Abstract
Serotonin (5-hydroxytryptamine, 5-HT) signaling plays an important role in dynamic control of peripheral and central nervous system physiology, with altered 5-HT homeostasis implicated in a significant number of disorders, ranging from pulmonary, bowel, and metabolic disease to depression, obsessive-compulsive disorder, and autism spectrum disorder (ASD). The presynaptic, 5-HT transporter (SERT) has a well-established role in regulating 5-HT signaling and is a target of widely prescribed psychotherapeutics, the 5-HT selective reuptake inhibitors (SSRIs). Although SSRI therapy provides symptom relief for many suffering from mood and anxiety disorders, response to these medications is slow (weeks), and too many receive modest or no benefit. At present, all prescribed SSRIs act as competitive SERT antagonists. Although non-serotonergic therapeutics for mood disorders deserve aggressive investigation, the development of agents that target SERT regulatory pathways have yet to be considered for their possible utility and may possibly offer improved efficacy and more rapid onset. Here, we focus attention on a significant body of evidence that SERT transport activity can be rapidly elevated by protein kinase G (PKG) and p38α mitogen activated protein kinase (MAPK) linked pathways, mechanisms that are impacted by disease-associated genetic variation. Here, we provide a brief overview of kinase-linked, posttranslational regulation of SERT, with a particular focus on evidence from pharmacological and genetic studies that the transporter's regulation by PKG/p38α MAPK associated pathways offers an opportunity to more subtly adjust, rather than eliminate, SERT function as a therapeutic strategy.
Keywords: Serotonin transporter, Regulation, Protein kinase G, p38α MAPK, Inflammation, IL-1β
1. Introduction
Serotonin (5-hydroxytryptamine, 5-HT) can be found throughout the body, including platelets, gut, placenta, and central nervous system (CNS), and thus contributes broadly to both systemic physiology and behavior (for an extended review on 5-HT, please see (The Serotonin System - History, 2019)). Given the broad actions of 5-HT, it comes as no surprise that perturbed 5-HT signaling has been linked to disorders of gastrointestinal, pulmonary, and cardiovascular function, as well as alterations in mood, motivation, anxiety, social behavior, and sleep. In the nervous system, 5-HT is released following vesicular fusion mechanisms. Subsequently, 5-HT is inactivated efficiently by the high-affinity, presynaptic 5-HT transporter (SERT). For more than 30 years, SERT has been targeted intentionally for the treatment of mood and compulsive disorders. Indeed, 5-HT selective reuptake inhibitors (SSRIs), whose members include fluoxetine (Prozac®), escitalopram (Lexapro®), and paroxetine (Paxil®), are among the most widely prescribed psychotherapeutics. These agents block SERT transport function by competitively inhibiting 5-HT binding to SERT, with the result being an increased availability of the neurotransmitter at somatodendritic, presynaptic, and postsynaptic 5-HT receptors. Evidence indicates that SSRIs achieve therapeutic effects only after significant SERT blockade (∼80% or more) attesting to the efficiency of the transporter in clearing 5-HT and/or the ability of compensatory mechanisms to offset the effects of reduced extracellular 5-HT levels (Meyer et al., 2004). Although SSRIs have brought relief from mood disorders to millions worldwide, side effects are evident, many do not achieve remission, and for those that do, significant delays in therapeutic onset occur. These issues have encouraged our team to seek to understand endogenous mechanisms that finely tune SERT activity, with the hope that these pathways might be targeted for improved efficacy.
High resolution structural studies, along with modeling of transport dynamics, has provided a mechanistic understanding of 5-HT transport and the competitive basis for SERT antagonism by SSRIs (Rudnick and Sandtner, 2019). Transport of 5-HT by SERT begins with the binding of extracellular Na+ and Cl− to the transporter when the transporter is in an “outward facing conformation”, followed by the binding of cationic 5-HT. The collective energy released in these binding reactions triggers a series of conformational changes that occludes 5-HT from both the extracellular and intracellular space. Subsequently, SERT moves to an “inward facing conformation”, which leads to a reduction in the affinity of substrates, promoting the release of 5-HT into the cytoplasm. Structural analysis and computational modeling studies of SERT in different conformations supports a “rocking bundle” mechanism of 5-HT transport, whereby mobile helices shift in relation to adjoining stable helices to allow the 5-HT binding site to move from outward to inward facing, leading to, sequentially, the binding of extracellular 5-HT and the release of 5-HT to the cytoplasm (Forrest et al., 2008). In the inward facing conformation, SERT binds intracellular K+ which promotes a shift of SERT back to an outward facing position where the ion is released to the extracellular space, and another cycle of 5-HT binding and transport can begin. As 5-HT is a cation at physiological pH, inward transport of one 5-HT+, one Na+, one Cl−, and one K+ outward means that no net charge moves across one full transport cycle, and thus membrane potential changes are predicted to have little to no effect on the rate of 5-HT transport. But, as noted below, biochemical mechanisms exist that can adjust SERT surface expression and the intrinsic cycle rate (catalytic activity). Evidence from in vivo genetic and biochemical studies indicates that both facets of SERT regulation are behaviorally significant and relevant to our understanding of serotonergic dysfunction, possibly representing novel opportunities for therapeutic “tuning” of 5-HT transport.
2. The ins and outs of SERT regulation
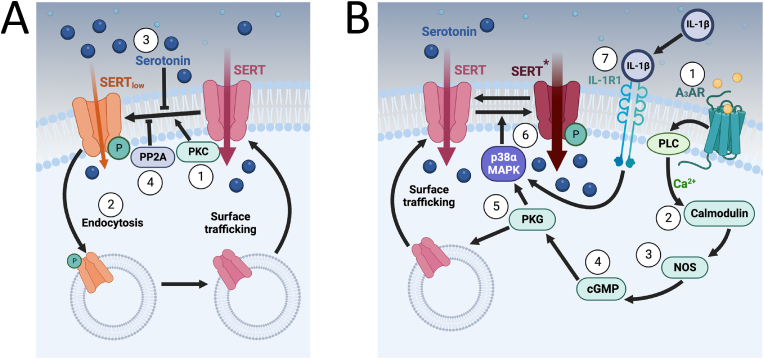
With the availability of cloned SERT cDNAs opportunities arose to determine whether the protein is subject to regulation, initially pursued using heterologous expression strategies (Blakely et al., 1991; Hoffman et al., 1991). Protein phosphorylation is a well-known, reversible mechanism for changing the activity and trafficking of membrane proteins including receptors, channels, and transporters. In this regard, an initial inspection of SERT sequence revealed the cytoplasmic N- and C-termini, as well as intracellular loops that link the transporter's twelve transmembrane domains, to possess a multitude of potential serine, threonine, and tyrosine phosphorylation sites (Bermingham and Blakely, 2016). As of this writing, multiple kinases have been identified as regulators of SERT, with isoforms of protein kinase C (PKC), protein kinase G (PKG), and p38 mitogen activated protein kinase (p38 MAPK) the most intensively studied (Fig. 1A and B). The first evidence that one or more of these sites might be utilized came from studies by Qian and colleagues (Qian et al., 1997) who found that β-phorbol 12-myristate 13-acetate (β-PMA) and β-phorbol 12,13-dibutyrate (β-PDBu), activators of multiple forms of PKC, produce a rapid (minutes) reduction in 5-HT transport activity in human SERT cDNA transfected HEK-293 cells. Subsequently, Ramamoorthy et al. demonstrated that PKC-dependent reductions in 5-HT uptake and transporter surface expression are paralleled temporally by elevations in SERT phosphorylation (Ramamoorthy et al., 1998). Notably, Ramamoorthy and Blakely demonstrated that the ability of PKC activation to phosphorylate SERT, to induce SERT surface endocytosis, and to diminish 5-HT uptake depends on whether SERT molecules are in the act of transporting substrates at the time of PKC activation, suggesting a “use it or lose it” process of SERT regulation (Ramamoorthy and Blakely, 1999). One mechanism that could support activity-dependent PKC regulation is the differential exposure of SERT phosphorylation sites across the 5-HT transport cycle. Alternatively, conformational transitions associated with 5-HT transport may change the probability that sites phosphorylated following PKC activation may become more readily dephosphorylated (Ramamoorthy and Blakely, 1999). Although not mutually exclusive, Bauman and colleagues provided support for the latter mechanism in demonstrating a PKC- and 5-HT-dependent physical association between SERT and the catalytic subunit of the Ser/Thr phosphatase PP2A (PP2Ac) (Bauman et al., 2000). One could envision that small molecule inhibitors of PP2Ac/SERT associations could lead to a promotion of SERT endocytosis, boosting extracellular 5-HT levels, and with specificity achieved by inhibition of the unique intersection of the as yet undefined PP2Ac/SERT binding site.
Fig. 1.
Regulation of SERT trafficking and intrinsic activity. 1A) ① Phosphorylation by PKC causes SERT to enter a “low” functioning state (SERTlow). ② SERTlow is targeted for endocytosis. ③ The active transport of 5-HT precludes the ability of PKC to cause the endocytosis of SERT (Forrest et al., 2008). Phosphatase activity (PP2A) also inhibit this process. 1B) ① Activation of the A3AR spurs PLC-mediated Ca2+ availability (likely from internal stores). ②The available Ca2+ leads to calmodulin activation, ③subsequent NOS activation, and production of ④ cGMP. ⑤ The increased cGMP activates PKG which can stimulate SERT surface trafficking as well as ⑥ p38α MAPK activation and subsequent phosphorylation of SERT (SERT*) which confers enhanced intrinsic activity. ⑦ Other pathways, such as those triggered by IL-1R1 activation by IL-1β, can also activate p38α MAPK to regulate SERT. Abbreviations: NOS, nitric oxide synthase); cGMP, cyclic guanine monophosphate; PKG, protein kinase G; MAPK, mitogen activated protein kinase; IL-1β, interleukin-1β, IL-1R1, interleukin-1 receptor type 1; PKC, protein kinase C, PP2A, protein phosphatase 2A; PLC, phospholipase C. All figures produced with BioRender.
Although studies in transfected cells clearly revealed SERT to be regulated through phosphorylation, whether these mechanisms are relevant for natively expressed SERT remained to be demonstrated. In an important study, Jayanthi and colleagues (Jayanthi et al., 2005) demonstrated that platelet SERT is phosphorylated and endocytosed following PKC activation as seen with transfected SERT, but with an important wrinkle. These investigators followed the process of platelet SERT endocytosis and changes in 5-HT transport kinetics over time and demonstrated that at 5 min post PKC activation, SERT activity is significantly reduced, with an increase in KM, often interpreted as lower apparent 5-HT affinity, and a reduction in transport VMAX. Surprisingly, no change in SERT surface levels occurred, suggesting that a portion of SERT had become functionally inactivated, with the remaining transporters shifted to a conformation that may exhibit a lower affinity for 5-HT. In contrast, when SERT was assessed at 30 min post PKC activation, the transporter demonstrated a reduction in surface expression, paralleled by a reduced VMAX and with no change in 5-HT KM. Together, these data suggest that following PKC activation, platelet SERT rapidly becomes catalytically inactivated followed later by transporter endocytosis, the first evidence of SERT catalytic activity as a modifiable component of transporter regulation (Fig. 1A, SERTlow). Notably, the group also showed that 5 min after PKC activation, SERT is phosphorylated at Ser residues, whereas after 30 min of PKC activation, SERT demonstrated both Ser and Thr phosphorylation. As of yet, the sites differentially phosphorylated in association with reduced transporter catalytic function or transporter endocytosis following PKC activation have yet to be defined, although Serine 277 (Ser277) and/or Threonine 276 (Thr 276) in intracellular loop 2 (IL2) are possible given the implication of analogous sites in the closely related norepinephrine transporter (NET) (Jayanthi et al., 2006).
3. Receptor regulation of 5-HT uptake exposes both trafficking-dependent and independent mechanisms of SERT regulation: contrasting contributions of PKG and p38α MAPK
Although the above studies clearly demonstrated a capacity of SERT for kinase-mediated posttranslational regulation, missing was an understanding as to how such mechanisms are normally initiated. They also revealed mechanisms by which SERT function can be rapidly diminished, leaving open the possibility that opposing mechanisms exist endogenously to increase SERT surface expression and/or catalytic activity. Evidence that the latter mechanisms may indeed exist arose first from studies by Miller and Hoffman who demonstrated that 5-HT transport activity was increased in RBL-2H3 cells following activation of cell surface adenosine receptors, with pharmacological approaches suggesting involvement of an A3 receptor subtype (A3AR) (Miller and Hoffman, 1994). These investigators also implicated nitric oxide synthetase (NOS) and a cGMP dependent pathway in the process. We further delineated this pathway, finding that A3AR activation leads to a NOS and cGMP dependent activation of PKG, leading to an increase in SERT trafficking to the cell surface (Zhu et al., 2004, 2005). Surprisingly, we also found that an elevation in surface trafficking induced by activated PKG does not lead to elevated SERT activity unless PKG also activates p38 MAPK, revealing the first pathway by which SERT catalytic function can be elevated (Fig. 1B). In our RBL-2H3 studies, we found that siRNAs specific to the α form of p38 MAPK could block anisomycin stimulation of SERT (Zhu et al., 2004), leading us to suspect that this isoform deserved prime attention in relation to SERT regulation. As such, our subsequent text describes and depicts p38α MAPK as the key SERT regulatory isoform. Interestingly, the ability of SERT catalytic activity to increase following p38α MAPK activation is shared with the norepinephrine transporter (NET), but not the dopamine transporter (DAT) (Zhu et al., 2005), possibly providing clues to conserved sites where such regulation occurs. Together, findings PKC and p38α MAPK reveal that SERT has the capacity to move between states of low activity (SERTlow), basal activity (SERT), and high activity (SERT*) that are temporally linked to trafficking away from or toward the cell surface (Fig. 1A and B).
Being most interested in the connection of SERT regulation to neurobehavioral disorders and their treatment, we extended our RBL-2H3 cell culture studies to native mouse brain preparations (Zhu et al., 2007). Here, again we found that A3AR activation rapidly triggered an increase in SERT activity, linked to a reduction in KM, effects that are absent from samples prepared from A3AR KO mice. Also, pretreatment with PKG or α/β selective p38 MAPK antagonists blocked the ability of A3AR activation to increase SERT activity. Interestingly, A3AR stimulated SERT activity was observed in synaptosomes prepared from the cortex, hippocampus, and midbrain, but not the striatum, indicating a unique region-specificity to SERT regulation that remains an important area of investigation in future studies. Subsequently, we established that SERT and A3ARs colocalized in midbrain serotonergic neurons and were found to be physically associated in co-transfected cells, with interactions regulated by A3AR activation (Zhu et al., 2011). Importantly, a translational relevance of A3AR regulation of SERT was suggested by our finding that an autism spectrum disorder (ASD)-associated hyperfunctional A3AR variant (L90V) disrupts SERT:A3AR interactions as well as SERT regulation (Zhu et al., 2011; Campbell et al., 2013).
Early on, we suspected that activation of p38α MAPK pathways to regulate SERT activity was likely to extend beyond A3ARs. A multitude of stimuli are known to activate p38α MAPKs, in a PKG-independent manner, such as oxidative stress, and we found these to also lead to elevated SERT activity in vitro (Zhu et al., 2005). To further probe this idea, two major inflammatory signals were assessed for their ability to regulate SERT in cultured serotonergic RN46A cells, interleukin-1 beta (IL-1β) and tumor necrosis factor alpha (TNFα) (Zhu et al., 2006). Indeed, both cytokines demonstrated an ability to rapidly modulate SERT activity, though they did so in different ways. Whereas IL-1β rapidly stimulated SERT activity in a fully p38α MAPK-dependent manner, solely decreasing KM, stimulation by TNF-α was only partially p38α MAPK-dependent and displayed changes in both KM and transport VMAX. As to the in vivo significance of these findings, similar observations were found in synaptosomes collected from multiple brain regions (Zhu et al., 2005, 2006, 2010). Importantly, multiple studies using either in situ hybridization of IL-1R1 mRNA (Takahashi et al., 2022) or IL-1R1 transcriptional reporter transgenic mice (Liu et al., 2019), have now demonstrated significant expression of the major signaling receptor for IL-1β, IL-1R1, in serotoninergic neurons of the dorsal raphe. These studies also provide a signaling basis for findings showing that these neurons in brain slices can be inhibited by bath application of IL-1β (Liu et al., 2019). Together, these findings demonstrate a strong connection between inflammatory cytokine signaling and SERT regulation and suggest that IL-1β likely acts acutely to reduce serotonergic transmission (Fig. 3). At a molecular level, it might be envisioned that catalytic activation of SERT reflects a subtle change in kinetic steps along the 5-HT transport cycle. However, work by Chang and colleagues in the Rosenthal group (Chang et al., 2012) has shown that IL-1β/p38α MAPK induces rapid lateral movement in quantum dot labeled SERT proteins that remained within a membrane microdomain, often called a “lipid raft.” We speculate that these movements reflect an untethering of SERT from interacting proteins to facilitate the conformational dynamics that govern the rate of the transport cycle.
Fig. 3.
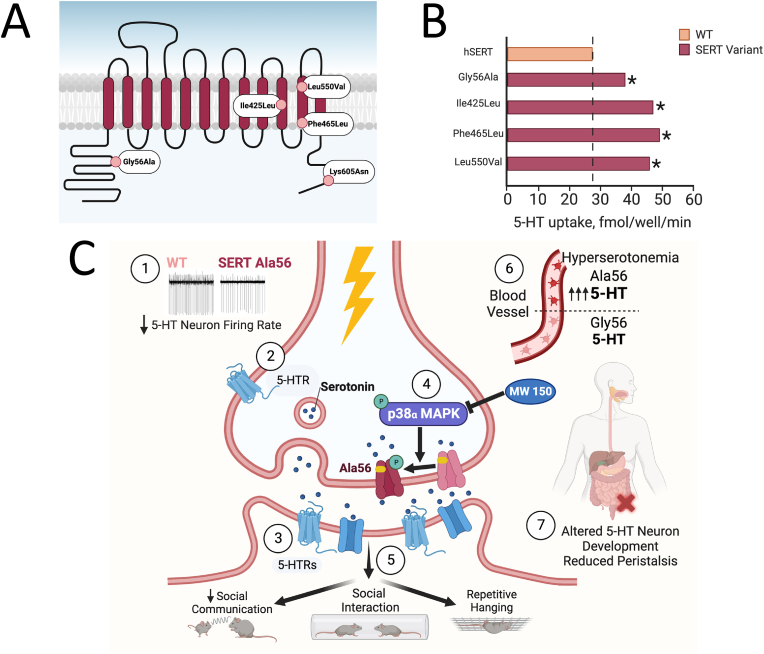
Multiple gain of function SERT mutations identified in autism spectrum disorder. A) The twelve transmembrane domains of SERT and cytoplasmic tails are illustrated to display the location of four mutations in the transporter found in individuals diagnosed with ASD (see main text). B) All four of these mutations were found to confer elevated 5-HT transport upon transfection into cells in culture or when assayed in native lymphoblasts from carriers. Although the Gly56Ala substitution did not reveal the largest change in SERT activity, it was the most common variant found, and does not induce elevated surface trafficking, indicative of catalytic activation. C) SERT Ala56 causes a decrease in basal firing rate of 5-HT neurons ①, along with increased sensitivity to 5-HT1A and 5HT2A receptors ②③. The increase in SERT activity due to the Ala56 mutation appears to be dependent on phosphorylation in a p38α MAPK-dependent manner ④. At baseline, the SERT Ala56 variant is phosphorylated to a higher level than WT and activation of p38α MAPK cannot increase it further. However, inhibition of p38α MAPK via MW150 and other inhibitors, brings the levels of SERT phosphorylation down to WT levels. Dysregulation in this manner leads to the manifestation of ASD-related behaviors, such as impaired social communication, social interaction, and repetitive behavior ⑤. The increased SERT activity in Ala56 causes an accumulation of 5-HT in platelet cells, which leads to hyperserotonemia ⑥. The SERT Ala56 mice show reduced intestinal motility, altered epithelial structure, hypo-innervation of the enteric nervous system, and decreased neuron number ⑦. Abbreviations: ASD, autism spectrum disorder.
4. Systemic inflammation regulates SERT activity and modulates behavior via IL-1R1 activation
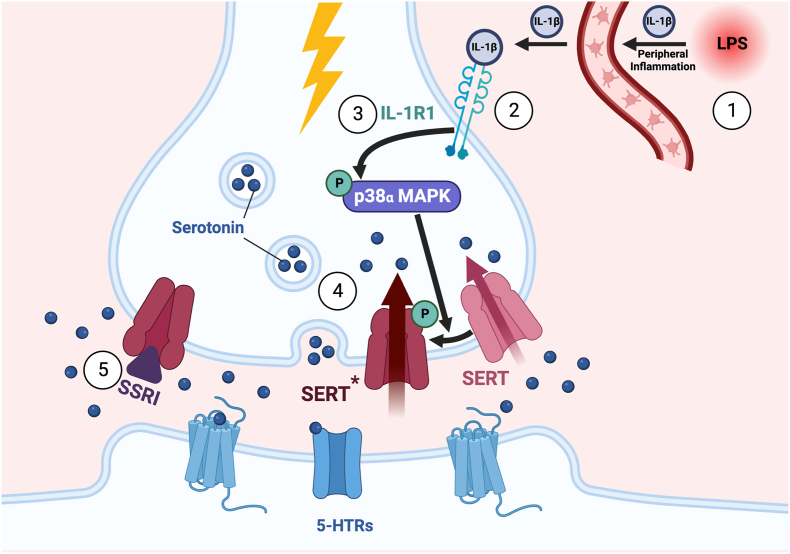
The interplay between immune and nervous systems exists in a delicate balance with disruptions to this equilibrium triggering the development of psychiatric conditions such as major depressive disorder (for review: ref Dantzer et al., 2010 (Dantzer et al., 2011)). Clinical studies examining patients diagnosed with depression have found elevated blood levels of proinflammatory cytokines including IL-1β and TNFα (Sluzewska et al., 1996). Moreover, peripheral immune activation in both animal models and humans leads to behavioral alterations characteristic of depression (Zhu et al., 2010; Pinto and Andrade, 2016). Proinflammatory cytokines rapidly increase SERT activity, effects opposite to, and treatable with SSRIs (Fig. 2). To complement our ex vivo studies of cytokine regulation of SERT, we sought a paradigm that can mimic inflammatory states and increase CNS cytokine production in vivo under temporal control. Peripherally administered lipopolysaccharide (LPS) generates a “cytokine storm” which includes proinflammatory signals like IL-1β and TNFα, thus generating an immune stimulation more akin to infection. We found that i.p. administration of a low dose of LPS (0.2 mg/kg) that did not induce long-term sickness behavior rapidly (minutes) increased CNS 5-HT uptake ex vivo and in extracellular 5-HT clearance in vivo (Zhu et al., 2010). These changes were accompanied by despair-like behavior, as assessed in the tail suspension test (TST) and forced swim test (FST). IL-1R1 knockout mice demonstrated no LPS-mediated change in SERT activity, nor alterations in the TST and FST. Importantly, mice pretreated with a p38α/β MAPK inhibitor also showed no LPS-mediated increase in SERT activity (Zhu et al., 2010). As systemic drug administration and constitutive gene elimination present confounds as to the specific location of relevant p38α MAPK expression, we opted next to implement a conditional genetic approach. Consistent with our surmise, elimination of p38α MAPK specifically in serotonergic neurons eliminated the LPS-induced increase in SERT activity, as well as subsequent behavioral changes (Baganz et al., 2015). Together these studies reveal that induction of peripheral inflammation by LPS requires serotonergic IL-1R1, p38α MAPK, and SERT to produce despair behavior. Moreover, they provide support for the idea that components of the “cytokine storm” that reaches the brain following peripheral inflammation can communicate through neurotransmitter-specific brain circuits to modulate discrete behaviors. Although our emphasis here is on 5-HT releasing pathways, we must remember that these projections are themselves under local control by other signaling molecules, such as histamine, that rise extracellularly as a consequence of inflammation (Hersey et al., 2021).
Fig. 2.
Effect of peripheral inflammation as modeled with LPS on CNS SERT activity. ① Peripheral LPS triggers innate immune system activation, such as the production and secretion of IL-1β. This signal travels through the circulatory system and leads to system-wide inflammation. ② Inflammation is sensed by the brain, either by directly receiving the cytokines, or by more indirect stimulation. Inflammatory signals are then made directly in the brain, which leads to binding to their receptors. ③ Binding of IL-1β to its receptor activates an intracellular signaling cascade that phosphorylates and activates p38α MAPK. ④ Activated p38α MAPK is responsible for the phosphorylation of SERT and leads to a hypermorphic transporter (SERT*). ⑤ One way to combat this is the use of pharmacological agents known to directly inhibit SERT such as with SSRIs. Abbreviations: LPS, lipopolysaccharide; SSRI; selective 5-HT reuptake inhibitor.
5. ASD associated SERT coding variants: role for p38α MAPK in transporter hyperactivity
Multiple rare missense coding variants in the human SERT gene (SLC6A4) have been identified (Sutcliffe et al., 2005; Prasad et al., 2005), with several of these shown to impact SERT protein function, regulation, and to be associated with 5-HT linked neurobehavioral disorders such as OCD and depression (Di Bella et al., 1996; Ozaki et al., 2003). Prasad and colleagues screened a panel of naturally occurring SERT coding variants and identified multiple constitutively hyperfunctional variants (Prasad et al., 2005). Interestingly, the four SNPs found on intracellular portions of SERT (Thr4Ala, Gly56Ala, Lys605Asn, Pro621Ser) were the only variants screened that did not respond to PKG or p38α MAPK activation, suggesting that these cytoplasmic sites may contribute to regulation through their interactions with accessory proteins such as syntaxin 1A or PP2Ac (Bauman et al., 2000; Haase et al., 2001) (Fig. 3A). The SERT Gly56Ala variant is discussed more below due to its relatively simultaneous discovery as associated with ASD.
Contemporaneously with the studies of human SERT coding variants by Prasad and colleagues that were not archived based on a clinical diagnosis, Sutcliffe et al. (2005) identified a set of rare, human SERT coding variants, underlying a male-specific linkage signal for ASD. Surprisingly, each of these variants conferred enhanced 5-HT uptake (Fig. 3B) (Prasad et al., 2009). Observations from transfected cell studies revealed that three of the variants led to increased uptake due to elevated surface density, whereas one SERT variant (Ala56) conferred enhanced 5-HT uptake in transfected cells via an increase in SERT catalytic activity. To evaluate the impact of SERT Ala56 in vivo, Veenstra-VanderWeele and colleagues (Veenstra-VanderWeele et al., 2012) generated a knock-in mouse line that expresses the coding variant from the native mouse SERT gene. These investigators found that, at baseline, synaptosomes from Ala56 mice demonstrate hyperphosphorylation under basal conditions that can be blocked with a p38α/β MAPK antagonist. This suggests the variant's increased activity may arise from changes in conformation or protein interactions that enhance access to the kinase and/or that limits phosphatase interactions. Quinlan et al. (2019) would later demonstrate that indeed, the Ala56 variant shifts transporter conformation to one more biased toward the outward facing state, consistent with an elevated rate of 5-HT transport. Moreover, LC-MS/MS analysis of immunoprecipitates derived from the Ala56 knock-in mice revealed multiple changes in protein associations as compared to wildtype (WT) mice, including a scaffolding protein of the PP2A complex, though their functional significance remains to be directly demonstrated.
One of the more striking effects observed in SERT Ala56 mice was hyperserotonemia, or increased whole-blood 5-HT (Fig. 3C). Hyperserotonemia is one of the most consistent biomarkers seen in ASD cases, although it only occurs in approximately 30% of subjects (Mulder et al., 2004). Moreover, the SERT Ala56 mice demonstrate reduced colonic motility (Veenstra-VanderWeele et al., 2012) due to alterations in 5-HT dependent development of late born neural architecture (Margolis et al., 2016), consistent with the high comorbidity of ASD with gastrointestinal (GI) dysfunction. Interestingly, brain slices from SERT Ala56 mice indicate reduced basal firing of 5-HT that in vivo likely underlies altered 5-HT receptor sensitivity and impairments in social behavior and communication as well as the repetitive behaviors (Fig. 3C).
In an effort to further solidify the pathways responsible for the functional upregulation of SERT Ala56 in vivo, and to specify the site of SERT regulation by p38α MAPK, Robson et al. implemented a conditional genetic elimination strategy, deleting the kinase selectively from 5-HT neurons (Robson et al., 2018). In this background, the investigators demonstrated a normalization of both social behavior and gut motility. To move these efforts to a context that might offer possibilities for therapeutic intervention, the group also found that five days of treatment with the highly selective, brain penetrant p38α MAPK inhibitor, MW150 (Roy et al., 2019) was able to normalize a host of altered traits in the Ala56 mice, including both 5HT1A and 5HT2A dependent behaviors as well as social avoidance. In vivo chronoamperometry studies confirmed that MW150 normalized hippocampal 5-HT clearance rates, with western blots demonstrating a lack of changes in SERT protein, consistent with a failure to catalytically activate the transporter. Moreover, colonic function was normalized. Finally, O'Reilly and colleagues demonstrated that multi-site changes in gene expression arise in the context of social encounters in the SERT Ala56 mice (O'Reilly et al., 2020), suggesting that a fuller understanding of the in vivo impact of the Ala56 variant will benefit from assessments in the context of sensory, physiological, behavioral or pharmacological stimuli. In this regard, SERT Ala56 mice have been found to display deficits in multi-sensory integration, a phenotype also observed in association with ASD (Siemann et al., 2017).
6. Conclusion and future directions
SERT can no longer be considered a quantitatively static contributor to extracellular 5-HT homeostasis, and as such, a deeper analysis of how SERT trafficking and activity is normally regulated is likely to inform our analysis of the true contribution made by SERT to the risk for neurobehavioral disorders and possibly to improved therapeutics. However, many questions remain. We also need to better understand when and where the in vivo changes detected in SERT Ala56 mice emerge. We have found that SERT Ala56 imparts a multitude of CNS changes that ultimately leads to a decrease in 5-HT signaling (Veenstra-VanderWeele et al., 2012; Robson et al., 2018). 5-HT also plays important roles in many peripheral tissues and as such the variant can be expected to result in additional peripheral effects. Prominent among these are enteric nervous system hypoplasia, decreased GI motility, and reduced peristalsis (Margolis et al., 2016). Importantly, the ability of MW150 to normalize both gut and brain phenotypes observed in SERT Ala56 mice indicates an ongoing and reversible contribution of elevated SERT activity to multiple aspects of systemic physiology and behavior. It is likely that the impact of Ala56 in vivo will depend not just on the intrinsic structural effects of the variant, but also on conditions that engage specific serotonergic pathways involved in stress response, reinforcement, and social behavior.
Last, might novel and faster acting therapeutics arise from further elucidating mechanisms of SERT regulation, such as agents useful in the treatment of OCD or ASD? SERT Ala56 and other hyperfunctional mutations to SERT have been shown to alter binding of various drugs to the transporter – for example, Ala56 lowers the affinity of SERT for cocaine (Prasad et al., 2005) – and while an exhaustive investigation of other SERT antagonists needs to be performed, this finding suggests that the conformational bias associated with a catalytically modulated transporter may specify a therapeutic pharmacology distinct from that simply based on 5-HT competition. Recently, manipulation of SERT trafficking has been shown to normalize behavioral changes arising from stress (Sun et al., 2022). Specifically, Sun and colleagues described a somatodendrititc-specific SERT/nNOS interaction that drives intracellular sequestration of the transporter, with pharmacological suppression of this interaction able to restore surface SERT levels and rescue depressive-like behaviors in mice. As modeled by these investigators, agents that specifically increase SERT surface density in the cell body vs axonal compartments may prove to be an additional target for antidepressant treatment. Since activation of nNOS activation leads to cGMP production and PKG activation, a consequence of disassembling the SERT/nNOS complex may allow for SERT catalytic enhancement via PKG/p38α MAPK, maximizing SERT activity once the transporter reaches the surface. Conversely, agents that catalytically inactivate SERT in axonal regions or selectively diminish axonal SERT surface expression, might also prove useful as antidepressant agents, diminishing 5-HT clearance as observed for SSRIs.
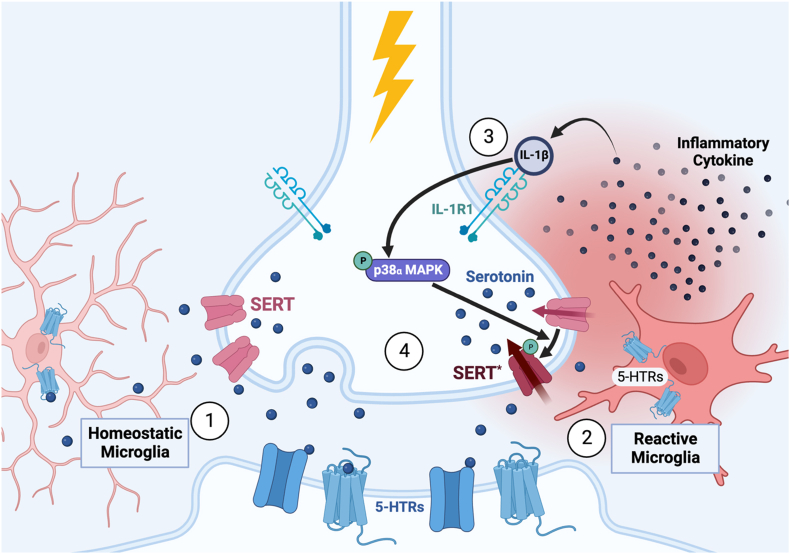
Finally, the studies reviewed above also argue that one important therapeutic opportunity involves disrupting the links between peripheral inflammation, peripheral and CNS IL-1β, p38α MAPK activation, and stimulation of SERT catalytic activity. Although the transient use of this pathway is likely of survival benefit, our work with SERT Ala56 mice indicates that developmental and/or prolonged activation may have unwanted and even pathological consequences. For example, 5-HT has been reported to diminish activation of microglia (Mariani et al., 2022). As such, catalytic activation of SERT (by mutation or chronic stress) could enhance microglial activation via a loss of immunosuppressive 5-HT, leading to the enhanced production of additional inflammatory cytokines, creating a self-sustaining cycle that allows CNS cytokine activation to remain elevated even when peripheral inflammatory stimuli or stress is relieved (Fig. 4). Indeed, increased reactive microglia have been found in the brains of postmortem individuals and in preclinical models of ASD (Vargas et al., 2005; Guneykaya et al., 2023). MW150 presents one pharmacological tool to interrupt this cycle, but as we continue to elucidate modes of SERT regulation, we suspect the list will grow.
Fig. 4.
The role of microglia in modulating SERT activity and positive feedback of enhanced clearance on sustaining microglia in a reactive state. ① Homeostatic microglia are constantly surveying their surrounding environment and respond to the release of neurotransmitters and other stimuli. They are highly branched and capable of modulating synaptic plasticity and neuronal activity and are maintained in this state by 5-HT signaling. ② In the context of pro-inflammatory stimuli (particularly in the absence of anti-inflammatory molecules), they express and secrete inflammatory cytokines and chemokines and alter their morphology, displaying decreased length of processes, branching, and enlargement of the cell body which assist in their mobility. This increase in inflammatory signaling in the brain can have a direct effect on 5-HT neurons, as these neurons can directly respond to IL-1β via their expression of IL-1R1. ③ Activation of this pathway ultimately leads to a significant increase in SERT catalytic activity (SERT*), elevating 5-HT clearance and reducing the amount of extracellular 5-HT for signaling, producing downstream 5-HT signaling alterations ④that impact 5-HT-related behaviors. Diminished 5-HT availability has also been reported to sustain the reactive state of microglia, creating a feedback loop that can maintain a pro-inflammatory state in the absence of the originating stimulus.
CRediT authorship contribution statement
Paula A. Gajeswski-Kurdziel: Conceptualization, Writing – original draft, Writing – review & editing, Funding acquisition. Allison E. Walsh: Conceptualization, Writing – original draft, Writing – review & editing, Visualization. Randy D. Blakely: Conceptualization, Writing – original draft, Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by National Institute of Mental Health for awards MH94527 (RDB) and MH120966 (PAG-K) and predoctoral support from the Stiles-Nicholson Brain Institute (AEW).
Contributor Information
Paula A. Gajeswski-Kurdziel, Email: pgajewski@health.fau.edu.
Randy D. Blakely, Email: rblakely@health.fau.edu.
Data availability
No data was used for the research described in the article.
References
- Baganz N.L., et al. A requirement of serotonergic p38alpha mitogen-activated protein kinase for peripheral immune system activation of CNS serotonin uptake and serotonin-linked behaviors. Transl. Psychiatry. 2015;5:e671. doi: 10.1038/tp.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman A.L., et al. Cocaine and antidepressant-sensitive biogenic amine transporters exist in regulated complexes with protein phosphatase 2A. J. Neurosci. 2000;20:7571–7578. doi: 10.1523/JNEUROSCI.20-20-07571.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham D.P., Blakely R.D. Kinase-dependent regulation of monoamine neurotransmitter transporters. Pharmacol. Rev. 2016;68:888–953. doi: 10.1124/pr.115.012260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely R.D., et al. Cloning and expression of a functional serotonin transporter from rat brain. Nature. 1991;354:66–70. doi: 10.1038/354066a0. [DOI] [PubMed] [Google Scholar]
- Campbell N.G., et al. Rare coding variants of the adenosine A3 receptor are increased in autism: on the trail of the serotonin transporter regulome. Mol. Autism. 2013;4:28. doi: 10.1186/2040-2392-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J.C., et al. Single molecule analysis of serotonin transporter regulation using antagonist-conjugated quantum dots reveals restricted, p38 MAPK-dependent mobilization underlying uptake activation. J. Neurosci. 2012;32:8919–8929. doi: 10.1523/JNEUROSCI.0048-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R., O'Connor J.C., Lawson M.A., Kelley K.W. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology. 2011;36:426–436. doi: 10.1016/j.psyneuen.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bella D., Catalano M., Balling U., Smeraldi E., Lesch K.P. Systematic screening for mutations in the coding region of the human serotonin transporter (5-HTT) gene using PCR and DGGE. Am. J. Med. Genet. 1996;67:541–545. doi: 10.1002/(SICI)1096-8628(19961122)67:6<541::AID-AJMG5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Forrest L.R., et al. Mechanism for alternating access in neurotransmitter transporters. P Natl Acad Sci USA. 2008;105:10338–10343. doi: 10.1073/pnas.0804659105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guneykaya D., et al. Sex-specific microglia state in the Neuroligin-4 knock-out mouse model of autism spectrum disorder. Brain Behav. Immun. 2023;111:61–75. doi: 10.1016/j.bbi.2023.03.023. Fig. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase J., Killian A.M., Magnani F., Williams C. Regulation of the serotonin transporter by interacting proteins. Biochem. Soc. Trans. 2001;29:722–728. doi: 10.1042/0300-5127:0290722. [DOI] [PubMed] [Google Scholar]
- Hersey M., et al. Inflammation-induced histamine impairs the capacity of escitalopram to increase hippocampal extracellular serotonin. J. Neurosci. 2021;41:6564–6577. doi: 10.1523/JNEUROSCI.2618-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman B.J., Mezey E., Brownstein M.J. Cloning of a serotonin transporter affected by antidepressants. Science. 1991;254:579–580. doi: 10.1126/science.1948036. [DOI] [PubMed] [Google Scholar]
- Jayanthi L.D., Samuvel D.J., Blakely R.D., Ramamoorthy S. Evidence for biphasic effects of protein kinase C on serotonin transporter function, endocytosis, and phosphorylation. Mol. Pharmacol. 2005;67:2077–2087. doi: 10.1124/mol.104.009555. [DOI] [PubMed] [Google Scholar]
- Jayanthi L.D., Annamalai B., Samuvel D.J., Gether U., Ramamoorthy S. Phosphorylation of the norepinephrine transporter at threonine 258 and serine 259 is linked to protein kinase C-mediated transporter internalization. J. Biol. Chem. 2006;281:23326–23340. doi: 10.1074/jbc.M601156200. [DOI] [PubMed] [Google Scholar]
- Liu X., et al. Cell-type-specific interleukin 1 receptor 1 signaling in the brain regulates distinct neuroimmune activities. Immunity. 2019;50:764–766. doi: 10.1016/j.immuni.2019.02.012. [DOI] [PubMed] [Google Scholar]
- Margolis K.G., et al. Serotonin transporter variant drives preventable gastrointestinal abnormalities in development and function. J. Clin. Invest. 2016;126:2221–2235. doi: 10.1172/JCI84877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani N., Everson J., Pariante C.M., Borsini A. Modulation of microglial activation by antidepressants. J. Psychopharmacol. 2022;36:131–150. doi: 10.1177/02698811211069110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J.H., et al. Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11C]DASB positron emission tomography study. Am. J. Psychiatr. 2004;161:826–835. doi: 10.1176/appi.ajp.161.5.826. [DOI] [PubMed] [Google Scholar]
- Miller K.J., Hoffman B.J. Adenosine A3 receptors regulate serotonin transport via nitric oxide and cGMP. J. Biol. Chem. 1994;269:27351–27356. [PubMed] [Google Scholar]
- Mulder E.J., et al. Platelet serotonin levels in pervasive developmental disorders and mental retardation: diagnostic group differences, within-group distribution, and behavioral correlates. J. Am. Acad. Child Adolesc. Psychiatry. 2004;43:491–499. doi: 10.1097/00004583-200404000-00016. [DOI] [PubMed] [Google Scholar]
- O'Reilly K.C., et al. A social encounter drives gene expression changes linked to neuronal function, brain development, and related disorders in mice expressing the serotonin transporter Ala56 variant. Neurosci. Lett. 2020;730 doi: 10.1016/j.neulet.2020.135027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki N., et al. Serotonin transporter missense mutation associated with a complex neuropsychiatric phenotype. Mol. Psychiatr. 2003;8:933–936. doi: 10.1038/sj.mp.4001365. [DOI] [PubMed] [Google Scholar]
- Pinto E.F., Andrade C. Interferon-related depression: a primer on mechanisms, treatment, and prevention of a common clinical problem. Curr. Neuropharmacol. 2016;14:743–748. doi: 10.2174/1570159X14666160106155129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad H.C., et al. Human serotonin transporter variants display altered sensitivity to protein kinase G and p38 mitogen-activated protein kinase. Proc. Natl. Acad. Sci. U. S. A. 2005;102:11545–11550. doi: 10.1073/pnas.0501432102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad H.C., Steiner J.A., Sutcliffe J.S., Blakely R.D. Enhanced activity of human serotonin transporter variants associated with autism. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:163–173. doi: 10.1098/rstb.2008.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y., et al. Protein kinase C activation regulates human serotonin transporters in HEK-293 cells via altered cell surface expression. J. Neurosci. 1997;17:45–57. doi: 10.1523/JNEUROSCI.17-01-00045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan M.A., et al. Human serotonin transporter coding variation establishes conformational bias with functional consequences. ACS Chem. Neurosci. 2019;10:3249–3260. doi: 10.1021/acschemneuro.8b00689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S., Blakely R.D. Phosphorylation and sequestration of serotonin transporters differentially modulated by psychostimulants. Science. 1999;285:763–766. doi: 10.1126/science.285.5428.763. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S., Giovanetti E., Qian Y., Blakely R.D. Phosphorylation and regulation of antidepressant-sensitive serotonin transporters. J. Biol. Chem. 1998;273:2458–2466. doi: 10.1074/jbc.273.4.2458. [DOI] [PubMed] [Google Scholar]
- Robson M.J., et al. p38alpha MAPK signaling drives pharmacologically reversible brain and gastrointestinal phenotypes in the SERT Ala56 mouse. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E10245–E10254. doi: 10.1073/pnas.1809137115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S.M., et al. A selective and brain penetrant p38alphaMAPK inhibitor candidate for neurologic and neuropsychiatric disorders that attenuates neuroinflammation and cognitive dysfunction. J. Med. Chem. 2019;62:5298–5311. doi: 10.1021/acs.jmedchem.9b00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnick G., Sandtner W. Serotonin transport in the 21st century. J. Gen. Physiol. 2019;151:1248–1264. doi: 10.1085/jgp.201812066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemann J.K., et al. An autism-associated serotonin transporter variant disrupts multisensory processing. Transl. Psychiatry. 2017;7 doi: 10.1038/tp.2017.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluzewska A., et al. Indicators of immune activation in major depression. Psychiatr. Res. 1996;64:161–167. doi: 10.1016/s0165-1781(96)02783-7. [DOI] [PubMed] [Google Scholar]
- Sun N., et al. Design of fast-onset antidepressant by dissociating SERT from nNOS in the DRN. Science. 2022;378:390–398. doi: 10.1126/science.abo3566. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J.S., et al. Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors. Am. J. Hum. Genet. 2005;77:265–279. doi: 10.1086/432648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A., et al. Neuromodulatory effect of interleukin 1beta in the dorsal raphe nucleus on individual differences in aggression. Mol. Psychiatr. 2022;27:2563–2579. doi: 10.1038/s41380-021-01110-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Serotonin System - History . Elsevier; 2019. Neuropharmacology, and Pathology. [Google Scholar]
- Vargas D.L., Nascimbene C., Krishnan C., Zimmerman A.W., Pardo C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J., et al. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc. Natl. Acad. Sci. U. S. A. 2012;109:5469–5474. doi: 10.1073/pnas.1112345109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C.B., Hewlett W.A., Francis S.H., Corbin J.D., Blakely R.D. Stimulation of serotonin transport by the cyclic GMP phosphodiesterase-5 inhibitor sildenafil. Eur. J. Pharmacol. 2004;504:1–6. doi: 10.1016/j.ejphar.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Zhu C.B., Carneiro A.M., Dostmann W.R., Hewlett W.A., Blakely R.D. p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J. Biol. Chem. 2005;280:15649–15658. doi: 10.1074/jbc.M410858200. [DOI] [PubMed] [Google Scholar]
- Zhu C.B., Blakely R.D., Hewlett W.A. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology. 2006;31:2121–2131. doi: 10.1038/sj.npp.1301029. [DOI] [PubMed] [Google Scholar]
- Zhu C.B., et al. Rapid stimulation of presynaptic serotonin transport by A(3) adenosine receptors. J. Pharmacol. Exp. Therapeut. 2007;322:332–340. doi: 10.1124/jpet.107.121665. [DOI] [PubMed] [Google Scholar]
- Zhu C.B., et al. Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology. 2010;35:2510–2520. doi: 10.1038/npp.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C.B., et al. Colocalization and regulated physical association of presynaptic serotonin transporters with A(3) adenosine receptors. Mol. Pharmacol. 2011;80:458–465. doi: 10.1124/mol.111.071399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.