Abstract
Ten phenols were selected as natural laccase mediators after screening 44 different compounds with a recalcitrant dye (Reactive Black 5) as a substrate. Their performances were evaluated at different mediator/dye ratios and incubation times (up to 6 h) by the use of Pycnoporus cinnabarinus and Trametes villosa laccases and were compared with those of eight known synthetic mediators (including -NOH- compounds). Among the six types of dyes assayed, only Reactive Blue 38 (phthalocyanine) was resistant to laccase-mediator treatment under the conditions used. Acid Blue 74 (indigoid dye), Reactive Blue 19 (anthraquinoid dye), and Aniline Blue (triarylmethane-type dye) were partially decolorized by the laccases alone, although decolorization was much more efficient and rapid with mediators, whereas Reactive Black 5 (diazo dye) and Azure B (heterocyclic dye) could be decolorized only in the presence of mediators. The efficiency of each natural mediator depended on the type of dye to be treated but, with the only exception being Azure B (<50% decolorization), nearly complete decolorization (80 to 100%) was attained in all cases. Similar rates were attained with the best synthetic mediators, but the reactions were significantly slower. Phenolic aldehydes, ketones, acids, and esters related to the three lignin units were among the best mediators, including p-coumaric acid, vanillin, acetovanillone, methyl vanillate, and above all, syringaldehyde and acetosyringone. The last two compounds are especially promising as ecofriendly (and potentially cheap) mediators for industrial applications since they provided the highest decolorization rates in only 5 to 30 min, depending on the type of dye to be treated.
Laccases are multicopper oxidases that catalyze the one-electron oxidation of substituted phenols, anilines, and aromatic thiols to their corresponding radicals with the concomitant reduction of molecular oxygen to water. Laccases are produced by plants and fungi, including white-rot basidiomycetes responsible for lignin degradation in nature (36, 46), although some bacterial laccases have been recently described and fully characterized (17). It is because of the involvement of laccases in lignin degradation that they were first investigated for applications in the pulp and paper industry as substitutes for chlorine-containing reagents for pulp bleaching (38, 41).
The broad substrate specificities of laccases, together with the fact that they use molecular oxygen as the final electron acceptor instead of the hydrogen peroxide used by ligninolytic peroxidases (38), make these enzymes highly interesting for the pulp and paper industry as well as for other industrial and environmental applications. However, the low redox potentials of laccases (0.5 to 0.8 V) compared to those of ligninolytic peroxidases (>1 V) only allow the direct degradation by laccases of low-redox-potential phenolic compounds and not the oxidation of the most recalcitrant aromatics, including different industrial dyes (49). Nevertheless, insights into lignin degradation by some white-rot fungi that only produce laccase showed a way to a new type of laccase applications involving enzyme mediators.
The basis of the laccase-mediator concept is the use of low-molecular-weight compounds that, once oxidized by the enzyme to stable radicals, act as redox mediators, oxidizing other compounds that in principle are not substrates of laccase. From the description of the first laccase mediator, 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) (4), to the more recent use of the -NOH- type, synthetic mediators, including 1-hydroxybenzotriazole (HBT), violuric acid (VIO), and N-hydroxyacetanilide (NHA), a large number of studies have been produced on the mechanisms of oxidation of nonphenolic substrates (2, 3, 48), the search for new mediators (5, 18), and their use in pulp bleaching (7, 20, 39). More recently, applications of the laccase-mediator system in the degradation of aromatic xenobiotics (including polychlorinated biphenyls, fungicides, industrial dyes, and polycyclic and other aromatic hydrocarbons) have been investigated (26, 27, 28, 37, 42). Nevertheless, the laccase-mediator system has yet to be applied at the mill scale due to the cost of mediators and the lack of studies that guarantee the absence of toxic effects of these compounds or their derivatives.
The use of naturally occurring laccase mediators would present environmental and economic advantages. Compounds involved in the natural degradation of lignin by white-rot fungi may be derived from oxidized lignin units or directly from fungal metabolism (16, 25, 34). In addition to enabling the oxidation of compounds that are not oxidized by laccases (e.g., the nonphenolic lignin moiety), the mediators can diffuse far away from the mycelium to sites that are difficult to reach by the enzyme itself (e.g., the lignin macromolecule inside the plant cell wall).
The purpose of the present study was to evaluate the potential of several naturally occurring (mostly phenolic) compounds to mediate the oxidative reactions catalyzed by laccase with the aim of identifying cheaper, more efficient and ecofriendly mediators for the decolorization of recalcitrant dyes and for other industrial and environmental applications. To evaluate the mediating capabilities of these compounds, we used a test based on the decolorization of Reactive Black 5, a recalcitrant dye that is not oxidized by laccase alone (12). Once selected, the new natural mediators were assayed for the decolorization of different types of industrial dyes by laccase.
MATERIALS AND METHODS
Chemicals.
The compounds mentioned in Fig. 1 as well as the synthetic compounds HBT, VIO, promazine (PZ), 2-nitroso-1-naphthol-4-sulfonic acid (HNNS), 1-nitroso-2-naphthol-3,6-disulfonic acid (NNDS), and 2,2,6,6-tetramethylpiperidin-1-yloxy (TEMPO) were purchased from Sigma-Aldrich. ABTS was supplied by Roche. 3-Hydroxyanthranilic acid (HAA), a Pycnoporus cinnabarinus metabolite (16), was also obtained from Sigma-Aldrich.
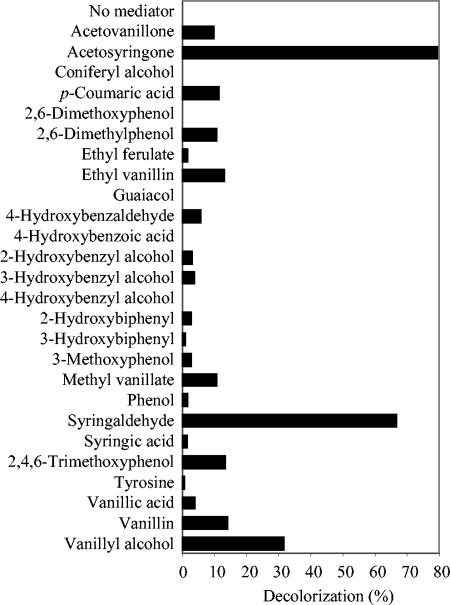
FIG. 1.
Screening for natural mediators based on decolorization of Reactive Black 5 (50 μM) after a 2-h treatment with P. cinnabarinus laccase in the presence of 44 phenolic compounds (50 μM) monitored at 598 nm. No decolorization was obtained with the following 18 compounds (even with a 500 μM concentration): 4-aminobenzoic acid, 4-aminophenol, catechol, 3,5-diaminobenzoic acid, ferulic acid, histidine, homovanillyl alcohol, hydroxylamine, 4-hydroxyacetophenone, 1,4-hydroquinone, 2-methoxyhydroquinone, 4-methoxyphenol, 4-(methylamino)benzoic acid, 2-methyl-1,4-hydroquinone, methyl 4-hydroxybenzoate, phenylalanine, protocatechuic acid, and vanillylacetone.
The Acid Blue 74, Aniline Blue, Reactive Blue 19, and Azure B dyes were obtained from Sigma-Aldrich, whereas Reactive Black 5 and Reactive Blue 38 were supplied by Dye Star (Frankfurt, Germany). Decolorization of the different dyes was monitored at their absorption maxima as described below.
Enzymes.
P. cinnabarinus laccase was produced by Beldem (Andenne, Belgium) from the monokaryotic hyperproducing strain ss3 obtained from strain I-937 (23). Commercial laccase from Trametes villosa (“Polyporus pinsitus”) was supplied by Novozymes (Denmark). Both laccase preparations were used for dye decolorization without further purification. Laccase activities were determined by monitoring 5 mM ABTS oxidation to its cation radical (ɛ436, 29,300 mM−1 cm−1) in 100 mM acetate buffer, pH 5. One activity unit was defined as the amount of enzyme that releases 1 μmol of product per min.
The optimum pH for substrate (100 μM) oxidation by laccases was estimated by the use of 100 mM sodium citrate (pH 3, 4, 5, and 6) and 100 mM sodium citrate-phosphate-borate (pH 2) buffers at 24°C. Laccase activities on phenols (50 μM) were estimated spectrophotometrically by using 100 mU of enzyme/ml in 50 mM sodium citrate buffer, pH 5. Oxidation rates were estimated by decreases in substrate absorbance in the cases of acetovanillone (ɛ304, 7,100 M−1 cm−1), acetosyringone (ɛ320, 6,000 M−1 cm−1), p-coumaric acid (ɛ312, 11,100 M−1 cm−1), ethyl vanillin (ɛ312, 6,466 M−1 cm−1), vanillin (ɛ308, 9,200 M−1 cm−1), and syringaldehyde (ɛ320, 8,500 M−1 cm−1) or by increases in product absorbance in the cases of 2,4,6-trimethoxyphenol (ɛ310, 5,502 M−1 cm−1), 2,6-dimethylphenol (ɛ300, 4,717 M−1 cm−1), vanillyl alcohol (ɛ320, 2,933 M−1 cm−1), and methyl vanillate (ɛ322, 3,217 M−1 cm−1). The molar absorbances of the substrates were estimated under reaction conditions. The molar absorbances of the reaction products were obtained after complete substrate oxidation with laccase.
Purified P. cinnabarinus laccase was used for estimations of kinetic constants on phenols. Mean values and 95% confidence limits of Km and Vmax values were obtained. Laccase purification included (i) the removal of glycerol, present in the preparation supplied by Beldem, by centrifugation at 13,000 rpm (Sorval SS-34 rotor) for 9 h at 4°C; (ii) dialysis and concentration by ultrafiltration (Amicon 3-kDa-cutoff columns) with 25 mM sodium acetate buffer, pH 5; (iii) Q-Resource chromatography in the same buffer with a linear NaCl gradient (0 to 0.5 M in 6 min) at a flow rate of 6 ml/min; and (iv) a final dialysis step against 100 mM sodium citrate buffer, pH 5, and conservation at −80°C.
Mediator screening.
Screening for natural mediators was based on the decolorization of Reactive Black 5 (monitored at 598 nm) by P. cinnabarinus laccase in the presence of each compound. The compounds mentioned in Fig. 1 were investigated as new mediators. For this purpose, 100 mU of P. cinnabarinus laccase/ml, 50 μM Reactive Black 5, and two concentrations of each potential mediator (50 and 500 μM) were incubated in the buffer described above at pH 5 for 2 and 6 h at 24°C in 1-ml plastic cuvettes.
Mediator concentration and pH.
The effects of the mediator concentration and reaction time were studied by the use of 25 μM Reactive Black 5 in the presence of 0.1, 1, 10, 25, 50, 100, 250, and 500 μM concentrations of mediator for different times (from 10 min to 6 h) with 100 mU of P. cinnabarinus laccase/ml in the buffer described above at 24°C in 1-ml cuvettes.
The effect of pH on dye decolorization was studied by treating 25 μM Reactive Black 5 with 100 mU of P. cinnabarinus laccase/ml in the presence of a 100 μM concentration of mediator (syringaldehyde or acetosyringone) in the buffer described above (pH 3, 4, 5, and 6) at 24°C in 1-ml cuvettes with 160-rpm agitation.
Decolorization of different dye types.
Assays of decolorization of 25 μM Azure B, Reactive Blue 19, Acid Blue 74, Reactive Blue 38, and Aniline Blue were performed with 100 mU of a laccase preparation from P. cinnabarinus or T. villosa and 25, 50, and 100 μM concentrations of mediator in the buffer described above (pH 5) for 6 h with agitation (160 rpm) in 1-ml cuvettes at 24°C. Decreases in the absorbance maxima characteristic of Azure B (647 nm), Reactive Blue 19 (592 nm), Acid Blue 74 (608 nm), Reactive Blue 38 (620 nm), and Aniline Blue (592 nm) were measured at different incubation times. Ten natural compounds selected from the previous screening, HAA, and the seven synthetic compounds listed above were used as mediators in these experiments. Absorption spectra between 275 and 800 nm were recorded during dye decolorization with selected mediators by use of a diode-array spectrophotometer.
RESULTS
Mediator screening and dye decolorization conditions.
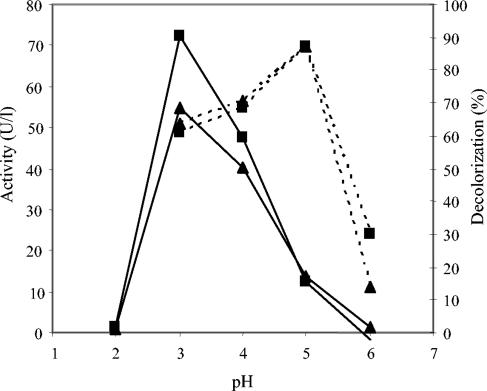
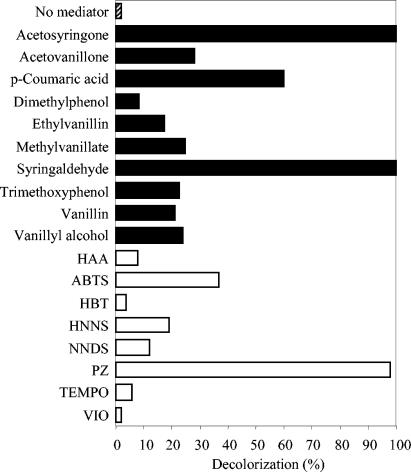
Among the 44 compounds screened as laccase mediators (phenolic alcohols, aldehydes, ketones, acids, esters, and some amines), 22 promoted the decolorization of Reactive Black 5 by P. cinnabarinus laccase with the same concentrations of mediator and dye (Fig. 1). The 10 phenols shown in Fig. 2a to j produced decolorization rates of 10 to 80% and were selected for additional studies with different types of dyes. The laccase activities on these compounds at the concentrations used for mediators are shown in Table 1. Among them, acetosyringone and syringaldehyde provided the highest decolorization rates. The kinetic constants of P. cinnabarinus laccase (purified to a final yield of 39%) were calculated for syringaldehyde (Km, 116 ± 3 μM; Vmax, 244 ± 0 U/mg), acetosyringone (Km, 1,404 ± 407 μM; Vmax, 122 ± 38 U/mg), vanillin (Km, 143 ± 15 μM; Vmax, 2 ± 0 U/mg), acetovanillone (Km, 102 ± 18 μM; Vmax, 2 ± 0 U/mg), and p-coumaric acid (Km, 301 ± 63 μM; Vmax, 201 ± 33 U/mg). The optimum pH for acetosyringone and syringaldehyde oxidation by laccase was about pH 3 (Fig. 3). In contrast, the most (and fastest) decolorization of Reactive Black 5 with these two mediators was obtained at pH 5. Therefore, pH 5 was used for subsequent decolorization assays.
FIG. 2.
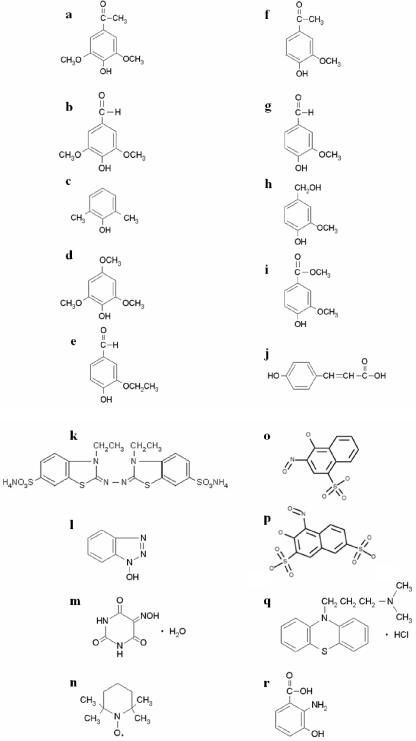
Chemical structures of the 10 phenolic compounds (a to j) selected in the mediator screening (Fig. 1) and of 7 synthetic mediators and HAA (k to r). (a) Acetosyringone; (b) syringaldehyde; (c) 2,6-dimethylphenol; (d) 2,4,6-trimethoxyphenol; (e) ethyl vanillin; (f) acetovanillone; (g) vanillin; (h) vanillyl alcohol; (i) methyl vanillate; (j) p-coumaric acid; (k) ABTS; (l) HBT; (m) VIO; (n) TEMPO; (o) HNNS; (p) NNDS; (q) PZ; (r) HAA.
TABLE 1.
Activity of P. cinnabarinus laccase on the 10 phenols selected, relative to ABTS (50 μM concentration)
| Substrate | % Activity |
|---|---|
| ABTS | 100 |
| Acetosyringone | 21 |
| Acetovanillone | 2 |
| p-Coumaric acid | 5 |
| 2,6-Dimethylphenol | 1 |
| Ethyl vanillin | 1 |
| Methyl vanillate | 2 |
| Syringaldehyde | 30 |
| 2,4,6-Trimethoxyphenol | 13 |
| Vanillin | 1 |
| Vanillyl alcohol | 18 |
FIG. 3.
Effect of pH on mediator (100 μM) oxidation (continuous lines) and initial (5 min) Reactive Black (25 μM) decolorization (dashed lines) by P. cinnabarinus laccase monitored at 598 nm. Mediators: ▪, acetosyringone; ▴, syringaldehyde.
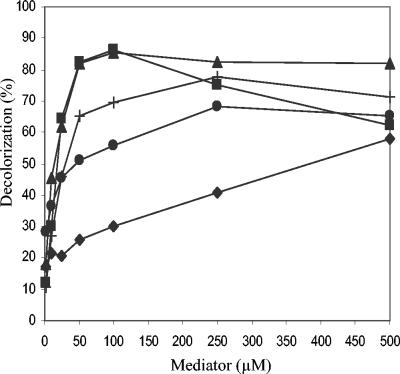
The influences of the natural mediator concentrations on the decolorization of Reactive Black 5 (2-h treatment) are shown in Fig. 4. Laccase in the presence of acetosyringone and syringaldehyde produced the strongest decolorization rate (>80%) at a mediator/dye ratio of only 2:1. In contrast, the p-coumaric acid decolorization rate was directly proportional to the mediator concentration. Reactions with the synthetic mediators HBT and VIO (data not shown) required much higher mediator concentrations than those for acetosyringone and syringaldehyde (up to 250 μM) to attain similar decolorization rates.
FIG. 4.
Effect of mediator concentration on dye decolorization (%) by P. cinnabarinus laccase (2-h treatment of 25 μM Reactive Black 5 monitored at 598 nm). Mediators: ▪, acetosyringone; ▴, syringaldehyde; +, 2,4,6-trimethoxyphenol; •, vanillin; ♦, p-coumaric acid.
Decolorization of different types of dyes.
The 10 natural mediators described above were evaluated for the oxidation of six dyes with different chemical structures (Fig. 5). Three mediator concentrations and both P. cinnabarinus and T. villosa laccases were used for 6-h treatments, although the most significant differences were observed during the first 2 h. The results were compared with those obtained with HAA and seven synthetic mediators, i.e., the two mentioned above and ABTS, PZ, HNNS, NNDS, and TEMPO (Fig. 2k to r).
FIG. 5.
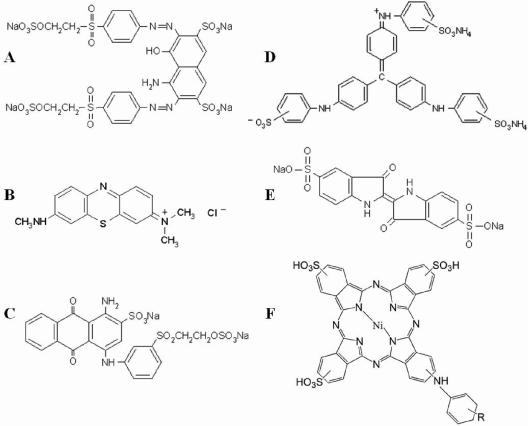
Chemical structures of the different dyes assayed. (A) Reactive Black 5 (diazo type); (B) Azure B (heterocyclic); (C) Reactive Blue 19 (anthraquinoid); (D) Aniline Blue (triarylmethane type); (E) Acid Blue 74 (indigoid); (F) Reactive Blue 38 (phthalocyanine type).
(i) Reactive Black 5.
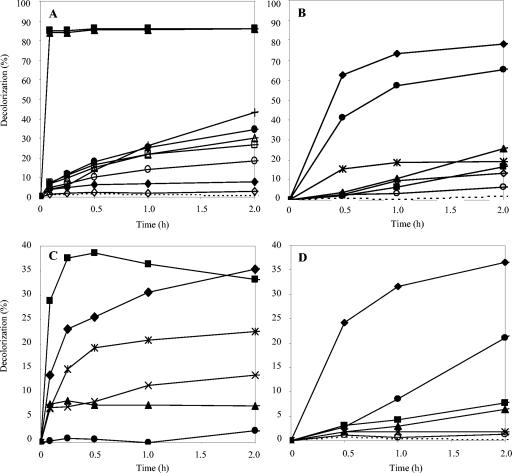
The diazo dye Reactive Black 5 (Fig. 5A) had been previously used for the screening and optimization of treatment conditions. Its decolorization by laccase and natural mediators (50 μM) as a function of time is shown in Fig. 6A. Acetosyringone and syringaldehyde not only produced the largest amounts of decolorization but also were extremely rapid at decolorizing the dye (>80% decolorization in 5 min). In contrast, the most effective synthetic mediators, NNDS and HNNS, required 4 h to attain 70 to 80% decolorization, and HBT and VIO only reached 50 to 60% after 6 h (Fig. 6B). Among the natural mediators, 2,4,6-trimethoxyphenol and vanillin also required several hours to attain 70 and 50% decolorization, respectively.
FIG. 6.
Reactive Black 5 (A and B) and Azure B (C and D) (25 μM) decolorization by P. cinnabarinus laccase, monitored at 598 and 647 nm, respectively, in the presence of natural (left) and synthetic (right) mediators (50 μM in panels A and B; 100 μM in panels C and D). Natural mediators: ▪, acetosyringone; ▴, syringaldehyde; ○, 2,6-dimethylphenol; +, 2,4,6-trimethoxyphenol; ▵, ethyl vanillin; □, acetovanillone; •, vanillin; ⋄, vanillyl alcohol; ×, methyl vanillate; ♦, p-coumaric acid; , HAA. Synthetic mediators: ♦, HNNS; •, NNDS;  , PZ; ▪, HBT; ▴, VIO; ⋄, ABTS; ○, TEMPO. Dashed lines, no mediator.
, PZ; ▪, HBT; ▴, VIO; ⋄, ABTS; ○, TEMPO. Dashed lines, no mediator.
(ii) Azure B.
The decolorization of Azure B, a heterocyclic dye (Fig. 5B) which, like Reactive Black 5, is not oxidized by laccase alone, is shown in Fig. 6C and D. In the presence of mediators, Azure B was decolorized to a lesser extent than Reactive Black 5. Effective mediating capabilities were observed for acetosyringone and p-coumaric acid, with both attaining 40% decolorization at a 100 μM mediator concentration, but decolorization occurred significantly more rapidly (15 min) with acetosyringone. Less than 10% color removal was obtained with syringaldehyde, whereas HAA produced about 25% decolorization. When the mediator concentration was raised above 100 μM, an enhancement of decolorization (about 60% in 2 h) was observed with p-coumaric acid (data not shown), but no effect was produced on acetosyringone or syringaldehyde decolorization (as also found for Reactive Black 5 decolorization). HNNS and NNDS were the most effective synthetic mediators, although they produced slower Azure B decolorization than the most effective natural mediators.
(iii) Reactive Blue 19.
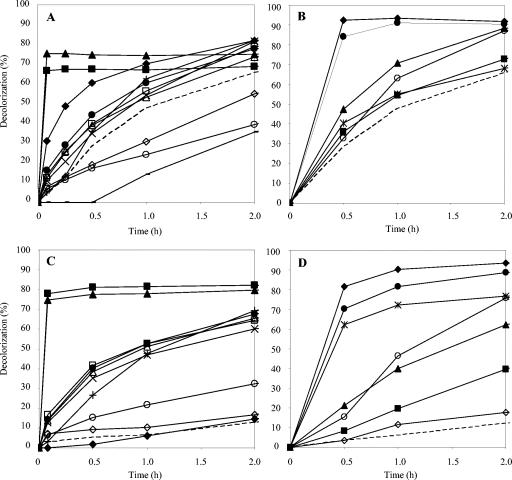
The anthraquinoid dye Reactive Blue 19 (Fig. 5C) was 90% decolorized by laccase alone in 6 h, and the effect of the different mediators was more easily observed after short reaction times (Fig. 7A and B). Acetosyringone and syringaldehyde strongly accelerated decolorization during the first minutes of reaction (e.g., a nearly 20-fold increase by 25 μM syringaldehyde after 5 min). p-Coumaric acid also improved decolorization during the first 15 min. Among the synthetic mediators, HNNS and NNDS enabled >80% decolorization in 30 min, whereas HBT and VIO did not significantly improved Reactive Blue 19 decolorization after the same amount of time.
FIG. 7.
Reactive Blue 19 (A and B) and Aniline Blue (C and D) (25 μM) decolorization by P. cinnabarinus laccase in the presence of natural (left) and synthetic (right) mediators (25 μM in panels A and B; 100 μM in panels C and D) monitored at 592 nm. Natural mediators: ▪, acetosyringone; ▴, syringaldehyde; ○, 2,6-dimethylphenol; +, 2,4,6-trimethoxyphenol; ▵, ethyl vanillin; □, acetovanillone; •, vanillin; ⋄, vanillyl alcohol; ×, methyl vanillate; ♦, p-coumaric acid; −, HAA. Synthetic mediators: ♦, HNNS; •, NNDS;  , PZ; ▪, HBT; ▴, VIO; ⋄, ABTS; ○, TEMPO. Dashed lines, no mediator.
, PZ; ▪, HBT; ▴, VIO; ⋄, ABTS; ○, TEMPO. Dashed lines, no mediator.
(iv) Aniline Blue.
The triarylmethane-type dye Aniline Blue (Fig. 5D) was decolorized up to 35% by laccase alone in 6 h, with the rate being strongly increased by natural mediators (Fig. 7C and D). Up to 80% decolorization was obtained with acetosyringone and syringaldehyde within 5 min. In fact, most of the natural mediators tested strongly enhanced Aniline Blue decolorization by laccase (up to 70% decolorization was attained with vanillin, acetovanillone, ethyl vanillin, and methyl vanillate), except for p-coumaric and vanillyl alcohol. Synthetic mediators also gave adequate decolorization rates, except for ABTS, which did not mediate the reaction. PZ, NNDS, and HNNS were the most efficient synthetic mediators.
(v) Acid Blue 74.
The indigoid dye Acid Blue 74 (Fig. 5E) was decolorized 50% by laccase alone in 6 h, but the presence of any of the natural mediators increased this rate to 100% at the lowest concentration in less than 1 h (except for 2,6-dimethylphenol and vanillyl alcohol, which required 2 to 4 h). The decolorization rates after 30 min are shown in Fig. 8. Complete decolorization was achieved during the first 5 min with acetosyringone and syringaldehyde. In general, synthetic mediators were less efficient. Among them, PZ was the most rapid and efficient, followed by HNNS and NNDS, enabling 100% decolorization after 1 h. In contrast, ABTS did not enhance decolorization.
FIG. 8.
Decolorization of Acid Blue 74 (25 μM) after 5-min reactions with laccase and mediators (25 μM) monitored at 608 nm. Bars represent the percentages of decolorization obtained by P. cinnabarinus laccase in the presence of synthetic (white bars) and natural (black bars) mediators and in the absence of mediators (hatched bar).
(vi) Reactive Blue 38.
The phthalocyanine-type dye Reactive Blue 38 (Fig. 5F) was not oxidized by laccase alone or in the presence of any of the natural or synthetic mediators assayed.
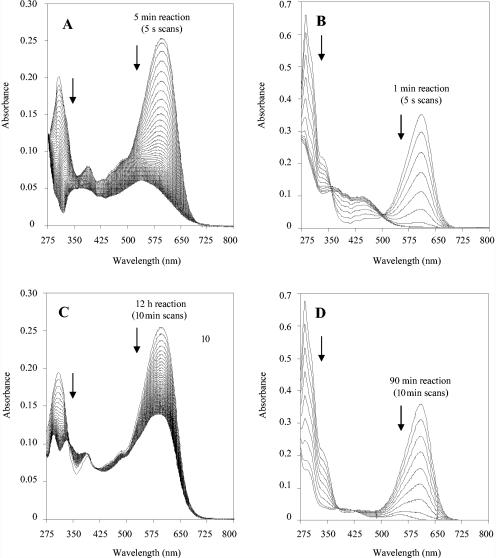
From the above results, acetosyringone appeared to be one of the most efficient natural mediators of laccase decolorization of the five dyes assayed. The decolorization rates attained with the two laccases assayed at different acetosyringone/dye molar ratios and treatment times are summarized in Table 2. The UV-visible spectra of Reactive Black 5 and Acid Blue 74 and the changes produced during fast decolorization (scans every 5 s) with P. cinnabarinus laccase and acetosyringone are shown in Fig. 9A and B, in contrast to the slow decolorization (scans every 10 min) in the presence of vanillin (Fig. 9C and D).
TABLE 2.
Decolorization of five dyes after different treatment times with laccases from P. cinnabarinus and T. villosa using different acetosyringone/dye molar ratiosa
| Dye and treatment time | % Decolorization at indicated acetosyringone/dye ratio
|
|||||||
|---|---|---|---|---|---|---|---|---|
|
P. cinnabarinus laccase
|
T. villosa laccase
|
|||||||
| 0 | 1 | 2 | 4 | 0 | 1 | 2 | 4 | |
| Azure B | ||||||||
| 5 min | 0 | 14 | 23 | 29 | 0 | 17 | 24 | 34 |
| 30 min | 0 | 18 | 28 | 39 | 0 | 20 | 30 | 41 |
| 2 h | 0 | 16 | 26 | 33 | 0 | 17 | 26 | 36 |
| Reactive Black 5 | ||||||||
| 5 min | 0 | 84 | 85 | 84 | 0 | 84 | 85 | 84 |
| 30 min | 0 | 86 | 86 | 85 | 0 | 85 | 85 | 84 |
| 2 h | 0 | 87 | 86 | 85 | 0 | 87 | 87 | 84 |
| Acid Blue 74 | ||||||||
| 5 min | 2 | 100 | 100 | 100 | 3 | 100 | 100 | 100 |
| 30 min | 7 | 100 | 100 | 100 | 8 | 100 | 100 | 100 |
| 2 h | 25 | 100 | 100 | 100 | 24 | 100 | 100 | 100 |
| Reactive Blue 19 | ||||||||
| 5 min | 17 | 67 | 64 | 57 | 21 | 64 | 61 | 57 |
| 30 min | 44 | 68 | 64 | 50 | 53 | 66 | 60 | 50 |
| 2 h | 78 | 69 | 63 | 47 | 83 | 67 | 59 | 47 |
| Aniline Blue | ||||||||
| 5 min | 2 | 43 | 66 | 77 | 3 | 40 | 63 | 78 |
| 30 min | 3 | 47 | 68 | 81 | 5 | 43 | 66 | 81 |
| 2 h | 10 | 53 | 72 | 82 | 13 | 49 | 69 | 82 |
Decolorization was monitored at the absorption maxima of Azure B (647 nm), Reactive Black 5 (598 nm), Reactive Blue 19 (592 nm), and Aniline Blue (592 nm).
FIG. 9.
Changes in absorption spectra (275 to 800 nm) of two dyes (25 μM) during decolorization with P. cinnabarinus laccase (100 mU/ml) in the presence of acetosyringone (25 μM) or vanillin (25 μM) (reactions were carried out at 24°C and pH 5). (A) Reactive Black 5 decolorization with acetosyringone (spectra were recorded every 5 s for a total time of 5 min). (B) Acid Blue 74 decolorization with acetosyringone (spectra were recorded every 5 s for a total time of 1 min). (C) Reactive Black 5 decolorization with vanillin (spectra were recorded every 10 min for a total time of 12 h). (D) Acid Blue 74 decolorization with vanillin (spectra were recorded every 10 min for a total time of 90 min).
DISCUSSION
Natural mediator screening.
Reactive Black 5 is not oxidized by laccases from P. cinnabarinus and T. villosa, despite both being high-redox-potential laccases (32, 43). This is probably due to the high redox potential of this dye, which is not oxidized by chemical oxidizers such as Mn3+ (22), but steric hindrances may also reduce the accessibility of the -OH and -NH2 groups to laccases. Only methyl or methoxy ortho-substituted diazo dyes have been shown to be oxidized by laccases (11). However, the decolorization of Reactive Black 5 by a laccase is possible in the presence of redox mediators (12). This reaction was used as a test to evaluate the mediating capabilities of naturally occurring phenols, amines, and other compounds under study. The 10 mediators selected among 44 different compounds included phenols derived from syringyl (S), guaiacyl (G), and p-hydroxyphenyl (H) lignin units, characterized by the presence of two, one, or no methoxy substituents, respectively (in ortho positions with respect to the phenolic hydroxyl) (24), such as syringaldehyde, acetosyringone, vanillin, acetovanillone, methyl vanillate, and p-coumaric acid. Recently, some phenols, including syringaldehyde and acetosyringone, have been described as laccase mediators for indigo decolorization (9) as well as for the transformation of a fungicide (27) and hydrocarbon degradation (25). However, the present study provides the first comprehensive screening for natural mediators, which were then compared with synthetic artificial mediators for the ability to decolorize different types of dyes. Moreover, the capabilities of natural phenols to act as laccase mediators were demonstrated by the use of mediator/substrate molar ratios of 1 to 4, which are much lower than those used in the studies mentioned above (ratios of 19 to 40) (25, 27). Both the possibility of obtaining mediators from natural sources (lignin) and the low mediator/substrate ratios used strongly increased the feasibility of the laccase-mediator system for industrial or environmental applications. In fact, syringaldehyde and acetosyringone are among the main products from both biological and enzymatic degradation and chemical (alkaline) depolymerization of S-rich lignins (14, 29), such as lignins of eucalypt and some nonwoody plants (15, 21).
Enzymatic decolorization of dyes.
P. cinnabarinus laccase (33, 44) has been investigated for use in paper pulp bleaching (8, 43), but we showed in the present study that it is also efficient for dye decolorization. No significant differences from T. villosa (50) laccase were observed in the presence of the same mediators. This shows that once an enzyme posses a redox potential that is high enough to oxidize the mediator (those of both laccases used here exceed 0.7 V) (32, 43), the efficiency of the laccase-mediator system mainly depends on the mediator used. Except for the phthalocyanine-type dye Reactive Blue 38, all other dyes assayed could be decolorized by the use of laccases and mediators. Efficient decolorization was confirmed by UV-visible spectroscopy showing a general decrease in dye absorption (in the 275- to 800-nm region) during laccase-mediator treatments. The decolorization of Reactive Blue 38 by ligninolytic peroxidases was reported previously (22). The anthraquinoid (Reactive Blue 19), indigoid (Acid Blue 74), and triarylmethane-type (Aniline Blue) dyes assayed could be directly decolorized by the P. cinnabarinus and T. villosa laccases. However, the presence of phenolic mediators strongly accelerated the reaction and increased the decolorization rates attained. The most recalcitrant dyes were the heterocyclic dye (Azure B), which is used to detect lignin peroxidase activity because it is not oxidized by laccase or manganese peroxidase alone, but it was partially decolorized in the presence of the mediators (1). The diazo dye Reactive Black 5 was oxidized by laccase only in the presence of mediators, but high decolorization rates were attained. Most of the phenolic mediators tested here were much more effective than HAA, a metabolite of P. cinnabarinus that was described as a natural laccase mediator (16), although more recent studies have shown that it does not play a role in lignin biodegradation as initially suggested (31). On the other hand, the most efficient natural mediators identified, i.e., syringaldehyde and acetosyringone, were always more rapid at decolorizing dyes (only 5 to 30 min were required) than the synthetic mediators, including the best-known mediators HBT and VIO as well as HNNS and NNDS (which provided the best results among synthetic mediators).
Natural mediator oxidation by laccase.
The limiting step in the oxidation of phenols by laccase is the first electron transfer from the substrate to T1 copper, a reaction that is mainly governed by differences in redox potential between the phenol and the enzyme (47). The electron donor effect of methoxy substituents at the benzenic ring enhances laccase activity due to a decreased redox potential. In this way, acetosyringone and syringaldehyde (two S-type compounds) were rapidly oxidized by laccase (50- to 100-fold higher Vmax values than the G-type phenols acetovanillone and vanillin) and were also found to be the most rapid and efficient enzyme mediators. However, neither syringol nor syringic acid, two other S-type phenols, were as effective as the corresponding ketone and aldehyde. This was due to other factors influencing the oxidation rate by laccase, especially the pKa, since phenol oxidation to a phenoxy radical is favored by the presence of the phenolate form (13). In this way, syringaldehyde and acetosyringone possess lower pKa values (7.34 and 7.88, respectively) than syringic acid (9.49 for the phenolate) and syringol (9.98) which promote faster oxidation (40).
Dye oxidation by phenol radicals.
The specificity of mediators towards different functional groups must also be taken into account in laccase-mediator reactions. For example, laccase-ABTS is not reactive towards benzylic ethers or alkylbenzenes, but it is effective on benzyl alcohols (2, 10), and oxidized HBT and VIO also seem to be more competent towards some specific groups (2, 45). In the present study, we obtained different decolorization rates depending on the type of dye and mediator used. Thus, p-coumaric acid efficiently mediated Azure B decolorization but was unable to decolorize Aniline Blue. In contrast, syringaldehyde was not very effective on Azure B but was highly efficient on the other dyes. Finally, acetosyringone seemed to be highly competent to mediate the oxidation of all dyes tested. Recently, two different mechanisms for the oxidation of nonphenolic compounds by laccase-mediator systems have been proposed: (i) an electron transfer route for mediators such as ABTS and (ii) a radical hydrogen atom transfer route for mediators of the -NOH- type (2). It is very likely that the phenoxy radicals formed during the oxidation of natural mediators by laccase act similarly to the -NO·- radicals from -NOH- compounds, i.e., they extract a hydrogen atom from the substrate (13). Therefore, the dissociation energy of the corresponding bond should govern their reaction with the laccase mediators.
The feasibility of the laccase-mediator systems depends on the redox reversibility of the reaction of the radical with the substrate as well as on the balance between the stability and reactivity of the mediator radical which, in addition, should not inhibit the enzyme activity. The two former conditions have been confirmed with acetosyringone, since voltametric and electron paramagnetic resonance studies have shown the true reversibility of the substrate reaction (19) and the long half-life of its phenoxy radical (35). In contrast, the -NO·- radical from HBT inactivates laccase, and due to its high reactivity, decays rapidly to benzotriazole and other inactive compounds (30). As mentioned above, syringaldehyde and acetosyringone are more easily oxidized by laccase than are vanillin and acetovanillone due to their lower redox potentials (their pKa values are very similar). These two S-type phenols also form more stable radicals due to the presence of two methoxyl groups on the aromatic ring that prevent the formation of biphenyl-type structures by radical condensation, as produced after the laccase oxidation of G-type phenols. The higher stability of acetosyringone (and also syringaldehyde) phenoxy radicals in slightly acidic aqueous media (6) explains why dye decolorization in the presence of this mediator was better at pH 5, despite the optimal oxidation by the enzyme at pH 3.
Conclusion.
After natural mediator screening and an evaluation of the chosen mediators' performances on different types of dyes (using low mediator concentrations), we concluded that several lignin-derived phenols (such as syringaldehyde and acetosyringone) represent ecofriendly alternatives to synthetic (including -NOH- type) mediators for laccase degradation of different types of dyes and other recalcitrant compounds in terms of both efficiency and velocity of oxidation.
Acknowledgments
This work was supported by a CSIC-ENCE contract, the Spanish Biotechnology Programme (BIO 2002-1166), and EU project QLK3-99-590. S.C. was supported by an R&C contract of the Spanish MEC, and D.I. was supported by an I3P fellowship of the Spanish CSIC.
We thank Novozymes (Bagsvaerd, Denmark) and Beldem (Andenne, Belgium) for preparations of laccases from T. villosa and P. cinnabarinus, respectively.
REFERENCES
- 1.Archibald, F. S. 1992. A new assay for lignin-type peroxidases employing the dye Azure-B. Appl. Environ. Microbiol. 58:3110-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baiocco, P., A. M. Barreca, M. Fabbrini, C. Galli, and P. Gentili. 2003. Promoting laccase activity towards non-phenolic substrates: a mechanistic investigation with some laccase-mediator systems. Org. Biomol. Chem. 1:191-197. [DOI] [PubMed] [Google Scholar]
- 3.Bourbonnais, R., D. Leech, and M. G. Paice. 1998. Electrochemical analysis of the interactions of laccase mediators with lignin model compounds. Biochim. Biophys. Acta 1379:381-390. [DOI] [PubMed] [Google Scholar]
- 4.Bourbonnais, R., and M. G. Paice. 1990. Oxidation of non-phenolic substrates. An expanded role for laccase in lignin biodegradation. FEBS Lett. 267:99-102. [DOI] [PubMed] [Google Scholar]
- 5.Bourbonnais, R., M. G. Paice, B. Freiermuth, E. Bodie, and S. Borneman. 1997. Reactivities of various mediators and laccases with kraft pulp and lignin model compounds. Appl. Environ. Microbiol. 63:4627-4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caldwell, E. S., and C. Steelink. 1969. Phenoxy radical intermediates in the enzymatic degradation of lignin model compounds. Biochim. Biophys. Acta 184:420-431. [DOI] [PubMed] [Google Scholar]
- 7.Call, H.-P. 1994. Verfahren zur Veränderung, Abbau oder Bleichen von Lignin, ligninhaltigen Materialien oder ähnlichen Stoffen. International patent WO 94/29510.
- 8.Camarero, S., O. García, T. Vidal, J. Colom, J. C. del Río, A. Gutiérrez, J. M. Gras, R. Monje, M. J. Martínez, and A. T. Martínez. 2004. Efficient bleaching of non-wood high-quality paper pulp using laccase-mediator system. Enzyme Microb. Technol. 35:113-120. [Google Scholar]
- 9.Campos, R., A. Kandelbauer, K. H. Robra, A. Cavaco-Paulo, and G. M. Gübitz. 2001. Indigo degradation with purified laccases from Trametes hirsuta and Sclerotium rolfsii. J. Biotechnol. 89:131-139. [DOI] [PubMed] [Google Scholar]
- 10.Cantarella, G., C. Galli, and P. Gentili. 2003. Free radical versus electron-transfer routes of oxidation of hydrocarbons by laccase-mediator systems. Catalytic and stoichiometric procedures. J. Mol. Biocatalysis B 22:135-144. [Google Scholar]
- 11.Chivukula, M., and V. Renganathan. 1995. Phenolic azo dye oxidation by laccase from Pyricularia oryzae. Appl. Environ. Microbiol. 61:4374-4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claus, H., G. Faber, and H. König. 2002. Redox-mediated decolorization of synthetic dyes by fungal laccases. Appl. Microbiol. Biotechnol. 59:672-678. [DOI] [PubMed] [Google Scholar]
- 13.d'Acunzo, F., and C. Galli. 2003. First evidence of catalytic mediation by phenolic compounds in the laccase-induced oxidation of lignin models. Eur. J. Biochem. 270:3634-3640. [DOI] [PubMed] [Google Scholar]
- 14.Dean, J. F. D. 1997. Lignin analysis, p. 199-215. In W. V. Dashek (ed.), Methods in plant biochemistry and molecular biology. CRC Press, Boca Raton, Fla.
- 15.del Río, J. C., A. Gutiérrez, M. J. Martínez, and A. T. Martínez. 2001. Py-GC-MS study of Eucalyptus globulus wood treated with different fungi. J. Anal. Appl. Pyrolysis 58/59:441-453. [Google Scholar]
- 16.Eggert, C., U. Temp, J. F. D. Dean, and K.-E. L. Eriksson. 1996. A fungal metabolite mediates degradation of non-phenolic lignin structures and synthetic lignin by laccase. FEBS Lett. 391:144-148. [DOI] [PubMed] [Google Scholar]
- 17.Enguita, F. J., P. M. Matias, L. O. Martins, D. Placido, A. O. Henriques, and M. A. Carrondo. 2002. Spore-coat laccase CotA from Bacillus subtilis: crystallization and preliminary X-ray characterization by the MAD method. Acta Crystallogr. D 58:1490-1493. [DOI] [PubMed] [Google Scholar]
- 18.Fabbrini, M., C. Galli, and P. Gentili. 2002. Comparing the catalytic efficiency of some mediators of laccase. J. Mol. Cat. B 16:231-240. [Google Scholar]
- 19.Fernández-Sánchez, C., T. Tzanov, G. M. Gübitz, and A. Cavaco-Paulo. 2002. Voltammetric monitoring of laccase-catalysed mediated reactions. Bioelectrochemistry 58:149-156. [DOI] [PubMed] [Google Scholar]
- 20.García, O., S. Camarero, J. Colom, A. T. Martínez, M. J. Martínez, R. Monje, and T. Vidal. 2003. Optimization of a laccase-mediator stage for TCF bleaching of flax pulp. Holzforschung 57:513-519. [Google Scholar]
- 21.Gutiérrez, A., I. M. Rodríguez, and J. C. del Río. 2004. Chemical characterization of lignin and lipid fractions in kenaf bast fibers used for manufacturing high-quality papers. J. Agric. Food Chem. 52:4764-4773. [DOI] [PubMed] [Google Scholar]
- 22.Heinfling, A., M. J. Martínez, A. T. Martínez, M. Bergbauer, and U. Szewzyk. 1998. Transformation of industrial dyes by manganese peroxidase from Bjerkandera adusta and Pleurotus eryngii in a manganese-independent reaction. Appl. Environ. Microbiol. 64:2788-2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herpoël, I., S. Moukha, L. Lesage-Meessen, J. C. Sigoillot, and M. Asther. 2000. Selection of Pycnoporus cinnabarinus strains for laccase production. FEMS Microbiol. Lett. 183:301-306. [DOI] [PubMed] [Google Scholar]
- 24.Higuchi, T. 1997. Biochemistry and molecular biology of wood. Springer Verlag, London, United Kingdom.
- 25.Johannes, C., and A. Majcherczyk. 2000. Natural mediators in the oxidation of polycyclic aromatic hydrocarbons by laccase mediator systems. Appl. Environ. Microbiol. 66:524-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johannes, C., A. Majcherczyk, and A. Hüttermann. 1998. Oxidation of acenaphthene and acenaphthylene by laccase of Trametes versicolor in a laccase-mediator system. J. Biotechnol. 61:151-156. [Google Scholar]
- 27.Kang, K. H., J. Dec, H. Park, and J. M. Bollag. 2002. Transformation of the fungicide cyprodinil by a laccase of Trametes villosa in the presence of phenolic mediators and humic acid. Water Res. 36:4907-4915. [DOI] [PubMed] [Google Scholar]
- 28.Keum, Y. S., and Q. X. Li. 2004. Fungal laccase-catalyzed degradation of hydroxy polychlorinated biphenyls. Chemosphere 56:23-30. [DOI] [PubMed] [Google Scholar]
- 29.Kirk, T. K., and R. L. Farrell. 1987. Enzymatic “combustion”: the microbial degradation of lignin. Annu. Rev. Microbiol. 41:465-505. [DOI] [PubMed] [Google Scholar]
- 30.Li, K. C., R. F. Helm, and K. E. L. Eriksson. 1998. Mechanistic studies of the oxidation of a non-phenolic lignin model compound by the laccase/1-hydroxybenzotriazole redox system. Biotechnol. Appl. Biochem. 27:239-243. [Google Scholar]
- 31.Li, K. C., P. S. Horanyi, R. Collins, R. S. Phillips, and K. E. L. Eriksson. 2001. Investigation of the role of 3-hydroxyanthranilic acid in the degradation of lignin by white-rot fungus Pycnoporus cinnabarinus. Enzyme Microb. Technol. 28:301-307. [DOI] [PubMed] [Google Scholar]
- 32.Li, K. C., F. Xu, and K. E. L. Eriksson. 1999. Comparison of fungal laccases and redox mediators in oxidation of a nonphenolic lignin model compound. Appl. Environ. Microbiol. 65:2654-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lomascolo, A., E. Record, I. Herpoël-Gimbert, M. Delattre, J. L. Robert, J. Georis, T. Dauvrin, J.-C. Sigoillot, and M. Asther. 2003. Overproduction of laccase by a monokaryotic strain of Pycnoporus cinnabarinus using ethanol as inducer. J. Appl. Microbiol. 94:618-624. [DOI] [PubMed] [Google Scholar]
- 34.Martínez, M. J., C. Muñoz, F. Guillén, and A. T. Martínez. 1994. Studies on homoveratric acid transformation by the ligninolytic fungus Pleurotus eryngii. Appl. Microbiol. Biotechnol. 41:500-504. [Google Scholar]
- 35.Marzullo, L., R. Cannio, P. Giardina, M. T. Santini, and G. Sannia. 1995. Veratryl alcohol oxidase from Pleurotus ostreatus participates in lignin biodegradation and prevents polymerization of laccase-oxidized substrates. J. Biol. Chem. 270:3823-3827. [DOI] [PubMed] [Google Scholar]
- 36.Mayer, A. M., and R. C. Staples. 2002. Laccase: new functions for an old enzyme. Phytochemistry 60:551-565. [DOI] [PubMed] [Google Scholar]
- 37.Nyanhongo, G. S., J. Gomes, G. M. Gübitz, R. Zvauya, J. Read, and W. Steiner. 2002. Decolorization of textile dyes by laccases from a newly isolated strain of Trametes modesta. Water Res. 36:1449-1456. [DOI] [PubMed] [Google Scholar]
- 38.Paice, M. G., R. Bourbonnais, I. D. Reid, F. S. Archibald, and L. Jurasek. 1995. Oxidative bleaching enzymes: a review. J. Pulp Paper Sci. 21:J280-J284. [Google Scholar]
- 39.Poppius-Levlin, K., W. Wang, T. Tamminen, B. Hortling, L. Viikari, and M.-L. Niku-Paavola. 1999. Effects of laccase/HBT treatment on pulp and lignin structures. J. Pulp Paper Sci. 25:90-94. [Google Scholar]
- 40.Ragnar, M., C. T. Lindgren, and N.-O. Nilvebrant. 2000. pKa values of guaiacyl and syringyl phenols related to lignin. J. Wood Chem. Technol. 20:277-305. [Google Scholar]
- 41.Reid, I. D., and M. G. Paice. 1994. Biological bleaching of kraft pulps by white-rot fungi and their enzymes. FEMS Microbiol. Rev. 13:369-376. [Google Scholar]
- 42.Rodríguez, E., O. Nuero, F. Guillén, A. T. Martínez, and M. J. Martínez. 2004. Degradation of phenolic and non-phenolic aromatic pollutants by four Pleurotus species: the role of laccase and versatile peroxidase. Soil Biol. Biochem. 36:909-916. [Google Scholar]
- 43.Sigoillot, C., E. Record, V. Belle, J. L. Robert, A. Levasseur, P. J. Punt, C. A. M. J. J. van den Hondel, A. Fournel, J.-C. Sigoillot, and M. Asther. 2003. Natural and recombinant fungal laccases for paper pulp bleaching. Appl. Microbiol. Biotechnol. 64:346-352. [DOI] [PubMed] [Google Scholar]
- 44.Sigoillot, J. C., I. Herpoël, P. Frasse, S. Moukha, L. Lesage-Meessen, and M. Asther. 1999. Laccase production by a monokaryotic strain of Pycnoporus cinnabarinus derived from a dikaryotic strain. World J. Microbiol. Biotechnol. 15:481-484. [Google Scholar]
- 45.Soares, G. M. B., M. T. P. Amorim, A. M. Oliveira-Campos, R. Hrdina, and M. Costa-Ferreira. 2002. Specificity of phenolic disazo dyes in relation to transformation by laccase. Enzyme Microb. Technol. 30:607-612. [Google Scholar]
- 46.Thurston, C. F. 1994. The structure and function of fungal laccases. Microbiology 140:19-26. [Google Scholar]
- 47.Xu, F. 1996. Oxidation of phenols, anilines, and benzenethiols by fungal laccases: correlation between activity and redox potentials as well as halide inhibition. Biochemistry 35:7608-7614. [DOI] [PubMed] [Google Scholar]
- 48.Xu, F., J. J. Kulys, K. Duke, K. C. Li, K. Krikstopaitis, H.-J. W. Deussen, E. Abbate, V. Galinyte, and P. Schneider. 2000. Redox chemistry in laccase-catalyzed oxidation of N-hydroxy compounds. Appl. Environ. Microbiol. 66:2052-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu, F., W. S. Shin, S. H. Brown, J. A. Wahleithner, U. M. Sundaram, and E. I. Solomon. 1996. A study of a series of recombinant fungal laccases and bilirubin oxidase that exhibit significant differences in redox potential, substrate specificity, and stability. Biochim. Biophys. Acta 1292:303-311. [DOI] [PubMed] [Google Scholar]
- 50.Yaver, D. S., F. Xu, E. J. Golightly, K. M. Brown, S. H. Brown, M. W. Rey, P. Schneider, T. Halkier, K. Mondorf, and H. Dalboge. 1996. Purification, characterization, molecular cloning, and expression of two laccase genes from the white rot basidiomycete Trametes villosa. Appl. Environ. Microbiol. 62:834-841. [DOI] [PMC free article] [PubMed] [Google Scholar]