Abstract
Pseudomonas putida CA-3 is capable of converting the aromatic hydrocarbon styrene, its metabolite phenylacetic acid, and glucose into polyhydroxyalkanoate (PHA) when a limiting concentration of nitrogen (as sodium ammonium phosphate) is supplied to the growth medium. PHA accumulation occurs to a low level when the nitrogen concentration drops below 26.8 mg/liter and increases rapidly once the nitrogen is no longer detectable in the growth medium. The depletion of nitrogen and the onset of PHA accumulation coincided with a decrease in the rate of substrate utilization and biochemical activity of whole cells grown on styrene, phenylacetic acid, and glucose. However, the efficiency of carbon conversion to PHA dramatically increased once the nitrogen concentration dropped below 26.8 mg/liter in the growth medium. When supplied with 67 mg of nitrogen/liter, the carbon-to-nitrogen (C:N) ratios that result in a maximum yield of PHA (grams of PHA per gram of carbon) for styrene, phenylacetic acid, and glucose are 28:1, 21:1, and 18:1, respectively. In cells grown on styrene and phenylacetic acid, decreasing the carbon-to-nitrogen ratio below 28:1 and 21:1, respectively, by increasing the nitrogen concentration and using a fixed carbon concentration leads to lower levels of PHA per cell and lower levels of PHA per batch of cells. Increasing the carbon-to-nitrogen ratio above 28:1 and 21:1 for cells grown on styrene and phenylacetic acid, respectively, by decreasing the nitrogen concentration and using a fixed carbon concentration increases the level of PHA per cell but results in a lower level of PHA per batch of cells. Increasing the carbon and nitrogen concentrations but maintaining the carbon-to-nitrogen ratio of 28:1 and 21:1 for cells grown on styrene and phenylacetic acid, respectively, results in an increase in the total PHA per batch of cells. The maximum yields for PHA from styrene, phenylacetic acid, and glucose are 0.11, 0.17, and 0.22 g of PHA per g of carbon, respectively.
Styrene, the starting material for polystyrene synthesis, is a major toxic environmental pollutant. Worldwide, millions of kilograms of styrene are released each year as industrial effluents into the environment (33). In the United States alone, over 25 million kilograms of styrene waste is annually released, primarily into the air and through underground injection (29). Styrene is associated with respiratory tract irritation, central nervous system depression, muscle weakness, and narcosis in humans and other mammals (24, 29). The conversion of styrene to polyhydroxyalkanoate (PHA) by Pseudomonas putida CA-3 provides a new and unique link between an aromatic environmental pollutant and aliphatic PHA accumulation.
Due to its biodegradable nature, PHA has a broad range of applications, including medical applications such as wound management, drug delivery, and tissue engineering (12, 36). Furthermore, PHA is composed of chiral hydroxy acids that have potential as synthons for anti-human immunodeficiency virus drugs, anticancer drugs, antibiotics, and vitamins (4, 17).
Investigations into the biodegradation of styrene have resulted in the elucidation of biochemical pathways and molecular control of styrene degradation (19). P. putida CA-3 is capable of the complete mineralization of styrene (14). It does so by epoxidation of styrene and isomerization of the epoxide to phenylacetaldehyde, which is further oxidized to phenylacetic acid (14). Phenylacetic acid is converted to phenylacetyl-coenzyme A (CoA), which is further oxidized to acetyl-CoA (18, 19).
In this study, we report on the conversion of aromatic hydrocarbons to aliphatic PHA. We determine PHA formation, the biochemical activity of whole cells, and the utilization of carbon and nitrogen throughout the growth cycle of P. putida CA-3 with styrene, phenylacetic acid, and glucose as the sole sources of carbon and energy. Furthermore, we determine the effects of altering the carbon and nitrogen supply as well as the ratio of carbon to nitrogen on PHA accumulation. Finally, we investigate the metabolic link between substrate utilization and PHA accumulation in P. putida CA-3 as well as the properties of the PHA polymer accumulated from styrene.
MATERIALS AND METHODS
Media and growth conditions.
P. putida CA-3 cultures were grown in 250-ml conical flasks containing 50 ml of E2 medium (32) at 30°C, with shaking at 200 rpm. For PHA accumulation studies, the inorganic nitrogen source (NaNH4HPO4· ·4H2O) was supplied at between 0.125 g/liter (8.4 mg of nitrogen/liter) and 2.0 g/liter (134 mg of nitrogen/liter) (E2 N-lim). For PHA accumulation studies, P. putida CA-3 was grown in batch culture with 50 ml of E2 N-lim medium for 48 h unless otherwise stated. Phenylacetic acid and glucose were added directly to the growth medium after autoclaving. For inhibition experiments, the required concentration of 2-bromooctanoic acid was added to the medium before inoculation.
Styrene is a volatile liquid that is poorly soluble in water (maximum solubility, 0.3 g/liter at 30°C). Therefore, styrene (20 to 200 mg, equivalent to 0.4 to 4.0 g per liter of medium) was placed in a glass tube (10 mm in diameter by 60 mm in length) fused to the central base of the flask and transferred to the culture through the vapor phase. The concentration of styrene in the air and liquid was measured by gas chromatography analysis.
Analysis of styrene concentration in the headspace and growth medium.
Styrene concentration in the headspace was analyzed by taking 100-μl samples from the headspace and manually injecting them into a Fisons GC-8000 series gas chromatograph (GC) equipped with a 30-m by 0.25-mm HP-1-0.25 μm column (Hewlett-Packard) operating in split mode (split ratio, 8:1) with temperature programming (50°C for 1 min, increments of 10°C/min up to 140°C, and 1 min at 140°C). The retention time obtained for styrene with this method was 5.2 min. The styrene concentration in the liquid medium was analyzed by removing 1-ml samples and extracting the styrene into a 1-ml mixture of hexane and ethanol in the ratio of 95:5. The organic layer was then removed, and a sample was run on a GC as above. The limit of detection of styrene with gas chromatography is 0.001 g/liter.
PHA monomer determination.
PHA monomer composition was determined by using a previously described method (9). Samples were analyzed on a GC. For peak identification, PHA standards from Pseudomonas oleovorans were used. PHA monomer composition was confirmed by GC-mass spectrometry as described previously (10). The composition of PHA was also confirmed by 13C and 1H nuclear magnetic resonance (NMR) analysis of the polymer.
HPLC.
The concentration of phenylacetic acid in the growth medium at various points in the growth curve of P. putida CA-3 was analyzed by high-performance liquid chromatography (HPLC). A 1-ml sample was taken from the flask and centrifuged at 20,000 × g for 10 min at 4°C. The supernatant was filtered prior to HPLC analysis by using a 0.45-μm-pore-size nylon filter. Samples were analyzed by HPLC using a C8 Lichrospher 125- by 4.0-mm column and a Hewlett-Packard HP1100 instrument equipped with an Agilent 1100 Series diode array detector. The samples were eluted with a 90% 0.2 M K2HPO4-10% isopropanol mix at a flow rate of 1.0 ml/min. The retention time for phenylacetic acid was 7.1 min.
Glucose determination assays.
Glucose concentrations in the growth medium were measured by using a 3,5-dinitrosalicylic acid method as described previously (2).
Nitrogen determination assays.
The nitrogen concentration (as ammonium ion) in the medium was measured by the method described by Scheiner (25).
Biochemical assays.
The oxygen consumption of P. putida CA-3 cells was monitored in a biological oxygen monitor (Rank Brothers Ltd., Cambridge, England) as reported previously (16). The cell dry weight (CDW) in oxygen consumption assays varied between 0.15 and 0.3 g/liter. The activity of the styrene monooxygenase enzyme was monitored by measuring indigo formation from indole by P. putida CA-3 whole cells as previously described (15). Unwashed cells were added to a 0.25 mM solution of indole at a CDW concentration of approximately 0.1 g/liter. The formation of indigo was measured at 5-min intervals over 30 min.
Isolation and analysis of PHA polymers.
PHA polymers were isolated by using a previously described method (9). 13C NMR spectrum analysis was performed in a Bruker advance 400-MHz NMR spectrometer operating at 111 MHz for 13C measurements at room temperature. NMR analysis was obtained from 25% (wt/vol) chloroform solutions, and a delay time between pulses of 2.0 s was applied. Differential scanning calorimetry experiments were carried out in a Perkin-Elmer Pyris Diamond system. Two scans were performed by using a 20°C/min heating rate and a 200°C/min cooling rate (quenching) between runs. Thermograms were obtained in the range of −60 to +75°C under helium purge. The glass transition temperature (Tg) values were taken at the inflection point on the transition of second scans. Thermogravimetric analysis investigation was carried out with a Perkin-Elmer Pyris 1 TGA over a temperature range of 30 to 600°C and a rate of 10°C/min. The average molecular weights, molecular weight distribution, and polydispersity index were determined by using a Waters gel permeation chromatograph equipped with a refractive index detector (series 410). Polymer Laboratories gel columns (500 Å) conditioned at 30°C were used to elute samples (1 g/liter) at a 1-ml/min HPLC grade tetrahydrofuran flow rate. Ten samples of polystyrene standards having molecular masses ranging from 950,000 to 1,340 Da were used for calibration.
RESULTS AND DISCUSSION
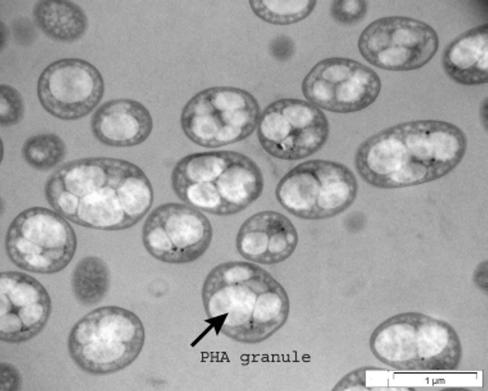
P. putida CA-3 is capable of transforming styrene into PHA when supplied with E2 mineral medium with a low nitrogen concentration of 67 mg of nitrogen/liter. Under these conditions, P. putida CA-3 was found to accumulate PHA intracellularly in discrete granules (Fig. 1). The PHA accumulated from styrene, as measured by GC-mass spectrometry, is composed of (R)-3-hydroxyhexanoic acid, (R)-3-hydroxyoctanoic acid, and (R)-3-hydroxydecanoic acid monomers in a ratio of 3:27:70. The monomer composition was further confirmed by 1H and 13C NMR analysis of the polymer (data not shown). P. putida CA-3 is also capable of transforming phenylacetic acid and glucose to PHA. The monomer composition of the PHA accumulated from these substrates is virtually identical to that of PHA accumulated from styrene.
FIG. 1.
Transmission electron micrograph of P. putida CA-3 cells containing PHA granules accumulated from styrene. The arrow indicates a PHA granule.
Monitoring microbial cellular activity and PHA accumulation over time.
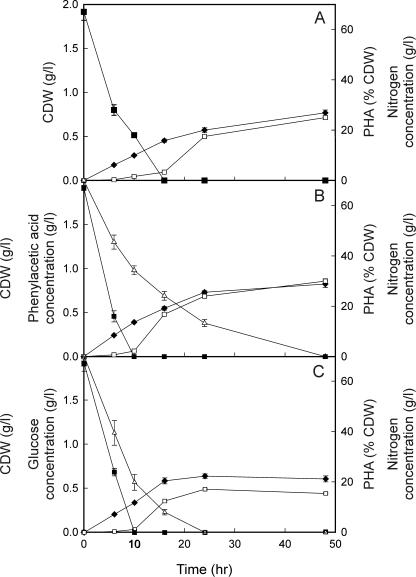
PHA accumulation by P. putida CA-3 was monitored over a 48-h period when either styrene (1.85 g of carbon/liter), phenylacetic acid (1.4 g of carbon/liter), or glucose (0.8 g of carbon/liter) was supplied at 2.0 g/liter to medium containing nitrogen at a concentration of 67 mg/liter (Fig. 2). The depletion of the nitrogen concentration below approximately 26.8 mg/liter coincided with the onset of PHA accumulation from all three carbon sources tested. This observation is in agreement with other studies where a limiting concentration of nitrogen resulted in the onset of PHA accumulation (9, 21, 35). However, it was not until the nitrogen concentration in the growth medium fell below the detectable limit (0.1 mg of nitrogen/liter) that a dramatic increase in the level of PHA accumulation occurred (Fig. 2). Interestingly, the rate of nitrogen depletion was lower in incubations with cells grown on styrene than in incubations with cells grown on phenylacetic acid and glucose (Fig. 2).
FIG. 2.
PHA accumulation by P. putida CA-3 when 2.0 g of styrene (A), phenylacetic acid (B), or glucose (C)/liter is supplied to 50 ml of growth medium containing 67 mg of nitrogen/liter. Biomass (in grams per liter) (♦), PHA accumulation (□), concentration of carbon source in the medium (in grams per liter) (▵), and nitrogen (supplied as sodium ammonium phosphate) concentration (in milligrams per liter) (▪) were all monitored over a 48-h period. All data are averages of results from at least three independent determinations. The concentrations of styrene in the growth medium and in the air above the growth medium were experimentally determined to be 0.02 and 0.004 g/liter, respectively, at time zero. While the concentration of styrene in the air above the medium remained at 0.004 g/liter throughout the growth curve, styrene was no longer detectable in the growth medium after time zero.
Nitrogen accounts for approximately 12% of the dry weight of Pseudomonas cells not containing PHA (35). After 48 h of growth, the total CDWs achieved by cells grown on styrene, phenylacetic acid, and glucose were 0.768, 0.824, and 0.608 g/liter, respectively (Fig. 2). However, 0.195, 0.247, and 0.091 g of these CDWs are accounted for by PHA (Fig. 2). Thus, the total CDWs minus the weight of PHA correspond to CDWs between 0.52 and 0.58 g/liter, which are in good agreement with the CDW value of 0.56 g/liter expected for Pseudomonas cells grown with 67 mg of nitrogen/liter but not containing PHA. The rate of carbon utilization was monitored over the 48-h growth period. Due to the insoluble nature of styrene, it was supplied as a gas. The concentration of styrene in the growth medium at time zero was 0.02 g/liter. At each time point in the growth curve after time zero, styrene was not detectable in the growth medium (Fig. 2A). The styrene concentration in the headspace remained constant throughout the growth curve at 0.004 g/liter. Thus, it was not possible to monitor styrene depletion in the growth medium over time. The rates of phenylacetic acid and glucose depletion were 0.11 and 0.12 g/liter/h, respectively, during the initial 6 h of growth (Fig. 2B and C). The rates of substrate depletion fell to 0.05 and 0.056 g/liter/h for cells grown on phenylacetic acid and glucose, respectively, during the first 16 h of growth (Fig. 2B and C).
In an attempt to establish the biochemical activity of P. putida CA-3 cells before and during PHA accumulation, biochemical assays measuring rates of oxygen consumption and styrene monooxygenase activity by whole cells were performed at various time points in the growth curve (Tables 1 and 2). On all of the carbon sources tested, the rate of oxygen consumption fell between 3.09- and 3.78-fold when the nitrogen concentration was no longer detectable and PHA accumulation began to increase dramatically (Table 1 and Fig. 2). The rate of oxygen consumption continued to decrease for all carbon sources during PHA accumulation. A similar pattern was observed for styrene monooxygenase activity of cells grown on styrene, where a 2.1-fold decrease in styrene monooxygenase coincided with the depletion of nitrogen in the growth medium (Table 2 and Fig. 2).
TABLE 1.
The rate of oxygen consumption of whole cells of P. putida CA-3 harvested at various time points after inoculationa
| Time elapsed (h) | Rate of oxygen consumption by washed whole-cell suspensions (nmol/min/mg of CDW) grown onb:
|
||
|---|---|---|---|
| Styrene | Phenylacetic acid | Glucose | |
| 6 | 270 | 250 | 210 |
| 10 | 265 | 75 | 68 |
| 16 | 70 | 20 | 36 |
| 24 | 20 | 9 | 20 |
| 48 | 23 | 13 | 25 |
All cells were grown in 50 ml of E2 medium containing 67 mg of nitrogen/liter and supplied with 2 g of the relevant growth substrate/liter.
All values are the means of results from at least two independent determinations. The standard error is less than 10%.
TABLE 2.
Styrene monooxygenase activity of whole cells of P. putida CA-3 harvested at various time points after inoculationa
| Rate of indigo formation by whole cells (nmol/min/mg of CDW) of P. putida CA-3b at:
| ||||
|---|---|---|---|---|
| 6 h | 10 h | 16 h | 24 h | 48 h |
| 12.5 | 12.2 | 5.8 | 2.1 | 1.8 |
All cells were grown in 50 ml of E2 medium containing 67 mg of nitrogen/liter and supplied with 2 g of styrene/liter. The molar extinction coefficient value for indigo at 610 nm is 15,900 M−1 cm−1.
All values are the means of results from at least two independent determinations. The standard error is less than 10%.
Interestingly, while the biochemical activity of the cells with respect to carbon utilization, oxygen utilization, and styrene monooxygenase activity decreased over time, the rate of carbon conversion to PHA increased over time (Fig. 2 and Tables 1 and 2); e.g., the rate of phenylacetic acid consumption by growing cells decreases 2.2-fold in the first 16 h of growth, while the rate of carbon-to-PHA conversion increases approximately 100-fold in the same time period (Fig. 2B). This result implies that the efficiency of carbon source-to-PHA conversion increases over time under inorganic nitrogen limitation. The change in biochemical activity is in keeping with previous reports for changes in enzyme activity in the late log or early stationary phases of growth or under nutrient limitation (26). However, this is to our knowledge the first time that the catabolic activity of PHA-accumulating cells has been shown to dramatically decrease during PHA accumulation.
When the efficiencies of the conversion of carbon source to PHA were compared over the entire growth curve across the three growth substrates, 0.099, 0.118, and 0.046 g of PHA per g of styrene, phenylacetic acid, and glucose, respectively, was converted to PHA by P. putida CA-3 (Fig. 2). This is equivalent to 0.11, 0.17, and 0.115 g of PHA per g of carbon supplied. The differences in the efficiencies of carbon source-to-PHA conversion across the three substrates may be explained by the influence of carbon concentration or carbon-to-nitrogen ratio. Thus, the effect of carbon supply to the growth medium was examined.
The effect of carbon supply on PHA accumulation by P. putida CA-3.
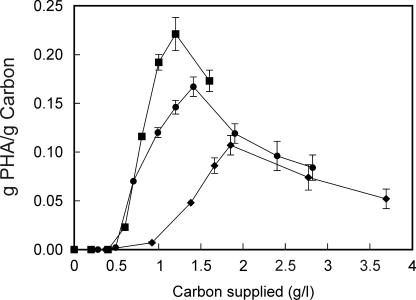
PHA accumulation was measured in cultures containing 67 mg of nitrogen/liter while the concentration of the carbon sources supplied was varied. Little PHA was observed on any of the carbon sources until the C:N ratios exceeded approximately 9:1 for cells grown on glucose, 10:1 for cells grown on phenylacetic acid, and 14:1 for cells grown on styrene (Fig. 3). Thereafter, PHA accumulation increased to maximal levels of 0.11 g/g of carbon for styrene, 0.17 g/g of carbon for phenylacetic acid, and 0.22 g/g of carbon for glucose (Fig. 3). These results correspond to optimum C:N ratios for PHA accumulation of 28:1, 21:1, and 18:1 for cells grown on styrene, phenylacetic acid, and glucose, respectively, when supplied with 67 mg of nitrogen/liter.
FIG. 3.
PHA accumulation by P. putida CA-3 as a function of the amount of carbon (in grams per liter) supplied in the form of styrene (♦), phenylacetic acid (•), or glucose (▪) to 50 ml of growth medium containing 67 mg of nitrogen/liter. PHA accumulation is expressed as the total number of grams of PHA formed per gram of carbon supplied. There was minimal variation in monomer composition of PHA accumulated with various amounts of styrene, phenylacetic acid, or glucose supplied. Data are averages of results from at least three independent determinations.
The PHA levels in cells grown on styrene are always lower than those in cells grown on phenylacetic acid (Fig. 3). This result is surprising, as phenylacetic acid is a known intermediate in the styrene catabolic pathway. However, styrene is present in the growth medium at a much lower concentration than phenylacetic acid due to its supply as a gas and its low solubility in aqueous solution. The concentration of substrates in the growth medium is known to affect the concentration of key PHA intermediates, such as (R)-3-hydroxyalkanoic acids in the cytoplasm, and ultimately the PHA content of the cell (28).
Effect of sodium ammonium phosphate concentration on PHA accumulation in P. putida CA-3.
In an attempt to determine the maximum yield of PHA from aromatic carbon substrates, the initial nitrogen concentration in the growth medium was altered while the initial concentration of the carbon source was kept constant (2.0 or 4.0 g/liter).
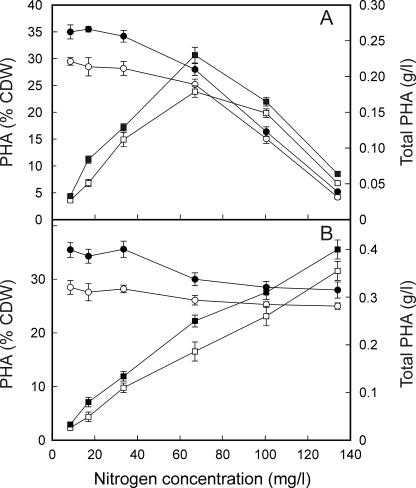
When supplied with 2 g of the carbon source/liter, the highest PHA content of cells (percent CDW) was observed at low nitrogen concentrations (Fig. 4A). However, PHA yields from styrene and phenylacetic acid were low due to poor growth of the cells. Total PHA yields increased dramatically to a maximum at a nitrogen concentration of 67 mg/liter, establishing an optimal C:N ratio of 28:1 when 2 g of styrene/liter was supplied and 21:1 when 2 g of phenylacetic acid/liter was supplied (Fig. 4A).
FIG. 4.
Effects of nitrogen concentration on the level of PHA accumulated from 2.0 g of styrene or phenylacetic acid/liter (A) and 4.0 g of styrene or phenylacetic acid/liter (B) added to 50 ml of growth medium. Shown are PHA as a percentage of CDW (○) and total PHA (in grams per liter) (□) for cells grown on styrene and PHA as a percentage of CDW (•) and total PHA (in grams per liter) (▪) for cells grown on phenylacetic acid. All data are averages of results from at least three independent determinations.
When the carbon source supplied was doubled to 4.0 g/liter, the PHA content of the cells (percent CDW) was still highest at a low nitrogen concentration and decreased slightly with increasing nitrogen concentration (Fig. 4B). The total PHA yield (expressed in grams of PHA per liter of growth medium) increased with increasing nitrogen supply through the range examined, with a maximum PHA yield observed at a nitrogen concentration of 134 mg/liter (Fig. 4B). This is equivalent to C:N ratios of 28:1 for cells grown on styrene and 21:1 for cells grown on phenylacetic acid.
Metabolic link between aromatic hydrocarbon metabolism and PHA accumulation by P. putida CA-3.
The accumulation of PHA during growth of pseudomonads on nonrelated carbon sources, such as glucose, is known to proceed through fatty acid de novo biosynthesis (7, 22, 23). An important metabolic link between fatty acid de novo biosynthesis and biosynthesis of PHA in pseudomonads has been shown to be catalyzed by a 3-hydroxy-acyl-ACP:CoA transacylase (PhaG) (5, 22, 23). We employed a known inhibitor of PhaG, 2-bromooctanoic acid (11), to look at the effect on PHA accumulation from styrene. The levels of PHA accumulated by P. putida CA-3 from styrene (2.0 g/liter) and phenylacetic acid (2.0 g/liter) are 73- and 60-fold lower (<0.3% PHA), respectively, when cells are incubated with 15 mM 2-bromooctanoic acid. A similar level of inhibition is evident when glucose is employed as the growth substrate.
PHA accumulation from alkanoic acids is known to proceed via β-oxidation (7, 11). However, 15 mM 2-bromoocatanoic acid inhibited PHA accumulation in P. putida CA-3 from octanoic acid by 20%, suggesting the inhibition of other cellular functions as well as PhaG by 2-bromooctanoic acid.
Polymer properties of PHA from styrene.
The monomer composition of the PHA accumulated from styrene results in the formation a thermostable plastic with a destruction temperature of 265°C (Table 3). The polymer is partially crystalline, as evidenced by the presence of a melting peak. The melting temperature of the crystalline PHA is 38.14°C (Table 3). The rapidly cooled PHA polymer is totally amorphous, with a glass transition temperature of −43.3°C. The molecular mass and polydispersity of the polymer were 76,500 Da and 3.03, respectively. These polymer properties are comparable to those of a typical elastomer (31). However, the molecular weight of the PHA accumulated from styrene is relatively low, and the polydispersity (Q) is relatively high (Table 3), when compared with other medium-chain-length PHAs or synthetic polyesters (13, 20, 31). A high molecular weight with a low polydispersity is usually desired in the production of commodity thermoplastics. A molecular weight of 600,000 or above is considered acceptable for the thermoplastic applications of the short-chain-length PHA copolymer poly(3-hydroxybutyate-co-3-hydroxyvalerate) (3). However, medium-chain-length PHA polymers have potential as specialty polymers, such as coatings, pressure-sensitive adhesives, polymer binding agents in organic-solvent-free paints, and a range of medical applications (21, 30, 31, 34), where low molecular weights may be advantageous to the potential applications of the polymer. Future investigations will look at the effect of growth conditions on PHA polydispersity and molecular weight.
TABLE 3.
Polymer properties of PHA accumulated from styrenea
| MW | Mn | Q (MW/Mn) | Tg (°C) | Tm (°C) | DT (°C) |
|---|---|---|---|---|---|
| 76,500 | 25,200 | 3.03 | −41.7 | 38.14 | 265 |
All values are the means of results from two independent determinations. MW, molecular weight; Mn, molecular number; Q (MW/Mn), polydispersity; Tg, glass transition temperature; Tm, melting temperature; DT, decomposition temperature.
Several bacteria have the ability to convert aromatic substrates with acyl side chains of five or more carbons to aromatic PHA (1, 6, 8, 27). However, these bacteria do not accumulate aliphatic PHA from these aromatic carbon sources. To the best of our knowledge, this is the first reported case of aliphatic PHA from an aromatic substrate. The conversion of aromatic hydrocarbons, such as styrene, to aliphatic PHA is interesting from a microbial biochemistry viewpoint but may also have a biotechnological application. However, a significant increase in the yield of PHA from styrene will have to be achieved if any biotechnological potential is to be investigated. Consequently, a greater understanding of the biochemistry and molecular regulation of PHA accumulation from styrene in continuous and fed-batch fermentation is required.
Acknowledgments
P.G.W. is the recipient of a Ph.D. scholarship from the Environmental Protection Agency Ireland and Enterprise Ireland.
We thank David John, Trinity College Dublin, Dublin, Ireland, for the transmission electron micrograph and Cormac Murphy, Ciaran O'Beirne, Wim Meijer, and Wouter Duetz for helpful discussions.
REFERENCES
- 1.Abraham, G. A., A. Gallardo, J. San Roman, E. R. Olivera, R. Jodra, B. Garcia, B. Minambres, J. L. Garcia, and J. M. Luengo. 2001. Microbial synthesis of poly(β-hydroxyalkanoates) bearing phenyl groups from Pseudomonas putida: chemical structure and characterization. Biomacromolecules 2:562-567. [DOI] [PubMed] [Google Scholar]
- 2.Bernfield, P. 1955. Amylases -α and -β. Methods Enzymol. 1:149-158. [Google Scholar]
- 3.Braunegg, G., G. Lefebvre, and K. F. Genser. 1998. Polyhydroxyalkanoates, biopolyesters from renewable resources: physiological and engineering aspects. J. Biotechnol. 65:127-161. [DOI] [PubMed] [Google Scholar]
- 4.de Roo, G., M. B. Kellerhals, Q. Ren, B. Witholt, and B. Kessler. 2002. Production of chiral R-3-hydroxyalkanoic acids and R-3-hydroxyalkanoic acid methylesters via hydrolytic degradation of polyhydroxyalkanoate synthesized by Pseudomonads. Biotechnol. Bioeng. 77:717-722. [DOI] [PubMed] [Google Scholar]
- 5.Fielder, S., A. Steinbüchel, and B. H. A. Rehm. 2000. PhaG-mediated synthesis of poly(3-hydroxyalkanoates) consisting of medium-chain-length constituents from nonrelated carbon sources in recombinant Pseudomonas fragi. Appl. Environ. Microbiol. 66:2117-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia, B., E. R. Olivera, B. Minambres, M. Fernandez-Valverde, L. M. Canedo, M. A. Prieto, J. L. Garcia, M. Martinez, and J. M. Luengo. 1999. Novel biodegradable aromatic plastics from a bacterial source. J. Biol. Chem. 274:29228-29241. [DOI] [PubMed] [Google Scholar]
- 7.Huijberts, G. N. M., T. C. de Rijk, R. de Waard, and G. Eggink. 1995. 13C nuclear magnetic resonance studies of Pseudomonas putida fatty acid metabolic routes involved in poly(3-hydroxyalkanoate) synthesis. J. Bacteriol. 176:1661-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim, Y. B., D. Y. Kim, and Y. H. Rhee. 1999. PHAs produced by Pseudomonas putida and Pseudomonas oleovorans grown with n-alkanoic acids containing aromatic groups. Macromolecules 32:6058-6064. [Google Scholar]
- 9.Lageveen, R. G., G. W. Huisman, H. Preusting, P. Ketelaar, G. Eggink, and B. Witholt. 1988. Formation of polyesters by Pseudomonas oleovorans: effect of substrates on formation and composition of poly-(R)-3-hydroxyalkanoates and poly-(R)-3-hydroxyalkenoates. Appl. Environ. Microbiol. 54:2924-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee, E. Y., and C. Y. Choi. 1995. Gas chromatography mass spectrometric analysis and its application to a screening procedure for novel bacterial polyhydroxyalkanoic acid containing long chain saturated and unsaturated monomers. J. Ferment. Bioeng. 49:1-14. [Google Scholar]
- 11.Lee, H. J., M. H. Choi, T. U. Kim, and S. C. Yoon. 2001. Accumulation of polyhydroxyalkanoic acid containing large amounts of unsaturated monomers in Pseudomonas fluorescens BM07 utilizing saccharides and its inhibition by 2-bromooctanoic acid. Appl. Environ. Microbiol. 67:4963-4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee, S. Y. 1996. Bacterial polyhydroxyalkanoates. Biotechnol. Bioeng. 49:1-14. [DOI] [PubMed] [Google Scholar]
- 13.Madison, L., and G. W. Huisman. 1999. Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic. Microbiol. Mol. Biol. Rev. 63:21-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Connor, K., C. M. Buckley, S. Hartmans, and A. D. W. Dobson. 1995. Possible regulatory role for non-aromatic carbon sources in styrene degradation by Pseudomonas putida CA-3. Appl. Environ. Microbiol. 61:544-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Connor, K., A. D. W. Dobson, and S. Hartmans. 1997. Indigo formation by microorganisms expressing styrene monooxygenase activity. Appl. Environ. Microbiol. 63:4287-4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Connor, K. E., W. Duetz, B. Wind, and A. D. W. Dobson. 1996. The effect of nutrient limitation on styrene metabolism in Pseudomonas putida CA-3. Appl. Environ. Microbiol. 62:3594-3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohashi, T., and J. Hasegawa. 1992. New preparative methods for optically active β-hydroxycarboxylic acids, p. 249-268. In G. N. Sheldrake, A. N. Collins, and J. Crosby (ed.), Chirality in industry. John Wiley & Sons, New York, N.Y.
- 18.O'Leary, N. D., K. E. O'Connor, W. Duetz, and A. D. W. Dobson. 2001. Transcriptional regulation of styrene degradation in Pseudomonas putida CA-3. Microbiology 147:973-979. [DOI] [PubMed] [Google Scholar]
- 19.O'Leary, N., W. A. Duetz, A. D. W. Dobson, and K. E. O'Connor. 2002. Induction and repression of the sty operon in Pseudomonas putida CA-3 during growth on phenylacetic acid under organic and inorganic nutrient limiting continuous culture conditions. FEMS Microbiol. Lett. 208:263-268. [DOI] [PubMed] [Google Scholar]
- 20.Preusting, H., A. Nijenhuis, and B. Witholt. 1990. Physical characteristics of poly(3-hydroxyalkanoates) and poly(3-hydroxyalkenoates) produced by Pseudomonas oleovorans grown on aliphatic hydrocarbons. Macromolecules 23:4220-4224. [Google Scholar]
- 21.Reddy, C. S. K., R. Ghai, Rashmi, and V. C. Kalia. 2003. Polyhydroxyalkanoates: an overview. Bioresour. Technol. 87:137-146. [DOI] [PubMed] [Google Scholar]
- 22.Rehm, B. H. A., N. Kruger, and A. Steinbüchel. 1998. A new metabolic link between fatty acid de novo synthesis and polyhydroxyalkanoic acid synthesis. The PHAG gene from Pseudomonas putida KT2440 encodes a 3-hydroxyacyl-acyl carrier protein-coenzyme A transferase. J. Biol. Chem. 273:24044-24051. [DOI] [PubMed] [Google Scholar]
- 23.Rehm, B. H. A., T. A. Mitsky, and A. Steinbüchel. 2001. Role of fatty acid de novo biosynthesis in polyhydroxyalkanoic acid (PHA) and rhamnolipid synthesis by pseudomonads: establishment of the transacylase (PhaG)-mediated pathway for PHA biosynthesis in Escherichia coli. Appl. Environ. Microbiol. 67:3102-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sax, N. I., and R. J. Lewis, Sr. (ed.). 1987. Hawley's condensed chemical dictionary, 11th ed. Van Nostrand Reinhold Company, New York, N.Y.
- 25.Scheiner, D. 1976. Determination of ammonia and Kjeldahl nitrogen by indophenol method. Water Res. 10:31-36. [Google Scholar]
- 26.Siegele, D. A., and R. Kolter. 1992. Life after log. J. Bacteriol. 174:345-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song, J. J., M. H. Choi, S. C. Yoon, and N. E. Huh. 2001. Cometabolism of phenylalkanoic acids with butyric acid for the efficient production of aromatic polyesters in Pseudomonas putida BM01. J. Microbiol. Biotechnol. 11:435-442. [Google Scholar]
- 28.Steinbüchel, A., and T. Lütke-Eversloh. 2003. Metabolic engineering and pathway construction for biotechnological production of relevant polyhydroxyalkanoates in microorganisms. Biochem. Eng. J. 16:81-96. [Google Scholar]
- 29.U.S. Inventory of Toxic Compounds. 2001. TRI92. Toxics release inventory public data. Office of Pollution Prevention and Toxics, U.S. EPA, Washington, D.C. [Online.] http://www.epa.gov/tri/.
- 30.van der Walle, G. A. M., G. J. H. Buisman, R. A. Weusthuis, and G. Eggink. 1999. Development of environmentally friendly coatings and paints using medium-chain-length poly(3-hydroxyalkanoates) as the polymer binder. Int. J. Biol. Macromol. 25:123-128. [DOI] [PubMed] [Google Scholar]
- 31.van der Walle, G. A. M., G. J. M. de Koning, R. A. Weusthuis, and G. Eggink. 2001. Properties, modifications and applications of biopolyesters. Adv. Biochem. Eng. Biotechnol. 71:264-291. [DOI] [PubMed] [Google Scholar]
- 32.Vogel, H. J., and D. M. Bonner. 1956. DM acetylornithinase of E. coli: partial purification and some properties. J. Biol. Chem. 218:97-106. [PubMed] [Google Scholar]
- 33.Westblad, C., Y. A. Levindis, H. Richter, J. B. Howard, and J. Carlson. 2002. A study on toxic emissions from batch combustion of styrene. Chemosphere 49:395-412. [DOI] [PubMed] [Google Scholar]
- 34.Williams, S. F., D. P. Martin, D. M. Horowitz, and O. P. Peoples. 1999. PHA applications: addressing the price performance issue. I. Tissue engineering. Int. J. Biol. Macromol. 25:111-121. [DOI] [PubMed] [Google Scholar]
- 35.Zinn, M. 1998. Dual (C,N) nutrient limited growth of Pseudomonas oleovorans. Ph.D. thesis 12987. Swiss Federal Institute of Technology, Zurich, Switzerland.
- 36.Zinn, M., B. Witholt, and T. Egli. 2001. Occurrence, synthesis and medical application of bacterial polyhydroxyalkanoate. Adv. Drug Delivery Rev. 53:5-21. [DOI] [PubMed] [Google Scholar]