Abstract
The occurrence of “Xanthomonas axonopodis pv. phaseoli var. fuscans” (proposed name) populations as biofilms on bean leaves was investigated during three field experiments on plots established with naturally contaminated bean seeds. Behavior of aggregated versus solitary populations was determined by quantification of culturable cells in different fractions of the epiphytic population separated by particle size. X. axonopodis pv. phaseoli var. fuscans population dynamic studies confirmed an asymptomatic and epiphytic colonization of the bean phyllosphere. For all years of experiment and cultivars tested, biofilms and solitary components of the populations were always detected. Biofilm population sizes remained stable throughout the growing season (around 105 CFU/g of fresh weight) while solitary population sizes were more abundant and varied with climate. According to enterobacterial repetitive intergenic consensus fingerprinting, aggregated bacterial isolates were not different from solitary isolates. In controlled conditions, application of a hydric stress resulted in a decrease of the solitary populations on the leaf surface while the biofilm fraction remained stable. Suppression of the hydric stress allowed solitary bacterial populations to increase again. Aggregation in biofilms on leaf surfaces provides protection to the bacterial cells against hydric stress.
Numerous plant-pathogenic bacteria multiply or survive on aerial parts of plants without causing any visible symptoms. Among other consequences, this asymptomatic phase allows bacterial populations to attain sizes permitting, in favorable environments, disease development (16). Sizes of these epiphytic populations are predictive of the amount of disease in some cases (34), and in other cases a threshold of population sizes is necessary to produce symptoms (44).
The common bean (Phaseolus vulgaris L.) is one of the most important crops worldwide in both economic and nutritional aspects (4). Common and fuscous blights of bean caused by Xanthomonas axonopodis pv. phaseoli and its variant “Xanthomonas axonopodis pv. phaseoli var. fuscans” (proposed name) occur frequently in temperate and tropical climates (14). Common blight, including fuscous blight, is one of the five major diseases of beans leading to important yield losses (4). Symptoms and epidemiology of these two diseases and ecology of the pathogens are thought to be similar, fuscous strains being generally more aggressive than X. axonopodis pv. phaseoli strains (41). Sanitary control of this seed-borne disease is complicated by epiphytic and asymptomatic growth of the bacteria on the bean canopy (43).
It has been reported from Michigan that symptom development on leaves requires an inoculum density of at least 5 × 106 CFU/20 cm2 of leaf tissue after inoculation (44). Expression of some traits implicated in plant-microbe interactions is density dependent through quorum sensing (42). It has been shown for Xanthomonas campestris pv. campestris that epiphytic survival is also dependent on the production of a diffusible factor (DF) implicated in xanthomonadin and exopolysaccharide production in a cell-density-dependent manner (30, 32). It has recently been hypothesized for X. campestris pv. campestris that a regulatory system is implicated in biofilm dispersal and in planta in transition to the planktonic lifestyle (11). Quorum sensing has also been suggested to play a pivotal role in epiphytic survival of Pseudomonas syringae, another important bean pathogen, by inducing the expression of fitness genes during initial phases of colonization of bean leaves and then subsequently downregulating these genes at later stages of colonization (42).
Epiphytic multiplication is affected by environmental factors and/or host physiology (18, 44). To colonize aerial parts of plants, bacteria need to deal with frequent and major environmental changes (17). Localization in protected sites and clustering in structures such as biofilms leading to enhanced resistance to stresses are thought to be adaptive traits of epiphytic bacteria (26). The most common sites of bacterial colonization are stomates, the base of trichomes, and depressions along veins (21, 38). In some of these locations, large epiphytic aggregates of bacteria embedded in an exopolymeric matrix have been observed and resemble biofilms found in aquatic and medical environments (27, 28). In those environments, biofilms offer microorganisms protection from external environmental stresses (desiccation, for example) and a favorable milieu for multiplication (5, 8). The aggregation of microorganisms in biofilms on leaf surfaces of a wide range of plant species has been reported (12, 23, 27). However, these punctual observations do not provide information on the dynamics of biofilm populations under field conditions, and their significance for plant-pathogenic bacteria is largely unknown. The existence of a dynamic exchange of cells between biofilm and planktonic components of the population (19) has been only partially explored in the phyllosphere (3). Elucidating these issues is of valuable importance for understanding the ecology and biology of an epiphytic plant-pathogenic bacterium such as X. axonopodis pv. phaseoli.
The overall objective of this study was to determine the occurrence of X. axonopodis pv. phaseoli in biofilms and as solitary cells on bean leaves during seed-borne epidemics in the field. The dynamics of naturally occurring X. axonopodis pv. phaseoli var. fuscans populations were monitored during three independent field experiments. We showed that cells of X. axonopodis pv. phaseoli var. fuscans aggregated in biofilms constitute a more stable population than do solitary populations. Further experiments were conducted under controlled conditions with a rifamycin-resistant strain of X. axonopodis pv. phaseoli var. fuscans to evaluate the impact of hydric stress on aggregated and solitary components of X. axonopodis pv. phaseoli var. fuscans epiphytic populations. We demonstrated that biofilms offered more protection to the bacterial populations than did the solitary state; multiplication of the solitary fraction of the population was significantly (P < 0.05) altered after stress application, while biofilm population sizes were not affected. Suppression of the hydric stress allowed solitary bacterial populations to increase again. The potential role of biofilms in colonization of the phyllosphere by bacteria is discussed.
MATERIALS AND METHODS
Bacterial strains and culture media.
CFBP4834-R is a spontaneous rifamycin-resistant derivative of X. axonopodis pv. phaseoli var. fuscans CFBP4834 (stored at the French Collection of Plant Pathogenic Bacteria, INRA, Angers, France) which was isolated from an epiphytic biofilm on an asymptomatic field-grown bean leaflet (cv. Michelet) in 1998 grown from naturally contaminated bean seeds. CFBP4834-R was selected on TSA (tryptone, 17 g/liter; Bacto Soytone, 3 g/liter; glucose, 2.5 g/liter; NaCl, 5 g/liter; K2HPO4, 5 g/liter; agar, 15 g/liter) containing 200 mg of rifamycin/liter. In vitro growth of this derivative on TSA and 10% TSA (1:10 dilution of TSA except for agar [15 g/liter]), its aggressiveness on beans, and its physiological characteristics were similar to those of the wild type, as was its in planta growth (data not shown).
Isolation of CFBP4834-R from inoculated plants incubated in growth chambers was routinely done on 10% TSA containing rifamycin (50 mg/liter). Furthermore, production of the typical fuscous pigment on TSA was used to confirm identification of the isolated yellow mucoid rifamycin-resistant colonies (9).
Isolation of naturally occurring X. axonopodis pv. phaseoli var. fuscans on field-grown bean leaflets or seeds was performed on semiselective modified MXP medium (9). Its composition is identical to the original MXP medium except that insoluble potato starch was used instead of soluble potato starch to visualize starch hydrolysis. Several selective antibiotics, stains, and chemicals are included in this medium, among which are crystal violet, which limits gram-positive bacterial growth; cephalexin, an antibiotic that inhibits enterobacteria; and kasugamycin, which limits pseudomonad development. Bromine is lethal to bacteria other than xanthomonads. Furthermore, a translucent area surrounding yellow mucoid colonies of X. axonopodis pv. phaseoli indicates starch hydrolysis, which is a characteristic of this pathogenic bacterium. A specific PCR test (kit DGN046203; D-Genos, Angers, France) was used to confirm preliminary identification of those X. axonopodis pv. phaseoli strains. These primers are specific for X. axonopodis pv. phaseoli strains pathogenic on beans (20).
To determine the sizes of the indigenous bacterial populations in samples, the same diluted homogenates were plated on 10% TSA containing cycloheximide (50 mg/liter).
Bacterial strains were stored at −80°C in 40% glycerol and routinely grown on 10% TSA containing, if necessary, rifamycin (50 mg/liter).
Dynamics of X. axonopodis subsp. fuscans pv. phaseoli bacterial populations.
Biofilm and solitary epiphytic bacterial populations were isolated from leaflets according to the method described by Morris et al. (28). Briefly, the leaflets were washed (Stomacher 80; Seward, London, United Kingdom) for 1 min at medium power in 40 ml of phosphate buffer. An aliquot (10 ml) of this washing liquid was dilution plated to estimate the size of the epiphytic population size before subsequent treatments to separate biofilm and solitary components. This washing liquid was then filtered with Isopore polycarbonate filters (pore diameter, 5 μm) to separate the biofilm and the solitary components. The number of cells in the washing liquid present as solitary bacteria was estimated by dilution plating the filtrate. The biofilm component was recovered by dilution plating the bacterial suspension resulting from ultrasonication of filters as described in the work of Morris et al. (28). Total populations were quantified by adding 10 ml of phosphate buffer to the washed leaflets and stomaching for 2 min at maximum power. As an internal control for nonspecific retention rates of bacteria on membranes, suspensions of solitary bacteria were refiltered with the same type of filter as used to isolate biofilms, and the number of adherent bacteria was determined. Platings were performed on 10% TSA medium supplemented with cycloheximide (50 mg/liter) for indigenous bacterial populations, on modified MXP medium for naturally occurring X. axonopodis pv. phaseoli from field samples, and on 10% TSA medium supplemented with rifamycin for CFBP4834-R-inoculated plants. Plates were incubated for 5 days at 28°C.
When counts of only total X. axonopodis pv. phaseoli population sizes were needed, bean leaflets were washed (Stomacher 80) in 10 ml of phosphate buffer (K2HPO4, 8.75 g; KH2PO4, 6.75 g/liter; pH 7) for 2 min at maximum power. Serial dilutions of washings were spread on plates of the appropriate medium.
Recovery of X. axonopodis pv. phaseoli var. fuscans from naturally contaminated seeds.
Common blight-infested bean seeds were incubated in sterile phosphate buffer (2 ml/g of seeds) for 12 h at 4°C. Serial dilutions of homogenates were spread on plates of modified MXP and 10% TSA media. The most probable number method (24, 37), a statistical procedure using samples of decreasing size, was used to estimate contamination rates of seed lots. Knowledge of contamination rates is necessary to determine the size of the plots in order to be able to see between 5 and 10 foci of disease in the field. This estimated number of foci allowed us to obtain at least one focus of disease in the field and hence the ability to monitor contaminated but asymptomatic plants in the vicinity of this focus.
Field experiments and sampling.
To determine the occurrence of seed-borne X. axonopodis pv. phaseoli in biofilms and as solitary cells on naturally contaminated beans, field experiments were conducted in 1998 (cultivar Michelet), 1999 (cultivar Michelet), and 2000 (cultivar Contender) at two sites (Domaine Bois l'Abbé, INRA Beaucouzé, and GEVES-SEV experimental station at Brion, Maine et Loire, France) with naturally contaminated seed lots. Due to the low availability of naturally contaminated seed lots in the primary laboratory (SNES, Angers, France), experiments were conducted on two bean varieties highly susceptible to common blight, Michelet and Contender. Seeds were sown (2,400, 720, and 5,300 seeds in 1998, 1999, and 2000 field experiments, respectively) and cultivated under traditional agricultural practices for beans in the Loire Valley (France). For experiments to determine dynamics of bacterial population sizes, asymptomatic plants were sampled after the first symptoms appeared in 1998 in the vicinity of identified diseased plants. In 1999 and 2000 samples were collected before the appearance of symptoms at specific sites in the field that corresponded to foci of relatively high bacterial populations and eventually disease. These sites were identified in preliminary experiments by systematic sampling in the field. Two leaf samples were collected in every third row in 1999, and three leaf samples were collected in every fifth row in 2000. These samples were analyzed for the presence of X. axonopodis pv. phaseoli. At dates subsequent to this preliminary sampling, two to three leaflets were sampled at each sampling date on each identified contaminated asymptomatic plant.
Plant inoculation and growth chamber conditions.
To evaluate the effect of hydric stress on the relative dynamics of biofilm and solitary bacterial population sizes, an experiment was conducted with plants of common bean (P. vulgaris cv. Michelet). Seeds used were not contaminated by X. axonopodis pv. phaseoli (30,000 seeds from the seed lot were analyzed, courtesy of R. Germain, Vilmorin, France). Plants at the two-trifoliate stage were grown in the greenhouse and were acclimated for 1 day before inoculation under controlled conditions in growth chambers. Plants were incubated under conditions of 16 h of light at 28°C and 8 h of darkness at 22°C. Plants were spray inoculated until runoff with suspensions (106 CFU/ml) of strain CFBP4834-R. Half of the plants were then subjected to one of two different hydric treatments: (i) plants were incubated under a relative humidity (RH) of 95% during the entire experiment (treatment 1) or (ii) plants were incubated for 4 days at an RH of 95%, then transferred to a lower RH (50 to 80% RH during the day and 70 to 90% RH at night) for 2 days, and then returned to an RH of 95% (treatment 2). Treatment 1 was used as the control for the hydric stress applied in treatment 2. This experiment was repeated twice.
Production of diffusible signal molecules by solitary and aggregated strains.
The biosensors Agrobacterium tumefaciens NT1(pDCI41E33) (29), X. campestris pv. campestris B24-B2 (30), and X. campestris pv. campestris 8523 (1) and respective control strains were supplied by the respective authors and used to detect potential production of different diffusible molecules, i.e., N-acylhomoserine lactones (AHLs) and butyrolactones (DF and diffusible signal factor [DSF]) by X. axonopodis pv. phaseoli var. fuscans strains isolated from solitary and biofilm fractions. Six and 14 biofilm strains and 10 and 11 solitary strains isolated from 1998 and 1999 field experiments, respectively, were assayed according to the original authors' procedures (1, 29, 30) including positive and negative controls.
ERIC fingerprinting of solitary and aggregated strains of X. axonopodis pv. phaseoli.
Characterization of genotypic DNA of X. axonopodis pv. phaseoli strains was based on enterobacterial repetitive intergenic consensus (ERIC)-PCR (40). ERIC1R and ERIC2 primer sequences (Bioprobe Systems, Montrevil, France) were as described by Versalovic et al. (40). Amplification reactions were performed as previously described (22) in volumes of 25 μl in a GeneAmp PCR System 9700 (Applied Biosystems PE, Courtaboeuf, France). PCR amplification products were detected by horizontal electrophoresis in 1.4% agarose gels as previously described (22). Ten biofilm and 10 solitary fuscous X. axonopodis pv. phaseoli strains isolated from 10 different plants from each of the last sampling dates in 1999 and 2000 field experiments were assayed.
Preparation of samples for SEM.
Scanning electron microscopy (SEM) was used to observe surfaces of diseased leaves sampled in 1999 and 2000 field experiments. To ensure the presence of X. axonopodis pv. phaseoli on observed leaf surfaces, samples were cut from the margin of symptomatic areas. They were degassed, fixed, and dehydrated as previously described (27). Samples were observed with a JEOL 6301 F scanning electron microscope.
Statistical analyses.
Data were analyzed with Microsoft Excel (Microsoft Corp., Redmond, Wash.) and Statbox Pro (version 2.5; Grimmer Logiciels, Optima, Mérignac, France) software. When assumptions of analysis of variance were verified (confirmation of homogeneity of variances with Bartlett's test and verification of log10-transformed population size distribution with Pearson coefficients), one-way analyses of variance were tested and mean population behaviors were compared with the Newman-Keuls statistical test. Kolmogorov-Smirnov or Mann-Whitney statistical tests were used when the assumptions of analysis of variance were not met (36).
RESULTS
Seed-borne X. axonopodis subsp. fuscans pv. phaseoli population sizes on field-grown beans.
Isolation of X. axonopodis pv. phaseoli from seed lots revealed the presence of only fuscous strains. Nonfuscous yellow mucoid starch-hydrolyzing colonies were not X. axonopodis pv. phaseoli. All fuscous strains tested were confirmed to be bean-pathogenic X. axonopodis pv. phaseoli according to PCR. Contamination levels of naturally contaminated bean seed lots used in field experiments were 1.1% in 1999 and 0.1% in 2000 (Table 1). Due to the small size of the 1998 seed lot, we did not determine the contamination rate (seeds were provided by a primary laboratory with a probable contamination rate of about 20%). In 1998, more than 10 disease foci developed in the field. In 1999, six foci of contaminated plants were identified in the field and in 2000 nine were identified, values comprised in the confidence interval of the estimated percentage of seed infection with a transmission rate close to 1:1. Asymptomatic leaves from sampled contaminated plants harbored maximum population sizes of X. axonopodis pv. phaseoli var. fuscans of 3.6 × 106 CFU/g (fresh weight) in 1998, 2.9 × 106 CFU/g (fresh weight) in 1999, and 2.4 × 106 CFU/g (fresh weight) in 2000. Among all the asymptomatic leaves analyzed X. axonopodis pv. phaseoli var. fuscans population sizes never exceeded these values. Indigenous total bacterial populations were always about 10 times higher than total X. axonopodis pv. phaseoli var. fuscans population sizes with a maximal population size detected on a sample of 1.45 × 107 CFU/g (fresh weight).
TABLE 1.
Contamination rates of naturally infested seed lots used for the 1999 and 2000 field experiments
| Expt yr | Samplinga | No. of positive samples | MPN in 100 seedsb | Confidence intervalb |
|---|---|---|---|---|
| 1999 | 1 batch of 500 | 1 | 1.1 | 0.3-2.7 |
| 5 batches of 100 | 3 | |||
| 5 batches of 10 | 1 | |||
| 2000 | 5 batches of 800 | 3 | 0.1 | <0.1-0.3 |
Number of batches each containing a defined number of seeds.
Most probable number (MPN) and associated confidence interval were obtained from reference tables (36).
Proportion and dynamics of epiphytic components in field experiments.
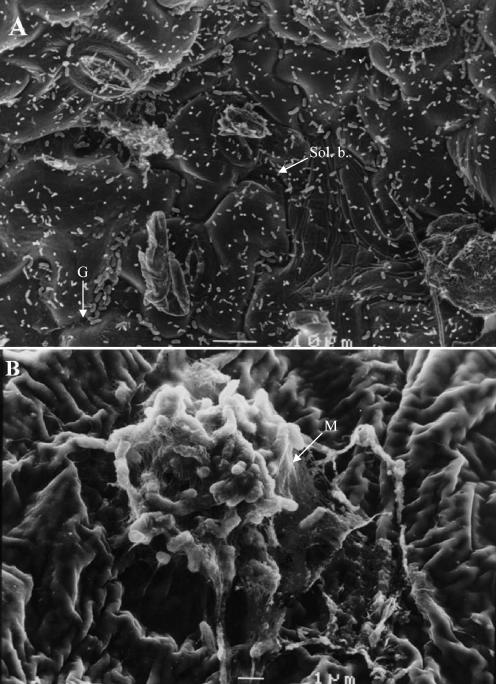
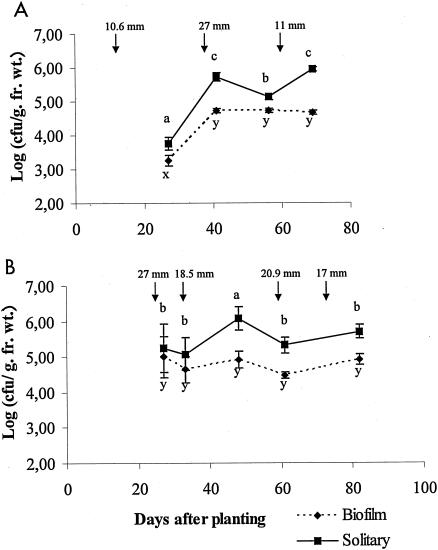
Epiphytic populations of X. axonopodis pv. phaseoli var. fuscans were differentiated into solitary and biofilm components during bean growing seasons. SEM observations were made on leaf samples taken at the margin of symptomatic areas. Solitary and aggregated bacteria were visible on these leaf surfaces from which X. axonopodis pv. phaseoli var. fuscans was isolated (Fig. 1). An extensive exopolymeric matrix (Fig. 1B), similar to that found in biofilms, embedded bacterial cells. The presence of X. axonopodis pv. phaseoli in biofilms and as solitary cells was confirmed by isolation on asymptomatic field samples (Fig. 2; Table 2). In both 1999 and 2000 field experiments, population sizes of aggregated X. axonopodis pv. phaseoli were more constant than those of the solitary component of the epiphytic population on bean leaves. X. axonopodis pv. phaseoli biofilm populations were not significantly different (P < 0.05) on days 41, 56, and 69 after sowing of cultivar Michelet in 1999 and on days 27, 33, 48, 61, and 82 after sowing of cultivar Contender in 2000. The population size of aggregated X. axonopodis pv. phaseoli tended to stabilize around 5.0 × 104 CFU/g (fresh weight) on cultivar Michelet in 1999 and around 5.7 × 104 CFU/g (fresh weight) on cultivar Contender in 2000. However, sizes of solitary populations of X. axonopodis pv. phaseoli were statistically (P < 0.05) different for leaves sampled at each sampling time. No relationship could be identified between leaf position or leaf age and biofilm frequency in X. axonopodis pv. phaseoli epiphytic populations. Peaks of X. axonopodis pv. phaseoli population sizes tended to occur generally soon after rainstorms (Fig. 2).
FIG. 1.
Scanning electron microscopic micrographs of field-grown bean leaf surfaces colonized by seed-borne X. axonopodis pv. phaseoli. (A) Leaf surface showing mostly solitary bacterial populations (Sol. b.). Note the accumulation of bacterial cells in grooves (G) between epidermal cells. Bar, 10 μm. (B) Focus on a bacterial biofilm. Note the matrix (M) embedding bacterial cells constituting a typical biofilm. Bar, 1 μm.
FIG. 2.
X. axonopodis pv. phaseoli population dynamics on bean leaflets for two field experiments (1999 [A] and 2000 [B]) and two cultivars (Michelet [A] and Contender [B]). Epiphytic bacterial populations are divided into solitary and biofilm fractions. Error bars represent the standard error of the mean. For a given fraction of the population in each experiment mean population sizes followed by different letters are significantly (P < 0.05) different on the basis of the Newman-Keuls test. Water amounts of every rainfall higher than 10 mm are indicated by arrows. fr. wt., fresh weight.
TABLE 2.
Population sizes and proportions of the epiphytic population of X. axonopodis pv. phaseoli aggregated in biofilms under three field conditions
| Bean cv., yr of field expt | Plant age (days after sowing) | Sample size (no. of leaves) | Mean log10 (X. axonopodis pv. phaseoli CFU/g [fresh wt]) (SEM)a
|
Mean B/(B + S)b (SEM) | |||
|---|---|---|---|---|---|---|---|
| Epiphytic | Solitary | Biofilm | Total | ||||
| Michelet, 1998 | 56 | 12 | 3.20 (0.23) | 3.90 (0.28) | 3.74 (0.22) | 3.52 (0.33) | 14.44 (5.56) |
| 62 | 8 | 6.42 (0.32) | 6.21 (0.37) | 5.24 (0.27) | 6.56 (0.33) | 30.99 (11.59) | |
| Michelet, 1999 | 27 | 27 | 3.49 (0.22) | 3.75 (0.18) | 3.26 (0.16) | 3.75 (0.21) | 27.28 (2.86) |
| 41 | 18 | 5.23 (0.07) | 5.11 (0.07) | 4.73 (0.06) | 5.55 (0.07) | 31.22 (3.36) | |
| 56 | 18 | 5.52 (0.09) | 5.61 (0.07) | 4.71 (0.07) | 5.91 (0.08) | 14.55 (3.18) | |
| 69 | 18 | 6.00 (0.07) | 5.94 (0.06) | 4.66 (0.06) | 6.47 (0.07) | 6.08 (1.14) | |
| Contender, 2000 | 27 | 6 | 5.41 (0.91) | 5.25 (0.68) | 5.00 (0.58) | 5.96 (0.66) | 42.75 (17.97) |
| 33 | 12 | 4.38 (0.43) | 5.07 (0.46) | 4.64 (0.38) | 4.58 (0.35) | 43.94 (13.58) | |
| 48 | 12 | 6.14 (0.27) | 6.07 (0.33) | 4.92 (0.24) | 6.38 (0.30) | 12.88 (5.54) | |
| 61 | 12 | 5.74 (0.07) | 5.32 (0.23) | 4.47 (0.08) | 6.09 (0.08) | 16.65 (6.16) | |
| 82 | 12 | 5.33 (0.17) | 5.70 (0.19) | 4.91 (0.14) | 5.54 (0.09) | 16.01 (4.07) | |
The epiphytic bacterial population corresponds to the washing liquid of the leaflets. Filtration of this suspension leads to separation of the solitary component from the biofilm populations retained on filters. Total bacterial populations were quantified by stomaching leaflets for 2 min at maximum power.
B/(B + S) represents the frequency of biofilm (B) bacterial component in the theoretical epiphytic population: biofilm + solitary (S). The epiphytic population size directly measured from leaves is given in the “Epiphytic” column.
Epiphytic X. axonopodis pv. phaseoli populations recovered by the washing step varied among samples from a minimum of 3 × 102 CFU/g (fresh weight) to a maximum of 4.3 × 106 CFU/g (fresh weight). Means are presented in Table 2. In 1999, the fraction of the population that could not be dislodged by washing (difference between total and epiphytic population sizes) of cultivar Michelet leaves tended to increase during the growing season. However, no obvious trend was observed for this fraction on Contender leaves during 2000 field cultivation. Mean efficiencies of washing to remove epiphytic X. axonopodis pv. phaseoli bacteria (percentage of epiphytic bacterial component in the total population) were 43% for both sampling dates in 1998. In 1999, these values were 60, 49, 42, and 35% for sampling on days 27, 41, 56, and 69 after sowing, respectively, and in 2000 they were 39, 70, 59, 46, and 66% for sampling on days 27, 33, 48, 61, and 82 after sowing, respectively. Nonspecific retention of bacteria on filters varied between 0.03 and 0.93% of the prefiltered solitary bacterial populations, i.e., always well below the fraction of bacteria identified as being in biofilms. It did not depend on bean cultivar or year of the experiment.
Effect of a desiccation stress on aggregated versus solitary epiphytic populations of X. axonopodis pv. phaseoli.
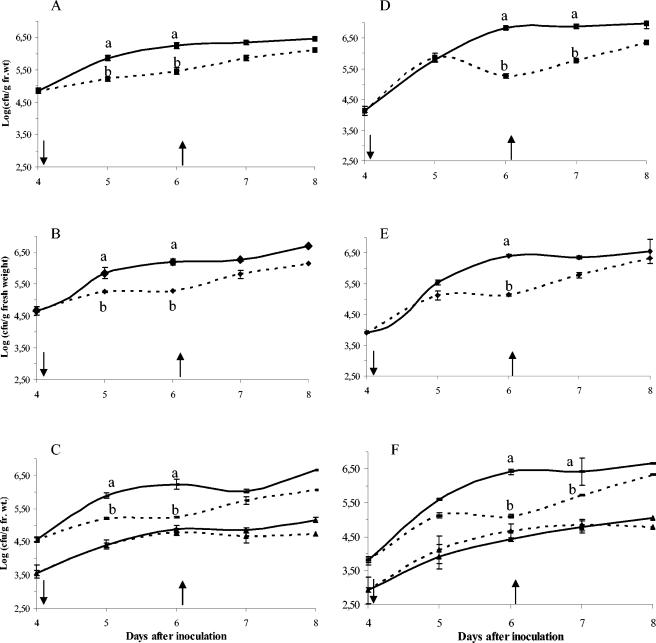
Decrease of epiphytic and total population sizes of CFBP4834-R on plants placed in stressful conditions could be observed in the first trial 24 h after stress had begun (Fig. 3A and B), but differences were significant 48 h poststress in the two trials (Fig. 3). Populations increased to control-level population sizes when high-RH conditions were restored for the two independent repetitions of this experiment (Fig. 3A and B for the first trial and Fig. 3D and E for the second one).
FIG. 3.
Dynamics of different fractions of X. axonopodis pv. phaseoli populations on bean leaflets following application of a hydric stress. The figure shows total (A and D), epiphytic (B and E), solitary (C and F [rectangles]), and biofilm (C and F [triangles]) X. axonopodis pv. phaseoli var. fuscans CFBP4834-R population dynamics on plants submitted to hydric stress (dashed line) applied (↓) 4 days after spray inoculation compared to plants maintained in high-RH conditions (solid line) for two repetitions of the experiment (first trial, A to C; second trial, D to F). Stress was suppressed (↑) after sampling on day 6. Error bars represent the standard error of the mean. For a sampling date mean population sizes followed by different letters are significantly (P < 0.05) different on the basis of the Newman-Keuls test.
A differential behavior was noticed for solitary versus biofilm populations under stressful conditions (Fig. 3C and F). Decrease of solitary populations was significant as early as 24 h after initiation of desiccation stress in the first trial and was significant 48 h after stress application in both trials. Biofilm population sizes were not significantly (P > 0.05) affected by desiccation stress and tended to stabilize around 4.5 × 104 CFU/g (fresh weight). Sizes of solitary populations varied significantly (P < 0.05) as a function of RH conditions. Furthermore, fluctuations in total and epiphytic population sizes were an indication of the response of the solitary population to the changing conditions.
Production of diffusible signal molecules and genomic characterization of solitary and aggregated strains.
Nine days after inoculation of plates, all the X. axonopodis pv. phaseoli var. fuscans strains tested produced DF; DSF was produced by all strains 4 days after inoculation as revealed by biosensor assays. No AHL activity was detected in the tested strains except for the positive controls. All 40 solitary and aggregated strains tested showed the same genomic profile based on ERIC-PCR analysis.
DISCUSSION
X. axonopodis pv. phaseoli is a seed-borne pathogen able to undergo a long epiphytic phase on beans. It was shown earlier that this bacterium could survive on leaf surfaces and endophytically (44). We show here that the epiphytic phase under field conditions has two components: biofilms and solitary fractions. This is the first report of the relative population dynamics of biofilm and solitary fractions of a plant-pathogenic bacterium under field conditions. X. axonopodis pv. phaseoli biofilm population sizes are stable in the field after an initial phase of growth. They stabilize around 105 CFU/g of fresh weight of leaf on two different susceptible cultivars and under different growing seasons for which symptoms were observed. This population size is just under the population threshold leading to symptom expression (44). Under controlled conditions, we observed the same trends.
In the field, rainstorms are often related to increases in bacterial population sizes to the threshold level and to rapid disease development (18). We observed the same tendency during the summer of 1999. The effect of rainstorms could be consequent to a sudden decrease of temperature or a rapid inflow of water or raindrop momentum (18). Further experiments are needed to test whether water could also act via RH or via water potential of the soil (33).
We show that biofilm population sizes do not decrease when plants are exposed to a desiccation stress. In contrast, solitary population sizes decreased drastically under those conditions. However, returning plants to high-RH conditions allowed solitary population sizes to increase de novo. Hence, aggregation in biofilms could offer protection from external stress to epiphytic cells of X. axonopodis pv. phaseoli, as has been shown for P. syringae (25). Under field conditions, solitary population sizes are sensitive to rain and temperature fluctuations. However, biofilm populations remain stable under those environmental variations. Hence, protection against harsh environmental conditions could arise either from endophytic localization (2, 46) or from aggregation in biofilms. A stable population size in a variable environment and a population size close to the threshold necessary for symptom induction lead us to hypothesize that biofilms constitute a reservoir of cells for X. axonopodis pv. phaseoli in the field. It has been shown for plant-associated Pseudomonas fluorescens that strains in the two fractions of the population are genetically identical, indicating mixing of solitary and aggregated components of the population (3). We obtained similar results using ERIC fingerprinting for strains of X. axonopodis pv. phaseoli var. fuscans isolated in the studies presented here.
Biofilm population sizes are always lower than solitary population sizes, and differences can reach more than 102 CFU/g of fresh weight. This result is different from data obtained in water-saturated environments (10) and on bean leaves after spray inoculation of P. syringae pv. syringae (25, 26) where the population aggregated in biofilms represents most of the bacterial population. P. syringae pv. syringae viable populations were enumerated on leaf surfaces maintained in controlled conditions while in the study presented here culturable populations were from field-grown plants. In fact, differences observed in the biofilm/solitary ratios could be consequent to induction of a viable but nonculturable (VNC) state for a part of the bacterial populations related to differences in biofilm enumeration methods or to differences in bacterial ecology between these two different bacteria.
Concerning enumeration method bias, the technique used here for quantification of biofilm and solitary components of culturable bacterial population size in the phyllosphere ecosystem is based on a combined use of leaf washing, filtration, and ultrasonication (28). Numerous methods are available to quantify biofilm population sizes in aquatic environments; however, the particular conditions of the leaf surface compared to aquatic systems where solitary bacteria are planktonic and generally attached to inorganic substrates imply first the removal of epiphytic bacterial populations before the separation of solitary from biofilm cells and their disintegration. In the technique adapted to the phyllosphere, the rate of bacterial removal by the first washing step can be measured, and it reflects the presence of bacteria on leaf surfaces. For our samples this rate varies from 35 to 70%, indicating that an important fraction of the population is either firmly attached to the leaf surface or endophytic. Partial dislocation of biofilms during this washing step might occur and could lead to a potential overestimation of the solitary cell fraction. This dislocation is, however, thought to be low (3).
Another difference between enumeration methods used in these studies is the physiological state of enumerated cells. Indeed, we quantified cultivable bacteria, while in the study by Monier and Lindow (25) viable cells were enumerated. In the only published investigation of the existence of VNC cells of foliar bacterial pathogens, it was estimated that half of the population of P. syringae became nonculturable but remained viable 6 days after foliar inoculation (45). Direct estimates of P. syringae cell viability showed that cell viability increased with aggregate size when plants were exposed to desiccation conditions, but no information is available concerning their culturability (25). It is known that biofilms can harbor strains in a VNC state (7). To our knowledge no data are available concerning viability of X. axonopodis pv. phaseoli on beans. However, this VNC state can be induced in X. campestris pv. campestris cells in liquid microcosms and in soil (13). Experimental data are awaited to determine if such a state occurs for X. axonopodis pv. phaseoli on the bean leaf surface. Differences in the ratios of P. syringae and X. axonopodis pv. phaseoli biofilm to solitary components could result from conditions of plant incubation. Indeed, conditions used in the study by Monier and Lindow (25) involved a desiccation stress that could be lethal for solitary cells, as suggested in this work.
Another explanation could arise from the ecology of P. syringae, for example in terms of regulation of biofilm maturation or of dispersal of cells. Biofilm formation has been shown to be regulated by AHLs in different bacteria including presumably P. syringae (42). AHLs were not found in Xanthomonas sp. (6); instead butyrolactones have been shown to act as signal molecules in two quorum-sensing-like systems (31, 42). While AHLs have divergent regulatory roles in different bacteria (11), it is suspected that for P. syringae this system operates positively in a later stage of colonization, being correlated with increasing proportions of cell aggregates (42). For X. campestris pv. campestris production of DSF is suspected to act as a signal to trigger in planta transition to the planktonic lifestyle from aggregates, allowing xylem vessel colonization (11). In contrast, the other signal molecule, DF, has been shown to be implicated in epiphytic fitness on the cabbage phyllosphere (39). We have shown that all tested isolates of X. axonopodis pv. phaseoli coming from solitary and biofilm components from 1998 and 1999 field experiments synthesize both these signal molecules (DF and DSF).
The importance of epiphytic life in the biological cycle may be higher for X. axonopodis pv. phaseoli than X. campestris pv. campestris, which is known to be mainly a vascular pathogen (35). Hence, we hypothesize that, for X. axonopodis pv. phaseoli, the role of DSF could be similar but not in the same plant compartment. Indeed, it could ensure bacterial dispersal by solitary populations on the leaf surface from aggregates but not in the vascular vessels, as for X. campestris pv. campestris. Its role could be demonstrated by mutagenesis, as was used to demonstrate the role of some hrp (hypersensitive reaction and pathogenicity) genes in bean colonization by P. syringae pv. syringae (15). This issue is part of the numerous open questions concerning the determinants of phyllosphere fitness for plant-pathogenic bacteria.
Acknowledgments
This work was supported by a grant from CER Pays de la Loire.
We thank F. Boulineau from GEVES-SEV Brion for bean field cultivation and R. Germain from Vilmorin Clause et Cie for the gift of bean seeds. We thank C. Audrain, M. Lesourd, and R. Filmon from SCIAM, Angers, for help in SEM. We thank J. Gibeaud, A. Morin, and A. Zaborowski for assistance and C. E. Morris and C. Manceau for critical reviews of the manuscript.
REFERENCES
- 1.Barber, C. E., J. L. Tang, J. X. Feng, M. Q. Pan, T. J. G. Wilson, H. Slater, J. M. Dow, P. Williams, and M. J. Daniels. 1997. A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Mol. Microbiol. 24:555-566. [DOI] [PubMed] [Google Scholar]
- 2.Beattie, G. A., and S. E. Lindow. 1994. Comparison of the behavior of epiphytic fitness mutants of Pseudomonas syringae under controlled and field conditions. Appl. Environ. Microbiol. 60:3799-3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boureau, T., M.-A. Jacques, R. Berruyer, Y. Dessaux, H. Dominguez, and C. E. Morris. 2004. Comparison of the phenotypes and genotypes of biofilm and solitary epiphytic bacterial populations on broad-leaved endive. Microb. Ecol. 47:87-95. [DOI] [PubMed] [Google Scholar]
- 4.Broughton, W. J., G. Hernandez, M. Blair, S. Beebe, P. Gepts, and J. Vanderleyden. 2003. Beans (Phaseolus spp.)—model food legumes. Plant Soil 252:55-128. [Google Scholar]
- 5.Carmichael, I., I. S. Harper, M. J. Coventry, P. W. J. Taylor, J. Wan, and M. W. Hickey. 1999. Bacterial colonization and biofilm development on minimally processed vegetables. J. Appl. Microbiol. Symp. Suppl. 85:45S-51S. [DOI] [PubMed] [Google Scholar]
- 6.Cha, C., P. Gao, Y. C. Chen, P. D. Shaw, and S. K. Farrand. 1998. Production of acyl-homoserine lactone quorum sensing signals by gram-negative plant-associated bacteria. Mol. Plant-Microbe Interact. 11:1119-1129. [DOI] [PubMed] [Google Scholar]
- 7.Chae, M. S., and H. Schraft. 2001. Cell viability of Listeria monocytogenes biofilms. Food Microbiol. 18:103-112. [Google Scholar]
- 8.Characklis, W. G., and K. C. Marshall. Biofilms. John Wiley & Sons, New York, N.Y.
- 9.Claflin, L. E., A. K. Vidaver, and M. Sasser. 1987. MXP, a semi-selective medium for Xanthomonas campestris pv. phaseoli. Phytopathology 77:730-734. [Google Scholar]
- 10.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711-745. [DOI] [PubMed] [Google Scholar]
- 11.Dow, J. M., L. Crossman, K. Findlay, Y.-Q. He, J.-X. Feng, and J.-L. Tang. 2003. Biofilm dispersal in Xanthomonas campestris is controlled by cell-cell signaling and is required for full virulence to plants. Proc. Natl. Acad. Sci. USA 100:10995-11000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fett, W. F. 2000. Naturally occurring biofilms on alfalfa and other types of sprouts. J. Food Prot. 63:625-635. [DOI] [PubMed] [Google Scholar]
- 13.Ghezzi, J. I., and T. R. Steck. 1999. Induction of the viable but non-culturable condition in Xanthomonas campestris pv. campestris in liquid microcosms and sterile soil. FEMS Microbiol. Ecol. 30:203-209. [DOI] [PubMed] [Google Scholar]
- 14.Gilbertson, R. L., and D. P. Maxwell. 1992. Common bacterial blight of bean, p. 18-39. In H. S. Chaube and J. Kumar (ed.), Plant diseases of international importance, vol. II. Diseases of vegetables and oil seed crop. Prentice Hall, Englewood Cliffs, N.J. [Google Scholar]
- 15.Hirano, S. S., A. O. Charkowski, A. Collmer, D. K. Willis, and C. D. Upper. 1999. Role of the Hrp type III protein secretion system in growth of Pseudomonas syringae pv. syringae B728a on host plants in the field. Proc. Natl. Acad. Sci. USA 96:9851-9856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirano, S. S., and C. D. Upper. 1983. Ecology and epidemiology of foliar bacterial plant pathogens. Annu. Rev. Phytopathol. 21:243-269. [Google Scholar]
- 17.Hirano, S. S., and C. D. Upper. 1989. Diel variation in population size and ice nucleation activity of Pseudomonas syringae on snap bean leaflets. Appl. Environ. Microbiol. 55:623-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirano, S. S., L. S. Baker, and C. D. Upper. 1996. Raindrop momentum triggers growth of leaf-associated populations of Pseudomonas syringae on field-grown snap bean plants. Appl. Environ. Microbiol. 62:2560-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawrence, J. R., D. R. Korber, G. M. Wolfaardt, and D. E. Caldwell. 1995. Behavioral strategies of surface-colonizing bacteria. Adv. Microb. Ecol. 14:1-75. [Google Scholar]
- 20.Manceau, C., and A. Horvais. 1998. Strategies for identification of oligonucleotidic sequences specific to plant pathogenic bacteria useful to design primers for PCR, p. 89-97. In C. Manceau and J. Spack (ed.), Advances in the detection of plant pathogens by polymerase chain reaction. Office for Official Publications of the European Communities, Luxembourg, Luxembourg.
- 21.Mansvelt, E. L., and M. J. Hattingh. 1989. Scanning electron microscopy of invasion of apple leaves and blossoms by Pseudomonas syringae pv. syringae. Appl. Environ. Microbiol. 55:533-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marques, A. S. D., R. Corbière, L. Gardan, C. Tourte, C. Manceau, J. D. Taylor, and R. Samson. 2000. Multiphasic approach for the identification of the different classification levels of Pseudomonas savastanoi pv. phaseolicola. Eur. J. Plant Pathol. 106:715-734. [Google Scholar]
- 23.Marques, L. L. R., H. Ceri, G. P. Manfio, D. M. Reid, and M. E. Olson. 2002. Characterization of biofilm formation by Xylella fastidiosa in vitro. Plant Dis. 86:633-638. [DOI] [PubMed] [Google Scholar]
- 24.Maury, Y., C. Duby, J.-M. Bossennec, and G. Boudazin. 1985. Group analysis using ELISA: determination of the level of transmission of soybean mosaic virus in soybean seed. Agronomie 5:405-415. [Google Scholar]
- 25.Monier, J.-M., and S. E. Lindow. 2004. Differential survival of solitary and aggregated bacterial cells promotes aggregate formation on leaf surfaces. Proc. Natl. Acad. Sci. USA 100:15977-15982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris, C. E., and J.-M. Monier. 2003. The ecological significance of biofilm formation by plant-associated bacteria. Annu. Rev. Phytopathol. 41:429-453. [DOI] [PubMed] [Google Scholar]
- 27.Morris, C. E., J.-M. Monier, and M.-A. Jacques. 1997. Methods for observing microbial biofilms directly on leaf surfaces and recovering them for isolation of culturable microorganisms. Appl. Environ. Microbiol. 63:1570-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris, C. E., J.-M. Monier, and M.-A. Jacques. 1998. A technique to quantify the population size and composition of the biofilm component in communities of bacteria in the phyllosphere. Appl. Environ. Microbiol. 64:4789-4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piper, K. R., S. Beck von Bodman, and S. K. Farrand. 1993. Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature 362:448-450. [DOI] [PubMed] [Google Scholar]
- 30.Poplawsky, A. R., and W. Chun. 1998. Xanthomonas campestris pv. campestris requires a functional pigB for epiphytic survival and host infection. Mol. Plant-Microbe Interact. 11:466-475. [DOI] [PubMed] [Google Scholar]
- 31.Poplawsky, A. R., W. Chun, H. Slater, M. J. Daniels, and J. M. Dow. 1998. Synthesis of extracellular enzymes, and xanthomonadin in Xanthomonas campestris: evidence for the involvement of two intercellular regulatory signals. Mol. Plant-Microbe Interact. 11:68-70. [Google Scholar]
- 32.Poplawsky, A. R., S. C. Urban, and W. Chun. 2000. Biological role of xanthomonadin pigments in Xanthomonas campestris pv. campestris. Appl. Environ. Microbiol. 66:5123-5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts, S. J., M. S. Ridout, L. Peach, and J. Brough. 1996. Transmission of pea bacterial blight (Pseudomonas syringae pv. pisi) from seed to seedling: effects of inoculum dose, inoculation method, temperature and soil moisture. J. Appl. Bacteriol. 81:65-72. [Google Scholar]
- 34.Rouse, D. I., E. V. Nordheim, S. S. Hirano, and C. D. Upper. 1985. A model relating the probability of foliar disease incidence to the population frequencies of bacterial plant pathogens. Phytopathology 75:505-509. [Google Scholar]
- 35.Rudolph, K. 1993. Infection of the plant by Xanthomonas, p. 193-264. In J. G. Swings and E. L. Civerolo (ed.), Xanthomonas. Chapman & Hall, London, United Kingdom.
- 36.Sokal, R. R., and F. J. Rohlf. Biometry. The principles and practice of statistics in biological research. W. H. Freeman & Co., San Francisco, Calif.
- 37.Swaroop, S. 1951. The range of variation of the most probable number of organisms estimated by the dilution method. Indian J. Med. Res. 39:107-134. [PubMed] [Google Scholar]
- 38.Timmer, L. W., J. J. Marois, and D. Achor. 1987. Growth and survival of xanthomonads under conditions nonconducive to disease development. Phytopathology 77:1341-1345. [Google Scholar]
- 39.Tombolini, R. A., M. E. Unge, M. E. Davey, F. J. de Bruijn, and J. K. Jansson. 1997. Flow cytometric and microscopic analysis of GFP-tagged Pseudomonas fluorescens bacteria. FEMS Microbiol. Ecol. 22:17-28. [Google Scholar]
- 40.Versalovic, J., T. Koeuth, and J. R. Lupski. 1991. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 19:6823-6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vidaver, A. K. 1993. Xanthomonas campestris pv. phaseoli: cause of common bacterial blight, p. 121-146. In J. G. Swings and E. L. Civerolo (ed.), Xanthomonas. Chapman & Hall, London, United Kingdom.
- 42.von Bodman, S. B., W. Dietz Bauer, and D. L. Coplin. 2003. Quorum sensing in plant-pathogenic bacteria. Annu. Rev. Phytopathol. 41:455-482. [DOI] [PubMed] [Google Scholar]
- 43.Weller, D. M., and A. W. Saettler. 1980. Evaluation of seedborne Xanthomonas phaseoli and X. phaseoli var. fuscans as primary inocula in bean blights. Phytopathology 70:148-152. [Google Scholar]
- 44.Weller, D. M., and A. W. Saettler. 1980. Colonization and distribution of Xanthomonas phaseoli and Xanthomonas phaseoli var. fuscans in field-grown navy beans. Phytopathology 70:500-506. [Google Scholar]
- 45.Wilson, M., and S. E. Lindow. 1992. Relationship of total viable and culturable cells in epiphytic populations of Pseudomonas syringae. Appl. Environ. Microbiol. 58:3908-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson, M., S. S. Hirano, and S. E. Lindow. 1999. Location and survival of leaf-associated bacteria in relation to pathogenicity and potential for growth within the leaf. Appl. Environ. Microbiol. 65:1435-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]