Abstract
Background
Aneurysmal bone cysts (ABCs) are infrequent, benign, and locally destructive lesions that most commonly occur during the first two decades of life. They usually affect the metaphysis of the long bones, but the pelvis is involved in 8%–12% of the cases. The management of pelvic ABCs is a challenging issue due to difficulties in choosing the appropriate approach, adjacent neurovascular bundles, the risk of intraoperative bleeding with difficulty achieving good hemostasis, and the risk of injury to the hip or sacroiliac joints. Limited data exist concerning the use of denosumab as a non-surgical treatment for pelvic ABCs. Our hypothesis was that denosumab might be an effective and safe solo treatment of cases with ABCs in the pelvis.
Methods
We retrospectively assessed 20 patients with ABCs in the pelvis, who were treated by denosumab as a solo agent without surgery. Patients were assessed regarding disease control, the incidence of recurrence and non-oncological complications, and functional outcome.
Results
The mean follow-up period was 38.5 months. Disease control was achieved in 16 patients (80%), with no local recurrence. Tolerable drug-related complications occurred in 15% of cases. The mean Musculoskeletal Tumor Society score was 92.3%.
Conclusions
Denosumab may provide a reliable option in the nonsurgical treatment of ABCs of pelvic origin with expected lower morbidity than the surgical solution and tolerable complications. Further studies on the safety profile and long-term effects of denosumab especially in skeletally immature patients are required.
Keywords: Denosumab, Aneurysmal bone cyst, Pelvis
Aneurysmal bone cysts (ABCs; International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM]: M85.50) are benign and locally destructive lesions that mostly occur in the first two decades1) and usually affect the metaphysis of long bones. Less commonly affected sites include the vertebral column2) and the pelvis (8%–12%).3) The nature and pathogenesis of ABCs are still unclear; they are classified as locally aggressive neoplastic lesions.4) Etiology was first attributed to abnormal venous circulation causing intraosseous or subperiosteal hemorrhage that recruits osteoclasts and induces bone resorption with local remodeling. This theory is no longer valid for primary ABCs, where a chromosomal translocation has been found responsible for the etiology. This translocation t (16;17) (q22;p13) causes malfunction in the ubiquitin carboxyl-terminal hydrolase 6 (USP6).4) Secondary ABCs do not exhibit such a translocation.5)
The lesions consist of hemorrhagic tissue with cavitary spaces separated by fibrous septa containing spindle cells, inflammatory cells, and a smaller percentage of giant cells, which are positive for markers of true osteoclasts.6) These cells produce receptor activators of nuclear factor kappa-B ligand (RANKL) in a similar manner found in giant cell tumors (GCTs).7) The optimal management of ABCs continues to be a subject of controversy.8) Wide resection is not an acceptable option due to the resultant morbidity.2) Open curettage with or without bone grafting is the standard line of treatment. This procedure is associated with a high recurrence rate that mounts up to 30%. Recurrence can be reduced to 15% when a high-speed burr and adjuvant elements are used.9) Larger or irresectable lesions can be treated by selective arterial embolization or sclerotherapy alone or with surgery.10)
The management of pelvic ABCs is a challenging issue, due to several factors including difficulties in choosing the appropriate approach, adjacent neurovascular structures, the risk of intraoperative bleeding with difficulty achieving good hemostasis, and the risk of injury to the hip or sacroiliac joints that may cause early osteoarthritis or impact pelvic stability.3) Denosumab, a human monoclonal antibody, blocks osteoclasts activation by binding to the receptor activator of the nuclear factor kappa-b ligand, inhibiting bone resorption.11) Several studies showed the efficacy of denosumab in reducing tumor mass in refractory, recurrent, or inoperable GCT lesions.12,13) However, limited data exist on its use with ABCs.2,14) This study aimed to report the short-term results of denosumab administration as a solo treatment line for cases with pelvic ABCs and to compare our results to the available published literature.
METHODS
This study was approved by the Institutional Review Board (IRB) of Ain Shams University (No. FMASU R 208/2021). Informed consent was obtained from all individual participants included in the study. Patients signed informed consent regarding publishing their data and photographs.
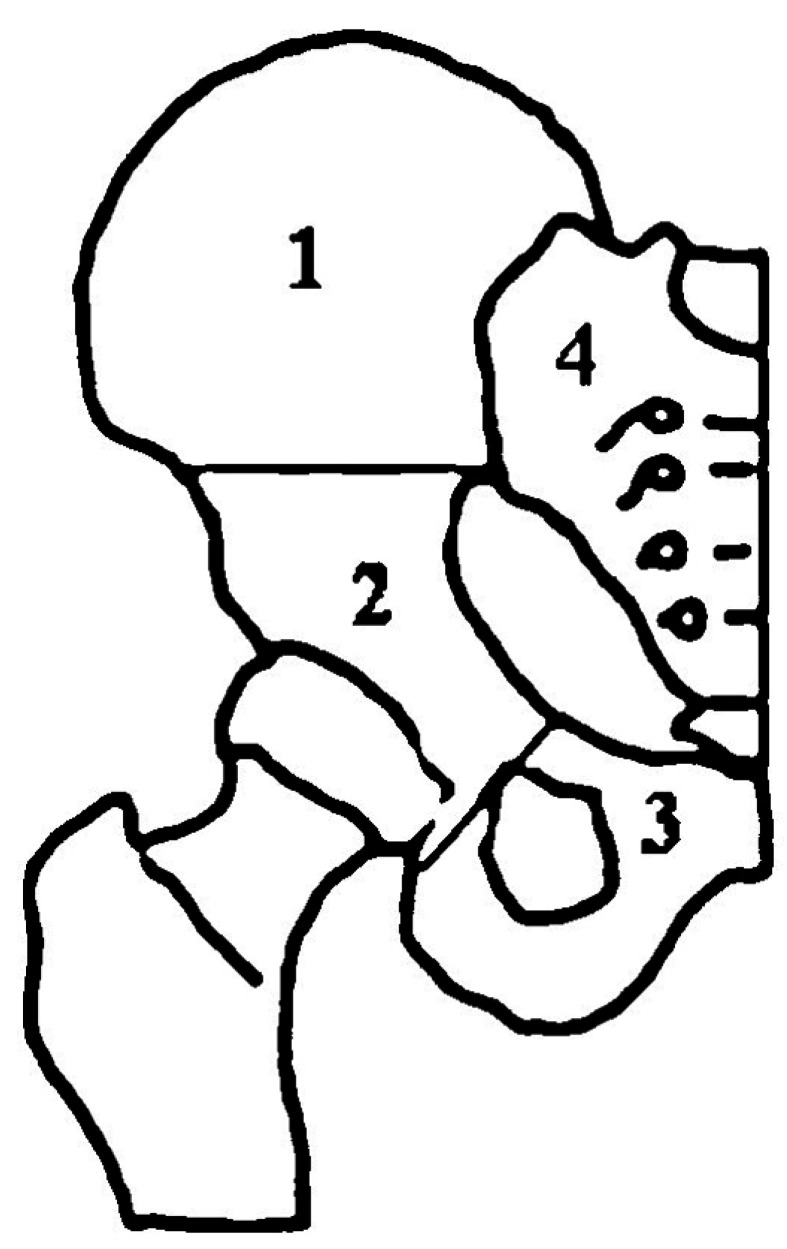
This is a retrospective study aiming to assess patients with pelvic ABCs who received treatment using denosumab in our institution. The study included 9 males and 11 females with a mean age of 18.8 years (range, 7–27 years). Location of the lesions was described according to Enneking and Dunham pelvic zones15) (Fig. 1): 4 cases in zone 1(iliac), 5 cases in zone 2 (periacetabular), 6 cases in zone 3 (pubic), and 5 cases in zone 4 (sacral). The off-label use of denosumab was approved by the IRB based on the previous Food and Drug Administration (FDA) approval for its use in GCTs and available literature on its use in ABC cases, and the IRB also approved our proposed dosage and recommended close follow-up of the patients.
Fig. 1. Pelvic zones according to Enneking and Dunham pelvic zones.15) 1: Iliac, 2: periacetabular, 3: pubic, 4: sacrum.
Dosage
No specific guidelines exist for denosumab dosage in the management of ABCs; so we utilized the regimen described by Ntalos et al.16): denosumab 60 mg (Prolia, Amgen) subcutaneous injection every 6 weeks. We viewed this dosage as a balance between safety and efficacy, considering the off-label nature of the treatment. We planned to use a minimum of 6 doses, after which if no response was achieved, we stopped the treatment. If a radiological response, which usually appears within 3–4 months, was noted, we continued to use it for a maximum of 9 months. During this interval (6–9 months), medication would be stopped if intolerable adverse effects were encountered or complete healing occurred. Patients included were those with symptomatic benign lesions in the pelvis, which were diagnosed as pelvic ABCs based on radiological features (with or without biopsy) in X-ray and magnetic resonance imaging (MRI) suggestive of cystic expansile lesions in the pelvis either in a primary, on top of another benign lesion, or in a recurrent lesion.
Once a secondary ABC is suspected, proper relevant investigations are required to exclude a more aggressive entity (e.g., telangiectatic osteosarcoma, which would require a totally different protocol). A secondary lesion was diagnosed based on previous medical history and radiographs, MRI suggestive of a concomitant lesion (whenever MRI findings were suspicious, a computed tomography-guided biopsy was done prior to any intervention.) Chromosomal translocation t(16;17) (q22;p13) can be detected in 75% of cases of primary ABCs;4) however, this test was not available at our institution.
It is worth mentioning that denosumab was prescribed to patients with symptomatic lesions (pain/limping, etc.). Those with accidentally discovered cystic pelvis lesions but without symptoms were just notified and given regular follow-up dates to pick up any disease progression. Serum calcium and creatinine were assessed before each dose. Based on previous studies, oral calcium (600 mg) and vitamin D (400 IU) daily doses were given during the treatment period to prevent hypocalcaemia.17) The treatment duration was tailored individually depending on the response clinically and radiologically as well as the participants’ tolerance. The patients received a mean of 6.95 doses (range, 6–9 doses). Follow-up was done monthly through clinical assessment and X-ray, which is the routine follow-up process used in our Ortho-Oncology outpatient clinic for patients treated by novel techniques. At the final follow-up, clinical assessment was done using the Musculoskeletal Tumor Society score18) and radiological assessment was done using the modified Neer Classification by Capanna et al. (Table 1).19,20)
Table 1. The Modified Neer Classification19) by Capanna et al.20).
| Score | Classification | Description |
|---|---|---|
| I | Healed | Consolidation with newly formed bone, thickened borders, with or without small radiolucent areas < 1 cm in size |
| II | Healed with defects | Consolidation with newly formed bone, radiolucent areas < 50% of the bone diameter |
| III | Recurrent cyst | Despite consolidation after treatment, osteolysis appeared in follow-up. |
| IV | Persistent cyst | No signs of cyst healing; radiolucent areas > 50% of bone diameter |
No available literature defines the term “disease control,” so we arbitrarily defined it as achieving ossification enough to support full weight-bearing/function in the absence of pain or risk of pathological fractures and in the absence of any radiological signs of disease progression or recurrence at the end of the follow-up. The National Institute of Health defines a locally recurrent tumor as a tumor that has recurred at or near the same place as the original (primary) tumor, usually after a period during which cancer could not be detected.21) We defined local recurrence as the recurrence of symptoms that already disappeared or the occurrence of new symptoms (pain, limitation of range of motion [ROM], and pathological fractures) that can be attributed to radiological changes in a previously healed lesion (grade I, II) in the form of osteolysis in X-ray or appearance of characteristic signal changes in MRI.
Stanulovic et al.22) defined drug tolerability as the degree to which drugs overt adverse effects can be tolerated by patients. We considered that Denosumab adverse effects were tolerable whenever they did not affect the vital managed without the need for hospitalization or affect the ability of the patient to perform the daily activities. The whole follow-up process was carried out by the same team. The literature states that ABCs can be considered completely healed if recurrence does not occur within 2 years after the end of therapy.4,23)
Statistical Analysis
Statistical analysis was done using IBM SPSS Statistics ver. 23 (IBM Corp.). Continuous numerical variables were presented as mean and standard deviation, and differences among groups were compared using one-way analysis of variance. Discrete data were presented as median and interquartile range and differences were compared using the Jonckheere-Terpstra test. Categorical variables were presented as counts and percentages and intergroup differences were compared using the chi-square test for trend. A p-value < 0.05 was considered statistically significant.
RESULTS
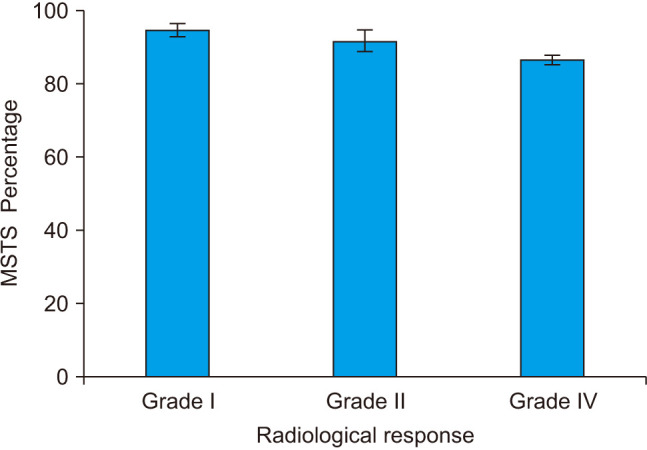
The mean follow-up period was 38.5 ± 4.5 months (range, 30–48 months). The follow-up period started after the cessation of the medication. At the final follow-up, 11 cases (55%) were scored as grade I (Fig. 2), 5 cases (25%) were grade II, and 4 cases (20%) were grade IV (Fig. 3). None of the cases had recurrence till the final follow-up. Table 2 summarizes the characteristics of the study population. Drug-related complications occurred in 3 cases (15%) in the form of gastrointestinal upset in 1 case (5%), and bony pain in 2 cases (10%). These patients suffered from dull aching pain in the back and the limbs, more at night and with activity. Clinical examination revealed generalized tenderness but preserved ROM. X-ray was done to exclude insufficiency fractures. We estimated serum calcium and vitamin D; they were borderline. So our management was reassurance, rest for 1 week, paracetamol and proper calcium and vitamin D intake, and proper hydration. The mean Musculoskeletal Tumor Society (MSTS) score was 92.3% (Fig. 4).
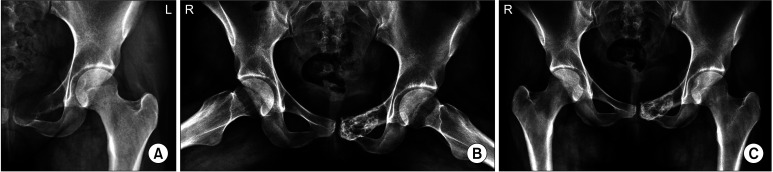
Fig. 2. Case 9: aneurysmal bone cyst in the pubic region treated by 7 doses of denosumab. (A) X-ray at presentation. (B) X-ray after completing the treatment course: evident calcification within the lesion. (C) Final follow-up. Grade I response: complete consolidation of the lesion at 42 months after denosumab therapy.
Fig. 3. Radiological response in the study cohort.
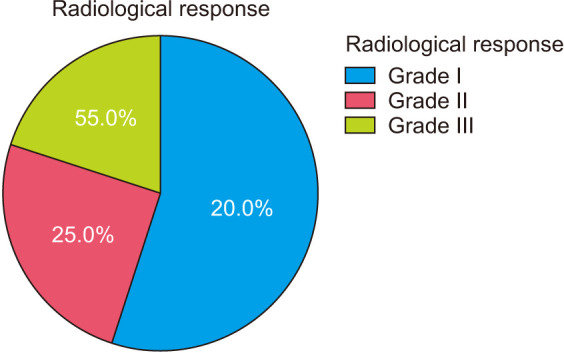
Table 2. Characteristics of the Study Cohort.
| Variable | Value | |
|---|---|---|
| Age (yr) | 9 ± 5 (7–27) | |
| Sex | ||
| Female | 11 (55.0) | |
| Male | 9 (45.0) | |
| Involved zone | ||
| Zone 1 | 4 (20.0) | |
| Zone 2 | 5 (25.0) | |
| Zone 3 | 6 (30.0) | |
| Zone 4 | 5 (25.0) | |
| Number of doses | 7 (6–9) | |
| Radiological response | ||
| Grade I | 11 (55.0) | |
| Grade II | 5 (25.0) | |
| Grade IV | 4 (20.0) | |
| Recurrence | 0 | |
| MSTS score (%) | 92.3 ± 6.2 (80–100) | |
| Treatment-related adverse effects | ||
| Gastrointestinal tract upset | 1 (5.0) | |
| Bone pain | 2 (10.0) | |
| Follow-up period (mo) | 38.5 ± 4.5 (30–48) | |
Values are presented as mean ± standard deviation (range), number (%), or median (range).
MSTS: Musculoskeletal Tumor Society.
Fig. 4. Mean Musculoskeletal Tumor Society (MSTS) score in patients with grade I, grade II, or grade IV radiological response. Error bars represent the standard error of the mean.
One case was skeletally immature. The patient’s parents wanted to use the medication as an alternative to surgery and they signed an informed consent to the use of denosumab despite the lack of information regarding this age group. We used 6 doses and obtained a very good response. During the follow-up period, no clinical or radiological evidence of denosumab effect on the physeal growth could be detected. The parents understood that the patient would require close observation till skeletal maturity.
Four cases (20%) showed no response to denosumab (grade IV). In three cases, the patients underwent surgical intervention in the form of extended curettage with adjuvant (phenol) and autogenous fibula bone graft. The lesion showed complete consolidation within 9 months. Histopathological assessment of the specimen confirmed the diagnosis of ABCs. In the fourth case, despite the absence of a radiological response, the patient showed complete absence of symptoms (pain/limitation of movement), so the patient refused the surgical treatment and close follow-up was done. Radiological follow-up showed a stationary course of the disease till the last follow-up. The response to denosumab could not be correlated to age, sex, location of the lesion within the pelvis, or the doses received (Table 3).
Table 3. Relation between Radiological Responses and Clinicopathological Characteristics of the Disease.
| Variable | Radiological response | p-value | |||
|---|---|---|---|---|---|
| Grade I (n = 11) | Grade II (n = 5) | Grade IV (n = 4) | |||
| Age (yr) | 18.0 ± 4.8 | 19.2 ± 5.0 | 20.8 ± 4.6 | 0.619* | |
| Sex | 0.089† | ||||
| Female | 8 (72.7) | 1 (20.0) | 1 (25.0) | ||
| Male | 3 (27.3) | 4 (80.0) | 3 (75.0) | ||
| Involved zone | 0.119† | ||||
| Zone 1 | 3 (27.3) | 1 (20.0) | 0 | ||
| Zone 2 | 2 (18.2) | 3 (60.0) | 0 | ||
| Zone 3 | 4 (36.4) | 0 | 2 (50.0) | ||
| Zone 4 | 2 (18.2) | 1 (20.0) | 2 (50.0) | ||
| Number of doses | 8 (6–8) | 7 (7–7) | 8 (7–9) | 0.656‡ | |
Values are presented as mean ± standard deviation, number (%), or median (interquartile range).
*One-way analysis of variance. †Chi-square test for trend. ‡Jonckheere-Terpstra test.
DISCUSSION
The management of pelvic ABCs has always been considered more laborious than long bone lesions with difficult accessibility due to the complex anatomy and adjacent important neurovascular structures. Treatment options for pelvic ABCs are mainly surgical in the form of curettage (with up to 30% recurrence rate). Minimally invasive options include sclerotherapy, radionuclide ablation, and selective arterial embolization, which is expensive and requires special equipment not available in all centers.16) Denosumab is an alluring solution for such a challenge.
Several authors reported on the use of denosumab in the management of ABC. In a review of 65 patients with ABCs in different locations, Grahneis et al.24) suggested that denosumab can be considered as an additional option in the management of ABCs especially in locations with difficult accessibility such as the spine or the pelvis. Savvidou et al.11) reported in their review that denosumab can be a nonoperative alternative for ABCs located at the spine and for cases in which selective arterial embolization is not applicable. Similar reports by Skubitz et al.25) and Pelle et al.26) observed pain relief, good drug tolerance, and radiographic evidence of healing in patients with sacral ABCs.
Ntalos et al.16) reported a 35-year-old female patient with a recurrent ABC affecting the pelvis. The patient was treated with denosumab therapy and got favorable clinical and radiological responses. At the final follow-up, the patient was clinically and radiologically stable. The authors concluded that denosumab could be considered as a reliable therapy in the management of patients with ABCs. Kulkarni and Patel27) reported 2 sacral ABCs (in a series of 8 spinal cases) treated by denosumab. Pain relief and new bone formation on CT scan were achieved with follow-up of 12 months. The authors concluded denosumab would be a promising novel alternative for ABC management, especially in patients where surgical resection is not possible and in relapsing patients where potential morbidities can thus be evaded. Pelle et al.26) described a case of a 5-year-old boy with a sacral ABC treated with denosumab. Improvement of pain and neurologic disease occurred with a significant reduction of tumor volume by MRI. The authors concluded that denosumab could provide therapeutic benefits and serve as a reasonable alternative to surgery in a specific population of ABC patients.
Dosage
Throughout literature search, we could find only case reports and case series with different denosumab regimens for ABCs, but no consensus on a unified protocol. There are two commercially available formulations of denosumab: Prolia, administered 60 mg subcutaneously and Xgeva, administered 120 mg subcutaneously.28) In 2013, the FDA approved Denosumab 120 mg SCI (Xgeva) every 4 weeks for unresectable GCTs with an additional dose of 120 mg on days 8 and 15 of the first month of therapy.29) However, this dose has a considerable risk of hypocalcemia (5%–70%), even more in patients with renal disease and preexisting hypocalcemia.14,17,30,31) The 60 mg dose is not expected to cause such severe hypocalcemia due to the lower dose,32) considering a lower tumor burden in ABCs than GCTs. In our study, we used the dose described by Ntalos et al.16): 60 mg every 6 weeks. With this dose, tolerable drug-related complications were encountered in 3 cases only, while achieving disease control in 80% of cases.
Functional Outcomes and Complications
At the final follow-up, the mean MSTS score was 92.3%, which is comparable with the reported mean MSTS score achieved with surgical treatment of pelvic ABCs (93%).33) It is worth noting that there was no statistically significant difference in MSTS scores among the three groups of radiological response, most probably due to marked improvement of pain, which was the main presenting symptom, even with the absence of lesion consolidation. No grave complications were encountered throughout the treatment course, while 3 patients had mild to moderate side effects. This may be attributed to the lower dose and longer interval between the doses as compared to those used in treating GCTs.
Local Control and Recurrence
In our study, disease control was achieved in 80% of the cases (grades I and II) at the final follow-up. Durr et al.2) stated that a consensus in the literature is that “ABCs should be considered as completely healed if recurrence does not occur within 2 years after the end of therapy.” Singh and Puri34) stated that recurrences mainly arise within 7–9 months of denosumab stoppage in GCT cases. In a metanalysis study by Maximen et al.,4) recurrence occurred at a mean time of 11 ± 6 months after denosumab cessation and the authors stressed the impact of the 2-year period in the validity of the assessment of the cases.
In our series, the mean follow-up was 38 months, with no evidence of local recurrence at the final follow-up. No correlation could be detected between the response to denosumab and either age, sex, location of the lesion within the pelvis, or the doses received. Despite the fact that all grade IV responses were in zones 3 and 4, no statistical significance could be detected. Lesions in zone 3 had 67% good response (grade I) and zone 4 had 60% grade I or II response. Considering the small sample size, we cannot reach a conclusion regarding the response to medication in zones 3 and 4. However, we will have to be more cautious regarding the expected prognosis for lesions in these areas.
Skeletally Immature Patients
Bisphosphonate therapy in the pediatric age group temporarily inhibits epiphyseal activity, forming sclerotic metaphyseal bands on X-ray, which was attributed to retained calcified cartilage. A similar effect may occur in children treated with denosumab, with dense sclerotic bands that fade away once denosumab is stopped.28) However, there are still insufficient data in the literature about the detailed effect of denosumab on growth plates. A single study observed that sclerotic metaphyseal bands, which appeared during therapy, migrated away from the growth plates after cessation of 7-month denosumab treatment in a 9-year-old with fibrous dysplasia. The authors concluded that growth plates remained functional and linear growth was not affected. Many other series confirmed preserved linear growth during denosumab treatment.28) Severe hypercalcemia may develop once treatment is stopped owing to a rapid loss of newly acquired bone caused by rebound formation and activation of osteoclasts after cessation of denosumab.35) This adverse effect is more common in pediatric patients. Mineral release from these bands may contribute to rebound hypercalcemia phenomenon.28)
Our study included only 1 patient who was skeletally immature (7 years old). With this patient, we opted to give the minimum dose (6 months). The follow-up period was 38 months. No signs of physeal affection (growth arrest, physeal bars, and arrest lines) were detected. To our knowledge, this is the largest series of patients assessing the use of denosumab in treating pelvis ABCs, giving the hope of tackling such a challenge in a non-invasive, cost-effective way.
Study Limitations
A major limitation of this study is that it is a retrospective study. Another limitation is the relatively small specimen size, which requires the results of the study to be elaborated with caution. This is related also to the retrospective nature of the study and the rarity of the disease in that location. We would recommend a multi-centric randomized control study as well as further studies regarding its safety profile in skeletally immature patients. Denosumab may provide a reliable option in nonsurgical treatment of ABCs of pelvic origin with expected lower morbidity than the surgical solution and tolerable complications. Further studies on the safety profile and long-term effects of denosumab especially in skeletally immature patients are required.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Rapp TB, Ward JP, Alaia MJ. Aneurysmal bone cyst. J Am Acad Orthop Surg. 2012;20(4):233–241. doi: 10.5435/JAAOS-20-04-233. [DOI] [PubMed] [Google Scholar]
- 2.Durr HR, Grahneis F, Baur-Melnyk A, et al. Aneurysmal bone cyst: results of an off label treatment with denosumab. BMC Musculoskelet Disord. 2019;20(1):456. doi: 10.1186/s12891-019-2855-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alalawi HH, Alfadhel S, Khan M, Bobseit A. Pelvic aneurysmal bone cyst in an adolescent: a case report and literature review. Cureus. 2020;12(8):e9534. doi: 10.7759/cureus.9534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maximen J, Robin F, Tronchot A, Rossetti A, Ropars M, Guggenbuhl P. Denosumab in the management of aneurysmal bone cyst. Joint Bone Spine. 2022;89(1):105260. doi: 10.1016/j.jbspin.2021.105260. [DOI] [PubMed] [Google Scholar]
- 5.Mascard E, Gomez-Brouchet A, Lambot K. Bone cysts: unicameral and aneurysmal bone cyst. Orthop Traumatol Surg Res. 2015;101(1 Suppl):S119–S127. doi: 10.1016/j.otsr.2014.06.031. [DOI] [PubMed] [Google Scholar]
- 6.Pauli C, Fuchs B, Pfirrmann C, Bridge JA, Hofer S, Bode B. Response of an aggressive periosteal aneurysmal bone cyst (ABC) of the radius to denosumab therapy. World J Surg Oncol. 2014;12:17. doi: 10.1186/1477-7819-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lange T, Stehling C, Frohlich B, et al. Denosumab: a potential new and innovative treatment option for aneurysmal bone cysts. Eur Spine J. 2013;22(6):1417–1422. doi: 10.1007/s00586-013-2715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan KS, de Castro LF, Roszko KL, et al. Successful intravascular treatment of an intraosseous arteriovenous fistula in fibrous dysplasia. Calcif Tissue Int. 2020;107(2):195–200. doi: 10.1007/s00223-020-00712-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brosjo O, Pechon P, Hesla A, Tsagozis P, Bauer H. Sclerotherapy with polidocanol for treatment of aneurysmal bone cysts. Acta Orthop. 2013;84(5):502–505. doi: 10.3109/17453674.2013.850013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park HY, Yang SK, Sheppard WL, et al. Current management of aneurysmal bone cysts. Curr Rev Musculoskelet Med. 2016;9(4):435–444. doi: 10.1007/s12178-016-9371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savvidou OD, Bolia IK, Chloros GD, Papanastasiou J, Koutsouradis P, Papagelopoulos PJ. Denosumab: current use in the treatment of primary bone tumors. Orthopedics. 2017;40(4):204–210. doi: 10.3928/01477447-20170627-04. [DOI] [PubMed] [Google Scholar]
- 12.Palmerini E, Chawla NS, Ferrari S, et al. Denosumab in advanced/unresectable giant-cell tumour of bone (GCTB): for how long? Eur J Cancer. 2017;76:118–124. doi: 10.1016/j.ejca.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 13.Niu X, Yang Y, Wong KC, Huang Z, Ding Y, Zhang W. Giant cell tumour of the bone treated with denosumab: how has the blood supply and oncological prognosis of the tumour changed? J Orthop Translat. 2019;18:100–108. doi: 10.1016/j.jot.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raux S, Bouhamama A, Gaspar N, Brugieres L, Entz-Werle N, Mallet C, et al. Denosumab for treating aneurysmal bone cysts in children. Orthop Traumatol Surg Res. 2019;105(6):1181–1185. doi: 10.1016/j.otsr.2019.04.028. [DOI] [PubMed] [Google Scholar]
- 15.Enneking WF, Dunham WK. Resection and reconstruction for primary neoplasms involving the innominate bone. J Bone Joint Surg Am. 1978;60(6):731–746. [PubMed] [Google Scholar]
- 16.Ntalos D, Priemel M, Schlickewei C, Thiesen DM, Rueger JM, Spiro AS. Therapeutic management of a substantial pelvic aneurysmatic bone cyst including the off-label use of denosumab in a 35-year-old female patient. Case Rep Orthop. 2017;2017:9125493. doi: 10.1155/2017/9125493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmerini E, Ruggieri P, Angelini A, Boriani S, Campanacci D, Milano GM, et al. Denosumab in patients with aneurysmal bone cysts: a case series with preliminary results. Tumori. 2018;104(5):344–351. doi: 10.1177/0300891618784808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;(286):241–246. [PubMed] [Google Scholar]
- 19.Neer CS, 2nd, Francis KC, Marcove RC, Terz J, Carbonara PN. Treatment of unicameral bone cyst: a follow-up study of one hundred seventy-five cases. J Bone Joint Surg Am. 1966;48(4):731–745. [PubMed] [Google Scholar]
- 20.Capanna R, Dal Monte A, Gitelis S, Campanacci M. The natural history of unicameral bone cyst after steroid injection. Clin Orthop Relat Res. 1982;(166):204–211. [PubMed] [Google Scholar]
- 21.National Cancer Institute. NCI dictionary of cancer terms: locally recurrent cancer [Internet] National Cancer Institute; 2020. [cited 2023 Nov 10]. Available from: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/locally-recurrent-cancer . [Google Scholar]
- 22.Stanulovic V, Hodolic M, Mitsikostas DD, Papadopoulos D. Drug tolerability: how much ambiguity can be tolerated? A systematic review of the assessment of tolerability in clinical studies. Br J Clin Pharmacol. 2022;88(2):551–565. doi: 10.1111/bcp.15016. [DOI] [PubMed] [Google Scholar]
- 23.Reddy KI, Sinnaeve F, Gaston CL, Grimer RJ, Carter SR. Aneurysmal bone cysts: do simple treatments work? Clin Orthop Relat Res. 2014;472(6):1901–1910. doi: 10.1007/s11999-014-3513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grahneis F, Klein A, Baur-Melnyk A, Knosel T, Birkenmaier C, Jansson V, et al. Aneurysmal bone cyst: a review of 65 patients. J Bone Oncol. 2019;18:100255. doi: 10.1016/j.jbo.2019.100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skubitz KM, Peltola JC, Santos ER, Cheng EY. Response of aneurysmal bone cyst to denosumab. Spine (Phila Pa 1976) 2015;40(22):E1201–E1204. doi: 10.1097/BRS.0000000000001027. [DOI] [PubMed] [Google Scholar]
- 26.Pelle DW, Ringler JW, Peacock JD, et al. Targeting receptoractivator of nuclear kappaB ligand in aneurysmal bone cysts: verification of target and therapeutic response. Transl Res. 2014;164(2):139–148. doi: 10.1016/j.trsl.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Kulkarni AG, Patel A. Denosumab: a potential new treatment option for recurrent aneurysmal bone cyst of the spine. SICOT J. 2019;5:10. doi: 10.1051/sicotj/2019007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyce AM. Denosumab: an emerging therapy in pediatric bone disorders. Curr Osteoporos Rep. 2017;15(4):283–292. doi: 10.1007/s11914-017-0380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dahiya N, Khadka A, Sharma AK, Gupta AK, Singh N, Brashier DB. Denosumab: a bone antiresorptive drug. Med J Armed Forces India. 2015;71(1):71–75. doi: 10.1016/j.mjafi.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurucu N, Akyuz C, Ergen FB, Yalcin B, Kosemehmetoglu K, Ayvaz M, et al. Denosumab treatment in aneurysmal bone cyst: evaluation of nine cases. Pediatr Blood Cancer. 2018;65(4):e26926. doi: 10.1002/pbc.26926. [DOI] [PubMed] [Google Scholar]
- 31.Laskowski LK, Goldfarb DS, Howland MA, Kavcsak K, Lugassy DM, Smith SW. A RANKL wrinkle: denosumab-induced hypocalcemia. J Med Toxicol. 2016;12(3):305–308. doi: 10.1007/s13181-016-0543-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muqeet Adnan M, Bhutta U, Iqbal T, AbdulMujeeb S, Haragsim L, Amer S. Severe hypocalcemia due to denosumab in metastatic prostate cancer. Case Rep Nephrol. 2014;2014:565393. doi: 10.1155/2014/565393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novais EN, Zimmerman AK, Lewallen LW, Rose PS, Sim FH, McIntosh AL. Functional outcomes and quality of life following surgical treatment of aneurysmal bone cysts of the pelvis in children. J Child Orthop. 2014;8(3):281–288. doi: 10.1007/s11832-014-0588-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh VA, Puri A. The current standing on the use of denosumab in giant cell tumour of the bone. J Orthop Surg (Hong Kong) 2020;28(3):2309499020979750. doi: 10.1177/2309499020979750. [DOI] [PubMed] [Google Scholar]
- 35.Wang HD, Boyce AM, Tsai JY, et al. Effects of denosumab treatment and discontinuation on human growth plates. J Clin Endocrinol Metab. 2014;99(3):891–897. doi: 10.1210/jc.2013-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]