Abstract
Background
A growing number of athletes are using synthetic anabolic–androgenic steroids (AAS), comprised of testosterone and other derivatives, to enhance athletic performance and muscle mass. Over the years, numerous reports elucidated the side effects of the illegal use of AAS, such as infertility, and liver disorders. The effect of AAS on the hepatic and reproductive systems in Saudi athletes has not yet been studied. Therefore, this study examined the liver function and sex hormone parameters of AAS users as compared to non-users.
Methods
Fasting blood samples were collected from 16 male Saudi athletes, 10 AAS-users (cases) and 6 non-users (controls) to measure liver function tests (ALT, AST, GGT, ALP, total protein, albumin, direct and total bilirubin) and muscle enzymes (CK, LDH), Fertility hormones (LH, FSH, total testosterone, estradiol, and prolactin) were included also. Furthermore, a self-reported questionnaire was obtained to identify the type of AAS used, the dosage, and the length of the course before sample collection.
Results
The results show a statistically significant increase in ALT (P < 0.001), AST (P < 0.001), CK (P < 0.05), and a significant decrease (P < 0.05) in albumin (P < 0.001) and total bilirubin levels (P < 0.01) in AAS-users. Total testosterone increased significantly among AAS (P < 0.05), along with a significant decrease in LH (P < 0.01), and FSH (P < 0.001) levels, while serum prolactin and estradiol levels were significantly increased (P < 0.05).
Conclusion
AAS can enhance physical performance and appearance, its potential adverse effects on the hepatic and reproductive systems necessitate careful consideration. Our research demonstrates an increase in the liver-specific enzyme ALT in AAS users relative to non-users and the possibility that short-term AAS usage increases the risk of liver injury.
Keywords: Anabolic-androgenic steroids, AAS, Athletes, Steroids, Liver enzymes, Fertility hormones, Testosterone
1. Introduction
Testosterone was discovered in 1932 and has been used medically since the mid to early 1940 s for post-surgery or trauma (Basaria,Wahlstrom & Dobs, 2001). In World War II, Germany used synthetic derivatives of testosterone to increase the endurance and aggressiveness of soldiers (Mottram & George, 2000). During the 1950′s Olympic games, Russian athletes used performance-enhancing drugs derived from testosterone for the first time, and by the 1970 s, two-thirds of Olympic runners were using them (Loughton & Ruhling, 1977). So recreational use of AAS started to increase in the mid-1950 s among athletes, weightlifters and bodybuilders. By the 1960 s, athletes in a variety of Olympic sports were using AAS, making them more popular and their availability more widespread. Then, in the early 1980′s, AAS had become a recreational drug used by athletes to improve their muscular composition and body image. (Yesalis & Bahrke, 1995). The most utilized AAS are methyltestosterone, methenolone acetate, mibolerone, oxandrolone, stanozolol, and oxymetholone. Injectable forms of AAS are boldenone undecylenate, clostebol, drostanolone propionate, nandrolone decanoate, trenbolone, testosterone cypionate, and stanozolol (Harvey, Keen, Parrish, & van Teijlingen, 2019).
Anabolic-androgenic steroids (AAS) are derivatives of testosterone and can be described as synthetic compounds that athletes utilize to enhance athletic performance and muscle tone. (Awai, Yu, Ellis, & Schwimmer, 2014). They function by increasing the physiologic concentrations of testosterone responsible for stimulating the synthesis of proteins, increasing muscle and body mass, and enhancing strength (Kicman, 2008). Testosterone additionally leads to secondary effects such as the initiation of the maturation of male secondary sexual characteristics in the form of increased body hair, masculine voice, premature baldness, and a decrease in libido and sperm production (El Osta et al., 2016). In addition, increased bone and mineral density, erythropoiesis, hemoglobin, hematocrit, glycogen storage, lean body mass, neural transmission, and enhanced recovery between workouts as well as increased aggressiveness (O'Connor, Archer, & Wu, 2004).
AAS can have adverse impacts on the body's reproductive, liver, kidney, cardiac, and central neurological systems, among other systems (Büttner & Thieme, 2010). The type of steroid used, the amount taken, the length of use, and the administration method all affect these side effects.(Salas-Ramirez et al., 2010, Santora et al., 2006). Athletes typically utilize AAS through the simultaneous administration of several different drugs. This is administered in such a way as to increase the potency of the steroid (Nakhaee, Pakravan, & Nakhaee, 2013). The administration of AAS is performed in an alternate manner where individuals will utilize the drugs by steadily increasing the concentrations for several weeks or months. Towards the end of the cycle of usage, the drug concentration is gradually reduced to decrease the possibility of adverse side effects that are commonly observed with the prolonged long-term utilization of the drug (Neri et al., 2011). Many studies over the years have clarified the adverse effects of illicit AAS use, including liver problems and infertility. A study carried out by Dickerman et al. (1999) analyzing the serum of bodybuilders who reported excessive use of AAS demonstrated that there was an increased correlation to elevated levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT) and creatine kinase (CK). However, there were no changes in gamma-glutamyl transpeptidase (GGT) levels upon the excessive use of AAS. (Dickerman, Pertusi, Zachariah, Dufour, & McConathy, 1999). On the other hand, the use of AAS has been correlated to an increased incidence of gynecomastia in recreational and non-recreational athletes. The normal functioning of the gonads depends on a normal hypothalamic/pituitary activity regulated by the gonadotrophin-releasing hormone secreted by the hypothalamus (Christou et al., 2017). Hormones such as FSH and LH synthesized and secreted by the pituitary gland contribute as well to the normal functioning of the gonads (Christensen et al., 2012). The use of AAS by male bodybuilders has been observed to cause dysfunctions in the reproductive systems in terms of the decrease in gonadotrophin hormones through its action on the pituitary gland or its ability to suppress the release of the gonadotrophin hormone (Fronczak, Kim, & Barqawi, 2012).
There is a higher prevalence of AAS users in Western countries than in Middle Eastern countries (Alharbi et al., 2019), and the prevalence is less among females than in males (Sagoe & Pallesen, 2018). In general, the percentage of athletes in the United States that use AAS was estimated to be approximately 79.6 % (Baker, Graham, & Davies, 2006), while in the United Kingdom that number is around 69 % (Tahtamouni et al., 2008). Recently, there has been a growing number of AAS users in many countries in the Middle East (Alharbi et al., 2019). The highest percentage is estimated in Jordan at 26 % (Tahtamouni et al., 2008), followed by Kuwait and the United Arab Emirates (UAE) sharing similar percentages of approximately 22.7 % and 22 %, respectively. In Iran, around 13 % of athletes are AAS-users. While in Saudi Arabia, that number is 9.8 % (Althobiti, Alqurashi, Alotaibi, Alharthi, & Alswat, 2018). Markedly, a recent study found that the percentage of AAS users among the Jeddah, Saudi Arabia, population was about 4.7 % (Ahmed et al., 2019).
The objective of the study was to investigate the serum biochemical changes among AAS users compared with a control group of male Saudi athlete volunteers and to elucidate the adverse effect of AAS on liver functions and reproduction.
2. Materials and methods
2.1. Sample collection
The study was a case-control study that included athletes who were AAS-users (10 subjects) and non-AAS-users (6 subjects). After receiving “the-right-to-know” document and signing consent forms in agreement to volunteer for this study, self-reported data regarding AAS use, type, and dosage using a questionnaire were collected from 10 AAS users. In addition, information including age, height, and weight were taken from all subjects.
2.2. Study venue and sample preparation
The study was carried out at the phlebotomy area of King Khalid Hospital (National Guard Health Affairs (NGHA)) and samples were analyzed in the pathology department which was accredited by the College of American Pathologists (CAP). Fasting samples were collected from 10 current AAS users and from 6 non-AAS-user athletic volunteers in two 5 mL vacutainer plain tubes.
2.3. Inclusion and exclusion criteria
The inclusion criteria in this study were set to include male athletes between 18 and 50 years of age who use AAS. Those with chronic diseases such as diabetes or high blood pressure and those who use AAS for therapeutic purposes were excluded from the study, as well as hemolyzed samples. Written informed consent was obtained from all participants included in this study following standard protocols and the guideline approved by King Abdullah International Medical Research Center (KAIMRC) and IRB Approval number SP20/393/J. The control individuals recruited in this study were male athletes in the same age range and never used AAS.
All participant’s data, including age, weight and height, type of AAS (oral or injectable), and dosage, were taken from the self-reported questionnaire. BMI was calculated using the formula BMI = kg/m2. Table 1 lists the AAS name used by the subjects, type of AAS, dosages, mode of use, and weeks of use before sampling.
Table 1.
The type and dosage of AAS used by the participants.
| Participant | Type IM | IM Dosage (mg/week) | Type Oral | On AAS dosage before sampling (weeks) |
|---|---|---|---|---|
| 1 | Testosterone Propionate | 700 | NA | 8 |
| 2 | Testosterone cypionate | 700 | NA | 10 |
| 3 | Testosterone Propionate | 300 | NA | 8 |
| 4 | Testosterone Propionate | 300 | Oxandrolone | 9 |
| Methenolone Acetate | 300 | Clenbuterol | ||
| Nandrolone decanoate | 750 | |||
| 5 | Testosterone Propionate | 200 | Oxandrolone | 13 |
| Stanozolol | 300 | Clenbutoral | ||
| Trenbolone acetate | 300 | |||
| 6 | Testosterone Sustanon | 750 | Oxandrolone | 6 |
| Testosterone Enanthate | 500 | |||
| Nandrolone decanoate | 750 | |||
| Boldenone Undecylenate | 500 | |||
| 7 | Testosterone Propionate | 300 | Clenbuterol | 12 |
| 8 | Testosterone Propionate | 1000 | NA | 6 |
| 9 | Testosterone Propionate | 300 | Oxandrolone | 8 |
| 10 | Testosterone Enanthate | 600 | NA | 7 |
A fasting blood serum sample in the plain yellow top tube was collected for general chemistry and hormone analysis. The serum was separated from the clot by centrifugation at 3500 rpm for 5 min and analyzed by ARCHITECT c8000 and c1000 (Abbott Laboratories, Abbott Park, IL, USA) immediately thereafter.
3. Results
3.1. Participants data analysis
According to the frequency of AAS use and average dosage, testosterone propionate represented the majority of AAS use among the participants (41.18 %), then testosterone enanthate and nandrolone decanoate (11.76 %). The AAS-users' age (34 ± 4 years) and the control group's age (28 ± 2 years) were similar (Mean ± SD; P > 0.05). However, BMI was higher in AAS users (29 ± 2 kg/m2) compared to the control group (25 ± 4 kg/m2) (P < 0.05).
3.2. Liver transaminases
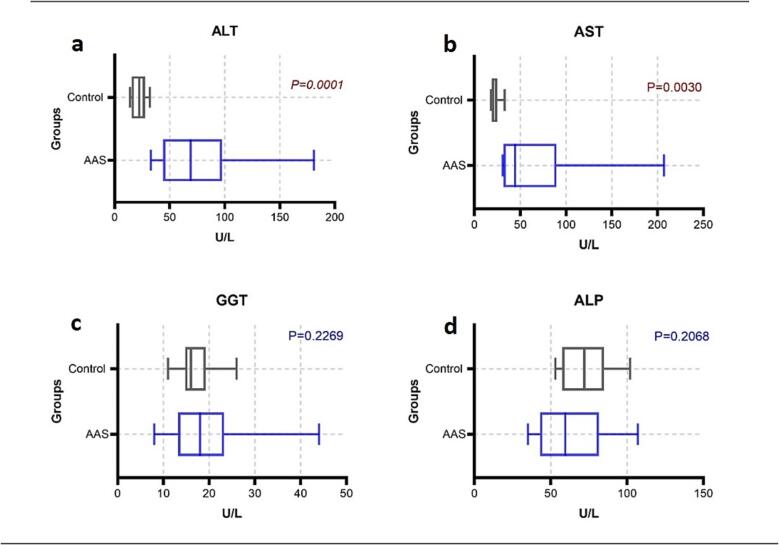
Liver enzyme level was presented with median (25th –75th percentiles), aspartate amino-transferase (AST) in AAS users was significantly higher 44.50 (31.75 – 89.50) U/L when compared to the control group 20.00 (18.17 – 24.75 U/L) (P < 0.05). Likewise, alanine amino-transferase (ALT) in the AAS-users was significantly higher 69 (44 – 97.50) U/L when compared to the control group 22.50 (15.50 – 27.50 U/L) (P < 0.05). Alkaline phosphatase (ALP) and gamma-glutamyl transferase (GGT) showed no significant difference (P > 0.05) (Fig. 1).
Fig. 1.
Comparison of liver enzymes ALT, AST, GGT and ALP (a, b, c and d, respectively) between AAS-users and control group.
3.3. Muscle enzymes
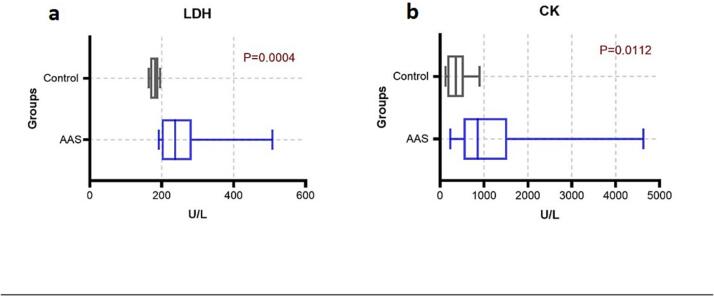
The lactate dehydrogenase (LDH) and creatine kinase (CK) levels were presented with median (25th – 75th percentiles) LDH in AAS-users of 237.5 (199.8 – 283.3) U/L, which increased significantly more than the control group 181.5 (176.8 – 190.5) U/L (P < 0.05). Also, CK in AAS-users 856 (531.5 – 1530) U/L were significantly higher than control group 362 (165 – 542.3) U/L (P < 0.05) (Fig. 2).
Fig. 2.
Comparison of muscle enzymes LDH and CK (a and b) between AAS-users and control group.
3.4. Liver function tests
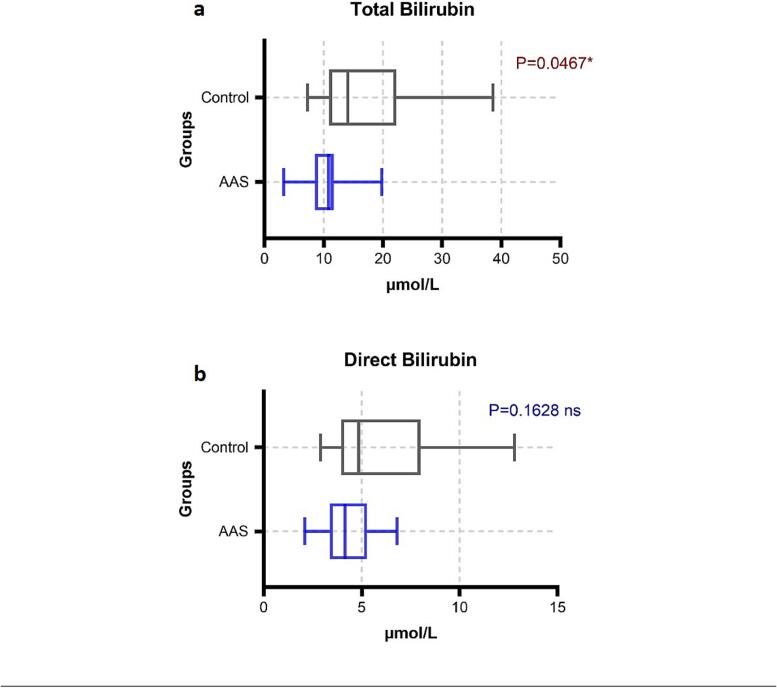
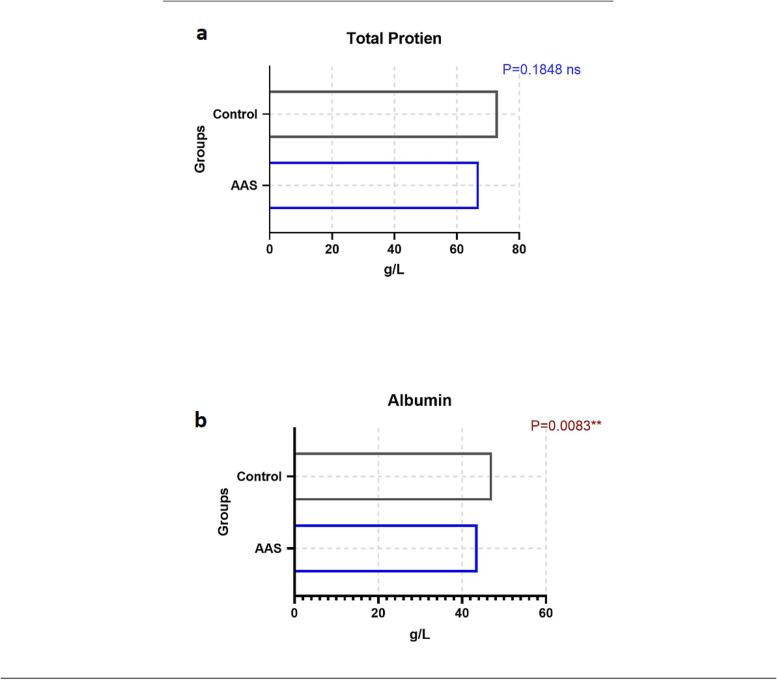
The data of total protein and albumin were parametric data a t-test for equal variance was used to determine the difference. The mean ± SD of total protein in AAS-users (67 ± 15 g/L) when compared to the control group (73 ± 1 g/L) showed no significant difference (P > 0.05). However, Albumin in AAS-users (43 ± 2 g/L) was significantly decreased in AAS-users when compared to the control group (47 ± 2 g/L) (P < 0.05). Two-tail Mann-Whitney (U test) was used to determine the groups' changes. Presented with median (25th – 75th percentiles) the total bilirubin in AAS-users was significantly lower 10.8 (8.5 – 11.68) µmol/L than the control group 14.1 (10.9 – 22.25) µmol/L, P < 0.05. While direct bilirubin has no difference between the groups (P > 0.05) (Fig. 3, Fig. 4.).
Fig. 3.
Comparison of total and direct (a and b) bilirubin between AAS-users and control group.
Fig. 4.
Comparison of total protein and albumin (a and b) between AAS-users and control group.
3.5. Reproductive hormones
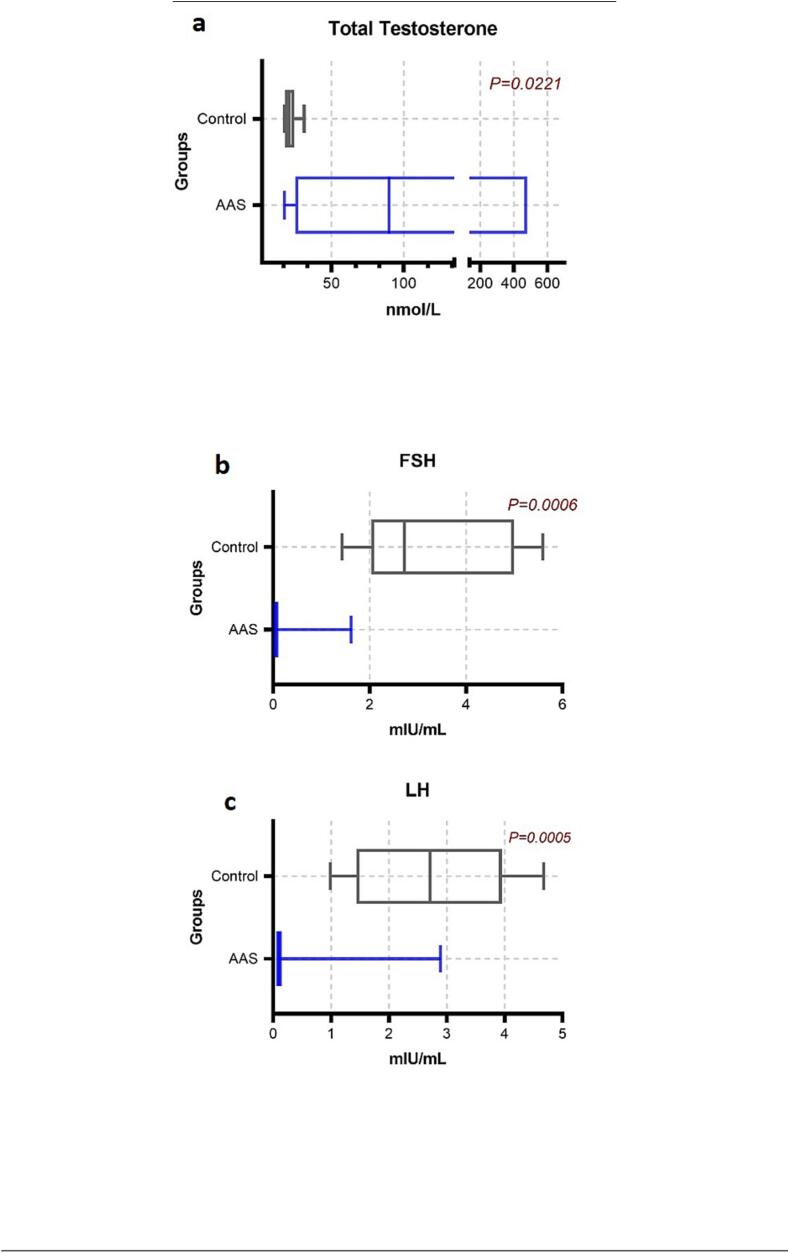
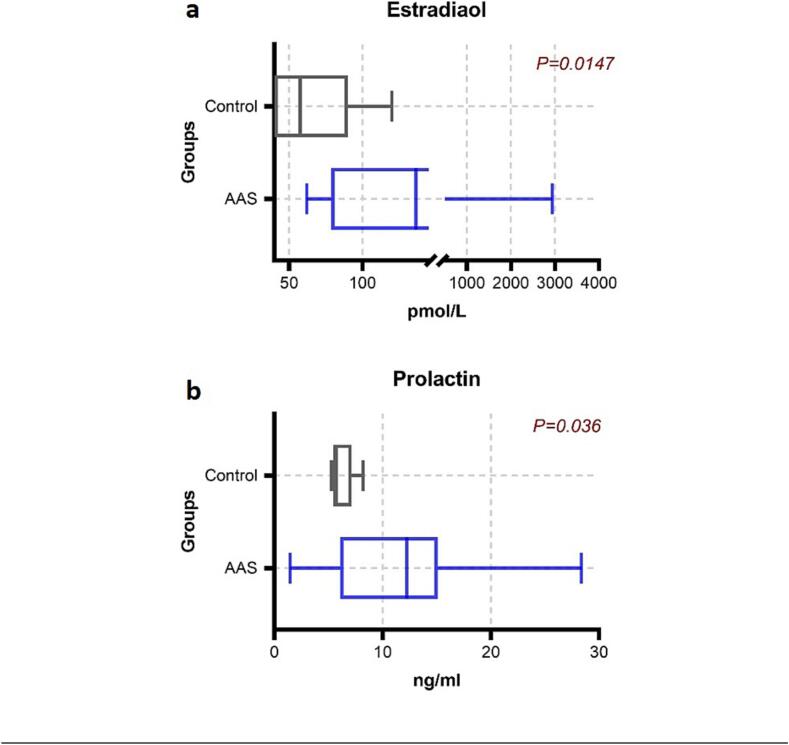
The level of hormones was not the same in the participants, and there is a variation in the dosage of AAS as mentioned in Table 1. Accordingly, the level of hormones will vary depending on their use. The level of hormones presented as median (25th – 75th percentiles) showed a highly significant decrease in luteinizing hormone (LH) in AAS-users 0.1 (0.1 – 0.97 mIU/mL), while the normal level of LH on control group 2.7 (1.44 – 3.95) mIU/mL (P < 0.05). As well as a decrease in Follicle-Stimulating Hormone (FSH) in AAS-users 0.05 (0.05 – 0.07) mIU/mL compared with the control group 2.71 (2.30 – 4.99) mIU/mL (P < 0.05). Serum prolactin was significantly increased in AAS-user 12.2 (6.07 – 15.09 ng/mL) (P < 0.05), and estradiol was significantly higher in AAS-users 136.5 (75.7 – 312 pmol/L) compared with the control group 57.5 (37 – 90 pmol/L) (P < 0.05). While significant increase in the total testosterone among AAS-users 89.9 (24.8 – 135) nmol/L and control group 20.5 (17.8 – 24.3) nmol/L (P < 0.05). (Fig. 5, Fig. 6.).
Fig. 5.
Comparison of testosterone, FSH and LH (a, b and c) between AAS-users and control group.
Fig. 6.
Comparison of estradiol and prolactin (a and b) between AAS-users and control group.
4. Discussion
The increased risk of liver problems related to AAS usage in athletes is one of the main areas of concern. A temporary increase in blood enzyme levels is a form of hepatic damage. (Bergasa, 2022). Elevation of ALT, AST, ALP, LDH, and GGT reflects hepatocellular injury or partially increases the permeability of the hepatocyte’s membrane (Dickerman et al., 1999, Ozer et al., 2008). Since AST is found in the heart, skeletal muscle, kidneys, brain, and red blood cells, and since ALT is found in low concentrations in skeletal muscle and kidney (Wróblewski, 1958), physicians frequently underestimate the role of muscle injury in elevated enzyme activity, leading to overestimation of anabolic steroid-induced hepatotoxicity based on transit liver transaminase elevations (Pertusi, Dickerman, & McConathy, 2001). Moreover, Dickerman et al. (1999) reported that elevated levels of AST, ALT and CK but not GGT in AAS-users and summarized that liver damage due to anabolic steroids cannot be confirmed along with regular heavy exercise (Pertusi et al., 2001). Weightlifting alone can cause a slight increase in serum transaminase activity, mild liver damage, muscular injury, or both (Dickerman et al., 1999, Pertusi et al., 2001). However, hepatocellular damage is evaluated with an increase in both transaminases AST and ALT as reported (Stępień et al., 2015, Štimac et al., 2002) Here, the examination of these parameters was one of the objectives in this study to evaluate the integrity of the liver in athletes who were AAS users compared with non-AAS users.
Although ALT levels are typically increased with the early onset of liver toxicity and injury (Giannini, Testa, & Savarino, 2005), hepatotoxicity is also related to time and dosage level of AAS (Solimini et al., 2017). Here, our results show significantly higher levels of ALT and AST in AAS-users than the control group. Our results are also in agreement with a more recent study that showed an increase of ALT and AST in AAS users and inferred a degree of hepatotoxicity (Fett, Maruyama, Brandao, & Fett, 2018). Although our study focused on current or short-term use among athletes, many studies have shown that long term use and high dosage over years may increase the risk of nodular transformation and growth of hepatic tumors. (Velazquez & Alter, 2004). The continuous use of AAS is correlated to the increase in serum levels of liver enzymes due to AAS metabolites, such as danazol and oxymetholone and can cause liver toxicity (Niedfeldt, 2018). Urhausen et al. in 2003 reported that liver transaminase levels in current AAS users were considerably greater than those athletes who had ceased using AAS for at least one year (Urhausen, Torsten, & Wilfried, 2003).
Even though CK and LDH are elevated in muscle injury, these enzymes increased above the normal level in both groups showing a significant difference between their mean serum levels. Interestingly, other studies reported that there are no differences in these enzymes amongst users in a study by Schwingel (Schwingel et al., 2011) as well as a study on endurance of athletes as AAS users and non-AAS users (Baume et al., 2006). Nonetheless, the AAS-associated elevation of LDH and CK was clear in our study, therefore we suggest further investigation to explain if this elevation may be associated with cardiovascular side effects.
ALP level was not statistically different among the two groups similar to previously published work (O'Sullivan et al., 2000). The mechanism of cholestasis caused by C-17 substituted androgens is unknown, however, serum levels of hepatic enzymes are observed to be at the normal range or slightly increased in cases of peliosis hepatis.(Neri et al., 2011) Since the cause of the two conditions cannot be explained, other methods such as liver biopsy and radiological examination are needed (Nakao et al., 2000). Nonetheless, large dosages have been shown to produce cholestasis in animal models (Rodríguez-Garay, 2003). In addition, for AAS to exert its effects, it binds to the androgen receptor in target organs. Then, it is converted to 5-dihydrotestosterone, which also binds to androgen receptor, to exert estrogenic effects. Specifically, testosterone is aromatized to become estradiol leading to an increase in estradiol level as seen in our AAS group. Thus, the condition of cholestasis is comparable to pregnancy cholestasis and the jaundice associated with high estradiol or birth control pill dosages, and it might be caused by a partial absence or variation of bile salt transporter proteins, as seen in certain individuals with androgenic anabolic steroid-related cholestasis (Pauli-Magnus, Meier, & Stieger, 2010). Expectedly, total bilirubin levels in AAS users were shown to be significantly lower than in the control group (P < 0.05), resulting in bile stagnation and suggesting why the total bilirubin level decreased. However, since AAS users are asymptomatic for liver damage, it was expected to show normal levels of direct bilirubin as previously shown in a study that analyzed the correlation between the onset of hepatotoxicity and the illicit use of AAS in bodybuilders (Robles-Diaz et al., 2015). The most widely reported clinical symptoms among bodybuilders in the previous study were hepatocellular injury, jaundice, and increased levels of bilirubin(Robles‐Diaz et al., 2015); these findings contradict the findings in our study. One possible explanation of this discrepancy is that our sample was collected during AAS dosing whereas in the previous studies, samples were collected after AAS cessation.
Normally, 53–55 % of testosterone is bound to serum albumin, 43–45 % is bound to sex hormone binding globulin, and the remaining is free in the blood (Södergard, Bäckström, Shanbhag, & Carstensen, 1982). Here, we measured serum albumin and total protein and found that albumin is drastically reduced (P < 0.01), while total protein remains within the normal range among AAS-users. Elevation of testosterone decreased serum albumin in our study is consistent with the previous study outcomes (Fett et al., 2018). It is also likely that using AAS at a certain phase of the cycle changes endogenous testosterone and albumin synthesis, according to a systematic review (Handelsman, 2020).
In our study, only male participants were tested and selected due to the limited number of female users in our population. However, the effect of AAS on the reproductive system in males, including hypogonadism, testicular atrophy, azoospermia, oligozoospermia, erectile dysfunction, and loss of libido(Pope et al., 2000, Schmidt et al., 2004) was frequently observed in previous studies.(Andrews, Magee, Combest, Allard, & Douglas, 2018). Most studies carried out to determine the impact of AAS on the levels of reproductive hormones in both males and female athletes demonstrate a reduction in LH and FSH levels and both are correlated to dysfunctions observed in the reproductive system. Our findings in AAS users were below the reference range with a significant decrease in LH and FSH after 8 weeks of using different types of AAS when compared to the control group. Since the test subjects are current users and samples were collected during their AAS dosage course, the amount of total testosterone increased up to 89.9 nmol/L due to the exogenous administration. As a resulting negative feedback mechanism to counter AAS use, the anterior pituitary gland reduces the production of LH and FSH. Likewise, AAS has a suppressive effect on the testicular and adrenal androgen production (Sheriff, 1984).
Our findings of high serum prolactin in AAS users as compared to the control group are consistent with the conclusion that testosterone and its metabolites enhance prolactin release. Estradiol has been shown to stimulate prolactin release from the anterior pituitary, and non-aromatize AAS-like stanozolol and methandrostenolone have been shown to activate estrogen receptors via interactions between the parent compound and its metabolites, suggesting a possible mechanism for increased prolactin. However, a case-control study to evaluate the effect of former abuse of AAS on testosterone levels and the symptoms of hypogonadism several years after cessation of the administration of steroids was performed by Rasmussen et al. (2016). Testosterone levels and the symptoms of hypogonadism were assessed in 33 former AAS abusers compared to 30 healthy volunteers.(Jon Jarløv Rasmussen et al., 2016) The levels of reproductive hormones LH, FSH, and testosterone were measured in all subjects to ascertain the effect of AAS on the natural synthesis and secretion of these hormones. Total free testosterone was significantly lower in AAS abusers compared to the control group, and levels of gonadotrophin were significantly reduced compared to the control group (J. J. Rasmussen et al., 2016). The study provides solid evidence of the changes that take place after years of chronic use. In addition, Torres-Calleja et al. (2001) conducted a case-control study to assess the effect of AAS on sperm and serum reproductive hormone levels in 30 male bodybuilders. Serum FSH and prolactin were significantly lower in the group using AAS compared to the control group. There were no significant changes in the serum levels of estradiol and testosterone. In contrast, our finding showed significant increases in both estradiol and prolactin. While the use of current testosterone showed an increase in testosterone concentration, this can be explained by the current administration of AAS. The small size of participants in this study limits our ability to reflect whether AAS played an etiologic role in all the AAS users reported.
5. Conclusion
Anabolic-androgenic steroids (AAS) remain a controversial topic in sports, particularly in Saudi Arabia, where their use among athletes is a growing concern. While AAS can enhance physical performance and appearance, its potential adverse effects on the hepatic and reproductive systems necessitate careful consideration. Our research demonstrates an increase in the liver-specific enzyme ALT in AAS users relative to non-users and the possibility that short-term AAS usage increases the risk of liver injury. However, further studies of liver function tests such as prothrombin time, ultrasound, and radiological examination are recommended, together with sperm analysis to evaluate spermatogenesis. Urine analysis, blood samples, and sperm analysis after 3 months of course cessations. Finally, the Ministry of Health in Saudi Arabia should enforce strict regulations and improve understanding and awareness of the potential health risks among recreational AAS users and bodybuilders.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmed M.H., Al-Saud N.S., Omar A.M., Magadmi R.M., Hassan S.M., Al-Qudsi F.M. Knowledge of and attitudes toward the use of anabolic-androgenic steroids among the population of Jeddah, Saudi Arabia. J. Microsc. Ultrastruct. 2019;7(2):78–83. doi: 10.4103/JMAU.JMAU_64_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alharbi F.F., Gamaleddin I., Alharbi S.F., Almodayfer O., Allohidan F., Alghobain M., Al-Surimi K. Knowledge, attitudes and use of anabolic-androgenic steroids among male gym users: A community based survey in Riyadh, Saudi Arabia. Saudi Pharm. J. 2019;27(2):254–263. doi: 10.1016/j.jsps.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althobiti S.D., Alqurashi N.M., Alotaibi A.S., Alharthi T.F., Alswat K.A. Prevalence, attitude, knowledge, and practice of Anabolic Androgenic Steroid (AAS) use among gym participants. Mater. Sociomed. 2018;30(1):49–52. doi: 10.5455/msm.2018.30.49-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews M.A., Magee C.D., Combest T.M., Allard R.J., Douglas K.M. Physical effects of anabolic-androgenic steroids in healthy exercising adults: A systematic review and meta-analysis. Curr. Sports Med. Rep. 2018;17(7):232–241. doi: 10.1249/jsr.0000000000000500. [DOI] [PubMed] [Google Scholar]
- Awai H.I., Yu E.L., Ellis L.S., Schwimmer J.B. Liver toxicity of anabolic androgenic steroid use in an adolescent with nonalcoholic fatty liver disease. J. Pediatr. Gastroenterol. Nutr. 2014;59(3):e32–e33. doi: 10.1097/MPG.0b013e3182952e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J.S., Graham M.R., Davies B. Steroid and prescription medicine abuse in the health and fitness community: A regional study. Eur. J. Intern. Med. 2006;17(7):479–484. doi: 10.1016/j.ejim.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Baume N., Schumacher Y.O., Sottas P.E., Bagutti C., Cauderay M., Mangin P., Saugy M. Effect of multiple oral doses of androgenic anabolic steroids on endurance performance and serum indices of physical stress in healthy male subjects. Eur. J. Appl. Physiol. 2006;98(4):329–340. doi: 10.1007/s00421-006-0271-0. [DOI] [PubMed] [Google Scholar]
- Bergasa N.V. Drug induced liver injury. Clin. Cases Hepatol. 2022:411–442. [Google Scholar]
- Büttner A., Thieme D. Side effects of anabolic androgenic steroids: pathological findings and structure–activity relationships. Doping Sports: Biochem. Principles Eff. Anal. 2010:459–484. doi: 10.1007/978-3-540-79088-4_19. [DOI] [PubMed] [Google Scholar]
- Christensen A., Bentley G.E., Cabrera R., Ortega H.H., Perfito N., Wu T.J., Micevych P. Hormonal regulation of female reproduction. Horm. Metab. Res. 2012;44(8):587–591. doi: 10.1055/s-0032-1306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou M.A., Christou P.A., Markozannes G., Tsatsoulis A., Mastorakos G., Tigas S. Effects of anabolic androgenic steroids on the reproductive system of athletes and recreational users: A systematic review and meta-analysis. Sports Med. 2017;47(9):1869–1883. doi: 10.1007/s40279-017-0709-z. [DOI] [PubMed] [Google Scholar]
- Dickerman R.D., Pertusi R.M., Zachariah N.Y., Dufour D.R., McConathy W.J. Anabolic steroid-induced hepatotoxicity: is it overstated? Clin. J. Sport Med. 1999;9(1):34–39. doi: 10.1097/00042752-199901000-00007. [DOI] [PubMed] [Google Scholar]
- El Osta R., Almont T., Diligent C., Hubert N., Eschwège P., Hubert J. Anabolic steroids abuse and male infertility. Basic Clin. Androl. 2016;26(1):2. doi: 10.1186/s12610-016-0029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett, W. C. R., Maruyama, M., Brandao, C., & Fett, W. (2018). Blood Biochemical Markers of Competitive Bodybuilding Athletes Users of Anabolic Androgenic Steroids and/or Growth Hormone (AAS/GH), Strength Athletes Drugs Free and Sedentary Persons. Strength Athletes Drugs Free and Sedentary Persons.
- Fronczak C.M., Kim E.D., Barqawi A.B. The insults of illicit drug use on male fertility. J. Androl. 2012;33(4):515–528. doi: 10.2164/jandrol.110.011874. [DOI] [PubMed] [Google Scholar]
- Giannini E.G., Testa R., Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ : Canad. Med. Assoc. J. = J. Assoc. Med. Canad. 2005;172(3):367–379. doi: 10.1503/cmaj.1040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handelsman, D. J. (2020). Androgen Physiology, Pharmacology, Use and Misuse. In Endotext [Internet]: MDText. com, Inc.
- Kicman A.T. Pharmacology of anabolic steroids. Br. J. Pharmacol. 2008;154(3):502–521. doi: 10.1038/bjp.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao A., Sakagami K., Nakata Y., Komazawa K., Amimoto T., Nakashima K., Tanaka N. Multiple hepatic adenomas caused by long-term administration of androgenic steroids for aplastic anemia in association with familial adenomatous polyposis. J. Gastroenterol. 2000;35(7):557–562. doi: 10.1007/s005350070081. [DOI] [PubMed] [Google Scholar]
- Neri M., Bello S., Bonsignore A., Cantatore S., Riezzo I., Turillazzi E., Fineschi V. Anabolic androgenic steroids abuse and liver toxicity. Mini Rev. Med. Chem. 2011;11(5):430–437. doi: 10.2174/138955711795445916. [DOI] [PubMed] [Google Scholar]
- Niedfeldt M.W. Anabolic steroid effect on the liver. Curr. Sports Med. Rep. 2018;17(3):97–102. doi: 10.1249/JSR.0000000000000467. [DOI] [PubMed] [Google Scholar]
- O'Connor D.B., Archer J., Wu F.C. Effects of testosterone on mood, aggression, and sexual behavior in young men: a double-blind, placebo-controlled, cross-over study. J. Clin. Endocrinol. Metab. 2004;89(6):2837–2845. doi: 10.1210/jc.2003-031354. [DOI] [PubMed] [Google Scholar]
- O'Sullivan A.J., Kennedy M.C., Casey J.H., Day R.O., Corrigan B., Wodak A.D. Anabolic-androgenic steroids: medical assessment of present, past and potential users. Med. J. Aust. 2000;173(6):323–327. doi: 10.5694/j.1326-5377.2000.tb125667.x. [DOI] [PubMed] [Google Scholar]
- Ozer J., Ratner M., Shaw M., Bailey W., Schomaker S. The current state of serum biomarkers of hepatotoxicity. Toxicology. 2008;245(3):194–205. doi: 10.1016/j.tox.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Pauli-Magnus, C., Meier, P. J., & Stieger, B. (2010). Genetic determinants of drug-induced cholestasis and intrahepatic cholestasis of pregnancy. Paper presented at the Semin Liver Dis. [DOI] [PubMed]
- Pertusi R., Dickerman R., McConathy W. Evaluation of aminotransferase elevations in a bodybuilder using anabolic steroids: hepatitis or rhabdomyolysis? J. Osteop. Med. 2001;101(7):391–394. [PubMed] [Google Scholar]
- Pope H.G., Kouri E.M., Hudson J.I. Effects of supraphysiologic doses of testosterone on mood and aggression in normal men: a randomized controlled trial. Arch. Gen. Psychiatry. 2000;57(2):133–140. doi: 10.1001/archpsyc.57.2.133. [DOI] [PubMed] [Google Scholar]
- Rasmussen J.J., Selmer C., Ostergren P.B., Pedersen K.B., Schou M., Gustafsson F., Kistorp C. Former abusers of anabolic androgenic steroids exhibit decreased testosterone levels and hypogonadal symptoms years after cessation: A case-control study. PLoS One. 2016;11(8):e0161208. doi: 10.1371/journal.pone.0161208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen J.J., Selmer C., Østergren P.B., Pedersen K.B., Schou M., Gustafsson F., Kistorp C. Former abusers of anabolic androgenic steroids exhibit decreased testosterone levels and hypogonadal symptoms years after cessation: A case-control study. PLoS One. 2016;11(8):e0161208–e. doi: 10.1371/journal.pone.0161208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles-Diaz M., Gonzalez-Jimenez A., Medina-Caliz I., Stephens C., Garcia-Cortes M., Garcia-Munoz B., Network S.L. Distinct phenotype of hepatotoxicity associated with illicit use of anabolic androgenic steroids. Aliment. Pharmacol. Ther. 2015;41(1):116–125. doi: 10.1111/apt.13023. [DOI] [PubMed] [Google Scholar]
- Robles-Diaz M., Gonzalez-Jimenez A., Medina-Caliz I., Stephens C., García-Cortes M., García-Muñoz B., Jimenez-Perez M. Distinct phenotype of hepatotoxicity associated with illicit use of anabolic androgenic steroids. Aliment. Pharmacol. Ther. 2015;41(1):116–125. doi: 10.1111/apt.13023. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Garay E.A. Cholestasis: human disease and experimental animal models. Ann. Hepatol. 2003;2(4):150–158. [PubMed] [Google Scholar]
- Sagoe D., Pallesen S. Androgen abuse epidemiology. Curr. Opin. Endocrinol. Diabetes Obes. 2018;25(3):185–194. doi: 10.1097/MED.0000000000000403. [DOI] [PubMed] [Google Scholar]
- Salas-Ramirez K.Y., Montalto P.R., Sisk C.L. Anabolic steroids have long-lasting effects on male social behaviors. Behav. Brain Res. 2010;208(2):328–335. doi: 10.1016/j.bbr.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santora L.J., Marin J., Vangrow J., Minegar C., Robinson M., Mora J., Frieds G. Coronary calcification in body builders using anabolic steroids. Prev. Cardiol. 2006;9(4):198–201. doi: 10.1111/j.1559-4564.2006.05210.x. [DOI] [PubMed] [Google Scholar]
- Schmidt P.J., Berlin K.L., Danaceau M.A., Neeren A., Haq N.A., Roca C.A., Rubinow D.R. The effects of pharmacologically induced hypogonadism on mood in HealthyMen. Arch. Gen. Psychiatry. 2004;61(10):997–1004. doi: 10.1001/archpsyc.61.10.997. [DOI] [PubMed] [Google Scholar]
- Schwingel, P. A., Cotrim, H. P., Salles, B. R., Almeida, C. E., dos Santos, C. R., Jr., Nachef, B., Zoppi, C. C. (2011). Anabolic-androgenic steroids: a possible new risk factor of toxicant-associated fatty liver disease. Liver Int, 31(3), 348-353. doi:10.1111/j.1478-3231.2010.02346.x. [DOI] [PubMed]
- Sheriff D.S. The Male Factor in Human Infertility Diagnosis and Treatment. Springer; 1984. Hyperprolactinaemia and abnormal seminal cytology; pp. 73–77. [Google Scholar]
- Södergard R., Bäckström T., Shanbhag V., Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17β to human plasma proteins at body temperature. J. Steroid Biochem. 1982;16(6):801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Solimini R., Rotolo M., Mastrobattista L., Mortali C., Minutillo A., Pichini S., Palmi I. Hepatotoxicity associated with illicit use of anabolic androgenic steroids in doping. Eur. Rev. Med. Pharmacol. Sci. 2017;21(1 Suppl):7–16. [PubMed] [Google Scholar]
- Stępień P.M., Reczko K., Wieczorek A., Zarębska-Michaluk D., Pabjan P., Król T., Kryczka W. Severe intrahepatic cholestasis and liver failure after stanozolol usage–case report and review of the literature. Clin. Exp. Hepatol. 2015;1(1):30–33. doi: 10.5114/ceh.2015.51376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Štimac D., Milic S., Dintinjana R.D., Kovac D., Ristic S. Androgenic/Anabolic steroid-induced toxic hepatitis. J. Clin. Gastroenterol. 2002;35(4):350–352. doi: 10.1097/00004836-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Tahtamouni L.H., Mustafa N.H., Alfaouri A.A., Hassan I.M., Abdalla M.Y., Yasin S.R. Prevalence and risk factors for anabolic-androgenic steroid abuse among Jordanian collegiate students and athletes. Eur. J. Public Health. 2008;18(6):661–665. doi: 10.1093/eurpub/ckn062. [DOI] [PubMed] [Google Scholar]
- Urhausen A., Torsten A., Wilfried K. Reversibility of the effects on blood cells, lipids, liver function and hormones in former anabolic–androgenic steroid abusers. J. Steroid Biochem. Mol. Biol. 2003;84(2–3):369–375. doi: 10.1016/s0960-0760(03)00105-5. [DOI] [PubMed] [Google Scholar]
- Velazquez I., Alter B.P. Androgens and liver tumors: Fanconi's anemia and non-Fanconi's conditions. Am. J. Hematol. 2004;77(3):257–267. doi: 10.1002/ajh.20183. [DOI] [PubMed] [Google Scholar]
- Wróblewski F. The clinical significance of alterations in transaminase activities of serum and other body fluids. Adv. Clin. Chem. 1958;1:313–351. [PubMed] [Google Scholar]