (km) | |||
| 0-0.44 | 35 | 0.37 | 0.78(0.38) |
| 0.44-0.87 | 41 | 0.37 | |
| 0.87-1.31 | 28 | 0.46 | |
| 1.31-1.97 | 15 | 0.47 | |
| Amt of runoff received (arbitary units) | |||
| 1 | 47 | 0.45 | 0.41 (0.77) |
| 2-10 | 37 | 0.32 | |
| 11-60 | 21 | 0.52 | |
| 61-1,278 | 15 | 0.33 | |
| Catchment area | |||
| 0 | 66 | 0.39 | 0.22 |
| 1 | 20 | 0.50 | |
| 2 | 11 | 0.45 | |
| 3 | 1 | 1.00 | |
| 4 | 8 | 0.63 | |
| 5 | 10 | 0.10 | |
| 6 | 4 | 0.25 | |
| Aspect | |||
| No aspect | 47 | 0.32 | 0.11 |
| North facing | 43 | 0.53 | |
| South facing | 29 | 0.38 |
P values were derived from chi-square tests for differences between groups. P values from chi-square tests for trend are in parentheses.
The distances to the nearest built land, hedgerow, and woodland for each water source and the soil type in the sampling square were determined with a GIS, Idrisi (Clark Labs, Worcester, Mass.). The variables runoff, catchment area, and aspect were calculated from a digital elevation map of the area with the GIS. Runoff represents the predicted accumulation of rainfall units per pixel from one unit of rainfall being dropped on every location. The variable catchment area describes the division of the area into separately draining areas. A value of zero indicates that no catchment area could be assigned because of edge effects. Aspect refers to the direction of slope of the land surrounding each water source.
Data on the laboratory processing of samples were recorded (see Table 3). The volume filtered refers to the amount of water, up to 500 ml, that could be passed through the filter before it became blocked by particles suspended in the water. Other variables refer to the presence or absence of wildlife feces and the isolation of Campylobacter spp. from cattle, bird, and rabbit samples and are described in more detail elsewhere (4).
TABLE 3.
Results of univariable analyses examining the relationship between isolation of Campylobacter spp. from environmental water samples and variables related to the laboratory method, the presence of wildlife feces, and the isolation of Campylobacter spp. from environmental samples taken in the adjacent area
| Variable and category | No. of samples | Proportion positive | P valuea |
|---|---|---|---|
| Time to processing (days; date processed − date sampled) | |||
| 0 | 40 | 0.38 | 0.73 (0.48) |
| 1-2 | 24 | 0.33 | |
| 3-4 | 25 | 0.48 | |
| 5+ | 21 | 0.43 | |
| pH of filtrate | |||
| 6-8.3 | 53 | 0.45 | 0.32 |
| 8.4-11 | 53 | 0.36 | |
| Vol filtered (ml) | |||
| 0-49 | 22 | 0.32 | 0.74 (0.28) |
| 50-149 | 35 | 0.40 | |
| 150-249 | 21 | 0.43 | |
| 250+ | 32 | 0.47 | |
| No. of bovine fecal samples positive for Campylobacter spp. | |||
| 0 | 54 | 0.39 | 0.47 (0.53) |
| 1-4 | 15 | 0.27 | |
| 5-10 | 21 | 0.52 | |
| 11+ | 31 | 0.42 | |
| Campylobacter spp. isolated from bird | |||
| No | 90 | 0.40 | 0.85 |
| Yes | 31 | 0.42 | |
| Bird feces found | |||
| No | 45 | 0.38 | 0.64 |
| Yes | 76 | 0.42 | |
| Campylobacter spp. isolated from rabbit | |||
| No | 106 | 0.42 | 0.55 |
| Yes | 15 | 0.33 | |
| Rabbit feces found | |||
| No | 57 | 0.39 | 0.69 |
| Yes | 64 | 0.42 | |
| E. coli isolated from sample | |||
| No | 37 | 0.38 | 0.69 |
| Yes | 84 | 0.42 |
P values were derived from chi-square tests for differences between groups. P values from chi-square tests for trend are in parentheses.
Data analysis.
Statistical analysis was done with the statistical software package STATA (Stata Corporation). The distribution of C. jejuni and. C. coli between different water types was compared with the chi-square test, and differences between individual water types were examined with Fisher's exact test. The relationships between Campylobacter spp. and categorical variables were examined with chi-square tests. Continuous variables were also categorized into quartiles, unless another classification was more biologically relevant, and examined with chi-square tests and chi-square tests for trend. The relationship between continuous variables and isolation of Campylobacter spp. was also examined by fitting linear and nonlinear terms in logistic regression models (11). A significance level of 0.05 was set for univariable analysis, and a P value of 0.2 was used as a cutoff for entry into multivariable analysis. A multivariable logistic regression model was built with forward stepwise regression (11). The fit of the final model was assessed by examining the standardized delta-betas, the model sensitivity and specificity (area under the receiver operating characteristic curve), and the Hosmer-Lemeshow test statistic (10).
Spatial analysis.
Maps were produced with the Arcview (ESRI) GIS software to allow visual exploration of the distribution of Campylobacter spp. over the area. The second-order spatial properties of the distribution of Campylobacter spp. were examined by constructing a semivariogram (6) with the statistical package R (12). This was done to test whether water sources that were closer together were more similar with respect to being positive or negative for Campylobacter spp. than sources that were farther apart. The lower the semivariance, the more similar the points are at that particular spatial separation. A semivariogram was also plotted for the deviance residuals generated from the final logistic regression model for Campylobacter spp. to test whether, having allowed for other covariates, the residual variation was spatially correlated.
RESULTS
Samples were taken from 267 of the projected 270 sampling squares. Of the three squares that were not sampled, one was in the middle of a town and permission was not given by landowners to sample the other two. One hundred twenty-one water samples were included in the analysis. Two of these were mixed samples and could not be classified as trough, running, or standing water. The overall prevalence of isolation of Campylobacter spp. in water samples was 40.5% (Table 1).
TABLE 1.
Proportion of environmental water samples positive for Campylobacter spp. in a 100-km2 area of predominantly dairy farmland in the United Kingdom
| Water source | No. of samples | % of samplesa
|
|||||
|---|---|---|---|---|---|---|---|
| C. jejuni | C. coli | C. lari | C. hyointestinalis | Other spp. | All campylobacters | ||
| Trough | 28 | 7.1 | 0.0 | 0.0 | 0 | 3.6 | 10.7 |
| Running | 30 | 36.7 | 10.0 | 6.7 | 0 | 6.7 | 56.7 |
| Standing | 61 | 6.6 | 31.1 | 4.9 | 0 | 8.2 | 45.9 |
| All | 119 | 14.3 | 18.5 | 4.2 | 0 | 6.7 | 40.5 |
Five samples were positive for more than one species.
Relationship between isolation of Campylobacter spp. and explanatory variables.
The results of univariable analysis are shown in Tables 2 and 3. The presence of Campylobacter spp. was associated with the source of water (P = 0.001). Fifty-seven percent of running-water, 46% of standing-water, and 11% of trough-water samples were positive for Campylobacter spp. The differences between trough-water and running-water samples and between trough-water and standing-water samples were significant (P < 0.001 for both comparisons), but the differences between running-water and standing-water samples were not significant (P = 0.34). Campylobacter spp. were found almost twice as often in areas with clay soil than in areas with nonclay soil (P = 0.04). The presence of E. coli showed no evidence of association with the presence of Campylobacter spp. (P = 0.69).
The final multivariable model is shown in Table 4. Water source and soil type were retained in the model; both running-water and standing-water samples were more likely to be positive for Campylobacter spp. compared to trough water samples, and water in areas of clay soil was more likely to be positive than water in nonclay areas. The number of bovine fecal pats found in the surrounding sampling square was also nonlinearly associated with the presence of Campylobacter spp. (P = 0.02), with the predicted probability based on the model coefficients increasing up to a count of 117 and decreasing thereafter. Water samples taken from north-facing areas were more likely to contain Campylobacter spp. than those taken from areas with no aspect, i.e., flat areas (P = 0.003).
TABLE 4.
Results of multivariable analyses examining relationships between explanatory variables and isolation of Campylobacter spp. from environmental water samples
| Variable | Coefficient | SE | P value |
|---|---|---|---|
| Running vs trough water | 2.99 | 0.80 | <0.001 |
| Standing vs trough water | 2.19 | 0.72 | 0.003 |
| Clay vs nonclay soil | 1.56 | 0.67 | 0.02 |
| North facing vs flat | 1.73 | 0.57 | 0.003 |
| South facing vs flat | 0.79 | 0.60 | 0.19 |
| Pat count (continuous) | 0.01 | 0.005 | 0.02 |
| Pat count squared | −0.0001 | 0.00005 | 0.01 |
Removal of three observations associated with the most extreme standardized delta-beta values (>0.4 or <−0.4) did not alter the direction or significance of the parameter estimates in the final model. The Hosmer-Lemeshow chi-square test for fit produced a P value of 0.50, and the area under the receiver operating characteristic curve for this model was 0.82, suggesting excellent discrimination (11).
Assignment to species.
C. jejuni was the species most frequently isolated from trough water (7.1%) and running water (36.7%), while C. coli was the species most frequently isolated from standing water (31.1%). The difference in distribution between C. jejuni and C. coli across the three water sources (Table 1) was significant (P < 0.001). The difference between trough water and running water was not significant, but the differences between trough-water and standing-water samples and between running-water and standing-water samples were significant (P = 0.05 and P < 0.001, respectively). C. coli was the most commonly isolated species, followed by C. jejuni and then C. lari. C. hyointestinalis was not isolated.
Five water samples contained more than one Campylobacter sp. One sample was positive for both C. jejuni and unspecified Campylobacter spp., two samples were positive for both C. jejuni and C. lari, and two samples were positive for both C. coli and C. lari.
Strain typing results.
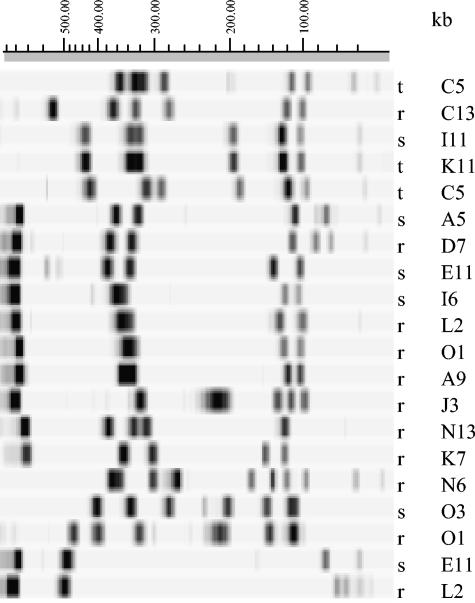
The 20 C. jejuni isolates successfully typed by PFGE-RFLP showed 17 distinct restriction patterns (RPs) (Fig. 1). A standing-water sample and a trough-water sample had the same unique RP, two running-water samples had the same unique RP, and a running-water and a standing-water isolate had the same unique RP. The latter two groups were closely related, showing 90% band similarity. Isolates from grid locations A9 and I6 (approximately 5 km apart), were within this 90% similar group and were indistinguishable by flaA typing. The isolates with the same RPs were from samples that were separated in space. The 17 C. jejuni isolates that were flaA typed showed 15 distinct banding patterns (Fig. 2). Two unique RPs were shared by two isolates; in both cases, one isolate was from running water and the other was from standing water. The isolates showing indistinguishable RPs were again spatially distant. PFGE-RFLP analysis of 22 C. coli isolates produced 20 distinct banding patterns, with 3 standing-water isolates being indistinguishable (Fig. 3). Two of these isolates came from adjacent squares; the third was from a spatially distant sample. These three isolates were also indistinguishable by flaA typing. flaA typing of 23 isolates produced 13 distinct banding patterns with two indistinguishable groupings comprising 10 and 2 isolates (Fig. 4). The larger grouping was found throughout the study area. Nine of the isolates making up this clonal group were from standing water; the remainder were from running water. The two isolates in the smaller clonal grouping were both from standing-water samples from distant locations. The five C. lari isolates typed by PFGE-RFLP were all distinguishable.
FIG. 1.
PFGE-RFLP (with the SmaI restriction enzyme) profiles of C. jejuni isolates from environmental water samples taken from a 100-km2 predominantly dairy farming area. More than one isolate from the same sample was included where either the PFGE-RFLP or flaA typing method distinguished between the isolates. r, running water; s, standing water; t, trough water; A to O, x coordinate; 1 to 15, y coordinate.
FIG. 2.
flaA profiles of C. jejuni isolates from environmental water samples taken from a 100-km2 predominantly dairy farming area. More than one isolate from the same sample was included where either the PFGE-RFLP or flaA typing method distinguished between the isolates. r, running water; s, standing water; t, trough water; A to O, x coordinate; 1 to 15, y coordinate.
FIG. 3.
PFGE-RFLP (with the SmaI restriction enzyme) profiles of C. coli isolates from environmental water samples taken from a 100-km2 predominantly dairy farming area. More than one isolate from the same sample was included where either the PFGE-RFLP or flaA typing method distinguished between the isolates. r, running water; s, standing water; t, trough water; A to O, x coordinate; 1 to 15, y coordinate.
FIG. 4.
flaA profiles of C. coli isolates from environmental water samples taken from a 100-km2 predominantly dairy farming area. More than one isolate from the same sample was included where either the PFGE-RFLP or flaA typing method distinguished between the isolates. r, running water; s, standing water; t, trough water; A to O, x coordinate; 1 to 15, y coordinate.
Spatial analysis.
Visual exploration of the distribution of Campylobacter spp. over the study area showed no clear trends. The semivariogram for the presence of Campylobacter spp. in water showed no evidence of spatial dependency—all points fell within the 95% credible interval (Fig. 5). The semivariogram for the deviance residuals from the multivariable model also showed no suggestion of spatial dependency.
FIG. 5.
Semivariograms for the distribution of Campylobacter spp. in environmental water samples and for the deviance residuals from the multivariable logistic regression model describing that distribution. The dashed lines indicate the 95% credible intervals.
DISCUSSION
Campylobacter spp. were commonly isolated from water in this study. The water samples tested were all from actual or potential water sources for cattle, so there are clear implications for the transmission of Campylobacter spp. to cattle. Direct human exposure is also possible within the study area, as it is widely used for recreational purposes.
The source of water was strongly associated with isolation of Campylobacter spp. Trough water was less likely to be positive for Campylobacter spp. (11% of samples) than either running water (57%) or standing water (46%). This may be due to the use of chlorinated mains water on the majority of the farms in the area.
Three environmental factors were found to be associated with the presence of Campylobacter spp. in water: soil type, aspect, and bovine fecal pat count. The probability of isolating Campylobacter spp. in water samples from clay soil areas was greater than in those from other areas. The reason for the association of the soil type with the presence of Campylobacter spp. in water is unclear, although various possible explanations exist. For example, poorer drainage in clay soils may produce a local environment in which Campylobacter spp. can survive for longer periods. The local soil type might affect the pH of the water in a pond or stream, but there was no evidence of any confounding because of this—pH was measured and showed no evidence of association with soil type or with the isolation of Campylobacter spp. Samples from north-facing areas were more likely to contain Campylobacter spp. than those from flat areas. North-facing areas are relatively shaded, so water sources in such areas are exposed to less light and hence a reduced biocidal effect from ultraviolet radiation, to which Campylobacter spp. have been shown to be sensitive (14). The number of bovine fecal pats in the area adjacent to where water samples were taken was nonlinearly associated with the probability of isolating Campylobacter spp. The probability of isolating Campylobacter spp. increased up to a pat count of 117 and decreased thereafter. The peak probability of isolating Campylobacter spp. was at the upper end of the observed values, suggesting that the observed decline may have been a modeling artifact. The pat count is a measure of bovine fecal contamination of the area and hence also of the recent presence of cattle. Contamination of water with Campylobacter spp. might therefore arise either through runoff from pasture or through direct contamination from cattle. Three of the PFGE-RFLP RPs obtained from water isolates in this study were also identified in isolates from bovine fecal samples from the same study area (data not shown) (15). However, the number of bovine fecal pats positive for Campylobacter spp. in the adjacent area was not related to the probability of isolating Campylobacter spp. from water samples. This may have been due to the additional measurement error involved in a further isolation procedure and because only a relatively small fraction of the bovine fecal pats enumerated were examined for Campylobacter spp.
Isolation of E. coli from water samples did not increase the likelihood of isolating Campylobacter spp. The association between E. coli and Campylobacter spp. was also examined, excluding trough-water samples. This removed any potential effect of chlorination, but again no association was seen (chi-square test, P = 0.9). The failure to detect an association between these bacteria although E. coli is considered an important fecal indicator (2) may be due to a lack of statistical power, although some earlier studies also failed to show an association between the presence of Campylobacter spp. and fecal indicators (5). Another study looking at the presence of Campylobacter spp. and fecal indicators in water from the River Lune in northwestern England found significant seasonal variation in numbers of Campylobacter spp. but no corresponding variation in the numbers of fecal indicators. One earlier study considered season, water temperature, fecal coliform counts, fecal streptococcus counts, and sulfite-reducing clostridium counts as possible indicators of the presence or absence of Campylobacter spp. (22). The final logistic regression model consisted of fecal coliform count and water temperature. The examination of fecal coliforms may have been useful in the present study as an alternative indicator of recent fecal contamination.
The spatial investigation showed no evidence of local clustering of Campylobacter spp. There was also no evidence of clustering of the deviance residuals from the final model; in other words, allowing for the covariates examined in the model, there was no evidence of spatial dependency in the distribution of Campylobacter spp. in water.
The distribution of C. jejuni differed from that of C. coli across the water sources. C. coli was more common in standing water than in other water sources, relative to C. jejuni. This is likely to represent a difference in survival between the species in the different types of water sampled. Different water sources may to some extent be exposed to different sources of fecal contamination; for example, ponds may be more prone to contamination through waterfowl and other wildlife. However, this seems unlikely to produce the species distribution seen. Strain typing showed the presence of a C. coli flaA type that was found throughout the study area. In an earlier study in the area that examined a range of samples including bovine and wildlife feces, this flaA type was only found in water samples (15). Another study examining C. jejuni clones by multilocus sequence typing found evidence of a similar clonal specificity for an environmental sample type, in this case beach sand (7). The findings of the present study may suggest the possibility of a water-adapted C. coli strain persisting in the environment. The isolates of either species that were indistinguishable by flaA or PFGE-RFLP were often from distant locations within the study area. This suggests wide-scale dissemination of these strains.
The high prevalence of Campylobacter spp. seen in this study, which was done in an area with considerable recreational use, highlights the possibility of direct human exposure taking place in the rural environment. The strain-typing results, coupled with the species distribution seen in the different types of sample taken, show the potential importance of environmental water in the epidemiology of Campylobacter spp. Furthermore, the associations seen with geographical factors suggest that the epidemiology of Campylobacter spp. in environmental water may be more than just a function of recent fecal contamination.
Acknowledgments
This work was funded by the Department for Environment, Food and Rural Affairs.
We thank the farmers and landowners who helped with this study.
REFERENCES
- 1.Adak, G. K., S. M. Long, and S. J. O'Brien. 2002. Trends in indigenous food-borne disease and deaths, England and Wales: 1992 to 2000. Gut 51:832-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baudisova, D. 1997. Evaluation of Escherichia coli as the main indicator of faecal pollution. Water Sci. Technol. 35:333-336. [Google Scholar]
- 3.Blaser, M. J., D. N. Taylor, and R. A. Feldman. 1983. Epidemiology of Campylobacter jejuni infections. Epidemiol. Rev. 5:157-176. [DOI] [PubMed] [Google Scholar]
- 4.Brown, P. E., O. F. Christensen, H. E. Clough, P. J. Diggle, C. A. Hart, S. Hazel, R. Kemp, A. J. H. Leatherbarrow, A. Moore, J. Sutherst, J. Turner, N. J. Williams, E. J. Wright, and N. P. French. 2004.. Frequency and spatial distribution of environmental Campylobacter spp. Appl. Environ. Microbiol. 70:6501-6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter, A. M., R. E. Pacha, G. W. Clark, and E. A. Williams. 1987. Seasonal occurrence of Campylobacter spp. in surface waters and their correlation with standard indicator bacteria. Appl. Environ. Microbiol. 53:523-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cressie, N. A. C. 1993. Statistics for spatial data. John Wiley & Sons, Inc., New York, N.Y.
- 7.Dingle, K. E., F. M. Colles, R. Ure, J. A. Wagenaar, B. Duim, F. J. Bolton, A. J. Fox, D. R. A. Wareing, and M. C. J. Maiden. 2002. Molecular characterization of Campylobacter jejuni clones: a basis for epidemiologic investigation. Emerg. Infect. Dis. 8:949-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furtado, C., G. K. Adak, J. M. Stuart, P. G. Wall, H. S. Evans, and D. P. Casemore. 1998. Outbreaks of waterborne infectious intestinal disease in England and Wales, 1992-5. Epidemiol. Infect. 121:109-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez, I., K. A. Grant, P. T. Richardson, S. F. Park, and M. D. Collins. 1997. Specific identification of the enteropathogens Campylobacter jejuni and Campylobacter coli by using a PCR test based on the ceuE gene encoding a putative virulence determinant. J. Clin. Microbiol. 35:759-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris, N. V., N. S. Weiss, and C. M. Nolan. 1986. The role of poultry and meats in the aetiology of Campylobacter jejuni enteritis. Am. J. Public Health 76:407-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosmer, D. W., and S. Lemeshow. 2000. Applied logistic regression. Wiley-Interscience, New York, N.Y.
- 12.Ihaka, R., and R. Gentleman. 1996. R: a language for data analysis and graphics. J. Comput. Graphical Stat. 5:299-314. [Google Scholar]
- 13.Jones, K. 2001. Campylobacters in water, sewage and the environment. J. Appl. Microbiol. 90:68s-79s. [DOI] [PubMed] [Google Scholar]
- 14.Jones, K., M. Betaieb, and D. R. Telford. 1990. Thermophilic campylobacters in surface waters around Lancaster, UK—negative correlation with campylobacter infections in the community. J. Appl. Microbiol. 69:758-764. [DOI] [PubMed] [Google Scholar]
- 15.Leatherbarrow, H. L., C. A. Hart, R. Kemp, N. J. Williams, A. Ridley, M. Sharma, M. Sharma, P. J. Diggle, E. J. Wright, J. Sutherst, and N. P. French. 2004. Genotypic and antibiotic susceptibility characteristics of a population of Campylobacter coli isolated from dairy farmland in the United Kingdom. Appl. Environ. Microbiol. 70:822-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linton, D., R. J. Owen, and J. Stanley. 1996. Rapid identification by PCR of the genus Campylobacter and of five Campylobacter species enteropathogenic for man and animals. Res. Microbiol. 147:707-718. [DOI] [PubMed] [Google Scholar]
- 17.Nachamkin, I., H. Ung, and C. M. Patton. 1996. Analysis of HL and O serotypes of Campylobacter strains by the flagellin gene typing system. J. Clin. Microbiol. 34:277-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ribot, E. M., C. Fitzgerald, K. Kubota, B. Swaminathan, and T. J. Barrett. 2001. Rapid pulsed-field gel electrophoresis protocol for subtyping of Campylobacter jejuni. J. Clin. Microbiol. 39:1889-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson, D. A., W. J. Edgar, G. L. Gibson, A. A. Matchett, and L. Robertson. 1979. Campylobacter enteritis associated with consumption of unpasteurised milk. Br. Med. J. 1:1171-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skirrow, M. B. 1987. A demographic survey of Campylobacter, Salmonella and Shigella infections in England—a Public Health Laboratory Service survey. Epidemiol. Infect. 99:647-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skirrow, M. B. 1991. Epidemiology of campylobacter enteritis. Int. J. Food Microbiol. 12:9-16. [DOI] [PubMed] [Google Scholar]
- 22.Skjerve, E., and O. Brennhovd. 1992. A multiple logistic model for predicting the occurrence of Campylobacter jejuni and Campylobacter coli in water. J. Appl. Bacteriol. 73:94-98. [DOI] [PubMed] [Google Scholar]