Abstract
The spatial distribution of an uncultured clade of marine diazotrophic γ-proteobacteria in the Arabian Sea was investigated by the development of a specific primer pair to amplify an internal fragment of nifH by PCR. These organisms were most readily detected in highly oligotrophic surface waters but could also be found in deeper waters below the nutricline. nifH transcripts originating from this clade were detected in oligotrophic surface waters and, in addition, in the deeper and the more productive near-coastal waters. The nifH sequences most closely related to the unidentified marine bacterial group are from environmental clones amplified from the Atlantic and Pacific Oceans. These findings suggest that these γ-proteobacteria are widespread and likely to be an important component of the heterotrophic diazotrophic microbial community of the tropical and subtropical oceans.
Over the past decade, biological oceanographers have increasingly recognized the importance of biological nitrogen fixation in supporting primary production in the oligotrophic open oceans (3, 6, 7). Based on the extrapolated contribution of the best-known marine diazotrophs, the cyanobacterial Trichodesmium spp., present estimates put the marine contribution to global annual nitrogen fixation at ∼80 Tg of N per annum (2). Recent work has shown, however, that as well as the symbiotic cyanobacteria found in tropical diatoms (14), there are planktonic unicellular cyanobacteria that fix nitrogen at rates comparable to or even in excess of those of Trichodesmium spp. (5, 21). Furthermore, culture-independent assessments of marine diazotroph diversity targeting nifH (encoding the Fe protein of nitrogenase) have revealed that heterotrophic nitrogen fixers are also present in the tropical and subtropical oceans (20). Their overall contribution to marine nitrogen fixation remains to be assessed, but that they are actively expressing nitrogenase in situ has been confirmed (4). It seems likely, therefore, that nitrogen fixation is of even greater importance in oceanic waters than has been considered hitherto.
Nitrogenase genes derived from diazotrophic α- and γ-proteobacteria have been amplified from samples from both the Atlantic and Pacific Oceans (20). The γ-proteobacteria-related sequences were found numerous times and include a group of closely related phylotypes that are most similar to nifH from Azotobacter and Vibrio spp. Since diazotrophic Vibrio spp. are frequently encountered in coastal waters (12), Zehr and coworkers (20) have proposed that they or very closely related γ-proteobacteria are major nitrogenase-containing phylotypes in the marine environment. To date, however, no studies have been carried out to establish how widely distributed these organisms are or to investigate the environmental conditions that are conducive to nitrogenase expression.
The present study is part of a wider investigation of the diversity and transcriptional activity of diazotrophic bacteria along a transect of contrasting biogeochemical conditions in the Arabian Sea. These conditions ranged from surface waters near the equator that were almost devoid of N and P to nutrient-rich regions off the coast of Oman that were influenced by a recent upwelling driven by the SW monsoon. During the course of the study, we isolated a number of partial nifH clones belonging to a cluster of closely related uncultured marine bacteria (UMB) which were most similar phylogenetically to γ-proteobacterial environmental clones from the tropical and subtropical Atlantic and Pacific Oceans.
To assess the distribution of the UMB group and its potential contribution to nitrogen fixation, primers that specifically amplify a fragment of nifH from members of this clade were designed. We show that members of this group of diazotrophic heterotrophic bacteria are widely distributed in the Arabian Sea and that they are transcriptionally active in nutrient-rich as well as oligotrophic waters. Whereas nitrogenase expression is repressed in the presence of combined nitrogen in most diazotrophic bacteria, our findings suggest that this was not the case for the UMB group.
MATERIALS AND METHODS
Study site, collection, and storage of plankton samples.
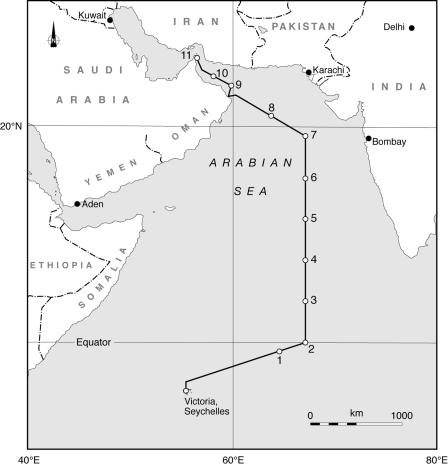
Observations were made in the northern Indian Ocean in September 2001 during the Natural Environment Research Council AMBITION cruise aboard the RRS Charles Darwin. The cruise departed in late August from Victoria (Seychelles Islands) toward the end of the SW monsoon season. Over the following month, the research vessel occupied 11 stations (Fig. 1), ranging in nutrient status from the ultraoligotrophic waters at the southern end of the transect to the more productive northerly stations influenced by seasonal upwelling.
FIG. 1.
Location of the stations (1 to 11) occupied in the Arabian Sea during the AMBITION cruise in September 2001.
Seawater samples were obtained from discrete depths with 30-liter GoFlo (General Oceanics) bottles or during conductivity-temperature-depth hydrocasts by using a rosette of 30-liter Niskin bottles. Stand-alone pumps (SAPS) equipped with 192-mm-diameter, 0.2-μm-pore-size polycarbonate filters (Osmonics) were deployed at some stations to filter large-volume (>100 liters) seawater samples in situ. All other samples (5 to 10 liters) were filtered at negative vacuum pressures of 10 to 20 cm of Hg through 90-mm-diameter, 0.2-μm-pore-size polycarbonate filters immediately following recovery. All samples for RNA analysis were processed within 10 to 15 min of collection, and the filtered samples were stored in RNALater (Ambion) at 4°C. Plankton samples for DNA analysis were lysed in DNA extraction buffer (250 mM NaCl, 100 mM EDTA, 100 mM Tris-HCl [pH 8.0], and 1% [wt/vol] sodium dodecyl sulfate [SDS]) and stored at −70°C. After the research vessel was docked in Muscat (Oman) at the end of the cruise, the stored samples were shipped on dry ice by air to the United Kingdom.
Isolation and purification of nucleic acids.
DNA was extracted and purified with a DNeasy tissue kit (QIAGEN) following the manufacturer's protocol for bacterial DNA with minor modifications. Cell lysate (180 μl) was amended with 25 μl of proteinase K solution (Roche) and incubated at 70°C for 30 min. Buffer AL (200 μl; QIAGEN) and 200 μl of 95% (vol/vol) ethanol were added to the digested lysate, and the recommended protocol was followed thereafter. Plankton samples stored in RNALater were centrifuged briefly at 13, 000 × g, washed in artificial seawater medium (16), and RNA isolated by using an acid phenol extraction protocol (15). The integrity of DNA and RNA samples was verified by electrophoresis through nuclease-free agarose gels stained with ethidium bromide, and any degraded samples were discarded.
Amplification of nifH by PCR.
A nested PCR protocol was used to amplify nifH from DNA samples with the primer pair NifH3 and NifH4 (18) in the first reaction, and equal quantities of modified versions of the CDPKAD and SGEMMA primers described by Zehr and McReynolds (19) were used for the nested reaction (Table 1). PCRs containing 4 mM MgCl2 and a 0.5 μM concentration of each primer were performed with a Taq PCR Master Mix kit (QIAGEN). After the reaction mix was heated to 94°C, 0.2 to 10 ng of DNA was added to the primary reaction and 1 μl of a 1-in-100 dilution of the resulting amplicons was added to the nested reaction. Cycling conditions for the primary PCR were as follows: initial denaturation at 94°C for 2 min; 40 cycles at 94°C (1 min), 55°C (1 min), and 72°C (1 min); and a final extension at 72°C for 10 min. The same cycling and reaction conditions were used for the nested reaction, except that the primer annealing temperature was increased to 57°C.
TABLE 1.
Nucleotide sequences of primers used in this study
| Primer name | Primer nucleotide sequence (5′-3′) |
|---|---|
| CDPKAD1 | TGYGAYCCNAANGCNGAT |
| CDPKAD2 | TGYGAYCCNAANGCNGAC |
| SGEMMA1 | GCCATCATYTCNCCNGA |
| SGEMMA2 | GCCATCATYTCNCCNCT |
| TOPOforw | TAGTAACGGCCGCCAGTGTG |
| TOPOrev | GGCCGCCAGTGTGATGGATA |
| UMBforw | CACAATAATGGAGATGGCTGCAC |
| UMBrev | CAAATCCGCCACAAACCACATCA |
For the amplification of nifH from the UMB clade, specific primers (UMBforw and UMBrev) were designed (Table 1) and used in the nested reaction containing 2 mM MgCl2 and a 0.5 μM concentration of each primer. The cycling conditions were as follows: initial denaturation at 94°C for 2 min; 40 cycles at 94°C (1 min), 53°C (1 min), and 72°C (1 min); and a final extension at 72°C for 10 min. The UMB primers were designed following inspection of a Clustal X alignment (9) of all of the DNA sequences retrieved in this study, and they amplify a 242-bp region of UMB nifH. Equal quantities of DNA or RNA were used in experiments to investigate the horizontal and vertical distribution of diazotrophs, including members of the UMB clade or nifH gene expression, respectively.
Cloning, purification of plasmid DNA, sequencing, and phylogenetic analysis of PCR products.
PCR products were electrophoresed through a 1% (wt/vol) agarose gel and purified by using a QIAquick gel extraction kit (QIAGEN). Purified DNA fragments were inserted into pCR2.1 TOPO and transformed into TOP10 One Shot Escherichia coli cells (Invitrogen) following the supplier's recommended protocol. Following initial screening experiments, the purified PCR products were digested with the restriction endonuclease XbaI at 37°C for 2 h prior to cloning. The nifH genes of Trichodesmium spp. have a recognition site for XbaI toward the 3′ end of the nested fragment that a search of the GenBank database showed was absent from other nifH genes from representative cultured and uncultured diazotrophic bacteria. Digestion with XbaI cleaves the additional A residue added by Taq DNA polymerase plus a short stretch of flanking sequence at the 3′ end of the PCR product, making it unlikely that the digested fragment will be inserted successfully into the T-tailed vector used. This procedure was adopted to reduce the sequence redundancy of the libraries which, in samples from the southern end of the transect in particular, were found to be dominated by nifH genes from Trichodesmium spp. unless predigested. Digested PCR products were electrophoresed through a 1% (wt/vol) agarose gel and purified by using a QIAquick gel extraction kit (QIAGEN). Purified DNA fragments were inserted into pCR2.1 TOPO and transformed into TOP10 One Shot E. coli cells (Invitrogen) following the supplier's recommended protocol.
Recombinant colonies were screened for inserts of the expected size (∼360 bp) by PCR. E. coli cells were resuspended in 11.5 μl of deionized water amended with a 0.5 μM concentration (1.0 μl) of each primer and incubated at 99°C for 10 min. The primer pair used (TOPO forw and TOPO rev) are complementary to vector sequences flanking the insert (Table 1). QIAGEN Taq PCR master mix (12.5 μl) was added to each reaction, and the PCR was performed by using the following cycling conditions: 25 cycles at 94°C (1 min), 64°C (1 min), and 72°C (1 min) followed by a final extension at 72°C for 10 min. Recombinants containing inserts of the expected size were grown in Luria-Bertani broth plus 100 μg ml of ampicillin−1 at 37°C overnight, and plasmid DNA was purified by using a QIAprep Spin Miniprep kit (QIAGEN).
Sequencing of plasmid DNA was performed with M13 primers and a DYEnamic sequencing kit (Amersham) on an ABI Prism model 377 automated sequencer. Sequences were manually trimmed of the primer sequences, and the derived peptide sequences were aligned by using Clustal X. Phylogenetic analysis of the UMB DNA sequences was performed following alignment of the 242-bp internal region of nifH amplified from members of this clade and the corresponding region of the coding sequences of four other environmental clones isolated previously (20). Genetic distances were estimated by using the Kimura correction, and phylogenetic analysis was performed by using a neighbor-joining routine from the TREECON for Windows software package (13). The trees are based on 1,000 bootstrap replicates.
Determination of nifH gene expression by RT-PCR.
Reverse transcription (RT)-PCR was performed with a QIAGEN OneStep RT-PCR kit. RT was carried out at 50°C in a 50-μl reaction containing 1 μl of a 1-in-10 dilution of RNA and 0.5 μM (each) concentrations of the NifH3 and NifH4 primers. HotStar Taq DNA polymerase was activated by incubation at 95°C for 15 min, and cDNAs were amplified for 40 cycles by using the reaction conditions given above for the this primer pair. A second nested PCR was performed with the CDPKAD and SGEMMA primer pair by using 1 μl of a 1-in-100 dilution of cDNA and reaction conditions as described for DNA samples. The purity of each sample was verified by omitting the RT step in parallel reactions performed under otherwise identical conditions. Only those positive samples that failed to produce a PCR product in the absence of the RT step were included in this study.
Agarose gel electrophoresis, blotting, and hybridization of DNA and cDNA.
PCR and RT-PCR products were electrophoresed through 1.5% agarose gels containing 10 μg ml of ethidium bromide−1. After denaturation in 0.5 M NaOH-1.5 M NaCl, the products were transferred to positively charged nylon membranes (Roche) in 10× SSC (1× SSC is 0.15 M NaCl and 15 mM sodium citrate [pH 7.5]). DNA was immobilized by UV irradiation, and the blots were prehybridized in 5× SSC, 1% blocking reagent (Roche), 0.1% Sarkosyl, and 0.02% SDS at 68°C for 1 h. The prehybridization solution was discarded and replaced with a fresh aliquot of prewarmed prehybridization solution amended with 25 ng ml−1 of denatured UMB probe DNA labeled with digoxigenin-dUTP by PCR using the reaction and cycling conditions given above. After overnight hybridization at 68°C, the blots were washed in 2× SSC-0.1% SDS at room temperature followed by two 30-min washes in 0.001× SSC-0.1% SDS. Hybrids were detected by using alkaline phosphatase-conjugated antidigoxigenin and the chemiluminescent substrate CDP-Star as recommended by the supplier (Roche). Luminographs were obtained by exposure of the blots to Kodak ML film. Cross hybridization experiments confirmed that under the stringency conditions applied in this investigation, the UMB probe does not detect nifH amplicons from any other clade of marine diazotrophs that were recovered from the Arabian Sea as part of a wider study.
Nucleotide sequence accession numbers.
The nifH sequences reported in this paper have been deposited in the GenBank database under accession numbers AY800134 to AY800143.
RESULTS
Phylogenetic analysis of UMB clade nifH and design of specific PCR primers.
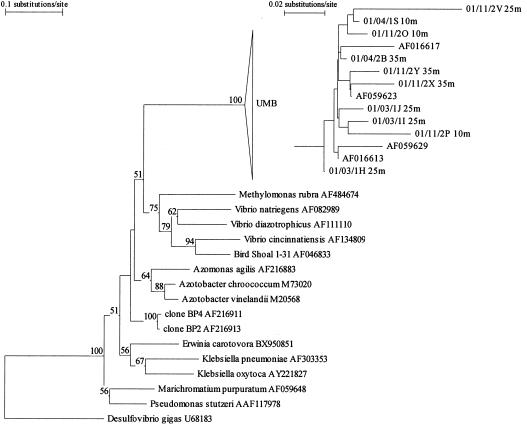
Phylogenetic analysis of the nifH clones retrieved in this study revealed a lineage of closely related (>98% DNA identity) sequences that had greatest similarity to members of the γ-proteobacteria. DNA sequences sharing identity to UMB nifH were retrieved by using the NCBI sequence similarity search tool BLASTN (Basic Local Alignment Search Tool) (1) and aligned, and a neighbor-joining tree was generated (Fig. 2). The analysis revealed that the most closely related previously published sequences were derived from environmental clones amplified from subtropical oligotrophic waters from the Atlantic and Pacific Oceans (20). For example, the DNA sequence of the clone 01/11/2O is 98% identical to that of an environmental clone originating from a depth of 125 m in the Atlantic Ocean (GenBank accession number AF016613). The most similar nifH fragments from cultured organisms are from a group that includes Vibrio natriegens and Azotobacter vinelandii (75 to 81% DNA identity).
FIG. 2.
Phylogram showing the relationship between the nifH gene of the UMB cluster and other diazotrophic members of the γ-proteobacteria. The percentages (>50%) of 1,000 bootstrap replicates supporting the topography of the tree are shown at the nodes. The nifH gene from the δ-proteobacterium Desulfovibrio gigas was used as an outgroup. The inset phylogram shows the relationship between the nifH gene of the UMB clade including four previously reported environmental clones. The tree is based on all 242 bp of the nifH DNA sequences recovered and the corresponding coding regions of the previously reported clones.
A Clustal X alignment of the UMB sequences with related nifH genes from γ-proteobacteria was used to design specific oligonucleotide primers (UMBforw and UMBrev) for the UMB clade. The primer regions selected target a 242-bp region of UMB nifH. They differ in at least four positions for the forward primer and five positions for the reverse primer from the corresponding regions of the next most closely related nifH sequences in the GenBank database, excluding the previously recovered environmental clones. An NCBI BLASTN sequence similarity search did not reveal any greater similarity to the primers among nifH genes from other taxonomic groupings of bacteria.
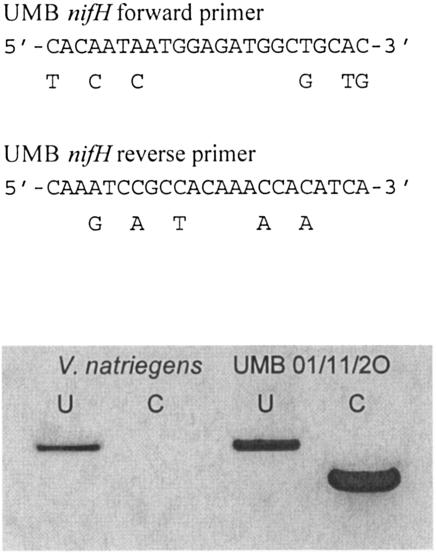
The specificity of the primers was verified by amplifying the cloned region of nifH from V. natriegens and a member of the UMB clade (clone 01/11/2O) with both the nested universal primer set and the UMB primer pair by using the reaction conditions described above. As expected, both templates produced a band of ∼360 bp with the universal primers, whereas only the UMB nifH clone produced the expected band of 242 bp with the specific primers (Fig. 3). Sequencing of several randomly chosen clones amplified with the UMB primers from DNA samples from station 1 showed that they shared >98% DNA sequence identity.
FIG. 3.
Alignment of the UMB primers with the corresponding regions of nifH from the γ-proteobacterium V. natriegens (top). The nucleotide positions where the V. natriegens sequence differs are indicated by the corresponding bases below the UMB sequences. The analytical agarose gel shows the PCR products obtained from either V. natriegens or the UMB clone 01/11/2O with either the universal nested primer set (U) or UMB primers (C) (bottom). The same template concentrations were used for each reaction.
Depth distribution of diazotrophs including the UMB cluster and nifH mRNA.
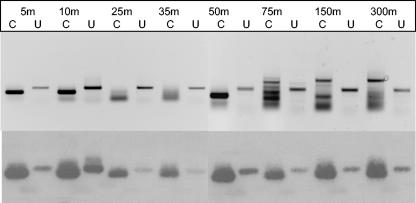
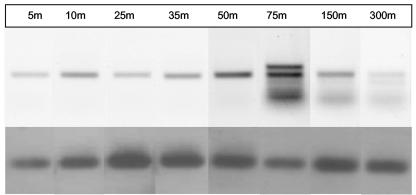
Nitrogen-fixing organisms were found throughout the upper 300 m of the water column at station 1 (Fig. 4). In comparison to the signal obtained with universal primers, the most intense bands for the UMB clade were observed in surface waters (5 m) and at a depth of 50 m that was coincident with the thermocline. Perhaps because of the low abundance of UMB target DNA, several nonspecific PCR products were amplified from samples from deeper waters. Nevertheless, Southern blotting revealed that the members of the UMB clade were present at every depth.
FIG. 4.
Depth distribution of nifH-containing organisms at station 1 detected with either the universal (U) or UMB-specific (C) nested primers (upper panel) and Southern blot of the same gel probed with the UMB probe (lower panel).
RT-PCR revealed that nifH was being actively expressed in the upper water column (Fig. 5) even though combined nitrogen increased from low nanomolar concentrations in surface waters to concentrations of >5 μM below the thermocline. While libraries generated from waters shallower than 75 m were dominated by clones derived from Trichodesmium spp. at this station, sequences from these cyanobacteria were rarely encountered in samples from greater depths (data not shown). However, Southern blotting of the cDNAs showed that members of the UMB clade were present and actively transcribing nitrogenase at every depth sampled.
FIG. 5.
Depth distribution of transcriptionally active nifH-containing organisms at station 1 amplified by RT-PCR with the universal nested primers (upper panel) and Southern blot of nifH cDNAs probed with the UMB probe (lower panel).
Horizontal distribution of diazotrophs including the UMB cluster and nifH mRNA.
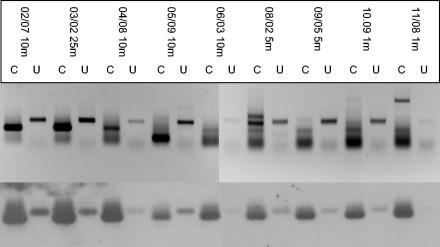
Despite the large variation in combined nitrogen concentrations between the southern and northern ends of the transect, nifH-containing organisms, including members of the UMB clade, were detected in surface waters at all stations (Fig. 4 and 6). The most intense product bands were found at the most oligotrophic southerly stations (stations 1 to 3), while only very faint products were detected at coastal station 11. Southern blotting confirmed, however, that the UMB clade was present at this station, albeit at much reduced concentrations compared to those in open waters at the start of the transect.
FIG. 6.
Horizontal distribution of nifH-containing organisms in the Arabian Sea amplified by using either the universal (U) or UMB-specific (C) nested primers (upper panel). The station and cast numbers are shown (as station number/cast number) for each sample and are followed by the depth from which the sample was obtained. Lower panel, Southern blot of the same gel probed with the UMB probe.
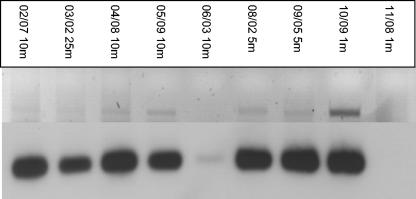
With the exception of station 11, nifH RT-PCR products were amplified from surface waters at all stations (Fig. 4 and 7). A very faint product was detected at station 6, and this finding may reflect the generally lower abundance of diazotrophs found at this station (Fig. 6). Southern blotting using the UMB probe, however, showed that this clade was transcriptionally active at this and the other stations where nifH mRNA was detected. While the UMB clade was more abundant at the southern stations, equally intense cDNA hybridization signals were detected at the more northerly stations, indicating active transcription over a wide range of nutrient concentrations.
FIG. 7.
Horizontal distribution of transcriptionally active nifH-containing organisms in the Arabian Sea amplified by RT-PCR with the universal nested primers (upper panel) and Southern blot of nifH cDNAs probed with the UMB probe (lower panel).
DISCUSSION
A previous pioneering study revealed that there is a rich diversity of heterotrophic diazotrophs in the oligotrophic oceans (20) in addition to the well-known colonial cyanobacteria, Trichodesmium spp. The potential contribution of these heterotrophs to the oceanic nitrogen budget is unknown, however, since very few studies have attempted to quantify heterotrophic nitrogen fixation in open waters directly. Transcriptionally active phylotypes of nitrogen-fixing proteobacteria have been recovered from both daytime and nighttime samples from the Pacific Ocean (4). Rates of nitrogenase activity for the <10-μm fraction (i.e., excluding Trichodesmium spp.) measured during the day, however, were negligible compared to those recorded at night, when newly described unicellular cyanobacteria are known to be actively fixing N2 (21). Due to the low abundance of natural populations of nitrogen-fixing bacteria in the open ocean and the comparatively low sensitivity of the acetylene reduction technique, the nitrogenase enzyme assays conducted by Falcón et al. (4) required prior concentration (>100-fold) of the bacterioplankton community. Given the potential for C limitation and a myriad of other in vitro effects, it remains uncertain whether this procedure may have differentially influenced the activity of diazotrophic heterotrophs compared to that accounted for by phototrophic unicellular cyanobacteria.
The present study has shown that there is an active clade of diazotrophic γ-proteobacteria that is both present and actively transcribing nitrogenase over a wide area in the Arabian Sea. These nifH phylotypes are closely related to previously described environmental clones from the Atlantic and Pacific Oceans (20), indicating that they are globally distributed in the tropical and subtropical ocean. Most of the RNA samples used for this study were collected during daylight, i.e., at times of day when Trichodesmium spp. should be actively expressing nitrogenase (17). These large colonial cyanobacteria dominated the unscreened nifH clone libraries at the more southerly stations. However, even at these stations, UMB nifH cDNAs were detected by hybridization of the RT-PCR products amplified with the universal nested primers. Hence, this clade made a significant contribution to the community nifH mRNA pool which was readily detectable even though the pool was expected to be dominated by nifH cDNAs amplified from Trichodesmium spp.
The question of whether these organisms are fixing atmospheric N2 in situ remains, however. From what is known of the regulation of nitrogenase synthesis in cultured organisms, it seems likely that the detection of nifH mRNA is a reasonable proxy indicator of active N2 fixation. Nitrogenase expression is regulated principally by the availability of combined nitrogen and oxygen, and each of these environmental factors regulates expression at a number of different genetic levels. For example, in many heterotrophic diazotrophic bacteria, the nitrogen fixation apparatus is expressed only under low oxygen tensions (10). Exposure to higher oxygen concentrations not only shuts down transcription of the nitrogenase structural genes but also inactivates the enzyme via a posttranslational modification of the Fe protein. Likewise, the addition of ammonium leads to the rapid cessation of both transcription and activity (10).
One of the surprising features of nitrogenase expression by the UMB cluster, however, is that nifH mRNA was detected in samples from below the nutricline at station 1 and also in surface waters at the very productive coastal sites impacted by upwelling. While the availability of combined N might be expected to down-regulate nitrogenase expression, it may be that members of the UMB clade lack the assimilatory enzymes to utilize either nitrate or nitrite or, as has been observed in salt marsh communities (8), that the presence of combined N has little effect on nitrogenase expression. Even in those diazotrophic organisms known to have the genetic capacity to utilize both nitrate and nitrite, the effect of combined N on nitrogen fixation is variable. For example, Trichodesmium spp. are able to take up combined nitrogen and fix N2 simultaneously, albeit at lower rates than in the absence of combined N (11). Likewise, there is very little effect of low (<5 μM) ammonium concentrations on nitrogenase expression or activity. Given the low concentrations of combined N that are typical of tropical and subtropical open waters, it may be that a lack of tight ammonium regulation is a general feature of diazotrophic organisms from these waters.
Acknowledgments
This research was supported by a project grant (NER/T/S/2000/00633) from the Natural Environment Research Council of the United Kingdom as part of the Marine and Freshwater Microbial Diversity (M&FMB) thematic program.
We thank the officers, crew, and technicians of RRS Charles Darwin for their excellent support during the AMBITION cruise.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capone, D. G., J. P. Zehr, H. W. Paerl, B. Bergman, and E. J. Carpenter. 1997. Trichodesmium, a globally significant marine cyanobacterium. Science 276:1221-1229. [Google Scholar]
- 3.Carpenter, E. J., and K. Romans. 1991. Major role of the cyanobacterium Trichodesmium in nutrient cycling in the North Atlantic Ocean. Science 254:1356-1358. [DOI] [PubMed] [Google Scholar]
- 4.Falcón, L. I., E. J. Carpenter, F. Cipriano, B. Bergman, and D. G. Capone. 2004. N2 fixation by unicellular bacterioplankton from the Atlantic and Pacific Oceans: phylogeny and in situ rates. Appl. Environ. Microbiol. 70:765-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falcón, L. I., F. Cipriano, A. Y. Christoserdov, and E. J. Carpenter. 2002. Diversity of diazotrophic unicellular cyanobacteria in the tropical North Atlantic Ocean. Appl. Environ. Microbiol. 68:5760-5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falkowski, P. G. 1997. Evolution of the nitrogen cycle and its influence on the biological sequestration of CO2 in the ocean. Nature 387:272-275. [Google Scholar]
- 7.Gruber, N., and J. L. Sarmiento. 1997. Global patterns of marine nitrogen fixation and denitrification. Global Biogeochem. Cycles 11:235-266. [Google Scholar]
- 8.Hanson, R. B. 1977. Nitrogen fixation (acetylene reduction) in a salt marsh amended with sewage sludge and organic carbon and nitrogen compounds. Appl. Environ. Microbiol. 33:846-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeanmougin, F., J. D. Thompson, M. Gouy, D. G. Higgins, and T. J. Gibson. 1998. Multiple sequence alignment with Clustal X. Trends Biochem. Sci. 23:403-405. [DOI] [PubMed] [Google Scholar]
- 10.Merrick, M. J. 1992. Regulation of nitrogen fixation genes in free-living and symbiotic bacteria, p. 835-877. In G. Stacey, H. J. Evans, and R. H. Burris (ed.), Biological nitrogen fixation. Chapman and Hall, New York, N.Y.
- 11.Mulholland, M. R., and D. G. Capone. 2000. The physiology of the marine N2 fixing cyanobacteria Trichodesmium. Trends Plant Sci. 5:148-153. [DOI] [PubMed] [Google Scholar]
- 12.Tibbles, B. J., and D. E. Rawlings. 1994. Characterization of nitrogen-fixing bacteria from a temperate saltmarsh lagoon, including isolates that produce ethane from acetylene. Microb. Ecol. 27:65-80. [DOI] [PubMed] [Google Scholar]
- 13.Van de Peer, Y., and R. de Wachter. 1994. TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput. Applic. Biosci. 10:569-570. [DOI] [PubMed] [Google Scholar]
- 14.Villareal, T. G. 1992. Marine nitrogen-fixing diatom-cyanobacterial symbioses, p. 163-175. In E. J. Carpenter, D. G. Capone, and J. G. Reuter (ed.), Marine pelagic cyanobacteria: Trichodesmium and other diazotrophs. NATO ASI series, vol. 362. Kluwer Academic Publishers, Dordrecht, The Netherlands. [Google Scholar]
- 15.Wyman, M. 1999. Diel rhythms in ribulose-1,5-bisphosphate carboxylase/oxygenase and glutamine synthetase gene expression in a natural population of marine picoplanktonic cyanobacteria (Synechococcus spp.). Appl. Environ. Microbiol. 65:3651-3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wyman, M., R. P. F. Gregory, and N. G. Carr. 1985. A novel role for phycoerythrin in the marine cyanobacterium, Synechococcus sp. DC2. Science 230:818-820. [DOI] [PubMed] [Google Scholar]
- 17.Wyman, M., J. P. Zehr, and D. G. Capone. 1996. Temporal variability in nitrogenase gene expression in natural populations of the marine cyanobacterium Trichodesmium thiebautii. Appl. Environ. Microbiol. 62:1073-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zani, S., M. T. Mellon, J. L. Collier, and J. P. Zehr. 2000. Expression of nifH genes in natural microbial assemblages in Lake George, New York, detected by reverse transcriptase PCR. Appl. Environ. Microbiol. 66:3119-3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zehr, J. P., and L. A. McReynolds. 1989. Use of degenerate oligonucleotides for amplification of the nifH gene from the marine cyanobacterium Trichodesmium thiebautii. Appl. Environ. Microbiol. 55:2522-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zehr, J. P., M. T. Mellon, and S. Zani. 1998. New nitrogen-fixing microorganisms detected in oligotrophic oceans by amplification of nitrogenase (nifH) genes. Appl. Environ. Microbiol. 64:3444-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zehr, J. P., J. B. Waterbury, P. J. Turner, J. P. Montoya, E. Omoregie, G. F. Steward, A. Hansen, and D. M. Karl. 2001. Unicellular cyanobacteria fix nitrogen in the subtropical North Pacific Ocean. Nature 412:635-638. [DOI] [PubMed] [Google Scholar]