Abstract
Isolated outer membranes of Borrelia burgdorferi were used in immunoblotting experiments with sera from immune mice to identify new putative Lyme disease vaccine candidates. One immunoreactive polypeptide migrated on polyacrylamide gels just proximal to outer surface protein C and comigrated with [3H]palmitate-labeled polypeptides. A degenerate oligonucleotide primer based upon internal amino acid sequence information was used to detect the corresponding gene within a B. burgdorferi total genomic library. The relevant open reading frame (ORF) encoded a polypeptide comprised of a 24-amino-acid putative signal peptide terminated by LLISC, a probable consensus sequence for lipoprotein modification, and a mature protein of 163 amino acids. Immunoblots of a recombinant fusion protein corresponding to this ORF supported the idea that the encoded protein was a previously reported decorin-binding protein (DBP) of B. burgdorferi N40 (B. P. Guo, S. J. Norris, L. C. Rosenberg, and M. Höök, Infect. Immun. 63:3467–3472, 1995). However, further DNA sequencing revealed the presence of a second ORF, designated ORF-1, whose termination codon was 119 bp upstream of the dbp gene. ORF-1 also encoded a putative lipoprotein with a mature length of 167 amino acids. Northern blots, Southern blots, and primer extension analyses indicated that ORF-1 and dbp comprised a two-gene operon located on the 49-kb linear plasmid. Both proteins, which were 40% identical and 56% similar, partitioned into Triton X-114 detergent extracts of B. burgdorferi isolated outer membranes. Mice infected with B. burgdorferi produced high titers of antibodies against the ORF-1-encoded protein and DBP during both early and later stages of chronic infection. Both DBP and the ORF-1-encoded protein were sensitive to proteinase K treatment of intact borreliae, suggesting that they were surface exposed. In active immunization experiments, 78% of mice immunized with recombinant DBP were immune to challenge. While it is not clear whether the two lipoproteins encoded by the ORF-1-dbp operon have analogous decorin-binding functions in vivo, the combined studies implicate DBP as a new candidate for a human Lyme disease vaccine.
Lyme disease, a multisystem infectious disorder caused by the spirochetal bacterium Borrelia burgdorferi (61), is the most prevalent arthropod-borne disease in the United States (43). In 1996, more than 16,000 cases of Lyme disease were reported to the Centers for Disease Control and Prevention, an increase of 41% above 1995 and a record high (43). Therefore, the development of an efficacious Lyme disease vaccine continues to be a public health priority.
Human clinical trials have generated optimism regarding the efficacy of a Lyme disease vaccine comprised of recombinant DNA-derived outer surface protein A (OspA) of B. burgdorferi (54, 63). However, improvements to this univalent formulation may be warranted given the heterogeneity (and even absence) of OspA among some American isolates of B. burgdorferi (20, 40), the waning of protective anti-OspA antibodies after vaccination (45), and the fact that the OspA vaccine is predicated solely upon killing of B. burgdorferi within the tick vector (24, 55). One way of potentially enhancing the efficacy of a Lyme disease vaccine would be to expand the number of vaccinogens in the formulation, particularly by incorporating immunogens known to be expressed during the mammalian phase of infection. This type of multivalent vaccine would elicit antibodies having immune targets during both the arthropod and the mammalian phases of the zoonotic life cycle of B. burgdorferi.
Technological advancements for the isolation of B. burgdorferi outer membranes (15, 49) have provided new opportunities for identifying outer membrane proteins that may have antibody-accessible epitopes. In the present study, we used the procedure of Radolf et al. (49) to survey the contents of B. burgdorferi outer membranes, with emphasis on selecting putative new vaccine candidates that were immunoreactive with antibodies present in the sera of immune mice. These efforts led to the identification and molecular characterization of the B. burgdorferi decorin-binding protein (DBP), a molecule previously reported by Guo et al. (29). Further experiments revealed a second open reading frame (ORF), ORF-1, encoding a related lipoprotein and located just upstream of the dbp gene. These two genes comprise an operon located on the 49-kb linear plasmid. While it is unclear whether the ORF-1-encoded protein and DBP have analogous decorin-binding functions in vivo, evidence was garnered to support surface exposure for DBP in B. burgdorferi and to establish its vaccinogenic potential in the murine model of Lyme borreliosis. The combined studies suggest that the DBP of B. burgdorferi may represent a new candidate component for a human Lyme disease vaccine.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Low-passage uncloned B. burgdorferi 297 and N40 were obtained from Russell Johnson (Minneapolis, Minn.) and Stephen Barthold (New Haven, Conn.), respectively. Low-passage uncloned B. burgdorferi B31 and high-passage B313 (52) were provided by Alan Barbour (San Antonio, Tex.). All low-passage isolates were cultivated in BSKII medium (7) for not more than four successive transfers before experimental manipulations. The virulence of all isolates was confirmed by induction of arthritis and carditis following intradermal needle inoculation of 3-week-old C3H/HeJ (Jackson Laboratory, Bar Harbor, Maine) mice with 104 bacteria and/or by recovery from organs and tissues of infected mice (59). The 50% infective dose for B. burgdorferi 297 was between 10 and 100 organisms per mouse (30). Escherichia coli XL1-Blue (Stratagene, La Jolla, Calif.) and INV-αF′ (Invitrogen, San Diego, Calif.) were used as cloning hosts and were cultivated either in yeast-tryptone broth or on yeast-tryptone agar supplemented with 100 μg of ampicillin per ml. Cloning vectors were either pProEX-1 (GIBCO-BRL, Gaithersburg, Md.) or pCRII (Invitrogen).
Intrinsic radiolabeling of spirochetes with [3H]palmitate.
B. burgdorferi was intrinsically radiolabeled with [9,10(n)-3H]palmitate according to the method of Belisle et al. (14).
Fractionation of B. burgdorferi outer membranes.
Outer membranes of B. burgdorferi 297 were isolated as previously described (49).
SDS-PAGE and immunoblotting.
Samples for protein analysis were boiled for 10 min in final sample buffer (62.5 mM Tris-HCl [pH 6.8], 10% [vol/vol] glycerol, 5% [vol/vol] 2-mercaptoethanol, 2.0% sodium dodecyl sulfate [SDS], 0.001% [vol/vol] bromophenol blue) prior to polyacrylamide gel electrophoresis (PAGE) through 2.4% polyacrylamide stacking and 12.5 or 15% polyacrylamide resolving gels. Gels were then stained with either Coomassie brilliant blue or silver nitrate. Alternatively, proteins were transferred electrophoretically to a 0.45-μm-pore-size nitrocellulose filter (Schleicher & Schuell, Inc., Keene, N.H.) for immunoblotting. Immunoblots were incubated with either 10−2 to 10−3 dilutions of sera from B. burgdorferi-infected mice or 1:1,000 dilutions of rat polyclonal antisera. This incubation was followed by sequential incubations with 1:1,000 dilutions of either goat anti-mouse or goat anti-rat immunoglobulin G (heavy- and light-chain specific)–horseradish peroxidase conjugates and rabbit anti-goat immunoglobulin G–horseradish peroxidase conjugates (Jackson ImmunoResearch, West Grove, Pa.). Immunoblots were developed with 4-chloro-1-naphthol as the substrate.
Amino acid sequencing of individual borrelial outer membrane proteins.
Amino acid sequencing of borrelial polypeptides was performed in the Protein Chemistry Core Facility (University of Texas Southwestern Medical Center). Briefly, B. burgdorferi outer-membrane-associated polypeptides were separated by SDS-PAGE, transferred to nitrocellulose or polyvinylidene difluoride membranes, and subjected to amino acid microsequencing by standard methods (1, 2, 5, 41, 66); automated Edman degradation was used in attempts to derive N-terminal amino acid sequences. Internal amino acid sequences were obtained after trypsin digestion and separation of the peptides by high-performance liquid chromatography.
Construction of a Lambda-ZAPII B. burgdorferi 297 genomic DNA library.
A genomic DNA library of uncloned, low-passage (virulent) strain 297 DNA was constructed by Stratagene as previously described (37).
Southern hybridization analysis.
Hybridization probes for genes encoding OspA and OspC were generated by PCR amplification (37). A probe for DBP also was generated by PCR with the primer pair (18K PCR 5′ and 18K PCR 3′) listed in Table 1. DNA probes were gel purified with a QIAEX gel extraction kit (Qiagen, Chatsworth, Calif.) and labeled with [α-32P]dCTP with a Boehringer Mannheim Biochemicals (Indianapolis, Ind.) random-primer DNA labeling kit.
TABLE 1.
Primers used for PCR amplification
| Gene | Primera | Designation | Purpose |
|---|---|---|---|
| dbp | GARAAYCCWTTYATHYTb | 18K Deg. | Degenerate primer (ENPFIL) |
| dbp | GGAATTCCATATGGGACTAACAGGAGCAACAAAAATC | 18K-5′-His | 5′ primer for His-tagged fusion (strain 297) |
| dbp | GCTCTAGATTATTACGATTTAGCAGTGCTGTC | 18K-3′-His | 3′ primer for His-tagged fusion (strain 297) |
| dbp | GGAATTCCATATGTGCGGATTAAAAGGAGAAACAAAAATC | N40-18K-5′-His | 5′ primer for His-tagged fusion (strain N40) |
| dbp | GCTCTAGAAGCCCAAACCCAACCAGATGGATTTGTTTG | N40-18K-5′-His | 3′ primer for His-tagged fusion (strain N40) |
| dbp | CATCAGCTAAAGCCATTGTAGATG | 297-NB-5′ | 5′ primer for Northern blot probe |
| dbp | TTACACCCATAGAAGCAGCC | 18K-NB-3′ | 3′ primer for Northern blot probe |
| dbp | CGTCCTAATATTTACAATTTAATAATATTGG | 18K PCR 5′ | 5′ primer for Southern blot probe |
| dbp | GAAATTCCAAATAACATCAAAAAGG | 18K PCR 3′ | 3′ primer for Southern blot probe |
| ORF-1 | GGAATTCCATATGTGTAGTATTGGATTAGTAGAAAGAAC | ORF-1-His-5′ | 5′ primer for His-tagged fusion and Northern blot probe (strains 297 and N40) |
| ORF-1 | GCTCTAGAATTTTACTTTTAGTCCATTCTAATGAC | ORF-1-His-3′ | 3′ primer for His-tagged fusion (strains 297 and N40) |
| ORF-1 | CACCTTTTCCCGTGGCATC | ORF-1-NB-3′ | 3′ primer for Northern blot probe |
Direction of each sequence is from 5′ to 3′.
Mixed sites: R, A/G; Y, C/T; W, A/T; H, A/T/C.
B. burgdorferi total genomic DNA was isolated with a DNA extraction kit (Stratagene). Borrelial DNAs were digested to completion with Sau3A; 1.5-μg quantities of each sample were then loaded into wells of 1% agarose gels. After electrophoresis, the gels were partially depurinated by soaking in 0.25 M HCl (with rocking) for 30 min at room temperature. The gels were subsequently denatured for 30 min in 1.5 M NaCl–0.5 M NaOH (with constant agitation), rinsed in water, and neutralized for 1 h with 1 M Tris (pH 7.4)–1.5 M NaCl. DNA in the gels was then transferred to nylon membranes (Micron Separations, Westboro, Mass.). The membranes were hybridized overnight in 5× Denhardt’s solution–5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–1.0% SDS–1 mM EDTA–100 μg of sonicated salmon sperm DNA per ml at 65°C in a rotating hybridization oven (Robbins Scientific Corp., Sunnyvale, Calif.). After hybridization, the membranes were washed twice for 15 min each time at 65°C in 2× SSC containing 0.1% SDS, followed by four washes at room temperature in 0.1× SSC containing 0.1% SDS. The membranes then were subjected to autoradiography at −70°C for 4 to 48 h.
Northern blot analysis.
Northern blotting of B. burgdorferi 297 RNA was carried out as described by Porcella et al. (47). The hybridization probes, corresponding to the ORF-1 and dbp genes, were generated by PCR with the primer pairs listed in Table 1.
Pulsed-field gel electrophoresis analysis.
Pulsed-field gel electrophoresis of borrelial genetic contents was performed as described previously (37).
DNA sequencing and computer analyses.
Nucleotide sequencing was performed with an Applied Biosystems Inc. model 373A automated DNA sequencer and PRISM Ready Reaction DyeDeoxy Terminator cycle sequencing kits according to the manufacturer’s instructions (Applied Biosystems Inc., Foster City, Calif.). Nucleotide and deduced amino acid sequences were analyzed and manipulated with University of Wisconsin Genetics Computer Group version 7.3 (GenBank database release 82.0) (25), Lasergene (DNASTAR, Madison, Wis.), and MacVector version 4.1.1 (International Biotechnologies Inc.-Kodak, New Haven, Conn.) software packages. The nucleotide sequences for the ORF-1 and dbp genes of B. burgdorferi 297 recently appeared in GenBank as dbpB (U75867) and dbpA (U75866), respectively. Recently published genetic sequences for the 49-kb (lp54) linear plasmid of strain B31 also contained regions homologous to ORF-1 (BBA25) and dbp (BBA24) (28).
Fusion proteins.
A glutathione S-transferase (GST) fusion protein of OspA was previously described (37). Fusion proteins containing six histidines at the N termini of the ORF-1-encoded protein and DBP (six-His fusion proteins) were generated by PCR amplification of the DNA encoding the predicted mature portions of the proteins; the respective forward and reverse oligonucleotide primer pairs are shown in Table 1. Conditions for PCR were 35 cycles at 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min. Amplification products were purified with a QIAEX gel extraction kit. The fragments were then ligated into the appropriate polylinker sites of pProEX-1 and used for transformation of XL1-Blue host cells. The DNA of all fusion constructs was sequenced to confirm that the cloning junctions were as intended. The expression of recombinant fusion proteins was induced by the addition of isopropyl-β-d-thiogalactopyranoside (IPTG). The resultant fusion proteins were purified by affinity chromatography on a nickel-nitrilotriacetic acid resin according to the manufacturer’s instructions (GIBCO-BRL). In some experiments, fusion protein bound to the nickel-nitrilotriacetic acid resin was cleaved with recombinant tobacco etch virus (GIBCO-BRL).
Antisera.
Rabbit antiserum directed against the DBP of B. burgdorferi 297 was generously provided by Mark Hanson (MedImmune, Gaithersburg, Md.). Guo et al. (29) termed this lipoprotein DBP-A (GenBank accession no. U75866). Rat polyclonal antisera directed against fusion proteins generated in this study were prepared according to a previously published protocol (37). Polyclonal antiserum directed against the recombinant DBP was of a high titer (>1:200,000) (determined by immunoblotting of either purified recombinant antigen or whole-cell lysates of B. burgdorferi 297) and highly specific when used at a dilution of 1:1,000 (data not shown). Polyclonal antiserum directed against the recombinant ORF-1-encoded protein also was of a high titer (>1:200,000) but was slightly cross-reactive with the recombinant DBP when used at a dilution of 1:1,000 (data not shown). Rat antiserum directed against B. burgdorferi 297 was described previously (37).
Triton X-114 phase partitioning.
Triton X-114 extraction of borrelial whole cells and outer membranes was carried out as described previously (17). Protein concentrations were determined by the bicinchoninic acid method (Pierce Chemical Co., Rockford, Ill.).
Proteinase K accessibility of DBP and the ORF-1-encoded protein.
Treatment of intact borreliae with 400 μg of proteinase K per ml was performed (44) as an indicator of potential surface exposure of these proteins in B. burgdorferi.
Indirect immunofluorescence assays of either acetone-fixed (disrupted) or intact spirochetes.
Antisera were either reacted with acetone-fixed (disrupted) borreliae on glass slides or added directly to 1-ml portions of mid-logarithmic-phase cultures of B. burgdorferi; spirochetes were processed for an indirect immunofluorescence assay as previously described (23). Antiserum against B. burgdorferi endoflagella, used as a probe for a subsurface marker, was described previously (23).
B. burgdorferi growth inhibition assays.
In vitro borreliacidal activities of mouse and rat antisera were determined according to the method of Lahdenne et al. (37) by use of modifications of previously described procedures (39, 51). Quantitative assessment of growth inhibition was performed with the aid of a Thermomax enzyme-linked immunosorbent assay (ELISA) reader (Molecular Devices, Sunnyvale, Calif.) and dual-wavelength readings at 562 and 620 nm (39); an adjusted absorbance value (A562 − A620) of ≥0.30 was reflective of borrelial growth in the assay system.
Murine model of Lyme borreliosis.
The well-characterized murine model of Lyme borreliosis (10–13, 27) was used to assess the ontogeny of the antibody response during chronic infection as well as for passive and active immunization experiments. Briefly, groups of 10 3-week-old C3H/HeJ mice were needle inoculated intradermally with 104 B. burgdorferi 297 cells in 50 μl of BSKII medium. At various intervals, mice within each group were sacrificed by CO2 narcosis. Specimens of ear pinna, heart, and urinary bladder from each sacrificed animal were cultured in BSKII medium supplemented with rifampin (50 μg/ml) and amphotericin B (25 μg/ml). Disseminated B. burgdorferi infection of mice was confirmed by recovery of spirochetes from any of the cultured sites. Sera from culture-positive mice within the same group were pooled and stored at −20°C until use.
Pooled mouse sera from the above-described study were tested for their ability to passively protect naive mice against challenge with virulent B. burgdorferi (10). Pooled mouse sera were first serially (twofold) diluted. Groups of two to four 3-week-old mice were then injected subcutaneously with 100 μl of the various dilutions of pooled mouse sera or with rat antisera directed against either OspA-GST (positive control) or GST alone (negative control). At 18 h, mice were needle inoculated intradermally with 103 low-passage B. burgdorferi 297 cells harvested from a mid-logarithmic-phase culture. At 2 weeks postchallenge, mice were sacrificed and specimens of ear pinna, heart, and urinary bladder were cultured in BSKII medium. Protection was indicated by the inability to recover spirochetes from any of the cultured sites. Passive protection activity of a particular serum dilution was considered to be present when at least 67% of passively immunized animals were protected from challenge. The 2-week-postchallenge pooled mouse serum sample was tested in six separate passive immunization experiments; all other pooled sera were tested in eight separate experiments. The protective titer (expressed as mean ± standard error of the mean) for each pooled serum sample (corresponding to each time point of infection) was derived by averaging the reciprocal protective titers from the multiple (six or eight) passive protection experiments.
For active immunization experiments (12, 27), mice were immunized intraperitoneally with 50 μg of either GST or recombinant fusion proteins corresponding to the ORF-1-encoded protein, DBP, or OspA in phosphate-buffered saline mixed 1:1 with complete Freund’s adjuvant. This procedure was followed at 2-week intervals by two booster injections of 20 μg of the respective proteins in phosphate-buffered saline–incomplete Freund’s adjuvant. At 2 weeks after the final booster, mice were bled to assess antibody titers and then were challenged with 103 low-passage B. burgdorferi 297 cells harvested from a mid-logarithmic-phase culture. At 2 weeks postchallenge, the level of immunoprotection was assessed by culturing tissue specimens (as described above).
Nucleotide sequence accession number.
The nucleotide sequences for the ORF-1 and dbp genes of strain N40 were submitted to GenBank under accession no. AF042796.
RESULTS
Mice chronically infected with B. burgdorferi produce antibodies with both neutralization and passive immunization properties.
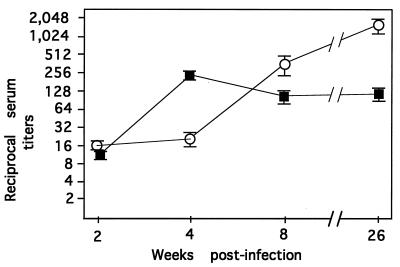
Kochi and colleagues (35, 36) first established that B. burgdorferi can be neutralized (killed) by antibodies in vitro. Sadziene et al. (51) later extended these observations by showing that antibodies from patients with Lyme disease also inhibited the growth of B. burgdorferi in vitro. Barthold and Bockenstedt (10) subsequently reported that antibodies with passive immunization properties appeared in the sera of mice inoculated intradermally with low doses (104) of B. burgdorferi N40. To corroborate this finding in our laboratory, groups of C3H/HeJ mice were needle inoculated intradermally with low doses of B. burgdorferi 297, blood was collected at various times postinoculation, and sera were tested in parallel for both growth inhibition and passive immunization activities. Sera obtained from mice 2 weeks postinfection displayed both growth inhibition and passive immunization titers of about 1:16 (Fig. 1). During the next 2 weeks serum passive immunization titers increased and stabilized to about 1:256, whereas growth inhibition titers continued to increase during the next 26 weeks to levels of about 1:2,000.
FIG. 1.
Comparison of passive immunization (▪) and growth inhibition (○) titers in sera of mice infected with B. burgdorferi. Values are reciprocal titers (means ± standard errors of the means).
Identification of B. burgdorferi outer membrane proteins immunoreactive with sera from chronically infected mice.
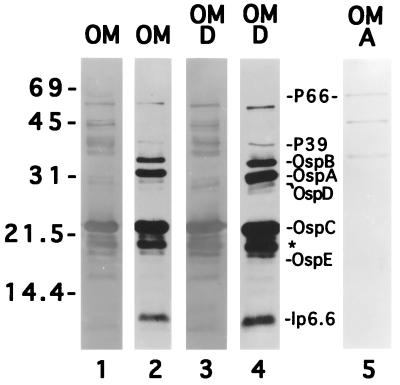
We reasoned that antibodies in mouse sera displaying growth inhibition and passive immunization properties (Fig. 1) could be used to probe B. burgdorferi isolated outer membranes (49) for the identification of previously uncharacterized outer membrane proteins as potential vaccine candidates. Upon immunoblotting, antibodies in sera from mice 8 weeks postinfection (Fig. 1) reacted strongly with a number of polypeptides in isolated outer membranes (Fig. 2, lane 1). With the exception of p66, which partitioned anomalously in Triton X-114 (48) (Fig. 2, lane 5), virtually all of the immunoreactive polypeptides partitioned into the detergent phase of Triton X-114 (lane 3). When various specific polyclonal and monoclonal antibody preparations were used, the major Triton X-114-extractable polypeptides that were immunoreactive with mouse serum (Fig. 2, lane 3) and/or that became palmitate labeled (lane 4) were identified as either p39 (58), OspB (18), OspA (9), OspD (44), OspC (65), OspE (38), or lp6.6 (37). Among the polypeptides not identifiable, one or two polypeptides with apparent molecular masses of about 18 kDa migrated just proximal to OspC and comigrated with palmitate-labeled polypeptides (Fig. 2, lane 4, asterisk). This region of a separate polyacrylamide gel was harvested for amino acid sequence analysis. The 18-kDa molecule(s) was protected from Edman degradation; however, upon digestion with trypsin and separation of the resultant peptides, one peptide fragment yielded the sequence ENPFIL.
FIG. 2.
SDS-PAGE analysis of isolated outer membranes (OM) of B. burgdorferi. Spirochetes were labeled with radioactive palmitate, OM were isolated, and a portion of the OM material was phase partitioned with Triton X-114. Lane 1 (OM) and lane 3 (detergent-phase [D] OM proteins) were immunoblotted with pooled sera from mice 8 weeks postinfection. Other lanes: 5, immunoblot of OM material partitioning into the aqueous phase after Triton X-114 extraction (note the presence of p66); 2 and 4, OM or D OM proteins, respectively, subjected to autoradiography to assess the incorporation of radioactive palmitate. Protein designations to the right of lane 4 are according to convention. The asterisk denotes the region of the gel harvested for amino acid sequence procedures. Numbers at left correspond to apparent molecular weights (in thousands).
A degenerate oligonucleotide based upon the ENPFIL sequence was synthesized (Table 1) and radiolabeled as a hybridization probe. Screening of a B. burgdorferi 297 Lambda-ZAPII genomic library by Southern blotting yielded a number of hybridizing clones. Hybridizing clones were isolated and processed for in vivo excision of the pBluescript phagemid from the Lambda-ZAPII vector. Rescued plasmids were purified and used as templates for automated DNA sequence analysis.
DNA and deduced amino acid sequences.
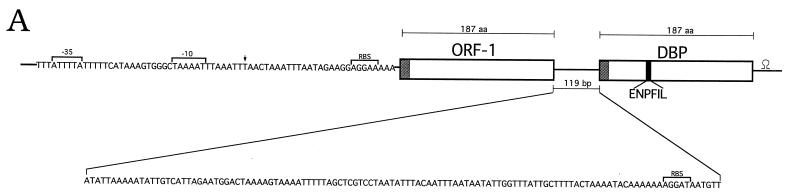
The relevant ORF, designated dbp, encoded a polypeptide comprised of 187 amino acids (Fig. 3A). The deduced amino acid sequence contained the sequence ENPFIL, which corresponded to the oligonucleotide probe used to isolate the original clone. The first 24 amino acids were representative of a signal peptide terminated by LLISC, a probable consensus sequence for lipoprotein modification (31). As such, the putative mature protein of 163 amino acids would have a predicted molecular mass of 17.8 kDa (Fig. 3A) and a pI of 8.52. Assuming that the molecule is modified via the configuration typical of other bacterial lipoproteins (31) and that the three acyl chains most likely are palmitates (14), the actual molecular mass of the mature, lipid-modified molecule would be approximately 18.6 kDa. A putative ribosome-binding site (AGGAT) (56) was present beginning at nucleotide −11, but putative −10 and −35 promoter sequences were not readily apparent. Subsequent immunoblotting of a recombinant DNA-derived fusion protein corresponding to this ORF with a polyclonal antiserum obtained from Mark Hanson revealed that the encoded protein was the previously reported DBP (29) (now termed DBP-A [GenBank accession no. U75866]) (see Fig. 5B).
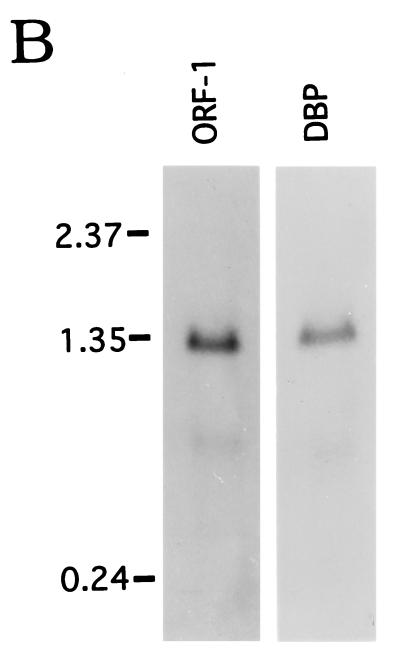
FIG. 3.
Schematic diagram (A) and Northern blot (B) of the ORF-1-dbp operon of B. burgdorferi 297. (A) The putative −10 and −35 sites and ribosome-binding site (RBS) are indicated. The arrow denotes a transcriptional initiation site determined from primer extension analysis of B. burgdorferi RNA. Predicted leader peptides culminating in signal peptidase II cleavage sites are denoted by shaded boxes. The black box within dbp corresponds to the amino acid (aa) sequence obtained from internal sequencing of trypsin fragments. The intergenic region is 119 bp long. A putative stem-loop structure for termination is present downstream of dbp. (B) The hybridization probes used to detect transcripts for ORF-1 and dbp were generated by PCR with the primer pairs listed in Table 1. Molecular size markers (kilobases) are shown at left.
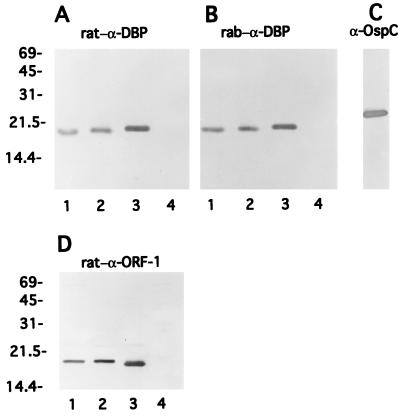
FIG. 5.
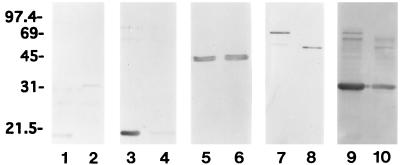
Immunoblots of native and recombinant DBP and ORF-1-encoded protein. Lanes: 1, whole-cell lysates of B. burgdorferi 297; 2, Triton X-114-extracted outer membranes; 3, recombinant DBP (cleaved from its six-His fusion partner) (A and B) or recombinant ORF-1-encoded protein (cleaved) (D); 4 (and panel C), recombinant OspC (cleaved from GST) as a control. Antibody probes are indicated at the top of each panel; rab, rabbit. Numbers at left denote apparent molecular weights (in thousands).
Additional DNA sequencing revealed the presence of a second ORF, designated ORF-1, whose termination codon (TAA) was 119 bp upstream of the dbp gene (Fig. 3A). ORF-1, encoding a putative 20-amino-acid leader peptide terminated by a plausible signal peptidase II recognition sequence (LLVAC) (31), was predicted to encode a 167-amino-acid mature protein with a mass of 18.2 kDa (ca. 19.0 kDa for the processed, acylated form) and a pI of 9.35. A presumptive ribosome-binding site (AGGAA) (56) was present beginning at nucleotide −8. Putative −10 and −35 sequences were present at nucleotides −41 and −64, respectively.
Linkage of the ORF-1 and dbp genes (Fig. 3A), the absence of readily identifiable promoter sequences upstream of the dbp gene, and the presence of putative −10 and −35 sequences upstream of ORF-1 prompted the hypothesis that the two genes may be cotranscribed. Northern blots of RNA from B. burgdorferi 297 with PCR-generated probes specific for ORF-1 and dbp revealed major transcripts of approximately 1.35 kb (Fig. 3B), consistent with the theoretical minimum polycistronic transcript size of about 1.3 kb. In each case, faint smaller transcripts of about 700 bp could be visualized, perhaps as a result of either degradation or residual synthesis from each of the individual genes. In any event, primer extension experiments confirmed a strong transcriptional initiation site at nucleotide −28 (T) upstream of ORF-1 (data not shown; noted on Fig. 3A, arrow), but a transcriptional initiation site could not be identified within the 119-bp intergenic region between ORF-1 and dbp. At 34 nucleotides downstream of the termination codon (TAA) was a potential stem-loop structure (Fig. 3A) that may serve as a rho-independent terminator (46).
The ORF-1-encoded protein and DBP of B. burgdorferi 297 were 40% identical and 56% similar to one another. BLASTp searches (4) via the National Center for Biotechnology Information initially did not reveal any other significant homologies between the ORF-1-encoded protein or DBP and other protein sequences in the databases. However, at the time of this writing, repeat searches revealed exact matches between ORF-1 and a gene from strain 297 designated dbpB (GenBank accession no. U75867) and between dbp and a gene from strain 297 designated dbpA (GenBank accession no. U75866). dbpB and dbpA genes of strain B31 also were noted in a recently published work on the B. burgdorferi genome (28).
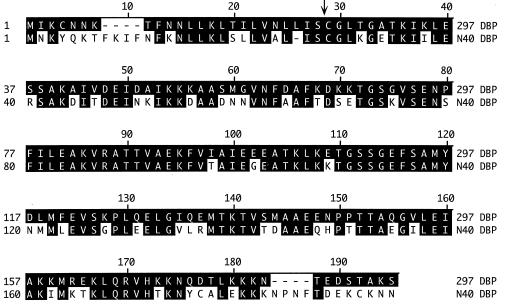
Given that Guo et al. (29) reported on the presence of a DBP(s) in B. burgdorferi N40, we sought to determine the presence and degree of similarity between the DBPs of strains 297 and N40. Oligonucleotide primers (Table 1) were used to amplify in strain N40 an analogous locus, which was then sequenced. The deduced amino acid sequences for the mature ORF-1-encoded polypeptides differed between the two B. burgdorferi strains by only one amino acid (Asp→Glu at position 30) (>99% identity) (data not shown). In contrast, the two mature DBPs were only 69% identical and 76% similar (Fig. 4).
FIG. 4.
Deduced amino acid sequences of DBPs from B. burgdorferi 297 and N40. The arrow indicates the predicted site of cleavage by signal peptidase II. Dashes indicate sequence gaps used to align the two DBPs. Black boxes on the lower line indicate identity to the upper line.
The dbp operon is located on the 49-kb linear plasmid.
Initial hybridization studies with two-dimensional pulsed-field gel electrophoresis, a technique which separates linear and circular plasmids of B. burgdorferi (52), indicated that the dbp gene was located exclusively on a linear plasmid(s) which comigrated with the 49-kb linear plasmid carrying the ospA gene of B. burgdorferi 297 (8) (data not shown). Subsequent experiments with one-dimensional pulsed-field gel electrophoresis (26) of strains 297, N40, and B31 also showed that the dbp gene hybridized to linear plasmids which comigrated with the 49-kb linear plasmid in each of the three strains (data not shown). Consistent with this finding, the dbp gene was absent in B. burgdorferi B313, which lacks the 49-kb linear plasmid (data not shown) (52), confirming that the dbp locus and the ospA-ospB operon are located on the same genetic element. Finally, the recently published genome and selected plasmid sequences for B. burgdorferi B31 confirmed that the ORF-1 (dbpB) and dbp (dbpA) genes were present in tandem on the single-copy 49-kb (lp54) linear plasmid (28, 32). Southern blots of total genomic DNA from B. burgdorferi 297 were consistent with the presence of these two elements as single-copy genes (data not shown).
Characterization of DBP.
The dbp gene (lacking the portion encoding the leader peptide) was cloned as a six-His fusion protein, and the resultant gene fusion was inducibly expressed in E. coli. This genetic construct was sequenced in its entirety to verify the DNA sequence. The resultant fusion protein was purified from E. coli (Fig. 5, lanes 3) and used to generate rat polyclonal antiserum. When the nonlipidated recombinant DBP was cleaved from its six-His partner and subjected to SDS-PAGE and immunoblot analysis with this highly specific antiserum (Fig. 5A, lane 3), it migrated only slightly slower than its native counterpart in B. burgdorferi (lane 1). Of note, native DBP also was readily detectable in immunoblots of isolated, Triton X-114 phase-partitioned outer membranes (Fig. 5A, lane 2). The highly similar electrophoretic mobilities of this DBP and the previously described DBP of B. burgdorferi (29) provided the impetus to determine whether these two polypeptides were identical. As shown in Fig. 5B, rabbit antiserum directed against the DBP of B. burgdorferi 297 (provided by Mark Hanson) bound strongly to the respective recombinant DNA-derived molecule (Fig. 5B, lane 3) as well as to native DBP from strain 297 (Fig. 5B, lanes 1 and 2). Similar to the case for DBP (Fig. 5A), rat antiserum directed against the recombinant ORF-1-encoded protein reacted with the native ORF-1-encoded protein found in either whole-cell lysates or Triton X-114 phase-partitioned outer membranes of B. burgdorferi 297 (Fig. 5D).
Mice chronically infected with B. burgdorferi produce high titers of antibodies directed against the ORF-1-encoded protein and DBP.
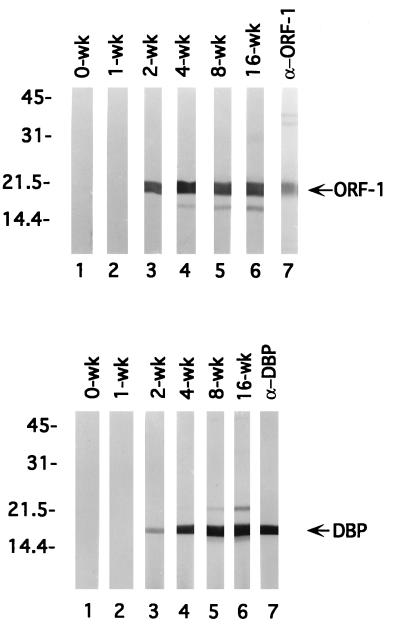
Recent studies showed that the lack of an antibody response against some borrelial lipoproteins, such as OspA and OspB, after low-dose needle or tick inoculation (6, 11, 50, 53) is due to down-regulation during borrelial infection of the mammalian host (24, 42, 55). These observations prompted us to investigate whether ORF-1 and dbp were expressed in vivo by examining the antibody responses of chronically infected mice to these proteins. Groups of mice were needle inoculated with low doses (104) of virulent B. burgdorferi 297 or N40 and housed for various intervals prior to sacrifice. Consistent with previous observations (3, 11, 53), antibodies directed against OspA or OspB were not detected in any of the mouse sera (data not shown). In contrast, antibodies directed against the ORF-1-encoded protein and DBP were readily detectable (immunoblotting of recombinant antigens) as early as 2 weeks postinfection, and the levels peaked after about 16 weeks (titers, approximately 1:50,000) (Fig. 6; data not shown for strain N40). Levels of antibodies directed against both proteins remained elevated even after 1 year postinfection (data not shown).
FIG. 6.
Appearance of antibodies against ORF-1-encoded protein and DBP during the course of experimental B. burgdorferi infection of C3H/HeJ mice. Recombinant ORF-1-encoded protein (top panel) and recombinant DBP (bottom panel) were subjected to SDS-PAGE. Nitrocellulose strips (containing 750 ng of protein) were immunoblotted with sera harvested from mice at various weeks (wk) (denoted at tops of strips) postinfection with B. burgdorferi. Lanes 7 contain strips probed with rat antiserum directed against either protein. Numbers at left denote apparent molecular weights (in thousands).
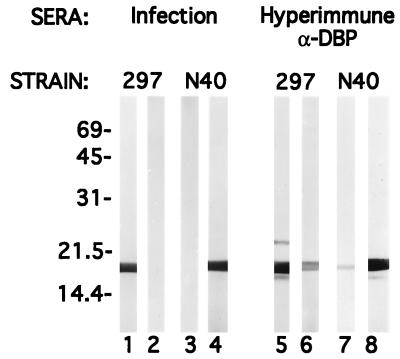
Immunological cross-reactivity between DBPs of B. burgdorferi 297 and N40.
The mature ORF-1-encoded proteins of B. burgdorferi 297 and N40 differ by only one amino acid. However, the fact that the DBPs of strains 297 and N40 differ significantly (Fig. 4) motivated us to assess further the immunological relatedness between these DBPs. To do this, sera from mice chronically infected for 8 weeks with either strain 297 or strain N40 were immunoblotted against recombinant fusion proteins derived from either strain 297 or strain N40. As shown in Fig. 7, sera from 297- or N40-infected mice reacted only with their homologous antigens. Interestingly, sera from animals artificially immunized with the respective recombinant DBPs contained antibodies which showed low-level cross-reactivity with the heterologous antigens (Fig. 7); this result underscored differences between antibody repertoires elicited during natural infection and those produced during artificial immunization.
FIG. 7.
Assessment of the immunological cross-reactivity between DBPs of B. burgdorferi 297 and N40. Recombinant DBPs from strain 297 (lanes 1, 3, 5, and 7) or strain N40 (lanes 2, 4, 6, and 8) were subjected to SDS-PAGE. Individual nitrocellulose strips (containing 750 ng of protein) were then immunoblotted with sera from either 297-infected (lanes 1 and 2) or N40-infected (lanes 3 and 4) mice. Note that during experimental infection, mice did not produce cross-reactive antibodies. For lanes 5 to 8, proteins were probed with either rat anti-297 DBP antibody (lanes 5 and 6) or rat anti-N40 DBP antibody (lanes 7 and 8); artificial immunization of rats with recombinant proteins produced low-level cross-reactive antibodies. Numbers at left denote apparent molecular weights (in thousands).
Proteinase K accessibility of DBP and the ORF-1-encoded protein.
Given the propensity for certain lipoproteins of B. burgdorferi to be surface exposed, treatment of intact borreliae with proteinase K was performed (44) as a potential indicator of surface exposure of DBP and the ORF-1-encoded protein. As shown in Fig. 8, both p66 (lane 8) and OspA (lane 10) were sensitive to proteinase K treatment. Proteinase K digestion of p66 yielded the typical 50-kDa cleavage fragment (19). OspA was only partially sensitive to proteinase K digestion, compatible with previous findings and the notion that a significant proportion of OspA is located beneath the borrelial outer membrane (23, 60). Both the ORF-1-encoded protein and DBP (Fig. 8, lanes 2 and 4, respectively) were accessible to proteinase K. In contrast, the Fla protein, a component of the periplasmic endoflagella, was completely resistant to proteinase K treatment (Fig. 8, lane 6).
FIG. 8.
Proteinase K accessibility of DBP and ORF-1-encoded protein in B. burgdorferi 297. Growing spirochetes were divided, and one-half of the population was treated with proteinase K. Borrelial proteins were separated by SDS-PAGE. Proteins on nitrocellulose strips from either untreated (lanes 1, 3, 5, 7, and 9) or proteinase K-treated (lanes 2, 4, 6, 8, and 10) borreliae were then immunoblotted with rat antisera as follows: lanes 1 and 2, anti-ORF-1; lanes 3 and 4, anti-DBP; lanes 5 and 6, anti-Fla; lanes 7 and 8, anti-p66; and lanes 9 and 10, anti-OspA. Numbers at left denote apparent molecular weights (in thousands).
Indirect immunofluorescence and growth inhibition assays.
Indirect immunofluorescence and growth inhibition assays were used in attempts to corroborate the results of proteinase K accessibility assays. Either acetone-fixed (disrupted) or unfixed (intact) B. burgdorferi 297 was exposed to high-titer (>1:200,000) rat polyclonal antiserum substantially specific for the ORF-1-encoded protein or DBP. Indirect immunofluorescence assays of fixed B. burgdorferi routinely showed the presence of moderate levels of both proteins (data not shown), consistent with the results of earlier immunoblotting experiments. However, the results of indirect immunofluorescence assays of intact borreliae were variable; some experiments were negative, whereas others showed modest immunofluorescence for DBP in a beaded pattern (data not shown). In vitro growth inhibition assays also failed to yield reproducible results.
Active immunization of mice with DBP and the ORF-1-encoded protein.
Despite equivocal results from immunofluorescence and growth inhibition assays, the results of proteinase K accessibility experiments implied that DBP and the ORF-1-encoded protein were surface exposed in B. burgdorferi cultured in vitro, suggesting that they may serve as in vivo immune targets for bactericidal antibodies. To examine this idea further, C3H/HeJ mice were actively immunized with either recombinant DBP or recombinant ORF-1-encoded protein. After achieving serum titers of more than 1:100,000 (determined by immunoblotting of purified recombinant proteins or whole-cell lysates of B. burgdorferi with dilutions of sera; data not shown), mice were challenged with virulent B. burgdorferi 297. As shown in Table 2, 78% of mice immunized with recombinant DBP were immune to challenge. Mice immunized with recombinant ORF-1-encoded protein were not protected to any appreciable degree.
TABLE 2.
Immunization of C3H/HeJ mice with recombinant proteins and subsequent challenge with B. burgdorferi
| Vaccinogen | No. of mice challenged | No. (%) of mice protected |
|---|---|---|
| GST | 9 | 0 (0) |
| OspA | 9 | 9 (100) |
| DBP | 9 | 7 (78) |
| ORF-1-encoded protein | 4 | 1 (25) |
DISCUSSION
It has been established that antibodies are the principal effectors for the clearance of B. burgdorferi during mammalian infection (10, 33, 57). It has been generally assumed that the activities of these protective antibodies are reflected in passive immunization experiments with mice (10) as well as in agglutination and borreliacidal-antibody assays performed in vitro (22, 51). However, our studies comparing passive immunization properties (which were similar to studies by Barthold and Bockenstedt [10]) and growth inhibition capabilities of sera from chronically infected mice revealed differences in the times at which levels of antibodies associated with these two activities peaked. One potential interpretation of the differences is that distinct functional classes of antibodies may be involved in passive immunization and growth inhibition activities. The recently recognized propensity for B. burgdorferi to undergo profound changes in its antigenic repertoire as it cycles between its arthropod and mammalian hosts (3, 21, 34, 42, 55, 62, 64) lends credibility to the hypothesis that different functional classes of antibodies may be induced as differential antigen expression ensues during mammalian infection. It is also possible that whereas passive immunization involves spirochetes adapting to the surrounding mammalian tissue(s), the growth inhibition assay is confined to the use of spirochetes cultured exclusively in vitro. As such, the growth inhibition assay may not be an entirely valid correlate of antigen-antibody interactions transpiring in vivo. Until methods that make use of mammalian host-adapted B. burgdorferi in growth inhibition assays are developed, the validity of the use of in vitro-cultured B. burgdorferi in such assays will not be fully known.
Given the above caveats, sera from immune mice still represented the most reasonable immunological reagent(s), paired with the recent technological advance for the preparation of B. burgdorferi outer membranes (49), with which to approach the identification of borrelial outer membrane proteins as new vaccine candidates. A few polypeptides, most of which were present in relatively low abundances and only moderately reactive with immune mouse serum, were unidentifiable by use of antibodies to previously well-characterized B. burgdorferi integral membrane proteins. One of these (DBP) was selected for further study based upon its high abundance and great immunoreactivity compared with most of the other unidentifiable polypeptides. Evidence from our studies, including reactivity with antibody preparations from both our laboratory and Mark Hanson as well as cloning and sequence analyses, suggested that the molecule is one of the two DBPs of B. burgdorferi described by Guo et al. (29). Inasmuch as Guo et al. (29) initially proposed that either or both of the two DBPs may subserve the attachment of B. burgdorferi to a mammalian tissue matrix as part of its parasitic strategy, the molecule(s) would need to be surface exposed to have such a function. The presence of DBP in outer membrane preparations, the electrophoretic comigration of DBP with palmitate-labeled polypeptides, and the propensity of both DBP and the ORF-1-encoded protein to partition into Triton X-114 are consistent with the contention, at the very least, that the molecules are integral outer membrane lipoproteins of B. burgdorferi.
Our gene cloning studies revealed the presence of a dbp gene encoding a putative lipoprotein with a mass (acylated form) of 18.6 kDa as well as an additional, homologous gene (ORF-1) located just upstream of dbp and encoding another putative lipoprotein, of about 19.0 kDa. Further molecular studies, as well as recent DNA sequence analyses reported elsewhere (28), confirmed that the two genes were tandemly located on the 49-kb linear plasmid of B. burgdorferi. Northern blot analyses and primer extension studies indicated that the two genes are cotranscribed, with the upstream ORF-1 contributing the transcriptional initiation site. The results of the combined molecular studies are consistent with the observation by Guo et al. (29) that two DBPs of similar apparent molecular masses were detectable by solid-phase decorin-binding assays with B. burgdorferi N40.
Evidence to substantiate the presence of the ORF-1-dbp operon in B. burgdorferi 297, N40, and B31 was obtained and suggested that the dbp operon may be common among sensu stricto strains. It remains to be determined, however, whether the same is true for sensu lato strains of other Lyme disease spirochetes. Presently it is also not known whether the ORF-1-encoded protein and DBP have analogous decorin-binding functions in vivo (29). Furthermore, the amino acid homologies between these proteins (40% identity and 56% similarity) do not appear to provide further insights into whether these proteins have comparable or overlapping functions. Additional structure-function studies are required to elucidate more precisely the functional roles of the ORF-1-encoded protein and DBP in the pathogenesis of Lyme disease.
Immunoblotting experiments, particularly those done with rat antisera that were highly specific for either DBP or the ORF-1-encoded protein, established that both proteins are expressed during in vitro cultivation of low-passage B. burgdorferi. However, when high-passage (>30 passages) populations of strains 297, N40, and B31 were assayed for the expression of DBP, the level of expression was greatly reduced, with no obvious concomitant reduction in the 49-kb linear plasmid in these populations (30). Guo et al. (29) also noted that decorin binding could not be detected in a high-passage population of strain B31. The murine model of Lyme borreliosis unequivocally established, however, that the ORF-1-dbp operon is expressed during mammalian infection; antibodies directed against both proteins were detectable as early as 2 weeks postinfection, suggesting that the lipoproteins are expressed at the very outset of infection and maintained during later stages. Given the 76% similarity between the ORF-1-encoded protein and DBP, it remained possible, on the other hand, that some of the mouse antibodies directed against either protein might be cross-reactive and therefore might have complicated the interpretations of the immunoblots. Two lines of evidence appear to refute this possibility. First, rat antisera highly specific for either the ORF-1-encoded protein or DBP each appeared to react with a single B. burgdorferi polypeptide (Fig. 5A and D). Second, given that ORF-1 is the first gene within a two-gene operon, it is virtually certain that both lipoproteins are expressed. However, some degree of posttranscriptional regulation still may be operative for either of the lipoproteins.
Proteinase K accessibility was used as an initial assessment of surface exposure for DBP and the ORF-1-encoded protein. Under conditions which left the periplasmic Fla protein intact but cleaved surface-exposed molecules, such as OspA and p66 (19, 23, 60), both native ORF-1-encoded protein and DBP were proteinase K accessible. We attempted to corroborate these findings by performing indirect immunofluorescence assays on either disrupted (fixed) or intact (unfixed) B. burgdorferi 297, but the results were highly variable. In vitro growth inhibition assays also failed to yield consistent results, for unknown reasons. Nonetheless, DBP was an efficacious protective immunogen, whereas vaccination results for the ORF-1-encoded protein were unremarkable. The immunoprotection results obtained with DBP sound a cautionary note that the vaccinogenic potential of a borrelial immunogen should not necessarily be ruled out based solely upon inconsistent results from surface localization assays.
To the best of our knowledge, this is the first study to successfully probe isolated borrelial outer membranes with antisera from immune mice to identify a new protective immunogen of B. burgdorferi. It should be noted, however, that DBP (and/or the ORF-1-encoded protein) may not be exclusively associated with the borrelial outer membrane. There is now considerable evidence for a dual-membrane distribution of other B. burgdorferi lipoproteins (e.g., OspA and OspB) (15, 16, 23, 49); thus, it is possible that increased amounts of DBP are shuttled to the spirochete surface as it adapts to its mammalian environment (3, 21, 34, 42, 55, 62, 64). This scenario would explain why DBP was readily detectable on immunoblots of in vitro-cultivated B. burgdorferi but could not be localized to the spirochete surface by immunofluorescence microscopy or growth inhibition by conventional methods. Regardless of the precise mechanism(s) by which DBP becomes surface exposed in B. burgdorferi, our combined studies support the notion that DBP may represent a new candidate component for a human Lyme disease vaccine. Further analysis of B. burgdorferi isolated outer membranes may also reveal other new Lyme disease vaccine candidates.
ACKNOWLEDGMENTS
We thank Martin Goldberg, Esther Robinson, Leslie Arndt, and Ken Bourell for excellent technical assistance, Mark Hanson for providing rabbit antiserum to DBP, and George H. McCracken, Jr., for helpful discussions, support, and encouragement.
We gratefully acknowledge funding for this work provided by grant AI-29735 from the Lyme Disease Program of the National Institute of Allergy and Infectious Diseases (National Institutes of Health) and by grant I-0940 from the Robert A. Welch Foundation. P.L. was supported by a Pediatric Infectious Diseases Society fellowship award from Abbott Laboratories and by grants from the Finnish Academy and the Foundation for Pediatric Research, Helsinki, Finland. J.D.R. was the recipient of an Established Investigatorship award from the American Heart Association.
REFERENCES
- 1.Aebersold R H, David B T, Hood L E, Kent S B H. Electroblotting onto activated glass. J Biol Chem. 1986;261:4229–4238. [PubMed] [Google Scholar]
- 2.Aebersold R H, Leavitt J, Saavedra R A, Hood L E, Kent S B H. Internal amino acid sequence analysis of proteins separated by one- or two-dimensional gel electrophoresis by in situ protease digestion on nitrocellulose. Proc Natl Acad Sci USA. 1987;84:6970–6974. doi: 10.1073/pnas.84.20.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akins D R, Porcella S F, Popova T G, Shevchenko D, Li M, Norgard M V, Radolf J D. Evidence for in vivo but not in vitro expression of a Borrelia burgdorferi outer surface protein F (OspF) homologue. Mol Microbiol. 1995;18:507–520. doi: 10.1111/j.1365-2958.1995.mmi_18030507.x. [DOI] [PubMed] [Google Scholar]
- 4.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 5.Andrews P C, Dixon J E. A procedure for in situ alkylation of cysteine residues on glass fiber prior to protein microsequence analysis. Anal Biochem. 1987;161:524–528. doi: 10.1016/0003-2697(87)90484-2. [DOI] [PubMed] [Google Scholar]
- 6.Aydintug M K, Gu Y, Philipp M T. Borrelia burgdorferi antigens that are targeted by antibody-dependent, complement-mediated killing in the rhesus monkey. Infect Immun. 1994;62:4929–4937. doi: 10.1128/iai.62.11.4929-4937.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbour A G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 8.Barbour A G, Garon C F. Linear plasmids of the bacterium Borrelia burgdorferi have covalently closed ends. Science. 1987;237:409–411. doi: 10.1126/science.3603026. [DOI] [PubMed] [Google Scholar]
- 9.Barbour A G, Tessier S L, Todd W J. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect Immun. 1983;41:795–804. doi: 10.1128/iai.41.2.795-804.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barthold S W, Bockenstedt L K. Passive immunizing activity of sera from mice infected with Borrelia burgdorferi. Infect Immun. 1993;61:4696–4702. doi: 10.1128/iai.61.11.4696-4702.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barthold S W, deSouza M S, Janotka J L, Smith A L, Persing D H. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol. 1993;143:959–971. [PMC free article] [PubMed] [Google Scholar]
- 12.Barthold S W, Fikrig E, Bockenstedt L K, Persing D H. Circumvention of outer surface protein A immunity by host-adapted Borrelia burgdorferi. Infect Immun. 1995;63:2255–2261. doi: 10.1128/iai.63.6.2255-2261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barthold S W, Persing D H, Armstrong A L, Peeples R A. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am J Pathol. 1991;139:263–273. [PMC free article] [PubMed] [Google Scholar]
- 14.Belisle J T, Brandt M E, Radolf J D, Norgard M V. Fatty acids of Treponema pallidum and Borrelia burgdorferi lipoproteins. J Bacteriol. 1994;176:2151–2157. doi: 10.1128/jb.176.8.2151-2157.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bledsoe H A, Carroll J A, Whelchel T R, Farmer M A, Dorward D W, Gherardini F C. Isolation and partial characterization of Borrelia burgdorferi inner and outer membranes by using isopycnic centrifugation. J Bacteriol. 1994;176:7447–7455. doi: 10.1128/jb.176.24.7447-7455.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brusca J S, McDowall A W, Norgard M V, Radolf J D. Localization of outer surface proteins A and B in both the outer membrane and intracellular compartments of Borrelia burgdorferi. J Bacteriol. 1991;173:8004–8008. doi: 10.1128/jb.173.24.8004-8008.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brusca J S, Radolf J D. Isolation of integral membrane proteins by phase partitioning with Triton X-114. Methods Enzymol. 1994;228:182–193. doi: 10.1016/0076-6879(94)28019-3. [DOI] [PubMed] [Google Scholar]
- 18.Bundoc V G, Barbour A G. Clonal polymorphisms of outer membrane protein OspB of Borrelia burgdorferi. Infect Immun. 1989;57:2733–2741. doi: 10.1128/iai.57.9.2733-2741.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bunikis J, Noppa L, Ostberg Y, Barbour A G, Bergstrom S. Surface exposure and species specificity of an immunoreactive domain of a 66-kilodalton outer membrane protein (P66) of the Borrelia spp. that cause Lyme disease. Infect Immun. 1996;64:5111–5116. doi: 10.1128/iai.64.12.5111-5116.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caporale D A, Kocher T D. Sequence variation in the outer-surface-protein genes of Borrelia burgdorferi. Mol Biol Evol. 1994;11:51–64. doi: 10.1093/oxfordjournals.molbev.a040092. [DOI] [PubMed] [Google Scholar]
- 21.Champion C I, Blanco D R, Skare J T, Haake D A, Giladi M, Foley D, Miller J N, Lovett M A. A 9.0-kilobase-pair circular plasmid of Borrelia burgdorferi encodes an exported protein: evidence for expression only during infection. Infect Immun. 1994;62:2653–2661. doi: 10.1128/iai.62.7.2653-2661.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coleman J L, Rogers R C, Benach J L. Selection of an escape variant of Borrelia burgdorferi by use of bactericidal monoclonal antibodies to OspB. Infect Immun. 1992;60:3098–3104. doi: 10.1128/iai.60.8.3098-3104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cox D L, Akins D R, Bourell K W, Lahdenne P, Norgard M V, Radolf J D. Limited surface exposure of Borrelia burgdorferi outer surface lipoproteins. Proc Natl Acad Sci USA. 1996;93:7973–7978. doi: 10.1073/pnas.93.15.7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.deSilva A M, Telford S R, Brunet L R, Barthold S W, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferdows M S, Barbour A G. Megabase-sized linear DNA in the bacterium Borrelia burgdorferi, the Lyme disease agent. Proc Natl Acad Sci USA. 1989;86:5969–5973. doi: 10.1073/pnas.86.15.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fikrig E, Barthold S W, Kantor F S, Flavell R A. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science. 1990;250:553–556. doi: 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- 28.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J-F, Fleischmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams M D, Gocayne J, Weidman J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fujii C, Cotton M D, Horst K, Roberts K, Hatch B, Smith H O, Venter J C. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 29.Guo B P, Norris S J, Rosenberg L C, Höök M. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect Immun. 1995;63:3467–3472. doi: 10.1128/iai.63.9.3467-3472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hagman, K. E., P. Lahdenne, T. G. Popova, K. Bourell, D. R. Akins, J. D. Radolf, and M. V. Norgard. Unpublished observations.
- 31.Hayashi S, Wu H C. Lipoproteins in bacteria. J Bioenerg Biomembr. 1990;22:451–471. doi: 10.1007/BF00763177. [DOI] [PubMed] [Google Scholar]
- 32.Hinnebusch J, Barbour A G. Linear and circular plasmid copy numbers in Borrelia burgdorferi. J Bacteriol. 1992;174:5251–5257. doi: 10.1128/jb.174.16.5251-5257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson R C, Kodner C, Russell M. Passive immunization of hamsters against experimental infection with the Lyme disease spirochete. Infect Immun. 1986;53:713–714. doi: 10.1128/iai.53.3.713-714.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jonsson M, Bergstrom S. Transcriptional and translational regulation of the expression of the major outer surface proteins in Lyme disease Borrelia strains. Microbiology. 1995;141:1321–1329. doi: 10.1099/13500872-141-6-1321. [DOI] [PubMed] [Google Scholar]
- 35.Kochi S K, Johnson R C. Role of immunoglobulin G in killing of Borrelia burgdorferi by the classical complement pathway. Infect Immun. 1988;56:314–321. doi: 10.1128/iai.56.2.314-321.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kochi S K, Johnson R C, Dalmasso A P. Complement-mediated killing of the Lyme disease spirochete Borrelia burgdorferi: role of antibody in formation of an effective membrane attack complex. J Immunol. 1991;146:3964–3970. [PubMed] [Google Scholar]
- 37.Lahdenne P, Porcella S F, Hagman K E, Akins D R, Popova T G, Cox D L, Katona L I, Radolf J D, Norgard M V. Molecular characterization of a 6.6-kilodalton Borrelia burgdorferi outer membrane-associated lipoprotein (lp6.6) which appears to be downregulated during mammalian infection. Infect Immun. 1997;65:412–421. doi: 10.1128/iai.65.2.412-421.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lam T T, Nguyen T-P K, Montgomery R R, Kantor F S, Fikrig E, Flavell R A. Outer surface proteins E and F of Borrelia burgdorferi, the agent of Lyme disease. Infect Immun. 1994;62:290–298. doi: 10.1128/iai.62.1.290-298.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma J, Coughlin R T. A simple, colorimetric microtiter assay for borreliacidal activity of antisera. J Microbiol Methods. 1993;17:145–153. [Google Scholar]
- 40.Mathiesen D A, Oliver J H, Kolbert C P, Tullson E D, Johnson B J B, Campbell G L, Mitchell P D, Reed K D, Telford S R, Anderson J F, Lane R S, Persing D H. Genetic heterogeneity of Borrelia burgdorferi in the United States. J Infect Dis. 1997;175:98–107. doi: 10.1093/infdis/175.1.98. [DOI] [PubMed] [Google Scholar]
- 41.Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- 42.Montgomery R R, Malawista S E, Feen K J M, Bockenstedt L K. Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J Exp Med. 1996;183:261–269. doi: 10.1084/jem.183.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morbidity and Mortality Weekly Report. Lyme disease—United States, 1996. Morbid Mortal Weekly Rep. 1997;46:531–535. [PubMed] [Google Scholar]
- 44.Norris S J, Carter C J, Howell J K, Barbour A G. Low-passage-associated proteins of Borrelia burgdorferi B31: characterization and molecular cloning of OspD, a surface-exposed, plasmid-encoded lipoprotein. Infect Immun. 1992;60:4662–4672. doi: 10.1128/iai.60.11.4662-4672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Padilla M L, Callister S M, Schell R F, Bryant G L, Jobe D A, Lovrich S D, DuChateau B K, Jensen J R. Characterization of the protective borreliacidal antibody response in humans and hamsters after vaccination with Borrelia burgdorferi outer surface protein A vaccine. J Infect Dis. 1996;174:739–746. doi: 10.1093/infdis/174.4.739. [DOI] [PubMed] [Google Scholar]
- 46.Platt T. Transcription termination and the regulation of gene expression. Annu Rev Biochem. 1986;55:339–372. doi: 10.1146/annurev.bi.55.070186.002011. [DOI] [PubMed] [Google Scholar]
- 47.Porcella S F, Popova T G, Akins D R, Li M, Radolf J D, Norgard M V. Borrelia burgdorferi supercoiled plasmids encode multicopy tandem open reading frames and a lipoprotein gene family. J Bacteriol. 1996;178:3293–3307. doi: 10.1128/jb.178.11.3293-3307.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Probert W S, Allsup K M, LeFebvre R B. Identification and characterization of a surface-exposed, 66-kilodalton protein from Borrelia burgdorferi. Infect Immun. 1995;63:1933–1939. doi: 10.1128/iai.63.5.1933-1939.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Radolf J D, Goldberg M S, Bourell K, Baker S I, Jones J D, Norgard M V. Characterization of outer membranes isolated from Borrelia burgdorferi, the Lyme disease spirochete. Infect Immun. 1995;63:2154–2163. doi: 10.1128/iai.63.6.2154-2163.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roehrig J T, Piesman J, Hunt A R, Keen M G, Happ C M, Johnson B J B. The hamster immune response to tick-transmitted Borrelia burgdorferi differs from the response to needle-inoculated, cultured organisms. J Immunol. 1992;149:3648–3653. [PubMed] [Google Scholar]
- 51.Sadziene A, Thompson P A, Barbour A G. In vitro inhibition of Borrelia burgdorferi growth by antibodies. J Infect Dis. 1993;167:165–172. doi: 10.1093/infdis/167.1.165. [DOI] [PubMed] [Google Scholar]
- 52.Sadziene A, Wilske B, Ferdows M S, Barbour A G. The cryptic ospC gene of Borrelia burgdorferi B31 is located on a circular plasmid. Infect Immun. 1993;61:2192–2195. doi: 10.1128/iai.61.5.2192-2195.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schaible U E, Gern L, Wallich R, Kramer M D, Prester M, Simon M M. Distinct patterns of protective antibodies are generated against Borrelia burgdorferi in mice experimentally inoculated with high and low doses of antigen. Immunol Lett. 1993;36:219–226. doi: 10.1016/0165-2478(93)90056-8. [DOI] [PubMed] [Google Scholar]
- 54.Schoen R T, Meurice F, Brunet C M, Cretella S, Krause D S, Craft J E, Fikrig E. Safety and immunogenicity of an outer surface protein A vaccine in subjects with previous Lyme disease. J Infect Dis. 1995;172:1324–1329. doi: 10.1093/infdis/172.5.1324. [DOI] [PubMed] [Google Scholar]
- 55.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwartz J J, Gazumyan A, Schwartz I. rRNA gene organization in the Lyme disease spirochete, Borrelia burgdorferi. J Bacteriol. 1992;174:3757–3765. doi: 10.1128/jb.174.11.3757-3765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simon M M, Schaible U E, Kramer M D, Eckerskorn C, Museteanu C, Muller-Hermelink H K, Wallich R. Recombinant outer surface protein A from Borrelia burgdorferi induces antibodies protective against spirochetal infection in mice. J Infect Dis. 1991;164:123–132. doi: 10.1093/infdis/164.1.123. [DOI] [PubMed] [Google Scholar]
- 58.Simpson W J, Cieplak W, Schrumpf M E, Barbour A G, Schwan T G. Nucleotide sequence and analysis of the gene in Borrelia burgdorferi encoding the immunogenic P39 antigen. FEMS Microbiol Lett. 1994;119:381–388. doi: 10.1111/j.1574-6968.1994.tb06917.x. [DOI] [PubMed] [Google Scholar]
- 59.Sinsky R J, Piesman J. Ear punch biopsy method for detection and isolation of Borrelia burgdorferi from rodents. J Clin Microbiol. 1989;27:1723–1727. doi: 10.1128/jcm.27.8.1723-1727.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Skare J T, Shang E S, Foley D M, Blanco D R, Champion C I, Mirzabekov T, Sokolov Y, Kagan B L, Miller J N, Lovett M A. Virulent strain associated outer membrane proteins of Borrelia burgdorferi. J Clin Invest. 1995;96:2380–2392. doi: 10.1172/JCI118295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steere A C. Borrelia burgdorferi (Lyme disease, Lyme borreliosis) In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. New York, N.Y: Churchill Livingstone; 1995. pp. 2143–2155. [Google Scholar]
- 62.Stevenson B, Schwan T G, Rosa P A. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:4535–4539. doi: 10.1128/iai.63.11.4535-4539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Hoecke C, Comberbach M, De Grave D, Desmons P, Fu D, Hauser P, Lebacq E, Lobet Y, Voet P. Evaluation of the safety, reactogenicity and immunogenicity of three recombinant outer surface protein (OspA) Lyme vaccines in healthy adults. Vaccine. 1996;14:1620–1626. doi: 10.1016/s0264-410x(96)00146-6. [DOI] [PubMed] [Google Scholar]
- 64.Wallich R, Brenner C, Kramer M D, Simon M M. Molecular cloning and immunological characterization of a novel linear-plasmid-encoded gene, pG, of Borrelia burgdorferi expressed only in vivo. Infect Immun. 1995;63:3327–3335. doi: 10.1128/iai.63.9.3327-3335.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilske B, Preac-Mursic V, Jauris S, Hofmann A, Pradel I, Soutschek E, Schwab E, Will G, Wanner G. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect Immun. 1993;61:2182–2191. doi: 10.1128/iai.61.5.2182-2191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yuen S, Hunkapiller M W, Wilson K J, Yuan P M. SDS-PAGE electroblotting. Applied Biosystems User Bull. 1986;25:1–15. [Google Scholar]