Abstract
Restoring femoral rotation alignment and limb length after distal femur resection and endoprosthetic reconstruction is crucial to avoid poor outcomes. This technical note presents a simple and reliable intraoperative technique for restoring femoral rotation and length during distal femur resection and endoprosthetic reconstruction without the need for extensive preoperative planning or complex perioperative modalities. The method utilizes an external fixator frame as a guiding device to assess and restore the native alignment. This approach provides a practical alternative to relying solely on the position of the linea aspera, which has been shown to be an unreliable landmark for rotational alignment. Implementing this technique can contribute to improved functional outcomes in patients undergoing distal femur endoprosthetic reconstruction.
Keywords: Distal femur resection, Endoprosthesis, Malrotation, Linea aspera, Distal femur, Tumor resection, Tumor prostheses
Introduction
Limb salvage surgery for malignant bone tumors using a wide resection and reconstruction by endoprosthesis is now a widely accepted technique for restoring limb function and improving patient outcomes [1,2]. Endoprosthetic reconstruction has made limb-sparing procedures possible in many situations, which leads to the expansion in their indication even in nononcological cases such as articular fracture with massive bone loss, revision of complex total knee arthroplasty, fractures nonunion, and complex periprosthetic fractures [3,4]. Despite the significant decrease in the complications of the modern endoprosthesis, restoring the native femoral rotation and length is still challenging. Inadequate restoration of femoral rotation and length after distal femur endoprosthetic reconstruction can lead to poor outcomes. Component malrotation can lead to patellofemoral complications while inappropriate femoral length and joint line restoration can result in patella baja, gait abnormalities, and overall dissatisfaction [[5], [6], [7], [8]].
There are numerous techniques that help surgeons restore component rotation in the classic total knee arthroplasty, such as the posterior femoral condyles, epicondyles, and the trochlear groove [9,10]. However, restoring the native alignment could be challenging in the absence of these anatomic landmarks, such as in the case of distal femoral resection for malignant tumors. Unfortunately, in these situations, there are limited techniques that guide surgeons intraoperatively to restore the rotation, such as the linea aspera (LA), which is usually used as a landmark to identify the true posterior of the femur [11]. However, many authors have doubted if the LA is a reliable landmark of rotational alignment as true posterior [[12], [13], [14], [15]]. Previous studies suggested a systemic preoperative radiological assessment of the position of LA at planned resection level or the use of computer-assisted navigation to restore the native alignment. In this present study, we describe a simple technique that could help surgeons to restore rotation and length alignment perioperatively during distal femur resection and reconstruction without the need for preoperative planning.
Surgical technique
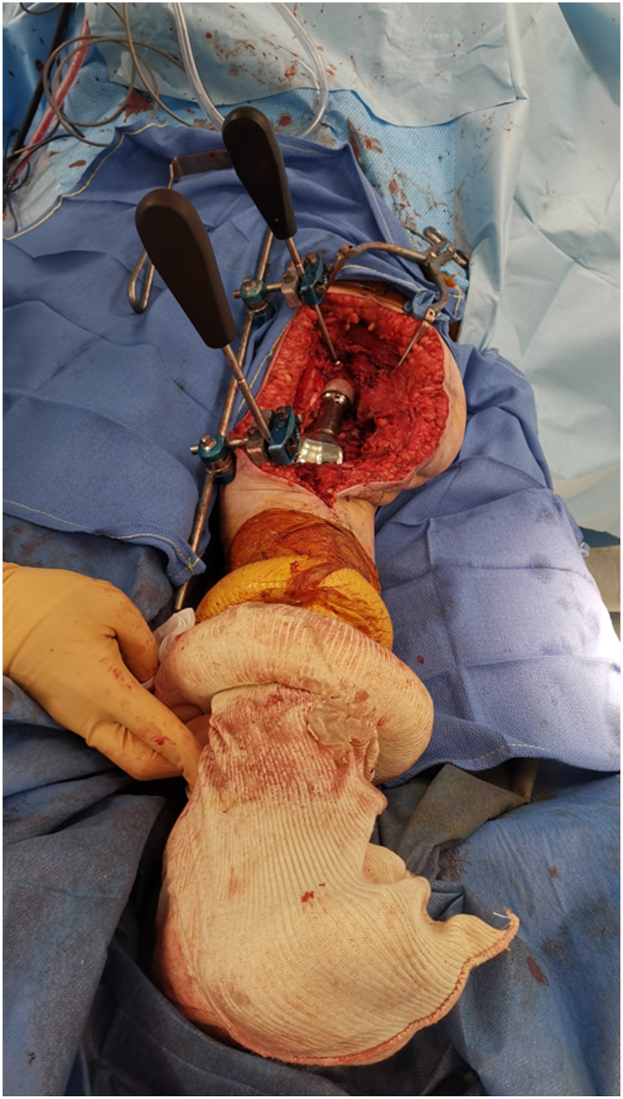
The intervention is carried out routinely depending on the indication of surgery. Before the femur osteotomy is performed, a Hoffman II (Stryker, Kalamazoo, MI, US) external fixator frame is built and used as a guiding device (Fig. 1). Two 5-hole pin clamps are attached to a long connecting rod by rod-to-rod coupling. First, the guidance device is held parallel to the limb. A 3.2 mm drill bit is passed from the same surgical incision through a selected hole by the surgeon of the 5-hole pin clamp (Fig. 2a). The drilling is performed on the anteromedial cortex of the femur, 2-4 cm proximal to the osteotomy level, and from the same site as the incision. We recommend this distance to prevent stress fractures near the resection level and to provide space in case a prophylactic cerclage wire application is desired. Subsequently, the drill is removed, and a 4.5 mm unicortical screw (12 mm in length) is inserted using a screwdriver that passes through the clamp from the same selected hole. The screwdriver is held in place, and the clamp is tightened (Fig. 2b). Afterward, a second drilling is performed in the tibia, using the same method, through the second 5-hole pin clamp and a selected hole of the guiding device (Fig. 2c). The drilling is performed on the anteromedial cortex of the tibia from the same incision. The guiding device is held as parallel as possible during this process. Once the drill bit is removed from the tibia, a second 4.5-mm unicortical screw (12-14 mm in length) is inserted using a screwdriver that passes through the clamp to maintain the same orientation. The screwdriver is kept in place, and the clamp is tightened. Herein, the surgeon will have an idea of the orientation of the preosteotomy rotational alignment by the 2 screwdrivers that are fixed to the guiding device, which are parallel (Fig. 2d). The distance between the 2 screwdrivers is measured using a long ruler, and it is recommended to double-check the length measurement. Additionally, inserting the 2 screwdrivers into the same selected holes (Figure 3, Figure 4, Figure 5) will provide an idea of the length. Afterward, the 2 unicortical screws are kept while the guiding device and the 2 screwdrivers are removed. The resection is then carried out as usual. It is important to emphasize the necessity of utilizing a short screw to prevent interference with the reamers or the stem during implant preparation. In the unlikely event that interference occurs, the screw can be simply rotated counterclockwise to ensure it does not protrude into the medullary canal. The screws can be reinserted completely while checking the length.
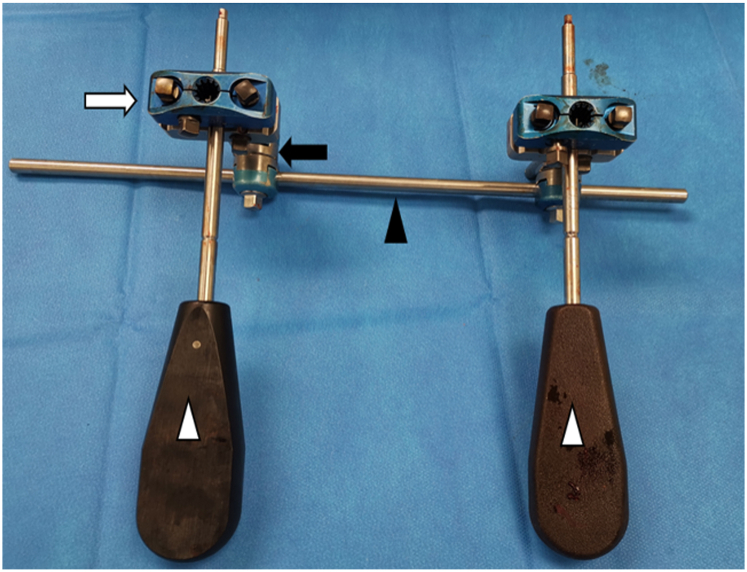
Figure 1.
A picture of the guiding device utilizing the external fixator. The device consists of 2 5-hole pin clamps (white arrows) attached to a connecting rod (black arrowhead) via rod-to-rod coupling (black arrow). Two screwdrivers (white arrowheads) are passed through a selected hole of the pin clamp.
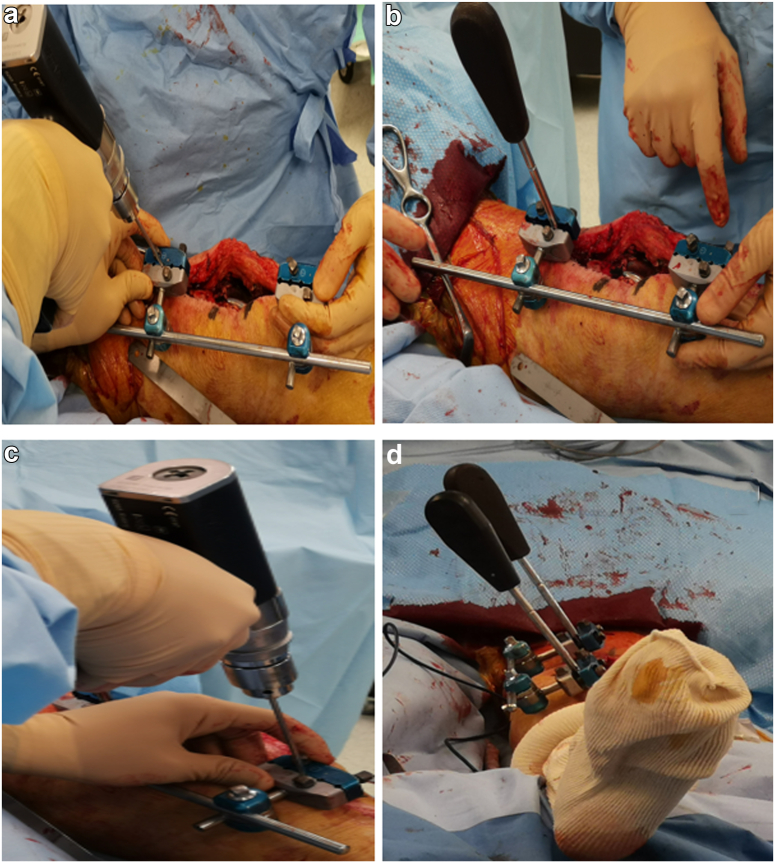
Figure 2.
(a) Drilling of the proximal femur through a selected hole of the pin clamps. (b) Insertion of a unicortical screw with the screwdriver attached to the screw head and fixed to the pin clamp. (c) Drilling of the tibia using the same method. (d) Distal view taken after the insertion of the second screw, which is held by a second screwdriver at the tibia, demonstrating the parallelism between the 2 screwdrivers.
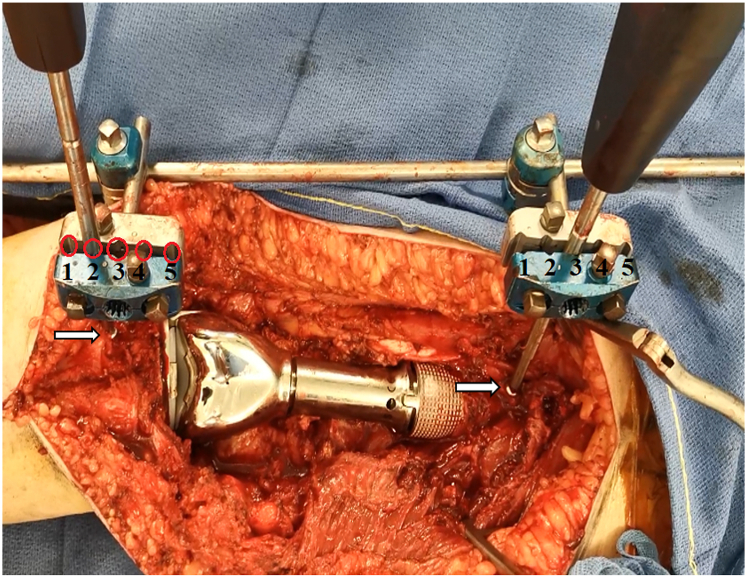
Figure 3.
Proximal view showing 2 screwdrivers inserted through selected holes of the pin clamp (indicated by red circles with numbers) and fixed to the guiding device, which is attached to 2 unicortical screws (indicated by white arrows).
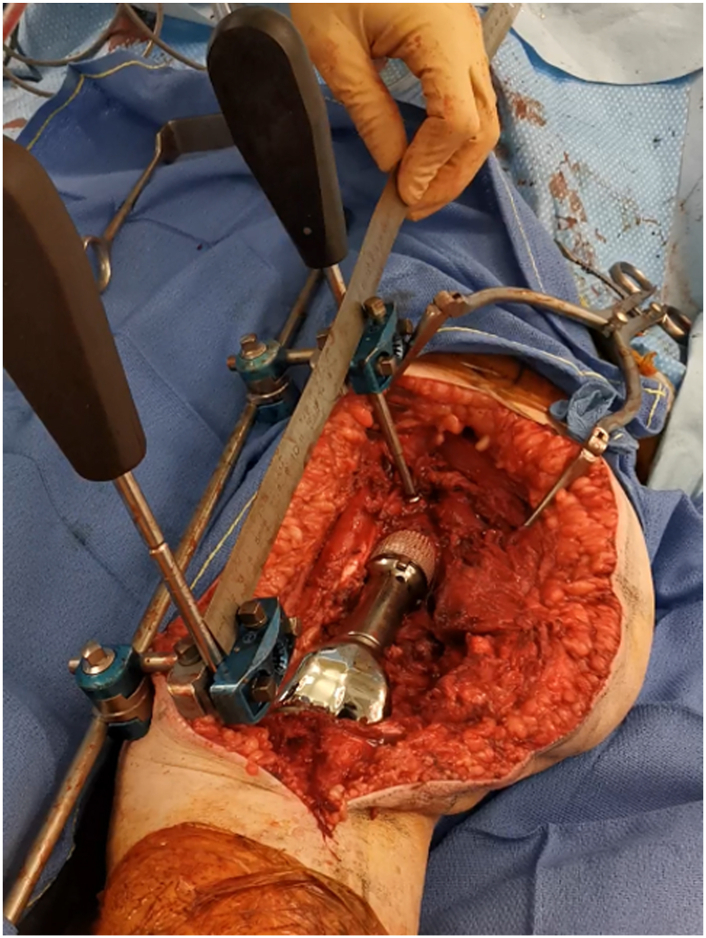
Figure 4.
Verification of limb lengths using a long ruler to double-check the length measurement.
Figure 5.
Distal view of lower limb with the guiding device.
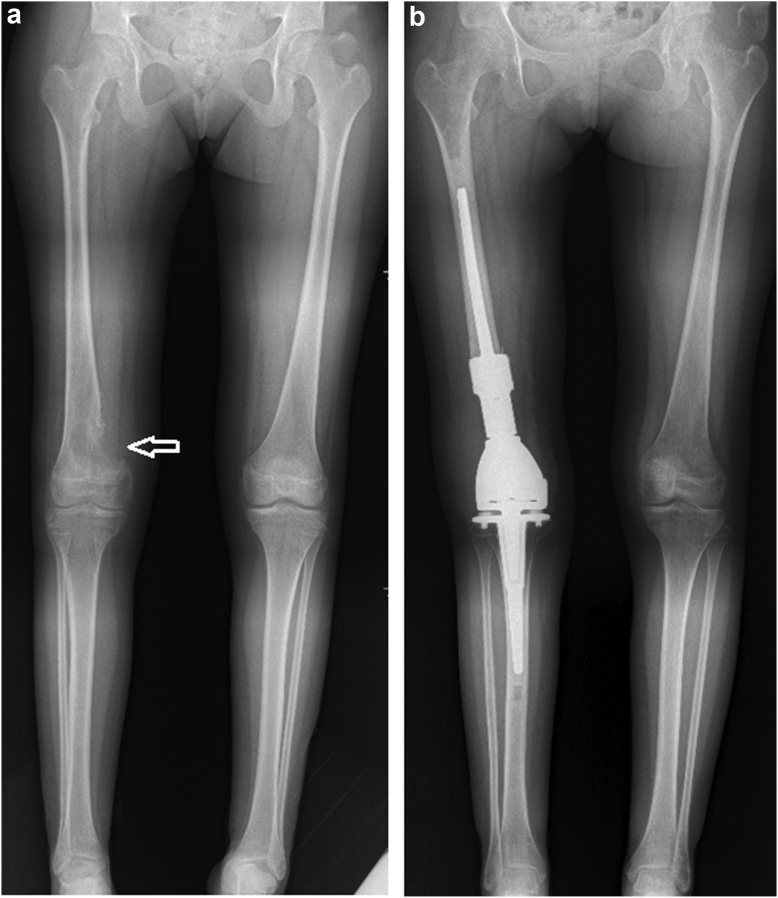
During the testing of component trials, the length and rotation are checked again using the guidance device. First, insert the screwdriver in the femoral unicortical screw through the guidance device on the middle hole of the pin clamp. Then the second screwdriver is attached to the screw head of the tibial unicortical screw through the distal pin clamp through the selected holes. If the screwdriver on the tibial side is well attached to the screw head through the selected pin clamp hole, the limb's length is well restored. If the screwdriver is passed through another hole to match the head screw, it means that the length is not well restored. For example, if the screwdriver is passed through the fourth hole distally of the clamp instead of the third hole in order to be attached to the screw head, it means that there is a shortening of the limb, while if the screwdriver is attached to the screw head, but through the second or first hole proximally, it means that limb is lengthened. We confirm this by using the ruler to remeasure the distance between the 2 screwdrivers as done before the resection. The same thing for the rotation, the second screwdriver will not attach to the screw head when passed through the guidance device until the good rotation is restored. Here, the surgeon will try to modify the rotation until the second screwdriver that passed via the guiding device is attached to the screw head of the tibial unicortical screw. When the optimal rotation is obtained, the surgeon can make a mark by drawing a line on the femoral bone using electrocautery, which additionally helps the control of the rotation when inserting the definitive implant. The same maneuver will be performed during the insertion of the definitive implant for final verification. We provide a case example of preoperative and postoperative x-rays for 15-year-old patients presented with conventional osteosarcoma of distal right femur, which was treated by distal femur resection and reconstruction with an endoprosthesis. The described technique was employed to restore the pre-existing normal alignment (Fig. 6).
Figure 6.
A case example of a 15-year-old patient presented with conventional osteosarcoma of distal right femur (white arrow), which was treated by distal femur resection and reconstruction with an endoprosthesis. The described technique was employed to restore the pre-existing normal alignment. (a) Preoperative long-film X-ray. (b) Postoperative long-film X-ray at 6 months.
Discussion
Restoring femoral rotation and length after distal femur resection and endoprosthetic reconstruction is essential to avoid poor outcomes. In case of missing these anatomical landmarks, most surgeons rely intraoperatively on the position of LA as a landmark of the true posterior of the femur, as suggested by Martin Malawer [11]. However, recent studies have shown that the LA is not true posterior, and it is not a reliable landmark for axial rotation of femoral implants [[12], [13], [14], [15]].
Reple et al. evaluated the anatomical position of LA at multiple axial levels on a magnetic resonance imaging-based study of 50 femurs. They concluded that when performing a distal femoral replacement, the component should not be inserted in line with the LA; otherwise, it will lead to malrotation alignment. Tuy et al. [15] examined the position of LA based on a computed tomography (CT) study. They found that the LA is positioned laterally at the distal femur, medially at the midshaft, and then laterally at the proximal third of the femur. Abdelaal et al. [12] evaluated the position of LA on 133 femurs by CT scan. They measured the angle of rotation of the LA at 4 different levels. They demonstrated that only 4.5% had an exact posterior position of the LA at all 4 measured levels. The LA was externally rotated to varying degrees in 74% of the femurs and was never exactly posterior at any of the 4 levels. A recent study demonstrated similar results as previously published studies, and they found that 78% of femoral implants would exceed the accepted deviation when surgeons blindly depend on the LA as a posterior landmark [14]. Another study evaluated the efficacy of computer-assisted navigation to restore the rotation and length alignment after distal femur resection. They demonstrated that computer-assisted navigation permitted reapproximate femoral rotation and length. The limb-length discrepancy was evaluated by postoperative radiological measures, while rotation was evaluated only clinically [16].
Prior studies have consistently recommended preoperative assessment of the LA orientation at the planned resection level to prevent malrotation alignment, which varies based on factors such as resection level, patient's age, and femoral anteversion [[12], [13], [14], [15]]. Although these studies evaluated LA position using radiological measures and emphasized preoperative determination, they did not assess postoperative outcomes or measure the validity of these techniques.
We believe that preoperative assessment can provide a general idea of the LA position at the planned resection level and assist surgeons in accurately positioning the implant. However, we doubt the reproducibility of these techniques since restoring the native rotation precisely is challenging, and there is a risk of deviation from the intended resection height in the preoperative plan. This deviation may affect the orientation of the LA and subsequently impact rotational alignment.
The indication of the described technique encompasses its use for restoring pre-existing alignment and normal rotation after distal femur resection reconstruction where normal anatomy is not compromised such as in oncological cases and in cases of total knee arthroplasty revision with endoprosthesis. There are no specific contraindications for the technique. In our experience, we have not encountered any complications related to patellar maltracking or malrotation. However, this technique has some limitations. The first limitation of the technique arises when it cannot be used in cases of fractures requiring endoprosthesis, such as periprosthetic fractures, or revisions for nonunion, where this technique cannot be applied due to compromised normal length and rotation unless an anatomic reduction is performed before applying this technique.
Another concern of this technique arises in case of a pre-existing varus or valgus deformity; controlling the length by this technique could be minimally affected. In these scenarios, we recommend inserting screws in the center of the anterior cortex to decrease measuring variation and using adjunct techniques such as comparing the length with the contralateral limb. Despite this limitation, this technique can still be valuable in restoring rotation.
The final limitation is that this technique could minimally increase operative time (5-10 minutes), which can be significantly reduced by having the operative assistants assemble the guide while the surgery is in progress. We believe that this technique does not significantly extend the surgical duration when considering the length of these surgeries, which typically take more than 2 hours. We have created a specialized set of surgical instruments for preparing the guiding device, avoiding the need to open a complete external fixator instrument set.
It is noteworthy that even if the surgical duration is minimally increased, it will prevent unnecessary preoperative CT planning and save time for surgeons by eliminating the need for these preparations. Moreover, this technique could spare patients unnecessary radiation and prevent the overutilization of healthcare resources. Furthermore, this technique might require a similar or even shorter duration than computer-assisted navigation.
Despite these limitations, we believe that our technique is a simple, reproducible, and cost-effective tool that can assist surgeons in these challenging situations.
Summary
This technique is effective for restoring rotation and limb length after a distal femoral resection and endoprosthetic reconstruction without the need for rigorous sophisticated preoperative planning and radiation exposure by CT or fancy techniques such as computer-assisted navigation. It can be used alone or as an adjunctive technique along with previously mentioned techniques. The surgeons must use different methods that help in restoring the anatomic rotation and length of patients to improve their functional outcomes.
Conflicts of interest
The authors declare there are no conflicts of interest.
For full disclosure statements refer to https://doi.org/10.1016/j.artd.2023.101284.
Informed patient consent
The author(s) confirm that written informed consent has been obtained from the involved patient(s) or if appropriate from the parent, guardian, power of attorney of the involved patient(s); and, they have given approval for this information to be published in this case report (series).
Author contributions
Louis-Romée Le Nail contributed to conceptualization, supervision, writing – review and editing. Ramy Samargandi contributed to conceptualization, data curation, writing – original draft, writing – review and editing.
Appendix A. Supplementary data
References
- 1.Eckardt J.J., Kabo J.M., Kelly C.M., Ward W.G., Cannon C.P. Endoprosthetic reconstructions for bone metastases. Clin Orthop Relat Res. 2003;(415 Suppl):S254–S262. doi: 10.1097/01.blo.0000093044.56370.94. [DOI] [PubMed] [Google Scholar]
- 2.Biau D., Faure F., Katsahian S., Jeanrot C., Tomeno B., Anract P. Survival of total knee replacement with a megaprosthesis after bone tumor resection. J Bone Joint Surg Am. 2006;88:1285–1293. doi: 10.2106/JBJS.E.00553. [DOI] [PubMed] [Google Scholar]
- 3.Vaishya R., Singh A.P., Hasija R., Singh A.P. Treatment of resistant nonunion of supracondylar fractures femur by megaprosthesis. Knee Surg Sports Traumatol Arthrosc. 2011;19:1137–1140. doi: 10.1007/s00167-011-1416-1. [DOI] [PubMed] [Google Scholar]
- 4.Harrison R.J., Thacker M.M., Pitcher J.D., Temple H.T., Scully S.P. Distal femur replacement is useful in complex total knee arthroplasty revisions. Clin Orthop. 2006;446:113–120. doi: 10.1097/01.blo.0000214433.64774.1b. [DOI] [PubMed] [Google Scholar]
- 5.Schwab J.H., Agarwal P., Boland P.J., Kennedy J.G., Healey J.H. Patellar complications following distal femoral replacement after bone tumor resection. J Bone Joint Surg Am. 2006;88:2225–2230. doi: 10.2106/JBJS.E.01279. [DOI] [PubMed] [Google Scholar]
- 6.Iversen M.D., Chudasama N., Losina E., Katz J.N. Influence of self-reported limb length discrepancy on function and satisfaction 6 years after total hip replacement. J Geriatr Phys Ther. 2011;34:148–152. doi: 10.1519/JPT.0b013e31820e16dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhattee G., Moonot P., Govindaswamy R., Pope A., Fiddian N., Harvey A. Does malrotation of components correlate with patient dissatisfaction following secondary patellar resurfacing? Knee. 2014;21:247–251. doi: 10.1016/j.knee.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Kessler O., Patil S., Colwell C.W., D’Lima D.D. The effect of femoral component malrotation on patellar biomechanics. J Biomech. 2008;41:3332–3339. doi: 10.1016/j.jbiomech.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 9.Victor J. Rotational alignment of the distal femur: a literature review. Orthop Traumatol Surg Res. 2009;95:365–372. doi: 10.1016/j.otsr.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Katz M.A., Beck T.D., Silber J.S., Seldes R.M., Lotke P.A. Determining femoral rotational alignment in total knee arthroplasty: reliability of techniques. J Arthroplasty. 2001;16:301–305. doi: 10.1054/arth.2001.21456. [DOI] [PubMed] [Google Scholar]
- 11.Malawer M.M. In: Musculoskeletal cancer surgery treatment of sarcomas and allied diseases. Malawer M.M., Sugarbaker P., editors. Kluwer Academic Publishers; Berlin: 2001. Distal femoral resection with endoprosthetic reconstruction; pp. 457–481. [Google Scholar]
- 12.Abdelaal A.H.K., Yamamoto N., Hayashi K., Takeuchi A., Miwa S., Morsy A.F., et al. The linea aspera as a guide for femoral rotation after tumor resection: is it directly posterior? A technical note. J Orthop Trauma. 2016;17:255–259. doi: 10.1007/s10195-016-0399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reple G., Felden A., Feydy A., Anract P., Biau D. The linea aspera as a rotational landmark: an anatomical MRI-based study. Surg Radiol Anat. 2016;38:1069–1074. doi: 10.1007/s00276-016-1661-6. [DOI] [PubMed] [Google Scholar]
- 14.De Smet A., Verrewaere D., Sys G. Enhancing rotational placement of reconstruction prostheses of the distal femur after sarcoma resection. Med Eng Phys. 2020;81:47–57. doi: 10.1016/j.medengphy.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Tuy B.E., Patterson F.R., Beebe K.S., Sirkin M., Rivero S.M., Benevenia J. Linea aspera as rotational landmark for tumor Endopostheses: a computed tomography study. Am J Orthop (Belle Mead NJ) 2016;45:E198–E203. [PubMed] [Google Scholar]
- 16.Palumbo B.T., Henderson E., Rizer J., Letson D.G., Cheong D. Computer navigation and distal femoral reconstruction in the oncologic patient. J Orthop. 2017;14:257–263. doi: 10.1016/j.jor.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.