Abstract
In experiments performed using graphite electrodes poised by a potentiostat (+200 mV versus Ag/AgCl) or in a microbial fuel cell (with oxygen as the electron acceptor), the Fe(III)-reducing organism Geothrix fermentans conserved energy to support growth by coupling the complete oxidation of acetate to reduction of a graphite electrode. Other organic compounds, such as lactate, malate, propionate, and succinate as well as components of peptone and yeast extract, were utilized for electricity production. However, electrical characteristics and the results of shuttling assays indicated that unlike previously described electrode-reducing microorganisms, G. fermentans produced a compound that promoted electrode reduction. This is the first report of complete oxidation of organic compounds linked to electrode reduction by an isolate outside of the Proteobacteria.
Published reports and patents claiming to harness microbial activity for electricity production date at least to the early 1960s (9, 23, 24). Most designs used dense suspensions of nongrowing bacteria in combination with a redox-active dye, or an inorganic compound such as sulfide, to shuttle a small percentage of electrons from fermentative metabolism to an electrode surface (5, 6, 8, 10, 16, 21, 27, 28). However, microorganisms that can completely oxidize organic compounds and quantitatively transfer electrons directly to an electrode have recently been described. These include several members of the Geobacteraceae (1, 2, 11) as well as Rhodoferax ferrireducens (3). Lower levels of electron recovery have also recently been reported for organisms that incompletely oxidize their substrates but still do not require a mediator for electricity production (12-15, 22).
Molecular analysis of microbial communities on the anode surfaces of sediment-based microbial fuel cells have revealed a specific enrichment of members of the proteobacterial families Geobacteraceae and, in one instance, Desulfobulbaceae (1, 25). Representatives of both of these groups have since been shown to have the capacity to transfer electrons directly to an electrode surface. In electrodes deployed in freshwater sediments, an enrichment of microorganisms closely related to Geothrix fermentans was recently noted (12a). G. fermentans was previously shown to oxidize acetate and other organic substrates to carbon dioxide with Fe(III) as the sole electron acceptor (7). However, complete oxidation at the expense of significant electricity production by an organism unrelated to members of the Proteobacteria family has yet to be observed. Thus, the ability of this organism to use an electrode as the sole electron acceptor was evaluated.
Growth of G. fermentans with an electrode as the sole electron acceptor.
G. fermentans was cultured with acetate as the electron donor and fumarate as the electron acceptor as previously described (2, 7). Cells were collected via centrifugation and resuspended in an equal volume of medium lacking electron donors or acceptors. Cells (5%) were inoculated into 250-ml anaerobic glass chambers which contained only a graphite electrode (62 cm2) as an electron acceptor. The medium in the chambers was identical to that used for growth, except that fumarate was omitted and 1 mM acetate was provided as the electron donor (2, 12). The anode in the growth chamber was connected via a 500-Ω resistor to an identical electrode (cathode) in a second chamber, which was flushed with air. The two chambers were linked by a Nafion 117 apparatus, as previously described (2).
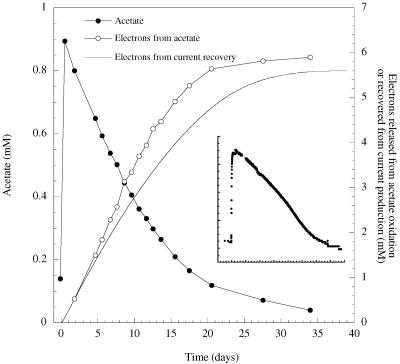
After a lag (typically 3 to 5 days), current production increased exponentially. When current production declined, due to exhaustion of the donor (verified by high-pressure liquid chromatography) (2), more acetate (1 mM) was added, and current production resumed. Typically, a second addition of acetate resulted in higher rates of current production (peak values of 0.08 mA), but subsequent additions of acetate typically did not increase current output significantly. In mature cultures, acetate declined as power was produced (Fig. 1), and recovery of electrons (integrated current over time versus acetate disappearance) was 94 ± 6% (mean ± standard deviation; n = 3). Acetate (open symbols; Fig. 1) initially disappeared faster than could be accounted for by current recovery (solid line; Fig. 1), suggesting storage rather than oxidation of some of the acetate, but as acetate concentrations declined, near-complete electron recovery levels were observed.
FIG. 1.
Acetate disappearance (•), electron recovery predicted on the basis of acetate disappearance (○), electron recovery on the basis of integrated amperage (solid line), and current production (inset) for a representative microbial fuel cell containing G. fermentans attached to a graphite electrode. The anode was connected to a graphite cathode (in aerated water) via a 500-Ω resistor.
Addition of propionate (5 mM), malate (5 mM), lactate (5 mM), or succinate (5 mM) resulted in higher rates of current production than addition of acetate. Propionate and lactate produced the highest peak rates of current flow (0.31 and 0.26 mA, respectively), with malate and succinate producing less (0.11 and 0.15 mA). Propionate and lactate were only partially oxidized to acetate, likely due to the additional substrate-level ATP gain associated with acetate production. Electron recovery data (comparison of integrated current and high-pressure liquid chromatography data) indicated that acetate produced from these compounds was further oxidized in the latter stages of the incubation, when propionate and lactate were depleted. Succinate and malate were completely oxidized.
While ΔE values (calculated from ΔGf values) (26) indicated that all of these organic acids were stronger electron donors than acetate, increases in the rate of current flow did not correlate with their formal potentials. For instance, the ΔE′ of the CO2-malate half-reaction is −0.35 V, while that for the acetate-propionate half-reaction is −0.28 V. Current production correlated more strongly with the compounds which were partially (and more rapidly) oxidized than with compounds for which only a complete oxidation pathway was available. Peptone (1 g/liter) or yeast extract (1 g/liter) also resulted in short periods of current production for ca. 48 h that peaked at rates lower than those observed with acetate (0.04 and 0.03 mA, respectively), suggesting that some components of each could be oxidized by the organism. Flushing of chambers with hydrogen as a potential electron donor did not result in any current production, an observation consistent with the fact that G. fermentans cannot use hydrogen to reduce other electron acceptors.
To reduce limitations imposed by the resistive load and oxygen reduction reactions at the cathode and to measure current production by attached G. fermentans cells under controlled conditions, the potential of the anode was poised with a potentiostat (2). G. fermentans could be grown on an anode surface with the anode poised at +200 mV versus an Ag/AgCl reference electrode, and the counter electrode chamber was flushed with 80:20 N2/CO2. Under these conditions, the rate of current production was 0.6 mA at 1 mM acetate, with an electron recovery of 97 ± 4% (n = 3).
An average of 3.3 ± 0.7 mg of protein/electrode (n = 4) was recovered when electrode surfaces from acetate-grown cultures were treated with 0.2 N NaOH and scraped to remove attached biomass. When electrode surfaces were fixed with 1% glutaraldehyde, dehydrated in a series of buffers with increasing ethanol concentrations, subjected to CO2 critical-point drying, and visualized via scanning electron microscopy (SEM) (2), the cells attached to the electrodes appeared to be coated in a thick matrix (Fig. 2). This differed from previous studies with members of the Proteobacteria family in which individual cells appeared to be attached to the electrode surface without substantial extracellular material (2, 11).
FIG. 2.
SEM image of a graphite anode after growth of G. fermentans in a microbial fuel cell.
Evidence for an electron shuttle in G. fermentans fuel cells.
When the medium in the anode chamber of G. fermentans was replaced, power production dropped by at least 50% and then slowly recovered to its original peak level within 10 days. This strongly contrasted with results of experiments performed with other organisms in which replacing the medium in the anode chamber had no impact on power production (1-3, 11). In those studies, the ability to remove or replace the medium without affecting power production was interpreted as evidence that no extracellular components were released to the medium that were assisting electron transfer to the electrode.
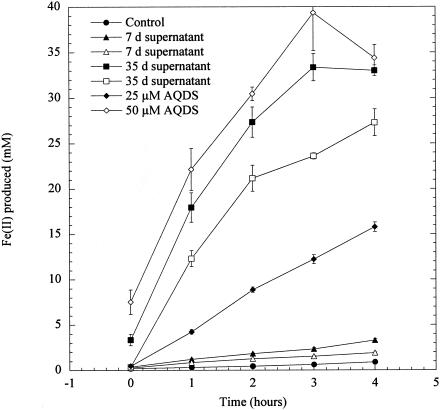
The potential presence of an electron shuttle in the G. fermentans electrode cultures was first investigated using washed cell suspensions of G. sulfurreducens as a test organism. As previously described (18, 19), G. sulfurreducens cells grown with fumarate as the electron acceptor were suspended in nongrowth medium with acetate as the electron donor and poorly crystalline Fe(III) oxide (100 mmol/liter) as the electron acceptor. As expected from previous studies (18, 19), these washed cells slowly reduced Fe(III) unless the electron-shuttling compound anthraquinone-2,6,-disulfonate (AQDS) was added (Fig. 3). Suspension of the same washed cells in 0.2-μm-pore-diameter-filtered medium from anode chambers of G. fermentans cultures that had been producing current for only 7 days had little impact on Fe(III) reduction. However, the filtrate of medium from anode chambers of cultures that had been producing power for 35 days stimulated Fe(III) reduction better than the addition of 25 μM AQDS.
FIG. 3.
Fe(III)-oxide reduction by G. sulfurreducens resting cell suspensions incubated with supernatants from G. fermentans fuel cells. Effects of supernatants from two 7-day-old fuel cells (▵, ▴) and two 35-day-old fuel cells (□, ▪) are shown, with the effects of 25 μM (♦) and 50 μM (⋄) AQDS shown for comparison.
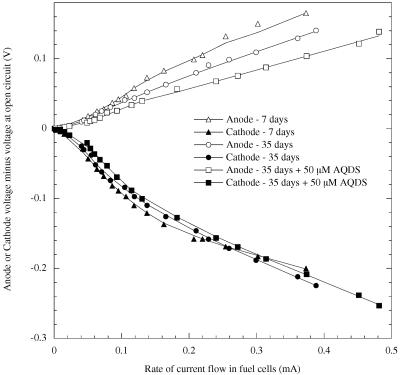
Characterization of power production also suggested that an electron shuttle was present in older cultures. Active fuel cells (5 mM acetate) were allowed to equilibrate at open circuit (no current flow) for 16 h. Resistance between electrodes was then decreased stepwise at 5-min intervals, and voltage between electrodes as well as between electrodes and a reference electrode (Ag/AgCl) was measured. This provided estimates of what is commonly termed polarization, or overvoltage, which can generally be described as the amount of energy lost to limitations at and between electrode surfaces (17).
In general, such current-voltage profiles of G. fermentans fuel cells demonstrated lower total power outputs at all rates of current flow in comparison to G. sulfurreducens fuel cells (2) (Fig. 4). Each individual fuel cell demonstrated variations in peak performance (as much as 30%) between replicates, likely due to differences such as the amount of biomass attached to electrodes and electrode roughness. However, when current-voltage relationships were recorded over a 35-day period following medium replacement, there was a clear trend in the anode overvoltages at higher current densities (a representative chamber is shown in Fig. 4). Results showed that 35-day-old cultures had substantially higher anode potentials at higher rates of current flow, manifested by the reduction in anode overvoltage, compared to 7-day-old cultures (Fig. 4). This suggests that there was an improvement in electron transfer to the anode as the culture aged. For comparison, when 50 μM AQDS was added to the 35-day-old fuel cells, further reductions in overvoltage were observed, demonstrating that additional electron shuttle in the anode chamber could produce the observed results.
FIG. 4.
Overvoltages for anodes and cathodes in a representative G. fermentans fuel cell over a range of current densities. Characteristics were measured 7 days after medium replacement (▵, ▴), in the same fuel cell 35 days after medium replacement (○, •), and with 50 μM AQDS addition 1 day later (□, ▪). Values closer to zero as the rate of current flow increases indicate improvements in operating cell voltage.
Implications.
The results presented here represent the first report of a organism from outside the Proteobacteria family able to completely oxidize a variety of substrates coupled to electrode reduction and provides the first evidence of an organism synthesizing a soluble compound that promotes electrode reduction. The finding that G. fermentans can oxidize acetate and other substrates with an electrode serving as the sole electron acceptor suggests that the enrichment of organisms closely related to G. fermentans on energy-harvesting electrodes in freshwater sediments may be due to these organisms gaining an energetic advantage by growth on or near the electrode.
Previous studies have suggested that G. fermentans releases an as-yet-unidentified soluble electron shuttle which permits it to reduce Fe(III) oxides that it cannot directly access (19). Thus, it is not surprising that this organism may use a similar strategy to enhance transfer of electrons to electrodes, which also represent an insoluble, extracellular electron acceptor. The finding that removal of the medium surrounding the G. fermentans cultures only decreased power production ca. 50%, and that significant evidence of a putative shuttling compound was not detected in the surrounding medium until weeks had elapsed, suggests that cells on the electrode surface may have a mechanism to prevent loss of the compound into the external medium. Previously described electrode-reducing organisms which do not produce a shuttle do not appear to be coated in a visible extracellular matrix, on the basis of SEM images, and one hypothesis involves retention of the shuttling compound near the cell layer via adsorption or increased affinity. Alternatively, Geothrix cells may be able to directly reduce electrodes in the absence of an electron shuttle, but the accumulation of a mediator over time further enhances the rate of electron transfer. In either event, the results demonstrate that just as there are different strategies for microorganisms to reduce insoluble Fe(III) oxides (4, 18, 20), microbial electron transfer to electrodes may proceed through different pathways. This provides alternatives that may have applications in the future design of microbial fuel cells.
Acknowledgments
This research was supported by the Office of Naval Research (ONR) (grant N00014-00-1-0776), the Defense Advanced Research Projects Agency (DARPA) Defense Sciences Office (DSO) (grant N66001-02C-8044), and the Office of Science (Biological and Environmental Research), U.S. Department of Energy (cooperative agreement DEFC02-02ER63446).
We thank Dale Callaham at the UMass Central Microscopy Facility for expertise in preparing SEM images.
REFERENCES
- 1.Bond, D. R., D. E. Holmes, L. M. Tender, and D. R. Lovley. 2002. Electrode-reducing microorganisms harvesting energy from marine sediments. Science 295:483-485. [DOI] [PubMed] [Google Scholar]
- 2.Bond, D. R., and D. R. Lovley. 2003. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 69:1548-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaudhuri, S. K., and D. R. Lovley. 2003. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat. Biotechnol. 21:1229-1232. [DOI] [PubMed] [Google Scholar]
- 4.Childers, S. E., S. Ciufo, and D. R. Lovley. 2002. Geobacter metallireducens accesses insoluble Fe(III) oxide by chemotaxis. Nature 416:767-769. [DOI] [PubMed] [Google Scholar]
- 5.Choi, Y., N. Kim, S. Kim, and S. Jung. 2003. Dynamic behaviors of redox mediators within the hydrophobic layers as an important factor for effective microbial fuel cell operation. Bull. Korean Chem. Soc. 24:437-440. [Google Scholar]
- 6.Choi, Y., J. Song, S. Jung, and S. Kim. 2001. Optimization of the performance of microbial fuel cells containing alkalophillic Bacillus sp. J. Microbiol. Biotechnol. 11:863-869. [Google Scholar]
- 7.Coates, J. D., D. J. Ellis, and D. R. Lovley. 1999. Geothrix fermentans gen. nov. sp. nov., an acetate-oxidizing Fe(III) reducer capable of growth via fermentation. Int. J. Syst. Bacteriol. 49:1615-1622. [DOI] [PubMed] [Google Scholar]
- 8.Cooney, M. J., E. Roschi, I. W. Marison, C. Comninellis, and U. von Stockar. 1996. Physiologic studies with the sulfate-reducing bacterium Desulfovibrio desulfuricans: evaluation for use in a biofuel cell. Enzyme Microb. Technol. 18:358-365. [DOI] [PubMed] [Google Scholar]
- 9.Davis, J. B., and H. F. Yarbrough. 1962. Preliminary experiments on a microbial fuel cell. Science 137:615-616. [DOI] [PubMed] [Google Scholar]
- 10.Delaney, G. M., H. P. Bennetto, J. R. Mason, S. D. Roller, J. L. Stirling, and C. F. Thurston. 1984. Electron-transfer coupling in microbial fuel cells: 2. Performance of fuel cells containing selected microorganism-mediator combinations. J. Chem. Tech. Biotechnol. 34B:13-27. [Google Scholar]
- 11.Gregory, K. B., D. R. Bond, and D. R. Lovley. 2004. Graphite electrodes as electron donors for anaerobic respiration. Environ. Microbiol. 6:596-604. [DOI] [PubMed] [Google Scholar]
- 12.Holmes, D. E., D. R. Bond, and D. R. Lovley. 2004. Electron transfer by Desulfobulbus propionicus to Fe(III) and graphite electrodes. Appl. Environ. Microbiol. 70:1234-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a.Holmes, D. E., D. R. Bond, R. A. O’Neil, C. E. Reimers, L. R. Tender, and D. R. Lovley. 2004. Microbial communities associated with electrodes harvesting electricity from a variety of aquatic sediments. Microb. Ecol. 48:178-190. [DOI] [PubMed] [Google Scholar]
- 13.Kim, B. H., T. Ikeda, H. S. Park, H. J. Kim, M. S. Hyun, K. Kano, K. Takagi, and H. Tatsumi. 1999. Electrochemical activity of an Fe(III)-reducing bacterium, Shewanella putrefaciens IR-1, in the presence of alternative electron acceptors. Biotechnol. Tech. 13:475-478. [Google Scholar]
- 14.Kim, H. J., M. S. Hyun, I. S. Chang, and B. H. Kim. 1999. A microbial fuel cell type lactate biosensor using a metal-reducing bacterium, Shewanella putrefaciens. J. Microbiol. Biotechnol. 9:365-367. [Google Scholar]
- 15.Kim, H. J., H. S. Park, M. S. Hyun, I. S. Chang, M. Kim, and B. H. Kim. 2002. A mediator-less microbial fuel cell using a metal reducing bacterium, Shewanella putrefaciens. Enzyme Microb. Technol. 30:145-152. [Google Scholar]
- 16.Kim, N., Y. Choi, S. Jung, and S. Kim. 2000. Effect of initial carbon sources on the performance of microbial fuel cells containing Proteus vulgaris. Biotechnol. Bioeng. 70:109-114. [DOI] [PubMed] [Google Scholar]
- 17.Larminie, J., and A. Dicks. 2003. Fuel cell systems explained, 2nd ed. John Wiley & Sons, West Sussex, England.
- 18.Nevin, K. P., and D. R. Lovley. 2000. Lack of production of electron-shuttling compounds or solubilization of Fe(III) during reduction of insoluble Fe(III) oxide by Geobacter metallireducens. Appl. Environ. Microbiol. 66:2248-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nevin, K. P., and D. R. Lovley. 2002. Mechanisms for accessing insoluble Fe(III) oxide during dissimilatory Fe(III) reduction by Geothrix fermentans. Appl. Environ. Microbiol. 68:2294-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nevin, K. P., and D. R. Lovley. 2002. Mechanisms for Fe(III) oxide reduction in sedimentary environments. Geomicrobiol. J. 19:141-159. [Google Scholar]
- 21.Park, D. H., and J. G. Zeikus. 2000. Electricity generation in microbial fuel cells using neutral red as an electronophore. Appl. Environ. Microbiol. 66:1292-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park, H. S., B. H. Kim, H. S. Kim, H. J. Kim, G. T. Kim, M. Kim, I. S. Chang, Y. H. Park, and H. I. Chang. 2001. A novel electrochemically active and Fe(III)-reducing bacterium phylogenetically related to Clostridium butyricum isolated from a microbial fuel cell. Anaerobe 7:297-306. [Google Scholar]
- 23.Sisler, F. D. Nov. 11 1969. Biochemical Fuel Cell. USA patent 3,477,879.
- 24.Sisler, F. D. 1971. Biochemical fuel cells, p. 1-11. In D. J. D. Hockenhull (ed.), Progress in industrial microbiology, vol. 9. J. & A. Churchill, London, United Kingdom. [PubMed] [Google Scholar]
- 25.Tender, L. M., C. E. Reimers, H. A. Stecher, D. E. Holmes, D. R. Bond, D. L. Lowy, K. Pilobello, S. J. Fertig, and D. R. Lovley. 2002. Harnessing microbial power generation on the seafloor. Nat. Biotechnol. 20:821-825. [DOI] [PubMed] [Google Scholar]
- 26.Thauer, R. K., K. Jungermann, and K. Decker. 1977. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol. Rev. 41:100-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wan, B. Y., and A. C. Tseung. 1974. Some studies related to electricity generation from biological fuel cells and galvanic cells, in vitro and in vivo. Med. Biol. Eng. 12:14-28. [DOI] [PubMed] [Google Scholar]
- 28.Wingard, L. B., Jr., C. H. Shaw, and J. F. Castner. 1982. Bioelectrochemical fuel cells. Enzyme Microb. Technol. 4:137-142. [Google Scholar]