Highlights
-
•
Charred bone (CB) induced the maximum promotion of soil P availability.
-
•
Addition of CB (heated at ∼300 °C) increased the abundance of PSF in soil.
-
•
Fungal community composition was more sensitive to CB addition than that of bacteria.
-
•
Fungal community assembly process shifted from stochastic to deterministic.
-
•
Positive feedback between P supply and PSF abundance was initiated by CB.
Keywords: Available phosphorus, Charred bones, Community assembly, Microbial community, P dissolving function
Abstract
Phosphorus (P) is one of the most common limited nutrients in terrestrial ecosystems. Animal bones, with abundant bioapatite, are considerable P sources in terrestrial ecosystems. Heating significantly promotes P release from bone bioapatite, which may alleviate P limitation in soil. This study aimed to explore P release from charred bone (CB) under heating at various temperatures (based on common natural heating). It showed that heating at ∼300 °C significantly increased the P release (up to ∼30 mg/kg) from CB compared with other heating temperatures. Then, the subsequent changes of available P and pH induced evident alternation of soil microbial community composition. For instance, CB heated at ∼300 °C caused elevation of phosphate-solubilizing fungi (PSF) abundance. This further stimulated P mobility in the soil. Meanwhile, the fungal community assembly process was shifted from stochastic to deterministic, whereas the bacterial community was relatively stable. This indicated that the bacterial community showed fewer sensitive responses to the CB addition. This study hence elucidated the significant contribution of heated bone materials on P supply. Moreover, functional fungi might assist CB treated by natural heating (e.g., fire) to construct P “Hot Spots”.
Graphical abstract
Introduction
Phosphorus (P) is an essential element for all living organisms (Butler et al., 2018). It is a critical element in nucleic acids, enzymes, phosphoproteins, phospholipids, etc. (Tamburini et al., 2012; Kamerlin et al., 2013; Li et al., 2023a). However, it has been predicted that current global reserves of phosphate rocks may be depleted within 50–100 years due to the increasing demands and dwindling stocks (Cordell et al., 2009; Gilbert, 2009). A better understanding of P biogeochemistry would hence help us to improve P utilization (Childers et al., 2011; Tonini et al., 2019; Walton et al., 2023).
Apatite is the most common P-bearing mineral and hence is the dominant P source in soils (Li et al., 2023b). Bioapatite (BAp) is a subgroup of hydroxylapatite (Cazalbou et al., 2004; Li et al., 2013), which has also been accepted as the main inorganic component of animal teeth and bones (Liu et al., 2013). Many substitutions in BAp, e.g., PO43− by CO32−, significantly enhance its solubility (Legeros et al., 1995). Therefore, bone materials might act as P “Hot Spots” in the ecosystem (Tang et al., 2019). However, the impact of bone minerals on the P dynamic in soil ecosystems has still not been fully understood.
Terrestrial ecosystem fires occur frequently, especially during the intensifying global warming (Alonso-Canas and Chuvieco, 2015). The fire-heating animal carcasses in the ecosystem promoted P release from their bones via disorganization of minerals (Ansley et al., 1998; Zwetsloot et al., 2015; Tang et al., 2019). Meanwhile, the different heating temperatures and durations of fires may cause variable P release from charred bone (CB) (Chen et al., 2017). Therefore, the role of the temperature in altering efficiency of P release should be addressed.
It has been observed that phosphate-solubilizing microorganisms (PSM) can significantly promote P release from apatite (up to ∼1000 times) (Wu et al., 2013; Mendes Gde et al., 2015; Li et al., 2016). In addition, as one of the major limiting elements for production of biomass, P input increases soil microbial biomass (Turner and Wright, 2014). Meanwhile, it could alter soil microbial community composition (Ducousso-Détrez et al., 2022) and diversity (Yao et al., 2018). It has been reported that P fertilization decreased Chao richness of soil bacterial community (Ling et al., 2017; Yao et al., 2018). Furthermore, P accumulation might indirectly impact various ecological services by changing the microbial community (Beauregard et al., 2010). For example, inorganic P addition reduced microbial secretion of phosphatase, which weakens the ability of soil microorganisms to solubilizing organic P (Olander and Vitousek, 2000; Sinsabaugh et al., 2008; Marklein and Houlton, 2012). Considering the important role of soil microorganisms and their sensitivity to P, it is essential to evaluate the responses of soil microorganisms to P utilization.
This study investigated the changes in soil available phosphorus (AP) and pH caused by the addition of CB heated at 0–300 °C, together with the responses of soil microbial community (including community composition, diversity, assembly processes, and functions). The heating temperature of 0–300 °C was referred to the typical heating temperature in 0–10 cm depth soil under nature fire (Dayamba et al., 2010).
Materials and methods
Preparation of CB
The bone samples were collected from the femur of an adult male pig (Wang et al., 2017). Elemental compositions of the bone samples were shown in Table S1 (Chen et al., 2017). Approximately, 10 g blocks bone samples (analog to natural bone debris) were heated at 100, 200, and 300 °C for 12 h in a B180 muffle furnace (Nabertherm Industrial Inc.) with air isolation. Three replicates were conducted for the bone samples heated under each temperature. The temperature range (up to 300 °C) was selected based on typical nature fire heating on top soil. The efficiencies of P release from different CB in solution were shown in Table S2 (Tang et al., 2019).
Field experiment
The field experiment was conducted at Nanjing Zhongshan Botanical Garden (N 32° 3′ 15′', E 118° 49′ 52′') in Nanjing, Jiangsu Province, China. The local climate is north subtropical monsoon with a mean annual temperature of 14.7 °C and means annual precipitation of ∼1100 mm.
Four treatments were performed in this study, i.e., Control (without CB), T-100 (addition of CB heated at 100 °C), T-200 (adding CB heated at 200 °C), and T-300 (adding CB heated at 300 °C). Each treatment had three replicates. For CB addition treatments, CBs were buried at a depth of 3 cm (each replicate was performed in a 0.5 × 1 m region). The soils around CB were collected from the top layer soils after one year. Each soil sample was homogenized with sterile tools and then split into two parts. One part was air-dried at room temperature. Then, it was ground and sieved with 10 mesh (2 mm) for soil characterization analysis (including measuring soil AP concentrations and pH). Another part was used for soil microbial DNA extraction.
Instrumentation
The concentrations of soluble P in water were analyzed by ICP-OES (Agilent 710, Agilent Technology). The pH values were measured by SG98 InLab pH meter (Mettler Toledo Int. Inc.) with an Expert Pro-ISM-IP67 probe (soil/water weight ratio of 2/5). The soil AP concentration was measured by using a UV spectrophotometer (Shimadzu UV mini-1240) at the wavelength of 880 nm, based on the ascorbic acid method. Soil total carbon (TC) and nitrogen (TN) content were analyzed using a CN analyzer (Elementar Vario Micro cube, Germany).
DNA extraction and high-throughput sequencing
Microbial DNA was extracted from 10 g fresh soil samples using the E.Z.N.A.® Soil DNA Kit (Omega Bio-tek, Norcross, GA, U.S.). The V4-V5 region of the bacteria 16S ribosomal RNA gene were amplified by PCR using primers 515F 5′-barcode- GTGCCAGCMGCCGCGG)−3′ and 907R 5′-CCGTCAATTCMTTTRAGTTT-3′. Fungi were amplified using primers ITS1F 5′- CTTGGTCATTTAGGAAGTAA-3′ and ITS2 5′- GCTGCGTTCTTCATCGATGC-3′. These primers provide reliable classification accuracy and coverage for bacterial and fungal sequences (Mello et al., 2011; Sun et al., 2013). PCR reactions were performed in triplicate 20 μL mixture containing 4 μL of 5 × FastPfu Buffer, 2 μL of 2.5 mM dNTPs, 0.8 μL of each primer (5 μM), 0.4 μL of FastPfu Polymerase, and 1 μL of template DNA. Amplicons were extracted from 2 % agarose gels and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, U.S.). Purified PCR products were quantified by Qubit®3.0 (Life Invitrogen) and every twenty-four amplicons whose barcodes were different were mixed equally, and at last served as DNA samples to be sequenced on the Illumina MiSeq System platform (Shanghai BIOZERON Co., Ltd) according to the manufacturer's procedures.
Bioinformatical analyses
Raw fastq files were demultiplexed and quality-filtered using QIIME (version 1.17). The 250 bp reads were truncated at any site receiving an average quality score of < 20 over a 10 bp sliding window. Truncated reads that were shorter than 50 bp were discarded. Any cases of exact barcode matching, 2 nucleotide mismatch in primer matching, and reads containing ambiguous characters, were all removed. Usearch (version 10 http://drive5.com/uparse/) was used to pick the operational taxonomic units (OTUs) to obtain an OTU table at a 97 % identity threshold. The Shannon–Wiener curves tended to be flat, indicating that the amount of sequencing data is large enough to reflect the vast majority of microbial information in the samples (Fig. S1A and B).
Statistical analysis
Rarefaction curves and other OTU-based analyses such as the abundance-based coverage estimators (ACE), Chao 1, Shannon-Wiener index (H0), and Simpson's index (D) were applied to represent the diversity of fungi and bacteria. To examine the effects of different treatments on fungi/bacteria species, one-way permutational analysis of variance (PERMANOVA) were conducted. Additionally, nonmetric multidimensional scaling (NMDS) analysis was used based on the same data. The results were plotted in a two-dimensional space to illustrate the differences in fungal or bacterial species composition among plots and treatments. Redundancy analysis (RDA) was used to explore the effects of environmental factors (soil pH, AP, TC, and TN) on the community compositions of bacteria and fungi under different treatments.
Non-parametric factorial Kruskal–Wallis (KW) sum-rank test detection showed the differences of top 10 genus abundance in all treatments. Community assembly processes of bacteria and fungi under different treatments were estimated by applying a null model. Briefly, the null model-based β-nearest taxon index (βNTI) was employed to determine the divergences in taxonomic and phylogenetic diversity.
The FUNGuild annotation platform (http://www.stbates.org/guilds/app.php) was used to analyze the ecological function categories of fungi (Nguyen et al., 2016). The FAPROTAX database was used to analyze the function categories of bacteria (Louca et al., 2016).
All analyses were conducted in R (version 3.6.1) (R Core Team, 2019). One-way ANOVAs were used to test the effects of different treatments on soil pH value, AP, TC, and TN.
Geochemical modeling
The BAp has a similar stoichiometry with hydroxylapatite. Therefore, this study used hydroxylapatite to simulated the dissolution of BAp by Geochemist's® Workbench (GWB Version 12, USA) software. This study simulated the dissolution of hydroxylapatite after 2000 days under 0.1, 0.5, 1, and 5 mm/month flow rates of soil runoff, respectively. The rate constant of hydroxylapatite is 2.52e−16 (Manecki et al., 2000).
Results
Soil properties after addition of CB
The AP concentrations in the Control, T-100, T-200, and T-300 treatments were 47.99 mg/kg, 55.31 mg/kg, 51.33 mg/kg, and 78.74 mg/kg, respectively (Fig. 1A). The soil pH values of Control, T-100, T-200, and T-300 treatments were 6.86, 6.41, 6.61 and 6.27, respectively (Fig. 1B). In all the treatments, only T-300 treatment showed significant changes in both AP concentrations and pH values. Meanwhile, the TC and TN values showed no significant difference across all the treatments (Table S3). In addition, the SE (standard error) values of TC and TN data were ≤ 0.02 which has high reliability.
Fig. 1.
Soil available P concentrations (A) and soil pH (B) in different treatments.
The residual hydroxylapatite simulated by GWB modeling of soil runoff scouring of 0.1, 0.5, 1, and 5 mm/month was 4.97 g, 4.93 g, 4.88 g, and 4.79 g, respectively (Fig. S2A). In addtion, the concentration of H2PO4− dissolved from hydroxylapatite (after soil runoff scouring of 0.1, 0.5, 1, and 5 mm/month) was 0.57 mg/kg, 1.07 mg/kg, 1.65 mg/kg, and 4.78 mg/kg, respectively (Fig. S2B). Such slow dissolution confirmed the low P release rate from the apatite.
Soil microbial α diversity
The high query coverages of all the treatments (> 95.0 %) suggested that the dominant OTUs of microbiota were successfully captured (Table 1). The numbers of bacterial OTUs in the Control, T-100, T-200, and T-300 treatments were 44,750, 40,934, 41,644, and 44,323, respectively. For fungal OTUs, the maximum number (54,475) of OTUs was observed in the T-100 treatment. Meanwhile, the minimum number (35,771) of OTUs was recorded in the T-200 treatment. There was a significant difference between them (p < 0.05). In the Control and T-300 treatments, the numbers of OTUs were 45,603 and 46,512. Additionally, bacterial and fungal alpha diversity indices (i.e., Shannon-Wiener index, Simpson, ACE, and Chao1) have no significant change in all the treatments (Table 1).
Table 1.
Responses of microbial alpha diversity indexes to different treatments.
| Diversity |
Richness |
||||||
|---|---|---|---|---|---|---|---|
| Microbe | Treatment | Coverage% | OTUs | Shannon | Simpson | Chao1 | Ace |
| Bacteria | Control | 95.38 ± 0.32 a | 44,750 ± 3595 a | 7.39 ± 0.01 a | 1.00 ± 0.00 a | 5414.46 ± 226.03 a | 5457.97 ± 236.82 a |
| T-100 | 95.29 ± 0.04 a | 40,934 ± 3106 a | 7.38 ± 0.03 a | 1.00 ± 0.00 a | 5474.17 ± 83.96 a | 5502.41 ± 31.64 a | |
| T-200 | 95.13 ± 0.21 a | 41,644 ± 2331 a | 7.31 ± 0.14 a | 1.00 ± 0.00 a | 5552.29 ± 226.57 a | 5624.78 ± 240.69 a | |
| T-300 | 95.14 ± 0.14 a | 44,323 ± 8008 a | 7.34 ± 0.07 a | 1.00 ± 0.00 a | 5533.05 ± 145.91 a | 5619.52 ± 150.87 a | |
| Fungi | Control | 99.57 ± 0.13 a | 45,603 ± 5101 ab | 4.81 ± 0.05 a | 0.98 ± 0.00 a | 762.07 ± 106.74 a | 751.27 ± 110.30 a |
| T-100 | 99.60 ± 0.10 a | 54,475 ± 3863 a | 4.49 ± 0.20 a | 0.97 ± 0.01 a | 655.16 ± 74.56 a | 664.15 ± 85.64 a | |
| T-200 | 99.53 ± 0.12 a | 35,771 ± 3070 b | 4.66 ± 0.17 a | 0.98 ± 0.00 a | 798.56 ± 56.81 a | 800.65 ± 65.45 a | |
| T-300 | 99.52 ± 0.13 a | 46,512 ± 2070 ab | 4.51 ± 0.09 a | 0.97 ± 0.00 a | 788.08 ± 110.78 a | 778.63 ± 106.25 a | |
Values are means of three replicates ± SE.
Soil microbial community composition
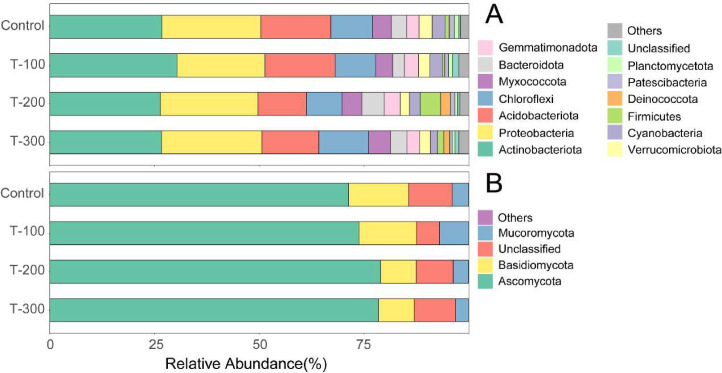
All effective bacterial and fungal sequences were assigned to phylum level (Fig. 2). Bacterial OTUs detected in all treatments were belonged to thirteen phylas (i.e., Actinobacteriota, Proteobacteria, Acidobacteriota, Chloroflexi, Myxococcota, Bacteroidota, Gemmatimonadota, Verrucomicrobiota, Cyanobacteria, Firmicutes, Deinococcota, Patescibacteria, and Planctomycetota) (Fig. 2A). Actinobacteriota and Proteobacteria were the two most abundant bacterial phyla. The sum of their abundance accounts for > 50 % of the total number of OTUs. In addition, fungal OTUs observed in all the treatments were classified to three phylas (Ascomycota, Basidiomycota, Mucoromycota). The phyla Ascomycota was the predominant fungal phyla under all the treatments (∼75 % of total OTUs) (Fig. 2B).
Fig. 2.
Average relative abundance of dominant (A) bacterial and (B) fungal phyla (>1.0 %) in different treatments. Note: “Others” refer to those identified phyla with lower than 1.0 % relative abundance under each treatment.
The NMDS figures showed that the bacterial and fungal community compositions were significantly different across all the treatments (p < 0.05) (Fig. 3). The addition of CB caused evident changes in fungal and bacterial community composition.
Fig. 3.
The dissimilarities of bacterial (A) and fungal (B) community composition among different treatments, which were visualized by non-metric multidimensional scaling (NMDS) analysis.
Differences in the abundance of the top 10 genera among different treatments were shown in Fig. 4 and Table S4. For bacteria, the Acidobacteriales and Vicinamibacteraceae have significant differences (p < 0.05) and Gaiellales have a marginally significant difference (0.05 < p < 0.1) under all the treatments (Fig. 4A). For fungi, Neonectria, Coniochaeta, Trichocladium, Atractium, Ceratobasidium, Humicola, and Fusarium have significant difference (p < 0.05). Meanwhile, the Mortierella and Plectosphaerella have marginally significant differences (0.05 < p < 0.1) in all the treatments (Fig. 4B).
Fig. 4.
The abundance of the top 10 bacterial (A) and fungal (B) genera in different treatments. (“ * ” : p 〈 0.05, “ · ” : 0.05 < p < 0.1, “ ” : p 〉 0.1).
Microbial community assembly processes and functional composition
In the bacterial community, βNTI values under all the treatments were within the range of −2 and 2. This suggested that stochastic processes were dominant during the assembly of bacterial community (Fig. 5A). For the fungal community, the βNTI values of the Control, T-100, and T-200 treatments were also between −2 and 2 (Fig. 5B). However, βNTI values of the fungal community under the T-300 treatment were over 2, which indicated that deterministic processes determined the assembly of fungal community (Fig. 5B).
Fig. 5.
The null model-based β-nearest taxon index (βNTI) of bacterial (A) and fungal (B) in different treatments.
According to redundant analysis, four environmental variables (i.e., pH, AP concentration, TC, and TN) all showed different influences on bacterial and fungal community compositions (Fig. 6). Either for the bacterial or fungal community, soil pH was significantly correlated with the community compositions (p = 0.001 and 0.007). Moreover, soil AP concentration (p = 0.063 and 0.098) was marginally correlated with the community compositions (Fig. 6). However, soil TC and TN contents did not show significant correlation with the community compositions, whether in fungal or bacterial communities (Fig. 6).
Fig. 6.
Redundancy analysis showing effects of environmental variables on the community composition of bacterial (A) and fungal (B) among different treatments. (“ *** ” : p < 0.001, “ ** ” : 0.001< p < 0.01, “ * ” : 0.01< p < 0.05, “ · ” : 0.05 < p < 0.1).
For the bacterial species involved in N cycle, the abundance of bacteria with denitrification function (nitrous oxide denitrification, nitrate denitrification, and nitrite denitrification) was increased in the T-100 treatment (Fig. 7A). In contrast, the abundance of bacteria with denitrification function were decreased in both the T-200 and T-300 treatments. Meanwhile, the abundance of bacteria with nitrate respiration, nitrate reduction, and nitrogen respiration function was significantly increased in the T-300 treatment (Fig. 7A). Moreover, the abundance of bacteria with N fixation and nitrate ammonification function decreased in all the treatments after CB addition (Fig. 7A).
Fig. 7.
Relative abundance of bacteria related to N cycle (A), phosphate-solubilizing bacteria and fungi (B), fungi with different trophic modes (C) and different mycorrhizal fungi (D). Bacteria related to N cycle were identified based on FAPROTAX database. Fungi with different trophic modes and different mycorrhizal fungi were identified based on FUNGuild database.
The genera and abundance of phosphate-solubilizing bacteria (PSB) were significantly declined in the T-100 treatment (Fig. 7B). In the T-300 treatment, the abundance of phosphate-solubilizing fungi (PSF) significantly increased (Fig. 7B). For the fungi with different trophic mode, the abundance of saprotroph increased in T-300 treatment (decreased in the T-100 treatment). This was reversed to the abundance of pathotroph fungi (see Fig. 7C). The abundance of symbiotroph fungi increased in T-100 treatment (decreased in the T-200 treatment). For mycorrhizal fungi, the abundance of arbuscular mycorrhizal fungi (AMF) declined in the T-100, T-200, and T-300 treatments. Meanwhile, ectomycorrhizal fungi (ECM) increased in the T-200 treatment and endophyte fungi decreased in Control treatment (Fig. 7D).
Discussion
BAp mineral in CB is relatively stable below 200 oC. Decrystallization is emerging when the heating temperature raised to ∼300 °C (Amin, 2023). Simultaneously, heating increased BET (Brunauer, Emmett, and Teller) surface area of CB, together with abundant pores with enlarged diameters (Tang et al., 2019). These could provide abundant sites for microbial occupation (Saquing et al., 2016; Liao et al., 2021). In addition, the organic P compounds in bone could be cleft into inorganic P after ∼300 °C heating. This would provide additional nutrition for PSM adhered to CB surface/pore system (Ahmed et al., 2021). Therefore, the P release from bone heated at ∼300 °C was significantly increased by ∼60 % (Fig. 1A).
In many ecosystems with low primary productivity, the fires usually cannot maintain heating for long-term, due to a shortage of flammable sources (Korfmacher et al., 2003). This would lead to a decrease in the P release of from bone materials. Simultaneously, this study observed that insufficient heating resulted in a decline of the richness and abundance of PSB (Fig. 7B), which will further reducing P release from CB. In addition, after addition of the CB heated at 100 °C, the abundance of pathotroph fungi was elevated (Fig. 7D), which intensified the risk of transmitting pathogens. When the ecosystem has high primary productivity (e.g., grasslands and shrubland), the fire could fully heat the debris and thus promote P release from CB (Bowman et al., 2014; Zwetsloot et al., 2016). Therefore, CB may act as a natural P “Hot Spot”, but possibly only in high primary productivity ecosystems.
The low availability of P is usually the major constraint on microbial biomass (Turner et al., 2007; Meng et al., 2022). In addition, P availability was also closely tied to soil microbial community composition (Fig. 3) (Yang et al., 2022). In this study, redundant analyses (based on CB heated at ∼300 °C, Fig. 6) suggested that these changes in the microbial community were both tightly correlated with AP concentration and pH. These results were consistent with previous studies based on P addition (not CB) to soils (Li et al., 2015; Nielsen et al., 2015; Ling et al., 2017). Additionally, the changes in microbial community composition may seriously affect ecological functions and services. For example, the abundance of denitrifiers is positively related to N2O emission, which would promote global warming (Wang et al., 2023). Previous studies proposed that the utilization of P could stimulate the growth of most denitrifiers (Sun et al., 2015; Tang et al., 2016). In contrast, this study showed that CB heated at ∼300 °C decreased the abundance of soil microbes involved in denitrification. This is due to that CB induced the decline of soil pH and then inhibited the growth of denitrifiers. This inhibitory effect overpassed CB's positive role of alleviating phosphorus limitation (Mehnaz and Dijkstra, 2016).
The assembly of microbial community could affect the soil P cycling capacity and stability of ecosystems (Wei et al., 2019). In this study, fungi and bacteria showed different responses to CB addition. Specifically, community assembly process shifted from stochastic to deterministic for the fungal community, while the bacterial community showed no significant change (Fig. 5). Stochastic and deterministic processes are two opposite ecological forces in shaping microbial community composition (Zhou and Ning, 2017). The shift in the assembly process of the fungal community indicated that CB exerted an environmental filtering force (Xiong et al., 2010; Luan et al., 2020). Additionally, the significant abundance of the top ten fungal genera confirmed that the fungal community composition was more sensitive to P availability than the bacterial community (Li et al., 2015). This is consistent with that P addition would stimulate fungal growth (Hagerberg et al., 2003; Li et al., 2015). The relatively high stimulation for fungi (compared with bacteria) can be attributed to that fungi require more nutrients to support their high biomass (Gulis and Suberkropp, 2003).
The addition of CB heated at ∼300 °C also increased the abundance of PSF (Fig. 7B). PSF are critical in bridging P release and biosphere (Tian et al., 2021). The abiotic weathering rate of P-minerals is usually slow (Fig. S2) (Xu et al., 2020). The phosphate-solubilizing ability of PSF is primarily attributed to their secretion of a variety of low molecular weight organic acids, e.g., gluconic acid, citric acid, oxalic acid, etc. (Zhu et al., 2018). These organic acids can increase the solubility of CB by reducing soil pH. In addition, organic acids also can chelate the cations from phosphates, then converting phosphates into soluble P species (Kpomblekoua and Tabatabai, 1994). Subsequently, as a positive feedback, the P released from CB into the environment is also beneficial to the growth of PSF (Zhang et al., 2019). This, again, promotes the production of organic acids. Furthermore, although P release after such moderate promotion is still not comparable to that due to typical chemical fertilizers, it would promise long-term sustainable usage of P source.
Conclusions
Charred bone materials changed the composition of soil microbial communities, and the fungal community was more sensitive than bacteria. Moreover, this study further observed that CB heated at ∼100 °C bring negative influences to the microbial community, thus increasing the risk of plants and the environments. CB heated at ∼300 °C, as P “Hot Spots”, caused the fungal community assembly process changed from stochastic to detSerministic. Furthermore, it increased the available P and abundance of PSF, which initiated positive feedback between P release and PSF in soil system. Future studies should be encouraged to test the above conclusions in other soil types, particularly based on long-term field experiments.
Funding sources
This study was supported by State Key Laboratory of Lake Science and Environment (2022SKL016), the Fundamental Research Funds for the Central Universities (KYCYXT2022004), State Key Laboratory of Biogeology and Environmental Geology, China University of Geosciences (No. GBL22102), and the Funds for Education Reform at Nanjing Agricultural University (2021Y012). Ms. Lingzi Meng were financially supported by Postgraduate Research & Practice Innovation Program of Jiangsu Province (SJCX22-0202).
CRediT authorship contribution statement
Lingzi Meng: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. Yunhui Chen: Investigation, Writing – review & editing. Lingyi Tang: Formal analysis, Writing – review & editing. Xiaoqin Sun: Conceptualization, Writing – review & editing. Hongxun Huo: Formal analysis, Writing – review & editing. Yuxin He: Formal analysis, Writing – review & editing. Yinan Huang: Formal analysis, Writing – review & editing. Qi Shao: Formal analysis, Writing – review & editing. Shang Pan: Investigation, Writing – review & editing. Zhen Li: Conceptualization, Writing – review & editing.
Declaration of competing interest
The authors have no conflict of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.crmicr.2024.100221.
Appendix. Supplementary materials
Data availability
Data will be made available on request.
References
- Ahmed M., Nigussie A., Addisu S., Belay B., Sato S. Valorization of animal bone into phosphorus biofertilizer: effects of animal species, thermal processing method, and production temperature on phosphorus availability. Soil Sci. Plant Nutr. 2021;67(4):471–481. doi: 10.1080/00380768.2021.1945403. [DOI] [Google Scholar]
- Alonso-Canas I., Chuvieco E. Global burned area mapping from ENVISAT-MERIS and MODIS active fire data. Remote Sens. Environ. 2015;163:140–152. doi: 10.1016/j.rse.2015.03.011. [DOI] [Google Scholar]
- Amin A.A. Effects of pyrolysis temperatures on bone char characterization and its releasing phosphorus in sandy soil. Arch. Agron. Soil Sci. 2023;69(2):304–313. doi: 10.1080/03650340.2021.1988940. [DOI] [Google Scholar]
- Ansley R.J., Jones D.L., Tunnell T.R., Kramp B.A., Jacoby P.W. Honey mesquite canopy responses to single winter fires: relation to herbaceous fuel, weather and fire temperature. Int. J. Wildland Fire. 1998;8(4):241–252. doi: 10.1071/Wf9980241. [DOI] [Google Scholar]
- Beauregard M.S., Hamel C., Nayyar A., St-Arnaud M. Long-term phosphorus fertilization impacts soil fungal and bacterial diversity but not AM Fungal Community in Alfalfa. Microb. Ecol. 2010;59(2):379–389. doi: 10.1007/s00248-009-9583-z. [DOI] [PubMed] [Google Scholar]
- Bowman D.M.J.S., Murphy B.P., Williamson G.J., Cochrane M.A. Pyrogeographic models, feedbacks and the future of global fire regimes. Glob. Ecol. Biogeogr. 2014;23(7):821–824. doi: 10.1111/geb.12180. [DOI] [Google Scholar]
- Butler O.M., Elser J.J., Lewis T., Mackey B., Chen C.R. The phosphorus-rich signature of fire in the soil-plant system: a global meta-analysis. Ecol. Lett. 2018;21(3):335–344. doi: 10.1111/ele.12896. [DOI] [PubMed] [Google Scholar]
- Cazalbou S., Combes C., Eichert D., Rey C. Adaptative physico-chemistry of bio-related calcium phosphates. J. Mater. Chem. 2004;14(14):2148–2153. doi: 10.1039/b401318b. [DOI] [Google Scholar]
- Chen W.K., Wang Q.Z., Meng S.T., Yang P., Jiang L., Zou X., Li Z., Hu S.J. Temperature-related changes of Ca and P release in synthesized hydroxylapatite, geological fluorapatite, and bone bioapatite. Chem. Geol. 2017;451:183–188. doi: 10.1016/j.chemgeo.2017.01.014. [DOI] [Google Scholar]
- Childers D.L., Corman J., Edwards M., Elser J.J. Sustainability challenges of phosphorus and food: solutions from closing the human phosphorus cycle. Bioscience. 2011;61(2):117–124. doi: 10.1525/bio.2011.61.2.6. [DOI] [Google Scholar]
- Cordell D., Drangert J.O., White S. The story of phosphorus: global food security and food for thought. Glob. Environ. Change. 2009;19(2):292–305. doi: 10.1016/j.gloenvcha.2008.10.009. [DOI] [Google Scholar]
- Dayamba S.D., Savadogo P., Zida D., Sawadogo L., Tiveau D., Oden P.C. Fire temperature and residence time during dry season burning in a Sudanian savanna-woodland of West Africa with implication for seed germination. J. For. Res. (Harbin) 2010;21(4):445–450. doi: 10.1007/s11676-010-0095-y. [DOI] [Google Scholar]
- Ducousso-Détrez, A., Fontaine, J., Sahraoui, A.L.H., Hijri, M., 2022. Diversity of phosphate chemical forms in soils and their contributions on soil microbial community structure changes. Microorganisms 10 (3). 609. 10.3390/microorganisms10030609. [DOI] [PMC free article] [PubMed]
- Gilbert N. Environment: the disappearing nutrient. Nature. 2009;461(7265):716–718. doi: 10.1038/461716a. [DOI] [PubMed] [Google Scholar]
- Gulis V., Suberkropp K. Effect of inorganic nutrients on relative contributions of fungi and bacteria to carbon flow from submerged decomposing leaf litter. Microb. Ecol. 2003;45(1):11–19. doi: 10.1007/s00248-002-1032-1. [DOI] [PubMed] [Google Scholar]
- Hagerberg D., Thelin G., Wallander H. The production of ectomycorrhizal mycelium in forests: relation between forest nutrient status and local mineral sources. Plant Soil. 2003;252(2):279–290. doi: 10.1023/A:1024719607740. [DOI] [Google Scholar]
- Kamerlin S.C.L., Sharma P.K., Prasad R.B., Warshel A. Why nature really chose phosphate. Q. Rev. Biophys. 2013;46(1):1–132. doi: 10.1017/S0033583512000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korfmacher J.L., Chambers J.C., Tausch R.J., Roundy B.A., Meyer S.E., Kitchen S. Technical note: a technique for conducting small-plot burn treatments. J. Range Manage. 2003;56(3):251–254. doi: 10.2307/4003814. [DOI] [Google Scholar]
- Kpomblekoua K., Tabatabai M.A. Effect of organic-acids on release of phosphorus from phosphate rocks. Soil Sci. 1994;158(6):442–453. doi: 10.1097/00010694-199415860-00006. [DOI] [Google Scholar]
- Legeros R.Z., Kijkowska R., Bautista C., Legeros J.P. Synergistic effects of magnesium and carbonate on properties of biological and synthetic apatites. Connect Tissue Res. 1995;33(1–3):203–209. doi: 10.3109/03008209509017003. [DOI] [PubMed] [Google Scholar]
- Li H.P., Han Q.Q., Liu Q.M., Gan Y.N., Rensing C., Rivera W.L., Zhao Q., Zhang J.L. Roles of phosphate-solubilizing bacteria in mediating soil legacy phosphorus availability. Microbiol. Res. 2023;272 doi: 10.1016/j.micres.2023.127375. [DOI] [PubMed] [Google Scholar]
- Li J., Li Z.A., Wang F.M., Zou B., Chen Y., Zhao J., Mo Q.F., Li Y.W., Li X.B., Xia H.P. Effects of nitrogen and phosphorus addition on soil microbial community in a secondary tropical forest of China. Biol Fertil Soils. 2015;51(2):207–215. doi: 10.1007/s00374-014-0964-1. [DOI] [Google Scholar]
- Li Z., Pasteris J.D., Novack D. Hypermineralized whale rostrum as the exemplar for bone mineral. Connect Tissue Res. 2013;54(3):167–175. doi: 10.3109/03008207.2013.769973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Wang F.W., Bai T.S., Tao J.J., Guo J.Y., Yang M.Y., Wang S.M., Hu S.J. Lead immobilization by geological fluorapatite and fungus Aspergillus niger. J. Hazard Mater. 2016;320:386–392. doi: 10.1016/j.jhazmat.2016.08.051. [DOI] [PubMed] [Google Scholar]
- Li Z.L., Qiu Y., Zhao D.Y., Li J., Li G.L., Jia H., Du D.L., Dang Z., Lu G.N., Li X.F., Yang C.F., Kong L.J. Application of apatite particles for remediation of contaminated soil and groundwater: a review and perspectives. Sci. Total Environ. 2023;904 doi: 10.1016/j.scitotenv.2023.166918. [DOI] [PubMed] [Google Scholar]
- Liao J.Y., Hu A., Zhao Z.W., Liu X.R., Jiang C., Zhang Z.H. Biochar with large specific surface area recruits N2O-reducing microbes and mitigate N2O emission. Soil Biol. Biochem. 2021;156 doi: 10.1016/j.soilbio.2021.108212. [DOI] [Google Scholar]
- Ling N., Chen D., Guo H., Wei J., Bai Y., Shen Q., Hu S. Differential responses of soil bacterial communities to long-term N and P inputs in a semi-arid steppe. Geoderma. 2017;292:25–33. doi: 10.1016/j.geoderma.2017.01.013. [DOI] [Google Scholar]
- Liu Q., Huang S.S., Matinlinna J.P., Chen Z.F., Pan H.B. Insight into biological apatite: physiochemical properties and preparation approaches. Biomed. Res. Int. 2013;2013 doi: 10.1155/2013/929748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louca S., Parfrey L.W., Doebeli M. Decoupling function and taxonomy in the global ocean microbiome. Science (1979) 2016;353(6305):1272–1277. doi: 10.1126/science.aaf4507. [DOI] [PubMed] [Google Scholar]
- Luan L., Liang C., Chen L.J., Wang H.T., Xu Q.S., Jiang Y.J., Sun B. Coupling bacterial community assembly to microbial metabolism across soil profiles. mSystems. 2020;5(3) doi: 10.1128/mSystems.00298-20. e00298-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manecki M., Maurice P.A., Traina S.J. Kinetics of aqueous Pb reaction with apatites. Soil Sci. 2000;165(12):920–933. doi: 10.1097/00010694-200012000-00002. [DOI] [Google Scholar]
- Marklein A.R., Houlton B.Z. Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol. 2012;193(3):696–704. doi: 10.1111/j.1469-8137.2011.03967.x. [DOI] [PubMed] [Google Scholar]
- Mehnaz K.R., Dijkstra F.A. Denitrification and associated N2O emissions are limited by phosphorus availability in a grassland soil. Geoderma. 2016;284:34–41. doi: 10.1016/j.geoderma.2016.08.011. [DOI] [Google Scholar]
- Mello A., Napoli C., Murat C., Morin E., Marceddu G., Bonfante P. ITS-1 versus ITS-2 pyrosequencing: a comparison of fungal populations in truffle grounds. Mycologia. 2011;103(6):1184–1193. doi: 10.3852/11-027. [DOI] [PubMed] [Google Scholar]
- Mendes Gde O., da Silva N.M., Anastacio T.C., Vassilev N.B., Ribeiro J.I., Jr., da Silva I.R., Costa M.D. Optimization of Aspergillus niger rock phosphate solubilization in solid-state fermentation and use of the resulting product as a P fertilizer. Microb. Biotechnol. 2015;8(6):930–939. doi: 10.1111/1751-7915.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L.Z., Pan S., Zhou L.M., Santasup C., Su M., Tian D., Li Z. Evaluating the survival of Aspergillus niger in a highly polluted red soil with addition of Phosphogypsum and bioorganic fertilizer. Environ. Sci. Pollut Res. Int. 2022 doi: 10.1007/s11356-022-21243-5. [DOI] [PubMed] [Google Scholar]
- Nguyen N.H., Song Z.W., Bates S.T., Branco S., Tedersoo L., Menke J., Schilling J.S., Kennedy P.G. FUNGuild: an open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016;20:241–248. doi: 10.1016/j.funeco.2015.06.006. [DOI] [Google Scholar]
- Nielsen U.N., Prior S., Delroy B., Walker J.K.M., Ellsworth D.S., Powell J.R. Response of belowground communities to short-term phosphorus addition in a phosphorus-limited woodland. Plant Soil. 2015;391(1–2):321–331. doi: 10.1007/s11104-015-2432-6. [DOI] [Google Scholar]
- Olander L.P., Vitousek P.M. Regulation of soil phosphatase and chitinase activity by N and P availability. Biogeochemistry. 2000;49(2):175–190. doi: 10.1023/A:1006316117817. [DOI] [Google Scholar]
- Saquing J.M., Yu Y.H., Chiu P.C. Wood-derived black carbon (Biochar) as a microbial electron donor and acceptor. Environ. Sci. Technol. Lett. 2016;3(2):62–66. doi: 10.1021/acs.estlett.5b00354. [DOI] [Google Scholar]
- Sinsabaugh R.L., Lauber C.L., Weintraub M.N., Ahmed B., Allison S.D., Crenshaw C., Contosta A.R., Cusack D., Frey S., Gallo M.E., Gartner T.B., Hobbie S.E., Holland K., Keeler B.L., Powers J.S., Stursova M., Takacs-Vesbach C., Waldrop M.P., Wallenstein M.D., Zak D.R., Zeglin L.H. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008;11(11):1252–1264. doi: 10.1111/j.1461-0248.2008.01245.x. [DOI] [PubMed] [Google Scholar]
- Sun D.L., Jiang X., Wu Q.L., Zhou N.Y. Intragenomic heterogeneity of 16S rRNA genes causes overestimation of prokaryotic diversity. Appl. Environ. Microbiol. 2013;79(19):5962–5969. doi: 10.1128/AEM.01282-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun R.B., Guo X.S., Wang D.Z., Chu H.Y. Effects of long-term application of chemical and organic fertilizers on the abundance of microbial communities involved in the nitrogen cycle. Appl. Soil Ecol. 2015;95:171–178. doi: 10.1016/j.apsoil.2015.06.010. [DOI] [Google Scholar]
- Tamburini F., Pfahler V., Bunemann E.K., Guelland K., Bernasconi S.M., Frossard E. Oxygen isotopes unravel the role of microorganisms in phosphate cycling in soils. Environ. Sci. Technol. 2012;46(11):5956–5962. doi: 10.1021/es300311h. [DOI] [PubMed] [Google Scholar]
- Tang L.Y., Shen Z.T., Duan X.F., Wang Z.J., Wu Y.Y., Shao X.Q., Song X.W., Hu S.J., Li Z. Evaluating the potential of charred bone as P hotspot assisted by phosphate-solubilizing bacteria. Sci. Total Environ. 2019;696 doi: 10.1016/j.scitotenv.2019.133965. [DOI] [PubMed] [Google Scholar]
- Tang Y.C., Zhang X.Y., Li D.D., Wang H.M., Chen F.S., Fu X.L., Fang X.M., Sun X.M., Yu G.R. Impacts of nitrogen and phosphorus additions on the abundance and community structure of ammonia oxidizers and denitrifying bacteria in Chinese fir plantations. Soil Biol. Biochem. 2016;103:284–293. doi: 10.1016/j.soilbio.2016.09.001. [DOI] [Google Scholar]
- Tian J., Ge F., Zhang D.Y., Deng S.Q., Liu X.W. Roles of phosphate solubilizing microorganisms from managing soil phosphorus deficiency to mediating biogeochemical P cycle. Biology-Basel. 2021;10(2):158. doi: 10.3390/biology10020158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonini D., Saveyn H.G.M., Huygens D. Environmental and health co-benefits for advanced phosphorus recovery. Nat. Sustain. 2019;2(11):1051–1061. doi: 10.1038/s41893-019-0416-x. [DOI] [Google Scholar]
- Turner B.L., Condron L.M., Richardson S.J., Peltzer D.A., Allison V.J. Soil organic phosphorus transformations during pedogenesis. Ecosystems. 2007;10(7):1166–1181. doi: 10.1007/s10021-007-9086-z. [DOI] [Google Scholar]
- Turner B.L., Wright S.J. The response of microbial biomass and hydrolytic enzymes to a decade of nitrogen, phosphorus, and potassium addition in a lowland tropical rain forest. Biogeochemistry. 2014;117(1):115–130. doi: 10.1007/s10533-013-9848-y. [DOI] [Google Scholar]
- Walton C.R., Ewens S., Coates J.D., Blake R.E., Planavsky N.J., Reinhard C., Ju P.C., Hao J.H., Pasek M.A. Phosphorus availability on the early Earth and the impacts of life. Nat. Geosci. 2023 doi: 10.1038/s41561-023-01167-6. [DOI] [Google Scholar]
- Wang H., Qiu Y.P., Zhang K.C., Zhao Y.X., Li Y.T., Wang Y., Bai Y.F., Zhang Y., Hu S.J. Alterations in substrate stoichiometry control the responses of soil diazotrophs to nutrient enrichment. Soil Biol. Biochem. 2023;179 [Google Scholar]
- Wang S.J., Zhang P.H., Kong X.F., Xie S.D., Li Q., Li Z., Zhou Z.L. Delicate changes of bioapatite mineral in pig femur with addition of dietary xylooligosaccharide: evidences from Raman spectroscopy and ICP. Anim. Sci. J. 2017;88(11):1820–1826. doi: 10.1111/asj.12837. [DOI] [PubMed] [Google Scholar]
- Wei Z., Gu Y., Friman V.P., Kowalchuk G.A., Xu Y.C., Shen Q.R., Jousset A. Initial soil microbiome composition and functioning predetermine future plant health. Sci. Adv. 2019;5(9):eaaw0759. doi: 10.1126/sciadv.aaw0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.H., Zhou J., Yu D., Sun S.Q., Luo J., Bing H.J., Sun H.Y. Phosphorus biogeochemical cycle research in mountainous ecosystems. J. Mt. Sci. 2013;10(1):43–53. doi: 10.1007/s11629-013-2386-1. [DOI] [Google Scholar]
- Xiong J.B., Wu L.Y., Tu S.X., Van Nostrand J.D., He Z.L., Zhou J.Z., Wang G.J. Microbial communities and functional genes associated with soil arsenic contamination and the rhizosphere of the arsenic-hyperaccumulating plant Pteris vittata L. Appl. Environ. Microbiol. 2010;76(21):7277–7284. doi: 10.1128/Aem.00500-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Mao X., Van Zwieten L., Niazi N.K., Lu K., Bolan N.S., Wang H. Wetting-drying cycles during a rice-wheat crop rotation rapidly (im)mobilize recalcitrant soil phosphorus. J. Soils Sediments. 2020;20(11):3921–3930. doi: 10.1007/s11368-020-02712-1. [DOI] [Google Scholar]
- Yang Z., Zhang Y., Wang Y., Zhang H., Zhu Q., Yan B., Fei J., Xiangmin R., Peng J., Luo G. Intercropping regulation of soil phosphorus composition and microbially-driven dynamics facilitates maize phosphorus uptake and productivity improvement. Field Crops Res. 2022;287 doi: 10.1016/j.fcr.2022.108666. [DOI] [Google Scholar]
- Yao Q.M., Li Z., Song Y., Wright S.J., Guo X., Tringe S.G., Tfaily M.M., Pasa-Tolic L., Hazen T.C., Turner B.L., Mayes M.A., Pan C.L. Community proteogenomics reveals the systemic impact of phosphorus availability on microbial functions in tropical soil. Nat. Ecol. Evol. 2018;2(3):499–509. doi: 10.1038/s41559-017-0463-5. [DOI] [PubMed] [Google Scholar]
- Zhang L., Song X.W., Shao X.Q., Wu Y.L., Zhang X.Y., Wang S.M., Pan J.J., Hu S.J., Li Z. Lead immobilization assisted by fungal decomposition of organophosphate under various pH values. Sci. Rep. 2019;9:13353. doi: 10.1038/s41598-019-49976-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J.Z., Ning D.L. Stochastic community assembly: does it matter in microbial ecology? Microbiol. Mol. Biol. Rev. 2017;81(4):e00002–e00017. doi: 10.1128/MMBR.00002-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Li M., Whelan M. Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: a review. Sci. Total Environ. 2018;612:522–537. doi: 10.1016/j.scitotenv.2017.08.095. [DOI] [PubMed] [Google Scholar]
- Zwetsloot M.J., Lehmann J., Bauerle T., Vanek S., Hestrin R., Nigussie A. Phosphorus availability from bone char in a P-fixing soil influenced by root-mycorrhizae-biochar interactions. Plant Soil. 2016;408(1–2):95–105. doi: 10.1007/s11104-016-2905-2. [DOI] [Google Scholar]
- Zwetsloot M.J., Lehmann J., Solomon D. Recycling slaughterhouse waste into fertilizer: how do pyrolysis temperature and biomass additions affect phosphorus availability and chemistry? J. Sci. Food Agric. 2015;95(2):281–288. doi: 10.1002/jsfa.6716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.