Abstract
We developed a metabolically engineered yeast which produces lactic acid efficiently. In this recombinant strain, the coding region for pyruvate decarboxylase 1 (PDC1) on chromosome XII is substituted for that of the l-lactate dehydrogenase gene (LDH) through homologous recombination. The expression of mRNA for the genome-integrated LDH is regulated under the control of the native PDC1 promoter, while PDC1 is completely disrupted. Using this method, we constructed a diploid yeast transformant, with each haploid genome having a single insertion of bovine LDH. Yeast cells expressing LDH were observed to convert glucose to both lactate (55.6 g/liter) and ethanol (16.9 g/liter), with up to 62.2% of the glucose being transformed into lactic acid under neutralizing conditions. This transgenic strain, which expresses bovine LDH under the control of the PDC1 promoter, also showed high lactic acid production (50.2 g/liter) under nonneutralizing conditions. The differences in lactic acid production were compared among four different recombinants expressing a heterologous LDH gene (i.e., either the bovine LDH gene or the Bifidobacterium longum LDH gene): two transgenic strains with 2μm plasmid-based vectors and two genome-integrated strains.
Plant- and crop-based renewable plastics (23), including polylactic acid, are polymeric materials that could be produced with a fermentation process and would be biodegraded to H2O and CO2 finally (25). In the sustainable society to come, renewable plastics should be available at a lower price than petroleum-based plastics currently in use. Lactic acid, which is used as a monomer for polymerization into polylactic acid, has a global market in excess of 100,000 tons per year (11), and further increased demand is predicted. Lactic acid is generally produced with lactic acid bacteria (22), such as Lactobacillus species, which are hard to cultivate at high density and show high auxotrophy regarding growth (13). During lactic acid fermentation, a low pH has inhibitory effects on cell growth and lactic acid production. While chemicals (CaCO3, NaOH, or NH4OH) are added to neutralize lactic acid, the processes are limited by the difficulty in the regeneration of precipitated lactates. With the potential demand for an increase in lactic acid production comes a greater interest in finding another approach to producing lactic acid.
Yeasts, such as Saccharomyces cerevisiae, are more tolerant to low pHs than lactic acid bacteria. Recently, new methods for producing lactic acid with genetically engineered yeast have been developed and applied for large-scale production on a trial basis. In ethanol fermentation, pyruvic acid is converted into acetaldehyde by pyruvate decarboxylase (PDC; EC 4.1.1.1), and then the acetaldehyde is converted into ethanol by alcohol dehydrogenase (EC 1.1.1.1) (29). A transgenic yeast expressing exogenous l-lactate dehydrogenase (l-LDH; EC 1.1.1.27) could produce lactic acid from pyruvic acid. Such metabolically engineered yeasts were first reported by Dequin and Barre (7) and Porro et al. (4, 27), who showed that the recombinants yielded about 10 to 20 g of lactate/liter. In both cases, a considerable amount of ethanol was produced concurrently because S. cerevisiae predominantly produces ethanol under anaerobic conditions. The by-product ethanol has become a problem in lactic acid fermentation with a transgenic yeast.
There are three structural PDC genes, PDC1, PDC5 (33, 34), and PDC6 (15), in the S. cerevisiae genome. To increase the metabolic flow from pyruvic acid to lactic acid, a mutant strain, such as the pdc1, pdc5 (1), or adh1 (35) mutant strain, was utilized as the genetic background for obtaining an l-LDH gene-expressing yeast. However, a remarkable improvement in l-lactic acid production has not been observed. In addition to the fact that the PDC activity in yeast is due mainly to PDC1, PDC5 was observed to compensate for a PDC1 deficit, because PDC1 deletion led to a great increase in PDC5 promoter-driven mRNA expression (14). A double mutant strain with pdc1 and pdc5 exhibited significantly impaired growth on glucose medium (9, 10). The other approach for lactic acid production involved the use of a Crabtree-negative yeast, such as Kluyveromyces lactis (28). K. lactis has a single PDC1 gene, KlPDC1, expressing PDC activity (2). Bianchi et al. used K. lactis strains lacking either PDC activity or PDC and PDH activities, transformed with the LDH gene placed under the control of the promoter of KlPDC1 gene and cloned into stable multicopy vector. Transgenic K. lactis strains showed remarkable improvement under the fed-batch condition (3).
To achieve mass production of lactic acid by using S. cerevisiae, we have developed a recombinant strain that produces lactic acid efficiently. In this study, we found, firstly, genome-integrated LDH was regulated under the control of the PDC1 promoter. Secondly, PDC1 was completely inactive. Also, two LDHs, i.e., bovine and Bifidobacterium longum LDHs, were compared, and they were compared with lactic acid-producing yeasts reported so far.
MATERIALS AND METHODS
Strains and media.
The Escherichia coli strain used for molecular cloning was JM109 (TOYOBO, Osaka, Japan). E. coli cultivation and medium were as described previously (32). The S. cerevisiae OC-2T (a/α trp1/trp1) was derived from the wine yeast strain IFO2260 (30). The culture medium used for S. cerevisiae was YPD medium (1% Bacto yeast extract, 2% Bacto peptone, 2% glucose, wt/vol).
Synthesis of an l-LDH gene.
Bovine (GenBank accession number D90141) (17) and B. longum (GenBank accession number M33585) (24) LDH sequences were designed based on major codon usage according to the database on S. cerevisiae (Codon Usage Database [http://www.kazusa.or.jp/codon/]). The amino acids sequences encoded by these sequences were the same as those in the database. The experimental method was that of Horton et al. (16). Each oligonucleotide primer was synthesized at 100-mer intervals (QIAGEN GmbH, Hilden, Germany) and then fused with the overlapping region by PCR. In the PCR, KOD DNA polymerase (TOYOBO) was used for amplification. Each fragment was subcloned into the pBluescript II SK(+) vector (Stratagene, La Jolla, Calif.) according to a previous report (32). The ligase reaction was performed with a Lig Fast rapid DNA ligation system (Promega, Madison, Wis.), and the competent cells used for transformation were of the E. coli JM109 strain. To confirm the subcloning of the vector, the nucleotide sequence was determined with an ABI PRISM 310 genetic analyzer (Applied Biosystems, Foster City, Calif.). The resulting fragments were named LDHKCB (bovine LDH) and LDHKCL (B. longum LDH).
Construction of plasmid vectors.
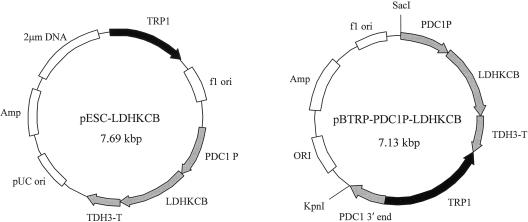
Maps of the plasmids used in this study are shown in Fig. 1, and the vectors in which the l-LDH gene was replaced were pBTRP-PDC1P-LDHKCB and pBTRP-PDC1P-LDHKCL. 2μm-based multicopy plasmid vectors, including pESC-LDHKCB (Fig. 1A) and pESC-LDHKCY, consisted of the PDC1 promoter and LDH within the pESC-TRP vector (Stratagene). Also, the pBBLE-Δpdc1 vector consisted of the PDC1 promoter, the Tn5 BLE gene cassette, and a PDC1 downstream fragment. The phleomycin resistance gene was the bleomycin gene (Tn5 BLE) of bacterial transposon Tn5 (11), which was fused downstream from the S. cerevisiae TDH3 (glyceraldehyde-3-phosphate dehydrogenase 3) promoter. Integration vectors, including pBTRP-PDC1-LDHKCB (Fig. 1B) and pBTRP-PDC1-LDHKCY, consisted of the PDC1 promoter, LDH, TRP1 (tryptophan requiring 1), and the PDC1 downstream fragment. TRP1 was obtained by treating pRS404 (Stratagene) with AatII and SspI and ligating it to the pBluescript II SK(+) vector treatment with T4 DNA polymerase. Each fragment was isolated by PCR using the genomic DNA of the S. cerevisiae OC-2T strain as a template. Genomic DNA was prepared using a Fast DNA kit (Qbiogene, Carlsbad, Calif.), and the concentration was determined with an Ultrospec 300 spectral photometer (Pharmacia Biotech, Uppsala, Sweden). KOD DNA polymerase was used for PCR amplification, and the primers and their oligonucleotide sequences were as follows. For the PDC1 promoter fragment, PDC1P-U had the sequence 5′-ATATATGGATCCGCGTTTATTTACCTATCTC-3′, containing a BamHI restriction site (underlined), and PDC1P-D had the sequence 5′-ATATATGAATTCTTTGATTGATTTGACTGTG-3′, containing an EcoRI restriction site (underlined). For the PDC1 downstream fragment, PDC1D-U had the sequence 5′-ATATATCTCGAGGCCAGCTAACTTCTTGGTCGAC-3′, containing a XhoI restriction site (underlined). This fragment was upstream −705 bp from the PDC1 open reading frame (ORF) start codon. PDC1D-D had the sequence 5′-ATATATGGGCCCCTCGTCAGCAATAGTGGTCAAC-3′, containing an ApaI restriction site (underlined). For the PDC1 3′ end, the PDC1 downstream fragment of 518 bp in length was between +501 and +1,018 from the PDC1 ORF start codon. The amplification fragments were treated with each restriction enzyme (TaKaRa BIO, Otsu, Japan) and were ligated to a vector, and all plasmids constructed in this work were obtained by using standard techniques (32).
FIG. 1.
Maps of the plasmid vectors used for LDH expression. PDC1 P, PDC1 promoter from S. cerevisiae OC-2T. This fragment was −705 bp upstream from the PDC1 ORF start codon. TDH3-T, TDH3 terminator fragment. LDHKCB, bovine LDH. PDC1 3′ end, PDC1 downstream fragment of 518 bp in length was between +501 and +1,018 from the PDC1 ORF start codon. Two other vectors (pESC-LDHKCY and pBTRP-PDC1P-LDHKCY) were obtained by substituting the LDHKCB in these vectors for LDHKCL (B. longum LDH).
Yeast transformation.
S. cerevisiae transformation was performed by the lithium acetate procedure of Ito et al. (18). Host strain OC-2T is a diploid and homothallic strain (30). Spore formation was performed on sporulation plates (1% acetate, 0.1% d-glucose, 0.1% yeast extract, and 2% agar, wt/vol). Diploid formation was performed using the homothallic property, and tetrads were dissected under an optical microscope (Olympus, Tokyo, Japan) and a micromanipulator (Narishige Science, Tokyo, Japan). After the colonies had been isolated, the target gene introduction was confirmed by PCR.
Construction of YEp multicopy control strains.
Recombinant strains, such as YEBL-8A and YEBO-1B, were constructed in the following way. By use of the pBBLE-Δpdc1 vector, a PDC1 deletion strain was constructed. After the colonies had been isolated, the PDC1 deletion was confirmed by PCR. Next, the pESC-LDHKCY and pESC-LDHKCB vectors were introduced into these strains, and the recombinants were named YEBL-8A and YEBO-1B, respectively.
Construction of PDC1 integration strains.
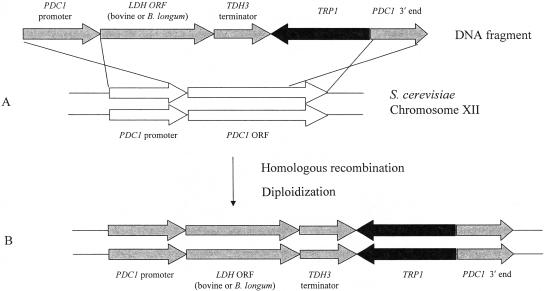
Recombinant strains, such as YIBL-2D and YEBO-7A, were constructed in the following way (Fig. 2A). By use of the pBTRP-PDC1-LDHKCB vector, the heterozygous integrated strain was constructed. But the PDC1 gene in this transformant was not completely deleted, because the OC-2T strain is a homothallic yeast. The LDH gene on one side of the chromosome could be amplified, and the PDC1 gene was completely deleted upon spore formation (Fig. 2B).
FIG. 2.
The improved method for LDH gene expression. (A) The constructed vector was integrated into the PDC1 locus of S. cerevisiae chromosome XII through homologous recombination. (B) Firstly, one copy of the target LDH was introduced into the host, the OC-2T strain. Secondly, diploidization occurred through spore formation. The introduced LDH was amplified into two copies, and the PDC1 coding region was completely disrupted.
Enzyme-specific activity in cell extracts.
Cell extracts were prepared with a SONIFIER 250 (Branson) as described previously (29). PDC-specific activity in freshly prepared extracts, as described by Pronk et al. (29), was determined by using 6a Ubest-55 spectrophotometer at 340 nm (Japan Spectroscopic, Tokyo, Japan). LDH-specific activity was determined in freshly prepared extracts as described by Minowa et al. (24). Protein concentrations in cell extracts were determined with a DC protein assay kit (Bio-Rad, Richmond, Calif.) by using bovine serum albumin (Sigma, St. Louis, Mo.) as a standard.
Fermentation.
Fermentation experiments were performed at 30°C in 100-ml flasks with working volumes of 40 ml in YPD10 medium (1% Bacto yeast extract, 2% Bacto peptone, 10% d-glucose) containing 3% sterilized calcium carbonate. The inoculum was prepared by transferring strains from stock cultures to flasks containing 5 ml of YPD medium. Each culture was incubated for 72 h at 30°C on a shaker and then transferred to the fermentation medium of 0.1% packed cell volume at the inoculum size. The glucose, lactic acid, and ethanol concentrations were measured with a biosensor BF-4 S instrument (Oji Keisoku Kiki, Hyogo, Japan).
RESULTS
Lactate production with multicopy plasmids.
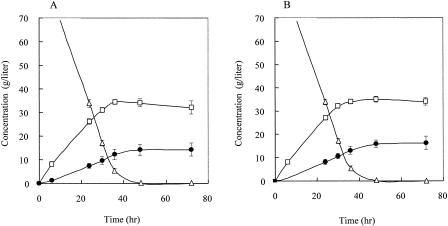
We constructed two kinds of recombinant yeasts expressing a heterologous LDH gene, bovine LDH or B. longum LDH (Table 1), under the control of the PDC1 promoter using a 2μm plasmid-based vector (Fig. 1A). The recombinants with the PDC1 deletion showed no significant differences in either PDC or LDH activities, and the level of PDC activity was one-third of that in the case in which OC-2T was used as the host strain (Table 2). The YEBO-1B strain, which expresses bovine LDH, was cultivated for 72 h, and the yields of lactate and ethanol were 16.3 g/liter and 34.3 g/liter, respectively (Fig. 3A). The yield of lactate for YEBL-2D, which expresses B. longum LDH, was almost the same as that for YEBO-1B (Fig. 3B), with no difference in yield being seen between the two kinds of LDH.
TABLE 1.
Yeast strains used in this study
| Strain | Relevant genotype | LDH expression cassette | Description or characteristic | Reference or source |
|---|---|---|---|---|
| OC-2T | a/α trp1/trp1 | Host strain | 31 | |
| YEBL-8A | a/α pdc1/pdc1 Phlr | PPDC1− B. longum LDH | YEp (2μm DNA) | This study |
| YEBO-1B | a/α pdc1/pdc1 Phlr | PPDC1− bovine LDH | YEp (2μm DNA) | This study |
| YIBL-2D | a/α pdc1/pdc1 | PPDC1− B. longum LDH | YIp (PDC1 locus) | This study |
| YIBO-7A | a/α pdc1/pdc1 | PPDC1− bovine LDH | YIp (PDC1 locus) | This study |
TABLE 2.
Specific activities of PDC and LDH
| Strain | Mean specific activity ± SD (mU/mg of protein)a
|
|
|---|---|---|
| PDC | LDH | |
| OC-2T | 225.0 ± 2.5 | ND |
| YEBL-8A | 87.0 ± 1.2 | 4.6 ± 3.3 |
| YEBO-1B | 88.2 ± 3.9 | 5.1 ± 4.8 |
| YIBL-2D | 84.0 ± 3.1 | 12.7 ± 2.2 |
| YIBO-7A | 89.7 ± 2.5 | 26.7 ± 3.1 |
These strains were cultivated for 24 h. Enzyme-specific activity in freshly prepared cell extracts was determined. The average and standard deviation were determined for three independent experiments. ND, not determined.
FIG. 3.
Comparison of fermentation with the S. cerevisiae YEBL-8A (A) and YEBO-1B (B) strains in YPD medium containing 100 g of glucose/liter and 50 g of CaCO3/liter. •, lactate; □, ethanol; ▵, glucose. Each strain was cultivated for 72 h under microanaerobic conditions at 30°C. The averages and standard deviations (error bars) for three independent experiments are presented.
Lactate production with PDC1 integration.
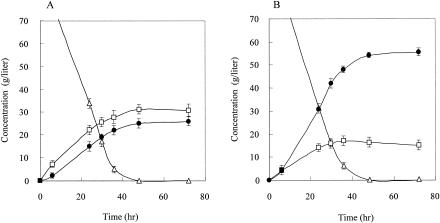
We constructed two other lactate-producing yeast recombinants, YIBL-2D (B. longum LDH) and YIBO-7A (bovine LDH), with integration in the PDC1 locus (Table 1). For the initial physiological characterization, the growth rates of these PDC1-disrupted recombinants were determined in shake flask cultures on YPD medium. On analysis of the absorbency at an optical density at 600 nm, both transgenic strains were found to grow exponentially, with a growth rate of 1.73/h, while that of OC-2T was 1.85/h. Compared to the host strain, suppression of the growth rate was not observed, although the PDC1 gene was disrupted. The LDH-specific activities of both YIBL-2D and YIBO-7A were clearly higher than those of the transgenic strains including a multicopy plasmid, although these recombinants have only two copies of the LDH genes (Table 2). The PDC-specific activities of the four recombinant strains were one-third of that of the OC-2T host strain and seemed to be the same. The YIBO-7A strain expressing bovine LDH under the control of the PDC1 promoter was observed to produce both lactate (55.6 g/liter) and ethanol (16.9 g/liter), with up to 62.2% of the glucose being transformed into lactic acid (Fig. 4B). YIBL-2D, the other strain which expresses the B. longum l-LDH gene, produced 25.7 g of l-lactate/liter and 31.1 g of ethanol/liter (Fig. 4A). Two genome integration recombinants, YIBL-2D and YIBO-7A, exhibited high levels of production of lactate compared with those of the YEBO-1B and YEBL-8A strains including a multicopy plasmid, although the genome-integrated strains maintained only two LDH genes. However, the yield of lactate with YEBO-7A expressing bovine LDH was almost twice that with YEBO-1B, and an effect of bovine LDH and B. longum LDH was observed clearly for the two genome-integrated strains. This difference in production on account of the different LDH genes was not observed for the multicopy strain.
FIG. 4.
Comparison of fermentation with the S. cerevisiae YIBL-2D (A) and YIBO-7A (B) strains in YPD medium containing 100 g of glucose/liter and 50 g of CaCO3/liter. •, lactate; □, ethanol; ▵, glucose. Each strain was cultivated for 72 h under microanaerobic conditions at 30°C. The averages and standard deviations (error bars) for three independent experiments are presented.
Lactic acid production under nonneutralizing conditions.
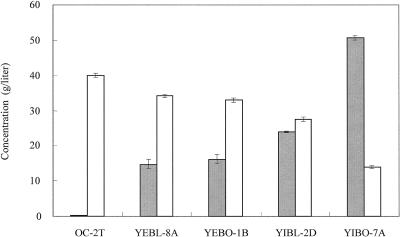
The production of lactic acid was examined by cultivation under nonneutralizing conditions without CaCO3. The accumulations of lactic acid and ethanol are shown in Fig. 5. The YIBO-7A strain, which had integrated the bovine LDH gene, gave yields of 50.2 g of lactic acid/liter and 16.7 g of ethanol/liter. The glucose was completely consumed in 72 h in the case of neutralizing conditions. The final pH was 2.8. The effect of changing the initial glucose concentration from 100 to 150 g/liter on the yield of the lactic acid production was examined. However, as judged by fermentation analysis, under the nonneutralizing conditions, an improvement of the lactic acid production was not observed; it did not reach 50 g/liter or more (data not shown).
FIG. 5.
Accumulations of lactic acid (gray bars) and ethanol (white bars) by S. cerevisiae recombinants in YPD medium containing 100 g of glucose/liter but without CaCO3. Each strain was cultivated for 72 h under microanaerobic conditions at 30°C. The glucose had been completely consumed after 72 h. The averages and standard deviations (error bars) for three independent experiments are presented.
DISCUSSION
In this study, we developed a recombinant yeast exhibiting efficient lactate production by substituting the coding region of PDC1 on chromosome XII for that of LDH through homologous recombination (Fig. 2). This method allowed robust gene expression under the control of the native PDC1 promoter. Steady lactate production was also maintained with the recombinant strain that was subcultured two times in nonselective medium (data not shown). For LDH expression in yeast, a YEp multicopy plasmid is generally used, but remarkable improvement of lactate production was not observed in this study (Table 3). In the case of multicopy strains, it was expected that unstable LDH expression would decrease the number of intracellular plasmids in nonselective medium. However, genome integration does not affect the number of LDH genes during fermentation; therefore, steady expression according to the promoter occurs. The comparison with transgenic yeast is shown in Table 4. In the batch culture condition, it can be confirmed that the strain of this report shows a high yield of lactic acid. A transgenic strain in which the Lactobacillus plantarum LDH gene was integrated into the genome was developed recently by Colombié et al.; this recombinant yields about 58 g of lactate/liter (6). The ADH1 promoter was used for the expression of the heterogeneous gene. In this study, we used the native PDC1 promoter on chromosome XII. PDC1 encoded pyruvate decarboxylase, which converts pyruvic acid to acetaldehyde and plays an important role in the production of ethanol. In general, host strain OC-2T was able to produce a high level of ethanol, even with a high glucose concentration; besides, only PDC1 usually worked for the production of ethanol. The choice to use this promoter was based on the fact that the expression of PDC1 is strongly induced by glucose (20), and glucose-responding elements in S. cerevisiae have already been reported (5, 21). It is advantageous to use the PDC1 promoter for fermentation with a high initial glucose level. Similarly, Bianchi et al. used KlPDC1 promoter for bovine LDH expression. Transgenic K. lactis strains showed 60 g of lactic acid/liter under the fed-batch condition, but it expended 500 h (28). Our S. cerevisiae strain disrupted only PDC1 and not PDC5, and genome-integrated LDH was regulated under the control of the native PDC1 promoter.
TABLE 3.
l-Lactic acid and ethanol productions from wild-type and transgenic strains during batch growth on 10% (wt/vol) glucose-based YPD medium
| Strain | Vector | LDH gene type | Neutralization
|
Yield of lactic acidb (%) | Nonneutralization
|
Yield of lactic acid (%) | ||
|---|---|---|---|---|---|---|---|---|
| Lactate (g/liter) | EtOH (g/liter)a | Lactic acid (g/liter) | EtOH (g/liter) | |||||
| OC-2T | 40.8 | 40.5 | ||||||
| YEBL-8A | YEp | B. longum | 14.1 | 33.9 | 17.2 | 14.7 | 34.8 | 17.6 |
| YEO-1B | YEp | Bovine | 16.3 | 34.3 | 19.2 | 16.1 | 33.1 | 19.6 |
| YIBL-2D | YIp | B. longum | 25.7 | 31.1 | 29.5 | 24.0 | 27.6 | 30.3 |
| YIBO-7A | YIp | Bovine | 55.6 | 16.9 | 62.2 | 50.6 | 13.8 | 64.7 |
EtOH, ethanol.
Yields are expressed as grams of lactate produced per liter divided by grams of glucose consumed per liter.
TABLE 4.
Study of l-lactic acid production by transgenic yeast
| Host | Vector | Promoter | LDH gene type | Maximum production (g/liter) | Yield of lactate (%) | Reference or source |
|---|---|---|---|---|---|---|
| Batch culture | ||||||
| S. cerevisiae | YEp | ADH1 | Lactobacillus casei | 12.0 | 24.0 | 7 |
| S. cerevisiae (pdc1, pdc5, pdc6) | YEp | ADH1 | Bovine | 7.9 | 26.3 | 1 |
| S. cerevisiae (wine yeast) | YEp | ADH1 | Lactobacillus casei | 8.6 | 4.3 | 8 |
| S. cerevisiae (pdc1 and adh1) | YEp | ADH1 | Rhizopus oryza | 38.0 | 41.3 | 35 |
| S. cerevisiae | YIp | ADH1 | L. plantarum | 58.0 | 29.0 | 6 |
| S. cerevisiae (wine yeast) | YIp | PDC1 | Bovine | 55.6 | 62.2 | This study |
| Fed-batch culture | ||||||
| S. cerevisiae | YEp | ADH1 | Bovine | 20.0 | 27 | |
| K. lactis (klpdc1) | YRp | KLPDC1 | Bovine | 26.7 | 3 | |
| K. lactis (klpdc1 and klpda1) | YRp | KLPDC1 | Bovine | 60.0 | 28 |
For batch culture, the yields are expressed as grams of lactate produced per liter divided by grams of glucose consumed per liter.
In the experiment using B. longum and bovine LDH, an improvement in lactate production was observed in the case of the integrated bovine LDH recombinant; however, a remarkable change was not observed in the case of a YEp-based multicopy plasmid (Table 4). The two LDHs were found to differ in amino acid level (40.3% identity; the calculation was performed with genetic analysis software GENETYX version 7.0.2). In order to determine the substrate affinity for pyruvic acid, bovine LDH was examined (Km, 0.13 mM; Vmax, 3,333 U/mg of protein) (data not shown). Also, the Km value for pyruvate of B. longum LDH has already been reported (Km, 1.05 mM; Vmax, 1,670 U/mg of protein) (19). The two LDHs are different in substrate affinities and enzyme activity levels. Because steady gene expression can be expected in the genome-integrated strain compared with a recombinant constructed with the YEp vector, it is considered that the differences in enzyme characteristics between the two LDHs were reflected by the lactic acid production. The combination of bovine LDH and the native PDC1 promoter on the genome is important for the efficient production of lactic acid.
The maximal concentration of lactate with YIBO-7A, which expresses bovine LDH under the control of the PDC1 promoter, was 58.8 g/liter under neutralizing conditions (Table 4). This recombinant, including two copies of the LDH gene on the genome, showed higher production than the transgenic strain, which had integrated one copy of the LDH gene on the genome (data not shown). It will be possible to improve the lactate production by increasing the number of LDH genes through genome integration. An analysis of a recombinant with an increased copy number of LDH is described elsewhere (31). On the other hand, if lactic acid is obtained directly under nonneutralizing conditions without a desalination process, lowering the cost on a manufacturing scale is possible. It has been pointed out that for a conventional method involving lactic acid bacteria, the process of desalination of lactate is a factor that increases the cost. In the case of lactic acid bacteria, it has been reported that the use of the genome-shuffling method improves the low pH tolerance (26). S. cerevisiae is well known to grow and survive at low pHs compared with lactic acid bacteria. Lactic acid production under nonneutralizing conditions was attempted by using a transgenic yeast (1, 7, 28, 35), but this production has not been reported so far. The present strain, which expresses bovine LDH under the control of the PDC1 promoter, exhibited high lactic acid production (50.2 g/liter) without pH control (Table 4). This result might lead to lactic acid fermentation that does not need the desalination process. But the level of lactic acid production did not become 50 g/liter or more. The final pH was 2.8, and it was expected that the low pH suppressed the lactic acid production. To improve the production under nonneutralizing conditions, it is important to make more progress regarding the low pH tolerance of intracellular LDH or host cells. S. cerevisiae is a suitable microorganism for an industrial scale. This strategy could be useful for applications for lactic acid production other than the use of lactic acid bacteria.
Acknowledgments
We thank Masana Hirai, Toru Onishi, Osamu Saotome, and Noriko Yasutani for valuable discussions and Takao Imaeda and Chikara Miyazaki for the synthesis of an l-LDH gene. We also thank Rie Yamaguchi, Miyoko Imoto, Wakana Takase, Junko Akimoto, and Keiko Uemura for technical assistance.
REFERENCES
- 1.Adachi, E., M. Torigoe, S. Sugiyama, J. Nikawa, and K. Shimizu. 1998. Modification of metabolic pathways of Saccharomyces cerevisiae by the expression of lactate dehydrogenase and deletion of pyruvate decarboxylase genes for the lactic acid fermentation at low pH value. J. Ferment. Bioeng. 86:284-289. [Google Scholar]
- 2.Bianchi, M. M., L. Tizzani, M. Destruelle, L. Frontali, and M. Wesolowski-Louvel. 1996. The “petite-negative” yeast Kluyveromyces lactis has a single gene expression pyruvate decarboxylase activity. Mol. Microbiol. 19:27-36. [DOI] [PubMed] [Google Scholar]
- 3.Bianchi, M. M., L. Brambilla, F. Protani, C. L. Liu, J. Lievense, and D. Porro. 2001. Efficient homolactic fermentation by Kluyveromyces lactis strains defective in pyruvate utilization and transformed with the heterologous LDH gene. Appl. Environ. Microbiol. 67:5621-5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brambilla, L., D. Bolzani, C. Compagno, V. Carrera, J. P. van Dijken, J. T. Pronk, B. M. Ranzi, L. Alberghina, and D. Porro. 1999. NADH reoxidation does not control glycolytic flux during exposure of respiring Saccharomyces cerevisiae cultures to glucose excess. FEMS Microbiol. Lett. 171:133-140. [DOI] [PubMed] [Google Scholar]
- 5.Butler, G., and D. J. McConnell. 1988. Identification of an upstream activation site in the pyruvate decarboxylase structural gene (PDC1) of Saccharomyces cerevisiae. Curr. Genet. 14:405-412. [DOI] [PubMed] [Google Scholar]
- 6.Colombié, S., S. Dequin, and J. M. Sablayrolles. 2003. Control of lactate production by Saccharomyces cerevisiae expressing a bacterial LDH gene. Enzyme Microb. Technol. 33:38-46. [Google Scholar]
- 7.Dequin, S., and P. Barre. 1994. Mixed lactic-alcoholic fermentation by Saccharomyces cerevisiae expressing the Lactobacillus casei L(+)-LDH. Bio/Technology 12:173-177. [DOI] [PubMed] [Google Scholar]
- 8.Dequin, S., E. Baptista, and P. Barre. 1999. Acidification of grape mutants by Saccharomyces cerevisiae wine yeast strains genetically engineered to produce lactic acid. Am. J. Enol. Vitic. 50:45-50. [Google Scholar]
- 9.Flikweert, M. T., L. Van Der Zanden, W. M. Janssen, H. Y. Steensma, J. P. Van Dijken, and J. T. Pronk. 1996. Pyruvate decarboxylase: an indispensable enzyme for growth of Saccharomyces cerevisiae on glucose. Yeast 12:247-257. [DOI] [PubMed] [Google Scholar]
- 10.Flikweert, M. T., M. Swaaf, J. P. Van Dijken, and J. T. Pronk. 1999. Growth requirements of pyruvate-decarboxylase-negative Saccharomyces cerevisiae. FEMS Microbiol. Lett. 174:73-79. [DOI] [PubMed] [Google Scholar]
- 11.Gatignol, A., M. Baron, and G. Tiraby. 1987. Phleomycin resistance encoded by ble gene from transposon Tn5 as a dominant selective marker in Saccharomyces cerevisiae. Mol. Gen. Genet. 207:342-348. [DOI] [PubMed] [Google Scholar]
- 12.Hester, A. 2000. IB market forecast. Ind. Bioprocess 22:4-5. [Google Scholar]
- 13.Hofvendahl, K., and B. Hahn-Hagerdal. 2000. Factors affecting the fermentative lactic acid production from renewable resources (1). Enzyme Microb. Technol. 26:87-107. [DOI] [PubMed] [Google Scholar]
- 14.Hohmann, S., and H. Cederberg. 1990. Autoregulation may control the expression of yeast pyruvate decarboxylase structural genes PDC1 and PDC5. Eur. J. Biochem. 188:615-621. [DOI] [PubMed] [Google Scholar]
- 15.Hohmann, S. 1991. Characterization of PDC6, a third structural gene for pyruvate decarboxylase in Saccharomyces cerevisiae. J. Bacteriol. 173:7963-7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horton, R. M., H. D. Hunt, S. N. Ho, J. K. Pullen, and L. R. Pease. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:61-68. [DOI] [PubMed] [Google Scholar]
- 17.Ishiguro, N., S. Osame, R. Kagiya, S. Ichijo, and M. Shinagawa. 1990. Primary structure of bovine lactate dehydrogenase-A isozyme and its synthesis in Escherichia coli. Gene 91:281-285. [DOI] [PubMed] [Google Scholar]
- 18.Ito, H., Y. Fukuda, K. Murata, and H. Kimura. 1983. Transformation of intact yeast cells with treated with alkalications. J. Bacteriol. 153:163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwata, S., T. Minowa, H. Saeki, and T. Ohta. 1989. Amino acid residues in the allosteric site of l-lactate dehydrogenase from Bifidobacterium longum. Agric. Biol. Chem. 53:3365-3366. [Google Scholar]
- 20.Kellermann, E., and C. P. Hollenberg. 1988. The glucose- and ethanol-dependent regulation of PDC1 from Saccharomyces cerevisiae are controlled by two distinct promoter regions. Curr. Genet. 14:337-344. [DOI] [PubMed] [Google Scholar]
- 21.Liesen, T., C. P. Hollenberg, and J. J. Heinisch. 1996. ERA, a novel cis-acting element required for autoregulation and ethanol repression of PDC1 transcription in Saccharomyces cerevisiae. Mol. Microbiol. 21:621-632. [DOI] [PubMed] [Google Scholar]
- 22.Marshall, V. M. 1987. Lactic acid bacteria: starters for flavor. FEMS Microbiol. Rev. 46:327-336. [Google Scholar]
- 23.McLaren, J., and D. Faulkner. 1999. The technology roadmap for plant/crop-based renewable resources 2020. U.S. Department of Energy. [Online] http://www.eere.energy.gov/biomass/pdfs/ag_vision.pdf.
- 24.Minowa, T., S. Iwata, H. Sakai, and T. Ohata. 1989. Sequence and characteristics of the Bifidobacterium longum gene encoding l-lactate dehydrogenase and primary structure of the enzyme; a new feature of the allosteric site. Gene 85:161-168. [DOI] [PubMed] [Google Scholar]
- 25.Ozeki, E. 1996. Characteristics of poly (l-lactide) as biodegradable plastics. Shimadzu Rev. 53:1-8. [Google Scholar]
- 26.Patnaik, R., S. Louie, V. Gavrilovic, K. Perry, W. P. C. Stemmer, C. M. Ryan, and S. del Cardayré. 2002. Genome shuffling of Lactobacillus for improved acid tolerance. Nat. Biotechnol. 20:707-712. [DOI] [PubMed] [Google Scholar]
- 27.Porro, D., L. Barmbilla, B. M. Ranzi, E. Martegani, and L. Alberghina. 1995. Development of metabolically engineered Saccharomyces cerevisiae cells for the production of lactic acid. Biotechnol. Prog. 11:294-298. [DOI] [PubMed] [Google Scholar]
- 28.Porro, D., M. M. Bianchi, L. Brambilla, R. Menghini, D. Bolzani, V. Carrera, J. Lievense, C. L. Liu, B. M. Ranzi, L. Frontali, and L. Alberghina. 1999. Replacement of a metabolic pathway for large scale production of lactic acid from engineered yeasts. Appl. Environ. Microbiol. 65:4211-4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pronk, J. T., H. Yde Steensma, and J. P. van Dijiken. 1996. Pyruvate metabolism in Saccharomyces cerevisiae. Yeast 12:1607-1633. [DOI] [PubMed] [Google Scholar]
- 30.Saitoh, S., Y. Mieno, T. Nagashima, C. Kumagai, and K. Kitamoto. 1996. Breeding of a new type of baker's yeast by δ-integration for overproduction of glucoamylase using a homothallic yeast. J. Ferment. Bioeng. 81:98-103. [Google Scholar]
- 31.Saitoh, S., N. Ishida, T. Onishi, K. Tokuhiro, E. Nagamori, K. Kitamoto, and H. Takahashi. Genetically engineered wine yeast produced a high concentration of l-lactic acid of extremely high optical purity. Appl. Environ. Microbiol., in press. [DOI] [PMC free article] [PubMed]
- 32.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 33.Schaalf, I., J. B. A. Green, D. Gozalbo, and S. Hohmann. 1989. A deletion of the PDC1 gene coding for pyruvate decarboxylase of yeast causes a different phenotype than previously isolated point mutations. Curr. Genet. 15:75-81. [DOI] [PubMed] [Google Scholar]
- 34.Schmitt, H. D., M. Ciriacy, and F. K. Zimmermann. 1983. The synthesis of yeast pyruvate decarboxylase is regulated by large variations in the messenger RNA level. Mol. Gen. Genet. 192:247-252. [DOI] [PubMed] [Google Scholar]
- 35.Skory, C. D. 2003. Lactic acid production by Saccharomyces cerevisiae expressing a Rhizopus oryzae lactate dehydrogenase gene. J. Ind. Microbiol. Biotechnol. 67:22-27. [DOI] [PubMed] [Google Scholar]